Reata Pharma Shares Climb Premarket After FDA OKs Prior-Approval Supplement for Skyclarys
28 Junio 2023 - 6:20AM
Noticias Dow Jones
By Robb M. Stewart
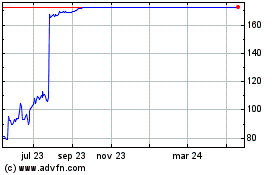
Reata Pharmaceuticals' shares look set for a strong open
Wednesday after the biopharmaceutical company received regulatory
approval to offer its drug for the treatment of Friedreich's ataxia
in the U.S.
In premarket trading, the shares were 12% higher after ending
the previous session at $92.39.
Reata after the market closed Tuesday said the U.S. Food and
Drug Administration approved the prior-approval supplement to
update the drug substance specification for Skyclarys.
Skyclarys is the first and only FDA-approved drug for the
treatment of Friedreich's ataxia in adults and adolescents aged 16
years and older. With this approval, the drug is now available to
patients with Friedreich's ataxia in the U.S., Reata said.
Friedreich's ataxia is an ultra-rare, genetic, life-shortening,
debilitating and degenerative neuromuscular disorder.
Write to Robb M. Stewart at robb.stewart@wsj.com
(END) Dow Jones Newswires
June 28, 2023 07:05 ET (11:05 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
Reata Pharmaceuticals (NASDAQ:RETA)
Gráfica de Acción Histórica
De May 2024 a Jun 2024
Reata Pharmaceuticals (NASDAQ:RETA)
Gráfica de Acción Histórica
De Jun 2023 a Jun 2024