Ambrx Biopharma Gets Fast Track Designation for ARX517 Cancer Treatment
19 Julio 2023 - 8:49AM
Noticias Dow Jones
By Chris Wack
Ambrx Biopharma said Wednesday that the U.S. Food and Drug
Administration has granted Fast Track designation to its
proprietary anti-PSMA antibody-drug conjugate investigational
therapy, ARX517.
The company's ARX517 is intended for the treatment of patients
with metastatic castration-resistant prostate cancer upon
progression on an androgen receptor pathway inhibitor.
Fast Track designation is a process designed to help the
development and expedite the review of drugs which may demonstrate
substantial improvement over available therapy for serious
conditions with unmet medical need.
Ambrx said ARX517 is currently being studied in a Phase 1/2,
first-in-human, open label dose escalation and dose expansion trial
enrolling patients with mCRPC whose tumors have progressed on at
least two prior FDA approved treatments for prostate cancer.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
July 19, 2023 09:34 ET (13:34 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
Ambrx Biopharma (NASDAQ:AMAM)
Gráfica de Acción Histórica
De May 2024 a Jun 2024
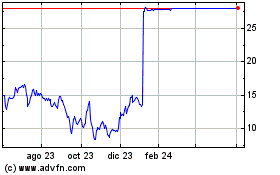
Ambrx Biopharma (NASDAQ:AMAM)
Gráfica de Acción Histórica
De Jun 2023 a Jun 2024