false 0001628171 0001628171 2023-10-22 2023-10-22
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): October 22, 2023
REVOLUTION MEDICINES, INC.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
| Delaware |
|
001-39219 |
|
47-2029180 |
| (State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification Number) |
700 Saginaw Drive
Redwood City, California 94063
(Address of principal executive offices, including Zip Code)
Registrant’s telephone number, including area code: (650) 481-6801
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading Symbol |
|
Name of each exchange on which registered |
| Common Stock, $0.0001 par value per share |
|
RVMD |
|
The Nasdaq Stock Market LLC (Nasdaq Global Select Market) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 |
Regulation FD Disclosure. |
On October 22, 2023, Revolution Medicines, Inc. (the “Company”) posted an updated corporate presentation to the investor section of the Company’s website at: ir.revmed.com/events-and-presentations. The Company’s updated corporate presentation is attached hereto as Exhibit 99.1.
The furnishing of the attached presentation is not an admission as to the materiality of any information therein. The information contained in the slides is summary information that is intended to be considered in the context of more complete information included in the Company’s filings with the U.S. Securities and Exchange Commission (the “SEC”) and other public announcements that the Company has made and may make from time to time by press release or otherwise. The Company undertakes no duty or obligation to update or revise the information contained in this Current Report on Form 8-K, although it may do so from time to time as its management believes is appropriate. Any such updating may be made through the filing of other reports or documents with the SEC, through press releases or through other public disclosures. For important information about forward looking statements, see the slide titled “Legal Disclaimer” in Exhibit 99.1 attached hereto.
The information furnished under this Item 7.01 and in the presentation attached as Exhibit 99.1 to this Current Report on Form 8-K shall not be deemed “filed” for purposes of Section 18 of the Exchange Act or otherwise subject to the liabilities of that section or Sections 11 or 12(a)(2) of the Securities Act. The information contained in this Item 7.01 and in the presentation attached as Exhibit 99.1 to this Current Report on Form 8-K shall not be incorporated by reference into any filing with the SEC made by the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
On October 22, 2023, the Company provided the following pipeline updates.
RMC-6236
The Company reported updated preliminary safety and anti-tumor data for RMC-6236, its RASMULTI(ON) Inhibitor.
In the Company’s ongoing RMC-6236-001 Phase 1/1b trial, a total of 111 patients with either non-small cell lung cancer (“NSCLC”) (n=46) or pancreatic ductal adenocarcinoma (“PDAC”) (n=65) treated across six dose cohorts ranging from 80 mg daily to 400 mg daily were evaluated for safety and tolerability as of a data cut-off date of October 12, 2023 (the “Data Cut-off Date”). Patients at dose levels below 80 mg daily were not included in this analysis based on the Company’s preclinical and pharmacokinetics predictions that these dose levels would not be associated with tumor regressions in patients and its clinical observations of these doses. Common RAS mutations in patients evaluated included G12D, G12V, G12R, G12A and G12S. Patients with KRASG12C mutations were excluded from the study due to the availability of currently approved KRASG12C(OFF) inhibitors. All of these patients had previously been treated with standard of care appropriate for tumor type and stage. Patients with NSCLC had received a median of two prior lines of therapy (range: 1–6) and patients with PDAC had received a median of three prior lines of therapy (range: 1–7).
As of the Data Cut-off Date, the Company observed that RMC-6236 demonstrated an acceptable safety profile that was generally well tolerated across the dose levels analyzed (Table 1). Median duration of treatment as of the Data Cut-off Date was 2.1 months (range: 0.2–10.9). The most common treatment-related adverse events (“TRAEs”) were rash and gastrointestinal (“GI”)-related toxicities that were primarily Grade 1 or 2 in severity. One previously reported Grade 4 TRAE occurred in a patient with PDAC at the 80 mg dose level who had a large intestine perforation at the site of an invasive tumor that reduced in size while on treatment, which resulted in treatment discontinuation. No fatal TRAEs were observed. No safety signals were observed that indicated an elevated risk of hepatotoxicity, which has been reported for some KRASG12C(OFF) inhibitors.
Table 1. RMC-6236-001: Select treatment-related adverse events for patients with NSCLC and PDAC Treated at ≥80 mg daily
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total (N=111) |
|
| Maximum Severity of Treatment-Related AEs (TRAEs) |
|
Grade 1 |
|
Grade 2 |
|
Grade 3 |
|
Grade 4 |
|
Any Grade |
| TRAEs occurring in ≥10% of patients, n (%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Rash‡ |
|
|
|
58(52) |
|
|
|
|
25(23) |
|
|
|
|
7(6) |
|
|
|
|
— |
|
|
|
|
90(81) |
|
| Nausea |
|
|
|
40(36) |
|
|
|
|
11(10) |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
51(46) |
|
| Diarrhea |
|
|
|
28(25) |
|
|
|
|
14(13) |
|
|
|
|
1(1) |
|
|
|
|
— |
|
|
|
|
43(39) |
|
| Vomiting |
|
|
|
30(27) |
|
|
|
|
7(6) |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
37(33) |
|
| Stomatitis |
|
|
|
13(12) |
|
|
|
|
9(8) |
|
|
|
|
2(2) |
|
|
|
|
— |
|
|
|
|
24(22) |
|
| Fatigue |
|
|
|
11(10) |
|
|
|
|
6(5) |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
17(15) |
|
| Other select TRAEs, n (%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ALT elevation |
|
|
|
8(7) |
|
|
|
|
1(1) |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
9(8) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Maximum Severity of Treatment-Related AEs (TRAEs) |
|
Grade 1 |
|
Grade 2 |
|
Grade 3 |
|
Grade 4 |
|
Any Grade |
| AST elevation |
|
|
|
8(7) |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
8(7) |
|
| Electrocardiogram QT prolonged |
|
|
|
1(1) |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
1(1) |
|
| TRAEs leading to dose reduction*, n (%) |
|
|
|
— |
|
|
|
|
10(9) |
|
|
|
|
5(5) |
† |
|
|
|
— |
|
|
|
|
15(14) |
|
| TRAEs leading to treatment discontinuation, n (%) |
|
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
|
1 |
(1)^ |
|
|
|
1(1) |
|
AE, adverse event; ALT, alanine transaminase; AST, aspartate transferase; TRAEs, treatment-related adverse events.
‡ Includes preferred terms of dermatitis acneiform, rash maculopapular, rash, rash pustular, erythema, rash erythematous; multiple types of rash may have occurred in the same patient.
* The most common reason for dose reduction was rash.
† Grade 3 TRAEs leading to reduction were rash (n=4), including one patient with a dose reduction due to rash and decreased appetite, and stomatitis (n=1).
^ One Grade 4 TRAE occurred in a patient with PDAC at the 80 mg dose level who had a large intestine perforation at the site of an invasive tumor that reduced in size while on treatment.
Clinical activity was evaluated as of the Data Cut-off Date in patients with NSCLC (n=40) and PDAC (n=46) who had received the first dose of RMC-6236 at least eight weeks prior to the Data Cut-off Date (n=86). Confirmed objective responses included tumors harboring KRAS mutations G12D, G12V or G12R, and disease control was observed across all KRAS mutations, including G12A and G12S.
As of the Data Cut-off Date, RMC-6236 demonstrated preliminary evidence of clinical activity in efficacy-evaluable NSCLC patients (Figure 1).
Figure 1. RMC-6236-001: Change in tumor burden from efficacy-evaluable KRASG12X NSCLC patients
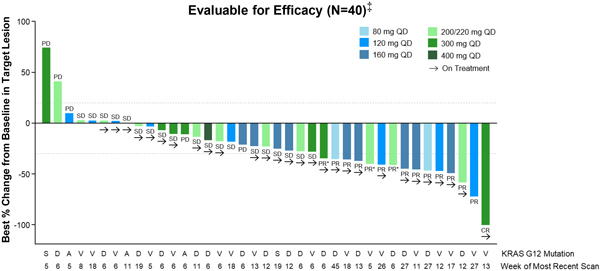
Data extracted October 12, 2023.
QD, daily dosing; PD, progressive disease; SD, stable disease; PR, partial response; PR*, unconfirmed PR per Response Evaluation Criteria in Solid Tumors (“RECIST”) 1.1; CR, complete response.
‡ Patients who received first dose of RMC-6236 at least eight weeks prior to data extract date.
Among the efficacy evaluable NSCLC patients, the objective response rate was 38 percent, with one patient achieving a complete response (“CR”) as a best response and 14 patients achieving a partial response (“PR”) (including three unconfirmed PRs) (Table 2). The disease control rate (“DCR”) in this NSCLC population was 85 percent.
Table 2. RMC-6236-001: Tumor Response per RECIST for efficacy-evaluable KRASG12X NSCLC patients
|
|
|
|
|
| Tumor Response (per RECIST 1.1) |
|
| Best Overall Response, n (%) |
|
|
|
|
| CR PR SD PD NE† |
|
|
1(3)
14(35) 19(48) 5(13) 1(3) |
|
| ORR, n (%) |
|
|
15(38) |
|
| Confirmed, n |
|
|
12 |
|
| DCR (CR+PR+SD), n (%) |
|
|
34(85) |
|
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluable; ORR, objective response rate; DCR, disease control rate.
† One subject withdrew from study without post-baseline scans.
As of the Data Cut-off Date, RMC-6236 demonstrated preliminary evidence of clinical activity in efficacy-evaluable PDAC patients (Figure 2).
Figure 2. RMC-6236-001: Change in tumor burden from efficacy-evaluable KRASG12X PDAC patients
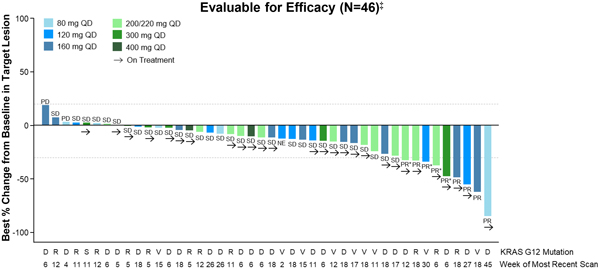
Data extracted October 12, 2023.
QD, daily dosing; PD, progressive disease; SD, stable disease; PR, partial response; PR*, unconfirmed PR per RECIST 1.1.
‡ Patients who received first dose of RMC-6236 at least eight weeks prior to data extract date.
Among the efficacy-evaluable PDAC patients, the objective response rate was 20 percent, with nine patients achieving a PR (including four unconfirmed PRs) as a best response (Table 3). The DCR in this PDAC population was 87 percent.
Table 3. RMC-6236-001: Tumor Response per RECIST for efficacy-evaluable KRASG12X PDAC patients
|
|
|
|
|
| Tumor Response (per RECIST 1.1) |
|
| Best Overall Response, n (%) |
|
|
|
|
| PR SD PD NE† |
|
|
9(20)
31(67) 3(7) 3(7) |
|
| ORR, n (%) |
|
|
9(20) |
|
| Confirmed, n |
|
|
5 |
|
| DCR (CR+PR+SD), n (%) |
|
|
40(87) |
|
PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluable; ORR, objective response rate; DCR, disease control rate.
† Two patients died prior to first post-baseline scan; one patient had scan after 11 days of treatment and subsequently died due to PD.
The Company is planning a global randomized phase 3 trial comparing RMC-6236 against docetaxel in patients with previously treated RAS-mutated NSCLC who have been treated with immunotherapy and platinum-containing chemotherapy. The study design for this planned trial is subject to change based on regulatory authority feedback. The Company is aiming to initiate this study in 2024.
The Company may potentially plan a global randomized phase 3 trial comparing RMC-6236 against a physician’s choice of chemotherapy regimens in patients with previously treated RAS-mutated PDAC. The study design for this potential trial is subject to change based on regulatory authority feedback. The Company expects to make a decision regarding plans for this study after additional patient follow-up regarding durability of response and dose optimization, but the Company believes the study could potentially be initiated in 2024.
Planning is underway for one or more combination pivotal clinical trials for RMC-6236 with standard of care therapies.
RMC-6291
Active recruitment of patients is underway for the Phase 1/1b clinical trial to evaluate the combination of RMC-6236 and RMC-6291.
RMC-4630
Based on the Company’s review of the complete data set from the RMC-4630-03 study, the Company concluded that the combination of RMC-4630 (200 mg intermittent dosing D1D2) with sotorasib (960 mg daily dosing) showed additive side effects compared to either agent alone and that tolerability was insufficient to confer a clinical benefit (objective response rate or durability) due to dose interruptions and discontinuations. The Company has no immediate plans for further development of RMC-4630, but believes this compound remains an option for potential evaluation in other combinations.
Forward-Looking Statements
This Current Report on Form 8-K contains forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. Any statements in this report that are not historical facts may be considered “forward-looking statements,” including, without limitation, statements regarding the scope, progress and results of developing the Company’s product candidates, and conducting clinical trials. Forward-looking statements are typically, but not always, identified by the use of words such as “may,” “will,” “would,” “believe,” “intend,” “plan,” “anticipate,” “estimate,” “expect” and other similar terminology indicating future results. Such forward-looking statements are subject to substantial risks and uncertainties that could cause the Company’s development programs, future results, performance or achievements to differ materially from those anticipated in the forward-looking statements. Such risks and uncertainties include, without limitation, risks and uncertainties inherent in the drug development process, including the Company’s programs’ early stage of development, the process of designing and conducting preclinical and clinical trials, the
regulatory approval processes, the timing of regulatory filings, the challenges associated with manufacturing drug products, the Company’s ability to successfully establish, protect and defend its intellectual property, other matters that could affect the sufficiency of the Company’s capital resources to fund operations, reliance on third parties for manufacturing and development efforts, changes in the competitive landscape and the effects on the Company’s business of global events and other macroeconomic conditions. For a further description of the risks and uncertainties that could cause actual results to differ from those anticipated in these forward-looking statements, as well as risks relating to the business of the Company in general, see the Company’s Quarterly Report on Form 10-Q filed with the SEC on August 8, 2023, and its future periodic reports to be filed with the SEC. Except as required by law, the Company undertakes no obligation to update any forward-looking statements to reflect new information, events or circumstances, or to reflect the occurrence of unanticipated events.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
REVOLUTION MEDICINES, INC. |
|
|
|
|
| Date: October 23, 2023 |
|
|
|
By: |
|
/s/ Mark A. Goldsmith |
|
|
|
|
|
|
Mark A. Goldsmith, M.D., Ph.D. President and Chief Executive Officer |

