As filed with the Securities and Exchange Commission on December 19, 2023
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
| GAIN THERAPEUTICS, INC. |
| (Exact name of registrant as specified in its charter) |
| Delaware |
|
2834 |
|
85-1726310 |
|
(State or other jurisdiction of
incorporation or organization) |
|
(Primary Standard Industrial
Classification Code Number) |
|
(I.R.S. Employer
Identification No.) |
4800 Montgomery Lane, Suite 220
Bethesda, Maryland 20814
Telephone: (301) 500-1556
(Address, including zip code, and telephone
number,
including area code, of principal executive
offices)
Matthias Alder
Chief Executive Officer
Gain Therapeutics, Inc.
4800 Montgomery Lane, Suite 220
Bethesda, Maryland 20814
(301) 500-1556
(Address, including zip code, and telephone
number,
including area code, of agent for service)
Copies to:
Steven M. Skolnick, Esq.
Lowenstein Sandler LLP
1251 Avenue of the Americas
New York, New York 10020
Telephone: (212) 262-6700
Approximate date of commencement of proposed sale to the public:
From time to time after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered
on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. x
If this Form is filed to register additional securities for an offering
pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration
statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under
the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective
registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under
the Securities Act, check the following box and list the Securities Act registration number of the earlier effective registration
statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated
filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated
filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 under the Securities
Exchange Act of 1934. (Check one):
| Large Accelerated Filer |
¨ |
Accelerated Filer |
¨ |
| Non-accelerated Filer |
x |
Smaller Reporting Company |
x |
| |
|
Emerging Growth Company |
x |
If an emerging growth company, indicate by check mark if the registrant
has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant
to Section 7(a)(2)(B) of the Securities Act. ¨
The Registrant hereby amends this registration statement on such
date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment that specifically states
that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended,
or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to
said Section 8(a), may determine.
The information in this prospectus is not complete and
may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is
effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state
where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED DECEMBER
19, 2023
PRELIMINARY PROSPECTUS

744,026 Shares of Common Stock
4,256,150 Shares of Common Stock Issuable Upon
Exercise of Outstanding Warrants
This prospectus relates to the resale from time
to time, by the selling stockholder identified in this prospectus under the section heading “Selling Stockholder,”
of up to (i) 744,026 shares (the “Private Shares”) of our common stock, par value
$0.0001 per share (“Common Stock”) that were issued in connection with a private placement (the “Private Placement”),
(ii) 1,756,062 shares of our Common Stock issuable upon the exercise of pre-funded warrants (the “Pre-Funded Warrants”
and the shares of Common Stock issuable upon the exercise thereof, the “Pre-Funded Warrant Shares”) issued in the Private
Placement, and (iii) 2,500,088 shares (the “Private Warrant Shares” and together with the Pre-Funded Warrant Shares,
the “Warrant Shares”) of our Common Stock issuable upon the exercise of warrants issued in the Private Placement (the
“Private Warrants” and together with the Pre-Funded Warrants, the “Warrants”). We issued
the Private Shares and the Warrants in connection with the Private Placement, which was conducted concurrently with a public offering
of 2,545,000 shares of Common Stock and warrants to purchase 1,272,500 shares of Common Stock, both of which closed on November 24, 2023.
The selling stockholder may, from time to time,
sell, transfer or otherwise dispose of any or all of his shares of Common Stock or interests in his shares of Common Stock on any stock
exchange, market or trading facility on which the shares of Common Stock are traded or in private transactions. These dispositions may
be at fixed prices, at prevailing market prices at the time of sale, at prices related to the prevailing market price, at varying prices
determined at the time of sale, or at negotiated prices. See “Plan of Distribution” in this prospectus for more information.
We will not receive any proceeds from the resale or other disposition of the shares of Common Stock by the selling stockholder. However,
we will receive the proceeds of any cash exercise of the Warrants. See “Use of Proceeds” beginning on page 9 and “Plan
of Distribution” beginning on page 18 of this prospectus for more information.
