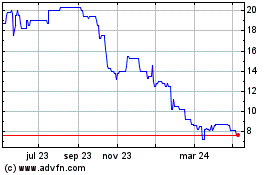
TIDMIXI
RNS Number : 6379V
IXICO plc
05 December 2023
5 December 2023
IXICO plc
("IXICO" or the "Group")
Financial Results for year ended 30 September 2023, Board chair
retirement & Notice of AGM
IXICO plc (AIM: IXI), the medical imaging advanced analytics
company delivering insights in neuroscience, announces its results
for the year ended 30 September 2023 ("FY23").
The Group has continued to develop its capabilities in 2023. The
validation of its next generation TrialTracker imaging platform, as
well as the launch of new MRI and PET imaging analysis solutions
exemplify investments that further strengthen the Group's offering
to the neurological clinical trials market. This infrastructure
also enables the integration of new, partner technologies that will
complement and augment the Group's internal capabilities to address
new markets and so expand the Group's addressable market.
Financial:
The Group has delivered financial earnings in line with market
expectations and retains a strong, debt free, cash balance in an
especially challenging year for the biopharmaceutical industry.
Financial performance marginally outperformed the trading update
provided in September following a strong final month of the
year.
-- GBP6.7 million revenues (2022: GBP8.6 million). The reduction
on the prior period principally reflects the final year of impact
of the previously announced early cessation of large client trials
coupled with a slower pace of new client contract wins reflecting a
challenging market backdrop;
-- Gross margin of 49% (2022: 61%) reflects the lower revenues
and an increased proportion of early phase trials requiring lower
volumes of automated, AI-assisted, image analysis;
-- EBITDA(1) loss of GBP0.8 million (2022: GBP1.5 million profit);
-- Closing, debt-free, cash of GBP4.0 million (2022: GBP5.8
million), after investments in long-term technology and data of
GBP1.9 million (2022: GBP2.2 million); and
-- Closing balance sheet value of GBP11.4 million (2022: GBP12.5 million).
Operational & Commercial:
-- Completed the PET analysis of the landmark 1,000 participant
BioHermes study for the Global Alzheimer's Platform (GAP);
-- Completed the first phase of an unprecedented harmonization
analysis of the c.6,000 HD-IH consortium MRI datasets and
onboarding of a third biopharma partner, Asklepios
BioPharmaceutical, Inc.
-- GBP8.0 million in new contracts won across 6 therapeutic
areas, a combination of new contracts across 7 clients and 13
client contract extensions;
-- Closing order book of GBP14.8 million (2022: GBP16.0
million), providing good visibility to future years' contracted
revenues; and
-- Funding secured to further progress the Group's "Precision in
Neuroscience" strategy, supporting diagnostic decision-support and
safety monitoring of ARIA(3) , the core side effect of recently
approved anti-amyloid therapies.
(1) Earnings before interest, tax, depreciation, and
amortisation
(2) Operating cash and R&D tax credit inflows
(3) Amyloid related imaging abnormality
Giulio Cerroni, CEO of IXICO, said: "I am proud of IXICO's
achievements in 2023, reflected most clearly by several important
successes in our scientific and technological capabilities. We have
purposefully and deliberately focussed investment over recent years
to ensure we develop and own our technology. In leveraging our
neurological expertise, together with cutting edge technologies, we
have built a truly unique, end-to-end, regulatory compliant,
medical imaging technology platform. Whilst IXICO was not immune to
the more challenging market conditions of 2023, we remain confident
that our "Precision in Neuroscience" strategy will gain market
traction as the importance and use of imaging biomarkers in a broad
range of CNS therapeutic areas continues to grow.
Board Chair Succession
Charles Spicer, having served for ten years as a Non-Executive
Director and seven as the Board Chair, will retire from the Board
at the AGM on 25 January 2024. As previously disclosed, Mark Warne,
a current Non-Executive Director, will subsequently assume the role
of Chair.
Giulio Cerroni, CEO of IXICO, said: "I and the rest of the Board
are tremendously grateful for all that Charles has given to IXICO.
His leadership of the Board has been balanced and considered and he
has been a wonderful adviser to me and to my leadership team. I
also want to thank Charles for continuing to work hard for IXICO
over the last few months whilst we have been undertaking a managed
transition of roles within the Board."
Notice of AGM
IXICO also announces that its 2024 Annual General Meeting
("AGM") will be held at CCT Venues Smithfield, Two East Poultry
Avenue, Smithfield, London EC1A 9PT on 25 January 2024 at
10.30am.
The full Annual Report and Accounts, along with Notice of AGM,
will be posted to shareholders on 15 December 2023 and at the same
time will be made available on the Company's website in accordance
with AIM Rule 20.
This announcement contains inside information as stipulated
under the retained EU law version of the Market Abuse Regulation
(EU) No. 596/2014 (the "UK MAR") which is part of UK law by virtue
of the European Union (Withdrawal) Act 2018. The information is
disclosed in accordance with the Company's obligations under
Article 17 of the UK MAR.
For further information please contact:
IXICO plc +44 (0) 20 3763 7499
Giulio Cerroni, Chief Executive Officer
Grant Nash, Chief Financial Officer
Cavendish Securities PLC (Nominated adviser and
sole broker) +44 (0) 20 7397 8900
Giles Balleny (Corporate Finance)
Charlie Combe (ECM)
Michael F Johnson / Tamar Cranford
Smith (Sales)
About IXICO
IXICO is dedicated to delivering insights in neuroscience to
help transform the advancement of investigational therapies for
neurological diseases, such as Huntington's disease, Parkinson's
disease and Alzheimer's disease. The Company's purpose is to
advance medicine and human health by turning data into clinically
meaningful information, providing valuable new insights in
neuroscience by supporting pharmaceutical companies across all
phases of CNS clinical research. IXICO's goal is to be a leading
advocate of artificial intelligence in medical image analysis.
IXICO has developed and deployed breakthrough data analytics, at
scale, through its remote access technology platform, to improve
the return on investment in drug development and reduce risk and
uncertainty in clinical trials for the Company's pharmaceutical
clients.
More information is available on www.IXICO.com and follow us on
Twitter @IXICOnews
Chair's statement
This is my last statement as Chair as, after ten years on the
Board and seven as Chair, I will step down at the AGM in January
2024 and be succeeded by my fellow non-executive director, Mark
Warne.
I am proud of all that the Group has achieved over the last
decade. IXICO now leads the market in neurological image analysis
capabilities. We support academic and industry partnerships
investigating and illuminating disease progression in the
challenging conditions of Huntingdon's disease (HD) and Alzheimer's
disease (AD) and have seamlessly delivered image analysis services
to the world's largest HD clinical trial.
This year, Eisai announced FDA approval for a drug to treat AD
and Eli Lilly reported positive late stage trial results that
together, by showing disease modification benefits, herald a new
era for neurological drug development. Such positive progress
towards mechanisms that slow, halt and potentially even reverse the
progression of AD, coupled with the global epidemic of dementia and
the rapid growth in associated international healthcare costs,
creates a compelling investment case for the biopharma industry
generally and IXICO in particular.
Despite the currently challenging neurological disease clinical
trials market, typified by high profile drug failures and decades
of research to achieve even minor progress, IXICO has progressed in
its technical, scientific and operational capabilities and is
strongly positioned to scale. In the last few months, we have
streamlined our cost base whilst sharpening our focus on the growth
opportunity ahead. The next generation TrialTracker platform is now
production ready and our recent investments in IT infrastructure
provide the Group with the highest levels of data security and
resilience.
Overview
Our purpose is to advance medicine and human health in
neuroscience by converting raw imaging data, captured as part of
the clinical trial process, into clinically meaningful information.
We accurately measure changes (which can be very small) in
biomarkers relevant to diseases of the brain. By doing so, our data
analytics services provide objective insights into the efficacy and
safety of the drugs being trialled and so deliver greater
efficiency and accuracy to the clinical development process. These
services are underpinned by our TrialTracker end-to-end technology
platform which supports the capture, management, analysis and
reporting of data on behalf of each clinical trial in a seamless,
centralised, regulatory compliant system that removes the need for
travel to global imaging sites.
A step backwards to move forwards.
It has been an especially challenging year for the
biopharmaceutical industry and the clinical trials service
providers that support it. Across the Contract Research
Organization (CRO) market we have seen fewer clinical trial
initiations as large pharma companies scrutinise their existing
trial pipelines. Meanwhile, biotech companies, facing tight capital
market conditions, are making cash conservation their primary
priority. Consequently, cost restructuring and market consolidation
have been widely reported.
In this context, IXICO has performed satisfactorily. Despite
reduced revenues (which we communicated this time last year) the
Group has delivered an earnings performance in line with market
expectations and retains a strong, debt-free, cash balance.
As the Group looks to return to growth, we have carefully
adjusted expectations based on the wider market challenges and the
Board took the important, but uncomfortable, decision to right-size
our cost base. I thank the IXICO team for the professional,
sensitive and respectful way in which this was managed by all
involved.
Governance and people
IXICO's future depends on our people and the Board thanks all
our employees for their hard work, dedication, and flexibility in
this particularly challenging year. We continue to promote our
values - Aspiration, Ability, Agility and Accountability - to
augment our culture and align our team with our purpose. The Group
uses the ten principles outlined in the Quoted Companies Alliance
('QCA') Corporate Governance Code to ensure it maintains
appropriate governance arrangements and the Board conducts itself
in a manner that places IXICO's values and the QCA principles at
the core of our culture. The Board met formally thirteen times
during the year with several additional ad-hoc meetings or calls to
discuss specific topics.
I am delighted that Dr Dipti Amin has joined us as a
Non-Executive Director. An experienced NED with an executive career
that includes more than twenty years with IQVIA, where she occupied
senior positions in compliance, drug safety and medical affairs.
Dipti brings significant additional pharmaceutical and CRO
experience to the Board.
At the 2024 Annual General Meeting ('AGM'), in accordance with
the Company's Articles of Association, Dipti Amin and Mark Warne
will stand for election, supported by the Board of Directors'
recommendation. I will retire from the Board on the same date and
Mark Warne will step up to the Board Chair position. I am delighted
that Mark has agreed to do so, thereby creating a smooth
succession, and wish him, our fellow directors and the wider IXICO
team the best of luck for the future.
Shareholders
The Group has an impressive list of leading institutional
investors, and we would like to thank all our shareholders for
their continued support and enthusiasm for IXICO's important
work.
Outlook
While we expect 2024 to be largely flat on 2023, we anticipate
growth in our orderbook of client contracts should position the
Group for a return to double digit revenue growth in 2025.
IXICO is well positioned in its wider market which we expect to
grow and therefore attract new investment in the global pursuit of
medical solutions to those neurological diseases so that impact the
lives of millions of patients and impose wider significant social
and economic burdens. We are a small but important part of the
solution to this high unmet medical need and the Board is proud of
the way that the Group approaches its business activities with this
significant responsibility held firmly at the front of mind.
Charles Spicer
Non-Executive Chair
Chief Executive's statement
Executive Summary
As I look back and reflect on 2023, it is with mixed feelings.
On the one hand, the Alzheimer's Disease (AD) research landscape
continues to experience a significant resurgence, with regulatory
approvals for new therapies with blockbuster potential imminent.
After nearly two decades of high-profile disappointing results in
Phase 3 trials, Biogen's Aducanumab approval in 2021 was followed
in 2023 by the accelerated and then full FDA approval granted for
Eisai's Lecanemab and positive Phase 3 readouts for Eli Lilly's
Donanemab. Our view is that these major milestones will be a
trigger for more investment in neurological disease areas by the
biopharmaceutical industry. With this favourable backdrop, we took
significant strides in 2023 in the delivery of our purpose of
harnessing medical imaging data to advance human health, investing
to strengthen our position as a specialist provider of neuroscience
imaging data analytics solutions to the clinical trials
industry.
Conversely, macro-economic impacts of a challenging political
landscape, high global inflation, and continuing regional conflicts
resulted in the biopharmaceutical industry cutting back on
development pipelines and/or delaying new clinical trial start-ups.
Like many other CRO companies supporting the clinical trials
market, IXICO was not immune to the financial impacts of these
conditions, resulting in a weaker year of new contract bookings
than had been anticipated. Despite lower than planned revenues, I
am pleased to report earnings that align with market expectations.
However, given that we expect the challenging business environment
to continue across 2024, we recently took the difficult decision to
reduce headcount to right size our cost base as we reset the
business for growth.
During the year we supported two important enhancements to
neurological disease knowledge in AD and Huntington's disease (HD).
We supported the Global Alzheimer's Platform (GAP) by providing PET
Amyloid visual reads for their 1,000 participant BioHermes trial,
completing data transfers ahead of the requested timelines. The
study was notable for achieving a secondary recruitment target
requiring a minimum of 20% of the study participants to be from
traditionally underrepresented populations, enabling IXICO to
report on initial findings on differences between racial and ethnic
groups at the CTAD opening symposium (Boston, October 2023). We
also led, alongside the CHDI Foundation, the Huntington's Disease
Imaging Harmonization Consortium (HD-IH) in securing funding to
ensure the full analysis of more than 6,000 HD datasets using
IXICO's leading IXIQ.Ai analytics platform. We anticipate that the
insights derived from the work of the HD-IH consortium will create
long term value to the biopharmaceutical partners to support them
in their clinical development programmes and to the broader HD
research community.
We delivered seamlessly for our clients, providing image
analysis services to more than 30 studies, broadening our offering
across therapeutic indications whilst improving our service level
metrics to exceed our clients' expectations. In tandem, we further
developed our PET imaging capabilities, including an enhanced
service offering for PET tracer management, deepening our reach
into the AD market. Our next generation TrialTracker platform is
production ready, and we are looking forward to deploying this
Microsoft Azure enabled technology in support of our client trials
during 2024 and in the years ahead.
We have made strides in executing our 2022 to 2027 "Precision in
Neuroscience" strategy. We enter our new financial year with an
order book of signed contracts valued at GBP14.8m and a stronger
pipeline of client opportunities, with visibility of new contracts
to provide a platform for double digit growth in 2025 and beyond.
Considering the longer term, I am also excited by the progress made
with the "Bridge Pillar" of our strategy, with a recent funding
award to develop our plans for post-market decision support; this
development will provide the bridge between clinical trials and
diagnostic tools to enable the right patients to safely access
novel therapies in AD. There remains significant opportunity in
this space, and our intention is to accelerate further development
in this area during 2024.
I would like to thank my team at IXICO for their incredible hard
work over the last twelve months, and for the approach and
professionalism that they have brought to both meeting the
opportunities and supporting the challenges that we and our current
and prospective clients face in developing new therapies across
many neurological diseases. As I look forward to 2024, it is with
cautious optimism underpinned by a deep-rooted confidence that
IXICO is better placed than it has ever been to deliver seamlessly
for our clients on our purpose of harnessing medical imaging data
to advance human health within neuroscience.