Exhibit 99.1 On Target to Outsmart Cancer October 22, 2023 © 2023
Revolution Medicines

2 Legal Disclaimer This presentation contains forward-looking statements
within the meaning of the Private Securities Litigation Reform Act. All statements other than statements of historical facts contained in this presentation, including statements regarding our future results of operations and financial position,
business strategy, prospective products, availability of funding, ability to manage existing collaborations and establish new strategic collaborations, licensing or other arrangements, the scope, progress, results and costs of developing our product
candidates or any other future product candidates, conducting clinical trials, the potential market size and size of the potential patient populations for our product candidates, the timing and likelihood of success of obtaining product approvals,
plans and objectives of management for future operations, the scope of protection we are able to establish and maintain for intellectual property rights covering our product candidates, future results of anticipated products the impact of global
events and other macroeconomic conditions on our business, the expected timing of closing of the proposed transaction with EQRx, Inc. (EQRx) and the expected benefits of the proposed transaction are forward-looking statements. These statements
involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the
forward-looking statements. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking
statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. The
information included in these materials is provided as of October 22, 2023 unless specified elsewhere herein, and is qualified as such. Except as required by applicable law, we undertake no obligation to update any forward-looking statements or
other information contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. For a further description of the risks and uncertainties that could cause actual results to differ from those
anticipated in these forward-looking statements, as well as risks relating to the business of Revolution Medicines in general, see Revolution Medicines’ Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August
8, 2023, and its future periodic reports to be filed with the Securities and Exchange Commission. This presentation concerns product candidates that are under clinical investigation and which have not yet been approved for marketing by the U.S. Food
and Drug Administration (FDA). These product candidates are currently limited by Federal law to investigational use, and no representation is made as to their safety or effectiveness for the purposes for which they are is being investigated. All
copyrights and trademarks used herein are the property of their respective owners.

