Allergan Gets FDA Fast-Track, Qualified Infectious Disease Status for ATM-AVI
11 Noviembre 2019 - 7:20AM
Noticias Dow Jones
By Colin Kellaher
Allergan PLC (AGN) on Monday said the U.S. Food and Drug
Administration granted fast-track and qualified infectious disease
product designations to its ATM-AVI investigational combination
antibiotic, which is under development for antibiotic-resistant
infections.
The Dublin-based pharmaceuticals company said the designations
cover ATM-AVI for the treatment of complicated intra-abdominal
infections, complicated urinary tract infections and
hospital-acquired bacterial pneumonia/ventilator-associated
bacterial pneumonia.
The FDA's fast-track program is designed to facilitate the
development and expedite the review of drugs to treat serious
conditions and fill an unmet medical need. The agency's qualified
infectious disease product designation provides incentives for the
development of new antibiotics, including priority review,
eligibility for fast-track designation and a five-year regulatory
exclusivity extension.
Allergan and Pfizer Inc. (PFE) are jointly developing ATM-AVI,
which is currently in phase III trials. Allergan holds the rights
to commercialize ATM-AVI in North America, while Pfizer holds the
commercial rights in the rest of the world.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
November 11, 2019 08:05 ET (13:05 GMT)
Copyright (c) 2019 Dow Jones & Company, Inc.
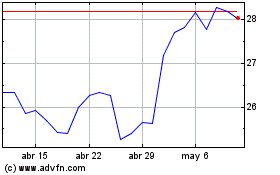
Pfizer (NYSE:PFE)
Gráfica de Acción Histórica
De Mar 2024 a Abr 2024
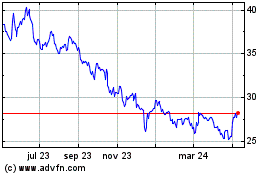
Pfizer (NYSE:PFE)
Gráfica de Acción Histórica
De Abr 2023 a Abr 2024