Filed Pursuant to Rule 424(b)(5)
Registration No. 333-264987
PROSPECTUS SUPPLEMENT
To Prospectus dated May 24, 2022
Up to $9,000,000

Common Stock
This prospectus supplement amends and restates our prospectus dated May 24, 2022.
We have previously entered into a sales agreement (the “Sales Agreement”) with Cowen and Company, LLC, TD Cowen, relating to shares of our common stock offered by this prospectus. In accordance with the terms of the sales agreement, we may offer and sell shares of our common stock having an aggregate offering price of up to $50.0 million from time to time through or to TD Cowen.
Pursuant to General Instruction I.B.6 of Form S-3, in no event will we sell our common stock registered on the registration statement of which this prospectus supplement forms a part in a public primary offering with a value exceeding more than one-third of our public float in any 12-month period so long as our public float remains below $75.0 million, as measured in accordance with General Instruction I.B.6 of Form S-3. We have not offered any securities pursuant to General Instruction I.B.6 of Form S-3 during the 12 calendar months prior to and including the date of this prospectus. As of October 4, 2023, we have sold 542,500 shares of our common stock for gross proceeds of approximately $4.3 million under the Sales Agreement, all of which shares were sold under General Instruction I.B.1 of Form S-3.
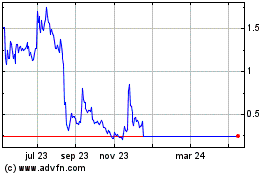
Our common stock is traded on The Nasdaq Global Market under the symbol “IMPL.” The last reported sales price of our common stock on The Nasdaq Global Market on October 4, 2023 was $0.411 per share.
Sales of our common stock, if any, under this prospectus may be made in sales deemed to be an “at the market offering” as defined in Rule 415(a)(4) promulgated under the Securities Act of 1933, as amended, or the Securities Act. TD Cowen is not required to sell any specific number or dollar amount of securities, but will act as the sales agent using commercially reasonable efforts consistent with their normal trading and sales practices, on mutually agreed terms between them and us. There is no arrangement for funds to be received in any escrow, trust or similar arrangement.
The compensation to TD Cowen for sales of common stock sold pursuant to the sales agreement will be equal to 3% of the aggregate gross proceeds of any shares of common stock sold under the Sales Agreement. In connection with the sale of the common stock on our behalf, TD Cowen will be deemed to be an “underwriter” within the meaning of the Securities Act and the compensation of TD Cowen will be deemed to be underwriting commissions or discounts. We have also agreed to provide indemnification and contribution to TD Cowen with respect to certain liabilities, including liabilities under the Securities Act or the Securities Exchange Act of 1934, as amended, or the Exchange Act.
Investing in our securities involves a high degree of risk. You should review carefully the risks and uncertainties referenced under the heading “Risk Factors” on page 6 of this prospectus as well as those contained in any accompanying prospectus and any related free writing prospectus or prospectus supplement we prepare or authorize in connection with this offering, and in the other documents that are incorporated by reference into this prospectus or the accompanying prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this Prospectus Supplement is October 5, 2023.
TABLE OF CONTENTS
PAGE
ABOUT THIS PROSPECTUS
WHERE YOU CAN FIND MORE INFORMATION; INCORPORATION BY REFERENCE
PROSPECTUS SUMMARY 3
THE OFFERING 7
RISK FACTORS 8
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS 38
USE OF PROCEEDS 39
DIVIDEND POLICY 40
DILUTION 41
DESCRIPTION OF CAPITAL STOCK 43
PLAN OF DISTRIBUTION 47
LEGAL MATTERS 48
EXPERTS 48
ABOUT THIS PROSPECTUS SUPPLEMENT
This prospectus supplement amends and restates the prospectus filed on May 24, 2022, which prospectus is part of a registration statement that we filed with the Securities and Exchange Commission, or the SEC, using a “shelf” registration process. Under this shelf registration process, we may from time to time sell shares of our common stock having an aggregate offering price of up to $200.0 million. Under this prospectus supplement, we may from time to time sell shares of our common stock having an aggregate offering price of up to $9.0 million, at prices and on terms to be determined by market conditions at the time of the offering. The $9.0 million of shares of our common stock that may be sold under this prospectus are included in the $200.0 million of shares of common stock that may be sold under the registration statement. Pursuant to General Instruction I.B.6, of Form S-3 in no event will we sell our common stock registered on the registration statement of which this prospectus supplement forms a part in a public primary offering with a value exceeding more than one-third of our public float in any 12-month period so long as our public float remains below $75.0 million, as measured in accordance with General Instruction I.B.6 of Form S-3.
This prospectus supplement describes the terms of this offering of common stock and also adds to and updates information contained in the documents incorporated by reference into this prospectus supplement. To the extent there is a conflict between the information contained in this prospectus supplement, on the one hand, and the information contained in any document incorporated by reference into this prospectus supplement that was filed with the SEC before the date of this prospectus supplement, on the other hand, you should rely on the information in this prospectus supplement. If any statement in one of these documents is inconsistent with a statement in another document having a later date—for example, a document incorporated by reference into this prospectus—the statement in the document having the later date modifies or supersedes the earlier statement.
We have not, and TD Cowen has not, authorized anyone to provide you with any information other than that contained or incorporated by reference in this prospectus supplement or any accompanying prospectus supplement or related free writing prospectus to which we have referred you. Neither we nor TD Cowen take any responsibility for, and can provide no assurance as to the reliability of, any other information others may give you. We are not, and TD Cowen is not, making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted or in which the person making that offer or solicitation is not qualified to do so or to anyone to whom it is unlawful to make an offer or solicitation. You should assume that the information appearing in this prospectus supplement, the documents incorporated by reference herein and any free writing prospectus that we have authorized for use in connection with this offering, is accurate only as of the date of those respective documents. Our business, financial condition, results of operations and prospects may have changed since those dates. You should read this prospectus supplement, the documents incorporated by reference herein and therein and any free writing prospectus that we have authorized for use in connection with this offering, in their entirety before making an investment decision.
When we refer to “Impel,” “Impel Pharmaceuticals” “we,” “our,” “us,” the “Registrant,” the “Company” and “our company” in this prospectus, we mean Impel Pharmaceuticals Inc., a Delaware corporation, unless otherwise specified.
The mark “Impel Pharmaceuticals Inc.”, the Impel Pharmaceuticals logo and all product candidate names are our common law trademarks. All other service marks, trademarks and tradenames appearing in this prospectus are the property of their respective owners. Solely for convenience, the trademarks and tradenames referred to in this prospectus appear without the ® and ™ symbols, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights, or the right of the applicable licensor to these trademarks and tradenames.
1
WHERE YOU CAN FIND MORE INFORMATION; INCORPORATION BY REFERENCE
Available Information
We file reports, proxy statements and other information with the SEC. The SEC maintains a web site that contains reports, proxy and information statements and other information about issuers, such as us, who file electronically with the SEC. The address of that website is www.sec.gov.
Our web site address is www.impelpharma.com. The information on our web site, however, is not, and should not be deemed to be, a part of this prospectus.
This prospectus is part of a registration statement that we filed with the SEC and does not contain all of the information in the registration statement. The full registration statement may be obtained from the SEC or us, as provided below. Documents establishing the terms of the offered securities are or may be filed as exhibits to the registration statement. Statements in this prospectus about these documents are summaries and each statement is qualified in all respects by reference to the document to which it refers. You should refer to the actual documents for a more complete description of the relevant matters. You may inspect a copy of the registration statement through the SEC’s website, as provided above, or at our principal executive offices, 201 Elliott Avenue West, Suite 260, Seattle, WA 98119, during normal business hours.
Incorporation by Reference
The SEC allows us to “incorporate by reference” information that we file with the SEC, which means that we can disclose important information to you by referring you to those other documents. The information incorporated by reference is an important part of this prospectus, and information we file later with the SEC will automatically update and supersede this information. A Current Report (or portion thereof) furnished, but not filed, on Form 8-K shall not be incorporated by reference into this prospectus. We incorporate by reference the documents listed below and any future filings we make with the SEC under Section 13(a), 13(c), 14, or 15(d) of the Exchange Act prior to the termination of any offering of securities made by this prospectus:
•our Quarterly Reports on Form 10-Q for the quarters ended March 31, 2023 and June 30, 2023, filed with the SEC on May 12, 2023 and August 18, 2023, respectively;
•the portions of our Definitive Proxy Statement on Schedule 14A (other than information furnished rather than filed) that are incorporated by reference into our Annual Report on Form 10-K, filed with the SEC on April 27, 2023;
•our Current Reports on Form 8-K filed on March 3, 2023, April 12, 2023, April 17, 2023, May 10, 2023, June 16, 2023, August 21, 2023, September 7, 2023 and October 4, 2023; and
•the description of our common stock contained in our registration statement on Form 8-A filed with the SEC on April 19, 2021 under Section 12 of the Exchange Act, including any amendment or report filed for the purpose of updating such description.
All reports and other documents we subsequently file pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act prior to the termination of this offering, including all such documents we may file with the SEC after the date of the initial registration statement and prior to the effectiveness of the registration statement, but excluding any information furnished to, rather than filed with, the SEC, will also be incorporated by reference in this prospectus and deemed to be part of this prospectus from the date of the filing of such reports and documents.
We will furnish without charge to you, on written or oral request, a copy of any or all of such documents that has been incorporated herein by reference (other than exhibits to such documents unless such exhibits are specifically incorporated by reference into the documents that this prospectus incorporates). Written or oral requests for copies should be directed to Impel Pharmaceuticals Inc., Attn: Investor Relations, 201 Elliott Avenue West, Suite 260, Seattle, WA 98119, and our telephone number is (206) 568-1466. See the section of
2
this prospectus entitled “Where You Can Find More Information” for information concerning how to obtain copies of materials that we file with the SEC.
Any statement contained in this prospectus, or in a document, all or a portion of which is incorporated by reference, shall be modified or superseded for purposes of this prospectus to the extent that a statement contained in this prospectus, any prospectus supplement or any document incorporated by reference modifies or supersedes such statement. Any such statement so modified or superseded shall not, except as so modified or superseded, constitute a part of this prospectus.
3
PROSPECTUS SUMMARY
Our Company
We are a commercial-stage biopharmaceutical company with a mission to develop transformative therapies for people suffering from diseases with high unmet medical needs, with an initial focus on diseases of the central nervous system, or CNS. Our company was founded on the premise that the upper nasal space can be an optimal treatment entry point for CNS and other diseases where rapid vascular absorption can result in superior clinical outcomes. Our strategy is to pair our proprietary Precision Olfactory Delivery, or POD, upper nasal delivery technology with well-established therapeutics or other therapeutics where rapid vascular absorption is preferred to drive therapeutic benefit, improve patient outcomes, reduce drug development risk and expand the commercial opportunity within our target diseases. In September of 2021, Trudhesa was approved by the U.S. Food and Drug Administration, or FDA, for the acute treatment of migraine headaches with or without aura in adult patients.
Recent Developments
Amended Credit Agreement
On September 5, 2023, we entered into the Second Amendment to Credit Agreement and Guaranty and Revenue Interest Financing Agreement, or the Second Amendment, with Oaktree Fund Administration, LLC, or Oaktree, as administrative agent, and the existing and new lenders party thereto, which we collectively refer to as the Secured Parties, which amends our (i) Credit Agreement and Guaranty dated March 17, 2022, as amended on August 21, 2023, and (ii) the Revenue Interest Financing Agreement dated March 17, 2022, as amended on August 21, 2023, or the RIF Agreement. Further, on October 2, 2023, we entered into the Third Amendment to Credit Agreement and Guaranty, which together with the Second Amendment we refer to as the Amended Credit Agreement. We refer to the RIF Agreement and the Credit Agreement and Guaranty as the Original Agreements. The Amended Credit Agreement provides for an aggregate principal loan amount to us by the existing and new lenders of approximately $121.5 million, including up to $20 million in additional cash proceeds to us from the making of additional term loans, the exchange of approximately $96.5 million of outstanding principal under the Original Agreements, and an in-kind forbearance fee of $5.0 million. Affiliates of KKR Iris Investors LLC, a greater than 10% holder of our common stock, are lenders of a portion of the new amount under the Credit Agreement.
Pursuant to the Amended Credit Agreement, as of October 4, 2023, we have drawn $10.0 million of tranche B term loans, including $2.5 million after September 30, 2023, and will have the right to draw up to $10.0 million more in additional tranche B term loans over the course of 2023, subject to our achievement of certain strategic transaction process milestones, satisfaction of minimum net revenue and product units sold covenants and satisfaction of certain other covenants and conditions specified in the Amended Credit Agreement.
Under the Amended Credit Agreement, the first lien tranche A provides for an aggregate original principal loan of $101.5 million, consisting of $51.4 million exchanged for existing tranche A-1 term loans, a $5.0 million forbearance fee, $9.1 million exchanged for existing tranche A-2 term loans, and $36.0 million exchanged for the right to future revenue interest payments under the RIF Agreement. In connection with the execution of the Amended Credit Agreement and the exchange of the future payments due under the RIF Agreement, the RIF Agreement was terminated.
Under the Amended Credit Agreement, the first lien tranche B provides for an aggregate original principal loan of $20.0 million. Further, tranche B investors received warrants to purchase shares of our common stock having an aggregate warrant coverage equal to an aggregate of approximately 19.99% of the outstanding shares and an exercise price of $0.01 per share. The warrants are issued to the tranche B lenders upon each borrowing of tranche B term loans in proportion to the amount of tranche B term loans funded.
Interest will be paid in kind, or PIK, on both the tranche A and tranche B term loans through the end of the forbearance period, which was extended to December 31, 2023 under the Amended Credit Agreement, and accrues at SOFR + 10.75%. The first lien tranche B is entitled to a 2x multiple on invested capital. The tranche A lenders and tranche B lenders will be entitled to be repaid a maximum aggregate amount of approximately $141.5 million (assuming the entire $20.0 million of tranche B loans are funded), plus PIK interest on the tranche A term loan.
The amounts outstanding under the Amended Credit Agreement are secured and collateralized by all of our assets. The Amended Credit Agreement also provides for certain modifications to the existing covenants, including additional reporting obligations, minimum net revenue and product units sold covenants and additional milestones. In addition, the Amended Credit Agreement includes customary events of default, the occurrence of which could result in termination of Oaktree’s commitments or the acceleration of our obligations under the Amended Credit Agreement.
Preliminary Financial and Corporate Information
After giving effect to the $2.5 million in draws under the Amended Credit Agreement after September 30, 2023, as described above, as of September 30, 2023, we had on a pro forma basis approximately $2.7 million in cash and cash equivalents, excluding amounts restricted under the Amended Credit Agreement. However, this estimate is preliminary and subject to the completion of our unaudited financial statements as of and for the three and nine months ended September 30, 2023. The actual amount that we report will be subject to the completion of our financial closing procedures and any final adjustments that may be made prior to the time our financial results for the quarter ended September 30, 2023, are finalized and filed with the SEC. Our independent registered public accounting firm has not audited, reviewed, compiled, or performed any procedures with respect to our cash, cash equivalents and marketable securities and, accordingly, does not express an opinion or any other form of assurance on it. This estimate should not be viewed as a substitute for financial statements prepared in accordance with accounting principles generally accepted in the United States and is not necessarily indicative of the results to be achieved in any future period. Accordingly, you should not draw any conclusions based on the foregoing estimate and should not place undue reliance on this preliminary estimate. We assume no duty to update this preliminary estimate except as required by law. Partially as a result of our ongoing liquidity issues as described herein, including increased attrition from our sales force, we currently expect our revenue for the three months ended September 30, 2023 to be lower than our revenue for the three months ended June 30, 2023.
Liquidity and Going Concern
There is substantial doubt about our ability to continue as a going concern. Due to the fact that we were unable to generate sufficient cash flows from operations, obtain funding to sustain operations, or reduce or stabilize expenses to the point where we could have realized a net positive cash flow, management and our board of directors determined that it was in the best interests of the stockholders to seek strategic alternatives. As part of this process, we plan to consider a wide range of options with a focus on maximizing shareholder value, including a potential sale of assets of the company, a sale of all of the company, a merger or other strategic transactions. We have engaged Moelis & Company LLC to act as our financial advisor in connection with the review.
There can be no assurance that this process will result in us pursuing any particular transaction or other strategic outcome. We currently expect to conduct this process over the remainder of 2023, with a goal of closing any such transactions no later than early 2024. However, if we are unable to complete a transaction, it will be necessary to seek additional financing or other alternatives for restructuring and resolving our liabilities, which may include the initiation of bankruptcy proceedings under Chapter 11 of the U.S. Bankruptcy Code in which case holders of our common stock will likely receive no recovery at all for the securities offered hereby. Even if we do complete a transaction, the proceeds may be insufficient to allow our stockholders to recognize any return on their investment.
Our ongoing liquidity issues also present significant challenges to current operations. We currently expect the financing availability under the Amended Credit Agreement to be sufficient to conduct the strategic review process, but we do not expect it to be sufficient to continue operations beyond this process without substantial additional investment. Our operations are also being impacted by the loss of sales representatives that we do not currently plan to replace, increased pressure from suppliers regarding payments from us that may delay or prevent our ability to obtain sufficient product from suppliers, and concern from prescribers regarding future product availability. Any of these issues can have a material impact on our sales and may further reduce our cash runway, accelerating the potential need to seek alternatives, including bankruptcy proceedings.