3 Mission: to revolutionize treatment for patients with RAS-addicted
cancers through the discovery, development and delivery of innovative, targeted medicines. • Pioneering class of drug candidates designed to serve RAS-addicted cancer patients by targeting oncogenic RAS(ON) drivers of common, life-threatening
cancers MULTI • Unprecedented RAS inhibitor (RMC-6236) and G12C RAS -selective inhibitor (RMC-6291) show highly differentiated and promising initial clinical profiles • Innovative single agent and combination development strategies aim
to deliver durable clinical benefit broadly to patients with RAS-addicted cancers

4 Excessive RAS(ON) Signaling Drives 30% of Human Cancers, Targeted by
Our RAS(ON) Inhibitors RAS-Mutant Cancers = RAS(OFF) (1) Normal New patients per year (U.S.) RAS Cancer Mutations = RAS(ON) >200,000 including Cell 60,000 Membrane Lung cancer (29% of NSCLC) Excessive Tightly regulated RAS(ON) signaling drives
RAS(ON) proteins 75,000 uncontrolled cell growth control cell growth Colorectal cancer (49% of CRC) Oncogenic mutations in 53,000 KRAS, NRAS or HRAS Pancreatic cancer are common at positions (92% of PDAC) G12, G13 and Q61 (1) Estimated using tumor
mutation frequencies from Foundation Medicine Insights March 2022 and scaled to estimated patient numbers using cancer incidence from ACS Cancer Facts and Figures 2023 (see appendix for additional detail); NSCLC = non-small cell lung cancer; CRC =
colorectal cancer; PDAC = pancreatic ductal adenocarcinoma

5 Pioneering Tri-complex RAS(ON) Inhibitors Designed to Deliver Robust
and Durable Anti-Tumor Activity • Direct inhibition of RAS(ON) Oncogenic RAS(ON) Inhibited RAS(ON) cancer drivers RAS(ON) Inhibitor • Deep and durable suppression of GTP RAS cancer signaling designed to defy common drug resistance
mechanisms • Clinical validation of first two RAS(ON) Inhibitors studied as single agents Cell Cyclophilin A Membrane

6 Development-Stage RAS(ON) Inhibitor Portfolio Designed to Treat Nearly
All Patients with RAS-Addicted Cancers >200,000 new patients (1) RAS Selectivity MULTI per year (U.S.) multiple mutations RMC-6236 G12C clinical (initial focus on G12X) and WT Mutant-Selective G12D RMC-6291 clinical G12C G12X RMC-9805 clinical
G12D G12V G12V IND-enabling RMC-5127 G12 IND-enabling Q61H RMC-0708 other G13X RMC-8839 IND-enabling G13C Q61X (1) Estimated using tumor mutation frequencies from Foundation Medicine Insights March 2022 and scaled to estimated patient numbers using
cancer incidence from ACS Cancer Facts and Figures 2023 (see appendix for additional detail).

7 Complementary RAS(ON) Inhibitors Designed for Monotherapy and
Combination Strategies Against RAS-Addicted Cancers MULTI RAS RAS Mutant-Selective • Monotherapy with broad • Alternative monotherapy potential for RAS-addicted approaches cancers MULTI • Complementary to RAS • Backbone of
RAS(ON) Inhibitor Inhibitor in RAS(ON) Inhibitor doublets with mutant-selective doublets RAS(ON) Inhibitors • Differentiated targeted agent • Targeted agent for SOC profiles for SOC combinations, combinations, including including
immunotherapies immunotherapies SOC = standard of care

8 MULTI RMC-6236: First-in-Class, RAS (ON) Inhibitor with Broad
Potential Against RAS-Addicted Cancers • Highly selective for RAS(ON) proteins with broad and deep anti-tumor activity in preclinical models • Orally bioavailable and generally well-tolerated in patients at active doses 166,000 •
Unprecedented clinical profile with anti-tumor G12X New KRAS patients (1) activity observed across diverse RAS cancer per year (U.S.) mutations; multiple potential monotherapy registrational paths • Profound combinatorial activity with mutant-
selective RAS(ON) Inhibitors in preclinical models; potential for targeted RAS(ON) Inhibitor doublets in patients (1) Estimated using tumor mutation frequencies from Foundation Medicine Insights March 2022 and scaled to estimated patient numbers
using cancer incidence from ACS Cancer Facts and Figures 2023 (see appendix for additional detail); NSCLC = non-small cell lung cancer; CRC = colorectal cancer; PDAC = pancreatic ductal adenocarcinoma

9 RMC-6236: Dose-Dependent Anti-Tumor Activity at Low Doses in
RAS-Addicted NSCLC Model End of Study Responses Control RMC-6236 1 mg/kg 3000 RMC-6236 10 mg/kg 500 Control RMC-6236 25 mg/kg RMC-6236 1 mg/kg 400 RMC-6236 10 mg/kg RMC-6236 25 mg/kg 2000 300 200 1000 Dosing 100 start 8/10 R 10/10 CR 0 0 20 30 40 50
-100 Days on Study RVMD preclinical research NSCLC = non-small cell lung cancer G12V/WT NCI-H441 CDX (NSCLC, KRAS ); All doses given orally, once daily R = number of regressions >10% from initial; CR = number of regressions ≥80% from
initial Each animal represented as a separate bar in waterfall plot 3 Mean Tumor Volume (mm ) % Change in Tumor Volume

10 RMC-6236: Highly Active with Durable Responses Across Models G12X of
Major Human Cancers with RAS Drivers NSCLC PDAC CRC PFS 53% ORR (8/15) 61% ORR (11/18) 44% ORR (8/18) RMC-6236 – Median not reached 100% DCR (15/15) 89% DCR (16/18) 56% DCR (10/18) Control – Median 9 days 300 300 300 100 200 200 200 75
mPD 100 100 100 50 mSD 25 0 0 0 mPR 0 mCR -100 -100 -100 0 20 40 60 80 100 Days on Treatment RMC-6236 (n=191, 51 models) Control (n=215, 51 models) RVMD preclinical research as of 06/01/22 NSCLC = non-small cell lung cancer; PDAC = pancreatic ductal
adenocarcinoma; CRC = colorectal cancer RMC-6236 dosed at 25 mg/kg po qd; n=1-10/group Progression defined as tumor doubling from baseline; Responses assigned according to mRECIST: mPD = progressive disease; mSD = stable disease; mPR = partial
response; mCR = complete response; ORR = objective response rate; DCR = disease control rate; PFS = progression-free survival Mean Tumor Volume % Change From Baseline LUN352 NCI-H2122 CTG-1903 LUN232 CTG-0743 CTG-1955 CTG-2393 CTG-1612 NCI-H2030
CTG-2803 NCI-H441 CTG-1358 NCI-H358 LUN020 LUN137 KP-4 PAN022 PAN026 PAN1001 PAN038 PAN020 PAN003 PAN010 PAN014 PAN039 Capan-2 PAN001 PAN045 PAN031 PAN028 HPAC PAN043 PAN009 CRC007 CRC043 CRC078 CRC022 CRC060 CRC1018 CRC050 CRC047 CRC044 CRC058 GP2D
CRC051 CRC039 CRC012 SW620 CRC1009 SW403 CRC1005 % Tumors Progression-Free

11 RMC-6236-001 Phase 1 Study Design Key Eligibility Criteria Dose
Escalation RMC-6236 administered orally QD • Advanced solid tumors (1) G12X with KRAS mutations 500 mg (currently excluding G12C 400 mg KRAS ) • Received prior standard 300 mg Dose Expansion / therapy appropriate for (2) 220 mg
Optimization tumor type and stage 160 mg • ECOG PS 0–1 Lowest dose/exposure • No active brain metastases 120 mg range projected to drive tumor regressions in 80 mg humans based on Key Endpoints preclinical models 40 mg •
Safety and tolerability 20 mg • Pharmacokinetics 10 mg Additional patients with PDAC or NSCLC were enrolled at dose • Anti-tumor activity levels that cleared DLT evaluation G12X (1) KRAS defined as mutation at codon 12 which encodes
glycine (G) to X where X = A, D, R, S, or V. (2) 220 mg cleared DLT evaluation and a dose of 200 mg was selected for further expansion/optimization. DLT, dose-limiting toxicity; ECOG PS, Eastern Cooperative Oncology Group Performance Status; QD,
once daily.