Our Common Stock is listed on the Nasdaq Global
Market LLC (“Nasdaq”) under the symbol “GANX”. On December 19, 2023, the last reported sale price of our
Common Stock as reported on Nasdaq was $2.56.
You should read this prospectus, together with
additional information described under the headings “Information Incorporated by Reference” and “Where You
Can Find More Information,” carefully before you invest in any of our securities.
An investment in our
securities involves a high degree of risk. Before deciding whether to invest in our securities, you should consider carefully the
risks and uncertainties described in the section captioned “Risk Factors” contained in (i) our Quarterly Reports on Form
10-Q for the quarterly periods ended September 30, 2023, June 30, 2023 and March 31, 2023, respectively, as filed with the
Securities and Exchange Commission (the “SEC”) on November 14, 2023, August 10, 2023 and May 12, 2023, and
(ii) our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, filed with the SEC on March 23, 2023, and our
other filings we make with the SEC from time to time, which are incorporated by reference herein in their entirety, together with
other information in this prospectus and the information incorporated by reference herein.
Neither the SEC nor any state securities commission
has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary
is a criminal offense.
The date of this Prospectus is , 2023
TABLE OF CONTENTS
PROSPECTUS SUMMARY
This summary highlights information contained
elsewhere in this prospectus and the documents incorporated by reference herein. This summary does not contain all of the information
that you should consider before deciding to invest in our securities. You should read this entire prospectus carefully, including the
section entitled “Risk Factors”, our consolidated financial statements and the related notes and the other information incorporated
by reference into this prospectus before making an investment decision.
This prospectus and the information incorporated
by reference herein contain references to trademarks, service marks and trade names owned by us or other companies. Solely for convenience,
trademarks, service marks and trade names referred to in this prospectus and the information incorporated by reference herein, including
logos, artwork, and other visual displays, may appear without the ® or ® symbols, but such references
are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights
of the applicable licensor to these trademarks, service marks and trade names. We do not intend our use or display of other companies’
trade names, service marks or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies. Other
trademarks, trade names and service marks appearing in this prospectus and the documents incorporated by reference herein are the property
of their respective owners.
Corporate Overview
We are a clinical stage biotechnology
company developing novel small molecule therapeutics to treat diseases across several therapeutics areas, including, lysosomal storage
disorders, or LSDs, central nervous system disorders, or CNS, metabolic disorders, and oncology. We use our exclusively in-licensed computational
target and drug discovery platform, Site-Directed Enzyme Enhancement Therapy, or SEE-Tx, to discover novel allosteric binding sites on
proteins implicated in a disease and to identify proprietary small molecules that bind these sites to modulate protein function and treat
the underlying cause of the disease. We believe that SEE-Tx is uniquely suited to identify allosteric binding sites on the protein surface,
which are different from the active (or orthosteric) binding site where the natural ligand of the protein binds. Targeting an allosteric
binding site instead of the active binding site of a protein provides numerous advantages, including: the ability to restore or disrupt
the function of proteins implicated in disease through several different mechanisms of action covering both functional and conformational
effects, including stabilization, destabilization, targeted degradation, allosteric inhibition, and allosteric activation of the targeted
protein; improved specificity of small molecules because binding to an allosteric binding site is non-competitive with the natural substrate
that binds to the active binding site; and the ability to identify small molecules with more favorable drug-like properties. The SEE-Tx
platform has been used to identify novel allosteric sites and small molecules for all of our pipeline programs. Discovering and targeting
novel allosteric sites with our platform not only reduces traditional drug discovery timelines but enables rational drug design and offers
the potential for superior small molecule drugs that are highly specific and that can penetrate hard to reach tissues and cross the blood-brain
barrier where necessary.
