UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington,
D.C. 20549
Form 6-K
REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13a-16 OR 15d-16 UNDER THE SECURITIES EXCHANGE
ACT OF 1934
Date of Report: August 1, 2024
Commission File Number: 001-40377
Valneva SE
(Translation of registrant's name
into English)
6 rue Alain Bombard
44800 Saint-Herblain, France
(Address of
principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F
[ X ] Form 40-F [ ]
On August 1, 2024, the Registrant issued a press release and a presentation, copies of which
are attached hereto as Exhibits 99.1 and 99.2, respectively, and are incorporated herein by reference. The information contained in this
Form 6-K, including Exhibits 99.1 and 99.2, are hereby incorporated by reference into the Registrant’s Registration Statement on
Form F-3 (File No. 333-266839).
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its
behalf by the undersigned, thereunto duly authorized.
| |
|
Valneva SE |
| |
|
(Registrant) |
| |
|
|
| |
|
|
| Date: August 1, 2024 |
|
/s/ Thomas Lingelbach |
| |
|
Thomas Lingelbach |
| |
|
Chief Executive Officer and President |
| |
|
|
EXHIBIT 99.1
Valneva and LimmaTech Enter into a Strategic Partnership to Accelerate the Development of the World’s Most Clinically Advanced Tetravalent Shigella Vaccine Candidate
- Valneva obtains exclusive worldwide license for LimmaTech’s S4V Shigella vaccine candidate and adds an attractive Phase 2 clinical asset to Valneva’s R&D pipeline
- LimmaTech to receive upfront payment, is eligible for future milestone and royalty payments, and will collaborate on S4V clinical development through Phase 2
- Valneva will host a live webcast on this announcement at 3 p.m. CEST/9 a.m. EDT today. Please refer to this link: https://edge.media-server.com/mmc/p/ck932u2n
Saint-Herblain (France) and Schlieren (Zurich), August 1, 2024 – Valneva SE (Nasdaq: VALN; Euronext Paris: VLA), a specialty vaccine company and LimmaTech Biologics AG, a clinical-stage biotech company developing vaccines for the prevention of life-threatening diseases, today announced that the companies have entered into a strategic partnership and exclusive licensing agreement for the development, manufacturing and commercialization of Shigella4V (S4V), a tetravalent bioconjugate vaccine candidate against shigellosis.
Shigellosis, caused by Shigella bacteria, is the second leading cause of fatal diarrheal disease worldwide. It is estimated that up to 165 million cases of disease and an estimated 600,000 deaths are attributed to Shigella each year1, particularly among children in Low- and Middle-Income Countries (LMICs). No approved Shigella vaccine is currently available and the development of Shigella vaccines has been identified as a priority by the World Health Organization (WHO)2. Shigellosis also affects international travelers from high-income countries and deployed military personnel in endemic regions. The global market for a vaccine against Shigella is estimated to exceed $500 million annually3.
Under the terms of the agreement with Valneva, LimmaTech will receive an upfront payment of €10 million and be eligible to receive additional regulatory, development and sales-based milestone payments as well as low double-digit royalties on sales. LimmaTech will be responsible for conducting a Phase 2 Controlled Human Infection Model (CHIM) and a Phase 2 pediatric study in LMICs. Both clinical trials are expected to begin in the second half of 2024. Valneva will assume all further development, including CMC (chemistry, manufacturing and controls) and regulatory activities, and be responsible for its commercialization worldwide if approved.
Thomas Lingelbach, Chief Executive Officer of Valneva, commented, “We are very pleased to partner with LimmaTech to advance a promising program in an area of high unmet medical need. The Shigella vaccine candidate enables a potential first-in-class vaccine solution for both LMICs and travelers and, as such, represents a potentially highly synergistic product for Valneva. The anticipated development path follows a staggered and risk-mitigated strategy, and hence allows an efficient capital allocation in line with our communicated plan of having a new R&D program in Phase 3 by 2027.”