12 RMC-6236: Patient Demographics and Baseline Characteristics Total
Tumor KRAS Genotypes, n (%) n=131 Age, median (range), years 64 (30–86) Male, n (%) 69 (53) Tumor type, n (%) G12V PDAC 69 (53) 37 (28) G12D NSCLC 47 (36) 67 (51) CRC 10 (7) Other* 5 (4) G12R 14 (11) ECOG PS, n (%) 0 40 (31) 1 91 (69) Number
of prior anti-cancer therapies, median (range) 2 (1–7) G12A G12S 8 (6) 5 (4) PDAC = pancreatic ductal adenocarcinoma; NSCLC = non-small cell lung cancer; CRC = colorectal cancer ECOG PS, Eastern Cooperative Oncology Group Performance Status
Data Extracted 11 Sep 2023.

13 RMC-6236: Dose-Dependent Increases in Exposure Mean Steady-State
Blood PK Individual Steady-State Blood AUC Profiles 400 mg Exposure in mice at 25 300 mg mg/kg* 200/220 mg Exposure in mice at 10 160 mg mg/kg* 120 mg 80 mg 40 mg 20 mg 10 mg Time (Hours) Dose Level • Dose-dependent increases in exposure with
minimal accumulation were observed after repeat daily dosing (1) G12X • Dose levels ≥80 mg achieved exposures that induced tumor regressions in human xenograft models with KRAS mutations in mice • 10 mg/kg QD induces tumor
regressions in sensitive models • 25 mg/kg QD induces tumor regressions in the majority of models *Exposure corrected with cross-species protein binding and blood/plasma partitioning. Left: steady-state concentrations from Cycle 1 Day 15.
Error bars represent standard deviation; right: steady-state AUC is Cycle 1 Day 15 AUC . Each circle represents an individual patient AUC. Horizontal bars represent mean AUC for each dose level (10 mg: n=2; 20 mg: n=4; last 40 mg: n=7; 80 mg: n=8;
120 mg: n=12; 160 mg: n=12, 200 mg: n=13; 220 mg: n=4; 300 mg: n=9; 400 mg: n=2); AUC, area under the curve; PK, pharmacokinetics. Data Extracted 22 Sep 2023. (1) Singh M, et al. Presentation at American Association for Cancer Research Annual
Meeting, 8–13 April 2022, New Orleans, USA; abstract #3597. Steady-State Whole Blood Concentration (nM) Whole Blood Steady-State AUC (nM x h)

14 RMC-6236: Summary of Treatment-Related Adverse Events Total (n=131)
Maximum severity of TRAEs Grade 1 Grade 2 Grade 3 Grade 4 Any Grade TRAEs occurring in ≥10% of patients, n (%) * Rash 57 (44) 29 (22) 6 (5) 0 92 (70) Nausea 41 (31) 14 (11) 0 0 55 (42) Diarrhea 32 (24) 9 (7) 1 (1) 0 42 (32) Vomiting 27 (21) 9
(7) 0 0 36 (28) Stomatitis 10 (8) 9 (7) 2 (2) 0 21 (16) Fatigue 12 (9) 4 (3) 0 0 16 (12) Other select TRAEs, n (%) ‡ ALT elevation 6 (5) 1 (1) 1 (1) 0 8 (6) ‡ AST elevation 6 (5) 0 1 (1) 0 7 (5) Electrocardiogram QT prolonged 1 (1) 0 0 0
1 (1) † TRAEs leading to dose reduction , n (%) 0 9 (7) 2 (2) 0 11 (8) TRAEs leading to treatment discontinuation, n (%) 0 0 0 1 (1) 1 (1) • Median duration of treatment at the time of data extraction was 2.27 months (range:
0.2–14) • One Grade 4 TRAE occurred in a patient with PDAC treated at 80 mg who had a large intestine perforation at the site of an invasive tumor that reduced in size while on treatment (TRAE leading to treatment discontinuation)
• No fatal TRAEs were observed. Two patients discontinued study treatment due to death: one patient with PDAC (120 mg) died due to PD; one patient with NSCLC (200 mg) died due to unknown cause reported as unrelated to RMC-6236 ‡
Post-data extraction, the Grade 3 ALT and AST elevations were associated with biliary obstruction and reported as unrelated to RMC-6236 *Includes preferred terms of dermatitis acneiform, rash maculopapular, rash, rash pustular, dermatitis
psoriasiform, erythema, rash erythematous; multiple types of rash may have occurred in the † same patient; The most common TRAE leading to dose reduction was rash (acneiform or maculopapular); there were no reductions at doses ≤80 mg.
AE, adverse event; ALT, alanine transaminase; AST, aspartate transferase; PD, progressive disease; TRAEs, treatment-related adverse events. Data Extracted 11 Sep 2023.

15 G12X KRAS NSCLC: Best Overall Response to RMC-6236 (a) Evaluable for
Efficacy (n = 40) Tumor Response (per RECIST 1.1) Best overall response, n (%) Complete response 1 (3) Partial response 14 (35) Stable disease 19 (48) Progressive disease 5 (13) (b) Not evaluable 1 (3) ORR, n (%) 15 (38) Confirmed, n 12 DCR
(CR+PR+SD), n (%) 34 (85) KRAS G12 Mutation Week of Most Recent scan *Unconfirmed PR per RECIST 1.1. (a) Patients who received first dose of RMC-6236 at least 8 weeks prior to data extract date. Data Extracted 12 Oct 2023. (b) One subject withdrew
from study without post-baseline scans. Best % Change from Baseline in Target Lesion

16 G12X KRAS NSCLC: Duration of Treatment and Responses to RMC-6236 (a)
Evaluable for Efficacy (n = 40) Median time to response: 1.4 months (range, 1.2–2.7 months) Median time on treatment: 3.1 months (range, 0.5–10.9 months) * Duration of Treatment (Weeks) (a) Patients who received first dose of RMC-6236 at
least 8 weeks prior to data extract date. Data Extracted 12 Oct 2023. *Death due to PD (n=1), Death due to unrelated AE (n=1), Death due to unknown cause reported as unrelated to RMC-6236 (n=1). G12X Patients with KRAS Mutation

17 G12V Case Report: Patient with KRAS NSCLC on RMC-6236 On Treatment
Baseline RMC-6236, Demographics and Baseline Week 6 Characteristics • 83-year-old woman • Former smoker (~60 pack years) • Diagnosed with NSCLC in 2021 Treatment History • Prior therapies: • Ipilimumab/nivolumab •
Paclitaxel Target Lesion: Lung, Left Lower Lobe • Carboplatin/pemetrexed Target Lesion Baseline On Treatment RMC-6236 Treatment Course • Started at 300 mg QD 1. Lung (left upper lobe) 11.6 mm 0 mm • Clinical improvement in cough 2.
Lung (left lower lobe) 49.8 mm 0 mm and dyspnea within one week of Sum of Diameters 61.4 mm 0 mm (−100% ↓) start of treatment • Dose reduced to 200 mg due to Overall Response -- CR Grade 2 fatigue (RECIST 1.1) • Complete
response achieved at Week 6 (confirmed); ongoing

18 G12X KRAS PDAC: Best Overall Response to RMC-6236 (a) Evaluable for
Efficacy (n = 46) Tumor Response (per RECIST 1.1) Best overall response, n (%) Partial response 9 (20) Stable disease 31 (67) Progressive disease 3 (7) (b) Not evaluable 3 (7) ORR, n (%) 9 (20) Confirmed, n 5 DCR (CR+PR+SD), n (%) 40 (87) KRAS G12
Mutation Week of Most Recent Scan *Unconfirmed PR per RECIST 1.1. (a) Patients who received first dose of RMC-6236 at least 8 weeks prior to data extract date. Data Extracted 12 Oct 2023. (b) Two patients died prior to first post-baseline scan; 1
patient had scan after 11 days of treatment and subsequently died due to PD. Best % Change from Baseline in Target Lesion

19 G12X KRAS PDAC: Duration of Treatment and Responses to RMC-6236 (a)
Evaluable for Efficacy (n = 46) Median time to response: 1.4 months (range, 1.2–4.1 months) Median time on treatment: 3.3 months (range, 0.2–10.9 months) * Duration of Treatment (Weeks) (a) Patients who received first dose of RMC-6236 at
least 8 weeks prior to data extract date. Data Extracted 12 Oct 2023. *Death due to PD (n = 9), Death due to unrelated AE (n = 2). G12X Patients with KRAS Mutation

20 KRAS Variant Allele Frequency in ctDNA Across Tumor Types and
Correlation with Clinical Response NSCLC PDAC • Patients with NSCLC or PDAC were dosed at 80–300 mg • Overall, 23/50 patients (46%) were evaluable for change in mutant KRAS VAF (a) while on-treatment • In total, 8/10 (80%)
patients with NSCLC and 12/13 (92%) patients with PDAC showed >50% reduction of the mutated KRAS allele G12X (a) KRAS VAF at Cycle 1 Day 1 (pre-treatment) to Cycle 2 Day 1 or Cycle 3 Day 1 (on-treatment) determined by Guardant Health ctDNA test;
G12X KRAS defined as mutation at codon 12 which encodes glycine (G) to X where X= A, D, R, or V; * Data Extracted 12 Oct 2023. Two patients were non-evaluable for best overall response; Unconfirmed partial response per RECIST 1.1.