Market overview
The burden of degenerative neurological disease continues to
increase, driven by an aging population. Research in this area
continues to advance; greater understanding of neurological disease
has been recently driven by insights derived from multi-modal
approaches combing genomics, biomarkers, diagnostics, and imaging
techniques, and this, along with emerging new drug mechanisms, is
changing the fundamentals of innovation in the sector.
Recent regulatory progress in AD for Biogen, Eisai and Eli Lilly
has given pharma companies greater certainty of commercial returns
in what has been historically a very challenging indication. This
has encouraged further investment in neuroscience portfolios and,
consequently, the market for clinical trials in neuroscience is
expected to continue to grow at pace.
Central Nervous System disorders account for the second largest
segment of pharmaceutical R&D investment, behind only that of
oncology. The CNS disorders segment accounted for 10.6% of the
clinical trial imaging market in 2020. This segment has been valued
at $108 million in 2021 and it is expected to grow to $157 million
in 2026 with a CAGR of 7.7%
Neuroimaging is widely deployed in CNS trials at all phases,
firstly to screen patients for safety and eligibility and then at
regular intervals to detect and measure changes in brain structure
caused by disease progression and interventions. Although many
imaging biomarkers are exploratory, objectively detecting and
measuring even small changes can deliver significant insights to
the sponsor on treatment mode of action and efficacy.
The core of IXICO's imaging offer is in its proprietary,
validated artificial intelligence technology which is deployed to
deliver standardised and repeatable analysis generating reliable
insights; IXIQ.Ai can more than double the amount of usable data
compared to widely used tools delivering greater value to clients
in extracting insights from its trial investments. In addition,
IXICO offers its clients deep expertise, in imaging techniques and
endpoints with specialist Biomarker Scientists dedicated to each
study to help clients plan the imaging approach and protocol, and
to provide quality assurance for the study output. IXICO's
TrialTracker platform ensures robust data management and security
which is of the highest importance to clients operating in the
highly regulated field of clinical trials, particularly when
deploying studies in multiple jurisdictions.
Outlook of the neurological drug development segment by
indication
Alzheimer's Disease (AD)
Historically, over 99% of novel treatments in development for AD
have failed to achieve regulatory approval, with many of these
failures occurring in the late stage of trial, representing a loss
of many billions of dollars of investment. More recently, Biogen
and Eisai have achieved approval by the FDA and, with a further
asset now in regulatory review (Eli Lilly), there is renewed
confidence in the validity of the research approach. This has paved
the way for even greater focus in this area of research and
development. The IXIQ.Ai brain segmentation platform can improve
success rates by providing more usable data, enabling more targeted
patient selection to lower the biggest driver of costs and
inefficiencies while increasing the chance of success. Currently
there are over 140 drugs in the global AD development pipeline;
many of these drugs are exploring new modes of action, increasing
the value of imaging biomarkers to closely monitor patients for
safety, as well as to study disease progression and drug
efficacy.
Parkinson's Disease (PD)
Until recently, treatments for PD have focused on reducing the
severity and impact of the physical symptoms of this debilitating
disorder. However, decades of research into the underlying causes
of PD are now bearing fruit with the development of newer drugs
that focus on slowing, halting, or even reversing disease
progression, particularly in the early stages. To date, IXICO has
provided neuroimaging services to 14 trials in parkinsonian
syndromes and more recently has introduced algorithms for DAT SPECT
imaging modalities, specifically to support the growing portfolio
of PD trials.
Multiple Sclerosis (MS)
MS tends to affect a younger patient population compared to AD
and PD, and a wide portfolio of therapies has been available for
treatment for many years. However, the disease is increasingly now
well-understood, and research has identified new clinical subtypes,
ushering in new approaches to treatment and management, and
fuelling an increasing development pipeline. IXICO is partnering
with leading clinical centres on the development of new algorithms
to support subtype detection and monitoring, providing leading-edge
services to MS studies. The Group also recently introduced an early
engagement programme to enable sponsors to fully take advantage of
novel imaging approaches on their studies to unlock new
insights.
Huntington's Disease (HD)
HD is a relatively rare neurodegenerative disease caused by a
faulty gene. Although there have been recent setbacks in the
progress of drug development for this indication, the genetic
nature of HD means that patients can be reliably identified earlier
in the disease pathway, long before symptoms are apparent. This may
enable earlier intervention and raises the possibility of gene
therapies, supporting the continued growth of the HD development
pipeline. IXICO is a leader in neuroimaging in HD, having supported
many HD studies in the past decade and has strengthened its
leadership position through its close collaboration with the CHDI
Foundation and the HD-IH consortium (see the Science review).
Orphan and Rare Diseases
Initiatives by the EU EMA and US FDA such as orphan drug
designation, and the increasing use of genomic sequencing
technology to screen newborns and to investigate early childhood
development disorders, have encouraged significant investment into
a wide range of rare diseases. In the past five years a new wave of
rare disease neurological treatments, including dozens with orphan
designation, have been approved. With its expertise in imaging and
biomarker development, IXICO has successfully adapted many
biomarkers for rare neurodegenerative diseases to support a wide
range of studies in rare indications such as Friedreich's Ataxia,
Multiple System Atrophy and Progressive Supranuclear Palsy.
Operational review
During 2023, the Group worked on more than 30 studies across a
broadening range of neurodegenerative indications, supporting all
phases of clinical research from small early phase studies to
larger Phase 3 trials.
Delivering operational excellence
As a highly specialised provider of tailored solutions, we
strive to deliver an excellent service to our biopharmaceutical
clients and to the imaging sites we work with. We achieve our high
standards by being agile in decision-making and through our ability
to customise the solutions we offer. Examples of 2023 performance
metrics include:
-- Project performance: we monitor our performance through
customer satisfaction, quality, resources and deliverables. Our
metrics have exceeded both those agreed with our sponsors and our
own internal targets.
-- Geographies: We supported more than 30 studies across 25
countries. During 2023 we added more than 10 SPECT sites to our
network of qualified sites, bringing our total qualified site
number for MRI, PET and SPECT to over 1,000 sites across the
globe.
-- Analysis units: we analysed over 30,000 image endpoints this
year for over 4,000 patient visits. The number of endpoints
analysed increased by 37% compared to FY22.
-- Turnaround timelines: fast turnarounds of radiology reports
are key to ensure patient eligibility and treatment decisions are
not delayed. During 2023, IXICO consistently delivered radiology
reports in an average of 3 working days or less, meeting or
exceeding the high standards of expectation by clients for brain
scans.
We strive to stay at the forefront when developing best practice
and have reduced the timelines for transferring data and images to
our clients. We know how critical it is for biopharmaceutical
companies to have quick access to the full set of high-quality data
collected in their study to allow regulatory filing, early
publication and conference presentations. We continue to streamline
our processes to ensure that client requirements are fulfilled or
exceeded to achieve these important milestones.
We are proud of our easy-to-work with operations processes,
exemplified by a webinar (December 2022) in collaboration with
Re:Cognition Health showcasing our best-in-class approach in
setting up imaging studies quickly and efficiently.
Enhancing operational capabilities
1. PET Tracer management
During 2023, IXICO enhanced its service offering to our sponsors
to provide PET Tracer management solution for AD and PD studies.
PET imaging uses a radioactive tracer during the scan. The tracer's
uptake provides information to help determine patient eligibility
and treatment efficacy. Due to their radioactive nature, these
tracers have a short half-life (e.g., 110 minutes for the most
widely used), and require special transportation, licencing and
storage conditions. They are complex to produce and therefore their
availability may be limited. Due to all these characteristics,
tracer management is increasingly of interest to biopharmaceutical
companies. IXICO is able to support these activities by
facilitating the coordination of the Tracer manufacturing company,
the logistics companies and the imaging sites.
2. Next generation of TrialTracker
Over the past three years, IXICO has utilised its in-house
development resources, augmented by contracted expertise, to bring
to market a next generation of its TrialTracker platform.
As with the Group's existing TrialTracker, this is an end-to-end
data management platform enabling imaging sites, wherever they are
in the world, to upload brain scans whilst automatically checking
scan quality and pseudonymising the scan. The platform seamlessly
transfers the scan to IXICO's radiological team, to provide image
reads and safety checks, and hosts its AI enabled, automated
proprietary analysis pipelines.
The next generation TrialTracker platform has been developed
using Microsoft's Azure cloud technologies, providing the Group and
its clients with state-of-the-art, secure, resilient, regulatory
compliant infrastructure benefitting from highly flexible
microservice capabilities. This enables the Group to adapt the
platform to suit the specific (and often bespoke) clinical trial
needs of its clients and partners.
The platform is ready to be deployed on client trials having
completed extensive testing and validation activities in 2023.
Science review
Significant progress was made across 2023 in further developing,
validating and positioning IXICO's clinical trial product portfolio
across therapeutic indications. In addition, the Group made further
progress in developing core technology to 'bridge' into potential
new markets in clinical applications as the field is experiencing
significant momentum in the approval of new therapies, specifically
in AD. Throughout 2023, IXICO has actively participated in the
scientific discussion across core therapeutic areas as demonstrated
by the attendance at eight conference and the (co-) presentation of
more than 20 posters and (invited) talks. IXICO has furthermore,
hosted three scientific webinars with key opinion leaders in AD,
gene therapies, and HD.
A key R&D focus was on the extension of IXICO's PET analysis
capabilities to extend the service offerings across both relevant
(MRI and PET) imaging modalities within a broadening range of
neuroscience clinical trials. Specifically, IXICO has now deployed
PET visual read and quantitative analysis solutions across core PET
tracers in AD and PD. During 2023, we have completed PET analysis
of the Bio-Hermes trial for the Global Alzheimer's Platform (GAP).
Co-sponsored by pharma companies with an interest in AD clinical
development, the landmark 1,000-participant Bio-Hermes study was
designed to evaluate the ability of blood-based and digital
biomarkers to reflect the presence of brain amyloid in participants
enrolled in AD clinical trials. A secondary objective of Bio-Hermes
was to include a significant representation of ethnic and racial
minority participants, building a highly valuable database as
regulatory requirements in AD clinical trials increasingly require
improved representation of traditionally underrepresented
populations. IXICO provided all PET data collection services as
well as the visual radiology read to determine gold-standard
amyloid pathology. Following study completion, IXICO's scientific
team led an initial analysis of the collected PET biomarkers,
specifically focusing on the analysis across ethnic and racial
groups. The work was selected for presentation and discussion in
the opening symposium at the high-impact CTAD (Clinical Trials in
Alzheimer's Disease) conference, held in Boston, Massachusetts,
between 24(th) and 27(th) October 2023. Selection by the organising
committee for this prestigious presentation slot highlights the
importance of the work performed for the AD community and provided
a significant opportunity for IXICO to demonstrate cutting edge
scientific and technical capabilities in AD PET imaging to
participating pharma sponsors and academic researchers. As a
Bio-Hermes partner with an R&D license on the data collected,
IXICO is well-positioned to further investigate the unique dataset
and thereby advance scientific knowledge in this critical field of
AD development.
Huntington's disease continues to be a key market for IXICO, and
good progress was made during 2023 to further underline the Group's
leading position by progressing the IXICO-initiated HD Imaging
Harmonization (HD-IH) consortium. HD-IH was founded in 2022 by
IXICO, the CHDI Foundation Inc. (CHDI) and pharma partners uniQure
and PTC Therapeutics to conduct an unprecedented harmonization
analysis of more than 6,000 participant-visit magnetic resonance
images (MRIs) acquired from over 2,000 research participants.
During 2023, the project has completed the initial phase and,
through the onboarding of a third pharma partner, Asklepios
BioPharmaceutical, Inc. (AskBio) and additional funding commitments
by both CHDI and IXICO, secured the necessary financing to complete
the project. These analyses are expected to support the development
of therapeutic strategies targeting specific HD sub-populations
("precision-medicine"), inform eligibility and dosing decisions for
clinical trials, and aid in associated trial design decisions and
biomarker development to enable interventional studies earlier in
the disease course. IXICO presented an update on progress of HD-IH
at the 18(th) Huntington's Disease Therapeutics Conference (HDTC)
held in Dubrovnik, Croatia, from 24-27 April 2023.
Further steps were taken in the development of an extended
offering for demyelinating disorders. The Group has previously
announced the award of two phase-III trials in MOG
antibody-associated disease (MOG-AD) and during 2023 we have
extended our service offering to provide automated lesion
quantification into one of those trials. With these extended
capabilities, the Group can now serve core imaging requirements
across demyelinating disorders, including Multiple Sclerosis (MS)
as the most prevalent and widely researched disease area. IXICO
presented a poster on its quantitative MS analysis at the 9(th)
conference by the European Committee for Treatment and Research in
MS, ECTRIMS, held in Milan, Italy, from 11-13 October 2023.
The Group continues an active R&D program exploring
opportunities to develop its core clinical trial analytics
technology for applications that support treatment-related
decision-making in new post-market applications. During 2023, we
have actively progressed several partnership opportunities on the
development of solutions to provide diagnostic decision-support in
clinical practice and safety monitoring of ARIA (amyloid related
imaging abnormality), the core side effect of recently approved
anti-amyloid therapy. To further support development of a
post-market decision support tool, the Group has secured funding
that will support and complement IXICO's internal R&D program
during 2024.
Growth strategy & Corporate outlook
Our focus remains on neuroscience with neurology global medicine
spending expected to grow at 3-6% to more than $140 billion by
2025. This includes much higher growth subsegments, as a range of
rare neurological diseases have had new treatments approved or
showing positive progression as well as the potential that large
population diseases like AD or PD will see further investment
consequent to the approval of new treatments.
During 2023, IXICO was impacted by a slow-down in trial
start-ups and near-term cutbacks in pharma R&D pipelines
resulting from a tighter funding environment affecting the
biopharmaceutical industry. Whilst we anticipate these conditions
continuing in 2024, we view them as near-term headwinds. We remain
resolute in our conviction that the unmet clinical need in
neurodegenerative diseases provides significant runway for growth
for IXICO, reflected in our "Precision in Neuroscience"
strategy.
Our 5-point Precision in Neuroscience strategy
IXICO continued to make good progress in several strategic
areas:
Build Pillar: Robust operational performance and cost control
measures for the period enabled financial earnings in line with
market expectations, despite lower year over year revenues.
Investments made during 2023 to build our commercial infrastructure
have led to notable increase in the client contract opportunities
pipeline providing management visibility to significantly larger
numbers of Requests for Proposal (RFP) over the next 24 months,
each valued at between GBP0.25m to GBP2.5m, across a broadening
range of therapeutic areas. This, combined with an order book at
the end of 2023 of GBP14.8m, provides good future revenue
visibility and a basis for targeting our commercial efforts to
return to double digit revenue growth in 2025 and beyond.
