UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): November 25, 2024
KINETA, INC.
(Exact name of registrant as specified in its charter)
|
|
|
Delaware |
001-37695 |
20-8436652 |
(State or other jurisdiction |
(Commission |
(IRS Employer |
of incorporation) |
File Number) |
Identification No.) |
7683 SE 27th Street, Suite 481 |
|
|
Mercer Island, WA |
|
98040 |
(Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: (206) 378-0400
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
|
|
|
☐ |
|
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
☒ |
|
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
☐ |
|
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
☐ |
|
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
|
Title of each class |
Trading |
Name of each exchange |
|
|
Symbol(s) |
on which registered |
|
Common Stock, par value $0.001 per share |
|
KANT |
|
OTC Pink Market |
|
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
|
|
Item 7.01 |
Regulation FD Disclosure. |
As previously disclosed, on July 3, 2024 (the “Effective Date”), Kineta, Inc. (“Kineta” or the “Company”) entered into an exclusivity and right of first offer agreement (the “TuHURA Agreement”) with TuHURA Biosciences, Inc., a Nevada corporation (“TuHURA”). Capitalized terms used but not otherwise defined herein have the meaning set forth in the TuHURA Agreement.
Pursuant to the TuHURA Agreement, among other things, the Company granted TuHURA an exclusive right during the Exclusivity Period to acquire the Company’s worldwide patents, patent rights, patent applications, product and development program assets, technical and business information, and other rights and assets associated with and derived from its development program related to KVA12123, the Company’s VISTA blocking immunotherapy.
In consideration for the Company’s compliance with its obligations set forth in the TuHURA Agreement, TuHURA paid the Company $5.0 million (the “Exclusivity Payment”) in July 2024. In October 2024, TuHURA exercised its right to extend the TuHURA Agreement and paid the Company $300,000 in Exclusivity Payments, which will be credited against the initial cash consideration that may be payable to the Company pursuant to a Definitive Agreement (if any) between the Company and TuHURA and/or its affiliates with respect to a Potential Transaction.
On November 25, 2024, TuHURA issued a press release announcing the entry into a non-binding letter of intent with the Company regarding a potential transaction in which TuHURA would acquire the rights to KVA12123 (the “potential transaction”). The press release is furnished as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
The information furnished under this Item 7.01, including Exhibit 99.1, will not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, and will not be deemed incorporated by reference into any filing under the Securities Act of 1933, as amended (the “Securities Act”), or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Cautionary Statements Regarding Forward-Looking Statements
This Current Report on Form 8-K contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. The use of words such as, but not limited to, “believe,” “expect,” “estimate,” “project,” “intend,” “future,” “potential,” “continue,” “may,” “might,” “plan,” “will,” “should,” “seek,” “anticipate,” or “could” and other similar words or expressions are intended to identify forward-looking statements. These forward-looking statements include, without limitation, statements relating to the terms and outcome of the potential transaction. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on the Company’s current beliefs, expectations and assumptions regarding the future of the Company’s business, future plans and strategies, and other future conditions. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements.
Such forward-looking statements are subject to a number of material risks and uncertainties including, but not limited to: whether the Company will be able to complete the potential transaction in a timely manner or at all or satisfy the conditions to the consummation of the potential transaction, including the negotiation, completion, and execution of a definitive acquisition or merger agreement (a “definitive agreement”) by the Company’s stockholders; the occurrence of any event, change or other circumstance that could give rise to the termination of the definitive agreement; the effect of the announcement or pendency of the potential transaction on the Company’s and TuHURA’s business relationships, performance, and business generally; or whether any transaction, if pursued, will be completed on attractive terms or at all; whether the Company’s cash resources will be sufficient to fund its foreseeable and unforeseeable operating expenses and capital requirements; and those risks set forth under the caption “Risk Factors” in the Company’s Annual Report on Form 10-K and Quarterly Reports on Form 10-Q as well as discussions of potential risks, uncertainties and other important factors in the Company’s subsequent filings with the U.S. Securities and Exchange Commission (the “SEC”). Any forward-looking statement speaks only as of the date on which it was made. Except as required by law, the Company undertakes no obligation to publicly update or revise any forward-looking statement, whether as result of new information, future events or otherwise.
