As filed with the Securities and Exchange
Commission on December 6, 2023
Registration No. 333-275579
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
Amendment No. 1 to
FORM S-3
REGISTRATION STATEMENT UNDER THE SECURITIES
ACT OF 1933
GAMIDA CELL LTD.
(Exact name of registrant as specified in its charter)
|
Israel |
|
Not Applicable |
|
(State or other jurisdiction of
incorporation or organization) |
|
(I.R.S. Employer
Identification No.) |
116 Huntington Avenue, 7th Floor
Boston, MA 02116
Tel: (617) 892-9080
(Address, including zip code, and telephone number,
including area code, of registrant’s principal executive offices)
Gamida Cell Inc.
116 Huntington Avenue
Boston, MA 02116
Tel: (617) 892-9080
(Name, address, including zip code, and telephone
number, including area code, of agent for service)
Copies to:
|
Divakar Gupta
Daniel I. Goldberg
Joshua A. Kaufman
Cooley LLP
55 Hudson Yards
New York, NY 10001
Telephone: (212) 479-6000
Facsimile: (212) 479-6275 |
|
Haim Gueta
Shachar Hadar
Meitar | Law Offices
16 Abba Hillel Road
Ramat Gan 5250608, Israel
Telephone: +972 (3) 610-3100
Facsimile: +972 (3) 610-3111 |
Approximate date of commencement of proposed sale
to the public: From time to time after the effective date of this Registration Statement.
If the only securities being registered on this
Form are being offered pursuant to dividend or interest reinvestment plans, please check the following box. ☐
If any of the securities being registered on this
Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, other than securities offered
only in connection with dividend or interest reinvestment plans, check the following box. ☒
If this Form is filed to register additional securities
for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration
statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed
pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of
the earlier effective registration statement for the same offering. ☐
If this Form is a registration statement pursuant
to General Instruction I.D. or a post-effective amendment thereto that shall become effective upon filing with the Commission pursuant
to Rule 462(e) under the Securities Act, check the following box. ☐
If this Form is a post-effective amendment to
a registration statement filed pursuant to General Instruction I.D. filed to register additional securities or additional classes of securities
pursuant to Rule 413(b) under the Securities Act, check the following box. ☐
Indicate by check
mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company,
or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller
reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange
Act.
| Large accelerated filer |
☐ |
Accelerated filer |
☐ |
| Non-accelerated filer |
☒ |
Smaller reporting company |
☒ |
| |
|
Emerging growth company |
☒ |
If an emerging growth company, indicate by check
mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting
standards† provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The Registrant hereby amends this Registration
Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which
specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities
Act or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may
determine.
EXPLANATORY NOTE
This registration statement is a replacement
registration statement being filed pursuant to Rule 415(a)(6) under the Securities Act of 1933, as amended (the “Securities Act”),
with respect to securities which remain unsold under the registration statement on Form F-3 (File No. 333-259472), originally filed on
September 13, 2021, initially declared effective on November 21, 2021, amended by Post-Effective Amendment No. 1 to Form F-3 on Form
S-3 registration statement on March 25, 2022 and again declared effective on April 1, 2022, which is due to expire on September 13, 2024
(the “Prior Registration Statement”). Pursuant to Rule 415(a)(5)(ii) under the Securities Act, by filing this registration
statement on Form S-3 (the “Registration Statement”), the Company may issue and sell securities covered by the Prior Registration
Statement until the earlier of the effective date of this Registration Statement or 180 days after September 21, 2024.
This registration statement of Gamida Cell
Ltd. contains two prospectuses:
| ● | a base prospectus, which covers the offering, issuance and
sale by the Registrant of ordinary shares, debt securities, warrants, rights, and units identified
above from time to time in one or more offerings, which together shall have an aggregate
initial offering price not to exceed $150,000,000; and |
| ● | an
Open Market Sale Agreement prospectus (the “Sale Agreement Prospectus”) covering
the offering, issuance and sale by the Registrant of up to a maximum aggregate offering price
of $50,000,000 of the Registrant’s ordinary shares that may be issued and sold under
the amended and restated open market sale agreement, dated June 5, 2023, between the Registrant
and Jefferies LLC. |
The base prospectus immediately follows this
explanatory note. The specific terms of any securities to be offered pursuant to the base prospectus will be specified in one or more
prospectus supplements to the base prospectus. The Sale Agreement Prospectus immediately follows the base prospectus. The ordinary shares
that may be offered, issued and sold by us under the Sale Agreement Prospectus are included in the $150,000,000 of securities that may
be offered, issued and sold by us under the base prospectus. In the event of the termination of the offering of ordinary shares under
the Sale Agreement Prospectus, any portion of the $50,000,000 aggregate offering price for the ordinary shares covered by the Sale Agreement
Prospectus that is not sold pursuant to the Sale Agreement Prospectus will be available for sale in other offerings pursuant to the base
prospectus.
This Pre-Effective
Amendment No. 1 to the Registration Statement is being filed solely to address certain comments of the Securities and Exchange Commission
in respect of Gamida Cell Ltd.’s business-related disclosures.
The information in this
prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities
and Exchange Commission is effective. This prospectus is not an offer to sell securities and it is not soliciting an offer to buy securities
in any jurisdiction where the offer or sale is not permitted.
SUBJECT
TO COMPLETION, DATED DECEMBER 6, 2023
PROSPECTUS
$150,000,000
Ordinary Shares, Debt, Warrants, Rights
and Units offered by the Company

Gamida Cell Ltd.
We may offer, issue and sell from time to time,
in one or more offerings, ordinary shares, debt, warrants, rights or units, which we collectively refer to as the “securities.”
The aggregate initial offering price of the securities that we may offer and sell under this prospectus will not exceed $150,000,000.
We may offer and sell any combination of the securities
described in this prospectus in different series, at times, in amounts, at prices and on terms to be determined at or prior to the time
of each offering. This prospectus describes the general terms of these securities and the general manner in which these securities will
be offered. Each time we sell securities pursuant to this prospectus, we will provide a supplement to this prospectus that contains specific
information about the offering and the specific terms of the securities offered. The prospectus supplement will also describe the specific
manner in which these securities will be offered and may also supplement, update or amend information contained in this prospectus. You
should read this prospectus and any applicable prospectus supplement before you invest.
The securities covered by this prospectus may
be offered through one or more underwriters, dealers and agents, or directly to purchasers. The names of any underwriters, dealers or
agents, if any, will be included in a supplement to this prospectus. For general information about the distribution of securities offered,
please see “Plan of Distribution” beginning on page 23.
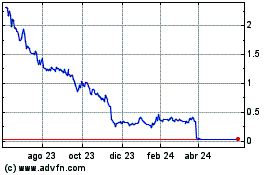
Our ordinary shares are
traded on the Nasdaq Global Market under the symbol “GMDA.” On December 5, 2023, the closing price of our ordinary shares
as reported by the Nasdaq Global Market was $0.32 per ordinary share.
We may sell these securities directly to investors,
through agents designated from time to time or to or through underwriters or dealers, on a continuous or delayed basis. For additional
information on the methods of sale, you should refer to the section titled “Plan of Distribution” in this prospectus. If any
agents or underwriters are involved in the sale of any securities with respect to which this prospectus is being delivered, the names
of such agents or underwriters and any applicable fees, commissions, discounts or over-allotment options will be set forth in a prospectus
supplement. The price to the public of such securities and the net proceeds we expect to receive from such sale will also be set forth
in a prospectus supplement.
Investing in our securities involves a high
degree of risk. You should review carefully the risks and uncertainties described under the heading “Risk Factors” contained
in the applicable prospectus supplement and any related free writing prospectus, and under similar headings in the other documents that
are incorporated by reference into this prospectus as described on page 3 of this prospectus.
Neither the U.S. Securities and Exchange Commission
nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete.
Any representation to the contrary is a criminal offense.
The date of this prospectus
is , 2023.
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement
on Form S-3 that we filed with the U.S. Securities and Exchange Commission, or the SEC, as part of a “shelf” registration
process.
Under this shelf registration, we may offer any
combination of the securities described in this prospectus from time to time in one or more offerings. This prospectus only provides you
with a general description of the securities we may offer. Each time we sell securities described herein, we will provide prospective
investors with a supplement to this prospectus that will contain specific information about the terms of that offering, including the
specific amounts, prices and terms of the securities offered. The prospectus supplement may also add to, update or change information
contained in this prospectus. We may also authorize one or more free writing prospectuses to be provided to you that may contain material
information relating to these offerings. The prospectus supplement and any related free writing prospectus that we may authorize to be
provided to you may also add, update or change information contained in this prospectus or in any documents that we have incorporated
by reference into this prospectus. Accordingly, to the extent inconsistent, information in this prospectus is superseded by the information
in any prospectus supplement or any related free writing prospectus that we may authorize. You should carefully read this prospectus,
any applicable prospectus supplement and any related free writing prospectus, together with the information incorporated herein by reference
as described under the heading “Incorporation of Certain Information by Reference,” before investing in any of the securities
offered.
THIS PROSPECTUS MAY NOT BE USED TO CONSUMMATE
A SALE OF SECURITIES UNLESS IT IS ACCOMPANIED BY A PROSPECTUS SUPPLEMENT.
We own various trademark registrations and applications,
and unregistered trademarks. Gamida Cell, omidubicel, Omisirge and GDA-201 are trademarks of ours that we use in this prospectus supplement
and the accompanying prospectus. This prospectus also includes trademarks, tradenames and service marks that are the property of other
organizations. Solely for convenience, our trademarks and tradenames referred to in this prospectus appear without the ® or ™
symbols, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable
law, our rights, or the right of the applicable licensor to our trademark and tradenames. We do not intend to use or display other companies’
trademarks and trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
The terms “shekel,” “Israeli
shekel” and “NIS” refer to New Israeli Shekels, the lawful currency of the State of Israel, and the terms “dollar,”
“U.S. dollar” or “$” refer to United States dollars, the lawful currency of the United States. All references
to “shares” in this prospectus refer to ordinary shares of Gamida Cell Ltd., par value NIS 0.01 per share.
References in this prospectus supplement to “Gamida
Cell,” “Gamida,” “the Company,” “we,” “us” and “our” refer to Gamida
Cell Ltd. and our wholly owned subsidiary Gamida Cell Inc., except where the context otherwise requires or as otherwise indicated. This
prospectus contains summaries of certain provisions contained in some of the documents described herein, but reference is made to the
actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some
of the documents referred to herein have been filed, will be filed or will be incorporated by reference as exhibits to the registration
statement of which this prospectus is a part, and you may obtain copies of those documents as described below under the heading “Where
You Can Find More Information.”
Neither we, nor any agent, underwriter or dealer
has authorized any person to give any information or to make any representation other than those contained or incorporated by reference
in this prospectus, any applicable prospectus supplement or any related free writing prospectus prepared by or on behalf of us or to which
we have referred you. This prospectus, any applicable supplement to this prospectus or any related free writing prospectus do not constitute
an offer to sell or the solicitation of an offer to buy any securities other than the registered securities to which they relate, nor
does this prospectus, any applicable supplement to this prospectus or any related free writing prospectus constitute an offer to sell
or the solicitation of an offer to buy securities in any jurisdiction to any person to whom it is unlawful to make such offer or solicitation
in such jurisdiction.
You should not assume that the information contained
in this prospectus, any applicable prospectus supplement or any related free writing prospectus is accurate on any date subsequent to
the date set forth on the front of the document or that any information we have incorporated by reference is correct on any date subsequent
to the date of the document incorporated by reference, even though this prospectus, any applicable prospectus supplement or any related
free writing prospectus is delivered, or securities are sold, on a later date.
For investors outside the United States: We
have not done anything that would permit the offering or possession or distribution of this prospectus in any jurisdiction where action
for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus
must inform themselves about, and observe any restrictions relating to, the offering of the securities described herein and the distribution
of this prospectus outside the United States.
SPECIAL NOTE REGARDING
FORWARD-LOOKING STATEMENTS
This prospectus, any prospectus supplement and
the information incorporated by reference in this prospectus and any prospectus supplement contain forward-looking statements within the
meaning of Section 27A of the Securities Act, and Section 21E of the Exchange Act of 1934, as amended, or the Exchange Act, that involve
substantial risks and uncertainties. Although our forward-looking statements reflect the good faith judgment of our management, these
statements can only be based on facts and factors currently known by us. Consequently, these forward-looking statements are inherently
subject to risks and uncertainties, and actual results and outcomes may differ materially from results and outcomes discussed in the forward-looking
statements.
All statements other than present and historical
facts and conditions contained in this prospectus, any prospectus supplement and the information incorporated by reference in this prospectus
and any prospectus supplement including statements regarding our future results of operations and financial positions, business strategy,
plans and our objectives for future operations, are forward-looking statements. The words “anticipate,” “believe,”
“continue” “could,” “estimate,” “expect,” “intend,” “may,” “might,”
“ongoing,” “objective,” “plan,” “potential,” “predict,” “should,”
“will” and “would,” or the negative of these and similar expressions identify forward-looking statements. Forward-looking
statements include, but are not limited to, statements about:
| ● | our estimates regarding the commercial potential of, and
our commercialization plans for, our allogenic cell therapy Omisirge (omidubicel-onlv), including our plans to manufacture Omisirge at
a commercial scale at our Kiryat Gat facility; |
| ● | the clinical utility and potential advantages of Omisirge
and any of our product candidates; |
| ● | the timing, progress and conduct of our clinical trial of
GDA-201; |
| ● | our plans regarding utilization of regulatory pathways that
would allow for accelerated marketing approval in the United States, the European Union and other jurisdictions; |
| ● | our recurring losses from operations, our estimates regarding
anticipated capital requirements and our needs for additional sources of financing or a commercial or strategic partnership to support
a more fulsome commercial launch of Omisirge; |
| ● | anticipated cost savings from our strategic restructuring
and our financial runway; |
| ● | our expectations regarding when certain patents may be issued
and the protection and enforcement of our intellectual property rights; |
| ● | our plans regarding the maintenance of intellectual property rights to our preclinical NK cell pipeline; |
| ● | our ability to manufacture Omisirge and any product candidates
at levels sufficient for commercialization or clinical development, as applicable; |
| ● | our ability to maintain relationships with certain third
parties; |
| ● | our planned level of capital expenditures; |
| ● | the impact of government laws and regulations; |
| ● | the impact of political, economic and military conditions
in Israel, including the recent attack by Hamas and other terrorist organizations from the Gaza Strip and Israel’s war against
them; and |
| ● | the effects that geopolitical events or economic conditions
may have on us. |
As a result of these factors, we cannot assure
you that the forward-looking statements in this prospectus, any prospectus supplement and the information incorporated by reference in
this prospectus and any prospectus supplement will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate,
the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these
statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time
frame or at all. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information,
future events or otherwise, except as required by law.
You should read this prospectus, any prospectus
supplement and the information incorporated by reference in this prospectus and any prospectus supplement completely and with the understanding
that our actual future results may be materially different from what we expect. We qualify all of our forward-looking statements by these
cautionary statements.
This prospectus, any prospectus supplement and
the information incorporated by reference in this prospectus and any prospectus supplement may contain market data and industry forecasts
that were obtained from industry publications. These data involve a number of assumptions and limitations, and you are cautioned not to
give undue weight to such estimates. While we believe the market position, market opportunity and market size information included in
this prospectus, any prospectus supplement and the information incorporated by reference in this prospectus and any prospectus supplement
is generally reliable, such information is inherently imprecise.
In addition, statements that “we believe”
and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available
to us as of the date the statements were made, and while we believed such information formed a reasonable basis for such statements at
the time they were made, such information may be limited or incomplete, and our statements should not be read to indicate that we have
conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain
and you are cautioned not to unduly rely upon these statements.
PROSPECTUS SUMMARY
The following summary highlights information
contained elsewhere in this prospectus. This summary is not complete and does not contain all of the information that you need to consider
in making your investment decision. You should carefully read the entire prospectus, the applicable prospectus supplement and any related
free writing prospectus, including the risks of investing in our securities discussed under the heading “Risk Factors” contained
in the applicable prospectus supplement and any related free writing prospectus, and under similar headings in the other documents that
are incorporated by reference into this prospectus. You should also carefully read the information incorporated by reference into this
prospectus, including our consolidated financial statements, and the exhibits to the registration statement of which this prospectus is
a part.
Company Overview
We are a cell therapy pioneer working to turn
cells into powerful therapeutics. We apply a proprietary expansion platform leveraging the properties of nicotinamide, or NAM, to allogeneic
cell sources including umbilical cord blood-derived cells and natural killer, or NK, cells to create cell therapy candidates, with the
potential to redefine standards of care. On April 17, 2023, the U.S. Food and Drug Administration, or FDA, approved our allogeneic cell
therapy, Omisirge (omidubicel-onlv), for use in adult and pediatric patients 12 years and older with hematologic malignancies who are
planned for umbilical cord blood transplantation following myeloablative conditioning to reduce the time to neutrophil recovery and the
incidence of infection.
Corporate Information
We are an Israeli corporation and were incorporated
in 1998. Our principal executive offices are located at 116 Huntington Avenue, Boston, Massachusetts 02116. Our telephone number is (617)
892-9080. Our website address is www.gamida-cell.com. The information contained on, or that can be accessed through, our website is not
incorporated by reference into this prospectus. We have included our website address as an inactive textual reference only.
Gamida Cell Inc., our wholly owned subsidiary,
was incorporated under the laws of the State of Delaware in October 2000 and is qualified to do business in Massachusetts and other states.
Our ordinary shares have been listed on the Nasdaq
Global Market under the symbol “GMDA” since October 26, 2018.
The Securities We May Offer
We may offer our ordinary shares, debt, warrants
to purchase ordinary shares, rights and units comprising any combination of these securities, up to a total aggregate offering price of
$150,000,000 from time to time in one or more offerings under this prospectus, together with any applicable prospectus supplement and
any related free writing prospectus, at prices and on terms to be determined by market conditions at the time of the relevant offering.
This prospectus provides you with a general description of the securities we may offer. Each time we offer a type or series of securities
under this prospectus, we will provide a prospectus supplement that will describe the specific amounts, prices and other important terms
of the securities, including, to the extent applicable:
| ● | designation or classification; |
| ● | aggregate principal amount or aggregate offering price; |
| ● | original issue discount; |
| ● | rates and times of payment of interest or dividends; |
| ● | redemption, conversion, exchange or sinking fund terms; |
| ● | conversion or exchange prices or rates and any provisions for changes to or adjustments in the conversion or exchange prices or rates
and in the securities or other property receivable upon conversion or exchange; and |
| ● | important tax considerations. |
The prospectus supplement and any related free
writing prospectus that we may authorize to be provided to you may also add, update or change information contained in this prospectus
or in documents we have incorporated by reference. However, no prospectus supplement or free writing prospectus will offer a security
that is not registered and described in this prospectus at the time of the effectiveness of the registration statement of which this prospectus
is a part.
We may sell the securities directly to investors
or through underwriters, dealers or agents. We, and our underwriters or agents, reserve the right to accept or reject all or part of any
proposed purchase of securities. If we do offer securities through underwriters or agents, we will include in the applicable prospectus
supplement:
| ● | the names of those underwriters or agents; |
| ● | applicable fees, discounts and commissions to be paid to them; |
| ● | details regarding over-allotment options, if any; and |
| ● | the estimated net proceeds to us. |
This prospectus may not be used to consummate
a sale of securities unless it is accompanied by a prospectus supplement.
RISK FACTORS
An investment in our securities involves a high
degree of risk. Before deciding whether to purchase our securities, you should carefully consider the risk factors incorporated by reference
from Part I, Item 1A. of our most recent Annual Report on Form 10-K, any subsequent Quarterly Reports on From 10-Q or Current Reports
on Form 8-K we file after the date of this prospectus, and all other information contained or incorporated by reference into this prospectus
or any applicable prospectus supplement, as updated by those subsequent filings with the SEC under the Exchange Act, that are incorporated
herein by reference. These risks could materially affect our business, results of operations or financial condition and cause the value
of our securities to decline, in which case you may lose all or part of your investment. For more information, see “Where You Can
Find More Information” and “Incorporation of Certain Information by Reference.”
USE OF PROCEEDS
Unless otherwise set forth in a prospectus supplement,
we currently intend to use the net proceeds of any offering of securities for working capital and other general corporate purposes. Accordingly,
we will have significant discretion in the use of any net proceeds. We may provide additional information on the use of the net proceeds
from the sale of the offered securities in an applicable prospectus supplement relating to the offered securities.
CAPITALIZATION
We intend to include information about our capitalization
and indebtedness in prospectus supplements.
DESCRIPTION OF
SECURITIES
The descriptions of the securities contained in
this prospectus, together with the applicable prospectus supplements, summarize the material terms and provisions of the various types
of securities that we may offer. We will describe in the applicable prospectus supplement relating to any securities the particular terms
of the securities offered by that prospectus supplement. If we so indicate in the applicable prospectus supplement, the terms of the securities
may differ from the terms we have summarized below.
We may sell from time to time, in one or more
offerings, ordinary shares, debt, warrants to purchase ordinary shares, rights and units comprising any combination of these securities.
In this prospectus, we refer to the ordinary shares,
debt, warrants to purchase ordinary shares, rights and units that may be offered by us collectively as “securities.” The total
dollar amount of all securities that we may issue under this prospectus will not exceed $150,000,000. The actual price per share of the
shares that will offer, or per security of the securities that we will offer, pursuant hereto will depend on a number of factors that
may be relevant as of the time of offer.
This prospectus may not be used to consummate
a sale of securities unless it is accompanied by a prospectus supplement.
DESCRIPTION OF
SHARE CAPITAL
The following descriptions of our share capital
and provisions of our amended and restated articles of association are summaries and do not purport to be complete.