We have generated an extensive
preclinical data package providing evidence of the mechanism of action and effect of our lead product candidate GT-02287 for the treatment
of misfolded beta-glucocerebrosidase, or GBA1 Parkinson’s disease, including restoration of the lysosomal enzyme β-Glucocerebrosidase
(GCase) function, reduction of toxic lipid substrates and toxic forms of alpha-synuclein, improved survival of dopaminergic neurons, increased
dopamine levels and improved locomotor function in animal models. In September 2023, we received approval from the Bellberry Human Research
Ethics Committee (HREC) to conduct a Phase 1 clinical trial in Australia. The Phase 1 clinical trial is intended to evaluate the administration
of both single and multiple ascending dose levels of GT-02287 in healthy volunteers to assess the safety, tolerability and pharmacokinetics
of GT-02287. In addition, in the multiple ascending dose part of the Phase 1 clinical trial, we will evaluate the pharmacodynamic response
to GT-02287 by analyzing GCase levels and activity. As of August 10, 2023, the first of five cohorts of the single ascending dose part
was completed, and the dose was escalated to the second dose level. No drug-related adverse effects have been observed as of the present
time. We expect to complete the Phase 1 clinical trial of GT-02287 in the first half of 2024.
We have filed four
patent applications for our novel small molecule drug candidates for the treatment of neurodegenerative diseases including GBA
Parkinson’s disease and idiopathic Parkinson’s disease. We announced the publication of two PCT patent applications with
the first directed at compounds targeting GBA1, addressing CNS diseases such as Parkinson’s disease, Gaucher disease,
Alzheimer’s disease, and Lewy body dementia. The second patent application was directed at compounds targeting
galactosylceramidase, or GALC, addressing demyelinating disorders such as Krabbe disease and multiple sclerosis.
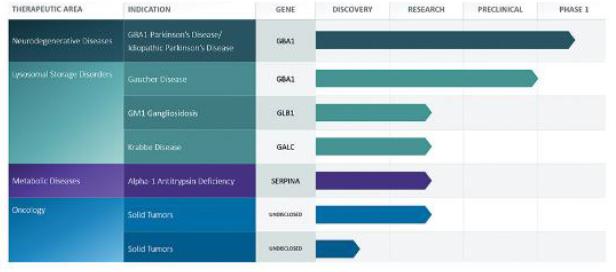
Our Pipeline
We are leveraging our SEE-Tx
technology platform to develop a pipeline of novel small molecule drug candidates to address complex diseases. The platform is disease
agnostic and provides us with the ability to expand our pipeline, quickly, efficiently and at low cost. We are currently focusing on progressing
our programs in Parkinson’s and Gaucher disease. We are also continuing to develop our program in Krabbe disease and liver and lung
disease, and applying our SEE-Tx platform to establish new programs in oncology. We are seeking non-dilutive funding in order to progress
our other research programs in additional lysosomal storage disorders and metabolic diseases.
The following table sets forth the status of our
product candidates:
Risks Associated with Our Business
Our business is subject to numerous risks. You should
read these risks before you invest in our securities. In particular, our risks include, but are not limited to, the following:
| · | there is substantial doubt about our ability to continue as a going concern.
We have a history of operating losses and expect to incur losses for the foreseeable future. We may never generate revenues or, if we
are able to generate revenues, achieve profitability; |
| · | we have a limited operating history, and we expect a number of factors to
cause our operating results to fluctuate on a quarterly and annual basis, which may make it difficult to predict our future performance; |
| · | if preclinical studies or clinical trials for our product candidates cannot
be initiated or completed or if they are delayed or unsuccessful, we will be unable to meet our future development and commercialization
goals; |
| · | the disorders we seek to treat have low prevalence and it may be difficult
to identify patients with these disorders, which may lead to delays in enrollment for our trials or slower commercial revenue if approved,
and we may also face enrollment challenges as a result of other factors; |
| · | our product candidates are novel and still in development. If we are unable
to successfully develop, receive regulatory approval for and commercialize our current or future product candidates, our business will
be harmed; |
| · | we have just started testing one of our product candidates in clinical trials.
