Evgen Pharma Climbs on FDA Orphan-Drug Designation for Brain Tumor Treatment
02 Septiembre 2021 - 5:46AM
Noticias Dow Jones
By Adam Clark
Evgen Pharma PLC shares surged on Thursday after it said that
its SFX-01 product to treat malignant glioma has been granted an
orphan drug designation by the U.S. Food and Drug
Administration.
The designation confers intellectual property cover to an
investigational drug in the form of data protection at the time of
approval of a new drug application. Tax credits are also possible
on eventual U.S. sales of an approved orphan drug.
"This is part of a wider strategy to access the U.S. market and
positions us well for further investigations of our lead asset in
this devastating brain cancer as we continue to optimize SFX-01 for
clinical trials and eventually partnering," Evgen Chief Executive
Huw Jones said.
Shares at 1007 GMT were up 2.50 pence, or 39%, at 8.90
pence.
Write to Adam Clark at adam.clark@dowjones.com
(END) Dow Jones Newswires
September 02, 2021 06:31 ET (10:31 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
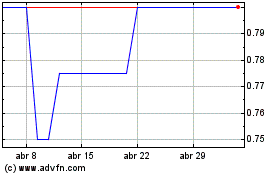
Evgen Pharma (LSE:EVG)
Gráfica de Acción Histórica
De Mar 2024 a Abr 2024
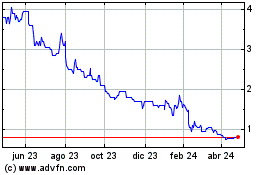
Evgen Pharma (LSE:EVG)
Gráfica de Acción Histórica
De Abr 2023 a Abr 2024