Lyra Therapeutics Announces Inducement Grants Under Nasdaq Listing Rule 5635(c)(4)
26 Abril 2024 - 3:51PM

Lyra Therapeutics, Inc. (Nasdaq: LYRA) (the “Company” or “Lyra”), a
clinical-stage biotech developing long-acting, anti-inflammatory
sinonasal implants for the treatment of chronic rhinosinusitis
(CRS), today announced that it has granted non-qualified stock
options to purchase a total of 78,400 shares of Lyra Therapeutics
common stock to 16 new non-executive employees as an inducement
material to their acceptance of employment with Lyra Therapeutics.
The employment inducement awards were approved by Lyra’s
independent directors serving on its Compensation Committee and
granted under Lyra’s 2022 Employment Inducement Award Plan, as
amended, and related form of stock option agreement in accordance
with Nasdaq Listing Rule 5635(c)(4).
The inducement plan is used exclusively for the
grant of equity awards to individuals who were not previously
employees of Lyra Therapeutics, or following a bona fide period of
non-employment, as an inducement material to such individuals
entering into employment with Lyra Therapeutics, pursuant to Nasdaq
Listing Rule 5635(c)(4).
Each option carries a ten-year term and an
exercise price per share equal to $4.72, which was the closing
price of Lyra’s common stock on April 26, 2024, the date of grant,
and vests over a four-year period as follows: 25% of the option
vests on the one year anniversary of the applicable employee’s
start date and an additional 1/48th of the option vests in equal
monthly installments over the following three years, subject to the
employee’s continued service through each vesting date.
About Lyra Therapeutics
Lyra Therapeutics, Inc. is a clinical-stage
biotechnology company developing therapies for the localized
treatment of patients with chronic rhinosinusitis (CRS), a highly
prevalent inflammatory disease of the paranasal sinuses which leads
to debilitating symptoms and significant morbidities. LYR-210 and
LYR-220 are bioabsorbable sinonasal implants designed to be
administered in a simple, in-office procedure and are intended to
deliver six months of continuous mometasone furoate drug therapy
(7500µg MF) to the sinonasal passages. LYR-210 is designed for
patients with narrow anatomy, primarily those who have not
undergone ethmoid sinus surgery, and is being evaluated in the
ENLIGHTEN Phase 3 clinical program, while LYR-220, an enlarged
implant, was evaluated in the BEACON Phase 2 clinical trial in
patients who have recurrent symptoms despite having had ethmoid
sinus surgery. These two product candidates are designed to treat
the estimated four million CRS patients in the United States who
fail medical management each year. For more information, please
visit www.lyratx.com and follow us on LinkedIn.
Contact Information:
Ellen Cavaleri, Investor
Relations615.618.6228ecavaleri@lyratx.com
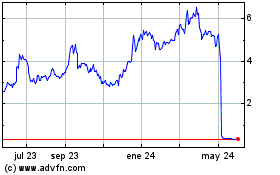
Lyra Therapeutics (NASDAQ:LYRA)
Gráfica de Acción Histórica
De Dic 2024 a Ene 2025
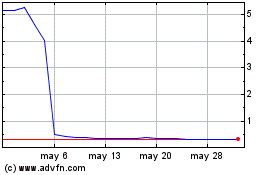
Lyra Therapeutics (NASDAQ:LYRA)
Gráfica de Acción Histórica
De Ene 2024 a Ene 2025