As
filed with the Securities and Exchange Commission on November 15, 2024
Registration
No. 333-__________
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
WASHINGTON,
D.C. 20549
FORM
S-1
REGISTRATION
STATEMENT
UNDER
THE
SECURITIES ACT OF 1933
Daré
Bioscience, Inc.
(Exact
name of registrant as specified in its charter)
| Delaware |
|
2834 |
|
20-4139823 |
| (State
or other jurisdiction of |
|
(Primary
Standard Industrial |
|
(I.R.S.
Employer |
| incorporation
or organization) |
|
Classification
Code Number) |
|
Identification
No.) |
3655
Nobel Drive, Suite 260
San
Diego, California 92122
(858)
926-7655
(Address,
including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Sabrina
Martucci Johnson
Chief
Executive Officer
Daré
Bioscience, Inc.
3655
Nobel Drive, Suite 260
San
Diego, California 92122
(858)
926-7655
(Name,
address, including zip code, and telephone number, including area code, of agent for service)
Copies
to:
Edwin
Astudillo, Esq.
Sheppard,
Mullin, Richter & Hampton LLP
12275
El Camino Real, Suite 100
San
Diego, California 92130
(858)
720-8953
Approximate
date of commencement of proposed sale to the public:
As soon as practicable after this registration statement becomes effective.
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the
Securities Act of 1933 check the following box: ☒
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the
following box and list the Securities Act registration statement number of the earlier effective registration statement for the same
offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company,
or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller
reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large
accelerated filer ☐ |
|
Accelerated
filer ☐ |
| Non-accelerated
filer ☒ |
|
Smaller
reporting company ☒ |
| |
|
Emerging
growth company ☐ |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
THE
REGISTRANT HEREBY AMENDS THIS REGISTRATION STATEMENT ON SUCH DATE OR DATES AS MAY BE NECESSARY TO DELAY ITS EFFECTIVE DATE UNTIL THE
REGISTRANT SHALL FILE A FURTHER AMENDMENT THAT SPECIFICALLY STATES THAT THIS REGISTRATION STATEMENT SHALL THEREAFTER BECOME EFFECTIVE
IN ACCORDANCE WITH SECTION 8(a) OF THE SECURITIES ACT OF 1933, AS AMENDED, OR UNTIL THE REGISTRATION STATEMENT SHALL BECOME EFFECTIVE
ON SUCH DATE AS THE SECURITIES AND EXCHANGE COMMISSION, ACTING PURSUANT TO SAID SECTION 8(a), MAY DETERMINE.
The
information in this prospectus is not complete and may be changed. These securities may not be sold until the registration statement
of which this prospectus forms a part filed with the Securities and Exchange Commission is effective. This preliminary prospectus is
not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale
is not permitted.
SUBJECT
TO COMPLETION, DATED NOVEMBER 15, 2024
PROSPECTUS
Daré
Bioscience, Inc.

2,750,000
Shares of Common Stock
This
prospectus relates to the resale, from time to time, of up to 2,750,000 shares of our common stock, par value $0.0001, by Lincoln
Park Capital Fund, LLC, or Lincoln Park or the selling stockholder.
The
shares of common stock to which this prospectus relates are shares that have been or may be issued to Lincoln Park
pursuant to the purchase agreement dated October 21, 2024 that we entered into with Lincoln Park, which we refer to in this prospectus
as the Purchase Agreement. See “Our Agreements with Lincoln Park” for a description of that agreement and “Selling
Stockholder” for additional information regarding Lincoln Park. The prices at which Lincoln Park may sell the shares will be determined
by the prevailing market price for the shares or in negotiated transactions.
We
are not selling any securities under this prospectus and will not receive any of the proceeds from the sale of shares by the selling
stockholder.
The
selling stockholder may sell or otherwise dispose of the shares of common stock described in this prospectus in a number of different
ways and at varying prices. See “Plan of Distribution” beginning on page 25 of this prospectus for more information about
how and the prices at which the selling stockholder may sell or otherwise dispose of the shares of our common stock being offered. The
selling stockholder is an “underwriter” within the meaning of Section 2(a)(11) of the Securities Act of 1933, as amended.
The
selling stockholder will pay all brokerage fees and commissions and similar expenses. We will pay all expenses (except brokerage fees
and commissions and similar expenses) relating to the registration of the shares with the Securities and Exchange Commission.
Our
common stock is listed on The Nasdaq Capital Market under the symbol “DARE.” On November 14, 2024, the last reported
sale price of our common stock was $3.57 per share.
Investing
in our common stock involves a high degree of risk. Before deciding whether to invest in our common stock, you should consider carefully
the risks described under the caption “Risk Factors” beginning on page 8 of this prospectus, and under similar headings
in the documents incorporated by reference in this prospectus, as well as in any amendments or supplements to this prospectus.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined
if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The
date of this prospectus is , 2024
TABLE
OF CONTENTS
ABOUT
THIS PROSPECTUS
This
prospectus does not contain all of the information included in the registration statement of which this prospectus forms a part. For
a more complete understanding of the offering of the securities described herein, you should refer to the registration statement, including
its exhibits. You should carefully read this prospectus, any prospectus supplement or free writing prospectus that we subsequently authorize
for use in connection with the offering of the securities described herein, the information and documents incorporated herein by reference
and the additional information under the heading “Where You Can Find More Information” before making an investment decision.
You should rely only on the information we have provided or incorporated by reference in this prospectus, or in any prospectus supplement
or free writing prospectus that we subsequently authorize for use in connection with the offering of the securities described herein.
Neither we, nor the selling stockholder, have authorized anyone to provide you with information different from that contained or incorporated
by reference in this prospectus. If anyone provides you with different or inconsistent information, you should not rely on it. You should
assume that the information in this prospectus, or any related prospectus supplement or free writing prospectus, is accurate only as
of the date set forth on the cover page of any such document or any earlier date as of which such information is given, as applicable,
and that any information we have incorporated herein by reference is accurate only as of the date set forth on the cover page of any
such document containing such information or any earlier date as of which such information is given, as applicable, regardless of the
time of delivery of this prospectus, or such prospectus supplement or free writing prospectus, or any sale of a security. Our business,
financial condition, results of operations and prospects may have changed since that date.
Neither
we, nor the selling stockholder, are offering to sell or seeking offers to purchase these securities in any jurisdiction where the offer
or sale is not permitted. We have not done anything that would permit this offering or possession or distribution of this prospectus
in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who
come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the securities
hereunder and the distribution of this prospectus outside the United States.
The
representations, warranties and covenants made by us in any agreement that is filed as an exhibit to the registration statement of which
this prospectus forms a part or to any document that is incorporated by reference in this prospectus were made solely for the benefit
of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties to such agreements,
and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants
were accurate only as of the date when made. Accordingly, such representations, warranties and covenants should not be relied on as accurately
representing the current state of our affairs.
Unless
otherwise indicated, information contained in this prospectus concerning our industry and the markets in which we operate, including
our general expectations and market position, market opportunity and market size, is based on information from various sources, including
peer reviewed journals, formal presentations at medical society meetings and third-parties commissioned by us or our licensors to provide
market research and analysis, and is subject to a number of assumptions and limitations. Although we are responsible for all of the disclosure
contained in this prospectus and we believe the information from industry publications and other third-party sources included in this
prospectus is reliable, such information is inherently imprecise. Information that is based on estimates, forecasts, projections, market
research or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from
events and circumstances that are assumed in this information. The industry in which we operate is subject to a high degree of uncertainty
and risk due to a variety of factors, including those described in the section captioned “Risk Factors.”
To
the extent there are inconsistencies between this prospectus, any related prospectus supplement or free writing prospectus, and any documents
incorporated by reference, the document with the most recent date will control.
Unless
the context otherwise requires, “Daré,” “Daré Bioscience,” “the Company,” “we,”
“us,” “our” and similar terms refer to Daré Bioscience, Inc. and its subsidiaries.
PROSPECTUS
SUMMARY
This
summary highlights information contained elsewhere in this prospectus. This summary provides an overview of selected information and
does not contain all of the information you should consider before investing in our securities. We urge you to read this entire prospectus,
including our consolidated financial statements, notes to the consolidated financial statements, the information in the section captioned
“Risk Factors,” and other information incorporated herein by reference to our other filings with the Securities and Exchange
Commission, or SEC, or included in any applicable prospectus supplement. Investing in our securities involves a high degree of risk and
uncertainty. Therefore, carefully consider the risk factors described in this prospectus, including those incorporated herein by reference
to our most recent annual and quarterly filings with the SEC, before purchasing our securities. Each of the risk factors could adversely
affect our business, operating results and financial condition, as well as adversely affect the value of an investment in our securities.
About
Daré Bioscience
We
are a biopharmaceutical company committed to advancing innovative products for women’s health. We are driven by a mission to identify,
develop and bring to market a diverse portfolio of differentiated therapies that prioritize women’s health and well-being, expand
treatment options, and improve outcomes, primarily in the areas of contraception, sexual health, pelvic pain, fertility, infectious disease
and menopause. Our business strategy is to in-license or otherwise acquire the rights to differentiated product candidates in our areas
of focus, some of which have existing clinical proof-of-concept data, to take those candidates through mid to late-stage clinical development
or regulatory approval, and to establish and leverage strategic collaborations to achieve commercialization. We and our wholly-owned
subsidiaries operate in one business segment.
The
first product approved by the United States Food and Drug Administration, or FDA, to emerge from our portfolio of women’s
health product candidates is XACIATO™ (clindamycin phosphate) vaginal gel 2%, or XACIATO (pronounced zah-she-AH-toe). We achieved
FDA approval of XACIATO three years after acquiring rights to the program. XACIATO was approved by the FDA in December 2021 as a single-dose
prescription medication for the treatment of bacterial vaginosis in females 12 years of age and older. In March 2022, we entered into
an agreement with an affiliate of Organon & Co., Organon International GmbH, or Organon, which became fully effective in June 2022,
whereby Organon licensed exclusive worldwide rights to develop, manufacture and commercialize XACIATO. In accordance with the license
agreement, as amended, we are no longer working on the development, manufacture or commercialization of XACIATO. Organon commenced U.S.
marketing of XACIATO in the fourth quarter of 2023 and, in January 2024, Organon announced that XACIATO was available nationwide.
Our
product pipeline includes diverse programs that target unmet needs in women’s health in the areas of contraception, sexual health,
pelvic pain, fertility, infectious disease and menopause, and aim to expand treatment options, enhance outcomes and improve ease of use
for women. We are primarily focused on progressing the development of our existing portfolio of product candidates. However, we also
explore opportunities to expand our portfolio by leveraging assets to which we hold rights or obtaining rights to new assets, with continued
focus solely on women’s health.
Our
current portfolio includes five product candidates in advanced clinical development (Phase 2-ready to Phase 3):
| ● | Ovaprene®,
a hormone-free, monthly intravaginal contraceptive; |
| ● | Sildenafil
Cream, 3.6%, a proprietary cream formulation of sildenafil for topical administration
to the female genitalia on demand for the treatment of female sexual arousal disorder (FSAD); |
| ● | DARE-HRT1,
an intravaginal ring designed to deliver both bio-identical estradiol and progesterone together,
continuously over a 28-day period, for the treatment of moderate-to-severe vasomotor symptoms,
as part of menopausal hormone therapy; |
| ● | DARE-VVA1,
a proprietary formulation of tamoxifen for intravaginal administration being developed as
a hormone-free alternative to estrogen-based therapies for the treatment of moderate-to-severe
dyspareunia, or pain during sexual intercourse, a symptom of vulvar and vaginal atrophy associated
with menopause; and |
| ● | DARE-HPV,
a proprietary, fixed-dose formulation of lopinavir and ritonavir in a soft gel vaginal insert
for the treatment of human papillomavirus (HPV)-related cervical diseases. |
Our
portfolio also includes five product candidates in Phase 1 clinical development or that we believe are Phase 1-ready:
| ● | DARE-PDM1,
a proprietary hydrogel formulation of diclofenac, a nonsteroidal anti-inflammatory drug,
for vaginal administration as a treatment for primary dysmenorrhea; |
| ● | DARE-204
and DARE-214, injectable formulations of etonogestrel designed to provide contraception
over 6-month and 12-month periods, respectively; |
| ● | DARE-FRT1,
an intravaginal ring designed to deliver bio-identical progesterone continuously for up to
14 days for luteal phase support as part of an in vitro fertilization treatment plan; and |
| ● | DARE-PTB1,
an intravaginal ring designed to deliver bio-identical progesterone continuously for up to
14 days for the prevention of preterm birth. |
In
addition, our portfolio includes five preclinical stage programs:
| ● | DARE-LARC1,
a contraceptive implant delivering levonorgestrel with a woman-centered design that has the
potential to be a long-acting, yet convenient and user-controlled contraceptive option; |
| ● | DARE-LBT,
a novel hydrogel formulation for vaginal delivery of live biotherapeutics to support vaginal
health; |
| ● | DARE-GML,
an intravaginally-delivered potential multi-target antimicrobial agent formulated with glycerol
monolaurate (GML), which has shown broad antimicrobial activity, killing bacteria and viruses; |
| ● | DARE-RH1,
a novel approach to non-hormonal contraception for both men and women by targeting the CatSper
ion channel; and |
| ● | DARE-PTB2,
a novel approach for the prevention and treatment of idiopathic preterm birth through inhibition
of a stress response protein. |
The
product candidates and potential product candidates in our portfolio will require review and approval from the FDA, or a comparable foreign
regulatory authority, prior to being marketed or sold.
Our
primary operations have consisted of research and development activities to advance our portfolio of product candidates through late-stage
clinical development and/or regulatory approval. We expect our research and development expenses will continue to represent the majority
of our operating expenses for at least the next twelve months. Until we secure additional capital to fund our operating needs, we will
focus our resources primarily on advancement of Ovaprene and Sildenafil Cream. In addition, we expect to incur significant research and
development expenses for the DARE-LARC1 and DARE-HPV programs, but we also expect such expenses will be supported, with respect to DARE-LARC1,
through at least 2026 by non-dilutive funding provided under a grant agreement we entered into in June 2021, and with respect to DARE-HPV,
through October 2026 by non-dilutive funding provided under a subaward agreement we entered into in October 2024.
We
will need to raise substantial additional capital to continue to fund our operations and execute our current business strategy. We are
also subject to a number of other risks common to biopharmaceutical companies, including, but not limited to, dependence on key employees,
reliance on third-party collaborators, service providers and suppliers, being able to develop commercially viable products in a timely
and cost-effective manner, dependence on intellectual property we own or in-license and the need to protect that intellectual property
and maintain those license agreements, uncertainty of market acceptance of products, uncertainty of third-party payor coverage, pricing
and reimbursement for products, rapid technology change, intense competition, compliance with government regulations, product liability
claims, and exposure to cybersecurity threats and incidents.
The
process of developing and obtaining regulatory approvals for prescription drug and drug/device products in the United States and in foreign
jurisdictions is inherently uncertain and requires the expenditure of substantial financial resources without any guarantee of success.
To the extent we receive regulatory approvals to market and sell our product candidates, the commercialization of any product and compliance
with subsequently applicable laws and regulations requires the expenditure of further substantial financial resources without any guarantee
of commercial success. The amount of post-approval financial resources required for commercialization and the potential revenue we may
receive from sales of any product will vary significantly depending on many factors, including whether, and the extent to which, we establish
our own sales and marketing capabilities and/or enter into and maintain commercial collaborations with third parties with established
commercialization infrastructure.