Success in early preclinical studies or clinical trials may not be indicative of results obtained in later preclinical studies and clinical
trials; |
| · | clinical trials required for our product candidates are expensive and time-consuming,
and their outcome is uncertain; |
| · | we will need to raise additional capital, which may cause dilution to our
stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates, and additional capital
may not be available on favorable terms or at all, which may force us to delay, reduce the scope of or eliminate our research and development
programs, reduce our commercialization efforts or curtail our operations; |
| · | we are subject to extensive and costly government regulation; |
| · | even if we obtain regulatory approval to market our product candidates, our
product candidates may not be accepted by the market and we might not receive coverage or adequate reimbursement from government and third-party
payors for any approved products; |
| · | we rely on third parties to manufacture the compounds used in our studies.
If these third parties do not manufacture these compounds in sufficient quantities and at acceptable cost, or if these third parties experience
delays in production or are required by government regulators to halt or modify their operations, progress of our clinical trials and
development and commercialization of our products could be delayed, prevented, or impaired; |
| · | we rely on a license to use the technology that is material to our business
and if the agreement underlying the license were to be terminated or if other rights that may be necessary for commercializing our intended
products cannot be obtained, it would halt our ability to market our products and technology, as well as have an immediate material adverse
effect on our business, operating results and financial condition; |
| · | we are subject to stringent and evolving U.S. and foreign laws, regulations,
rules, contractual obligations, policies and other obligations related to data privacy and security. Our actual or perceived failure to
comply with such obligations could lead to regulatory investigations or actions, litigation (including class actions) and mass arbitration
demands, fines and penalties, disruptions of our business operations, reputational harm, loss of revenue or profits and other adverse
business consequences; and |
| · | global and macroeconomic conditions, including worldwide economic, political
and social instability could adversely affect our revenue, financial condition, or results of operations. |
Recent Developments
Public Offering
On
November 21, 2023, we entered into an underwriting agreement (the “Underwriting Agreement”) with Newbridge
Securities Corporation, or Newbridge, as the underwriter relating to the offering, issuance and sale of an aggregate of (i)
2,545,000 shares of our Common Stock and (ii) warrants to purchase 1,272,500 shares of our Common Stock (the “Public
Warrants” and the shares of Common Stock underlying the Public Warrants, the “Public Warrant Shares”)
for the aggregate gross proceeds of $5.1 million (the “Public Offering”). The Public Warrants have an exercise
price of $2.75 per share, subject to adjustment as provided for therein, were exercisable immediately upon the closing of the Public
Offering and are exercisable for a period of five years from the closing of the Public Offering. The Public Warrants were offered
and sold at the rate of one Public Warrant to purchase one share of our Common Stock for every two shares of Common Stock purchased
in the Public Offering. The Public Offering price for each set of two shares of Common Stock and accompanying Public Warrant to
purchase one share of Common Stock was $4.01 per set of securities, yielding an effective price of $2.00 per share and $0.01 per
Public Warrant. Our net proceeds from the Public Offering were approximately $4.3 million, after deducting underwriting discounts
and commissions and other estimated offering expenses payable by us. Upon the closing of the Public Offering, we issued to
Newbridge, or its designees, warrants (the “Underwriter’s Warrants”) to purchase up to 154,913 shares of
Common Stock, or 7.0% of the total number of shares sold in the Public Offering. The Underwriter’s Warrants are exercisable at
an exercise price of $2.75 per share. The Underwriter’s Warrants are exercisable during the four-and-one-half year period
commencing six months after the closing date of the Public Offering.
The
Public Offering was made pursuant to our effective registration statement on Form S-3 (File No. 333-265061) that was initially filed on
May 18, 2022, and amended on May 23, 2022, and declared effective on June 1, 2022 (the “Registration Statement”) by
the SEC and a prospectus supplement and accompanying prospectus filed with the SEC.
Private Placement Offering
On November 21, 2023, the
Private Placement was completed simultaneously with the Public Offering. In the Private Placement we issued the Private Shares, and the
Warrants for aggregate gross proceeds of $5.0 million. The Private Warrants are exercisable at an exercise price of $2.75 per share, will
be exercisable six months after issuance and expire five years from the date of issuance. The Pre-Funded Warrants are each exercisable
for one share of Common Stock at an exercise price of $0.0001 per share and will expire when exercised in full. The offering price for
each of the Private Shares and each of the accompanying Private Warrants to purchase one Private Warrant Share was $2.00. The net proceeds
from the Private Placement, after deducting placement agent fees and other estimated offering expenses payable by us, was approximately
$4.65 million The Private Placement closed on November 24, 2023.