General
Our authorized share capital consists of 325,000,000
ordinary shares, par value NIS 0.01 per share, of which 131,931,600 shares are issued and outstanding as of September 30, 2023.
All of our outstanding ordinary shares are validly
issued, fully paid and non-assessable. Our ordinary shares are not redeemable and do not have any preemptive rights. We have no preferred
shares authorized or outstanding.
Registration Number and Purposes of the Company
We are registered with the Israeli Registrar of
Companies. Our registration number is 51-260120-4. Our purpose, as set forth in our amended and restated articles of association, is to
engage in any lawful act or activity.
Voting Rights
All ordinary shares have identical voting and
other rights in all respects.
Transfer of Shares
Our fully paid ordinary shares are issued in registered
form and may be freely transferred under our amended and restated articles of association, unless the transfer is restricted or prohibited
by another instrument, applicable law or the rules of a stock exchange on which the shares are listed for trade. The ownership or voting
of our ordinary shares by non-residents of Israel is not restricted in any way by our amended and restated articles of association or
the laws of the State of Israel, except for ownership by nationals of some countries that are, or have been, in a state of war with Israel.
Election of Directors
Under our amended and restated articles of association,
our board of directors must consist of not less than 5 but no more than 11 directors. We currently have six directors on our board of
directors. Pursuant to our amended and restated articles of association, each of our directors is appointed by a simple majority vote
of holders of our voting shares, participating and voting at an annual general meeting of our shareholders. In addition, our directors
are divided into three classes, one class being elected each year at the annual general meeting of our shareholders, and serve on our
board of directors until they are removed by a vote of 60% of the total voting power of our shareholders at a general meeting of our shareholders
or upon the occurrence of certain events, in accordance with the Israeli Companies Law – 1999 (the “Companies Law”),
and our amended and restated articles of association. In addition, our amended and restated articles of association allow our board of
directors to fill vacancies on the board of directors or to appoint new directors up to the maximum number of directors permitted under
our amended and restated articles of association. Such directors serve for a term of office equal to the remaining period of the term
of office of the directors(s) whose office(s) have been vacated or in the case of new directors, for a term of office according to the
class to which such director was assigned upon appointment.
Dividend and Liquidation Rights
We may declare a dividend to be paid to the holders
of our ordinary shares in proportion to their respective shareholdings. Under the Companies Law, dividend distributions are determined
by the board of directors and do not require the approval of the shareholders of a company unless the company’s articles of association
provide otherwise. Our amended and restated articles of association do not require shareholder approval of a dividend distribution and
provide that dividend distributions may be determined by our board of directors.
Pursuant to the Israeli Companies Law, the distribution
amount is limited to the greater of retained earnings or earnings generated over the previous two years, according to our then last reviewed
or audited financial statements, provided that the end of the period to which the financial statements relate is not more than six months
prior to the date of the distribution. If we do not meet such criteria, then we may distribute dividends only with court approval. In
each case, we are only permitted to distribute a dividend if our board of directors and the court, if applicable, determines that there
is no reasonable concern that payment of the dividend will prevent us from satisfying our existing and foreseeable obligations as they
become due.
In the event of our liquidation, after satisfaction
of liabilities to creditors, our assets will be distributed to the holders of our ordinary shares in proportion to their shareholdings.
This right, as well as the right to receive dividends, may be affected by the grant of preferential dividend or distribution rights to
the holders of a class of shares with preferential rights that may be authorized in the future.
Exchange Controls
There are currently no Israeli currency control
restrictions on remittances of dividends on our ordinary shares, proceeds from the sale of the shares or interest or other payments to
non-residents of Israel, except for shareholders who are subjects of countries that are, or have been, in a state of war with Israel.
Shareholder Meetings
Under Israeli law, we are required to hold an
annual general meeting of our shareholders once every calendar year that must be held no later than 15 months after the date of the previous
annual general meeting. All meetings other than the annual general meeting of shareholders are referred to in our amended and restated
articles of association as special general meetings. Our board of directors may call special general meetings whenever it sees fit, at
such time and place, within or outside of Israel, as it may determine. In addition, the Companies Law provides that our board of directors
is required to convene a special general meeting upon the written request of (i) any two or more of our directors or one-quarter or more
of the members of our board of directors or (ii) one or more shareholders holding, in the aggregate, either (a) 5% or more of our outstanding
issued shares and 1% or more of our outstanding voting power or (b) 5% or more of our outstanding voting power.
Subject to the provisions of the Companies Law
and the regulations promulgated thereunder, shareholders entitled to participate and vote at general meetings are the shareholders of
record on a date to be decided by the board of directors, which may generally be between four and 21 days prior to the date of the meeting,
and in certain circumstances, between four and 40 days prior to the date of the meeting. Furthermore, the Companies Law requires that
resolutions regarding the following matters must be passed at a general meeting of our shareholders:
| ● | amendments to our articles of association; |
| ● | appointment or termination of our auditors; |
| ● | appointment of external directors; |
| ● | approval of certain related party transactions; |
| ● | increases or reductions of our authorized share capital; |
| ● | the exercise of our board of director’s powers by a general meeting, if our board of directors is unable to exercise its powers
and the exercise of any of its powers is required for our proper management. |
The Companies Law requires that a notice of any
annual general meeting or special general meeting be provided to shareholders at least 21 days prior to the meeting and if the agenda
of the meeting includes the appointment or removal of directors, the approval of transactions with office holders or interested or related
parties, or an approval of a merger, notice must be provided at least 35 days prior to the meeting. Under the Companies Law and our amended
and restated articles of association, shareholders are not permitted to take action by way of written consent in lieu of a meeting.
Voting Rights
Quorum
Pursuant to our amended and restated articles
of association, holders of our ordinary shares have one vote for each ordinary share held on all matters submitted to a vote before the
shareholders at a general meeting. The quorum required for our general meetings of shareholders consists of one or more shareholders present
in person, by proxy or written ballot who hold or represent between them at least 33 1/3% of the total outstanding voting rights. A meeting
adjourned for lack of a quorum shall be adjourned either to the same day in the next week, at the same time and place, to such day and
at such time and place as indicated in the notice to such meeting, or to such day and at such time and place as the chairperson of the
meeting shall determine. At the reconvened meeting, one or more shareholders present in person, by proxy or written ballot who hold or
represent between them at least 33 1/3% of the total outstanding voting rights shall constitute a quorum.
Vote Requirements
Our amended and restated articles of association
provide that all resolutions of our shareholders require a simple majority vote, unless otherwise required by the Companies Law or by
our amended and restated articles of association. Under the Companies Law, each of (i) the approval of an extraordinary transaction with
a controlling shareholder, (ii) the terms of employment or other engagement of the controlling shareholder of the company or such controlling
shareholder’s relative (even if such terms are not extraordinary) requires the approval under “Management–Fiduciary
duties and approval of specified related party transactions under Israeli law” and (iii) approval of certain compensation-related
matters require the approval described in the final prospectus filed with our Form F-1 Registration Statement (No. 333-232302) on June
28, 2019 under “Management–Compensation Committee.” Under our amended and restated articles of association, the alteration
of the rights, privileges, preferences or obligations of any class of our shares requires a simple majority of the class so affected (or
such other percentage of the relevant class that may be set forth in the governing documents relevant to such class), in addition to the
ordinary majority vote of all classes of shares voting together as a single class at a shareholder meeting. Our amended and restated articles
of association also provide that the removal of any director from office or the amendment of the provisions relating to our staggered
board requires the vote of 60% of the total voting power of our shareholders. Another exception to the simple majority vote requirement
is a resolution for the voluntary winding up, or an approval of a scheme of arrangement or reorganization, of the company pursuant to
Section 350 of the Companies Law, which requires the approval of holders of 75% of the voting rights represented at the meeting and voting
on the resolution.
In addition, pursuant to our amended and restated
articles of association, in order to approve any amendment to our amended and restated articles of association, in addition and prior
to the approval of a general meeting of shareholders, the approval by affirmative vote of a majority of the members of our board of directors
then in office and entitled to vote thereon is required.
Access to Corporate Records
Under the Companies Law, all shareholders generally
have the right to review minutes of our general meetings, our shareholder register, including with respect to material shareholders, our
articles of association, our financial statements, other documents as provided in the Companies Law, and any document we are required
by law to file publicly with the Israeli Companies Registrar or the Israeli Securities Authority. Any shareholder who specifies the purpose
of its request may request to review any document in our possession that relates to any action or transaction with a related party which
requires shareholder approval under the Companies Law. We may deny a request to review a document if we determine that the request was
not made in good faith, that the document contains a commercial secret or a patent or that the document’s disclosure may otherwise
impair our interests.
Acquisitions under Israeli Law
Full Tender Offer
A person wishing to acquire shares of a public
Israeli company and who would as a result hold over 90% of the target company’s issued and outstanding share capital or that of
a certain class of shares is required by the Companies Law to make a tender offer to all of the company’s shareholders or the shareholders
who hold shares of the same class for the purchase of all of the issued and outstanding shares of the company or of the same class, as
applicable.
If the shareholders who do not respond to or accept
the offer hold less than 5% of the issued and outstanding share capital of the company or of the applicable class of the shares, all of
the shares that the acquirer offered to purchase will be transferred to the acquirer by operation of law (provided that a majority of
the offerees that do not have a personal interest in such tender offer shall have approved it, which condition shall not apply if offerees
holding less than 2% of the company’s issued and outstanding share capital failed to approve such tender offer).
Upon a successful completion of such a full tender
offer, any shareholder that was an offeree in such tender offer, whether the shareholder accepted the tender offer or not, may, within
six months from the date of acceptance of the tender offer, petition the Israeli court to determine whether the tender offer was for less
than fair value and that the fair value should be paid as determined by the court unless the acquirer stipulated that a shareholder that
accepts the offer may not seek appraisal rights. If the shareholders who did not respond or accept the tender offer hold at least 5% of
the issued and outstanding share capital of the company or of the applicable class, or the shareholders who did not accept the tender
offer hold 2% or more of the issued and outstanding share capital of the company (or of the applicable class), the acquirer may not acquire
shares of the company that will increase its holdings to more than 90% of the company’s issued and outstanding share capital or
of the applicable class from shareholders who accepted the tender offer.
Special Tender Offer
The Companies Law provides that an acquisition
of shares of a public Israeli company must be made by means of a special tender offer if as a result of the acquisition the purchaser
would become a holder of at least 25% of the voting rights in the company. This rule does not apply if there is already another holder
of at least 25% of the voting rights in the company. Similarly, the Companies Law provides that an acquisition of shares in a public company
must be made by means of a tender offer if as a result of the acquisition the purchaser would become a holder of more than 45% of the
voting rights in the company, if there is no other shareholder of the company who holds more than 45% of the voting rights in the company.
These requirements do not apply if the acquisition
(i) occurs in the context of a private placement, provided that the general meeting approved the acquisition as a private offering whose
purpose is to give the acquirer at least 25% of the voting rights in the company if there is no person who holds at least 25% of the voting
rights in the company, or as a private offering whose purpose is to give the acquirer 45% of the voting rights in the company, if there
is no person who holds 45% of the voting rights in the company, (ii) was from a shareholder holding at least 25% of the voting rights
in the company and resulted in the acquirer becoming a holder of at least 25% of the voting rights in the company, or (iii) was from a
holder of more than 45% of the voting rights in the company and resulted in the acquirer becoming a holder of more than 45% of the voting
rights in the company.
The special tender offer may be consummated only
if (i) at least 5% of the voting power attached to the company’s outstanding shares will be acquired by the offeror and (ii) the
special tender offer is accepted by a majority of the votes of those offerees who gave notice of their position in respect of the offer,
excluding the votes of a holder of control in the offeror, a person who has personal interest in acceptance of the special tender offer,
holders of 25% or more of the voting rights in the company or anyone on their behalf, including their relatives and entities controlled
by them.
In the event that a special tender offer is made,
a company’s board of directors is required to express its opinion on the advisability of the offer, or shall abstain from expressing
any opinion if it is unable to do so, provided that it gives the reasons for its abstention. In addition, the board of directors must
disclose any personal interest each member of the board of directors has in the offer or stems therefrom. An office holder in a target
company who, in his or her capacity as an office holder, performs an action the purpose of which is to cause the failure of an existing
or foreseeable special tender offer or is to impair the chances of its acceptance, is liable to the potential purchaser and shareholders
for damages resulting from his or her acts, unless such office holder acted in good faith and had reasonable grounds to believe he or
she was acting for the benefit of the company. However, office holders of the target company may negotiate with the potential purchaser
in order to improve the terms of the special tender offer, and may further negotiate with third parties in order to obtain a competing
offer.
If a special tender offer was accepted by a majority
of the shareholders who announced their stand on such offer, then shareholders who did not respond to the special tender offer or had
objected to the offer may accept the offer within four days of the last day set for the acceptance of the offer.
In the event that a special tender offer is accepted,
then the purchaser or any person or entity controlling it or under common control with the purchaser or such controlling person or entity
shall refrain from making a subsequent tender offer for the purchase of shares of the target company and cannot execute a merger with
the target company for a period of one year from the date of the offer, unless the purchaser or such person or entity undertook to effect
such an offer or merger in the initial special tender offer.
Merger
The Companies Law permits merger transactions
if approved by each party’s board of directors and, unless certain requirements described under the Companies Law are met, a majority
of each party’s shareholders and, in the case of the target company, a majority vote of each class of its shares, voted on the proposed
merger at a shareholders meeting. The board of directors of a merging company is required pursuant to the Companies Law to discuss and
determine whether in its opinion there exists a reasonable concern that as a result of a proposed merger, the surviving company will not
be able to satisfy its obligations towards its creditors, such determination taking into account the financial status of the merging companies.
If the board of directors has determined that such a concern exists, it may not approve a proposed merger. Following the approval of the
board of directors of each of the merging companies, the boards of directors must jointly prepare a merger proposal for submission to
the Israeli Registrar of Companies.
For purposes of the shareholder vote, unless a
court rules otherwise, the merger will not be deemed approved if a majority of the shares represented at the shareholders meeting that
are held by parties other than the other party to the merger, or by any person who holds 25% or more of the outstanding shares or the
right to appoint 25% or more of the directors of the other party, vote against the merger. In addition, if the non-surviving entity of
the merger has more than one class of shares, the merger must be approved by each class of shareholders. If the transaction would have
been approved but for the separate approval of each class or the exclusion of the votes of certain shareholders as provided above, a court
may still approve the merger upon the request of holders of at least 25% of the voting rights of a company, if the court holds that the
merger is fair and reasonable, taking into account the value of the parties to the merger and the consideration offered to the shareholders.
Pursuant to the Companies Law, if a merger is with a company’s controlling shareholder or if the controlling shareholder has a personal
interest in the merger, then the merger is instead subject to the same special majority approval that governs all extraordinary transactions
with controlling shareholders (as described in our final prospectus filed with our Form F-1 Registration Statement (No. 333-232302) on
June 28, 2019 under “Management–Fiduciary duties and approval of specified related party transactions under Israeli law.”).
Under the Companies Law, each merging company
must send a copy of the proposed merger plan to its secured creditors. Unsecured creditors are entitled to receive notice of the merger
pursuant to regulations promulgated under the Companies Law. Upon the request of a creditor of either party to the proposed merger, the
court may delay or prevent the merger if it concludes that there exists a reasonable concern that, as a result of the merger, the surviving
company will be unable to satisfy the obligations the target company. The court may further give instructions to secure the rights of
creditors.
In addition, a merger may not be completed unless
at least 50 days have passed from the date that a proposal for approval of the merger was filed with the Israeli Registrar of Companies
and 30 days from the date that shareholder approval of both merging companies was obtained.
Anti-Takeover Measures
The Companies Law allows us to create and issue
shares having rights different from those attached to our ordinary shares, including shares providing certain preferred rights with respect
to voting, distributions or other matters and shares having preemptive rights. We have no preferred shares authorized under our amended
and restated articles of association. In the future, if we do authorize, create and issue a specific class of preferred shares, such class
of shares, depending on the specific rights that may be attached to it, may have the ability to frustrate or prevent a takeover or otherwise
prevent our shareholders from realizing a potential premium over the market value of their ordinary shares. The authorization and designation
of a class of preferred shares will require an amendment to our amended and restated articles of association, which requires the prior
approval of the holders of a majority of the voting power attaching to our issued and outstanding shares at a general meeting. The convening
of the meeting, the shareholders entitled to participate and the majority vote required to be obtained at such a meeting will be subject
to the requirements set forth in the Companies Law as described above in “–Voting Rights.” In addition, as disclosed
under “–Election of directors”, we have a classified board structure which effectively limits the ability of any investor
or potential investor or group of investors or potential investors to gain control of our board of directors.
Significant Transactions
Under our amended and restated articles of association,
the affirmative vote of at least two-thirds (2/3) of the then serving directors is required in order to approve certain transactions
which may have a significant effect on the Company’s structure, assets or business, including mergers and acquisitions, a disposition
of all or substantially all of the assets of the Company, a voluntary dissolution and material changes to the principal business of the
Company. Any amendment or replacement of such provision is subject, in addition to the approval of the Company’s shareholders, to
the approval of at least two-thirds (2/3) of the then serving directors.
Business Combinations
Our amended and restated articles of association,
restrict certain business transactions for a period of three years following (i) with respect to any shareholder of the Company
holding twenty percent (20%) or more of the issued and outstanding voting power of the ordinary shares as of July 27, 2022 (the effective
date of the amended and restated articles of association), and (ii) with respect to any other shareholder of the Company, each time
as such shareholder (and its affiliates) (other than due to a buyback, redemption or cancellation of shares by the Company) becomes the
holder (beneficially or of record) of twenty percent (20%) or more of the issued and outstanding voting power of the ordinary shares.
The restricted business combinations include mergers, consolidations and dispositions of assets having a value of 10% or more of (i) the
Company’s assets or (ii) the market value of its outstanding shares. Any amendment or replacement of such provision is subject,
in addition to the approval of the Company’s shareholders, to an approval of at least two-thirds (2/3) of the then serving directors.
Borrowing Powers
Pursuant to the Companies Law and our amended
and restated articles of association, our board of directors may exercise all powers and take all actions that are not required under
law or under our amended and restated articles of association to be exercised or taken by our shareholders, including the power to borrow
money for company purposes.
Changes in Capital
Our amended and restated articles of association
enable us to increase or reduce our share capital. Any such changes are subject to the Companies Law and must be approved by a resolution
duly passed by our shareholders at a general meeting by voting on such change in the capital. In addition, transactions that have the
effect of reducing capital, such as the declaration and payment of dividends in the absence of sufficient retained earnings or profits,
require the approval of both our board of directors and an Israeli court.
Transfer Agent and Registrar
The transfer agent and registrar for our ordinary
shares is Broadridge Corporate Issuer Solutions, Inc. Its address is 1717 Arch Street, Suite 1300, Philadelphia, Pennsylvania 19103, and
its telephone number is (215) 553-5400.
Listing
Our ordinary shares are listed on The Nasdaq Global
Market under the symbol “GMDA.”
DESCRIPTION OF
DEBT SECURITIES
We may issue debt securities from time to time,
in one or more series, as either senior or subordinated debt or as senior or subordinated convertible debt. While the terms we have summarized
below will apply generally to any debt securities that we may offer under this prospectus, we will describe the particular terms of any
debt securities that we may offer in more detail in the applicable prospectus supplement. The terms of any debt securities offered under
a prospectus supplement may differ from the terms described below. Unless the context requires otherwise, whenever we refer to the indenture,
we also are referring to any supplemental indentures that specify the terms of a particular series of debt securities.
We will issue the debt securities under the indenture
that we will enter into with the trustee named in the indenture. The indenture will be qualified under the Trust Indenture Act of 1939,
as amended, or the Trust Indenture Act. We have filed the form of indenture as an exhibit to the registration statement of which this
prospectus is a part, and supplemental indentures and forms of debt securities containing the terms of the debt securities being offered
will be filed as exhibits to the registration statement of which this prospectus is a part or will be incorporated by reference from reports
that we file with the SEC.
The following summary of material provisions of
the debt securities and the indenture is subject to, and qualified in its entirety by reference to, all of the provisions of the indenture
applicable to a particular series of debt securities. We urge you to read the applicable prospectus supplements and any related free writing
prospectuses related to the debt securities that we may offer under this prospectus, as well as the complete indenture that contains the
terms of the debt securities.
General
The indenture does not limit the amount of debt
securities that we may issue. It provides that we may issue debt securities up to the principal amount that we may authorize and may be
in any currency or currency unit that we may designate. Except for the limitations on consolidation, merger and sale of all or substantially
all of our assets contained in the indenture, the terms of the indenture do not contain any covenants or other provisions designed to
give holders of any debt securities protection against changes in our operations, financial condition or transactions involving us.
We may issue the debt securities issued under
the indenture as “discount securities,” which means they may be sold at a discount below their stated principal amount. These
debt securities, as well as other debt securities that are not issued at a discount, may be issued with “original issue discount,”
or OID, for U.S. federal income tax purposes because of interest payment and other characteristics or terms of the debt securities. Material
U.S. federal income tax considerations applicable to debt securities issued with OID will be described in more detail in any applicable
prospectus supplement.