Nasdaq Compliance
As previously disclosed, on April 11, 2023, we received two written notifications from the Listing Qualifications Department of The Nasdaq Stock Market LLC, or Nasdaq, stating that we were no longer in compliance with certain minimum market value conditions to maintain continued listing as set forth in Nasdaq Marketplace Rule
5450(b)(2)(C). Under applicable Nasdaq rules, we have until October 9, 2023 to regain compliance by meeting the continued listing requirements, namely that the market value of our publicly held shares closes at $15,000,000 or more for a minimum of 10 consecutive business days and the market value of listed securities closes at $50,000,000 or more for a minimum of 10 consecutive business days. As of the date hereof, we do not expect to meet these criteria by such date, and we expect to receive a delisting determination from Nasdaq at such time. Upon receiving such determination, we intend to request a hearing to remain on the Nasdaq Stock Market, and we expect such request will suspend such delisting determination until a decision is issued by Nasdaq subsequent to the hearing, although we cannot predict whether Nasdaq will suspend a delisting determination or, if we are granted a hearing, what Nasdaq’s ultimate decision in this matter will be.
Additionally, on September 28, 2023, we received written notice from Nasdaq notifying us that we are not in compliance with the minimum bid price requirement set forth in Nasdaq Listing Rule 5550(a)(2) for continued listing. Nasdaq Listing Rule 5550(a)(2) requires listed securities to maintain a minimum bid price of $1.00 per share, and Listing Rule 5810(c)(3)(A) provides that a failure to meet the minimum bid price requirement exists if the deficiency continues for a period of 30 consecutive business days. Under applicable Nasdaq rules, we have a compliance period of 180 calendar days in which to regain compliance, expiring on March 26, 2024.
Company Information
We were incorporated under the laws of the State of Delaware in July 2008. Our principal executive offices are located at 201 Elliott Avenue West, Suite 260, Seattle, Washington 98119, and our telephone number is (206) 568-1466. Our website address is www.impelpharma.com. The information contained on, or that can be accessed through, our website is not part of, and is not incorporated by reference into, this prospectus. Investors should not rely on any such information in deciding whether to purchase our common stock.
THE OFFERING
|
|
Common stock offered by us |
Shares of our common stock having an aggregate offering price of up to $9.0 million. Pursuant to General Instruction I.B.6 of Form S-3, in no event will we sell our common stock registered on the registration statement of which this prospectus supplement forms a part in a public primary offering with a value exceeding more than one-third of our public float in any 12-month period so long as our public float remains below $75.0 million, as measured in accordance with General Instruction I.B.6 of Form S-3. |
Common stock to be outstanding immediately after this offering |
Up to 45,646,815 shares (as more fully described in the notes following this table), assuming sales of 21,897,810 shares of our common stock in this offering at an offering price of $0.411 per share, which was the last reported sale price of our common shares on The Nasdaq Global Market on October 4, 2023. The actual number of shares issued will vary depending on the sales price under this offering. |
Manner of Offering |
“At the market offering” that may be made from time to time through TD Cowen. See “Plan of Distribution.” |
Use of Proceeds |
We currently intend to use the net proceeds of this offering for general corporate purposes, which may include increasing our working capital, reducing indebtedness, ongoing commercialization activities and market development of Trudhesa, and capital expenditures. See “Use of Proceeds.” |
Risk Factors |
Investing in our common stock involves significant risks. See the disclosure under the heading “Risk Factors” in this prospectus and under similar headings in other documents incorporated by reference into this prospectus. |
The Nasdaq Global Market symbol |
IMPL |
The number of shares of our common stock to be outstanding after this offering is based on 23,749,005 shares of our common stock outstanding as of June 30, 2023, and excludes:
•4,629,027 shares of common stock issuable upon the exercise of options outstanding as of June 30, 2023, with a weighted-average exercise price of $6.82;
•20,000 shares of our common stock issuable upon the exercise of stock options granted after June 30, 2023, with a weighted-average exercise price of $1.31 per share;
•252,500 shares of our common stock issuable upon the vesting and settlement of restricted stock units, or RSUs, outstanding as of June 30, 2023;
•94,688 shares of our common stock issuable upon the exercise of warrants outstanding as of June 30, 2023, with a weighted-average exercise price of $9.51 per share;
•4,749,900 shares of our common stock issuable upon the exercise of warrants issued or issuable to our lenders pursuant to the Amended Credit Agreement after June 30, 2023 with an exercise price of $0.01 per share; and
•2,894,973 shares of common stock reserved for future issuance under our stock-based compensation plans as of June 30, 2023, consisting of (i) 2,150,350 shares of common stock reserved for future issuance under our 2021 Equity Incentive Plan as of June 30, 2023 and (ii) 744,623 shares of common stock reserved for future issuance under our 2021 Employee Stock Purchase Plan.
Except as otherwise indicated, all information in this prospectus does not assume or give effect to any exercise of outstanding options after June 30, 2023.
7
RISK FACTORS
Investment in any securities offered pursuant to this prospectus and the accompanying base prospectus involves risks. You should carefully consider the risk factors described below and in our most recent Annual Report on Form 10-K and any subsequent Quarterly Reports on Form 10-Q, which are incorporated by reference in this prospectus, any amendment or update thereto reflected in subsequent filings with the SEC, including in our Annual Reports on Form 10-K and Quarterly Reports on Form 10-Q, and all other information contained or incorporated by reference in this prospectus, as updated by our subsequent filings under the Exchange Act. The occurrence of any of these risks might cause you to lose all or part of your investment in the offered securities.
Summary Risk Factors
Our business is subject to numerous risks and uncertainties, including those highlighted in the section of this report captioned “Risk Factors.” The following is a summary of the principal risks we face:
•We are substantially restricted in our corporate activities by our existing debt facilities and are considering all strategic alternatives. If we do not maintain access to funding our debt facilities, we may be required to cease operations and seek relief under the U.S. Bankruptcy Code.
•There is substantial doubt about our ability to continue as a going concern, which may hinder our ability to obtain further financing.
•If we do not complete a strategic transaction, or do not receive sufficient financing from our debt facilities or from the offering of securities covered by this prospectus, we expect that we will likely file for bankruptcy protection.
•We are not currently in compliance with certain listing requirements of The Nasdaq Global Market. If our common stock is delisted, the market price and liquidity of our common stock and our ability to raise additional capital would be adversely impacted.
•If we fail to retain senior management and key scientific personnel, we may be unable to continue operations, meet sale milestones and complete our strategic review process.
•We are a commercial-stage biopharmaceutical company and have incurred net losses since our inception. We anticipate that we will continue to incur substantial operating losses for the foreseeable future and we may never achieve or sustain profitability.
•Statements in this report concerning our future plans and operations are dependent on our ability to secure adequate funding and the absence of unexpected delays or adverse developments. We may not be able to secure required funding.
•Our future commercial success depends upon attaining significant market acceptance of Trudhesa among physicians, patients, health care payors and others in the medical community necessary for commercial success.
•We rely entirely on third parties for the manufacturing of Trudhesa. If such third parties cease to provide services or require changes to our payment terms, or if we encounter difficulties in negotiating manufacturing and supply agreements with third-party manufacturers and suppliers of our POD device and the active ingredients in Trudhesa, our business and results of operation would be adversely impacted.
•If we are not able to obtain and enforce patent protection for our technologies, development and commercialization of our technology may be adversely affected.
•The market price of our common stock may be volatile, which could result in substantial losses for investors.
Risks Related to the Offering, Our Financial Position and Need for Additional Capital
We are substantially restricted in our corporate activities by our existing our debt facilities and are considering all strategic alternatives. If we do not maintain access to funding our debt facilities, we may be required to cease operations and seek relief under the U.S. Bankruptcy Code.
8
In September 2023 we entered into the Amended Credit Agreement with Oaktree and the Secured Parties. Prior to entry into such agreement, we were in breach of the covenant under our Original Agreements with Oaktree to maintain a minimum of $12.5 million in unrestricted cash and cash equivalents on hand. Pursuant to the Amended Credit Agreement, we have drawn an aggregate of $10.0 million as of the date of this prospectus supplement and will have the right to draw up to $10.0 million more in additional tranche B term loans over the course of 2023, subject to our achievement of certain strategic transaction process milestones, satisfaction of minimum net revenue and product units sold covenants and satisfaction of certain other covenants and conditions specified in the Amended Credit Agreement. We have recently failed to satisfy certain covenants while we have received waivers for such violations to date, if we fail to satisfy these covenants going forward, we may be unable to draw down remaining tranche B term loans. If we are unable to draw additional term loans on the schedule currently anticipated, raise additional capital, whether through this offering or otherwise, or complete a strategic transaction, we will need to cease operations and seek relief under Chapter 11 of the U.S. Bankruptcy Code.
There is substantial doubt about our ability to continue as a going concern, which may hinder our ability to obtain further financing.
Our consolidated financial statements are prepared using the generally accepted accounting principles applicable to a going concern, which contemplates the realization of assets and liquidation of liabilities in the normal course of business. However, as shown in our consolidated financial statements for the year ended December 31, 2022, included in our Annual Report on Form 10-K for the year ended December 31, 2022, and the condensed consolidated financial statements included in our most recent Quarterly Report on Form 10-Q, we have sustained substantial recurring losses from operations. In addition, we have used, rather than provided, cash in our continuing operations. After giving effect to the $2.5 million in draws under the Amended Credit Agreement after September 30, 2023, as described above, as of September 30, 2023, we had on a pro forma basis approximately $2.7 million in cash and cash equivalents, excluding amounts restricted under the Amended Credit Agreement. The above conditions raise substantial doubt about our ability to continue as a going concern within one year after the date that our financial statements are issued. Our consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts and classification of liabilities that might be necessary should we be unable to continue in existence.
Uncertainty concerning our ability to continue as a going concern, among other factors, may hinder our ability to obtain future financing. Continued operations and our ability to continue as a going concern are dependent, among other factors, on our ability to successfully commercialize Trudhesa and our ability to obtain additional required funding in the near term and thereafter. In addition the financing contemplated by this prospectus, we are exploring a wide range of options with a focus on maximizing shareholder value, including a potential sale of assets of the company, a sale of all of the company, a merger or other strategic transaction. Other potential strategic alternatives may include restructuring or refinancing our debt, seeking additional debt or equity capital, reducing or delaying our business activities and strategic, initiatives, mergers and acquisitions, licensing arrangements and partnerships, co-development agreements or a combination of these. We have engaged Moelis & Company LLC to act as our financial advisor in connection with the review of strategic alternatives, however, there can be no assurance that we will be able to complete additional or alternative financings, business development transactions or other strategic alternatives. If we cannot continue as a viable entity, we will likely be required to reduce or cease operations and seek relief under the U.S. Bankruptcy Code, and our stockholders would likely lose most or all of their investment in us.
Our ongoing liquidity issues also present significant challenges to current operations. We currently expect the financing availability under the Amended Credit Agreement to be sufficient to conduct the strategic review process, but we do not expect it to be sufficient to continue operations beyond this process without substantial additional investment. Our operations are also being impacted by the loss of sales representatives that we do not currently plan to replace, increased pressure from suppliers regarding payments from us that may delay or prevent our ability to obtain sufficient product from suppliers, and concern from prescribers regarding future product availability. Any of these issues can have a material impact on our sales and may further reduce our cash runway, accelerating the potential need to seek alternatives, including seeking relieve under the U.S. Bankruptcy Code.
If we do not complete a strategic transaction, or do not receive sufficient financing from our debt facilities or from the offering of securities covered by this prospectus or through any other financing, we expect that we will likely file for bankruptcy protection.
9
If we do not complete a strategic transaction or receive sufficient financing from our existing debt facilities or from the proceeds from the offering of securities covered by this prospectus or any other financing, we expect that we will file for bankruptcy protection. We believe that the present fair market value of our assets are less than the total amount required to pay our probable liabilities on our total existing debts and liabilities (including contingent liabilities) as they become absolute and matured. Subject to certain limitations in the sales agreement and compliance with applicable law, we have the discretion to deliver instruction to TD Cowen to use its commercially reasonable efforts to sell shares of our common stock at any time throughout the term of the sales agreement. The number of shares that are sold through TD Cowen after our instruction will fluctuate based on a number of factors, including the market price of our common stock during the sales period, the limits we set in any instruction to sell shares, and the demand for our common stock during the sales period. Because the price per share of each share sold will fluctuate during this offering, it is not currently possible to predict the gross proceeds to be raised in connection with those sales. In addition, the sales agreement requires that we meet certain conditions at each time of sale, including that our common stock shall remain listed on a national securities exchange. We may not satisfy the conditions in the sales agreement, and therefore we may not receive the potential proceeds from this offering. If we do not receive the proceeds from this offering, whether because we cannot satisfy the conditions in the sales agreement or otherwise, and we are unable to otherwise raise sufficient proceeds in the near term, then we expect that we will likely file for bankruptcy protection, in which case holders of our common stock will likely receive no recovery at all for the securities offered hereby.
We are not currently in compliance with certain listing requirements of The Nasdaq Global Market. If our common stock is delisted, the market price and liquidity of our common stock and our ability to raise additional capital would be adversely impacted.
Our common stock is currently listed on The Nasdaq Global Market (“Nasdaq”). Continued listing of a security on Nasdaq is conditioned upon compliance with various continued listing standards. On April 11, 2023, we received a letter from Nasdaq notifying us that the Company did not meet the $15 million minimum market value of publicly held shares required to maintain continued listing as set forth in Nasdaq Marketplace Rule 5450(b)(2)(C) (the “MVPHS Rule”) for the 30-business day period ended April 5, 2023, and, as a result, we no longer comply with the MVPHS Rule for continued listing on Nasdaq. Additionally, on April 11, 2023, we received a second notice from Nasdaq stating that, for the for the 30-business day period ended April 5, 2023, we had not met the $50 million minimum market value of listed securities required to maintain continued listing as set forth in Nasdaq Marketplace Rule 5450(b)(2)(A) (the “MVLS Rule” and together with the MVPHS Rule, the “Rules”).
As provided in the Nasdaq rules, we have 180 calendar days, or until October 9, 2023, to regain compliance. To regain compliance, the market value of our publicly held shares must be $15 million or more for a minimum of 10 consecutive business days and the market value of our listed securities must close at $50 million or more for a minimum of 10 consecutive business days at any time prior to October 9, 2023.
As of the date hereof, we do not expect to meet these criteria by such date, and we expect to receive a delisting determination from Nasdaq at such time. Upon receiving such determination, we intend to request a hearing to remain on the Nasdaq Stock Market, and we expect such request will suspend such delisting determination until a decision is issued by Nasdaq subsequent to the hearing, though we cannot be certain what decision Nasdaq will ultimately issue or the timing thereof.
Further, on September 28, 2023, we received a notice from Nasdaq notifying us that for 30 consecutive trading days, the bid price of our common stock had closed below the minimum $1.00 per share requirement. In accordance with Nasdaq’s listing rules, we were afforded a grace period of 180 calendar days, or until March 26, 2024, to regain compliance with the bid price requirement. In order to regain compliance, the bid price of our common stock must close at a price of at least $1.00 per share for a minimum of 10 consecutive trading days.
If we fail to regain compliance by March 26, 2024, we may be eligible for a second 180 day compliance period if we elect to transfer to The Nasdaq Capital market, provided that, on such date, we meet the continued listing requirement for market value of publicly held shares and all other applicable Nasdaq listing requirements (other than the minimum closing bid price requirement) and we provide written notice to Nasdaq of our intention to cure the deficiency during the second compliance period, by effecting a reverse stock split, if necessary. Such extension of the grace period would be subject to Nasdaq’s discretion, and there can be no guarantee that we would be granted an extension. Our failure to regain compliance with the minimum bid price requirement under the Nasdaq listing rules may result in the delisting of our common stock.
10
If we fail to retain senior management and key scientific personnel, we may be unable to continue operations, meet sale milestones and complete our strategic review process.
We are highly dependent on members of our senior management, including Adrian Adams, our Chairman, President and Chief Executive Officer, Michael Kalb, Chief Financial Officer, John Hoekman, Ph.D., Chief Technology and Development Officer and one of our founders, and Leonard S. Paolillo, our Chief Commercial Officer. Although we have entered into employment agreements and retention agreements with our executive officers, each of these persons may terminate their employment with us at any time. We do not maintain “key person” insurance for any of our executives or other employees.
Further, the reduction in employee and non-employee expenses announced in February 2023, and our ongoing liquidity issues makes retention of our current personnel both more important and more challenging. In particular, we are seeing increased loss of sales representatives, limiting our ability to meet our sales goals and revenue targets. We do not intend to fill these vacated positions for the foreseeable future and, as a result, retaining our current sales personnel will be critical to our immediate success. Further, the loss of the services of our executive officers or other key employees could impede the achievement of our research, development and commercialization objectives and seriously harm our ability to successfully implement our business strategy.
We are a commercial-stage biopharmaceutical company and have incurred net losses since our inception. We anticipate that we will continue to incur substantial operating losses for the foreseeable future and we may never achieve or sustain profitability.
We are a commercial-stage biopharmaceutical company formed in 2008. To date, we have financed our operations primarily through the sale and issuance of redeemable convertible preferred stock, convertible notes and warrants, common stock offerings, debt financings and royalty financings. Since 2021, we have also relied on revenues generated from net sales of Trudhesa.
