UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
OF THE SECURITIES EXCHANGE ACT OF 1934
Date of Report: May 23, 2024
(Commission File No. 001-39308)
CALLIDITAS THERAPEUTICS AB
(Translation of registrant’s name into
English)
Kungsbron 1, D5
SE-111 22
Stockholm, Sweden
(Address of registrant’s principal executive office)
Indicate by check mark whether the registrant files or will file annual
reports under cover of Form 20-F or Form 40-F.
Form 20-F x
Form 40-F ¨
Company Announcement and Interim Report
On May 23, 2024, the Company announced its unaudited results for the
three months ended March 31, 2024, which are further described in the Company’s Interim Report Q1 2024 and press release, copies
of which are attached hereto as Exhibits 99.1 and 99.2, respectively, and are incorporated by reference herein.
The information contained in this Form 6-K, including Exhibits 99.1
and 99.2 is hereby incorporated by reference into the registrant’s Registration Statements on Form F-3 (File No. 333-265881) and
Form S-8 (File Nos. 333-240126 and 333-272594).
EXHIBIT INDEX
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934,
the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| |
CALLIDITAS THERAPEUTICS AB |
| |
|
|
| Date: May 23, 2024 |
By: |
/s/ Fredrik Johansson |
| |
|
Fredrik Johansson |
| |
|
Chief Financial Officer |
Exhibit 99.1

| INTERIM REPORT
JANUARY – MARCH
2024
Q1 |

| Calliditas Therapeutics | Interim Report 2024: January – March 2
278 50% 810
Key takeaways from Q1, 2024
Outlook 2024: Unchanged
JANUARY – MARCH 2024
(COMPARED TO JANUARY – MARCH 2023)
“In Q1 we generated another record JAN – MAR 2024
quarter in terms of demand with 705
enrollments and 354 new prescribers.
We are very excited over this positive
trend and we continue to see strong
demand in Q2.”
JAN – MAR 2024 31 MAR 2024
MSEK
TARPEYO net sales
Renée Aguiar-Lucander / CEO
TARPEYO net sales
growth in SEK (vs Q1
2023)
MSEK
Cash position
Interim report
January – March 2024
• Net sales amounted to SEK 295.5 million, of which
TARPEYO® net sales amounted to SEK 278.3 million, for
the three months ended March 31, 2024. For the three
months ended March 31, 2023 net sales amounted to SEK
191.4 million, of which TARPEYO net sales amounted to
SEK 185.7 million.
• Operating loss amounted to SEK 203.8 million and SEK
180.1 million for the three months ended March 31, 2024
and 2023, respectively.
• Calliditas had a record quarter with 705 enrollments,
representing a 27% increase over Q4.
• In February, the United States Patent and Trademark
Office (USPTO) issued patent no. 11896719, entitled
“New Pharmaceutical Compositions”. This was Calliditas’
second patent for TARPEYO in the United States, and
provides product protection until 2043.
• In March, the FDA granted an orphan drug exclusivity
period of seven years for TARPEYO®, expiring in December
2030, based on when the company obtained full approval
with a new indication for this drug product.
• There was a negative impact on net TARPEYO revenues
in the quarter of approximately USD 4.7 million due to a
cyberattack on Change Health. The revenues we were not
able to record in Q1 because of this technical issue are
not lost, but are expected to roll forward over the next
several months. This is not expected to have any impact
on annual revenues.
• Preliminary net sales from TARPEYO for the second quarter
up until the date of this report amounts to USD 25.5 million.
• Positive read out of the Nefecon Open label Phase 3
extension trial.
• Positive topline results from the setanaxib Phase 2 trial in
head and neck cancer.
• Commercial launch of Nefecon in China by partner Everest
Medicines.
• Calliditas expects continued revenue growth:
Total net sales from the Nefecon franchise, including milestones, are estimated
to be USD 150-180 million for the year ending December 31, 2024.
Key events after the reporting period
• Loss per share before and after dilution amounted to SEK
4.59 and SEK 3.49 for the three months ended March 31,
2024 and 2023, respectively.
• Cash amounted to SEK 810.3 million and SEK 1,013.6
million as of March 31, 2024 and 2023, respectively.
• European Commission decision regarding a potential full
approval for Kinpeygo for Calliditas’ partner STADA.
• Full data read out of setanaxib Phase 2 trial in Primary
Biliary Cholangitis.
• Updated KDIGO guidelines.
Key events in upcoming 6 months |

| Calliditas Therapeutics | Interim Report 2024: January – March 3
Calliditas
– pioneering new
treatments for rare
diseases
Our values
Calliditas Therapeutics leverages scientific expertise and disease
specific insights to help improve the lives of patients. We are a
commercial-stage biopharma company that researches, develops
and commercializes novel therapies that seek to address significant
unmet needs in relation to the treatment of rare diseases. We are
committed to expanding treatment options and establishing new
standards of care for patients with rare diseases, reflected by our
pipeline of innovative medicines that target unmet medical needs.
Our lead product provides a treatment option that has been demon-strated to be disease-modifying for IgA nephropathy (IgAN) – also
known as Berger’s Disease – a progressive autoimmune disease of
the kidney that for many patients leads to end-stage renal disease
(ESRD), requiring dialysis or organ transplantation. This drug product,
developed under the name Nefecon®, was granted accelerated
approval by the FDA in 2021 and full approval in December 2023,
and is today marketed in the US under the brand name TARPEYO®.
TARPEYO is now the first and only fully approved treatment for
IgAN and is approved based on a measure of kidney function.
Nefecon has also been granted conditional marketing authorisation
by the European Commission under the brand name Kinpeygo®
in the European Economic Area (EEA) and in the UK. Kinpeygo is
currently being reviewed for full marketing authorization by the
European Commission and the MHRA.
Nefecon has been granted conditional approval in China, Singa-pore, and Macau, and is being reviewed by regulators in Hong Kong
and South Korea. Nefecon was launched commercially by Everest
AGILITY
We are flexible and able to rapidly pivot and adapt to
changing situations and requirements.
EXPERTISE
We leverage our strong internal experience and com-petencies while complementing our strengths through
knowledge sharing and external collaborations as
needed.
INTEGRITY
We take responsibility for our actions and hold ourselves
to the highest ethical standards, guided by our moral
principles to make the right decisions.
PIONEER
We explore novel approaches and empower each other
to find new ways of operating in a compliant, innovative
and pragmatic manner.
Medicines in China in May 2024. Calliditas has also entered into a
partnership to develop and commercialize Nefecon in Japan.
IgA nephropathy is the most common primary glomerulonephritis
worldwide, so the market potential for Nefecon is substantial, as
evidenced by our early commercial success and out-licensing deals
with potential payments exceeding USD 300 million, encompassing
upfront payments and predefined milestones, as well as ongoing
royalty obligations.
Our late-stage pipeline is based on a first-in-class platform of NOX
inhibitors. Our lead compound, setanaxib, inhibits enzymes involved
in inflammation and fibrosis pathways and is the first drug of this
class to reach the clinical stage. Setanaxib is currently undergoing
clinical trials targeting rare diseases characterized by inflammation
and fibrosis, including Primary Biliary Cholangitis (PBC) and Alport
syndrome, and there is also an investigator led trial ongoing in idio-pathic pulmonary fibrosis (IPF). Calliditas read out positive data from
a Phase 2 proof-of-concept trial with setanaxib in head and neck
cancer in May 2024.
While our headquarter is in Stockholm, Sweden, we maintain a
significant presence in the United States, with offices in New York
and New Jersey. We also have offices in France and Switzerland,
where our discovery team is based. Calliditas Therapeutics ordinary
shares were listed on NASDAQ Stockholm in 2018 (CALTX) and
subsequently American Depositary Shares representing our ordinary
shares were listed on the NASDAQ Global Select Market in the
United States in 2020 (CALT). |
| Calliditas Therapeutics | Interim Report 2024: January – March 4
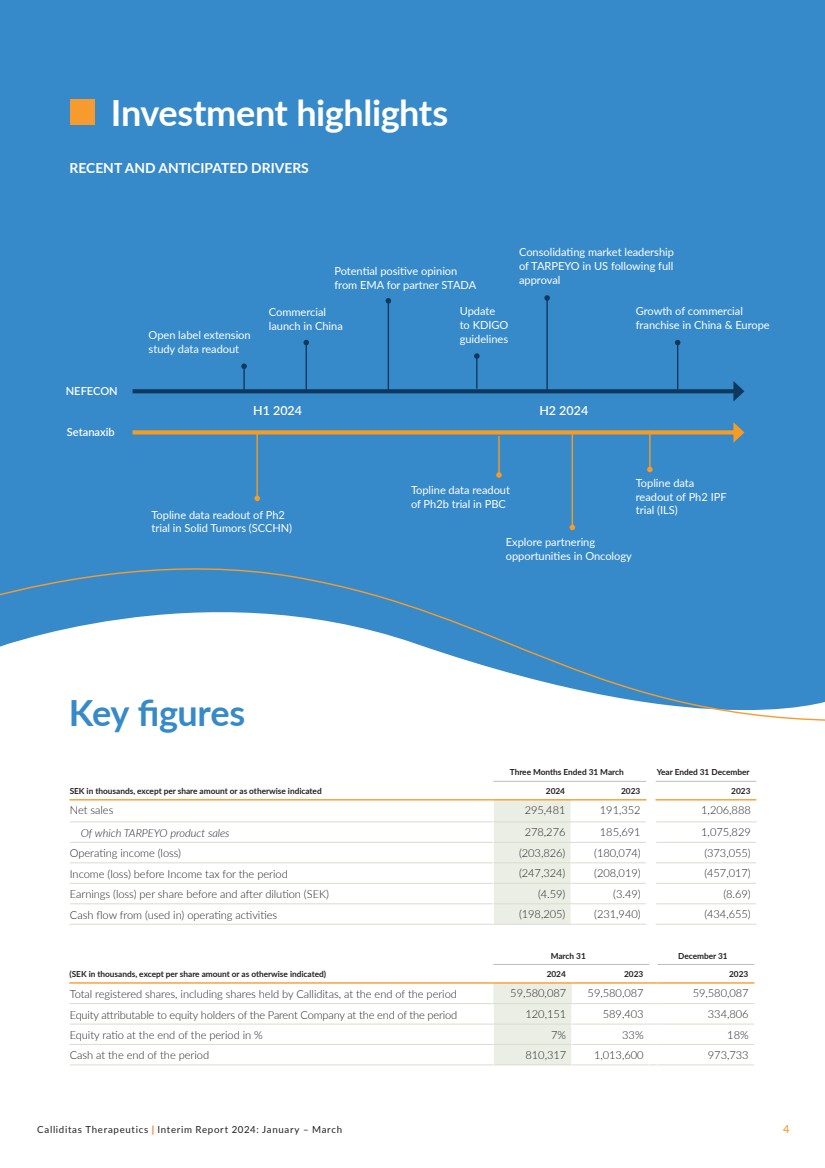
Key figures
Investment highlights
Three Months Ended 31 March Year Ended 31 December
SEK in thousands, except per share amount or as otherwise indicated 2024 2023 2023
Net sales 295,481 191,352 1,206,888
Of which TARPEYO product sales 278,276 185,691 1,075,829
Operating income (loss) (203,826) (180,074) (373,055)
Income (loss) before Income tax for the period (247,324) (208,019) (457,017)
Earnings (loss) per share before and after dilution (SEK) (4.59) (3.49) (8.69)
Cash flow from (used in) operating activities (198,205) (231,940) (434,655)
March 31 December 31
(SEK in thousands, except per share amount or as otherwise indicated) 2024 2023 2023
Total registered shares, including shares held by Calliditas, at the end of the period 59,580,087 59,580,087 59,580,087
Equity attributable to equity holders of the Parent Company at the end of the period 120,151 589,403 334,806
Equity ratio at the end of the period in % 7% 33% 18%
Cash at the end of the period 810,317 1,013,600 973,733
RECENT AND ANTICIPATED DRIVERS
NEFECON
Setanaxib
Topline data readout of Ph2
trial in Solid Tumors (SCCHN)
Topline data
readout of Ph2 IPF
trial (ILS)
Topline data readout
of Ph2b trial in PBC
Potential positive opinion
from EMA for partner STADA
H1 2024 H2 2024
Explore partnering
opportunities in Oncology
Growth of commercial
franchise in China & Europe
Open label extension
study data readout
Update
to KDIGO
guidelines
Commercial
launch in China
Consolidating market leadership
of TARPEYO in US following full
approval |