We will describe in the applicable prospectus
supplement the terms of the series of debt securities being offered, including:
| ● | the title of the series of debt securities; |
| ● | any limit upon the aggregate principal amount that may be issued; |
| ● | the maturity date or dates; |
| ● | the form of the debt securities of the series; |
| ● | the applicability of any guarantees; |
| ● | whether or not the debt securities will be secured or unsecured, and the terms of any secured debt; |
| ● | whether the debt securities rank as senior debt, senior subordinated debt, subordinated debt or any combination thereof, and the terms
of any subordination; |
| ● | if the price (expressed as a percentage of the aggregate principal amount thereof) at which such debt securities will be issued is
a price other than the principal amount thereof, the portion of the principal amount thereof payable upon declaration of acceleration
of the maturity thereof, or if applicable, the portion of the principal amount of such debt securities that is convertible into another
security or the method by which any such portion shall be determined; |
| ● | the interest rate or rates, which may be fixed or variable, or the method for determining the rate and the date interest will begin
to accrue, the dates interest will be payable and the regular record dates for interest payment dates or the method for determining such
dates; |
| ● | our right, if any, to defer payment of interest and the maximum length of any such deferral period; |
| ● | if applicable, the date or dates after which, or the period or periods during which, and the price or prices at which, we may, at
our option, redeem the series of debt securities pursuant to any optional or provisional redemption provisions and the terms of those
redemption provisions; |
| ● | the date or dates, if any, on which, and the price or prices at which we are obligated, pursuant to any mandatory sinking fund or
analogous fund provisions or otherwise, to redeem, or at the holder’s option to purchase, the series of debt securities and the
currency or currency unit in which the debt securities are payable; |
| ● | the denominations in which we will issue the series of debt securities, if other than denominations of $1,000 and any integral multiple
thereof; |
| ● | any and all terms, if applicable, relating to any auction or remarketing of the debt securities of that series and any security for
our obligations with respect to such debt securities and any other terms which may be advisable in connection with the marketing of debt
securities of that series; |
| ● | whether the debt securities of the series shall be issued in whole or in part in the form of a global security or securities; the
terms and conditions, if any, upon which such global security or securities may be exchanged in whole or in part for other individual
securities; and the depositary for such global security or securities; |
| ● | if applicable, the provisions relating to conversion or exchange of any debt securities of the series and the terms and conditions
upon which such debt securities will be so convertible or exchangeable, including the conversion or exchange price, as applicable, or
how it will be calculated and may be adjusted, any mandatory or optional (at our option or the holders’ option) conversion or exchange
features, the applicable conversion or exchange period and the manner of settlement for any conversion or exchange; |
| ● | if other than the full principal amount thereof, the portion of the principal amount of debt securities of the series which shall
be payable upon declaration of acceleration of the maturity thereof; |
| ● | additions to or changes in the covenants applicable to the particular debt securities being issued, including, among others, the consolidation,
merger or sale covenant; |
| ● | additions to or changes in the events of default with respect to the securities and any change in the right of the trustee or the
holders to declare the principal, premium, if any, and interest, if any, with respect to such securities to be due and payable; |
| ● | additions to or changes in or deletions of the provisions relating to covenant defeasance and legal defeasance; |
| ● | additions to or changes in the provisions relating to satisfaction and discharge of the indenture; |
| ● | additions to or changes in the provisions relating to the modification of the indenture both with and without the consent of holders
of debt securities issued under the indenture; |
| ● | the currency of payment of debt securities if other than U.S. dollars and the manner of determining the equivalent amount in U.S.
dollars; |
| ● | whether interest will be payable in cash or additional debt securities at our or the holders’ option and the terms and conditions
upon which the election may be made; |
| ● | the terms and conditions, if any, upon which we will pay amounts in addition to the stated interest, premium, if any and principal
amounts of the debt securities of the series to any holder that is not a “United States person” for U.S. federal income tax
purposes; |
| ● | any restrictions on transfer, sale or assignment of the debt securities of the series; and |
| ● | any other specific terms, preferences, rights or limitations of, or restrictions on, the debt securities, any other additions or changes
in the provisions of the indenture, and any terms that may be required by us or advisable under applicable laws or regulations. |
Conversion or Exchange Rights
We will set forth in the applicable prospectus
supplement the terms on which a series of debt securities may be convertible into or exchangeable for our ordinary shares or our other
securities. We will include provisions as to settlement upon conversion or exchange and whether conversion or exchange is mandatory, at
the option of the holder or at our option. We may include provisions pursuant to which the number of shares of our ordinary shares or
our other securities that the holders of the series of debt securities receive would be subject to adjustment.
Consolidation, Merger or Sale
Unless we provide otherwise in the prospectus
supplement applicable to a particular series of debt securities, the indenture will not contain any covenant that restricts our ability
to merge or consolidate, or sell, convey, transfer or otherwise dispose of our assets as an entirety or substantially as an entirety.
However, any successor to or acquirer of such assets (other than a subsidiary of ours) must assume all of our obligations under the indenture
or the debt securities, as appropriate.
Events of Default under the Indenture
Unless we provide otherwise in the prospectus
supplement applicable to a particular series of debt securities, the following are events of default under the indenture with respect
to any series of debt securities that we may issue:
| ● | if we fail to pay any installment of interest on any series of debt securities, as and when the same shall become due and payable,
and such default continues for a period of 90 days; provided, however, that a valid extension of an interest payment period by us in accordance
with the terms of any indenture supplemental thereto shall not constitute a default in the payment of interest for this purpose; |
| ● | if we fail to pay the principal of, or premium, if any, on any series of debt securities as and when the same shall become due and
payable whether at maturity, upon redemption, by declaration or otherwise, or in any payment required by any sinking or analogous fund
established with respect to such series; provided, however, that a valid extension of the maturity of such debt securities in accordance
with the terms of any indenture supplemental thereto shall not constitute a default in the payment of principal or premium, if any; |
| ● | if we fail to observe or perform any other covenant or agreement contained in the debt securities or the indenture, other than a covenant
specifically relating to another series of debt securities, and our failure continues for 90 days after we receive written notice of such
failure, requiring the same to be remedied and stating that such is a notice of default thereunder, from the trustee or holders of at
least 25% in aggregate principal amount of the outstanding debt securities of the applicable series; and |
| ● | if specified events of bankruptcy, insolvency or reorganization occur. |
If an event of default with respect to debt securities
of any series occurs and is continuing, other than an event of default specified in the last bullet point above, the trustee or the holders
of at least 25% in aggregate principal amount of the outstanding debt securities of that series, by notice to us in writing, and to the
trustee if notice is given by such holders, may declare the unpaid principal of, premium, if any, and accrued interest, if any, due and
payable immediately. If an event of default specified in the last bullet point above occurs with respect to us, the principal amount of
and accrued interest, if any, of each issue of debt securities then outstanding shall be due and payable without any notice or other action
on the part of the trustee or any holder.
The holders of a majority in principal amount
of the outstanding debt securities of an affected series may waive any default or event of default with respect to the series and its
consequences, except defaults or events of default regarding payment of principal, premium, if any, or interest, unless we have cured
the default or event of default in accordance with the indenture. Any waiver shall cure the default or event of default.
Subject to the terms of the indenture, if an event
of default under an indenture shall occur and be continuing, the trustee will be under no obligation to exercise any of its rights or
powers under such indenture at the request or direction of any of the holders of the applicable series of debt securities, unless such
holders have offered the trustee reasonable indemnity. The holders of a majority in principal amount of the outstanding debt securities
of any series will have the right to direct the time, method and place of conducting any proceeding for any remedy available to the trustee,
or exercising any trust or power conferred on the trustee, with respect to the debt securities of that series, provided that:
| ● | the direction so given by the holder is not in conflict with any law or the applicable indenture; and |
| ● | subject to its duties under the Trust Indenture Act, the trustee need not take any action that might involve it in personal liability
or might be unduly prejudicial to the holders not involved in the proceeding. |
A holder of the debt securities of any series
will have the right to institute a proceeding under the indenture or to appoint a receiver or trustee, or to seek other remedies only
if:
| ● | the holder has given written notice to the trustee of a continuing event of default with respect to that series; |
| ● | the holders of at least 25% in aggregate principal amount of the outstanding debt securities of that series have made written request, |
| ● | such holders have offered to the trustee indemnity satisfactory to it against the costs, expenses and liabilities to be incurred by
the trustee in compliance with the request; and |
| ● | the trustee does not institute the proceeding, and does not receive from the holders of a majority in aggregate principal amount of
the outstanding debt securities of that series other conflicting directions within 90 days after the notice, request and offer. |
These limitations do not apply to a suit instituted
by a holder of debt securities if we default in the payment of the principal, premium, if any, or interest on, the debt securities.
We will periodically file statements with the
trustee regarding our compliance with specified covenants in the indenture.
Modification of Indenture; Waiver
We and the trustee may change an indenture without
the consent of any holders with respect to specific matters:
| ● | to cure any ambiguity, defect or inconsistency in the indenture or in the debt securities of any series; |
| ● | to comply with the provisions described above under “Description of Debt Securities—Consolidation, Merger or Sale;” |
| ● | to provide for uncertificated debt securities in addition to or in place of certificated debt securities; |
| ● | to add to our covenants, restrictions, conditions or provisions such new covenants, restrictions, conditions or provisions for the
benefit of the holders of all or any series of debt securities, to make the occurrence, or the occurrence and the continuance, of a default
in any such additional covenants, restrictions, conditions or provisions an event of default or to surrender any right or power conferred
upon us in the indenture; |
| ● | to add to, delete from or revise the conditions, limitations, and restrictions on the authorized amount, terms, or purposes of issue,
authentication and delivery of debt securities, as set forth in the indenture; |
| ● | to make any change that does not adversely affect the interests of any holder of debt securities of any series in any material respect; |
| ● | to provide for the issuance of and establish the form and terms and conditions of the debt securities of any series as provided above
under “Description of Debt Securities—General” to establish the form of any certifications required to be furnished
pursuant to the terms of the indenture or any series of debt securities, or to add to the rights of the holders of any series of debt
securities; |
| ● | to evidence and provide for the acceptance of appointment under any indenture by a successor trustee; or |
| ● | to comply with any requirements of the SEC in connection with the qualification of any indenture under the Trust Indenture Act. |
In addition, under the indenture, the rights of
holders of a series of debt securities may be changed by us and the trustee with the written consent of the holders of at least a majority
in aggregate principal amount of the outstanding debt securities of each series that is affected. However, unless we provide otherwise
in the prospectus supplement applicable to a particular series of debt securities, we and the trustee may make the following changes only
with the consent of each holder of any outstanding debt securities affected:
| ● | extending the fixed maturity of any debt securities of any series; |
| ● | reducing the principal amount, reducing the rate of or extending the time of payment of interest, or reducing any premium payable
upon the redemption of any series of any debt securities; or |
| ● | reducing the percentage of debt securities, the holders of which are required to consent to any amendment, supplement, modification
or waiver. |
Discharge
Each indenture provides that we can elect to be
discharged from our obligations with respect to one or more series of debt securities, except for specified obligations, including obligations
to:
| ● | register the transfer or exchange of debt securities of the series; |
| ● | replace stolen, lost or mutilated debt securities of the series; |
| ● | pay principal of and premium and interest on any debt securities of the series; |
| ● | maintain paying agencies; |
| ● | hold monies for payment in trust; |
| ● | recover excess money held by the trustee; |
| ● | compensate and indemnify the trustee; and |
| ● | appoint any successor trustee. |
In order to exercise our rights to be discharged,
we must deposit with the trustee money or government obligations sufficient to pay all the principal of, any premium, if any, and interest
on, the debt securities of the series on the dates payments are due.
Form, Exchange and Transfer
We will issue the debt securities of each series
only in fully registered form without coupons and, unless we provide otherwise in the applicable prospectus supplement, in denominations
of $1,000 and any integral multiple thereof. The indenture provides that we may issue debt securities of a series in temporary or permanent
global form and as book-entry securities that will be deposited with, or on behalf of, The Depository Trust Company, or DTC, or another
depositary named by us and identified in the applicable prospectus supplement with respect to that series. To the extent the debt securities
of a series are issued in global form and as book-entry, a description of terms relating to any book-entry securities will be set forth
in the applicable prospectus supplement.
At the option of the holder, subject to the terms
of the indenture and the limitations applicable to global securities described in the applicable prospectus supplement, the holder of
the debt securities of any series can exchange the debt securities for other debt securities of the same series, in any authorized denomination
and of like tenor and aggregate principal amount.
Subject to the terms of the indenture and the
limitations applicable to global securities set forth in the applicable prospectus supplement, holders of the debt securities may present
the debt securities for exchange or for registration of transfer, duly endorsed or with the form of transfer endorsed thereon duly executed
if so required by us or the security registrar, at the office of the security registrar or at the office of any transfer agent designated
by us for this purpose. Unless otherwise provided in the debt securities that the holder presents for transfer or exchange, we will impose
no service charge for any registration of transfer or exchange, but we may require payment of any taxes or other governmental charges.
We will name in the applicable prospectus supplement
the security registrar, and any transfer agent in addition to the security registrar, that we initially designate for any debt securities.
We may at any time designate additional transfer agents or rescind the designation of any transfer agent or approve a change in the office
through which any transfer agent acts, except that we will be required to maintain a transfer agent in each place of payment for the debt
securities of each series.
If we elect to redeem the debt securities of any
series, we will not be required to:
| ● | issue, register the transfer of, or exchange any debt securities of that series during a period beginning at the opening of business
15 days before the day of mailing of a notice of redemption of any debt securities that may be selected for redemption and ending at the
close of business on the day of the mailing; or |
| ● | register the transfer of or exchange any debt securities so selected for redemption, in whole or in part, except the unredeemed portion
of any debt securities we are redeeming in part. |
Information Concerning the Trustee
The trustee, other than during the occurrence
and continuance of an event of default under an indenture, undertakes to perform only those duties as are specifically set forth in the
applicable indenture. Upon an event of default under an indenture, the trustee must use the same degree of care as a prudent person would
exercise or use in the conduct of his or her own affairs. Subject to this provision, the trustee is under no obligation to exercise any
of the powers given it by the indenture at the request of any holder of debt securities unless it is offered reasonable security and indemnity
against the costs, expenses and liabilities that it might incur.
Payment and Paying Agents
Unless we otherwise indicate in the applicable
prospectus supplement, we will make payment of the interest on any debt securities on any interest payment date to the person in whose
name the debt securities, or one or more predecessor securities, are registered at the close of business on the regular record date for
the interest.
We will pay principal of and any premium and interest
on the debt securities of a particular series at the office of the paying agents designated by us, except that unless we otherwise indicate
in the applicable prospectus supplement, we will make interest payments by check that we will mail to the holder or by wire transfer to
certain holders. Unless we otherwise indicate in the applicable prospectus supplement, we will designate the corporate trust office of
the trustee as our sole paying agent for payments with respect to debt securities of each series. We will name in the applicable prospectus
supplement any other paying agents that we initially designate for the debt securities of a particular series. We will maintain a paying
agent in each place of payment for the debt securities of a particular series.
All money we pay to a paying agent or the trustee
for the payment of the principal of or any premium or interest on any debt securities that remains unclaimed at the end of two years after
such principal, premium or interest has become due and payable will be repaid to us, and the holder of the debt security thereafter may
look only to us for payment thereof.
Governing Law
The indenture and the debt securities will be
governed by and construed in accordance with the internal laws of the State of New York, except to the extent that the Trust Indenture
Act of 1939 is applicable.
DESCRIPTION OF
WARRANTS
General
The following description, together with the additional
information we may include in any applicable prospectus supplement and in any related free writing prospectus that we may authorize to
be distributed to you, summarizes the material terms and provisions of the warrants that we may offer under this prospectus, which may
consist of warrants to purchase ordinary shares or debt securities and may be issued in one or more series. Warrants may be offered independently
or in combination with ordinary shares or debt securities offered by any prospectus supplement. While the terms we have summarized below
will apply generally to any warrants that we may offer under this prospectus, we will describe the particular terms of any series of warrants
in more detail in the applicable prospectus supplement. The following description of warrants will apply to the warrants offered by this
prospectus unless we provide otherwise in the applicable prospectus supplement. The applicable prospectus supplement for a particular
series of warrants may specify different or additional terms.
We have filed forms of the warrant agreements and
forms of warrant certificates as exhibits to the registration statement of which this prospectus is a part. We will file as exhibits to
the registration statement of which this prospectus is a part, or will incorporate by reference from reports that we file with the SEC,
the form of warrant agreement, if any, including a form of warrant certificate, that contains the terms of the particular series of warrants
we are offering, as well as any supplemental agreements, before the issuance of such warrants. The following summaries of material terms
and provisions of the warrants are subject to, and qualified in their entirety by reference to, all the provisions of the form of warrant
or the warrant agreement and warrant certificate, as applicable, and any supplemental agreements applicable to a particular series of
warrants that we may offer under this prospectus. We urge you to read the applicable prospectus supplement related to the particular series
of warrants that we may offer under this prospectus, as well as any related free writing prospectus, and the complete form of warrant
or the warrant agreement and warrant certificate, as applicable, and any supplemental agreements, that contain the terms of the warrants.
General
In
the applicable prospectus supplement, we will describe the terms of the series of warrants being offered, including, to the extent applicable:
| ● | the
offering price or prices and aggregate number of warrants offered; |
| ● | the
currency or currencies for which the warrants may be purchased; |
| ● | the
designation and terms of the securities with which the warrants are issued and the number
of warrants issued with each such security or each principal amount of such security; |
| ● | the
date on and after which the warrants and the related securities will be separately transferable; |
| ● | the
minimum or maximum amount of such warrants which may be exercised at any one time; |
| ● | in
the case of warrants to purchase debt securities, the principal amount of debt securities
purchasable on exercise of one warrant and the price at, and currency in which, this principal
amount of debt securities may be purchased on such exercise; |
| ● | in
the case of warrants to purchase ordinary shares, the number of equity securities purchasable
on the exercise of one warrant and the price at which, and the currency in which, these equity
securities may be purchased on such exercise; |
| ● | the
effect of any merger, consolidation, sale or other disposition of our business on the warrant
agreements and the warrants; |
| ● | the
terms of any rights to redeem or call the warrants; |
| ● | the
terms of any rights to force the exercise of the warrants; |
| ● | any
provisions for changes to or adjustments in the exercise price or number of securities issuable
on exercise of the warrants; |
| ● | the
dates on which the right to exercise the warrants will commence and expire; |
| ● | the
manner in which the warrant agreements and warrants may be modified; |
| ● | a
discussion of certain material U.S. federal income tax considerations and any applicable
material Israeli tax considerations of holding or exercising the warrants; |
| ● | the
terms of the securities issuable on exercise of the warrants; and |
| ● | any
other specific terms, preferences, rights or limitations of or restrictions on the warrants. |
Before
exercising their warrants, holders of warrants will not have any of the rights of holders of the securities purchasable on such exercise,
including:
| ● | in
the case of warrants to purchase ordinary shares, the right to receive dividends, if any,
or payments upon our liquidation, dissolution or winding up or to exercise voting rights,
if any; or |
| | | |
| ● | in
the case of warrants to purchase debt securities, the right to receive payments of principal
of, or premium, if any, or interest on, the debt securities purchasable upon exercise or
to enforce covenants in the applicable indenture. |
Exercise
of Warrants
Each
warrant will entitle the holder to purchase the securities that we specify in the applicable prospectus supplement at the exercise price
that we describe in the applicable prospectus supplement. The warrants may be exercised as described in the prospectus supplement relating
to the warrants offered. Unless we otherwise specify in the applicable prospectus supplement, warrants may be exercised at any time up
to the expiration date that we list in the applicable prospectus supplement. After the close of business on the expiration date, unexercised
warrants will become void.
Unless
we otherwise specify in the applicable prospectus supplement, holders of the warrants may exercise the warrants by delivering the warrant
certificate representing the warrants to be exercised together with specified information, and paying the required amount to the warrant
agent in immediately available funds, as provided in the applicable prospectus supplement. We will set forth on the reverse side of the
warrant certificate and in the applicable prospectus supplement the information that the holder of the warrant will be required to deliver
to the warrant agent in connection with the exercise of the warrant.
On
receipt of the required payment and the warrant certificate properly completed and duly executed at the corporate trust office of the
warrant agent, if any, or any other office, including ours, indicated in the applicable prospectus supplement, we will issue and deliver
the securities purchasable on such exercise. If fewer than all of the warrants represented by the warrant certificate are exercised,
then we will issue a new warrant certificate for the remaining amount of warrants. If we so indicate in the applicable prospectus supplement,
holders of the warrants may surrender securities as all or part of the exercise price for warrants.
Governing
Law
Unless
we provide otherwise in the applicable prospectus supplement, the warrants and warrant agreements will be governed by and construed in
accordance with the laws of the State of New York.
Transfer
Agent and Registrar
The
transfer agent and registrar for any warrants will be set forth in the applicable prospectus supplement.
DESCRIPTION OF
RIGHTS
The following description, together with the additional
information that we include in any applicable prospectus supplements summarizes the material terms and provisions of the rights that we
may offer under this prospectus and the related rights agreements. While the terms we have summarized below will apply generally to any
rights that we may offer under this prospectus, we will describe the particular terms of any rights in more detail in the applicable prospectus
supplement. The terms of any rights offered under a prospectus supplement may differ from the terms described below.
We will incorporate by reference from reports that
we file with the SEC the form of any rights certificate or rights agreement that describes the terms of the rights we are offering, and
any supplemental agreements, before the issuance of the related rights. The following summaries of material terms and provisions of the
rights are subject to, and qualified in their entirety by reference to, all the provisions of any rights certificate or rights agreement
and any supplemental agreements applicable to particular rights. We urge you to read the applicable prospectus supplements related to
the particular rights that we may offer under this prospectus, as well as any related free writing prospectuses and the complete rights
certificate or rights agreement and any supplemental agreements that contain the terms of the rights.