Innovate Pillar: We believe that IXICO will lead in our
addressable markets by developing proprietary algorithms that the
biopharmaceutical industry rely on to establish both the safety and
efficacy of new treatments. Data analysis undertaken by IXICO as
part of the GAP Bio-Hermes study (see Science review) completed in
2023 were presented in the opening symposium at the prestigious
CTAD (Boston, October 24th), AD clinical trials scientific
conference. Presenting important data at large disease specific
conferences is expected to increase awareness of IXICO's
capabilities to the global pharma industry and provide significant
potential future business development opportunities.
In 2023, the HD-IH consortium was expanded (see Science review).
The HD-IH consortium deploys IXICO's proprietary IXIQ.Ai brain
segmentation platform to conduct a harmonized analysis of more than
6,000 participant-visit magnetic resonance images (MRIs) acquired
from over 2,000 research participants. These analyses will support
the development of therapeutic strategies targeting more specific
sub-populations, inform eligibility and dosing decisions for HD
clinical trials, and aid in associated trial design decisions and
biomarker development to enable interventional studies earlier in
the disease course.
Penetrate Pillar: Despite delays in clinical trial start-ups, we
continue to make good progress in pursuing our strategy of
penetrating early stage (phase 1 and 2) programmes, accessing the
potential to stay with the client for several years as the asset,
if successful, moves along the development continuum. Broadening
the client base with more early phase studies reduces the risk
associated with any single large, late phase project, while
providing multiple opportunities to move into larger, later stage
(phases 3 and 4) clinical trials in the future.
Developing drugs takes a long time and is expensive. The overall
likelihood of approval (LOA) from Phase 1 for all developmental
candidates over 2011-2020 was 7.9%. However, rare disease therapies
(of which many are in neurology) are notably more successful with
an overall LOA of 17.0% and development programs with trials
employing patient preselection biomarkers have two-fold higher LOAs
(15.9%), driven by a Phase 2 success rate of nearly one-in-two.
This insight supports our strategy of becoming the partner of
choice across a broader range of rare diseases, as we have done in
HD. In addition, we will be looking to leverage our next generation
TrialTracker platform to increase commercial success in new trials
of large population diseases like AD, MS, or PD, in particular
those employing imaging biomarkers combined with potential analysis
of non-imaging biomarkers, such as blood-based and digital
biomarkers.
Bridge Pillar: There is incredible potential for real world
evidence (RWE) to play a much broader role in the advancement of
drug development, delivering insights that ensure efficacy and
safety, while increasing patient-centricity and trial feasibility.
With our recent and ongoing investments in artificial intelligence
(AI) and cloud analytics together with our next generation
TrialTracker data sharing and AI analysis platform, IXICO's
ambition is to address many of the challenges to analysing and
interpreting RWE Imaging data. We expect this to expand IXICO's
addressable clinical trials market whilst creating a bridge into
post marketing and clinical practice markets.
Enhance Pillar: With long lead times, significant regulatory and
procurement barriers to entry into the clinical trials market,
IXICO is uniquely positioned to partner with innovative analytics
organisations to provide a market channel to the global
biopharmaceutical industry. This partnering strategy supplements
our own innovation roadmap with additional enhancements to our
offering to provide cutting edge innovations across a broad range
of CNS disease areas. Progress made during 2023 means that we are
hopeful to be able to announce our first significant partnership
agreement early in 2024.
Giulio Cerroni
Chief Executive Officer
Financial review
Right sizing the Group for future growth.
In 2023, the Group has navigated a challenging trading
environment. This was anticipated, and the Group has delivered
financial earnings for 2023 in line with market expectations.
Looking to 2024, ongoing macro-economic and political challenges
continue to weigh down on the clinical trials market, and, in
particular, the availability of capital. This has led to cost
restructuring and consolidation across the pharma, biotech and CRO
space, and has softened the Group's revenue outlook for 2024, as
announced in September 2023. To anticipate this, the Group has
undertaken cost reduction measures, including a 'right sizing' of
the employee base seeking to balance the weaker short-term outlook
and the medium-long term market opportunity (which, if anything,
has strengthened in the year).
The Group expects to deliver flat revenue across 2024, before
returning to revenue growth in 2025.
This review includes a comparison of the financial KPIs used to
measure progress over the prior year, a summary of which is shown
below:
KPI 2023 result 2022 result Movement
------------------------------- ------------ ------------ ---------
Revenue GBP6.7m GBP8.6m GBP1.9m
Gross profit GBP3.3m GBP5.2m GBP1.9m
Gross margin 49.1% 60.7% 1,160bps
EBITDA (loss)/profit (GBP0.8m) GBP1.5m GBP2.3m
Operating (loss)/profit (GBP1.4m) GBP0.9m GBP2.3m
(Loss)/profit per share (2.44p) 2.14p 4.58p
Order book GBP14.8m GBP16.0m GBP1.2m
Net assets GBP11.4m GBP12.5m GBP1.1m
Cash GBP4.0m GBP5.8m GBP1.8m
Non-current asset investments GBP1.9m GBP2.3m GBP0.4m
------------------------------- ------------ ------------ ---------
Revenue
Revenue for the year of GBP6.7 million (2022: GBP8.6 million)
represents a year-on-year contraction of 23%. This contraction was
expected, and was caused by the final year impact of large client
trial cessations arising across 2021 and early 2022, each
materially impacting future revenues. Replacing the revenues lost
from these trials takes time, both in contracting and initiating
new trials and those new trials will tend to be lower value,
earlier phase trials compared to the large, failed trials (which
were predominantly late stage).
Across 2023, the Group has seen lower levels of new contract
bookings than it anticipated, a trend seen across the clinical
trials market. The Group will deliver approximately flat revenues
in 2024 before an expected return to revenue growth in 2025.
Gross profit
The Group reports gross profit of GBP3.3 million for the year
(2022: GBP5.2 million). This equates to a gross margin of 49%
(2022: 61%). Whilst this remains a strong gross margin, the
reduction on the prior year reflects both the reduction in revenues
and a revenue mix increasingly reflective of earlier phase trials,
which tend to be lower margin. Whilst in the short term, a
portfolio of early phase trials results in lower gross margins,
this portfolio also provides a strong base for future growth, as
those trials that successfully move from early to late phase
provide the Group with the opportunity to continue providing
services as these trials transition to larger, later phase
trials.
Earnings before interest, tax, depreciation, and amortisation
('EBITDA')
The Group delivered an EBITDA loss of GBP0.8 million in the year
(2022: GBP1.5 million profit). This reflects the reduction in
revenues, tighter margins, a lower level of grant income, several
non-recurring benefits that supported profitability in 2022 and a
reduced level of cost capitalisation. These negative impacts have
then been partially offset by careful cost management including the
completion of a headcount reduction exercise immediately post the
financial year end.
2023 2022
GBP000 GBP000
--------------------------------------- -------- --------
Profit attributable to equity holders (1,178) 1,032
--------------------------------------- -------- --------
Depreciation of fixed assets 400 451
Amortisation of fixed assets 225 188
Interest on lease liabilities 29 33
Interest on cash held at bank (105) (10)
Taxation (183) (147)
--------------------------------------- -------- --------
EBITDA (812) 1,547
--------------------------------------- -------- --------
Operating profit
Operating expenditure in the year reflected careful cost
management alongside targeted investment, specifically:
-- research and development expenses of GBP0.9 million (2022:
GBP1.2 million) included the development of new algorithms to
support image analysis in new and existing therapeutic indications.
In addition, the Group capitalised GBP1.2 million of internal
development expenditure primarily in respect of its next generation
TrialTracker platform (2022: GBP0.9 million);
-- sales and marketing expenses of GBP1.3 million (2022: GBP1.2
million) reflecting increased investment in this team in
particular, sales executives and marketing and product
capabilities; and
-- general and administrative expenses of GBP2.9 million (2022:
GBP2.6 million) reflecting several non-recurring positive impacts
on profit in the prior year that included positive foreign exchange
movements and the write back of long term incentive charges on
share options that did not, or were not expected to, meet their
performance conditions.
Operating losses totalled GBP1.4 million (2022: GBP0.9 million
profit) equated to an operating loss margin of 22% (2022: 11%
profit margin).
Order book
The Group continues to benefit from a healthy contracted order
book. On 30 September 2023 this totalled
GBP14.8 million (2022: GBP16.0 million), which takes account of
GBP6.7 million of revenues delivered during the financial year,
GBP8.0 million of new and expanded multi-year contracts secured
during the year and GBP2.6 million of trial descopes due to client
trial failures and minor foreign exchange movement in the year.
This net contraction in the order book reflects the notable
slowdown in new trial initiations during 2023. This is a short-term
challenge reflective of the tight capital markets and is counter to
the medium and longer term trends for increased investment in this
market driven by aging populations, increased global healthcare
costs and scientific breakthroughs in the area of neurological
disease.
New contracts won were across 7 clients with contract extensions
across 13 clients.
2023 2022
GBP000 GBP000
--------------------------------- -------- --------
Opening orderbook 16,019 18,776
---------------------------------- -------- --------
New wins 8,030 12,617
Revenue (6,665) (8,643)
Net descoping, inflation and FX (2,631) (6,731)
Closing orderbook 14,753 16,019
---------------------------------- -------- --------
Cash
The Group reported operating cash inflows after tax receipts of
GBP0.3 million in the year (2022: GBP1.4 million). This reflects
the timing of operational cash inflows and outflows with strong
client payment volumes early in the year supporting an overall
positive operational cash position.
The Group had a closing cash balance at 30 September 2023 of
GBP4.0 million (2022: GBP5.8 million) with the reduction in cash
reflecting GBP1.9 million (2022: GBP2.2 million) of investment in
data and technology assets designed to support future scalability
and GBP0.2m of lease payments on the Group offices (2022: GBP0.1m).
These investments were partially offset by the GBP0.3m of operating
cash and taxation inflows (2022: GBP1.4 million).
Non-current asset investments
The Group capitalised GBP1.9 million of non-current assets in
the year to 30 September 2023 (2022: GBP2.3 million). This decrease
in non-current assets reflects the 2023 investment in bringing to
operational readiness of the Group's next generation TrialTracker
platform totalling GBP1.6 million (2022: GBP2.0 million). 2024
capitalised investment in this platform to deliver additional
functionality is expected to be less than GBP1.0 million.
The next generation TrialTracker platform further enhances the
Group's capabilities to remotely collect, and centrally analyse,
brain images in support of clinical trials. The platform has been
developed on Microsoft Azure's cloud infrastructure supporting
further improvements in system resilience, security, scalability,
and efficiency.
Net assets
The Group's net asset position decreased by GBP1.1 million to
GBP11.4 million across the year (2022: GBP12.5 million). This
reflects the losses reported partially offset by the investments
made in technology assets to underpin long-term future growth
aspirations and market demands.
Loss per share
The Group reports a loss per share of 2.44p (2022: 2.14p profit
per share).
Grant Nash
Chief Financial Officer
Financial Statements
Consolidated Statement of Comprehensive Income
for the years ended 30 September 2023 and for 30 September
2022
30-Sep-23 30-Sep-22
Notes GBP000 GBP000
---------------------------------------------- ------ ---------- ----------
Revenue 5 6,665 8,643
Cost of sales (3,395) (3,400)
---------------------------------------------- ------ ---------- ----------
Gross profit 3,270 5,243
Other income 7 393 689
Operating expenses
Research and development expenses (925) (1,217)
Sales and marketing expenses (1,321) (1,226)
General and administrative expenses (2,854) (2,581)
Total operating expenses 10 (5,100) (5,024)
---------------------------------------------- ------ ---------- ----------
Operating (loss) / profit (1,437) 908
Finance income 105 10
Finance expense (29) (33)
(Loss) / profit on ordinary activities
before taxation (1,361) 885
Taxation 11 183 147
---------------------------------------------- ------ ---------- ----------
(Loss) / profit attributable to equity
holders for the period (1,178) 1,032
---------------------------------------------- ------ ---------- ----------
Other comprehensive income / (expense):
Items that will be reclassified subsequently
to profit or loss
Foreign exchange translation differences (21) 14
Movement in fair value of cash flow hedges 22 111 (214)
Cash flow hedges recycled to revenue 22 (27) 103
---------------------------------------------- ------ ---------- ----------
Total other comprehensive income / (expense) 63 (97)
Total comprehensive (expense) / income
attributable (1,115) 935
------
to equity holders for the period
---------------------------------------------- ------ ---------- ----------
(Loss) / profit per share (pence)
---------------------------------------------- ------ ---------- ----------
Basic (loss) / profit per share 12 (2.44) 2.14
Diluted (loss) / profit per share 12 (2.44) 2.03
---------------------------------------------- ------ ---------- ----------
Consolidated Statement of Financial Position
as at 30 September 2023 and 30 September 2022
30-Sep-23 30-Sep-22
Notes GBP000 GBP000
-------------------------------------- ------ ---------- ----------
Assets
Non-current assets
Property, plant and equipment 13 518 817
Intangible assets 14 6,147 4,587
Commission assets 16 39 -
Total non-current assets 6,704 5,404
Current assets
Trade and other receivables 16 1,706 3,029
Current tax receivables 11 549 453
Cash and cash equivalents 4,031 5,769
-------------------------------------- ------ ---------- ----------
Total current assets 6,286 9,251
Total assets 12,990 14,655
-------------------------------------- ------ ---------- ----------
Liabilities and equity
Non-current liabilities
Trade and other payables 17 2 33
Lease liabilities 18 275 394
-------------------------------------- ------ ---------- ----------
Total non-current liabilities 277 427
Current liabilities
Trade and other payables 17 1,142 1,502
Derivative financial liabilities 22 27 111
Lease liabilities 18 112 122
Total current liabilities 1,281 1,735
Total liabilities 1,558 2,162
-------------------------------------- ------ ---------- ----------
Equity
Ordinary shares 20 484 482
Share premium 20 84,802 84,802
Merger relief reserve 20 1,480 1,480
Reverse acquisition reserve 20 (75,308) (75,308)
Cash flow hedge reserve 20,22 (27) (111)
Foreign exchange translation reserve 20 (95) (74)
Capital redemption reserve 20 7,456 7,456
Accumulated losses 20 (7,360) (6,234)
-------------------------------------- ------ ---------- ----------
Total equity 11,432 12,493
Total liabilities and equity 12,990 14,655
-------------------------------------- ------ ---------- ----------
Company Statement of Financial Position
as at 30 September 2023 and 30 September 2022
30-Sep-23 30-Sep-22
Notes GBP000 GBP000
----------------------------------- ------ ---------- ----------
Assets
Non-current assets
Investments in Group undertakings 15 5,857 5,805
Total non-current assets 5,857 5,805
Current assets
Trade and other receivables 16 2,481 3,088
Cash and cash equivalents 1,469 1,590
----------------------------------- ------ ---------- ----------
Total current assets 3,950 4,678
Total assets 9,807 10,483
----------------------------------- ------ ---------- ----------
Liabilities and equity
Current liabilities
Trade and other payables 17 60 83
Total current liabilities 60 83
Equity
Ordinary shares 20 484 482
Share premium 20 84,802 84,802
Merger relief reserve 20 1,480 1,480
Capital redemption reserve 20 7,456 7,456
Accumulated losses 20 (84,475) (83,820)
----------------------------------- ------ ---------- ----------
Total equity 9,747 10,400
Total liabilities and equity 9,807 10,483
----------------------------------- ------ ---------- ----------
Parent Company Income Statement
As permitted by Section 408 of the Companies Act 2006, the
income statement of the Company is not presented as part of these
financial statements. The Company's loss for the financial year was
GBP707,000 (2022: GBP741,000).
