CytoSorbents to Present at the H.C. Wainwright 26ᵗʰ Annual Global Investment Conference
27 Agosto 2024 - 6:00AM

CytoSorbents Corporation (NASDAQ: CTSO), a leader in the treatment
of life-threatening conditions in the intensive care unit and
cardiac surgery using blood purification via its proprietary
polymer adsorption technology, today announced that the Dr. Phillip
Chan, Chief Executive Officer, and Peter J. Mariani, Chief
Financial Officer will be attending and presenting at the H.C.
Wainwright 26th Annual Global Investment Conference being held
September 9-11, 2024 at the Lotte New York Palace Hotel in New
York, NY.
A company presentation will be available on
demand here as of Monday, September 9 at 7:00 AM
ET. CytoSorbents’ management team will be available for one-on-one
meetings Monday, September 9, 2024. Interested investors should
contact their representative at H.C. Wainwright. A replay of the
presentation will be posted, when available, to CytoSorbents’s
website on the Events & Presentation page of
the investors section for 90 days.
About CytoSorbents Corporation (NASDAQ:
CTSO)
CytoSorbents Corporation is a leader in the
treatment of life-threatening conditions in the intensive care unit
and in cardiac surgery through blood purification. Its lead
product, CytoSorb®, is approved in the European
Union and distributed in 76 countries worldwide. It is an
extracorporeal cytokine adsorber that reduces “cytokine storm” or
“cytokine release syndrome” in common critical illnesses that can
lead to massive inflammation, organ failure and patient death. In
these diseases, the risk of death can be extremely high, and there
are few, if any, effective treatments. CytoSorb is also used during
and after cardiothoracic surgery to remove antithrombotic drugs and
inflammatory mediators that can lead to postoperative
complications, including severe bleeding and multiple organ
failure. As of June 30, 2024, more than 248,000 CytoSorb
devices have been used cumulatively. CytoSorb was originally
launched in the European Union under CE mark as the first
cytokine adsorber. Additional CE mark extensions were granted for
bilirubin and myoglobin removal in clinical conditions such as
liver disease and trauma, respectively, and
for ticagrelor and rivaroxaban removal in
cardiothoracic surgery procedures. CytoSorb has also
received FDA Emergency Use Authorization in the
United States for use in adult critically ill COVID-19
patients with impending or confirmed respiratory failure. CytoSorb
is not yet approved in the United States.
The DrugSorb™-ATR antithrombotic removal system,
an investigational device based on the same polymer technology as
CytoSorb, has received two FDA Breakthrough Device
Designations, one for the removal of ticagrelor and
another for the removal of the direct oral anticoagulants
(DOAC) apixaban and rivaroxaban in a cardiopulmonary bypass
circuit during urgent cardiothoracic procedures. The Company has
completed the FDA-approved, randomized, controlled STAR-T (Safe and
Timely Antithrombotic Removal-Ticagrelor) study of 140 patients at
approximately 30 centers in U.S. and Canada to
evaluate whether intraoperative use of DrugSorb-ATR can reduce the
perioperative risk of bleeding in patients receiving ticagrelor and
undergoing cardiothoracic surgery. This pivotal study is intended
to support U.S. FDA and Health Canada marketing
approval for DrugSorb-ATR in this application.
CytoSorbents’ purification technologies are
based on biocompatible, highly porous polymer beads that can
actively remove toxic substances from blood and other bodily fluids
by pore capture and surface adsorption. Its technologies have
received non-dilutive grant, contract, and other funding of
approximately $50 million from DARPA,
the U.S. Department of Health and Human Services (HHS),
the National Institutes of Health (NIH), National Heart,
Lung, and Blood Institute (NHLBI), the U.S. Army,
the U.S. Air Force, U.S. Special Operations Command
(SOCOM), Air Force Material Command (USAF/AFMC), and others. The
Company has numerous marketed products and products under
development based upon this unique blood purification technology
protected by many issued U.S. and international patents
and registered trademarks, and multiple patent applications
pending, including ECOS-300CY®, CytoSorb-XL™, HemoDefend-RBC™,
HemoDefend-BGA™, VetResQ®, K+ontrol™, DrugSorb™, ContrastSorb, and
others. For more information, please visit the Company’s websites
at www.cytosorbents.com and www.cytosorb.com or
follow us on Facebook and X.
Please Click to Follow Us
on Facebook and X
Company Contact:Peter J. Mariani, Chief
Financial Officer305 College Road EastPrinceton, NJ
08540pmariani@cytosorbents.com
Investor Relations Contact:Eric RibnerLifeSci
Advisors, LLC250 W 55th St, #3401New York, NY 10019+1 (646)
751-4363ir@cytosorbents.com
CytoSorbents (NASDAQ:CTSO)
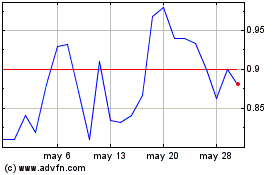
Gráfica de Acción Histórica
De Oct 2024 a Nov 2024
CytoSorbents (NASDAQ:CTSO)
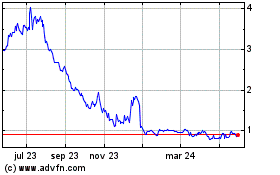
Gráfica de Acción Histórica
De Nov 2023 a Nov 2024