We have incurred significant net losses since our inception. Our net losses were $37.4 million and $106.3 million for the six months ended June 30, 2023 and the year ended December 31, 2022, respectively. As of June 30, 2023, we had an accumulated deficit of $358.5 million. We cannot predict when or whether we will become profitable. Our losses have resulted principally from costs incurred in our product candidate discovery and development activities. We expect to incur net losses for the foreseeable future.
Our financial position will depend, in part, on the rate of our future expenditures and our ability to obtain funding through equity or alternative financings, strategic collaborations, or additional grants. Although we have received approval for Trudhesa, the resulting revenue from its commercialization may not enable us to achieve profitability. Our future revenues will depend upon our ability to achieve sufficient market acceptance, reimbursement from third-party payors and adequate market share for Trudhesa.
Our expenses and net losses may increase as we continue to commercialize Trudhesa as well as hire additional personnel, protect our intellectual property and incur additional costs associated with operating as a public company. Our net losses may fluctuate significantly from quarter to quarter and year to year, depending on our commercialization activities and any expenditures on other research and development activities.
To become and remain profitable, we must expand the market for Trudhesa. We may not succeed in these activities and we may never generate revenue from product sales that are significant enough to achieve profitability. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. We may encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect our business for any reason, including as a result of pandemics or other public health emergencies. The size of our future net losses will depend, in part, on the rate of future growth of our expenses and our ability to generate revenue. Our prior losses and expected future losses have had and will continue to have an adverse effect on our stockholders’ equity and working capital. Our failure to become or remain profitable would depress our market value and could impair our ability to raise capital, expand our business, discover or develop other product candidates or continue our operations.
Statements in this report concerning our future plans and operations are dependent on our ability to secure adequate funding and the absence of unexpected delays or adverse developments. We may not be able to secure required funding.
11
Any statements contained in this report concerning future events or developments or our future activities, such as concerning commercialization activities or regulatory matters, commercial introduction of any further products that we may develop in the future, anticipated outcome of any legal proceedings in which we are involved, and other statements concerning our future operations and activities, are forward-looking statements that in each instance assume that we have or are able to obtain sufficient funding to support such activities and continue our operations and satisfy our liability and obligations in a timely manner. There can be no assurance that this will be the case. Also, such statements assume that there are no significant unexpected developments or events that delay or prevent such activities from occurring. Failure to timely obtain any required additional funding, or unexpected developments or events, could delay the occurrence of such events or prevent the events described in any such statements from occurring which could adversely affect our business, financial condition and results of operations.
Our ongoing liquidity issues also present significant challenges to current operations. We currently expect the financing availability under the Amended Credit Agreement to be sufficient to conduct the strategic review process, but we do not expect it to be sufficient to continue operations beyond this process without substantial additional investment. Our operations are also being impacted by the loss of sales representatives that we do not currently plan to replace, increased pressure from suppliers regarding payments from us that may delay or prevent our ability to obtain sufficient product from suppliers, and concern from prescribers regarding future product availability. Any of these issues can have a material impact on our sales and may further reduce our cash runway, accelerating the potential need to seek alternatives, including bankruptcy proceedings.
If you purchase shares of our common stock sold in this offering, you may experience immediate and substantial dilution in the net tangible book value of your shares. In addition, we may issue additional equity or convertible debt securities in the future, which may result in additional dilution to you.
The price per share of our common stock being offered may be higher than the net tangible book value per share of our outstanding common stock prior to this offering. Assuming that an aggregate of 21,897,810 shares of our common stock are sold at a price of $0.411 per share, the last reported sale price of our common stock on The Nasdaq Global Market on October 4, 2023, for aggregate gross proceeds of approximately $9.0 million, and after deducting commissions and estimated offering expenses payable by us, new investors in this offering will incur immediate dilution of $1.96 per share. For a more detailed discussion of the foregoing, see the section entitled “Dilution” below. To the extent outstanding stock options or warrants are exercised, there will be further dilution to new investors. We expect our net tangible book value to be negative following this offering, and if we initiate bankruptcy proceedings, holders of our common stock will likely receive no recovery at all for the securities offered hereby.
In addition, to the extent we need to raise additional capital in the future and we issue additional shares of common stock or securities convertible or exchangeable for our common stock, our then existing stockholders may experience dilution and the new securities may have rights senior to those of our common stock offered in this offering.
Our quarterly operating results may fluctuate significantly or may fall below the expectations of investors or securities analysts, each of which may cause our stock price to fluctuate or decline.
We expect our operating results to be subject to quarterly fluctuations. Our net loss and other operating results will be affected by numerous factors, including:
•quarterly fluctuations in product sales of Trudhesa;
•our ability to retain our sales force and the negative impact such attrition will have on our sales of Trudhesa;
•any reductions in supply of Trudhesa due to inability to timely pay our suppliers;
•variation in the level of expense related to the commercialization of Trudhesa;
•any intellectual property infringement lawsuit or opposition, interference or cancellation proceeding in which we may become involved;
•strategic decisions by us or our competitors, such as acquisitions, divestitures, spin-offs, joint ventures, strategic investments or changes in business strategy; and
•changes in general market and macroeconomic conditions.
12
If our quarterly operating results fall below the expectations of investors or securities analysts, the price of our common stock could decline substantially. Furthermore, any quarterly fluctuations in our operating results may, in turn, cause the price of our common stock to fluctuate substantially. We believe that quarterly comparisons of our financial results should not be relied upon as an indication of our future performance.
Our cash and cash equivalents could be adversely affected if the financial institutions in which we hold our cash and cash equivalents fail.
We regularly maintain cash balances at third-party financial institutions, including formerly with Silicon Valley Bank, in excess of the FDIC insurance limit and similar regulatory insurance limits outside the United States. Further, if we enter into a credit, loan or other similar facility with a financial institution, certain covenants included in such facility may require as security that we keep a significant portion of our cash with the institution providing such facility. If a depository institution where we maintain deposits fails or is subject to adverse conditions in the financial or credit markets, we may not be able to recover all, if any, of our deposits, which could adversely impact our operating liquidity and financial performance.
We have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
Our management will have broad discretion in the application of the net proceeds from this offering, including for any of the purposes described in the section entitled “Use of Proceeds,” and you will not have the opportunity as part of your investment decision to assess whether the net proceeds are being used appropriately. Because of the number and variability of factors that will determine our use of the net proceeds from this offering, their ultimate use may vary substantially from their currently intended use. Our management might not apply our net proceeds in ways that ultimately increase the value of your investment. We currently intend to use the net proceeds from this offering general corporate purposes, which may include increasing our working capital, reducing indebtedness, ongoing commercialization activities and market development of Trudhesa, and capital expenditures. Pursuant to the terms of the Amended Credit Agreement, if we raise proceeds in an equity financing, including this offering, that exceed $5,000,000, we will apply 50% of such excess proceeds to repay the tranche B term loans. The failure by our management to apply these funds effectively could harm our business. Pending their use, we plan to invest the net proceeds from this offering in short-term or long-term, investment-grade, interest-bearing securities. These investments may not yield a favorable return to our stockholders. If we do not invest or apply the net proceeds from this offering in ways that enhance stockholder value, we may fail to achieve expected financial results, which could cause our stock price to decline.
The actual number of shares we will issue under the sales agreement, at any one time or in total, is uncertain.
Subject to certain limitations in the sales agreement and compliance with applicable law, we have the discretion to deliver a placement notice to TD Cowen at any time throughout the term of the sales agreement. The number of shares that are sold by TD Cowen after delivering a placement notice will fluctuate based on the market price of the common shares during the sales period and limits we set with TD Cowen. Because the price per share of each share sold will fluctuate based on the market price of our common stock during the sales period, it is not possible at this stage to predict the number of shares that will be ultimately issued. Further, we are restricted pursuant to General Instruction I.B.6 of Form S-3 to selling securities with a value of no more than one-third of our public float in any 12-month period so long as our public float remains below $75.0 million.
The common stock offered hereby will be sold in “at the market offerings”, and investors who buy shares at different times will likely pay different prices.
Investors who purchase shares in this offering at different times will likely pay different prices, and so may experience different outcomes in their investment results. We will have discretion, subject to market demand, to vary the timing, prices, and numbers of shares sold, and there is no minimum or maximum sales price. Investors may experience a decline in the value of their shares as a result of share sales made at prices lower than the prices they paid.
Further, if we do not complete a strategic transaction or receive sufficient financing from our existing debt facilities or from the proceeds from the offering of securities covered by this prospectus or any other financing, we expect
13
that we will file for bankruptcy protection, in which case holders of our common stock will likely receive no recovery at all for the securities offered hereby. Even if we do complete a transaction, the proceeds may be insufficient to allow our stockholders to recognize any return on their investment.
The market price of our common stock may be volatile, which could result in substantial losses for investors.
The market price of our common stock has been and may continue to be volatile. The market price for our common stock may be influenced by many factors, including the other risks described in this section and the following:
•our potential insolvency;
•actual or anticipated variations in our financial results or those of companies that are perceived to be similar to us;
•market conditions in the life sciences and pharmaceutical sectors;
•announcements by us or our competitors of significant acquisitions, strategic collaborations, joint ventures or capital commitments;
•developments or disputes concerning patents or other proprietary rights, including patents and litigation matters;
•our ability or inability to raise additional capital and the terms on which we raise it;
•the recruitment or departure of key personnel, particularly our sales personnel;
•introductions and announcements of new product candidates by us, our future commercialization partners, or our competitors, and the timing of these introductions or announcements;
•regulatory or legal developments in the United States and other countries;
•material and adverse impact of pandemics or other public health emergencies on the markets and the broader global economy;
•the success of competitive products or technologies;
•actions taken by regulatory agencies with respect to any clinical trials, manufacturing process or sales and marketing terms;
•changes in the structure of healthcare payment systems;
•actual or anticipated changes in earnings estimates or changes in stock market analyst recommendations regarding our common stock, other comparable companies or our industry generally;
•our failure or the failure of our competitors to meet analysts’ projections or guidance that we or our competitors may give to the market;
•fluctuations in the valuation of companies perceived by investors to be comparable to us;
•announcement and expectation of additional financing efforts;
•speculation in the press or investment community;
•trading volume of our common stock;
•sales of our common stock by us or our stockholders;
•the concentration in ownership of our common stock;
•changes in accounting principles;
•potential litigation or the threat thereof;
•terrorist acts, acts of war or periods of widespread civil unrest;
•natural disasters and other calamities; and
•general economic, industry and market conditions.
In addition, the stock market in general, and the markets for pharmaceutical and medical device stocks in particular, have experienced extreme price and volume fluctuations that have been often unrelated or disproportionate to the operating performance of these companies, including as a result of the COVID-19 pandemic. These broad market and industry factors may seriously harm the market price of our common stock, regardless of our actual operating performance. The realization of any of the above risks or any of a broad range of other risks, including those described in this “Risk Factors” section, could have a dramatic and adverse impact on the market price of our common stock.
Risks Related to Commercialization of Trudhesa
14
Our future commercial success depends upon attaining significant market acceptance of Trudhesa among physicians, patients, health care payors and others in the medical community necessary for commercial success.
Trudhesa may not gain market acceptance among physicians, health care payors, patients and the medical community. There are several approved acute treatments for migraine currently on the market, including triptans, calcitonin gene-related peptides antagonists, or gepants, lasmiditan and alternative formulations of DHE, such as Migranal, which is also administered intranasally. All of these are competitive with Trudhesa and our level of market acceptance of Trudhesa for the acute treatment for migraine may be lower than we expect. Market acceptance of Trudhesa depends on a number of factors, including:
•the efficacy and safety of Trudhesa;
•perceived advantages of Trudhesa over alternative treatments, such as oral, IM and IV formulations;
•the indications for which the product candidates are approved and the labeling approved by regulatory authorities for use with the product candidates, including any warnings, limitations or contraindications contained in a product’s approved labeling;
•acceptance by physicians and patients of Trudhesa as a safe and effective treatment;
•the cost, safety and efficacy of treatment in relation to alternative treatments, including generic versions of the product candidates;
•the extent to which Trudhesa is included on formularies of hospitals and managed care organizations;
•the availability of coverage and adequate reimbursement and pricing by third-party payors and government authorities for Trudhesa;
•relative convenience and ease of administration of Trudhesa;
•the prevalence and severity of adverse side effects;
•the timing of market introduction of competitive products;
•restrictions on the distribution of Trudhesa;
•the effectiveness of our sales and marketing efforts;
•unfavorable publicity relating to Trudhesa; and
•the approval of other new therapies for the same indications.
Market acceptance is critical to our ability to generate significant revenue and become profitable. Trudhesa may be accepted in only limited capacities or not at all. If Trudhesa is not accepted by the market to the extent that we expect, we may not be able to generate significant revenue and our business would suffer.
The market for Trudhesa may not be as large as we expect and, as a result, our product revenues may be lower than expected and our stock price may decline.
Our estimates of the potential market opportunity for Trudhesa include several key assumptions based on our industry knowledge, industry publications, third-party research reports and other surveys, including surveys commissioned by us. These assumptions include the size of our target populations, the prevalence and incidence of addressable migraines, the number of patients receiving current treatment, the percentage of patients unsatisfied with the current treatments, the number of diagnosed but untreated patients, the compliance and adherence of patients in our target populations, the number of treatment centers and prescribing physicians and the percentage of payer acceptance. While we believe that our internal assumptions are reasonable, if any of these assumptions proves to be inaccurate, then the actual market for Trudhesa could be smaller than our estimates of our potential market opportunity. If the actual market for Trudhesa is smaller than we expect, our product revenue may be limited, and it may be more difficult for us to achieve or maintain profitability.
In addition, the FDA has required labeling restrictions for patients and uses of Trudhesa. For example, upper nasal space drug delivery may not be appropriate for use by patients with certain pre-existing conditions, such as chronic rhinitis with or without nasal polyposis or anatomical nasal obstruction.
If we are unable to maintain our commercial distribution capabilities, we will be unable to successfully commercialize Trudhesa.
15
Our ongoing liquidity issues have contributed, in part, to a loss of sales representatives that we do not currently plan to replace and we have also experienced increased pressure from suppliers and other commercial counterparties, including distributors, regarding payments from us. Our inability to retain sales representatives and distributor relationships, or an ability to obtain sufficient product from suppliers may delay or prevent our ability to make and fulfill sales, which would have a material adverse impact on our sales and may further reduce our cash runway, accelerating the potential need to seek alternatives, including bankruptcy proceedings.
Problems related to large-scale commercial manufacturing could cause delays in product launches, an increase in costs or shortages of Trudhesa.
Manufacturing finished drug products, especially in large quantities, is complex. The commercialization of Trudhesa requires several manufacturing steps and involves complex techniques to assure quality and sufficient quantity, especially as the manufacturing scale increases. Trudhesa will need to be made consistently and in compliance with a clearly defined manufacturing process pursuant to FDA regulations. Accordingly, it will be essential to be able to validate and control the manufacturing process to assure that it is reproducible. Slight deviations anywhere in the manufacturing process, including obtaining materials, filling, labeling, packaging, storage, shipping, quality control and testing, may result in lot failures, delay in the release of lots, product recalls or spoilage. Success rates can vary dramatically at different stages of the manufacturing process, which can lower yields and increase costs. We may experience deviations in the manufacturing process that may take significant time and resources to resolve and, if unresolved, may affect manufacturing output and cause us to fail to satisfy contractual commitments, lead to delays in our clinical trials or result in litigation or regulatory action. Such actions would hinder our ability to meet contractual obligations and could cause material adverse consequences for our business. In addition to the complexities of large-scale manufacturing, our ongoing liquidity issues also present significant challenges to current operations, including as a result of increased pressure from suppliers regarding payments from us, which may delay or prevent our ability to obtain sufficient product from suppliers.
Reimbursement for any approved products may be limited or unavailable, which could make it difficult for us to sell Trudhesa profitably.
In both domestic and foreign markets, sales of Trudhesa depend, in part, on the extent to which the costs of any future product candidates will be covered by third-party payors, such as government health care programs, commercial insurance and managed health care organizations. These third-party payors decide which drugs will be covered and establish reimbursement levels for those drugs. The containment of health care costs has become a priority of foreign and domestic governments as well as private third-party payors. The prices of drugs have been a focus in this effort. Governments and private third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications, which could affect our ability to sell any approved products profitably. Cost-control initiatives could cause us to decrease the price we might establish for any approved products, which could result in lower than anticipated product revenues.
Reimbursement by a third-party payor may depend upon a number of factors, including the third-party payor’s determination that use of a product is:
•a covered benefit under its health plan;
•safe, effective and medically necessary;
•appropriate for the specific patient;
•cost-effective relative to other alternatives, including generic products; and
•neither experimental nor investigational.
Adverse pricing limitations may hinder our ability to recoup our investment in historical products.
Obtaining coverage and reimbursement approval for a product from a government or other third-party payor is a time-consuming and costly process that could require us to provide supporting scientific, clinical and cost-effectiveness data for the use of any future product candidates to the payor. Further, there is significant uncertainty related to third-party payor coverage and reimbursement of newly approved product candidates. We may not be able to provide data sufficient to gain acceptance with respect to coverage and reimbursement. Also, we cannot be sure that reimbursement amounts will not reduce the demand for, or the price of, Trudhesa. In addition, in the United States, third-party payors are increasingly attempting to contain health care costs by limiting both coverage and the level of reimbursement of new product candidates. As a result, significant uncertainty exists as to whether
16
and how much third-party payors will reimburse patients for their use of newly approved product candidates, which in turn will put pressure on pricing.