| Calliditas Therapeutics | Interim Report 2024: January – March 5
CEO STATEMENT
Following the full approval of TARPEYO by the FDA in December of
2023, we were poised to bring the message of the disease modifying
potential of TARPEYO to nephrologists. New marketing materials and
training of everyone in the field to reflect the new indication and label
were initiated, and in February we rolled out the new program. In Q3
of 2023 we had made the decision to expand the US field force and
increase key field functions such as thought leader liaisons, medical
directors and field reimbursement managers to address the larger mar-ket potential. Our new indication – reduction of kidney loss in patients
with primary IgAN at risk of disease progression – now enables us to
address the full adult IgAN population at risk, an important change for
both patients and physicians. The potential that the drug has to pro-vide a clinically meaningful delay in the need for dialysis or transplanta-tion can now be discussed in interactions with treating nephrologists,
as we now can share the exciting and important eGFR data from
the Phase 3 trial. It was important to us that we invested in the best
possible team with the right focus in order to continue our important
pioneering work for IgAN patients. In January we also announced the
addition of an experienced senior executive, Maria Törnsén as Presi-dent North America, who brings over a decade of expertise from the
US rare disease market as well as a wealth of experience from account
management, optimization of field resources, and franchise building.
This was a key complement to our expanded field presence and we are
delighted to already see the benefits of her expertise.
This timely expansion leverages the expertise we have built over the
last two years and was instrumental in generating another record
quarter in terms of enrollments. The 705 enrollments received in Q1
2024 represent a 27% increase over Q4, which in turn was a 51%
increase over Q3. This continued strong growth in demand we believe
is the result of our strong data and the positive patient and physician
experiences with using the drug in a real-world setting, a key leading
indicator of expected revenue growth in 2024. We are very excited
about this positive trend and are continuing to see strong demand
in Q2. We believe that TARPEYO is poised to become the backbone
treatment in IgAN as the only disease specific and disease modifying
medication on the market, providing eGFR stabilization during
treatment which is durable post treatment. The ability to treat when
necessary to provide disease management without the high cost and
potential safety-related issues of many chronic treatments is important
both for patients and physicians dealing with this progressive disease,
especially if it potentially can keep patients out of dialysis or transplan-tation in their lifetime.
Total net revenues for the quarter from TARPEYO amounted to USD
26.8 million (SEK 278 million). Revenues in Q1 were impacted by
two important factors. The first was already communicated in our
Q4 presentation: namely, that Q1 is typically a somewhat slower
quarter due to the insurance reverification process taking place.
The second was completely unexpected, namely the cyberattack in
Target market
expansion following
full approval in the US
February on Change Health, a division of United Health, which is one
of three major claims processors in the US. This significant event had
a profound effect on the industry generally, and on our hub’s ability to
verify insurance coverage during the time that the system was down,
as our specialty pharmacy exclusively utilizes Change Health. This led
to a negative revenue impact of approximately USD 4.7 million for
the period. The revenues we were not able to record in Q1 because
of this technical issue are not lost, but are expected to roll forward
over the next several months, and this is not expected to have an
impact on annual revenues, which is also borne out by a strong start
to Q2 in terms of TARPEYO net sales, which quarter to date already
amount to approximately USD 25.5 million1 with an additional 5 weeks
remaining in the quarter. Our hub manager, Biologics by McKesson,
has subsequently implemented routines to better deal with this type of
unexpected situation in the future.
Our interactions with payors have continued as planned, and we have
had many interactions with P&T committees already. We expect the
updated rules to come into effect for many of the larger plans in their
next update cycle, which is slated for June/July.
Q1 also saw a significant strengthening of the product protection of
TARPEYO as we received seven years of orphan drug status for the
new indication, ending in late December of 2030. In addition, we com-plemented our existing TARPEYO patent portfolio with a new patent,
expiring in 2043. We will continue to work to both broaden the patent
portfolio as well as achieve greater geographical coverage.
Post period we were also very excited to report out topline data from
our setanaxib Phase 2 head and neck cancer trial. The highly relevant
and clinically meaningful measures of progression free survival and
overall survival came out as statistically significant in the patients who
received setanaxib and pembrolizumab, compared to the group receiv-ing placebo and pembrolizumab, and in addition we could see clinical
evidence of setanaxib’s anti fibrotic effect given the statistical signifi-cance on T cell activity in the tumors treated with setanaxib. This was
beyond our initial expectations for the trial and we are looking forward
to engaging in discussions with potential partners, as well as seeing the
results from the other Phase 2 trials from our rare disease pipeline.
Our cash position remains strong with SEK 810 million on the balance
sheet at the end of the quarter, which we believe is sufficient to take
us to profitability based on expected revenue growth of TARPEYO. We
reiterate our guidance of USD 150 – 180 million of net revenues for
the Nefecon franchise in 2024.
Renée Aguiar-Lucander, CEO
1Net sales for TARPEYO are preliminary, unaudited and refer to the period April 1 – May 23. |
| Calliditas Therapeutics | Interim Report 2024: January – March 6
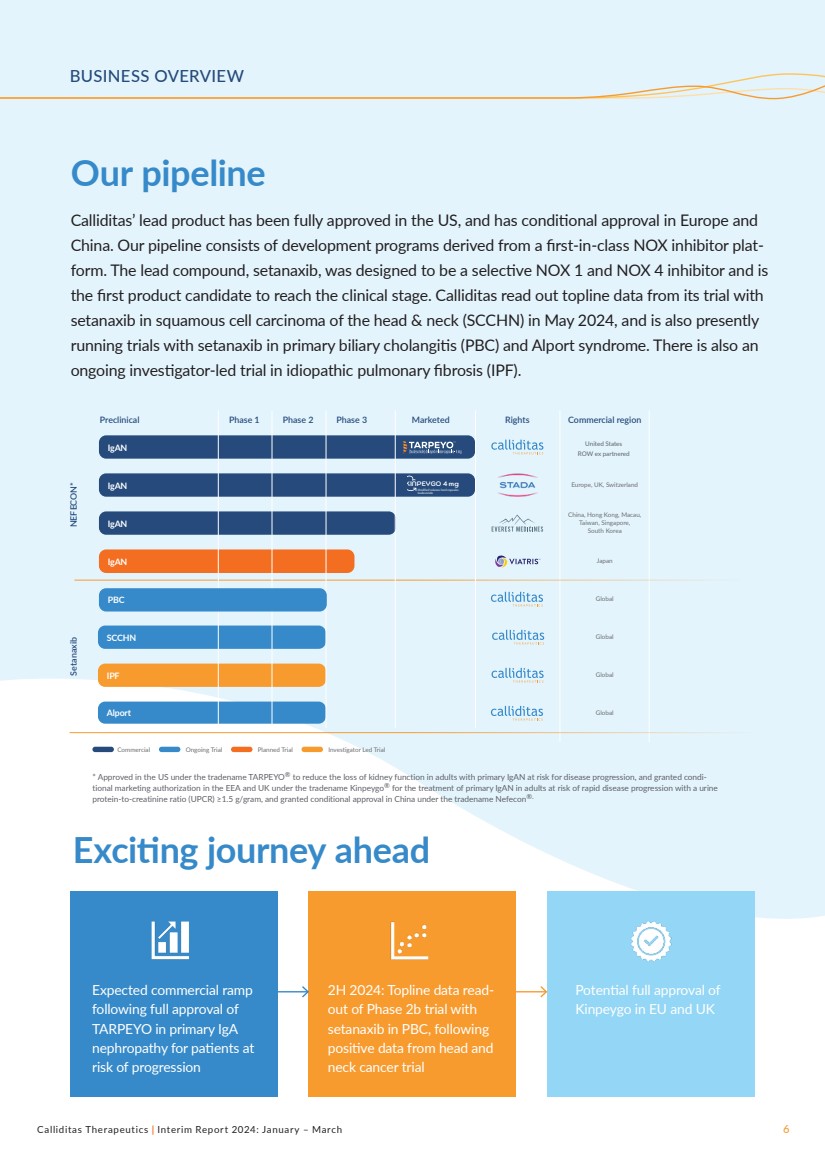
BUSINESS OVERVIEW
Our pipeline
Exciting journey ahead
Calliditas’ lead product has been fully approved in the US, and has conditional approval in Europe and
China. Our pipeline consists of development programs derived from a first-in-class NOX inhibitor plat-form. The lead compound, setanaxib, was designed to be a selective NOX 1 and NOX 4 inhibitor and is
the first product candidate to reach the clinical stage. Calliditas read out topline data from its trial with
setanaxib in squamous cell carcinoma of the head & neck (SCCHN) in May 2024, and is also presently
running trials with setanaxib in primary biliary cholangitis (PBC) and Alport syndrome. There is also an
ongoing investigator-led trial in idiopathic pulmonary fibrosis (IPF).
Expected commercial ramp
following full approval of
TARPEYO in primary IgA
nephropathy for patients at
risk of progression
2H 2024: Topline data read-out of Phase 2b trial with
setanaxib in PBC, following
positive data from head and
neck cancer trial
Potential full approval of
Kinpeygo in EU and UK
NEFECON* Setanaxib
Preclinical Phase 1 Phase 2 Phase 3 Marketed Rights Commercial region
* Approved in the US under the tradename TARPEYO® to reduce the loss of kidney function in adults with primary IgAN at risk for disease progression, and granted condi-tional marketing authorization in the EEA and UK under the tradename Kinpeygo® for the treatment of primary IgAN in adults at risk of rapid disease progression with a urine
protein-to-creatinine ratio (UPCR) ≥1.5 g/gram, and granted conditional approval in China under the tradename Nefecon®.
Commercial Ongoing Trial Planned Trial Investigator Led Trial
IgAN Japan
IgAN United States
ROW ex partnered
IgAN
China, Hong Kong, Macau,
Taiwan, Singapore,
South Korea
IgAN KINPEYGO 4 mg Modified-release hard capsules
budesonide
Europe, UK, Switzerland
Global
SCCHN Global
IPF Global
Alport Global
PBC |

| Calliditas Therapeutics | Interim Report 2024: January – March 7
BUSINESS OVERVIEW
Our commercial product
On December 20, 2023, Calliditas’ lead
product, TARPEYO, became the first and
only drug granted full approval by the US
Food and Drug Administration for patients
affected by IgA nephropathy (IgAN). It is
the only treatment specifically designed to
target the origin of IgAN and to be disease-modifying.
IgAN is a serious progressive disease, in which up to 50% of
patients end up at risk of developing end-stage renal disease
(ESRD) within ten to twenty years. This product, which was
developed under the name Nefecon®, is approved under the
brand name TARPEYO® in the United States. It was also granted
conditional approval by the European Commission under the
brand name Kinpeygo® in July 2022 and by the MHRA for the
UK in February 2023. Nefecon received conditional approval in
China by the China NMPA in November 2023.
Disease background
Although IgAN manifests in the kidney, the evidence indicates
that it is a disease that starts in the distal part of the intestine,
specifically in the ileum. Peyer’s patches, which are concen-trated within the gut-associated lymphoid tissue in the ileum,
have been identified as a major source of mucosal-type IgA
antibodies. Patients with IgA nephropathy have elevated levels
of mucosal-type IgA, which – in contrast to the majority of the
IgA in the blood – are predominately dimeric or polymeric and
are galactose-deficient. In IgAN patients, a combination of a
genetic predisposition and environmental, bacterial and dietary
factors is presumed to lead to an increased production of these
galactose-deficient IgA antibodies. This increased production,
potentially in conjunction with increased intestinal permeability,
leads to these secretory antibodies appearing in the blood.
Successful Phase 3 trial readout
NefIgArd is the first Phase 3 trial in IgA nephropathy to show a
statistically significant and clinically relevant kidney protective
effect as measured by eGFR. Calliditas’ full approval for Nefecon
from the FDA was based on the strong eGFR data from this trial.
The trial confirmed that targeting the origin of the disease with
a non chronic approach had a significant long-term impact on
kidney function.
The full Phase 3 NefIgArd trial consisted of a total of 364
patients, including 200 patients from the interim analysis, based
upon which Calliditas successfully filed for accelerated approval
with the FDA and for conditional approval with the European
Commission, UK MHRA, and China NMPA. The full trial included
9 months of treatment and a 15-month post-treatment obser-vational period for all study participants to confirm long-term
renal protection. The endpoint of the full Phase 3 trial assessed
the difference in kidney function between treated and placebo
patients, as measured by eGFR, over a two-year period from the
start of dosing of each patient. The data read-out took place in
March 2023, and in August 2023 was published in The Lancet.
The primary endpoint of the Phase 3 trial was a time-weighted
average of eGFR observed at each time point over two years.
The primary endpoint was successfully met with a highly statis-tical p value of <0.0001. At 9 months the absolute difference
in eGFR of the treatment arm was an improvement of 0.7
mL/min/1.73 m2 versus a loss of 4.6 mL/min/1.73 m2 for the
placebo arm. The treatment benefit was preserved during the
period of observation, reflected by a loss of kidney function at
two years in the placebo arm of 12.0 mL/min/1.73 m2 versus
6.1 mL/min/1.73 m2 for the treatment arm. This was also
confirmed by a difference in slope of 3 mL/min/year in favor of
TARPEYO.
There was a cumulative improvement in proteinuria in patients
treated with Nefecon versus placebo during the 9-month treat-ment period, which continued to significantly improve after end
of treatment, resulting in a decline of over 50% at 12 months.
At month 24, proteinuria levels in patients who had received
Nefecon were still at a reduced level, similar to that observed
at the 9-month time point, reflecting the durability of the
proteinuria reduction of a 9-month course of treatment.
Regulatory approvals
On the basis of this positive data, Calliditas submitted an
sNDA to the FDA seeking full approval of TARPEYO for the
complete study population from the Phase 3 NeflgArd study. On
December 20, 2023, the FDA approved TARPEYO (budesonide)
delayed release capsules to reduce the loss of kidney function in
adults with primary IgAN at risk for disease progression. Marking
a significant milestone, TARPEYO is now the first fully FDA-ap-proved treatment for IgAN reflecting the impact on a measure of
kidney function.
In September 2023, Calliditas’ partner STADA filed with Euro-pean Commission for full marketing authorisation of Kinpeygo in
the EU, and in October 2023 they also filed with the UK MHRA.
Nefecon received conditional approval in China in November
2023 and approval in the Macau administrative region in
October 2023. Calliditas’ partner Everest Medicines will be
commercialising this product in these territories. |

| Calliditas Therapeutics | Interim Report 2024: January – March 8
IgA nephropathy
- a significant market opportunity
Our commercial partnerships
• While IgAN is a rare disease, it is the most common form of pri-mary glomerulonephritis. Prevalence is estimated to range from
130,000 to 150,000 patients in the US, to be around 200,000
patients in Europe and up to 5 million patients in China.
• In the United States, we estimate there are around 12,000
nephrologists, of which up to two thirds treat patients with
IgAN. The majority of patients are seen by approximately
4,000 to 5,000 specialists. About 40% of the patients are
treated in academic settings while the remaining are treated
in community settings.1
BUSINESS OVERVIEW
Europe
Nefecon® was granted conditional
marketing authorisation (CMA) by the
European Commission in July 2022,
and subsequently by the Medicines and
Healthcare products Regulatory Agency
(MHRA) of the United Kingdom in February
2023, under the brand name Kinpeygo®
for the treatment of IgAN in adults at
risk of rapid disease progression with a
urine protein-to-creatinine ratio (UPCR)
>1.5 g/gram, becoming the first approved
treatment for IgAN in the EU. Kinpeygo
is marketed in the European Economic
Area (EEA), the UK and Switzerland, if
approved in this jurisdiction, exclusively
by STADA Arzneimittel AG, with whom
Calliditas entered into a license agreement
in July 2021 to register and commercialize
Kinpeygo in Europe. STADA launched
Kinpeygo in Germany in September 2022,
with additional European countries to
follow.
Following the positive data readout from
the full NefIgArd trial and the submission of
an sNDA to the FDA, Calliditas is collabo-rating with STADA to seek full approval of
Kinpeygo by the European Commission and
the MHRA in the full study population. An
opinion from the CHMP is expected in the
first half of 2024.
Greater China
In 2019, Calliditas entered into a license
agreement with Everest Medicines (HKEX
1952.HK) for Everest to develop and
commercialize Nefecon for IgAN in Greater
China and Singapore. In March 2022, this
agreement was expanded to include South
Korea.
Everest first launched Nefecon in China’s
Hainan Boao Pilot Zone as a First-in-Dis-ease therapy for IgA nephropathy in April
2023. This program allows innovative over-seas drugs and medical devices that have
been approved in other territories to be sold
and used in real-world clinical settings in
Hainan Province before regulatory approval
by the NMPA. Several hundreds of patients
signed up for this early access program,
making it one of the most successful EAP
programs launched in China.
Nefecon® was awarded conditional
approval in IgAN by China’s National
Medical Products Administration (NMPA) in
November 2023. Everest launched Nefecon
in mainland China in May 2024.
In addition to being approved and
commercially launched in Mainland China,
Nefecon® has also received approval in
Macau, Hong Kong and Singapore, and
was successfully commercially launched
and first prescribed in Macau at the end of
• The IgAN patient population at risk of disease progression
as defined by KDIGO guidelines is estimated to amount
to between 45,000 and 60,000 patients in the US.2
• Today the majority of these patients are treated principally with
supportive care such as generic ACEs and/or ARBs to control
blood pressure, complemented with other broadly indicated
cardio and kidney protective drugs.
• As availability and familiarity of approved drugs specifically
indicated and approved for IgAN increase and physicians
consider more active intervention to preserve kidney func-tion, we estimate the global IgAN market will grow to USD
5 – 8 billion.
2023. New Drug Applications (NDA) for
Nefecon® were also successfully accepted
for review in Taiwan and South Korea at the
end of 2023.
China has the highest prevalence of primary
glomerular diseases in the world, with an
estimated five million IgAN patients. Results
from the Chinese subpopulation analysis
of the Phase 3 NefIgArd trial, presented at
the American Society of Nephrology (ASN)
Kidney Week 2023, provided evidence
that the treatment effect of Nefecon in
the Chinese cohort was greater than in
the global data set with regards to kidney
function, proteinuria and microhematuria.
In the Chinese cohort, the mean absolute
change from baseline in eGFR at 24 months
showed an approximately 66% reduction in
loss of this measure of kidney function with
Nefecon compared with a 50% reduction in
loss of eGFR in the global data set.
Japan
At the end of 2022, Calliditas entered into
a partnership to commercialize Nefecon in
Japan with Viatris Pharmaceuticals Japan,
a subsidiary of Viatris Inc. (Nasdaq: VTRS).
Viatris is a global healthcare company which
is headquartered in the United States and
has a presence in over 165 countries.
1Veeva OpenData for 2023, including all active HCPs where the primary specialty is Nephrology
2Spherix RealWorld Dynamix |

