0001017491
false
0001017491
2023-09-20
2023-09-20
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): September 20, 2023
Seelos Therapeutics, Inc.
(Exact name of registrant as specified in its charter)
| Nevada |
|
000-22245 |
|
87-0449967 |
(State or Other Jurisdiction of
Incorporation) |
|
(Commission File Number) |
|
(I.R.S. Employer Identification
No.) |
| 300
Park Avenue, 2nd Floor,
New York, NY |
|
10022 |
| (Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: (646) 293-2100
Not Applicable
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
¨ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
¨ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
¨ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
¨ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities Registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
| Common Stock, $0.001 par value |
SEEL |
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter). Emerging growth company ¨
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Item 8.01. Other Events.
On September 20, 2023,
Seelos Therapeutics, Inc. (the “Company”) issued a press release announcing top line results from its double-blind, placebo-controlled
cohort (Part 2) of its Phase 2 study of SLS-002 (intranasal racemic ketamine) for Acute Suicidal Ideation and Behavior in adults
with Major Depressive Disorder. The press release further announced that the Company will host a webcast on September 20, 2023 at
8:00 a.m. ET to discuss the top line results.
On September 20, 2023,
the Company posted an updated corporate presentation (the “Presentation”) on the Company’s website at seelostherapeutics.com,
portions of which will be referenced by the Company’s management during the webcast.
Copies of the press release
and the Presentation are filed as Exhibits 99.1 and 99.2 to this Current Report on Form 8-K, respectively, and are incorporated herein
by reference. The Presentation is current as of September 20, 2023, and the Company expressly disclaims any obligation to update
this material in the future.
The Presentation contains
forward-looking statements that involve future risks and uncertainties as contemplated by the safe harbor provided by the Private Securities
Litigation Reform Act of 1995. All statements, other than statements of historical facts, included in the Presentation should be regarded
as “forward-looking statements.” These forward-looking statements involve known and unknown risks, uncertainties and other
important factors that may cause the actual results, performance or achievements of the Company to be materially different from any future
results, performance or achievements expressed or implied by the forward-looking statements. These forward-looking statements are based
on the expectations, estimates, projections, beliefs and assumptions of the Company based on information currently available to it, all
of which are subject to change. These forward-looking statements are not guarantees of future performance and are subject to risks and
uncertainties that could cause actual results to differ materially from the results contemplated by the forward-looking statements. Many
of the important factors that will determine these results and values are beyond the Company’s ability to control or predict. Except
as otherwise required by law, the Company does not assume any obligation to update any forward-looking statements.
For additional information
about factors that could cause actual results to differ materially from those described in the forward-looking statements, please refer
to the Company’s filings with the Securities and Exchange Commission, including the risk factors contained in the Company’s
Annual Report on Form 10-K for the year ended December 31, 2022.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits.
SIGNATURES
Pursuant to the requirements
of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto
duly authorized.
| |
Seelos Therapeutics, Inc. |
| |
|
| |
|
| Date: September 20, 2023 |
By: |
/s/ Michael Golembiewski |
| |
|
Name: Michael Golembiewski |
| |
|
Title: Chief Financial Officer |
Exhibit 99.1

Seelos Therapeutics Announces Top Line Results
from SLS-002 Phase II Study in Adults with Major Depressive Disorder at Imminent Risk of Suicide
| · | SLS-002
demonstrated early and persistent clinically meaningful reductions in symptoms of depression
and acute suicidality |
| · | Robust
Response and Remission Rates were observed using the Montgomery-Åsberg Depression Rating
Scale (MADRS) |
| · | Results
demonstrate the therapeutic potential of SLS-002 to address imminent suicidality |
| · | SLS-002
was well-tolerated, with no evidence of new or unique adverse events and there were no deaths
reported in the study |
| · | The
Company will host a webcast with Dr. David V Sheehan, today, Wednesday, September 20th
at 8:00 a.m. ET |
NEW YORK, September 20, 2023 /PRNewswire/ --
Seelos Therapeutics, Inc. (Nasdaq: SEEL) (“Seelos”), a clinical-stage biopharmaceutical company focused on the development
of therapies for central nervous system disorders and rare diseases, today announced top line data demonstrating clinically meaningful
treatment effects across multiple endpoints and a well-tolerated safety profile from the double-blind, placebo-controlled cohort (Part 2)
of its Phase II study of SLS-002 (intranasal racemic ketamine) for Acute Suicidal Ideation and Behavior (ASIB) in adults with Major Depressive
Disorder (MDD).
SLS-002 versus placebo demonstrated early and persistent
reductions in symptoms of depression as assessed by the Montgomery-Åsberg Depression Rating Scale (MADRS)1. The graph
below presents results from the mixed model for repeated measures (MMRM) analysis of change from baseline in MADRS total score.

Target enrollment of this study was 220 patients, however,
due to financial constraints, only 147 patients diagnosed with MDD requiring psychiatric hospitalization due to significant risk of suicide
were randomized. The data from the 147 subjects (67% of target enrollment) were evaluated using the protocol-defined methods of analysis.
Due to the limited sample size, the study did not meet the pre-defined primary endpoint (MADRS ANCOVA at 24 hours post dosing). However,
assuming the same treatment difference and standard deviation, analyses showed that the study would have achieved statistical significance
for the primary endpoint, had the study reached full enrollment (220 patients).
“The analyses of the 147 enrolled subjects in this multicenter,
double-blind placebo-controlled trial of SLS-002 demonstrated both meaningful early and persistent improvement in depressive symptoms,
as well as clinically meaningful reduction in acute suicidality symptoms relative to standard of care,” said Tim Whitaker, M.D.,
Chief Medical Officer of Seelos. “We believe these results demonstrate the therapeutic potential of SLS-002 to address this huge
unmet need and those at risk. We look forward to our discussions with the FDA to align on next steps. In addition, we want to thank the
study participants, as well as the clinical trial sites and staff, for their expert and careful care of these high-risk study patients.”
Detailed Summary of Key Efficacy Endpoints
| · | MADRS
results at 4 hours after dosing demonstrated a statistically significant change relative
to placebo (p <0.001, 5.9 point LS2 mean treatment difference) |
| · | MADRS
results at 24 hours after dosing utilizing 2-way ANCOVA3 with baseline MADRS as
a covariate (the pre-defined primary endpoint/analysis) demonstrated clinically meaningful
results, but did not achieve statistical significance under the methodology used (p=0.069,
3.3 point LS mean treatment difference) |
| o | MADRS
results at 24 hours after dosing utilizing an exploratory ANOVA (t-test) analysis demonstrated
statistically significant change relative to placebo (p=0.049, 3.6 point mean treatment difference) |
| · | MADRS
results at Day 16 demonstrated a statistically significant change relative to placebo (p=0.012,
4.4 point LS mean treatment difference) – demonstrating persistence of effect |
| · | Meaningful
results further supported by (proportion of SLS-002 subjects versus placebo, respectively): |
| o | MADRS
Response Rate, defined as ≥ 50% reduction from baseline, at Day 16 (75.7% versus 47.9%)
p<0.001 |
| o | MADRS
Remission Rate, defined as a total score ≤ 12, at Day 16 (62.2% versus 32.9%) p<0.001 |
| · | MADRS
at end of 2-week safety follow up (Day 29/30) revealed continued improvement, demonstrating
no evidence of return of symptoms. |
Clinically meaningful reduction in acute suicidality was demonstrated
with SLS-002 over placebo. Both groups continued to improve over time.
| · | Sheehan-Suicidality
Tracking Scale (S-STS) Total Score4: (Mean baseline total scores were 21.4 for
SLS-002 and 21.0 for placebo) |
| o | 4
hour change from baseline was -15.1 for SLS-002 and -12.0 for placebo (p=0.022) |
| o | 24
hour change from baseline was -15.5 for SLS-002 and -12.1 for placebo (p=0.008) |
| · | Clinical
Global Impression of Severity for Suicidal Ideation and Behavior (CGIS-S/IB)5:
(Mean baseline scores were 4.0 for both SLS-002 and placebo) |
| o | 4
hour change from baseline was -1.5 for SLS-002 and -1.1 for placebo (p=0.011) |
| o | 24
hour change from baseline was -1.7 for SLS-002 and -1.4 for placebo (p=0.102) |
The table below represents the observed MADRS summary statistics,
including the assessments collected at the safety follow-up visits.
Group Mean
MADRS Baseline /
Change from Baseline |
|
Placebo
(N=73) |
|
SLS-002
(N=74) |
|
Difference |
| Baseline |
|
37.4 |
|
38.0 |
|
0.6 |
| Day 2 (24 hours) |
|
-12.4 |
|
-16.1 |
|
-3.7 |
| |
n=73 |
|
n=74 |
|
| Day 16 |
|
-22.2 |
|
-27.9 |
|
-5.7 |
| |
n=55 |
|
n=63 |
|
| Day 22 (Safety Follow-up Week 1) |
|
-23.9 |
|
-29.3 |
|
-5.4 |
| |
n=54 |
|
n=61 |
|
| Day 29/30 (Safety Follow-up Week 2) |
|
-25.0 |
|
-30.3 |
|
-5.3 |
| |
n=52 |
|
n=60 |
|
*Note: Results presented in table are based on simple summary statistics
of observed MADRS assessments with differences calculated from group mean scores rounded to the tenth.
Detailed Summary of Safety Results
SLS-002 was well-tolerated with no new or unique safety signals identified
and there were no deaths reported in the study. At least one treatment-emergent adverse event was reported in 52.7% of subjects treated
with SLS-002 versus 39.7% treated with placebo; the majority of adverse events were mild or moderate and transient in nature. The most
common treatment-emergent adverse events (≥5% and >placebo) were dizziness (18.9% versus 2.7%), euphoric mood (6.8% versus 0%)
and suicidal ideation (5.4% versus 2.7%). There were 5 serious adverse events (3 with SLS-002, 2 with placebo), all for suicidality,
all judged unrelated to the study drug, and all resolved.
Specific scales were utilized to measure the three most common known
adverse events associated with ketamine treatments, which are dissociation, hemodynamic effects and sedation. The data below support
that SLS-002 may reduce the frequency and severity of these most common effects compared to what is reported with other ketamine treatments.
Clinician-Administered Dissociative States Scale (CADSS)6
| · | At
no point did the placebo-adjusted mean change from pre-dose baseline exceed the threshold
of clinically meaningful dissociation (>4) |
| · | Placebo-adjusted
mean change from pre-dose baseline (at 40 minutes – correlating roughly with maximum
plasma concentrations) = 3.9 after first dose and 1.8 at 1 hour post first dose |
| · | Placebo-adjusted
mean change from pre-dose baseline 2.5 on Day 4 (at 40 minutes) after second dose and 1.4
on Day 8 (at 40 minutes) after third dose |
Hemodynamic Effects
| · | 6
subjects who received SLS-002 had an adverse event reported of either increased blood pressure
or hypertension, all events were mild (except 1 moderate), all were transient and resolved |
| · | In
review of mean vital sign data for SLS-002, minimal changes were observed |
| o | Systolic
blood pressure at Baseline was 122.7 mmHg, with maximum mean values of 126.7 mmHg on days
1 and 11 (1 hour post dose) |
| o | Diastolic
blood pressure at Baseline was 77.7 mmHg, with maximum mean values of 81.1 mmHg on Day 8
(1 hour post dose) |
Modified Observer’s Assessment of Alertness/Sedation Scale
(MOAA/S)7
| · | Maximum
sedation (MOAA/S score < 5) occurred approximately 15 minutes after dosing on day 1 (placebo
adjusted % of subjects 25.5%) with attenuation over time |
“We believe these data are remarkable. We expect to move forward
with our development of SLS-002 after the end of Phase II meeting with the FDA,” said Raj Mehra Ph.D., Chairman and Chief Executive
Officer of Seelos. “The improvements with SLS-002 were robust and continued to improve across efficacy scales with 5 doses over
the two-week treatment period. In addition to the data on efficacy, the differentiated and well-tolerated safety profile of SLS-002 highly
underscores this product candidate’s uniqueness and potential for the treatment of ASIB in MDD. We look forward to advancing this
therapy toward a potential first approval for an important unmet need for this patient population, especially considering that 2022 experienced
the highest number of suicides in U.S. history.”
Webcast Information
Seelos will host a webcast today, Wednesday, September 20th,
at 8 a.m. ET, to discuss the results. On the call from Seelos will be Raj Mehra, Ph.D., Chairman and CEO, and Tim Whitaker, M.D.,
Chief Medical Officer.
Additionally, David V. Sheehan, M.D., the Distinguished University
Health Professor Emeritus at the University of South Florida College of Medicine, will be on the call as well to discuss this data.
Webcast for live and replay: https://lifescievents.com/event/seelos-2
A replay of the webcast will be available shortly following the presentation.
This study randomized 147 subjects diagnosed with MDD requiring psychiatric
hospitalization due to significant risk of suicide and severe depression as confirmed by the rating scales as discussed above. In addition,
subjects had to have at least one suicide attempt. For more information on entry criteria visit https://clinicaltrials.gov/study/NCT04669665?term=sls-002&rank=1.
After admission to the emergency room or hospital, each subject participated
in a 1- to 2-day screening phase, a 16-day treatment phase, including clinical standard of care, during which the study drug was administered
2 times per week (total of 5 doses), and a 2-week safety follow-up phase for a total of up to 5 weeks of study participation. Subjects
were treated as inpatients for approximately 7 days (including screening), and assuming the subject met readiness for discharge criteria,
were discharged on Day 6 to continue the trial as outpatients. At study completion, all subjects were well-connected with follow-up care
to ensure their safety.
Per
the Centers for Disease Control and Prevention (CDC) provisional data, the 49,449 suicides in the U.S. in 2022 represented
the highest number of suicides recorded in U.S. history and unfortunately, the medical community still lacks an FDA approved therapeutic
to treat the symptoms of suicidality. According to the CDC, in 2020, suicides and non-fatal self-harm cost the U.S. over $500
billion in medical and work-loss costs, value of statistical life, and quality of life costs. Suicidal patients who present
suicidal ideation and behavior symptoms at an Emergency Department and can be held in the Emergency Department for several days while
awaiting an inpatient psychiatric bed. Currently in the U.S., there is a shortage of over 120,000 inpatient psychiatric beds and the
average length of hospitalization for a suicidal patient is 10 days.
If you or a loved one are having thoughts of suicide, please seek
immediate medical help, go to your nearest emergency room, call the Suicide and Crisis Lifeline at 988 or 1-800-273-8255
(TALK).
1
Montgomery-Åsberg Depression Rating Scale (MADRS) scale range 0-60, higher scores indicating more severe depression.
2 Least
Squares (LS) Means are means estimated from a linear model that are adjusted for other effects defined in the model.
3 ANCOVA
is short for Analysis of Covariance. The analysis of covariance is a combination of an ANOVA and a regression analysis.
4 Sheehan-Suicidality
Tracking Scale (S-STS) is a clinician-rated scale, which includes 13 suicidality items that are rated on a scale ranging from 0 (not
at all) to 4 (extremely), which yields a total score ranging from 0 to 52.
5
Clinical Global Impression of Severity for Suicidal Ideation and Behavior (CGIS-SI/B) is a 5-point clinician-rated measure of suicidality-specific
symptom severity, ranging from 1 (not at all suicidal) to 5 (among the most extremely suicidal).
6
Clinician-Administered Dissociative States Scale (CADSS) is a standardized instrument used to measure present-state dissociative symptoms.
The scale includes 23 subjective items to be answered by the subject according to a 5-point scale (0 = not at all, 1 = mild,
2 = moderate, 3 = severe, and 4 = extreme). CADSS total score range is 0-92; a higher score reflects a more severe condition.
7
Modified Observer’s Assessment of Alertness/Sedation Scale (MOAA/S) is a 6 point scale with a score of 5 defined as responds readily
to name, and a score of 0 defined as does not respond to painful stimulus.
About SLS-002
SLS-002 is intranasal racemic ketamine with two investigational new
drug applications for the treatment of Acute Suicidal Ideation and Behavior in Major Depressive Disorder and in Post-Traumatic Stress
Disorder. SLS-002 was originally derived from a Javelin Pharmaceuticals, Inc./Hospira, Inc. program with 16 clinical studies
involving approximately 500 subjects. Seelos looks to address an unmet need for a therapy to treat suicidality in the U.S. with SLS-002.
Traditionally, anti-depressants have been used in this setting but many of the existing treatments are known to contribute to an increased
risk of suicidal thoughts in some circumstances, and if they are effective, it often takes weeks for the full therapeutic effect to be
manifested. Based on information gathered from the databases of the Agency for Healthcare Research and Quality, there were more than
1,000,000 visits to emergency rooms for suicide attempts in 2019 in the U.S. alone. Experimental studies suggest ketamine has the potential
to be a rapid, effective treatment for refractory depression and suicidality.
About Seelos Therapeutics
Seelos Therapeutics, Inc. is a clinical-stage biopharmaceutical
company focused on the development and advancement of novel therapeutics to address unmet medical needs for the benefit of patients with
central nervous system (CNS) disorders and other rare diseases. The Company's robust portfolio includes several late-stage clinical assets
targeting indications including Acute Suicidal Ideation and Behavior (ASIB) in Major Depressive Disorder (MDD), amyotrophic lateral sclerosis
(ALS) and spinocerebellar ataxia (SCA), as well as early-stage programs in Huntington's disease, Alzheimer's disease, and Parkinson's
disease.
For more information, please visit our website: http://seelostherapeutics.com,
the content of which is not incorporated herein by reference.
Forward Looking Statements
Statements made in this press release, which are not historical
in nature, constitute forward-looking statements for purposes of the safe harbor provided by the Private Securities Litigation Reform
Act of 1995. These statements include, among others, those regarding Part 2 of the Phase II study of SLS-002, statements regarding
SLS-002's prospects and potential, statements regarding any potential market opportunity for SLS-002, statements to the effect that Part 2
of the Phase II study of SLS-002 would have achieved statistical significance for the primary endpoint had the study reached full enrollment
and statements regarding Seelos’ development plans for SLS-002 and any planned meetings and discussions with the FDA. These statements
are based on Seelos' current expectations and beliefs and are subject to a number of risks and uncertainties that could cause actual
results to differ materially from those described in the forward-looking statements. Risks associated with Seelos' business and plans
described herein include, but are not limited to, the risk of not successfully executing its preclinical and clinical studies, not being
able to move forward with the development of SLS-002 after the anticipated end of Phase II meeting with the FDA, and not gaining marketing
approvals for SLS-002 and/or its other product candidates; the risk that prior clinical results may not be replicated in future studies
and trials (including the risk that the results from the prior studies of SLS-002 may not be replicated or may be materially different
from the results of Part 2 of the Phase II study of SLS-002); the risks that clinical study results may not meet any or all endpoints
of a clinical study and that any data generated from such studies may not support a regulatory submission or approval; the risks associated
with the implementation of Seelos' business strategy; the risks related to raising capital to fund its development plans and ongoing
operations; the risks related to Seelos' current stock price; as well as other factors expressed in Seelos' periodic filings with the
U.S. Securities and Exchange Commission, including its most recent Annual Report on Form 10-K and Quarterly Reports on Form 10-Q.
Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations
will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date
hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or
clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required
under applicable securities laws.
Contact Information:
Anthony Marciano
Chief Communications Officer
Seelos Therapeutics, Inc. (Nasdaq: SEEL)
300 Park Avenue
New York, NY 10022
(646) 293-2136
anthony.marciano@seelostx.com
https://seelostherapeutics.com/
https://twitter.com/seelostx
https://www.linkedin.com/company/seelos
Mike Moyer
Managing Director
LifeSci Advisors, LLC
250 West 55th St., Suite 3401
New York, NY 10019
(617) 308-4306
mmoyer@lifesciadvisors.com
Exhibit 99.2
SEELOS THERAPEUTICS September 2023 “We are a company focused on achieving the most efficient development of products that address significant unmet needs in CNS disorders and in rare diseases”