Price controls may be imposed in foreign markets, which may adversely affect our future profitability.
In some countries, including member states of the European Union, the pricing of prescription drugs is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after receipt of marketing approval for a product. In addition, there can be considerable pressure by governments and other stakeholders on prices and reimbursement levels, including as part of cost containment measures. Political, economic and regulatory developments may further complicate pricing negotiations, and pricing negotiations may continue after reimbursement has been obtained. Reference pricing used by various European Union member states and other countries and parallel distribution, or arbitrage between low-priced and high-priced member states, can further reduce prices. Publication of discounts by third-party payors or authorities may lead to further pressure on the prices or reimbursement levels within the country of publication and other countries.
We face substantial competition, which may result in others discovering, developing or commercializing product candidates before, or more successfully, than we do.
The development and commercialization of new and improved pharmaceutical products is highly competitive. There are many pharmaceutical companies, biotechnology companies, public and private universities, government agencies and research organizations actively engaged in research and development of product candidates which may target the same markets as Trudhesa. Our future success depends on our ability to demonstrate and maintain a competitive advantage with respect to the design, development and commercialization of any of Trudhesa within those markets.
For Trudhesa we are aware of the several competing efforts. Approved acute treatments for migraine include triptans, gepants (such as ZavzpretTM and Nurtec® both commercialized by Pfizer Inc.), lasmiditan and alternative formulations of DHE, such as Migranal, which is administered intranasally. Some of these competitor products have been launched. Some of these competitors are also developing product candidates that utilize alternative routes of administration, including Amneal Pharmaceuticals, Inc., Satsuma Pharmaceuticals, Inc. and Zosano Pharma Corporation, whose product candidates use nasal pumps or other drug delivery technologies.
One or more of our competitors may utilize their expertise in other methods of pharmaceutical drug delivery to develop and obtain approval for upper nasal space delivery products that may compete with Trudhesa. These competitors may include Aegis, Optinose and other smaller pharmaceutical companies. Many of our competitors have significantly greater financial, technical, manufacturing, marketing, sales and supply resources or experience than we have had to date. Our ability to compete effectively will depend, in part, on the timing and scope of regulatory approvals for these product candidates, the availability and cost of manufacturing, marketing and sales capabilities, price, reimbursement coverage and patent position, the safety and effectiveness of any of our future product candidates, the ease with which any of our future product candidates can be administered and the extent to which patients accept relatively new routes of administration. Competing products could present superior treatment alternatives, including by being more effective, safer, less expensive or marketed and sold more effectively than any product candidates we may develop. Competitive products may reduce the demand and price for Trudhesa, making it obsolete or noncompetitive before we recover the expense of developing and commercializing. Our competitors could also recruit our employees, which could negatively impact our level of expertise and our ability to execute our business plan.
We rely entirely on third parties for the manufacturing of Trudhesa. If such third parties cease to provide services or require changes to our payment terms, or if we encounter difficulties in negotiating manufacturing and supply agreements with third-party manufacturers and suppliers of our POD device and the active ingredients in Trudhesa would be impaired.
We do not own any manufacturing facilities and have limited experience in commercial manufacturing. We currently rely, and expect to continue to rely, on a limited number of experienced personnel and contract manufacturing organizations, or CMOs, and suppliers, including in some cases single-source suppliers, who would assist in the production, assembly, test, validation, supply, storage and distribution of Trudhesa, and we do not
17
control their activities. While we have developmental and commercial supply agreements in place with some of our key suppliers, we may not be able to obtain terms that are favorable to us or enter into commercial manufacturing and supply agreements at all with other necessary third parties. If we are unable to enter into such agreements on commercially reasonable terms, our ability to commercialize Trudhesa would be impaired, and our business, financial condition and results of operations would be materially adversely affected.
Further, given our current liquidity issues, we may be delayed in paying these third parties, or these third parties may demand accelerated payment in advance of providing necessary supplies of Trudhesa. Any such change to our payment obligations may decrease the availability of Trudhesa and materially impair our ability to further commercialize Trudhesa.
If product sales for Trudhesa grow, Trudhesa will require production processes to be scaled up. We will be dependent on external manufacturers and suppliers to ensure that their manufacturing processes can be scaled up adequately such that we are able to supply the market. If any of our key suppliers are unable or unwilling to scale up production, our product candidates would be impaired, and our business, financial condition and results of operations would be materially adversely affected.
If third-party manufacturers, wholesalers and distributors fail to perform as expected, our costs may be higher than expected.
Our reliance on third-party manufacturers, wholesalers and distributors exposes us to the following risks, any of which could result in higher costs or deprive us of potential product revenues:
•our CMOs, or other third parties we rely on, may require changes to our payment terms if they consider us unlikely to pay in a timely manner, accelerating our expected cash usage or delaying our access to Trudhesa supply;
•our CMOs, or other third parties we rely on, may encounter difficulties in achieving the volume of production needed to satisfy commercial demand, may experience technical issues that impact quality or compliance with applicable and strictly enforced regulations governing the manufacture of pharmaceutical products, and may experience shortages of qualified personnel to adequately staff production operations;
•our CMOs, wholesalers and distributors may misappropriate our proprietary information; and
•if our CMOs, wholesalers and distributors were to terminate our arrangements or fail to meet their contractual obligations, we may be forced to delay our commercial programs.
For example, we identified increased levels of impurities in some drug vials of certain drug lots used in our Trudhesa STOP 301 trial. Vials from those drug lots were removed from the trial and we conducted a root cause investigation, identifying the likely root cause as long stoppages in the production of two lots. If we encounter similar or other issues in connection with our commercial manufacturing of Trudhesa, we may face delays and shortages in production of Trudhesa, impacting our ability to fill prescriptions, and may face further scrutiny from the SEC.
Our reliance on third parties does not relieve us of our responsibility to ensure compliance with all required legal, regulatory and scientific standards. For example, the FDA and other regulatory authorities require that product candidates and any products that we may eventually commercialize be manufactured according to cGMP and QSR, and similar foreign standards. In addition, our third-party manufacturers will be subject to periodic inspections by the FDA and other regulatory authorities, and failure to comply with cGMP or QSR could be the basis for the FDA to issue a warning or untitled letter, withdraw approvals for product candidates previously granted to us, or take other regulatory or legal action, including request a recall or seize product candidates, total or partial suspension of production, suspension of clinical trials, refusal to approve pending applications or supplemental applications, detention of product, refusal to permit the import or export of product candidates, injunction, imposing civil penalties or pursuing criminal prosecution.
Risks Related to Government Regulation
18
If a drug delivery device in a future clinical trial similar to the drug delivery device used by Trudhesa demonstrates unanticipated biocompatibility, usability, performance or safety issues in a clinical or nonclinical study for one product candidate, our entire pipeline may be adversely affected.
Trudhesa utilizes our POD devices, which are designed to deliver the drug into the upper nasal space using a gas propellant. While our prior product candidates have been generally well tolerated in nonclinical studies and clinical trials, patients may in the future experience different or more severe adverse events due in part to our POD device. Any failure of our POD device to demonstrate adequate biocompatibility, usability, performance or safety could adversely affect the development, approval, or commercialization of Trudhesa.
Inadequate funding for the FDA, the SEC and other government agencies or other disruptions at these agencies could hinder these agencies’ ability to hire and retain key leadership and other personnel, prevent new products and services from being developed or commercialized in a timely manner or otherwise prevent those agencies from performing normal business functions on which the operation of our business may rely, which could negatively impact our business.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory, and policy changes. In addition, government funding of the SEC and other government agencies on which our operations may rely, including those that fund research and development activities, is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other agencies may also slow the time necessary for new drugs to be reviewed and/or approved by necessary government agencies, which would adversely affect our business. For example, over the last several years, the U.S. government has shut down several times, and certain regulatory agencies, such as the FDA and the SEC, have had to furlough critical employees and stop critical activities. If a prolonged government shutdown occurs, or if global health concerns continue to prevent the FDA or other regulatory authorities from conducting their regular inspections, reviews, or other regulatory activities, it could significantly impact the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business.
Further, in our operations as a public company, government shutdowns could impact our ability to access the public markets and obtain necessary capital in order to properly capitalize and continue our operations.
If we fail to obtain regulatory approval in jurisdictions outside the United States, we will not be able to market Trudhesa in those jurisdictions.
We intend to market Trudhesa in international markets either directly or through partnerships. Such marketing will require separate regulatory approvals in each jurisdiction and compliance with numerous and varying regulatory requirements. The approval procedures vary from jurisdiction to jurisdiction and may require additional testing that we are not required to perform to obtain regulatory approval in the United States. Moreover, the time required to obtain approval may differ from that required to obtain FDA approval. In addition, in many countries outside the United States, a product candidate must be approved for reimbursement before it can be approved for sale in that country. Approval by the FDA does not guarantee approval by regulatory authorities in other countries or jurisdictions, and approval by one foreign regulatory authority does not guarantee approval by regulatory authorities in other foreign jurisdictions or by the FDA. The foreign regulatory approval process may include all of the risks associated with obtaining FDA approval. We may not obtain foreign regulatory approvals on a timely basis, if at all. We may not be able to file for regulatory approvals and may not receive necessary approvals to commercialize Trudhesa in any foreign market. If we or any future partner are unable to obtain regulatory approval for Trudhesa in one or more significant foreign jurisdictions, then the commercial opportunity for Trudhesa, as well as our financial condition, will be adversely affected.
Trudhesa could be subject to labeling and other restrictions, and we may be subject to penalties if we fail to comply with regulatory requirements or experience unanticipated problems.
Any regulatory approvals that we receive for Trudhesa may also be subject to limitations on the approved indicated uses for which the product may be marketed, or to conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase 4 clinical trials, and surveillance to monitor safety and efficacy. For
19
example, under the Pediatric Research Equity Act, we are required to conduct certain juvenile animal and pediatric studies in accordance with the timelines set forth in our Trudhesa NDA approval letter. These studies will require significant resources. We cannot predict the outcome of these studies. In addition, the manufacturing processes, labeling, packaging, distribution, adverse event, or AE, reporting, storage, advertising, promotion and recordkeeping for any approved product will be subject to extensive and ongoing regulatory requirements. These requirements include submissions of safety and other post-marketing information and reports, including reporting of certain adverse events, malfunctions, corrections and removals related to the POD device, registration, as well as continued compliance with cGMP for the drug products, the quality system regulation, or QSR, for medical devices and GCP for any clinical trials that we conduct post-approval.
Later discovery of previously unknown problems with an approved product, including AEs of unanticipated severity or frequency, or with manufacturing operations or processes, or failure to comply with regulatory requirements, may result in, among other things:
•holds on clinical trials;
•restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market, or voluntary or mandatory product recalls;
•imposition of a REMS, which may include distribution or use restrictions;
•requirements to conduct additional post-market clinical trials to assess the safety of the product;
•revisions to the labeling, including limitation on approved uses or the imposition of additional warnings, contraindications or other safety information, including boxed warnings;
•manufacturing delays and supply disruptions where regulatory inspections identify observations of noncompliance requiring remediation;
•fines, warning or untitled letters;
•refusal by the FDA to approve pending applications or supplements to approved applications submitted by us, or withdrawal of product approvals;
•product seizure or detention, or refusal to permit the import or export of product candidates; and•
•injunctions or the imposition of civil or criminal penalties.
We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or not able to maintain regulatory compliance, we may lose any marketing approval that may have been obtained and we may not achieve or sustain profitability, which would adversely affect our business.
We may be subject to enforcement action by the FDA or other government agencies or competitor lawsuits or other claims, including litigation brought by the government, if we engage or are found to have engaged in improper promotion of our products.
Our promotional materials and training methods must comply with FDA and other applicable laws and regulations, including laws and regulations prohibiting marketing claims that promote the off-label use of our products or that omit material facts or make false or misleading statements about the safety or efficacy of our products. We are responsible for training our marketing and sales force not to promote any products for off-label uses, but healthcare providers may use our products off-label as the FDA does not restrict or regulate a physician’s choice of treatment within the practice of medicine. The FDA also could conclude that a claim is misleading if it determines that there are inadequate nonclinical and/or clinical data supporting the claim, or if a claim fails to reveal material facts about the safety or efficacy of our products. If the FDA determines that our promotional labeling or advertising materials promote an off-label use or make false or misleading claims, it could request that we modify our promotional materials or training content or subject us to regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, injunction, seizure, civil fines and criminal penalties.
The FDA closely regulates the pre and post-approval marketing and promotion of drugs to ensure they are promoted and marketed in compliance with the FDCA and its implementing regulations and only for the approved indications and in a manner consistent with the approved labeling. For example, our labeling for Trudhesa does not include any of the data from the exploratory efficacy endpoints that we evaluated in our Phase 3 safety clinical trial or contain any efficacy claims based on the results of this study. If the FDA disagrees with our claims or approach to describing the efficacy results from any data deemed as unreliable or uninterpretable, including our exploratory efficacy analyses, in our promotional materials, it may take enforcement action against us. The FDA
20
imposes stringent restrictions on manufacturers’ communications and promotion of their products, including specific restrictions for promotions of products with boxed warnings. If we promote any products in a manner inconsistent with the FDA-approved labeling or otherwise not in compliance with the FDCA or implementing regulations, we may be subject to enforcement action. Violations of the FDCA relating to improper promotion of prescription drugs may lead to warning letters, investigations, violations under federal and state healthcare fraud and abuse laws, including the False Claims Act, as well as state consumer protection laws.
It is also possible that other federal, state or foreign enforcement authorities might take action if they determine that our promotional or training materials promote an unapproved use or make false or misleading claims, which could result in significant fines or penalties. Although our policy is to refrain from statements that could be considered off-label promotion of our products or false or misleading claims, the FDA or another regulatory agency could disagree with the manner in which we advertise and promote our products. Violations of the FDCA may also lead to investigations alleging violations of federal and state health care fraud and abuse laws, as well as state consumer protection laws, which may lead to costly penalties and may adversely impact our business. Recent court decisions have impacted the FDA’s enforcement activity regarding off-label promotion in light of First Amendment considerations; however, there are still significant risks in this area, in part due to the potential for False Claims Act exposure. Competitors may also object to our promotional claims, which could lead to trade complaints to FDA or other actions related to unfair competition.
Many companies have also faced government investigations or lawsuits by whistleblowers who bring a qui tam action under the False Claims Act on behalf of themselves and the government for a variety of alleged improper marketing activities. In addition, the government and private whistleblowers have pursued False Claims Act cases against pharmaceutical companies for causing false claims to be submitted as a result of the marketing of their products for unapproved uses. If we are found to have improperly promoted our products, we may be subject to significant liability, including civil fines, criminal fines and penalties, civil damages, exclusion from federally funded healthcare programs and potential liability under the federal False Claims Act and any applicable state false claims act. In addition, we may incur liability from claims initiated under the Lanham Act or other federal and state unfair competition laws with respect to how our products are marketed and promoted. Furthermore, the off-label use of our products may increase the risk of product liability claims. The scope of potential liability with respect to any such claims, enforcement actions, or lawsuits is uncertain, and we cannot assure you that we will not receive claims from competitors or other third parties or be subject to enforcement actions in the future from regulatory agencies. Moreover, threatened or actual government enforcement actions or lawsuits by third parties could generate adverse publicity, which could decrease demand for our products and require that we devote substantial resources that could be used productively on other aspects of our business.
Our relationships with health care professionals, institutional providers, principal investigators, consultants, potential customers and third-party payors are, and will continue to be, subject, directly and indirectly, to federal and state health care fraud and abuse, false claims, marketing expenditure tracking and disclosure, government price reporting, and privacy, data protection and data security laws. If we are unable to comply, or have not fully complied, with such laws, we could face penalties, including, without limitation, civil, criminal, and administrative penalties, damages, monetary fines, disgorgement, possible exclusion from participation in Medicare, Medicaid and other federal and state health care programs, contractual damages, reputational harm, diminished profits and future earnings, and curtailment or restructuring of our operations.
