0001818331false00018183312024-07-302024-07-300001818331us-gaap:CommonClassAMember2024-07-302024-07-300001818331us-gaap:WarrantMember2024-07-302024-07-30
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 OR 15(d) of The Securities Exchange Act of 1934
Date of Report (date of earliest event reported): July 30, 2024
Commission file number 001-39482
GeneDx Holdings Corp.
(Exact name of registrant as specified in its charter)
| | | | | |
Delaware | 85-1966622 |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
| |
333 Ludlow Street, North Tower; 6th Floor Stamford, Connecticut 06902 |
| (Address of Principal Executive Offices) (Zip Code) |
Registrant's telephone number, including area code: (888) 729-1206
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | | | | | | | |
| Title of each class | | Trading Symbol | | Name of each exchange on which registered |
| Class A common stock, par value $0.0001 per share | | WGS | | The Nasdaq Stock Market LLC |
| Warrants to purchase one share of Class A common stock, each at an exercise price of $379.50 per share | | WGSWW | | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02 Results of Operations and Financial Condition.
On July 30, 2024, GeneDx Holdings Corp. (the “Company”) issued a press release (the “Press Release”) and will hold a conference call announcing the Company's financial results for the quarter ended June 30, 2024. Copies of the Press Release and Earnings Presentation are furnished as Exhibits 99.1 and 99.2, respectively, to this Current Report on Form 8-K.
The information furnished with this Item 2.02, including Exhibits 99.1 and 99.2 hereto, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference into any other filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
| | | | | |
Exhibit No | Description |
99.1 | |
99.2 | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | | | | | | | | | | |
| | GENEDX HOLDINGS CORP. |
| | |
| Date: | July 30, 2024 | By: | /s/ Katherine Stueland |
| | Name: | Katherine Stueland |
| | Title: | Chief Executive Officer |
GeneDx Reports Second Quarter 2024 Financial Results and Business Highlights
Reported second quarter 2024 revenue from continuing operations1 of $68.9M with 77% year-over-year growth of exome and genome test revenue
Expanded second quarter 2024 adjusted gross margins2 from continuing operations to 62%
Narrowed second quarter 2024 adjusted net loss2 to $2.7M
Raising guidance to deliver between $255M and $265M in FY 2024 revenue and reiterate path to profitability in 2025
GeneDx to host conference call today at 4:30 p.m. ET
STAMFORD, Conn., July 30, 2024 — GeneDx Holdings Corp. (Nasdaq: WGS), a leader in delivering improved health outcomes through genomic insights, today reported its financial results for the second quarter of 2024.
“Our continued organizational focus on execution fueled our strong second quarter results, giving us confidence to raise full year 2024 revenue guidance to between $255-$265 million and reiterate our expectation to reach profitability in the next several quarters,” said Katherine Stueland, President and Chief Executive Officer at GeneDx. “The strength in our performance across all measures of the business has enabled us to begin investments in future growth opportunities for our business with a focus on extending our leadership in whole exome sequencing to whole genome sequencing to ensure we continue to lead the charge in quickly providing the most accurate and actionable genetic information to diagnose disease for as many patients and families as possible.”
Second Quarter 2024 Financial Results (Unaudited)1,2
Revenues
•Revenues from continuing operations grew to $68.9 million, an increase of 52% year-over-year and 12% sequentially.
◦Total company revenues were $70.5 million.
•Exome and genome test revenue grew to $50.7 million, an increase of 77% year-over-year and 15% sequentially.
Exome and genome volume
•Exome and genome test results volume grew to 18,017, an increase of 52% year-over-year and 9% sequentially.
•Exome and genome represented 31% of all test results, up from 22% in the second quarter of 2023 and up from 30% in the first quarter of 2024.
Gross margin
•Adjusted gross margin from continuing operations expanded to 62%, up from 37% in the second quarter of 2023 and up from 61% in the first quarter of 2024.
◦Total company gross margin was 61%.
Operating expenses
•Adjusted total operating expenses reduced to $45.0 million, a decrease of 24% year-over-year and 1% sequentially.
◦Total GAAP operating expenses were $52.7 million.
Net loss
•Adjusted net loss narrowed to $2.7 million, an improvement of 93% year-over-year and 68% sequentially.
◦GAAP net loss was $29.2 million, inclusive of a one-time, net litigation charge of $13 million.
Cash burn and cash position
•Total net use of cash was $6.1 million in the second quarter of 2024, an improvement of 89% year-over-year and 65% sequentially.
•Cash, cash equivalents, marketable securities and restricted cash was $107.8 million as of June 30, 2024.
1Revenue and gross margin results from continuing operations, which we believe are representative of our ongoing business strategy exclude any revenue and cost of goods sold of the exited Legacy Sema4 diagnostic testing business for the current and all comparative periods. Total company results include GeneDx’s continuing operations and the financial impacts of exited Legacy Sema4 business activities for the current and all comparative periods.
2Adjusted gross margin, adjusted total operating expenses and adjusted net loss are non-GAAP financial measures. See appendix for a reconciliation of GAAP to Non-GAAP figures presented.
GeneDx Full Year 2024 Guidance
GeneDx has updated full year 2024 guidance. Management expects GeneDx to:
•Drive full year 2024 revenues1 between $255 and $265 million (previous guidance was between $235 and $245 million);
•Expand full year 2024 adjusted gross margin profile to at least 60% (no change);
•Use between $65 to $70 million of net cash for full year 2024 (previous guidance was between $70 to $80 million);
•Turn to profitability in 2025 (no change).
1Total company results include the combination of the GeneDx diagnostic business revenues and the data and information revenues from the Legacy Sema4 business.
Second Quarter 2024 Business Highlights
Driving sustainable growth, expanding access and improving the standard of care
•Announced collaboration with Epic Aura1 to expand access to rapid whole genome sequencing (“rWGS”) services to inform diagnosis for affected pediatric and neonatal patients, enhancing GeneDx’s commercial footprint within leading health systems.
•Launched first-of-its-kind Patient Access Program in collaboration with leading biopharma partners to expand access to exome testing for pediatric epilepsy patients.
•Grew biopharma partner programs to 32, predominantly with biotech companies who are relying on us to find patients with a specific variant for clinical trial purposes.
•North Carolina Medicaid has expanded its existing coverage of outpatient whole exome sequencing (“WES”) to include the analysis of family comparator samples, which increases the rate of diagnosis of WES, effective June 1, 2024.
•State Medicaid programs continue to expand coverage of rapid genome sequencing in the neonatal intensive care unit (“NICU”), bringing total states covering rapid whole genome in the acute care setting to 14, including new coverage announced in:
◦North Carolina (June 2024)
◦Tennessee (July 2024)
◦Connecticut (July 2024)
•Announced reinvestment in rapid and standard whole genome sequencing products, enabling:
◦Faster turnaround time for rWGS with a written report in as fast as 5 days
◦Buccal samples (cheek swab) allowing for easier and more accessible non-invasive sample collection method for even the youngest patients
◦Expanding the number of repeat expansions covered by whole genome sequencing to increase diagnostic yield and improve the provider and patient experience by decreasing the need for follow-up testing
1Epic and Aura are trademarks of Epic Systems Corporation.
Webcast and Conference Call Details
GeneDx will host a conference call today, July 30, 2024, at 4:30 p.m. Eastern Time. Investors interested in listening to the conference call are required to register online. A live and archived webcast of the event will be available on the “Events” section of the GeneDx investor relations website at https://ir.genedx.com/.
Forward-Looking Statements
This press release contains certain forward-looking statements within the meaning of the federal securities laws, including statements regarding our future performance and our market opportunity, including our expected full year 2024 reported revenue guidance, our expectations regarding our adjusted gross margin profile in 2024, our use of net cash in 2024 and our turning profitable in 2025. These forward-looking statements generally are identified by the words “believe,” “project,” “expect,” “anticipate,” “estimate,” “intend,” “strategy,” “future,” “opportunity,” “plan,” “may,” “should,” “will,” “would,” “will be,” “will continue,” “will likely result,” and similar expressions. Forward-looking statements are predictions, projections and other statements about future events that are based on current expectations and assumptions and, as a result, are subject to risks and uncertainties. Many factors could cause actual future events to differ materially from the forward-looking statements in this press release, including but not limited to: (i) our ability to implement business plans, goals and forecasts, and identify and realize additional opportunities, (ii) the risk of downturns and a changing regulatory landscape in the highly competitive healthcare industry, (iii) the size and growth of the market in which we operate, (iv) our ability to pursue our new strategic direction, and (v) our ability to enhance our artificial intelligence tools that we use in our clinical interpretation platform. The foregoing list of factors is not exhaustive. You should carefully consider the foregoing factors and the other risks and uncertainties described in the “Risk Factors” section of our Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with the U.S. Securities and Exchange Commission (the “SEC”) on February 23, 2024 and other documents filed by us from time to time with the SEC. These filings identify and address other important risks and uncertainties that could cause actual events and results to differ materially from those contained in the forward-looking statements. Forward-looking statements speak only as of the date they are made. Readers are cautioned not to put undue reliance on forward-looking statements, and we assume no obligation and do not intend to update or revise these forward-looking statements, whether as a result of new information, future events, or otherwise. We do not give any assurance that we will achieve our expectations.
About GeneDx
At GeneDx (Nasdaq: WGS), we believe that everyone deserves personalized, targeted medical care—and that it all begins with a genetic diagnosis. Fueled by one of the world’s largest rare disease data sets, our industry-leading exome and genome tests translate complex genomic data into clinical answers that unlock personalized health plans, accelerate drug discovery, and improve health system efficiencies. It all starts with a single test. For more information, please visit genedx.com and connect with us on LinkedIn, X, Facebook, and Instagram.
Investor Relations Contact:
Investors@GeneDx.com
Media Contact:
Press@GeneDx.com
Volume and revenue in the table below include the combination of the Legacy GeneDx diagnostic business with the data and information business of Legacy Sema4.
Volume & Revenue
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 2Q24 | | 1Q24 | | 4Q23 | | 3Q23 | | 2Q23 |
| Volumes | | | | | | | | | |
| Whole exome, whole genome | 18,017 | | 16,592 | | 15,663 | | 13,216 | | 11,855 |
| Hereditary cancer | 5,482 | | 6,868 | | 8,240 | | 8,556 | | 7,142 |
| Other panels | 34,204 | | 31,763 | | 33,692 | | 35,861 | | 35,931 |
| Total | 57,703 | | 55,223 | | 57,595 | | 57,633 | | 54,928 |
| | | | | | | | | |
| Revenue ($ millions) | | | | | | | | | |
| Whole exome, whole genome | $ | 50.7 | | | $ | 44.0 | | | $ | 39.2 | | | $ | 34.0 | | | $ | 28.7 | |
| Hereditary cancer | 3.8 | | | 5.5 | | | 5.5 | | | 4.5 | | | 3.8 | |
| Other panels | 13.3 | | | 10.7 | | | 11.2 | | | 10.6 | | | 10.6 | |
| Data information | 1.1 | | | 1.3 | | | 2.2 | | | 1.3 | | | 2.1 | |
| Total | $ | 68.9 | | | $ | 61.5 | | | $ | 58.1 | | | $ | 50.4 | | | $ | 45.2 | |
Unaudited Select Financial Information (in thousands)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
| | | | | | | | | | | |
| Three months ended June 30, 2024 | | Three months ended March 31, 2024 |
| GeneDx | | Legacy Sema4 | | Total | | GeneDx | | Legacy Sema4 | | Total |
| Revenue | $68,924 | | $1,590 | | $70,514 | | $61,461 | | $961 | | $62,422 |
| Adjusted cost of services | 26,523 | | 145 | | 26,668 | | 24,099 | | — | | 24,099 |
| Adjusted gross profit (loss) | $42,401 | | $1,445 | | $43,846 | | $37,362 | | $961 | | $38,323 |
| Adjusted gross margin % | 61.5% | | 90.9% | | 62.2% | | 60.8% | | 100.0% | | 61.4% |
| | | | | | | | | | | | | | | | | |
| Three months ended June 30, 2023 |
| GeneDx | | Legacy Sema4 | | Total |
| Revenue | $45,226 | | $3,480 | | $48,706 |
| Adjusted cost of services | 28,452 | | — | | 28,452 |
| Adjusted gross profit (loss) | $16,774 | | $3,480 | | $20,254 |
| Adjusted gross margin % | 37.1% | | 100.0% | | 41.6% |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Three months ended June 30, 2024 |
| Reported | | Depreciation and amortization | | Stock-based compensation expense | | Restructuring costs | | Change in FV of financial liabilities | | Charges related to business exit | | Other | | Adjusted |
| Diagnostic test revenue | $ | 69,439 | | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | 69,439 | |
| Other revenue | 1,075 | | | — | | | — | | | — | | | — | | | — | | | — | | | 1,075 | |
| Total revenue | 70,514 | | | — | | | — | | | — | | | — | | | — | | | — | | | 70,514 | |
| Cost of services | 27,562 | | | (808) | | | (86) | | | — | | | — | | | — | | | — | | | 26,668 | |
| Gross profit (loss) | 42,952 | | | 808 | | | 86 | | | — | | | — | | | — | | | — | | | 43,846 | |
| Gross margin | 60.9 | % | | | | | | | | | | | | | | 62.2 | % |
| | | | | | | | | | | | | | | |
| Research and development | 10,902 | | | (211) | | | (347) | | | (35) | | | — | | | — | | | — | | | 10,309 | |
| Selling and marketing | 16,585 | | | (1,225) | | | (368) | | | (63) | | | — | | | — | | | — | | | 14,929 | |
| General and administrative | 25,170 | | | (2,974) | | | (2,307) | | | (150) | | | — | | | — | | | — | | | 19,739 | |
| Impairment loss | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Other, net | 874 | | | — | | | — | | | — | | | — | | | — | | | — | | | 874 | |
| | | | | | | | | | | | | | | |
| Loss from operations | (10,579) | | | 5,218 | | | 3,108 | | | 248 | | | — | | | — | | | — | | | (2,005) | |
| | | | | | | | | | | | | | | |
| Interest income (expense), net | (894) | | | — | | | — | | | — | | | — | | | — | | | — | | | (894) | |
| Other income (expense), net | (17,890) | | | — | | | — | | | — | | | 4,409 | | | — | | | 13,450 | | | (31) | |
| Income tax benefit | 190 | | | — | | | — | | | — | | | — | | | — | | | — | | | 190 | |
| Net loss | $ | (29,173) | | | $ | 5,218 | | | $ | 3,108 | | | $ | 248 | | | $ | 4,409 | | | $ | — | | | $ | 13,450 | | | $ | (2,740) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Three months ended June 30, 2023 |
| Reported | | Depreciation and amortization | | Stock-based compensation expense | | Restructuring costs | | Change in FV of financial liabilities | | Charges related to business exit | | Other | | Adjusted |
| Diagnostic test revenue | $ | 46,635 | | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | 46,635 | |
| Other revenue | 2,071 | | | — | | | — | | | — | | | — | | | — | | | — | | | 2,071 | |
| Total revenue | 48,706 | | | — | | | — | | | — | | | — | | | — | | | — | | | 48,706 | |
| Cost of services | 29,949 | | | (1,233) | | | (251) | | | (13) | | | — | | | — | | | — | | | 28,452 | |
| Gross profit (loss) | 18,757 | | | 1,233 | | | 251 | | | 13 | | | — | | | — | | | — | | | 20,254 | |
| Gross margin | 38.5 | % | | | | | | | | | | | | | | 41.6 | % |
| | | | | | | | | | | | | | | |
| Research and development | 17,138 | | | (4,656) | | | 675 | | | (815) | | | — | | | — | | | — | | | 12,342 | |
| Selling and marketing | 15,182 | | | (1,225) | | | 143 | | | (326) | | | — | | | — | | | — | | | 13,774 | |
| General and administrative | 37,341 | | | (3,218) | | | (675) | | | (483) | | | — | | | — | | | — | | | 32,965 | |
| Impairment loss | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Other, net | 718 | | | — | | | — | | | — | | | — | | | 334 | | | 3,238 | | | 4,290 | |
| | | | | | | | | | | | | | | |
| Loss from operations | (51,622) | | | 10,332 | | | 108 | | | 1,637 | | | — | | | (334) | | | (3,238) | | | (43,117) | |
| | | | | | | | | | | | | | | |
| Interest income (expense), net | 1,074 | | | — | | | — | | | — | | | — | | | — | | | — | | | 1,074 | |
| Other income (expense), net | 3,633 | | | — | | | — | | | — | | | (3,547) | | | — | | | (86) | | | — | |
| Income tax benefit | 196 | | | — | | | — | | | — | | | — | | | — | | | — | | | 196 | |
| Net loss | $ | (46,719) | | | $ | 10,332 | | | $ | 108 | | | $ | 1,637 | | | $ | (3,547) | | | $ | (334) | | | $ | (3,324) | | | $ | (41,847) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Three months ended March 31, 2024 |
| Reported | | Depreciation and amortization | | Stock-based compensation expense | | Restructuring costs | | Change in FV of financial liabilities | | Charges related to business exit | | Other | | Adjusted |
| Diagnostic test revenue | $ | 61,104 | | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | 61,104 | |
| Other revenue | 1,318 | | | — | | | — | | | — | | | — | | | — | | | — | | | 1,318 | |
| Total revenue | 62,422 | | | — | | | — | | | — | | | — | | | — | | | — | | | 62,422 | |
| Cost of services | 25,011 | | | (816) | | | (48) | | | (48) | | | — | | | — | | | — | | | 24,099 | |
| Gross profit (loss) | 37,411 | | | 816 | | | 48 | | | 48 | | | — | | | — | | | — | | | 38,323 | |
| Gross margin | 59.9 | % | | | | | | | | | | | | | | 61.4 | % |
| | | | | | | | | | | | | | | |
| Research and development | 11,567 | | | (196) | | | 187 | | | (103) | | | — | | | — | | | — | | | 11,455 | |
| Selling and marketing | 16,085 | | | (1,225) | | | 20 | | | (400) | | | — | | | — | | | — | | | 14,480 | |
| General and administrative | 22,445 | | | (3,011) | | | 292 | | | (292) | | | — | | | — | | | — | | | 19,434 | |
| Impairment loss | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Other, net | 974 | | | — | | | — | | | — | | | — | | | — | | | — | | | 974 | |
| | | | | | | | | | | | | | | |
| Loss from operations | (13,660) | | | 5,248 | | | (451) | | | 843 | | | — | | | — | | | — | | | (8,020) | |
| | | | | | | | | | | | | | | |
| Interest income (expense), net | (597) | | | — | | | — | | | — | | | — | | | — | | | — | | | (597) | |
| Other income (expense), net | (6,064) | | | — | | | — | | | — | | | 6,101 | | | — | | | — | | | 37 | |
| Income tax benefit | 82 | | | — | | | — | | | — | | | — | | | — | | | — | | | 82 | |
| Net loss | $ | (20,239) | | | $ | 5,248 | | | $ | (451) | | | $ | 843 | | | $ | 6,101 | | | $ | — | | | $ | — | | | $ | (8,498) | |
GeneDx Holdings Corp.
Condensed Consolidated Balance Sheets
(in thousands, except share and per share amounts)
| | | | | | | | | | | |
| June 30, 2024 (Unaudited) | | December 31, 2023 |
| Assets: | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 56,076 | | | $ | 99,681 | |
| Marketable securities | 50,784 | | | 30,467 | |
| Accounts receivable | 25,500 | | | 32,371 | |
| Due from related parties | 693 | | | 445 | |
| Inventory, net | 10,322 | | | 8,777 | |
| Prepaid expenses and other current assets | 18,792 | | | 10,598 | |
| Total current assets | 162,167 | | | 182,339 | |
| Operating lease right-of-use assets | 25,624 | | | 26,900 | |
| Property and equipment, net | 31,339 | | | 32,479 | |
| Intangible assets, net | 165,613 | | | 172,625 | |
Other assets (1) | 4,357 | | | 4,413 | |
| Total assets | $ | 389,100 | | | $ | 418,756 | |
| Liabilities and Stockholders’ Equity: | | | |
| Current liabilities: | | | |
| Accounts payable and accrued expenses | $ | 51,959 | | | $ | 37,456 | |
| Due to related parties | 1,213 | | | 1,379 | |
| Short-term lease liabilities | 4,001 | | | 3,647 | |
| Other current liabilities | 11,097 | | | 16,336 | |
| Total current liabilities | 68,270 | | | 58,818 | |
| Long-term debt, net of current portion | 52,160 | | | 52,688 | |
| Long-term lease liabilities | 60,800 | | | 62,938 | |
| Other liabilities | 12,660 | | | 14,735 | |
| Deferred taxes | 1,167 | | | 1,560 | |
| Total liabilities | 195,057 | | | 190,739 | |
| Stockholders’ Equity: | | | |
| Preferred stock | — | | | — | |
| Class A common stock | 2 | | | 2 | |
| Additional paid-in capital | 1,543,182 | | | 1,527,778 | |
| Accumulated deficit | (1,349,600) | | | (1,300,188) | |
| Accumulated other comprehensive income | 459 | | | 425 | |
| Total stockholders’ equity | 194,043 | | | 228,017 | |
| Total liabilities and stockholders’ equity | $ | 389,100 | | | $ | 418,756 | |
(1)Other assets includes $987 thousand of restricted cash as of both June 30, 2024 and December 31, 2023.
GeneDx Holdings Corp.
Condensed Consolidated Statements of Operations (Unaudited)
(in thousands, except share and per share amounts)
| | | | | | | | | | | | | | | | | | | | | | | |
| June 30, | | Six months ended June 30, |
| 2024 | | 2023 | | 2024 | | 2023 |
| Revenue | | | | | | | |
| Diagnostic test revenue | $ | 69,439 | | | $ | 46,635 | | | $ | 130,543 | | | $ | 88,485 | |
| Other revenue | 1,075 | | | 2,071 | | | 2,393 | | | 3,360 | |
| Total revenue | 70,514 | | | 48,706 | | | 132,936 | | | 91,845 | |
| Cost of services | 27,562 | | | 29,949 | | | 52,573 | | | 57,852 | |
| Gross profit | 42,952 | | | 18,757 | | | 80,363 | | | 33,993 | |
| Research and development | 10,902 | | | 17,138 | | | 22,469 | | | 31,730 | |
| Selling and marketing | 16,585 | | | 15,182 | | | 32,670 | | | 28,634 | |
| General and administrative | 25,170 | | | 37,341 | | | 47,615 | | | 81,030 | |
| Impairment loss | — | | | — | | | — | | | 2,120 | |
| Other operating expenses, net | 874 | | | 718 | | | 1,848 | | | 2,465 | |
| Loss from operations | (10,579) | | | (51,622) | | | (24,239) | | | (111,986) | |
| | | | | | | |
| Non-operating income (expenses), net | | | | | | | |
| Change in fair value of warrants and earn-out contingent liabilities | (4,409) | | | 3,547 | | | (10,510) | | | 94 | |
| Interest expense, net | (894) | | | 1,074 | | | (1,491) | | | 1,039 | |
| Other expense, net | (13,481) | | | 86 | | | (13,444) | | | 2,802 | |
| Total non-operating income, net | (18,784) | | | 4,707 | | | (25,445) | | | 3,935 | |
| Loss before income taxes | (29,363) | | | (46,915) | | | $ | (49,684) | | | $ | (108,051) | |
| Income tax benefit | 190 | | | 196 | | | 272 | | | 343 | |
| Net loss | $ | (29,173) | | | $ | (46,719) | | | $ | (49,412) | | | $ | (107,708) | |
| | | | | | | |
| Weighted average shares outstanding of Class A common stock | 26,617,955 | | 25,418,358 | | 26,340,063 | | 22,754,948 |
| Basic and diluted net loss per share, Class A common stock | $ | (1.10) | | | $ | (1.84) | | | $ | (1.88) | | | $ | (4.73) | |
GeneDx Holdings Corp.
Condensed Consolidated Statements of Cash Flows (Unaudited)
(in thousands)
| | | | | | | | | | | | | |
| Six months ended June 30, |
| 2024 | | 2023 | | |
| Operating activities | | | | | |
| Net loss | $ | (49,412) | | | $ | (107,708) | | | |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | |
| Depreciation and amortization expense | 10,466 | | | 18,968 | | | |
| Stock-based compensation expense | 2,657 | | | 156 | | | |
| Change in fair value of warrants and contingent liabilities | 10,510 | | | (94) | | | |
| Deferred tax benefit | (272) | | | (343) | | | |
| Provision for excess and obsolete inventory | 109 | | | 2,620 | | | |
| Legal reserves | 13,450 | | | — | | | |
| Change in third party payor reserves | 1,066 | | | (4,308) | | | |
| Gain on sale of assets | — | | | (2,954) | | | |
| Gain on debt forgiveness | — | | | (2,750) | | | |
| Impairment loss | — | | | 2,120 | | | |
| Other | 1,738 | | | 412 | | | |
| Change in operating assets and liabilities: | | | | | |
| Accounts receivable | 6,871 | | | 10,174 | | | |
| Inventory | (1,654) | | | (486) | | | |
| Accounts payable and accrued expenses | (10,359) | | | (25,399) | | | |
| Other assets and liabilities | (6,088) | | | 531 | | | |
| Net cash used in operating activities | (20,918) | | | (109,061) | | | |
| Investing activities | | | | | |
| Consideration on escrow paid for GeneDx acquisition | — | | | (12,144) | | | |
| Purchases of property and equipment | (1,795) | | | (2,762) | | | |
| Proceeds from sales of assets | — | | | 3,634 | | | |
| Purchases of marketable securities | (29,381) | | | — | | | |
| Proceeds from sales of marketable securities | 598 | | | — | | | |
| Proceeds from maturities of marketable securities | 8,720 | | | — | | | |
| Development of internal-use software assets | — | | | (461) | | | |
| Net cash used in investing activities | (21,858) | | | (11,733) | | | |
| Financing activities | | | | | |
| Proceeds from offerings, net of issuance costs | — | | | 143,002 | | | |
| | | | | |
| Exercise of stock options | 161 | | | 266 | | | |
| Long-term debt principal payments | — | | | (2,000) | | | |
| Finance lease payoff and principal payments | (990) | | | (1,222) | | | |
| Net cash (used in) provided by financing activities | (829) | | | 140,046 | | | |
| Net (decrease) increase in cash, cash equivalents and restricted cash | (43,605) | | | 19,252 | | | |
| Cash, cash equivalents and restricted cash, at beginning of period | 100,668 | | | 138,303 | | | |
Cash, cash equivalents and restricted cash, at end of period (1) | $ | 57,063 | | | $ | 157,555 | | | |
| | | | | |
| Supplemental disclosures of cash flow information | | | | | |
| Cash paid for interest | $ | 4,033 | | | $ | 946 | | | |
| Cash paid for taxes | $ | 557 | | | $ | 1,003 | | | |
| Stock consideration paid for purchase of business | $ | — | | | $ | 6,692 | | | |
| Stock consideration paid pursuant to exercise of Perceptive warrant | $ | 12,586 | | | $ | — | | | |
| Purchases of property and equipment in accounts payable and accrued expenses | $ | 501 | | | $ | 109 | | | |
| Assets acquired under capital leases obligations | $ | 689 | | | $ | — | | | |
(1)Cash, cash equivalents and restricted cash at June 30, 2024 excludes marketable securities of $50.8 million.