FORWARD - LOOKING STATEMENTS This corporate presentation includes certain forward - looking statements within the meaning of Section 21E of the Securities Exch ange Act of 1934, as amended, and the Private Securities Litigation Reform Act of 1995. These forward - looking statements include, but are not limited to, statements r egarding the intent, belief or current expectations of Seelos Therapeutics, Inc. ("we," "us," "our," the "Company" or "Seelos") and our management team. These forwa rd - looking statements involve known and unknown risks, uncertainties and other important factors that may cause the actual results, performance or achievements of th e C ompany to be materially different from any future results, performance or achievements expressed or implied by the forward - looking statements. Words such as “anticipates,” “believes,” “forecasts,” “potential,” “goal,” “contemplates,” “expects,” “intends,” “plans,” “pr oje cts,” “hopes,” “seeks,” “estimates,” “strategy,” “continues,” “ongoing,” “opportunity,” “could,” “would,” “should,” “likely,” “will,” “may,” “can,” “designed to,” “future,” “ for eseeable future” and similar expressions and variations, and negatives of these words, identify forward - looking statements. These forward - looking statements are based on the expectations, estimates, projections, beliefs and assumptions of our management based on information currently available to us, all of which are subject to change. Th ese forward - looking statements are not guarantees of future performance and are subject to risks and uncertainties that could cause actual results to differ materia lly from the results contemplated by the forward - looking statements. Many of the important factors that will determine these results and values are beyond our ability to control or predict. You are cautioned not to put undue reliance on any forward - looking statements. Except as otherwise required by law, we do not assume any obligation to up date any forward - looking statements. For additional information about factors that could cause actual results to differ materially from those described in the for war d - looking statements, please refer to the Company’s filings with the Securities and Exchange Commission (the “SEC”), including the risk factors contained in the Compan y’s Annual Report on Form 10 - K for the year ended December 31, 2022, subsequent Quarterly Reports on Form 10 - Q and in the company’s other filings with the SEC . This corporate presentation is for informational purposes only and is neither an offer to purchase, nor solicitation of an of fer to sell, subscribe for or buy any securities or the solicitation of any vote in any jurisdiction pursuant to the proposed transaction, nor shall there be any sale, issuance or transfer of securities in any jurisdiction in contravention of applicable law. No offer of securities shall be made except by means of a prospectus meeting the requirement s o f Section 10 of the Securities Act of 1933, as amended. 2

EXPERIENCED MANAGEMENT TEAM Raj Mehra, PhD Chairman, Founder, and Chief Executive Officer 23 years experience as VC/investor Michael Golembiewski Chief Financial Officer 30 years experience in public company accounting and finance Tim Whitaker, MD Chief Medical Officer 26 years experience with 19 NDA and sNDAs and one BLA approved in CNS M ultiple global approvals in CNS and one NDA and one sNDA in GI space Warren Wasiewski, MD Senior Neuro Advisor 23 years of experience as pediatric neurologist, 18 years in pharma industry, and designed clinical trials for five orphan CNS programs Karen Fusaro SVP Head of Clinical Operations 27 years experience with four NDAs in infectious diseases Gopal Krishna, PhD Head of Manufacturing & Technical Operations 27 years experience in cGMP, GLP, and CMC regulations Anthony Marciano Chief Communications Officer 25 years experience in Healthcare Investor Engagement 3

Advancing multiple late - stage therapeutic candidates for CNS disorders Developing therapeutics with demonstrated mechanisms of action Identifying opportunities in large markets SEELOS IS A CNS AND RARE DISEASE FOCUSED BIOTECH COMPANY SLS - 004 Parkinson’s Disease (PD) Gene therapy program targeting the regulation of the SNCA gene, which encodes alpha - synuclein ( α - synuclein) SLS - 009 Huntington’s disease, Alzheimer’s disease, ALS protein targeted autophagy mechanism of action, in vivo studies underway in 2023 SLS - 005 Amyotrophic Lateral Sclerosis (ALS), Spinocerebellar Ataxia (SCA), Huntington’s Disease (HD), and Alzheimer’s Disease (AD) Fast - Track designation in SCA Encouraging Phase IIa open - label data shown in SCA type 3 Completed enrollment with the HEALEY ALS Platform Trial, led by Harvard, in Q1 2023, data readout expected in late 2023 SCA initiated a double - blind, placebo - controlled Phase II/III study in Q4 2022 and has Orphan Drug designation SLS - 002 Acute Suicidal Ideation and Behavior (ASIB) in Major Depressive Disorder (MDD) Fast - Track designation Rapid - acting intranasal administration Phase 1 completed June 2020 Phase 2 Study, Proof - of - Concept Part 1 completed in Q2 2021 Phase 2 Study, Part 2 (double - blind, placebo controlled) initiated in Q3 2021 with data readout in Q3 2023 4

SLS - 002 (INTRANASAL RACEMIC KETAMINE) 5

SUICIDAL IDEATION AND BEHAVIORS AMONG U.S. ADULTS 12.2 million adults had serious thoughts of committing suicide 2 1.2 million adults attempted suicide 2 3.2 million adults made suicide plans 2 920,000 adults made plans and attempted suicide 2 283,000 adults made no plans and attempted suicide 2 1. Owens PL (AHRQ), Fingar KR (IBM Watson Health), Heslin KC (AHRQ), Mutter R (SAMHSA), Booth CL (SAMHSA). Emergency Departme nt Visits Related to Suicidal Ideation, 2006 - 2013. HCUP Statistical Brief #220. January 2017. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup - us.ahrq.go v/reports/statbriefs/sb220 - Suicidal - Ideation - ED - Visits.pdf. 2. Center for Behavioral Health Statistics and Quality. (2020). Results from the 2020 National Survey on Drug Use and Health: Gr aphics from the Key Findings Report - Rockville, MD: Substance Abuse and Mental Health Services Administration. Retrieved from https://www.samhsa.gov/data/sites/default/files/rep ort s/rpt35325/2020NSDUHFFRSlides090821.pdf. Accessed December 7, 2022. 3. CDC Suicide Prevention: Suicide Data and Statistics. Available at: https://www. https://www.cdc.gov/suicide/suicide - data - stat istics.html/. Accessed September 6, 2023. ASIB overall accounts for over 900,000 emergency room visits annually, and over 40% of these patients are admitted 1 49,449 Deaths by suicide in the United States in 2022 (provisional total from CDC) 3 Highest annual number of suicides on record in the US 6

SLS - 002 - 201 STUDY TITLE A 2 - part Phase 2 Study To Assess The Efficacy, Safety, And Tolerability Of SLS - 002 (Intranasal Racemic Ketamine) Administered To Adults With Major Depressive Disorder At Imminent Risk Of Suicide 7
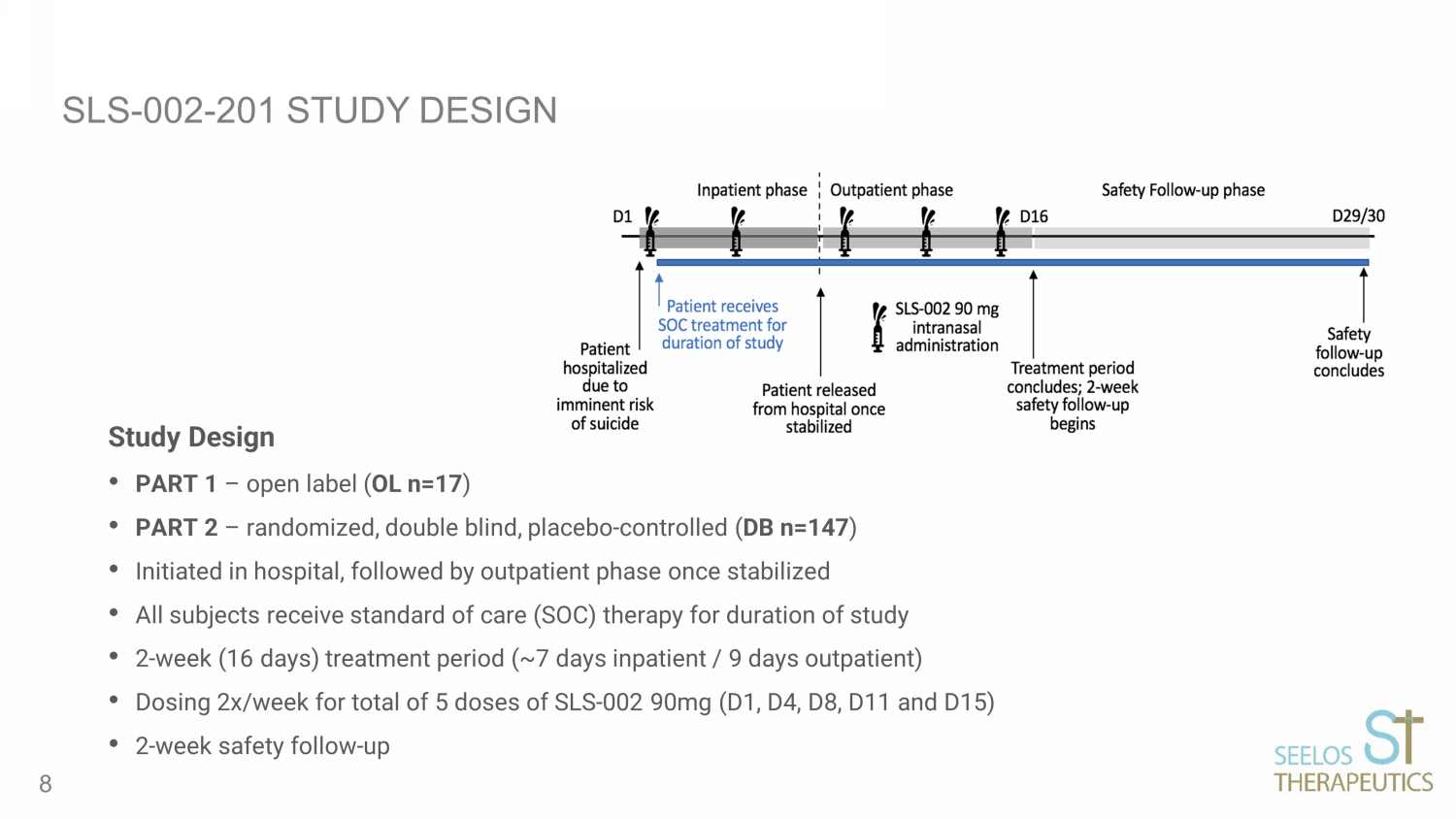
SLS - 002 - 201 STUDY DESIGN Study Design • PART 1 – open label ( OL n=17 ) • PART 2 – randomized, double blind, placebo - controlled ( DB n=147 ) • Initiated in hospital, followed by outpatient phase once stabilized • All subjects receive standard of care (SOC) therapy for duration of study • 2 - week (16 days) treatment period (~7 days inpatient / 9 days outpatient) • Dosing 2x/week for total of 5 doses of SLS - 002 90mg (D1, D4, D8, D11 and D15) • 2 - week safety follow - up 8
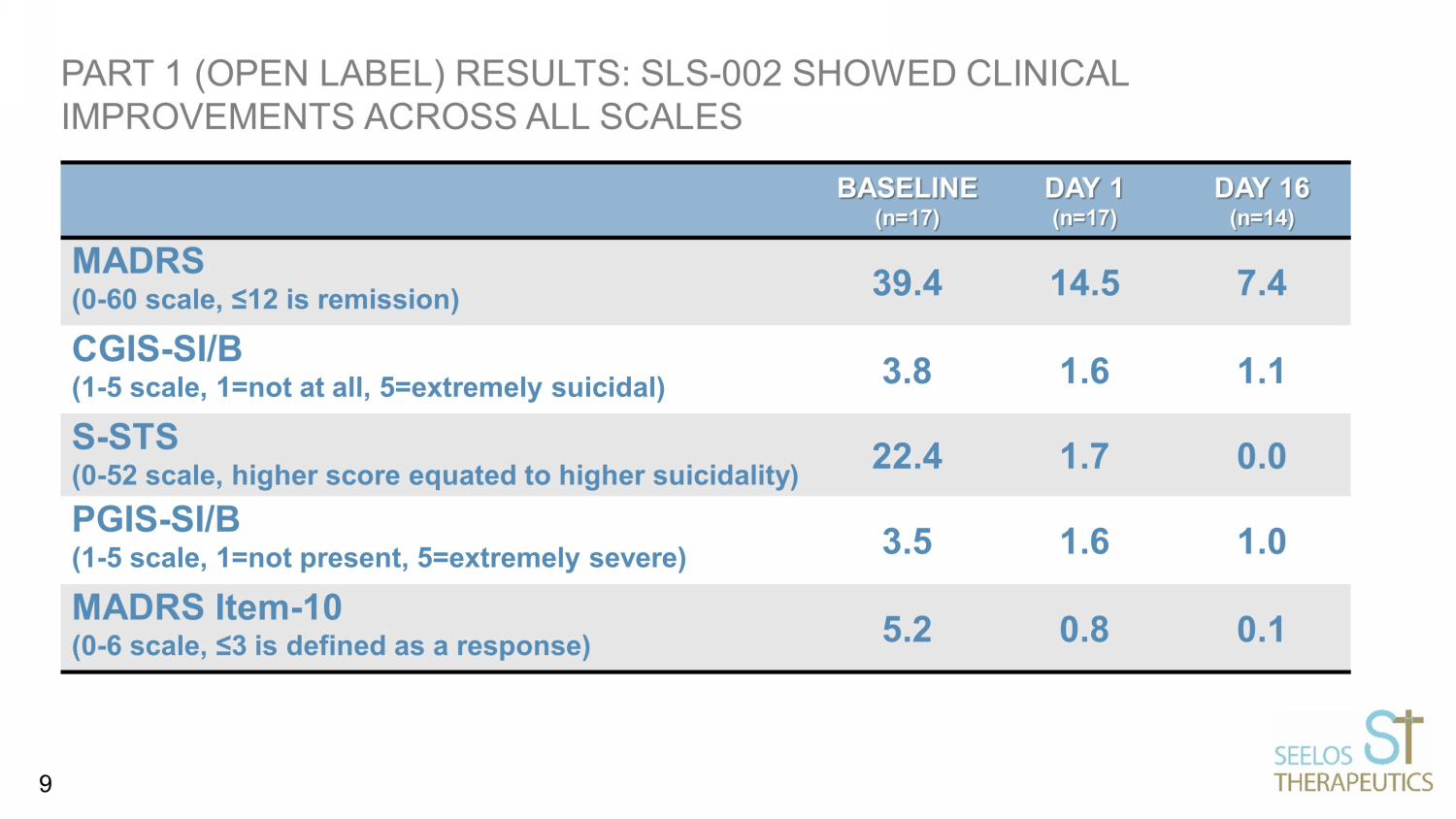
PART 1 (OPEN LABEL) RESULTS: SLS - 002 SHOWED CLINICAL IMPROVEMENTS ACROSS ALL SCALES BASELINE (n=17) DAY 1 (n=17) DAY 16 (n=14) MADRS (0 - 60 scale, ≤12 is remission) 39.4 14.5 7.4 CGIS - SI/B (1 - 5 scale, 1=not at all, 5=extremely suicidal) 3.8 1.6 1.1 S - STS (0 - 52 scale, higher score equated to higher suicidality) 22.4 1.7 0.0 PGIS - SI/B (1 - 5 scale, 1=not present, 5=extremely severe) 3.5 1.6 1.0 MADRS Item - 10 (0 - 6 scale, ≤3 is defined as a response) 5.2 0.8 0.1 9
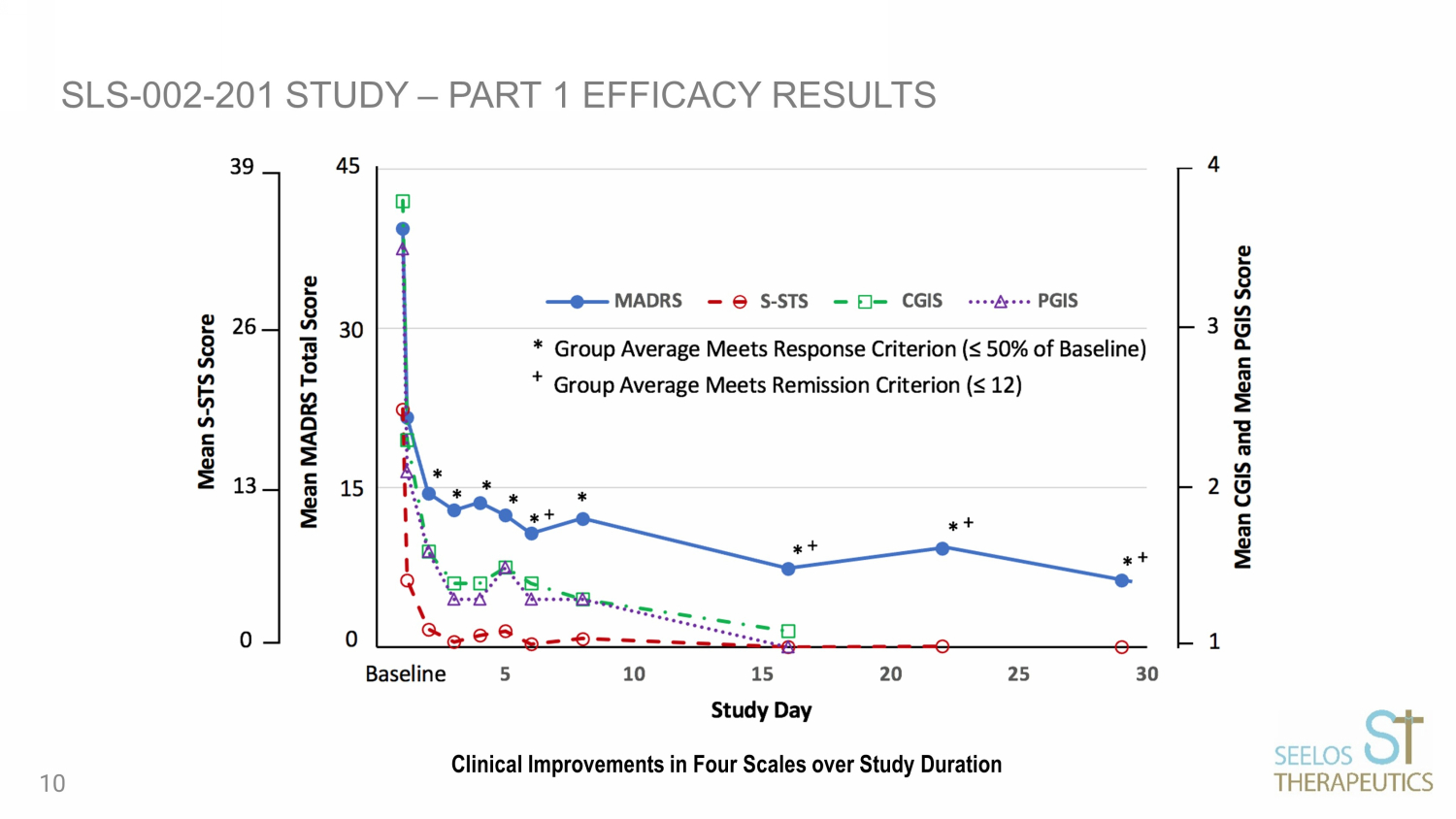
SLS - 002 - 201 STUDY – PART 1 EFFICACY RESULTS Clinical Improvements in Four Scales over Study Duration 10