Our business operations and activities may be directly or indirectly subject to various federal and state fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute and the federal False Claims Act. Our current and future arrangements with healthcare professionals, clinical investigators, CROs, third-party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our products. In addition, we may be subject to laws of the federal government and state governments in which we conduct our business relating to privacy, data protection and data security with respect to patient information. The laws that may affect our ability to operate include, but are not limited to:
•the federal Anti-Kickback Statute, which prohibits, among other things, knowingly and willfully soliciting, receiving, offering or paying any remuneration (including any kickback, bribe, or rebate), directly or
21
indirectly, overtly or covertly, in cash or in kind, to induce, or in return for, either the referral of an individual, or the purchase, lease, order or recommendation of any good, facility, item or service for which payment may be made, in whole or in part, under a federal health care program, such as the Medicare and Medicaid programs;
•federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment or approval from a federal health care program, such as Medicare, Medicaid, or other third-party payors that are false or fraudulent or knowingly making a false statement to improperly avoid, decrease or conceal an obligation to pay money to the federal government;
•the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created new federal criminal statutes that prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud any health care benefit program or obtain, by means of false or fraudulent pretenses, representations, or promises, any of the money or property owned by, or under the custody or control of, any health care benefit program, regardless of the payor (e.g., public or private) and knowingly and willfully falsifying, concealing, or covering up by any trick or device a material fact or making any materially false statements in connection with the delivery of, or payment for, health care benefits, items or services relating to health care matters;
•HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and their respective implementing regulations, which impose requirements on certain covered health care providers, health plans, and health care clearinghouses as well as their respective business associates that perform services for them that involve the use, or disclosure of, individually identifiable health information, relating to the privacy, security and transmission of individually identifiable health information without appropriate authorization;
•the federal physician self-referral law, commonly known as the Stark Law, which prohibits a physician from making a referral to an entity for certain designated health services reimbursed by Medicare or Medicaid if the physician or a member of the physician’s family has a financial relationship with the entity, and which also prohibits the submission of any claims for reimbursement for designated health services furnished pursuant to a prohibited referral;
•the federal Physician Payments Sunshine Act, created under Section 6002 of the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, collectively, or the ACA, and its implementing regulations require manufacturers of drugs, devices, biologicals and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually to the United States Department of Health and Human Services, Centers for Medicare & Medicaid Services information related to payments or other transfers of value made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) physician assistants, certain types of advanced practice nurses, and teaching hospitals, including ownership and investment interests held by the physicians described above and their immediate family members, with the information made publicly available on a searchable website;
•federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers;
•federal government price reporting laws, changed by the ACA to, among other things, increase the minimum Medicaid rebates owed by most manufacturers under the Medicaid Drug Rebate Program and offer such rebates to additional populations, that require us to calculate and report complex pricing metrics to government programs, where such reported prices may be used in the calculation of reimbursement or discounts on our marketed drugs (participation in these programs and compliance with the applicable requirements may subject us to increased infrastructure costs, and potentially limit our ability to offer certain marketplace discounts);
•the Foreign Corrupt Practices Act, a United States law which regulates certain financial relationships with foreign government officials (which could include, for example, certain medical professionals); and
•state law equivalents and adjuncts to many of the above federal laws, such as anti-kickback, false claims, consumer protection, unfair competition, and privacy and data security laws, which may apply to our business practices, including but not limited to, research, distribution, sales and marketing arrangements; state laws that require biotech companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government that otherwise restricts payments that may be made to health care providers; state laws that require drug manufacturers to file reports with states regarding marketing information, such as the tracking and
22
reporting of gifts, compensation and other remuneration and items of value provided to health care professionals and entities (compliance with such requirements may require investment in infrastructure to ensure that tracking is performed properly, and some of these laws result in the public disclosure of various types of payments and relationships, which could potentially have a negative effect on our business or increase enforcement scrutiny of our activities); state laws regarding the reporting of certain pricing information; and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways, with differing effects and obligations.
In addition to health information privacy, data security, and data protection laws that apply to some of the patient data we hold, other privacy, data security and data protection laws may also apply to such data, as well as to the personal data of our employees and other individuals generally. Many of these laws governing privacy, data protection and cybersecurity differ from each other in significant ways and may not have the same effects or obligations, thus complicating compliance efforts. We expect to incur increased costs of compliance with such laws and regulations as they continue to evolve, as well as the increased risk of regulatory investigations, enforcement actions, and other claims and litigation, with the potential for significant fines, penalties, and other liabilities in the event of actual or alleged noncompliance. Any of these could adversely affect our business, financial condition, and results of operations.
The ACA, among other things, amended the intent standard of the federal Anti-Kickback Statute and criminal health care fraud statutes to a stricter standard such that a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. In addition, the ACA codified case law that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act.
Efforts to ensure that our business arrangements with third parties will comply with applicable health care laws may involve substantial costs. It is possible that governmental and enforcement authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law interpreting applicable fraud and abuse or other health care laws and regulations. If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including, without limitation, civil, criminal, and administrative penalties, damages, monetary fines, disgorgement, imprisonment, loss of eligibility to obtain approvals from the FDA, qui tam actions, lawsuits, government investigations, exclusion from participation in government contracting, healthcare reimbursement, or other federal or state government healthcare programs, including Medicare and Medicaid, corporate integrity oversight and reporting obligations, contractual damages, reputational harm, diminished profits and future earnings, and curtailment or restructuring of our operations.
The impact of recent health care reform legislation and other changes in the health care industry and in healthcare spending on us is currently unknown, and may adversely affect our business model.
We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained and we may not achieve or sustain profitability.
In the United States, there have been and continue to be a number of legislative initiatives to contain healthcare costs.
On September 9, 2021, the Biden administration published a wide-ranging list of policy proposals, most of which would need to be carried out by Congress, to reduce drug prices and drug payment. The HHS plan includes, among other reform measures, proposals to lower prescription drug prices, including by allowing Medicare to negotiate prices and disincentivizing price increases, and to support market changes that strengthen supply chains, promote biosimilars and generic drugs, and increase price transparency. These proposals recently culminated in the enactment of the IRA in August 2022, which will, among other things, allow HHS to negotiate the selling price of certain drugs and biologics that CMS reimburses under Medicare Part B and Part D, although only high-expenditure single-source drugs that have been approved for at least 7 years (11 years for biologics) can be selected by CMS for negotiation. The negotiated prices, which will first become effective in 2026, will be
23
capped at a statutory ceiling price. Beginning in January 2023 for Medicare Part B and October 2022 for Medicare Part D, the IRA will also penalize drug manufacturers that increase prices of Medicare Part B and Part D drugs at a rate greater than the rate of inflation. The IRA permits the Secretary of HHS to implement many of these provisions through guidance, as opposed to regulation, for the initial years. Manufacturers that fail to comply with the IRA may be subject to various penalties, including civil monetary penalties. The IRA also extends enhanced subsidies for individuals purchasing health insurance coverage in ACA marketplaces through plan year 2025. These provisions will take effect progressively starting in 2023, although they may be subject to legal challenges. The full economic impact of the IRA is unknown at this time, but the law’s passage may affect the pricing of our products and product candidates. The adoption of restrictive price controls in new jurisdictions, more restrictive controls in existing jurisdictions or the failure to obtain or maintain timely or adequate pricing could also adversely impact revenue. We expect pricing pressures will continue globally.
At the state level, legislatures are increasingly passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
We expect that additional state and federal healthcare reform measures will be adopted in the future. Such reform measures may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for any approved product. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize Trudhesa. Complying with any new legislation and regulatory changes could be time-intensive and expensive, resulting in a material adverse effect on our business.
Risks Related to Our Intellectual Property
If we are not able to obtain and enforce patent protection for our technologies, development and commercialization of our technology may be adversely affected.
Our success depends in part on our ability to obtain, maintain, protect and enforce patents and other forms of intellectual property rights, including in-licenses of intellectual property rights of others, our technology such as our proprietary POD nasal drug delivery platform, and methods for treating patients, as well as our ability to preserve our trade secrets, to prevent third parties from infringing upon our proprietary rights and to operate without infringing upon the proprietary rights of others. Our patent portfolio as of February 1, 2023 contained 14 U.S. issued patents and 74 patents issued in ex-U.S. jurisdictions including Australia, Belgium, Brazil, Canada, China, Switzerland, Germany, France, Great Britain, India, Israel, Italy, Japan, Mexico, Luxembourg, Netherlands, New Zealand, Spain, and South Africa and 13 U.S. pending applications as well as 67 patent applications pending in ex-U.S. jurisdictions including Australia, Brazil, Canada, Chile, China, Europe, Hong Kong, Israel, India, Japan, Korea, Mexico, New Zealand, and Singapore, owned by us. We may not be able to apply for patents on certain aspects of our technology in a timely fashion or at all. Further, we may not be able to prosecute all necessary or desirable patent applications, or maintain, enforce and license any patents that may issue from such patent applications, at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. We may not have the right to control the preparation, filing and prosecution of any patent applications that we license from third parties, or the ability to maintain the rights to patents licensed to third parties, and should we decide to license any of our patents to third parties in the future, we may not retain sufficient rights to prosecute and enforce such patents. Our existing issued and granted patents and any future patents we obtain may not be sufficiently broad to prevent others from using our technology or from developing competing product candidates and technology. There is no guarantee that any of our pending patent applications will result in issued or granted patents, that any of our issued or granted patents will not later be found to be invalid or unenforceable or that any issued or granted patents will include claims that are sufficiently broad to cover our technology or to provide meaningful protection from our competitors. Moreover, the patent position of biotechnology and pharmaceutical companies can be highly uncertain because it involves complex legal and factual questions. We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our current and future proprietary technology and product candidates are covered by valid and enforceable patents or are effectively maintained as trade secrets. If third parties disclose or misappropriate our proprietary rights, it may materially and adversely affect our position in the market.
24
The U.S. Patent and Trademark Office, or USPTO, and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other provisions during the patent process. There are situations in which noncompliance can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, competitors might be able to enter the market earlier than would otherwise have been the case. The standards applied by the USPTO and foreign patent offices in granting patents are not always applied uniformly or predictably. For example, there is no uniform worldwide policy regarding patentable subject matter or the scope of claims allowable in biotechnology and pharmaceutical patents. As such, we do not know the degree of future protection that we will have on our drug delivery system. Accordingly, despite our efforts, we may be unable to prevent third parties from infringing upon or misappropriating our intellectual property. While we will endeavor to try to protect our technology with intellectual property rights such as patents, as appropriate, the process of obtaining patents is time consuming, expensive and sometimes unpredictable. The failure to adequately protect our intellectual property and other proprietary rights could materially harm our business.
We may be required to spend significant resources to monitor and protect our intellectual property rights. Monitoring unauthorized uses and disclosures is difficult and we do not know whether the steps we have taken to protect our proprietary technologies will be effective. The issuance of a patent is not conclusive as to its inventorship, scope, validity, or enforceability, and our patents may be challenged in the courts or patent offices in the U.S. and abroad. Any patents that are issued may subsequently be invalidated or otherwise limited, allowing other companies to develop offerings that compete with our offerings, which could adversely affect our competitive business position, business prospects and financial condition. In addition, issuance of a patent does not guarantee that we have a right to practice the patented invention. Once granted, patents may remain open to opposition, interference, re-examination, post-grant review, inter partes review, nullification or derivation action before patent offices for a given period after allowance or grant, during which time third parties can raise objections against such initial grant, or in court. In the course of such proceedings, which may continue for a protracted period of time, the patent owner may be compelled to limit the scope of the allowed or granted claims thus attacked, or may lose the allowed or granted claims altogether.
We may be subject to claims that former employees, collaborators or other third parties have an interest in our patents, trade secrets, or other intellectual property as an inventor or co-inventor. For example, we may have inventorship disputes arise from conflicting obligations of employees, consultants or others who are involved in developing our product candidates. Litigation may be necessary to defend against these and other claims challenging inventorship or our patents, trade secrets or other intellectual property. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, intellectual property that is important to our products. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees. Any of the foregoing could have a material adverse effect on our business, financial condition, and results of operations.
In addition, there can be no assurance that:
•others will not or may not be able to make, use or sell upper nasal space product candidates that are the same as or similar to any of our products but that are not covered by the claims of the patents that we own;
•we or our existing or future collaborators are the first to make the inventions covered by each of our issued patents and pending patent applications that we own;
•we, or our existing or future collaborators, are the first to file patent applications covering certain aspects of our inventions;
•others will not independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights;
•a third party will not challenge our patents and, if challenged, a court would hold that our patents are valid, enforceable and infringed;
•any issued patents that we own or have licensed will provide us with any competitive advantages, or will not be challenged by third parties;
•we may develop additional proprietary technologies that are patentable;
•the patents of others will not have a material or adverse effect on our business, financial condition, results of operations and prospects; and
25
•our competitors do not conduct research and development activities in countries where we do not have enforceable patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets.
If we, our licensor or collaborators fail to maintain the patents and patent applications covering our technology, our competitors might be able to enter the market, which could have a material and adverse effect on our business, financial condition, results of operations and prospects.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patent protection for certain aspects of our technology, we also consider trade secrets, including confidential and unpatented know-how, important to the maintenance of our competitive position. We protect trade secrets and confidential and unpatented know-how, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to such knowledge, such as our employees, corporate collaborators, outside scientific collaborators, CROs, CMOs, consultants, advisors and other third parties. We also enter into confidentiality and invention or patent assignment agreements with our employees and consultants that obligate them to maintain confidentiality and assign their inventions to us. Despite these efforts, we cannot be certain that such agreements have been entered into with all relevant parties. In addition, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts in the United States and certain foreign jurisdictions are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position would be harmed which could have a material and adverse effect on our business, financial condition, results of operations and prospects.
Other companies or organizations may challenge our or our licensor’s patent rights.
The issued patents and pending patent applications in the United States and in key markets around the world that we own or license claim many different devices, compositions and methods, including processes relating to the discovery, development, manufacture and commercialization of upper nasal space drug delivery. As the field of upper nasal space drug delivery continues to mature, patent applications are being processed by national patent offices around the world. There is uncertainty about which patents will issue and, if they do, as to when, to whom, and with what claims. In addition, third parties may attempt to invalidate our intellectual property rights. Even if our rights are not directly challenged, disputes could lead to the weakening of our intellectual property rights. Our defense against any attempt by third parties to circumvent or invalidate our intellectual property rights could be costly to us, could require significant time and attention of our management and could have a material and adverse effect on our business, financial condition, results of operations and prospects or our ability to successfully compete.
We may not be able to protect our intellectual property rights throughout the world.
Obtaining a valid and enforceable issued or granted patent covering our technology in the United States and worldwide can be extremely costly, and our or our licensors' or collaborators’ intellectual property rights may not exist in some countries outside the United States or may be less extensive in some countries than in the United States. In jurisdictions where we or our licensor or collaborators have not obtained patent protection, competitors may seek to use our or their technology to develop their own products and further, may export otherwise infringing products to territories where we or they have patent protection, but where it is more difficult to enforce a patent as compared to the United States. Competitor products may compete with our products in jurisdictions where we do not have issued or granted patents or where our or our licensors' or collaborators’ issued or granted patent claims or other intellectual property rights are not sufficient to prevent competitor activities in these jurisdictions. The legal systems of certain countries, particularly certain developing countries, make it difficult to enforce patents and such countries may not recognize other types of intellectual property protection, particularly relating to pharmaceuticals. This could make it difficult for us or our licensor or collaborators to prevent the infringement of our or their patents or marketing of competing products in violation of our or their proprietary rights generally in certain jurisdictions.
26
Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our and our licensor’s or collaborators’ efforts and attention from other aspects of our business, could put our and our licensor’s or collaborators’ patents at risk of being invalidated or interpreted narrowly, and our and our licensor’s or collaborators’ patent applications at risk of not issuing and could provoke third parties to assert claims against us or our licensor or collaborators. We or our licensor or collaborators may not prevail in any lawsuits that we or our licensor or collaborators initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful.
We have so far not filed for patent protection in all national and regional jurisdictions where such protection may be available. In addition, we may decide to abandon national and regional patent applications before grant. Finally, the grant proceeding of each national or regional patent is an independent proceeding which may lead to situations in which applications might in some jurisdictions be refused by the relevant registration authorities, while granted by others. It is also quite common that depending on the country, various scopes of patent protection may be granted on the same product candidate or technology.
The laws of some jurisdictions do not protect intellectual property rights to the same extent as the laws in the United States, and many companies have encountered significant difficulties in protecting and defending such rights in such jurisdictions. If we or our licensor or collaborators encounter difficulties in protecting, or are otherwise precluded from effectively protecting, the intellectual property rights important for our business in such jurisdictions, the value of these rights may be diminished and we may face additional competition from others in those jurisdictions. Many countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. If we or any of our licensor or collaborators are forced to grant a license to third parties with respect to any patents relevant to our business, our competitive position in the relevant jurisdiction may be impaired and our business, financial condition, results of operations and prospects may be adversely affected.
We, our collaborators, or any future strategic partners may need to resort to litigation to protect or enforce our patents or other proprietary rights, all of which could be costly, time consuming, delay or prevent the development and commercialization of our technology, or put our patents and other proprietary rights at risk.
Competitors may infringe our patents or other intellectual property. If we were to initiate legal proceedings against a third party to enforce a patent covering our technology, the defendant could counterclaim that our patent is invalid or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, for example, lack of novelty, obviousness or non-enablement. Grounds for an unenforceability assertion could be an allegation that an individual connected with prosecution of the patent withheld information material to patentability from the USPTO, or made a materially misleading statement, during prosecution. The outcome following legal assertions of invalidity and unenforceability during patent litigation is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of the patent protection on certain aspects of our platform technology. Such a loss of patent protection could have a material and adverse effect on our business, financial condition, results of operations and prospects.
Interference or derivation proceedings provoked by third parties or brought by us or declared by the USPTO may be necessary to determine the priority of inventions with respect to our patents or patent applications. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms or at all, or if a non-exclusive license is offered and our competitors gain access to the same technology. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions, or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of our common stock. Patents and other
27
intellectual property rights also will not protect our technology if competitors design around our protected technology without legally infringing our patents or other intellectual property rights.