| Calliditas Therapeutics | Interim Report 2024: January – March 9
TARPEYO: Moving from
supportive care to treating IgAN
BUSINESS OVERVIEW
Targeted B cell
immunomodulator
designed to local-ly target origin of
disease
In combination with
optimized RASi ther-apy; option of inter-mittent, rather than
chronic treatment
Durable eGFR benefit
and sustained pro-teinuria disease-mod-ifying effects in IgAN
Well characterized
active ingredient and
safety profile
Mechanism of action Patient focus Efficacy Safety
TARPEYO and Kinpeygo were the first-ever medications approved for IgAN by the FDA and
European Commission, respectively, and the only treatments specifically designed to target
the origin of IgAN and to be disease-modifying. TARPEYO is the only fully FDA-approved
treatment for IgAN and the only treatment approved based on protection of kidney function.
• A genetic predisposition is required
but not sufficient; most patients
are diagnosed in their 20s and 30s
• More than 50% are at risk of
developing ESRD within 10-20
years, leading to kidney transplant
• The treatment goal is to preserve
eGFR – kidney function
• Recently published longitudinal
data imply that disease progression
is faster and outlook worse than
previously thought1
IgAN Patients:
1 Pitcher D, Braddon F, Hendry B, et al. Long-Term Outcomes in IgA Nephropathy. Clin J Am Soc Nephrol. 2023;18(6):727-738. doi:10.2215/CJN.000000000000013
Kwon CS, Daniele P, Forsythe A, Ngai C. A Systematic Literature Review of the Epidemiology, Health-Related Quality of Life Impact, and Economic Burden of Immunoglobulin A Nephropathy. J Health Econ Outcomes Res. 2021 Sep 1;8(2):36-45. doi: 10.36469/001c.26129. PMID: 34692885; PMCID:
PMC8410133. |

| Calliditas Therapeutics | Interim Report 2024: January – March 10
Strong Demand for TARPEYO in Q1
BUSINESS OVERVIEW
During the first quarter, the Calliditas US team focused on leveraging TARPEYO’s full FDA
approval and new label to inform and engage nephrology healthcare professionals, payors and
patient communities regarding the latest clinical data.
In Q1 2024, TARPEYO set another quarterly record with 705
new patient enrollments, marking a substantial 27% quarter-over-quarter increase, following the 51% quarterly increase seen in
Q4. The increase in new prescribers of 354 was also a quarterly
record, which is another clear indicator of market acceptance and
demand for TARPEYO. The positive momentum is expected to
persist throughout 2024, supported by the new label and indica-tion, further reinforcing TARPEYO’s positioning as the backbone
treatment option in IgAN.
New Label Promotional Launch
First and only product FDA-approved
to reduce the loss of kidney function.
Patient Educational Webinar with
IgAN
Foundation & TARPEYO
Patient Ambassadors.
4 Presentations at WCN with analysis
from NeflgArd
Phase 3 trial and QoL data.
QUARTERLY HIGHLIGHTS Q1
New Patients enrolled in Q1
27% QoQ growth
New Prescribers in Q1
LTD Prescribers: 1,993
17% QoQ growth
Net sales of TARPEYO in Q1
2024
KEY METRICS Q1 2024
705 354 $26.8M
EXCITING JOURNEY AHEAD
The Q1 revenue was impacted by two factors: the seasonal
effect of the open enrollment period in the US, with insurance
changes for many patients, and a cyber-attack on the IT network
of our exclusive specialty pharmacy’s insurance claims processor
in the US, Change Healthcare. The estimated negative impact
on Q1 revenues of this unexpected disruption is ~$4.7m, which
we anticipate will be recorded over the next several months.
Importantly, this does not impact our revenue guidance for 2024.
We are also highly encouraged by a strong start to Q2 in terms
of TARPEYO net sales, which to the date of this report already
amount to approximately $25.5m with an additional 5 weeks
remaining in the quarter.
Continue US promotional
efforts to drive TARPEYO´s
positioning as a disease
modifying foundational
therapy in IgAN.
Drive scientific exchange
and data dissemination at
major scientific congress
and programs (e.g. ERA,
NKF,ASN).
Leverage KDIGO guidelines
expected in 2024.
Educate and inform US
payors on the full approval
to ensure TARPEYO payor
policies are reflecting new
label. |

| Calliditas Therapeutics | Interim Report 2024: January – March 11
BUSINESS OVERVIEW
Calliditas’ pipeline consists of development
programs based on a first-in-class NOX inhib-itor platform. Calliditas is presently running
clinical trials with lead compound setanaxib in
squamous cell carcinoma of the head & neck
(SCCHN), which read out positive topline data
data in May 2024, as well as in primary biliary
cholangitis (PBC) and Alport syndrome.
NOX Enzyme Inhibitors
NOX enzymes, also known as nicotinamide adenine dinucleotide
phosphate (NADPH) oxidases, are the only known enzymes
that are solely dedicated to producing reactive oxygen species
(ROS). At appropriate concentrations, ROS help regulate cell
proliferation, differentiation, and migration, as well as modulate
the innate immune response, inflammation, and fibrosis.
The disruption of redox homeostasis has been implicated in
multiple disease pathways, with oxidative stress caused by excess
ROS being a likely underlying mechanism for many disorders,
including cardiovascular diseases, neurodegenerative disorders,
and cancer. As such, NOX enzyme inhibitors emerged as
promising novel experimental drugs in a new therapeutic class.
Setanaxib, which is the first NOX inhibitor to reach the clinical
stage, inhibits NOX1 and NOX4, enzymes that are implicated in
fibrosis and inflammation pathways and that represent a high-potential therapeutic target.
Alport syndrome
Alport syndrome is a genetic disorder arising from the muta-tions in the genes that code for type IV collagen. The type IV
collagen alpha chains are primarily located in the kidneys, eyes,
and cochlea, and thus the condition is characterized by kidney
disease, loss of hearing, and eye abnormalities. Eventually,
patients present with proteinuria, hypertension, progressive loss
of kidney function (gradual decline in GFR), and ESRD.
It is estimated that approximately 67,000 people in the United
States have this disorder, and it is a significant cause of chronic
kidney disease (CKD), leading to ESRD in adolescents and young
adults and accounting for 1.5% to 3.0% of children on renal
replacement therapies in EU and the US.
Pipeline: NOX Inhibitor platform
Based on supportive pre-clinical work, Calliditas launched
a randomized, placebo-controlled Phase 2 study in Alport
syndrome including around 20 patients. The study will eval-uate overall safety as well as impact on proteinuria. The study
was initiated in November 2023 and on the basis of the data
readout we will decide on a full regulatory program in Alport.
Calliditas was granted orphan drug designation for the
treatment of Alport syndrome with setanaxib by the FDA in
September 2023, and by the EMA in November 2023.
Primary biliary cholangitis
PBC is a progressive and chronic autoimmune disease of the
liver that causes immune injury to biliary epithelial cells, resulting
in cholestasis and fibrosis. It is an orphan disease and, based on
its known prevalence rates, we estimate that there are approxi-mately 140,000 patients in the United States, where the annual
incidence ranges from 0.3 to 5.8 cases per 100,000. Calliditas
received FDA Fast Track Designation for setanaxib in PBC in
August 2021.
Ursodeoxycholic acid, a generic drug also known as ursodiol or
UDCA, and obeticholic acid, known as Ocaliva, are the only treat-ments for PBC approved by the FDA. However, despite these
treatment options, there is still an unmet medical need among
PBC patients, in particular when it comes to important quality of
life outcomes.
Phase 2 data from a trial with setanaxib in 111 patients with PBC
demonstrated that setanaxib had a more pronounced effect on
fibrosis and ALP reduction (alkaline phosphatase, an established
independent predictor of prognosis in PBC) in patients with
an estimated liver fibrosis stage of F3 or higher. Patients with
elevated liver stiffness are at greater risk of disease progression.
Calliditas is conducting a randomized, placebo-controlled, double-blind Phase 2b trial in PBC patients with elevated liver stiffness
We are expecting to read out data from approximately 75
patients in Q3 2024. |

| Calliditas Therapeutics | Interim Report 2024: January – March 12
BUSINESS OVERVIEW
No significant difference in the primary endpoint of best
percentage change from baseline in tumor size was observed.
Transcriptomic analysis of tumor biopsy samples showed a
statistically significant increase in CD8+ T-cells in tumor tissue
from patients treated with setanaxib, indicating an increase in
tumor immunological activity consistent with the mechanism of
action of setanaxib. The tolerability of setanaxib when given with
pembrolizumab was generally good, with no new safety signals
identified.
Setanaxib in squamous cell carcinoma of the head
and neck
In May 2024, Calliditas read out topline data from its proof-of-concept Phase 2 trial evaluating setanaxib in combination
with pembrolizumab in patients with recurrent or metastatic
squamous cell carcinoma of the head and neck (SCCHN). The
trial is a randomized, placebo-controlled, double-blind Phase 2
study investigating the effect of setanaxib 800mg twice daily in
conjunction with pembrolizumab 200mg IV, administered every
3 weeks, (a standard treatment regimen for SCCHN) with the
full dataset reflecting all patients having had the opportunity
to complete at least 15 weeks of treatment. The basis for the
analysis consisted of 55 enrolled patients with recurrent or
metastatic SCCHN and moderate or high CAF-density tumors.
A tumor biopsy was taken prior to randomization and then again
after at least 9 weeks of treatment.
Pipeline: NOX Inhibitor platform
Phase 2 data readout
The treatment groups were well-balanced with no clinically
relevant differences between the groups observed at baseline.
Patients treated with pembrolizumab and setanaxib showed
statistically significant improvements in the key secondary
endpoints of progression-free survival, (PFS median 5 months
versus 2.9 months; Hazard ratio= 0.58) and overall survival (OS
at 6 months 92% vs 68%; OS at 9 months 88% vs 58%; Hazard
ratio=0.45) compared to patients treated with pembrolizumab
and placebo.
There was also an improvement in disease-control rate in seta-naxib-treated patients, with 70% in the setanaxib arm showing a
best response of at least stable disease compared to 52% in the
placebo arm.
Expanded patent protection
In April 2024, Calliditas received a Notice of Allowance from the
United States Patent and Trademark Office (USPTO) for patent
application no. 16/760,910 entitled “Use of NOX Inhibitors for
Treatment of Cancer”. This Notice of Allowance is expected
to result in the issuance of a U.S. patent once administrative
processes are completed. The allowed claims cover a method
of treating a solid tumor presenting resistance to PD-1 inhibitor
immunotherapy by administering setanaxib in combination with
a PD-1 inhibitor. The patent, when issued, will have an antici-pated expiration date in 2038.
“It is very encouraging to see statistical significance on
important clinical outcomes in this relatively small study,
which provides an excellent basis for advancing setanaxib
in this hard-to-treat population.”
Kevin Harrington, Professor in Biological Cancer Therapies at
The Institute of Cancer Research (ICR) London, Consultant Clinical
Oncologist at The Royal Marsden NHS Foundation, London, and
Investigator on the trial. |