General
We may issue rights to purchase
our equity or debt securities described in this prospectus. We may offer rights separately or together with one or more securities offered
hereby and may or may not be transferable by the shareholder receiving the rights in such offering, as described in
the applicable prospectus supplement. In connection with any offering of rights, we may enter into a standby agreement with one or more
underwriters or other purchasers pursuant to which the underwriter or other purchasers may be required to purchase any securities remaining
unsubscribed for after such offering.
We
will describe in the applicable prospectus supplement the specific terms of any offering of rights for which this prospectus is being
delivered, including the following:
| ● | the
price, if any, for the rights; |
| ● | the
exercise price payable for our equity or debt securities upon the exercise of the rights; |
| ● | the
number of rights issued to each shareholder; |
| ● | the
amount of our equity or debt securities that may be purchased per each right; |
| ● | the
extent to which the rights are transferable; |
| ● | any
other terms of the rights, including the terms, procedures and limitations relating to the
exchange and exercise of the rights; |
| ● | the
date on which the right to exercise the rights shall commence, and the date on which the
rights shall expire; |
| ● | the
extent to which the rights may include an over-subscription privilege with respect to unsubscribed
securities; and |
| ● | if
applicable, the material terms of any standby underwriting or purchase arrangement entered
into by us in connection with the offering of rights. |
Rights
Agent
The
rights agent, if any, for any rights we offer will be set forth in the applicable prospectus supplement.
DESCRIPTION OF
UNITS
The following description, together with the additional
information that we include in any applicable prospectus supplement, summarizes the material terms and provisions of the units that
we may offer under this prospectus. While the terms we have summarized below will apply generally to any units that we may offer
under this prospectus, we will describe the particular terms of any series of units in more detail in the applicable prospectus supplement.
The terms of any units offered under a prospectus supplement may differ from the terms described below.
We will incorporate by reference from reports that
we file with the SEC, the form of unit agreement that describes the terms of the series of units we are offering, and any supplemental
agreements, before the issuance of the related series of units. The following summaries of material terms and provisions of the units
are subject to, and qualified in their entirety by reference to, all the provisions of the unit agreement and any supplemental agreements
applicable to a particular series of units. We urge you to read the applicable prospectus supplement related to the particular series
of units that we may offer under this prospectus, as well as any related free writing prospectuses and the complete unit agreement
and any supplemental agreements that contain the terms of the units.
General
We may issue units consisting of any combination
of the other types of securities offered under this prospectus in one or more series. Each unit will be issued so that the holder of the
unit is also the holder of each security included in the unit. Thus, the holder of a unit will have the rights and obligations of a holder
of each security included in the unit. The unit agreement under which a unit is issued may provide that the securities included in the
unit may not be held or transferred separately, at any time or at any time before a specified date or upon the occurrence of a specified
event or occurrence.
We
will describe in the applicable prospectus supplement the terms of the series of units being offered, including:
| ● | the
designation and terms of the units and of the securities comprising the units,
including whether and under what circumstances those securities may be held or transferred
separately; |
| ● | any
provisions of the governing unit agreement that differ from those described below; |
| ● | certain
material U.S. federal income tax considerations and material U.K. tax considerations relevant
to the units; and |
| ● | any
provisions for the issuance, payment, settlement, transfer or exchange of the units
or of the securities comprising the units. |
The provisions described in this section, as well
as those set forth in any prospectus supplement or as described under the sections entitled “Description of Share Capital,”
“Description of Debt Securities,” “Description of Warrants” and “Description of Rights” will apply
to each unit, as applicable, and to any ordinary shares debt security, warrant or right included in each unit, as applicable.
Unit Agent
The name and address of the unit agent, if any,
for any units we offer will be set forth in the applicable prospectus supplement.
Issuance in Series
We may issue units in such amounts and in such
numerous distinct series as we determine.
Enforceability of Rights by Holders of Units
Each unit agent will act solely as our agent under
the applicable unit agreement and will not assume any obligation or relationship of agency or trust with any holder of any unit. A single
bank or trust company may act as unit agent for more than one series of units. A unit agent will have no duty or responsibility in
case of any default by us under the applicable unit agreement or unit, including any duty or responsibility to initiate any proceedings
at law or otherwise, or to make any demand upon us. Any holder of a unit may, without the consent of the related unit agent or the holder
of any other unit, enforce by appropriate legal action its rights as holder under any security included in the unit.
PLAN OF DISTRIBUTION
We may sell the securities in one or more of the
following ways (or in any combination) from time to time:
| ● | through underwriters or dealers; |
| ● | directly to a limited number of purchasers or to a single purchaser; |
| ● | through any other method permitted by applicable law and described in the applicable prospectus supplement. |
The distribution of our securities may be carried
out, from time to time, in one or more transactions, including:
| ● | block transactions and transactions on the Nasdaq Global Market or any other organized market where the securities may be traded; |
| ● | purchases by a broker-dealer as principal and resale by the broker-dealer for its own account pursuant to a prospectus supplement; |
| ● | ordinary brokerage transactions and transactions in which a broker-dealer solicits purchasers; |
| ● | sales “at the market” or into an existing trading market, on an exchange or otherwise; or |
| ● | sales in other ways not involving market makers or established trading markets, including direct sales to purchasers. |
A prospectus supplement or supplements (and any
related free writing prospectus that we may authorize to be provided to you) will describe the terms of the offering of the securities,
including, to the extent applicable:
| ● | the name or names of any underwriters, dealers or agents; |
| ● | the method of distribution; |
| ● | the public offering price or purchase price and the proceeds to us from that sale; |
| ● | the expenses of the offering; |
| ● | any discounts or commissions to be allowed or paid to the underwriters, dealers or agents; |
| ● | all other items constituting underwriting compensation and the discounts and commissions to be allowed or paid to dealers, if any;
and |
| ● | any other information regarding the distribution of the securities that we believe to be material. |
Underwriters may offer and sell the securities
at a fixed price or prices, which may be changed, or from time to time at market prices prevailing at the time of sale, at prices related
to prevailing market prices or at negotiated prices. We may, from time to time, authorize agents acting on a best or reasonable efforts
basis as our agents to solicit or receive offers to purchase the securities upon the terms and conditions as are set forth in the applicable
prospectus supplement. In connection with the sale of securities, underwriters or agents may be deemed to have received compensation from
us in the form of underwriting discounts or commissions and may also receive commissions from purchasers of securities for whom they may
act as agent. Underwriters may sell securities to or through dealers, and dealers may receive compensation in the form of discounts, concessions
or commissions from the underwriters and/or commissions from the purchasers for whom they may act as agent.
Underwriters, dealers and agents who participate
in the distribution of securities and their controlling persons may be entitled, under agreements that may be entered into with us to
indemnification by us against certain liabilities, including liabilities under the Securities Act, or to contribution with respect to
payments that the underwriters, dealers or agents and their controlling persons may be required to make in respect of those liabilities.
We may also make direct sales through subscription
rights distributed to our existing shareholders on a pro rata basis, which may or may not be transferable. In any distribution of subscription
rights to our shareholders, if all of the underlying securities are not subscribed for, we may then sell the unsubscribed securities directly
to third parties or may engage the services of one or more underwriters, dealers or agents, including standby underwriters, to sell the
unsubscribed securities to third parties.
Certain persons participating in an offering may
engage in over-allotment, stabilizing transactions, short-covering transactions and penalty bids in accordance with Regulation M under
the Exchange Act that stabilize, maintain or otherwise affect the price of the offered securities. If any such activities will occur,
they will be described in the applicable prospectus supplement.
ENFORCEMENT OF
CIVIL LIABILITIES
We are incorporated under the laws of the State
of Israel. Service of process upon us and any Israeli experts named in this prospectus may be difficult to obtain within the United States.
Furthermore, because most of our assets are located outside the United States, any judgment obtained in the United States against us may
not be collectible within the United States.
We have irrevocably appointed Gamida Cell Inc.
as our agent to receive service of process in any action against us in any U.S. federal or state court arising out of this offering or
any purchase or sale of securities in connection with any offering described in this prospectus. The address of our agent is 116 Huntington
Avenue, 7th Floor, Boston, Massachusetts 02116.
We have been informed by our legal counsel in
Israel, Meitar | Law Offices, that it may be difficult to initiate an action with respect to U.S. securities law in Israel. Israeli courts
may refuse to hear a claim based on an alleged violation of U.S. securities laws reasoning that Israel is not the most appropriate forum
to hear such a claim. In Israeli courts, the content of applicable U.S. law must be proved as a fact by expert witnesses which can be
a time-consuming and costly process and certain matters of procedure may be governed by Israeli law.
Subject to certain time limitations and legal
procedures, Israeli courts may enforce a U.S. judgment in a civil matter which, subject to certain exceptions, is non-appealable, including
judgments based upon the civil liability provisions of the Securities Act and the Exchange Act and including a monetary or compensatory
judgment in a non-civil matter, provided that:
| ● | the judgment was rendered by a court which was, according to the laws of the state of the court, competent
to render the judgment; |
| ● | the obligation imposed by the judgment is enforceable according to the rules relating to the enforceability of judgments in Israel
and the substance of the judgment is not contrary to public policy; and |
| ● | the judgment is executory in the state in which it was given. |
Even if these conditions are met, an Israeli court
will not declare a foreign civil judgment enforceable if:
| ● | the judgment was given in a state whose laws do not provide for the enforcement of judgments of Israeli
courts (subject to exceptional cases); |
| ● | the enforcement of the judgment is likely to prejudice the sovereignty or security of the State of Israel; |
| ● | the judgment was obtained by fraud; |
| ● | the opportunity given to the defendant to bring its arguments and evidence before the court was not reasonable in the opinion of the
Israeli court; |
| ● | the judgment was rendered by a court not competent to render it according to the laws of private international law as they apply in
Israel; |
| ● | the judgment is contradictory to another judgment that was given in the same matter between the same parties and that is still valid;
or |
| ● | at the time the action was brought in the foreign court, a lawsuit in the same matter and between the same parties was pending before
a court or tribunal in Israel. |
LEGAL MATTERS
The validity of the issuance of our ordinary shares
offered in this prospectus and certain other matters of Israeli law will be passed upon for us by Meitar | Law Offices, Israel. Certain
matters of U.S. federal law and New York State law will be passed upon for us by Cooley LLP, New York, New York. Additional legal matters
may be passed upon for us or any underwriters, dealers or agents, by counsel that we will name in the applicable prospectus supplement.
EXPERTS
The consolidated financial statements as of December
31, 2022 and 2021 and for each of the three years in the period ended December 31, 2022, incorporated in this Prospectus by reference
to the Company’s Annual Report on Form 10-K filed on March 31, 2023, have been audited by Kost, Forer, Gabbay & Kasierer, a
member of Ernst & Young Global, independent registered public accounting firm, as set forth in their report thereon (which contain
an explanatory paragraph describing conditions that raise substantial doubt about the Company’s ability to continue as a going concern
as described in the notes to the consolidated financial statements) incorporated by reference herein, and are included in reliance upon
such report given on the authority of such firm as experts in accounting and auditing. The address of Kost, Forer, Gabbay & Kasierer
is Menachem Begin 144, Tel Aviv, Israel.
WHERE YOU CAN FIND
MORE INFORMATION
This prospectus is part of a registration statement
we filed with the SEC. This prospectus does not contain all of the information set forth in the registration statement and the exhibits
to the registration statement. For further information with respect to us and the securities we are offering under this prospectus, we
refer you to the registration statement and the exhibits and schedules filed as a part of the registration statement. Neither we nor any
agent, underwriter or dealer has authorized any person to provide you with different information. We are not making an offer of these
securities in any state where the offer is not permitted. You should not assume that the information in this prospectus is accurate as
of any date other than the date on the front page of this prospectus, regardless of the time of delivery of this prospectus or any sale
of the securities offered by this prospectus.
We file annual, quarterly and current reports,
proxy statements and other information with the SEC. Our SEC filings are available to the public at the SEC’s website at www.sec.gov.
You also may access these filings on our website at www.gamida-cell.com. We do not incorporate the information on our website into this
prospectus or any supplement to this prospectus and you should not consider any information on, or that can be accessed through, our website
as part of this prospectus or any supplement to this prospectus (other than those filings with the SEC that we specifically incorporate
by reference into this prospectus or any supplement to this prospectus).
INCORPORATION OF
CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference”
into this prospectus and any accompanying prospectus supplement the information we have filed with the SEC. This means that we can disclose
important information by referring you to another document filed separately with the SEC. The information incorporated by reference is
considered to be a part of this prospectus, and information that we file later with the SEC will also be deemed to be incorporated by
reference into this prospectus and to be a part hereof from the date of filing of such documents and will automatically update and supersede
previously filed information, including information contained in this document.
We incorporate by reference into this prospectus
and any accompanying prospectus supplement the following documents that we have filed with the SEC:
| ● | our Annual Report on Form 10-K for the year ended December 31, 2022, filed with the SEC on March 31, 2023; |
| ● | our Quarterly Reports on Form 10-Q for the quarters ended March 31, 2023, June 30, 2023, and September
30, 2023, filed with the SEC on May 15, 2023, August 14, 2023, and November 14, 2023, respectively; |
| ● | our
Current Reports on Form 8-K filed with the SEC on January
9, 2023, January
19, 2023, January
30, 2023, March
20, 2023, March
27, 2023, April
17, 2023, April
21, 2023, May
19, 2023, May
22, 2023, August
14, 2023, September
27, 2023, October
20, 2023, November
15, 2023 and November
24, 2023 to the extent the information in such reports is filed and not furnished; and |
| ● | the description of our ordinary shares contained in our Registration Statement on Form 8-A, filed with
the SEC on October 23, 2018, as amended on March 25, 2022, as the description therein has been updated and superseded by the description
of our capital stock contained in Exhibit 4.1 to our Annual Report on Form 10-K for the year ended December 31, 2022, including any further
amendments or reports filed for the purposes of updating this description. |
We also incorporate by reference into this prospectus
all documents (other than current reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on such form that are
related to such items) that are filed by us with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act (i) after
the date of the initial filing of the registration statement of which this prospectus forms a part and prior to effectiveness of the registration
statement, or (ii) after the date of this prospectus but prior to the termination of the offering.
We will furnish without charge to each person,
including any beneficial owner, to whom a prospectus is delivered, on written or oral request, a copy of any or all of the documents incorporated
by reference in this prospectus, including exhibits to these documents. You should direct any requests for documents, either in writing
to Gamida Cell Ltd., 116 Huntington Avenue Boston, MA 02116, Attn: General Counsel or by telephone (617) 892-9080.
Any statement contained in a document incorporated
or deemed to be incorporated by reference in this prospectus or any prospectus supplement will be deemed modified, superseded or replaced
for purposes of this prospectus or any prospectus supplement to the extent that a statement contained in any other subsequently filed
document that also is or is deemed to be incorporated by reference in this prospectus or any prospectus supplement modifies, supersedes
or replaces such statement. Any statement that is modified or superseded will not constitute a part of this prospectus or any prospectus
supplement, except as modified or superseded.
The information in this
prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities
and Exchange Commission is effective. This prospectus is not an offer to sell these securities, and we are not soliciting an offer to
buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION,
DATED DECEMBER 6, 2023
PROSPECTUS

Up to $50,000,000
Ordinary Shares
We have previously entered into that
certain Amended & Restated Open Market Sale AgreementSM, or the sale agreement, with
Jefferies LLC, or Jefferies, dated June 5, 2023, relating to the sale of our ordinary shares offered by this prospectus. In accordance
with the terms of the sale agreement, under this prospectus, we may offer and sell shares of our ordinary shares having an aggregate offering
price of up to $50,000,000 from time to time through Jefferies, acting as sales agent.
Our ordinary shares are traded on the Nasdaq
Global Market under the symbol “GMDA.” On December 5, 2023, the closing price of our ordinary shares as reported by the Nasdaq
Global Market was $0.32 per ordinary share.
Sales of our ordinary shares, if any, under this
prospectus and the accompanying base prospectus may be made by any method permitted that is deemed to be an “at the market offering”
as defined in Rule 415(a)(4) promulgated under the Securities Act of 1933, as amended, or the Securities Act. Jefferies is not required
to sell any specific amount of ordinary shares, but will act as our sales agent using commercially reasonable efforts to sell on our behalf
all of the ordinary shares requested to be sold by us, consistent with its normal trading and sales practices, on mutually agreed terms
between Jefferies and us. There is no arrangement for funds to be received in any escrow, trust or similar arrangement.
Jefferies will be entitled to compensation under
the terms of the sale agreement at a commission rate equal to 3.0% of the aggregate gross sales price of ordinary shares sold through
it. In connection with the sale of our ordinary shares on our behalf, Jefferies will be deemed to be an “underwriter” within
the meaning of the Securities Act and the compensation of Jefferies will be deemed to be underwriting commissions or discounts. We
have also agreed to provide indemnification and contribution to Jefferies with respect to certain liabilities, including liabilities under
the Securities Act. See “Plan of Distribution” beginning on page S-20 regarding the compensation to be paid to Jefferies.
Investing in our securities involves a high
degree of risk. You should review carefully the risks and uncertainties described and incorporated by reference under the heading “Risk
Factors” and any related free writing prospectus, and under similar headings in the other documents that are incorporated by reference
into this prospectus as described on page S-6 of this prospectus.
Neither the U.S. Securities and Exchange Commission
nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete.
Any representation to the contrary is a criminal offense.
Jefferies
The date of this prospectus is ,
2023
Table of Contents
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement
on Form S-3 that we filed with the Securities and Exchange Commission, or the SEC, using a “shelf” registration process. This
prospectus is not complete without, and may not be utilized except in connection with, the accompanying base prospectus. Under this shelf
registration process, we may sell any combination of the securities described in our base prospectus included in the shelf registration
statement in one or more offerings up to a total aggregate offering price of $150,000,000. The $50,000,000 of ordinary shares that may
be offered, issued and sold under this prospectus is included in the $150,000,000 of securities that may be offered, issued and sold by
us pursuant to our shelf registration statement.
In this prospectus, as permitted by law, we “incorporate
by reference” information from other documents that we file with the SEC. This means that we can disclose important information
to you by referring to those documents. The information incorporated by reference is considered to be a part of this prospectus,
and should be read with the same care. When we make future filings with the SEC to update the information contained in documents that
have been incorporated by reference, the information included or incorporated by reference in this prospectus is considered to be automatically
updated and superseded. However, if any statement in this prospectus is inconsistent with a statement in another document having a later
date (including a document incorporated by reference in the accompanying prospectus), the statement in the document having the later date
modifies or supersedes the earlier statement.
Neither we nor Jefferies have authorized anyone
to provide you with information different from that contained in this prospectus or any free writing prospectus we have authorized for
use in connection with this offering. Neither we nor Jefferies take any responsibility for, and can provide no assurance as to the reliability
of, any other information that others may give you. The information contained in, or incorporated by reference into, this prospectus,
and any free writing prospectus we have authorized for use in connection with this offering is accurate only as of the date of each such
document. Our business, financial condition, results of operations and prospects may have changed since those dates. You should read this
prospectus, the documents incorporated by reference in this prospectus and any free writing prospectus that we have authorized for use
in connection with this offering in their entirety before making an investment decision. You should also read and consider the information
in the documents to which we have referred you in the sections of the accompanying prospectus entitled “Where You Can Find More
Information” and “Incorporation of Certain Documents by Reference.” These documents contain important information that
you should consider when making your investment decision.
We are offering to sell, and seeking offers to
buy, our ordinary shares only in jurisdictions where offers and sales are permitted. The distribution of this prospectus or any free writing
prospectus we have authorized for use in connection with this offering in certain jurisdictions may be restricted by law. Persons outside
the United States who come into possession of this prospectus or any free writing prospectus we have authorized for use in connection
with this offering must inform themselves about, and observe any restrictions relating to, the offering of the ordinary shares and the
distribution of this prospectus and any free writing prospectus we have authorized for use in connection with this offering outside the
United States. This prospectus and any free writing prospectus we have authorized for use in connection with this offering do not
constitute, and may not be used in connection with, an offer to sell, or a solicitation of an offer to buy, any securities offered by
this prospectus or any such free writing prospectus by any person in any jurisdiction in which it is unlawful for such person to make
such an offer or solicitation.
We further note that the representations, warranties
and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated by reference in the prospectus
were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among
the parties to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations,
warranties or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and covenants should
not be relied on as accurately representing the current state of our affairs.
We own various trademark registrations and applications,
and unregistered trademarks. Gamida Cell, omidubicel, Omisirge® and GDA-201 are trademarks of ours that we use in this prospectus.
This prospectus also includes trademarks, tradenames and service marks that are the property of other organizations. Solely for convenience,
our trademarks and tradenames referred to in this prospectus appear without the ® or ™ symbols, but those references are not
intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights, or the right of the
applicable licensor to our trademark and tradenames. We do not intend to use or display other companies’ trademarks and trade names
to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
The financial statements incorporated by reference
in this prospectus have been prepared in accordance with accounting principles generally accepted in the United States, or GAAP,
as issued by the Financial Accounting Standards Board, FASB. We have made rounding adjustments to some of the figures included in
this prospectus. Accordingly, numerical figures shown as totals in some tables may not be an arithmetic aggregation of the figures that
preceded them.
Unless the context indicates otherwise, references
in this prospectus to “NIS” are to the legal currency of Israel, and “U.S. dollars,” “$” or “dollars”
are to United States dollars. References in this prospectus to “Gamida Cell,” “Gamida,” “the Company,”
“we,” “us” and “our” refer to Gamida Cell Ltd. and our wholly owned subsidiary Gamida Cell Inc., except
where the context otherwise requires or as otherwise indicated.
You should rely only on the information contained
or incorporated by reference in this prospectus or any “free writing prospectus” we may authorize to be delivered to you.