Purchase
Agreement with Lincoln Park
On
October 21, 2024, we entered into a purchase agreement with Lincoln Park, which we refer to in this prospectus as the Purchase Agreement,
pursuant to which Lincoln Park agreed to purchase from us up to an aggregate of $15,000,000 of our common stock (subject to certain limitations)
from time to time over the term of the Purchase Agreement. Also on October 21, 2024, we entered into a registration rights agreement
with Lincoln Park, which we refer to in this prospectus as the Registration Rights Agreement, pursuant to which we filed with the SEC
the registration statement of which this prospectus forms a part to register for resale under the Securities Act of 1933, as amended,
or the Securities Act, the shares of common stock that have been or may be issued to Lincoln Park under the Purchase Agreement.
We
do not have the right to commence any sales of our common stock to Lincoln Park under the Purchase Agreement until the conditions set
forth in the Purchase Agreement, all of which are outside of Lincoln Park’s control, have been satisfied, including that the SEC
has declared effective the registration statement of which this prospectus forms a part, which time we refer to in this prospectus as
the Commencement. From time to time after the Commencement, at our sole discretion, on any business day selected by us on which the closing
sale price of our common stock is not below $0.50 per share, we may direct Lincoln Park to purchase up to 30,000 shares of our common
stock (each, a “Regular Purchase”); provided that the share amount under a Regular Purchase may be increased to up to 35,000
shares or up to 40,000 shares if the closing sale price of our common stock is not below $5.00 or $7.50, respectively, on the business
day on which we initiate the purchase, subject to adjustment for any reorganization, recapitalization, non-cash dividend, stock split,
reverse stock split or other similar transaction as provided in the Purchase Agreement. However, Lincoln Park’s maximum commitment
in any single Regular Purchase may not exceed $500,000. The purchase price per share for each Regular Purchase will be the lower of (i)
the lowest sale price of our common stock on the business day on which we initiate the Regular Purchase and (ii) the average of the three
lowest closing sale prices of our common stock during the 10-business day period immediately preceding the business day on which we initiate
the Regular Purchase. In addition to Regular Purchases, we may also direct Lincoln Park to purchase other amounts of common stock as
accelerated purchases and as additional accelerated purchases, subject to limits specified in the Purchase Agreement, at a purchase price
per share calculated as specified in the Purchase Agreement, but in no case lower than the minimum price per share we stipulate in our
notice to Lincoln Park initiating these purchases. We will control the timing and amount of any sales of our common stock to Lincoln
Park.
On
October 21, 2024, in consideration for its commitment to purchase shares of our common stock under the Purchase Agreement, we issued
137,614 shares of our common stock to Lincoln Park, which we refer to in this prospectus as the “Commitment Shares.”
We
may terminate the Purchase Agreement at any time after the date of Commencement, at no penalty or cost, other than the Commitment Shares.
There are no restrictions on future financings, rights of first refusal, participation rights, penalties or liquidated damages in the
Purchase Agreement or Registration Rights Agreement, other than a prohibition on our issuing, or entering into any agreement to effect
the issuance of, shares of our common stock or common stock equivalents involving a transaction that is defined in the Purchase Agreement
as a “Variable Rate Transaction.” Lincoln Park may not assign or transfer its rights and obligations under the Purchase Agreement.
As
of September 30, 2024, there were 8,546,364 shares of our common stock outstanding, excluding the Commitment Shares, of which 8,444,775
shares were held by non-affiliates. Although the Purchase Agreement provides that we may sell up to $15,000,000 of our common stock to
Lincoln Park, only 2,750,000 shares of our common stock are being offered under this prospectus, which represents the Commitment Shares
and up to 2,612,386 shares which may be issued to Lincoln Park in the future under the Purchase Agreement, if and when we sell shares
to Lincoln Park under the Purchase Agreement. If all of the 2,750,000 shares offered by Lincoln Park under this prospectus were issued
and outstanding, such shares would represent approximately 24% of the total number of shares of our common stock outstanding and approximately
25% of the total number of outstanding shares held by non-affiliates, in each case as of September 30, 2024. Depending on the price per
share at which we sell shares to Lincoln Park under the Purchase Agreement, we may need to sell more shares to Lincoln Park than are
offered under this prospectus to receive aggregate gross proceeds equal to the $15,000,000 total commitment of Lincoln Park under the
Purchase Agreement, in which case we must first register for resale under the Securities Act additional shares of our common stock. The
number of shares ultimately offered for resale by Lincoln Park will depend upon the number of shares we elect to sell to Lincoln Park
under the Purchase Agreement. Sales of our common stock to Lincoln Park by us under the Purchase Agreement could result in substantial
dilution to our stockholders.
Under
applicable rules of The Nasdaq Stock Market, in no event may we issue or sell to Lincoln Park under the Purchase Agreement more than
19.99% of the shares of our common stock outstanding immediately prior to the execution of the Purchase Agreement, which is 1,711,172
shares based on 8,555,864 shares outstanding immediately prior to the execution of the Purchase Agreement (the “Exchange Cap”),
unless (i) we obtain stockholder approval to issue shares in excess of the Exchange Cap or (ii) the average price of all applicable sales
of our common stock to Lincoln Park under the Purchase Agreement equals or exceeds $3.59 per share (which represents the lower of (A)
the official closing price of our common stock on Nasdaq immediately preceding the signing of the Purchase Agreement and (B) the average
official closing price of our common stock on Nasdaq for the five consecutive trading days ending on the trading day immediately preceding
the date of the Purchase Agreement), such that issuances and sales of common stock to Lincoln Park under the Purchase Agreement would
not be subject to the Exchange Cap under applicable Nasdaq rules. In any event, the Purchase Agreement specifically provides that we
may not issue or sell any shares of our common stock under the Purchase Agreement if such issuance or sale would breach any applicable
Nasdaq rules.
The
Purchase Agreement also prohibits us from directing Lincoln Park to purchase any shares of common stock if those shares, when aggregated
with all other shares of our common stock then beneficially owned by Lincoln Park and its affiliates, would result in Lincoln Park and
its affiliates having beneficial ownership, at any single point in time, of more than 4.99% of the then total outstanding shares of our
common stock, as calculated pursuant to Section 13(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and Rule
13d-3 thereunder, which limitation we refer to as the Beneficial Ownership Cap. Lincoln Park, upon written notice to us, may increase
the Beneficial Ownership Cap to up to 9.99%. Any increase in the Beneficial Ownership Cap will not be effective until the 61st day after
such written notice is delivered to us.
Issuances
of our common stock to Lincoln Park will not affect the rights or privileges of our existing stockholders, except that the economic and
voting interests of each of our existing stockholders will be diluted as a result of any such issuance. Although the number of shares
of common stock that our existing stockholders own will not decrease, the shares owned by our existing stockholders will represent a
smaller percentage of our total outstanding shares after any such issuance to Lincoln Park.
Additional
Information
For
additional information related to our business and operations, please refer to the annual and quarterly reports incorporated herein by
reference, as described under the caption “Incorporation of Documents by Reference” on page 27 of this prospectus.
Corporate
Information
Our
principal executive offices are located at 3655 Nobel Drive, Suite 260, San Diego, California 92122, and our telephone number at that
address is (858) 926-7655. We maintain a website at www.darebioscience.com, to which we regularly post copies of our press releases as
well as additional information about us. The information contained on, or that can be accessed through, our website is not a part of
this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
Daré
Bioscience® is a registered trademark of Daré Bioscience, Inc. Ovaprene® is a registered trademark licensed to Daré
Bioscience, Inc. All brand names or trademarks appearing in this prospectus are the property of their respective holders. Use or display
by us of other parties’ trademarks, trade dress, or products in this prospectus is not intended to, and does not, imply a relationship
with, or endorsements or sponsorship of, us by the trademark or trade dress owners.
The
Offering
This
prospectus relates to the resale by Lincoln Park Capital Fund, LLC, the selling stockholder identified in this prospectus, of shares
of our common stock, par value $0.0001 per share, as follows:
| Common
stock offered by the selling stockholder |
|
Up
to 2,750,000 shares consisting of: |
| |
|
● |
137,614
shares issued to Lincoln Park in consideration for its commitment to purchase shares of our common stock under the Purchase Agreement;
and |
| |
|
|
|
| |
|
● |
2,612,386
shares we may sell to Lincoln Park under the Purchase Agreement from time to time after the date of this prospectus. |
| Common
stock outstanding prior to this offering (which excludes the 137,614 shares issued to Lincoln Park described above) |
|
8,546,364
shares |
| |
|
|
| Common
stock to be outstanding after giving effect to the issuance of 2,750,000 shares under the Purchase Agreement registered hereunder |
|
11,296,364
shares |
| |
|
| Use
of proceeds |
|
We
will receive no proceeds from the sale of shares of common stock by Lincoln Park in this offering. We may receive up to $15,000,000
in aggregate gross proceeds under the Purchase Agreement from any sales we make to Lincoln Park pursuant to the Purchase Agreement
after the date of this prospectus. Any such proceeds we receive will be used for working capital and general corporate purposes.
See “Use of Proceeds” on page 22 of this prospectus. |
| |
|
| Risk
factors |
|
Investment
in our common stock involves a high degree of risk. See “Risk Factors” beginning on page 8 of this prospectus, as well
as the other information included in or incorporated by reference in this prospectus, for a discussion of risks you should carefully
consider before investing in our common stock.
|
| Nasdaq
Capital Market symbol |
|
“DARE” |
The
actual number of shares of our common stock outstanding after this offering will vary depending on the actual number of shares we sell
to Lincoln Park under the Purchase Agreement. The number of shares of our common stock outstanding prior to and after this offering in
the table above is based on 8,546,364 shares outstanding as of September 30, 2024 and excludes:
| ● | 137,614
shares issued to Lincoln Park on October 21, 2024 in consideration for its commitment to
purchase shares under the Purchase Agreement; |
| ● | 903,424
shares of common stock issuable upon exercise of stock options outstanding as of September
30, 2024, with a weighted-average exercise price of $14.73 per share; |
| ● | 445,661
shares of common stock reserved and available for future issuance as of September 30, 2024
under our equity incentive plans; |
| ● | 1,268,572
shares of common stock issuable upon exercise of warrants outstanding as of September 30,
2024, with a weighted-average exercise price of $7.49 per share; and |
| ● | 16,408
shares of common stock issued after September 30, 2024 pursuant to our “at the market”
offering program. |
RISK
FACTORS
Investing
in our securities involves significant risk. In addition to the other information included or incorporated by reference in this prospectus,
including the risks, uncertainties and assumptions discussed under the heading “Risk Factors” included in our most recent
annual report on Form 10-K, as revised or supplemented by our subsequent quarterly reports on Form 10-Q or our current reports on Form
8-K that we have filed with the SEC, all of which are incorporated herein by reference (other than current reports on Form 8-K, or portions
thereof, furnished under Items 2.02 or 7.01 of Form 8-K), you should consider the risks described below before making an investment decision
with respect to the shares of our common stock in this offering. We expect to update these risks and our other risk factors from time
to time in periodic and current reports we file with the SEC in the future. Such updated risk factors will be incorporated by reference
in this prospectus. Please refer to our subsequently filed reports for additional information relating to the risks associated with investing
in our common stock. The risks and uncertainties we have described are not the only ones we face. Additional risks and uncertainties
not presently known to us or that we currently deem immaterial may also affect our operations. The occurrence of any of these risks might
cause you to lose all or part of your investment.
The
sale of our common stock to Lincoln Park may cause dilution and the subsequent sale of the shares of common stock acquired by Lincoln
Park, or the perception that such sales may occur, could cause the price of our common stock to fall.
On
October 21, 2024, we entered into the Purchase Agreement with Lincoln Park, pursuant to which Lincoln Park committed to purchase up to
$15,000,000 of our common stock, and we issued the Commitment Shares. Other than the Commitment Shares, the shares of our common stock
that may be issued under the Purchase Agreement may be sold by us to Lincoln Park from time to time at our discretion over a 24-month
period commencing on the date that the conditions set forth in the Purchase Agreement are satisfied, including that the registration
statement of which this prospectus forms a part is declared effective by the SEC. The purchase price for the shares that we may sell
to Lincoln Park under the Purchase Agreement will vary based on the price of our common stock at the time we initiate the sale. Depending
on market liquidity at the time, sales of such shares may cause the trading price of our common stock to fall.
We
generally have the right to control the timing and amount of any future sales of our shares to Lincoln Park. Sales of shares of our common
stock to Lincoln Park under the Purchase Agreement, if any, will depend upon market conditions and other factors to be determined by
us. We may ultimately decide to sell to Lincoln Park all, some or none of the shares of our common stock that may be available for us
to sell pursuant to the Purchase Agreement. If and when we do sell shares to Lincoln Park, after Lincoln Park has acquired the shares,
Lincoln Park may resell all, some or none of those shares at any time or from time to time in its discretion. Therefore, sales to Lincoln
Park by us could result in substantial dilution to the interests of other holders of our common stock. Additionally, the sale of a substantial
number of shares of our common stock to Lincoln Park, or the anticipation of such sales, could make it more difficult for us to sell
equity or equity-related securities in the future at a time and at a price that we might otherwise wish to effect sales. See also, “The
sale of our common stock in ATM offerings may cause substantial dilution to our existing stockholders, and such sales, or the anticipation
of such sales, may cause the price of our common stock to decline,” below.
It
is not possible to predict the actual number of shares of common stock we may sell to Lincoln Park under the Purchase Agreement, or the
actual gross proceeds resulting from those sales.
Because
the purchase price per share to be paid by Lincoln Park for the shares of common stock that we may elect to sell to Lincoln Park under
the Purchase Agreement, if any, will fluctuate based on the market prices of our common stock at the time we elect to sell shares to
Lincoln Park pursuant to the Purchase Agreement, if any, it is not possible for us to predict, as of the date of this prospectus and
prior to any such sales, the number of shares of common stock that we will sell to Lincoln Park under the Purchase Agreement, the purchase
price per share that Lincoln Park will pay for shares purchased from us under the Purchase Agreement, or the aggregate gross proceeds
that we will receive from those purchases by Lincoln Park under the Purchase Agreement.
Moreover,
although the Purchase Agreement provides that we may sell up to an aggregate of $15.0 million of our common stock to Lincoln Park, only
2,750,000 shares of common stock are being registered under the Securities Act for resale by Lincoln Park under the registration
statement of which this prospectus forms a part, consisting of (i) the 137,614 Commitment Shares that we issued to Lincoln Park as consideration
for its commitment to purchase shares of our common stock under the Purchase Agreement and (ii) up to 2,612,386 shares of our common
stock that we may elect to sell to Lincoln Park, in our sole discretion, from time to time from and after the Commencement under
the Purchase Agreement.
If
after the Commencement we elect to sell to the selling stockholder all of the 2,612,386 shares of common stock being registered for resale
by Lincoln Park under this prospectus that are available for sale by us to the selling stockholder under the Purchase Agreement, depending
on the market prices of our common stock at the time of such sales, the actual gross proceeds from the sale of all such shares of common
stock by us to Lincoln Park may be substantially less than the $15.0 million total purchase commitment available to us under the Purchase
Agreement, which could materially adversely affect our liquidity.