One test. Big picture. Brighter futures. July 30, 2024 GeneDx (Nasdaq: WGS) 2Q 2024 Earnings Presentation Exhibit 99.2

2 Disclaimer This presentation contains forward-looking statements under the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements are statements that do not relate to historical facts and events and such statements and opinions pertaining to the future that, for example, contain wording such as “may,” “might,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “objective,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “continue,” “ongoing,” or the negative of these terms, or other comparable terminology intended to identify statements about the future. Forward- looking statements contained in this presentation may include, but are not limited to, statements about: our future performance and our market opportunity, our expectations regarding full year 2024 revenue, adjusted gross margin profile and cash burn in 2024 and our expectation of turning profitable in 2025. We cannot assure that the forward-looking statements in this presentation will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. The forward-looking statements and opinions contained in this presentation are based on our management’s beliefs and assumptions and are based upon information currently available to our management as of the date of this presentation and, while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. Many factors could cause actual future events to differ materially from the forward-looking statements in this presentation, including but not limited to: (i) the ability to implement business plans, goals and forecasts, and identify and realize additional opportunities, (ii) the risk of downturns and a changing regulatory landscape in the highly competitive healthcare industry, (iii) the size and growth of the market in which we operate, (iv) our ability to pursue our new strategic direction, and (v) our ability to enhance our artificial intelligence tools that we use in our clinical interpretation platform. The information, opinions and forward-looking statements contained in this announcement speak only as of its date and are subject to change without notice. This presentation contains estimates, projections and other information concerning our industry, our business, and the markets for our products and services. Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties, and actual events or circumstances may differ materially from events and circumstances that are assumed in this information. Unless otherwise expressly stated, we obtained this industry, business, market and other data from our own internal estimates and research as well as from reports, research surveys, studies and similar data prepared by market research firms and other third parties, industry, medical and general publications, government data and similar sources. While we believe our internal company research as to such matters is reliable and the market definitions are appropriate, neither such research nor these definitions have been verified by any independent source. We discuss these and other risks and uncertainties in greater detail in the sections entitled “Risk Factors” and "Management's Discussion and Analysis of Financial Condition and Results of Operations" in our periodic reports and other filings we make with the SEC from time to time. Given these uncertainties, you should not place undue reliance on the forward-looking statements. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. Except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in our expectations. We file reports, proxy statements, and other information with the SEC. Such reports, proxy statements, and other information concerning us are available www.sec.gov. Requests for copies of such documents should be directed to our Investor Relations department at GeneDx Holdings Corp. 333 Ludlow Street, North Tower 6th Floor, Stamford, Connecticut, 06902. Our telephone number is 888-729-1206.