We have not, and the sales agent has not, authorized anyone to provide you with different information. If anyone provides you with different
or inconsistent information, you should not rely on it. You should assume that the information appearing in this prospectus and the documents
incorporated by reference herein and therein are accurate only as of their respective dates. Our business, financial condition, results
of operations and prospects may have changed since those dates.
This prospectus shall not constitute an offer or solicitation
by anyone in any jurisdiction in which such offer or solicitation is not authorized or in which the person making such offer or solicitation
is not qualified to do so or to anyone to whom it is unlawful to make such offer or solicitation.
PROSPECTUS SUMMARY
This summary highlights only some of the information
included or incorporated by reference in this prospectus. You should carefully read this entire prospectus and the accompanying base prospectus,
including the risks and uncertainties discussed under the heading “Risk Factors” beginning on page S-6 of this prospectus
and in the documents incorporated by reference into this prospectus, together with the additional information about us described in the
sections entitled “Where You Can Find Additional Information” and “Incorporation of Certain Information by Reference”,
including our financial statements, before making an investment decision. If you invest in our securities, you are assuming a high degree
of risk.
Overview
We are a cell therapy pioneer working to turn
cells into powerful therapeutics. We apply a proprietary expansion platform leveraging the properties of nicotinamide, or NAM, to allogeneic
cell sources including umbilical cord blood-derived cells and natural killer, or NK, cells to create cell therapy candidates, with the
potential to redefine standards of care. On April 17, 2023, the U.S. Food and Drug Administration, or FDA, approved our allogenic cell
therapy, Omisirge (omidubicel-onlv), for use in adult and pediatric patients 12 years and older with hematologic malignancies who are
planned for umbilical cord blood transplantation following myeloablative conditioning to reduce the time to neutrophil recovery and the
incidence of infection.
According to the Center for International Blood
and Marrow Transplant Research, in the United States, there are approximately 8,000 patients above the age of 12 with hematologic malignancies
who undergo an allogeneic stem cell transplant each year, and we believe that number of patients may grow over time. We estimate that
there are approximately 1,700 patients each year, who are above the age of 12 and are deemed eligible for an allogeneic stem cell transplant
but cannot find an appropriate donor.
We believe the commercial potential for Omisirge
consists of two key opportunities: potentially improving outcomes for patients, and potentially increasing access for patients who are
currently eligible for transplant and cannot find an appropriate donor. We estimate that in 2028 approximately 10,000 patients who are
ages 12 and above with hematologic malignancies will be eligible for transplant and that Omisirge could be the treatment of choice for
approximately 20% of this population.
Corporate Information
We are an Israeli corporation and were incorporated
in 1998. Our principal executive offices are located at 116 Huntington Avenue, 7th Floor, Boston, Massachusetts 02116. Our telephone number
is (617) 892-9080. Our website address is www.gamida-cell.com. The information contained on, or that can be accessed through, our website
is not incorporated by reference into this prospectus. We have included our website address as an inactive textual reference only.
Risks Associated with our Business
Our business is subject to numerous risks, as
described under the heading “Risk Factors” contained in this prospectus and in any free writing prospectuses that we have
authorized for use in connection with this offering, and under similar headings in the documents that are incorporated by reference herein.
You should be aware of these risks before making an investment decision. In particular, these risks include, among others:
| ● | We are heavily dependent on the success of Omisirge, including obtaining regulatory approvals in geographies
outside of the United States, and if Omisirge is not successfully commercialized, our business will be adversely affected. |
| ● | We have limited experience producing Omisirge at commercial levels and we have limited experience operating
a cGMP compliant manufacturing facility. |
| ● | We currently have a limited marketing and sales organization. If we are unable to establish adequate sales
and marketing capabilities to support the commercial launch of Omisirge or enter into agreements with third parties to market and sell
Omisirge, we may be unable to generate sufficient product revenue. |
| ● | Sales of Omisirge will be limited unless it achieves broad market acceptance by physicians, patients,
third-party payers, hospital pharmacists and others in the medical community. |
| ● | It may be difficult for us to profitably sell Omisirge if coverage and reimbursement for Omisirge is limited
by government authorities and/or third-party payer policies. |
| ● | Although we are exploring a range of strategic alternatives, there is no certainty that we will be able
to execute on any transaction or that such a transaction will enhance shareholder value, and any such transaction, if available and achieved,
may be highly dilutive to our stockholders. |
| ● | The costs associated with a potential strategic transaction may be significant. |
| ● | We have incurred significant losses since our inception. We anticipate that we will continue to incur
significant losses for the foreseeable future, and we may never achieve or maintain profitability. |
| ● | There is substantial doubt regarding our ability to continue as a going concern. Operating our business
and servicing our debt requires a significant amount of cash, and we will need to obtain additional funding or complete a strategic transaction
in the near-term to continue to sufficiently fund our operations and pay our substantial debt, including our 5.875% convertible senior
notes that mature in February 2026, or the 2021 Notes, and our first lien secured note that matures in December 2024, or the 2022 Note. |
| ● | The Indenture governing the 2021 Notes and the Loan and Security Agreement governing the 2022 Note each
contains restrictive and financial covenants and other provisions that adversely affect our liquidity and may make it more difficult to
execute our strategy or to effectively compete. |
| ● | We have generated limited revenue to date from product sales and may never be profitable. |
| ● | We may be unable to obtain regulatory approval for GDA-201 or any future product candidates. |
| ● | The results of earlier studies and trials may not be predictive of future trial results, and our clinical
trials may fail to adequately demonstrate the safety and efficacy of our product candidates. |
| ● | Interim, “topline” and preliminary data from our clinical trials that we announce or publish
from time to time may change as more patient data become available and are subject to audit and verification procedures that could result
in material changes in the final data. |
| ● | The success of our NAM technology platform and our product candidates is substantially dependent on developments
within the emerging field of cellular therapies, some of which are beyond our control. |
| ● | Because GDA-201 is based on novel technologies, it is difficult to predict the time and cost of development
and our ability to successfully complete clinical development of GDA-201 and obtain the necessary regulatory approvals for commercialization. |
| ● | We may find it difficult to enroll patients in our clinical studies, which could delay or prevent us from
proceeding with clinical trials. |
| ● | Omisirge, GDA-201, or any future product candidates and the administration process may cause undesirable
side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved
label or result in significant negative consequences following marketing approval, and result in costly and damaging product liability
claims against us. |
| ● | Even if we complete the necessary clinical trials, we cannot predict when, or if, we will obtain regulatory
approval to commercialize GDA-201 or any of our future product candidates, and the approval may be for a narrower indication than we seek
or be subject to other limitations or restrictions that limit its commercial profile. |
| ● | Enacted and future healthcare legislation may increase the difficulty and cost for us to commercialize
Omisirge and obtain marketing approval for and commercialize GDA-201 and may affect the prices we set. |
| ● | Our business operations and current and future relationships with investigators, healthcare professionals,
consultants, third-party payers, patient organizations and customers will be subject to applicable healthcare regulatory laws, which could
expose us to penalties. |
| ● | Legislative or regulatory healthcare reforms in the United States may make it more difficult and costly
for us to obtain regulatory clearance or approval of our product candidates and to produce, market and distribute our products after clearance
or approval is obtained. |
| ● | We may rely on third parties to conduct certain elements of our preclinical studies and clinical trials
and perform other tasks for us. If these third parties do not successfully carry out their contractual duties, meet expected deadlines
or comply with regulatory requirements, we may not be able to obtain regulatory approval for or commercialize our product candidates. |
| ● | We rely on a single facility to manufacture Omisirge that is located in Kiryat Gat, Israel, proximate
to the ongoing hostilities between Israel and Hamas in and around the Gaza Strip. Damage to this site from such hostilities or otherwise
could have a material adverse effect on our ability to manufacture Omisirge and generate revenue. |
| ● | We rely on our ability to import starting materials into Israel (including cord blood units, or CBUs)
to manufacture Omisirge and our ability to export Omisirge manufactured for a given patient. If the conflict between Israel and Hamas
affects the flow of air travel into and out of Ben Gurion Airport in Tel Aviv, it could have a material adverse impact on our ability
to manufacture and deliver Omisirge and generate revenue. |
| ● | We face a variety of challenges and uncertainties associated with our dependence on the availability of
human CBUs at cord blood banks for the manufacture of Omisirge. |
| ● | If we are unable to obtain, maintain or protect intellectual property rights related to Omisirge, GDA-201
or any future product candidates, we may not be able to compete effectively in our market. |
| ● | Due to our limited resources and access to capital, we must, and have in the past decided to, prioritize
development of certain product candidates over other potential candidates. These decisions may prove to have been wrong and may adversely
affect our revenue. |
| ● | The market price of our ordinary shares may fluctuate significantly, which could result in substantial
losses by our investors. |
| ● | The exchange of some or all of the 2021 Notes or 2022 Note into our ordinary shares could result in significant
dilution to existing shareholders, adversely affect the market price of our ordinary shares and impair our ability to raise capital through
the sale of additional equity securities. |
| ● | Significant
parts of our operations are located in Israel and, therefore, our results may be adversely
affected by political, economic and military conditions in Israel, including the recent attack
by Hamas and other terrorist organizations from the Gaza Strip and Israel’s war against
them. As of December 1, 2023, approximately 5% of our Israeli workforce had been called to serve
in the Israeli military; however, such employees are not so critical either individually
or in the aggregate to our operations so as to have a material adverse effect on our ability
to produce Omisirge. |
Implications of Being an “Emerging Growth Company”
We qualify as an “emerging growth company”
as defined in the Jumpstart our Business Startups Act of 2012, or the JOBS Act. As an emerging growth company, we may take advantage
of specified reduced reporting and other burdens that are otherwise applicable generally to public companies. These provisions include:
| ● | an exemption from the auditor attestation requirement in the assessment of our internal control over financial
reporting pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act; and |
| ● | reduced disclosure obligations regarding executive compensation in our periodic reports and other filings and exemptions from the
requirements of holding a non-binding advisory vote on executive compensation, including golden parachute compensation. |
We may take advantage of these provisions for
up to five years or such earlier time that we are no longer an emerging growth company. We will remain an emerging growth company
until the earliest of (i) December 31, 2023; (ii) the last day of the fiscal year in which we have total annual gross
revenues of $1.235 billion or more; (iii) the date on which we have issued more than $1.0 billion in non-convertible debt
during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under the rules of
the SEC, which means the market value of our ordinary shares that are held by non-affiliates equals or exceeds $700.0 million
as of the prior June 30.
In this prospectus, we have taken advantage of
certain of the reduced reporting requirements as a result of being an emerging growth company. Accordingly, the information contained
herein may be different than the information you receive from other public companies in which you hold equity securities.
THE OFFERING
|
Ordinary shares offered by us |
Ordinary shares, par value NIS 0.01 per share, having an aggregate offering price of up to $50,000,000. |
| |
|
| Plan of Distribution |
“At the market offering” that may be made from time to time through our sales agent, Jefferies LLC. See “Plan of Distribution.” |
| |
|
| Use of Proceeds |
We intend to use the net proceeds from this offering for (i) commercialization activities to support the launch of Omisirge; (ii) the continued clinical development of GDA-201; and (iii) general corporate purposes, including general and administrative expenses and working capital. See “Use of Proceeds” on page S-10 of this prospectus. |
| |
|
| Risk Factors |
Investing in our securities involves significant risks. See the information under the heading “Risk Factors” beginning on page S-6 of this prospectus and in the documents incorporated by reference into this prospectus for a discussion of factors you should carefully consider before deciding to invest in our securities. |
| |
|
| Nasdaq Global Market symbol |
Our ordinary shares are listed for trading on the Nasdaq Global Market under the symbol “GMDA.” |
RISK FACTORS
Investing in our ordinary shares may involve
a high degree of risk. Before deciding whether to invest in our ordinary shares, you should carefully consider the risks and uncertainties
described under the sections captioned “Risk Factors” contained in our most recent Annual Report on Form 10-K, and in our
subsequent Quarterly Reports on Form 10-Q, as well as any amendments thereto reflected in subsequent filings with the SEC, which are incorporated
by reference in this prospectus in their entirety, together with other information in this prospectus, the information and documents incorporated
by reference herein and therein, and in any free writing prospectus that we have authorized for use in connection with this offering.
If any of these risks actually occurs, our business, financial condition, cash flows and results of operations could be negatively impacted.
In that case, the trading price of our ordinary shares would likely decline and you might lose all or part of your investment. Additional
risks and uncertainties not presently known to us or that we currently deem immaterial also may impair our business operations.
Risks Related to our Securities and this Offering
Our management will have broad discretion
in the use of the net proceeds we receive in this offering and may allocate the net proceeds from this offering in ways that you and other
shareholders may not approve.
Our management will have broad discretion in the
use of the net proceeds, including for any of the purposes described in the section entitled “Use of Proceeds,” and you will
not have the opportunity as part of your investment decision to assess whether the net proceeds are being used appropriately. Because
of the number and variability of factors that will determine our use of the net proceeds from this offering, their ultimate use may vary
substantially from their currently intended use. The failure of our management to use these funds effectively could harm our business.
Pending their use, we may invest the net proceeds from this offering in short-term, investment-grade, interest-bearing securities
and depositary institutions. These investments may not yield a favorable return to our shareholders.
Investors in this offering may experience
immediate and substantial dilution.
The public offering price of the ordinary shares
offered pursuant to this prospectus may be higher than the net tangible book value per share of our ordinary shares. Therefore, if you
purchase securities in this offering, you may incur immediate and substantial dilution in the net tangible book value per share from the
price per share that you pay. If the holders of outstanding options to acquire ordinary shares exercise those options at prices below
the public offering price, you would incur further dilution. See the section entitled “Dilution” herein for a more detailed
discussion of the dilution associated with this offering.
Because we do not intend to declare cash dividends
on our ordinary shares in the foreseeable future, shareholders must rely on appreciation of the value of our ordinary shares for any return
on their investment and may not receive any funds without selling their ordinary shares.
We have never declared or paid cash dividends
on our ordinary shares and do not anticipate declaring or paying any cash dividends in the foreseeable future. As a result, we expect
that only appreciation of the price of our ordinary shares, if any, will provide a return to investors in this offering for the foreseeable
future. In addition, because we do not pay cash dividends, if our shareholders want to receive funds in respect of our ordinary shares,
they must sell their ordinary shares to do so.
You may experience future dilution
as a result of future equity offerings.
In order to raise additional capital, we expect
to in the future offer additional ordinary shares or other securities convertible into or exchangeable for our ordinary shares. We cannot
assure you that we will be able to sell shares or other securities in any other offering at a price per share that is equal to or greater
than the price per share paid by investors in this offering, and investors purchasing shares or other securities in the future could have
rights superior to existing stockholders. The price per share at which we sell additional ordinary shares or other securities convertible
into or exchangeable for our ordinary shares in future transactions may be higher or lower than the price per share in this offering.
Sales of a substantial number of our ordinary shares in the public
market could cause our share price to fall.
Sales of a substantial number of our ordinary
shares in the public market could occur at any time. These sales, or the perception in the market that we or the holders of a large number
of our shares intend to sell shares, could reduce the market price of our ordinary shares.
It is not possible to predict the aggregate proceeds resulting
from sales made under the sale agreement.
Subject to certain limitations in the sale agreement
and compliance with applicable law, we have the discretion to deliver a placement notice to Jefferies, at any time throughout the term
of the sale agreement. The number of shares that are sold through Jefferies after delivering a placement notice will fluctuate based on
a number of factors, including the market price of our ordinary shares during the sales period, any limits we may set with Jefferies in
any applicable placement notice and the demand for our ordinary shares. Because the price per ordinary share of each share sold pursuant
to the sale agreement will fluctuate over time, it is not currently possible to predict the aggregate proceeds to be raised in connection
with sales under the sale agreement.
Sales of ordinary shares offered hereby will be in “at
the market offerings,” and investors who buy shares at different times will likely pay different prices.
Investors who purchase ordinary shares in this
offering at different times will likely pay different prices, and accordingly may experience different levels of dilution and different
outcomes in their investment results. We will have discretion, subject to market demand, to vary the timing, prices and number of ordinary
shares sold in this offering. In addition, subject to the final determination by our board of directors or any restrictions we may place
in any applicable placement notice delivered to Jefferies, there is no minimum or maximum sales price for ordinary shares to be sold in
this offering. Investors may experience a decline in the value of the ordinary shares they purchase in this offering as a result of sales
made at prices lower than the prices they paid.
U.S. Holders may suffer adverse tax consequences
if we are characterized as a “passive foreign investment company” for U.S. federal income tax purposes.
Generally, for any taxable year, if at least 75%
of our gross income is passive income, or at least 50% of the value of our assets (generally determined based on a weighted quarterly
average) is attributable to assets that produce, or are held for the production of, passive income, we would be a “passive foreign
investment company,” or PFIC, for U.S. federal income tax purposes. For purposes of these tests, passive income generally includes
dividends, interest, certain gains from the sale or exchange of investment property, and rents and royalties (other than rents and royalties
received from unrelated parties in connection with the active conduct of a trade or business), and passive assets generally include cash.
Additionally, we generally are treated as holding and receiving directly our proportionate share of the assets and income, respectively,
of any corporation in which we own, directly or indirectly, 25% of its stock by value.
Our status as a PFIC generally will depend on
the nature and composition of our income, and the nature, composition, and value of our assets (which generally will be determined based
on the fair market value of each asset, with the value of goodwill and going concern value determined in large part by reference to the
market value of our ordinary shares from time to time, which may be volatile). If our market capitalization declines while we hold a substantial
amount of cash for any taxable year, we may be a PFIC for such taxable year. The manner and timeframe in which we spend the cash we raise
in this and any other offering, the transactions we enter into, and how our corporate structure may change in the future will affect the
nature and composition of our income and assets. Our PFIC status may depend, in part, on the treatment of payments we receive (including
government grants), which is uncertain, and the magnitude of such payments compared to passive income from investments. Based on the value
of our assets, including goodwill, and the nature and composition of our income and assets, we believe that we were not a PFIC for the
taxable year ended December 31, 2022, but due to changes in the nature and composition of our income and our market capitalization,
we may be a PFIC for our current taxable year. Because the determination of whether we are a PFIC for any taxable year is a factual determination
made annually after the end of each taxable year by applying principles and methodologies that in some circumstances are unclear and subject
to varying interpretation, there can be no assurance that we will not be a PFIC for any taxable year, and our U.S. counsel expresses
no opinion with respect to our PFIC status for any taxable year.
If we are a PFIC for any taxable year during which a U.S. Holder (as
defined below) holds our ordinary shares, such U.S. Holder may suffer adverse tax consequences, including having gains realized on the
sale of our ordinary shares treated as ordinary income, rather than capital gain, the loss of the preferential rate applicable to dividends
received on our ordinary shares by individual U.S. holders, and having interest charges apply to distributions by us and the proceeds
of sales of our ordinary shares. See the section entitled “Certain Material U.S. Federal Income Tax Consequences” for further
information.
SPECIAL NOTE REGARDING
FORWARD-LOOKING STATEMENTS
This prospectus, any prospectus supplement and
the information incorporated by reference in this prospectus and any prospectus supplement contain forward-looking statements within the
meaning of Section 27A of the Securities Act, and Section 21E of the Exchange Act of 1934, as amended, or the Exchange Act, that involve
substantial risks and uncertainties. Although our forward-looking statements reflect the good faith judgment of our management, these
statements can only be based on facts and factors currently known by us. Consequently, these forward-looking statements are inherently
subject to risks and uncertainties, and actual results and outcomes may differ materially from results and outcomes discussed in the forward-looking
statements.
All statements other than present and historical
facts and conditions contained in this prospectus, any prospectus supplement and the information incorporated by reference in this prospectus
and any prospectus supplement including statements regarding our future results of operations and financial positions, business strategy,
plans and our objectives for future operations, are forward-looking statements. The words “anticipate,” “believe,”
“continue” “could,” “estimate,” “expect,” “intend,” “may,” “might,”
“ongoing,” “objective,” “plan,” “potential,” “predict,” “should,”
“will” and “would,” or the negative of these and similar expressions identify forward-looking statements. Forward-looking
statements include, but are not limited to, statements about:
| ● | our estimates regarding the commercial potential of, and our commercialization plans for our
allogenic cell therapy, Omisirge (omidubicel-onlv), including our plans to manufacture Omisirge at a commercial scale at our Kiryat
Gat facility; |
| ● | the clinical utility and potential advantages of Omisirge and any of our product candidates; |
| ● | the timing, progress and conduct of our clinical trial of GDA-201; |
| ● | our plans regarding utilization of regulatory pathways that would allow for accelerated marketing approval in the United States, the
European Union and other jurisdictions; |
| ● | our recurring losses from operations, our estimates regarding anticipated capital requirements and our needs for additional sources
of financing or a commercial or strategic partnership to support a more fulsome commercial launch of Omisirge; |
| ● | anticipated cost savings from our strategic restructuring and our financial runway; |
| ● | our expectations regarding when certain patents may be issued and the protection and enforcement of our intellectual property rights; |
| ● | our plans regarding the maintenance of intellectual property rights to our preclinical NK cell pipeline; |
| ● | our ability to manufacture Omisirge and any product candidates at levels sufficient for commercialization or clinical development,
as applicable; |
| ● | our ability to maintain relationships with certain third parties; |
| ● | our planned level of capital expenditures; |
| ● | the impact of government laws and regulations; and |
| ● | the effects that geopolitical events or economic conditions may have on us. |
As a result of these factors, we cannot assure
you that the forward-looking statements in this prospectus will prove to be accurate. Furthermore, if our forward-looking statements prove
to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should
not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in
any specified time frame or at all. We undertake no obligation to publicly update any forward-looking statements, whether as a result
of new information, future events or otherwise, except as required by law.