Dr. Franz-Werner Haas, Chief Executive Officer of LimmaTech, said, “Having developed the S4V Shigella vaccine candidate from its early discovery phase to the promising clinical data we achieved to date, we are excited to accelerate the program with our partnership with Valneva. Their proven expertise in late-stage development and commercialization of vaccines will expedite potential market approval and bring a Shigella vaccine to people in need. This agreement underscores our capabilities to leverage LimmaTech’s proficiency in vaccine development with the best path to develop programs rapidly. We continue to expand our pipeline of vaccine candidates to combat microbial-based infectious diseases, providing protection against antimicrobial resistance, a dramatically increasing global health threat.”
LimmaTech initiated the tetravalent Shigella vaccine candidate and continued to lead its development as part of its ongoing collaboration with GSK, and later in-licensed the vaccine candidate from GSK. In February 2024, LimmaTech reported positive interim Phase 1/2 data for the S4V vaccine candidate, including a favorable safety and tolerability profile as well as robust data on immunogenicity against the four most common pathogenic Shigella serotypes, S. flexneri 2a, 3a, 6, and S. sonnei4. The results of the completed Phase 1/2 study confirmed the interim data.
About Shigellosis
Shigellosis is a global health threat caused by the Gram-negative Shigella bacteria. It is estimated that up to 165 million infections5 are due to Shigella of which 62.3 million occur in children younger than five years. Diarrheal infection is one of the major causes of morbidity and mortality in numerous countries as well as in travelers and deployed military personnel in endemic regions. There are an estimated 600,000 deaths attributed to Shigella each year and it is the second leading cause for diarrheal deaths6. The standard treatment for shigellosis is oral rehydration and antibiotic therapy, however, the bacteria have acquired resistance to many antibiotics with numerous reports of outbreaks of multidrug-resistant strains, making treatment extremely difficult. Currently, no licensed Shigella vaccine is available.
About Valneva SE
We are a specialty vaccine company that develops, manufactures, and commercializes prophylactic vaccines for infectious diseases addressing unmet medical needs. We take a highly specialized and targeted approach, applying our deep expertise across multiple vaccine modalities, focused on providing either first-, best- or only-in-class vaccine solutions.
We have a strong track record, having advanced multiple vaccines from early R&D to approvals, and currently market three proprietary travel vaccines, including the world’s first and only chikungunya vaccine, as well as certain third-party vaccines.
Revenues from our growing commercial business help fuel the continued advancement of our vaccine pipeline. This includes the only Lyme disease vaccine candidate in advanced clinical development, which is partnered with Pfizer, as well as vaccine candidates against the Zika virus and other global public health threats. More information is available at www.valneva.com.
About LimmaTech Biologics AG
LimmaTech Biologics is at the forefront of combating the global antimicrobial resistance epidemic based on its unparalleled track record in vaccine technology and clinical candidate development. The company is leveraging its proprietary self-adjuvanting and multi-antigen vaccine platform alongside additional disease-specific vaccine approaches to prevent increasingly untreatable microbial infections. With decades of expertise and an expanding, robust pipeline, the LimmaTech team is dedicated to generating protective solutions to deliver transformative value worldwide. LimmaTech Biologics is backed by specialist healthcare investors, including Adjuvant Capital, AXA IM Alts, Novo Holdings REPAIR Impact Fund, and Tenmile.
For more information, please visit www.lmtbio.com.
Contacts
For Valneva
Valneva Investor and Media Contacts
Laetitia Bachelot-Fontaine
VP Global Communications & European Investor Relations
M +33 (0)6 4516 7099
laetitia.bachelot-fontaine@valneva.com |
Joshua Drumm, Ph.D.
