- Etripamil, an investigational drug, showed
statistically significant reduction in ventricular rate by 30 beats
per minute for episodes of atrial fibrillation with rapid
ventricular rate (p < 0.0001) compared to
placebo
- Results also showed statistically significant rapid and
sustained reductions in ventricular rate and improvement in patient
reported symptoms
- Safety and tolerability data were generally consistent with
data from studies evaluating etripamil in PSVT
- Results support further clinical development in a Phase 3
clinical trial evaluating patient-administered etripamil for
management of AFib-RVR
MONTREAL and CHARLOTTE, N.C., Nov. 11, 2023 /CNW/ -- Milestone Pharmaceuticals
Inc. (Nasdaq: MIST) today announced positive Phase 2 data that show
etripamil nasal spray resulted in rapid and statistically superior
ventricular rate reduction and improved symptom-relief in patients
with atrial fibrillation with rapid ventricular rate (AFib-RVR)
compared to placebo. Safety and tolerability reported in the
56-patient safety population is generally consistent with that
observed in Milestone's much larger Phase 3 paroxysmal
supraventricular tachycardia (PSVT) program. The results were
presented as a Featured Science presentation at the American Heart
Association (AHA) Scientific Sessions 2023 and simultaneously
published in Circulation: Arrhythmia and Electrophysiology.
These data support further development of self-administered
etripamil for the treatment of AFib-RVR.
Incidence of atrial fibrillation (AFib) in the United States is expected to grow to
approximately 10 million by 2025 and up to about 12 million by
2030.[1],[2],[3] Patients with AFib-RVR continue to face
a significant unmet need for symptom relief. They can experience
the burden of symptomatic acute attacks and our market research
indicates 30-40 percent of patients with AFib experience one or
more symptomatic episodes of rapid ventricular rate (RVR) per year
requiring treatment.
"Breakthrough episodes of rapid ventricular rate in patients
with AFib are frequent and often symptomatic and may result in
increasing burdens in patients' everyday lives and disruptions to
the healthcare system. Today, there is an unmet need for a
portable, fast-acting treatment solution that can be easily
administered by patients when they experience a sudden
episode," said A. John Camm,
M.D., lead investigator, British Heart Foundation Emeritus
Professor of Clinical Cardiology, The Cardiology Clinical Academic
Group, Molecular and Clinical Sciences Research Institute,
St. George's University of
London, London, UK. "The results presented and
published today from the ReVeRA Phase 2 study are encouraging for
patients and healthcare providers who are seeking a treatment
solution that delivers significant symptom relief and helps reduce
potential visits to the emergency department."
"The results from the ReVeRA study are promising and demonstrate
the potential of etripamil nasal spray to rapidly reduce heart rate
and provide symptomatic benefit to patients suffering from
AFib-RVR," said David Bharucha, MD,
PhD, Chief Medical Officer, Milestone Pharmaceuticals. "We believe
our recent NDA submission for etripamil for the potential treatment
of PSVT, as well as FDA guidance received on AFib-RVR, provide a
strong foundation for the continued clinical development of
patient-administered etripamil with a Phase 3 study in
AFib-RVR."
Randomized, Controlled Study of the Efficacy and Safety of
Etripamil Nasal Spray: Findings from Phase 2 ReVeRA-201 Study (AHA
Featured Science Session)
The randomized, controlled Phase 2 ReVeRA study treated 56
patients aged 18 years and older presenting in an emergency
department or hospital with AFib with a ventricular rate of 110 or
more beats per minute (bpm) prior to receiving either etripamil
nasal spray or placebo. The study was designed to assess the
reduction in ventricular rate (primary endpoint), the time to
achieve maximum reduction in ventricular rate, the duration of
effect, and patient satisfaction with treatment using the Treatment
Satisfaction Questionnaire 9 (TSQM-9) patient reported outcome
(PRO) tool (secondary endpoints).
Data from the ReVeRA trial showed that delivery of etripamil
nasal spray significantly and rapidly reduced ventricular rate,
consistent with the drug's pharmacologic profile. The study
achieved its primary endpoint with high statistical significance
with patients experiencing a mean ventricular rate reduction of
29.91 bpm (95% confidence interval: -40.31, -19.52; p < 0.0001)
in the etripamil arm compared to placebo. The absolute maximum
reduction in rate was 35 bpm in the etripamil arm, compared with 5
bpm in the placebo arm. The median time to maximum reduction in
ventricular rate was 13 minutes in the etripamil arm, and time
course graphs of mean ventricular rate reduction illustrate onset
of etripamil within minutes after drug administration and lasting
approximately 150 minutes compared to placebo.