You should read this prospectus, any applicable
prospectus supplement and the information incorporated by reference completely and with the understanding that our actual future results
may be materially different from what we expect. We qualify all of our forward- looking statements by these cautionary statements.
This prospectus, any applicable prospectus supplement
and the information incorporated by reference in this prospectus and any applicable prospectus supplement may contain market data and
industry forecasts that were obtained from industry publications. These data involve a number of assumptions and limitations, and you
are cautioned not to give undue weight to such estimates. While we believe the market position, market opportunity and market size information
included in this prospectus, any applicable prospectus supplement and the information incorporated by reference in this prospectus and
any applicable prospectus supplement is generally reliable, such information is inherently imprecise.
In addition, statements that “we believe”
and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available
to us as of the date the statements were made, and while we believed such information formed a reasonable basis for such statements at
the time they were made, such information may be limited or incomplete, and our statements should not be read to indicate that we have
conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain
and you are cautioned not to unduly rely upon these statements.
USE OF PROCEEDS
We may issue and sell ordinary shares having aggregate
sales proceeds of up to $50,000,000 from time to time. Because there is no minimum offering amount required as a condition to close this
offering, the actual total public offering amount, commissions and proceeds to us, if any, are not determinable at this time. There can
be no assurance that we will sell any shares under or fully utilize the sales agreement with Jefferies as a source of financing.
We intend to use the net proceeds from this offering
as follows:
| ● | commercialization activities to support the launch of Omisirge; |
| ● | the continued clinical development of GDA-201; and |
| ● | the balance for general corporate purposes, including general and administrative expenses and working capital. |
Although we currently anticipate that we will
use the net proceeds from this offering as described above, there may be circumstances where a reallocation of funds is necessary. Due
to the uncertainties inherent in the clinical development and regulatory approval and commercialization process, it is difficult to estimate
with certainty the exact amounts of the net proceeds from this offering that may be used for any of the above purposes on a stand-alone
basis. Amounts and timing of our actual expenditures will depend upon a number of factors, including our sales, marketing and commercialization
efforts, regulatory approval and demand for our product candidates, operating costs and other factors described under “Risk Factors”
in this prospectus. Accordingly, our management will have flexibility in applying the net proceeds from this offering. An investor will
not have the opportunity to evaluate the economic, financial or other information on which we base our decisions on how to use the proceeds.
As of the date of this prospectus, we cannot specify
with certainty all of the particular uses of the net proceeds from this offering. Accordingly, we will retain broad discretion over the
use of such proceeds. Pending our application of the net proceeds from this offering, we plan to invest such proceeds in short-term, investment-grade,
interest-bearing securities and depositary institutions.
MATERIAL TAX CONSIDERATIONS
The following is a brief summary of certain material
tax consequences concerning the ownership and disposition of our securities by purchasers or holders of our securities. Because parts
of this discussion are based on new or existing tax or other legislation that has not been subject to judicial or administrative interpretation,
there can be no assurance that the views expressed herein will be accepted by the tax or other authorities in question. The summary below
does not address all of the tax consequences that may be relevant to all purchasers or holders of our securities in light of each purchaser’s
or holder’s particular circumstances and specific tax treatment. For example, the summary below does not address the tax treatment
of residents of Israel and traders in securities who are subject to specific tax regimes. As individual circumstances may differ, holders
of our securities should consult their tax advisors as to United States, Israeli or other tax consequences of the purchase, ownership
and disposition of our securities. This discussion is not intended, nor should it be construed, as legal or professional tax advice and
it is not exhaustive of all possible tax considerations. Each individual should consult his or her own tax or legal advisor.
Israeli Taxation
Taxation of Capital Gains Applicable
to Non-Israeli Shareholders
Capital gain tax is imposed on the disposal of
capital assets by a non-Israel resident if those assets are either (i) located in Israel, (ii) are shares or a right to a share in an
Israeli resident corporation, or (iii) represent, directly or indirectly, rights to assets located in Israel, unless a tax treaty between
Israel and the seller’s country of residence provides otherwise. The Israeli Income Tax Ordinance (“Ordinance”) distinguishes
between “Real Capital Gain” and the “Inflationary Surplus”. Real Capital Gain is the excess of the total capital
gain over Inflationary Surplus computed generally on the basis of the increase in the Israeli Consumer Price Index (“CPI”)
between the date of purchase and the date of disposal. The Inflationary Surplus is currently not subject to tax in Israel.
The Real Capital Gain accrued by individuals on
the sale of our ordinary shares (that were purchased after January 1, 2012, whether listed on a stock exchange or not) will be taxed at
the rate of 25%. However, if such shareholder is a “Substantial Shareholder” (i.e., a person who holds, directly or indirectly,
alone or together with such person’s relative or another person who collaborates with such person on a permanent basis, 10% or more
of one of the Israeli resident company’s means of control) at the time of sale or at any time during the preceding twelve (12) months
period and/or claims a deduction for interest and linkage differences expenses in connection with the purchase and holding of such shares,
such gain will be taxed at the rate of 30%. In addition, individual shareholders dealing in securities, or to whom such income is otherwise
taxable as ordinary business income are taxed in Israel at their marginal tax rates applicable to business income (up to 47% in 2023).
The Real Capital Gain derived by corporations
will be generally subject to ordinary corporate tax (23% in 2023).
Notwithstanding the foregoing, capital gain derived
from the sale of our ordinary shares by a non-Israeli resident shareholder (whether an individual or a corporation) generally may be exempt
under the Ordinance from Israeli taxation provided that the following cumulative conditions are met: (i) the shares were purchased upon
or after the Company was listed for trading on Nasdaq (this condition will not apply to shares purchased on or after January 1, 2009),
(ii) such gains were not derived from a permanent business or business activity that the non-Israeli resident maintains in Israel, and
(iii) neither such shareholders nor the particular gain are subject to the Israeli Income Tax Law (Inflationary Adjustments) 5745-1985
(this condition will not apply to shares purchased on or after January 1, 2009). These provisions dealing with capital gain are not applicable
to a person whose gains from selling or otherwise disposing of the shares are deemed to be business income. However, non-Israeli corporations
will not be entitled to the foregoing exemptions if an Israeli resident (i) has a controlling interest of more than 25% in such non-Israeli
corporation or (ii) is the beneficiary of or is entitled to 25% or more of the revenue or profits of such non-Israeli corporation, whether
directly or indirectly.
In addition, the sale of shares may be exempt
from Israeli capital gain tax under the provisions of an applicable tax treaty (subject to the receipt in advance of a valid certificate
from the Israel Tax Authority allowing for an exemption). For example, the U.S.-Israel Double Tax Treaty (the “Treaty”) generally
exempts U.S. resident holding the shares as a capital asset and who is entitled to claim the benefits afforded to such a resident by the
Treaty, (a “Treaty U.S. Resident”), from Israeli capital gain tax in connection with such sale, provided that (i) the U.S.
resident owned, directly or indirectly, less than 10% of an Israeli resident company’s voting power at any time within the 12 month
period preceding such sale, subject to certain conditions; (ii) the seller, being an individual, is present in Israel for a period or
periods of less than 183 days in the aggregate at the taxable year; and (iii) the capital gain from the sale, exchange or disposition
was not derived through a permanent establishment that the U.S. resident maintains in Israel, (iv) the capital gains arising from such
sale, exchange or disposition is not attributed to real estate located in Israel; or (v) the capital gains arising from such sale, exchange
or disposition is not attributed to royalties. If any such case occurs, the sale, exchange or disposition of our ordinary shares would
be subject to Israeli tax, to the extent applicable. However, under the Treaty, a Treaty U.S. Resident would be permitted to claim a credit
for such taxes against U.S. federal income tax imposed on any gain from such sale, exchange or disposition, under the circumstances and
subject to the limitations specified in the Treaty and the U.S. federal income tax laws applicable to foreign tax credits.
In some instances where our shareholders may be
liable for Israeli tax on the sale of their ordinary shares, the payment of the consideration may be subject to withholding of Israeli
tax at source. Shareholders may be required to demonstrate that they are exempt from tax on their capital gains in order to avoid withholding
at source at the time of sale. Specifically, in transactions involving a sale of all of the shares of an Israeli resident company, in
the form of a merger or otherwise, the Israel Tax Authority may require from shareholders who are not liable for Israeli tax to sign declarations
in forms specified by this authority or obtain a specific exemption from the Israel Tax Authority to confirm their status as non-Israeli
resident, and, in the absence of such declarations or exemptions, may require the purchaser of the shares to withhold taxes at source.
Either the purchaser, the Israeli stockbrokers
or financial institution through which the shares are held is obliged, to withhold tax upon the sale of securities on the amount of the
consideration paid upon the sale of the securities at the rate of 25% in respect of an individual, or at a rate of corporate tax, in respect
of a corporation (23% in 2023), unless a valid exemption certificate issued by the Israel Tax Authority is presented prior to the applicable
payment.
At the sale of securities traded on a stock exchange,
a detailed return, including a computation of the tax due, must be filed and an advanced payment must be paid on January 31 and July 31
of every tax year in respect of sales of securities made within the previous six months. However, if all tax due was withheld at source
according to applicable provisions of the Ordinance and regulations promulgated thereunder, the aforementioned return need not be filed
provided that (i) such income was not generated from business conducted in Israel by the taxpayer, (ii) the taxpayer has no other taxable
sources of income in Israel with respect to which a tax return is required to be filed and (iii) the taxpayer is not obliged to pay excess
tax (as further explained below); and no advance payment must be paid. Capital gain is also reportable on the annual income tax return.
Income Taxes on Dividend Distribution
to Non-Israeli Shareholders
The Ordinance generally provides that a non-Israeli
resident (either individual or corporation) is subject to an Israeli income tax on the receipt of dividends at the rate of 25% (30% if
the dividends recipient is a “Substantial Shareholder” (as defined above), at the time of distribution or at any time during
the preceding 12 months period) or 20% if the dividend is distributed from income attributed to a Preferred Enterprise. Such dividends
are generally subject to Israeli withholding tax at a rate of 25% so long as the shares are registered with a Nominee Company (whether
the recipient is a Substantial Shareholder or not), and 20% if the dividend is distributed from income attributed to a Preferred Enterprise
(subject to the receipt in advance of a valid certificate from the Israel Tax Authority allowing for a reduced tax rate); those rates
may be subject to a reduced tax rate under the provisions of an applicable double tax treaty (subject to the receipt in advance of a valid
certificate from the Israel Tax Authority allowing for a reduced tax rate).
For example, under the Treaty the following rates
will apply in respect of dividends distributed by an Israeli resident company to a Treaty U.S. Resident: (i) if the Treaty U.S. Resident
is a corporation which holds during that portion of the taxable year which precedes the date of payment of the dividend and during the
whole of its prior taxable year (if any), at least 10% of the outstanding shares of the voting shares of the Israeli resident paying corporation
and not more than 25% of the gross income of the Israeli resident paying corporation for such prior taxable year (if any) consists of
certain type of interest or dividends – the maximum tax rate of withholding is 12.5%, and (ii) in all other cases, the tax rate
is 25%, or the domestic rate (if such is lower). The aforementioned rates under the Treaty will not apply if the dividend income was derived
through a permanent establishment that the Treaty U.S. Resident maintains in Israel. U.S. residents who are subject to Israeli withholding
tax on a dividend may be entitled to a credit or deduction for United States federal income tax purposes in the amount of the taxes withheld,
subject to detailed rules contained in U.S. tax legislation.
A non-Israeli resident who receives dividend income
derived from or accrued from Israel, from which the full amount of tax was withheld at source, is generally exempt from the obligation
to file tax returns in Israel with respect to such income, provided that (i) such income was not generated from business conducted in
Israel by the taxpayer, and (ii) the taxpayer has no other taxable sources of income in Israel with respect to which a tax return is required
to be filed and (iii) the taxpayer is not obliged to pay excess tax (as further explained below).
Payers of dividends on our shares, including the
Israeli shareholder effectuating the transaction, or the financial institution through which the securities are held, are generally required,
subject to any of the foregoing exemption, reduced tax rates and the demonstration of a shareholder of his, her or its foreign residency,
to withhold taxes upon the distribution of dividends at a rate of 25% provided that the shares are registered with a Nominee Company (for
corporations and individuals).
Surcharge Tax
Individuals who are subject to tax in Israel are
also subject to an additional tax at a rate of 3%, on annual income exceeding a certain threshold (NIS 698,280 for 2023 which amount is
linked to the annual change in the Israeli consumer price index), including, but not limited to income derived from dividends, interest
and capital gains.
Certain Material U.S. Federal Income Tax Consequences
The following discussion describes certain material
U.S. federal income tax consequences relating to the ownership and disposition of our ordinary shares by U.S. Holders (as defined
below). This discussion applies to U.S. Holders that purchase our ordinary shares pursuant to the offering covered by the Sale Agreement
Prospectus and hold such ordinary shares as capital assets within the meaning of Section 1221 of the U.S. Internal Revenue Code
of 1986, as amended, or the Code. This discussion is based on the Code, U.S. Treasury regulations promulgated thereunder and administrative
and judicial interpretations thereof, all as in effect on the date hereof and all of which are subject to change or differing interpretation,
possibly with retroactive effect. This discussion does not address all of the U.S. federal income tax consequences that may be relevant
to specific U.S. Holders in light of their particular circumstances, or to U.S. Holders subject to special treatment under U.S. federal
income tax law (such as banks and certain other financial institutions, insurance companies, pension plans, cooperatives, broker-dealers
and traders in securities or other persons that generally mark their securities to market for U.S. federal income tax purposes, tax-exempt
entities (including private foundations), governmental organizations, retirement plans, regulated investment companies, real estate investment
trusts, certain former citizens or long-term residents of the United States, persons that hold our ordinary shares as part of a “straddle,”
“hedge,” “conversion transaction,” “synthetic security” or integrated investment, persons who received
our ordinary shares pursuant to the exercise of employee stock options or otherwise as compensation for services, persons that have a
“functional currency” other than the U.S. dollar, persons that own directly, indirectly or through attribution 10% or
more of our stock by vote or value, persons that hold our ordinary shares in connection with a trade or business, permanent establishment
or fixed place of business outside the United States, corporations that accumulate earnings to avoid U.S. federal income tax
and partnerships (or entities and arrangements that are classified as partnerships for U.S. federal income tax purposes) and other
pass-through entities (and investors therein). This discussion does not address any U.S. state or local or non-U.S. tax consequences,
any U.S. federal estate, gift, or alternative minimum tax consequences, the potential application of the Medicare contribution tax
on net investment income, or the special tax accounting rules under Section 451(b) of the Code.
As used in this discussion, the term “U.S. Holder”
means a beneficial owner of our ordinary shares that is eligible for the benefits of the Treaty and that is, for U.S. federal income
tax purposes, (1) an individual who is a citizen or resident of the United States, (2) a corporation (or entity treated
as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the United States, any state
thereof, or the District of Columbia, (3) an estate the income of which is subject to U.S. federal income tax regardless of
its source or (4) a trust (x) with respect to which a court within the United States is able to exercise primary supervision
over its administration and one or more United States persons have the authority to control all of its substantial decisions or (y) that
has elected under applicable U.S. Treasury regulations to be treated as a U.S. Person for U.S. federal income tax purposes.
If a partnership (or an entity or arrangement
treated as a partnership for U.S. federal income tax purposes) holds our ordinary shares, the U.S. federal income tax consequences
relating to an investment in our ordinary shares will depend in part upon the status of the partner, the activities of the partnership,
and certain determinations made at the partner level. Any partnership and partner should consult their tax advisors regarding the tax
consequences of the ownership and disposition of our ordinary shares in their particular circumstances.
Prospective investors considering an investment in our ordinary
shares should consult their tax advisors regarding the income and non-income tax consequences under U.S. federal, state, and local
tax laws and non-U.S. tax laws relating to the ownership and disposition of our ordinary shares in their particular circumstances.
Passive Foreign Investment Company
Consequences
Generally, for any taxable year, if at least 75%
of our gross income is passive income, or at least 50% of the value of our assets (generally determined based on a weighted quarterly
average) is attributable to assets that produce, or are held for the production of, passive income, we would be a “passive foreign
investment company,” or PFIC, for U.S. federal income tax purposes. For purposes of these tests, passive income generally includes
dividends, interest, certain gains from the sale or exchange of investment property, and rents and royalties (other than rents and royalties
received from unrelated parties in connection with the active conduct of a trade or business), and passive assets generally include cash.
Additionally, we generally are treated as holding and receiving directly our proportionate share of the assets and income, respectively,
of any corporation in which we own, directly or indirectly, 25% of its stock by value.
Our status as a PFIC generally will depend on
the nature and composition of our income, and the nature, composition, and value of our assets (which generally will be determined based
on the fair market value of each asset, with the value of goodwill and going concern value determined in large part by reference to the
market value of our ordinary shares from time to time, which may be volatile). If our market capitalization declines while we hold a substantial
amount of cash for any taxable year, we may be a PFIC for such taxable year. The manner and timeframe in which we spend the cash we raise
in this and any other offering, the transactions we enter into, and how our corporate structure may change in the future will affect the
nature and composition of our income and assets. Our PFIC status may depend, in part, on the treatment of payments we receive (including
government grants), which is uncertain, and the magnitude of such payments compared to passive income from investments. Based on the value
of our assets, including goodwill, and the nature and composition of our income and assets, we believe that we were not a PFIC for the
taxable year ended December 31, 2022, but due to changes in the nature and composition of our income and market capitalization, we
may be a PFIC for any taxable year is a factual determination made annually after the end of each taxable year by applying principles
and methodologies that in some circumstances are unclear and subject to varying interpretation, there can be no assurance that we will
not be a PFIC for any taxable year, and our U.S. counsel expresses no opinion with respect to our PFIC status for any taxable year.
If we are a PFIC for any taxable year during which
a U.S. Holder holds our ordinary shares, regardless of whether we continue to be a PFIC, the U.S. Holder could be liable for
additional taxes and interest charges under the “PFIC excess distribution regime” on (1) a distribution paid during a
taxable year that is greater than 125% of the average annual distributions paid in the three preceding taxable years, or, if shorter,
the U.S. Holder’s holding period for the ordinary shares and (2) any gain recognized on a sale, exchange or other disposition,
including a pledge, of the ordinary shares. Additionally, under the PFIC excess distribution regime, (i) the tax on such distribution
or gain would be determined by allocating such distribution or gain ratably over the U.S. Holder’s holding period for the ordinary
shares, (ii) the amount of such distribution or gain allocated to the taxable year in which such distribution occurs or such gain
is recognized and any taxable year prior to the first taxable year in which we are a PFIC will be taxed as ordinary income earned in the
taxable year in which such distribution occurs or such gain is recognized and (iii) the amount allocated to each other taxable year
will be taxed at the highest marginal rates in effect for individuals or corporations, as applicable, for each such taxable year and an
interest charge, generally applicable to underpayments of tax, will be added to the tax. The tax for the amount allocated to taxable years
prior to the taxable year in which such distribution occurs or such gain is recognized cannot be offset by any net operating losses for
such taxable years, and any such gain (but not loss) recognized cannot be treated as capital gain, even if the U.S. Holder holds
our ordinary shares as capital assets.
If we pay a dividend to a non-corporate U.S. Holder
and we are a PFIC for the taxable year in which the dividend is paid or the preceding taxable year, such dividend generally will not qualify
for taxation at preferential capital gains tax rate even if we are a “qualified foreign corporation” and certain other requirements
are met (see discussion below under “—Distributions”).
If we are a PFIC for any taxable year during which
a U.S. Holder holds our ordinary shares, we must generally continue to be treated as a PFIC by the U.S. Holder for all succeeding
taxable years during which the U.S. Holder holds the ordinary shares, unless we cease to meet the requirements for PFIC status
and the U.S. Holder makes a valid “deemed sale” election with respect to the ordinary shares. If the election is made,
the U.S. Holder will be deemed to sell the ordinary shares at their fair market value on the last day of the last taxable year
in which we were a PFIC, and any gain recognized from such deemed sale will be taxed under the PFIC excess distribution regime. After
the deemed sale election, the U.S. Holder’s ordinary shares will not be treated as shares in a PFIC unless we subsequently
become a PFIC. Each U.S. Holder of our ordinary shares is advised to consult its tax advisors regarding the availability and
desirability of making a deemed sale election.
If we are a PFIC for any taxable year during which
a U.S. Holder holds our ordinary shares and one of our non-U.S. corporate subsidiaries (if any) is also a PFIC, or a lower-tier
PFIC, the U.S. Holder generally will be treated as owning a proportionate amount (by value) of the shares of the lower-tier PFIC
and will be taxed under the PFIC excess distribution regime on distributions by the lower-tier PFIC and on gain from the disposition of
shares of the lower-tier PFIC even though the U.S. Holder might not receive the proceeds of such distributions or disposition. Each
U.S. Holder is advised to consult its tax advisors regarding the application of the PFIC rules to our non-U.S. subsidiaries.
If we are a PFIC for any taxable year during which
a U.S. Holder holds our ordinary shares, the U.S. Holder will not be subject to the PFIC excess distribution regime if the U.S. Holder
makes a valid “mark-to-market” election with respect to the ordinary shares. A mark-to-market election is available to the
U.S. Holder only if the ordinary shares are “marketable stock.” Our ordinary shares will be marketable stock as long
as they remain listed on the Nasdaq Global Market and are “regularly traded,” other than in de minimis quantities, on at least
15 days during each calendar quarter. If a mark-to-market election is in effect, the U.S. Holder generally will take into account,
as ordinary income for each taxable year, the excess of the fair market value of the ordinary shares over the adjusted tax basis of such
ordinary shares at the end of such taxable year. The U.S. Holder will also take into account, as ordinary loss for each taxable year,
the excess of the adjusted tax basis of the ordinary shares over the fair market value of such ordinary shares at the end of such taxable
year, but only to the extent of any net mark-to-market gain previously included in income. The U.S. Holder’s tax basis in the
ordinary shares will be adjusted to reflect any income or loss recognized as a result of the mark-to-market election. Any gain from a
sale, exchange or other disposition of the U.S. Holder’s ordinary shares in any taxable year in which we are a PFIC will be
treated as ordinary income and any loss from such sale, exchange or other disposition will be treated first as ordinary loss (to the extent
of any net mark-to-market gains previously included in income) and thereafter as capital loss. Once made, a mark-to-market election cannot
be revoked without the consent of the U.S. Internal Revenue Service, or the IRS, unless our ordinary shares cease to be marketable
stock.