If
it becomes necessary for us to issue and sell to Lincoln Park shares of common stock in excess of the Exchange Cap under the Purchase
Agreement in order to receive aggregate gross proceeds equal to $15.0 million under the Purchase Agreement, then for so long as the Exchange
Cap continues to apply to issuances and sales of common stock under the Purchase Agreement, we must first obtain stockholder approval
to issue shares of common stock in excess of the Exchange Cap in accordance with applicable Nasdaq listing rules. Furthermore, if we
elect to issue and sell to Lincoln Park more than the 2,612,386 shares of our common stock that we may elect to issue and sell to Lincoln
Park under the Purchase Agreement that are being registered for resale by Lincoln Park hereunder, which we have the right, but not the
obligation, to do, we must first file with the SEC one or more additional registration statements to register under the Securities Act
for resale by Lincoln Park such additional shares of our common stock we wish to sell from time to time under the Purchase Agreement,
which the SEC must declare effective, in each case before we may elect to sell any additional shares of our common stock to Lincoln Park
under the Purchase Agreement. Any issuance and sale by us under the Purchase Agreement of a substantial amount of shares of common stock
in addition to the 2,612,386 shares of common stock that we may elect to issue and sell to Lincoln Park under the Purchase Agreement
that are being registered for resale by Lincoln Park hereunder could cause additional substantial dilution to our stockholders. The number
of shares of our common stock ultimately offered for sale by Lincoln Park is dependent upon the number of shares of common stock, if
any, we ultimately sell to Lincoln Park under the Purchase Agreement, and the sale of common stock under the Purchase Agreement may cause
the trading price of our common stock to decline.
Investors
who buy shares at different times will likely pay different prices, and the sale of the shares of common stock acquired by Lincoln Park
could cause the price of our common stock to decline.
Pursuant
to the Purchase Agreement, we will have discretion, subject to market demand, to vary the timing, prices, and numbers of shares sold
to Lincoln Park. If and when we do elect to sell shares of our common stock to Lincoln Park pursuant to the Purchase Agreement, after
Lincoln Park has acquired such shares, Lincoln Park may resell all, some or none of such shares at any time or from time to time in its
discretion and at different prices. As a result, investors who purchase shares from Lincoln Park in this offering at different times
will likely pay different prices for those shares, and so may experience different levels of dilution and in some cases substantial dilution
and different outcomes in their investment results. Investors may experience a decline in the value of the shares they purchase from
Lincoln Park in this offering as a result of future sales made by us to Lincoln Park at prices lower than the prices such investors paid
for their shares in this offering. Further, the sale of a substantial number of shares of our common stock by Lincoln Park, or anticipation
of such sales, could cause the trading price of our common stock to decline or make it more difficult for us to sell equity or equity-related
securities in the future at a time and at a price that we might otherwise desire.
We
may not have access to the full amount available under the Purchase Agreement with Lincoln Park. We may require additional financing
to sustain our operations, without which we may not be able to continue operations, and the terms of subsequent financings may adversely
impact our stockholders.
We
may direct Lincoln Park to purchase up to $15.0 million worth of shares of our common stock in a Regular Purchase from time to time under
the Purchase Agreement over a 24-month period generally in amounts up to 30,000 shares of our common stock, which may be increased to
up to 40,000 shares of our common stock depending on the closing sale price of our common stock at the time of sale, provided that Lincoln
Park’s maximum purchase obligation under any single Regular Purchase shall not exceed $500,000. Moreover, under certain circumstances
as set forth in the Purchase Agreement, we may, in our sole discretion, also direct Lincoln Park to purchase additional shares of common
stock in “accelerated purchases,” and “additional accelerated purchases” as set forth in the Purchase Agreement.
Depending
on the prevailing market price of our common stock, we may not be able to sell shares to Lincoln Park for the maximum $15.0 million over
the term of the Purchase Agreement. We will need to seek stockholder approval before issuing more than the Exchange Cap limit of 1,711,172
shares of common stock under the Purchase Agreement, unless the average price per share of common stock for all shares of common stock
sold by us to Lincoln Park under the Purchase Agreement equals or exceeds $3.59 per share (which represents the lower of (A) the official
closing price of our common stock on Nasdaq immediately preceding the signing of the Purchase Agreement and (B) the average official
closing price of our common stock on Nasdaq for the five consecutive trading days ending on the trading day immediately preceding the
date of the Purchase Agreement), such that the Exchange Cap limitation would no longer apply to issuances and sales of common stock by
us to Lincoln Park under the Purchase Agreement under applicable Nasdaq listing rules. In addition, Lincoln Park will not be required
to purchase any shares of our common stock if such sale would result in Lincoln Park’s beneficial ownership of our common stock
exceeding the Beneficial Ownership Cap. Our inability to access a portion or the full amount available under the Purchase Agreement,
in the absence of any other financing sources, could have a material adverse effect on our business.
The
extent we rely on Lincoln Park as a source of funding will depend on a number of factors including the prevailing market price of our
common stock and the extent to which we are able to raise capital from other sources. Assuming a purchase price of $4.41 per share (which
represents the closing price of our common stock on November 12, 2024), the purchase by Lincoln Park of the entire 2,612,386 shares of
common stock issuable under the Purchase Agreement being registered for resale by Lincoln Park hereunder would result in gross proceeds
to us of approximately $11.5 million. If obtaining sufficient funding from Lincoln Park were to prove unavailable or prohibitively dilutive,
we will need to secure another source of capital in order to satisfy our working capital needs. Even if we sell all $15.0 million of
shares of our common stock to Lincoln Park under the Purchase Agreement, we will need to raise substantial additional capital to continue
to fund our operations and execute our current business strategy.
If
we fail to regain and maintain compliance with the continued listing requirements of The Nasdaq Capital Market, our common stock could
be suspended and delisted, which could, among other things, limit demand for our common stock, substantially impair our ability to raise
additional capital and have an adverse effect on the market price of, and the efficiency of the trading market for, our common stock.
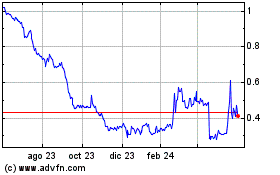
Our
common stock is listed on The Nasdaq Capital Market. On August 12, 2024, we received written notice from Nasdaq notifying us that we
do not meet the requirement in Nasdaq Listing Rule 5550(b)(2) to maintain a minimum Market Value of Listed Securities, or MVLS, of $35.0
million that is required for continued listing on The Nasdaq Capital Market. The notice has no effect at this time on the listing of
our common stock on The Nasdaq Capital Market.
We
have a period of 180 days, or until February 10, 2025, to regain compliance with the minimum MVLS rule. We will regain compliance if
at any time during the 180-day period, our MVLS closes at $35.0 million or more for a minimum of 10 consecutive business days. If we
do not regain compliance prior to February 10, 2025, Nasdaq will notify us that our securities are subject to delisting, at which time
we may appeal the delisting determination to a Nasdaq hearings panel. There can be no assurance that, if we were to appeal the delisting
determination, such an appeal would be successful.
There
are many factors that affect the trading price of our common stock, and many of those factors are outside of our control. We intend to
actively monitor our MVLS and may, if appropriate, consider implementing available options to regain compliance with the minimum MVLS
rule. There can be no assurance that we will be able to regain compliance with such rule or that we will be able to satisfy all other
continued listing requirements of The Nasdaq Capital Market and maintain the listing of our common stock on The Nasdaq Capital Market
even if we regain compliance with the minimum MVLS rule. For example, until we regained compliance on July 18, 2024, we were not in compliance
with the continued listing standard commonly referred to as the minimum bid price rule since July 19, 2023.
The
suspension or delisting of our common stock, for whatever reason, could, among other things, substantially impair our ability to raise
additional capital; result in the loss of interest from institutional investors, the loss of confidence in our company by investors and
employees, and in fewer financing, strategic and business development opportunities; and result in potential breaches of agreements under
which we made representations or covenants relating to our compliance with applicable listing requirements. Claims related to any such
breaches, with or without merit, could result in costly litigation, significant liabilities and diversion of our management’s time
and attention and could have a material adverse effect on our financial condition, business and results of operations. In addition, the
suspension or delisting of our common stock, for whatever reason, may materially impair our stockholders’ ability to buy and sell
shares of our common stock and could have an adverse effect on the market price of, and the efficiency of the trading market for, our
common stock.
The
sale of our common stock in ATM offerings may cause substantial dilution to our existing stockholders, and such sales, or the anticipation
of such sales, may cause the price of our common stock to decline.
We
have used at-the-market, or ATM, offerings to fund a significant portion of our operations in prior years, and we may continue to use
ATM offerings to raise additional capital in the future. For example, in 2021, we sold an aggregate of approximately 3.0 million shares
of our common stock in ATM offerings. We sold substantially fewer shares in ATM offerings in 2022 and 2023 and to-date in 2024, however,
we may sell significant amounts of shares in ATM offerings again in the future. While sales of shares of our common stock in ATM offerings
may enable us to raise capital at a lower cost compared with other types of equity financing transactions; such sales may result in substantial
dilution to our existing stockholders, and such sales, or the anticipation of such sales, may cause the trading price of our common stock
to decline.
Our
management will have broad discretion over the use of the net proceeds, if any, from sales of shares of our common stock to Lincoln Park,
you may not agree with how we use the proceeds and the proceeds may not be used effectively.
This
prospectus relates to shares of our common stock that may be offered and sold from time to time by Lincoln Park. We will not receive
any proceeds upon the sale of shares by Lincoln Park. However, we may receive gross proceeds of up to $15.0 million from the sale of
shares under the Purchase Agreement to Lincoln Park. The anticipated use of net proceeds from the sale of our common stock to Lincoln
Park under the Purchase Agreement represents our intentions based upon our current plans and business conditions. Because we have not
designated the amount of net proceeds from the sale of shares under the Purchase Agreement to be used for any particular purpose, our
management will have broad discretion as to the use of the net proceeds from our sale of shares of common stock to Lincoln Park. Accordingly,
you will be relying on the judgment of our management with regard to the use of those net proceeds, and you will not have the opportunity,
as part of your investment decision, to assess whether the proceeds are being used appropriately. It is possible that, pending their
use, we may invest those net proceeds in a way that does not yield a favorable, or any, return for us. Further, our management may use
the net proceeds for corporate purposes that may not improve our financial condition or market value. The failure of our management to
use such funds effectively could have a material adverse effect on our business, financial condition, operating results and cash flows.
SPECIAL
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus and the documents incorporated by reference in this prospectus contain or incorporate by reference forward-looking statements
that involve substantial risks and uncertainties. All statements, other than statements of historical fact, including statements regarding
our strategy, future operations, future financial position, projected revenue, funding and expenses, prospects, plans and objectives
of management, are forward-looking statements. Forward-looking statements, in some cases, can be identified by terms such as “believe,”
“may,” “will,” “estimate,” “continue,” “anticipate,” “design,”
“intend,” “expect,” “could,” “plan,” “potential,” “predict,”
“seek,” “pursue,” “should,” “would,” “contemplate,” “project,”
“target,” “tend to,” or the negative version of these words and similar expressions.
Forward-looking
statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements
to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements,
including those factors described under the headings “Risk Factors” of this prospectus and in our Annual Report on Form 10-K
for the year ended December 31, 2023 and in our subsequent Quarterly Reports on Form 10-Q, all of which are incorporated herein by reference,
as may be updated or superseded by the risks and uncertainties described under similar headings in the other documents that are filed
after the date hereof and incorporated by reference into this prospectus. Given these uncertainties, you should not place undue reliance
on any forward-looking statement. The following factors are among those that may cause such differences:
● Inability
to raise additional capital, under favorable terms or at all, to fund our operating needs and continue as a going concern;
● The
number and scope of product development programs we pursue;
● Difficulties
or delays in commencement or completion, or the termination or suspension, of our current or planned clinical or preclinical studies;
● Clinical
trial outcomes and results of preclinical development;
● Failure
to complete development of our product candidates or submit and obtain FDA or foreign
regulatory authority approval for our product candidates on projected timelines or budgets, or at all;
● Challenges
and delays in obtaining timely supplies of our product candidates, including their components as well as the finished product, in the
quantities needed in accordance with current good manufacturing practices, our specifications and other applicable requirements;
● The
performance of third parties on which we rely to conduct nonclinical studies and clinical trials of our product candidates;
● Our
failure, or a failure of a strategic collaborator, to successfully commercialize our product candidates, if approved, or our failure
to otherwise monetize our portfolio programs and assets;
● Termination
by a collaborator of our respective out-license agreements for commercialization of XACIATO and Ovaprene®, or, in the case of Ovaprene, a decision by the collaborator not to make the license grant fully effective
following its review of the results of the ongoing pivotal clinical trial of Ovaprene;
●
The timing and amount of future royalty, milestone or other payments to us, if any, under our out-license agreement for Ovaprene, and
of upside-sharing milestone payments from XOMA (US) LLC under our traditional and synthetic royalty purchase agreements, if any;
● The
performance of third parties on which we rely to commercialize, or assist us in commercializing, XACIATO and any future product;
● Difficulties
with maintaining existing collaborations relating to the development and/or commercialization of our product candidates, or establishing
new ones on a timely basis or on acceptable terms, or at all;
● The
terms and conditions of any future strategic collaborations relating to our product candidates;
● The
degree of market acceptance that XACIATO and any future product achieves;
● Coverage
and reimbursement levels for XACIATO and any future product by government health care programs, private health insurance companies and
other third-party payors;
● Our
loss of, or inability to attract, key personnel;
● A
change in the FDA’s prior determination that the Center for Devices and Radiological Health would lead the review of a premarket
approval application for potential marketing approval of Ovaprene;
● A
change in regulatory requirements for our product candidates, including the development pathway pursuant to Section 505(b)(2) of the
Federal Food, Drug, and Cosmetic Act, or the FDA’s 505(b)(2) pathway;
● Unfavorable
differences between preliminary, interim or topline clinical study data reported by us and final study results;
● Communication
from the FDA or another regulatory authority, including a complete response letter, that such agency does not accept or agree with our
assumptions, estimates, calculations, conclusions or analyses of clinical or nonclinical study data regarding a product candidate, or
that such agency interprets or weighs the importance of study data differently than we have in a manner that negatively impacts the candidate’s
prospects for regulatory approval in a timely manner, or at all;
● Failure
to select product candidates that capitalize on the most scientifically, clinically or commercially promising or profitable indications
or therapeutic areas within women’s health including due to our limited financial resources;
● Loss
or impairment of our in-licensed rights to develop and commercialize XACIATO and our product candidates;
● The
timing and amount of our payment and other obligations under our in-license and acquisition agreements for XACIATO and our product candidates;
● Developments
by our competitors that make XACIATO, or any potential product we develop, less competitive or obsolete;
● Unfavorable
or unanticipated macroeconomic factors, geopolitical events or conflicts, public health emergencies, or natural disasters;
● Weak
interest in women’s health relative to other healthcare sectors from the investment community or from pharmaceutical companies
and other potential development and commercialization collaborators;
● Cyber-attacks,
security breaches or similar events compromising our technology systems and data, our financial resources and other assets, or the technology
systems and data of third parties on which we rely;
● Difficulty
in introducing branded products in a market made up of generic products;
● Inability
to adequately protect or enforce our, or our licensor’s, intellectual property rights;
● Lack
of patent protection for the active ingredients in XACIATO and certain of our product candidates that expose them to competition from
other formulations using the same active ingredients;
● Higher
risk of failure associated with product candidates in preclinical stages of development that may lead investors to assign them little
to no value and make these assets difficult to fund;
● Dependence
on grants and other financial awards from governmental entities and private foundations to advance the development of several of our
product candidates;
● Disputes
or other developments concerning our intellectual property rights;
● Actual
and anticipated fluctuations in our quarterly or annual operating results or results that differ from investors’ expectations for
such results;
● Price
and volume fluctuations in the stock market, and in our stock in particular, which could cause investors to experience losses and subject
us to securities class-action litigation;
● Failure
to maintain the listing of our common stock on The Nasdaq Capital Market or another nationally recognized exchange;
● Development
of safety, efficacy or quality concerns related to our product or product candidates (or third-party products or product candidates that
share similar characteristics or drug substances), whether or not scientifically justified, leading to delays in or discontinuation of
product development, product recalls or withdrawals, diminished sales, and/or other significant negative consequences;
● Product
liability claims or governmental investigations;
● Changes
in government laws and regulations in the United States and other jurisdictions, including laws and regulations governing the research,
development, approval, clearance, manufacturing, supply, distribution, pricing and/or marketing of our products, product candidates and
related intellectual property, health care information and data privacy and security laws, transparency laws and fraud and abuse laws,
and the enforcement thereof affecting our business; and
● Increased
costs as a result of operating as a public company, and substantial time devoted by our management to compliance initiatives and corporate
governance practices.