Intellectual property rights of third parties could adversely affect our ability to commercialize our technology, and we, our licensor or collaborators, or any future strategic partners may become subject to third party claims or litigation alleging infringement of patents or other proprietary rights or seeking to invalidate patents or other proprietary rights. We might be required to litigate or obtain licenses from third parties in order to develop or market our technology. Such litigation or licenses could be costly or not available on commercially reasonable terms.
We, our collaborators, or any future strategic partners may be subject to third-party claims for infringement or misappropriation of patent or other proprietary rights. There is a substantial amount of litigation, both within and outside the United States, involving patent and other intellectual property rights in the biotechnology and pharmaceutical industries, including patent infringement lawsuits, interferences, oppositions, post grant review and inter partes review proceedings before the USPTO, and corresponding foreign patent offices. We have previously received communications from third parties claiming that our technology infringes on their patents. While we do not believe that these claims have merit, we cannot be certain that these third parties would not pursue infringement claims against us. There are issued and pending patents that might claim aspects of our technology, and modifications that we may need to apply to our technology. Thus, it is possible that one or more individuals or organizations will hold patent rights to which we will need a license. If those individuals or organizations refuse to grant us a license to such patent rights or refuse to grant us a license on reasonable terms, we may not be able to market product candidates or perform research and development or other activities covered by these patents which could have a material and adverse effect on our business, financial condition, results of operations and prospects. We are obligated under certain of our license and collaboration agreements to indemnify and hold harmless our licensor or collaborators for damages arising from intellectual property infringement by us. If we, our licensor or collaborators, or any future strategic partners are found to infringe a third-party patent or other intellectual property rights, we could be required to pay damages, potentially including treble damages, if we are found to have infringed willfully. In addition, we, our licensor or collaborators, or any future strategic partners may choose to seek, or be required to seek, a license from a third party, which may not be available on acceptable terms, if at all. Even if a license can be obtained on acceptable terms, the rights may be non-exclusive, which could give our competitors access to the same technology or intellectual property rights licensed to us. If we fail to obtain a required license, we or our existing or future collaborators may be unable to effectively market our technology, which could limit our ability to generate revenue or achieve profitability and possibly prevent us from generating revenue sufficient to sustain our operations. In addition, we may find it necessary to pursue claims or initiate lawsuits to protect or enforce our patent or other intellectual property rights. The cost to us in defending or initiating any litigation or other proceeding relating to patent or other proprietary rights, even if resolved in our favor, could be substantial, and litigation could divert our management’s attention. Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could delay our research and development efforts and limit our ability to continue our operations.
Because the upper nasal space therapeutics landscape is still evolving, it is difficult to conclusively assess our freedom to operate without infringing on third-party rights. Our competitive position may suffer if patents issued to third parties or other third-party intellectual property rights cover our technology or elements thereof, or our manufacture or uses relevant to our development plans. In such cases, we may not be in a position to develop or commercialize our technology until such patents expire or unless we successfully pursue litigation to nullify or invalidate the third-party intellectual property right concerned, or enter into a license agreement with the intellectual property right holder, if available on commercially reasonable terms. There may be issued patents held by third parties of which we are not aware that, if found to be valid and enforceable, could be alleged to be infringed by our POD nasal drug delivery platform and related technologies. There also may be pending patent applications of which we are not aware that may result in issued patents, which could be alleged to be infringed by our POD nasal drug delivery platform and related technologies. If such an infringement claim should be brought and be successful, we may be required to pay substantial damages, including potentially treble damages and attorneys’ fees for willful infringement, and we may be forced to abandon our technology or seek a license from any patent holders. No assurances can be given that a license will be available on commercially reasonable terms, if at all.
28
It is also possible that we have failed to identify relevant third-party patents or applications. For example, U.S. applications filed before November 29, 2000 and certain U.S. applications filed after that date that will not be filed outside the United States remain confidential until patents issue. Patent applications in the United States and elsewhere are published approximately 18 months after the earliest filing for which priority is claimed, with such earliest filing date being commonly referred to as the priority date. Therefore, patent applications covering any future platform technology could have been filed by others without our knowledge. Additionally, pending patent applications that have been published can, subject to certain limitations, be later amended in a manner that could cover our platform technology. Third-party intellectual property right holders may also actively bring infringement claims against us. We cannot guarantee that we will be able to successfully settle or otherwise resolve such infringement claims. If we are unable to successfully settle future claims on terms acceptable to us, we may be required to engage in or continue costly, unpredictable and time-consuming litigation. Parties making claims against us may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation or administrative proceedings, there is a risk that some of our confidential information could be compromised by disclosure. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have material adverse effect on our ability to raise additional funds or otherwise have a material adverse effect on our business, results of operations, financial condition and prospects. If we fail in any such dispute, in addition to being forced to pay damages, we may be temporarily or permanently prohibited from commercializing any of our technology that are held to be infringing. We might, if possible, also be forced to redesign our technology so that we no longer infringe the third-party intellectual property rights. Any of these events, even if we were ultimately to prevail, could require us to divert substantial financial and management resources that we would otherwise be able to devote to our business and could have a material and adverse effect on our business, financial condition, results of operations and prospects.
Intellectual property litigation could cause us to spend substantial resources and distract our personnel from their normal responsibilities.
Litigation or other legal proceedings relating to intellectual property claims, with or without merit, is unpredictable and generally expensive and time consuming and is likely to divert significant resources from our core business, including distracting our technical and management personnel from their normal responsibilities. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. Moreover, such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities.
We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources and more mature and developed intellectual property portfolios. Accordingly, despite our efforts, we may not be able to prevent third parties from infringing upon or misappropriating or from successfully challenging our intellectual property rights. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
We may be subject to claims that we or our employees, consultants or independent contractors have wrongfully used or disclosed confidential information or alleged trade secrets of third parties or their former employers. These claims may be costly to defend and if we do not successfully do so, we may be required to pay monetary damages and may lose valuable intellectual property rights or personnel.
Many of our employees were previously employed at universities or biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although no claims against us are currently pending, we may be subject to claims that these employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. A loss of key research personnel or their work product could hamper our ability to commercialize, or prevent us from commercializing, our technology, which could severely harm our
29
business. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
Patent terms may be inadequate to protect our competitive position on our technology for an adequate amount of time.
Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our technology are obtained, once the patent life has expired, we may be open to competition from competitive products, including generics or biosimilars. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to our products.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents or applications will be due to be paid to the USPTO and various governmental patent agencies outside of the United States in several stages over the lifetime of the patents or applications. We have systems in place to remind us to pay these fees, and we employ an outside firm or rely on our outside counsel to pay these fees due to the USPTO and non-U.S. patent agencies. The USPTO and various non-U.S. governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. We employ reputable law firms and other professionals to help us comply, and in many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules. However, there are situations in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, our competitors might be able to enter the market and this circumstance would have a material adverse effect on our business.
Changes in U.S. patent and ex-U.S. patent laws could diminish the value of patents in general..
Changes in either the patent laws or interpretation of the patent laws in the United States or in other ex-U.S. jurisdictions could increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. In the United States, numerous recent changes to the patent laws and proposed changes to the rules of the USPTO may have a significant impact on our ability to protect our technology and enforce our intellectual property rights. Additionally, the U.S. Supreme Court has ruled on several patent cases in recent years, some of which cases either narrow the scope of patent protection available in certain circumstances or weaken the rights of patent owners in certain situations. For example, the decision by the U.S. Supreme Court in Association for Molecular Pathology v. Myriad Genetics, Inc. precludes a claim to a nucleic acid having a stated nucleotide sequence that is identical to a sequence found in nature and unmodified. We currently are not aware of an immediate impact of this decision on our patents or patent applications because we may develop product candidates that contain modifications that we believe are not found in nature. However, this decision has yet to be unambiguously interpreted by courts and by the USPTO. We cannot assure you that the interpretations of this decision or subsequent rulings will not adversely impact our patents or patent applications. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts and the USPTO, and similar legislative and regulatory bodies in other countries in which may pursue patent protection, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
After March 2013, under the Leahy-Smith America Invents Act, or the America Invents Act, enacted in September 2011, the U.S. transitioned to a first inventor to file system in which, assuming that other requirements for patentability are met, the first inventor to file a patent application will be entitled to the patent on an invention regardless of whether a third party was the first to invent the claimed invention. Assuming that other requirements
30
for patentability are met, prior to March 2013, in the U.S., the first to invent the claimed invention was entitled to the patent, while outside the U.S., the first to file a patent application was entitled to the patent. A third party that files a patent application in the USPTO after March 2013, but before we do, could therefore be awarded a patent covering an invention of ours even if we had made the invention before it was made by such third party. This will require us to be cognizant going forward of the time from invention to filing of a patent application. Since patent applications in the U.S. and most other countries are confidential for a period of time after filing or until issuance, we cannot be certain that we were the first to invent any of the inventions claimed in our patents or patent applications. The America Invents Act also includes a number of significant changes that affect the way patent applications will be prosecuted and also may affect patent litigation. These include allowing third-party submission of prior art to the USPTO during patent prosecution and additional procedures to attack the validity of a patent by USPTO administered post-grant proceedings, including post-grant review, inter partes review, and derivation proceedings. Because of a lower evidentiary standard in USPTO proceedings compared to the evidentiary standard in U.S. federal courts necessary to invalidate a patent claim, a third party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party may attempt to use the USPTO procedures to invalidate our patent claims that would not have been invalidated if first challenged by the third party as a defendant in a district court action. Therefore, the America Invents Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business, financial condition, and results of operations.
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names or may be forced to stop using these names, which we need for name recognition by potential partners or customers in our markets of interest. If we are unable to establish name recognition based on our trademarks and trade names, we may not be able to compete effectively which could have a material and adverse effect on our business, financial condition, results of operations and prospects.
Risks Related to Our Employee Matters, Managing Growth and Other Risks Related to Our Business
We incur increased costs as a result of operating as a public company, and our management is required to devote substantial time to compliance initiatives and corporate governance practices.
As a public company, and particularly after we are no longer an emerging growth company, we will continue to incur significant legal, accounting and other expenses on an ongoing basis that we did not incur as a private company. The Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the listing requirements of The Nasdaq Global Market, or Nasdaq, and other applicable securities rules and regulations impose various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. We will need to hire additional accounting, finance and other personnel and make further investments in processes and systems in connection with these ongoing efforts. Our management and other personnel devote a substantial amount of time to these compliance initiatives. Moreover, we expect these rules and regulations to substantially increase our legal and financial compliance costs and to make some activities more time consuming and costly. For example, we expect that these rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to incur substantial costs to maintain sufficient coverage. We cannot predict or estimate the amount or timing of additional costs we may incur to respond to these requirements and future changes to such requirements. The impact of these requirements could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees or as executive officers. Moreover, these rules and regulations are often subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices.
Pursuant to Section 404, we are required to furnish a report by our management on our internal control over financial reporting. However, while we remain an emerging growth company or a non-accelerated filer, we will not
31
be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. To achieve compliance with Section 404 within the prescribed period, we will be engaged in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented and implement a continuous reporting and improvement process for internal control over financial reporting. Despite our efforts, there is a risk that we will not be able to conclude, within the prescribed timeframe or at all, that our internal control over financial reporting is effective as required by Section 404. If we identify one or more material weaknesses, it could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our financial statements. In addition, if we are not able to continue to meet these requirements, we may not be able to remain listed on Nasdaq.
Further, if we seek relief under Chapter 11 of the U.S. Bankruptcy Code, we will incur substantial additional professional service fees to assist us through such process. Such fees may be substantial and materially limit potential recovery, if any, for holders of our common stock in a bankruptcy proceeding.
Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements and insider trading.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include intentional failures to comply with FDA regulations, to provide accurate information to the FDA, to comply with manufacturing standards we have established, to comply with federal and state health care fraud and abuse laws and regulations, to report financial information or data accurately or to disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the health care industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. Employees may also misappropriate information in violation of applicable insider trading laws, which could also seriously harm our reputation even if we are not deemed to be at fault. It is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, disgorgement, possible exclusion from participation in Medicare, Medicaid and other federal health care programs, contractual damages, reputational harm, diminished profits and future earnings, and curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
If product liability lawsuits are brought against us, we may incur substantial liabilities.
We face an inherent risk of product liability as a result of the commercial sale of Trudhesa. For example, we may be sued if any product we develop allegedly causes injury or is found to be otherwise unsuitable during product testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability, and a breach of warranties. Claims could also be asserted under state consumer protection acts. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities. Even a successful defense would require significant financial and management resources.
Regardless of the merits or eventual outcome, liability claims may result in:
•injury to our reputation;
•withdrawal of clinical trial participants;
•costs to defend the related litigations;
•a diversion of management’s time and our resources;
•substantial monetary awards to trial participants or patients;
32
•product recalls, withdrawals, or labeling, marketing or promotional restrictions;
•a decline in our stock price.
Failure to obtain and retain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of our products. We currently carry product liability insurance covering the commercial sale of Trudhesa and our clinical trials. Although we maintain such insurance, any claim that may be brought against us could result in a court judgment or settlement in an amount that is not covered, in whole or in part, by our insurance or that is in excess of the limits of our insurance coverage. Our insurance policies also have various exclusions, and we may be subject to a product liability claim for which we have no coverage. We will have to pay any amounts awarded by a court or negotiated in a settlement that exceed our coverage limitations or that are not covered by our insurance, and we may not have, or be able to obtain, sufficient capital to pay such amounts. If we are unable to obtain or maintain sufficient insurance coverage at an acceptable cost or to otherwise protect against potential product liability claims, we could adversely affect our business, financial condition, and results of operations.
The security of the information technology systems used in our business may be compromised, and confidential information, including non-public personal information, could be improperly disclosed.
Our information technology systems, and those of our contractors, service providers and consultants, may be vulnerable to physical or electronic intrusions, computer viruses or other attacks, as well as employee, vendor, or contractor errors or malfeasance. As part of our business, we and our contractors and consultants maintain large amounts of confidential information, including non-public personal information on patients and our employees. Breaches in security and other information security events and incidents, including from ransomware, other malicious code, and other cyberattacks, could result in interruption to our systems and operations, or those of our contractors, consultants or our respective service providers, and the loss, unavailability, and unauthorized modification, use, acquisition or disclosure of information, including information subject to intellectual property protection or for which the loss or other compromise of such information may lead to the loss of intellectual property protection. Any such breach or other incident may result in significant costs to remediate and otherwise respond, including efforts to analyze, correct, eliminate, remediate or work around deficiencies in our systems or our security measures, recover and validate data, and to address any applicable legal or contractual obligations. Further, any actual or perceived breach in security or security incident may result in potential regulatory actions or litigation, including material claims for damages, interruption to our operations, delays in regulatory filings and approvals, damage to our reputation or otherwise have a material adverse effect on our business, financial condition and operating results. Like many businesses, we have been in the past, and may again be in the future, subject to phishing attacks. In 2018 we experienced a successful phishing attack. While we were able to swiftly contain and remediate this incident, without a material impact to our business, there can be no assurances that we will be able to defend against or successfully remediate any such attacks that may occur in the future. Further, companies have experienced an increase in phishing and social engineering attacks from third parties, including in connection with the recent COVID-19 pandemic. Also, due to the COVID-19 pandemic, the majority of our employees continue to work remotely as of September 30, 2023. As a result, we may have increased cybersecurity and data security risks, due to increased use of home wi-fi networks and virtual private networks, as well as increased disbursement of physical machines. While we have implemented IT controls to reduce the risk of a cybersecurity or data security breach or incident, there is no guarantee that these measures will be adequate to safeguard all systems, especially with an increased number of employees working remotely. While we expect to implement and maintain appropriate information security policies and systems in order to prevent unauthorized loss, unavailability, modification, use or disclosure of confidential information, including non-public personal information and other information relating to individuals, there can be no assurance that any such loss, unavailability, modification, use or disclosure will not occur. We incur significant costs in an effort to detect and prevent security breaches and other security-related incidents and we expect our costs will increase as we make improvements to our systems, policies and processes to prevent further breaches and incidents. In the event of a future breach or incident, we could be required to expend additional significant capital and other resources in an effort to prevent further breaches or incidents, which may require us to divert substantial resources. Moreover, we could be required or otherwise find it appropriate to expend significant capital and other resources to respond to, notify third parties of, and otherwise address the incident or breach and its root cause. Each of these could require us to divert substantial resources.
33
While we maintain insurance with respect to cybersecurity, our insurance may be insufficient to cover all liabilities incurred by us in connection with any privacy or cybersecurity incidents. We also cannot be certain that any insurance coverage will be adequate for data handling or data security liabilities actually incurred, that insurance will continue to be available to us on economically reasonable terms, or at all, or that any insurer will not deny coverage as to any future claim. The successful assertion of one or more large claims against us that exceed available insurance coverage, or the occurrence of changes in our insurance policies, including premium increases or the imposition of large deductible or co-insurance requirements, could have a material adverse effect on our business, including our financial condition, operating results and reputation.
Our ability to utilize our net operating loss carryforwards and certain other tax attributes may be limited.