| Calliditas Therapeutics | Interim Report 2024: January – March 13
INTERVIEW WITH MARIA TÖRNSÉN
Calliditas President North America Maria Törnsén
You have joined Calliditas with over 20 years of experience in the
pharmaceutical industry. Can you talk about your career and your
experiences in the industry thus far?
I started my career as a sales representative in the north of Sweden.
Looking back, I would say this was a bit of by luck, as I met represen-tatives from Eli Lilly Sweden at a career day at Lund University as I
was finishing up my Master’s degree. Little did I know that this initial
meeting would bring me the career I have enjoyed over the last 22
years and the majority spent outside my home country of Sweden.
From early days of sales and marketing with Eli Lilly and Merck Serono
in Sweden, I emigrated in 2008 when I got the opportunity to move
into a global role with Merck Serono in Switzerland. Since 2011, I have
almost exclusively spent my career in rare diseases at organizations
such as Shire, Sanofi Genzyme and Sarepta in global, European and US
roles. I have had the opportunity to work in diseases where there is no
therapy available, in areas with several approved therapies and with
programs at all stages of development and commercialization.
What experiences from your previous positions do you think have
been most valuable as you have taken over as President, North
America at Calliditas?
While all rare diseases are unique, I have through working on over
fifteen different rare diseases learned that there are many similarities,
which I have been able to bring from one organization to the next one.
First, patients with rare diseases typically face a long journey to diag-nosis which sometimes includes a misdiagnosis and seeing multiple
specialists. This is why understanding the patient journey is critical,
to ensure we put in place the right type of education, to the right
stakeholders, at the right time to shorten time to diagnosis and raise
the urgency to treat.
Second, once a patient has received a diagnosis, there are in most
cases no treatments available, or access is challenging. More than 95%
of rare diseases have no treatments available today, so the healthcare
provider may be limited to offering supportive care. At Calliditas, we
can provide a treatment for one of the rare diseases, TARPEYO for
IgA nephropathy (IgAN) and we also have an experienced access team
who can help ensure eligible patients gain access to TARPEYO.
Finally, I would also highlight the aspect of community support and
how important that is for someone living with a rare disease. As rare
diseases are not well known to most people, a person diagnosed with
a rare disease may be challenged with finding accurate information
and get the support they need. That’s why the patient organizations
play such a critical role for rare disease patients, and I am proud that
Calliditas is working with multiple patient organizations supporting
people living with IgAN and other kidney diseases.
What excited you about the prospect of leading
Calliditas’ commercial efforts in the US?
In December 2023, we gained full approval for TARPEYO for the
treatment of IgAN. This was a pivotal moment for Calliditas, but most
importantly for the IgAN community. This is the first time a product
has proven to reduce the loss of kidney function in IgAN, which is
something the community has been waiting for, for a long time. I am
excited about the opportunity to engage with healthcare providers,
patient organizations and payors to educate on the new indication
for TARPEYO and ensure appropriate patients can have access to our
therapy.
I am equally excited about our clinical
programs and our NOX enzyme inhib-itors platform. Over the next twelve
months, we will have multiple data read-outs in disease areas with high unmet
need. This may allow us an opportunity
to potentially help other patients in the
future.
What do you think the full approval
and expanded label will mean for
TARPEYO in the context of standard
of care?
For many years, the IgAN community
has not had an approved therapy which has shown to reduce the
loss of kidney function. Since the full approval of TARPEYO, they
now have that. We can not underestimate the importance of this full
approval, as it gives healthcare providers an opportunity to not only
manage proteinuria, but also tell their patients it can help preserve
their kidney function. It will also facilitate discussions with payors and
improve patient access. This is a benefit for IgAN patients, as many are
diagnosed before the age of 40 and will live with this disease for many
decades.
What key events and milestones in the upcoming year
do you think might drive TARPEYO sales and profile?
The full approval in December 2023 was the most important
milestone for Calliditas. This full approval provides access to a broader
IgAN population. During the first half of 2024, our focus is on
educating payors and healthcare providers on the new label, to ensure
broader access to TARPEYO.
The data from our open label extension (OLE) study is also important
in helping us better understand the potential benefit of providing a
second 9-month treatment of TARPEYO.
Another important milestone we are anticipating this year is the
update of the KDIGO guidelines. These guidelines were last updated
in 2021, prior to the full approval of TARPEYO. We know that many
nephrologists use these guidelines as they make decisions on how to
treat their patients with IgAN. We are anticipating that the updated
guidelines will include TARPEYO, and that they may also expand the
definition of the “at risk” population who should be treated.
What can you share with regards to progress related to payor
interactions on the new label in the first quarter of the year?
Our focus in Q1 has been on educating payors on the new label for
TARPEYO. We have invested in our field-based team, both in terms of
national account managers, whose focus is commercial and govern-ment payors, and our field reimbursement managers, who are focused
on educating nephrology offices on the prior authorization process
and facilitating patient access.
Our national account team, alongside our field based medical team,
have been engaging with payors since the full approval was granted.
This has included multiple scientific presentations on the new label,
providing payors with a summary of the differences compared to the
previous label, and answering their questions. With this information in
hand, we are expecting the major payors to update their policies over
the next six months, which should facilitate access to TARPEYO. |

| Calliditas Therapeutics | Interim Report 2024: January – March 14
Continued focus on
CSRD implementation
Calliditas Therapeutics | Interim Report 2024: January – March
During the first quarter, Calliditas took
further steps in the implementation of
the upcoming legal requirement, CSRD,
by establishing a roadmap for the project
and preparing several key performance
indicators to ensure progress in its
sustainability work.
During the first quarter, Calliditas continued the efforts to
develop its sustainability work with a focus on the issues that,
in accordance with the double materiality assessment, will guide
the strategic sustainability work and reporting going forward.
Based on current sustainability reporting, an analysis was made
to get a clear picture of the information and data that is avail-able, and what needs to be added to comply with CSRD and its
associated standards.
To ensure that the right priorities are set in the long-term
sustainability work, a roadmap was developed during the first
quarter. It defines what Calliditas needs to work on in order to
drive its sustainability work forward, together with how the work
is to be done, the timeline, as well as who, alternatively which
function, is responsible.
SUSTAINABILITY
Key figures for continuous follow-up
With the ambition to increase the pace of sustainability work
and data collection, several selected key performance indicators
were developed during the first quarter to continuously monitor
the progress of some of Calliditas’ material sustainability matters.
The selection was made based on the metrics and targets
included in a number of the ESRS on which Calliditas will report
starting in fiscal year 2025. An overview:
Environment
• Share of all purchased electricity from renewable sources
Social
• Number of incidents linked to work-related injuries, ill health
cases and fatalities
• Number of days lost due to work-related ill health
• Number of employees who left Calliditas/Employee turnover
Governance
• Percentage of employees trained in Calliditas’ Code of
Conduct
• Percentage of business partners who have signed Calliditas’
Code of Conduct
Continued work to drive transition
For Calliditas, the long-term work to develop policies, processes,
targets, and activities continues. In addition, Calliditas will grad-ually add key performance indicators to increase progress in the
sustainability work and the implementation of CSRD.
14
Environmental matters
• Climate change mitigation and adaptation
• Circular economy and waste
Social matters
• Employee health and safety
• Access to products
• Health and safety of end-users
Governance matters
• Anti-corruption and anti-bribery
• Animal protection
Calliditas’ material sustainability areas
The sustainability matters that are most important
for Calliditas to work with, monitor and report
on are gathered in seven main areas divided as
follows: |

| Calliditas Therapeutics | Interim Report 2024: January – March 15
January – March 2024
FINANCIAL OVERVIEW
Revenue
Net sales amounted to SEK 295.5 million and SEK 191.4
million for the three months ended March 31, 2024 and 2023,
respectively. Net sales primarily originated from net sales of
TARPEYO® in the US, which amounted to SEK 278.3 million and
SEK 185.7 million for the three months ended March 31, 2024
and 2023, respectively. Royalty income from our partnerships
amounted to SEK 13.0 million and SEK 4.4 million for the three
months ended March 31, 2024 and 2023, respectively.
For additional information see Note 4.
Cost of Sales
Cost of sales amounted to SEK 14.0 million and SEK 9.0 million for
the three months ended March 31, 2024 and 2023, respectively.
Total Operating Expenses
Total operating expenses amounted to SEK 485.3 million and SEK
362.4 million for the three months ended March 31, 2024 and
2023, respectively.
Research and Development Expenses
Research and development expenses amounted to SEK 150.6
million and SEK 126.7 million for the three months ended March
31, 2024 and 2023, respectively. The increase of SEK 23.9 million
for the period was primarily due to increased clinical activities for
the Nox-platform, including the ongoing setanaxib trials.
Marketing and Selling Expenses
Marketing and selling expenses amounted to SEK 240.1 million
and SEK 167.2 million for the three months ended March 31,
2024 and 2023, respectively. The increase of SEK 72.9 million was
primarily related to intensified marketing activities of TARPEYO
and increased US salesforce due to the TARPEYO full approval in
the US.
Administrative Expenses
Administrative expenses amounted to SEK 102.0 million and SEK
72.5 million for the three months ended March 31, 2024 and
2023, respectively. The increase of SEK 29.5 million for the period
was primarily related to increased costs from a larger organization
and increased regulatory requirements.
Other Operating Incomes/Expenses, net
Other operating income (expenses), net amounted to SEK 7.5
million and SEK 4.0 million for the three months ended March
31, 2024 and 2023, respectively. The improvement was primarily
attributable to movements in exchange rates related to operating
receivables and liabilities.
Net Financial Income and Expenses
Net financial income (expenses) amounted to (SEK 43.5 million)
and (SEK 27.9 million) for the three months ended March 31,
2024 and 2023, respectively. The change in the net amount of
(SEK 15.5 million) was primarily derived from interest expenses
and currency effects primarily related to translation effects.
Tax
Total income tax (expense) amounted to SEK 1.2 million and
SEK 20.5 million for the three months ended March 31, 2024
and 2023, respectively. The change in income tax was primarily
explained by carried-forward losses recognized regarding U.S.
subsidiaries in the first quarter of 2023. The Group’s tax losses
carried-forward have not been recognized as deferred tax assets,
other than to the extent such tax losses can be used to offset
temporary differences.
Result for the period
For the three months ended March 31, 2024 and 2023, loss
for the period amounted to SEK 246.2 million and SEK 187.5
million, and the corresponding loss per share before and after
dilution amounted to SEK 4.59 and SEK 3.49, respectively.
Cash Flow and Cash Position
Cash flow used in operating activities amounted to SEK 198.2
million and SEK 231.9 million for the three months ended March
31, 2024 and 2023, respectively. The decrease is mainly attrib-utable to the change in current receivables.
Cash flow used in investing activities amounted to SEK 3.9
million and SEK 2.9 million for the three months ended March
31, 2024 and 2023, respectively. The change was primarily
explained by acquisition of equipment.
Cash flow used in financing activities amounted to SEK 5.5
million and SEK 3.0 million for the three months ended March
31, 2024 and 2023, respectively.
Net decrease in cash amounted to SEK 207.5 million and SEK
237.8 million for the three months periods ended March 31,
2024 and 2023, respectively. Cash amounted to SEK 810.3
million and SEK 1,013.6 million as of March 31, 2024 and 2023,
respectively.
Personnel
The average number of employees were 219 and 170 for the
three months ended March 31, 2024 and 2023, respectively. |
| Calliditas Therapeutics | Interim Report 2024: January – March 16
FINANCIAL OVERVIEW
Changes in Shareholders’ Equity and Number of
Shares
Equity attributable to equity holders of the Parent Company
amounted to SEK 120.2 million and SEK 589.4 million as of
March 31, 2024 and 2023, respectively. The number of regis-tered shares amounted to 59,580,087 and 59,580,087 as of
March 31, 2024 and 2023, respectively.
Treasury Shares
As of March 31, 2024, Calliditas had 5,908,018 ordinary shares
held as treasury shares by the Parent Company. At the Annual
General Meeting 2023, authorization was given that Calliditas
can transfer (sale) these ordinary shares with the purpose to
finance an acquisition of operations, to procure capital to finance
the development of projects, repayment of loans or to commer-cialize Calliditas’ products. No transfer (sale) of treasury shares
have occurred as of March 31, 2024. See Note 7 and 8 for
further information.
Incentive Programs
During the three months ended March 31, 2024, 555,000
options have been allocated for the ESOP 2023 Program. For
more information on incentive programs, see Note 9.
Parent Company
Net sales for the Parent Company, Calliditas Therapeutics AB,
amounted to SEK 138.2 million and SEK 168.4 million for the
three months ended March 31, 2024 and 2023, respectively.
The decrease is primarily attributable to change in the price mix of
product sales compared to previous year.
Operating loss amounted to SEK 174.0 million and SEK 46.6
million for the three months ended March 31, 2024 and 2023,
respectively. The decrease of SEK 127.4 million was primarily
related to higher costs related to intensified marketing activities,
increased regulatory requirements, and the larger organization.
Executive Management
The Executive Management of Calliditas Therapeutics
AB consists of: CEO Renée Aguiar-Lucander, CFO Fredrik
Johansson, CMO Richard Philipson, Group General Counsel
Brian Gorman, President North America Maria Törnsén, Vice
President Regulatory Affairs Frank Bringstrup, Head of Technical
Operations Lars Stubberud and Head of Human Resources
Sandra Frithiof.
Nomination Committee AGM 2024
The nomination committee for the AGM 2024 consists of:
Patrick Sobocki, appointed by Stiftelsen Industrifonden, Karl
Tobieson, appointed by Linc AB and Spike Loy, appointed by
BVF.
Annual General Meeting 2024
The 2024 Annual General Meeting will be held 17 June at 14.00
p.m. CET, Klarabergsviadukten 90, Stockholm, Sweden. All docu-mentation will be published on the company’s website.
Unchanged Outlook 2024
For 2024, Calliditas expects continued revenue growth: Total
net sales from the Nefecon franchise, including milestones, are
estimated to be USD 150-180 million for the year ending 31
December, 2024.
The Share
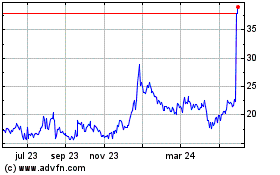
As of 31 March 2024, the number of shares amounted to
59,580,087 ordinary shares, of which, 5,908,018 are held as
treasury shares by the Parent Company. As of 28 March, 2024,
the closing price for the Calliditas Therapeutics share CALTX was
SEK 113.4. The total number of shareholders as of 31 March,
2024 was approximately 18,000.
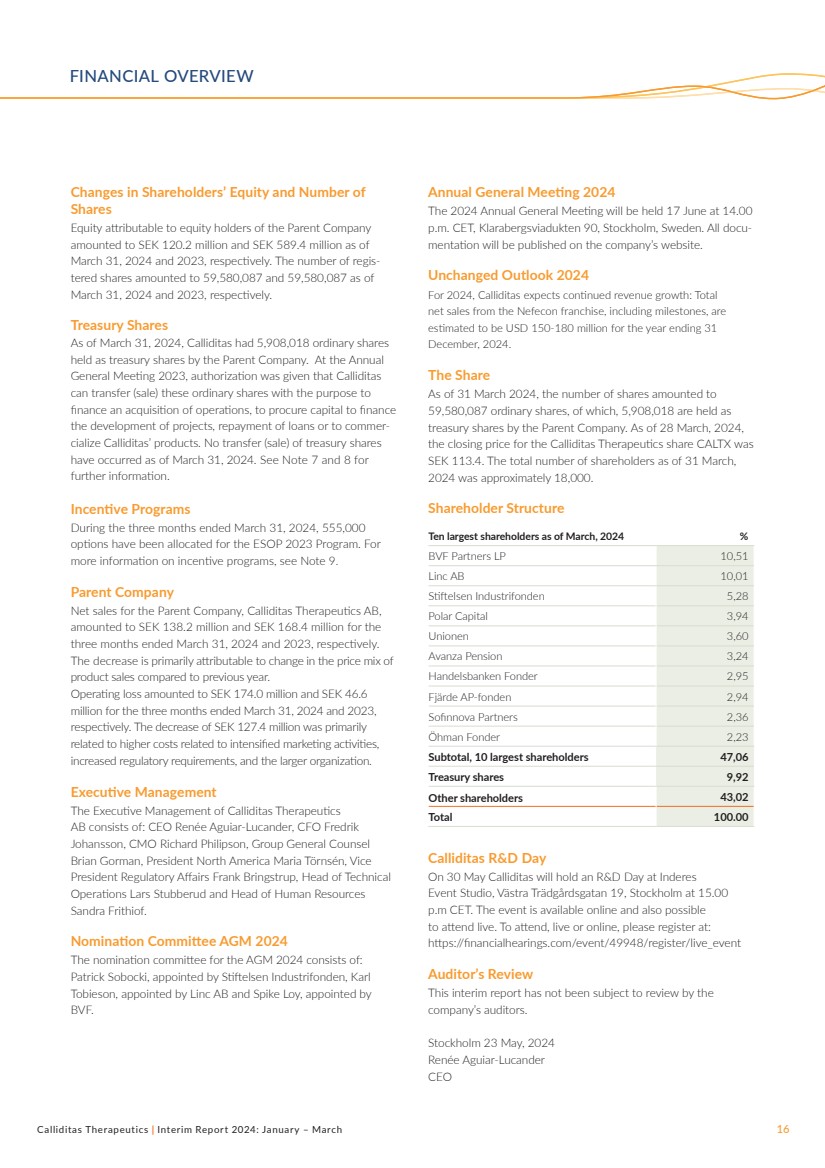
Shareholder Structure
Ten largest shareholders as of March, 2024 %
BVF Partners LP 10,51
Linc AB 10,01
Stiftelsen Industrifonden 5,28
Polar Capital 3,94
Unionen 3,60
Avanza Pension 3,24
Handelsbanken Fonder 2,95
Fjärde AP-fonden 2,94
Sofinnova Partners 2,36
Öhman Fonder 2,23
Subtotal, 10 largest shareholders 47,06
Treasury shares 9,92
Other shareholders 43,02
Total 100.00
Calliditas R&D Day
On 30 May Calliditas will hold an R&D Day at Inderes
Event Studio, Västra Trädgårdsgatan 19, Stockholm at 15.00
p.m CET. The event is available online and also possible
to attend live. To attend, live or online, please register at:
https://financialhearings.com/event/49948/register/live_event
Auditor’s Review
This interim report has not been subject to review by the
company’s auditors.
Stockholm 23 May, 2024
Renée Aguiar-Lucander
CEO |