Important Information About the Potential Transaction and Where to Find It
This Current Report on Form 8-K, including Exhibit 99.1 hereto, discloses that Kineta and TuHURA have entered into a non-binding letter of intent for the potential transaction. The potential transaction, if any, is subject to negotiation, completion, and execution of a definitive agreement, which will contain the full terms and conditions of the potential transaction. If a definitive agreement is entered into by Kineta and TuHURA in connection with the potential transaction, Kineta and TuHURA intend to file a proxy statement/prospectus with the SEC, which is expected to include a joint proxy statement of Kineta and TuHURA and a preliminary prospectus of TuHURA in connection with the potential transaction, referred to as a proxy statement/prospectus. If a proxy statement/prospectus is filed, after the proxy statement prospectus is cleared by the SEC, a definitive proxy statement/prospectus will
be mailed or made available to Kineta’s stockholders as of a record date to be established for voting on the transaction and to the stockholders of TuHURA. TuHURA also will file other documents regarding the potential transaction with the SEC. INVESTORS AND STOCKHOLDERS ARE URGED TO READ THE DEFINITIVE PROXY STATEMENT/PROSPECTUS AND OTHER MATERIALS, IF ANY, THAT MAY BE FILED WITH THE SEC IN CONNECTION WITH THE POTENTIAL TRANSACTION CAREFULLY AND IN THEIR ENTIRETY IF AND WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT THE POTENTIAL TRANSACTION. THIS CURRENT REPORT ON FORM 8-K IS NOT A SOLICITATION TO STOCKHOLDERS TO APPROVE ANY TRANSACTION.
Investors and stockholders will be able to obtain free copies of the proxy statement/prospectus and all other relevant documents filed or that will be filed with the SEC by Kineta through the website maintained by the SEC at www.sec.gov. The documents filed by Kineta with the SEC may also be obtained free of charge at Kineta’s website at visit www.kinetabio.com or upon written request to: Kineta, Inc. 7683 SE 27th Street, Suite 481, Mercer Island, WA 98040.
NEITHER THE SEC NOR ANY STATE SECURITIES REGULATORY AGENCY HAS APPROVED OR DISAPPROVED THE POTENTIAL TRANSACTION DESCRIBED IN THIS CURRENT REPORT ON FORM 8-K, PASSED UPON THE MERITS OR FAIRNESS OF THE POTENTIAL TRANSACTION OR RELATED TRANSACTIONS OR PASSED UPON THE ADEQUACY OR ACCURACY OF THE DISCLOSURE IN THIS CURRENT REPORT ON FORM 8-K. ANY REPRESENTATION TO THE CONTRARY CONSTITUTES A CRIMINAL OFFENSE.
Participants in the Solicitation
If we solicit proxies for the potential transaction, Kineta and TuHURA and their respective directors and officers and other members of management may, under the SEC rules, be deemed to be participants in the solicitation of proxies from stockholders in connection with the potential transaction and other matters that may be set forth in the proxy statement/prospectus. Information about Kineta’s directors and executive officers is set forth in Kineta’s filings with the SEC, including Kineta’s proxy statement filed with the SEC on April 26, 2024. Additional information regarding the direct and indirect interests, by security holdings or otherwise, of those persons and other persons who may be deemed participants in the solicitation of proxies in the potential transaction may be obtained by reading the proxy statement/prospectus when it becomes available. You may obtain free copies of these documents as described above under “Important Information About the Potential Transaction and Where to Find It.”