Consolidated Statement of Changes in Equity
for the years ended 30 September 2023 and 30 September 2022
Foreign Cash
Merger Reverse exchange flow Capital
Ordinary Share relief acquisition translation hedge redemption Accumulated
shares premium reserve reserve reserve reserve reserve Losses Total
GBP000 GBP000 GBP000 GBP000 GBP000 GBP000 GBP000 GBP000 GBP000
---------------- --------- -------- -------- ------------ ------------ -------- ----------- ------------ --------
Balance at 30
September
2021 482 84,802 1,480 (75,308) (88) - 7,456 (7,345) 11,479
---------------- --------- -------- -------- ------------ ------------ -------- ----------- ------------ --------
Total
comprehensive
income
Profit for the
period - - - - - - - 1,032 1,032
Other
comprehensive
income/expense
Foreign
exchange
translation - - - - 14 - - - 14
Movement in
fair value of
cash flow
hedges - - - - - (214) - - (214)
Cash flow
hedges
recycled
to revenue - - - - - 103 - - 103
---------------- --------- -------- -------- ------------ ------------ -------- ----------- ------------ --------
Total
comprehensive
income/expense - - - - 14 (111) - 1,032 935
Transactions
with owners
Charge in
respect of
share
options - - - - - - - 79 79
Balance at 30
September
2022 482 84,802 1,480 (75,308) (74) (111) 7,456 (6,234) 12,493
---------------- --------- -------- -------- ------------ ------------ -------- ----------- ------------ --------
Total
comprehensive
income
Loss for the
period - - - - - - - (1,178) (1,178)
Other
comprehensive
income/expense
Foreign
exchange
translation - - - - (21) - - - (21)
Movement in
fair value of
cash flow - - - - - 111 - - 111
Cash flow
hedges
recycled
to revenue - - - - - (27) - - (27)
---------------- --------- -------- -------- ------------ ------------ -------- ----------- ------------ --------
Total
comprehensive
income/expense - - - - (21) 84 - (1,178) (1,115)
---------------- --------- -------- -------- ------------ ------------ -------- ----------- ------------ --------
Transactions
with owners
---------------- --------- -------- -------- ------------ ------------ -------- ----------- ------------ --------
Charge in
respect of
share
options - - - - - - - 52 52
Exercise of
share options 2 - - - - - - - 2
---------------- --------- -------- -------- ------------ ------------ -------- ----------- ------------ --------
Total
transactions
with
owners 2 - - - - - - 52 54
---------------- --------- -------- -------- ------------ ------------ -------- ----------- ------------ --------
Balance at 30
September
2023 484 84,802 1,480 (75,308) (95) (27) 7,456 (7,360) 11,432
---------------- --------- -------- -------- ------------ ------------ -------- ----------- ------------ --------
Company Statement of Changes in Equity
for the years ended 30 September 2023 and 30 September 2022
Capital
Ordinary Share Merger relief redemption Accumulated
shares premium reserve reserve losses Total
GBP000 GBP000 GBP000 GBP000 GBP000 GBP000
------------------------------------ --------- -------- -------------- ----------- ------------ -------
Balance at 30 September 2021 482 84,802 1,480 7,456 (83,158) 11,062
Total comprehensive expense for
the period - - - - (741) (741)
Transactions with owners
Charge in respect of share options - - - - 79 79
Balance at 30 September 2022 482 84,802 1,480 7,456 (83,820) 10,400
Total comprehensive expense for
the period - - - - (707) (707)
Transactions with owners
Charge in respect of share options - - - - 52 52
Exercise of share options 2 - - - - 2
Total transactions with owners 2 - - - 52 54
Balance at 30 September 2023 484 84,802 1,480 7,456 (84,475) 9,747
------------------------------------ --------- -------- -------------- ----------- ------------ -------
Consolidated Statement of Cash Flows
for the years ended 30 September 2023 and 30 September 2022
30-Sep-23 30-Sep-22
GBP000 GBP000
---------------------------------------------------- ---------- ----------
Cash flows from operating activities
Profit / (loss) for the period (1,178) 1,032
Finance income (105) (10)
Finance expense 29 33
Taxation (183) (147)
Depreciation of fixed assets 400 451
Amortisation of intangibles 225 188
Research and development expenditure credit (355) (316)
Impairment of intangible assets 14 41
Share option charge 52 79
(1,101) 1,351
Changes in working capital
Decrease in trade and other receivables 1,290 280
(Decrease) / increase in trade and other payables (327) (696)
---------------------------------------------------- ---------- ----------
Cash (used in) / generated from operations (138) 935
Taxation received 456 499
Taxation paid (16) (10)
---------------------------------------------------- ---------- ----------
Net cash (used in) / generated from operating
activities 302 1,424
Cash flows from investing activities
Purchase of property, plant and equipment (100) (187)
Purchase of intangible assets including staff
costs capitalised (1,863) (2,058)
Finance income 99 6
Net cash (used in) / generated from investing
activities (1,864) (2,239)
Cash flows from financing activities
Issue of shares 2 -
Repayment of lease liabilities (158) (114)
Net cash (used in) / generated from financing
activities (156) (114)
Movements in cash and cash equivalents in the
period (1,718) (929)
---------------------------------------------------- ---------- ----------
Cash and cash equivalents at start of period 5,769 6,684
Effect of exchange rate fluctuations on cash
held (20) 14
---------------------------------------------------- ---------- ----------
Cash and cash equivalents at end of period 4,031 5,769
---------------------------------------------------- ---------- ----------
The financial information set out in these results does not
constitute the Group's consolidated statutory accounts for the
years ended 30 September 2023 or 2022. Statutory accounts for the
year ended 30 September 2022 have been filed with the Registrar of
Companies. The statutory accounts for the year ended 30 September
2023 will be delivered to the Registrar in due course. Those
accounts have been reported on by the Independent Auditors; their
report for the accounts for both financial years was (i)
unqualfied; (ii) did not include a reference of any matters to
which the auditor drew attention by way of emphasis without
qualifying their report; and (iii) did not contain a statement
under 498 (2) or 498 (3) of the Companies act 2006.
Copies of the Annual Report 2023 will be posted to shareholders
on or about 15 December 2023.
1. Presentation of the financial statements
a. General information
IXICO plc (the 'Company') is a public limited company
incorporated in England and Wales and is admitted to trading on the
AIM market of the London Stock Exchange under the symbol IXI. The
address of its registered office is 4th Floor, Griffin Court, 15
Long Lane, London EC1A 9PN.
The Company is the parent of the subsidiaries detailed in note
15 , together referred to throughout as 'the Group'. The Group is
an established provider of technology-enabled services to the
global biopharmaceutical industry. The Group's services are used to
select participants for clinical trials and assess the safety and
efficacy of new drugs in development within the field of
neurological disease.
b. Basis of preparation
The consolidated financial statements have been prepared on a
going concern basis and in accordance with international accounting
standards in conformity with the requirement of the Companies Act
2006.
The consolidated financial statements comprise a Statement of
Comprehensive Income, a Statement of Financial Position, a
Statement of Changes in Equity, a Statement of Cash Flows, and
accompanying notes. These financial statements have been prepared
under the historical cost convention modified by the revaluation of
certain financial instruments.
The consolidated financial statements are presented in Great
British Pounds ('GBP' or 'GBP') and are rounded to the nearest
thousand unless otherwise stated. This is the predominant
functional currency of the Group, and is the currency of the
primary economic environment in which it operates. Foreign currency
transactions are accounted in accordance with the policies set out
below.
The Company has elected to use Financial Reporting Standard -
'The Reduced Disclosure Framework' (FRS101). In preparing these
financial statements the Company has taken advantage of all
disclosure exemptions conferred by FRS 101. Therefore, these
financial statements do not include:
-- A statement of cash flows and related notes;
-- The requirement to produce a statement of financial position
at the beginning of the earliest comparative period;
-- The requirements of IAS 24 'Related Party Disclosures' to
disclose related party transactions entered in to between two or
more members of the group as they are wholly owned within the
group;
-- The effect of future accounting standards not adopted;
-- Paragraphs 45(b) and 46 to 52 of IFRS 2, 'Share-based
payment' (details of the number and weighted average exercise
prices of share options, and how the fair value of goods or
services received was determined);
-- Paragraphs 91 to 99 of IFRS 13, 'Fair value measurement'
(disclosure of valuation techniques and inputs used for fair value
measurement of assets and liabilities).
-- Disclosures in relation to impairment of assets
-- IFRS 7, 'Financial instruments: Disclosures'.
c. Basis of consolidation
The consolidated financial statements incorporate the accounts
of the Company and its subsidiary companies adjusted to eliminate
intra-Group balances and any unrealised gains and losses or income
and expenses arising from intra-Group transactions. The Company's
subsidiaries are detailed in note 15 . When necessary, adjustments
are made to the financial statements of subsidiaries to bring their
accounting policies into line with the Group's accounting
policies.
The Group controls a subsidiary when the Group is exposed to, or
has rights to, variable returns from its involvement with a
subsidiary and has the ability to affect those returns through its
power over a subsidiary. In assessing control, potential voting
rights that are currently exercisable or convertible are taken into
account.
The results of subsidiary companies are included in the
consolidated financial statements from the date that control
commences until the date that control ceases. The assets and
liabilities of foreign operations are translated into GBP at
exchange rates prevailing at the end of the reporting period.
Income statements and cash flows of foreign operations are
translated into GBP at average monthly exchange rates which
approximate foreign exchange rates at the date of the transaction.
Foreign exchange differences arising on retranslation are
recognised directly in a separate translation reserve.
d. Going concern
The ongoing conflict in eastern Europe and recent
re-commencement of hostilities in the middle east, accompanied by
rising inflation, interest rates and a broad degree of
macro-economic and political disruption continue to create
challenges for the global economy. These have resulted in a lowered
risk appetite which has impacted capital markets around the world,
reducing capital availability and investment in areas deemed higher
risk.
The impact of this has been visible in the clinical trials
market both through a slow down in the initiation of new clinical
trials, increased focus on development pipelines by the
biopharmaceutical companies and restructuring and consolidation
announcements within both biopharma and CROs.
The Group has seen the impact of this and, whilst it remains
well capitalised and debt-free, it has seen an elongation of the
timeframes to sign new clinical trials and therefore has lowered
its expectations for revenues in 2024 ahead of a return to growth
in 2025.
Irrespective, the Group has a strong balance sheet for its size
with financial year end net assets of GBP11.4 million, a GBP4.0
million cash balance and has secured GBP8.0 million of new
contracts in the year providing it with good visibility of future
revenues across a diversified portfolio of clients and
projects.
In assessing going concern, management has prepared detailed
sensitised forecasts which consider different scenarios through to
December 2024. These include the risk to current projects and
expected future sales pipelines. The Directors have considered
these forecasts, alongside the Group's strong balance sheet and
cash balance as well as the ability for the Group to mitigate costs
if necessary. After due consideration of these forecasts, as well
as the review completed by the Audit Committee (including a review
of a reverse stress test) the Directors concluded with confidence
that the Group has adequate financial resources to continue in
operation for the foreseeable future.
2. New and amended accounting standards and interpretations
a. Adoption of new accounting standards for the year ended 30 September 2023
The Group has adopted all new and amended accounting standards
and interpretations issued by the International Accounting
Standards Board ('IASB') that are mandatory for the current
reporting period.
There was no impact on the Group's financial statements as a
result of adopting these standards.
b. Accounting developments affecting financial statements in subsequent periods
At the date of authorisation of these financial statements,
several new, but not yet effective, standards and amendments to
existing standards and interpretations have been published by the
IASB. The standards and amendments that are not yet effective and
have not been adopted early by the Group include:
-- Classification of liabilities as current or non-current (Amendments to IAS 1)
-- Deferred Tax related to Assets and Liabilities arising from a Single Transaction
-- Definition of Accounting Estimates
-- Disclosure of Accounting Policies
The Directors anticipate, based on current business processes,
that the introduction of the above standards and amendments will
not have a material impact on the Group and Company financial
statements and therefore the impact of these changes on the
financial statements has not been assessed.
3. Significant accounting policies
3.1 Revenue
Revenue is principally derived from service revenue. Revenue
comprises the transaction price, being the amount of consideration
the Group expects to be entitled to in exchange for transferring
promised goods or services to a customer in the ordinary course of
business net of value-added tax, returns, rebates and discounts and
after eliminating sales within the Group.
In determining whether to recognise revenue, the Group follows a
5-step process:
1. Identifying the contract with a client;
2. Identifying the performance obligations;
3. Determining the transaction price;
4. Allocating the transaction price to the performance obligations; and
5. Recognising revenue when/as performance obligation(s) are satisfied.
All services provided to clients are agreed at the inception of
a project through contracts, wherein the transaction price is
determined and agreed for each performance obligation in the
schedule of work. The transaction price agreed at the outset is not
variable or subject to any refunds or warranties, and this is
consistent across all revenue streams. A critical part of the
contract is a detailed schedule of work that provides the list of
services to be provided by the Group. Under the requirements of
IFRS 15 - Revenue from Contracts with Customers, the Group is
required to identify individual and distinct performance
obligations within each contract. This represents a judgement, and
the Group has considered whether each individual service provided
meets these requirements in its own right and in the context of the
contract, by assessing in particular the level of interrelationship
between each type of service and the nature of the contract entered
in to with clients.
The Group has identified performance obligations within each of
the revenue streams as set out below. The transaction price
associated to each performance obligation is allocated based on
their relative stand-alone selling price. Revenue is recognised
once the performance obligation is met for each distinct service.
Deferred income and advanced payments are recognised where
consideration is received before all performance considerations
have been completed. They are then released in line with
contractual terms which dictate which performance obligations they
relate to. In some instances the Group invoices in advance of work
being completed, a corresponding contract liability is therefore
created to account for this. The Group also invoices on completion
of contractual milestone. In these instances accrued income is
recognised until the invoices are issued to reflect the Group's
right to compensation for these completed but not invoiced
performance obligations.