This study randomized 147 patients diagnosed with MDD requiring psychiatric hospitalization due to imminent risk of suicide and: • Baseline total score of ≥ 28 on the Montgomery - Åsberg Depression Rating Scale (MADRS) • S core of 5 or 6 on MADRS Item - 10 • Baseline CGIS - SI/B of ≥ 4 • Total s core of ≥ 15 on the Sheehan - Suicidality Tracking Scale (S - STS) • History of previous suicide attempt(s), as confirmed on the Columbia Suicide Severity Rating Scale (C - SSRS) with a history of at least one actual attempt, or if the attempt was interrupted or aborted, is judged to have been serious in intent * Criteria must have been met at screening and baseline SLS - 002 - 201 PART 2 ENROLLMENT CRITERIA – SEVERE SUICIDALITY REQUIRED 11
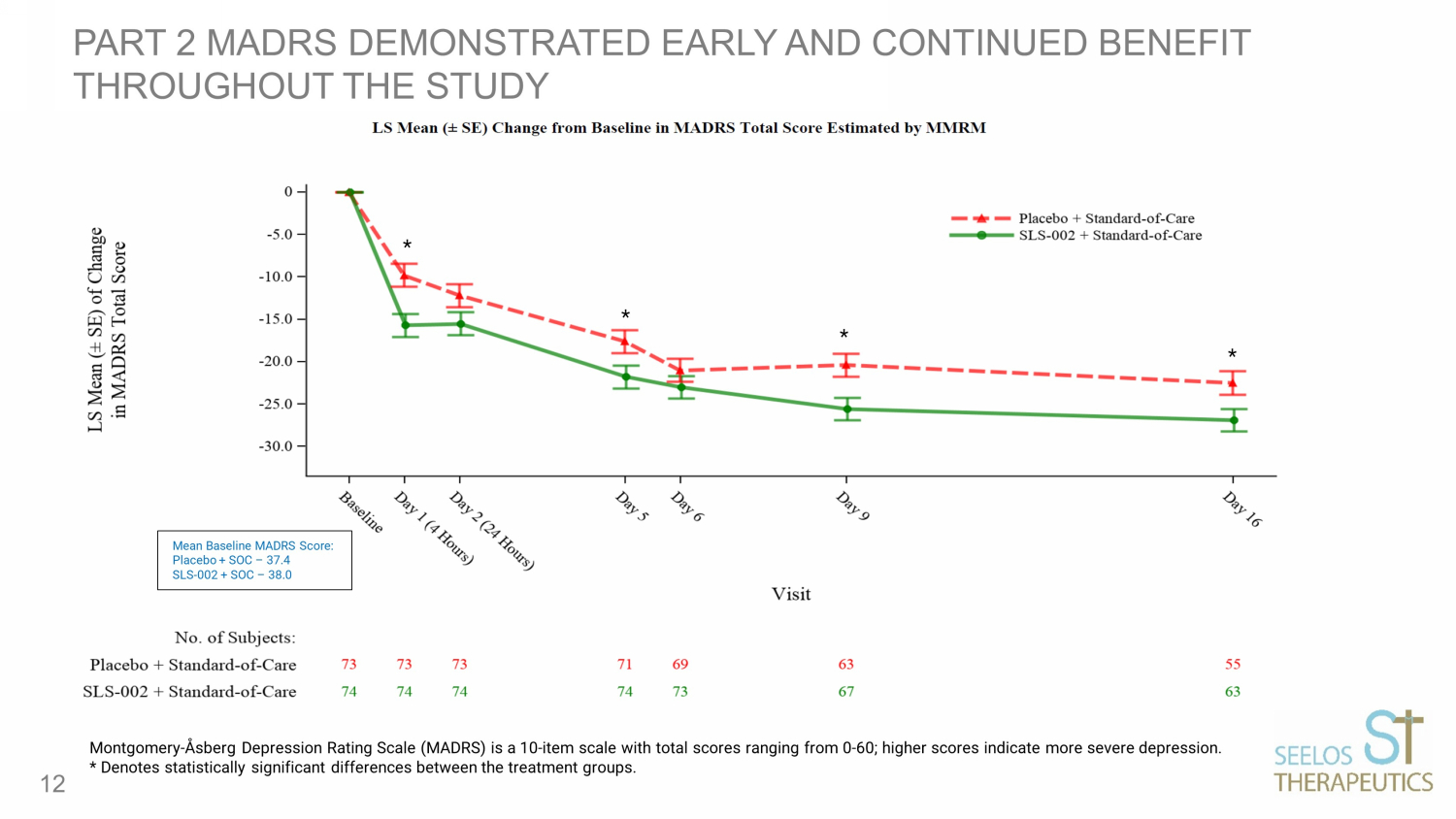
PART 2 MADRS DEMONSTRATED EARLY AND CONTINUED BENEFIT THROUGHOUT THE STUDY 12 Montgomery - Åsberg Depression Rating Scale (MADRS) is a 10 - item scale with total scores ranging from 0 - 60; higher scores indicate more severe depr ession. * Denotes statistically significant differences between the treatment groups. * * * * Mean Baseline MADRS Score: Placebo + SOC – 37.4 SLS - 002 + SOC – 38.0

MADRS DEMONSTRATED CLINICALLY MEANINGFUL DIFFERENCES BETWEEN GROUPS THROUGHOUT THE STUDY HOWEVER, STATISTICAL SIGNIFICANCE WAS NOT ACHIEVED FOR THE PREDE FINED PRIMARY ENDPOINT ( ANCOVA MADRS 24 HOURS POST DOSING) CHANGE FROM BASELINE MADRS TOTAL SCORE MMRM ESTIMATES PRESENTED HERE 13 Visit Placebo LS Means (SE) SLS - 002 LS Means (SE) Difference LS Mean (SE) P - value MADRS Day 1 (4 hours) - 9.8 (1.36) - 15.7 (1.34) - 5.9 (1.74) <0.001 MADRS Day 2 (24 hours) - 12.2 (1.38) - 15.5 (1.36) - 3.3 (1.77) 0.068 MADRS Day 16 - 22.5 (1.37) - 26.9 (1.29) - 4.4 (1.72) 0.012 Montgomery - Åsberg Depression Rating Scale (MADRS) is a 10 - item scale with total scores ranging from 0 - 60; higher scores indicate more severe depr ession.

SLS - 002 - 201 SUSTAINED IMPROVEMENT ON MADRS DURING SAFETY FOLLOW - UP 14 Group Mean MADRS Baseline / Change from Baseline Placebo (N=73) SLS - 002 (N=74) Difference Baseline 37.4 38.0 0.6 Day 2 (24 hours) - 12.4 n=73 - 16.1 n=74 - 3.7 Day 16 - 22.2 n=55 - 27.9 n=63 - 5.7 Day 22 (Safety Follow - up Week 1) - 23.9 n=54 - 29.3 n=61 - 5.4 Day 29/30 (Safety Follow - up Week 2) - 25.0 n=52 - 30.3 n=60 - 5.3 Note: Results presented are based on simple summary statistics, not adjusted (least - square) means as presented for the MMRM anal yses, with differences calculated from group mean scores rounded to the tenth.

SLS - 002 - 201 PRIMARY ANALYSIS AND AD HOC SENSITIVITY ANALYSIS 15 MADRS Day 2 (24 hours) Treatment Difference Standard Error p - Value 2 - Way ANCOVA 1 (Primary Analysis) - 3.3 1.78 0.069 1 - Way ANOVA 2 (t - test) - 3.6 1.82 0.049 1 Based on model with Treatment and Standard of Care as fixed effects and baseline MADRS score as a covariate 2 While not a planned analysis in the study, shown here for illustrative purposes. Based on model with treatment as a fixed e ffe ct

MADRS RESPONSE AND REMISSION RATES AT END OF TREATMENT 16 DAY 16 Placebo DAY 16 SLS - 002 MADRS Response (≥50% reduction from baseline) 47.9% 75.7% P - value <0.001 MADRS Remission (≤12 is remission) 32.9% 62.2% P - value <0.001 Montgomery - Åsberg Depression Rating Scale (MADRS) is a 10 - item scale with total scores ranging from 0 - 60; higher scores indicate more severe depr ession.
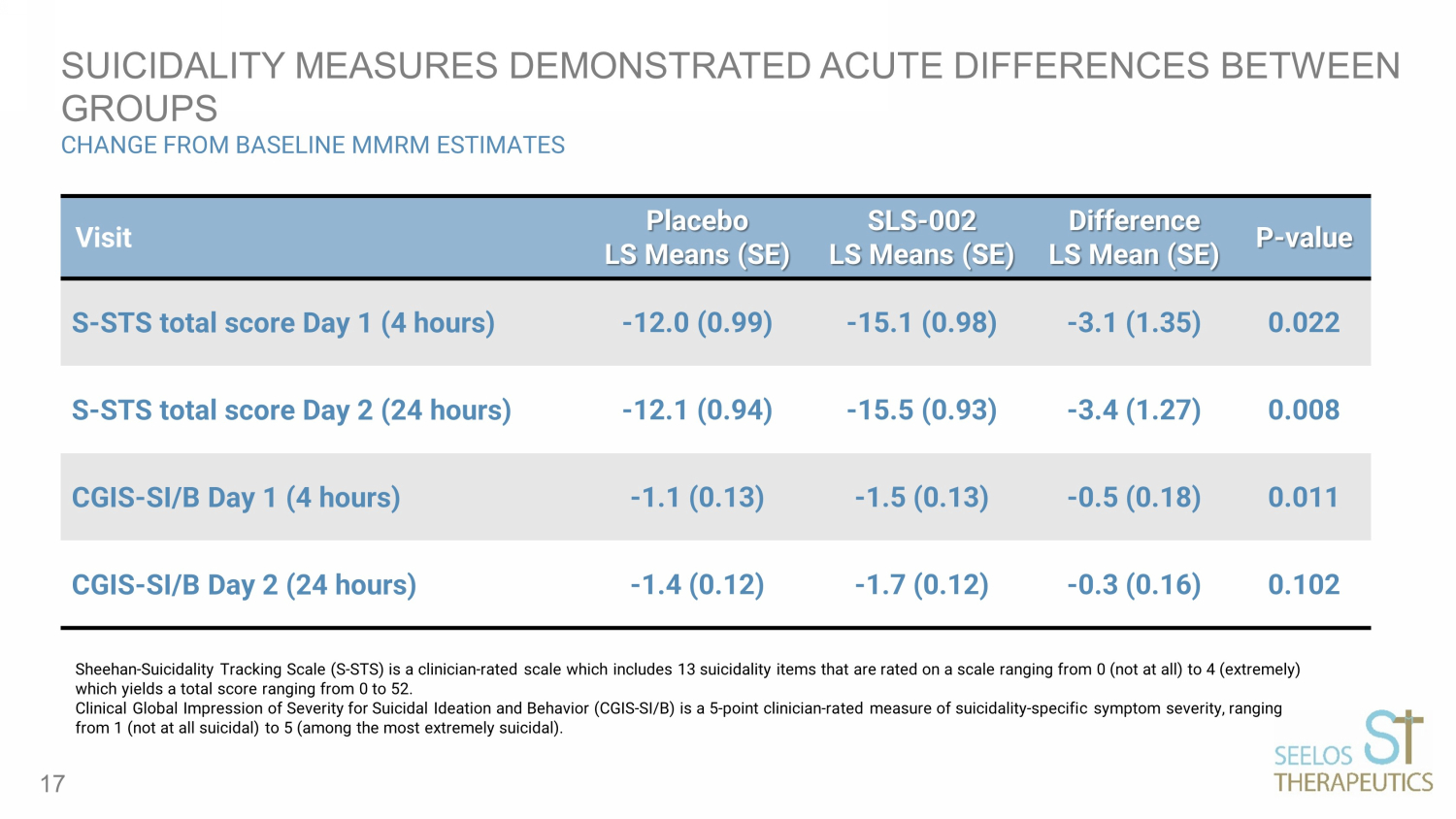
SUICIDALITY MEASURES DEMONSTRATED ACUTE DIFFERENCES BETWEEN GROUPS CHANGE FROM BASELINE MMRM ESTIMATES 17 Visit Placebo LS Means (SE) SLS - 002 LS Means (SE) Difference LS Mean (SE) P - value S - STS total score Day 1 (4 hours) - 12.0 (0.99) - 15.1 (0.98) - 3.1 (1.35) 0.022 S - STS total score Day 2 (24 hours) - 12.1 (0.94) - 15.5 (0.93) - 3.4 (1.27) 0.008 CGIS - SI/B Day 1 (4 hours) - 1.1 (0.13) - 1.5 (0.13) - 0.5 (0.18) 0.011 CGIS - SI/B Day 2 (24 hours) - 1.4 (0.12) - 1.7 (0.12) - 0.3 (0.16) 0.102 Sheehan - Suicidality Tracking Scale (S - STS) is a clinician - rated scale which includes 13 suicidality items that are rated on a sc ale ranging from 0 (not at all) to 4 (extremely) which yields a total score ranging from 0 to 52. Clinical Global Impression of Severity for Suicidal Ideation and Behavior (CGIS - SI/B) is a 5 - point clinician - rated measure of su icidality - specific symptom severity, ranging from 1 (not at all suicidal) to 5 (among the most extremely suicidal).

SAFETY DATA SUMMARY 18 • SLS - 002 showed a favorable safety profile and was well tolerated • There were no deaths • Most AEs were mild or moderate, short term/transient • No new/unique AEs identified • 52.7% (vs. 39.7% on placebo) of subjects had at least one TEAE • Most common TEAEs (≥5% and >placebo) • dizziness (18.9% vs. 2.7%) • euphoric mood (6.8% vs. 0%) • suicidal ideation (5.4% vs. 2.7%) • TEAEs leading to discontinuation 8.1% (vs. 4.1% placebo) • 3 SAEs (vs. 2 on placebo), all for suicidality, all judged unrelated to study drug, all resolved

GROUP MEAN CADSS CHANGE FROM PRE - DOSE RESULTS 19 Placebo SLS - 002 SLS - 002 Placebo adjusted Day 1 - 40 mins 0.2 4.1 3.9 Day 1 - 1hr Day 1 - 2hr - 0.3 - 0.6 1.5 - 0.2 1.8 0.4 Day 4 - 40 mins 0.3 2.8 2.5 Day 4 - 1hr 0.04 0.8 0.76 Day 8 - 40 mins 0.1 1.5 1.4 CADSS (Clinician - Administered Dissociative States Scale) includes 23 subjective items to be answered by the subject according to a 5 - point scale (0 = not at all, 1 = mild, 2 = moderate, 3 = severe, and 4 = extreme). Scores are totaled overall; a higher score reflects a more severe condition. Total score range is 0 - 92. CADSS scores > 4 represent clinically meaningful dissociation. The Day 1, 40 min timepoint was only timepoint with a SLS - 002 mean CADSS greater than 4.
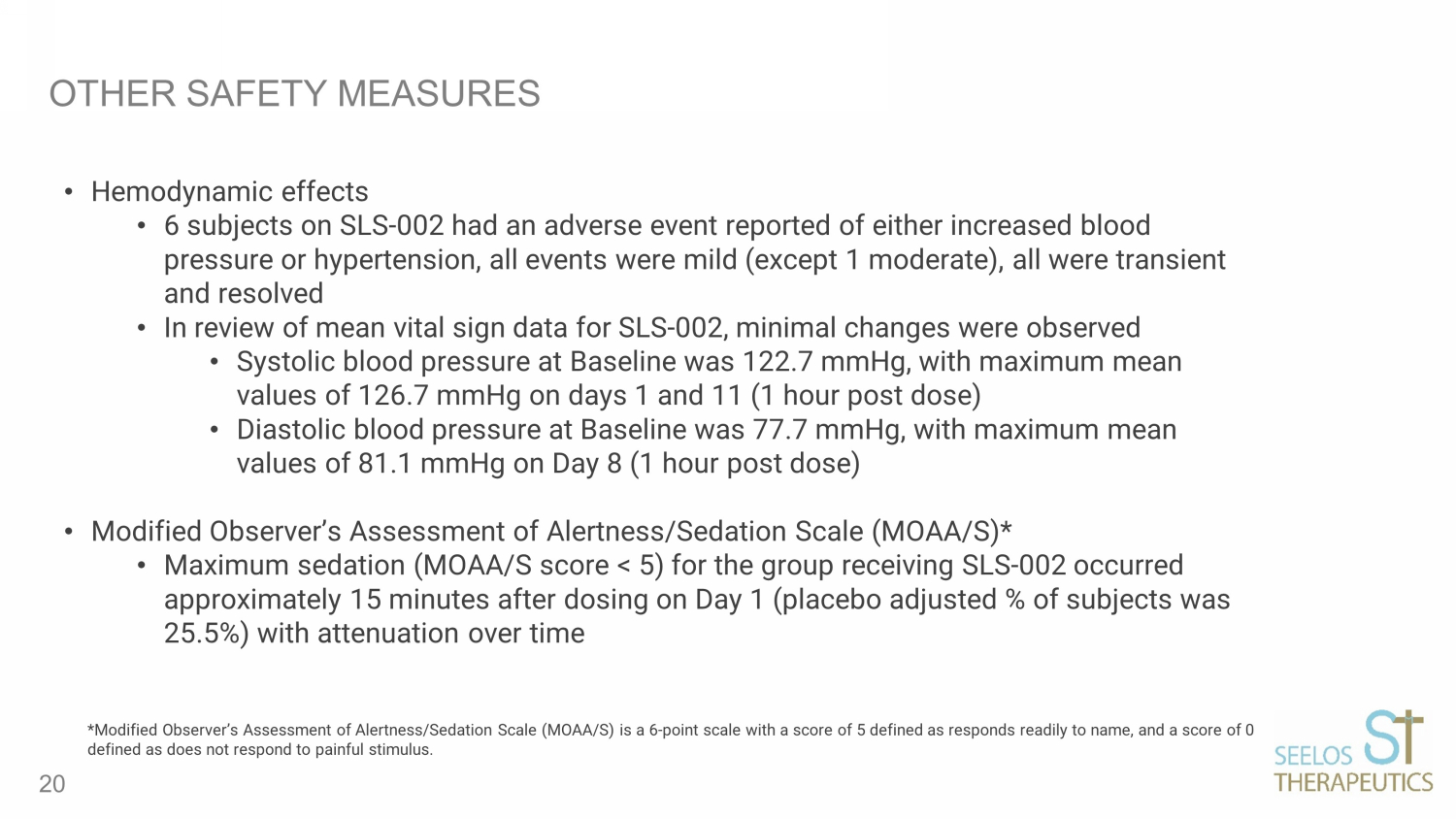
OTHER SAFETY MEASURES • Hemodynamic effects • 6 subjects on SLS - 002 had an adverse event reported of either increased blood pressure or hypertension, all events were mild (except 1 moderate), all were transient and resolved • In review of mean vital sign data for SLS - 002, minimal changes were observed • Systolic blood pressure at Baseline was 122.7 mmHg, with maximum mean values of 126.7 mmHg on days 1 and 11 (1 hour post dose) • Diastolic blood pressure at Baseline was 77.7 mmHg, with maximum mean values of 81.1 mmHg on Day 8 (1 hour post dose) • Modified Observer’s Assessment of Alertness/Sedation Scale (MOAA/S)* • Maximum sedation (MOAA/S score < 5) for the group receiving SLS - 002 occurred approximately 15 minutes after dosing on Day 1 (placebo adjusted % of subjects was 25.5%) with attenuation over time 20 *Modified Observer’s Assessment of Alertness/Sedation Scale (MOAA/S) is a 6 - point scale with a score of 5 defined as responds re adily to name, and a score of 0 defined as does not respond to painful stimulus.