In
addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These
statements are based upon information available to us as of the date they are made, and while we believe such information forms a reasonable
basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have
conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain
and investors are cautioned not to unduly rely upon these statements.
Investors
are cautioned not to place undue reliance on forward-looking statements, which speak only as of the date of this prospectus or the date
of the document incorporated by reference in this prospectus. We do not undertake any obligation to publicly update any forward-looking
statement to reflect events or circumstances after the date on which any statement is made or to reflect the occurrence of unanticipated
events, except as required by law.
OUR
AGREEMENTS WITH LINCOLN PARK
Overview
On
October 21, 2024, we entered into the Purchase Agreement and the Registration Rights Agreement with Lincoln Park. Pursuant to the terms
of the Purchase Agreement, Lincoln Park has agreed to purchase from us up to $15,000,000 of our common stock (subject to certain limitations)
from time to time during the 24-month term of the Purchase Agreement. Pursuant to the terms of the Registration Rights Agreement, we
filed with the SEC the registration statement of which this prospectus forms a part to register for resale under the Securities Act shares
that have been or may be issued to Lincoln Park under the Purchase Agreement.
We
do not have the right to commence sales of our shares of common stock to Lincoln Park under the Purchase Agreement until the Commencement
has occurred, which is the time at which all of the conditions set forth in the Purchase Agreement, all of which are outside of Lincoln
Park’s control, have been satisfied, including that the SEC has declared effective the registration statement of which this prospectus
forms a part. On or after the Commencement, from time to time, at our sole discretion, we may direct Lincoln Park to purchase shares
of our common stock in amounts of up to 30,000 shares on any single business day as a Regular Purchase. The amount of a Regular Purchase
may be increased to up to 40,000 shares depending on the market price of our common stock at the time we initiate the sale, subject to
a maximum commitment by Lincoln Park of $500,000 per single Regular Purchase. In addition, at our discretion, Lincoln Park has committed
to purchase other “accelerated amounts” and/or “additional accelerated amounts” under certain circumstances.
The purchase price per share sold in any Regular Purchase will be the lower of (i) the lowest sale price of our common stock on the business
day on which we initiate the purchase and (ii) the average of the three lowest closing sale prices of our common stock during the 10
consecutive business day period immediately preceding the business day on which we initiate the purchase. The purchase price per share
will be equitably adjusted for any reorganization, recapitalization, non-cash dividend, stock split, reverse stock split or other
similar transaction occurring during the business days used to compute such price. Lincoln Park may not assign or transfer its rights
and obligations under the Purchase Agreement.
Under
applicable rules of The Nasdaq Stock Market, in no event may we issue or sell to Lincoln Park under the Purchase Agreement more than
19.99% of the shares of our common stock outstanding immediately prior to the execution of the Purchase Agreement (which is 1,711,172
shares based on 8,555,864 shares outstanding immediately prior to the execution of the Purchase Agreement), unless (i) we obtain stockholder
approval to issue shares of common stock in excess of the Exchange Cap or (ii) the average price per share of all applicable sales of
our common stock to Lincoln Park under the Purchase Agreement equals or exceeds $3.59 (which represents the lower of (A) the official
closing price of our common stock on Nasdaq immediately preceding the signing of the Purchase Agreement and (B) the average official
closing price of our common stock on Nasdaq for the five consecutive trading days ending on the trading day immediately preceding the
date of the Purchase Agreement), such that issuances and sales of our common stock to Lincoln Park under the Purchase Agreement would
not be subject to the Exchange Cap under applicable Nasdaq rules. In any event, the Purchase Agreement specifically provides that we
may not issue or sell any shares of our common stock under the Purchase Agreement if such issuance or sale would breach any applicable
Nasdaq rules.
The
Purchase Agreement also prohibits us from directing Lincoln Park to purchase any shares of common stock if those shares, when aggregated
with all other shares of our common stock then beneficially owned by Lincoln Park and its affiliates, would result in Lincoln Park exceeding
the Beneficial Ownership Cap.
On
October 21, 2024, the day we entered into the Purchase Agreement, we issued the Commitment Shares to Lincoln Park in consideration for
its commitment to purchase shares of our common stock under the Purchase Agreement.
Purchase
of Shares Under the Purchase Agreement
On
or after the Commencement, from time to time, at our sole discretion, on any business day selected by us on which the closing sale price
of the common stock is not below $0.50 per share, we may direct Lincoln Park to purchase shares of our common stock in amounts up to
30,000 shares on any single business day as a Regular Purchase; provided that such share limit increases to up to 35,000 shares if the
closing sale price of our common stock is not below $5.00 per share on the business day on which we initiate the purchase, and
to up to 40,000 shares if the closing sale price of our common stock is not below $7.50 per share on the business day on which we initiate
the purchase. We refer to such share amount limitation in this prospectus as the “Regular Purchase Share Limit.” In any
case, Lincoln Park’s maximum commitment in any single Regular Purchase may not exceed $500,000. The Regular Purchase Share
Limit is subject to proportionate adjustment in the event of a reorganization, recapitalization, non-cash dividend, stock split, reverse
stock split, or other similar transaction; provided, that if, after giving effect to such full proportionate adjustment, the adjusted
Regular Purchase Share Limit would preclude us from requiring Lincoln Park to purchase common stock at an aggregate purchase price equal
to or greater than $50,000 in any single Regular Purchase, then the Regular Purchase Share Limit will not be fully adjusted, but rather
the Regular Purchase Share Limit for such Regular Purchase shall be adjusted as specified in the Purchase Agreement, such that, after
giving effect to such adjustment, the Regular Purchase Share Limit will be equal to (or as close as can be derived from such adjustment
without exceeding) $50,000.
The
purchase price per share for the shares that may be sold to Lincoln Park in any Regular Purchase will be the lesser of:
| ● | the
lowest sale price of our common stock on the business day on which we initiate the purchase;
or |
| ● | the
arithmetic average of the three lowest closing sale prices for our common stock during the
10-consecutive-business-day period immediately preceding the business day on which we initiate
the purchase. |
In
addition to Regular Purchases, on any business day on which we have properly submitted a notice directing Lincoln Park to purchase the
then applicable Regular Purchase Share Limit and properly delivered all the shares purchased in all prior purchases under the Purchase
Agreement, we may direct Lincoln Park to purchase an additional amount of shares of our common stock, which we refer to as an “Accelerated
Purchase,” not to exceed the lesser of:
| ● | 30%
of the total volume of shares of our common stock traded during the Accelerated Purchase
Period (as defined in the Purchase Agreement); and |
| ● | three
times the number of shares of common stock purchased pursuant to the corresponding Regular
Purchase. |
We
may also direct Lincoln Park, by notice delivered before 1:00 p.m. Eastern time on a business day on which an Accelerated Purchase has
been completed and all of the shares to be purchased thereunder (and under the corresponding Regular Purchase) have been properly delivered
to Lincoln Park in accordance with the Purchase Agreement prior to such time on such business day, to purchase an additional amount of
shares of our common stock, which we refer to as an Additional Accelerated Purchase, of up to the lesser of:
| ● | 30%
of the total volume of shares of our common stock traded during the Additional Accelerated
Purchase Period (as defined in the Purchase Agreement); and |
| ● | three
times the number of shares of common stock purchased pursuant to the corresponding Regular
Purchase. |
We
may, in our sole discretion, submit multiple Additional Accelerated Purchase notices to Lincoln Park on a single Accelerated Purchase
date, provided that all prior Accelerated Purchases and Additional Accelerated Purchases (including those that have occurred earlier
on the same day) have been completed and all of the shares to be purchased thereunder (and under the corresponding Regular Purchase)
have been properly delivered to Lincoln Park in accordance with the Purchase Agreement.
The
purchase price per share for each Accelerated Purchase and each Additional Accelerated Purchase will be equal to 95% of the lesser of:
| ● | the
volume weighted average price of our common stock during the applicable Accelerated Purchase
Period or Additional Accelerated Purchase Period; and |
| ● | the
closing sale price of our common stock on the applicable Accelerated Purchase date. |
In
the case of any Regular Purchase, Accelerated Purchase and Additional Accelerated Purchase, the purchase price per share will be equitably
adjusted for any reorganization, recapitalization, non-cash dividend, stock split, reverse stock split or other similar transaction
occurring during the business days used to compute such price.
Other
than as described above, there are no trading volume requirements or restrictions under the Purchase Agreement, and we will control the
timing and amount of any sales of our common stock to Lincoln Park.
Suspension
Events
Suspension
Events under the Purchase Agreement include,
among others, the following:
| ● | a
lapse in the effectiveness of the registration statement of which this prospectus forms a
party for any reason (including, without limitation, the issuance of a stop order or similar
order) or any required prospectus is unavailable for the resale by Lincoln Park of the shares
of our common stock offered hereby and such lapse or unavailability continues for a period
of 10 consecutive business days or for more than an aggregate of 30 business days in any
365-day period, subject to certain exceptions described in the Purchase Agreement; |
| ● | the
suspension of our common stock from trading on The Nasdaq Capital Market (or such other nationally
recognized market or exchange on which our common stock may be listed or traded) for a period
of one business day; |
| ● | the
delisting of our common stock from The Nasdaq Capital Market, unless our common stock is
immediately thereafter trading or quoted on the New York Stock Exchange, The Nasdaq Global
Market, The Nasdaq Global Select Market, the NYSE American, the NYSE Arca, the OTCQX Best
Market or the OTCQB Venture Market operated by OTC Markets Group, Inc. (or any nationally
recognized successor to any of the foregoing); |
| ● | the
failure by our transfer agent to issue to Lincoln Park the shares purchased by Lincoln Park
under the Purchase Agreement within two business days after the applicable date on which
Lincoln Park is entitled to receive such shares; |
| | | |
| ● | any
breach by us of any of our representations, warranties or covenants in the Purchase Agreement
or Registration Rights Agreement if such breach would reasonably be expected to have a Material
Adverse Effect (as defined in the Purchase Agreement) and, in the case of a breach of
a covenant that is reasonably curable, only if such breach continues for a period of at least
five business days; |
| | | |
| ● | we
become insolvent or we, voluntarily or involuntarily, become the subject of any insolvency
or bankruptcy proceeding or any such proceeding is threatened against us, as more fully described
in the Purchase Agreement; |
| | | |
| ● | if
at any time we are not eligible to transfer shares of our stock electronically through the
Depository Trust Company’s (DTC) Deposit and Withdrawal at Custodian (DWAC) service,
or any similar program hereafter adopted by DTC performing substantially the same function;
or |
| | | |
| ● | if
at any time the Exchange Cap is reached (to the extent applicable) and our stockholders have
not approved the issuance and sale to Lincoln Park of shares in excess of the Exchange Cap. |
Lincoln
Park does not have the right to terminate the Purchase Agreement upon any of the Suspension Events set forth above. During a
Suspension Event, or if any event has occurred which, after notice and/or lapse of time, would reasonably be expected to become a
Suspension Event under the Purchase Agreement, all of which are outside of Lincoln Park’s control, we may not direct Lincoln
Park to purchase any shares of our common stock under the Purchase Agreement.
Term
of the Purchase Agreement; Termination Rights
The
Purchase Agreement will automatically terminate upon the earlier of (a) the date we sell all $15,000,000 in shares of our common stock
to Lincoln Park under the Purchase Agreement, and (b) the first day of the month immediately following the 24-month anniversary of the Commencement. The Purchase Agreement will also automatically terminate upon commencement of a voluntary or involuntary bankruptcy
proceeding by or against us, as more fully described in the Purchase Agreement.
If
the Commencement shall not have occurred on or before January 31, 2025 due to the failure to satisfy the conditions required for the
Commencement to occur, subject to customary exceptions, either we or Lincoln Park may terminate the Purchase Agreement at the close of
business on such date or thereafter.
After
the Commencement, we may, at any time, for any reason or for no reason, terminate the Purchase Agreement upon one business
day prior notice to Lincoln Park.
No
Short-Selling or Hedging by Lincoln Park
Lincoln
Park has represented to us that at no time prior to the time of execution of the Purchase Agreement has Lincoln Park or its agents, representatives
or affiliates engaged in or effected, in any manner whatsoever, directly or indirectly, any short sale (as such term is defined in Rule
200 of Regulation SHO under the Exchange Act) of our common stock or any hedging transaction, which establishes a net short position
with respect to our common stock. Lincoln Park agreed that during the term of the Purchase Agreement, it, its agents, representatives
or affiliates will not enter into or effect, directly or indirectly, any of the foregoing transactions.
Prohibitions
on Variable Rate Transactions
There
are no restrictions or limitations on our ability to raise capital from other sources at our sole discretion, except that for so long
as the Purchase Agreement is in effect, we agreed not to effect any issuance of, or enter into any agreement to effect any issuance of,
shares of common stock or common stock equivalents involving a “variable rate transaction,” which is defined in the Purchase
Agreement as an “equity line of credit” or substantially similar transaction whereby an investor is irrevocably bound to
purchase securities over a period of time from us at a price based on the market price of our common stock at the time of each such purchase;
provided that foregoing prohibition does prohibit the issuance and sale of our common stock pursuant to an “at-the-market offering”
by us exclusively through a registered broker-dealer acting as agent of ours pursuant to a written agreement between us and such registered
broker-dealer.
Effect
of Performance of the Purchase Agreement on Our Stockholders
All
shares registered in this offering which have been or may be issued or sold by us to Lincoln Park under the Purchase Agreement are expected
to be freely tradable. Other than the Commitment Shares, which were issued to Lincoln Park on October 21, 2024, the shares registered
in this offering may be sold to Lincoln Park at our discretion over a period of up to 24-months commencing after the satisfaction of
certain conditions set forth in the Purchase Agreement, including that the SEC has declared effective the registration statement of which
this prospectus forms a part. The sale by Lincoln Park of a significant amount of the shares registered in this offering at any given
time could cause the market price of our common stock to decline and to be highly volatile. Sales of our common stock to Lincoln Park,
if any, will depend upon market conditions and other factors to be determined by us. We may ultimately decide to sell to Lincoln Park
all, some or none of the shares of our common stock that may be available for us to sell pursuant to the Purchase Agreement. If and when
we do sell shares to Lincoln Park, after Lincoln Park has acquired the shares, Lincoln Park may resell all, some or none of those shares
at any time or from time to time in its discretion. Therefore, sales to Lincoln Park by us under the Purchase Agreement may result in
substantial dilution to the interests of other holders of our common stock. In addition, if we sell a substantial number of shares to
Lincoln Park under the Purchase Agreement, or if investors expect that we will do so, the actual sales of shares or the mere existence
of our arrangement with Lincoln Park may make it more difficult for us to sell equity or equity-related securities in the future at a
time and at a price that we might otherwise wish to effect such sales. However, we have the right to control the timing and amount of
any additional sales of our shares to Lincoln Park and the Purchase Agreement may be terminated by us at any time at our discretion without
any additional cost to us.