VP Global Investor Relations
M +001 917 815 4520
joshua.drumm@valneva.com |
For LimmaTech
LimmaTech Biologics AG
Franz-Werner Haas, CEO
E-mail: media@lmtbio.com
For media enquiries
Trophic Communications
Sara Ortiz or Jacob Verghese
Phone: +49 151 7441 6179
E-mail: limmatech@trophic.eu
Forward-Looking Statements
This press release contains certain forward-looking statements relating to the business of Valneva, including with respect to business partnerships, the progress, timing, results and completion of research, development and clinical trials for product candidates, to regulatory approval of product candidates and review of existing products. In addition, even if the actual results or development of Valneva are consistent with the forward-looking statements contained in this press release, those results or developments of Valneva may not be sustained in the future. In some cases, you can identify forward-looking statements by words such as “could,” “should,” “may,” “expects,” “anticipates,” “believes,” “intends,” “estimates,” “aims,” “targets,” or similar words. These forward-looking statements are based largely on the current expectations of Valneva as of the date of this press release and are subject to a number of known and unknown risks and uncertainties and other factors that may cause actual results, performance or achievements to be materially different from any future results, performance or achievement expressed or implied by these forward-looking statements. In particular, the expectations of Valneva could be affected by, among other things, uncertainties and delays involved in the development and manufacture of vaccines, unexpected clinical trial results, unexpected regulatory actions or delays, competition in general, currency fluctuations, the impact of the global and European financing environment, and the ability to obtain or maintain patent or other proprietary intellectual property protection. Success in preclinical studies or earlier clinical trials may not be indicative of results in future clinical trials. In light of these risks and uncertainties, there can be no assurance that the forward-looking statements made in this press release will in fact be realized. Valneva is providing this information as of the date of this press release and disclaims any intention or obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise.
1 Shigellosis | CDC Yellow Book 2024
2 Immunization, Vaccines and Biologicals (who.int)
3 Valneva’s Initial internal assessment
4 20240221_LimmaTech_Shigella-Interim-Data-PR_Final.pdf (lmtbio.com)
5Shigellosis | CDC Yellow Book 2024
6 Shigellosis | CDC Yellow Book 2024
Exhibit 99.2

Valneva and LimmaTech Enter into a Strategic Partnership to Accelerate the Development of the World’s Most Clinically Advanced Tetravalent Shigella Vaccine Candidate (“S4V”) August 01, 2024