CONCLUSIONS • While trial did not meet its predefined primary endpoint, early and persistent improvements were observed on depressive symptoms (MADRS) – all timepoints showed clinically meaningful differences over placebo and statistically significant differences at the majority of timepoints • Statistical significance at 4hr (p=0.001) • Statistical significance at 24hr with exploratory ANOVA (p=0.049), differences at 24hr with pre - defined ANCOVA (p=0.069) would have reached statistical significance with larger sample size • Statistical significance at Day 16 (p=0.012) – required by FDA to demonstrate persistence of effect • Improvements on MADRS maintained during safety follow - up • Clinically meaningful reduction in acute suicidality was demonstrated with SLS - 002 over placebo and was statistically significant at key timepoints (4 and 24 hrs after dosing) • S - STS – statistically significant at 4hr (p=0.022) and 24hr (p=0.008) • CGIS - SI/B – statistically significant at 4hr (p=0.011) and at 24hr numeric improvement • Continued improvement in both treatment groups over time as would be expected with SOC • Results demonstrate clinical benefit on key measures of depression and suicidality in the study • Treatment showed a favorable safety profile and was generally well tolerated, with no new/unique AEs identified • All subjects were maintained safely throughout the study • Overall safety profile suggests better tolerability (e.g. sedation and dissociation) with potential differentiation over other ketamine options 21

SLS - 002 INTRANASAL ADMINISTRATION DEVICE Manufactured by Aptar Pharma (NYSE:ATR) Seelos has Co - Exclusive use of Bidose (BDS) Liquid System Bi - dose device administers 0.1 mL sprays in each nostril Disposable device for single use Convenient, ready to use administration 22
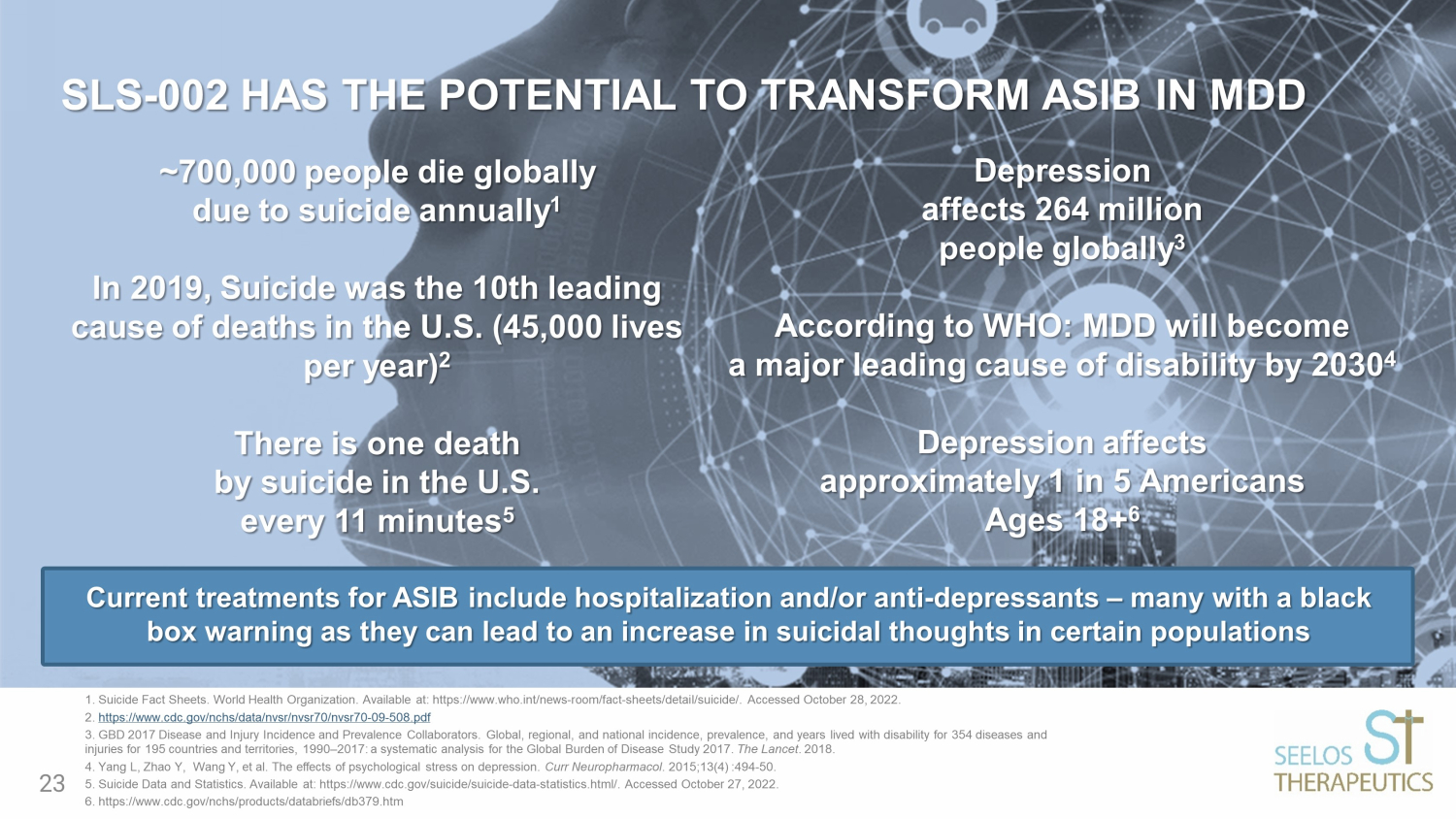
~700,000 people die globally due to suicide annually 1 In 2019, Suicide was the 10th leading cause of deaths in the U.S. (45,000 lives per year) 2 There is one death by suicide in the U.S. every 11 minutes 5 Depression affects 264 million people globally 3 According to WHO: MDD will become a major leading cause of disability by 2030 4 Depression affects approximately 1 in 5 Americans Ages 18+ 6 SLS - 002 HAS THE POTENTIAL TO TRANSFORM ASIB IN MDD 1. Suicide Fact Sheets. World Health Organization. Available at: https://www.who.int/news - room/fact - sheets/detail/suicide/. Acce ssed October 28, 2022. 2. https://www.cdc.gov/nchs/data/nvsr/nvsr70/nvsr70 - 09 - 508.pdf 3. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990 – 2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet . 2018. 4. Yang L, Zhao Y, Wang Y, et al. The effects of psychological stress on depression. Curr Neuropharmacol . 2015;13(4) :494 - 50. 5. Suicide Data and Statistics. Available at: https://www.cdc.gov/suicide/suicide - data - statistics.html/. Accessed October 27, 20 22. 6. https://www.cdc.gov/nchs/products/databriefs/db379.htm Current treatments for ASIB include hospitalization and/or anti - depressants – many with a black box warning as they can lead to an increase in suicidal thoughts in certain populations 23

SLS - 005 (TREHALOSE) 24
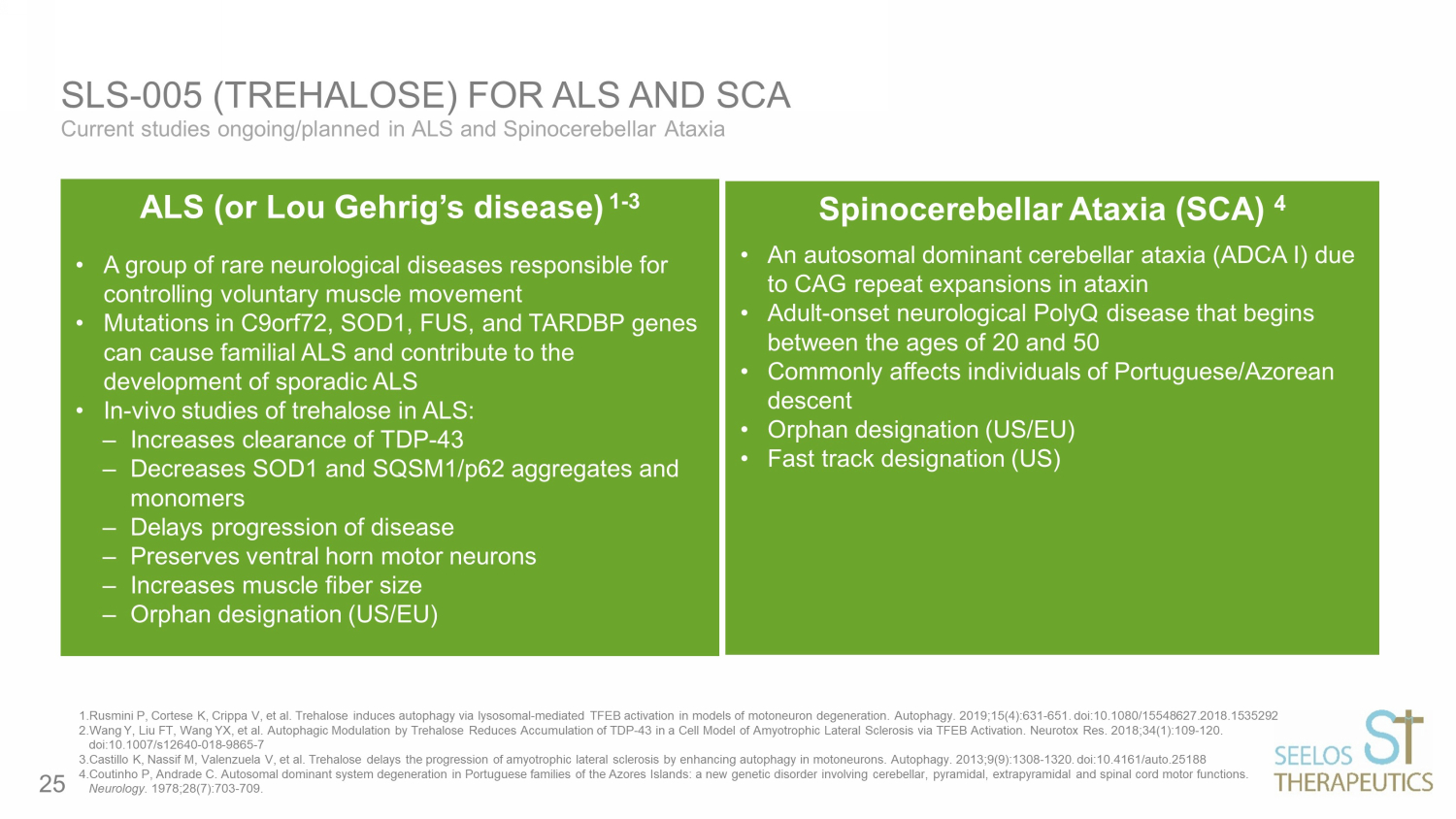
Current studies ongoing/planned in ALS and Spinocerebellar Ataxia SLS - 005 (TREHALOSE) FOR ALS AND SCA Dose IN ketamine hydrochloride (50 mg) or saline solution Duration Each treatment period (ketamine or placebo) consisted of 7 days. Assessments occurred at 40 min, 120 min, 240 min, 24 hrs, 48 hrs, 72 hrs and 7 days after the start of treatment intervention Results Patients showed significant improvement in depressive symptoms at 24 hours after ketamine compared to placebo (p<0.001) 1.Rusmini P, Cortese K, Crippa V, et al. Trehalose induces autophagy via lysosomal - mediated TFEB activation in models of motoneu ron degeneration. Autophagy. 2019;15(4):631 - 651. doi:10.1080/15548627.2018.1535292 2.Wang Y, Liu FT, Wang YX, et al. Autophagic Modulation by Trehalose Reduces Accumulation of TDP - 43 in a Cell Model of Amyotroph ic Lateral Sclerosis via TFEB Activation. Neurotox Res. 2018;34(1):109 - 120. doi:10.1007/s12640 - 018 - 9865 - 7 3.Castillo K, Nassif M, Valenzuela V, et al. Trehalose delays the progression of amyotrophic lateral sclerosis by enhancing a uto phagy in motoneurons. Autophagy. 2013;9(9):1308 - 1320. doi:10.4161/auto.25188 4.Coutinho P, Andrade C. Autosomal dominant system degeneration in Portuguese families of the Azores Islands: a new genetic d iso rder involving cerebellar, pyramidal, extrapyramidal and spinal cord motor functions. Neurology. 1978;28(7):703 - 709. ALS (or Lou Gehrig’s disease) 1 - 3 • A group of rare neurological diseases responsible for controlling voluntary muscle movement • Mutations in C9orf72, SOD1, FUS, and TARDBP genes can cause familial ALS and contribute to the development of sporadic ALS • In - vivo studies of trehalose in ALS: ‒ Increases clearance of TDP - 43 ‒ Decreases SOD1 and SQSM1/p62 aggregates and monomers ‒ Delays progression of disease ‒ Preserves ventral horn motor neurons ‒ Increases muscle fiber size ‒ Orphan designation (US/EU) Spinocerebellar Ataxia (SCA) 4 • An autosomal dominant cerebellar ataxia (ADCA I) due to CAG repeat expansions in ataxin • Adult - onset neurological PolyQ disease that begins between the ages of 20 and 50 • Commonly affects individuals of Portuguese/Azorean descent • Orphan designation (US/EU) • Fast track designation (US) 25
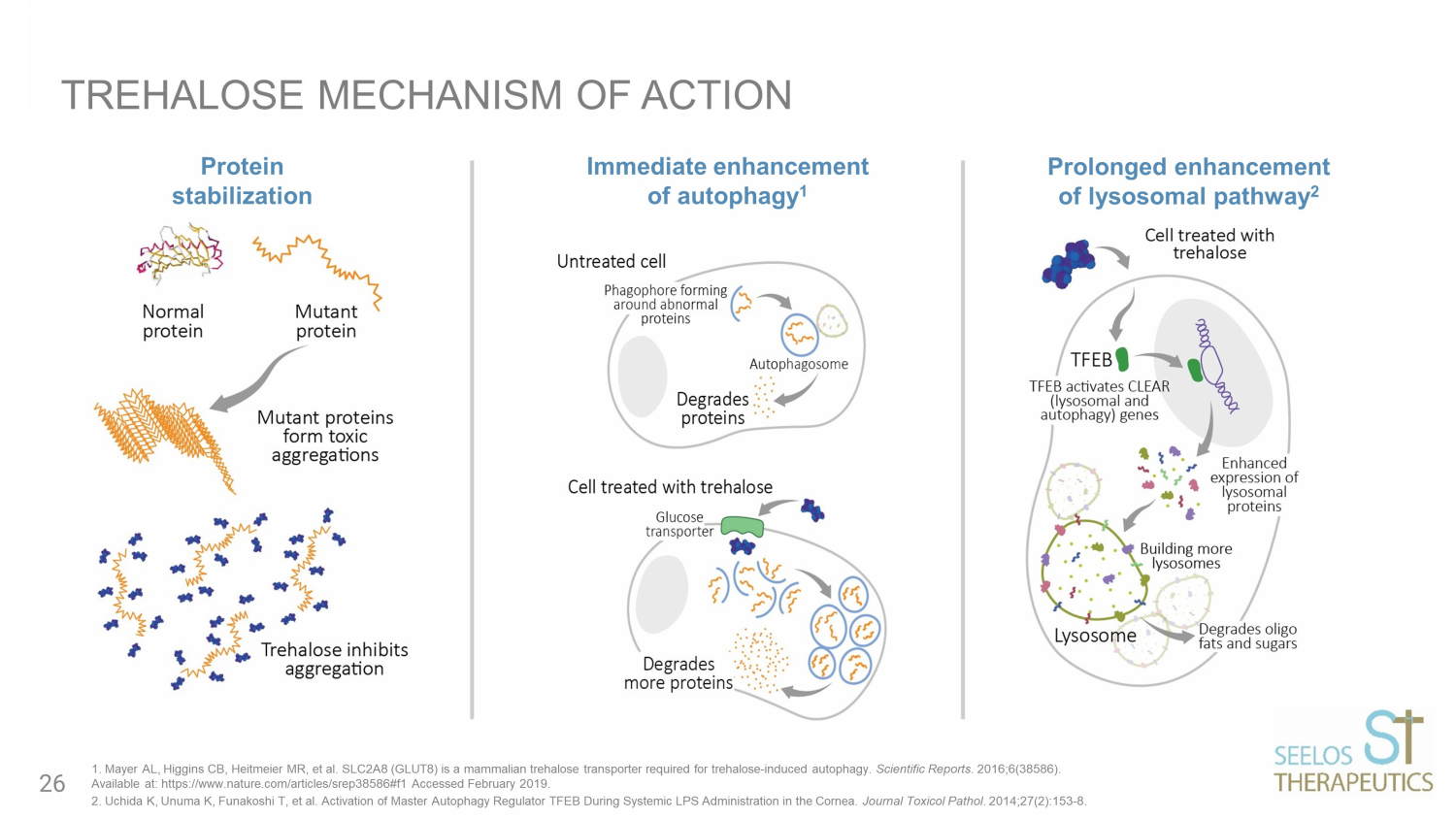
TREHALOSE MECHANISM OF ACTION Immediate enhancement of autophagy 1 Prolonged enhancement of lysosomal pathway 2 Protein stabilization 1. Mayer AL, Higgins CB, Heitmeier MR, et al. SLC2A8 (GLUT8) is a mammalian trehalose transporter required for trehalose - induced autophagy. Scientific Reports. 2016;6(38586). Available at: https://www.nature.com/articles/srep38586#f1 Accessed February 2019. 2. Uchida K, Unuma K, Funakoshi T, et al. Activation of Master Autophagy Regulator TFEB During Systemic LPS Administration in th e Cornea. Journal Toxicol Pathol . 2014;27(2):153 - 8. 26
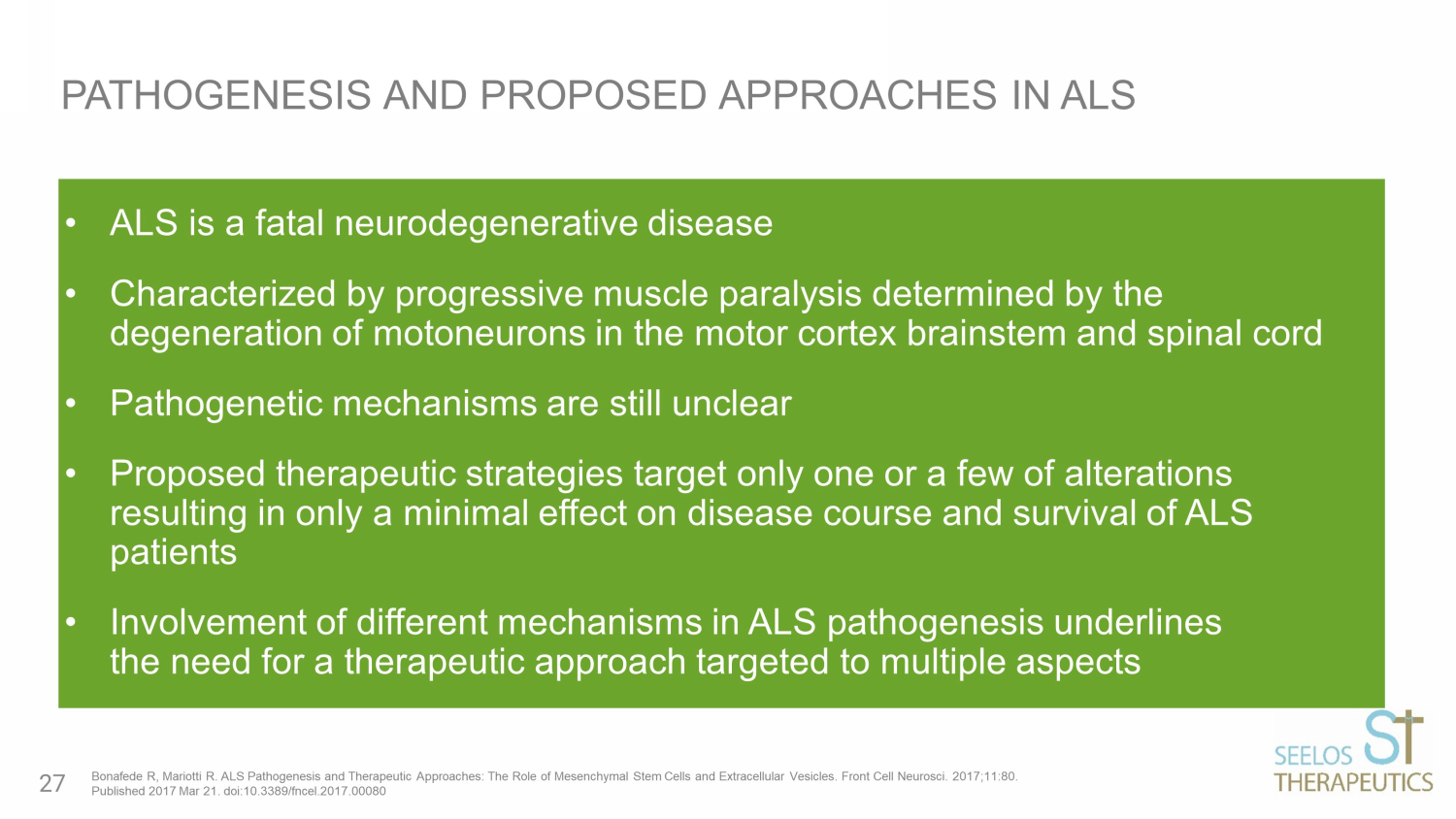
• ALS is a fatal neurodegenerative disease • C haracterized by progressive muscle paralysis determined by the degeneration of motoneurons in the motor cortex brainstem and spinal cord • P athogenetic mechanisms are still unclear • P roposed therapeutic strategies target only one or a few of alterations resulting in only a minimal effect on disease course and survival of ALS patients • I nvolvement of different mechanisms in ALS pathogenesis underlines the need for a therapeutic approach targeted to multiple aspects PATHOGENESIS AND PROPOSED APPROACHES IN ALS Bonafede R, Mariotti R. ALS Pathogenesis and Therapeutic Approaches: The Role of Mesenchymal Stem Cells and Extracellular Ves icl es. Front Cell Neurosci. 2017;11:80. Published 2017 Mar 21. doi:10.3389/fncel.2017.00080 27
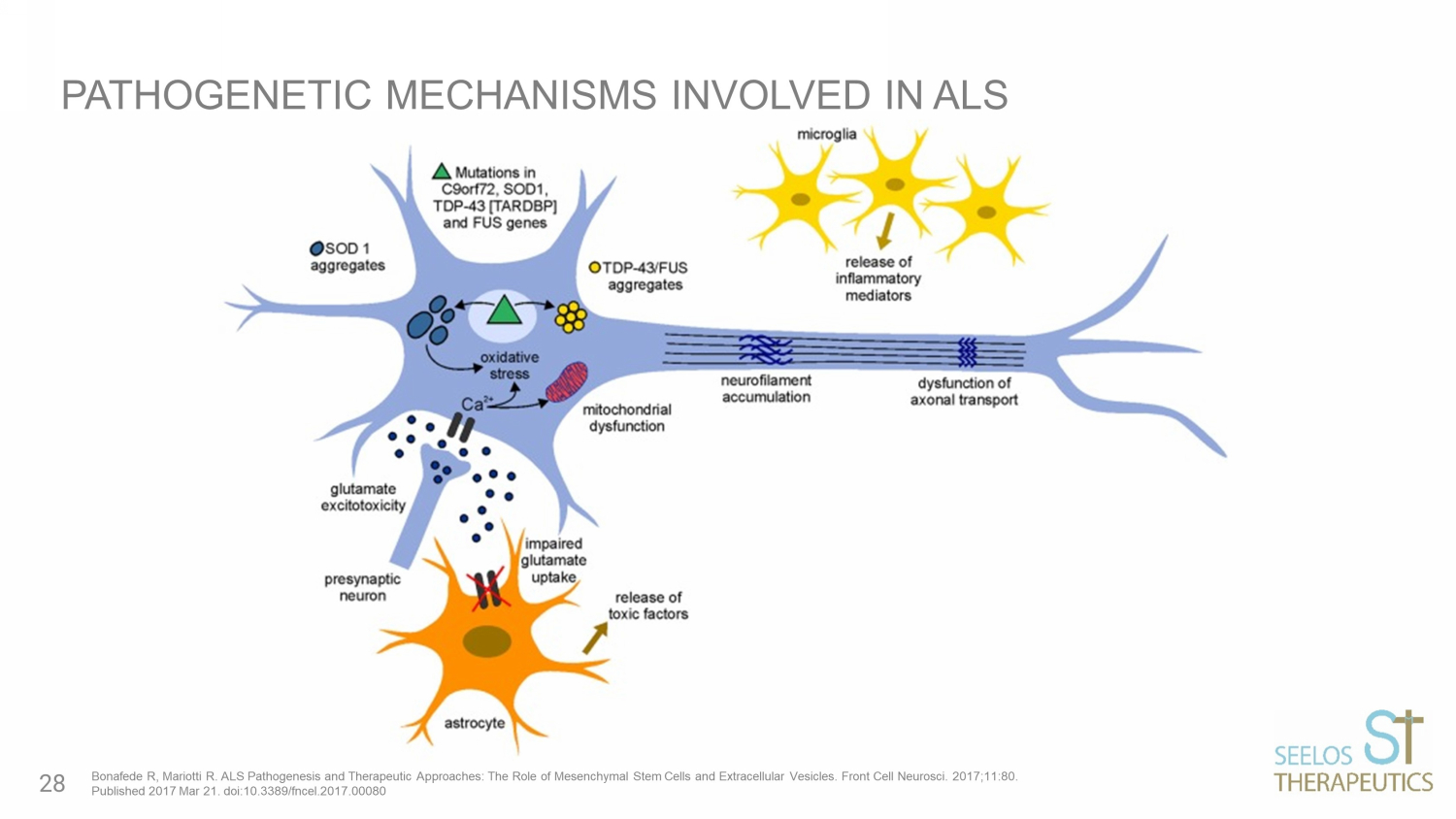
PATHOGENETIC MECHANISMS INVOLVED IN ALS Bonafede R, Mariotti R. ALS Pathogenesis and Therapeutic Approaches: The Role of Mesenchymal Stem Cells and Extracellular Ves icl es. Front Cell Neurosci. 2017;11:80. Published 2017 Mar 21. doi:10.3389/fncel.2017.00080 28
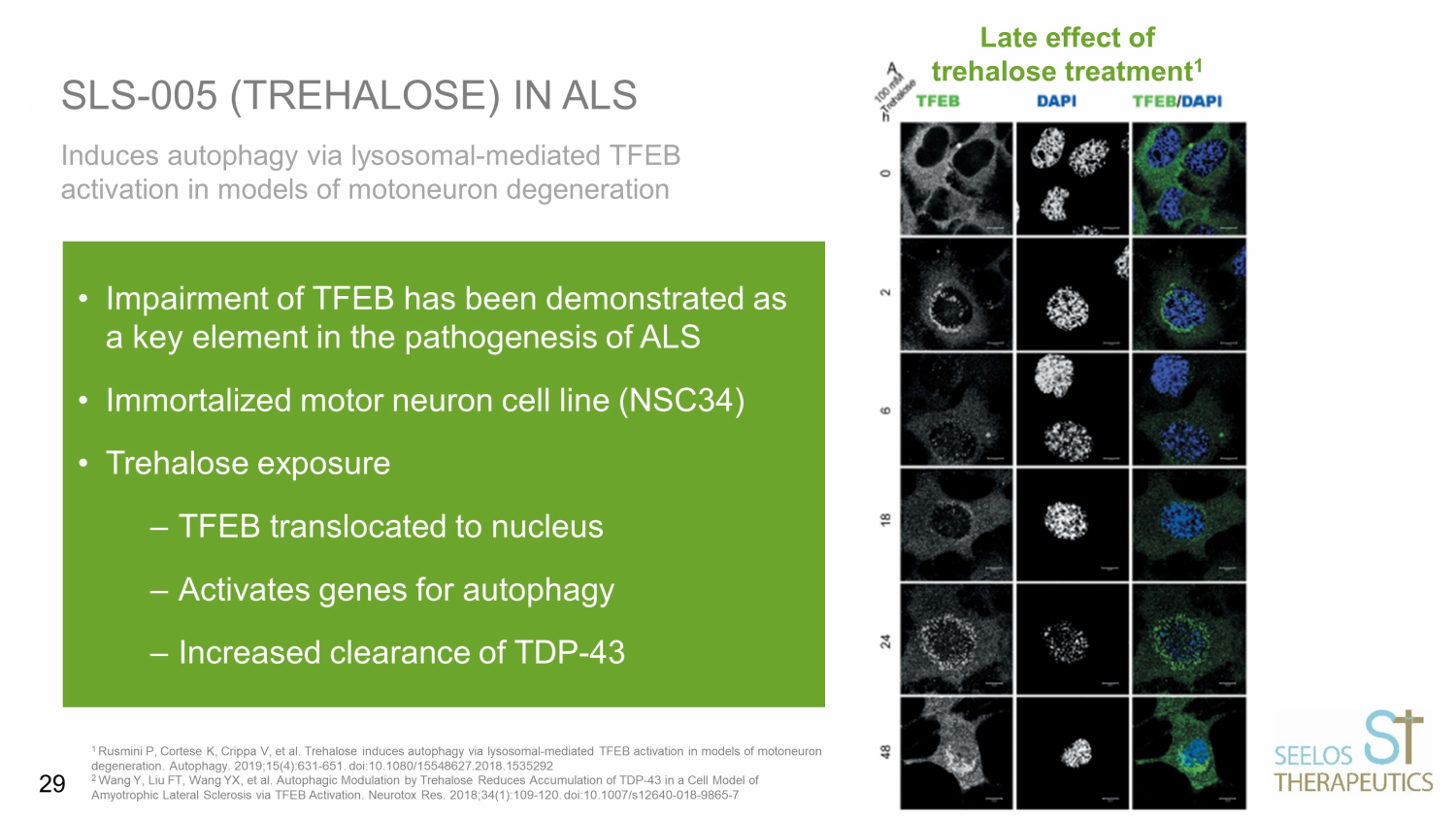
Induces autophagy via lysosomal - mediated TFEB activation in models of motoneuron degeneration SLS - 005 (TREHALOSE) IN ALS 1 Rusmini P, Cortese K, Crippa V, et al. Trehalose induces autophagy via lysosomal - mediated TFEB activation in models of motoneuron degeneration. Autophagy. 2019;15(4):631 - 651. doi:10.1080/15548627.2018.1535292 2 Wang Y, Liu FT, Wang YX, et al. Autophagic Modulation by Trehalose Reduces Accumulation of TDP - 43 in a Cell Model of Amyotrophic Lateral Sclerosis via TFEB Activation. Neurotox Res. 2018;34(1):109 - 120. doi:10.1007/s12640 - 018 - 9865 - 7 • Impairment of TFEB has been demonstrated as a key element in the pathogenesis of ALS • Immortalized motor neuron cell line (NSC34) • Trehalose exposure – TFEB translocated to nucleus – Activates genes for autophagy – Increased clearance of TDP - 43 Late effect of trehalose treatment 1 29

