Valneva Shares Slump After FDA Postpones Chikungunya Vaccine Review Date
14 Agosto 2023 - 2:55AM
Noticias Dow Jones
By Maitane Sardon
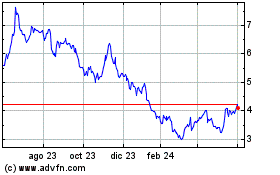
Shares in Valneva plunged in early trading Monday after the
French biotech company said the U.S. Food and Drug Administration
has delayed the target date for completing the regulatory review of
the marketing application for its Chikungunya vaccine
candidate.
At 0713 GMT, shares were down 8.5% at EUR6.14 after falling as
much as 12% at open.
The review is now set for the end of November and not the end of
August as initially planned, Valneva said.
The FDA extended the date to allow sufficient time to align and
agree on the Phase 4 trial program required under the accelerated
approval pathway, the company said.
The agency hasn't requested any additional clinical data for the
approval process, Valneva added.
Write to Maitane Sardon at maitane.sardon@wsj.com
(END) Dow Jones Newswires
August 14, 2023 03:40 ET (07:40 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
Valneva (EU:VLA)
Gráfica de Acción Histórica
De Abr 2024 a May 2024
Valneva (EU:VLA)
Gráfica de Acción Histórica
De May 2023 a May 2024