Revenue types
The Group's contracts comprise a variety of performance
obligations. These obligations are all considered streams of a
single revenue type, being service revenue. Most of the Group's
revenue is recognised at a point in time; the Group recognises this
revenue once control is passed to the client, or once the service
has been delivered on behalf of the client.
The Group's most significant streams of service revenue are
outlined below and have the respective recognition criteria:
Service type Performance obligations Revenue recognition
policy
-------------------- ----------------------------------------------------------------- -----------------------------
Project & site This service type includes the initial Revenue for this service
set up project set up documentation, such is recognised at a
Training materials as scientific protocols and operational point in time once
and delivery guides, and close out activities such the Group has delivered
Scientific reports as scientific reports. Where a tangible the relevant material
product is created, the performance on behalf of the client.
obligation is met once the item is
transferred to the client. For training materials
and delivery, revenue
In respect of training, materials are is recognised at the
prepared in advance and provided to point in time when
clients as tools for site training. a site has completed
Site training is provided either through its training.
live online training or through a self-paced
training module. The performance obligation
is met once each individual site has
completed the training.
-------------------- ----------------------------------------------------------------- -----------------------------
Project management Each contract requires various project The services provided
Site management management activities. These services for project and site
are provided throughout the duration management represents
of a contract. Site management services a provision of ongoing
are provided throughout the duration services. As the fee
of a site being operational and would is charged monthly
typically be shorter than the project to the client over
management cycle. For both activities, the duration for which
the costs and time spent delivering management services
these services are generally spread are provided, revenue
evenly over the project lifetime. As for these items is
such the performance obligation is recognised over a
met when the specific service is provided series of points in
each month. time across the contract.
-------------------- ----------------------------------------------------------------- -----------------------------
TrialTracker The TrialTracker platform delivers The deployment of
configuration a robust and comprehensive set of centralised TrialTracker is recognised
and access imaging services designed to efficiently over time as the platform
manage the complex imaging workflow, is configured for
including image upload, quality control, the customer. This
reading and analysis. The platform is because an asset
also allows for reporting and data is being created that
transfer. This involves the initial has no alternative
configuration and deployment of TrialTracker, use for the Group
and access granted to client trial and there is an enforceable
sites for upload of clinical information. entitlement to receive
payment for the work
Due to the lack of interrelationship completed to date.
between the two distinct services provided,
each are recognised independently. The ongoing access
The performance obligations for each fee is charged monthly
are: to the client and
so revenue is recognised
* The performance obligation for deployment is met over over a series of points
a period of time during the configuration and in time across the
development of TrialTracker. contract.
* The performance obligation for ongoing access to
TrialTracker for the upload of data by client trial
sites is recognised over the duration of the project
once TrialTracker is deployed.
-------------------- ----------------------------------------------------------------- -----------------------------
Data management Ensuring data are managed appropriately In respect of data
and quality and that the data are of a high quality quality control, revenue
control is critical in the delivery of the will be recognised
Group's service. The data management at the point in time
and imaging teams work in collaboration when data is quality
to ensure ongoing integrity of data. checked.
The data will go through a series of The services provided
quality control reviews prior to being for data management
used in the Group's performance of represents a provision
reading and analysis. Therefore, the of ongoing services.
performance obligation is met once As the fee is charged
the data is quality checked. monthly to the client
over the duration
Data management is an ongoing service for which data management
performed throughout the duration of is required, revenue
a project whilst data is being received for these items is
and managed on a project. The respective recognised over a
costs and time spent delivering this series of points in
service is generally spread evenly time across the contract.
over the duration in which data is
being managed and as such the performance
obligation is met when the specific
service is provided each month.
-------------------- ----------------------------------------------------------------- -----------------------------
Data reading The Group provides data analysis services Revenue from reading
and analysis across a range of biomarkers, providing and analysis of clinical
high-quality, clinically meaningful data is recognised
data. The performance obligation for at the point in time
these services is met once the analysis when the work is complete.
is completed.
-------------------- ----------------------------------------------------------------- -----------------------------
Licence revenue Revenue relating to licencing is entirely Revenue for both the
attributable to TrialTracker. Each licencing and support
agreement will grant the user rights are recognised on
to access the software for their own a straight-line basis
use and receive associated technical over the duration
support during the licence period. of the contract and
is therefore recognised
The granting of the licence and its over time. Licence
associated support are distinct performance revenue in the current
obligations and are met on a straight-line year is not material.
basis over the contract term.
-------------------- ----------------------------------------------------------------- -----------------------------
Change orders
Throughout the duration of a contract, the client may request
additional services or service changes to be made. For revenue
recognition purposes, the Group treats a change order or contract
modification to a client agreement as a separate contract, if
both:
-- the scope changes due to the addition, or reduction, of 'distinct' services; and
-- the price change reflects the services stand-alone selling
prices ('SSP') under the circumstances of the modified
contract.
The revenue recognition for the change order is applied in the
same way as the original contract, as detailed above, with the
original client agreement remaining unchanged.
In line with note 5, the Group has determined that it acted as
an agent in one material contract in the year. The Group charges a
management fee and recognises this as revenue. This contract
delivered GBP13,000 (GBP192,000) of revenues in the year.
3.2 Other income
Government grants and assistance
A government grant is recognised only when there is reasonable
assurance that the Group will comply with any conditions attached
to the grant and the grant will be received. The grants are
recognised as income over the period necessary to match them with
the related costs, for which they are intended to compensate, on a
systematic basis. The Group recognises grant income as an item of
other income.
Research and Development Expenditure Credit ('RDEC')
The Group has elected to take advantage of the RDEC introduced
in the Finance Act 2013. A company may surrender corporation tax
losses on research and development expenditure incurred on or after
1 April 2013 for a corporation tax refund. Relief is given as a
taxable credit on 13% of qualifying research and development
expenditure, with the rate increasing to 20% for expenses incurred
from 1 April 2023. The Group recognises research and development
expenditure credit as an item of other income, taking advantage of
the 'above the line' presentation, and is recognised in the year
for which the research and development relates.
3.3 Research and development expenditure
In all instances across the Group, research expenditure is
expensed through the income statement. For development expenditure,
items will be expensed where the recognition criteria for
internally generated intangible assets is not met.
The main criteria used to assess this, as required under IAS 38
- Intangible Assets, are:
- Demonstrating technical feasibility of completing the intangible asset;
- Intention to complete the asset;
- Ability to use or sell the asset in order to generate future economic benefit;
- Availability of adequate technical or other resources to complete development; and
- Ability to measure reliably the expenditure attributable to the asset.
It was determined that the Group continued to meet the above
criteria in respect of specific developments to its TrialTracker
platform and data analytics service offering. As a result,
associated development costs are capitalised in the year and an
intangible asset is recognised as set out in note 14 .
3.4 Share-based payments
Equity-settled share-based payments are measured at the fair
value of the equity instruments at the grant date. The fair value
determined at the grant date of the equity-settled share-based
payment is expensed on a straight-line basis over the performance
period, based on the Group's estimate of equity instruments that
will eventually vest. At each reporting date, the Group revises its
estimate of the number of equity instruments expected to vest as a
result of the effect of any non-market-based performance
conditions.
Any changes that impact the original estimates, for example the
effect of employees who have left the Group in the year and have
forfeited their options, is recognised in the Consolidated
Statement of Comprehensive Income such that the cumulative expense
reflects the revised estimate, with a corresponding adjustment to
equity reserves.
Details regarding the determination of the fair value of
equity-settled share-based transactions are set out in note 21 of
the consolidated financial statements.
3.5 Employee benefits
All employee benefit costs are recognised in the Consolidated
Statement of Comprehensive Income as they are incurred. These
principally relate to holiday pay and contributions to the Group
defined contribution pension plan.
The assets of the Group pension scheme are held separately from
those of the Group in independently administered pension funds. The
Group does not offer any other post-retirement benefits.
3.6 Leased assets
A lease is defined as a contract that gives the Group the right
to use an asset for a period of time in exchange for consideration.
The Group identifies from the contract the total length and cost of
the lease contract, and determines whether it meets the definition
of a right-of-use asset. Recognition of a right-of-use asset is met
if it is longer than 12 months and of a high value. For those
leases that do not meet these criteria, the rental charge payable
under these leases are charged to the Consolidated Statement of
Comprehensive Income on a straight-line basis over the lease
term.
The initial recognition and subsequent measurement of
right-of-use asset leases are:
Initial recognition
At the commencement date, the Group measures the lease liability
at the present value of future lease payments, discounted using the
Group's incremental borrowing rate. The Group also recognises a
right-of-use asset which is measured at cost, which is made up of
the initial measurement of the lease liability, any initial direct
costs and an estimate of any costs to reinstate the asset to its
original condition.
Subsequent measurement
The lease liability is reduced for payments made and increased
for interest accrued, and is remeasured for any modifications made
to the lease. The right-of-use asset is depreciated on a
straight-line basis over the expected lease term. The asset is also
assessed for impairment when such indicators exist.
On the statement of financial position, right-of-use assets are
included in property, plant and equipment and lease liabilities are
shown separately. Please see note 18 for more information.
3.7 Property, plant and equipment
Property, plant and equipment is stated at cost less accumulated
depreciation and, where appropriate, less provisions for
impairment. The initial recognition and subsequent measurement of
property, plant and equipment are:
Initial recognition
Property, plant and equipment is initially recognised at
acquisition cost, including any costs directly attributable to
bringing the assets to the location and condition necessary for
them to be capable of operating. In most circumstances, the cost
will be its purchase cost, together with the cost of delivery.
Subsequent measurement
An asset will only be depreciated once it is ready for use.
Depreciation is charged so as to write off the cost of property,
plant and equipment, less its estimated residual value, over the
expected useful economic lives of the assets.
Depreciation is charged on a straight-line basis as follows:
Office buildings over expected lease term
Leasehold improvements shorter of 5 years or the
lease term
Fixtures and fittings 3 years
Equipment 3 years
The disposal or retirement of an asset is determined by
comparing the sales proceeds with the carrying amount. Any gains or
losses are recognised within the Consolidated Statement of
Comprehensive Income.
3.8 Intangible assets
Acquired intangibles
Intangible assets that are acquired through business
combinations are recognised as intangible assets if they are
separable from the acquired business or arise from contractual or
legal rights. These assets will only be recognised if they are also
expected to generate future economic benefits and their fair value
can be reliably measured.
Initial recognition
Intangible assets acquired separately are measured on initial
recognition at cost. The cost of intangible assets acquired in a
business combination is their fair value at the date of
acquisition.
Subsequent measurement
Following capitalisation, the intangible assets are carried at
cost less any accumulated amortisation, and where appropriate, less
provisions for impairment.
Intangible assets are amortised using the straight-line method
over their estimated useful economic life as follows:
5 years
* Intangibles acquired through business combinations
3 years
* Computer software
5 years
* Data acquisition
Amortisation is charged to the Consolidated Statement of
Comprehensive Income and is included within cost of sales for those
items directly related to project activities, or otherwise within
general and administrative expenses.
Internally generated intangible assets
Intangible assets that are capitalised internally are deemed to
have met the recognition criteria set out in IAS 38. These items
relate to research and development costs and are considered in note
3.3.
Initial recognition
Internally generated intangible assets are initially recognised
at cost once the recognition criteria of IAS 38 are met.
Subsequent measurement
Any assets that are not yet ready for use will be capitalised as
assets under construction and will not be amortised. Once the asset
is ready for use, amortisation will begin. The amortisation rates
adopted are based on the expected useful economic life of the
projects to which they relate, with the charges recognised in
accordance with how the Group receives the benefit from the
technology. The assets useful economic life is as follows:
Internally generated technology 3 - 5 years
Proprietary clinical trial platform 15 years
3.9 Impairment of non-current assets
Each category of non-current assets is reviewed for impairment
annually when under construction or when there is an indication
that an asset may be impaired, being when events or changes in
circumstances indicate that the carrying value may not be
recoverable. An impairment loss is recognised in the Consolidated
Statement of Comprehensive Income for the amount by which the
asset's carrying value exceeds its recoverable amount.
The recoverable amount is the higher of an asset's fair value
less cost to sell and value in use. Non-financial assets, other
than goodwill, which have suffered an impairment are reviewed for
possible reversal of the impairment at each reporting date.
3.10 Investments in Group undertakings
Investments in Group undertakings are initially recognised at
cost and subsequently measured at cost less any impairment
provision. Investments are subject to an annual impairment review,
with any impairment charge being recognised through the
Consolidated Statement of Comprehensive Income. Additions to
investments are amounts relating to share options for the services
performed by employees of the subsidiaries of the Company and are
classified as capital contributions within note 15 .
3.11 Trade and other receivables
Trade and other receivables are initially recognised at fair
value and subsequently stated at amortised cost using the effective
interest method, less any expected credit losses. The Group makes
use of a simplified approach in accounting for trade and other
receivables as well as contract assets and records the loss
allowance as lifetime expected credit losses. These are the
expected shortfalls in contractual cash flows, considering the
potential for default at any point during the life of the financial
instrument. In calculating, the Group uses its historical
experience, external indicators and forward-looking information to
calculate the expected credit losses.
The Group assess impairment of trade receivables on an
individual basis as they possess individual credit risk
characteristics based on each client. Refer to note 16 for further
information on aging of trade receivables and an analysis of any
expected credit losses.
The Group recognises commission payments as incremental costs
from obtaining a contract. Those that are paid immediately are
capitalised under IFRS 15 and amortised over 3 years (2022: 3
years), being the average length of contracts entered into by the
Group, as opposed to using a tailored time period for each project.
Management reviews this assessment annually to determine that there
are no material variances. Those not paid immediately are accrued
over a period of time as this element of the commission payment
requires the respective employee to remain in service for a
specific period. Commission assets.
3.12 Taxation
Current tax
Current tax represents amounts recoverable within the United
Kingdom and is provided at amounts expected to be recovered using
the tax rates and laws that have been enacted at the Statement of
Financial Position date.
Research and development credits
The benefit associated with UK-based research and development is
recognised under the UK's Research and Development Expenditure
Credit scheme. Details of the recognition are set out in note
3.2.
Deferred taxation
Deferred tax is provided in full, using the liability method, on
temporary differences arising between the tax bases of assets and
liabilities and their carrying amounts in the consolidated
financial statements in accordance with IAS 12 -
Income taxes. Deferred tax liabilities are recognised for all
taxable temporary differences. A deferred tax asset is recognised
only to the extent that it is probable that sufficient taxable
profit will be available in future years to utilise the temporary
difference. Deferred tax is not accounted for if it arises from
initial recognition of an asset or liability in a transaction,
other than a business combination, that at the time of the
transaction affects neither the accounting, nor taxable profit or
loss.
Deferred income tax is determined using tax rates (and laws)
that have been enacted or substantively enacted by the Statement of
Financial Position date and are expected to apply when the related
deferred income tax asset is realised or the deferred income tax
liability is settled.