MTOR - independent, autophagic enhancer trehalose prolongs motor neuron survival and ameliorates the autophagic flux defect in a mouse model of ALS SLS - 005 (TREHALOSE) IN ALS *Mice can be treated orally since they do not have gut trehalase Zhang X, Chen S, Song L, et al. MTOR - independent, autophagic enhancer trehalose prolongs motor neuron survival and ameliorates t he autophagic flux defect in a mouse model of amyotrophic lateral sclerosis. Autophagy. 2014;10(4):588 - 602. doi:10.4161/auto.27710 Treatment with oral trehalose* • Delayed onset of disease • Prolonged survival • Reduced skeletal muscle denervation • Reduced motor neuron loss and skeletal denervation • Improved autophagy flux • Decreases aggregation of SOD1 and SQSTM1/p62 E 30
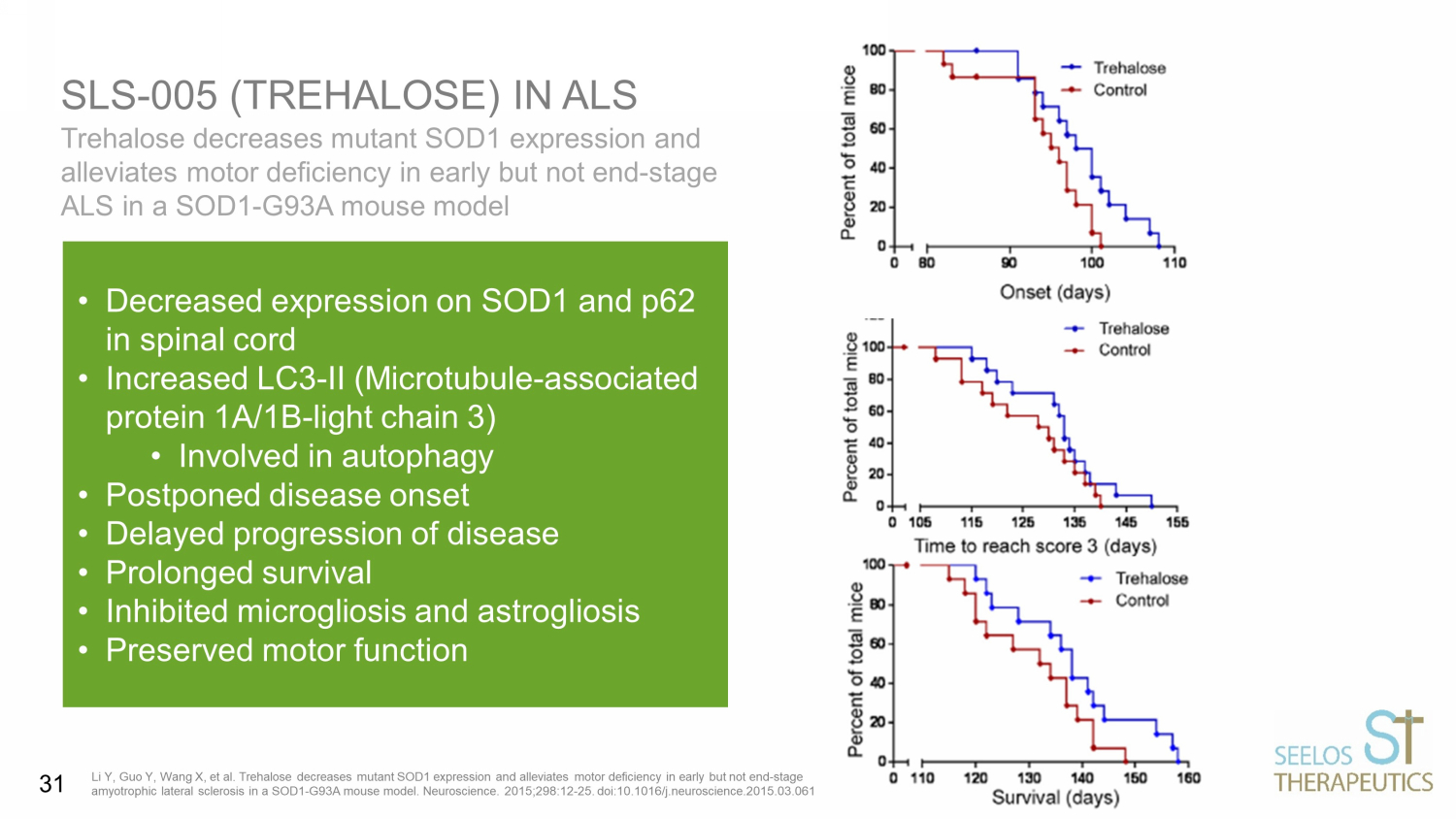
Trehalose decreases mutant SOD1 expression and alleviates motor deficiency in early but not end - stage ALS in a SOD1 - G93A mouse model SLS - 005 (TREHALOSE) IN ALS Li Y, Guo Y, Wang X, et al. Trehalose decreases mutant SOD1 expression and alleviates motor deficiency in early but not end - stag e amyotrophic lateral sclerosis in a SOD1 - G93A mouse model. Neuroscience. 2015;298:12 - 25. doi:10.1016/j.neuroscience.2015.03.061 • Decreased expression on SOD1 and p62 in spinal cord • Increased LC3 - II (Microtubule - associated protein 1A/1B - light chain 3) • Involved in autophagy • Postponed disease onset • Delayed progression of disease • Prolonged survival • Inhibited microgliosis and astrogliosis • Preserved motor function 31