21 RMC-6291: Mutant-Selective, Covalent RAS(ON) Inhibitor with G12C
Differentiated Clinical Profile for KRAS Cancers G12C • Highly selective for KRAS (ON) with potent CRC activity across a range of preclinical models 17% • Orally bioavailable and generally well-tolerated in PDAC patients at active doses
3% • Promising and differentiated initial clinical profile in Other 32,000 previously-treated NSCLC and treatment-naïve 7% G12C New KRAS patients CRC (1) per year (U.S.) • Profound combinatorial activity with RMC-6236 in preclinical
models, potential for targeted RAS(ON) NSCLC doublets 73% • High potential for checkpoint inhibitor combination given low evidence of hepatotoxicity (1) Estimated using tumor mutation frequencies from Foundation Medicine Insights March 2022
and scaled to estimated patient numbers using cancer incidence from ACS Cancer Facts and Figures 2023 (see appendix for additional detail); NSCLC = non-small cell lung cancer; CRC = colorectal cancer; PDAC = pancreatic ductal
adenocarcinoma

22 RMC-6291 Drives Tumor Regressions at Low Doses in Preclinical G12C
Models of KRAS NSCLC Control End of Study Responses RMC-6291 3 mg/kg 2500 300 RMC-6291 10 mg/kg RMC-6291 25 mg/kg 2000 RMC-6291 100 mg/kg 200 1500 100 4/8 R 1/8 R 1000 1/8 CR 6/8 CR 8/8 CR 8/8 CR Dosing start 0 500 -100 0 10 20 30 40 Days on Study
RVMD preclinical research NSCLC = non-small cell lung cancer G12C/WT NCI-H358 CDX (NSCLC, KRAS ); all doses given orally, once daily R = number of regressions >10% from initial; CR = number of regressions ≥80% from initial Each animal
represented as a separate bar in waterfall plot 3 Mean Tumor Volume (mm ) % Change in Tumor Volume

23 RMC-6291 Drives Tumor Regressions in Preclinical Models of G12C KRAS
(OFF) Inhibitor Clinical Resistance Mechanisms (1) KRAS Amplification (2) KRAS and RTK Amplification 3000 Control 1500 Control Sotorasib Adagrasib RMC-6291 RMC-6291 2000 1000 Dosing start 1000 500 0 0 0 10 20 30 40 50 60 70 80 90 0 10 20 30 40 Days
on Study Days on Study RVMD preclinical research NSCLC = non-small cell lung cancer; PDAC = pancreatic ductal adenocarcinoma G12C/G12C amp (1) Sotorasib-Resistant MIA PaCa-2 CDX (PDAC, KRAS , KRAS ). RMC-6291 dosed at 100 mg/kg po qd; Sotorasib
dosed at 100 mg/kg po qd G12C/WT amp amp (2) LUN055 PDX (NSCLC, KRAS , ERBB3 , KRAS ). RMC-6291 dosed at 200 mg/kg po qd; Adagrasib dosed at 100 mg/kg po qd 3 Mean Tumor Volume (mm ) 3 Mean Tumor Volume (mm )

24 RMC-6236 + RMC-6291 Doublet Overcomes Resistance and G12C Prolongs
Durability in KRAS NSCLC Models 100 75 Control RMC-6291 50 RMC-6236 RMC-6291 + RMC-6236 25 0 0 20 40 60 80 100 Days on Treatment • RAS(ON) Inhibitor doublet evaluated across seven models, including five identified as resistant to RMC-6291
monotherapy RVMD preclinical research NSCLC = non-small cell lung cancer RMC-6236 dosed at 25 mg/kg po qd (n=52); RMC-6291 dosed at 100 or 200 mg/kg po qd (n=52); Combination (n=51). For each group, n = total number of animals from the seven models
that comprise the dataset. Progression defined as tumor doubling from baseline. % Tumors Progression-Free

25 RMC-6291-001 Phase 1 Study Design Key Eligibility Criteria Dose
Escalation RMC-6291 administered orally QD or BID • Advanced solid tumors G12C with KRAS mutations 400 mg BID • Received prior standard therapy including treatment 300 mg BID G12C with KRAS (OFF) 200 mg BID Dose Expansion / inhibitors
Optimization 100 mg BID • ECOG PS 0–1 • No active brain metastases 200 mg QD 100 mg QD Key Endpoints Lowest dose projected to drive 50 mg QD tumor regressions in humans • Safety and tolerability based on preclinical models
• Pharmacokinetics • Anti-tumor activity Additional patients with NSCLC or CRC were enrolled at dose levels that cleared DLT evaluation (backfill enrollment and dose optimization) DLT=dose-limiting toxicity; ECOG PS=Eastern Cooperative
Oncology Group Performance Status; QD=once daily; BID=twice daily

26 RMC-6291: Patient Demographics and Baseline Characteristics NSCLC
CRC Other All Histologies n=23 n=33 n=7 n=63 Age, median (range), years 65 (45–85) 54 (26–84) 66 (52–78) 64 (26–85) Male, n (%) 13 (57) 21 (64) 2 (29) 36 (57) ECOG PS, n (%) 0 8 (35) 13 (39) 3 (43) 24 (38) 1 15 (65) 20 (61) 4
(57) 39 (62) Smoking status, n (%) Current 5 (22) 2 (6) 0 7 (11) Past 18 (78) 12 (36) 1 (14) 31 (49) Never 0 19 (58) 6 (86) 25 (40) Number of prior therapies, median (range) 3 (1–7) 3 (1–7) 4 (2–6) 3 (1–7) G12C Prior KRAS
inhibitor, n (%) Yes 13 (57) 8 (24) 4 (57) 25 (40) No 10 (44) 25 (76) 3 (43) 38 (60) G12C Time between prior KRAS inhibitor and RMC-6291 6 (2–86) 10 (3–31) 9 (8–128) 9 (2–128) first dose, median (range), weeks Prior
checkpoint inhibitor within 12 weeks of RMC- 6291 first dose Yes 9 (39) 0 1 (14) 10 (16) No 14 (61) 32 (97) 6 (86) 52 (83) Data Extracted 05 October 2023. NSCLC = non-small cell lung cancer; CRC = colorectal cancer
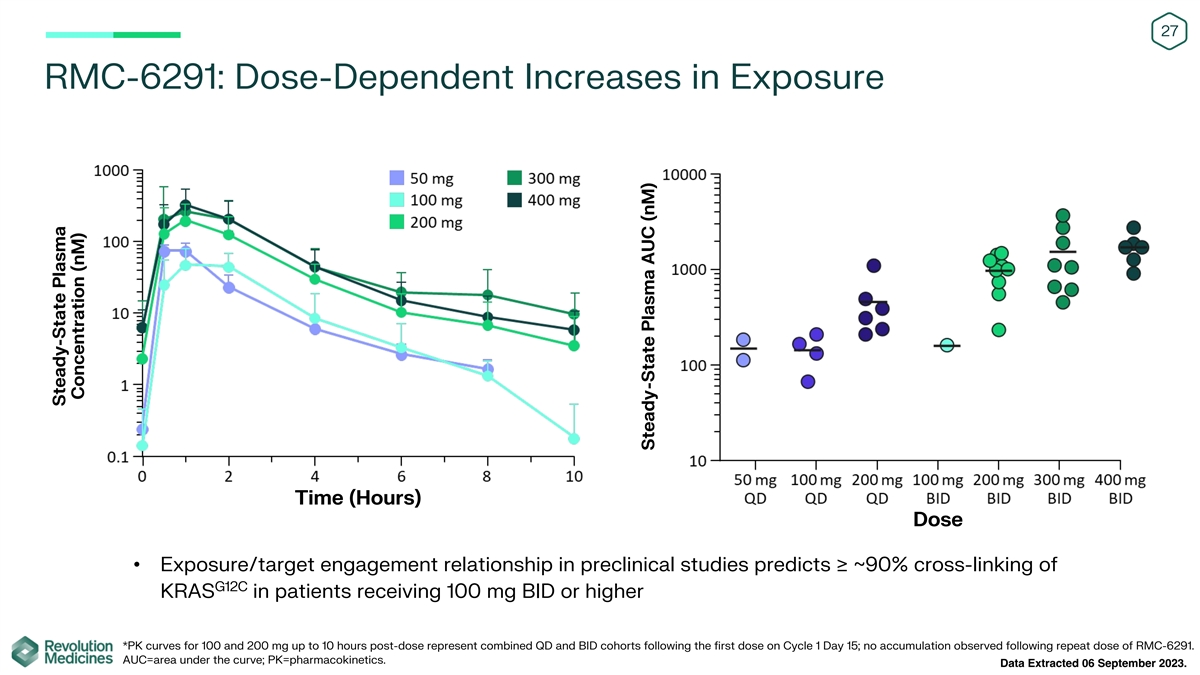
27 RMC-6291: Dose-Dependent Increases in Exposure Time (Hours) Dose
• Exposure/target engagement relationship in preclinical studies predicts ≥ ~90% cross-linking of G12C KRAS in patients receiving 100 mg BID or higher *PK curves for 100 and 200 mg up to 10 hours post-dose represent combined QD and BID
cohorts following the first dose on Cycle 1 Day 15; no accumulation observed following repeat dose of RMC-6291. AUC=area under the curve; PK=pharmacokinetics. Data Extracted 06 September 2023. Steady-State Plasma Concentration (nM) Steady-State
Plasma AUC (nM)