A mark-to-market election will not apply to our
ordinary shares for any taxable year during which we are not a PFIC, but will remain in effect with respect to any subsequent taxable
year in which we become a PFIC. Such election generally will not apply to any of our non-U.S. subsidiaries (if any), unless
the shares in such non-U.S. subsidiaries are themselves marketable stock. Accordingly, a U.S. Holder of our ordinary shares
may continue to be subject to tax under the PFIC excess distribution regime with respect to any lower-tier PFICs, notwithstanding the
U.S. Holder’s mark-to-market election with respect to our ordinary shares. Each U.S. Holder of our ordinary shares is
advised to consult its tax advisors regarding the availability and desirability of making a mark-to-market election, including the impact
of such election with respect to any lower-tier PFICs.
The tax consequences that would apply if we were
a PFIC would also be different from those described above if a U.S. Holder of our ordinary shares were able to make a valid “qualified
electing fund,” or QEF, election. At this time, if we are a PFIC for our current taxable year, we intend to provide U.S. Holders
of our ordinary shares a “PFIC Annual Information Statement” with the information necessary to make a QEF election.
If a U.S. Holder makes a QEF election, the U.S.
Holder will be subject to current taxation on its pro rata share of our ordinary earnings and net capital gain for each taxable year that
we are classified as a PFIC. Furthermore, any distributions paid by us out of our earnings and profits that were previously included in
the U.S. Holder’s income under the QEF election will not be taxable to the U.S. Holder. The U.S. Holder will increase its tax basis
in its ordinary shares by an amount equal to any income included under the QEF election and will decrease its tax basis by any amount
distributed on the ordinary shares that is not included in the U.S. Holder’s income. In addition, the U.S. Holder will recognize
capital gain or loss on the disposition of its ordinary shares in an amount equal to the difference between the amount realized and the
U.S. Holder’s adjusted tax basis in the ordinary shares. If the U.S. Holder does not make and maintain a QEF election for the U.S.
Holder’s entire holding period for our ordinary shares by making the election for the first taxable year in which the U.S. Holder
acquired our ordinary shares, the U.S. Holder will be subject to the PFIC excess distribution regime discussed above, unless the U.S.
Holder can properly make a “purging election” with respect to our ordinary shares in connection with the U.S. Holder’s
QEF election. A purging election may require the U.S. Holder to recognize taxable gain on the U.S. Holder’s ordinary shares. No
purging election is necessary for a U.S. Holder that timely makes a QEF election for the first taxable year in which the U.S. Holder acquired
our ordinary shares.
If we are a PFIC, U.S. Holders should consult
their tax advisors to determine whether any of the elections discussed above would be available and, if so, what the tax consequences
of the alternative tax treatments would be in their particular circumstances.
Each U.S. person that is an investor of a
PFIC is generally required to file an annual information return on IRS Form 8621 containing such information as the U.S. Treasury
Department may require. The failure to file IRS Form 8621 could result in the imposition of penalties and the extension of the statute
of limitations with respect to U.S. federal income tax.
The U.S. federal income tax rules relating to PFICs are very
complex. Prospective U.S. investors in our ordinary shares are strongly urged to consult their tax advisors regarding our PFIC status,
the impact of such status on the ownership and disposition of our ordinary shares, any elections that might be available with respect
to our ordinary shares if we were a PFIC and all related information reporting obligations.
Distributions
Subject to the discussion above under “—Passive
Foreign Investment Company Consequences,” a U.S. Holder of our ordinary shares that receives a distribution with respect to
our ordinary shares generally will be required to include the gross amount of such distribution (before reduction for any Israeli withholding
taxes withheld therefrom) in gross income as a dividend when actually or constructively received to the extent of the U.S. Holder’s
pro rata share of our current and/or accumulated earnings and profits (as determined under U.S. federal income tax principles). To
the extent a distribution received by the U.S. Holder is not a dividend because it exceeds the U.S. Holder’s pro rata
share of our current and accumulated earnings and profits, it will be treated first as a tax-free return of capital and reduce (but not
below zero) the adjusted tax basis of the U.S. Holder’s ordinary shares. To the extent the distribution exceeds the adjusted
tax basis of the U.S. Holder’s ordinary shares, the remainder will be taxed as capital gain. We may not account for our earnings
and profits in accordance with U.S. federal income tax principles, and U.S. Holders of our ordinary shares should expect all
distributions from us to be reported to them as dividends. Such dividends will not be eligible for the dividends-received deduction generally
allowed to corporate shareholders with respect to dividends received from U.S. corporations.
Dividends paid to non-corporate U.S. Holders
by a “qualified foreign corporation” are currently eligible, as “qualified dividend income,” for taxation at preferential
capital gains rate rather than the marginal tax rates generally applicable to ordinary income, provided that certain requirements (including
conditions relating to holding period) are met. However, as discussed above under “—Passive Foreign Investment Company Consequences”),
if we are a PFIC for the taxable year in which the dividend is paid or the preceding taxable year, we will not be treated as a qualified
foreign corporation, and therefore the preferential capital gains tax rate will not apply.
A non-U.S. corporation (other than a corporation
that is a PFIC for the taxable year in which the dividend is paid or the preceding taxable year) generally will be a qualified foreign
corporation (1) if it is eligible for the benefits of a comprehensive tax treaty with the United States that the Secretary of
Treasury of the United States determines is satisfactory for purposes of this provision and that includes an exchange of information
provision or (2) with respect to any dividend it pays on its ordinary shares that are readily tradable on an established securities
market in the United States. We believe that we qualify as a resident of Israel for purposes of, and are eligible for the benefits
of, the Treaty, although there can be no assurance in this regard. Further, the IRS has determined that the Treaty is satisfactory for
purposes of the qualified dividend income rules and that it includes an exchange of information provision. Therefore, subject to the discussion
above under “—Passive Foreign Investment Company Consequences,” if the Treaty is applicable, dividends paid by us generally
will be qualified dividend income in the hands of individual U.S. Holders of our ordinary shares, provided that certain requirements
(including conditions relating to holding period) are met. Each U.S. Holder is advised to consult its tax advisors regarding the
availability of the preferential capital gains tax rate on dividends paid by us. Distributions on our ordinary shares that are treated
as dividends generally will be included in the income of a U.S. Holder of our ordinary shares on the date of the U.S. Holder’s
actual or constructive receipt of such dividends. The amount of any dividend income paid in foreign currency will be the U.S. dollar
amount calculated by reference to the exchange rate in effect on the date of such receipt, regardless of whether such dividend income
is in fact converted into U.S. dollars. If such dividend income is converted into U.S. dollars on the date of such receipt,
the U.S. Holder will not be required to recognize foreign currency gain or loss. If such dividend income is converted into U.S. dollars
after the date of such receipt, the U.S. Holder may have foreign currency gain or loss.
Distributions on our ordinary shares that are
treated as dividends generally will constitute income from sources outside the United States for U.S. foreign tax credit purposes
and generally will be categorized as passive category income. Subject to applicable limitations, some of which vary depending on a U.S. Holder’s
particular circumstances, Israeli income taxes withheld from dividends on our ordinary shares at a tax rate not exceeding the tax rate
provided by the Treaty will be creditable against the U.S. Holder’s U.S. federal income tax liability. The rules governing
U.S. foreign tax credits are complex and U.S. Holders of our ordinary shares should consult their tax advisers regarding the
creditability of foreign taxes in their particular circumstances. Recently issued U.S. Treasury regulations may in some circumstances
prohibit a U.S. person from claiming a foreign tax credit with respect to certain non-U.S. taxes that are not creditable under
applicable income tax treaties. In lieu of claiming a U.S. foreign tax credit, a U.S. Holder of our ordinary shares may, at
such U.S. Holder’s election, deduct foreign taxes, including any Israeli income tax, in computing their taxable income, subject
to generally applicable limitations under U.S. law. An election to deduct foreign taxes instead of claiming U.S. foreign tax
credits applies to all foreign taxes paid or accrued in the taxable year.
Sale, Exchange or Other Disposition
of Ordinary Shares
Subject to the discussion above under “—Passive
Foreign Investment Company Consequences,” a U.S. Holder of our ordinary shares generally will recognize capital gain or loss
for U.S. federal income tax purposes on the sale, exchange or other disposition of the ordinary shares in an amount equal to the
difference, if any, between the amount realized (i.e., the amount of cash plus the fair market value of any property received) on the
sale, exchange or other disposition and the U.S. Holder’s adjusted tax basis in the ordinary shares. Such capital gain or loss
generally will be long-term capital gain taxable at a preferential tax rate for non-corporate U.S. Holders or long-term capital loss
if, on the date of sale, exchange or other disposition, the ordinary shares were held by the U.S. Holder for more than one year.
Any capital gain of a non-corporate U.S. Holder of our ordinary shares that is not long-term capital gain is taxed at ordinary income
rates. The deductibility of capital losses is subject to limitations. Any gain or loss recognized from the sale, exchange or other disposition
of our ordinary shares generally will be gain or loss from sources within the United States for U.S. foreign tax credit purposes.
If the proceeds received by the U.S. Holder
are not paid in U.S. dollars, the amount realized will be the U.S. dollar value of the payment received determined by reference
to the spot rate of exchange on the date of the sale, exchange or other disposition. However, if the ordinary shares are traded on an
established securities market and the U.S. Holder is either a cash basis taxpayer or an accrual basis taxpayer that has made a special
election to determine the amount realized using the spot rate on the settlement date (which must be consistently applied from year to
year and cannot be changed without the consent of the IRS), the U.S. Holder will determine the U.S. dollar value of the amount
realized in a non-U.S. dollar currency by translating the amount received at the spot rate of exchange on the settlement date of
the sale, exchange or other disposition. If the U.S. Holder is an accrual basis taxpayer that is not eligible to make or does not
make the special election, the U.S. Holder will recognize foreign currency gain or loss to the extent of any difference between the
U.S. dollar amount realized on the date of sale, exchange or other disposition and the U.S. dollar value of the amount received
at the spot rate of exchange on the settlement date of the sale, exchange or other disposition.
Information Reporting and Backup Withholding
U.S. Holders of our ordinary shares may be
required to file certain U.S. information returns with the IRS with respect to an investment in our ordinary shares, including, among
others, IRS Form 8938 (Statement of Specified Foreign Financial Assets). As described above under “Passive Foreign Investment
Company Consequences,” each U.S. Holder who is a shareholder of a PFIC must file an annual report containing certain information.
U.S. Holders paying more than $100,000 for ordinary shares may be required to file IRS Form 926 (Return by a U.S. Transferor
of Property to a Foreign Corporation) reporting this payment. Substantial penalties may be imposed on a U.S. Holder that fails to
comply with the required information reporting.
Dividends on and proceeds from the sale or other
disposition of our ordinary shares may be reported to the IRS unless the U.S. Holder of our ordinary shares establishes a basis for
exemption. Backup withholding (currently at a rate of 24%) may apply to amounts subject to reporting if the U.S. Holder (1) fails
to provide an accurate United States taxpayer identification number or otherwise establish a basis for exemption or (2) is described
in certain other categories of persons. However, U.S. Holders of our shares that are corporations are generally excluded from these
information reporting and backup withholding tax rules. Backup withholding is not an additional tax. Any amounts withheld under the backup
withholding rules generally will be allowed as a refund or a credit against the U.S. Holder’s U.S. federal income tax
liability if the required information is furnished by the U.S. Holder on a timely basis to the IRS.
U.S. Holders should consult their tax advisors
regarding the backup withholding and information reporting rules in their particular circumstances.
THE DISCUSSION ABOVE IS FOR GENERAL INFORMATIONAL
PURPOSES ONLY AND IS NOT TAX ADVICE. PROSPECTIVE INVESTORS IN OUR ORDINARY SHARES SHOULD CONSULT THEIR TAX ADVISORS REGARDING THE
U.S. FEDERAL, STATE, AND LOCAL AND NON-U.S. INCOME AND NON-INCOME TAX CONSEQUENCES OF THE OWNERSHIP AND DISPOSITION OF OUR ORDINARY
SHARES IN THEIR PARTICULAR CIRCUMSTANCES, INCLUDING ALL RELATED INFORMATION REPORTING REQUIREMENTS AND THE IMPACT OF ANY POTENTIAL CHANGE
IN LAW.
DILUTION
If you invest in our ordinary shares in this offering,
your ownership interest will be immediately diluted to the extent of the difference between the public offering price per share and the
as adjusted net tangible book value per ordinary share after this offering. Net tangible book value per ordinary share is calculated by
dividing the net tangible book value, which is tangible assets less total liabilities, by the number of ordinary shares outstanding. Our
net tangible book value as of September 30, 2023 was $0.4 million, or $0.003 per ordinary share.
After giving effect to the sale of 84,745,763
ordinary shares in this offering at an assumed public offering price of $0.59 per share, which was the last reported sale price of our
ordinary shares on the Nasdaq Global Market on November 13, 2023, and after deducting commissions and other estimated offering expenses
payable by us, our as adjusted net tangible book value as of September 30, 2023 would have been approximately $216.7 million, or $0.22
per ordinary share. This amount would represent an immediate increase in the net tangible book value of $0.22 per ordinary share to our
existing shareholders and an immediate and substantial dilution in net tangible book value of $0.37 per ordinary share to new investors
purchasing ordinary shares in this offering. The following table illustrates this per share dilution:
| Assumed offering price per share | |
| | | |
$ | 0.59 | |
| Net tangible book value per share as of September 30, 2023 | |
$ | 0.003 | | |
| | |
| Increase in net tangible book value per share attributable to new investors | |
$ | 0.22 | | |
| | |
| As adjusted net tangible book value per share after this offering | |
| | | |
$ | 0.22 | |
| Net dilution per share to new investors in this offering | |
| | | |
$ | 0.37 | |
The table above assumes for illustrative purposes
that an aggregate of 84,745,763 ordinary shares are sold at a price of $0.59 per share, the last reported sales price of our ordinary
shares on the Nasdaq Global Market on November 13, 2023, for aggregate proceeds of approximately $48.2 million, net of issuance expenses.
The shares sold in this offering, if any, will be sold from time to time at various prices.
The number of ordinary shares issued and outstanding,
actual and as adjusted shown in the foregoing table and calculations excludes:
| |
● |
7,108,234 ordinary shares reserved for issuance upon the exercise of outstanding options as of September 30, 2023, at a weighted average exercise price of 3.85 per share; |
| |
● |
1,330,282 ordinary shares reserved for issuance upon the settlement of restricted share units as of September 30, 2023; |
| |
● |
2,025,064 ordinary shares reserved for future issuance under our 2017 Share Incentive Plan, as of September 30, 2023, as well as any automatic increases in the number of ordinary shares reserved for future issuance under this plan; |
| |
● |
4,542,369 ordinary shares reserved for potential issuance upon exchange of outstanding notes under a first lien secured note, or the 2022 Note, held by certain funds managed by Highbridge Capital Management, LLC, or Highbridge; |
| |
● |
6,334,455 ordinary shares reserved for issuance upon the exchange of outstanding convertible notes held by certain funds managed by Highbridge as of September 30, 2023; and |
| |
● |
17,466,730 ordinary shares issuable upon exercise of warrants as of September 30, 2023; and |
| |
● |
718,726 ordinary shares issued pursuant to our “at-the-market” offering program since September 30, 2023. |
To the extent that options outstanding as of
September 30, 2023 may be exercised or any ordinary shares are issued to funds managed by Highbridge upon the exchange of the notes held
by such funds, investors purchasing our securities in this offering may experience further dilution.
PLAN OF DISTRIBUTION
We have previously entered into a sale agreement
with Jefferies, under which we may offer and sell our ordinary shares from time to time through Jefferies acting as agent. In accordance
with the terms of the sale agreement, under this prospectus, we may offer and sell shares of our ordinary shares having an aggregate offering
price of up to $50,000,000. Sales of our ordinary shares, if any, under this prospectus will be made by any method that is deemed to be
an “at the market offering” as defined in Rule 415(a)(4) under the Securities Act.
Each time we wish to issue and sell our ordinary
shares under the sale agreement, we will notify Jefferies of the number of shares to be issued, the dates on which such sales are anticipated
to be made, any limitation on the number of shares to be sold in any one day and any minimum price below which sales may not be made.
Once we have so instructed Jefferies, unless Jefferies declines to accept the terms of such notice, Jefferies has agreed to use its commercially
reasonable efforts consistent with its normal trading and sales practices to sell such shares up to the amount specified on such terms.
The obligations of Jefferies under the sale agreement to sell our ordinary shares are subject to a number of conditions that we must meet.
The settlement of sales of shares between us and
Jefferies is generally anticipated to occur on the second trading day following the date on which the sale was made. Sales of our ordinary
shares as contemplated in this prospectus will be settled through the facilities of The Depository Trust Company or by such other means
as we and Jefferies may agree upon. There is no arrangement for funds to be received in an escrow, trust or similar arrangement.
We will pay Jefferies a commission equal to 3.0%
of the aggregate gross proceeds we receive from each sale of our ordinary shares. Because there is no minimum offering amount required
as a condition to close this offering, the actual total public offering amount, commissions and proceeds to us, if any, are not determinable
at this time. In addition, we agreed to reimburse Jefferies for the fees and disbursements of its counsel, payable upon execution of the
sale agreement, in an amount not to exceed $95,000, in addition to certain ongoing disbursements of its legal counsel. We estimate that
the total expenses for the offering, excluding any commissions or expense reimbursement payable to Jefferies under the terms of the sale
agreement, will be approximately $400,000. The remaining sale proceeds, after deducting any other transaction fees, will equal our net
proceeds from the sale of such shares.
Jefferies will provide written confirmation to
us before the open on The Nasdaq Global Market on the day following each day on which our ordinary shares are sold under the sale agreement.
Each confirmation will include the number of shares sold on that day, the aggregate gross proceeds of such sales and the proceeds to us.
In connection with the sale of our ordinary shares
on our behalf, Jefferies will be deemed to be an “underwriter” within the meaning of the Securities Act, and the compensation
of Jefferies will be deemed to be underwriting commissions or discounts. We have agreed to indemnify Jefferies against certain civil liabilities,
including liabilities under the Securities Act. We have also agreed to contribute to payments Jefferies may be required to make in respect
of such liabilities.
The offering of our ordinary shares pursuant to
the sale agreement will terminate upon the earlier of (i) the sale of all ordinary shares subject to the sale agreement and (ii) the termination
of the sale agreement as permitted therein. We and Jefferies may each terminate the sale agreement at any time upon ten trading days’
prior notice.
This summary of the material provisions of the
sale agreement does not purport to be a complete statement of its terms and conditions. A copy of the sale agreement is filed as an exhibit
to the registration statement of which this prospectus forms a part.
Jefferies and its affiliates may in the future
provide various investment banking, commercial banking, financial advisory and other financial services for us and our affiliates, for
which services they may in the future receive customary fees. In the course of its business, Jefferies may actively trade our securities
for its own account or for the accounts of customers, and, accordingly, Jefferies may at any time hold long or short positions in such
securities.
A prospectus in electronic format may be made
available on a website maintained by Jefferies, and Jefferies may distribute the prospectus electronically.
ENFORCEMENT OF
CIVIL LIABILITIES
We are incorporated under the laws of the State
of Israel. Service of process upon us and any Israeli experts named in this prospectus, may be difficult to obtain within the United States.
Furthermore, because most of our assets are located outside the United States, any judgment obtained in the United States against us may
not be collectible within the United States.
We have irrevocably appointed Gamida Cell Inc.
as our agent to receive service of process in any action against us in any U.S. federal or state court arising out of this offering or
any purchase or sale of securities in connection with any offering described in this prospectus. The address of our agent is 116 Huntington
Avenue, 7th Floor, Boston, Massachusetts 02116.
We have been informed by our legal counsel in
Israel, Meitar | Law Offices, that it may be difficult to initiate an action with respect to U.S. securities law in Israel. Israeli courts
may refuse to hear a claim based on an alleged violation of U.S. securities laws reasoning that Israel is not the most appropriate forum
to hear such a claim. In Israeli courts, the content of applicable U.S. law must be proved as a fact by expert witnesses which can be
a time-consuming and costly process and certain matters of procedure may be governed by Israeli law.