• Randomized double blind placebo - controlled trial • 160 Patients with sporadic or familial ALS with onset of ALS < 36 months • Randomized 3:1 trehalose to placebo and 24 - week treatment period • ~90% power to detect a slowing in the rate of ALSFRS - R progression of 25% Primary Efficacy Endpoint • Change in disease severity measured by the ALSFRS - R at 24 weeks (Approval based on this EP) Secondary Efficacy Endpoints • Change in respiratory function assessed by slow vital capacity (SVC) • Change in muscle strength using a handheld dynamometer • Survival SLS - 005 PHASE II/III IN ALS 32
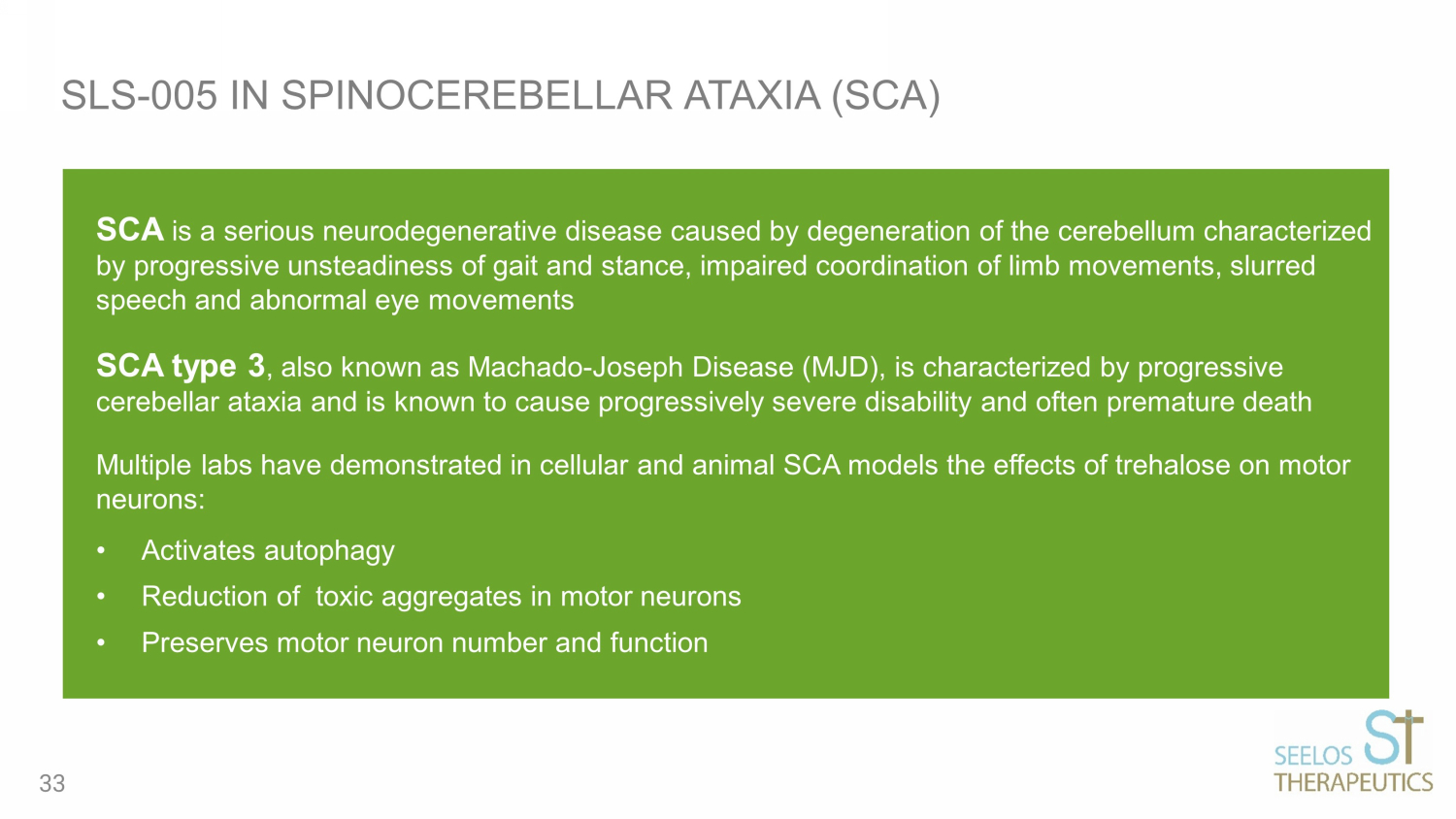
SCA is a serious neurodegenerative disease caused by degeneration of the cerebellum characterized by progressive unsteadiness of gait and stance, impaired coordination of limb movements, slurred speech and abnormal eye movements SCA type 3 , also known as Machado - Joseph Disease (MJD), is characterized by progressive cerebellar ataxia and is known to cause progressively severe disability and often premature death Multiple labs have demonstrated in cellular and animal SCA models the effects of trehalose on motor neurons: • Activates autophagy • Reduction of toxic aggregates in motor neurons • Preserves motor neuron number and function SLS - 005 IN SPINOCEREBELLAR ATAXIA (SCA) 33
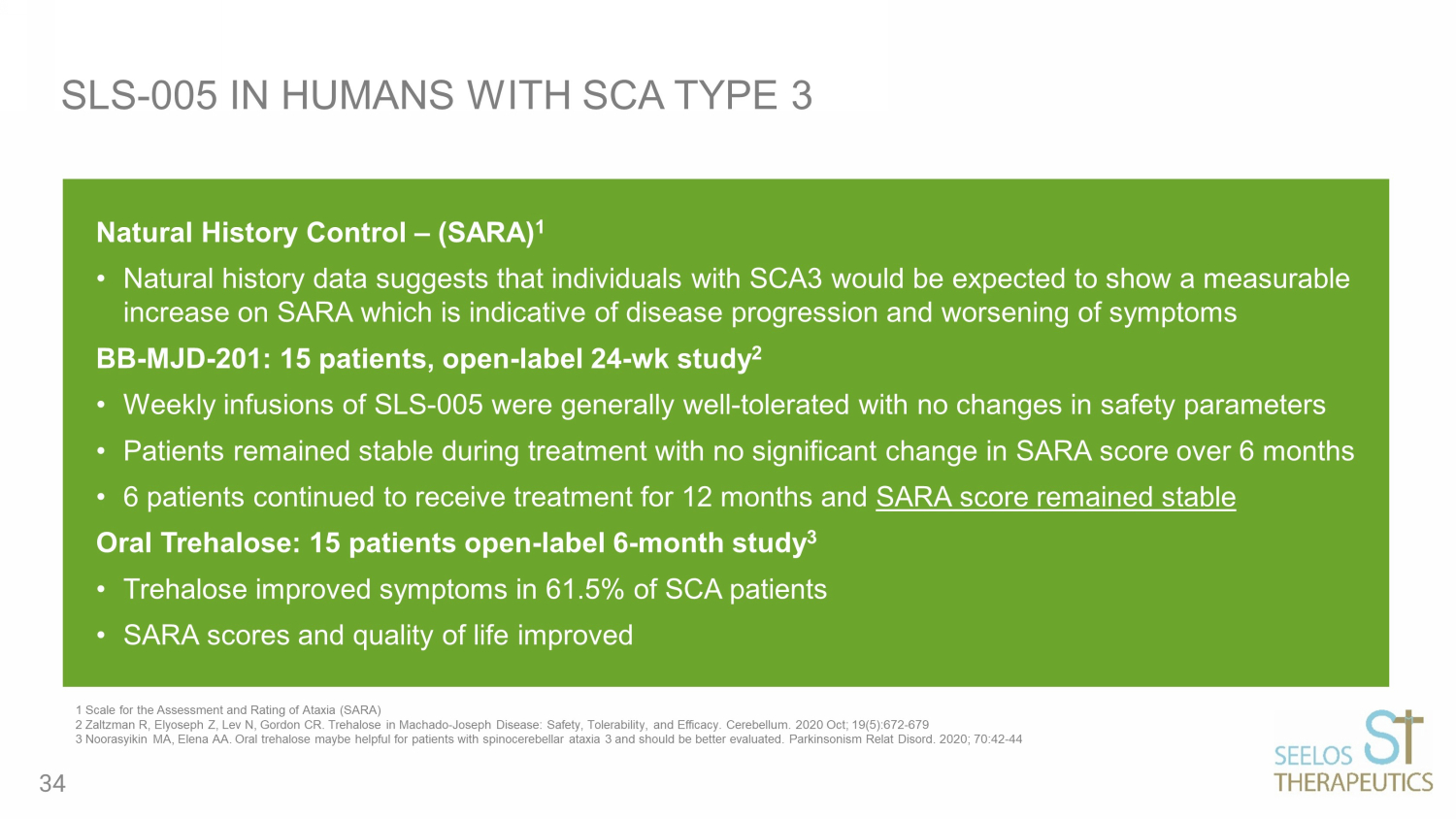
Natural History Control – (SARA) 1 • Natural history data suggests that individuals with SCA3 would be expected to show a measurable increase on SARA which is indicative of disease progression and worsening of symptoms BB - MJD - 201: 15 patients, open - label 24 - wk study 2 • Weekly infusions of SLS - 005 were generally well - tolerated with no changes in safety parameters • Patients remained stable during treatment with no significant change in SARA score over 6 months • 6 patients continued to receive treatment for 12 months and SARA score remained stable Oral Trehalose: 15 patients open - label 6 - month study 3 • Trehalose improved symptoms in 61.5% of SCA patients • SARA scores and quality of life improved SLS - 005 IN HUMANS WITH SCA TYPE 3 1 Scale for the Assessment and Rating of Ataxia (SARA) 2 Zaltzman R, Elyoseph Z, Lev N, Gordon CR. Trehalose in Machado - Joseph Disease: Safety, Tolerability, and Efficacy. Cerebellum. 2020 Oct; 19(5):672 - 6 79 3 Noorasyikin MA, Elena AA. Oral trehalose maybe helpful for patients with spinocerebellar ataxia 3 and should be better evaluated. Parkins on ism Relat Disord . 2020; 70:42 - 44 34
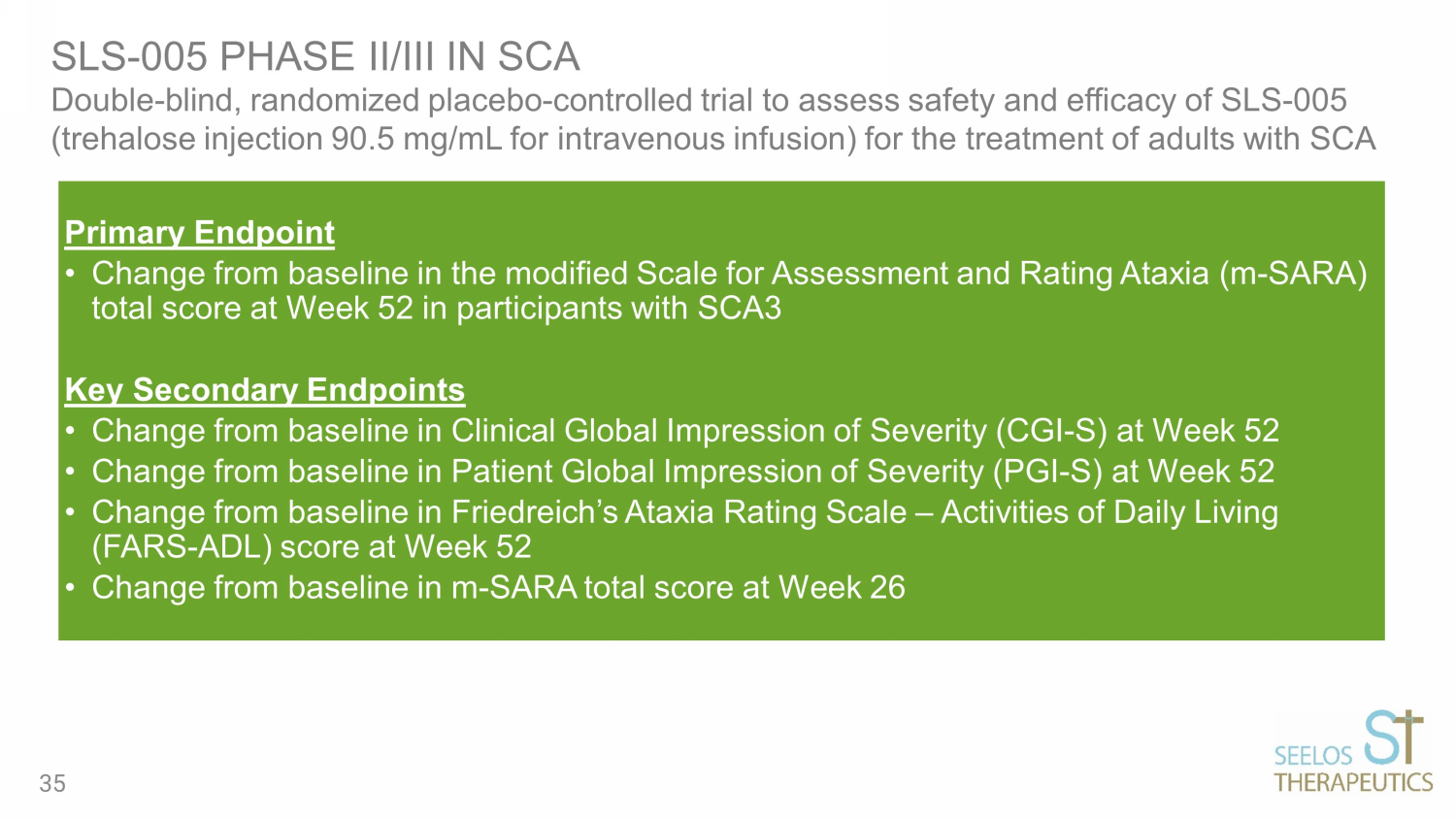
Primary Endpoint • Change from baseline in the modified Scale for Assessment and Rating Ataxia (m - SARA) total score at Week 52 in participants with SCA3 Key Secondary Endpoints • Change from baseline in Clinical Global Impression of Severity (CGI - S) at Week 52 • Change from baseline in Patient Global Impression of Severity (PGI - S) at Week 52 • Change from baseline in Friedreich’s Ataxia Rating Scale – Activities of Daily Living (FARS - ADL) score at Week 52 • Change from baseline in m - SARA total score at Week 26 SLS - 005 PHASE II/ III IN SCA Double - blind, randomized placebo - controlled trial to assess safety and efficacy of SLS - 005 (trehalose injection 90.5 mg/mL for intravenous infusion) for the treatment of adults with SCA 35