Deferred tax assets and liabilities are offset only when there
is a legally enforceable right to set off current tax assets
against current tax liabilities, they relate to income taxes levied
by the same taxation authority and the Group intends to settle
these on a net basis.
Deferred tax assets are recognised to the extent it is probable
that the underlying tax loss or deductible temporary difference
will be utilised against future taxable income. This is assessed
based on the Group's forecast of future operating results, adjusted
for significant non-taxable income and expenses and specific limits
on the use of any unused tax loss or credit. As such, the Group
does not recognise any deferred tax assets, see note 19 .
3.13 Cash and cash equivalents
Cash and cash equivalents comprise cash at bank and in hand with
original maturities at inception of 3 months or less.
3.14 Foreign currency translation
Transactions denominated in foreign currencies are translated
into Great British Pounds at actual rates of exchange prevailing at
the date of transaction. Monetary assets and liabilities expressed
in foreign currencies are translated into Great British Pounds at
rates of exchange prevailing at the end of the financial year. All
foreign currency exchange differences are taken to the Consolidated
Statement of Comprehensive Income in the year in which they
arise.
Non-monetary items are not retranslated at year end and are
measured at historical cost (translated using the exchange rates at
the transaction date), except for non-monetary items measured at
fair value which are translated using the exchange rates at the
date when fair value was determined.
3.15 Trade and other payables
Trade and other payables are non-interest-bearing, unless
significantly overdue, and are initially recognised at fair value
and subsequently stated at amortised cost.
3.16 Provisions, contingent assets and contingent
liabilities
Provisions are recognised when the Group has a present legal or
constructive obligation as a result of a past event, it is probable
that an outflow of economic resources will be required from the
Group and amounts can be estimated reliably. The timing of such
outflows may still be uncertain. Such provisions are measured at
the estimated expenditure required to settle the present obligation
based on the most reliable estimate available at the reporting
date, discounted to the present value where material.
Any reimbursement that the Group is virtually certain to collect
from a third party in relation to the related provision will be
recognised as a separate asset.
Liabilities are not recognised where the outflow of economic
resources is not probable, but are instead disclosed as contingent
liabilities.
3.17 Equity instruments
Equity instruments issued by the Group are recorded at the
proceeds received, net of direct issue costs.
3.18 Financial instruments
Financial assets and financial liabilities are recognised on the
Consolidated Statement of Financial Position when the Group or the
Company becomes a party to the contractual provisions of the
instrument. Debt and equity instruments are classified as either
financial liabilities or as equity in accordance with the substance
of the contractual arrangement.
The Group holds one type of derivative financial instrument -
forward contracts used for the purposes of hedging. These are
designated as cash flow hedges and held at fair value with changes
held in the cash flow hedge reserve. On crystallisation the gain or
loss is recycled to revenue to reflect the risks being hedged. The
ineffective portion of the hedging instrument is recognised in the
profit or loss account immediately.
Further information relating to financial instruments and the
policies adopted by the Group to manage risk is found in note 22
.
4. Significant management judgement in applying accounting policies and estimation uncertainty
When preparing the consolidated financial statements, the
Directors make a number of judgements, estimates and assumptions
about the recognition and measurement of assets, liabilities,
income and expenses.
Significant management judgements
The following are significant management judgements in applying
the accounting policies of the Group that have the most significant
effect on the consolidated financial statements.
Determination of acting as agent or principal
The scope of a client project or its contract terms are reviewed
to determine whether the Group is acting as principal or agent.
This determination depends on the facts and circumstances of each
individual project or contract and requires judgement, which are
made in accordance with the applicable standards. The primary
indicator used to determine whether the Group is acting as a
principal is whether control of the good or service is gained prior
to the good or service transferring to the client. If control is
gained, revenue is recognised on a gross basis. If no control is
achieved, then revenue is recognised on a net basis. During the
year, the Group had a contract with a client to arrange the
delivery of products from a third party to various client trial
sites. The Group determined this was an agency relationship. If
this judgement was incorrect and the Group was acting as principal,
it would result in an immaterial increase in revenue and cost of
sales recognised in the year and a decrease in profit margins
achieved. In the prior year the effect would have been
material.
Capitalisation of internally developed software
Distinguishing the research and development phases of a new
software product and determining whether the requirements for the
capitalisation of development costs are met requires judgement.
Management will assess whether a project meets the recognition
criteria as set out in IAS 38 based on an individual project basis.
More detail is included in note 3.3 as to the specific
considerations given to each project when determining whether to
capitalise internally developed software. Where the criteria are
not met, the research and development expenditure will be expensed
in the Consolidated Statement of Comprehensive Income. Where the
recognition criteria are met, the items will be capitalised as an
intangible asset.
During the year ended 30 September 2023, research and
development expenses totalled GBP2,152,000 (2022: GBP2,129,000). Of
this amount, GBP1,211,000 (2022: GBP912,000) was capitalised as an
intangible asset relating to employee costs. The balance of
expenditure being GBP925,000 (2022: GBP1,217,000) is recognised in
the Consolidated Statement of Comprehensive Income as an
expense.
Recovery of deferred tax assets
Deferred tax assets have not been recognised for deductible
temporary differences and tax losses. The Directors consider that
there is not sufficient certainty that future taxable profits will
be available to utilise those temporary differences and tax losses.
Further information on the Group's deferred tax asset can be found
in note 19 of the consolidated financial statements.
Estimation uncertainty
Information about estimates and assumptions that have the most
significant effect on recognition and measurement of assets,
liabilities, income and expenses is provided below. Changes to
these estimations may result in substantially different results for
the year.
Determination of transaction prices in revenue recognition
Client contracts include an agreed work order so the transaction
price for a contract is allocated against each distinct performance
obligations for each service, based on their relative stand-alone
selling prices. For legacy contracts prior
to the adoption of IFRS 15, management were required to estimate
the standalone price allocated to each distinct service that were
previously grouped in a single price. For new contracts, the fair
value of individual components is based on actual amounts charged
by the Group on a stand-alone basis. Management have determined
that for items recognised on a straight-line basis, including
project, site and data management, the demands of this on the Group
are spread evenly over the life of the revenue stream. This was
determined through an understanding of the work required to deliver
the various revenue streams and the obligations within the contract
needing to be met.
Share-based payments
The Group measures the cost of equity-settled transactions with
employees by reference to the fair value of the equity instruments
at the date at which they are granted. The fair value of the
options granted is measured using an option valuation model, taking
into account the terms and conditions upon which the options were
granted. Details of the estimations used in determining the fair
value of the options in issue are detailed in note 22. In line with
IAS 2, management assess whether non-market conditions will be
achieved and adjusts appropriately.
Useful lives of depreciable assets
The useful lives of depreciable assets are determined by
management at the date of purchase based on the expected useful
lives of the assets. These are subsequently monitored and reviewed
annually and where there is objective evidence of changes in the
useful economic lives, these estimates are adjusted. Any changes to
these estimates may result in significantly different results for
the period.
5. Revenue
An analysis of the Group's revenue by type is as follows:
2023 2022
GBP000 GBP000
----------------- ------- -------
Service revenue 6,665 8,643
--------------------- ------- -------
All material revenue streams derived by the Group relate to the
delivery of services in support of clinical trials. As such, all
revenue is deemed to belong to one stream, being service
revenue.
Revenue derived from services provided over time do not
constitute a material portion of revenue and therefore disclosure
distinguishing between revenue recognised at a point in time versus
over time is not made.
For the year ended 30 September 2023, revenue includes
GBP214,000 (2022: GBP499,000) held in contract liabilities within
trade and other payables at the beginning of the period. This
amount also includes performance obligations relating to advance
payments that were not yet complete at the end of the prior year.
Advance payments are charged to clients to de-risk start-up
activities and are recognised at a point in time once an activities
performance obligation is met. At 30 September 2023, GBP343,000
(2022: GBP575,000) of advanced payments were recognised on the
balance sheet.
6. Segmental information
The Board considers there to be only one core operating segment
for the Group's activities. This is based on the Group's
development, commercial and operational delivery teams operating
across the entirety of the Group, which is primarily based in the
United Kingdom. The projects undertaken by the Group are managed by
project managers, who receive inputs for each project from other
team members. Performance information is reported as a single
business unit to the management team.
The information gathered for each project is subsequently
reported to the Group's Chief Executive Officer, who is considered
to be the chief operating decision-maker. This information is used
for resource allocation and assessment of performance. Therefore,
the entirety of the Group's revenue and assets can be attributed
wholly to this operating segment with reference to the Consolidated
Statement of Comprehensive Income and Consolidated Statement of
Financial Position.
During the year ended 30 September 2023, the Group had five
clients (2022: three clients) that exceeded 10% of total revenue.
In 2023 the individual percentage revenue associated with these
clients was 14% (GBP966,000), 14% (GBP949,000), 13% (GBP862,000),
12% (GBP792,000) and 10% (GBP699,000). In 2022, the individual
percentage revenue associated with the three largest clients was
38% (GBP3,320,000), 14% (GBP1,175,000) and 11% (GBP976,000).
Geographical information
The Group's revenue can be categorised by country, based on the
location of the contracting client. Sometimes clients of the Group,
which include global biopharmaceutical companies with offices in
multiple locations across the world, request the Group to contract
directly with their regional offices in the United Kingdom or
European locations. In such circumstances the associated revenues
are reported as being based in the contracting location even though
much of the operational execution of the contract will include
entities or partners of the client based elsewhere in the
world.
2023 2022
GBP000 GBP000
------------------ ------- -------
United States of
America 3,053 2711
United Kingdom 952 2057
Netherlands 862 436
Switzerland 816 2077
Ireland 689 724
Other - Europe 293 638
Revenue 6,665 8,643
---------------------- ------- -------
As the Group is domiciled in the United Kingdom, the entirety of
the revenue originates from this location.
7. Other income
Items of other income principally relate to government grants
received. Grants are recognised as income over the period required
to match them with the related costs, for which they are intended
to compensate, on a systematic basis.
The Group also recognises Research and Development Expenditure
Credit ('RDEC') as other income.
2023 2022
GBP000 GBP000
-------------- ------- -------
Grant income 3 8 373
RDEC 355 316
-------------- ------- -------
Other income 393 689
-------------- ------- -------
8. Auditor's remuneration
2023 2022
GBP000 GBP000
------------------------------ ------- -------
Audit services
- Group and Parent Company 56 38
- subsidiary companies 37 26
Total audit fees 93 64
Audit-related assurance
services 8 7
Total auditor's remuneration 101 71
---------------------------------- ------- -------
9. Employees and Directors
The average monthly number of persons (including Executive and
Non-Executive Directors) employed by the Group was:
2023 2022
Number
Number
-------------------------------- --------- -------
Administration 14 15
Operations, research and
development 75 75
----------------------------------- --------- -------
Average total persons employed 89 90
----------------------------------- --------- -------
The aggregate remuneration of employees in the Group was:
2023 2022
GBP000 GBP000
----------------------------- -------- -------
Wages and salaries 5,944 5,851
Social security costs 702 610
Other pension costs 303 286
Share-based payments charge 52 79
-------------------------------- -------- -------
Total remuneration for
employees 7,001 6,826
-------------------------------- -------- -------
Employee costs capitalised (1,211) (912)
-------------------------------- -------- -------
Net employee costs 5,790 5,914
-------------------------------- -------- -------
The Group operates a defined contribution pension scheme for
employees. The assets of the scheme are held separately from those
of the Group in independently administered funds. The amounts
outstanding at 30 September 2023 in respect of pension costs were
GBP46,000 (2022: GBP46,000).
The remuneration of the Group's Directors is set out in the
Directors' Remuneration Report in the full annual report, as well
as in note 23 under related party transactions.
The Company did not directly employ any staff and therefore
there is no cost recognised in respect of staff costs.
10. Operating loss / profit
The Group's operating loss (2022: profit) has been achieved
after charging:
2023 2022
GBP000 GBP000
------------------------------------------ ------- -------
Research and development expenses 903 1,176
Research and development related
impairment 14 41
Research and development related 8 -
amortisation
Sales and marketing expenses 1,262 1,173
Amortisation of commission assets 59 53
Operating lease charges: land, buildings
and printers 1 1
Depreciation of tangible assets 400 451
Amortisation of intangible assets 24 23
Foreign exchange (gain) / loss 85 (149)
Administrative expenses 2,344 2,255
Total operating expenses 5,100 5,024
-------------------------------------------- ------- -------
There is a further amortisation charge of GBP193,000 (2022:
GBP165,000) recognised in cost of sales for those items directly
related to project activities. The total amortisation charge for
the year is GBP225,000 (2022: GBP188,000).
11. Taxation
The tax charge for each period can be reconciled to the result
per the Consolidated Statement of Comprehensive Income as
follows:
2023 2022
GBP000 GBP000
------------------------------------------------------------ -------- -------
(Loss) / profit on ordinary activities before taxation (1,361) 885
(Loss) / profit before tax at the effective rate
of corporation tax
in the United Kingdom of 22% (2022: 19%) (299) 168
Effects of:
Expenses not deductible for tax purposes (17) 4
Origination and reversal of temporary differences (291) (332)
Research and development uplifts net of losses surrendered
for tax credits 406 17
Overseas taxation 16 -
Prior period adjustment 2 (4)
Tax credit for the period (183) (147)
------------------------------------------------------------ -------- -------
The tax credit for each period can be reconciled as follows:
2023 2022
GBP000 GBP000
----------------------------------------------------- ------- -------
Small or medium enterprise research and development
credit (276) (200)
Deduction for corporation tax on RDEC 75 57
Overseas taxation 16 -
Prior period adjustment 2 (4)
Tax credit for the period (183) (147)
----------------------------------------------------- ------- -------
The Group has elected to take advantage of the RDEC, introduced
in the Finance Act 2013 whereby a company may surrender corporation
tax losses on research and development expenditure incurred on or
after 1 April 2013 for a corporation tax refund.
The following is a reconciliation between the tax charge and the
tax receivable within the Consolidated Statement of Financial
Position:
2023 2022
GBP000 GBP000
------------------------------------------- ------- -------
Current tax receivable at start of period 453 480
Current period credit 552 472
Corporation tax repayment (456) (499)
Current tax receivable at end of period 549 453
------------------------------------------- ------- -------
The tax credit for each period can be reconciled to the current
period credit recognised in tax receivable within the Consolidated
Statement of Financial Position in each period as follows:
2023 2022
GBP000 GBP000
----------------------------------------- ------- -------
Tax credit for the year 183 147
RDEC gross of corporation tax deduction 355 316
Overseas taxation 15 -
Tax recoverable (1) 9
----------------------------------------- ------- -------
Current period credit 552 472
----------------------------------------- ------- -------
12. Earnings per share
The calculation of basic and diluted earnings per share ('EPS')
of the Group is based on the following data:
2023 2022
Earnings
Earnings for the purposes of basic and diluted
EPS, being net profit attributable to the owners
of the Company (GBP000) (1,178) 1,032
Number of shares
Weighted average number of shares for the purposes
of basic EPS 48,309,181 48,151,373
Effect of potentially dilutive ordinary shares:
- Weighted average number of share options - 2,606,350
Weighted average number of shares for the purposes
of diluted EPS 48,309,181 50,757,723
Basic earnings per share is calculated by dividing earnings
attributable to the owners of the Company by the weighted average
number of shares in issue during the year. The diluted EPS is
calculated by dividing earnings attributable to the owners of the
Company by the weighted average number of shares in issue taking
into account the share options outstanding during the year. For the
year ended to 30 September 2023, there was no dilutive effect as
the share options in issue would have decreased the loss per
share.