| Calliditas Therapeutics | Interim Report 2024: January – March 17
Significant Events
FINANCIAL COMMENTS
Significant Events During the Period
1 January – 31 March, 2024
• On 7 January, Calliditas announced that Maria Törnsén
was appointed to the position of President North America.
Ms. Törnsén is responsible for all US based operations and
reports to the CEO.
• On 13 February, Calliditas announced that the United States
Patent and Trademark Office (USPTO) issued patent no.
11896719, entitled “New Pharmaceutical Compositions”, on 24
January, 2024 with validity 13 February, 2024. This is Calliditas’
second patent for TARPEYO in the United States, and provides
product protection until 13 February 2043.
• On 6 March, Calliditas announced that the FDA granted an
orphan drug exclusivity period of seven years for TARPEYO®,
expiring in December 2030, based on when the company
obtained full approval with an expanded indication for this drug
product.
Significant Events After the end of the Period
• On 8 April, Calliditas announced that the Company received a
Notice of Allowance from the United States Patent and Trade-mark Office (USPTO) for patent application no. 16/760,910
entitled “Use of NOX Inhibitors for Treatment of Cancer”. This
Notice of Allowance is expected to result in the issuance of a U.S.
patent once administrative processes are completed.
• On 24 April, Calliditas announced that the global open-label
extension (OLE) study to the Phase 3 NefIgArd study showed a
treatment response consistent with the NefIgArd study across
endpoints of urine protein to creatinine ration (UPCR) and
estimated glomerular filtration rate (eGFR) at 9 months across
all IgAN patients, including those who had previously received
Nefecon in the NefIgArd study.
• On 6 May, Calliditas announced topline data from the proof-of-concept Phase 2 trial evaluating setanaxib, its lead NOX enzyme
inhibitor, in combination with pembrolizumab, in patients with
squamous cell carcinoma of the head and neck (SCCHN). The
analysis showed statistically significant improvements in progres-sion-free survival (PFS), as well as in overall survival (OS), with
statistically significant changes in tumor biology consistent with
the mechanism of action of setanaxib.
• On 14 May, Calliditas announced that its partner Everest
Medicines launched Nefecon® in China, which is estimated
to have up to 5 million patients suffering from the progressive
autoimmune disease.
• Preliminary net sales from TARPEYO for the second quarter up
until the date of this report amounts to USD 25.5 million. |

