Form 8-K - Current report
15 Noviembre 2023 - 7:19AM
Edgar (US Regulatory)
FALSE0001641489NASDAQ00016414892023-11-152023-11-15
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(D) OF THE
SECURITIES EXCHANGE ACT OF 1934
Date of Report (date of earliest event reported): November 15, 2023
vTv Therapeutics Inc.
(Exact name of registrant as specified in its charter)
| | | | | | | | |
Delaware | 001-37524 | 47-3916571 |
(State or other jurisdiction of incorporation) | (Commission File No.) | (IRS Employer Identification No.) |
3980 Premier Drive, Suite 310
High Point, NC 27265
(Address of principal executive offices)
(336) 841-0300
(Registrant’s telephone number, including area code)
NOT APPLICABLE
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| | | | | |
| ¨ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | |
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Class A common stock, par value $0.01 per share | VTVT | NASDAQ Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ¨
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Item 7.01 Regulation FD Disclosure
On November 15, 2023, vTv Therapeutics, Inc., (the "Company") posted on its website an updated slide presentation, which is attached as Exhibit 99.1 to this Current Report on Form 8-K and incorporated by reference herein. Representatives of the Company will use the presentation in various meetings with investors, analysts and other parties from time to time. This presentation may be amended or updated at any time and from time to time through another Current Report on Form 8-K, a later Company filing or other means.
The information in this Item 7.01 (including Exhibit 99.1) shall not be deemed to be “filed” for purposes of, or otherwise subject to the liabilities of, Section 18 of the Exchange Act, nor shall it be deemed to be incorporated by reference in any filing under the 33 Act or the Exchange Act, except as shall be expressly set forth by specific reference in any such filing.
Item 9.01 Financial Statements and Exhibits
(d)Exhibits
| | | | | | | | |
| Exhibit No. | | Description |
| 99.1 | | |
| 104 | | Cover Page Interactive Data File (embedded within Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, hereunto duly authorized.
| | | | | | | | | | | |
| VTV THERAPEUTICS INC. | |
| | | |
| By: | /s/ Paul J. Sekhri | |
| Name: | Paul J. Sekhri | |
| Title: | President and Chief Executive Officer | |
Dated: November 15, 2023