Disclaimer This presentation does not contain or constitute an offer of, or the solicitation of an offer to buy or subscribe for, Valneva SE shares to any person in the USA or in any jurisdiction to whom or in which such offer or solicitation is unlawful . Valneva is a European company . Information distributed is subject to European disclosure requirements that are different from those of the United States . Financial statements and information may be prepared according to accounting standards which may not be comparable to those used generally by companies in the United States . This presentation includes only summary information provided as of the date of this presentation only and does not purport to be comprehensive . Any information in this presentation is purely indicative and subject to modification at any time without notice . Valneva does not warrant the completeness, accuracy or correctness of the information or opinions contained in this presentation . None of Valneva, or any of its affiliates, directors, officers, advisors and employees is under any obligation to update such information or shall bear any liability for any loss arising from any use of this presentation . The information has not been subject to independent verification and is qualified in its entirety by the business, financial and other information that Valneva is required to publish in accordance with the rules, regulations and practices applicable to companies listed on Euronext Paris and the NASDAQ Global Select Market, including in particular the risk factors described in Valneva’s universal registration document filed with the French Financial Markets Authority ( Autorité des Marchés Financiers , or AMF) on March 22 , 2024 ( document d’enregistrement universel 2023 ) under number D . 24 - 0157 (the “ 2023 URD”), and in the Form 20 - F filed with the U . S . Securities and Exchange Commission (SEC) on March 22 , 2024 , as well as the information in any other periodic report and in any other press release, which are available free of charge on the websites of Valneva (www . valneva . com) and/or the AMF (www . amf - france . org) and SEC ( www . sec . gov ) . Certain information and statements included in this presentation are not historical facts but are forward - looking statements, including with respect to business partnerships, the progress, timing, results and completion of research, development and clinical trials for product candidates, to regulatory approval of product candidates and review of existing products . The forward - looking statements (a) are based on current beliefs, expectations and assumptions, including, without limitation, assumptions regarding present and future business strategies and the environment in which Valneva operates, and involve known and unknown risk, uncertainties and other factors, which may cause actual results, performance or achievements to be materially different from those expressed or implied by these forward - looking statements, (b) speak only as of the date this presentation is released, and (c) are for illustrative purposes only . Investors are cautioned that forward - looking information and statements are not guarantees