Subject to certain time limitations and legal
procedures, Israeli courts may enforce a U.S. judgment in a civil matter which, subject to certain exceptions, is non-appealable, including
judgments based upon the civil liability provisions of the Securities Act and the Exchange Act and including a monetary or compensatory
judgment in a non-civil matter, provided that:
| ● | the judgment was rendered by a court which was, according to the laws of the state of the court, competent
to render the judgment; |
| ● | the obligation imposed by the judgment is enforceable according to the rules relating to the enforceability of judgments in Israel
and the substance of the judgment is not contrary to public policy; and |
| ● | the judgment is executory in the state in which it was given. |
Even if these conditions are met, an Israeli court
will not declare a foreign civil judgment enforceable if:
| ● | the judgment was given in a state whose laws do not provide for the enforcement of judgments of Israeli
courts (subject to exceptional cases); |
| ● | the enforcement of the judgment is likely to prejudice the sovereignty or security of the State of Israel; |
| ● | the judgment was obtained by fraud; |
| ● | the opportunity given to the defendant to bring its arguments and evidence before the court was not reasonable in the opinion of the
Israeli court; |
| ● | the judgment was rendered by a court not competent to render it according to the laws of private international law as they apply in
Israel; |
| ● | the judgment is contradictory to another judgment that was given in the same matter between the same parties and that is still valid;
or |
| ● | at the time the action was brought in the foreign court, a lawsuit in the same matter and between the same parties was pending before
a court or tribunal in Israel. |
LEGAL MATTERS
The validity of the issuance of our ordinary shares
offered in this prospectus and certain other matters of Israeli law will be passed upon for us by Meitar | Law Offices, Israel. Certain
matters of U.S. federal law will be passed upon for us by Cooley LLP, New York, New York. Jefferies LLC is being represented in connection
with this offering by Latham & Watkins LLP, San Diego, California.
EXPERTS
The consolidated financial statements as of December 31,
2022 and 2021 and for each of the three years in the period ended December 31, 2022 incorporated by reference into this prospectus
have been audited by Kost, Forer, Gabbay & Kasierer, a member of Ernst & Young Global, independent registered public
accounting firm, as set forth in their report thereon (which contain an explanatory paragraph describing conditions that raise substantial
doubt about the Company’s ability to continue as a going concern as described in the notes to the consolidated financial statements)
incorporated by reference herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting
and auditing. The address of Kost Forer Gabbay & Kasierer is 144 Menachem Begin Road, Tel Aviv, Israel.
WHERE YOU CAN FIND
MORE INFORMATION
This prospectus is part of the registration statement
on Form S-3 we filed with the SEC under the Securities Act. This prospectus does not contain all of the information set forth in the registration
statement and the exhibits to the registration statement. For further information with respect to us and the securities we are offering
under this prospectus, we refer you to the registration statement and the exhibits and schedules filed as a part of the registration statement.
You should rely only on the information contained in this prospectus or incorporated by reference. We have not authorized anyone else
to provide you with different information. We are not making an offer of these securities in any state where the offer is not permitted.
You should not assume that the information in this prospectus is accurate as of any date other than the date on the front cover of this
prospectus, regardless of the time of delivery of this prospectus or any sale of the securities offered by this prospectus.
We file annual, quarterly and current reports,
proxy statements and other information with the SEC. The SEC maintains a website that contains reports, proxy and information statements
and other information regarding issuers that file electronically with the SEC, including Gamida Cell. The address of the SEC website is
www.sec.gov.
We maintain a website at www.gamida-cell.com.
Information contained in or accessible through our website does not constitute a part of this prospectus.
INCORPORATION OF
CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference”
into this prospectus the information we have filed with the SEC. This means that we can disclose important information by referring you
to another document filed separately with the SEC. The information incorporated by reference is considered to be a part of this prospectus,
and information that we file later with the SEC will also be deemed to be incorporated by reference into this prospectus and to be a part
hereof from the date of filing of such documents and will automatically update and supersede previously filed information, including information
contained in this document.
We incorporate by reference into this prospectus
the following documents that we have filed with the SEC:
| ● | Our Annual Report on Form 10-K for the year ended December 31, 2022, filed with the SEC on March 31, 2023; |
| ● | Our Quarterly Reports on Form 10-Q for the quarters ended March 31, 2023, June 30, 2023 and September 30, 2023, filed with the SEC
on May 15, 2023, August 14, 2023, and November 14, 2023, respectively; |
| ● | our Current Reports on Form 8-K filed with the SEC on January
9, 2023, January
19, 2023, January
30, 2023, March 20, 2023,
March
27, 2023, April
17, 2023, April
21, 2023, May
19, 2023, May
22, 2023, August
14, 2023, September
27, 2023, October
20, 2023 November
15, 2023 and November
24, 2023, to the extent the information in such reports is filed and not furnished; and |
| ● | The description of our ordinary shares contained in our Registration Statement on Form 8-A, filed with the SEC on October
23, 2018, as amended on March
25, 2022, as the description therein has been updated and superseded by the description of our capital stock contained
in Exhibit 4.1 to our Annual Report on Form 10-K for the year ended December 31, 2022, including any further
amendments or reports filed for the purposes of updating this description. |
We also incorporate by reference into this prospectus
all documents (other than current reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on
such form that are related to such items) that are filed by us with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the
Exchange Act (i) after the date of the initial filing of the registration statement of which this prospectus forms a part and
prior to effectiveness of the registration statement, or (ii) after the date of this prospectus but prior to the termination of the
offering.
We will furnish without charge to each person,
including any beneficial owner, to whom a prospectus is delivered, on written or oral request, a copy of any or all of the documents incorporated
by reference in this prospectus, including exhibits to these documents. You should direct any requests for documents, either in writing
to Gamida Cell Ltd., 116 Huntington Avenue, 7th Floor, Boston, MA 02116, Attn: Chief Financial Officer or by telephone (617) 892-9080.
Any statement contained in a document incorporated
or deemed to be incorporated by reference in this prospectus or any applicable prospectus supplement will be deemed modified, superseded
or replaced for purposes of this prospectus or any applicable prospectus supplement to the extent that a statement contained in any other
subsequently filed document that also is or is deemed to be incorporated by reference in this prospectus or any applicable prospectus
supplement modifies, supersedes or replaces such statement. Any statement that is modified or superseded will not constitute a part of
this prospectus or any applicable prospectus supplement, except as modified or superseded.

Up to $50,000,000
Ordinary Shares
PROSPECTUS
Jefferies
,
2023
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 14. Other Expenses of Issuance and Distribution
The following table sets forth an estimate of
the fees and expenses, other than the underwriting discounts and commissions, payable by us in connection with the issuance and distribution
of the securities being registered. All the amounts shown are estimates, except for the SEC registration fee and the FINRA filing fee.
| SEC registration fee | |
$ | 22,140 | |
| Printing and engraving expenses | |
| (1 | ) |
| Legal fees and expenses | |
| (1 | ) |
| Accounting fees and expenses | |
| (1 | ) |
| Miscellaneous | |
| (1 | ) |
| Total | |
$ | (1 | ) |
| (1) | The
amount of securities and number of offerings are indeterminable and the expenses cannot be estimated at this time. |
Item 15. Indemnification of Directors, Officers and Employees
Under the Israeli Companies Law, a company may
not exculpate an office holder from liability for a breach of the duty of loyalty. A company may exculpate an office holder in advance
from liability to the company, in whole or in part, for damages caused to the company as a result of a breach of the duty of care but
only if a provision authorizing such exculpation is included in its articles of association. Our amended and restated articles of association
include such a provision. An Israeli company may not exculpate a director from liability arising out of a breach of the duty of care with
respect to a dividend or distribution to shareholders.
Under the Israeli Companies Law and the Securities
Law, 5728-1968, or the Securities Law, a company may indemnify an office holder in respect of the following liabilities, payments and
expenses incurred for acts performed as an office holder, either pursuant to an undertaking made in advance of an event or following an
event, provided a provision authorizing such indemnification is contained in its articles of association:
| ● | a monetary liability incurred by or imposed on him or her in favor of another person pursuant to a judgment,
including a settlement or arbitrator’s award approved by a court. However, if an undertaking to indemnify an office holder with
respect to such liability is provided in advance, then such undertaking must be limited to certain events which, in the opinion of the
board of directors, can be foreseen based on the company’s activities when the undertaking to indemnify is given, and to an amount
or according to criteria determined by the board of directors as reasonable under the circumstances, and such undertaking shall detail
the foreseen events and described above amount or criteria; |
| ● | reasonable litigation expenses, including reasonable attorneys’ fees, incurred by the office holder as (1) a result of an investigation
or proceeding instituted against him or her by an authority authorized to conduct such investigation or proceeding, provided that (i)
no indictment was filed against such office holder as a result of such investigation or proceeding; and (ii) no financial liability was
imposed upon him or her as a substitute for the criminal proceeding as a result of such investigation or proceeding or, if such financial
liability was imposed, it was imposed with respect to an offense that does not require proof of criminal intent; or (2) in connection
with a monetary sanction; a monetary liability imposed on him or her in favor of an injured party at an Administrative Procedure (as defined
below) pursuant to Section 52(54)(a)(1)(a) of the Securities Law; |
| ● | expenses incurred by an office holder in connection with an Administrative Procedure under the Securities Law, including reasonable
litigation expenses and reasonable attorneys’ fees; and |
| ● | reasonable litigation expenses, including attorneys’ fees, incurred by the office holder or imposed by a court in proceedings
instituted against him or her by the company, on its behalf or by a third party or in connection with criminal proceedings in which the
office holder was acquitted or as a result of a conviction for an offense that does not require proof of criminal intent. |
“Administrative Procedure” is defined
as a procedure pursuant to chapters H3 (Monetary Sanction by the Israeli Securities Authority), H4 (Administrative Enforcement Procedures
of the Administrative Enforcement Committee) or I1 (Arrangement to prevent Procedures or Interruption of procedures subject to conditions)
to the Securities Law.
Under the Israeli Companies Law and the Securities
Law, a company may insure an office holder against the following liabilities incurred for acts performed by him or her as an office holder
if and to the extent provided in the company’s articles of association:
| ● | a breach of duty of care to the company or to a third party, to the extent such a breach arises out of
the negligent conduct of the office holder; |
| ● | a breach of duty of loyalty to the company, provided that the office holder acted in good faith and had a reasonable basis to believe
that the act would not harm the company; |
| ● | a monetary liability imposed on the office holder in favor of a third party; |
| ● | a monetary liability imposed on the office holder in favor of an injured party at an Administrative Procedure pursuant to Section
52(54)(a)(1)(a) of the Securities Law; and |
| ● | expenses incurred by an office holder in connection with an Administrative Procedure, including reasonable litigation expenses and
reasonable attorneys’ fees. |
Under the Israeli Companies Law, a company may
not indemnify, exculpate or insure an office holder against any of the following:
| ● | a breach of duty of loyalty, except for indemnification and insurance for a breach of the duty of loyalty
to the company to the extent that the office holder acted in good faith and had a reasonable basis to believe that the act would not harm
the company; |
| ● | a breach of the duty of care committed intentionally or recklessly, excluding a breach arising out of the negligent conduct of the
office holder; |
| ● | an act or omission committed with intent to derive illegal personal benefit; or |
| ● | a fine or forfeit levied against the office holder. |
Under the Israeli Companies Law, exculpation,
indemnification and insurance of office holders must be approved by the compensation committee and the board of directors and, with respect
to certain office holders or under certain circumstances, also by the shareholders. See “Item 10.—Directors, Executive Officers
and Corporate Governance—Fiduciary duties and approval of specified related party transactions under Israeli law” of our Annual
Report on Form 10-K filed on March 31, 2023.
Our amended and restated articles of association
permit us to, exculpate, indemnify and insure our office holders as permitted under the Israeli Companies Law. Our office holders are
currently covered by a directors and officers’ liability insurance policy. As of the date of this registration statement, no claims
for directors’ and officers’ liability insurance have been filed under this policy, we are not aware of any pending or threatened
litigation or proceeding involving any of our directors or officers in which indemnification is sought, nor are we aware of any pending
or threatened litigation that may result in claims for indemnification by any director or officer.
We have entered into agreements with each of our
directors and executive officers exculpating them, to the fullest extent permitted by law, from liability to us for damages caused to
us as a result of a breach of duty of care, and undertaking to indemnify them to the fullest extent permitted by law. The insurance is
subject to our discretion depending on its availability, effectiveness and cost. Effective as November 17, 2021, the maximum amount set
forth in such agreements is (1) with respect to indemnification in connection with a public offering by the Company of our securities,
the gross proceeds raised by us and/or any selling shareholder in such public offering, and (2) with respect to all other permitted indemnification,
the greater of (i) an amount equal to 25% of our shareholders’ equity on a consolidated basis, according to the Company’s
most recent financial statements as of the time of the actual payment of indemnification; (ii) $150 million and (iii) 40% of the Company
Total Market Cap, which means the average closing price of the Company’s ordinary shares over the 30 trading days prior to the actual
payment of indemnification multiplied by the total number of issued and outstanding shares of the Company as of the date of actual payment).
In the opinion of the SEC, indemnification of directors and executive officers for liabilities arising under the Securities Act however,
is against public policy and therefore unenforceable.
Item 16. Exhibits.
The following exhibits are filed herewith:
| |
|
|
|
Incorporation by Reference |
|
|
| Exhibit Number |
|
Exhibit Description |
|
Form |
|
File No. |
|
Exhibit Number |
|
Filing Date |
|
Filed Herewith |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 1.1 |
|
Form of Underwriting Agreement |
|
|
|
|
|
|
|
|
|
** |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 1.2 |
|
Amended & Restated Open Market Sale AgreementSM, dated June 5, 2023, by and between Jefferies LLC and Gamida Cell Ltd. |
|
10-Q |
|
001-38716 |
|
10.7 |
|
August 14, 2023 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 3.1 |
|
Amended and Restated Articles of Association of the Registrant, as currently in effect |
|
10-Q |
|
001-38716 |
|
3.1 |
|
November 14, 2023 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 4.1 |
|
Form of Indenture |
|
S-3 |
|
333-275579 |
|
4.2 |
|
November 15, 2023 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 4.2 |
|
Form of Debt Securities |
|
|
|
|
|
|
|
|
|
** |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 4.3 |
|
Form
of Ordinary Share Warrant Agreement and Warrant Certificate |
|
S-3 |
|
333-275579 |
|
4.4 |
|
November
15, 2023 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 4.4 |
|
Form
of Debt Securities Warrant Agreement and Warrant Certificate |
|
S-3 |
|
333-275579 |
|
4.5 |
|
November
15, 2023 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 4.5 |
|
Form of Rights Agreement |
|
|
|
|
|
|
|
|
|
** |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 4.6 |
|
Form of Unit Agreement and Unit Certificate |
|
|
|
|
|
|
|
|
|
** |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 5.1 |
|
Opinion
of Meitar | Law Offices |
|
S-3 |
|
333-275579 |
|
5.1 |
|
November
15, 2023 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 5.2 |
|
Opinion
of Cooley LLP |
|
S-3 |
|
333-275579 |
|
5.2 |
|
November
15, 2023 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 23.1 |
|
Consent of Kost, Forer, Gabbay & Kasierer, a member of Ernst & Young Global |
|
|
|
|
|
|
|
|
|
* |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 23.2 |
|
Consent
of Meitar | Law Offices (included in Exhibit 5.1) |
|
S-3 |
|
333-275579 |
|
23.2 |
|
November
15, 2023 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 23.3 |
|
Consent
of Cooley LLP (included in Exhibit 5.2) |
|
S-3 |
|
333-275579 |
|
23.3 |
|
November
15, 2023 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 24.1 |
|
Power
of Attorney of certain directors of the registrant |
|
S-3 |
|
333-275579 |
|
24.1 |
|
November
15, 2023 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 25.1 |
|
Statement of Eligibility
of Trustee under the Indenture |
|
|
|
|
|
|
|
|
|
*** |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 107 |
|
Filing
Fee Table |
|
S-3 |
|
333-275579 |
|
107 |
|
November
15, 2023 |
|
|
| ** | To be filed by amendment or as an exhibit to a report
filed under the Securities Exchange Act of 1934 and incorporated herein by reference. |
| *** | To be filed, if applicable, in accordance with the
requirements of Section 305(b)(2) of the Trust Indenture Act of 1939 and Rule 5b-3 thereunder. |
Item 17. Undertakings
(a) The
undersigned Registrant hereby undertakes:
(1) To
file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To
include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
(ii) To
reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective
amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration
statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities
offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range
may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume
and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration
Fee” table in the effective registration statement.
(iii) To
include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any
material change to such information in the registration statement.
provided, however, that:
(A) the
undertakings set forth in Paragraphs (a)(1)(i), (a)(1)(ii) and (a)(1)(iii) of this section do not apply if the information required to
be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the SEC by the registrant
pursuant to section 13 or section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration
statement, or is contained in a form of prospectus filed pursuant to Rule 424(b) that is part of the registration statement.
(2) That,
for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be
a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed
to be the initial bona fide offering thereof.
(3) To
remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination
of the offering.
(4) That,
for the purpose of determining liability under the Securities Act to any purchaser,
(i) each
prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date
the filed prospectus was deemed part of and included in the registration statement; and
(ii) each
prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance on Rule
430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information required by
section 10(a) of the Securities Act shall be deemed to be part of and included in the registration statement as of the earlier of the
date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering
described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter,
such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement
to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering
thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement
or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the
registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement
that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately
prior to such effective date.
(5) That,
for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution
of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant
to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities
are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to
the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any
preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any
free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the
undersigned registrant;
(iii) The
portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant
or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any
other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(6) For
purposes of determining any liability under the Securities Act, each filing of the registrant’s annual report pursuant to Section
13(a) or Section 15(d) of the Exchange Act (and, where applicable, each filing of an employee benefit plan’s annual report pursuant
to Section 15(d) of the Exchange Act) that is incorporated by reference in the registration statement shall be deemed to be a new registration
statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial
bona fide offering thereof.
Insofar as indemnification for liabilities arising
under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing
provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification
is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification
against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling
person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling
person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been
settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against
public policy as expressed in the Securities Act of 1933, as amended, and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes to
file an application for the purpose of determining the eligibility of the trustee to act under subsection (a) of section 310 of the Trust
Indenture Act in accordance with the rules and regulations prescribed by the Commission under section 305(b)(2) of the Trust Indenture
Act.
SIGNATURES
Pursuant to the requirement of the Securities
Act of 1933, the Registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form
S-3 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, the City
of Boston, State of Massachusetts on December 6, 2023.
| |
GAMIDA CELL LTD. |
| |
|
|
| |
By: |
/s/ Abigail Jenkins |
| |
|
Abigail Jenkins
President and Chief Executive Officer |
POWER OF ATTORNEY
Each person whose signature appears below constitutes
and appoints Abigail Jenkins, Terry Coelho and Josh Patterson, and each of them singly, his or her true and lawful attorneys-in-fact and
agents, with full power to any of them, and to each of them singly, to sign for him or her and in his or her name in the capacities indicated
below the registration statement on Form S-3 filed herewith, and any and all pre-effective and post-effective amendments to said registration
statement, and any registration statement filed pursuant to Rule 462(b) under the Securities Act, as amended, in connection with the said
registration under the Securities Act, as amended, and to file or cause to be filed the same, with all exhibits thereto and other documents
in connection therewith, with the SEC, granting unto said attorneys-in-fact, and each of them, full power and authority to do and perform
each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as each
of them might or could do in person, and hereby ratifying and confirming all that said attorneys, and each of them, shall do or cause
to be done by virtue of this Power of Attorney.
Pursuant to the requirements of the Securities
Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature |
|
Title |
|
Date |
| |
|
|
|
|
| /s/ Abigail Jenkins |
|
President, Chief Executive Officer and Director |
|
December 6, 2023 |
| Abigail Jenkins |
|
(Principal Executive Officer) |
|
|
| |
|
|
|
|
| /s/ Terry Coelho |
|
Chief Financial Officer |
|
December 6, 2023 |
| Terry Coelho |
|
(Principal Financial Officer and Principal Accounting Officer) |
|
|
| |
|
|
|
|
| * |
|
Chairwoman of the Board of Directors |
|
December 6, 2023 |
| Shawn Tomasello |
|
|
|
|
| |
|
|
|
|
| * |
|
Director |
|
December 6, 2023 |
| Julian Adams |
|
|
|
|
| |
|
|
|
|
| * |
|
Director |
|
December 6, 2023 |
| Ivan Borrello |
|
|
|
|
| |
|
|
|
|
| * |
|
Director |
|
December 6, 2023 |
| Kenneth I. Moch |
|
|
|
|
| |
|
|
|
|
| * |
|
Director |
|
December 6, 2023 |
| Stephen T. Wills |
|
|
|
|
| *By: |
/s/
Abigail Jenkins |
|
|
|
December 6, 2023 |
| |
Abigail Jenkins |
|
|
|
|
| |
Attorney-in-Fact |
|
|
|
|
Gamida Cell Inc.
| By: |
/s/ Abigail Jenkins |
|
Authorized U.S. Representative |
|
December 6, 2023 |
| |
Abigail Jenkins, President and Chief Executive Officer |
|
|
|
|
II-6
Exhibit 23.1
CONSENT OF INDEPENDENT REGISTERED PUBLIC
ACCOUNTING FIRM
We consent to the reference to our firm under
caption “Experts” in the Registration Statement (Form S-3) and related Prospectus of Gamida Cell Ltd. for the registration of
ordinary shares, debt, warrants, rights or units and to the incorporation by reference therein of our report dated March 31, 2023 (which
contain an explanatory paragraph describing conditions that raise substantial doubt about the Company’s ability to continue as a
going concern as described in the notes to the consolidated financial statements), with respect to the consolidated financial statements
of Gamida Cell Ltd., included in its Annual Report (Form 10-K) for the year ended December 31, 2022, filed with the Securities and Exchange
Commission.
| /s/ Kost Forer Gabbay & Kasierer |
|
| Kost Forer Gabbay & Kasierer |
|
| A Member of Ernst & Young Global |
|
| |
|
| December 6, 2023 |
|
| Tel-Aviv, Israel |
|
Gamida Cell (NASDAQ:GMDA)
Gráfica de Acción Histórica
De Nov 2024 a Dic 2024
Gamida Cell (NASDAQ:GMDA)
Gráfica de Acción Histórica
De Dic 2023 a Dic 2024