Form 424B3 - Prospectus [Rule 424(b)(3)]
01 Octubre 2024 - 7:07AM
Edgar (US Regulatory)
Filed
pursuant to 424(b)(3)
Registration
Statement No. 333-276487
PROSPECTUS
SUPPLEMENT NO. 6
(To
Prospectus dated June 21, 2024)
Microbot
Medical Inc.
1,769,966
Shares of Common Stock
This
prospectus supplement (this “Prospectus Supplement”) is being filed to update and supplement our prospectus dated June 21,
2024 as supplemented (the “Prospectus”), relating to the resale of up to 1,769,966 shares of our common stock, $0.01 par
value per share, representing shares issuable upon the exercise of outstanding preferred investment options held by the selling stockholders
named in the Prospectus, including their transferees, pledgees, donees or successors.
Specifically,
this Prospectus Supplement is being filed to update and supplement the information included in the Prospectus with certain information
reported by us with the Securities and Exchange Commission. Accordingly, we have included such information in this Prospectus Supplement
below. Any statement contained in the Prospectus shall be deemed to be modified or superseded to the extent that information in this
Prospectus Supplement modifies or supersedes such statement.
Capitalized
terms used but not defined herein have the meanings ascribed to them in the Prospectus.
This
Prospectus Supplement is not complete without, and may not be utilized except in connection with, the Prospectus, including any supplements
and amendments thereto.
We
may further amend or supplement the Prospectus and this Prospectus Supplement from time to time by filing amendments or supplements as
required. You should read the entire Prospectus, this Prospectus Supplement and any amendments or supplements carefully before you make
your investment decision.
Our
common stock is listed on The Nasdaq Capital Market under the symbol “MBOT”. On September 30, 2024, the closing price of
our common stock was $0.894.
Investing
in our common stock involves significant risks. You should read the section entitled “Risk Factors” beginning on page 11
of the Prospectus for a discussion of certain risk factors that you should consider before investing in our common stock.
Neither
the Securities and Exchange Commission nor any state securities commission or other regulatory body has approved or disapproved of these
securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The
date of this Prospectus Supplement is September 30, 2024
The
Company is currently experiencing an acceleration of patient enrollment for ACCESS-PVI human clinical trial, a prospective, multi-center,
single-arm trial to evaluate the performance and safety of LIBERTY® in human subjects undergoing Peripheral Vascular Interventions.
As a result of the increased rate of patient enrollment, 80% of the patients have completed the follow up period, and the Company now
anticipates completing the trial ahead of its prior expectation. The Company remains on track to file its 510(k) submission with the
U.S. Food and Drug Administration (FDA) by of the end of 2024. The Company also announced that is has successfully completed all biocompatibility
tests, as required by its Investigational Device Exemption (IDE) application and received full approval for the IDE study from the FDA.
In parallel with the clinical trial, the Company is performing additional customary bench testing, and these final results will be included
in the Company’s 510(k) submission.
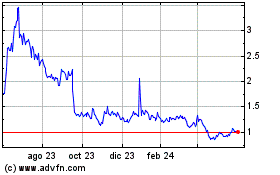
Microbot Medical (NASDAQ:MBOT)
Gráfica de Acción Histórica
De Oct 2024 a Nov 2024
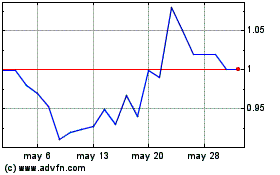
Microbot Medical (NASDAQ:MBOT)
Gráfica de Acción Histórica
De Nov 2023 a Nov 2024