WGS Q2 2024 Results Second quarter 2024 revenue from continuing operations1 of $68.9M with 77% year-over- year revenue growth for exome and genome test revenue Raising guidance to deliver between $255M and $265M in FY 2024 revenue and reiterate path to profitability in 2025 Expanded second quarter 2024 adjusted gross margin from continuing operations1,2 to 62% Second quarter 2024 total cash burn of $6M; ending June 30, 2024 with cash, cash equivalents, marketable securities and restricted cash of $108M 1. Results from continuing operations, which representatives our ongoing business strategy, exclude any revenue and cost of goods sold of the exited Legacy Sema4 diagnostic testing business for the current and all comparative periods. Total company results include GeneDx's continuing operations and the financial impacts of exited Legacy Sema4 business activities. 2. Adjusted gross margin is a non-GAAP financial measure. For a reconciliation of GAAP and non-GAAP results, please refer to the reconciliation contained at the end of this earnings presentation.3
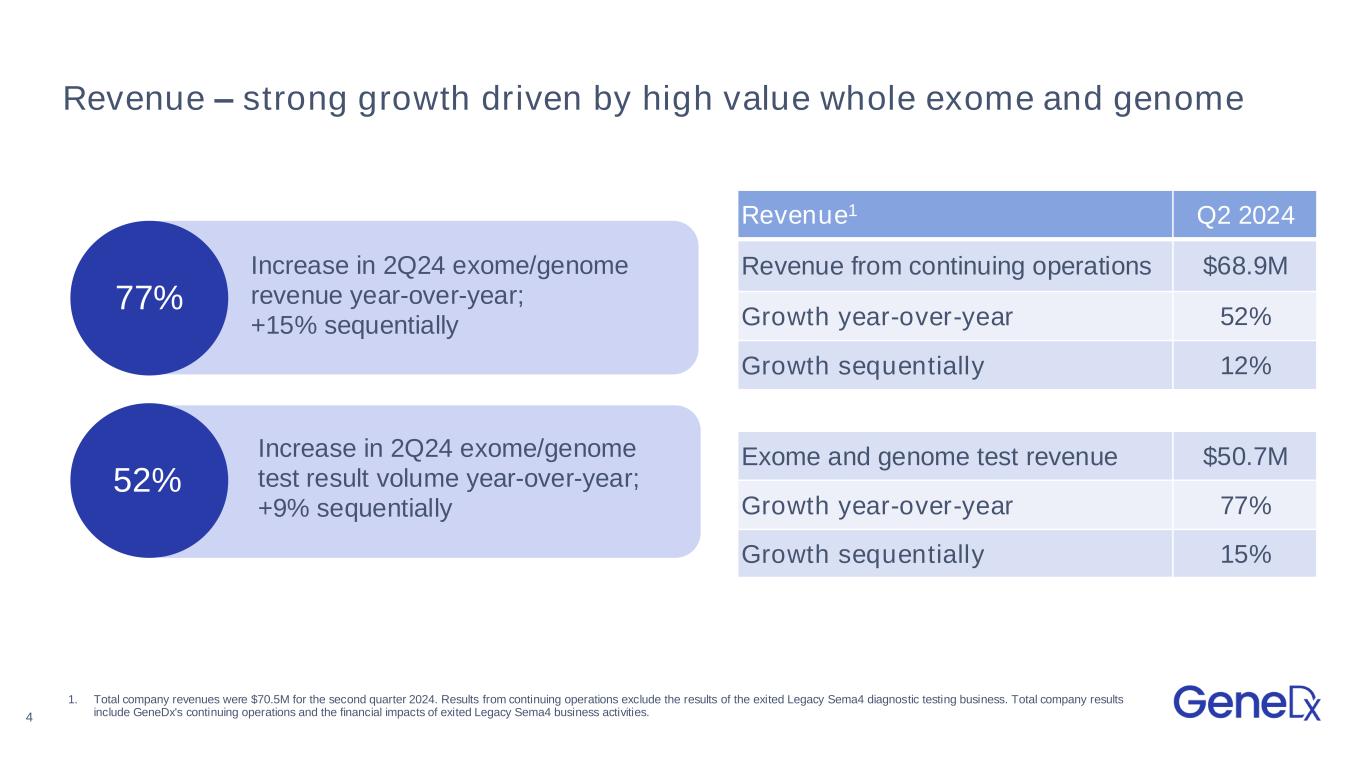
4 77% Revenue – strong growth driven by high value whole exome and genome Revenue1 Q2 2024 Revenue from continuing operations $68.9M Growth year-over-year 52% Growth sequentially 12% Exome and genome test revenue $50.7M Growth year-over-year 77% Growth sequentially 15% Increase in 2Q24 exome/genome test result volume year-over-year; +9% sequentially 1. Total company revenues were $70.5M for the second quarter 2024. Results from continuing operations exclude the results of the exited Legacy Sema4 diagnostic testing business. Total company results include GeneDx's continuing operations and the financial impacts of exited Legacy Sema4 business activities. 52% Increase in 2Q24 exome/genome revenue year-over-year; +15% sequentially
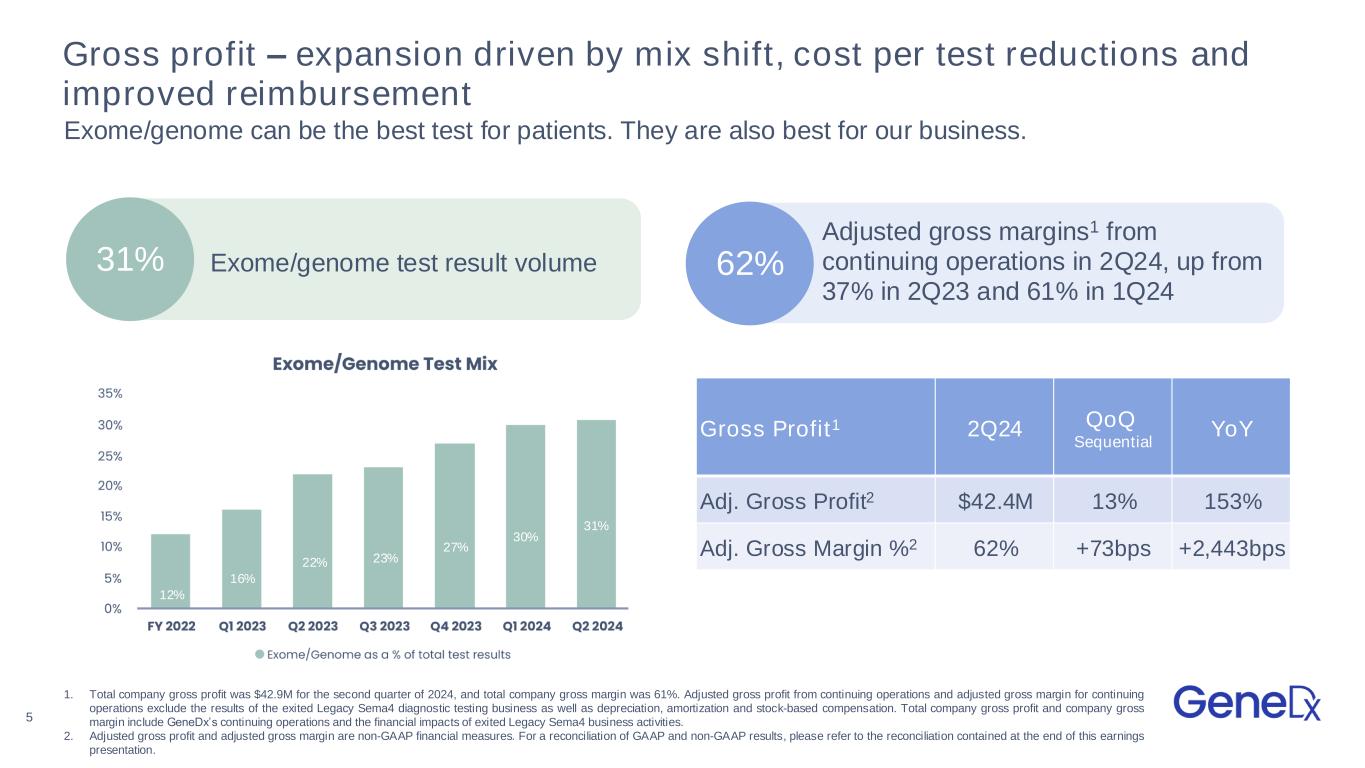
5 Gross profit – expansion driven by mix shift, cost per test reductions and improved reimbursement Exome/genome can be the best test for patients. They are also best for our business. 31% Exome/genome test result volume Adjusted gross margins1 from continuing operations in 2Q24, up from 37% in 2Q23 and 61% in 1Q24 62% 12% 16% 22% 23% 27% 30% 1. Total company gross profit was $42.9M for the second quarter of 2024, and total company gross margin was 61%. Adjusted gross profit from continuing operations and adjusted gross margin for continuing operations exclude the results of the exited Legacy Sema4 diagnostic testing business as well as depreciation, amortization and stock-based compensation. Total company gross profit and company gross margin include GeneDx’s continuing operations and the financial impacts of exited Legacy Sema4 business activities. 2. Adjusted gross profit and adjusted gross margin are non-GAAP financial measures. For a reconciliation of GAAP and non-GAAP results, please refer to the reconciliation contained at the end of this earnings presentation. Gross Profit1 2Q24 QoQ Sequential YoY Adj. Gross Profit2 $42.4M 13% 153% Adj. Gross Margin %2 62% +73bps +2,443bps 31%
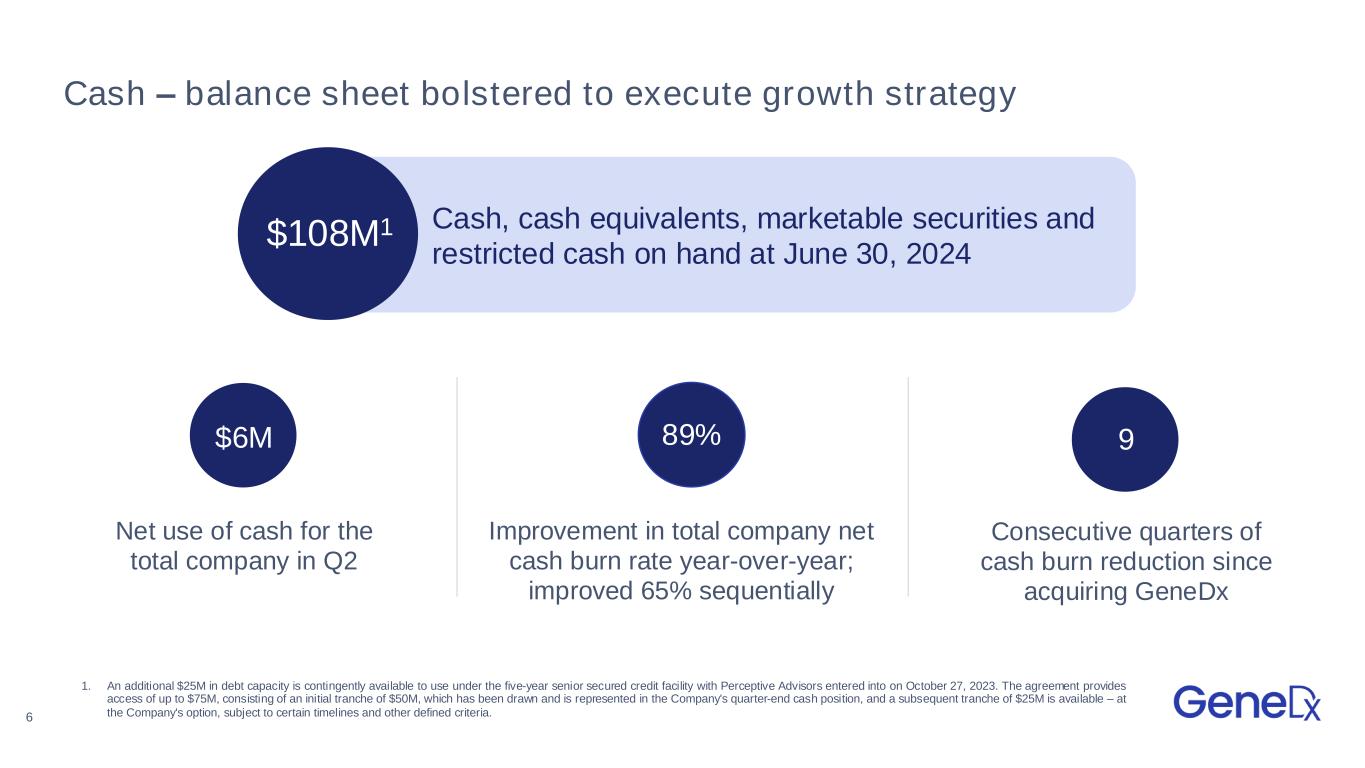
6 Cash – balance sheet bolstered to execute growth strategy 1 $108M1 Cash, cash equivalents, marketable securities and restricted cash on hand at June 30, 2024 9 Consecutive quarters of cash burn reduction since acquiring GeneDx Net use of cash for the total company in Q2 Improvement in total company net cash burn rate year-over-year; improved 65% sequentially $6M 89% 1. An additional $25M in debt capacity is contingently available to use under the five-year senior secured credit facility with Perceptive Advisors entered into on October 27, 2023. The agreement provides access of up to $75M, consisting of an initial tranche of $50M, which has been drawn and is represented in the Company's quarter-end cash position, and a subsequent tranche of $25M is available – at the Company's option, subject to certain timelines and other defined criteria.