of future performances and are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Valneva This presentation presents information about investigational vaccine candidates that have not been approved for use and have not been determined by any regulatory authority to be safe or effective . Management uses and presents IFRS results, as well as the non - IFRS measure of Adjusted EBITDA to evaluate and communicate its performance . While non - IFRS measures should not be construed as alternatives to IFRS measures, management believes non - IFRS measures are useful to further understand Valneva's current performance, performance trends, and financial condition . Adjusted EBITDA is a supplemental measure of performance used by investors and financial analysts . Management believes this measure provides additional analytical tools . 2 Valneva / LimmaTech - Shigella Partnership
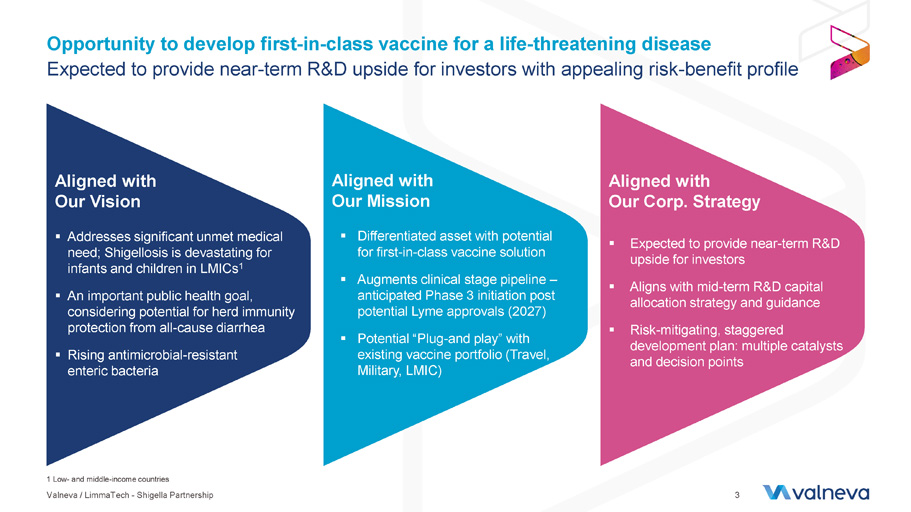
1 Low - and middle - income countries Opportunity to develop first - in - class vaccine for a life - threatening disease Expected to provide near - term R&D upside for investors with appealing risk - benefit profile 3 Aligned with Our Vision ▪ Addresses significant unmet medical need; Shigellosis is devastating for infants and children in LMICs 1 ▪ An important public health goal, considering potential for herd immunity protection from all - cause diarrhea ▪ Rising antimicrobial - resistant enteric bacteria Aligned with Our Mission ▪ Differentiated asset with potential for first - in - class vaccine solution ▪ Augments clinical stage pipeline – anticipated Phase 3 initiation post potential Lyme approvals (2027) ▪ Potential “Plug - and play” with existing vaccine portfolio (Travel, Military, LMIC) Aligned with Our Corp. Strategy ▪ Expected to provide near - term R&D upside for investors ▪ Aligns with mid - term R&D capital allocation strategy and guidance ▪ Risk - mitigating, staggered development plan: multiple catalysts and decision points Valneva / LimmaTech - Shigella Partnership
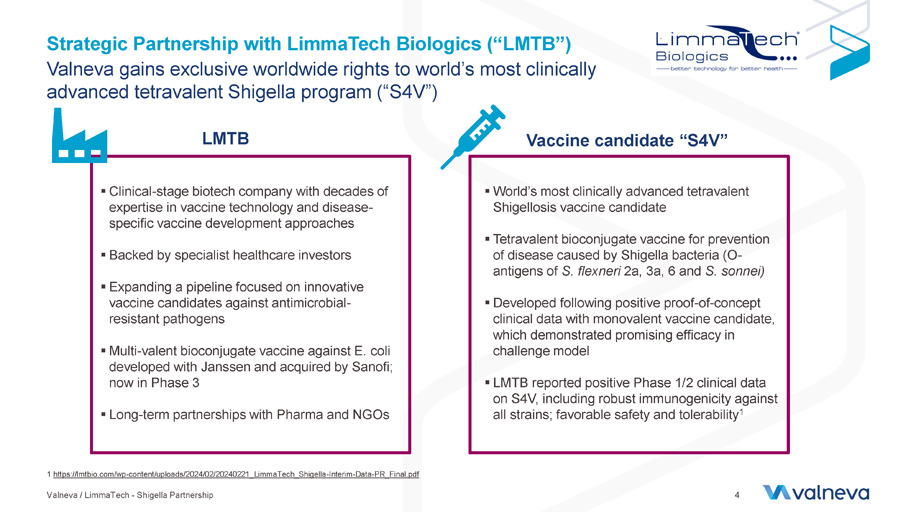
Strategic Partnership with LimmaTech Biologics (“LMTB”) Valneva / LimmaTech - Shigella Partnership 4 1 https://lmtbio.com/wp - content/uploads/2024/02/20240221_LimmaTech_Shigella - Interim - Data - PR_Final.pdf Valneva gains exclusive worldwide rights to world’s most clinically advanced tetravalent Shigella program (“S4V”) LMTB Vaccine candidate “S4V” ▪ World’s most clinically advanced tetravalent Shigellosis vaccine candidate ▪ Tetravalent bioconjugate vaccine for prevention of disease caused by Shigella bacteria ( O - antigens of S. flexneri 2a, 3a, 6 and S. sonnei ) ▪ Developed following positive proof - of - concept clinical data with monovalent vaccine candidate, which demonstrated promising efficacy in challenge model ▪ LMTB reported positive Phase 1/2 clinical data on S4V, including robust immunogenicity against all strains; favorable safety and tolerability 1 ▪ Clinical - stage biotech company with decades of expertise in vaccine technology and disease - specific vaccine development approaches ▪ Backed by specialist healthcare investors ▪ Expanding a pipeline focused on innovative vaccine candidates against antimicrobial - resistant pathogens ▪ Multi - valent bioconjugate vaccine against E. coli developed with Janssen and acquired by Sanofi; now in Phase 3 ▪ Long - term partnerships with Pharma and NGOs