Pursuant to a letter agreement
dated as of November 14, 2023, we engaged Newbridge to act as our exclusive placement agent in connection with the Private Placement.
We paid Newbridge a cash fee equal to 7.0% of the aggregate gross proceeds of the Private Placement, excluding the proceeds, if any, from
the exercise of the Private Warrant and the Pre-Funded Warrants. In addition, we issued to Newbridge or its designees, warrants to purchase
up to 175,006 shares of our Common Stock. Newbridge’s warrants are exercisable at any time beginning on May 24, 2024 and expire
on November 24, 2028.
This prospectus covers the
resale or other disposition by the selling stockholder of the Private Shares and the Warrant Shares.
Corporate Information
We were incorporated under
the laws of the state of Delaware on June 26, 2020 under the name Gain Therapeutics, Inc. Our principal executive offices are located
at 4800 Montgomery Lane, Suite 220, Bethesda, M.D. 20814. Our telephone number is (301) 500-1556. Our website address is http://www.gaintherapeutics.com.
Information contained in,
or accessible through, our website does not constitute part of this prospectus or registration statement and inclusions of our website
address in this prospectus or registration statement are inactive textual references only. You should not rely on any such information
in making your decision whether to purchase our securities.
The Offering
This prospectus relates to the resale or other
disposition from time to time by the selling stockholder identified in this prospectus of up to (i) 744,026 Private Shares, (ii) 1,756,062
shares of our Common Stock issuable upon the exercise of the Pre-Funded Warrants, and (iii) 2,500,088 shares of our Common Stock issuable
upon the exercise of the Private Warrants a. None of the shares of Common Stock registered hereby are being offered for sale by us.
| Shares of Common Stock offered by the selling stockholder |
|
Up to 5,000,176 shares of Common Stock issuable upon exercise of the Warrants. |
| |
|
|
| Shares of Common Stock outstanding after this offering |
|
20,455,518 shares, assuming the exercise in full of the Warrants. |
| |
|
|
| Use of proceeds |
|
We
will not receive any proceeds from the shares of Common Stock offered by the selling stockholder pursuant to this prospectus. However,
we will receive the proceeds of any cash exercise of the Warrants. We intend to use the net proceeds from any cash exercise of the Warrants
for working capital and general corporate purposes. Please see the section entitled see “Use of Proceeds” on page
9 of this prospectus for a more detailed discussion. |
| |
|
|
| National Securities Exchange Listing |
|
Our Common Stock is currently listed on Nasdaq under the symbol “GANX”. There is no established public trading market for the Warrants and we do not expect a market to develop. In addition, we do not intend to apply to list the Warrants on any national securities exchange or other nationally recognized trading system. Without an active trading market, the liquidity of the Warrants will be limited. |
| |
|
|
| Risk Factors |
|
An
investment in our securities involves a high degree of risk. Please see the section entitled “Risk Factors”
beginning on page 6 of this prospectus. In addition before deciding whether to invest in our securities, you should consider
carefully the risks and uncertainties described in the section captioned “Risk Factors” contained in our Annual
Report on Form 10-K for the fiscal year ended December 31, 2022 filed with the SEC on March 23, 2023, and other filings we make
with the SEC from time to time, which are incorporated by reference herein in their entirety, together with other information in
this prospectus and the information incorporated by reference herein. |
The number of shares of Common Stock to be outstanding
upon completion of this offering is based on 20,455,518 of our shares of Common Stock outstanding as of December 18, 2023 and excludes
the following:
| · | 425,387 shares of Common Stock issuable upon the exercise of outstanding
warrants to purchase shares of Common Stock with a weighted-average exercise price of $9.15 per share; |
| · | 506,188 shares of Common Stock issuable upon the vesting of outstanding restricted
stock units; |
| · | 2,599,751 shares issuable upon the exercise of outstanding stock options
with a weighted-average exercise price of $4.53 per share; |
| · | 314,077 shares reserved for future issuances under our equity compensation
plans; |
| · | 1,272,500 shares of our Common Stock issuable upon exercise of the Public
Warrants issued in connection with the Public Offering; |
| · | 178,150 shares of our Common Stock issuable upon exercise of the warrants