Depending
on the price per share at which we sell our common stock to Lincoln Park pursuant to the Purchase Agreement, we may need to sell more
shares to Lincoln Park than are offered under this prospectus to receive aggregate gross proceeds equal to the $15,000,000 total commitment
of Lincoln Park under the Purchase Agreement, which could cause additional substantial dilution to our stockholders. If we choose to
sell more shares to Lincoln Park than are offered under this prospectus, we must first register for resale under the Securities Act such
additional shares of our common stock. The number of shares ultimately offered for resale by Lincoln Park will depend upon the number
of shares we elect to sell to Lincoln Park under the Purchase Agreement.
The
following table sets forth the amount of gross proceeds we would receive from Lincoln Park from our sale of up to 2,612,386 shares of
our common stock (which excludes the Commitment Shares that we issued to Lincoln Park for which we will receive no cash proceeds) that
we are registering hereby that we may issue and sell to Lincoln Park in the future under the Purchase Agreement at varying purchase prices:
Assumed
Average
Purchase Price
Per Share | | |
Number
of Shares
to be Issued
if Full Purchase (1) | | |
Percentage
of
Outstanding Shares
After Giving Effect
to the Sales to
Lincoln Park (2) | | |
Gross
Proceeds from
the Sales to
Lincoln Park Under
the Purchase
Agreement | |
| $ | 1.00 | | |
| 1,573,558 | | |
| 17 | % | |
$ | 1,573,558 | |
| $ | 2.00 | | |
| 1,573,558 | | |
| 17 | % | |
$ | 3,147,116 | |
| $ | 3.00 | | |
| 1,573,558 | | |
| 17 | % | |
$ | 4,720,674 | |
| $ | 4.00 | | |
| 2,612,386 | | |
| 24 | % | |
$ | 10,449,544 | |
| $ | 4.41 | (3) | |
| 2,612,386 | | |
| 24 | % | |
$ | 11,520,622 | |
| $ | 5.00 | | |
| 2,612,386 | | |
| 24 | % | |
$ | 13,061,930 | |
| $ | 6.00 | | |
| 2,500,000 | | |
| 24 | % | |
$ | 15,000,000 | |
| $ | 7.00 | | |
| 2,142,857 | | |
| 21 | % | |
$ | 14,999,999 | |
| (1) | Although
the Purchase Agreement provides that we may sell up to $15,000,000 of our common stock to
Lincoln Park, we are only registering the Commitment Shares (137,614 shares) and 2,612,386
shares that may be sold to Lincoln Park under this prospectus, which may or may not cover
all the shares we ultimately sell to Lincoln Park under the Purchase Agreement, depending
on the purchase price per share. The number of shares included in this column reflects only
those shares that we are registering in this offering, excluding the Commitment Shares because
no proceeds are attributable to the Commitment Shares, giving effect to the Exchange Cap,
and without regard for the Beneficial Ownership Cap. |
| | |
| (2) | The
denominator is based on 8,700,389 shares outstanding as of November 12, 2024, which includes
the Commitment Shares, adjusted to include the number of shares set forth in the adjacent
column which we would have sold to Lincoln Park, assuming the purchase price in the adjacent
column. The numerator reflects the number of shares set forth in the adjacent column which
we would have sold under the Purchase Agreement at the corresponding assumed average purchase
price set forth in the adjacent column, and the Commitment Shares. |
| | |
| (3) | The
closing sale price of our shares on November 12, 2024. |
USE
OF PROCEEDS
This
prospectus relates to shares of our common stock that may be offered and sold from time to time by Lincoln Park. See “Plan of Distribution”
elsewhere in this prospectus for more information. We are not selling any securities under this prospectus and we will not receive any
proceeds from the sale of shares by Lincoln Park under this prospectus.
We
may receive up to $15,000,000 in aggregate gross proceeds from sales of shares of our common stock we make to Lincoln Park under the
Purchase Agreement. We may choose to sell fewer than $15,000,000 in shares of our common stock, or, due to the Exchange Cap and/or the
Beneficial Ownership Cap, we may not be able to sell all $15,000,000 in shares of our common stock under the Purchase Agreement, in which
case we would raise less than $15,000,000 in aggregate gross proceeds under the Purchase Agreement. It is also possible that we do not
sell any shares under the Purchase Agreement. We did not and will not receive any proceeds from the issuance of the Commitment Shares
to Lincoln Park.
We
will have broad discretion in the use of the net proceeds from any sale of shares of our common stock to Lincoln Park under the Purchase
Agreement. Based upon our current plans and business conditions, we intend to use net proceeds from such sales for working capital and
general corporate purposes, which include, but are not limited to, research and development expenses and general and administrative expenses.
We have not determined the amount of net proceeds to be used specifically for such purposes. The amounts and timing of our actual expenditures
may vary significantly and will depend on numerous factors, including market conditions, cash generated or used by our operations, business
developments and opportunities that may arise. We may find it necessary or advisable to use portions of the proceeds we receive from
our sale of shares of common stock to Lincoln Park under the Purchase Agreement for other purposes. Pending the use of any net proceeds,
we expect to invest the net proceeds in interest-bearing, marketable securities.
We
will bear all of the costs, fees and expenses incurred in effecting the registration of the shares covered by this prospectus, including,
without limitation, the registration and filing fees, printing fees, Nasdaq listing fees and fees and expenses of our counsel and our
accountants, but all selling and other expenses incurred by the selling stockholder will be paid by the selling stockholder.
SELLING
STOCKHOLDER
This
prospectus relates to the possible resale by the selling stockholder, Lincoln Park, of shares of our common stock that have been or may
be issued to Lincoln Park pursuant to the Purchase Agreement. We are filing the registration statement of which this prospectus forms
a part pursuant to the provisions of the Registration Rights Agreement, which we entered into with Lincoln Park on October 21, 2024 concurrently
with our execution of the Purchase Agreement, in which we agreed to provide certain registration rights with respect to resales by Lincoln
Park of the shares of our common stock that have been or may be issued to Lincoln Park under the Purchase Agreement.
Lincoln
Park, as the selling stockholder, may, from time to time, offer and sell pursuant to this prospectus any or all of the shares that we
have issued or may issue and sell to Lincoln Park under the Purchase Agreement. Lincoln Park may sell some, all or none of those shares.
We do not know how long Lincoln Park will hold the shares before selling them, and we currently have no agreements, arrangements or understandings
with Lincoln Park regarding its sale of any of the shares. See “Plan of Distribution.”
The
table below sets forth, to our knowledge, information concerning the beneficial ownership of shares of our common stock by the selling
stockholder as of November 12, 2024. The percentages of shares owned prior to and after the offering are based on 8,700,389 shares of
common stock outstanding as of November 12, 2024, which includes the Commitment Shares we issued to Lincoln Park on October 21, 2024.
The information in the table below with respect to the selling stockholder has been obtained from the selling stockholder. Other than
as described under “Our Agreements With Lincoln Park,” above, neither Lincoln Park nor any of its affiliates has held a position
or office, or had any other material relationship, with us or any of our predecessors or affiliates. Beneficial ownership is determined
in accordance with Section 13(d) of the Exchange Act and Rule 13d-3 thereunder.
| Selling Stockholder | |
Shares
Beneficially Owned Prior to this Offering | | |
Percentage
of Outstanding Shares Beneficially Owned Prior to this Offering | | |
Shares
to be Sold in this Offering Assuming the Company issues the Maximum Number of Shares Under the Purchase Agreement | | |
Percentage
of Outstanding Shares Beneficially Owned After this Offering | |
| Lincoln Park Capital Fund, LLC (1) | |
| 137,614
(2) | | |
| 1.6%
(2) | | |
| 2,750,000
(3) | | |
| | *(4) |
*Less
than 1%
| (1) | Josh
Scheinfeld and Jonathan Cope, the Managing Members of Lincoln Park Capital, LLC, the manager
of Lincoln Park Capital Fund, LLC, are deemed to be beneficial owners of all of the shares
of common stock owned by Lincoln Park Capital Fund, LLC. Messrs. Cope and Scheinfeld have
shared voting and investment power over the shares being offered under this prospectus. Neither
Lincoln Park Capital, LLC nor Lincoln Park Capital Fund, LLC is a licensed broker dealer
or an affiliate of a licensed broker dealer. |
| | |
| (2) | Represents
the Commitment Shares issued to Lincoln Park on October 21, 2024 in consideration for its
commitment to purchase shares of our common stock under the Purchase Agreement. In accordance
with Rule 13d-3(d) of the Exchange Act, we have excluded from the number of shares beneficially
owned prior to this offering all the shares of common stock that we may issue and sell to
Lincoln Park under the Purchase Agreement from and after the Commencement that are being
registered for resale under the registration statement of which this prospectus forms a part,
because the issuance of such shares is solely at our discretion and is subject to certain
conditions, the satisfaction of all of which are outside of Lincoln Park’s control,
including the registration statement of which this prospectus forms a part becoming and remaining
effective. Furthermore, under the terms of the Purchase Agreement, issuances and sales of
shares of our common stock to Lincoln Park are subject to certain limitations on the amounts
we may sell to Lincoln Park at any time, including the Exchange Cap and the Beneficial Ownership
Cap. See the description under the heading “Our Agreements with Lincoln Park”
for more information about the Purchase Agreement. |
| (3) | Assumes
issuance of the maximum 2,750,000 shares being registered hereby, 137,614 of which were issued
to Lincoln Park on October 21, 2024. Although the Purchase Agreement provides that we may
sell up to $15,000,000 in shares of our common stock to Lincoln Park, only 2,612,386 shares
of our common stock are being offered under this prospectus that may be sold by us to Lincoln
Park at our discretion from time to time over a 24-month period following the Commencement.
Depending on the price per share at which we sell our common stock to Lincoln Park pursuant
to the Purchase Agreement, we may need to sell to Lincoln Park under the Purchase Agreement
more shares of our common stock than are offered under this prospectus to receive aggregate
gross proceeds equal to the $15,000,000 total commitment of Lincoln Park under the Purchase
Agreement. If we choose to do so, we must first register for resale under the Securities
Act such additional shares. The number of shares ultimately offered for resale by Lincoln
Park will depend upon the number of shares we elect to sell to Lincoln Park under the Purchase
Agreement. |
| | |
| (4) | Assumes
the sale by Lincoln Park of all 2,750,000 shares of our common stock registered hereby, although
the selling stockholder is under no obligation known to us to sell any shares of common stock
at any particular time. |
DILUTION
Our
sale of shares of our common stock to Lincoln Park pursuant to the Purchase Agreement will have a dilutive impact on our stockholders.
In addition, the lower our stock price is at the time we elect to sell shares to Lincoln Park under the Purchase Agreement, the more
shares of our common stock we will have to sell to Lincoln Park to achieve the same gross proceeds amount, in which case our existing
stockholders would experience greater dilution.
The
amount that Lincoln Park will receive for our common stock when resold pursuant to this prospectus will depend upon the timing of sales
and will fluctuate based on the trading price of our common stock.
Our
net tangible book value (deficit) as of September 30, 2024 was approximately ($1.5 million), or ($0.17) per share of common stock. Net
tangible book value per share is determined by dividing our total tangible assets, excluding goodwill and intangible assets, less total
liabilities, by the number of shares of our common stock outstanding as of September 30, 2024.
After
giving effect to (a) the sale and issuance of 2,612,386 shares of common stock to Lincoln Park pursuant to the Purchase Agreement at
an assumed average sale price of $4.41 per share of common stock (which was the closing sale price of our common stock on November 12,
2024) and deducting estimated offering expenses payable by us of approximately $47,000, and (b) the issuance of 137,614 shares
of our common stock to Lincoln Park on October 21, 2024 in consideration for its commitment to purchase shares of our common stock under
the Purchase Agreement, our as adjusted net tangible book value as of September 30, 2024 would have been approximately $10.0 million,
or $0.90 per share. This represents an immediate increase in net tangible book value of $1.07 per share to existing stockholders
and an immediate dilution of $3.51 per share to new investors. Dilution in net tangible book value per share represents the difference
between the amount per share paid by purchasers of common stock in this offering, assuming a purchase price of $4.41 per share of common
stock (which was the closing sale price of our common stock on November 12, 2024), and our as adjusted net tangible book value per share
after giving effect to this offering.
The
following table illustrates this dilution on a per share basis:
| Assumed offering price per share of common stock | |
| | | |
$ | 4.41 | |
| Net tangible book value (deficit) per share as of September 30, 2024 | |
$ | (0.17 | ) | |
| | |
| Increase in net tangible book value per share attributable to this offering | |
$ | 1.07 | | |
| | |
| As adjusted net tangible book value per share after giving effect to this offering | |
| | | |
$ | 0.90 | |
| Dilution per share to new investors | |
| | | |
$ | 3.51 | |
The
number of shares of our common stock to be outstanding as shown above is based on 8,546,364 shares outstanding as of September 30, 2024,
and excludes:
| ● | 137,614
shares issued to Lincoln Park on October 21, 2024 in consideration for its commitment to
purchase shares under the Purchase Agreement; |
| | | |
| ● | 903,424
shares of common stock issuable upon exercise of stock options outstanding as of September
30, 2024, with a weighted-average exercise price of $7.36 per share; |
| | | |
| ● | 445,661
shares of common stock reserved and available for future issuance as of September 30, 2024
under our equity incentive plans; and |
| | | |
| ● | 1,268,572
shares of common stock issuable upon exercise of warrants outstanding as of September 30,
2024, with a weighted-average exercise price of $7.49 per share; and |
| | | |
| ● | 16,408
shares of common stock issued after September 30, 2024 pursuant to our “at the market”
offering program. |
To
the extent that outstanding options or warrants have been or may be exercised, new equity awards were are or issued, shares of our common
stock are sold under our employee stock purchase plan, or we otherwise issued or issue additional shares of common stock, including in
our “at the market” offering program, investors purchasing our common stock in this offering may experience further dilution.
PLAN
OF DISTRIBUTION
The
shares of our common stock offered by this prospectus are being offered by the selling stockholder, Lincoln Park. The shares may be sold
or distributed from time to time by the selling stockholder directly to one or more purchasers or through brokers, dealers, or underwriters
who may act solely as agents at market prices prevailing at the time of sale, at prices related to the prevailing market prices, at negotiated
prices, or at fixed prices, which may be changed. The sale of the shares offered by this prospectus could be effected in one or more
of the following methods:
| ● | ordinary
brokers’ transactions; |
| | | |
| ● | transactions
involving cross or block trades; |
| | | |
| ● | through
brokers, dealers, or underwriters who may act solely as agents; |
| | | |
| ● | “at
the market” into an existing market for the shares of our common stock; |
| | | |
| ● | in
other ways not involving market makers or established business markets, including direct
sales to purchasers or sales effected through agents; |
| | | |
| ● | in
privately negotiated transactions; or |
| | | |
| ● | any
combination of the foregoing. |
In
order to comply with the securities laws of certain states, if applicable, the shares of our common stock offered by this prospectus
may be sold only through registered or licensed brokers or dealers. In addition, in certain states, the shares of our common stock offered
by this prospectus may not be sold unless they have been registered or qualified for sale in the state or an exemption from the state’s
registration or qualification requirement is available and complied with.