We have incurred substantial losses during our history and do not expect to become profitable in the near future, and we may never achieve profitability. Unused losses incurred in taxable years beginning on or prior to December 31, 2017, will carry forward to offset future taxable income, if any, until such unused losses expire. Under the Tax Reform Act, as modified by the Coronavirus Aid, Relief and Economic Security Act, or the CARES Act, unused U.S. federal net operating losses generated in tax years beginning after December 31, 2017, will not expire and may be carried forward indefinitely but the deductibility of such federal net operating losses is limited to 80% of current year taxable income in taxable years beginning after December 31, 2020. As a result, our net operating loss carryforwards generated in taxable years beginning on or before December 31, 2017, may expire prior to being used, and the deductibility of our net operating loss carryforwards generated in taxable years beginning after December 31, 2017 in taxable years beginning after December 31, 2020, may be limited. It is uncertain if and to what extent various states will conform to the Tax Reform Act or the CARES Act. In addition, both our current and our future unused losses and other tax attributes may be subject to limitation under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended (the Code) if we undergo, or have undergone, an “ownership change,” generally defined as a greater than 50 percentage point change (by value) in our equity ownership by certain stockholders over a three-year period. We have not completed a Section 382 study to assess whether an ownership change has occurred or whether there have been multiple ownership changes since our formation due to the complexity and cost associated with such a study and the fact that there may be additional ownership changes in the future. If we undergo an ownership change (or if we previously underwent such an ownership change), our ability to use all of our pre-change net operating loss carryforwards and other pre-change tax attributes (such as research tax credits) to offset our post-change income or taxes may be limited. Similar provisions of state tax law may also apply to limit our use of accumulated state tax attributes. In addition, at the state level, there may be periods during which the use of net operating losses is suspended or otherwise limited, which could accelerate or permanently increase state taxes owed. As a result, even if we attain profitability, we may be unable to use all or a material portion of our net operating losses and other tax attributes, which could adversely affect our future cash flows.
Changes in U.S. tax law could adversely affect our financial condition and results of operations.
The rules dealing with U.S. federal, state, and local income taxation are constantly under review by persons involved in the legislative process and by the Internal Revenue Service, or IRS, and the U.S. Treasury Department. Changes to tax laws (which changes may have retroactive application) could adversely affect us or holders of our common stock. In recent years, many such changes have been made and changes are likely to continue to occur in the future. For example, on March 27, 2020, the CARES Act was enacted, which included certain changes in tax law intended to stimulate the U.S. economy in light of the COVID-19 coronavirus outbreak, including temporary beneficial changes to the treatment of net operating losses, interest deductibility limitations and payroll tax matters. Future changes in U.S. tax laws could have a material adverse effect on our business, cash flow, financial condition or results of operations. We urge investors to consult with their legal and tax advisors regarding the implications of potential changes in U.S. tax laws on an investment in our common stock.
Risks Related to Our Common Stock
Our principal stockholders and management own a significant percentage of our stock and will be able to exert significant control over matters subject to stockholder approval.
As of September 30, 2023, our executive officers, directors and their respective affiliates owned a substantial portion of our voting stock. As a result, these stockholders, if acting together, have significant influence over the outcome of corporate actions requiring stockholder approval, including the election of directors, amendment of
34
our organizational documents, any merger, consolidation or sale of all or substantially all of our assets and any other significant corporate transaction. The interests of these stockholders may not be the same as or may even conflict with your interests. For example, these stockholders could delay or prevent a change of control of our company, even if such a change of control would benefit our other stockholders, which could deprive our stockholders of an opportunity to receive a premium for their common stock as part of a sale of our company or our assets and might affect the prevailing market price of our common stock. The significant concentration of stock ownership may adversely affect the trading price of our common stock due to investors’ perception that conflicts of interest may exist or arise.
We are an “emerging growth company” and we cannot be certain if the reduced reporting requirements applicable to emerging growth companies will make our common stock less attractive to investors.
We are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including (i) not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, (ii) reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and (iii) exemptions from the requirements of holding nonbinding advisory stockholder votes on executive compensation and stockholder approval of any golden parachute payments not approved previously. In addition, as an emerging growth company, we are only required to provide two years of audited financial statements and two years of selected financial data in the annual reports.
We could be an emerging growth company until December 31, 2026, although circumstances could cause us to lose that status earlier, including if we are deemed to be a “large accelerated filer,” which occurs when the market value of our common stock that is held by non-affiliates exceeds $700 million as of the prior September 30, or if we have total annual gross revenue of $1.07 billion or more during any fiscal year before that time, in which cases we would no longer be an emerging growth company as of the following December 31, or if we issue more than $1.0 billion in non-convertible debt during any three-year period before that time, in which case we would no longer be an emerging growth company immediately. Even after we no longer qualify as an emerging growth company, we may still qualify as a “smaller reporting company,” which would allow us to take advantage of many of the same exemptions from disclosure requirements, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act and reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements. We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our share price may be more volatile.
Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have elected to take advantage of the benefits of this extended transition period. Our financial statements may therefore not be comparable to those of companies that comply with such new or revised accounting standards. Until the date that we are no longer an “emerging growth company” or affirmatively and irrevocably opt out of the exemption provided by Section 7(a)(2)(B) of the Securities Act, upon issuance of a new or revised accounting standard that applies to our financial statements and that has a different effective date for public and private companies, we will disclose the date on which adoption is required for non-emerging growth companies and the date on which we will adopt the recently issued accounting standard.
Anti-takeover provisions in our restated certificate of incorporation and our restated bylaws and under Delaware or Washington law could make an acquisition of our business, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Our restated certificate of incorporation and our restated bylaws contain provisions that could delay or prevent a change in control of our company. These provisions could also make it difficult for stockholders to elect directors who are not nominated by current members of our board of directors or take other corporate actions, including effecting changes in our management. These provisions:
35
•establish a classified board of directors so that not all members of our board are elected at one time;
•permit only the board of directors to establish the number of directors and fill vacancies on the board;
•provide that directors may only be removed “for cause” and only with the approval of two-thirds of our stockholders;
•require super-majority voting to amend some provisions in our restated certificate of incorporation and restated bylaws;
•authorize the issuance of “blank check” preferred stock that our board could use to implement a stockholder rights plan;
•eliminate the ability of our stockholders to call special meetings of stockholders;
•prohibit stockholder action by written consent, which requires all stockholder actions to be taken at a meeting of our stockholders;
•prohibit cumulative voting; and
•establish advance notice requirements for nominations for election to our board or for proposing matters that can be acted upon by stockholders at annual stockholder meetings.
Moreover, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, or DGCL, which prohibits a person who owns in excess of 15% of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner. Likewise, because our principal executive offices are located in Washington, the anti-takeover provisions of the Washington Business Corporation Act may apply to us under certain circumstances now or in the future. These provisions prohibit a “target corporation” from engaging in any of a broad range of business combinations with any stockholder constituting an “acquiring person” for a period of five years following the date on which the stockholder became an “acquiring person.” Any of these provisions of our charter documents or Delaware or Washington law could, under certain circumstances, depress the market price of our common stock.
Our restated certificate of incorporation designates the Court of Chancery of the State of Delaware as the sole and exclusive forum for certain types of actions and proceedings that may be initiated by our stockholders and our restated bylaws designate federal district courts as the sole and exclusive forum for actions under the Securities Act, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers, employees, or agents.
Our restated certificate of incorporation provides that the Court of Chancery of the State of Delaware will be the exclusive forum for the following types of actions or proceedings under the DGCL: any derivative action or proceeding brought on our behalf; any action asserting a breach of fiduciary duty; any action asserting a claim against us arising pursuant to the DGCL, our restated certificate of incorporation, or our restated bylaws; or any action asserting a claim against us that is governed by the internal affairs doctrine. This exclusive forum provision does not apply to suits brought to enforce a duty or liability created by the Exchange Act or any other claim for which the U.S. federal courts have exclusive jurisdiction. It could apply, however, to a suit that falls within one or more of the categories enumerated in the exclusive forum provision.
Our restated bylaws also provide that the federal district courts of the United States of America is the exclusive forum for the resolution of any complaint asserting a cause of action under the Securities Act. The enforceability of similar exclusive federal forum provisions in other companies’ organizational documents has been challenged in legal proceedings, and while the Delaware Supreme Court has ruled that this type of exclusive federal forum provision is facially valid under Delaware law, there is uncertainty as to whether other courts would enforce such provisions and that investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder.
These choice of forum provisions may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or any of our directors, officers, or other employees, which may discourage lawsuits with respect to such claims. Alternatively, if a court were to find the choice of forum provisions contained in our restated certificate of incorporation or restated bylaws to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could harm our business, operating results and financial condition.
36
General Risk Factors
Natural disasters, catastrophic events and calamities including epidemics and pandemics may disrupt our business.
Natural disasters or other catastrophic events may damage or disrupt our operations and thus could harm our business. For example, our headquarters are located in Seattle, Washington, an earthquake-prone area. A natural disaster or catastrophic event in Seattle could interrupt our operations and impair access to internal systems, documents, and materials critical to the operation and growth of our business.
Further, occurrences of epidemics or pandemics, depending on their scale, may result in damage to the national and local economies within our geographic area. Global economic conditions may be disrupted by widespread outbreaks of infectious or contagious diseases, and such disruption may adversely affect clinical development plans.
As we grow, the need for business continuity planning and disaster recovery plans will become increasingly important. We may incur substantial expenses as a result of the limited nature of our disaster recovery and business continuity plans, which could have a material adverse effect on our business. If we are unable to develop adequate plans to ensure that our business functions continue to operate during and after a disaster, and successfully execute on those plans in the event of a disaster or emergency, our business could be harmed.
We and our CMOs must comply with environmental, health and safety laws and regulations, and failure to comply with these laws and regulations could expose us to significant costs or liabilities.
We and our CMOs are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the use, generation, manufacture, distribution, storage, handling, treatment, remediation and disposal of hazardous materials and wastes. Hazardous chemicals, including flammable and biological materials, are involved in certain aspects of our business, and we cannot eliminate the risk of injury or contamination from the use, generation, manufacture, distribution, storage, handling, treatment or disposal of hazardous materials and wastes. In the event of contamination or injury, or failure to comply with environmental, health and safety laws and regulations, we could be held liable for any resulting damages and any such liability could exceed our assets and resources. We could also incur significant costs associated with civil or criminal fines and penalties for failure to comply with such laws and regulations. We are uninsured for third-party injury from contamination.
Environmental, health and safety laws and regulations are becoming increasingly more stringent. We may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Our failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
Further, with respect to the operations of our CMOs, it is possible that if they fail to operate in compliance with applicable environmental, health and safety laws and regulations or properly dispose of wastes, we could be held liable for any resulting damages, suffer reputational harm or experience a disruption in the manufacture and supply of our products.
We may be subject to securities litigation, which is expensive and could divert management attention.
The market price of our common stock may be volatile and, in the past, companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs and divert our management’s attention from other business concerns, which could seriously harm our business.
If securities or industry analysts do not publish research or reports about our business, or if they issue an adverse or misleading opinion regarding our common stock, our stock price and trading volume could decline.
The trading market for our common stock can be influenced by the research and reports that industry or securities analysts publish about us or our business. We do not currently have and may never obtain research coverage by
37
securities and industry analysts. If no or few securities or industry analysts commence coverage of us, the trading price for our common stock could be impacted negatively. In the event we obtain securities or industry analyst coverage, if any of the analysts who cover us issue an adverse or misleading opinion regarding us, our business model, our intellectual property or our stock performance, or if our nonclinical studies and clinical trials and operating results fail to meet the expectations of analysts, our stock price would likely decline. If one or more of such analysts cease coverage of us or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause a decline in our stock price or trading volume.
38
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and documents incorporated herein by reference contain “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements involve a number of risks and uncertainties. We caution readers that any forward-looking statement is not a guarantee of future performance and that actual results could differ materially from those contained in the forward-looking statement. These statements are based on current expectations of future events.
Such statements include, but are not limited to, statements regarding expectations and intentions, costs and expenses, our expectations regarding our plans to explore strategic alternatives, including halting development on certain product candidates, our need to raise substantial additional capital, our statements regarding the outcome of contingencies, financial condition, results of operations, liquidity, cost savings, objectives of management, debt financing, our future results of operations and financial position, our commercialization strategy, business strategies, market size, potential growth opportunities, clinical development activities, efficacy and safety profile of our product candidates, timing and results of our development activities, preclinical studies and clinical trials, the receipt and timing of potential regulatory designations, the achievement of clinical and commercial milestones, the advancement of our technologies and our proprietary product candidates, the approvals and commercialization of Trudhesa, our ability to attract and retain qualified employees and key personnel and other statements that are not historical facts. In some cases you can identify these statements by forward-looking words such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “could,” “would,” “project,” “plan,” “expect” or the negative or plural of these words or similar expressions, in documents incorporated by reference into this prospectus or any free writing prospectus. We intend that such forward-looking statements be subject to the safe harbors created thereby.
These forward-looking statements are based on the current beliefs and expectations of our management and are subject to significant risks and uncertainties. If underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results may differ materially from current expectations and projections. Factors that might cause such a difference include those discussed in our most recent Annual Report on Form 10-K and any subsequent Quarterly Report on Form 10-Q, as well as those discussed in this prospectus, the documents incorporated by reference into this prospectus and any free writing prospectus. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this prospectus or, in the case of documents referred to or incorporated by reference, the date of those documents.
All subsequent written or oral forward-looking statements attributable to us or any person acting on our behalf are expressly qualified in their entirety by the cautionary statements contained or referred to in this section. We do not undertake any obligation to release publicly any revisions to these forward-looking statements to reflect events or circumstances after the date of this prospectus or to reflect the occurrence of unanticipated events, except as may be required under applicable U.S. securities law. If we do update one or more forward-looking statements, no inference should be drawn that we will make additional updates with respect to those or other forward-looking statements.
39
USE OF PROCEEDS
We may issue and sell shares of our common stock having aggregate sales proceeds of up to $9.0 million from time to time. Because there is no minimum offering amount required as a condition to close this offering, the actual total public offering amount, commissions and proceeds to us, if any, are not determinable at this time. There can be no assurance that we will sell any shares under or fully utilize the Sales Agreement as a source of financing.
We currently intend to use the net proceeds from the sale of securities under this prospectus for general corporate purposes, which may include increasing our working capital, reducing indebtedness, ongoing commercialization activities and market development of Trudhesa, and capital expenditures. Pending the application of the net proceeds, we intend to invest the net proceeds in short-term or long-term, investment-grade, interest-bearing securities. Pursuant to the terms of the Amended Credit Agreement, if we raise proceeds in an equity financing, including this offering, that exceed $5,000,000, we will apply 50% of such excess proceeds to repay the tranche B term loans.
The amounts and timing of our actual expenditures will depend on numerous factors, including the progress of our clinical trials and other development efforts and other factors described under “Risk Factors” in this prospectus, the accompanying base prospectus and the documents incorporated by reference herein and therein, as well as the amount of cash used in our operations. We may find it necessary or advisable to use the net proceeds for other purposes, and we will have broad discretion in the application of the net proceeds. Pending the uses described above, we plan to invest the net proceeds from this offering in short-term or long-term, investment-grade, interest-bearing securities.
40
DIVIDEND POLICY
We have never declared or paid any cash dividends on our capital stock. We intend to retain future earnings, if any, to finance the operation and expansion of our business and do not anticipate paying any cash dividends in the foreseeable future. Any future determination related to our dividend policy will be made at the discretion of our board of directors after considering our financial condition, results of operations, capital requirements, business prospects and other factors the board of directors deems relevant, and subject to the restrictions contained in any future financing instruments. Our future ability to pay cash dividends on our capital stock is limited by the terms of our existing loan and security agreement with Oaktree Fund Administration, LLC as administrative agent, and the lenders party thereto.
41
DILUTION
If you invest in our common stock, your interest will be diluted to the extent of the difference between the price per share you pay in this offering and the net tangible book value per share of our common stock immediately after this offering.
Our net tangible book value of our common stock as of June 30, 2023 was approximately $(79.2) million, or approximately $(3.33) per share of common stock based upon 23,749,005 shares outstanding. Net tangible book value per share is equal to our total tangible assets, less our total liabilities, divided by the total number of shares outstanding as of June 30, 2023.
After giving effect to the sale of our common stock in the aggregate amount of $9.0 million at an assumed offering price of $0.411 per share, the last reported sale price of our common stock on The Nasdaq Global Market on October 4, 2023, and after deducting commissions and estimated offering expenses payable by us, our as adjusted net tangible book value as of June 30, 2023 would have been $(70.7) million, or $(1.55) per share of common stock. This represents an immediate increase in net tangible book value of $1.78 per share to our existing stockholders and an immediate dilution in net tangible book value of $1.96 per share to new investors in this offering.