Improving the Lives of Millions of Patients with Type 1 Diabetes NASDAQ: VTVT

THE STATEMENTS MADE IN THIS PRESENTATION AND THE ACCOMPANYING ORAL COMMENTARY MAY INCLUDE FORWARD-LOOKING STATEMENTS REGARDING (I) DIABETES MARKET AND OTHER MARKETS, (II) THE DEVELOPMENT, CLINICAL TRIAL PROCESS, REGULATORY APPROVAL PROCESS AND ATTRIBUTES OF INVESTIGATIONAL AND MARKETED PRODUCTS TO TREAT THESE DISEASES AND OTHER CONDITIONS, (III) THE ECONOMIC POTENTIAL OF THOSE PRODUCTS AND (IV) THE FUTURE OPERATIONS, FUND-RAISING ACTIVITIES, EXPENDITURES, OPPORTUNITIES, AND FINANCIAL PERFORMANCE OF VTV THERAPEUTICS INC. FORWARD-LOOKING STATEMENTS INCLUDE ALL STATEMENTS THAT ARE NOT HISTORICAL FACTS AND CAN BE IDENTIFIED BY TERMS SUCH AS “ANTICIPATES,” “BELIEVES,” “COULD,” “SEEKS,” “ESTIMATES,” “EXPECTS,” “INTENDS,” “MAY,” “PLANS,” “POTENTIAL,” PREDICTS,” “PROJECTS,” “SHOULD,” “WILL,” “WOULD” OR SIMILAR EXPRESSIONS AND THE NEGATIVES OF THOSE TERMS. THESE FORWARD-LOOKING STATEMENTS ARE ONLY ESTIMATES BASED UPON THE INFORMATION AVAILABLE TO VTV THERAPEUTICS INC. (OR THE PARTY PREPARING SUCH FORWARD-LOOKING STATEMENTS) AS OF THE DATE OF THIS PRESENTATION. THE FORWARD-LOOKING STATEMENTS INCLUDED HEREIN INVOLVE KNOWN AND UNKNOWN RISKS AND UNCERTAINTIES AND OTHER IMPORTANT FACTORS SUCH THAT ACTUAL FUTURE OPERATIONS, OPPORTUNITIES, PRODUCT DEVELOPMENT PROCESSES AND OUTCOMES, CLINICAL TRIAL PROCESSES AND OUTCOMES, REGULATORY APPROVAL PROCESSES AND OUTCOMES, ECONOMIC PERFORMANCE OF PRODUCTS, FUND-RAISING ACTIVITIES AND FINANCIAL PERFORMANCE MAY DIFFER MATERIALLY FROM THOSE SET FORTH IN OR IMPLIED IN THESE FORWARD-LOOKING STATEMENTS. THESE RISKS, UNCERTAINTIES, AND OTHER FACTORS, WHICH MAY NOT BE WITHIN OUR CONTROL, ARE DISCUSSED IN MORE DETAIL IN OUR QUARTERLY, ANNUAL AND CURRENT REPORTS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION, INCLUDING, WITHOUT LIMITATION, UNDER THE CAPTIONS, “RISK FACTORS,” “CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS” AND “MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.” THEREFORE, YOU SHOULD READ THIS PRESENTATION IN CONJUNCTION WITH SUCH MEANINGFUL CAUTIONARYSTATEMENTS. UNDUE RELIANCE SHOULD NOT BE PLACED ON FORWARD-LOOKING STATEMENTS, WHICH SPEAK ONLY AS OF THE DATE HEREOF. EXCEPT AS REQUIRED BY LAW, WE EXPRESSLY DISCLAIM ANY RESPONSIBILITY TO PUBLICLY UPDATE OR REVISE OUR FORWARD-LOOKING STATEMENTS, WHETHER AS A RESULT OF NEW INFORMATION, FUTURE EVENTS OR OTHERWISE. ALL FORWARD-LOOKING STATEMENTS CONTAINED HEREIN ARE QUALIFIED IN THEIR ENTIRETY BY THE FOREGOING CAUTIONARY STATEMENTS. THIS PRESENTATION IS BEING PROVIDED TO YOU FOR INFORMATION PURPOSES ONLY. THIS PRESENTATION DOES NOT CONSTITUTE AN OFFER OR SALE OF (OR THE SOLICITATION OF AN OFFER TO BUY) ANY SECURITIES OF VTV THERAPEUTICS INC. OR ANY OF ITS SUBSIDIARIES. BY ACCEPTING THIS PRESENTATION, YOU ACKNOWLEDGE AND AGREE THAT (I) YOU WILL NOT RELY ON THIS PRESENTATION FOR MAKING ANY INVESTMENT DECISION WITH RESPECT TO ANY SECURITIES OF VTV THERAPEUTICS INC. OR ANY OF ITS SUBSIDIARIES, AND (II) ANY INVESTMENT DECISION MADE BY YOU WITH RESPECT TO ANY SUCH SECURITIES WILL BE BASED SOLELY ON AN OFFERING DOCUMENT RELATING TO SUCH SECURITIES (IF ANY), INCLUDING THE INFORMATION INCORPORATED BY REFERENCE THEREIN. 2vTv Therapeutics

New Leadership Builds upon Decades of Scientific &Clinical Expertise Paul Sekhri President & CEO Rich Nelson Head of CorpDev StevenTuch CFO Carmen Valcarce,PhD Thomas Strack,MD Chief Scientific Officer Chief Medical Officer Jon Isaacsohn,MD Chairman Martin Lafontaine Commercial Consultant 3vTv Therapeutics

4vTv Therapeutics Justin Gregory, MD, MSci Asst. Professor of Pediatrics Pediatric Endocrinology Gary Koch, PhD Professor, Department of Biostatistics Director, Biometric Consulting Laboratory G. Alexander “Zan” Fleming, MD Founder & Executive Chairman, Kinexum Former FDA Supervisory Physician for Diabetes Robert Rizza, MD Emeritus Professor of Medicine Division of Endocrinology, Diabetes, Metabolism & Nutrition John Buse, MD, PhD Verne S. Caviness Distinguished Professor Director, Diabetes Center Director, NC Translational & Clinical Sciences Institute Jay Skyler, MD, MACP, FRCP Professor of Medicine, Pediatrics, & Psychology Division of Endocrinology, Diabetes, & Metabolism Deputy Director for Clinical Research & Academic Programs, Diabetes Research Institute Distinguished SAB Continues to Support Development of Cadisegliatin (TTP399)

5vTv Therapeutics Living with T1D is Like Driving Too Fast on a Dangerous Road TOO MUCH INSULIN ~80% of patients fail to achieve adequate blood glucose control. Fear of hypoglycemia is so intense that many accept high blood glucose, risking long-term health consequences and diabetic ketoacidosis (DKA). TOO LITTLE INSULIN