| Calliditas Therapeutics | Interim Report 2024: January – March 18
Presentation to investors,
analysts and press
For further information
please contact
Upcoming events
• Calliditas invites investors, analysts and press to a presentation of the
Q1 Report 2024 at 14:30 p.m. CET on 23 May, 2024. The report was
published on 23 May at 7:00 a.m. CET.
• Calliditas’ CEO Renée Aguiar-Lucander will present the report together
with CFO Fredrik Johansson, CMO Richard Philipson and President
North America Maria Törnsén. The presentations will be given in English.
• Time: Thursday 14:30 p.m. CET on 23 May, 2024
• Link to webcast
https://ir.financialhearings.com/calliditas-therapeutics-q1-report-2024
• To participate via conference call register via this link:
https://conference.financialhearings.com/teleconference/?id=50047214
After registration, you will receive a phone number and a conference ID
to log in to the conference call. Via the telephone conference, there is an
opportunity to ask oral questions.
Calliditas R&D Day 2024
Västra Trädgårdsgatan 19, Stockholm
30 May
ANNUAL GENERAL MEETING 2024
Klarabergsviadukten 90, Stockholm
17 June
INTERIM REPORT Q2
January – June 2024
13 August
INTERIM REPORT Q3
January – September 2024
11 November
Renée Aguiar-Lucander / CEO
+46 (0)8 411 30 05
renee.lucander@calliditas.com
Åsa Hillsten / Head of IR & Sustainability
+46 (0) 764 03 35 43
asa.hillsten@calliditas.com
Supplemental Information
This interim report has not been reviewed or audited by the Company’s auditors.
The information in the report is information that Calliditas is obliged to make public pursuant to the EU
Market Abuse Regulation. The information was sent for publication, through the agency of the contact
persons set out above, on May 23, 2024, at 7:00 a.m. CET.
Registered office
Calliditas Therapeutics AB
Kungsbron 1
SE 111 22 Stockholm, Sweden
calliditas.com / ir@calliditas.com
Forward looking statements
This Interim Report contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended,
including, without limitation, statements regarding Calliditas’ strategy, business plans, revenue and other financial projections, and focus. The words “may,”
“will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” and similar
expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words.
Any forward-looking statements in this Interim Report are based on management’s current expectations and beliefs and are subject to a number of risks,
uncertainties and important factors that may cause actual events or results to differ materially from those expressed or implied by any forward-looking
statements contained in this Interim Report, including, without limitation, any related to Calliditas’ business, operations, commercialization of TARPEYO,
Kinpeygo and Nefecon, clinical trials, supply chain, strategy, goals and anticipated timelines for development and potential approvals, competition from
other biopharmaceutical companies, revenue and product sales projections or forecasts, including 2024 total net sales guidance and cash runway and
preliminary net sales for the second quarter of 2024 to date, and other risks identified in the section entitled “Risk Factors” in Calliditas’ reports filed with
the Securities and Exchange Commission.
Calliditas cautions you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. Calliditas disclaims
any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which
any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements.
Any forward-looking statements contained in this Interim Report represent Calliditas’ views only as of the date hereof and should not be relied upon as
representing its views as of any subsequent date.
This Interim Report has been prepared in a Swedish original and has been translated into English. In case of differences between the two, the Swedish
version shall apply. |
| Calliditas Therapeutics | Interim Report 2024: January – March 19
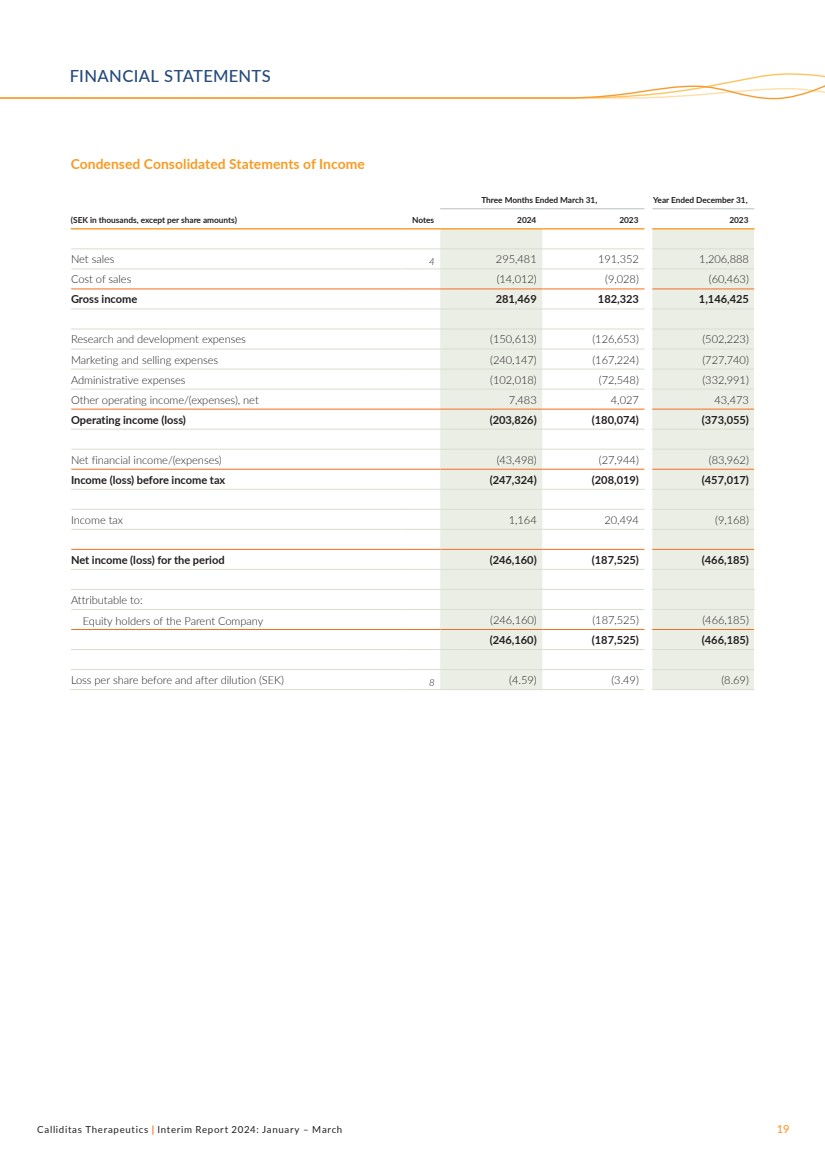
Condensed Consolidated Statements of Income
Three Months Ended March 31, Year Ended December 31,
(SEK in thousands, except per share amounts) Notes 2024 2023 2023
Net sales 4 295,481 191,352 1,206,888
Cost of sales (14,012) (9,028) (60,463)
Gross income 281,469 182,323 1,146,425
Research and development expenses (150,613) (126,653) (502,223)
Marketing and selling expenses (240,147) (167,224) (727,740)
Administrative expenses (102,018) (72,548) (332,991)
Other operating income/(expenses), net 7,483 4,027 43,473
Operating income (loss) (203,826) (180,074) (373,055)
Net financial income/(expenses) (43,498) (27,944) (83,962)
Income (loss) before income tax (247,324) (208,019) (457,017)
Income tax 1,164 20,494 (9,168)
Net income (loss) for the period (246,160) (187,525) (466,185)
Attributable to:
Equity holders of the Parent Company (246,160) (187,525) (466,185)
(246,160) (187,525) (466,185)
Loss per share before and after dilution (SEK) 8 (4.59) (3.49) (8.69)
FINANCIAL STATEMENTS |
| Calliditas Therapeutics | Interim Report 2024: January – March 20
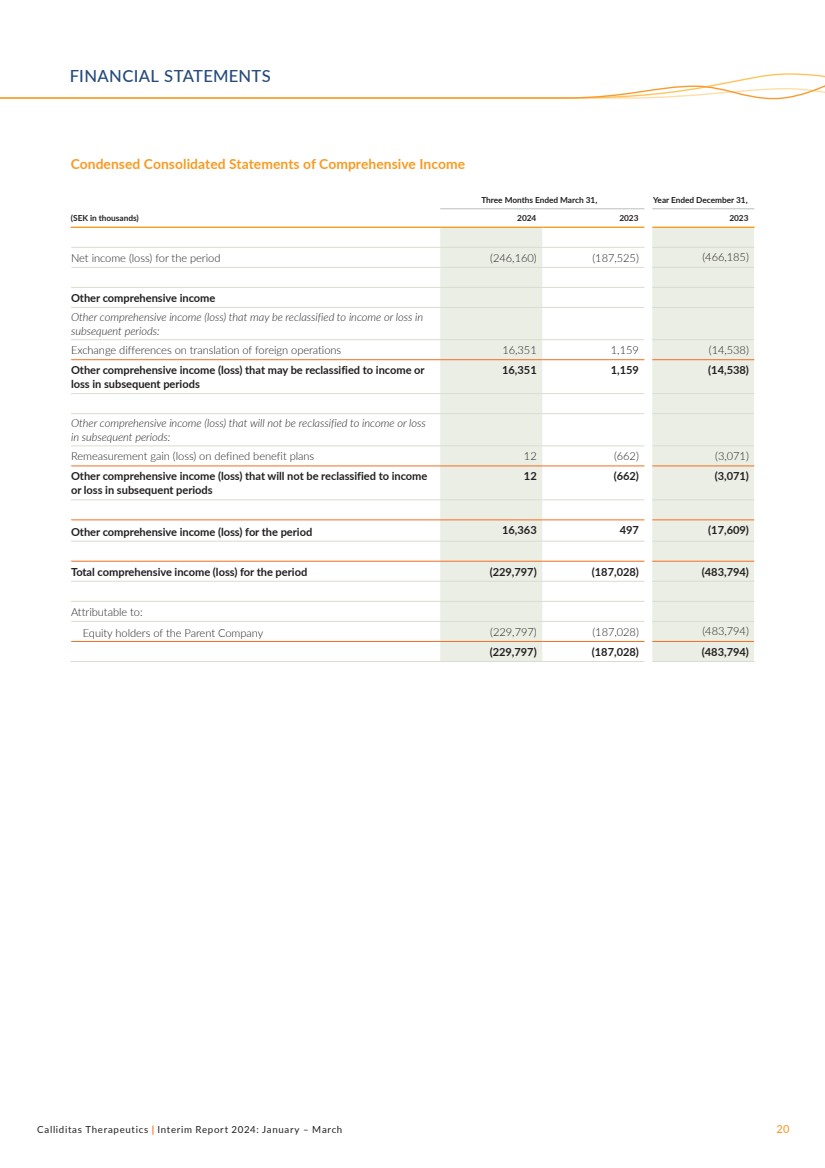
Condensed Consolidated Statements of Comprehensive Income
Three Months Ended March 31, Year Ended December 31,
(SEK in thousands) 2024 2023 2023
Net income (loss) for the period (246,160) (187,525) (466,185)
Other comprehensive income
Other comprehensive income (loss) that may be reclassified to income or loss in
subsequent periods:
Exchange differences on translation of foreign operations 16,351 1,159 (14,538)
Other comprehensive income (loss) that may be reclassified to income or
loss in subsequent periods
16,351 1,159 (14,538)
Other comprehensive income (loss) that will not be reclassified to income or loss
in subsequent periods:
Remeasurement gain (loss) on defined benefit plans 12 (662) (3,071)
Other comprehensive income (loss) that will not be reclassified to income
or loss in subsequent periods
12 (662) (3,071)
Other comprehensive income (loss) for the period 16,363 497 (17,609)
Total comprehensive income (loss) for the period (229,797) (187,028) (483,794)
Attributable to:
Equity holders of the Parent Company (229,797) (187,028) (483,794)
(229,797) (187,028) (483,794)
FINANCIAL STATEMENTS |
| Calliditas Therapeutics | Interim Report 2024: January – March 21
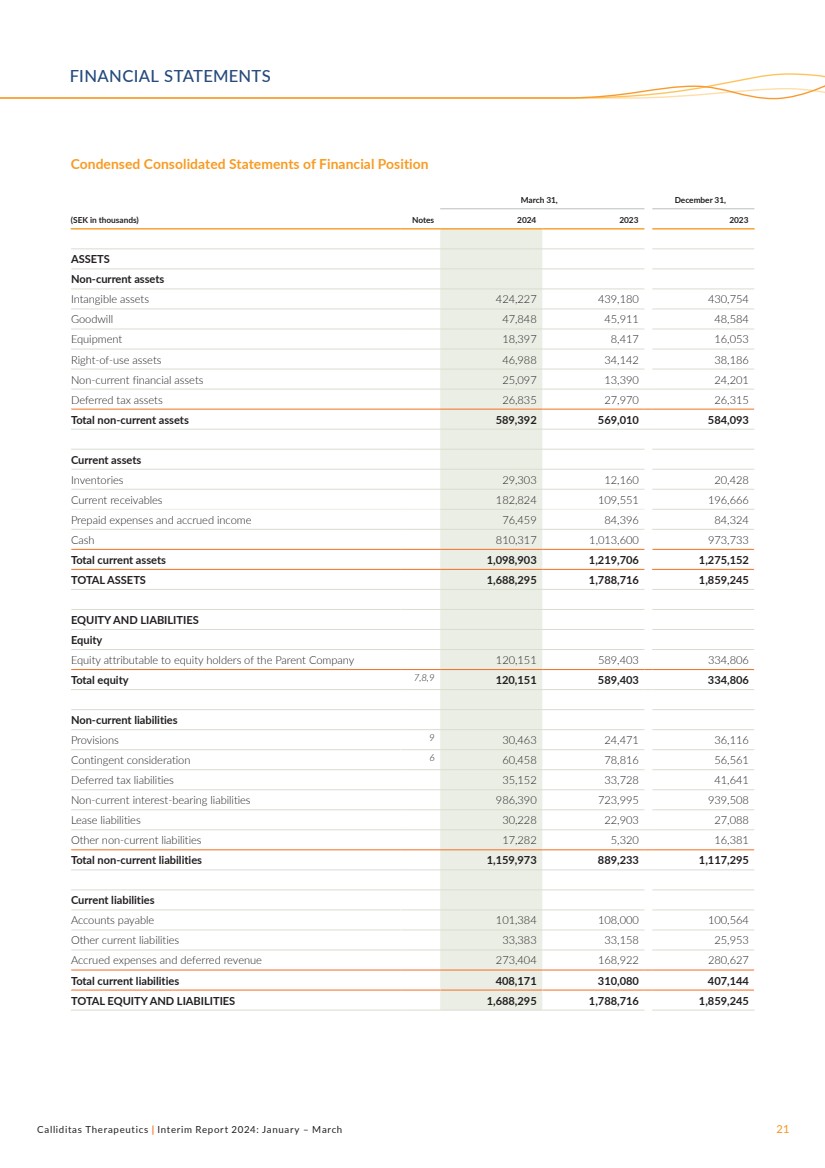
March 31, December 31,
(SEK in thousands) Notes 2024 2023 2023
ASSETS
Non-current assets
Intangible assets 424,227 439,180 430,754
Goodwill 47,848 45,911 48,584
Equipment 18,397 8,417 16,053
Right-of-use assets 46,988 34,142 38,186
Non-current financial assets 25,097 13,390 24,201
Deferred tax assets 26,835 27,970 26,315
Total non-current assets 589,392 569,010 584,093
Current assets
Inventories 29,303 12,160 20,428
Current receivables 182,824 109,551 196,666
Prepaid expenses and accrued income 76,459 84,396 84,324
Cash 810,317 1,013,600 973,733
Total current assets 1,098,903 1,219,706 1,275,152
TOTAL ASSETS 1,688,295 1,788,716 1,859,245
EQUITY AND LIABILITIES
Equity
Equity attributable to equity holders of the Parent Company 120,151 589,403 334,806
Total equity 7,8,9 120,151 589,403 334,806
Non-current liabilities
Provisions 9 30,463 24,471 36,116
Contingent consideration 6 60,458 78,816 56,561
Deferred tax liabilities 35,152 33,728 41,641
Non-current interest-bearing liabilities 986,390 723,995 939,508
Lease liabilities 30,228 22,903 27,088
Other non-current liabilities 17,282 5,320 16,381
Total non-current liabilities 1,159,973 889,233 1,117,295
Current liabilities
Accounts payable 101,384 108,000 100,564
Other current liabilities 33,383 33,158 25,953
Accrued expenses and deferred revenue 273,404 168,922 280,627
Total current liabilities 408,171 310,080 407,144
TOTAL EQUITY AND LIABILITIES 1,688,295 1,788,716 1,859,245
Condensed Consolidated Statements of Financial Position
FINANCIAL STATEMENTS |
| Calliditas Therapeutics | Interim Report 2024: January – March 22
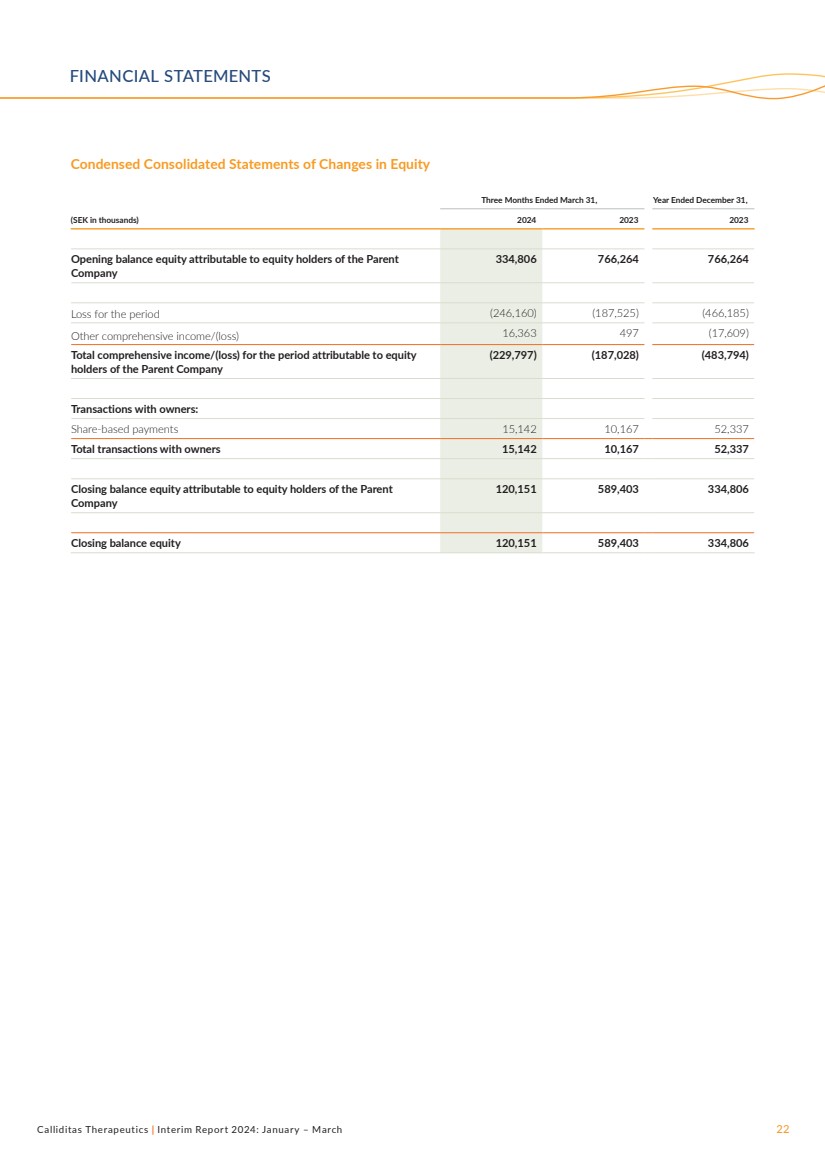
Condensed Consolidated Statements of Changes in Equity
Three Months Ended March 31, Year Ended December 31,
(SEK in thousands) 2024 2023 2023
Opening balance equity attributable to equity holders of the Parent
Company
334,806 766,264 766,264
Loss for the period (246,160) (187,525) (466,185)
Other comprehensive income/(loss) 16,363 497 (17,609)
Total comprehensive income/(loss) for the period attributable to equity
holders of the Parent Company
(229,797) (187,028) (483,794)
Transactions with owners:
Share-based payments 15,142 10,167 52,337
Total transactions with owners 15,142 10,167 52,337
Closing balance equity attributable to equity holders of the Parent
Company
120,151 589,403 334,806
Closing balance equity 120,151 589,403 334,806
FINANCIAL STATEMENTS |
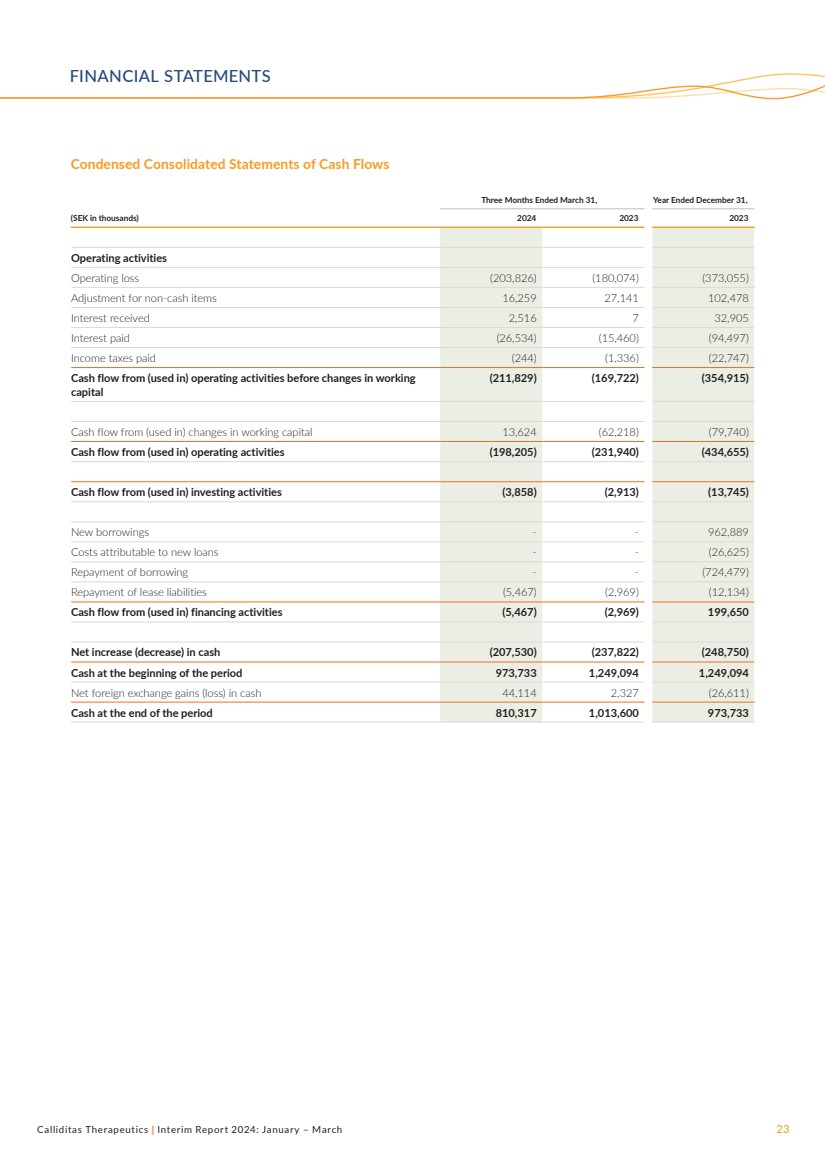
| Calliditas Therapeutics | Interim Report 2024: January – March 23
Three Months Ended March 31, Year Ended December 31,
(SEK in thousands) 2024 2023 2023
Operating activities
Operating loss (203,826) (180,074) (373,055)
Adjustment for non-cash items 16,259 27,141 102,478
Interest received 2,516 7 32,905
Interest paid (26,534) (15,460) (94,497)
Income taxes paid (244) (1,336) (22,747)
Cash flow from (used in) operating activities before changes in working
capital
(211,829) (169,722) (354,915)
Cash flow from (used in) changes in working capital 13,624 (62,218) (79,740)
Cash flow from (used in) operating activities (198,205) (231,940) (434,655)
Cash flow from (used in) investing activities (3,858) (2,913) (13,745)
New borrowings - - 962,889
Costs attributable to new loans - - (26,625)
Repayment of borrowing - - (724,479)
Repayment of lease liabilities (5,467) (2,969) (12,134)
Cash flow from (used in) financing activities (5,467) (2,969) 199,650
Net increase (decrease) in cash (207,530) (237,822) (248,750)
Cash at the beginning of the period 973,733 1,249,094 1,249,094
Net foreign exchange gains (loss) in cash 44,114 2,327 (26,611)
Cash at the end of the period 810,317 1,013,600 973,733
Condensed Consolidated Statements of Cash Flows
FINANCIAL STATEMENTS |
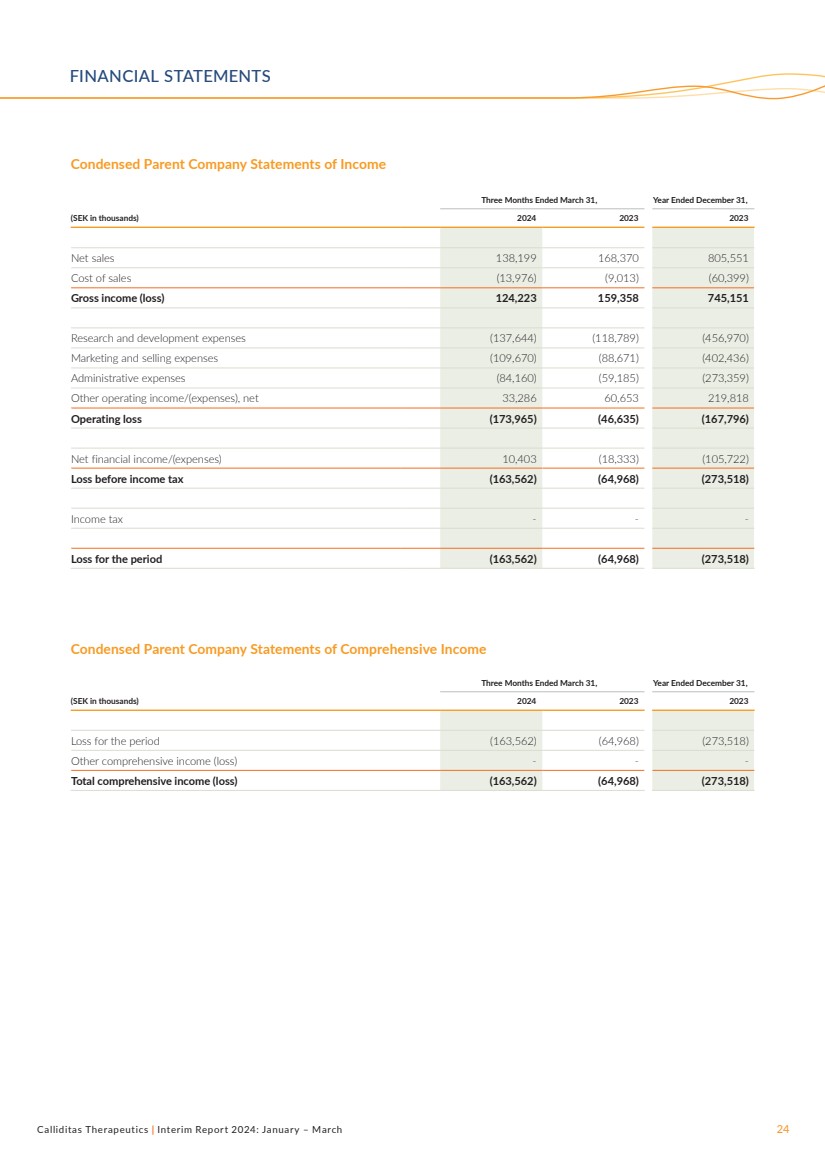
| Calliditas Therapeutics | Interim Report 2024: January – March 24
Condensed Parent Company Statements of Income
Three Months Ended March 31, Year Ended December 31,
(SEK in thousands) 2024 2023 2023
Net sales 138,199 168,370 805,551
Cost of sales (13,976) (9,013) (60,399)
Gross income (loss) 124,223 159,358 745,151
Research and development expenses (137,644) (118,789) (456,970)
Marketing and selling expenses (109,670) (88,671) (402,436)
Administrative expenses (84,160) (59,185) (273,359)
Other operating income/(expenses), net 33,286 60,653 219,818
Operating loss (173,965) (46,635) (167,796)
Net financial income/(expenses) 10,403 (18,333) (105,722)
Loss before income tax (163,562) (64,968) (273,518)
Income tax - - -
Loss for the period (163,562) (64,968) (273,518)
Three Months Ended March 31, Year Ended December 31,
(SEK in thousands) 2024 2023 2023
Loss for the period (163,562) (64,968) (273,518)
Other comprehensive income (loss) - - -
Total comprehensive income (loss) (163,562) (64,968) (273,518)
Condensed Parent Company Statements of Comprehensive Income
FINANCIAL STATEMENTS |
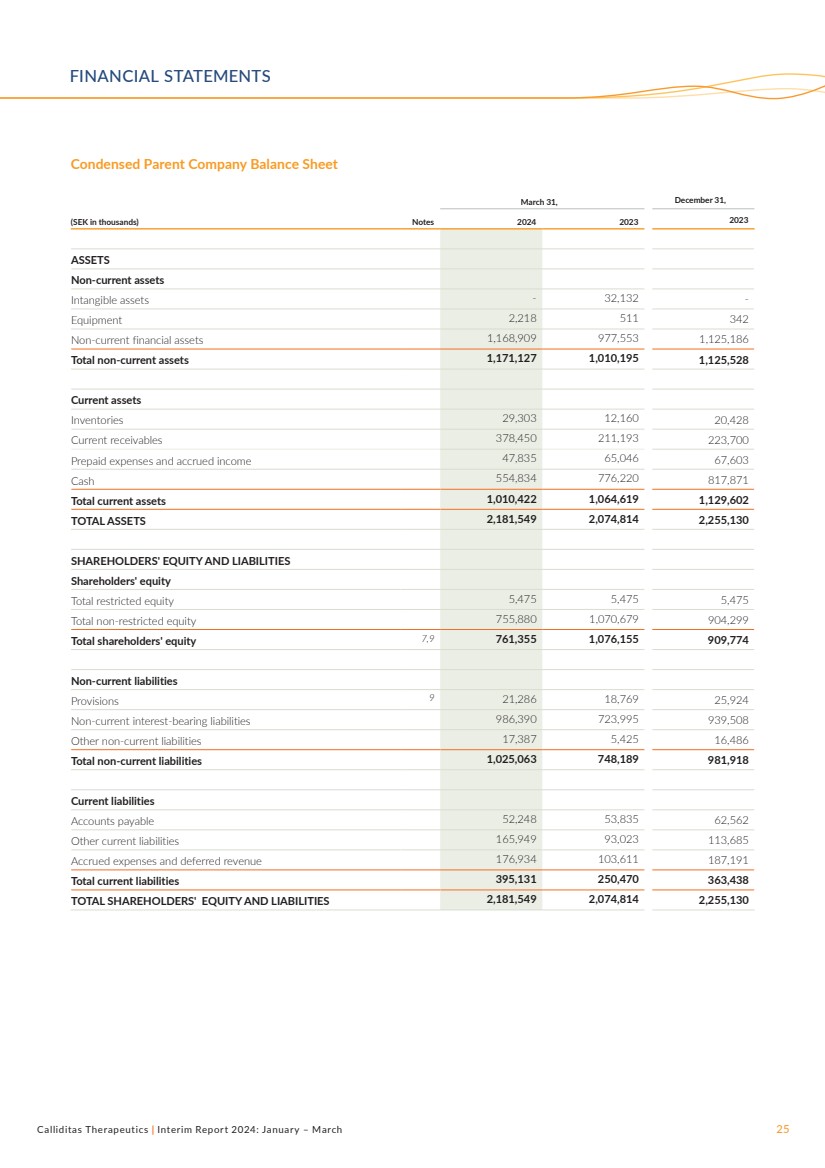
| Calliditas Therapeutics | Interim Report 2024: January – March 25
Condensed Parent Company Balance Sheet
March 31, December 31,
(SEK in thousands) Notes 2024 2023 2023
ASSETS
Non-current assets
Intangible assets - 32,132 -
Equipment 2,218 511 342
Non-current financial assets 1,168,909 977,553 1,125,186
Total non-current assets 1,171,127 1,010,195 1,125,528
Current assets
Inventories 29,303 12,160 20,428
Current receivables 378,450 211,193 223,700
Prepaid expenses and accrued income 47,835 65,046 67,603
Cash 554,834 776,220 817,871
Total current assets 1,010,422 1,064,619 1,129,602
TOTAL ASSETS 2,181,549 2,074,814 2,255,130
SHAREHOLDERS' EQUITY AND LIABILITIES
Shareholders' equity
Total restricted equity 5,475 5,475 5,475
Total non-restricted equity 755,880 1,070,679 904,299
Total shareholders' equity 7,9 761,355 1,076,155 909,774
Non-current liabilities
Provisions 9 21,286 18,769 25,924
Non-current interest-bearing liabilities 986,390 723,995 939,508
Other non-current liabilities 17,387 5,425 16,486
Total non-current liabilities 1,025,063 748,189 981,918
Current liabilities
Accounts payable 52,248 53,835 62,562
Other current liabilities 165,949 93,023 113,685
Accrued expenses and deferred revenue 176,934 103,611 187,191
Total current liabilities 395,131 250,470 363,438
TOTAL SHAREHOLDERS' EQUITY AND LIABILITIES 2,181,549 2,074,814 2,255,130
FINANCIAL STATEMENTS |

