Hemogenyx Pharmaceuticals Addresses FDA Concern on Leukemia Treatment; Shares Rise
10 Julio 2023 - 3:15AM
Noticias Dow Jones
By Elena Vardon
Hemogenyx Pharmaceuticals shares rose on Monday after the
company said it is remanufacturing the lentivirus used in its acute
myeloid leukemia treatment product candidate after the U.S. Food
and Drug Administration raised concerns about it.
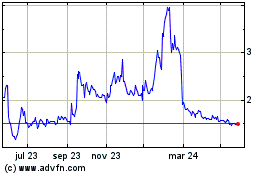
At 0729 GMT, shares were up 0.17 pence, or 11%, at 1.63
pence.
The FDA concerns were related to splicing that occurs during the
manufacturing of the lentivirus, the company said.
The biopharmaceutical group--that develops new therapies and
treatments for blood diseases--said it identified the source of the
splicing deficiency and has developed a method to eliminate it
after the regulator explained in a letter its rationale behind
placing the application on clinical hold.
The group said the FDA also shared suggestions on how to improve
the safety of the treatment, adding they don't relate to the
clinical hold and can be dealt with quickly.
Write to Elena Vardon at elena.vardon@wsj.com
(END) Dow Jones Newswires
July 10, 2023 04:00 ET (08:00 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
Hemogenyx Pharmaceuticals (LSE:HEMO)
Gráfica de Acción Histórica
De Abr 2024 a May 2024
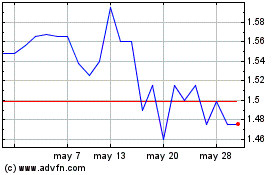
Hemogenyx Pharmaceuticals (LSE:HEMO)
Gráfica de Acción Histórica
De May 2023 a May 2024