No Offer or Solicitation
This Current Report on Form 8-K is not a proxy statement or solicitation of a proxy, consent or authorization with respect to any securities or in respect of the potential transaction and is not intended to and does not constitute an offer to sell or the solicitation of an offer to buy the securities of Kineta or TuHURA, nor shall there be any sale of any such securities in any state or jurisdiction in which such offer, solicitation, or sale would be unlawful prior to registration or qualification under the securities laws of such state or jurisdiction. No offer of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act.
|
|
Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits
|
|
|
|
|
|
Exhibit No. |
|
Description |
|
|
99.1 |
|
Press release issued by TuHURA Biosciences, Inc., dated November 25, 2024. |
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
Date: November 25, 2024
Kineta, Inc.
|
|
By: |
/s/ Craig Philips |
Name: |
Craig Philips |
Title: |
President |
Exhibit 99.1

TuHURA Biosciences, Inc. (Nasdaq: HURA) Outlines Development Pathway for Single Phase 3 Accelerated Approval Registration Trial in First Line Treatment of Advanced or Metastatic Merkel Cell Carcinoma and Provides Business Update
IFx-2.0, a first-in-class innate immune response agonist, entering single Phase 3 accelerated approval registration trial in first half of 2025 for first line treatment of Merkel Cell Carcinoma (MCC) under Special Protocol Assessment (SPA) agreement with U.S. Food and Drug Administration (FDA)
Entered into a non-binding letter of intent with Kineta regarding a potential transaction in which TuHURA would acquire the rights to KVA12123 expanding its pipeline with a Phase 2 ready, novel VISTA inhibiting antibody
Advancing IFx-3.0, the first systemically administered tumor-targeted mRNA innate immune response agonist for aggressive B Cell Lymphoma
Early discovery programs designing first-in-class tumor microenvironment modulating bi-specific antibody drug conjugates (ADCs) or peptide drug conjugates (PDCs) targeting Myeloid Derived Suppressor Cells (MDSCs)
Company expands leadership team with key clinical and regulatory appointments to drive strategy and operational execution
TAMPA, FL, November 25, 2024 – TuHURA Biosciences, Inc. (Nasdaq: HURA) (“TuHURA” or the “Company”), a Phase 3 registration-stage immune-oncology company developing novel technologies to overcome resistance to cancer immunotherapy, today provided a business update and outlined upcoming targeted milestones.
“We have made significant progress toward accomplishing our 2024 corporate objectives, including reaching a Special Protocol Assessment agreement with the FDA by working with the Oncology Center of Excellence (OCE) and FDA’s Project Front Runner initiative for a novel Phase 3 trial design for IFx-2.0 under the FDA’s accelerated approval pathway. This single registration directed trial, in addition to its primary endpoint of Overall Response Rate (ORR), it will also incorporate a key secondary endpoint of Progression-Free Survival (PFS) that, if achieved, can satisfy the requirement for a post-approval confirmatory trial, potentially converting accelerated approval to full approval,” commented James Bianco, M.D., President and Chief Executive Officer of TuHURA. “We have also advanced our efforts toward the potential acquisition of KVA12123, a novel VISTA inhibiting antibody, with a non-binding letter of intent, which would bring to our pipeline a Phase 2 ready candidate with therapeutic synergies across our pipeline and
technologies, all while becoming a NASDAQ-listed Company after having raised significant capital adequate to advance our current programs late into the second half of 2025.”
Advancing Novel Technologies to Overcome Resistance to Cancer Immunotherapy
Innate Immune Response Agonists: TuHURA’s IFx technology utilizes a proprietary plasmid DNA or messenger RNA (“mRNA”) which, when introduced into or targeted to a tumor, results in the expression of a highly immunogenic gram-positive, bacterial protein (Emm55) on the surface of the tumor cell, making the tumor look like a bacterium. Gram-positive bacterium has molecular patterns, or motifs, preserved over evolution which are recognized by receptors on our immune cells called toll like receptors (TLR). TLR 2 specifically recognizes the pattern of gram-positive bacterial proteins, like Emm55 leading to the activation of antigen presenting cells (APCs). Once activated, APCs digest the tumor cell and present non-self, tumor neoantigens to newly produced T and B cells, activating a tumor-specific adaptive immune response. Through its activation of tumor specific T cells, IFx-2.0 administration can potentially overcome primary resistance to checkpoint inhibitors.