| Calliditas Therapeutics | Interim Report 2024: January – March 26
Note 1 - Description of Business
Calliditas Therapeutics AB (publ) (“Calliditas” or the “Parent Company”), with corporate registration number 556659-9766, and its
subsidiaries (collectively, the “Group”) conducts commercial and development activities in pharmaceuticals. These interim condensed
consolidated financial statements encompass the Group, domiciled in Stockholm, Sweden, and its subsidiaries for the three months
ended March 31, 2024 and 2023.
Calliditas is a Swedish public limited company registered in and with its registered office in Stockholm. The registered address of the
corporate headquarters is Kungsbron 1, D5, Stockholm, Sweden. Calliditas is listed at Nasdaq Stockholm in the Mid Cap segment with
ticker “CALTX” and, in the form of ADSs, on the Nasdaq Global Select Market in the United States with the ticker “CALT”.
These interim condensed consolidated financial statements were approved by the Board of Directors (the “Board”) for publication on
May 23, 2024. This report may include forward-looking statements. Actual outcomes may deviate from what has been stated. Internal
factors such as successful management of research projects, and intellectual property rights may affect future results. There are also
external conditions, (e.g. the economic climate, political changes, and competing research projects) that may affect the Group’s results.
Note 2 - Accounting Policies
These interim condensed consolidated financial statements have been prepared in accordance with International Accounting Standard
No. 34 (IAS 34), “Interim Financial Reporting”. The Parent Company applies the Swedish Financial Reporting Board recommendation
RFR2, Accounting for legal entities. The accounting policies adopted in the preparation of the interim condensed consolidated financial
statements are consistent with those followed in the preparation of the Annual Report for 2023. None of the new or amended standards
and interpretations that became effective January 1, 2024, have had a significant impact on the Group’s financial reporting. Significant
accounting policies can be found in the Annual Report 2023, from pages 45 and onwards including disclosures at respective note.
The ESMA (European Securities and Markets Authority) guidelines on alternative key performance ratios are applied, which means
disclosure requirements regarding financial measures that are not defined in accordance with IFRS. For key ratios not defined by IFRS,
see the Definitions and reconciliations of alternative performance measures on page 30.
Note 3 - Risks and Uncertainties in the Group and the Parent Company
Operational Risks
Research and drug development up to approved registration and marketing is subject to considerable risk and is a capital-intensive
process. The majority of all initiated projects will never reach market registration due to the technological risks, such as a failure to
demonstrate efficacy or a favorable risk/benefit profile, or manufacturing problems. Competing pharmaceuticals can capture market
share or reach the market faster, or if competing research projects achieve better product profiles, the future value of the product
portfolio may be lower than expected. The operations may also be impacted negatively by regulatory decisions, such as lack of approvals
and price changes.
Calliditas has a commercialized product, which has received full approval in the US under the brand name TARPEYO and has received
conditional marketing authorization in the EU and the UK under the brand name Kinpeygo, and in China under the brand name
Nefecon, and are dependent on renewal of the conditional marketing authorizations. There is a risk that commercialization will not go
according to plan or that the uptake of prescribing physicians will be worse than planned or that the drug will not have sufficient effect,
or show unwanted side effects, which may affect the sales negatively. The impact on the financial statements is described in the Finan-cial overview.
Financial Risks
Calliditas’ financial policy governing the management of financial risks has been designed by the Board of Directors and represents
the framework of guidelines and rules in the form of risk mandated and limits for financial activities. The Group is primarily affected
by foreign exchange risk, since the development costs for Nefecon and setanaxib are mainly paid in USD and EUR. Further, the Group
holds account receivables in USD and EUR and cash in USD and EUR to meet future expected costs in USD and EUR in connection
with commercialization of TARPEYO in the US and the clinical development programs. Regarding the Group and the Parent Company’s
financial risk management, the risks are essentially unchanged compared with the description in the Annual Report for 2023.
For more information and full disclosure regarding the operational and financial risks, reference is made to the Annual Report for 2023
and the Annual Report on Form 20-F, filed with the SEC in April 2024.
Notes to Condensed Consolidated Financial Statements
NOTES |
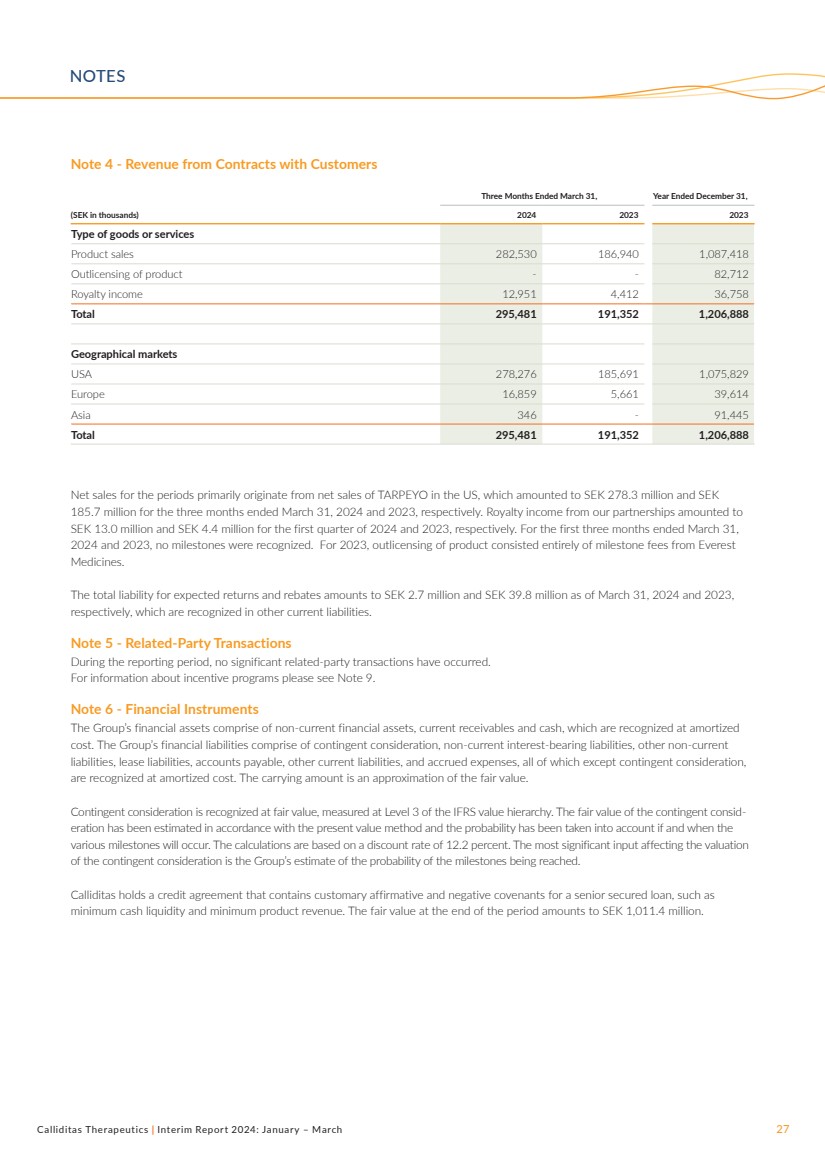
| Calliditas Therapeutics | Interim Report 2024: January – March 27
Note 4 - Revenue from Contracts with Customers
Three Months Ended March 31, Year Ended December 31,
(SEK in thousands) 2024 2023 2023
Type of goods or services
Product sales 282,530 186,940 1,087,418
Outlicensing of product - - 82,712
Royalty income 12,951 4,412 36,758
Total 295,481 191,352 1,206,888
Geographical markets
USA 278,276 185,691 1,075,829
Europe 16,859 5,661 39,614
Asia 346 - 91,445
Total 295,481 191,352 1,206,888
Net sales for the periods primarily originate from net sales of TARPEYO in the US, which amounted to SEK 278.3 million and SEK
185.7 million for the three months ended March 31, 2024 and 2023, respectively. Royalty income from our partnerships amounted to
SEK 13.0 million and SEK 4.4 million for the first quarter of 2024 and 2023, respectively. For the first three months ended March 31,
2024 and 2023, no milestones were recognized. For 2023, outlicensing of product consisted entirely of milestone fees from Everest
Medicines.
The total liability for expected returns and rebates amounts to SEK 2.7 million and SEK 39.8 million as of March 31, 2024 and 2023,
respectively, which are recognized in other current liabilities.
Note 5 - Related-Party Transactions
During the reporting period, no significant related-party transactions have occurred.
For information about incentive programs please see Note 9.
Note 6 - Financial Instruments
The Group’s financial assets comprise of non-current financial assets, current receivables and cash, which are recognized at amortized
cost. The Group’s financial liabilities comprise of contingent consideration, non-current interest-bearing liabilities, other non-current
liabilities, lease liabilities, accounts payable, other current liabilities, and accrued expenses, all of which except contingent consideration,
are recognized at amortized cost. The carrying amount is an approximation of the fair value.
Contingent consideration is recognized at fair value, measured at Level 3 of the IFRS value hierarchy. The fair value of the contingent consid-eration has been estimated in accordance with the present value method and the probability has been taken into account if and when the
various milestones will occur. The calculations are based on a discount rate of 12.2 percent. The most significant input affecting the valuation
of the contingent consideration is the Group’s estimate of the probability of the milestones being reached.
Calliditas holds a credit agreement that contains customary affirmative and negative covenants for a senior secured loan, such as
minimum cash liquidity and minimum product revenue. The fair value at the end of the period amounts to SEK 1,011.4 million.
NOTES |
| Calliditas Therapeutics | Interim Report 2024: January – March 28
Note 7 - Treasury Shares
As of March 31, 2024, Calliditas had 5,908,018 ordinary shares held as treasury shares by the Parent Company. At the Annual General
Meeting 2023, authorization was given that Calliditas can transfer (sale) these ordinary shares with the purpose to finance an acquisition
of operations, to procure capital to finance the development of projects, repayment of loans or to commercialize Calliditas’ products.
No transfer (sale) of treasury shares have occurred as of March 31, 2024. The total number of issued shares as of March 31, 2024, is
disclosed in Note 8.
Note 8 - Shareholders’ Equity
March 31, December 31,
(SEK in thousands, except per share amounts and number of shares) 2024 2023 2023
Total registered shares at the beginning of the period 59,580,087 59,580,087 59,580,087
Total registered and subscribed but not registered shares
at the end of the period
59,580,087 59,580,087 59,580,087
Shares
Ordinary shares 59,580,087 59,157,587 59,580,087
Total 59,580,087 59,157,587 59,580,087
- of which shares are held by Calliditas 5,908,018 5,908,018 5,908,018
Total registered and subscribed but not registered shares
at the end of the period, net of shares held by Calliditas
53,672,069 53,249,569 53,672,069
Share capital at the end of the period 2,383 2,383 2,383
Equity attributable to equity holders of the Parent Company 120,151 589,403 334,806
Total equity at the end of the period 120,151 589,403 334,806
Three Months Ended
March 31, Year Ended December 31,
(SEK in thousands, except per share amounts and number of shares) 2024 2023 2023
Loss per share before and after dilution, SEK (4.59) (3.49) (8.69)
Weighted-average number of ordinary shares outstanding for the period,
before and after dilution
53,672,069 53,672,069 53,672,069
Reserves for translation from foreign operations amounted to SEK 11.1 million and SEK 10.5 million which are included in retained
earnings in equity as of March 31, 2024 and 2023, respectively.
NOTES |
| Calliditas Therapeutics | Interim Report 2024: January – March 29
Note 9 - Incentive Programs
Board LTIP 2021:
This is a performance-based long-term incentive program for Calliditas Board members. The share awards are subject to perfor-mance-based earnings, which are dependent on the development of Calliditas’ share price from the date of the 2021 Annual General
Meeting to July 1, 2024.
Board LTIP 2022:
This is a performance-based long-term incentive program for Calliditas Board members. The share awards are subject to perfor-mance-based earnings, which are dependent on the development of Calliditas’ share price from the date of the 2022 Annual General
Meeting to July 1, 2025.
Board LTIP 2023:
This is a performance-based long-term incentive program for Calliditas Board members. The share awards are subject to perfor-mance-based earnings, which are dependent on the development of Calliditas’ share price from the date of the 2023 Annual General
Meeting to July 1, 2026.
ESOP Programs
Calliditas implements option programs for employees and key consultants in Calliditas. The options are granted free of charge to
participants of the program. The options have a three-year vesting period calculated from the grant date, provided that, with customary
exceptions, the participants remain as employees of, or continue to provide services to, Calliditas. Once the options are vested, they can
be exercised within a one-year period. Each vested option entitles the holder to acquire one share in Calliditas at a predetermined price.
The price per share is to be equivalent to 115% of the weighted average price that the company’s shares were traded for on Nasdaq
Stockholm during the ten trading days preceding the grant date. The options have, at the time of each issue, been valued according to
the Black-Scholes valuation model.
March 31, 2024 March 31, 2023
Options
Outstanding
Share Awards
Outstanding
Total
Outstanding
Options
Outstanding
Share Awards
Outstanding
Total
Outstanding
Incentive Programs
Board LTIP 2020 - - - - 29,928 29,928
Board LTIP 2021 - 22,882 22,882 - 24,244 24,244
Board LTIP 2022 - 37,136 37,136 - 40,706 40,706
Board LTIP 2023 - 40,957 40,957 - - -
ESOP 2020 1,364,730 - 1,364,730 1,364,730 - 1,364,730
ESOP 2021 1,390,500 - 1,390,500 1,479,500 - 1,479,500
ESOP 2022 1,826,000 - 1,826,000 1,548,000 - 1,548,000
ESOP 2023 1,880,000 - 1,880,000 - - -
Total Outstanding 6,461,230 100,975 6,562,205 4,392,230 94,878 4,487,108
NOTES |
| Calliditas Therapeutics | Interim Report 2024: January – March 30
Definitions and Reconciliations of Alternative Performance Measures
Definitions of Alternative Performance Measures
Alternative
Key Performance Indicator Definitions Reason for Inclusion
Equity ratio at the end of the period in % The ratio at the end of respective period
is calculated by dividing total shareholders’
equity by total assets.
The equity ratio measures the proportion of
the total assets that are financed by share-holders.
Reconciliations of Alternative Performance Measures
March 31, December 31,
(SEK in thousands or otherwise indicated) 2024 2023 2023
Equity ratio at the end of the period in %
Total shareholders' equity at the end of the period 120,151 589,403 334,806
Total assets at the end of the period 1,688,295 1,788,716 1,859,245
Equity ratio at the end of the period in % 7% 33% 18%
NOTES |
Exhibit 99.2

