Shield Therapeutics PLC KOL Investor Event on Iron Deficiency
04 Febrero 2022 - 1:00AM
RNS Non-Regulatory
TIDMSTX
Shield Therapeutics PLC
04 February 2022
Shield Therapeutics plc
("Shield" or the "Company" or the "Group")
KOL Investor Event on Iron Deficiency
Innovation in Iron Deficiency Treatment with a Focus on Clinical
Utility of Accrufer(R)/Feraccru(R)
Webinar on Thursday 10 February at 10am EST / 3pm GMT
LONDON, UK - 4 February 2022 - Shield Therapeutics plc (LSE:
STX), a commercial stage specialty pharmaceutical company with a
focus on addressing iron deficiency with its lead product
Accrufer(R)(ferric maltol), announces it will host a Key Opinion
Leader ("KOL") event for Investors on "Iron Deficiency Treatment
Innovation" with a focus on clinical utility of
Accrufer(R)/Feraccru(R) on Thursday 10 February 2022 at 10am (EST)
/ 3pm (GMT).
The live webinar will feature presentations from:
-- Dr. Lee P. Shulman MD FACMG FACOG (Professor of Obstetrics
and Gynecology at the Feinberg School of Medicine at Northwestern
University in Chicago, Illinois), who will discuss the current
treatment landscape and unmet medical need in treating patients
with iron deficiency.
-- Dr. Carsten Schmidt, MA, FEBGH (Director of Medical Clinic II - Gastroenterology, Hepatology, Endocrinology, Diabetology and Infectious Diseases - Fulda Hospital, Marburg University Medicine), who will discuss Shield Therapeutics' potential treatment solution Accrufer(R), a stable non-salt based oral therapy for adults with iron deficiency with or without anaemia.
Additionally, members of the Shield senior management team will
also be in attendance.
Registration for this event is available through LifeSci Events
. A live video webcast will be available in the "Events" section of
the Shield corporate website. An archived version of the event will
be available on the website for 60 days.
For speaker biographies please visit the website here:
https://www.shieldtherapeutics.com/events/
For further information please contact:
Shield Therapeutics plc www.shieldtherapeutics.com
Greg Madison, CEO +44 (0) 191 511 8500
Hans-Peter Rudolf, CFO
Nominated Adviser and Joint
Broker
Peel Hunt LLP
James Steel/Christopher Golden +44 (0)20 7418 8900
Joint Broker
finnCap Ltd
Geoff Nash/Alice Lane/George
Dollemore +44 (0)20 7220 0500
Financial PR & IR Advisor
Walbrook PR (UK Advisor)
Paul McManus/Lianne Applegarth/Alice +44 (0)20 7933 8780 or shield@walbrookpr.com
Woodings
Investor Contact (US Advisor)
LifeSci Advisors, LLC
John Mullaly +1 617 429 3548 or jmullaly@lifesciadvisors.com
About Accrufer(R)/Feraccru(R)
Shield's lead product Accrufer(R)/Feraccru(R), has been approved
for use in the United States, European Union, UK, Switzerland and
Australia and has exclusive IP rights until the mid-2030s. The
Group has recently launched Accrufer(R) in the US and Feraccru(R)
is already being commercialized in the UK and European Union by
Norgine B.V., who also have the marketing rights in Australia and
New Zealand. Shield also has an exclusive license agreement with
Beijing Aosaikang Pharmaceutical Co., Ltd., for the development and
commercialization of Accrufer(R) / Feraccru (R) in China, Hong
Kong, Macau and Taiwan, with Korea Pharma Co., Ltd. in the Republic
of Korea, and with KYE Pharmaceuticals Inc. in Canada.
About Shield
Shield is a commercial stage, pharmaceutical company with a
focus on addressing iron deficiency with its lead product
Accrufer(R)/Feraccru(R) (ferric maltol), a novel, stable, non-salt
based oral therapy for adults with iron deficiency with or without
anaemia.
For more information, please visit www.shieldtherapeutics.com or
follow Shield on Twitter @ShieldTx.
This information is provided by Reach, the non-regulatory press
release distribution service of RNS, part of the London Stock
Exchange. Terms and conditions relating to the use and distribution
of this information may apply. For further information, please
contact rns@lseg.com or visit www.rns.com.
Reach is a non-regulatory news service. By using this service an
issuer is confirming that the information contained within this
announcement is of a non-regulatory nature. Reach announcements are
identified with an orange label and the word "Reach" in the source
column of the News Explorer pages of London Stock Exchange's
website so that they are distinguished from the RNS UK regulatory
service. Other vendors subscribing for Reach press releases may use
a different method to distinguish Reach announcements from UK
regulatory news.
RNS may use your IP address to confirm compliance with the terms
and conditions, to analyse how you engage with the information
contained in this communication, and to share such analysis on an
anonymised basis with others as part of our commercial services.
For further information about how RNS and the London Stock Exchange
use the personal data you provide us, please see our Privacy
Policy.
END
NRABLGDDXUGDGDX
(END) Dow Jones Newswires
February 04, 2022 02:00 ET (07:00 GMT)
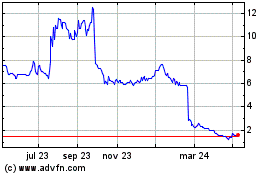
Shield Therapeutics (LSE:STX)
Gráfica de Acción Histórica
De Mar 2024 a Abr 2024
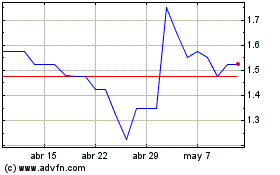
Shield Therapeutics (LSE:STX)
Gráfica de Acción Histórica
De Abr 2023 a Abr 2024