The basic and diluted earnings per share for the Group and
Company is:
2023 2022
Basic earnings per share (2.44p) 2.14p
Diluted earnings per share (2.44p) 2.03p
13. Property, plant and equipment
Group
Fixtures
Office Leasehold and
building improvement fittings Equipment Total
Cost GBP000 GBP000 GBP000 GBP000 GBP000
At 30 September 2021 777 185 5 955 1,922
---------------------- --------- ------------ ---------- ---------- -------
Additions - - - 187 187
Disposals - - - (25) (25)
---------------------- --------- ------------ ---------- ---------- -------
At 30 September 2022 777 185 5 1,117 2,084
Additions - 7 - 94 101
Disposals - - - (20) (20)
At 30 September 2023 777 192 5 1,191 2,165
---------------------- --------- ------------ ---------- ---------- -------
Accumulated depreciation
At 30 September 2021 277 98 5 461 841
Charge for the period 102 59 - 290 451
Disposals - - - (25) (25)
-------------------------- ---- ---- ----- ------
At 30 September 2022 379 157 5 726 1,267
Charge for the period 102 19 - 279 400
Disposals - - - (20) (20)
At 30 September 2023 481 176 5 985 1,647
-------------------------- ---- ---- ----- ------
Net book value
At 30 September 2022 398 28 - 391 817
At 30 September 2023 296 16 - 206 518
---------------------- ---- --- ---- ----
The only right-of-use asset is held within the office building
category. At 30 September 2023, the carrying amount of the
right-of-use asset was GBP296,000 (2022: GBP398,000).
Company
At 30 September 2023 and 30 September 2022, the Company had no
property, plant and equipment.
14. Intangible assets
Group
Other acquired Other Internally Next generation Total
intangibles developed technology TrialTracker
platform
GBP000 GBP000 GBP000 GBP000
Cost
---------------------- --------------- ---------------------- ---------------- -------
At 30 September 2021 210 638 2,137 2,985
Additions 11 121 1,974 2,106
Impairment - (41) - (41)
Disposals - (8) - (8)
---------------------- --------------- ---------------------- ---------------- -------
At 30 September 2022 221 710 4,111 5,042
Additions 121 89 1,589 1,799
Impairment - (14) - (14)
At 30 September 2023 342 785 5,700 6,827
---------------------- --------------- ---------------------- ---------------- -------
Accumulated amortisation
-------------------------- ---- ---- ----
At 30 September 2021 104 171 - 275
Amortisation 37 151 - 188
Disposals - (8) - (8)
-------------------------- ---- ---- ----
At 30 September 2022 141 314 - 455
Amortisation 47 178 - 225
At 30 September 2023 188 492 - 680
-------------------------- ---- ---- ----
Net book value
---------------------- ---- ---- ------ ------
At 30 September 2022 80 396 4,111 4,587
At 30 September 2023 154 293 5,700 6,147
---------------------- ---- ---- ------ ------
Amortisation is charged to the Consolidated Statement of
Comprehensive Income and is included within cost of sales for those
items directly related to project activities, research and
development for those items directly related to the research
activities of the company or otherwise within general and
administrative expenses.
Internally developed technology
The Group has capitalised research and development costs during
the year in relation to the development of its proprietary
TrialTracker software. Development includes TrialTracker platform
upgrades as well as additional algorithm development. The costs
capitalised include time and expenses in relation to staff costs.
In recognising these assets, the Group has applied the recognition
criteria of IAS 38 relating to internally generated intangible
assets, where costs in relation to the development phase must be
capitalised under certain circumstances. More information in
relation to this is included in the accounting policies of the
Group in notes 4 and 5.
Assets under construction
Assets that are still under construction undergo an annual
impairment test which is carried out at the end of the reporting
period. This impairment test considers the carrying amount of the
asset and compares it with its recoverable amount, with an
impairment being recognised if the recoverable amount is lower than
the carrying amount. Management have determined the recoverable
amount as being the value-in-use, which is calculated using
management expectations of future revenues, discounted at an
applicable rate. Whilst the asset remains under construction,
amortisation is not charged.
Company
At 30 September 2023 and 30 September 2022, the Company had no
intangible assets.
15. Investments
The consolidated financial statements of the Group as at 30
September 2023 and at 30 September 2022 include:
Class of Country
Name of subsidiary share of incorporation Principal activities
------------------- --------- ------------------ -----------------------------
Directly held:
IXICO Technologies Ordinary United Kingdom Data collection and analysis
Limited of neurological diseases
Indirectly held:
IXICO Technologies Ordinary United States Sales and marketing
Inc.
The Company and Group has no investments other than the holdings
in the above subsidiaries that are all 100% owned. The carrying
amounts of the investments in subsidiaries for the Company are:
2023 2022
GBP000 GBP000
Investments in subsidiary
undertakings
At beginning of the period 5,805 5,748
Capital contribution 52 57
Total investments at end
of the period 5,857 5,805
----------------------------- -------- -------
The capital contribution represents the charge in the year for
share-based awards issued by the Company to employees of IXICO
Technologies Limited and IXICO Technologies Inc.
16. Trade and other receivables
Group Company
2023 2022 2023 2022
Current receivables GBP000 GBP000 GBP000 GBP000
------------------------------------------ ------- ------- ------- -------
Trade receivables 945 2,247 - -
Less provision for bad and doubtful - - - -
debts
------------------------------------------ ------- ------- ------- -------
Net carrying amount of trade
receivables 945 2,247 - -
Other taxation and social security 40 30 6 2
Prepayments and accrued income 684 652 20 28
Commission assets 27 96 - -
Other receivables 10 4 5 1
Amounts due from subsidiary undertakings - - 2,450 3,057
------------------------------------------ ------- ------- ------- -------
Current receivables 1,706 3,029 2,481 3,088
------------------------------------------ ------- ------- ------- -------
Non-current receivables
----------------------------------- ------ ------ ------ ------
Commission assets 39 - - -
----------------------------------- ------ ------ ------ ------
Total trade and other receivables 1,745 3,029 2,481 3,088
----------------------------------- ------ ------ ------ ------
All amounts are classified as short-term and are expected to be
received within one year. The average credit period granted to
clients ranges from 30 to 90 days (2022: 30 to 90 days).
A provision for expected credit losses is made when there is
uncertainty over the ability to collect the amounts outstanding
from clients. This is determined based on specific circumstances
relating to each individual client. The Directors consider that
there are immaterial credit losses (2022: immaterial credit losses)
due to the calibre of customers the Group has and so the carrying
amount of trade and other receivables approximates their fair
value.
Within the Company, there are expected to be immaterial credit
losses (2022: immaterial credit losses) from subsidiary companies
due to the level of cash available in the subsidiaries which would
allow the repayment of these receivables immediately.
As at the year-end, the ageing of trade receivables which are
past due but not impaired is as follows:
Group Company
2023 2022 2023 2022
GBP000 GBP000 GBP000 GBP000
------------------------- ------- ------- ------- -------
Amounts not past due 864 2,189 - -
Past due:
Less than 30 days 81 58 - -
Total trade receivables 945 2,247 - -
------------------------- ------- ------- ------- -------
The maximum exposure to credit risk at the reporting date is the
carrying value of each class of financial assets disclosed in note
22 .
17. Trade and other payables
Group Company
2023 2022 2023 2022
GBP000 GBP000 GBP000 GBP000
Current liabilities
Trade payables 86 254 - -
Other taxation and social security 58 56 - -
Contract liabilities 529 673 - -
Accrued expenses 464 508 60 83
Other payables 5 11 - -
------------------------------------ ------- ------- ------- -------
1,142 1,502 60 83
Non-current liabilities
Accrued expenses 2 33 - -
Total trade and other payables 1,144 1,535 60 83
------------------------------------ ------- ------- ------- -------
Trade payables and accrued expenses principally comprise amounts
outstanding for trade purchases and ongoing costs. No interest is
charged on the trade payables. The Group's policy is to ensure that
payables are paid within the pre-agreed credit terms and to avoid
incurring penalties and/or interest on late payments.
The fair value of trade and other payables approximates their
current book values.
Reconciliation of liabilities arising from financing
activities
The only liabilities affecting financing activities arise solely
from the recognition of the lease liability:
GBP000
------------------------------------ -------
Lease liability as at 1 October
2021 597
Cash-flow: Repayment of lease (114)
Non-cash: Interest charge 33
Lease liability as at 1 October
2022 516
-------------------------------------- -------
Lease liability as at 1 October
2022 516
Cash-flow: Repayment of lease (158)
Non-cash: Interest charge 29
Lease liability as at 30 September
2023 387
-------------------------------------- -------
18. Leases
All lease liabilities are presented in the statement of
financial position as follows:
2023 2022
GBP000 GBP000
------------- ------- -------
Current 112 122
Non-current 275 394
------------------ ------- -------
387 516
---------------- ------- -------
The Group uses leases throughout the business for office space
and IT equipment. With the exception of short-term leases and
leases of low value, each lease is reflected on the balance sheet
as a right-of-use asset in property, plant and equipment and a
lease liability.
Each lease generally imposes a restriction that, unless there is
a contractual right for the Group to sublet the asset to another
party, the right-of-use asset can only be used by the Group. For
leases over office buildings, the Group must keep those properties
in a good state of repair.
The Group has identified one lease relating to the office
building that meets the definition of a right-of-use asset. There
is no option to purchase and payments are not linked to an index.
The remaining lease term is 36 months (2022: 48 months). The lease
has the ability to be extended at the end of this term and can be
terminated on the break date being after 3.5 years from the date
the lease was renegotiated.
The Group has elected to not recognise a lease liability for
short-term leases, being 12 months or less, or for leases of low
value. Payments for these are expensed on a straight-line
basis.
Right-of-use asset and lease liability
Additional information on the right-of-use asset is as
follows:
Carrying
Asset Depreciation amount
GBP000 GBP000 GBP000
----------------- ------- ------------- ---------
Office building 398 (102) 296
------------------ ------- ------------- ---------
The various elements recognised in the financial statements are
as follows:
2023 2022
GBP000 GBP000
----------------------------- ------- -------
Statement of Comprehensive
Income
Depreciation charge in the
year 102 102
Interest expense on lease
liability 29 33
Low value leases expensed
in the year 1 1
Statement of Cash Flows
Capital repayments on lease
agreements 158 114
------------------------------- ------- -------
The undiscounted maturity analysis of lease liabilities for the
office building is as follows:
Within 1 - 2 years 2 - 3 years 3 - 4 years Total
1 year
-------------------- -------- ------------ ------------ ------------ ------
30 September
2023
Lease payments 132 166 127 - 425
Finance charges (20) (14) (4) - (38)
--------------------- -------- ------------ ------------ ------------ ------
Net present values 112 152 123 - 387
--------------------- -------- ------------ ------------ ------------ ------
30 September
2022
Lease payments 151 132 166 134 583
Finance charges (29) (20) (14) (4) (67)
--------------------- -------- ------------ ------------ ------------ ------
Net present values 122 112 152 130 516
--------------------- -------- ------------ ------------ ------------ ------
At 30 September 2023, the Group's commitment to short-term and
low-value leases was GBPnil (2022: GBPnil).
19. Deferred tax
Deferred tax asset (unrecognised)
Group Company
2023 2022 2023 2022
GBP000 GBP000 GBP000 GBP000
------------------------------------------ --------- ---------- -------- --------
Tax effect of temporary differences:
Tax allowances in excess of depreciation 1,581 1,316 (1) (1)
Accumulated losses (17,618) (17,310) (3,331) (3,217)
Losses on financial instruments
debited to equity 5 28 - -
Accelerated commission charge 14 - - -
Deductible temporary differences (13) (14) - (5)
------------------------------------------- --------- ---------- -------- --------
Deferred tax asset (unrecognised) (16,031) (15,980) (3,332) (3,223)
------------------------------------------- --------- ---------- -------- --------
The unrecognised deferred tax asset predominantly arises due to
unused tax losses carried forward that have originated but not
reversed at the Consolidated Statement of Financial Position date
and from transactions or events that result in an obligation to pay
more tax in the future or a right to pay less tax in the
future.
The unrecognised deferred tax asset is measured on an
undiscounted basis at the tax rates that are expected to apply in
the periods in which temporary differences will reverse. Based on
tax rates and laws enacted or substantively enacted at the latest
balance sheet date, the rate when the above temporary differences
are expected to reverse is currently 25% (2022: 25%).
20. Issued capital and reserves
Ordinary shares and share premium
The Company has one class of ordinary shares. The share capital
issued has a nominal value of GBP0.01 and each share carries the
right to one vote at shareholders' meetings and all shares are
eligible to receive dividends. Share premium is recognised when the
amount paid for a share is in excess of the nominal value.
The Group and Company's opening and closing share capital and
share premium reserves are:
Group and Company
Ordinary Share Share
shares capital premium
Number GBP000 GBP000
----------------------------------- ----------- -------- --------
Authorised, issued and fully paid
At 30 September 2022 48,151,373 482 84,802
Share issue in the year 200,000 2 -
At 30 September 2023 48,351,373 484 84,802
----------------------------------- ----------- -------- --------
Exercise of share options
During the year, the following share options were exercised:
Date of exercise Key management personnel shares Other employee shares Total shares Exercise price Value
Pence GBP000
------------------ -------------------------------- ---------------------- ------------- --------------- --------
16/12/2022 200,000 - 200,000 1.0 2
------------------ -------------------------------- ---------------------- ------------- --------------- --------
200,000 - 200,000 2
Other reserves
Accumulated losses
This reserve relates to the cumulative results made by the Group
and Company in the current and prior periods.
Merger relief reserve
In accordance with Section 612 'Merger Relief' of the Companies
Act 2006, the Company issuing shares as consideration for a
business combination, accounted at fair value, is obliged, once the
necessary conditions are satisfied, to record the share premium to
the merger relief reserve.
Reverse acquisition reserve
Reverse accounting under IFRS 3 'Business Combinations' requires
that the difference between the equity of the legal parent and the
issued equity instruments of the legal subsidiary, pre-combination,
is recognised as a separate component of equity.