Lincoln
Park is an “underwriter” within the meaning of Section 2(a)(11) of the Securities Act.
Lincoln
Park has informed us that it intends to use an unaffiliated broker-dealer to effectuate all sales, if any, of the common stock that it
has acquired and may purchase from us pursuant to the Purchase Agreement. Such sales will be made at prices and at terms then prevailing
or at prices related to the then current market price. Each such unaffiliated broker-dealer will be an underwriter within the meaning
of Section 2(a)(11) of the Securities Act. Lincoln Park has informed us that each such broker-dealer will receive commissions from Lincoln
Park that will not exceed customary brokerage commissions.
Brokers,
dealers, underwriters or agents participating in the distribution of the shares of our common stock offered by this prospectus may receive
compensation in the form of commissions, discounts, or concessions from the purchasers, for whom the broker-dealers may act as agent,
of shares of our common stock sold by Lincoln Park through this prospectus. The compensation paid to any such particular broker-dealer
by any such purchasers of shares of our common stock sold by Lincoln Park may be less than or in excess of customary commissions. Neither
we nor Lincoln Park can presently estimate the amount of compensation that any agent will receive from any purchasers of shares of our
common stock sold by Lincoln Park.
We
know of no existing arrangements between Lincoln Park or any other stockholder, broker, dealer, underwriter or agent relating to the
sale or distribution of the shares offered by this prospectus.
We
may from time to time file with the SEC one or more supplements to this prospectus or amendments to the registration statement of which
this prospectus forms a part to amend, supplement or update information contained in this prospectus, including, if and when required
under the Securities Act, to disclose certain information relating to a particular sale of shares of our common stock offered by this
prospectus by the selling stockholder, including the names of any brokers, dealers, underwriters or agents participating in the distribution
of such shares of our common stock by the selling stockholder, any compensation paid by Lincoln Park to any such brokers, dealers, underwriters
or agents, and any other required information.
We
will pay the expenses incident to the registration under the Securities Act of the offer and sale of the shares of our common stock covered
by this prospectus by Lincoln Park. We estimate the total of such expenses will be approximately $47,000.
We
have agreed to indemnify Lincoln Park and certain other persons against certain liabilities in connection with the offering of shares
of common stock offered hereby, including liabilities arising under the Securities Act or, if such indemnity is unavailable, to contribute
amounts required to be paid in respect of such liabilities. Lincoln Park has agreed to indemnify us against liabilities under the Securities
Act that may arise from certain written information furnished to us by Lincoln Park specifically for use in this prospectus or, if such
indemnity is unavailable, to contribute amounts required to be paid in respect of such liabilities.
Lincoln
Park has represented to us that at no time prior to the Purchase Agreement has Lincoln Park or its agents, representatives or affiliates
engaged in or effected, in any manner whatsoever, directly or indirectly, any short sale (as such term is defined in Rule 200 of Regulation
SHO of the Exchange Act) of our common stock or any hedging transaction, which establishes a net short position with respect to our common
stock. Lincoln Park agreed that during the term of the Purchase Agreement, it, its agents, representatives or affiliates will not enter
into or effect, directly or indirectly, any of the foregoing transactions.
We
have advised Lincoln Park that it is required to comply with Regulation M promulgated under the Exchange Act. With certain exceptions,
Regulation M precludes the selling stockholder, any affiliated purchasers, and any broker-dealer or other person who participates in
the distribution from bidding for or purchasing, or attempting to induce any person to bid for or purchase any security which is the
subject of the distribution until the entire distribution is complete. Regulation M also prohibits any bids or purchases made in order
to stabilize the price of a security in connection with the distribution of that security. All of the foregoing may affect the marketability
of the securities offered by this prospectus.
This
offering will terminate on the earlier of (i) termination of the Purchase Agreement or (ii) the date that all shares offered by this
prospectus have been sold by Lincoln Park.
LEGAL
MATTERS
Sheppard,
Mullin, Richter & Hampton LLP, San Diego, California, will pass upon the validity of the common stock being offered by this prospectus.
EXPERTS
Haskell
& White LLP, an independent registered public accounting firm, has audited our consolidated financial statements included in our
Annual Report on Form 10-K for the year ended December 31, 2023, as set forth in its report (which report includes an explanatory
paragraph regarding the existence of substantial doubt about our ability to continue as a going concern), which is incorporated by
reference in this prospectus and the registration statement. Our financial statements are incorporated by reference in reliance on Haskell
& White LLP’s report, given on the authority of said firm as experts in accounting and auditing.
CBIZ
CPAs P.C. (formerly Mayer Hoffman McCann P.C.),
an independent registered public accounting firm, has audited our consolidated financial statements included in our Annual Report
on Form 10-K for the year ended December 31, 2022, as set forth in its report (which report includes an explanatory paragraph regarding
the existence of substantial doubt about our ability to continue as a going concern), which is incorporated by reference in this prospectus
and the registration statement. Our financial statements are incorporated by reference in reliance on CBIZ CPAs P.C.’s report,
given on the authority of said firm as experts in accounting and auditing.
WHERE
YOU CAN FIND MORE INFORMATION
We
are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and file annual, quarterly
and current reports, proxy statements and other information with the SEC. The SEC maintains a website that contains reports, proxy and
information statements, and other information regarding issuers, such as our company, that file documents electronically with the SEC.
Our SEC filings are available to the public at the SEC’s website address at http://www.sec.gov. You may also request a copy
of these filings, at no cost, by writing us at the address below or telephoning us at number below.
We
also maintain a website at www.darebioscience.com, through which you can access our SEC filings. The information set forth on our website
is not part of this prospectus.
This
prospectus is only part of a registration statement on Form S-1 that we have filed with the SEC. This prospectus omits some information
contained in the registration statement in accordance with SEC rules and regulations. You should review the information in and schedules
and/or exhibits to the registration statement for further information about us and the securities being offered pursuant to this prospectus.
Statements in this prospectus concerning any document we filed as an exhibit or schedule to the registration statement or that we otherwise
filed with the SEC are not intended to be comprehensive and are qualified by reference to these filings. You should review the complete
document to evaluate these statements.
INCORPORATION
OF CERTAIN INFORMATION BY REFERENCE
The
SEC allows us to “incorporate by reference” into this prospectus information from other documents that we file with the SEC,
which means that we can disclose important information to you by referring you to those documents. The information that we incorporate
by reference into this prospectus is an important part of this prospectus. This prospectus incorporates by reference the documents listed
below and any future filings we make with the SEC pursuant to Sections 13(a), 13,(c), 14 or 15(d) of the Exchange Act after the effective
date of the registration statement of which this prospectus is a part and prior to the termination or completion of this offering, other
than information furnished to, rather than filed with the SEC:
| ● | our
Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with the SEC
on March 28, 2024 (the “Annual Report”), including all material incorporated
by reference therein, which includes the portions of our definitive proxy statement on Schedule 14A filed with the SEC on April 26, 2024 incorporated by reference into Part III of the Annual
Report; |
| | | |
| ● | our
Quarterly Reports on Form 10-Q for the quarters ended March 31, 2024, June 30, 2024 and September
30, 2024, filed with the SEC on May 14, 2024, August 12, 2024 and November 14, 2024, respectively; |
| ● | our
Current Reports on Form 8-K filed with the SEC on January 19, 2024, January 26, 2024, April 30, 2024, June 7, 2024, June 27, 2024, July 19, 2024, October 21, 2024, and October 23, 2024
(except for the information furnished under Items 2.02 or 7.01 and the exhibits furnished
thereto); |
| | | |
| ● | the
description of our common stock contained in our Registration Statement on Form 8-A filed
on April 4, 2014, including any amendments thereto or reports filed for the purpose of updating
such description, including the description of our common stock in Exhibit 4.6 of the Annual
Report; |
The
SEC file number for each of the documents listed above is 001-36395.
Any
statement contained in this prospectus or in a document incorporated or deemed to be incorporated by reference into this prospectus will
be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained in this prospectus or
any other subsequently filed document that is deemed to be incorporated by reference into this prospectus modifies or supersedes the
statement. Any statement so modified or superseded will not be deemed, except as so modified or superseded, to constitute a part of this
prospectus.
We
will provide to each person, including any beneficial owner, to whom this prospectus is delivered, upon written or oral request and at
no cost to the requester, a copy of any or all reports or documents that are incorporated by reference into this prospectus, but not
delivered with the prospectus. Such written or oral requests should be directed to:
Daré
Bioscience, Inc.
3655
Nobel Drive, Suite 260
San
Diego, CA 92122
Attn:
Chief Accounting Officer
Telephone:
(858) 926-7655
You
may also access these incorporated reports and other documents on our website, www.darebioscience.com. The information contained on,
or that can be accessed through, our website is not a part of this prospectus. We have included our website address in this prospectus
solely as an inactive textual reference.
You
should rely only on information contained in, or incorporated by reference into, this prospectus and any related prospectus supplement.
We have not authorized anyone to provide you with information different from that contained in this prospectus or incorporated by reference
in this prospectus. We are not making offers to sell the securities in any jurisdiction in which such an offer or solicitation is not
authorized or in which the person making such offer or solicitation is not qualified to do so or to anyone to whom it is unlawful to
make such offer or solicitation.
DISCLOSURE
OF COMMISSION POSITION ON INDEMNIFICATION FOR SECURITIES ACT LIABILITY
Insofar
as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling the
registrant pursuant to the foregoing provisions, the registrant has been informed that in the opinion of the SEC such indemnification
is against public policy as expressed in the Securities Act and is therefore unenforceable.

2,750,000
Shares of Common Stock
PROSPECTUS
,
2024
PART
II
INFORMATION
NOT REQUIRED IN PROSPECTUS
Item
13. Other Expenses of Issuance and Distribution
The
following table sets forth the fees and expenses incurred or expected to be incurred by us in connection with the sale and issuance of
the securities being registered hereby. Other than the SEC registration fee, the amounts stated are estimates.
| SEC registration fee | |
$ | 1,734 | |
| Legal fees and expenses | |
| 15,000 | |
| Accounting fees and expenses | |
| 30,000 | |
| Total | |
$ | 46,734 | |
Item
14. Indemnification of Directors and Officers
Delaware
Law
Section
102 of the Delaware General Corporation Law, or the DGCL, permits a corporation to provide in its certificate of incorporation that a
director or officer of the corporation shall not be personally liable to the corporation or its stockholders for monetary damages for
breach of fiduciary duty as a director or officer, except for liability (i) for any breach of the director’s or officer’s
duty of loyalty to the corporation or its stockholders, (ii) for acts or omissions not in good faith or which involve intentional misconduct
or a knowing violation of law, (iii) for payments of unlawful dividends or unlawful stock purchases or redemptions, (iv) for any transaction
from which the director or officer derived an improper personal benefit, or (v) of officers in any action by or in the right of the corporation.
Section
145 of the DGCL provides that a corporation has the power to indemnify a director, officer, employee, or agent of the corporation, or
a person serving at the request of the corporation for another corporation, partnership, joint venture, trust or other enterprise in
related capacities against expenses, including attorneys’ fees, judgments, fines and amounts paid in settlement actually and reasonably
incurred by the person in connection with an action, suit or proceeding to which he was or is a party or is threatened to be made a party
to any threatened, ending or completed action, suit or proceeding by reason of such position, if such person acted in good faith and
in a manner he reasonably believed to be in or not opposed to the best interests of the corporation, and, in any criminal action or proceeding,
had no reasonable cause to believe his or her conduct was unlawful, except that, in the case of actions brought by or in the right of
the corporation, no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been
adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery or other adjudicating court determines
that, despite the adjudication of liability but in view of all of the circumstances of the case, such person is fairly and reasonably
entitled to indemnity for such expenses which the Court of Chancery or such other court shall deem proper.
Restated
Certificate of Incorporation
Our restated certificate of incorporation provides that no director of our corporation shall be personally liable to us or our stockholders
for monetary damages for any breach of fiduciary duty as a director, notwithstanding any provision of law imposing such liability, except
to the extent that the DGCL prohibits the elimination or limitation of liability of directors for breaches of fiduciary duty.
In
addition, our restated certificate of incorporation provides that we will indemnify each individual who was or is a party or threatened
to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative
(other than an action by or in the right of us), by reason of the fact that such individual is or was, or has agreed to become, our director
or officer, or is or was serving, or has agreed to serve, at our request as a director, officer, partner, employee or trustee of, or
in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise (all such individuals being referred
to as an Indemnitee), or by reason of any action alleged to have been taken or omitted in such capacity, against all expenses (including
attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with such action,
suit or proceeding and any appeal therefrom if such Indemnitee acted in good faith and in a manner the Indemnitee reasonably believed
to be in, or not opposed to, our best interests, and, with respect to any criminal action or proceeding, such Indemnitee had no reasonable
cause to believe the Indemnitee’s conduct was unlawful.
Our
restated certificate of incorporation also provides that we will indemnify any Indemnitee who was or is a party to an action or suit
by or in the right of us to procure a judgment in our favor by reason of the fact that the Indemnitee is or was, or has agreed to become,
our director or officer, or is or was serving, or has agreed to serve, at our request as a director, officer, partner, employee or trustee
or, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise, or by reason of any action
alleged to have been taken or omitted in such capacity, against all expenses (including attorneys’ fees) and, to the extent permitted
by law, amounts paid in settlement actually and reasonably incurred by Indemnitee in connection with such action, suit or proceeding
and any appeal therefrom, if the Indemnitee acted in good faith and in a manner which the Indemnitee reasonably believed to be in, or
not opposed to, our best interests, except that no indemnification shall be made in respect of any claim, issue or matter as to which
the Indemnitee shall have been adjudged to be liable to us, unless, and only to the extent, that the Court of Chancery of Delaware or
the court in which such action or suit was brought shall determine upon application that, despite the adjudication of such liability
but in view of all the circumstances of the case, the Indemnitee is fairly and reasonably entitled to indemnity for such expenses (including
attorneys’ fees) which the Court of Chancery of Delaware or such other court shall deem proper. Notwithstanding the foregoing,
to the extent that any Indemnitee has been successful, on the merits or otherwise, such Indemnitee will be indemnified by us against
all expenses (including attorneys’ fees) actually and reasonably incurred by or on behalf of the Indemnitee in connection therewith.
Without limiting the foregoing, if any action, suit or proceeding is disposed of, on the merits or otherwise (including a disposition
without prejudice), without (i) the disposition being adverse to the Indemnitee, (ii) an adjudication that the Indemnitee was liable
to us, (iii) a plea of guilty or nolo contendere by the Indemnitee, (iv) an adjudication that the Indemnitee did not act in good faith
and in a manner the Indemnitee reasonably believed to be in or not opposed to our best interests, and (v) with respect to any criminal
proceeding, an adjudication that Indemnitee had reasonable cause to believe his or her conduct was unlawful, the Indemnitee shall be
considered for the purposes hereof to have been wholly successful with respect thereto. If we do not assume the defense, expenses must
be advanced to an Indemnitee under certain circumstances.
Indemnification
Agreements
We
have entered into indemnification agreements with our directors and executive officers. In general, these agreements provide that we
will indemnify the director or executive officer to the fullest extent permitted by law for claims arising in his or her capacity as
a director or officer of our company or in connection with their service at our request for another entity. The indemnification agreements
also provide for procedures that will apply in the event that a director or executive officer makes a claim for indemnification and establish
certain presumptions that are favorable to the director or executive officer.
