Merck Says Verquvo Granted Marketing Authorization in European Union
21 Julio 2021 - 7:15AM
Noticias Dow Jones
By Michael Dabaie
Merck & Co. Inc. said the European Commission granted
marketing authorization for Verquvo to Bayer AG.
In the EU, Verquvo is indicated for the treatment of symptomatic
chronic heart failure. Verquvo is being jointly developed by Merck
and Bayer. Merck has the commercial rights in the U.S. and Bayer
has the exclusive commercial rights in the rest of world.
The U.S. Food and Drug Administration in January approved
Verquvo to reduce the risk of cardiovascular death and heart
failure hospitalization.
Write to Michael Dabaie at michael.dabaie@wsj.com
(END) Dow Jones Newswires
July 21, 2021 08:10 ET (12:10 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
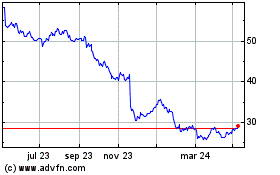
Bayer (TG:BAYN)
Gráfica de Acción Histórica
De Mar 2024 a Abr 2024
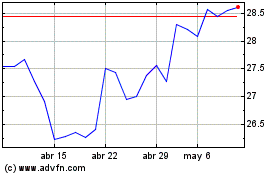
Bayer (TG:BAYN)
Gráfica de Acción Histórica
De Abr 2023 a Abr 2024