TuHURA is preparing to initiate a single, randomized, placebo-controlled Phase 3 accelerated approval trial of IFx-2.0 administered as an adjunctive therapy to Keytruda® (pembrolizumab) versus pembrolizumab plus placebo in first line treatment for checkpoint inhibitor-naïve patients with advanced or metastatic MCC. The data from the Company’s Phase 1b trial in patients with advanced or metastatic MCC who exhibited primary resistance to CPI was used to support a potential single registration directed trial. Consistent with the FDA’s Project Front Runner Initiative, the FDA’s OCE recommended investigating IFx-2.0 in the first line setting rather than in patients progressing on first line therapy.
Project Front Runner is an FDA OCE initiative to encourage drug sponsors to consider when it may be appropriate to first develop and seek approval of new cancer drugs for advanced or metastatic disease, in an earlier clinical setting rather than the usual approach to develop and seek approval of a new drug for treatment of patients who have received numerous prior lines of therapies or have exhausted available treatment options.
The FDA also encouraged the Company to consider designing the trial to include a key secondary endpoint shown to be of clinical benefit like PFS allowing this accelerated approval trial to potentially satisfy both the requirements for accelerated approval based on ORR, while satisfying the requirement for a post-approval confirmatory trial if the secondary PFS endpoint is achieved. The trial will be conducted under an SPA agreement with the FDA.
Tumor Microenvironment Modulators: Leveraging its Delta receptor technology, TuHURA is developing bi-specific immune modulating ADCs or PDCs targeting MDSCs to inhibit their immune suppressing effects on the tumor microenvironment to prevent T cell exhaustion and acquired resistance to checkpoint inhibitors and cellular therapies.
Potential Acquisition of Novel Anti-VISTA Checkpoint Inhibitor: As previously announced on July 8, 2024, TuHURA entered into an Exclusivity and Right of First Offer Agreement with Kineta, Inc. (OTC Pink: KANT) for the potential acquisition of Kineta's KVA12123 VISTA inhibiting antibody.
TuHURA has continued to engage in discussions and negotiations with Kineta and has entered into a non-binding letter of intent regarding a potential transaction in which TuHURA would acquire the rights to KVA12123 for a combination of cash and shares of TuHURA common stock via a merger transaction structure.
KVA12123 has completed enrollment in its monotherapy arm, demonstrating safety at its highest dose level (1000mg). Kineta anticipates completion of enrollment in the combination therapy arm where KVA12123 is administered with Merck's anti-PD1 therapy, KEYTRUDA® (pembrolizumab). Initial results were reported earlier this year at the American Association of Cancer Research (AACR) Annual Meeting 2024 and recently at the Society for Immunotherapy of Cancer (SITC) meeting.
Upcoming Milestone Targets
TuHURA is targeting the achievement of the following potential milestones for the remainder of 2024 and 2025:
•Q4 2024: Reach a potential definitive agreement for acquisition of KVA12123 VISTA inhibiting antibody via merger with Kineta, subject to continuing diligence and negotiations with Kineta
•H1 2025: Initiate IFx-2.0 Phase 3 trial
•H1 2025: IFx-2.0 Inhibitor Resistant “basket” trial
•H1 2025: Complete potential acquisition of transaction involving Kineta’s KVA12123
•H2 2025: Commence VISTA inhibiting Mab Phase 2 trials (if Kineta merger transaction has been completed)
•H2 2025: Bi-specific MDSC targeted ADC in vivo POC
•H2 2025: IFx-3.0: CD22+ tumor targeted mRNA in vivo POC studies
REM-001
The legacy asset from the completed merger with Kintara Therapeutics, Inc., REM-001, is a late-stage photodynamic therapy ("PDT”) currently being evaluated in an open-label 15- patient study for the treatment of cutaneous metastatic breast cancer. As of October 7, 2024, four patients had been dosed. The Company currently expects to complete enrollment in Q4 2024. Based on the Company’s strategic focus on advancing its technologies that seek to overcome the major obstacles that limit the effectiveness of current immunotherapies in treating cancer, TuHURA plans to seek licensing opportunities for REM-001.