2024 Guidance Update Drive full year 2024 revenues between $255 to $265 million (previous guidance was between $235 to $245 million) Expand full year 2024 adjusted gross margin profile to at least 60% (no change) Use between $65 to $70 million of net cash for full year 2024 (previous guidance was between $70 to $80 million) Turn profitable in 2025 (no change) 7 1. Total company results include the combination of the GeneDx diagnostic business revenues and the data and information revenues from the Legacy Sema4 business.

8 Appendix

GeneDx is a leader in improving health outcomes through genomic insights.
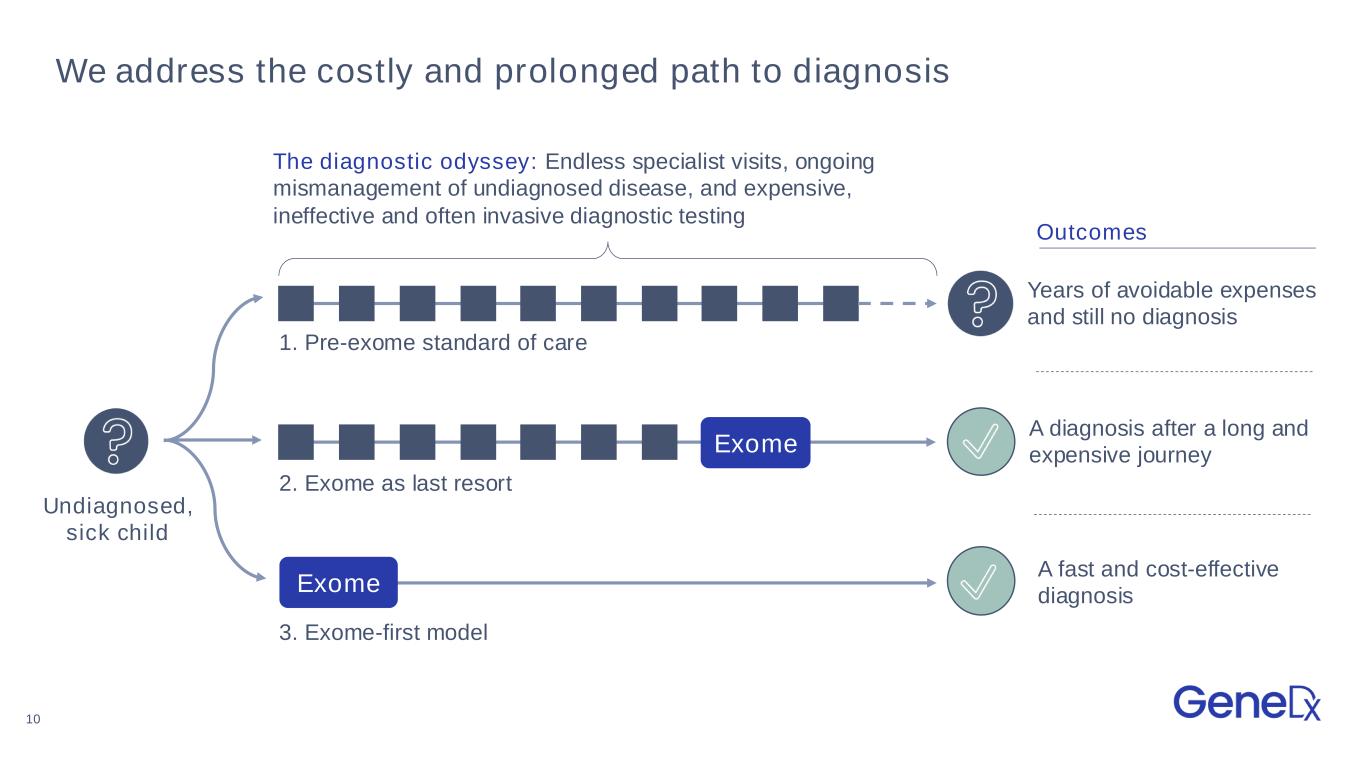
10 We address the costly and prolonged path to diagnosis Years of avoidable expenses and still no diagnosis A diagnosis after a long and expensive journey A fast and cost-effective diagnosis 1. Pre-exome standard of care 2. Exome as last resort 3. Exome-first model The diagnostic odyssey: Endless specialist visits, ongoing mismanagement of undiagnosed disease, and expensive, ineffective and often invasive diagnostic testing Undiagnosed, sick child Outcomes Exome Exome
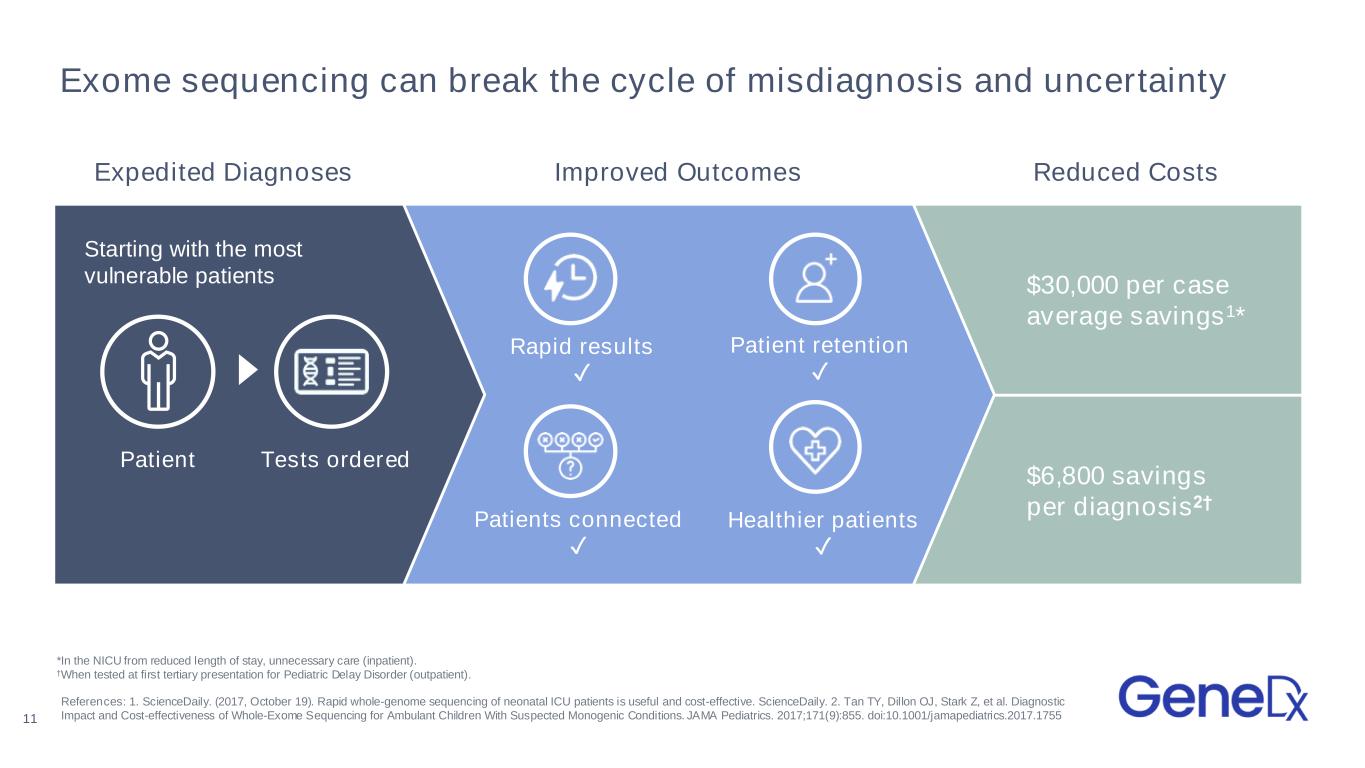
Exome sequencing can break the cycle of misdiagnosis and uncertainty $6,800 savings per diagnosis2† $30,000 per case average savings1* Rapid results ✓ Patient retention ✓ Patients connected ✓ Healthier patients ✓ Tests ordered Starting with the most vulnerable patients References: 1. ScienceDaily. (2017, October 19). Rapid whole-genome sequencing of neonatal ICU patients is useful and cost-effective. ScienceDaily. 2. Tan TY, Dillon OJ, Stark Z, et al. Diagnostic Impact and Cost-effectiveness of Whole-Exome Sequencing for Ambulant Children With Suspected Monogenic Conditions. JAMA Pediatrics. 2017;171(9):855. doi:10.1001/jamapediatrics.2017.1755 *In the NICU from reduced length of stay, unnecessary care (inpatient). †When tested at first tertiary presentation for Pediatric Delay Disorder (outpatient). 11 Patient Expedited Diagnoses Improved Outcomes Reduced Costs
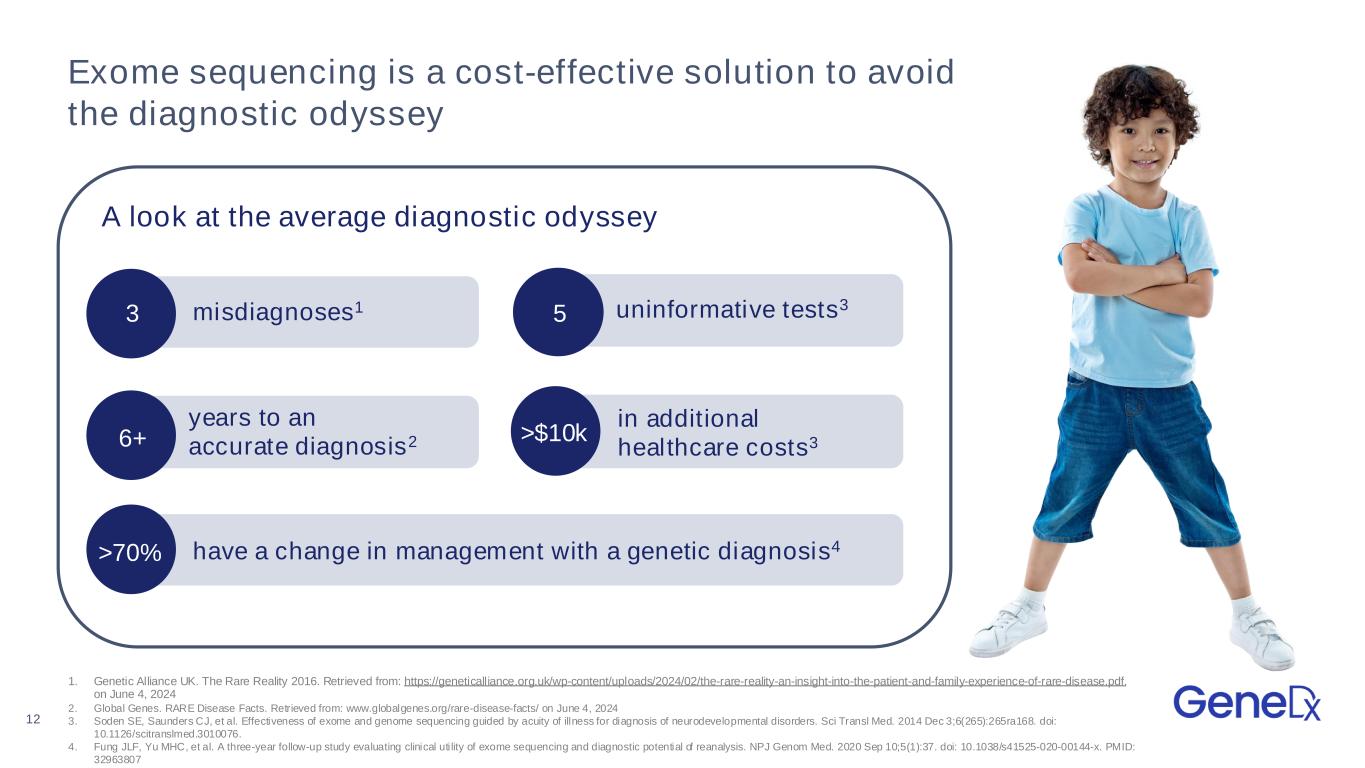
12 Exome sequencing is a cost-effective solution to avoid the diagnostic odyssey A look at the average diagnostic odyssey 1. Genetic Alliance UK. The Rare Reality 2016. Retrieved from: https://geneticalliance.org.uk/wp-content/uploads/2024/02/the-rare-reality-an-insight-into-the-patient-and-family-experience-of-rare-disease.pdf. on June 4, 2024 2. Global Genes. RARE Disease Facts. Retrieved from: www.globalgenes.org/rare-disease-facts/ on June 4, 2024 3. Soden SE, Saunders CJ, et al. Effectiveness of exome and genome sequencing guided by acuity of illness for diagnosis of neurodevelopmental disorders. Sci Transl Med. 2014 Dec 3;6(265):265ra168. doi: 10.1126/scitranslmed.3010076. 4. Fung JLF, Yu MHC, et al. A three-year follow-up study evaluating clinical uti lity of exome sequencing and diagnostic potential of reanalysis. NPJ Genom Med. 2020 Sep 10;5(1):37. doi: 10.1038/s41525-020-00144-x. PMID: 32963807 misdiagnoses1 uninformative tests3 years to an accurate diagnosis26+ in additional healthcare costs3>$10k have a change in management with a genetic diagnosis4>70% 53