SLS - 004 (gene therapy) 36
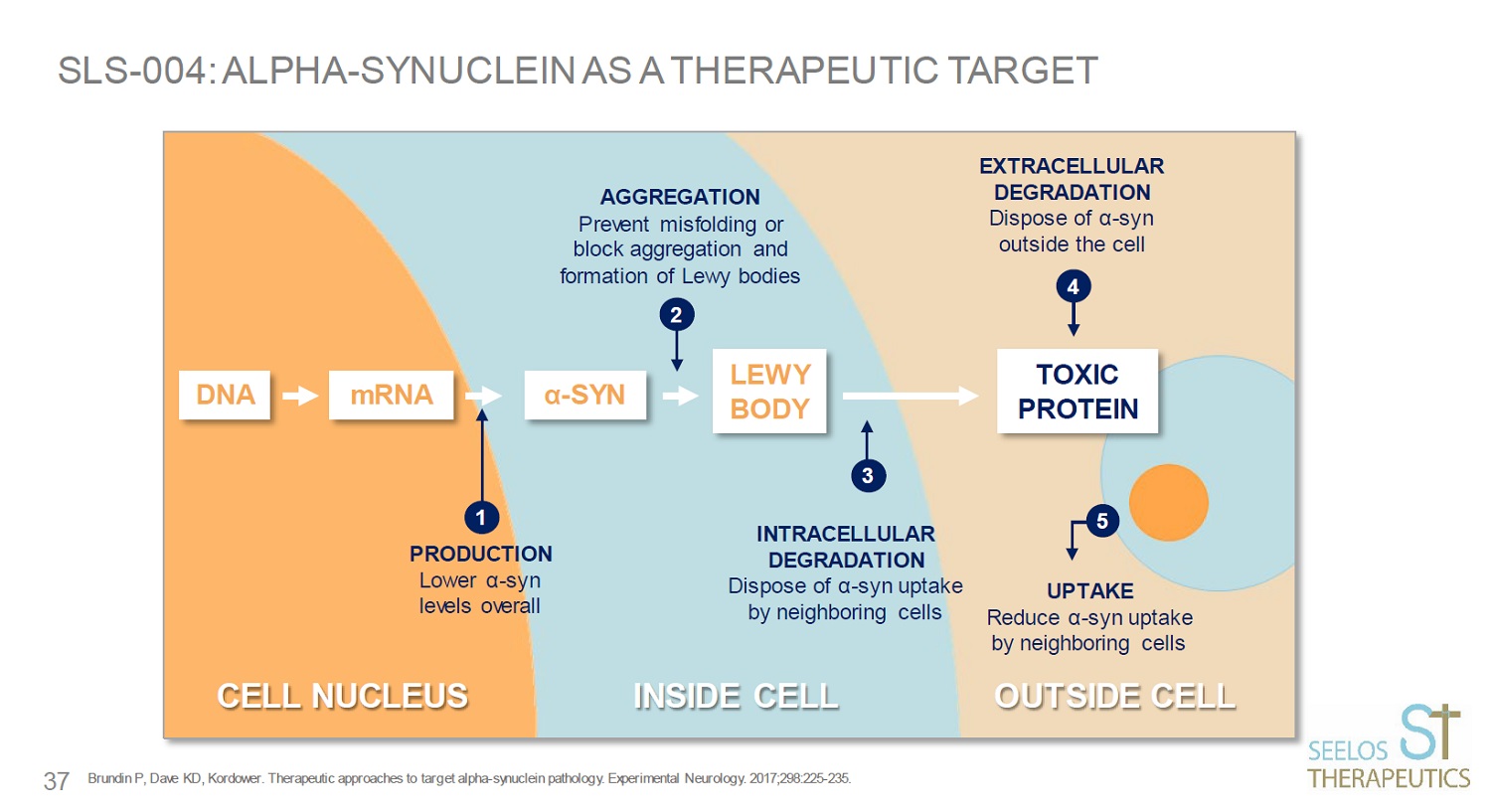
SLS - 004: ALPHA - SYNUCLEIN AS A THERAPEUTIC TARGET Brundin P, Dave KD, Kordower. Therapeutic approaches to target alpha - synuclein pathology. Experimental Neurology. 2017;298:225 - 2 35. α - SYN LEWY BODY TOXIC PROTEIN DNA mRNA 1 2 3 4 5 37
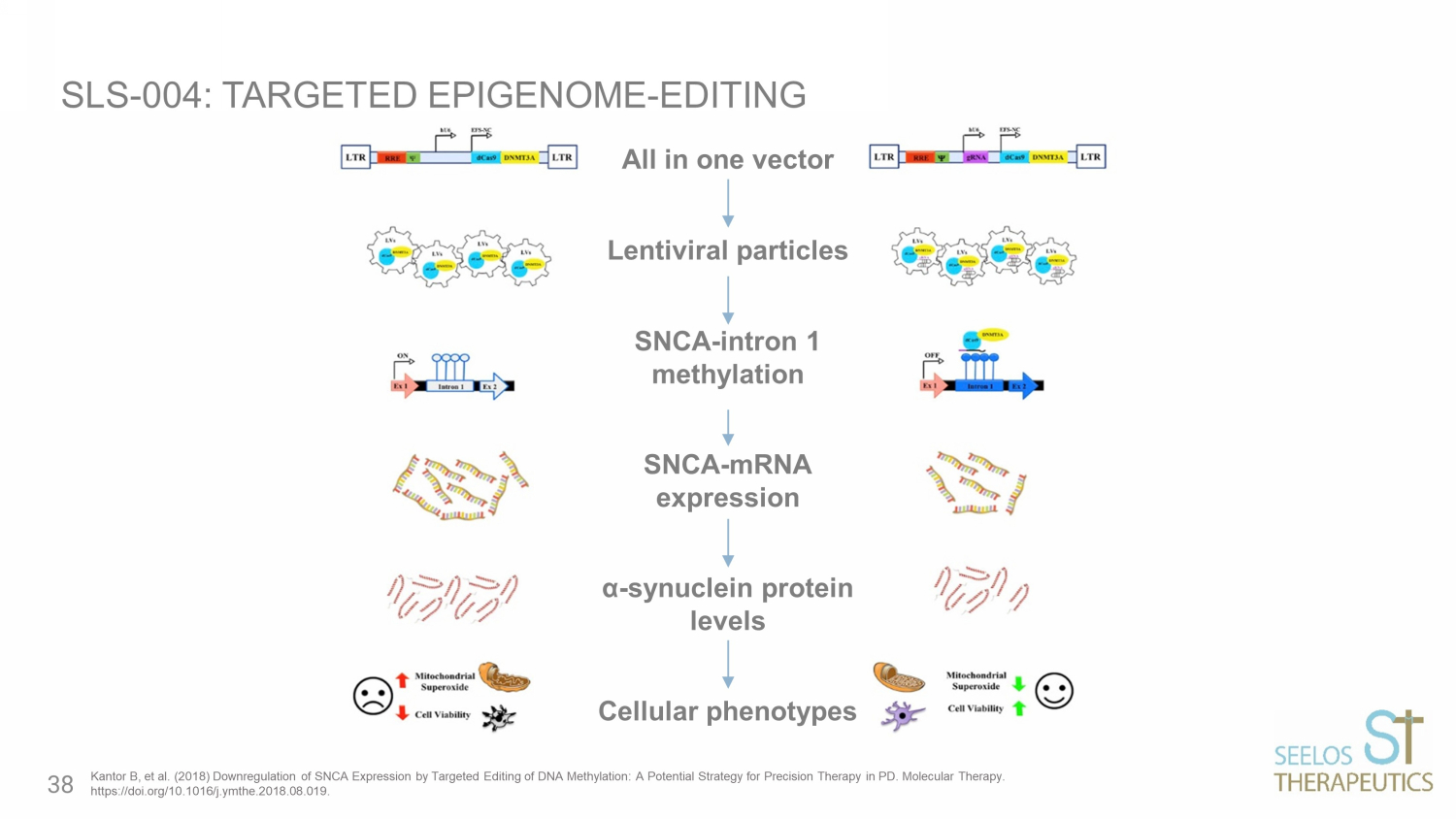
All in one vector Lentiviral particles SNCA - intron 1 methylation SNCA - mRNA expression α - synuclein protein levels Cellular phenotypes SLS - 004: TARGETED EPIGENOME - EDITING Kantor B, et al. (2018) Downregulation of SNCA Expression by Targeted Editing of DNA Methylation: A Potential Strategy for Pr eci sion Therapy in PD. Molecular Therapy. https://doi.org/10.1016/j.ymthe.2018.08.019. 38
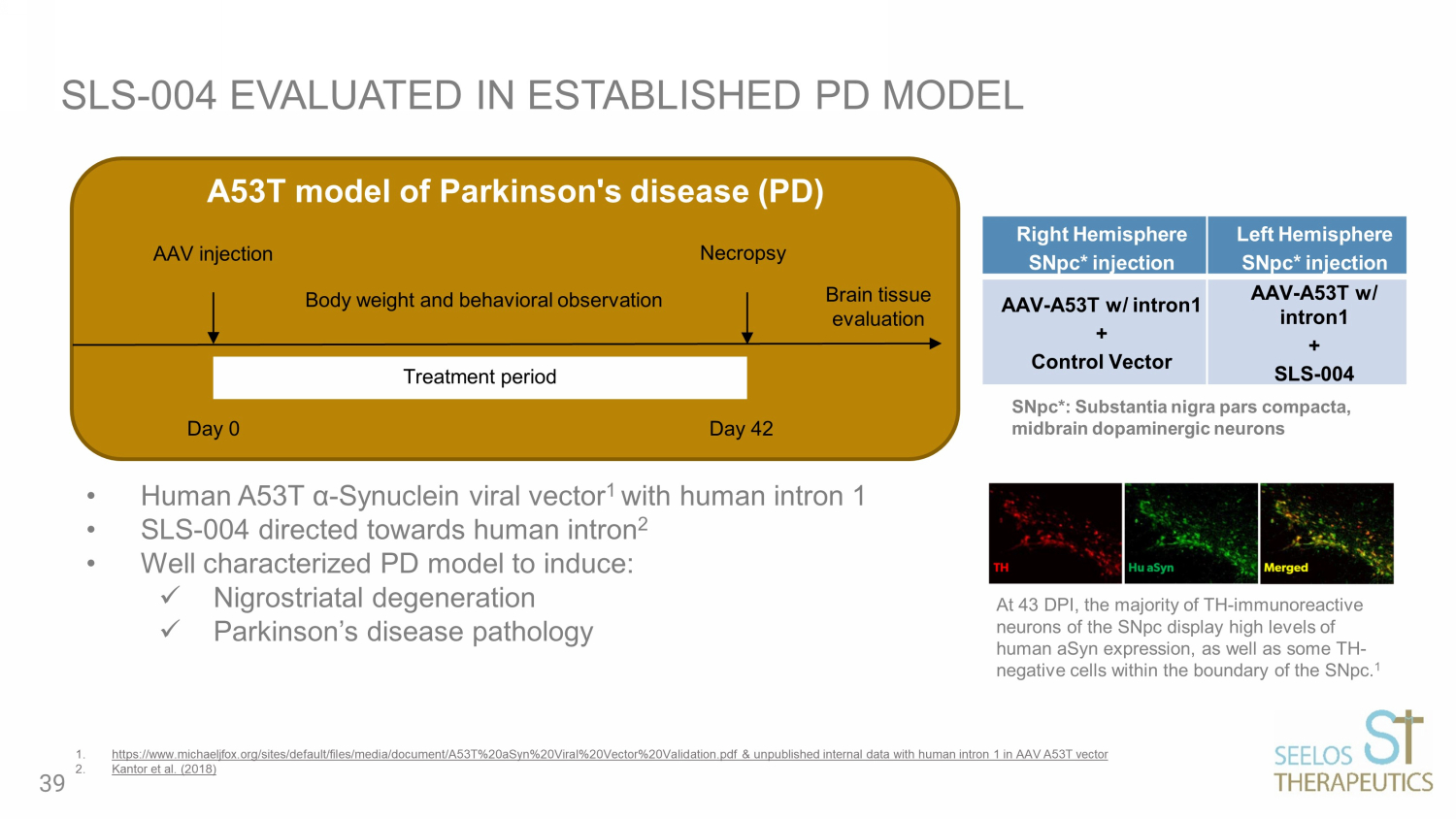
SLS - 004 EVALUATED IN ESTABLISHED PD MODEL Right Hemisphere SNpc* injection Left Hemisphere SNpc* injection AAV - A53T w/ intron1 + Control Vector AAV - A53T w/ intron1 + SLS - 004 • Human A53T α - Synuclein viral vector 1 with human intron 1 • SLS - 004 directed towards human intron 2 • Well characterized PD model to induce: x Nigrostriatal degeneration x Parkinson’s disease pathology 1. https://www.michaeljfox.org/sites/default/files/media/document/A53T%20aSyn%20Viral%20Vector%20Validation.pdf & unpublished in ter nal data with human intron 1 in AAV A53T vector 2. Kantor et al. (2018) Treatment period Body weight and behavioral observation Necropsy AAV injection Brain tissue evaluation A53T model of Parkinson's disease (PD) Day 0 Day 42 At 43 DPI, the majority of TH - immunoreactive neurons of the SNpc display high levels of human aSyn expression, as well as some TH - negative cells within the boundary of the SNpc. 1 SNpc*: Substantia nigra pars compacta, midbrain dopaminergic neurons 39
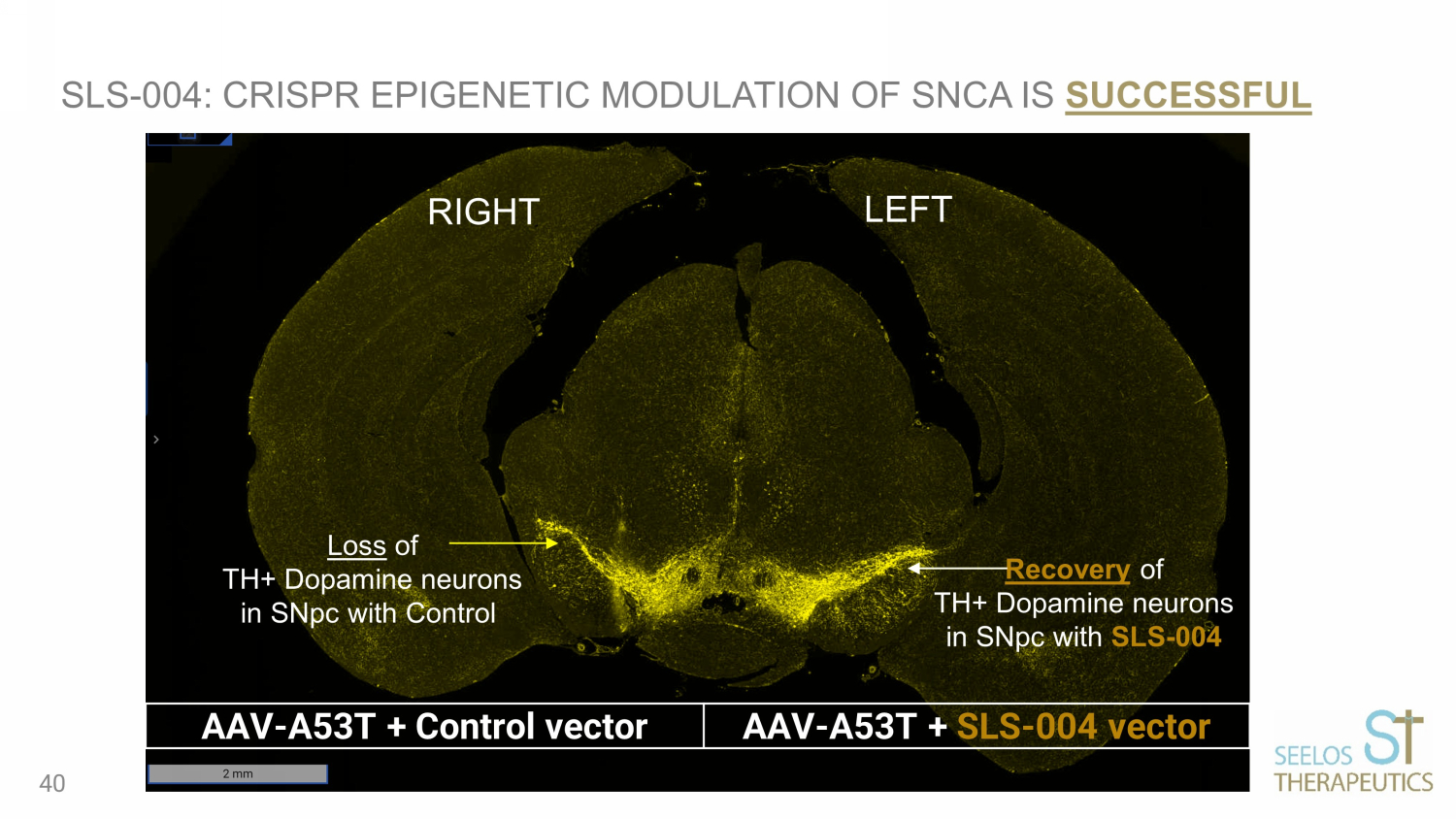
RIGHT LEFT Loss of TH+ Dopamine neurons in SNpc with Control Recovery of TH+ Dopamine neurons in SNpc with SLS - 004 SLS - 004: CRISPR EPIGENETIC MODULATION OF SNCA IS SUCCESSFUL AAV - A53T + Control vector AAV - A53T + SLS - 004 vector 40
SLS - 009 ( protein targeted autophagy ) 41
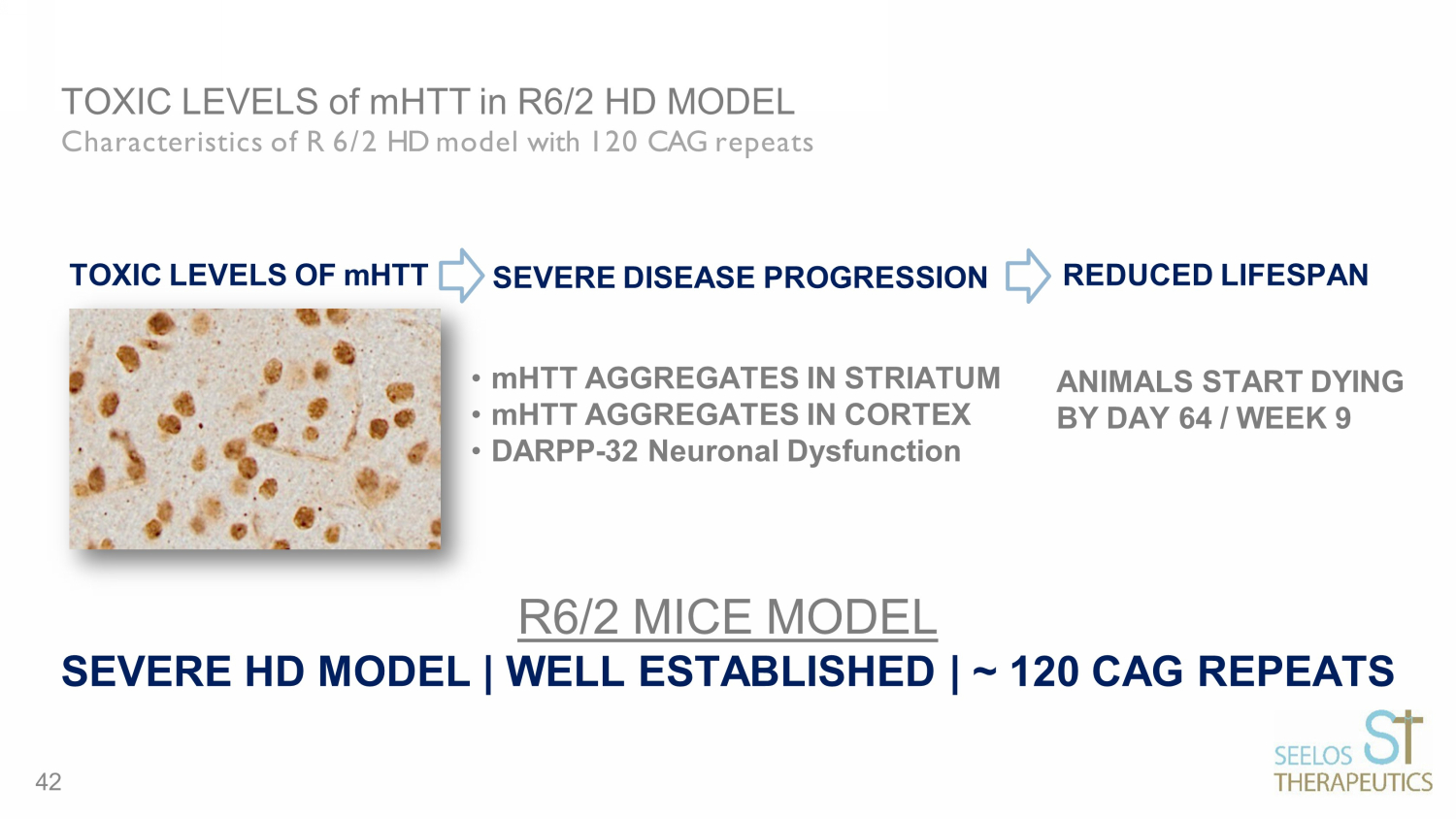
TOXIC LEVELS of mHTT in R6/2 HD MODEL Characteristics of R 6/2 HD model with 120 CAG repeats ANIMALS START DYING BY DAY 64 / WEEK 9 TOXIC LEVELS OF mHTT • mHTT AGGREGATES IN STRIATUM • mHTT AGGREGATES IN CORTEX • DARPP - 32 Neuronal Dysfunction SEVERE DISEASE PROGRESSION REDUCED LIFESPAN R6/2 MICE MODEL SEVERE HD MODEL | WELL ESTABLISHED | ~ 120 CAG REPEATS 42
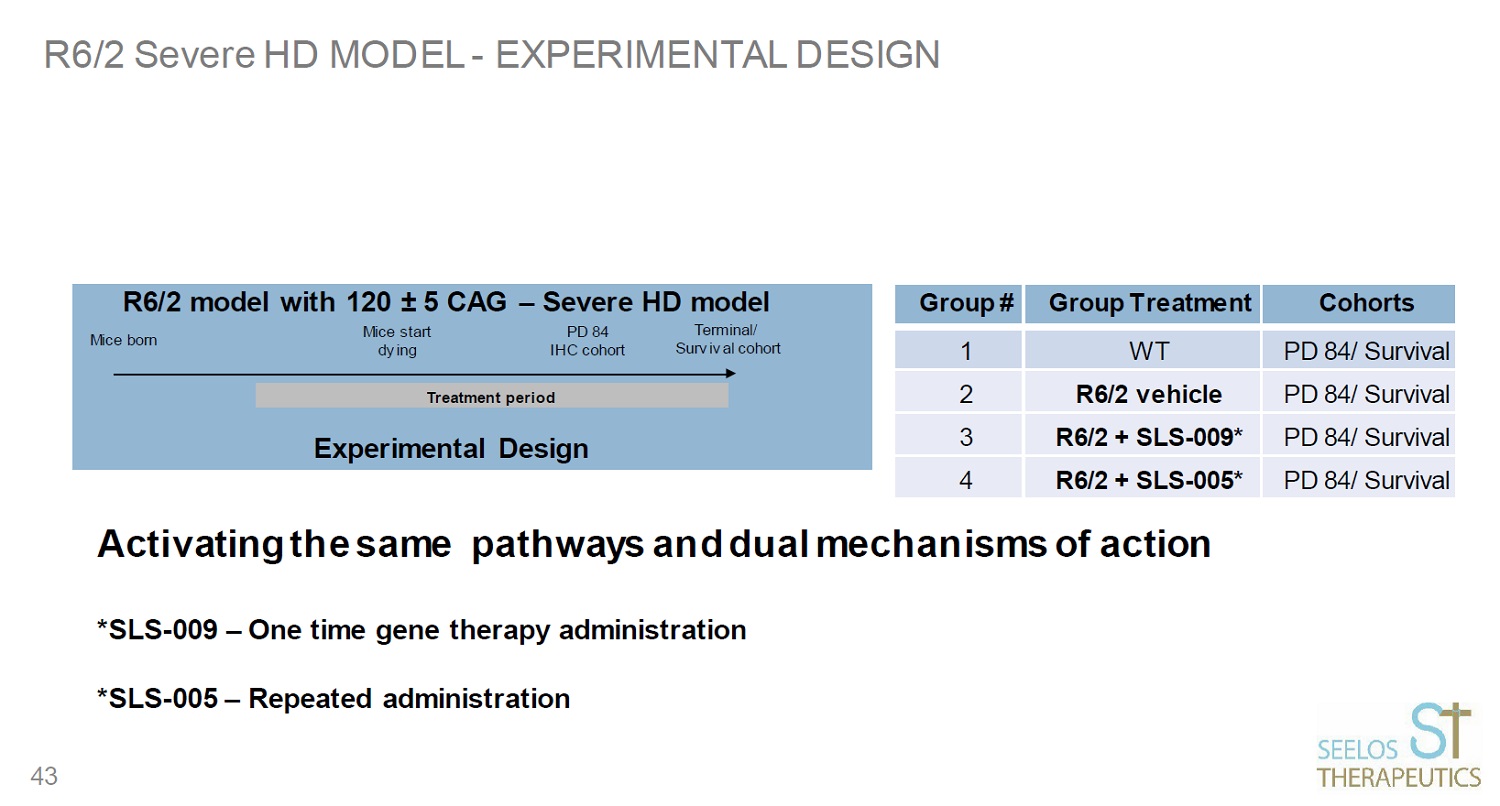
R6/2 Severe HD MODEL - EXPERIMENTAL DESIGN Treatment period Group # Group Treatment Cohorts 1 WT PD 84/ Survival 2 R6/2 vehicle PD 84/ Survival 3 R6/2 + SLS - 009* PD 84/ Survival 4 R6/2 + SLS - 005* PD 84/ Survival 43
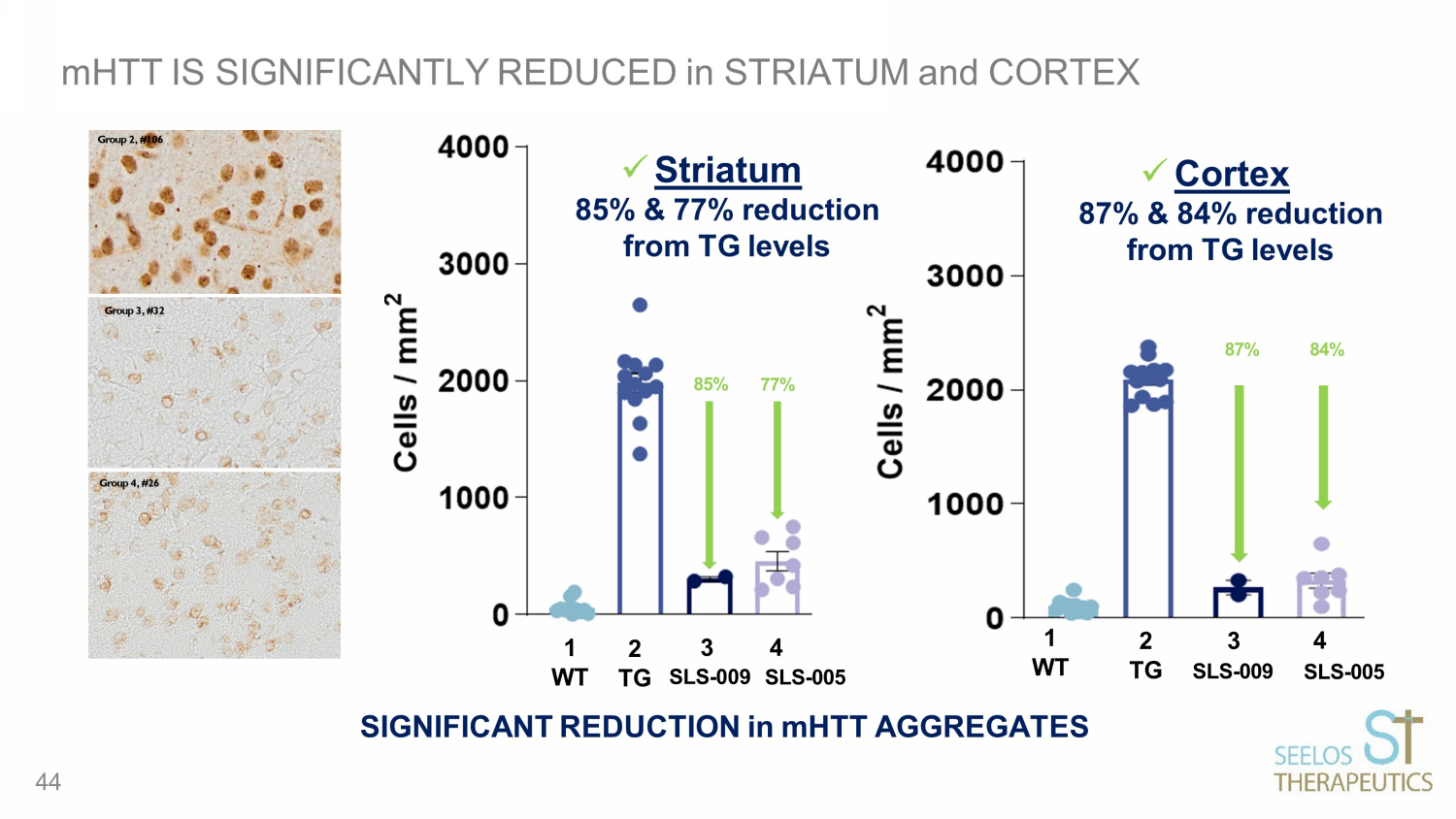
mHTT IS SIGNIFICANTLY REDUCED in STRIATUM and CORTEX SIGNIFICANT REDUCTION in mHTT AGGREGATES 1 2 WT TG 3 4 SLS - 009 SLS - 005 85% 77% Group 2, #106 Group 4, #26 Group 3, #32 1 WT 4 SLS - 005 2 3 TG SLS - 009 84% 87% x Striatum 85% & 77% reduction from TG levels 44 x Cortex 87% & 84% reduction from TG levels
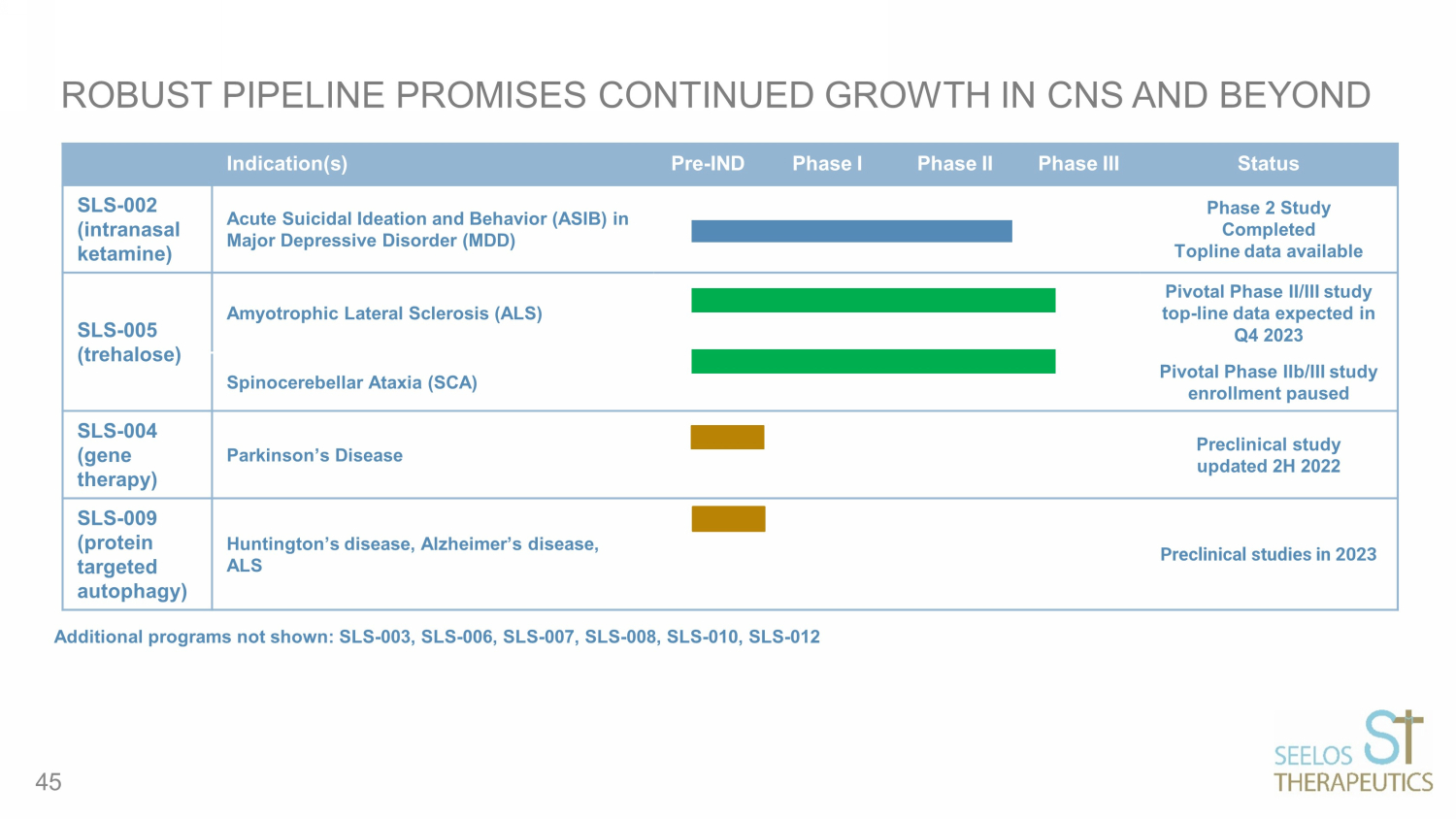
ROBUST PIPELINE PROMISES CONTINUED GROWTH IN CNS AND BEYOND Indication(s) Pre - IND Phase I Phase II Phase III Status SLS - 002 (intranasal ketamine ) Acute Suicidal Ideation and Behavior (ASIB) in Major Depressive Disorder (MDD) Phase 2 Study Completed Topline data available SLS - 005 (trehalose) Amyotrophic Lateral Sclerosis (ALS) Pivotal Phase II/III study top - line data expected in Q4 2023 Spinocerebellar Ataxia (SCA) Pivotal Phase IIb/III study enrollment paused SLS - 004 (gene therapy) Parkinson’s Disease Preclinical study updated 2H 2022 SLS - 009 (protein targeted autophagy) Huntington’s disease, Alzheimer’s disease, ALS Preclinical studies in 2023 Additional programs not shown: SLS - 003, SLS - 006, SLS - 007, SLS - 008, SLS - 010, SLS - 012 45
SEELOS IS SEEKING TO ADVANCE INNOVATIVE THERAPEUTICS TO ADDRESS UNMET MEDICAL NEEDS FOR PATIENTS WITH PSYCHIATRIC AND MOVEMENT DISORDERS Developing a broad portfolio of late - stage CNS product candidates with demonstrated mechanism of action Assembling a team of established industry leaders Advancing clinical studies in multiple indications with large market opportunities and orphan indications Anticipating initiation of various trials and data releases in 2023 Applying clinical expertise to transform the lives of patients with neurological and psychiatric disorders, including orphan diseases Securing strategic partnerships with companies like Ligand Pharmaceuticals, who have a history of success with licensing foundational assets at the early stage of company formation 46