We
maintain a general liability insurance policy that covers certain liabilities of directors and officers of our corporation arising out
of claims based on acts or omissions in their capacities as directors or officers.
Insofar
as the forgoing provisions permit indemnification of our directors and officers, or persons controlling us, for liability arising under
the Securities Act we have been informed that, in the opinion of the SEC, such indemnification is against public policy as expressed
in the Securities Act and is therefore unenforceable.
Item
15. Recent Sales of Unregistered Securities
The
following is a summary of all securities that we have sold within the past three years without registration under the Securities Act:
● In December 2023, in connection with entering into a royalty interest financing agreement with United in Endeavour, LLC, we issued to United in Endeavour, LLC a warrant to purchase shares of our common stock. The warrant is exercisable to purchase 422,804 shares of our common stock and has a five-year term and a current exercise price of $0.3467 per share, which is subject to customary adjustment in the event of stock dividends, stock splits and other similar transactions.
● In October 2024, we issued 137,614 shares of our common stock to Lincoln Park Capital Fund, LLC, in consideration for its commitment to purchase shares under the Purchase Agreement dated October 21, 2024, between us and Lincoln Park Capital Fund, LLC.
The
shares of common stock described above were issued in reliance on an exemption from registration under Section 4(a)(2) of the Securities
Act in that such transactions did not involve a public offering and/or Regulation D promulgated thereunder.
Item
16. Exhibits and Financial Statement Schedules
(a)
Exhibits
Exhibits
not filed or furnished herewith are incorporated by reference to exhibits previously filed with the SEC, as reflected in the table below.
| |
|
|
|
Incorporated by Reference |
|
|
Exhibit
Number |
|
Description
of Exhibit |
|
Form |
|
File
No. |
|
Filing
Date |
|
Exhibit
No. |
|
Filed
Herewith |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| PLANS
OF ACQUISITION |
2.1§
Δ |
|
Agreement and Plan of Merger, dated as of April 30, 2018, by and among Daré Bioscience, Inc., Daré Merger Sub, Inc., Pear Tree Pharmaceuticals, Inc., and Fred Mermelstein and Stephen C. Rocamboli, as Holders’ Representatives |
|
10-Q |
|
001-36395 |
|
8/13/2018 |
|
10.10 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 2.2+ |
|
Agreement and Plan of Merger, dated November 10, 2019, Dare Bioscience, Inc., MC Merger Sub, Inc., Microchips Biotech, Inc., and Shareholder Representative Services LLC, as the stockholders’ representative |
|
8-K |
|
001-36395 |
|
11/12/2019 |
|
2.1 |
|
|
ARTICLES OF INCORPORATION AND BYLAWS |
| |
| 3.1 |
|
Restated Certificate of Incorporation, as amended to date
|
|
10-Q |
|
001-36395 |
|
08/12/2024 |
|
3.1 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 3.2 |
|
Third Amended and Restated By-laws |
|
10-Q |
|
001-36395 |
|
05/14/2024 |
|
3.1 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| INSTRUMENTS
DEFINING RIGHTS OF SECURITY HOLDERS |
| |
| 4.1 |
|
Specimen stock certificate evidencing the shares of common stock |
|
10-K |
|
001-36395 |
|
03/28/2018 |
|
4.1 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 4.2 |
|
Warrant Agreement to purchase shares of common stock of the registrant with Aquilo Partners, L.P., entered into as of October 16, 2016. |
|
10-K |
|
001-36395 |
|
03/31/2022 |
|
4.2 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 4.3 |
|
Form of common stock purchase warrants issued on September 1, 2023 |
|
8-K |
|
0001-36395 |
|
08/30/2023 |
|
4.1 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 4.4 |
|
Form of common stock purchase warrants issued on December 21, 2023 |
|
10-K |
|
001-36395 |
|
03/28/2024 |
|
4.4 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 4.5 |
|
Description of securities of the registrant |
|
10-K |
|
001-36395 |
|
03/27/2020 |
|
4.6 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| OPINION
RE LEGALITY |
| |
| 5.1 |
|
Opinion of Sheppard, Mullin, Richter & Hampton LLP |
|
|
|
|
|
|
|
|
|
X |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| COMMERCIAL
AGREEMENTS |
| |
| 10.1(a)+ |
|
Exclusive License Agreement dated March 31, 2022 between Organon International GmbH and Dare Bioscience, Inc., effective as of June 30, 2022 |
|
10-Q |
|
001-36395 |
|
05/12/2022 |
|
10.1 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.1(b)+ |
|
First Amendment to License Agreement by and between Organon International GmbH and Dare Bioscience, Inc. entered into as of July 4, 2023 |
|
10-Q |
|
0001-36395 |
|
11/09/2023 |
|
10.2 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.2+ |
|
Consent, Waiver and Stand-By License Agreement, dated March 30, 2022, by and among TriLogic Pharma, LLC, and MilanaPharm LLC, Dare Bioscience, Inc., and Organon International GmbH. |
|
10-Q |
|
001-36395 |
|
05/12/2022 |
|
10.2 |
|
|
| 10.3Δ |
|
License and Collaboration Agreement dated February 11, 2018 between Daré Bioscience, Inc., Strategic Science and Technologies-D, LLC and Strategic Science Technologies, LLC |
|
10-K/A |
|
001-36395 |
|
04/30/2018 |
|
10.1 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.4Δ |
|
License Agreement dated March 19, 2017, between Daré Bioscience Operations, Inc. and ADVA-Tec, Inc. |
|
10-Q |
|
001-36395 |
|
11/13/2017 |
|
10.1 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.5Δ |
|
Exclusive License Agreement made as April 24, 2018 by and between Catalent JNP, Inc. (fka Juniper Pharmaceuticals, Inc.), and Daré Bioscience, Inc. |
|
10-Q |
|
001-36395 |
|
8/13/2018 |
|
10.1 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.6(a)Δ |
|
Amended and Restated Exclusive License Agreement for Atrophic Vaginitis Technology, effective as of July 14, 2006, dated August 15, 2007, by and between Fred Mermelstein, Ph.D., and Janet Chollet, M.D., and Pear Tree Women’s Health Care, Inc. |
|
10-Q |
|
001-36395 |
|
8/13/2018 |
|
10.5 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.6(b)Δ |
|
Amendment No. 1 to the Amended and Restated Exclusive License Agreement, dated as of October 10, 2007, by and among Fred Mermelstein, Ph.D. and Janet Chollet, M.D., and Pear Tree Pharmaceuticals, Inc. |
|
10-Q |
|
001-36395 |
|
8/13/2018 |
|
10.6 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.6(c)Δ |
|
Amendment No. 2 to the Amended and Restated Exclusive License Agreement, dated as of February 13, 2017, by and among Fred Mermelstein, Ph.D., and Janet Chollet, M.D., Pear Tree Pharmaceuticals, Inc. and Bernadette Klamerus |
|
10-Q |
|
001-36395 |
|
8/13/2018 |
|
10.7 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.6(d)+ |
|
Amendment No. 3 to the Amended and Restated Exclusive License Agreement, effective as of February 13, 2017, by and among Fred Mermelstein, Ph.D., and Janet Chollet, M.D., Pear Tree Pharmaceuticals, Inc. and Bernadette Klamerus |
|
10-K |
|
001-36395 |
|
3/30/2023 |
|
10.6(d) |
|
|
| 10.6(e)Δ |
|
Exclusive License Agreement, dated as of February 13, 2017, by and between GYN Holdings, Inc., a wholly-owned subsidiary of Pear Tree Pharmaceuticals, Inc. and Bernadette Klamerus |
|
10-Q |
|
001-36395 |
|
8/13/2018 |
|
10.8 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.6(f)Δ |
|
Exclusive License Agreement, effective as of September 15, 2017, by and between Fred Mermelstein, Ph.D., Janet Chollet, M.D., Pear Tree Pharmaceuticals, Inc., and Stephen C. Rocamboli |
|
10-Q |
|
001-36395 |
|
8/13/2018 |
|
10.9 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.7(a)Δ |
|
Assignment Agreement by and between Daré Bioscience, Inc. and Hammock Pharmaceuticals, Inc. effective as of December 5, 2018 |
|
10-K |
|
001-36395 |
|
04/01/2019 |
|
10.10(a) |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.7(b)Δ |
|
First Amendment to the License Agreement effective as of December 5, 2018 by and among Daré Bioscience, Inc., TriLogic Pharma, LLC and MilanaPharm LLC |
|
10-K |
|
001-36395 |
|
04/01/2019 |
|
10.10(b) |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.7(c) |
|
Amendment No. 1 to Assignment Agreement entered into as of December 4, 2019 between Daré Bioscience, Inc. and Hammock Pharmaceuticals, Inc. |
|
10-K |
|
001-36395 |
|
03/27/2020 |
|
10.10(c) |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.7(d) |
|
Amendment No. 2 to the License Agreement entered into as of December 3, 2019 between Daré Bioscience, Inc., TriLogic Pharma, LLC and MilanaPharm LLC |
|
10-K |
|
001-36395 |
|
03/27/2020 |
|
10.10(d) |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.7(e) |
|
Amendment to License Agreement effective as of September 21, 2021 by and among Daré Bioscience, Inc., TriLogic Pharma, LLC and MilanaPharm LLC |
|
10-Q |
|
001-36395 |
|
11/10/2021 |
|
10.1 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.8+ |
|
License Agreement dated as of January 10, 2020 between Bayer HealthCare LLC and Daré Bioscience, Inc. |
|
10-K |
|
001-36395 |
|
03/27/2020 |
|
10.16 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.9(a)+ |
|
Grant Agreement between Daré Bioscience, Inc. and the Bill & Melinda Gates Foundation effective as of June 30, 2021 |
|
10-Q |
|
001-36395 |
|
08/12/2021 |
|
10.1 |
|
|
| 10.9(b)+ |
|
Amendment No. 3 to Grant Agreement between Daré Bioscience, Inc. and the Bill & Melinda Gates Foundation, dated as of April 18, 2024 |
|
10-Q |
|
001-36395 |
|
08/12/2024 |
|
10.3 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.10+ |
|
Cooperative Research and Development Agreement entered into as of July 8, 2021 between Daré Bioscience, Inc. and the Eunice Kennedy Shriver National Institutes of Child Health and Human Development Institute |
|
10-Q |
|
001-36395 |
|
11/10/2021 |
|
10.2 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.11 |
|
Form of Securities Purchase Agreement dated as of August 29, 2023, between Dare Bioscience, Inc. and each purchaser identified on the signature pages thereto |
|
8-K |
|
0001-36395 |
|
08/30/2023 |
|
10.1 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.12 |
|
Royalty Interest Financing Agreement entered into as of December 21, 2023 between Dare Bioscience, Inc. and United in Endeavor, LLC |
|
10-K |
|
001-36395 |
|
03/28/2024 |
|
10.12 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.13 |
|
Traditional Royalty Purchase Agreement between Daré Bioscience, Inc. and XOMA (US) LLC, dated as of April 29, 2024 |
|
10-Q |
|
001-36395 |
|
08/12/2024 |
|
10.1 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.14 |
|
Traditional Royalty Purchase Agreement between Daré Bioscience, Inc. and XOMA (US) LLC, dated as of April 29, 2024
|
|
10-Q |
|
001-36395 |
|
08/12/2024 |
|
10.2 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| MANAGEMENT
CONTRACTS AND COMPENSATORY PLANS |
| |
| 10.13(a)* |
|
Daré Bioscience, Inc. Amended and Restated 2014 Stock Incentive Plan |
|
8-K |
|
001-36395 |
|
7/12/2018 |
|
10.1 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.13(b)* |
|
Form of Incentive Stock Option Agreement for grants under the Daré Bioscience, Inc. Amended and Restated 2014 Stock Incentive Plan |
|
10-Q |
|
001-36395 |
|
8/13/2018 |
|
10.3 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.13(c)* |
|
Form of Nonstatutory Stock Option Agreement for grants under the Daré Bioscience, Inc. Amended and Restated 2014 Stock Incentive Plan |
|
10-Q |
|
001-36395 |
|
8/13/2018 |
|
10.4 |
|
|
| 10.14* |
|
2014 Employee Stock Purchase Plan |
|
S-1/A |
|
333-194442 |
|
3/31/2014 |
|
10.26 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.15(a)* |
|
Daré Bioscience, Inc. 2022 Stock Incentive Plan |
|
10-Q |
|
001-36395 |
|
08/12/2024 |
|
10.7 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.15(b)* |
|
Form of Incentive Stock Option Agreement for Grants under the Daré Bioscience, Inc. 2022 Stock Incentive Plan |
|
8-K |
|
001-36395 |
|
6/24/2022 |
|
10.1(b) |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.15(c)* |
|
Form of Nonstatutory Stock Option Agreement for Grants under the Daré Bioscience, Inc. 2022 Stock Incentive Plan |
|
8-K |
|
001-36395 |
|
6/24/2022 |
|
10.2(c) |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.16* |
|
Daré Bioscience, Inc. Performance Bonus Plan, as amended |
|
10-Q |
|
001-36395 |
|
11/9/2023 |
|
10.3 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.17* |
|
Form of indemnification agreement between the registrant and each of its executive officers and directors |
|
S-1 |
|
333-194442 |
|
03/10/2014 |
|
10.16 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.18* |
|
Amended and Restated Non-Employee Director Compensation Policy (as amended on April 2024) |
|
10-Q |
|
001-36395 |
|
08/12/2024 |
|
10.4 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.19(a)* |
|
Employment Agreement by and between Daré Bioscience, Inc. and Sabrina Martucci Johnson dated as of August 15, 2017 |
|
8-K |
|
001-36395 |
|
08/18/2017 |
|
10.1 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.19(b)* |
|
Amendment No. 1 to Employment Agreement between Daré Bioscience, Inc. and Sabrina Martucci Johnson dated as of March 9, 2020 |
|
10-Q |
|
001-36395 |
|
05/14/2020 |
|
10.13(b) |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.19(c)* |
|
Amendment No. 2 to Employment Agreement between Daré Bioscience, Inc. and Sabrina Martucci Johnson, dated as of May 20, 2024 |
|
10-Q |
|
001-36395 |
|
08/12/2024 |
|
10.5 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.20(a)* |
|
Employment Agreement by and between Daré Bioscience, Inc. and Lisa Walters-Hoffert dated as of August 15, 2017 |
|
8-K |
|
001-36395 |
|
08/18/2017 |
|
10.2 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.20(b)* |
|
Amendment No. 1 to Employment Agreement between Daré Bioscience, Inc. and Lisa Walters-Hoffert dated as of March 9, 2020 |
|
10-Q |
|
001-36395 |
|
05/14/2020 |
|
10.14(b) |
|
|
| 10.20(c)* |
|
Consulting Agreement by and between Daré Bioscience, Inc. and Lisa Walters-Hoffert, dated as of January 26, 2024 |
|
10-Q |
|
001-36395 |
|
05/14/2024 |
|
10.1 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.21* |
|
Daré Bioscience, Inc. Employment Offer Letter to John Fair, dated April 24, 2018 |
|
S-1 |
|
333-251599 |
|
01/05/2021 |
|
10.19 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.22* |
|
Daré Bioscience, Inc. Change in Control Policy (as amended on April 29, 2024) |
|
10-Q |
|
001-36395 |
|
08/12/2024 |
|
10.6 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| OTHER
EXHIBITS |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 21.1 |
|
Subsidiaries of the registrant |
|
10-K |
|
001-36395 |
|
03/28/2024 |
|
21.1 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 23.1 |
|
Consent of Haskell & White LLP |
|
|
|
|
|
|
|
|
|
X |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 23.2 |
|
Consent of CBIZ CPAs P.C. |
|
|
|
|
|
|
|
|
|
X |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 24.1 |
|
Power of Attorney (included in the signature page hereto) |
|
|
|
|
|
|
|
|
|
X |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 101.INS |
|
XBRL
Instance Document |
|
|
|
|
|
|
|
|
|
X |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 101.SCH |
|
XBRL
Taxonomy Extension Schema Document |
|
|
|
|
|
|
|
|
|
X |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 101.CAL |
|
XBRL
Taxonomy Calculation Linkbase Document |
|
|
|
|
|
|
|
|
|
X |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 101.DEF |
|
XBRL
Taxonomy Extension Definition Linkbase Document |
|
|
|
|
|
|
|
|
|
X |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 101.LAB |
|
XBRL
Taxonomy Label Linkbase Document |
|
|
|
|
|
|
|
|
|
X |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 101.PRE |
|
XBRL
Taxonomy Presentation Linkbase Document |
|
|
|
|
|
|
|
|
|
X |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 107 |
|
Filing Fee Table |
|
|
|
|
|
|
|
|
|
X |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| § |
|
All
schedules (or similar attachments) have been omitted from this filing pursuant to Item 601(b)(2) of Regulation S-K. The registrant
will furnish copies of any schedules to the Securities and Exchange Commission upon request. |
| Δ |
|
Confidential
treatment has been requested or granted to certain confidential information contained in this exhibit. |
| + |
|
Portions
of this exhibit have been redacted in compliance with Regulation S-K Item 601(b)(10). The omitted information is not material and
would likely cause competitive harm to the Company if publicly disclosed. |
| * |
|
Management
contract or compensatory plan or arrangement |
| # |
|
Furnished
herewith. This certification is being furnished solely to accompany this report pursuant to U.S.C. § 1350, and is not being
filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and is not to be incorporated herein by reference
into any filing of the registrant whether made before or after the date hereof, regardless of any general incorporation language
in such filing. |
(b)
Financial statement schedules
No
financial statement schedules are provided because the information called for is not required or is shown either in the financial statements
or related notes, which are incorporated herein by reference.