The following table illustrates this calculation on a per share basis. The as-adjusted information is illustrative only and will adjust based on the actual price to the public, the actual number of shares sold and other terms of the offering determined at the time shares of our common stock are sold pursuant to this prospectus. The as-adjusted information assumes that all of our common stock in the aggregate amount of $9.0 million is sold at the assumed offering price of $0.411 per share, the last reported sale price of our common stock on The Nasdaq Global Market on June 30, 2023. The shares sold in this offering, if any, will be sold from time to time at various prices.
|
|
|
|
|
|
|
|
|
Assumed public offering price per share |
|
|
|
|
|
$ |
0.411 |
|
Historical net tangible book value per share as of June 30, 2023 |
|
|
$(3.33) |
|
|
|
|
|
Increase in net tangible book value per share attributable to the offering |
|
|
1.78 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As adjusted net tangible book value per share after giving effect to the offering |
|
|
|
|
|
|
(1.55) |
|
|
|
|
|
|
|
|
|
|
Dilution per share to new investors participating in the offering |
|
|
|
|
|
$ |
1.96 |
|
|
|
|
|
|
|
|
|
|
The number of shares of our common stock to be outstanding after this offering is based on 23,749,005 shares of our common stock outstanding as of June 30, 2023, and excludes:
•4,629,027 shares of common stock issuable upon the exercise of options outstanding as of June 30, 2023, with a weighted-average exercise price of $6.82;
•20,000 shares of our common stock issuable upon the exercise of stock options granted after June 30, 2023, with a weighted-average exercise price of $1.31 per share;
•252,500 shares of our common stock issuable upon the vesting and settlement of restricted stock units, or RSUs, outstanding as of June 30, 2023;
•94,688 shares of our common stock issuable upon the exercise of warrants outstanding as of June 30, 2023, with a weighted-average exercise price of $9.51 per share;
•4,749,900 shares of our common stock issuable upon the exercise of warrants issued or issuable to our lenders pursuant to the Amended Credit Agreement after June 30, 2023 with an exercise price of $0.01 per share; and
•2,894,973 shares of common stock reserved for future issuance under our stock-based compensation plans as of June 30, 2023, consisting of (i) 2,150,350 shares of common stock reserved for future
42
issuance under our 2021 Equity Incentive Plan as of June 30, 2023 and (ii) 744,623 shares of common stock reserved for future issuance under our 2021 Employee Stock Purchase Plan.
Except as otherwise indicated, all information in this prospectus does not assume or give effect to any exercise of outstanding options after June 30, 2023.
43
DESCRIPTION OF CAPITAL STOCK
General
The following is a summary description of the material terms of our capital stock. The description of capital stock is intended as a summary and is qualified in its entirety by reference to our restated certificate of incorporation, as amended (“certificate of incorporation”), and our restated bylaws, as amended (“bylaws”), copies of which are filed as exhibits to our most recent Annual Report on Form 10-K and are incorporated by reference herein. We encourage you to read our certificate of incorporation, our bylaws and the applicable provisions of the Delaware General Corporation Law, or DGCL, for additional information.
Our certificate of incorporation authorizes us to issue up to 300,000,000 shares of common stock, $0.001 par value per share, and 10,000,000 shares of preferred stock, $0.001 par value per share.
As of June 30, 2023, there were 23,749,005 shares of our common stock outstanding, and no shares of preferred stock outstanding.
Common Stock
Voting Rights
Holders of our common stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders. We have not provided for cumulative voting for the election of directors in our restated certificate of incorporation, which means that holders of a majority of the shares of our common stock will be able to elect all of our directors.
Dividends
Subject to preferences that may apply to any shares of preferred stock outstanding at the time, the holders of our common stock are entitled to receive dividends out of funds legally available if our board of directors, in its discretion, determines to issue dividends and then only at the times and in the amounts that our board of directors may determine. See the section titled “Dividend Policy.”
Right to Receive Liquidation Distributions
Upon our liquidation, dissolution or winding-up, the assets legally available for distribution to our stockholders would be distributable ratably among the holders of our common stock and any participating preferred stock outstanding at that time, subject to prior satisfaction of all outstanding debt and liabilities and the preferential rights of and the payment of liquidation preferences, if any, on any outstanding shares of preferred stock.
No Preemptive or Similar Rights
Our common stock is not entitled to preemptive rights, and is not subject to conversion, redemption or sinking fund provisions.
Preferred Stock
Our board of directors is authorized, subject to limitations prescribed by Delaware law, to issue preferred stock in one or more series, to establish from time to time the number of shares to be included in each series and to fix the designation, powers, preferences and rights of the shares of each series and any of their qualifications, limitations or restrictions, in each case without further vote or action by our stockholders. Our board of directors is able to increase or decrease the number of shares of any series of preferred stock, but not below the number of shares of that series then outstanding, without any further vote or action by our stockholders. Our board of directors may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of our common stock. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change in control of our company and might adversely affect the market price of our common stock and the voting and other rights of the holders of our common stock.
44
Anti-Takeover Provisions
The provisions of DGCL, our restated certificate of incorporation and our restated bylaws could have the effect of delaying, deferring or discouraging another person from acquiring control of our company. These provisions, which are summarized below, may have the effect of discouraging takeover bids. They are also designed, in part, to encourage persons seeking to acquire control of us to negotiate first with our board of directors. We believe that the benefits of increased protection of our potential ability to negotiate with an unfriendly or unsolicited acquirer outweigh the disadvantages of discouraging a proposal to acquire us because negotiation of these proposals could result in an improvement of their terms
Washington Law
We may also be subject to the provisions of Chapter 23B.19 of the Washington Business Corporation Act, or the WBCA, which imposes restrictions on certain transactions between a corporation and certain significant stockholders. The WBCA generally prohibits a “target corporation” (as defined in the WBCA) from engaging in certain significant business transactions with an “acquiring person,” which is defined as a person or group of persons that beneficially owns 10% or more of the voting securities of the target corporation, for a period of five years after such acquisition, unless the transaction or acquisition of shares is approved by a majority of the members of the target corporation’s board of directors prior to the time of the acquisition or at or subsequent to the acquiring person’s share acquisition time, such significant business transaction is approved by a majority of the members of the target corporation’s board of directors and authorized at an annual or special meeting of stockholders by the affirmative vote of at least 662/3% of the outstanding voting shares, except for shares beneficially owned by or under the voting control of the acquiring person. Such prohibited transactions include, among other things:
•a merger or consolidation with, disposition of assets to, or issuance or redemption of stock to or from the acquiring person;
•termination of five percent or more of the employees of the target corporation as a result of the acquiring person’s acquisition of 10% or more of the shares; or
•allowing the acquiring person to receive any disproportionate benefit as a stockholder.
After the five-year period, a “significant business transaction” may occur if it complies with “fair price” provisions specified in the statute. A corporation may not opt out of this statute and, therefore, we anticipate this statute will apply to us. Depending upon whether we meet the definition of a target corporation, Chapter 23B.19 of the WBCA may have the effect of delaying, deferring, or preventing a change in control.
Delaware Law
We are subject to the provisions of Section 203 of the DGCL regulating corporate takeovers. In general, Section 203 prohibits a publicly held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years following the date on which the person became an interested stockholder unless:
•prior to the date of the transaction, the board of directors of the corporation approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder;
•the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding, but not the outstanding voting stock owned by the interested stockholder, (i) shares owned by persons who are directors and also officers and(ii) shares owned by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or
•at or subsequent to the date of the transaction, the business combination is approved by the board of directors of the corporation and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 66.67% of the outstanding voting stock that is not owned by the interested stockholder.
45
Generally, a business combination includes a merger, asset or stock sale, or other transaction or series of transactions together resulting in a financial benefit to the interested stockholder. An interested stockholder is a person who, together with affiliates and associates, owns or, within three years prior to the determination of interested stockholder status, did own 15% or more of a corporation’s outstanding voting stock. We expect the existence of this provision to have an anti-takeover effect with respect to transactions our board of directors does not approve in advance. We also anticipate that Section 203 may also discourage attempts that might result in a premium over the market price for the shares of common stock held by stockholders.
Restated Certificate of Incorporation and Restated Bylaw Provisions
Our restated certificate of incorporation and our restated bylaws include a number of provisions that could deter hostile takeovers or delay or prevent changes in control of our company, including the following:
•Board of Directors Vacancies. Our restated certificate of incorporation and restated bylaws authorize only our board of directors to fill vacant directorships, including newly created seats. In addition, the number of directors constituting our board of directors is permitted to be set only by a resolution adopted by a majority vote of our entire board of directors. These provisions would prevent a stockholder from increasing the size of our board of directors and then gaining control of our board of directors by filling the resulting vacancies with its own nominees. This makes it more difficult to change the composition of our board of directors but promotes continuity of management.
•Classified Board. Our restated certificate of incorporation and restated bylaws provide that our board of directors is classified into three classes of directors, each with staggered three-year terms. A third party may be discouraged from making a tender offer or otherwise attempting to obtain control of us as it is more difficult and time consuming for stockholders to replace a majority of the directors on a classified board of directors. See the section titled “Management—Board Composition.”
•Stockholder Action; Special Meetings of Stockholders. Our restated certificate of incorporation provides that our stockholders may not take action by written consent but may only take action at annual or special meetings of our stockholders. As a result, a holder controlling a majority of our capital stock would not be able to amend our restated bylaws or remove directors without holding a meeting of our stockholders called in accordance with our restated bylaws. Further, our restated bylaws provide that special meetings of our stockholders may be called only by a majority of our board of directors, the chair of our board of directors, our Chief Executive Officer or our President, thus prohibiting a stockholder from calling a special meeting. These provisions might delay the ability of our stockholders to force consideration of a proposal or for stockholders controlling a majority of our capital stock to take any action, including the removal of directors.
•Advance Notice Requirements for Stockholder Proposals and Director Nominations. Our restated bylaws provide advance notice procedures for stockholders seeking to bring business before our annual meeting of stockholders or to nominate candidates for election as directors at our annual meeting of stockholders. Our restated bylaws also specify certain requirements regarding the form and content of a stockholder’s notice. These provisions might preclude our stockholders from bringing matters before our annual meeting of stockholders or from making nominations for directors at our annual meeting of stockholders if the proper procedures are not followed. We expect that these provisions might also discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to obtain control of our company.
•No Cumulative Voting. The DGCL provides that stockholders are not entitled to the right to cumulate votes in the election of directors unless a corporation’s certificate of incorporation provides otherwise. Our restated certificate of incorporation and restated bylaws do not provide for cumulative voting.
•Directors Removed Only for Cause. Our restated certificate of incorporation provides that stockholders may remove directors only for cause and only by the affirmative vote of the holders of at least two-thirds of our outstanding common stock.
46
•Amendment of Charter Provisions. Any amendment of the above expected provisions in our restated certificate of incorporation would require approval by holders of at least two-thirds of our outstanding common stock.
•Issuance of Undesignated Preferred Stock. Our board of directors has the authority, without further action by the stockholders, to issue up to 10,000,000 shares of undesignated preferred stock with rights and preferences, including voting rights, designated from time to time by our board of directors. The existence of authorized but unissued shares of preferred stock would enable our board of directors to render more difficult or to discourage an attempt to obtain control of us by merger, tender offer, proxy contest or other means.
•Choice of Forum. Our restated certificate of incorporation provides that, to the fullest extent permitted by law, the Court of Chancery of the State of Delaware will be the exclusive forum for any derivative action or proceeding brought on our behalf, any action asserting a breach of fiduciary duty, any action asserting a claim against us arising pursuant to the DGCL, our restated certificate of incorporation, or our restated bylaws, or any action asserting a claim against us that is governed by the internal affairs doctrine. Our restated bylaws will provide that the federal district courts of the United States of America will, to the fullest extent permitted by law, be the exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act, which we refer to as a Federal Forum Provision. Our decision to adopt a Federal Forum Provision followed a decision by the Supreme Court of the State of Delaware holding that such provisions are facially valid under Delaware law. While there can be no assurance that federal courts or state courts will follow the holding of the Delaware Supreme Court or determine that the Federal Forum Provision should be enforced in a particular case, application of the Federal Forum Provision means that suits brought by our stockholders to enforce any duty or liability created by the Securities Act must be brought in federal court and cannot be brought in state court. While neither the exclusive forum provision nor the Federal Forum Provision applies to suits brought to enforce any duty or liability created by the Exchange Act, Section 27 of the Exchange Act creates exclusive federal jurisdiction over all claims brought to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder. Accordingly, actions by our stockholders to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder also must be brought in federal court. Our stockholders will not be deemed to have waived our compliance with the federal securities laws and the regulations promulgated thereunder. Any person or entity purchasing or otherwise acquiring or holding any interest in any of our securities shall be deemed to have notice of and consented to our exclusive forum provisions, including the Federal Forum Provision. These provisions may limit a stockholder’s ability to bring a claim in a judicial forum of their choosing for disputes with us or our directors, executive officers or other employees, which may discourage lawsuits against us and our directors, executive officers, and other employees.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is American Stock Transfer and Trust Company, LLC. The transfer agent’s address is 6201 15th Avenue, Brooklyn, New York 11219 and its telephone number is (800) 937-5449.
Exchange Listing
Our common stock is listed on The Nasdaq Global Market under the symbol “IMPL.”
47
PLAN OF DISTRIBUTION
We have entered into a sales agreement with TD Cowen, under which we may issue and sell from time to time up to $50,000,000 of our common stock through or to TD Cowen as our sales agent or principal. Sales of our common stock, if any, will be made at market prices by any method that is deemed to be an “at the market offering” as defined in Rule 415 under the Securities Act.
TD Cowen will offer our common stock subject to the terms and conditions of the sales agreement on a daily basis or as otherwise agreed upon by us and TD Cowen. We will designate the maximum amount of common stock to be sold through TD Cowen on a daily basis or otherwise determine such maximum amount together with TD Cowen. Subject to the terms and conditions of the sales agreement, TD Cowen will use its commercially reasonable efforts to sell on our behalf all of the shares of common stock requested to be sold by us. We may instruct TD Cowen not to sell common stock if the sales cannot be effected at or above the price designated by us in any such instruction. TD Cowen or we may suspend the offering of our common stock being made through TD Cowen under the sales agreement upon proper notice to the other party. TD Cowen and we each have the right, by giving written notice as specified in the sales agreement, to terminate the sales agreement in each party’s sole discretion at any time.
The aggregate compensation payable to TD Cowen as sales agent equals 3.0% of the gross sales price of the shares sold through it pursuant to the sales agreement. We also agreed to reimburse TD Cowen up to $75,000 of TD Cowen’s actual outside legal expenses incurred by TD Cowen in connection with the Sales Agreement. We estimate that the total expenses of the offering payable by us, excluding commissions payable to TD Cowen under the sales agreement, will be approximately $300,000.
The remaining sales proceeds, after deducting any expenses payable by us and any transaction fees imposed by any governmental, regulatory, or self-regulatory organization in connection with the sales, will equal our net proceeds for the sale of such common stock.
TD Cowen will provide written confirmation to us following the close of trading on The Nasdaq Global Market on each day in which common stock is sold through it as sales agent under the sales agreement. Each confirmation will include the number of shares of common stock sold through it as sales agent on that day, the volume weighted average price of the shares sold, the percentage of the daily trading volume and the net proceeds to us.
We will report at least quarterly the number of shares of common stock sold through TD Cowen under the sales agreement, the net proceeds to us and the compensation paid by us to TD Cowen in connection with the sales of common stock. Settlement for sales of common stock will occur, unless the parties agree otherwise, on the second business day that is also a trading day following the date on which any sales were made in return for payment of the net proceeds to us. There is no arrangement for funds to be received in an escrow, trust or similar arrangement.
Under the sales agreement, we may also sell shares of common stock to TD Cowen as principal for its own account, at a price to be agreed upon at the time of sale. If we sell shares to TD Cowen as principal, we will enter into a separate terms agreement with TD Cowen, and we will describe the agreement in a separate prospectus supplement or pricing supplement. In connection with the sales of our common stock on our behalf, TD Cowen will be deemed to be an “underwriter” within the meaning of the Securities Act, and the compensation paid to TD Cowen will be deemed to be underwriting commissions or discounts. We have agreed in the sales agreement to provide indemnification and contribution to TD Cowen against certain liabilities, including liabilities under the Securities Act. As sales agent, TD Cowen will not engage in any transactions that stabilizes our common stock.
Our common stock is listed on The Nasdaq Global Market and trades under the symbol “IMPL.” The transfer agent of our common stock is American Stock Transfer & Trust Company, LLC.
48
TD Cowen and/or its affiliates have provided, and may in the future provide, various investment banking and other financial services for us for which services they have received and, may in the future receive, customary fees.
Pursuant to General Instruction I.B.6 of Form S-3, in no event will we sell our common stock in a public primary offering with a value exceeding more than one-third of our public float in any 12-month period so long as our public float remains below $75.0 million. We have not offered any securities pursuant to General Instruction I.B.6 of Form S-3 during the 12 calendar months prior to and including the date of this prospectus.
LEGAL MATTERS
Fenwick & West LLP, Seattle, Washington, will pass upon certain legal matters relating to the issuance and sale of the securities offered hereby on behalf of Impel Pharmaceuticals Inc. TD Cowen is being represented in connection with this offering by Wilson Sonsini Goodrich & Rosati, Professional Corporation, Seattle, Washington.
EXPERTS
Ernst & Young LLP, independent registered public accounting firm, has audited our consolidated financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2022, as set forth in their report (which contains an explanatory paragraph describing the conditions that raise substantial doubt about the Company’s ability to continue as a going concern as described in Note 1 to the consolidated financial statements), which is incorporated by reference in this prospectus and elsewhere in the registration statement. Our financial statements are incorporated by reference in reliance on Ernst & Young LLP’s report, given on their authority as experts in accounting and auditing.
49
Up to $9,000,000

Common Stock
PROSPECTUS
TD Cowen
October 5, 2023
50
Impel Pharmaceuticals (NASDAQ:IMPL)
Gráfica de Acción Histórica
De Abr 2024 a May 2024
Impel Pharmaceuticals (NASDAQ:IMPL)
Gráfica de Acción Histórica
De May 2023 a May 2024