13 GeneDx offers leading exome and genome products • Genome sequencing – Analyzes the entirety of an individual’s DNA, which is known as the genome. The genome includes ~20,000 genes. • Exome sequencing – Analyzes the protein coding regions of the ~20,000 genes in an individual’s genome, which is known as the exome. The exome is thought to contain a majority of disease-causing genetic variants. Translating complex genomic data into definitive diagnoses for patients
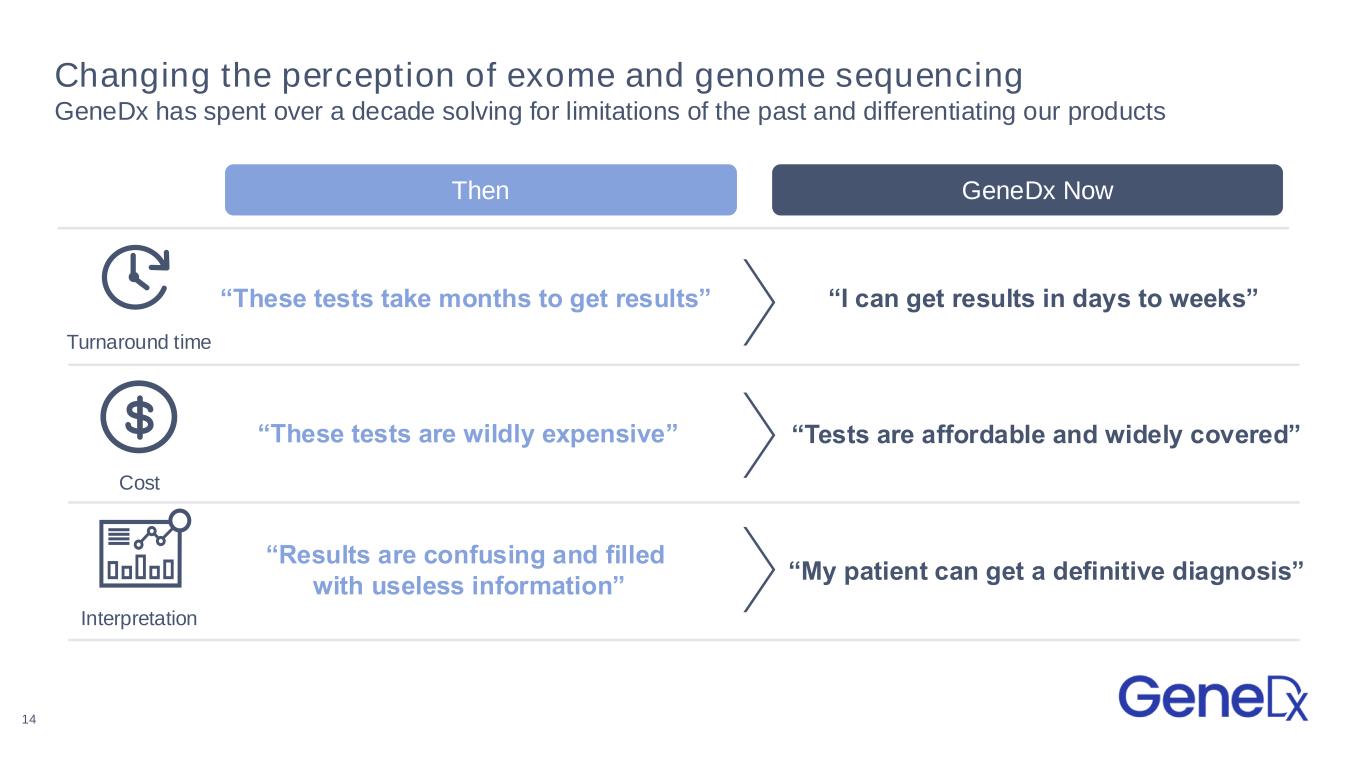
Changing the perception of exome and genome sequencing GeneDx has spent over a decade solving for limitations of the past and differentiating our products Then GeneDx Now “I can get results in days to weeks”“These tests take months to get results” “These tests are wildly expensive” “Tests are affordable and widely covered” “Results are confusing and filled with useless information” “My patient can get a definitive diagnosis” Turnaround time Cost Interpretation 14

15 Patients we serve today are difficult to diagnose and have complex needs o Congenital abnormalities (birth defects) o Significant Intellectual disability o Global developmental delay o Seizures/epilepsy o Failure to thrive or other growth concerns o Autism spectrum disorder o Complex neurodevelopmental disorder o Severe neuropsychiatric condition o Cerebral palsy o Dysmorphic features o Significant hearing or visual impairment o Period of unexplained developmental regression o Biochemical findings suggesting inborn error of metabolism o Family history strongly suggestive of a genetic etiology Patients typically have 2+ of the indications below
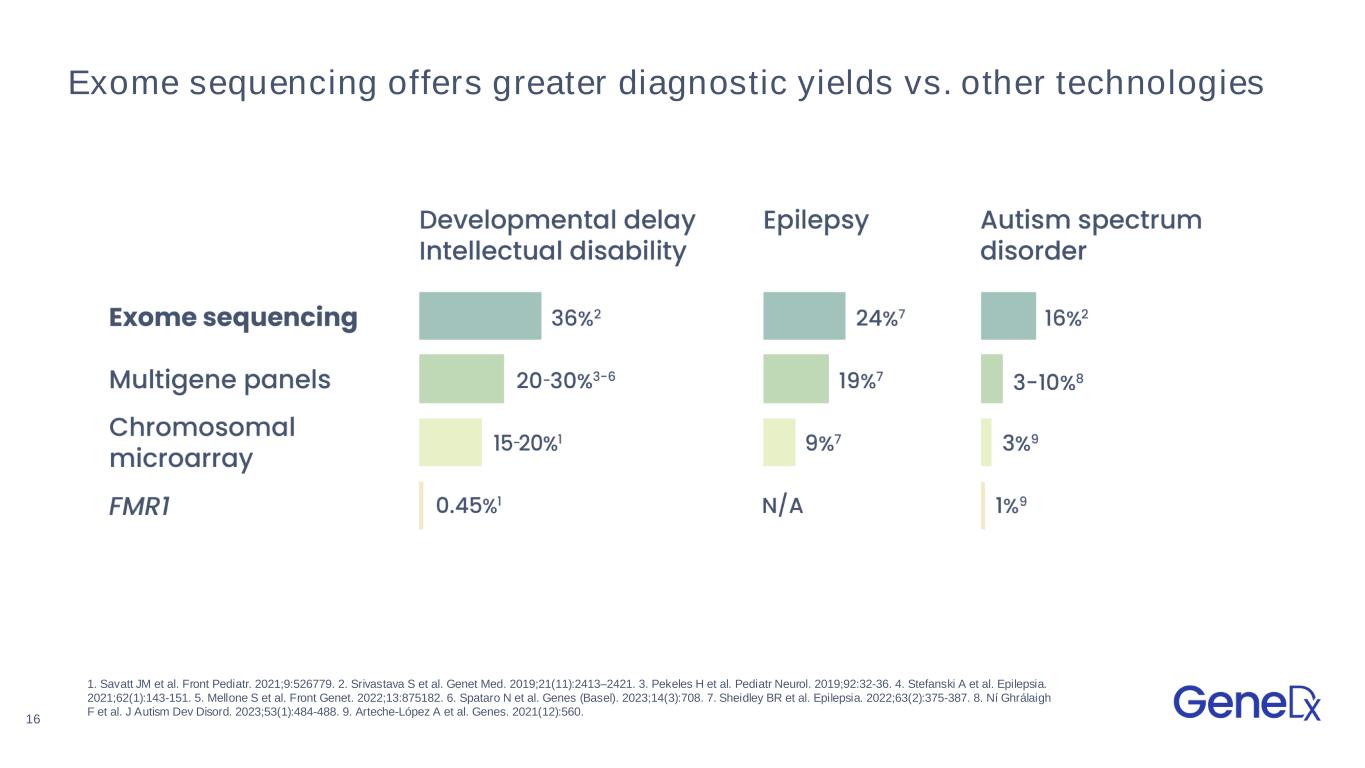
16 1. Savatt JM et al. Front Pediatr. 2021;9:526779. 2. Srivastava S et al. Genet Med. 2019;21(11):2413–2421. 3. Pekeles H et al. Pediatr Neurol. 2019;92:32-36. 4. Stefanski A et al. Epilepsia. 2021;62(1):143-151. 5. Mellone S et al. Front Genet. 2022;13:875182. 6. Spataro N et al. Genes (Basel). 2023;14(3):708. 7. Sheidley BR et al. Epilepsia. 2022;63(2):375-387. 8. Ní Ghrálaigh F et al. J Autism Dev Disord. 2023;53(1):484-488. 9. Arteche-López A et al. Genes. 2021(12):560. Exome sequencing offers greater diagnostic yields vs. other technologies