Item
17. Undertakings
(a)
The undersigned registrant hereby undertakes:
(1)
To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i)
To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
(ii)
To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective
amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration
statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities
offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range
may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume
and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration
Fee” table in the effective registration statement; and
(iii)
To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or
any material change to such information in the registration statement;
provided,
however, that paragraphs (a)(1)(i), (a)(1)(ii) and (a)(1)(iii) do not apply if the information required to be included in a post-effective
amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the registrant pursuant to section
13 or section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration statement.
(2)
That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed
to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall
be deemed to be the initial bona fide offering thereof.
(3)
To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the
termination of the offering.
(4)
That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser: each prospectus filed pursuant to Rule
424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other
than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the
date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus
that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration
statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such
first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration
statement or made in any such document immediately prior to such date of first use.
(b)
The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act of 1933, each filing
of the registrant’s annual report pursuant to section 13(a) or section 15(d) of the Securities Exchange Act of 1934 (and, where
applicable, each filing of an employee benefit plan’s annual report pursuant to section 15(d) of the Securities Exchange Act of
1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to
the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering
thereof.
(c)
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling
persons of the registrant, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification
is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against
such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person
of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person
in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled
by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public
policy as expressed in the Act and will be governed by the final adjudication of such issue.
SIGNATURES
Pursuant
to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf
by the undersigned, thereunto duly authorized, in the City of San Diego, State of California, on November 15, 2024.
| |
Daré Bioscience, Inc. |
| |
|
|
| |
By: |
/s/ Sabrina Martucci Johnson |
| |
|
Sabrina Martucci Johnson |
| |
|
Chief Executive Officer |
POWER
OF ATTORNEY
We,
the undersigned officers and directors of Daré Bioscience, Inc., hereby severally constitute and appoint Sabrina Martucci Johnson
and MarDee Haring-Layton, and each of them singly (with full power to each of them to act alone), our true and lawful attorneys-in-fact
and agents, with full power of substitution and resubstitution in each of them for her or him and in her or his name, place and stead,
and in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement (or
any other registration statement for the same offering that is to be effective upon filing pursuant to Rule 462(b) under the Securities
Act of 1933), and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange
Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every
act and thing requisite or necessary to be done in and about the premises, as full to all intents and purposes as she or he might or
could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents or any of them or their or her or his
substitute or substitutes may lawfully do or cause to be done by virtue hereof.
Pursuant
to the requirements of the Securities Act of 1933, this registration statement has been signed below by the following persons in the
capacities and on the dates indicated.
| Signature |
|
Title |
|
Date |
| /s/
Sabrina Martucci Johnson |
|
President,
Chief Executive Officer, Secretary and Director |
|
November
15, 2024 |
| Sabrina
Martucci Johnson |
|
(Principal
Executive and Financial Officer) |
|
|
| |
|
|
|
|
| /s/
MarDee Haring-Layton |
|
Chief
Accounting Officer |
|
November
15, 2024 |
| MarDee
Haring-Layton |
|
(Principal
Accounting Officer) |
|
|
| |
|
|
|
|
| /s/
William H. Rastetter |
|
Chairman
of the Board |
|
November
15, 2024 |
| William
H. Rastetter, Ph.D. |
|
|
|
|
| |
|
|
|
|
| /s/
Jessica D. Grossman |
|
Director |
|
November
15, 2024 |
| Jessica
D. Grossman, M.D. |
|
|
|
|
| |
|
|
|
|
| /s/
Susan L. Kelley |
|
Director |
|
November
15, 2024 |
| Susan
L. Kelley, M.D. |
|
|
|
|
| |
|
|
|
|
| /s/
Gregory W. Matz |
|
Director |
|
November
15, 2024 |
| Gregory
W. Matz |
|
|
|
|
| |
|
|
|
|
| /s/
Robin J. Steele |
|
Director |
|
November
15, 2024 |
| Robin
J. Steele, J.D., L.L.M. |
|
|
|
|
| |
Exhibit
5.1 |
| |
|
 |
Sheppard,
Mullin, Richter & Hampton LLP
12275
El Camino Real, Suite 100
San
Diego, CA 92130
www.sheppardmullin.com |
November
15, 2024
Daré
Bioscience, Inc.
Attn:
Board of Directors
3655
Nobel Drive, Suite 260
San
Diego, CA 92122.
Re:
Registration Statement on Form S-1
Ladies
and Gentlemen:
We
have acted as counsel to Daré Bioscience, Inc., a Delaware corporation (the “Company”), in connection with
the filing with the U.S. Securities and Exchange Commission (the “Commission”) on or about the date hereof of a registration
statement on Form S-1 (the “Registration Statement”) under the Securities Act of 1933, as amended (the “Act”).
The Registration Statement registers the resale of up to 2,750,000 shares (the “Shares”) of the Company’s common
stock, par value $0.0001 per share (“Common Stock”), by the selling stockholder (the “Selling Stockholder”)
identified in the Registration Statement. The Shares consist of 137,614 shares of Common Stock issued by the Company to the Selling
Stockholder on October 21, 2024 (the “Commitment Shares”), and up to 2,612,386 shares of Common Stock (the “Purchase
Shares”) that the Company may issue and sell to the Selling Stockholder from time to time, in each case, pursuant to the purchase
agreement dated October 21, 2024 between the Selling Stockholder and the Company (the “Agreement”).
This
opinion is being furnished in connection with the requirements of Item 601(b)(5) of Regulation S-K under the Act. It is understood that
this opinion is to be used only in connection with the offer and sale of the Shares while the Registration Statement is effective under
the Act.
In
connection with this opinion letter, we have examined and relied upon originals or copies, certified or otherwise identified to our satisfaction,
of the Registration Statement and the Prospectus, the Company’s certificate of incorporation and bylaws, each as currently in effect,
the Agreement, and such records, documents, certificates, memoranda and other instruments as in our judgment are necessary or appropriate
to enable us to render the opinion expressed below. We have assumed: the genuineness of all signatures, including endorsements; the legal
capacity and competency of all natural persons; the authenticity of all documents submitted to us as originals; the conformity to originals
of all documents submitted to us as copies, including facsimile, electronic, certified or photostatic copies; the authenticity of the
originals of all documents submitted to us as copies; the accuracy, completeness and authenticity of certificates of public officials;
and the due authorization, execution and delivery of all documents by all persons other than the Company where authorization, execution
and delivery are prerequisites to the effectiveness thereof. As to any facts relevant to the opinions stated herein that we did not independently
establish or verify, we relied upon statements and representations of officers and other representatives of the Company and others and
of public officials and have not independently verified such facts.
Based
upon the foregoing and subject to the qualifications and assumptions stated herein, we are of the opinion that, (i) the Commitment
Shares are validly issued, fully paid and nonassessable and (ii) the Purchase Shares, when sold and issued against payment therefor
in accordance with the Agreement, and when evidence of the issuance thereof is duly recorded in the Company’s books and
records, will be validly issued, fully paid and non-assessable.
In
rendering the foregoing opinion, we assumed that (i) the Company will comply with all applicable notice requirements regarding uncertificated
shares provided in the General Corporation Law of the State of Delaware (the “DGCL”), (ii) each sale of Purchase Shares
will be duly authorized by the Company’s board of directors or a duly authorized committee thereof in accordance with the DGCL,
(iii) that the price at which the Purchase Shares are sold will equal or exceed the par value of the Common Stock, and (iv) upon the
issuance of any Purchase Shares, the total number of shares of Common Stock issued and outstanding will not exceed the total number
of shares of Common Stock the Company is then authorized to issue under its certificate of incorporation. We express no opinion to the
extent that future issuances of securities of the Company, anti-dilution adjustments to outstanding securities of the Company and/or
other matters cause the number of shares of Common Stock issuable under the Agreement to be more than the number of shares of Common
Stock available for issuance by the Company.
The
opinion which we render herein is expressly limited solely to those matters governed by the DGCL and is based on the DGCL as in
effect on the date hereof. We express no opinion to the extent that any other laws are applicable to the subject matter hereof and we
express no opinion and provide no assurance with respect to any other laws or as to compliance with any federal or state securities law,
rule or regulation or as to any matter pertaining to the contents of the Registration Statement or the Prospectus, other than as expressly
stated herein with respect to the issue of the Shares.
We
hereby consent to the filing of this opinion letter as an exhibit to the Registration Statement. We also hereby consent to the reference
to our firm in the “Legal Matters” section in the Prospectus. In giving this consent, we do not thereby admit that we are
within the category of persons whose consent is required under Section 7 of the Act or the General Rules and Regulations under the Act.
This
opinion letter is rendered as of the date first written above and we disclaim any obligation to advise you of facts, circumstances, events
or developments which hereafter may be brought to our attention and which may alter, affect or modify the opinion expressed herein. Our
opinion is expressly limited to the matters set forth above and we render no opinion, whether by implication or otherwise, as to any
other matters relating to the Company, the Shares or any other agreements or transactions that may be related thereto or contemplated
thereby. We are expressing no opinion as to any obligations that parties other than the Company may have under or in respect of the Shares,
or as to the effect that their performance of such obligations may have upon any of the matters referred to above. No opinion may be
implied or inferred beyond the opinion expressly stated above.
| |
Respectfully, |
| |
|
| |
/s/
Sheppard, Mullin, Richter & Hampton LLP |
| |
|
| |
Sheppard,
Mullin, Richter & Hampton LLP |
Exhibit
23.1
CONSENT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We
consent to the incorporation by reference in the Registration Statement on Form S-1 of Daré Bioscience, Inc. (the “Company”)
of our report dated March 28, 2024, relating to the consolidated financial statements as of December 31, 2023 and for the year then ended
(which includes an explanatory paragraph expressing substantial doubt regarding the Company’s ability to continue as a going
concern), which appears in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023.
We
also consent to the reference to our Firm under the heading “Experts” in this Registration Statement on Form S-1.
| |
/s/
Haskell & White LLP |
| |
HASKELL
& WHITE LLP |
Irvine,
California
November
15, 2024
Exhibit
23.2
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We hereby consent to the incorporation by reference in this Registration
Statement on Form S-1 and related Prospectus of Daré Bioscience, Inc. and Subsidiaries (the “Company”) of our report
dated March 30, 2023 (which includes an explanatory paragraph relating to the uncertainty of the Company’s ability to continue as
a going concern), relating to the consolidated financial statements of the Company appearing in the Company’s Annual Report on Form
10-K for the year ended December 31, 2023. We also consent to the reference to our Firm under the caption “Experts” in this
Prospectus which is a part of said Registration Statement.
/s/
CBIZ CPAs P.C.1
San
Diego, California
November
15, 2024
1
In certain jurisdictions, CBIZ CPAs P.C. operates under its previous name, Mayer Hoffman McCann P.C.
Exhibit
107
Calculation
of Filing Fee Table
FORM
S-1
(Form
Type)
DARÉ
BIOSCIENCE, INC.
(Exact
Name of Registrant as Specified in its Charter)
Table
1: Newly Registered Securities
| | |
Security Type | |
Security Class Title | |
Fee Calculation Rule | |
Amount Registered(1) | | |
Proposed Maximum Offering Price Per Unit(2) | | |
Maximum Aggregate Offering Price (1)(2) | | |
Fee Rate | | |
Amount of Registration Fee | |
| Fees to Be Paid | |
Equity | |
Common stock, par value $0.0001 per share | |
Other(2) | |
| 2,750,000 | (3) | |
$ | 4.1178 | | |
$ | 11,323,950 | | |
| 0.0001531 | | |
$ | 1,733.70 | |
| | |
Total Offering Amounts | | |
| | | |
$ | 11,323,950 | | |
| | | |
$ | 1,733.70 | |
| | |
Total Fees Previously Paid | | |
| | | |
| | | |
| | | |
| — | |
| | |
Total Fee Offsets | | |
| | | |
| | | |
| | | |
| — | |
| | |
Net Fee Due | | |
| | | |
| | | |
| | | |
$ | 1,733.70 | |
| (1) |
In
addition to the common stock set forth in this table, pursuant to Rule 416, this registration statement also registers such additional
number of shares of common stock as may become issuable by reason of any stock splits, stock dividends or similar transactions. |
| |
|
| (2) |
Estimated
solely for purposes of calculating the registration fee in accordance with Rule 457(c) under the Securities Act and based upon the
average of the high and low sales prices of a share of common stock as reported on The Nasdaq Capital Market on November 8, 2024. |
| |
|
| (3) |
Consists
of 137,614 shares issued on October 21, 2024 pursuant to the purchase agreement between the registrant and the selling stockholder,
and up to 2,612,386 shares that the registrant may sell to the selling stockholder pursuant to such purchase agreement. |
Dare Bioscience (NASDAQ:DARE)
Gráfica de Acción Histórica
De Nov 2024 a Dic 2024
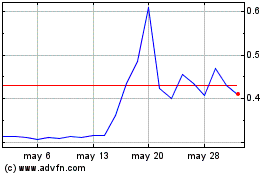
Dare Bioscience (NASDAQ:DARE)
Gráfica de Acción Histórica
De Dic 2023 a Dic 2024