28 RMC-6291: Summary of Treatment-Related Adverse Events Total (n=63)
Maximum Severity of TRAEs Grade 3 Any Grade Grade 1 Grade 2 TRAEs occurring in ≥10% of patients, n (%) Diarrhea 1 (2) 18 (29) 10 (16) 7 (11) Nausea 0 17 (27) 14 (22) 3 (5) ECG QT prolonged 7 (11) 16 (25) 8 (13) 1 (2) – – –
QTcF* ≥ 501 ms 1 (2) Fatigue 0 8 (13) 4 (6) 4 (6) Vomiting 0 8 (13) 6 (10) 2 (3) AST increased 0 7 (11) 7 (11) 0 TRAEs leading to dose reduction, n (%) 9 (14) 0 1 (2) 8 (13) 1 (2) TRAEs leading to treatment discontinuation, n (%) 1 (2) 0 0
• No treatment-related Grade 4 or 5 AEs or SAEs have been reported. • No patients had cardiac sequelae (e.g., torsade de pointes) associated with an ECG QT prolonged event *QTcF refers to QT interval corrected for heart rate by
Fridericia's formula. AE, adverse event; AST, aspartate transferase; ECG, electrocardiogram; SAE, serious adverse event, TRAE, treatment-related adverse event. Data Extracted 05 October 2023.

29 G12C G12C KRAS NSCLC Previously Treated with or Naïve to a KRAS
(OFF) Inhibitor: Best Overall Response to RMC-6291 Evaluable for Efficacy* (n=17) Tumor Response (per RECIST 1.1) Best overall Prior Naïve to response, G12Ci G12Ci n (%) (n=10) (n=7) † Partial response 5 (50) 3 (43) Stable disease 5 (50)
4 (57) Progressive disease 0 0 ORR, n (%) 5 (50) 3 (43) DCR (CR+PR+SD), 10 (100) 7 (100) n (%) † *All treated patients who received a first dose of RMC-6291 at least 8 weeks prior to data extract date; PR includes 5 confirmed and 3
unconfirmed. CR, complete response; DCR, disease control rate; G12Ci, G12C inhibitor; PD, progressive disease; PR, partial response; PRu, unconfirmed partial response; SD, stable disease; SOD, sum of diameters; ORR objective response rate; DCR,
disease control rate; RECIST, response evaluation criteria in solid tumors. Data Extracted 05 October 2023. Best % Change in SOD from Baseline in Target Tumor Burden

30 G12C G12C KRAS CRC Naïve to KRAS (OFF) Inhibitor: Best Overall
Response to RMC-6291 Evaluable for Efficacy* (n=19) Tumor Response (per RECIST 1.1) Best overall response, n † n=20 (%) ‡ Partial response 8 (40) Stable disease 8 (40) † Progressive disease 4 (20) ORR, n (%) 8 (40) DCR (CR+PR+SD),
n (%) 16 (80) *All treated patients who received first dose of RMC-6291 at least 8 weeks prior to data extract date † ‡ One patient had PD due to a new lesion and target lesion measurements were not available; PR includes 5 confirmed and
3 unconfirmed. Data Extracted 05 October 2023. Best % Change in SOD from Baseline in Target Tumor Burden

31 G12C Duration of Treatment and Responses to RMC-6291 for KRAS
Inhibitor-Treated or Naïve NSCLC and Naïve CRC NSCLC Median time to response: 1.3 months (range: 1.1–4.1 months) Median time on treatment: 3.5 months (range: 0.3–9.8 months) CRC Median time to response: 1.4 months (range:
1.2–4.1 months) Median time on treatment: 2.4 months (range: 0.3–7.9 months) Duration of Treatment (Weeks) 50 mg QD 100 mg QD 200 mg QD 200 mg BID 100 mg BID 300 mg BID 400 mg BID G12C # = KRAS inhibitor-treated; *The date of treatment
discontinuation due to PD was missing as of data extract date. Data Extracted 05 October 2023.

32 G12C Reduction in ctDNA of the KRAS Allele Across Doses Correlates
with Clinical Response Visit G12C KRAS VAF at Cycle 1 Day 1 (pre-treatment) to Cycle 2 Day 1 or Cycle 3 Day 1 (on-treatment) determined by Guardant Health ctDNA (circulating tumor DNA) test. ctDNA, circulating tumor DNA; VAF, variant allele
frequency. Data Extracted 05 October 2023. G12C KRAS VAF (%)

33 RMC-9805: Clinical Stage, Mutant-Selective, Covalent RAS(ON) G12D
Inhibitor for KRAS Cancers G12D Selective Covalent Binding to KRAS (ON) WT G12D G12C G13C G13D KRAS: X-linked KRAS KRAS H/NRAS G12D In Vivo Anti-Tumor Activity across KRAS Cancer Models 200 61,000 CRC 150 G12D New KRAS patients NSCLC mPD 100 (1)
PDAC per year (U.S.) 50 mSD 0 -50 mPR mCR -100 (1) Estimated using tumor mutation frequencies from Foundation Medicine Insights March 2022 and scaled to estimated patient numbers using cancer incidence from ACS Cancer Facts and Figures 2023 (see
appendix for additional detail); RVMD preclinical research as of 11/02/22; NSCLC = non-small cell lung cancer; PDAC = pancreatic ductal adenocarcinoma; CRC = colorectal cancer RMC-9805 dosed at 100 mg/kg po qd; n=3-8/group; Responses assigned
according to mRECIST: mPD = progressive disease; mSD = stable disease; mPR = partial response; mCR = complete response Mean Tumor Volume % Change From Baseline KP-4 CRC1018 CTG-2393 CRC043 LUN232 CRC054 PAN026 CRC044 CRC047 CRC012 CTG-1932 PAN038
LXFL625 PAN010 GP2d AsPC-1 PAN031 PAN009 LXFA2889 HPAC LXFA2204 CTG-2803 LUN137 LUN020 PAN001