GeneDx is positioned to enable a data-informed future for healthcare.
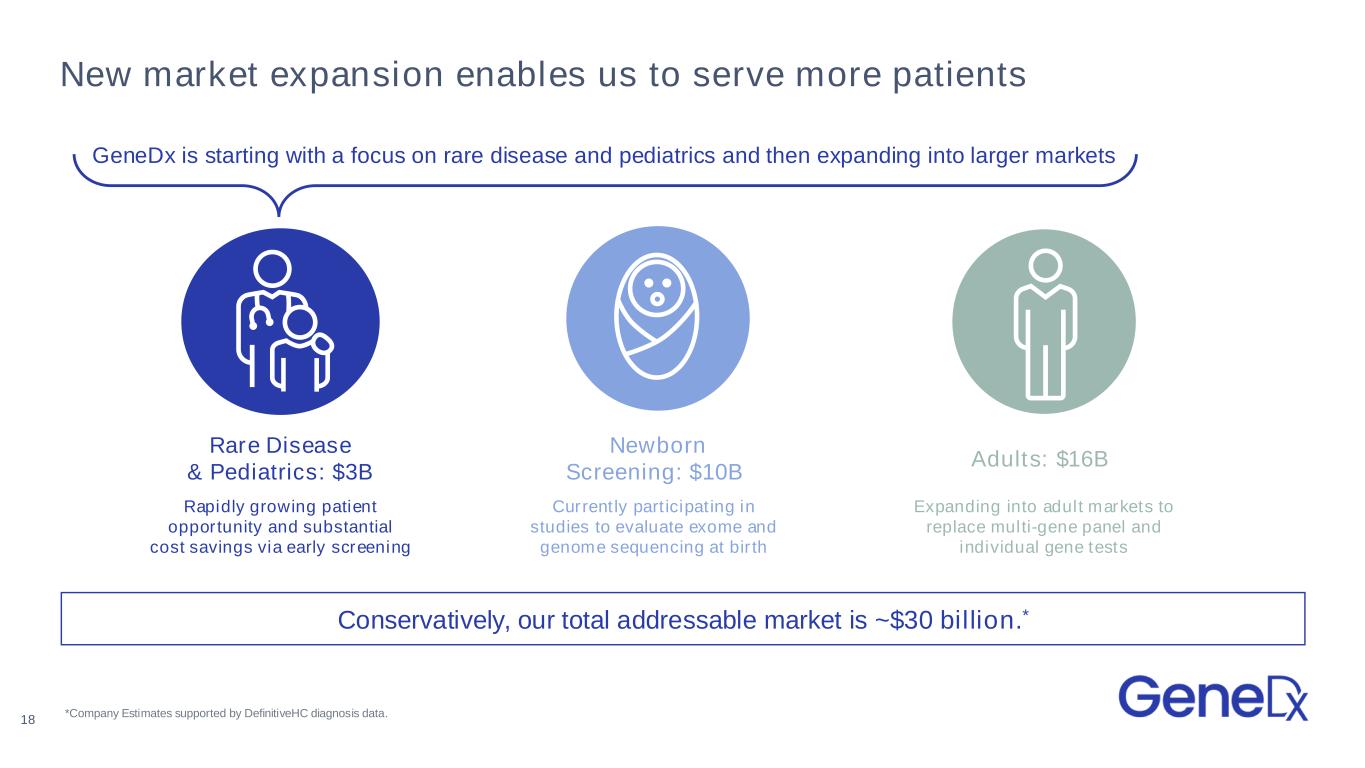
Adults: $16B Expanding into adult markets to replace multi-gene panel and individual gene tests New market expansion enables us to serve more patients Rapidly growing patient opportunity and substantial cost savings via early screening Rare Disease & Pediatrics: $3B *Company Estimates supported by DefinitiveHC diagnosis data. Conservatively, our total addressable market is ~$30 billion.* Newborn Screening: $10B Currently participating in studies to evaluate exome and genome sequencing at birth 18 GeneDx is starting with a focus on rare disease and pediatrics and then expanding into larger markets
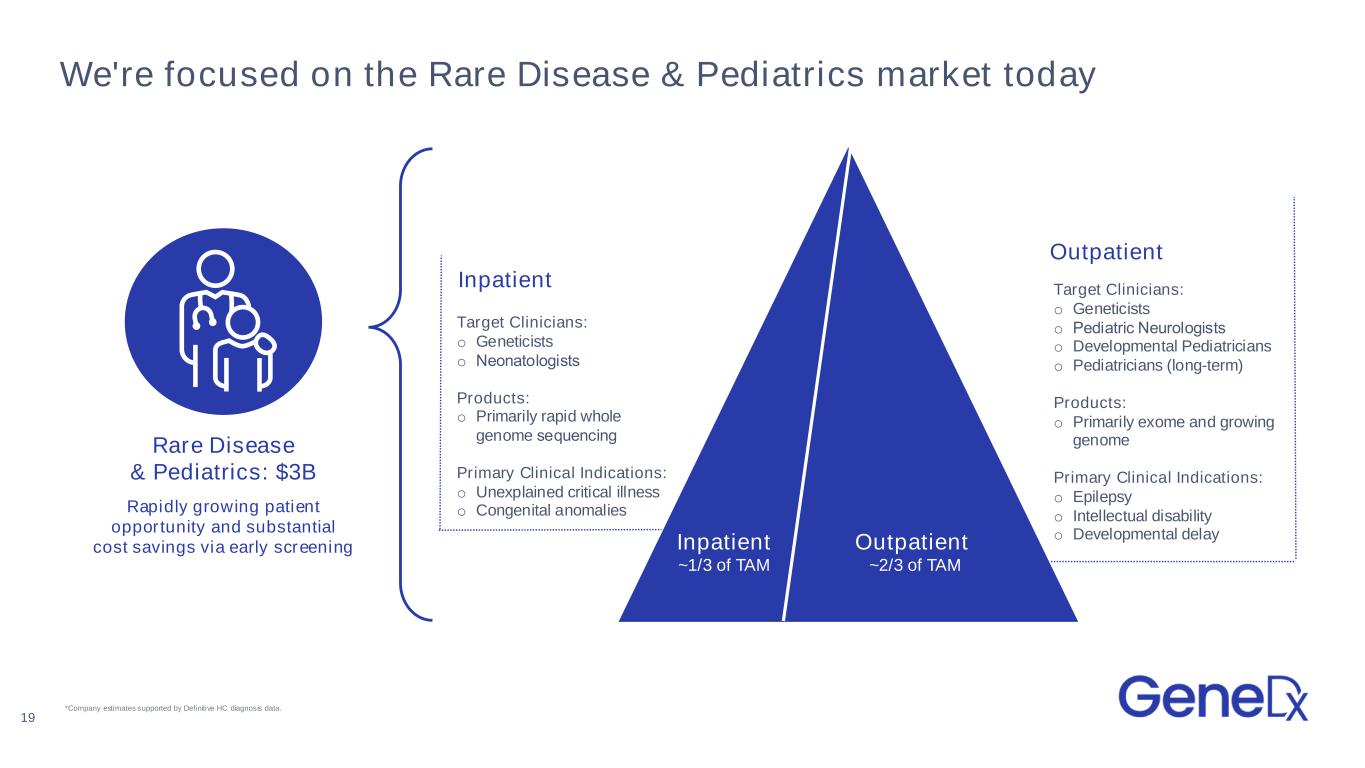
We're focused on the Rare Disease & Pediatrics market today Rapidly growing patient opportunity and substantial cost savings via early screening Rare Disease & Pediatrics: $3B 19 Inpatient ~1/3 of TAM Outpatient ~2/3 of TAM Target Clinicians: o Geneticists o Pediatric Neurologists o Developmental Pediatricians o Pediatricians (long-term) Products: o Primarily exome and growing genome Primary Clinical Indications: o Epilepsy o Intellectual disability o Developmental delay Target Clinicians: o Geneticists o Neonatologists Products: o Primarily rapid whole genome sequencing Primary Clinical Indications: o Unexplained critical illness o Congenital anomalies Inpatient Outpatient *Company estimates supported by Definitive HC diagnosis data.
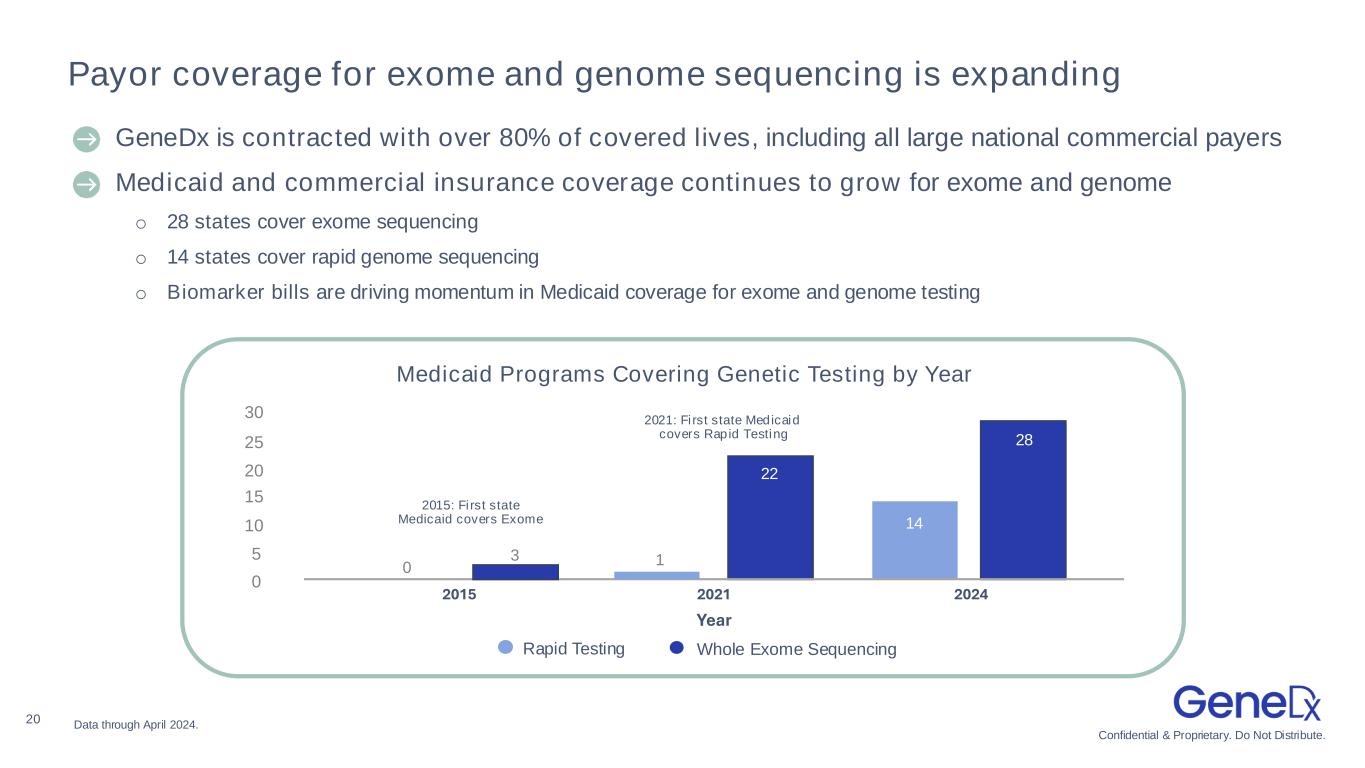
Confidential & Proprietary. Do Not Distribute. 20 Payor coverage for exome and genome sequencing is expanding Data through April 2024. 〉 GeneDx is contracted with over 80% of covered lives, including all large national commercial payers 〉Medicaid and commercial insurance coverage continues to grow for exome and genome o 28 states cover exome sequencing o 14 states cover rapid genome sequencing o Biomarker bills are driving momentum in Medicaid coverage for exome and genome testing Medicaid Programs Covering Genetic Testing by Year 2015: First state Medicaid covers Exome 2021: First state Medicaid covers Rapid Testing 30 25 20 15 10 5 0 0 3 1 28 2015 2021 2024 Year Rapid Testing Whole Exome Sequencing 22 14
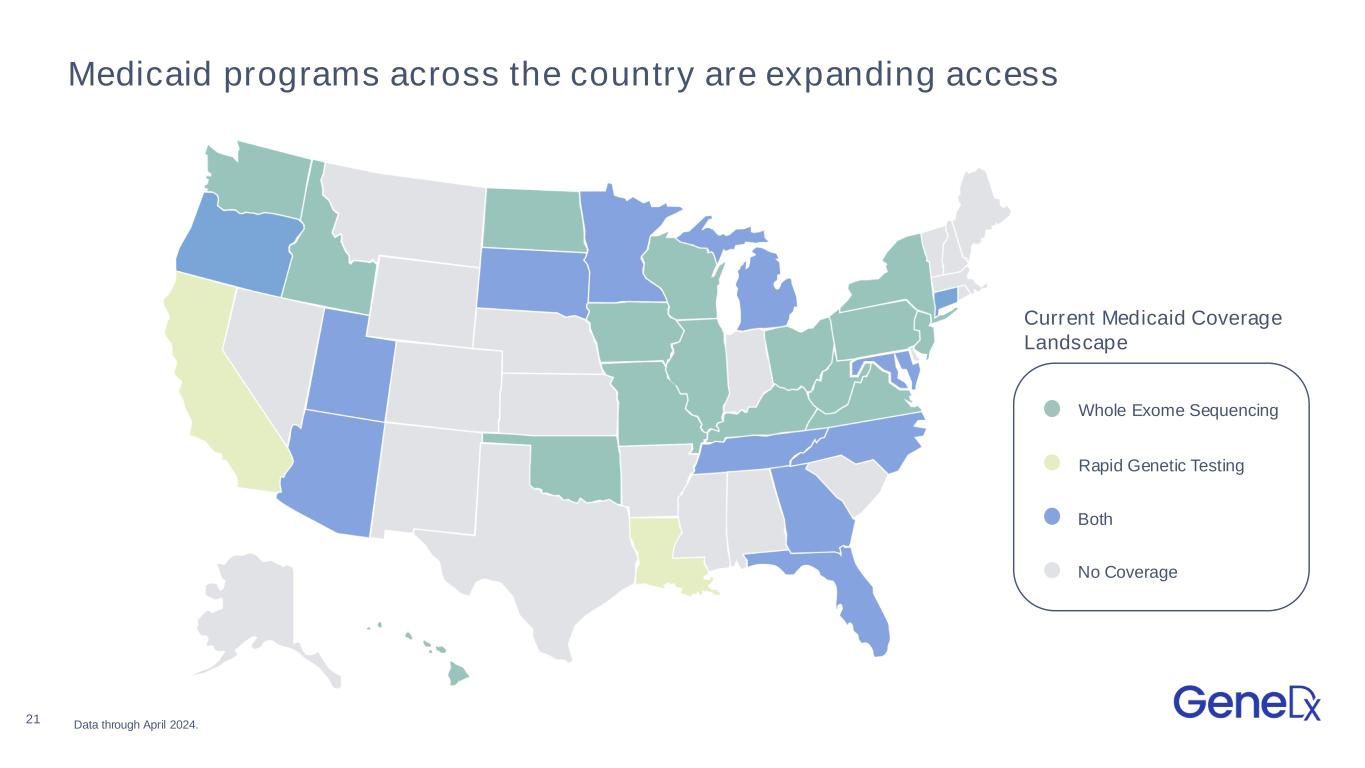
21 Medicaid programs across the country are expanding access Whole Exome Sequencing Rapid Genetic Testing Both No Coverage Current Medicaid Coverage Landscape Data through April 2024.