APPENDIX 47
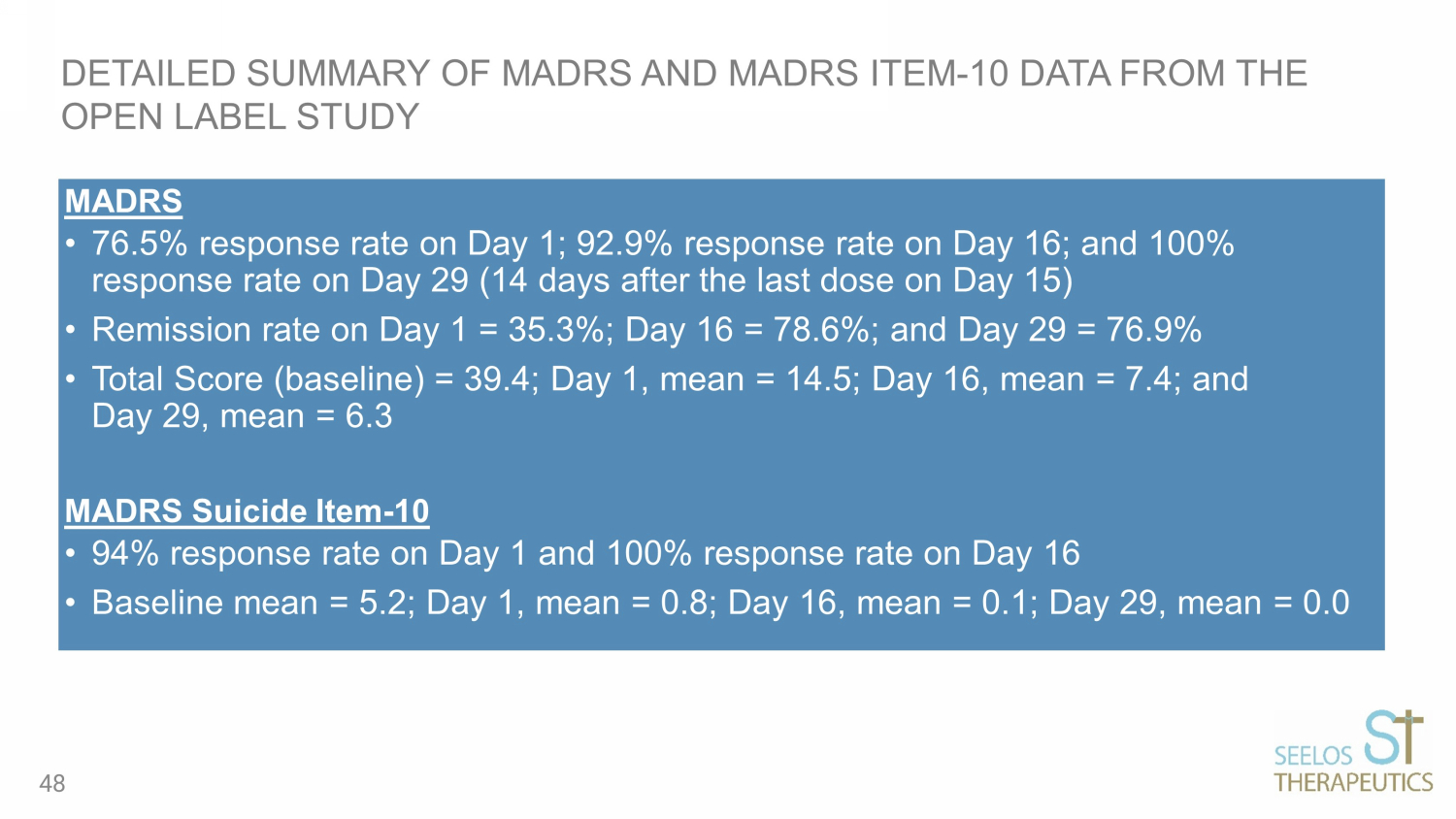
MADRS • 76.5% response rate on Day 1; 92.9% response rate on Day 16; and 100% response rate on Day 29 (14 days after the last dose on Day 15) • Remission rate on Day 1 = 35.3%; Day 16 = 78.6%; and Day 29 = 76.9% • Total Score (baseline) = 39.4; Day 1, mean = 14.5; Day 16, mean = 7.4; and Day 29, mean = 6.3 MADRS Suicide Item - 10 • 94% response rate on Day 1 and 100% response rate on Day 16 • Baseline mean = 5.2; Day 1, mean = 0.8; Day 16, mean = 0.1; Day 29, mean = 0.0 DETAILED SUMMARY OF MADRS AND MADRS ITEM - 10 DATA FROM THE OPEN LABEL STUDY 48
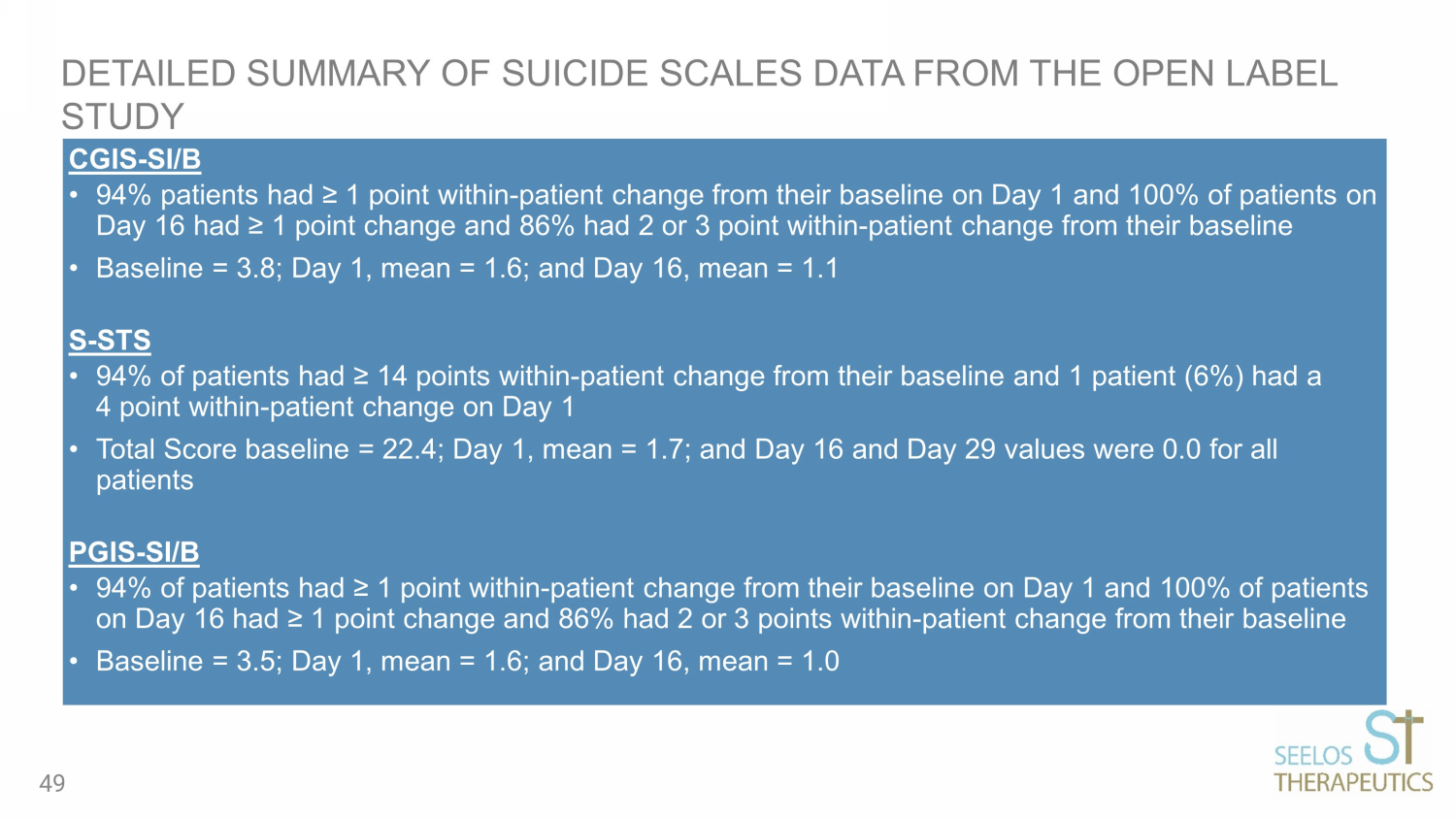
CGIS - SI/B • 94% patients had ≥ 1 point within - patient change from their baseline on Day 1 and 100% of patients on Day 16 had ≥ 1 point change and 86% had 2 or 3 point within - patient change from their baseline • Baseline = 3.8; Day 1, mean = 1.6; and Day 16, mean = 1.1 S - STS • 94% of patients had ≥ 14 points within - patient change from their baseline and 1 patient (6%) had a 4 point within - patient change on Day 1 • Total Score baseline = 22.4; Day 1, mean = 1.7; and Day 16 and Day 29 values were 0.0 for all patients PGIS - SI/B • 94% of patients had ≥ 1 point within - patient change from their baseline on Day 1 and 100% of patients on Day 16 had ≥ 1 point change and 86% had 2 or 3 points within - patient change from their baseline • Baseline = 3.5; Day 1, mean = 1.6; and Day 16, mean = 1.0 DETAILED SUMMARY OF SUICIDE SCALES DATA FROM THE OPEN LABEL STUDY 49
v3.23.3
Cover
|
Sep. 20, 2023 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Sep. 20, 2023
|
| Entity File Number |
000-22245
|
| Entity Registrant Name |
Seelos Therapeutics, Inc.
|
| Entity Central Index Key |
0001017491
|
| Entity Tax Identification Number |
87-0449967
|
| Entity Incorporation, State or Country Code |
NV
|
| Entity Address, Address Line One |
300
Park Avenue
|
| Entity Address, Address Line Two |
2nd Floor
|
| Entity Address, City or Town |
New York
|
| Entity Address, State or Province |
NY
|
| Entity Address, Postal Zip Code |
10022
|
| City Area Code |
646
|
| Local Phone Number |
293-2100
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, $0.001 par value
|
| Trading Symbol |
SEEL
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Seelos Therapeutics (NASDAQ:SEEL)
Gráfica de Acción Histórica
De Abr 2024 a May 2024
Seelos Therapeutics (NASDAQ:SEEL)
Gráfica de Acción Histórica
De May 2023 a May 2024