In connection with the completed merger with Kintara, existing stockholders of Kintara received contingent value rights entitling them to receive additional shares of TuHURA common stock
upon achievement of enrollment of a minimum of 10 patients in the REM-001 Study, with such patients each completing 8 weeks of follow-up on or before December 31, 2025.
Key Management Hires to Advance Development
The Company also announced the key management appointments of Peter O’Neill as Vice President, Clinical Operations and Michael Krsulich as Head of Quality Assurance.
Peter O’Neill
Mr. O’Neill brings over 25 years of clinical trial experience, having led large and small Clinical Operations teams at sponsors (Biotech/Pharma), CROs and research hospitals. As a former cancer patient and survivor of malignant melanoma, Mr. O’Neill is passionate about developing advanced immunotherapies for patients with cancer and other debilitating diseases and is focused on innovative strategies to enhance the clinical trial experience for patients and their care teams. He joins TuHURA having most recently served as Senior Director, Clinical Operations at Cellectis, where he was responsible for clinical trial programs of allogeneic “off-the-shelf” gene-edited CAR T-cell products for the treatment of relapsed or refractory B-cell acute lymphoblastic leukemia and multiple myeloma. Prior to Cellectis, he served in multiple roles, including most recently as Senior Director, Clinical Operations at Incyte, where he helped lead the pivotal trials and subsequent supporting trials that resulted in the marketing approval and label expansion of Jakafi® (ruxolitinib), Incyte’s first approved therapy and the first JAK inhibitor approved by FDA. He also served as the Innovation Lead for Incyte’s Clinical Development Operations organization. Additional appointments over the course of his career include roles at Sanofi, AAI Pharma, and Beth Israel Deaconess Medical Center.
Mr. O’Neill holds a Bachelor of Science in Biology from Fairfield University and a Master of Business Administration in Pharmaceutical and Healthcare Marketing from St. Jospeh’s University – Haub School of Business. He has since received a Leadership & Management Certificate from The Wharton School, and Certificates in Disruptive Strategy and Digital Health from Harvard Business School. Additionally, he was awarded “Emerging Pharma Leader” by Pharmaceutical Executive as a special recognition to rising leaders in the life sciences industry (May 2020 edition of Pharmaceutical Executive magazine).
Michael Krsulich
Mr. Krsulich has established diverse international expertise supporting progression of therapies from clinical development through commercialization and leading GxP Quality oversight. Mr. Krsulich joins TuHURA having most recently served as Vice President, Global Quality Assurance (CRO) at Reaction Biology Corporation, an industry-leading provider of drug discovery and development services where he led the global quality team ensuring compliance, designed and implemented a global quality management strategy for all sites across the US and EU and implemented a global quality management system and risk management program. Prior to that, Mr. Krsulich served as Senior Director, GxP Quality Assurance (Gene Therapy) at Renovacor, Inc. (acquired by Rocket Pharmaceuticals). Other career appointments include Senior Director, Quality Assurance GMP (small molecule) at Galera Therapeutics, Inc.; Associate Director, R&D
Quality Assurance (small / large molecule, biosimilars) at Teva Pharmaceuticals Industries, where he led the GMP QA activities for Teva’s first commercially approved biologic (AJOVY® – fremanezumab-vfrm); Senior Manager, Development Quality Assurance (small/large molecule, ADC) at Eisai, Inc.; Quality Site Lead (large molecule) at OPK Biotech, LLC.; and Quality Assurance Specialist Team Leader (medical device, biomaterials) at Global Medical, Inc.
Mr. Krsulich holds a Bachelor of Science in Psychology from the University of Pittsburgh. He is also a member of the American Society for Quality.
“We are excited to strengthen our experienced leadership team with both Peter and Michael. We believe their skillsets are valuable additions as we prepare to launch our Phase 3 accelerated approval trial,” commented Dr. Bianco.