| Stockholm, Sweden | | May 23, 2024 |
Calliditas
Q1 report, January – March 2024
Calliditas
Therapeutics AB (Nasdaq Stockholm: CALTX):
Target
market expansion following full approval in the US
JANUARY – MARCH 2024 (COMPARED TO JANUARY –
MARCH 2023)
| · | Net sales amounted to SEK 295.5 million, of which TARPEYO® net sales amounted to SEK 278.3 million,
for the three months ended March 31, 2024. For the three months ended March 31, 2023, net sales amounted to SEK 191.4 million, of which TARPEYO net sales amounted to SEK
185.7 million. |
| · | Operating loss amounted to SEK 203.8 million and SEK 180.1 million for the three months ended March
31, 2024, and 2023, respectively. |
| · | Loss per share before and after dilution amounted to SEK 4.59 and SEK 3.49 for the three months ended
March 31, 2024, and 2023, respectively. |
| · | Cash amounted to SEK 810.3 million and SEK 1,013.6 million as of March 31, 2024, and 2023, respectively. |
“In
Q1 we generated another record quarter in terms of demand with 705 enrollments and 354 new prescribers. We are very excited over this
positive trend, and we continue to see strong demand in Q2.” – CEO Renée Aguiar-Lucander.
KEY TAKEAWAYS FROM Q1, 2024
| ● | Calliditas
had a record quarter with 705 enrollments, representing a 27% increase over Q4. |
| | | |
| | ● | In February, the United States Patent and Trademark Office (USPTO) issued patent no. 11896719, entitled “New Pharmaceutical
Compositions”. This was Calliditas’ second patent for TARPEYO in the United States and provides product protection until 2043. |
| | | |
| | ● | In March, the FDA granted an orphan drug exclusivity period of seven years for TARPEYO®, expiring in December 2030, based on
when the company obtained full approval with a new indication for this drug product. |
| | | |
| | ● | There was a negative impact on net TARPEYO revenues in the quarter of approximately USD 4.7 million due to a cyberattack on Change Health. The revenues we were not able to record in Q1 because of this technical issue are not lost, but are expected to roll forward over the next several months. This is not expected to have any impact on annual revenues. |
KEY EVENTS AFTER THE REPORTING PERIOD
| |
● |
Preliminary net sales from TARPEYO for the second quarter to date amounts to USD 25.5 million. |
| |
|
|
| |
● |
Positive read out of the Nefecon Open label Phase 3 extension trial. |
| |
|
|
| |
● |
Positive topline results from the setanaxib Phase 2 trial in head and neck cancer. |
| |
|
|
| |
● |
Commercial launch of Nefecon in China by partner Everest Medicines. |
| |
|
|
| KEY EVENTS UPCOMING 6 MONTHS |
| |
|
|
| |
● |
European Commission decision regarding a potential full approval for Kinpeygo for Calliditas’ partner STADA. |
| |
|
|
| |
● |
Full data read out of Phase 2 trial in Primary Biliary Cholangitis. |
| |
|
|
| |
● |
Updated KDIGO guidelines expected in 2024. |

Outlook for 2024: Unchanged
Calliditas expects continued revenue growth:
Total net sales from the Nefecon franchise, including
milestones, are estimated to be USD 150-180 million for the year ending December 31, 2024.
Investor Presentation:
May 23, 2024 14:30 CET
Link
to webcast: Calliditas Therapeutics Q1 Report 2024 (financialhearings.com)
To
participate via conference call register via this link: Call Access (financialhearings.com)
For further information,
please contact:
Åsa Hillsten, Head of
IR & Sustainability, Calliditas
Tel.: +46 76 403 35 43, Email:
asa.hillsten@calliditas.com
The information in the report
is information that Calliditas is obliged to make public pursuant to the EU Market Abuse Regulation. The information was sent for publication,
through the agency of the contact person set out above, on May 23, 2024, at 7:00 a.m. CET.
About Calliditas
Calliditas Therapeutics is
a biopharma company headquartered in Stockholm, Sweden, focused on identifying, developing, and commercializing novel treatments in orphan
indications with significant unmet medical needs. Calliditas’ common shares are listed on Nasdaq Stockholm (ticker: CALTX) and
its American Depositary Shares are listed on the Nasdaq Global Select Market (ticker: CALT). Visit Calliditas.com for further information.
Forward-Looking Statements
This press release contains forward-looking statements
within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, without limitation, statements regarding
Calliditas’ strategy, business plans, revenue and other financial projections, and focus. The words “may,” “will,”
“could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,”
“believe,” “estimate,” “predict,” “project,” “potential,” “continue,”
“target” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements
contain these identifying words.
Any forward-looking statements in this press release are
based on management’s current expectations and beliefs and are subject to a number of risks, uncertainties and important factors
that may cause actual events or results to differ materially from those expressed or implied by any forward-looking statements contained
in this Interim Report, including, without limitation, any related to Calliditas’ business, operations, commercialization of TARPEYO,
Kinpeygo and Nefecon, clinical trials, supply chain, strategy, goals and anticipated timelines for development and potential approvals,
competition from other biopharmaceutical companies, revenue and product sales projections or forecasts, including 2024 total net sales
guidance and cash runway and preliminary TARPEYO net sales for the second quarter of 2024 to date, and other risks identified in the section
entitled “Risk Factors” in Calliditas’ reports filed with the Securities and Exchange Commission.
Calliditas cautions you not to place undue reliance
on any forward-looking statements, which speak only as of the date they are made. Calliditas disclaims any obligation to publicly update
or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements
may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward- looking statements.
Any forward-looking statements contained in this Interim Report represent Calliditas’ views only as of the date hereof and should
not be relied upon as representing its views as of any subsequent date.
Calliditas Therapeutics AB (NASDAQ:CALT)
Gráfica de Acción Histórica
De May 2024 a Jun 2024
Calliditas Therapeutics AB (NASDAQ:CALT)
Gráfica de Acción Histórica
De Jun 2023 a Jun 2024