Medical practice guidelines recommend exome and genome sequencing for patients ACMG Practice Guideline1: “Strong recommendation based on the available evidence to support the use of ES/GS as either a first- (or second-) line test in patients …. ES/ GS demonstrates clinical utility for the patients and their families with limited evidence for negative outcomes and the ever-increasing emerging evidence of therapeutic benefit.” NSGC Guideline2: “Recommending Exome Sequencing as a First-Tier Genetic Test for Unexplained Epilepsies” 22 American Epilepsy Society: “Exome or genome sequencing are favored for most scenarios, as they are more likely to provide a diagnosis.” 1. Manickam K, McClain MR, Demmer LA, et al. Exome and genome sequencing for pediatric patients with congenital anomalies or intellectual disability: an evidence-based clinical guideline of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2021 Nov;23(11):2029-2037. doi: 10.1038/s41436-021-01242-6. Epub 2021 Jul 1. 2. Smith L, Malinowski J, Ceulemans S, et al. Genetic testing and counseling for the unexplained epilepsies: An evidence-based practice guideline of the National Society of Genetic Counselors. J Genet Couns. 2022 Oct 24. doi.org/10.1002/jgc4.1646
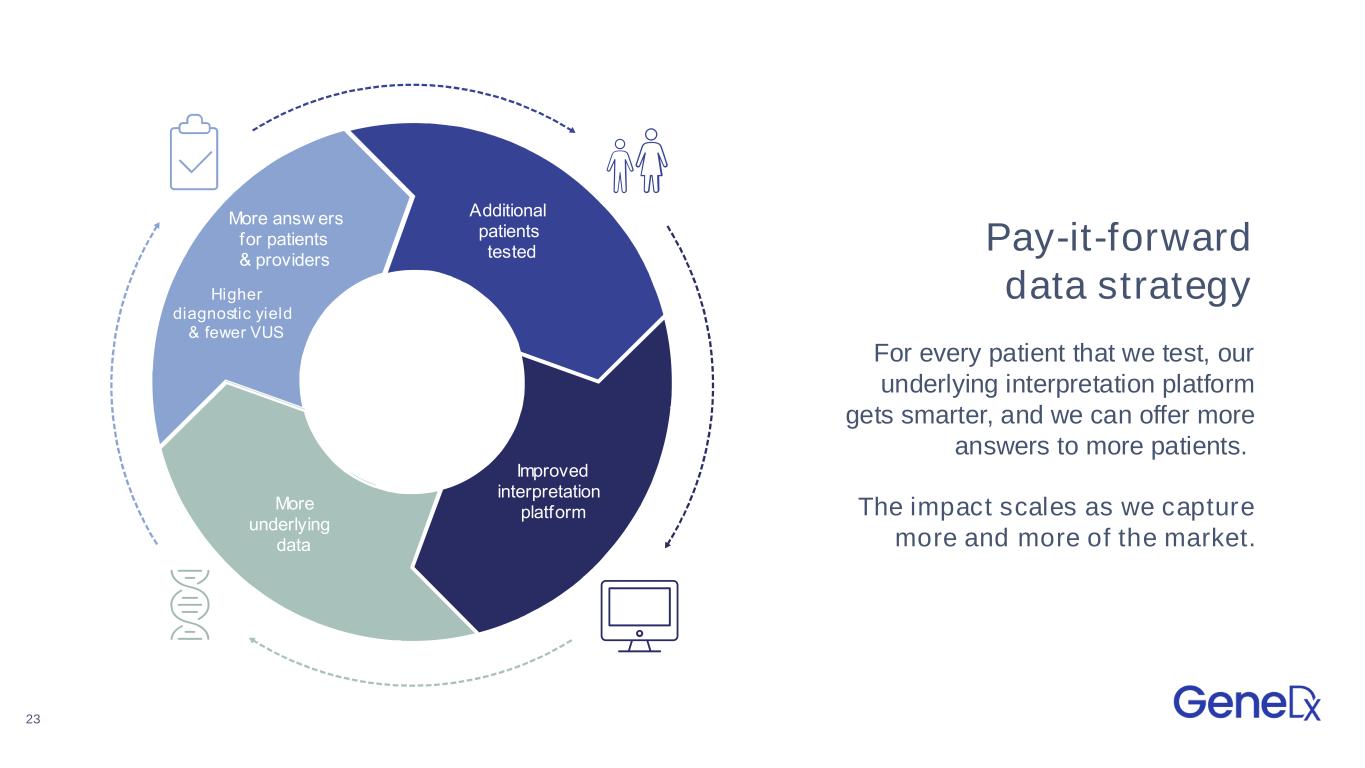
23 Pay-it-forward data strategy For every patient that we test, our underlying interpretation platform gets smarter, and we can offer more answers to more patients. The impact scales as we capture more and more of the market. Additional patients tested mproved interpretation platform ore underlying data ore answ ers for patients providers igher diagnostic y ield f ewer S Additional patients tested mproved interpretation platform ore underlying data ore answ ers for patients providers igher diagnostic yield fewer S
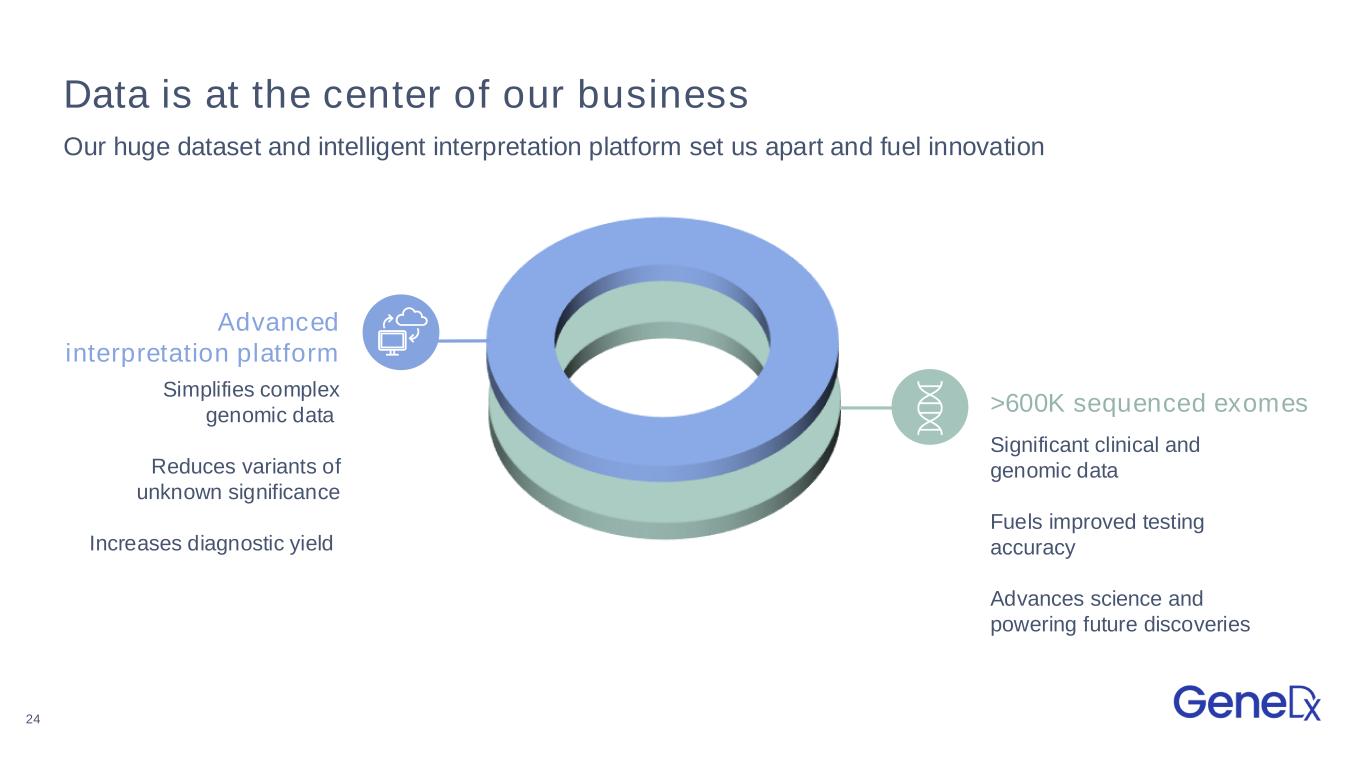
24 >600K sequenced exomes Data is at the center of our business Our huge dataset and intelligent interpretation platform set us apart and fuel innovation Simplifies complex genomic data Reduces variants of unknown significance Increases diagnostic yield Significant clinical and genomic data Fuels improved testing accuracy Advances science and powering future discoveries Advanced interpretation platform
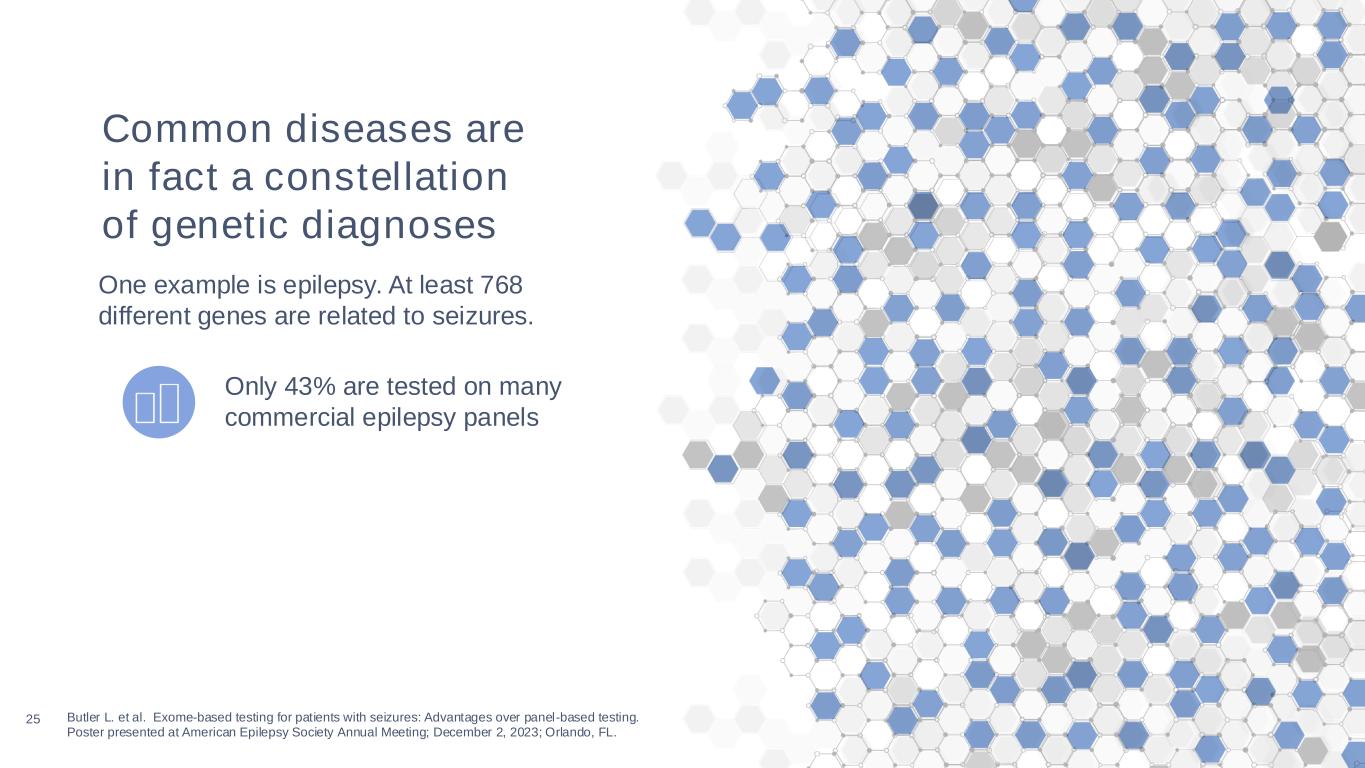
25 Butler L. et al. Exome-based testing for patients with seizures: Advantages over panel-based testing. Poster presented at American Epilepsy Society Annual Meeting; December 2, 2023; Orlando, FL. Only 43% are tested on many commercial epilepsy panels Common diseases are in fact a constellation of genetic diagnoses One example is epilepsy. At least 768 different genes are related to seizures.
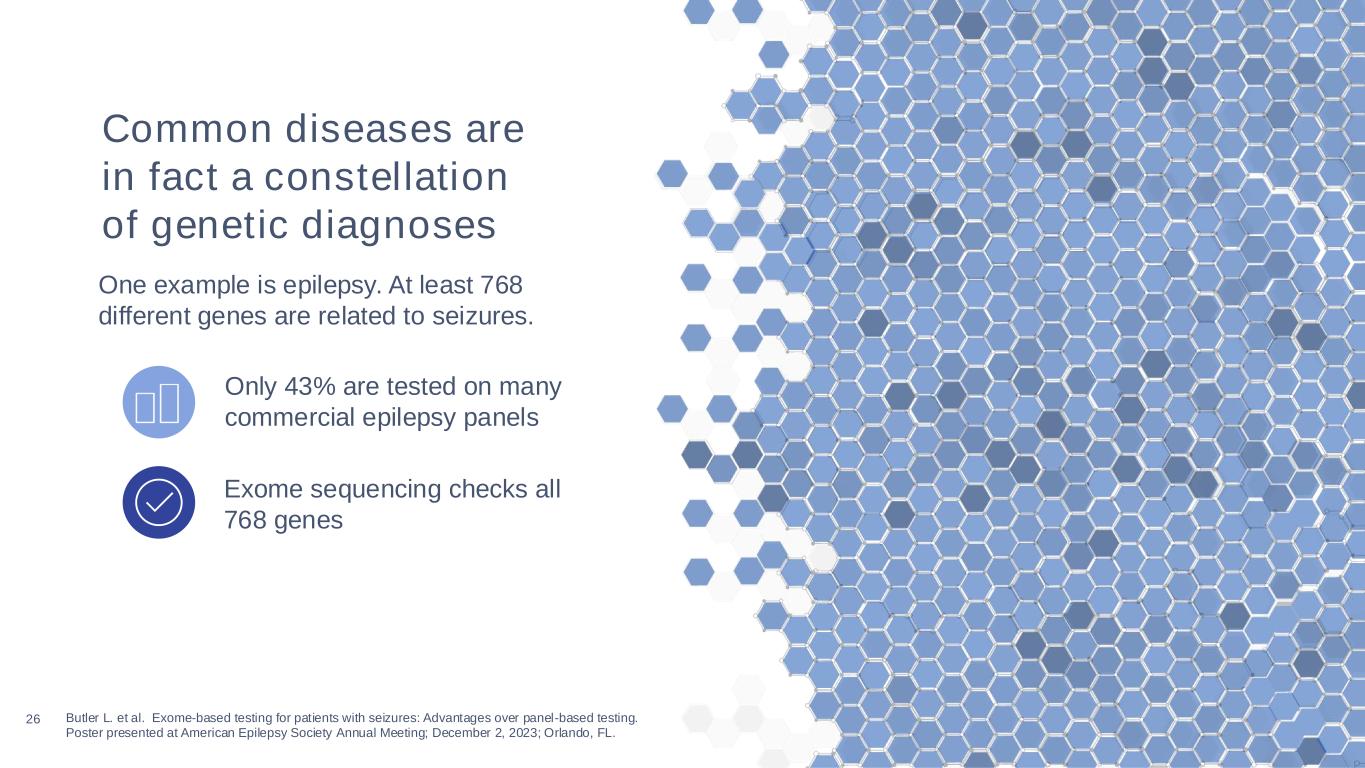
26 Butler L. et al. Exome-based testing for patients with seizures: Advantages over panel-based testing. Poster presented at American Epilepsy Society Annual Meeting; December 2, 2023; Orlando, FL. Exome sequencing checks all 768 genes Only 43% are tested on many commercial epilepsy panels Common diseases are in fact a constellation of genetic diagnoses One example is epilepsy. At least 768 different genes are related to seizures.
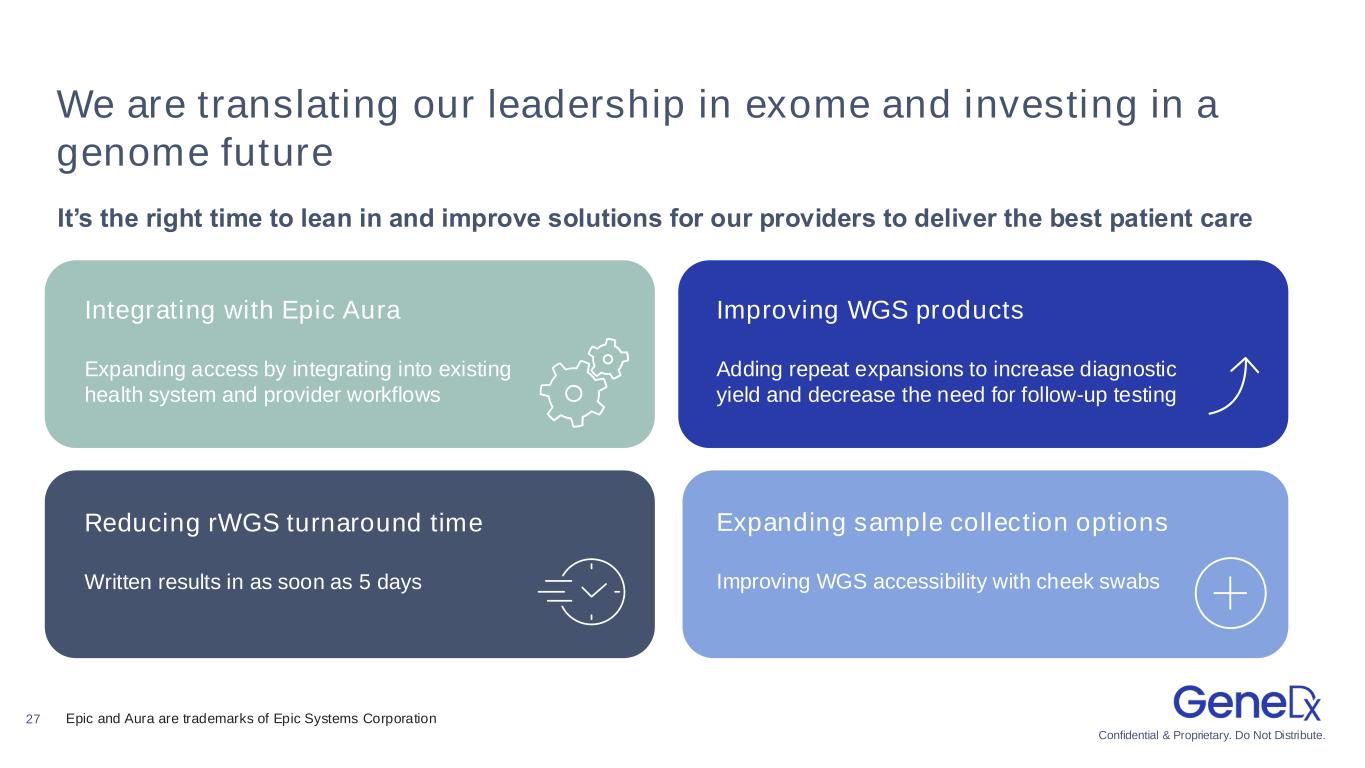
Confidential & Proprietary. Do Not Distribute. 27 We are translating our leadership in exome and investing in a genome future It’s the right time to lean in and improve solutions for our providers to deliver the best patient care Integrating with Epic Aura Expanding access by integrating into existing health system and provider workflows Improving WGS products Adding repeat expansions to increase diagnostic yield and decrease the need for follow-up testing Reducing rWGS turnaround time Written results in as soon as 5 days Expanding sample collection options Improving WGS accessibility with cheek swabs Epic and Aura are trademarks of Epic Systems Corporation
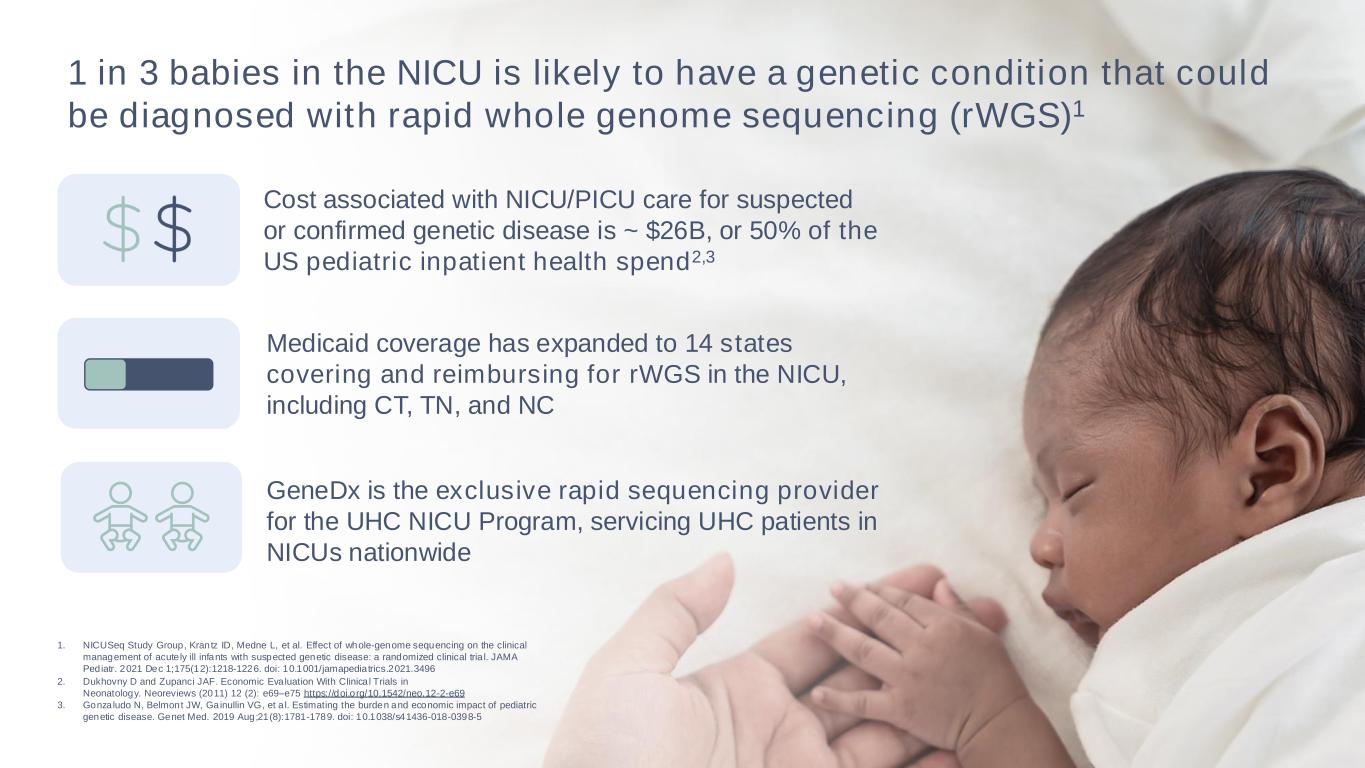
28 1 in 3 babies in the NICU is likely to have a genetic condition that could be diagnosed with rapid whole genome sequencing (rWGS)1 Cost associated with NICU/PICU care for suspected or confirmed genetic disease is ~ $26B, or 50% of the US pediatric inpatient health spend2,3 Medicaid coverage has expanded to 14 states covering and reimbursing for rWGS in the NICU, including CT, TN, and NC 1. NICUSeq Study Group, Krantz ID, Medne L, et al. Effect of whole-genome sequencing on the clinical management of acute ly ill infants with suspected genetic disease: a randomized clinical tria l. JAMA Pediatr. 2021 Dec 1;175(12):1218-1226. doi: 10.1001/jamapediatrics.2021.3496 2. Dukhovny D and Zupanci JAF. Economic Evaluation With Clinica l Trials in Neonatology. Neoreviews (2011) 12 (2): e69–e75 https://doi.org/10.1542/neo.12-2-e69 3. Gonzaludo N, Belmont JW, Gainullin VG, et a l. Estimating the burden and economic impact of pediatric genetic disease. Genet Med. 2019 Aug;21(8):1781-1789. doi: 10.1038/s41436-018-0398-5 GeneDx is the exclusive rapid sequencing provider for the UHC NICU Program, servicing UHC patients in NICUs nationwide