Shigellosis: Significant Unmet Medical Need 1 No approved Shigella vaccine is currently available Valneva / LimmaTech - Shigella Partnership 5 ▪ Second - leading cause of fatal diarrheal disease worldwide ▪ Estimated to cause up to 165 million cases and 600,000 deaths each year, particularly among children in LMICs 2 ▪ Caused by species of Shigella bacteria 1 . Shigellosis | CDC Yellow Book 2024 ; 2. Low - and - middle - income countries ▪ Highly contagious; person - to - person (directly or by contaminated materials), food - and water - borne transmissions are common ▪ Illness typically begins 1 – 2 days after exposure with symptoms lasting 5 – 7 days. Symptoms include diarrhea, fever and stomach cramps between others. Long term consequences can develop in children (linear growth faltering, stunting) a nd adults (arthritis). ▪ Considering the potential for herd immunity and protection from all - cause diarrhea, the development of a Shigella vaccine is an important goal for public health - priority for the World Health Organization (WHO) ▪ Shigella is a rising antimicrobial - resistant (AMR) enteric bacteria – hence a vaccine may indirectly impact the emergence of AMR
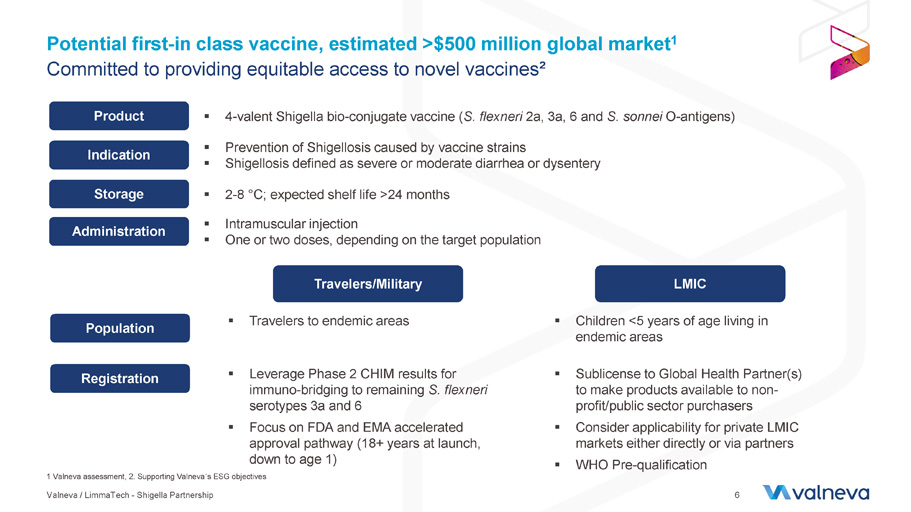
Potential first - in class vaccine, estimated >$500 million global market 1 Committed to providing equitable access to novel vaccines² Product ▪ 4 - valent Shigella bio - conjugate vaccine ( S. flexneri 2a, 3a, 6 and S. sonnei O - antigens) Indication ▪ Prevention of Shigellosis caused by vacci ne strains ▪ Shigellosis defi ned as severe or moderate diarrhea or dysentery Storage ▪ 2 - 8 ° C; expected shelf life >24 months Travelers/Military LMIC Population ▪ Travelers to endemic areas Registration Administration ▪ Intramuscular injection ▪ One or two doses, dependin g on the target population ▪ Leverage Phase 2 CHIM results for immuno - bridging to remaining S. flexneri serotypes 3a and 6 ▪ F ocus on FDA and EMA accelerated approval pathway (18+ years at launch, down to age 1) ▪ Children <5 years of age living in endemic areas ▪ Sublicense to Global Health Partner(s) to make products available to non - profit/public sector purchasers ▪ Consider applicability for private LMIC markets either directly or via partners ▪ WHO Pre - qualification Valneva / LimmaTech - Shigella Partnership 6 1 Valneva assessment, 2. Supporting Valneva ´ s ESG objectives
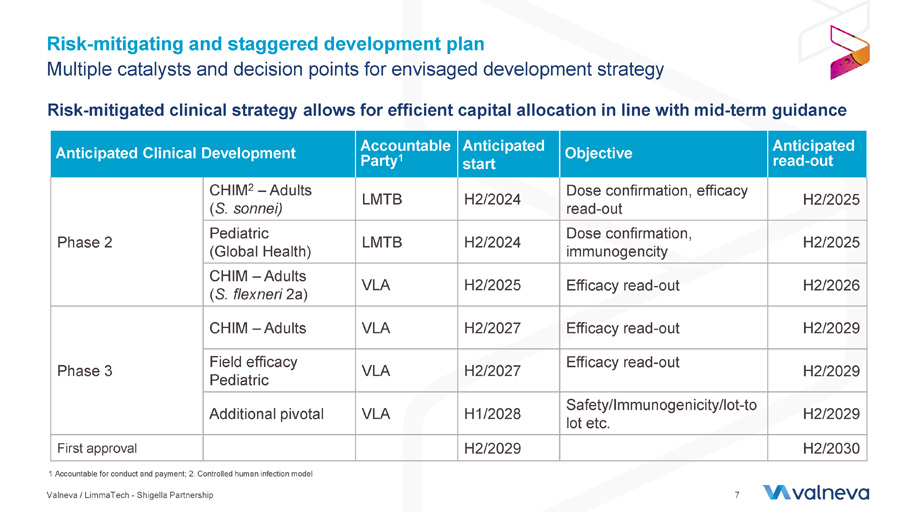