6vTv Therapeutics 1.Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57(12):3169–76. 2. Foster NC, Beck RW, Miller KM, Clements MA, Rickels MR, DiMeglio LA, Maahs DM, Tamborlane WV, Bergenstal R, Smith E, Olson BA, Garg SK. State of Type 1 Diabetes Management and Outcomes from the T1D Exchange in 2016-2018. Diabetes Technol Ther. 2019. 3. dQ&A Market Research 2019. 4. Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med. 2012 May;29(5):682-9. doi: 10.1111/j.1464-5491.2012.03605.x. PMID: 22313123; PMCID: PMC3433794 Hypoglycemia: The Plague of T1D PATIENTS PROVIDERS PAYORS Prevalent & Disruptive 85% Suffer from 1-2 episodes every week 1 Worrisome & Life-Threatening 3-7% of CGM users will suffer from a severe episode resulting in seizure or coma every 3 months 2 Counter-Productive >21% of CGM users exhibit high avoidance behaviors (e.g. keeping elevated BG) 3 Barrier to Treatment 76% Would treat patients more aggressively if not for risk of hypoglycemia 4 High Direct & Indirect Costs

Cadisegliatin is the First Liver-Selective Glucokinase Activator to Reach Phase 3 Pancreas Effect Lowers threshold for insulin production Triggers storage of glucose as glycogen Result Rapid decline in blood sugar levels: Induces Hypoglycemia Reduces elevated blood sugar levels; Glucose released when needed: Reduces Hypoglycemia Glucokinase is present in both pancreatic β-cells & the liver. Past efforts to target have failed due to an increase in hypoglycemic events among other issues* Glucokinase Activation Liver *Other factors: Loss of potency over time; hypertriglyceridemia; fatty liver. None of these have been observed with cadisegliatin preclinically or in clinical studies up to 6 months. 7vTv Therapeutics

Cadisegliatin: A First In Class Adjunctive Therapy to Insulin for the Treatment of Type 1 Diabetes A Liver-Selective Glucokinase Activatorthat: 1) Reduces the Risk of Hypoglycemia 2) Improves Glycemic Control 8vTv Therapeutics
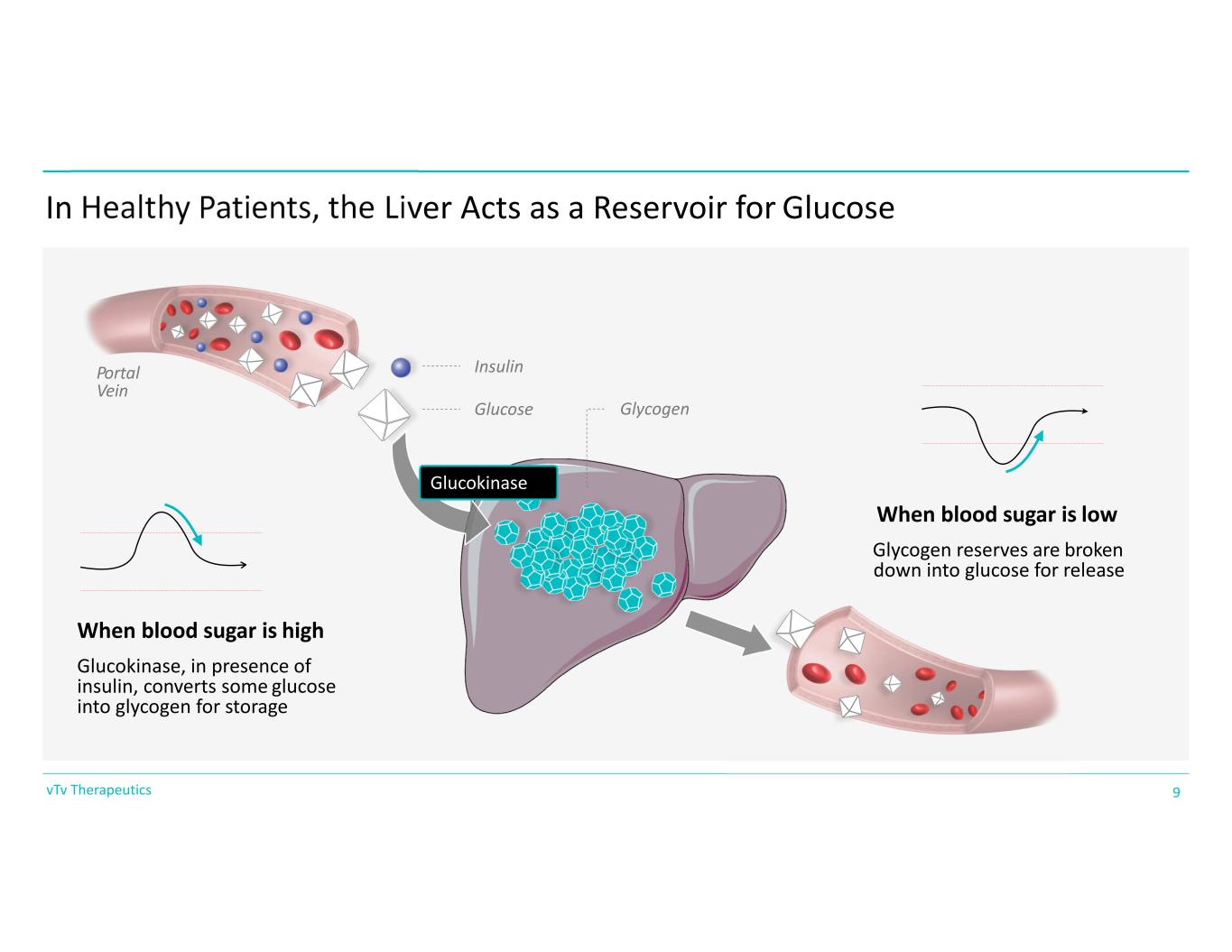
In Healthy Patients, the Liver Acts as a Reservoir for Glucose Glucokinase When blood sugar is high Glucokinase, in presence of insulin, converts some glucose into glycogen for storage Insulin Glucose When blood sugar is low Glycogen reserves are broken down into glucose for release Glycogen Portal Vein 9vTv Therapeutics