Financial Update
In connection with the Company’s recently completed merger with Kintara, a $31 million fully-funded financing was closed and is expected to fund planned operations late into the second half of 2025. Prior to the merger, the Company raised an additional $5 million to fund the Exclusivity and Right of First Offer Agreement with Kineta relating to KVA12123, its Phase 2 ready VISTA inhibiting antibody, which would be applied to the acquisition consideration if a definitive agreement is entered into with Kineta.
About TuHURA Biosciences, Inc.
TuHURA Biosciences, Inc. is a Phase 3 registration-stage immuno-oncology company developing novel technologies to overcome primary and acquired resistance to cancer immunotherapy, two of the most common reasons cancer immunotherapies fail to work in the majority of patients with cancer.
TuHURA’s lead innate immune response agonist candidate, IFx-2.0, is designed to overcome primary resistance to checkpoint inhibitors. TuHURA is preparing to initiate a single randomized placebo-controlled Phase 3 registration trial of IFx-2.0 administered as an adjunctive therapy to Keytruda® (pembrolizumab) in first line treatment for advanced or metastatic Merkel Cell Carcinoma.
In addition to its innate immune response agonist candidates, TuHURA is leveraging its Delta receptor technology to develop first-in-class bi-specific ADCs and PDCs targeting Myeloid Derived Suppressor Cells to inhibit their immune suppressing effects on the tumor microenvironment to prevent T cell exhaustion and acquired resistance to checkpoint inhibitors and cellular therapies.
For more information, please visit tuhurabio.com and connect with TuHURA on Facebook, X, and LinkedIn.
Forward-Looking Statements
This news release contains forward-looking statements that are not historical facts within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and other future conditions. In some cases you can identify these statements by forward-looking words such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “could,” “should,” “would,” “project,” “plan,” “expect,” “goal,” “seek,” “future,” “likely” or the negative or plural of these words or similar expressions. Examples of such forward-looking statements include but are not limited to express or implied statements regarding TuHURA’s expectations, hopes, beliefs, intentions or strategies regarding the future including, without limitation, statements regarding clinical trials and research and development programs, in particular with respect to TuHURA’s IFx-Hu2.0 product candidate and its ADC and PDC development program, and any developments or results in connection therewith; the anticipated timing of the results from those studies and trials; a potential acquisition of rights to Kineta Inc.’s KVA12123 product candidate; expectations regarding the use of capital resources, including the net proceeds from TuHURA’s financing; and the time period over which the combined company’s capital resources will be sufficient to fund its anticipated operations. In addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. You are cautioned that such statements are not guarantees of future performance and that actual results or developments may differ materially from those set forth in these forward-looking statements. Factors that could cause actual results to differ materially from these forward-looking statements are described in the “Risk Factors” section of the Company’s Annual Report on Form 10-K for the fiscal year ended June 30, 2023 filed with the Securities and Exchange Commission (“SEC”) and the proxy statement/prospectus dated August 19, 2024, as supplemented. Additional assumptions, risks and uncertainties are described in detail in our registration statements, reports and other filings with the SEC, which are available on the combined company’s website, and at www.sec.gov.
You are cautioned that such statements are not guaranteed of future performance and that our actual results may differ materially from those set forth in the forward-looking statements. The forward-looking statements and other information contained in this news release are made as of the date hereof, and TuHURA does not undertake any obligation to update publicly or revise any forward-looking statements or information, whether as a result of new information, future events or otherwise, unless so required by applicable securities laws. Nothing herein shall constitute an offer to sell or the solicitation of an offer to buy any securities.