Today, we shorten the diagnostic journey. Tomorrow, we hope to prevent it.

30 Building the future: The SeqFirst Study Shorter hospital stays. Less uncertainty. Better care. 63% of infants had abnormal rapid WGS results, and 88% of these cases resulted in a change in management In phase one of the SeqFirst study, 125 infants were offered rapid WGS: 90% of diagnoses made by WGS would not have been predicted by clinical features Families of enrolled infants reported an overall positive experience, regardless of rapid WGS test outcome
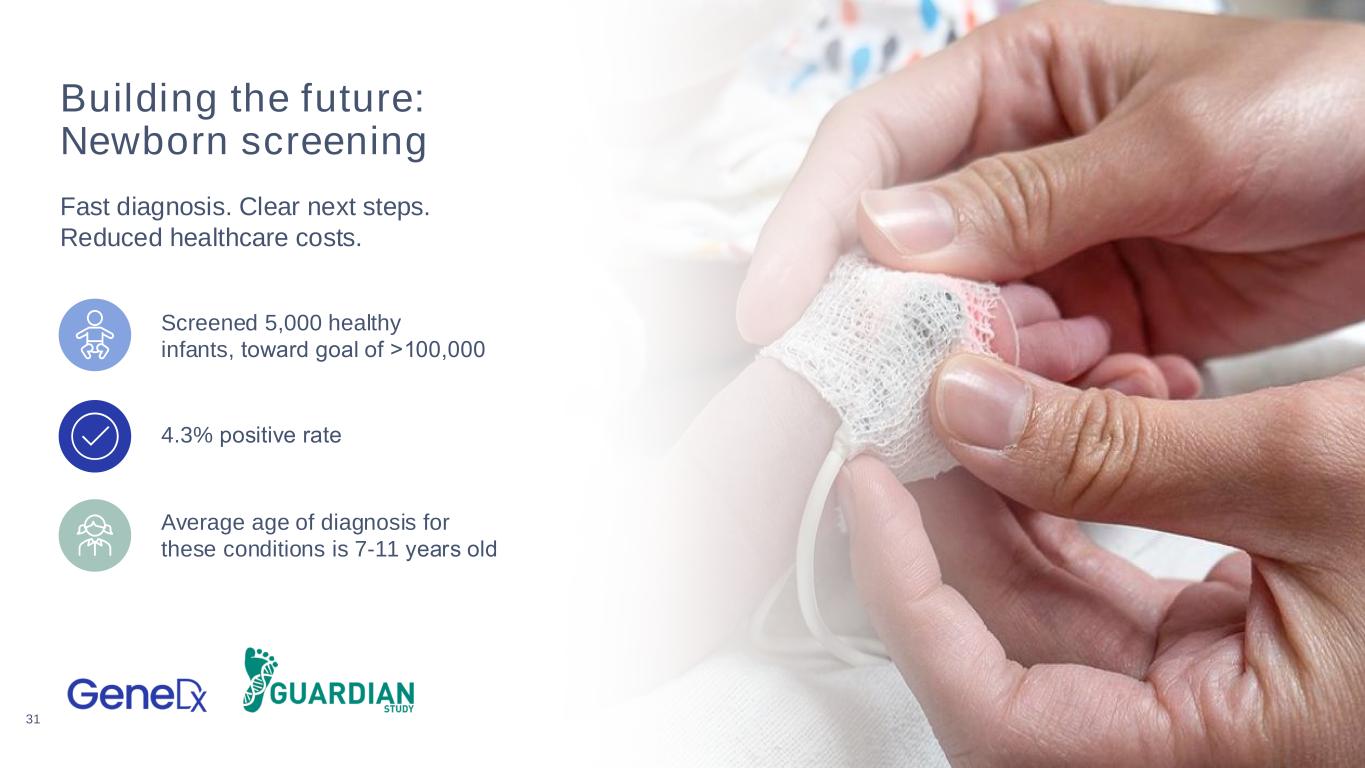
31 Building the future: Newborn screening Fast diagnosis. Clear next steps. Reduced healthcare costs. Screened 5,000 healthy infants, toward goal of >100,000 4.3% positive rate Average age of diagnosis for these conditions is 7-11 years old

32 Building the future: Partnerships Enriched data. Empowered drug discovery. mproved outcomes. GeneDx offers solutions across the pharma drug development pipeline Find Connect Understand

33 One test. Big picture. Brighter futures.
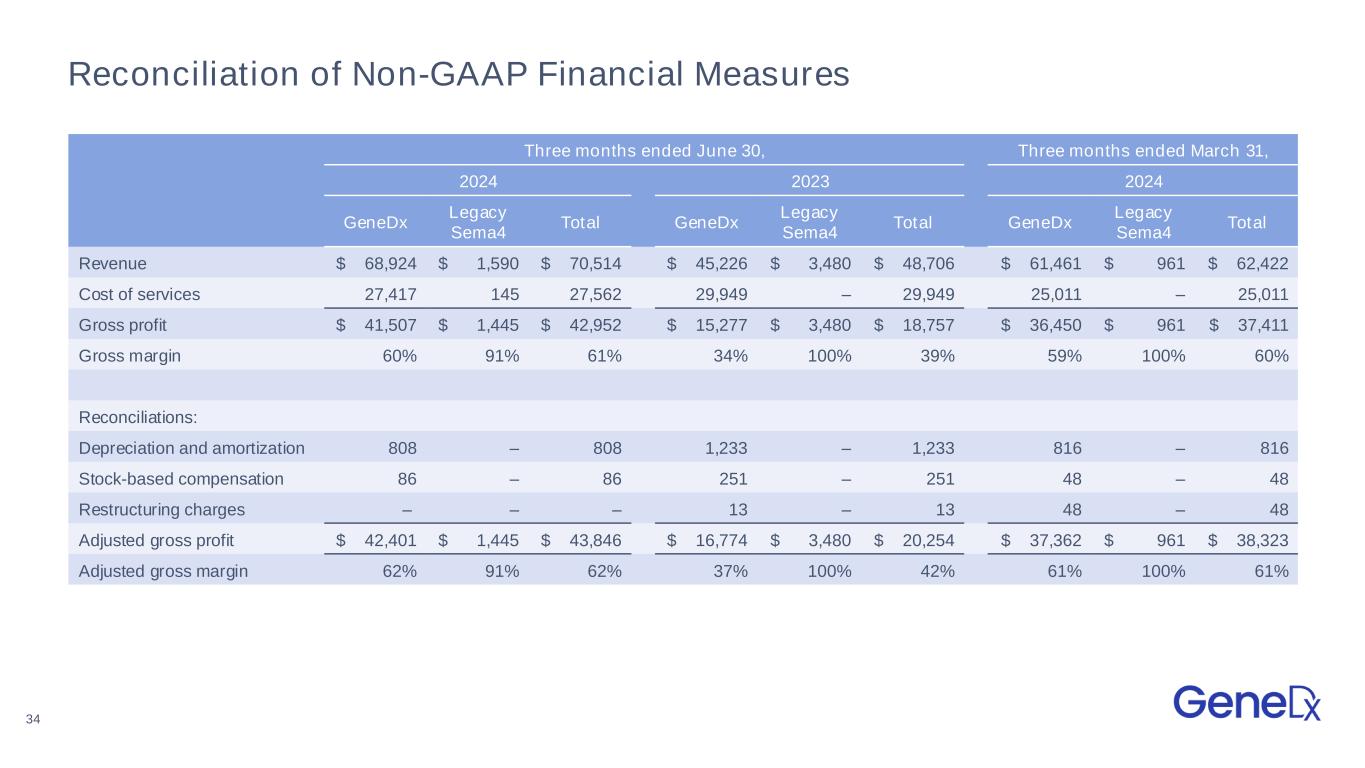
34 Reconciliation of Non-GAAP Financial Measures Three months ended June 30, Three months ended March 31, 2024 2023 2024 GeneDx Legacy Sema4 Total GeneDx Legacy Sema4 Total GeneDx Legacy Sema4 Total Revenue $ 68,924 $ 1,590 $ 70,514 $ 45,226 $ 3,480 $ 48,706 $ 61,461 $ 961 $ 62,422 Cost of services 27,417 145 27,562 29,949 – 29,949 25,011 – 25,011 Gross profit $ 41,507 $ 1,445 $ 42,952 $ 15,277 $ 3,480 $ 18,757 $ 36,450 $ 961 $ 37,411 Gross margin 60% 91% 61% 34% 100% 39% 59% 100% 60% Reconciliations: Depreciation and amortization 808 – 808 1,233 – 1,233 816 – 816 Stock-based compensation 86 – 86 251 – 251 48 – 48 Restructuring charges – – – 13 – 13 48 – 48 Adjusted gross profit $ 42,401 $ 1,445 $ 43,846 $ 16,774 $ 3,480 $ 20,254 $ 37,362 $ 961 $ 38,323 Adjusted gross margin 62% 91% 62% 37% 100% 42% 61% 100% 61%
v3.24.2
Document and Entity Information Document
|
Jul. 30, 2024 |
| Entity Information [Line Items] |
|
| Document Type |
8-K
|
| Document Period End Date |
Jul. 30, 2024
|
| Entity Registrant Name |
GeneDx Holdings Corp.
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity File Number |
001-39482
|
| Entity Tax Identification Number |
85-1966622
|
| Entity Address, Address Line One |
333 Ludlow Street
|
| Entity Address, City or Town |
Stamford
|
| Entity Address, State or Province |
CT
|
| Entity Address, Postal Zip Code |
06902
|
| City Area Code |
888
|
| Local Phone Number |
729-1206
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity Emerging Growth Company |
true
|
| Entity Ex Transition Period |
false
|
| Entity Address, Address Line Two |
North Tower
|
| Entity Address, Address Line Three |
6th Floor
|
| Entity Central Index Key |
0001818331
|
| Amendment Flag |
false
|
| Common Class A |
|
| Entity Information [Line Items] |
|
| Title of 12(b) Security |
Class A common stock, par value $0.0001 per share
|
| Trading Symbol |
WGS
|
| Security Exchange Name |
NASDAQ
|
| Warrant |
|
| Entity Information [Line Items] |
|
| Title of 12(b) Security |
Warrants to purchase one share of Class A common stock, each at an exercise price of $379.50 per share
|
| Trading Symbol |
WGSWW
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 3 such as an Office Park
| Name: |
dei_EntityAddressAddressLine3 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassAMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_WarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
GeneDx (NASDAQ:WGS)
Gráfica de Acción Histórica
De Jun 2024 a Jul 2024

GeneDx (NASDAQ:WGS)
Gráfica de Acción Histórica
De Jul 2023 a Jul 2024