With Type 1 Diabetes, Glucokinase Activity in the Liver is Impaired Glucokinase When blood sugar is high Glucokinase activity is diminished due to low insulin in the liver, reducing glycogen storage When blood sugar is low Insufficient glycogen reserves to maintain glucose homeostasis Hypoglycemia Patient must take exogenous insulin Glucose Portal Vein 10vTv Therapeutics

Cadisegliatin Reactivates Innate Glucose-Regulating Capacity of the Liver When blood sugar is high Glucokinase is activated despite lower insulin levels When blood sugar is low Glycogen reserves available to prevent hypoglycemia Portal Vein cadisegliatin Glucokinase 11vTv Therapeutics

Our SimpliciT1 Trial Demonstrated Statistically & Clinically Significant Efficacy & Confirmed Safety Trials sponsored in part by: Randomized, Double-Blind, Placebo Controlled 2-Part Study of ~100 patients. A total of 46 patients in the treatment groups received 800mg daily of cadisegliatin. Study Details: https://diabetesjournals.org/care/article/44/4/960/138590/The-SimpliciT1-Study-A-Randomized-Double-Blind & https://clinicaltrials.gov/ct2/show/NCT03335371 Fewer Hypoglycemic Events Improved Glycemic Control 12vTv Therapeutics

13vTv Therapeutics Cadisegliatin MoA of reducing hyperglycemic stress could be complementary to other interventions in T1D, in addition to preventing the long-term sequelae of both hyper- and hypoglycemia

1 Type 1 Diabetes Index website. Available at 1dindex.org. Accessed on September 28th, 2023. 2 Prescription Drug Disclosure program. Texas HHS. Available at https://www.dshs.texas.gov/prescription-drug-price-disclosure-program 3 Internal, vTv Therapeutics High Level Commercial Opportunity Assessment Further Upside: Ex-US Markets; T2D indication adds ~2.8M lives Base Case Cadisegliatin Product Profile (≥ 30% reduction hypoglycemic episodes; Δ HbA1c ≥ -0.5%) 1.6M people, growing 2.9% annually 1 70% adherence rate 3 $600 Monthly WAC 2 2028 Product Launch 3 15% - 25% peak population uptake 3 $1.7B - $2.8B Peak U.S. Sales Opportunity 14vTv Therapeutics

Pipeline Creates Additional Upside vTv Partner vTv + Partner 15vTv Therapeutics

We Have the Opportunity to Ease the Burden of Managing T1D and Improve the Lives of Patients Living with Type 1 Diabetes 16vTv Therapeutics

17vTv Therapeutics Cadisegliatin significantly improved glycemic control Cadisegliatin had a favorable safety profile: reduction of hypoglycemia and ketosis events FDA has granted cadisegliatin Breakthrough Designation for the Treatment of T1D Poised to initiate phase 3 trials for cadisegliatin Conclusions
v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
vTv Therapeutics (NASDAQ:VTVT)
Gráfica de Acción Histórica
De Nov 2024 a Dic 2024

vTv Therapeutics (NASDAQ:VTVT)
Gráfica de Acción Histórica
De Dic 2023 a Dic 2024