Important Information About the Potential transaction and Where to Find It
This press release discloses that TuHURA and Kineta have entered into a non-binding letter of intent for a transaction in which TuHURA would acquire Kineta’s KVA12123 VISTA inhibiting antibody via a merger transaction structure (the “potential transaction”). The potential transaction, if any, is subject to negotiation, completion, and execution of a definitive acquisition or merger agreement (a “definitive agreement”), which will contain the full terms and conditions of the potential transaction. If a definitive agreement is entered into by TuHURA and Kineta in connection with the potential transaction, TuHURA and Kineta intend to file a proxy statement/prospectus with the SEC, which is expected to include a preliminary prospectus of
TuHURA and a proxy statement of Kineta in connection with the potential transaction, referred to as a proxy statement/prospectus. If a proxy statement/prospectus is filed, after it is cleared by the SEC, a definitive proxy statement/prospectus will be mailed or made available to Kineta’s stockholders as of a record date to be established for voting on the transaction and to the stockholders of TuHURA. TuHURA also will file other documents regarding the potential transaction with the SEC. INVESTORS AND STOCKHOLDERS ARE URGED TO READ THE DEFINITIVE PROXY STATEMENT/PROSPECTUS AND OTHER MATERIALS, IF ANY, THAT MAY BE FILED WITH THE SEC IN CONNECTION WITH THE POTENTIAL TRANSACTION CAREFULLY AND IN THEIR ENTIRETY IF AND WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT THE POTENTIAL TRANSACTION. THIS PRESS RELEASE IS NOT A SOLICITATION TO STOCKHOLDERS TO APPROVE ANY TRANSACTION.
Investors and stockholders will be able to obtain free copies of the proxy statement/prospectus and all other relevant documents filed or that will be filed with the SEC by TuHURA through the website maintained by the SEC at www.sec.gov. The documents filed by TuHURA with the SEC may also be obtained free of charge at TuHURA’s website at visit www.tuhurabio.com or upon written request to: TuHURA, 10500 University Drive, Suite 110, Tampa, Florida 33612.
NEITHER THE SEC NOR ANY STATE SECURITIES REGULATORY AGENCY HAS APPROVED OR DISAPPROVED THE POTENTIAL TRANSACTION DESCRIBED IN THIS PRESS RELEASE, PASSED UPON THE MERITS OR FAIRNESS OF THE POTENTIAL TRANSACTION OR RELATED TRANSACTIONS OR PASSED UPON THE ADEQUACY OR ACCURACY OF THE DISCLOSURE IN THIS PRESS RELEASE. ANY REPRESENTATION TO THE CONTRARY CONSTITUTES A CRIMINAL OFFENSE.
Participants in the Solicitation
If we solicit proxies for the potential transaction, TuHURA and Kineta and their respective directors and officers and other members of management may, under SEC rules, be deemed to be participants in the solicitation of proxies from stockholders in connection with the potential transaction and other matters that may be set forth in the proxy statement/prospectus. Information about TuHURA’s directors and executive officers is set forth in TuHURA’s filings with the SEC, including TuHURA’s Current Report on Form 8-K filed with the SEC on October 21, 2024. Additional information regarding the direct and indirect interests, by security holdings or otherwise, of those persons and other persons who may be deemed participants in the solicitation of proxies in the potential transaction may be obtained by reading the proxy statement/prospectus when it becomes available. You may obtain free copies of these documents as described above under “Important Information About the Potential transaction and Where to Find It.”
No Offer or Solicitation
This press release is not a proxy statement or solicitation of a proxy, consent or authorization with respect to any securities or in respect of the potential transaction and is not intended to and does not constitute an offer to sell or the solicitation of an offer to buy the securities of TuHURA or Kineta, nor shall there be any sale of any such securities in any state or jurisdiction in which such offer, solicitation, or sale would be unlawful prior to registration or qualification under the
securities laws of such state or jurisdiction. No offer of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended.
Investor/Media Contact:
Jenene Thomas
JTC Team, LLC
908.824.0775
tuhura@jtcir.com
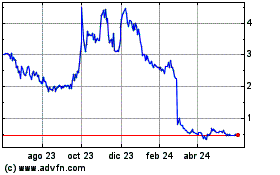
Kineta (NASDAQ:KA)
Gráfica de Acción Histórica
De Nov 2024 a Dic 2024
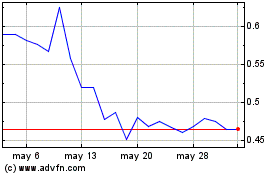
Kineta (NASDAQ:KA)
Gráfica de Acción Histórica
De Dic 2023 a Dic 2024