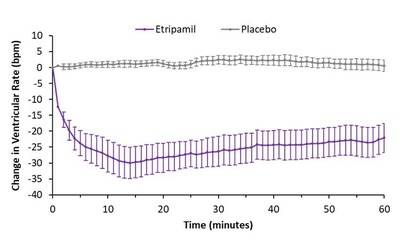
A greater number of patients receiving etripamil achieved a
ventricular rate of less than 100 bpm (58.3%) than those receiving
placebo (4%). Furthermore, 67% of patients receiving etripamil
achieved at least 20% reductions in ventricular rate and 96%
achieved at least 10% reductions in ventricular rate in the first
60 minutes compared to 0% and 20% on placebo, respectively. Using
the TSQM-9, compared to placebo, patients treated with etripamil
demonstrated significant improvements in two satisfaction ratings:
effectiveness (p < 0.0001) and relief of symptoms (p =
0.0002).
Serious adverse events (SAEs) occurring in the 24 hours after
drug were rare, with two occurring in one patient in the etripamil
arm (3.7%) and four occurring in two patients in the placebo arm
(6.9%). The SAEs in the etripamil arm were transient severe
bradycardia and syncope, assessed as due to hyper-vagotonia, which
occurred in a patient with a history of vagal events, and fully
resolved by placing the patient supine and without sequelae. The
most common (≥ 5%) adverse events were mild or moderate in
intensity and included nasal discomfort and congestion, rhinorrhea
("runny nose"), and dizziness.
Investor and Analyst Call and Webcast
The Company will host an investor and analyst call and webcast on
Monday, November 13, 2023, at
8:00 a.m. Eastern Time. The event
will feature a review of the ReVeRA data, an overview of AFib-RVR
and current treatment landscape, characteristics of etripamil, and
commentary on next steps for Milestone's clinical development
program for etripamil. To join the live call by phone, dial (877)
870-4263 (domestic) or (412) 317-0790 (international) and ask to be
connected to the Milestone Pharmaceuticals call. To access the live
or recorded webcast and accompanying slides, please visit the News
& Events section of Milestone's investor relations website
at investors.milestonepharma.com.
About Atrial Fibrillation with Rapid Ventricular
Rate
An estimated five million Americans suffer from atrial
fibrillation (AFib), a common arrhythmia marked by an irregular,
disruptive and often rapid heartbeat. The incidence of AFib is
expected to grow to approximately 10 million by 2025 and up to
about 12 million by 2030.1,2,3 A subset of patients with
AFib experience episodes of abnormally high heart rate most often
accompanied by palpitations, shortness of breath, dizziness, and
weakness. While these episodes, known as AFib-RVR, may be treated
by oral calcium channel blockers and/or beta blockers, patients
frequently seek acute care in the emergency department to address
symptoms. In 2016, nearly 800,000 patients were admitted to the
emergency department due to AFib symptoms where treatment includes
medically supervised intravenous administration of calcium channel
blockers or beta blockers, or electrical cardioversion. With little
available data for AFib-RVR, Milestone's initial market research
indicates that 30 to 40% of patients with AFib experience one or
more symptomatic episodes of RVR per year that require treatment,
suggesting a target addressable market of approximately three to
four million patients in 2030 for etripamil in patients with
AFib-RVR.
About Etripamil
Etripamil is Milestone's lead
investigational product. It is a novel calcium channel blocker
nasal spray under clinical development for elevated and often
highly symptomatic heart-rate attacks associated with PSVT and
AFib-RVR. It is designed to be a rapid-response therapy that is
self-administered by the patient, without the need for direct
medical oversight. If approved, etripamil is intended to provide
health care providers with a new treatment option to enable on
demand care and patient self-management. If approved, the portable
treatment, studied as self-administered, may provide patients with
active management and a greater sense of control over their
condition. CARDAMYST™, the conditionally approved brand
name for etripamil nasal spray, is well studied with a robust
clinical trial program that includes a completed Phase 3
clinical-stage program for the treatment of PSVT and Phase 2
proof-of-concept trial for the treatment of patients with
AFib-RVR.
About Milestone Pharmaceuticals
Milestone
Pharmaceuticals Inc. (Nasdaq: MIST) is a biopharmaceutical
company developing and commercializing innovative cardiovascular
medicines to benefit people living with certain heart conditions.