Capital redemption reserve
This reserve holds shares that were repurchased and cancelled by
the Company.
Foreign exchange translation reserve
This reserve represents the impact of retranslation of overseas
subsidiaries on consolidation.
Cash flow hedge reserve
This reserve represents the movement in designated hedging
instruments in the year that have not yet crystallised.
21. Share-based payments
Certain Directors and employees of the Group hold options to
subscribe for shares in the Company under share option schemes. All
share options relate to a single scheme outlined in the EMI Share
Option Plan 2014.
The scheme is open, by invitation, to both Executive Directors
and employees. Participants are granted share options in the
Company which contain vesting conditions. These are subject to the
achievement of individual employee and Group performance criteria
as determined by the Board. The vesting period varies by award and
the conditions approved by the Board. Options are usually forfeited
if the employee leaves the Group before the options vest.
Total share options outstanding have a range of exercise prices
from GBP0.01 to GBP0.70 per option and the weighted average
contractual life is 6.7 years (2022: 7.2 years). The total charge
for each period relating to employee share-based payment plans for
continuing operations is disclosed in note 10 of the consolidated
financial statements.
Details of the share options under the scheme outstanding during
the period are as follows:
2023 2022
---------------------------------- ----------------------------- -----------------------------
Number Weighted average Number Weighted average
exercise price exercise price
Outstanding at start of the
period 4,490,931 GBP0.18 3,815,931 GBP0.18
Granted - - 900,000 GBP0.20
Exercised (200,000) GBP0.01 - -
Lapsed (761,250) GBP0.29 (225,000) GBP0.35
Outstanding at end of the period 3,529,681 GBP0.15 4,490,931 GBP0.18
Exercisable at end of the period 1,949,680 GBP0.08 1,719,680 GBP0.07
---------------------------------- ---------- ----------------- ---------- -----------------
22. Financial risk management
In common with all other areas of the business, the Group is
exposed to risks that arise from the use of financial instruments.
This note describes the Group's objectives, policies and processes
for managing those risks and the methods used to measure them.
The main risks arising from the Group's financial instruments
are liquidity, interest rate, foreign currency and credit risk. The
Group's financial instruments comprise cash and various items such
as trade receivables and trade payables, which arise directly from
its operations.
Categories of financial instruments
2023 2022
GBP000 GBP000
--------------------------------------------------- ------- -------
Financial assets held at amortised cost
Trade and other receivables excluding prepayments 1,795 2,943
Cash and cash equivalents 4,031 5,769
--------------------------------------------------- ------- -------
5,826 8,712
--------------------------------------------------- ------- -------
Financial liabilities held at amortised cost
Trade and other payables excluding statutory liabilities 1,144 1,535
Lease liabilities 387 516
---------------------------------------------------------- ------ ------
1,531 2,051
---------------------------------------------------------- ------ ------
Financial liabilities held at fair value
Forward contracts held at fair value (Level 2) 27 111
------------------------------------------------ --- ---------
27 111
------------------------------------------------ --- ---------
Fair value of financial assets and liabilities
There is no material difference between the fair values and the
carrying values of the financial instruments held at amortised cost
because of the short maturity period of these financial instruments
or their intrinsic size and risk.
Liquidity risk management
Liquidity risk is the risk that the Group will not be able to
meet its obligations as they fall due through having insufficient
resources. The Group monitors its levels of working capital to
ensure that it can meet its liabilities as they fall due. Ultimate
responsibility for liquidity risk management rests with the Board,
which has built an appropriate framework for the management of the
Group's short-, medium- and long-term funding and liquidity
requirements.
The principal current asset of the business is cash and cash
equivalents and is therefore the principal financial instrument
employed by the Group to meet its liquidity requirements. The Board
ensures that the business maintains surplus cash reserves to
minimise any liquidity risk.
The financial liabilities of the Group and Company are all
mostly due within 3 months (2022: 3 months) of the Consolidated
Statement of Financial Position date, with the exception of the
lease liability. Further analysis of the lease liability is
provided in note 18 . All other non-current liabilities are due
between 1 to 3 years after the period end. The Group does not have
any borrowings or payables on demand which would increase the risk
of the Group not holding sufficient reserves for repayment.
Market risk
Interest rate risk management
Interest rate risk is the risk that the fair value or future
cash flows of a financial instrument will fluctuate because of
changes in market interest rate. The Group operates an interest
rate policy designed to minimise interest costs and reduce
volatility in reported earnings.
The Group holds all cash and cash equivalents with institutions
with a recognised high credit rating. Interest rates on current
accounts are floating. Changes in interest rates may increase or
decrease the Group's finance income.
The Group does not have any committed interest-bearing borrowing
facilities and consequently there is no material exposure to
interest rate risk in respect of financial liabilities.
Foreign currency risk management
Foreign currency risk is the risk that the fair value of future
cash flows of a foreign currency exposure will fluctuate because of
changes in foreign exchange rates.
The Group's exposure to the risk of changes in foreign exchange
rates relates to the Group's overseas operating activities,
primarily denominated in US Dollars, Euros and Swiss Francs. There
is also an investment by the Company in a foreign subsidiary. The
Group's exposure to foreign currency changes for all other
currencies is not material. The Group seeks to minimise the
exposure to foreign currency risk by matching local currency income
with local currency costs where possible. The Group utilises US
Dollar forward contracts to mitigate the risk of US Dollar
fluctuations on client contracts. It agrees forward contracts based
on forecasts of its US Dollar inflows and applies hedge accounting
to minimise currency risk.
The Group enters into forward contracts to sell US Dollars at
quarterly intervals and applies hedge accounting to these
contracts. Under hedge accounting, unrealised gains or losses are
recognised in other comprehensive income and the cash flow hedge
reserve, with the ineffective portion being recognised in the
profit and loss as soon as they occur. The gains or losses arising
on these are allocated to revenue on settlement. The item hedged
was a portion of highly probable forecast US Dollar inflows. The
hedged item is the receipt of US Dollars, and the hedging
instrument is the sale of a portion of these. The Group has
determined that a 1:1 ratio exists between the instrument and items
as the underlying risks of both are the same - the exchange rate of
USD:GBP. The Group uses the dollar offset method to monitor
effectiveness, which compares the change in fair value of the
underlying derivative and the change in fair value of future cash
flows. Ineffectiveness can arise due to the counterparties credit
risk and inaccurate forecasting, which
could leave the Group over hedged. In the year some
ineffectiveness arose where the Group's actual inflows were below
that of the hedging instrument. This ineffective portion was
recognised in general and administrative expenses.
At year end the Group had contracts to sell $750,000, these
hedges are designated as effective under IFRS 9 and hence the fair
value of these is recognised in other comprehensive income. These
balances are removed from the Group's US Dollar exposure as there
is deemed to be no foreign exchange exposure. At 30 September 2023,
$750,000 is hedged to period of March 2024, at an average rate of
1.2785. The contracts are valued based on observable market
exchange rates.
The hedging transactions in the year had the following effect on
the Group's results:
Without Hedging
hedge accounting movements 2023
GBP000 GBP000 GBP000
-------------------------------------------- ------------------ ----------- --------
Statement of Comprehensive Income
Revenue 6,638 27 6,665
Gross profit 3,243 27 3,270
General and administrative expenses (2,743) (111) (2,854)
Profit for the year (1,094) (84) (1,178)
Total other comprehensive expense (21) 84 63
Total comprehensive income attributable to
equity holders for the period (1,115) - (1,115)
Statement of financial position
Derivative financial liabilities 27 - 27
Cash flow hedge reserve - (27) (27)
Accumulated losses (7,387) 27 (7,360)
-------------------------------------------- ------------------ ----------- --------
Without Hedging
hedge accounting movements 2022
GBP000 GBP000 GBP000
-------------------------------------------- ------------------ ----------- --------
Statement of Comprehensive Income
Revenue 8,746 (103) 8,643
Gross profit 5,346 (103) 5,243
General and administrative expenses (2,795) 214 (2,581)
Profit for the year 921 111 1,032
Total other comprehensive expense 14 (111) (97)
Total comprehensive income attributable to
equity holders for the period 935 - 935
Statement of financial position
Derivative financial liabilities 111 - 111
Cash flow hedge reserve - (111) (111)
Accumulated losses (6,345) 111 (6,234)
-------------------------------------------- ------------------ ----------- --------
The carrying amounts of the Group's foreign currency denominated
monetary assets and monetary liabilities as at 30 September are as
follows:
2023 2022
US Dollar exposure USD'000 USD'000
------------------------------------------------------- -------- --------
Balance at end of period
Monetary assets 1 4 704
Monetary liabilities (27) (135)
------------------------------------------------------- -------- --------
Total exposure ( 13 ) 569
------------------------------------------------------- -------- --------
2023 2022
Euro exposure EUR'000 EUR'000
------------------------------------------------------- -------- ---------
Balance at end of period
Monetary assets 156 480
Monetary liabilities (13) (15)
------------------------------------------------------- -------- ---------
Total exposure 143 465
------------------------------------------------------- -------- ---------
2023 2022
Swiss Franc exposure CHF'000 CHF'000
------------------------------------------------------- -------- ---------
Balance at end of period
Monetary assets 33 113
Monetary liabilities - -
------------------------------------------------------- -------- ---------
Total exposure 33 113
------------------------------------------------------- -------- ---------
The Company had no foreign currency exposure at the year end
(2022: nil).
Foreign currency sensitivity analysis
As at 30 September 2023, the sensitivity analysis assumes a
+/-10% change of the USD/GBP, EUR/GBP and CHF/GBP exchange rates,
which represents management's assessment of a reasonably possible
change in foreign exchange rates (2022: 10%). The sensitivity
analysis was applied on the fair value of financial assets and
liabilities.
2023 2022
10% weaker(1) 10% stronger 10% weaker 10% stronger
GBP000 GBP000
------------- -------------- ------------- ----------- -------------
US Dollar 1 (1) (51) 51
Euro (12) 12 (41) 41
Swiss Franc (3) 3 (10) 10
------------- -------------- ------------- ----------- -------------
(14) 14 (102) 102
------------- -------------- ------------- ----------- -------------
(1) 10% weaker relates to the Great British Pound strengthening
against the currency and therefore the Group would be in a weaker
monetary position.
Credit risk management
Credit risk refers to the risk that a counterparty will default
on its contractual obligations resulting in financial loss to the
Group. The Group's financial assets are cash and cash equivalents
and trade and other receivables. The carrying value of these assets
represents the Group's maximum exposure to credit risk in relation
to financial assets.
The Group's credit risk is primarily attributable to its trade
receivables. The amounts presented in the Consolidated Statement of
Financial Position are net of allowances for any expected credit
losses, estimated by the Group's management based on prior
experience and their assessment of the current economic
environment, and any specific criteria identified in respect of
individual trade receivables. An allowance for expected credit
losses is made where there is an identified loss event, which,
based on previous experience, is evidence of a reduction in the
recoverability of future cash flows. There are no outstanding
expected credit losses identified at 30 September 2023 (2022:
nil).
Prior to entering into an agreement to provide services, the
Group makes appropriate enquiries of the counterparty and
independent third parties to determine creditworthiness. The Group
has not identified any significant credit risk exposure to any
single counterparty or Group of counterparties as at the period
end.
The Group and Company continually reviews client credit limits
based on market conditions and historical experience. Any provision
for impairment, as well as the ageing analysis of overdue trade
receivables, is set out in note 16 .
The Group and Company's policy is to minimise the risks
associated with cash and cash equivalents by placing these deposits
with institutions with a recognised high credit rating.
Capital risk management
The Group considers capital to be shareholders' equity as shown
in the Consolidated Statement of Financial Position, as the Group
is primarily funded by equity finance and is not yet in a position
to pay a dividend. The Group had no borrowings at 30 September 2023
(2022: GBPnil).
The objectives when managing capital are to safeguard the
Group's ability to continue as a going concern in order to provide
returns for shareholders and for other stakeholders. In order to
maintain or adjust the capital structure the Group may return
capital to shareholders or issue new shares.
23. Related party transactions
Transactions between the Company and its subsidiaries, which are
related parties, have been eliminated on consolidation and are not
disclosed in this note.
Remuneration and transactions of Directors and key management
personnel
Key management remuneration:
2023 2022
GBP000 GBP000
------------------------------ ------- -------
Short-term employee benefits 1,113 1,269
Post-employment benefits 29 33
Other long-term benefits (44) (115)
Share-based payments 19 77
---------------------------------- ------- -------
Total remuneration 1,117 1,264
---------------------------------- ------- -------
Key management includes Executive Directors, Non-Executive
Directors and senior management who have the responsibility for
managing, directly or indirectly, the activities of the Group.
The aggregate Directors' remuneration, including employers'
National Insurance and share-based payments' expense, was
GBP687,000 (2022: GBP658,000) and aggregate pension of GBP16,000
(2022: GBP15,000). Further detail of Directors' remuneration is
disclosed in the Directors' Remuneration Report in the full annual
report.
Transactions with group companies
The Company is responsible for financing and setting Group
strategy. The Company's subsidiaries carry out the Group's research
and development strategy, employ all employees, including the
Executive Directors, and manage the Group's intellectual property.
As a result, a management charge is made between the subsidiaries
and the Company for the services provided by the subsidiaries on
behalf of the Company. Similarly, as share options are issued in
the Company for employees of the subsidiaries, a charge is made
between the Company and its subsidiaries.
Intercompany balances are unsecured and are interest bearing at
6%, with no fixed date of repayment but are repayable on demand.
The intercompany balance also includes specific funding provided by
the Company, which attracts a 0% interest rate.
Outstanding balances related to subsidiary undertakings are
disclosed in note 17 . During the year, the following transactions
occurred with related parties:
2023 2022
GBP000 GBP000
--------------------------------------- ------- -------
Charges from subsidiaries:
Management recharge from subsidiaries 530 416
Net interest charged (100) (68)
Charges to subsidiaries:
Share option charge 52 57
This information is provided by RNS, the news service of the
London Stock Exchange. RNS is approved by the Financial Conduct
Authority to act as a Primary Information Provider in the United
Kingdom. Terms and conditions relating to the use and distribution
of this information may apply. For further information, please
contact rns@lseg.com or visit www.rns.com.
RNS may use your IP address to confirm compliance with the terms
and conditions, to analyse how you engage with the information
contained in this communication, and to share such analysis on an
anonymised basis with others as part of our commercial services.
For further information about how RNS and the London Stock Exchange
use the personal data you provide us, please see our Privacy
Policy.
END
FR EANALEDEDFFA
(END) Dow Jones Newswires
December 05, 2023 02:00 ET (07:00 GMT)
Ixico (LSE:IXI)
Gráfica de Acción Histórica
De Mar 2024 a Abr 2024
Ixico (LSE:IXI)
Gráfica de Acción Histórica
De Abr 2023 a Abr 2024