34 RMC-5127: First-in-Class Mutant-Selective RAS(ON) Inhibitor for G12V
KRAS Cancers G12V NSCLC In Vivo Anti-Tumor Activity across KRAS Cancer Models 23% CRC CRC NSCLC 33% PDAC 50 48,000 G12V New KRAS patients mSD 0 (1) Other per year (U.S.) 10% -50 mPR mCR -100 PDAC 34% (1) Estimated using tumor mutation frequencies
from Foundation Medicine Insights March 2022 and scaled to estimated patient numbers using cancer incidence from ACS Cancer Facts and Figures 2023 (see appendix for additional detail); RVMD preclinical research as of 09/27/23; NSCLC = non-small cell
lung cancer; PDAC = pancreatic ductal adenocarcinoma; CRC = colorectal cancer RMC-5127 dosed at 100 mg/kg po qd; n=3-8/group; Responses assigned according to mRECIST: mPD = progressive disease; mSD = stable disease; mPR = partial response; mCR =
complete response Mean Tumor Volume % Change From Baseline CRC058 CRC060 LUN352 Capan-1 SW620 NCI-H441 Capan-2
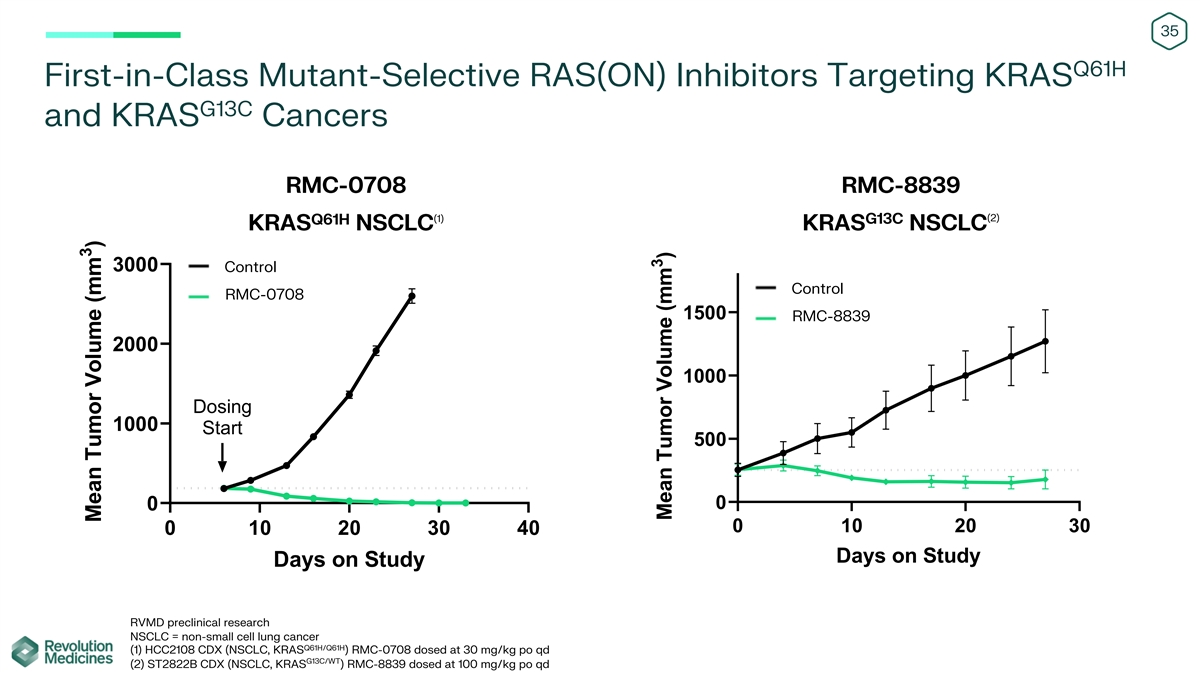
35 Q61H First-in-Class Mutant-Selective RAS(ON) Inhibitors Targeting
KRAS G13C and KRAS Cancers RMC-0708 RMC-8839 (2) (1) G13C Q61H KRAS NSCLC KRAS NSCLC 3000 Control Control RMC-0708 1500 RMC-8839 2000 1000 Dosing 1000 Start 500 0 0 0 10 20 30 0 10 20 30 40 Days on Study Days on Study RVMD preclinical research NSCLC
= non-small cell lung cancer Q61H/Q61H (1) HCC2108 CDX (NSCLC, KRAS ) RMC-0708 dosed at 30 mg/kg po qd G13C/WT (2) ST2822B CDX (NSCLC, KRAS ) RMC-8839 dosed at 100 mg/kg po qd 3 Mean Tumor Volume (mm ) 3 Mean Tumor Volume (mm )

36 Deep Pipeline of Targeted Therapies for Majority of RAS-Addicted
Cancers PRECLINICAL IND-ENABLING PHASE 1 PHASE 2 PHASE 3 RAS(ON) INHIBITORS MULTI RMC-6236 G12C RMC-6291 RMC-9805 G12D G12V RMC-5127 Q61H RMC-0708 G13C RMC-8839 Pipeline G12R, G13D, other Expansion RAS COMPANION INHIBITORS (1) RMC-4630 SHP2 RMC-5552
mTORC1/4EBP1 (1) Development paused subject to potential future evaluation in combination with RAS(ON) Inhibitors

37 Clinical Development Vision for RMC-6236 Proven to be broadly active
in patients in multiple common tumor types and RAS genotypes Parallel strategies to prioritize speed + breadth including both late-stage monotherapy trial(s) and combinations Pursuing multiple dimensions for efficient expansion: RAS genotypes, lines
of therapy, tumor types Confidential © 2023 Revolution Medicines

38 RMC-6236 Clinical Development Plan Designed to Benefit the Greatest
Number of Patients RAS Mutations KRAS G12-mutant (K/N/H) RAS-mutant Randomized trials with nested design and hierarchical testing G13 Q61 G12 G12 Lines of Therapy Focused exploration of RAS(ON) 1L / Adjuvant Patients Inhibitor doublets, combinations
with 2L+ Patients with Monotherapy or SOC, including checkpoint inhibitors with Monotherapy Combinations and other agents Tumor Types Potential conversion of RMC-6236- Tumor specific Tissue Agnostic 001 study to single-arm Phase 2 NSCLC NSCLC, PDAC,
CRC PDAC Tissue Agnostic Registration Trial Other Solid Tumors CRC Expand in three dimensions:

39 Efficacy Benchmarks from Standard of Care for Patients with MUT
Previously-Treated RAS NSCLC (1) Efficacy 2L+ Therapy Docetaxel Sotorasib (2) Benchmarks All patients N 174 171 Docetaxel ORR (%) 13.2 28.1 Docetaxel + ramucirumab mDOR (m) 6.8 8.6 G12C KRAS only mPFS (m) 4.5 5.6 Sotorasib (accelerated approval) mOS
(m) 11.3 10.6 Adagrasib (accelerated approval) (1) Sotorasib PDUFA date: 24 December 2023. Target assumptions to be discussed with regulatory authorities. (2) Efficacy benchmarks for docetaxel and sotorasib taken from CodeBreaK 200, Lancet (2023)
401: 733-746.

40 Proposed Global Randomized Phase 3 Trial in Patients with MUT
Previously-Treated RAS NSCLC (1) (1,2) Trial Design Potential Patient Populations Endpoints RMC-6236 PFS R Core Population: G12X-C OS G12X Patients 13% Docetaxel Patient Reported Outcomes • N > 400 patients • Prior therapies:
Anti-PD-(L)1 and platinum-containing G12C regimen in metastatic setting; RAS inhibitor 12% naïve (including G12C inhibitor) • Biomarker: RAS G12X, G13X, or Q61X mutation Expanded Population: • Study Initiation: Aiming for 2024 G13X
- 2% G12X + G13X + Q61X Q61X - 3% Patients (1) • Potential for nested trial design to enable evaluation of core and expanded patient populations R = Randomized (1) Study design subject to change based on regulatory authority feedback (2)
Percentages of all NSCLC patients with tumors bearing RAS G12X, G13X, or Q61X genotypes; estimated using tumor mutation frequencies from Foundation Medicine Insights March 2022 and scaled to estimated patient numbers using cancer incidence from ACS
Cancer Facts and Figures 2023 (see appendix for additional detail); G12X - 25%

41 Efficacy Benchmarks from Standard of Care in Patients with
Previously-Treated PDAC (1) Efficacy lipo-irino + 2L+ Therapy GnP (2) Benchmarks 5-FU FOLFIRINOX ORR (%) 7.7 11 mFOLFIRINOX Gemcitabine+nab-Paclitaxel (GnP) mPFS (m) 3.1 3.5 FOLFOX mOS (m) 6.1 7.1 FOLFIRI post-GnP only liposomal irinotecan + 5-FU
(1) No clearly established standard of care. (2) Efficacy benchmarks for lipo-irino+5-FU taken from Lancet (2016) 387: 545-557; GnP taken from Br J Cancer (2022) 126:1394-1400.