Milestone recently submitted a New Drug Application (NDA) to the
U.S. Food and Drug Administration (FDA) for etripamil for treatment
of an abnormal heart rhythm, paroxysmal supraventricular
tachycardia or PSVT. Find out more
at www.milestonepharma.com.
Forward-Looking Statements
This press release
contains forward-looking statements within the meaning of the
Private Securities Litigation Reform Act of 1995. Words such as
"believe," "continue," "could," "demonstrate," "designed,"
"develop," "estimate," "expect," "may," "pending," "plan,"
"potential," "progress," "will" and similar expressions (as well as
other words or expressions referencing future events, conditions,
or circumstances) are intended to identify forward-looking
statements. These forward-looking statements are based on
Milestone's expectations and assumptions as of the date of this
press release. Each of these forward-looking statements involves
risks and uncertainties. Actual results may differ materially from
these forward-looking statements. Forward-looking statements
contained in this press release include statements regarding the
anticipated growth of incidence of AFib and AFib-RVR by 2030; the
ability of etripamil to provide patients with a treatment solution
that delivers significant symptom relief and helps reduce potential
visits to the emergency department; the ability of etripamil nasal
spray to rapidly reduce heart rate and provide symptomatic benefit
to patients suffering from AFib-RVR; the continued ability of
etripamil provided superior time to conversion to normal heart
rhythm compared to placebo; the timing of the anticipated launch of
etripamil; the success of the NDA submission for etripamil nasal
spray and the timing of the FDA's approval of the NDA; and timing
of the Phase 2 proof-of-concept trial of etripamil for the
treatment of patients with AFib-RVR. Important factors that could
cause actual results to differ materially from those in the
forward-looking statements include, but are not limited to, the
risks inherent in biopharmaceutical product development and
clinical trials, including the lengthy and uncertain regulatory
approval process; uncertainties related to the timing of
initiation, enrollment, completion, evaluation and results of our
clinical trials; risks and uncertainty related to the complexity
inherent in cleaning, verifying and analyzing trial data; and
whether the clinical trials will validate the safety and efficacy
of etripamil for PSVT or other indications, among others, general
economic, political, and market conditions, including deteriorating
market conditions due to investor concerns regarding inflation and
Russian hostilities in Ukraine and
ongoing disputes in Israel and
Gaza and overall fluctuations in
the financial markets in the United
States and abroad, risks related to pandemics and public
health emergencies, and risks related the sufficiency of
Milestone's capital resources and its ability to raise additional
capital in the current economic climate. These and other risks are
set forth in Milestone's filings with the U.S. Securities and
Exchange Commission, including in its annual report on Form 10-K
for the year ended December 31, 2022,
and its Quarterly Report on Form 10-Q for the quarter ended
June 30, 2023 under the caption "Risk
Factors," as such discussion may be updated from time to time by
subsequent filings, we may make with the U.S. Securities and
Exchange Commission. Except as required by law, Milestone assumes
no obligation to update any forward-looking statements contained
herein to reflect any change in expectations, even as new
information becomes available.
1 Colilla S, Crow A, Petkun W, Singer DE, Simon
T, Liu X. Estimates of current and future incidence and prevalence
of atrial fibrillation in the U.S. adult population. Am J
Cardiol. 2013;112:1142–1147.
2 Miyasaka Y, Barnes ME, Gersh BJ, et
al. Secular trends in incidence of atrial fibrillation in
Olmsted County, Minnesota, 1980 to
2000, and implications on the projections for future
prevalence. Circulation. 2006;114:199–225.
3 Benjamin, E. J., Muntner, P., et al. American
Heart Association Council on Epidemiology and Prevention Statistics
Committee and Stroke Statistics Subcommittee (2019). Heart Disease
and Stroke Statistics-2019 Update: A Report From the American Heart
Association. Circulation, 139(10), e56–e528.
Contact:
Kim Fox, Vice
President, Communications
kfox@milestonepharma.com
704-803-9295
 View original content to download
multimedia:https://www.prnewswire.com/news-releases/milestone-pharmaceuticals-presents-positive-results-from-revera-phase-2-study-of-etripamil-in-afib-rvr-at-the-american-heart-association-scientific-sessions-2023-301985199.html
View original content to download
multimedia:https://www.prnewswire.com/news-releases/milestone-pharmaceuticals-presents-positive-results-from-revera-phase-2-study-of-etripamil-in-afib-rvr-at-the-american-heart-association-scientific-sessions-2023-301985199.html
SOURCE Milestone Pharmaceuticals, Inc.