Philips Receives US FDA Clearance for Covid-19 Biosensor
26 Mayo 2020 - 9:47AM
Noticias Dow Jones
By Adriano Marchese
Koninklijke Philips NV said Tuesday that it has received
clearance from the U.S. Food and Drug Administration for its
wearable biosensor for confirmed and suspected coronavirus
patients.
The Dutch technology company said the solution has already
received CE mark, and is currently in use with the first install at
the OLVG Hospital--a clinical, referral and training hospital in
the Netherlands--to help manage the triage and clinical
surveillance of Covid-19 patients.
Write to Adriano Marchese at adriano.marchese@wsj.com
(END) Dow Jones Newswires
May 26, 2020 10:32 ET (14:32 GMT)
Copyright (c) 2020 Dow Jones & Company, Inc.
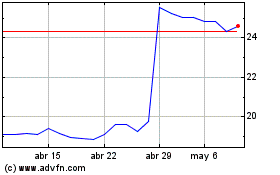
Koninklijke Philips NV (EU:PHIA)
Gráfica de Acción Histórica
De Mar 2024 a Abr 2024
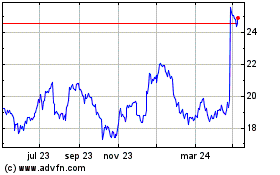
Koninklijke Philips NV (EU:PHIA)
Gráfica de Acción Histórica
De Abr 2023 a Abr 2024