42 Potential Global Randomized Phase 3 Trial of RMC-6236 in Patients
with Previously-Treated PDAC (1) (1,2) Trial Design Potential Patient Populations RMC-6236 Endpoints R PFS Physician’s choice: OS e.g., GnP or G12X - 85% Patient Reported Outcomes mFOLFIRINOX Core Population: G12X Patients • N > 500
patients • Prior therapies: fluoropyrimidine or gemcitabine-based regimen; RAS inhibitor naïve (including G12C inhibitor) • Biomarker: All comers, RAS mutation testing (G12X, G13X, or Q61X) to allow stratification • Study
Initiation: Potentially in 2024 Expanded Populations: G13X/Q61X - 7% • G12X + G13X + Q61X patients WT - 8% • All PDAC patients (1) • Potential for nested trial design to enable evaluation of core and expanded patient populations R
= Randomized; WT=wild-type (1) Study design subject to change based on regulatory authority feedback (2) Percentages of all PDAC patients with tumors bearing RAS G12X, G13X, Q61X or WT genotypes; estimated using tumor mutation frequencies from
Foundation Medicine Insights March 2022 and scaled to estimated patient numbers using cancer incidence from ACS Cancer Facts and Figures 2023 (see appendix for additional detail);
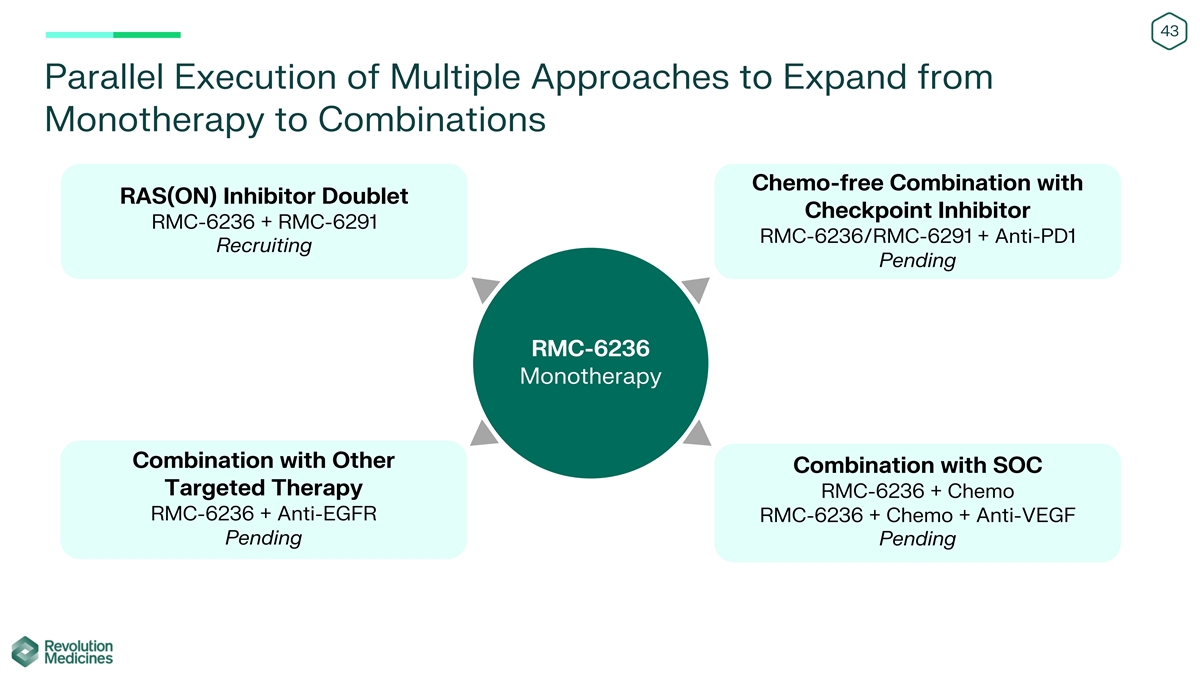
43 Parallel Execution of Multiple Approaches to Expand from Monotherapy
to Combinations Chemo-free Combination with RAS(ON) Inhibitor Doublet Checkpoint Inhibitor RMC-6236 + RMC-6291 RMC-6236/RMC-6291 + Anti-PD1 Recruiting Pending RMC-6236 Monotherapy Combination with Other Combination with SOC Targeted Therapy RMC-6236
+ Chemo RMC-6236 + Anti-EGFR RMC-6236 + Chemo + Anti-VEGF Pending Pending

44 G12X First Two RAS(ON) Inhibitors Clinically Validated Against RAS
Cancer Drivers and Major Tumor Types 80,000 Q61X G12X Validated - RAS 60,000 G13X Q61X • 150,000 new patients per year among (1,2) G13X Q61X PDAC, NSCLC and CRC * 40,000 Expansion – additional tumor types and G13X Q61X RAS + RAS G12X
G12X G12X 20,000 • > 200,000 new patients combined across (1) RAS genotypes and tumor types broadly 0 CRC NSCLC PDAC MUT (1) New RAS patients per year (U.S.) estimated using tumor mutation frequencies from Foundation Medicine Insights March
2022 and scaled to estimated patient numbers using cancer incidence from ACS Cancer Facts and Figures 2023 (see appendix for additional detail); *G13X < 1000 new PDAC patients per year (U.S.) (2) G12X genotypes validated based on clinical data
from RMC-6236-001 (data extracted 12 Oct 2023) and RMC-6291-001 (data extracted 05 October 2023) MUT new RAS patients per (1) year (U.S.)

45 Financial Information Financial Position Cash, cash equivalents and
(1) $909.5 million marketable securities as of June 30, 2023 2023 Financial Guidance (2) 2023 GAAP net loss of $360 million to $400 million (1) With current cash, cash equivalents and marketable securities, the company projects it can fund planned
operations into 2025 (2) Includes non-cash stock-based compensation expense of approximately $40 million to $50 million Financial guidance does not include impact of proposed EQRx acquisition

46 Summary of Pending EQRx Transaction • RVMD to acquire EQRx in
an all-stock transaction to gain more than $1B in additional capital • Strengthened balance sheet intended to support RVMD’s parallel, late-stage development for RAS(ON) Inhibitor pipeline • Shareholder meeting to vote on the
transaction scheduled for November 8, 2023 at 11:00am Eastern time • Deal expected to close shortly following shareholder vote, subject to satisfaction of customary closing conditions • Stock exchange ratio to be determined using a
blended average share price to account for developments in our business and potential movement in RVMD share price • ~20% based on a determined RVMD share price at signing • ~80% based on RVMD share price as determined in close proximity
to shareholder vote (subject to 6% discount) • RVMD to continue focus on mission to revolutionize treatment for patients with RAS-addicted cancers through the discovery, development and delivery of innovative, targeted medicines

47 On Target to TM Outsmart Cancer

48 Appendix • All RAS cancer epidemiology statistics are
estimated using tumor mutation frequencies from Foundation Medicine Insights March 2022 and scaled to estimated patient numbers using cancer incidence from ACS Cancer Facts and Figures 2023: • RAS mutations include: KRAS G12(A,C,D,F,L,R,S,V),
KRAS G13(C,D,R,V), KRAS Q61(E,H,K,L,P,R) NRAS G12(A,C,D,R,S,V), NRAS G13(C,D,R,V), NRAS Q61(H,K,L,R), HRASG12(C,D,S,V), HRASG13(C,D,N,R,S,V), HRASQ61(K,L,R). • Includes 13 major solid cancer types: non-small cell lung cancer, colorectal,
pancreatic ductal adenocarcinoma, renal, esophageal, head and neck squamous cell, ovarian, stomach, biliary, and carcinomas of unknown primary (CUP), and advanced melanoma, bladder and endometrial cancers causing mortality. • KRASQ61H
epidemiology statistics include multiple myeloma in addition to 13 major solid cancer types named above • RAS mutations drive 30% of human cancers per Prior et al., Cancer Research 2020 • Mouse tumor responses assigned according to
mRECIST (modified from Gao et al. Nat Med. 2015): • mPD = progressive disease; mSD = stable disease; mPR = partial response; mCR = complete response
v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Revolution Medicines (NASDAQ:RVMD)
Gráfica de Acción Histórica
De Nov 2024 a Dic 2024

Revolution Medicines (NASDAQ:RVMD)
Gráfica de Acción Histórica
De Dic 2023 a Dic 2024