Risk - mitigating and staggered development plan Risk - mitigated clinical strategy allows for efficient capital allocation in line with mid - term guidance 1 Accountable for conduct and payment; 2. Controlled human infection model Valneva / LimmaTech - Shigella Partnership 7 Anticipated read - out Objective Anticipated start Accountable Party 1 Anticipated Clinical Development H2/2025 Dose confirmation, efficacy read - out H2/2024 LMTB CHIM 2 – Adults ( S. sonnei ) Phase 2 H2/2025 Dose confirmation, immunogencity H2/2024 LMTB Pediatric (Global Health) H2/2026 Efficacy read - out H2/2025 VLA CHIM – Adults ( S. flexneri 2a) H2/2029 Efficacy read - out H2/2027 VLA CHIM – Adults Phase 3 H2/2029 Efficacy read - out H2/2027 VLA Field efficacy Pediatric H2/2029 Safety/Immunogenicity/lot - to lot etc. H1/2028 VLA Additional pivotal H2/2030 H2/2029 First approval Multiple catalysts and decision points for envisaged development strategy

Strategic Partnership with LMTB on Shigella candidate S4V Key terms ▪ VLA receives global exclusive license to develop, manufacture and commercialize “S4V ” (4 valent (flexneri 2a, 3a, 6 and sonnei O - antigens) bio - conjugate vaccine for the prevention of a disease caused by Shigella) ▪ LMTB to receive upfront, is eligible for future milestone and royalty payments €10 million upfront payment Future development, regulatory and sales - based milestone payments totaling up to €40 million Low double - digit royalty on net sales in the travel segment Additional payments and single - digit royalties based on commercialization in LMICs ▪ Parties to collaborate through Phase 2 LMTB to conduct first Phase 2 “human challenge” study (CHIM trial ( S. sonnei )) and pediatric immunogenicity study in LMICs Valneva to initiate second Phase 2 “human challenge” study (CHIM trial)( S. flexneri 2a) LMTB to conduct technology transfer and transfer of IND 1 to Valneva once all Phase 2 studies are fully enrolled ▪ Valneva to lead and manage all future development activities 1 Investigational New Drug Application Valneva / LimmaTech - Shigella Partnership 8
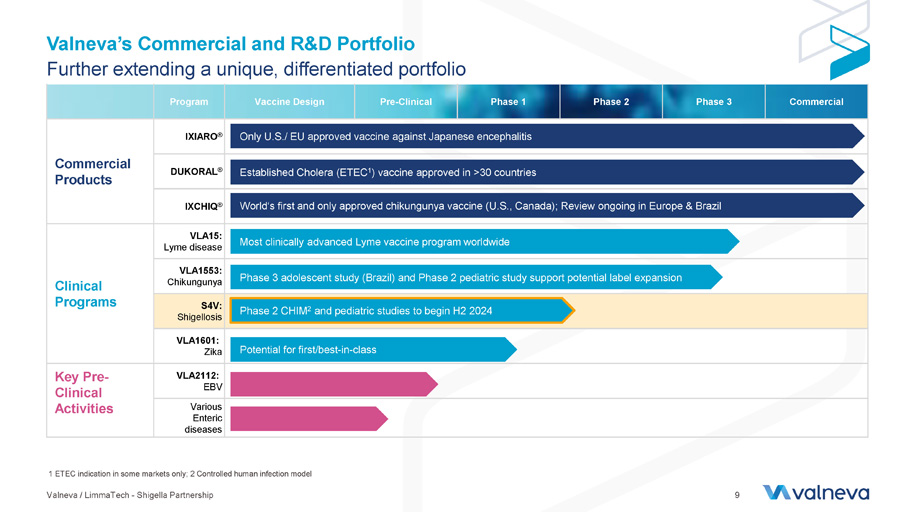
Commercial Phase 3 Phase 2 Phase 1 Pre - Clinical Vaccine Design Program IXIARO ® Commercial Products DUKORAL ® IXCHIQ ® VLA15: Lyme disease Clinical Programs VLA1553: Chikungunya S4V: Shigellosis VLA1601: Zika VLA2112: EBV Key Pre - Clinical Activities Various Enteric diseases Valneva’s Commercial and R&D Portfolio Further extending a unique, differentiated portfolio Only U.S./ EU approved vaccine against Japanese encephalitis Most clinically advanced Lyme vaccine program worldwide Potential for first/best - in - class Established Cholera (ETEC 1 ) vaccine approved in >30 countries World‘s first and only approved chikungunya vaccine (U.S., Canada); Review ongoing in Europe & Brazil Phase 3 adolescent study (Brazil) and Phase 2 pediatric study support potential label expansion 1 ETEC indication in some markets only; 2 Controlled human infection model Phase 2 CHIM 2 and pediatric studies to begin H2 2024 Valneva / LimmaTech - Shigella Partnership 9

Thank you Merci Danke Tack
Valneva (PK) (USOTC:INRLF)
Gráfica de Acción Histórica
De Jul 2024 a Ago 2024

Valneva (PK) (USOTC:INRLF)
Gráfica de Acción Histórica
De Ago 2023 a Ago 2024
