Amazon Among Companies Issued FDA Warning Letter Over Unapproved Products
09 Agosto 2022 - 10:37AM
Noticias Dow Jones
By Will Feuer
The U.S. Food and Drug Administration said it has issued warning
letters to three companies, including Amazon.com Inc., for
allegedly facilitating the sale of mole and skin tag removal
products that aren't approved.
The FDA said it also sent warning letters to Ariella Naturals
and Justified Laboratories. The agency posted copies of the letters
online.
In one letter, dated Aug. 4 and addressed to Amazon Chief
Executive Andy Jassy, an FDA official said Amazon has an
opportunity to address the agency's concerns, but failure to do so
"may result in legal action."
Representatives for Amazon didn't immediately return a request
for comment.
"We will continue to work diligently to ensure that online
retailers do not sell products that violate federal law," said
Donald Ashley, director of the Office of Compliance in the FDA's
Center for Drug Evaluation and Research.
Write to Will Feuer at Will.Feuer@wsj.com
(END) Dow Jones Newswires
August 09, 2022 11:22 ET (15:22 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
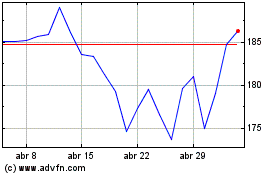
Amazon.com (NASDAQ:AMZN)
Gráfica de Acción Histórica
De Mar 2024 a Abr 2024
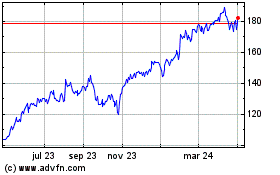
Amazon.com (NASDAQ:AMZN)
Gráfica de Acción Histórica
De Abr 2023 a Abr 2024