issued to the underwriter in the Public Offering; and |
| · | 175,006 shares of our Common Stock issuable upon exercise of the warrants
issued to the placement agent in the Private Placement. |
RISK FACTORS
An investment in our securities involves a high
degree of risk. Before deciding whether to invest in our securities, you should consider carefully the risks and uncertainties described
in the section captioned “Risk Factors” contained in our Annual Report on Form 10-K for the fiscal year ended December 31, 2022 filed with the SEC on March 23, 2023, and our other filings we make with the Securities and Exchange Commission from time to
time, which are incorporated by reference herein in their entirety, together with other information in this prospectus and the information
incorporated by reference herein. If any of these risks actually occurs, our business, financial condition, results of operations or cash
flow could suffer materially. In such an event, the trading price of our shares of Common Stock could decline, and you might lose all
or part of your investment.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Except for historical information, this prospectus
contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, (the “Securities
Act”) and Section 21E of the Exchange Act of 1934, as amended (the “Exchange Act”). Forward-looking statements
include statements with respect to our beliefs, plans, objectives, goals, expectations, anticipations, assumptions, estimates, intentions
and future performance, and involve known and unknown risks, uncertainties and other factors, which may be beyond our control, and which
may cause our actual results, performance or achievements to be materially different from future results, performance or achievements
expressed or implied by such forward-looking statements. All statements other than statements of historical fact are statements that could
be forward-looking statements. You can identify these forward-looking statements through our use of words such as “may,” “can,”
“anticipate,” “assume,” “should,” “indicate,” “would,” “believe,”
“contemplate,” “expect,” “seek,” “estimate,” “continue,” “plan,”
“point to,” “project,” “predict,” “could,” “intend,” “target,”
“potential” and other similar words and expressions of the future. Without limiting the broader description of forward-looking
statements above, we specifically note that statements regarding potential drug candidates, their potential therapeutic effect, the possibility
of obtaining regulatory approval, our expected timing for completing clinical trials and clinical trial milestones for our drug candidates,
our ability or the ability of our collaborators to manufacture and sell any products, market acceptance or our ability to earn a profit
from sales or licenses of any drug candidate or to discover new drugs in the future are all forward-looking in nature.
There are a number of important factors that could
cause the actual results to differ materially from those expressed in any forward-looking statement made by us. These factors include,
but are not limited to:
| · | our ability to continue as a going concern; |
| · | our needs for additional financing; |
| · | the success of our efforts, and those of our
advisors, in exploring, and possibly executing on, our strategic alternatives, while preserving our cash balance to the extent practicable; |
| · | the initiation, timing, progress and results
of our current and future preclinical studies and clinical trials and our research and development programs; |
| · | the success of our efforts to expand our pipeline
of product candidates and develop marketable products through the use of our in-licensed Site-Directed Enzyme Enhancement Therapy, or
SEE-Tx®, platform; |
| · | our ability to develop, obtain regulatory approval
for and commercialize our current and future product candidates; |
| · | our expectations regarding collaborations and
other agreements with third parties and their potential benefits; |
| · | the timing of investigational new drug, or IND,
submissions, initiation of preclinical studies and clinical trials, and timing of expected clinical results for our product candidates; |
| · | our success in early preclinical studies, which
may not be indicative of results obtained in later studies or clinical trials; |
| · | the potential benefits of our product candidates; |
| · | our ability to identify patients with the diseases
treated by our product candidates, and to enroll healthy volunteers and patients in clinical trials; |
| · | our ability to obtain, maintain and protect our
intellectual property; |
| · | our reliance upon intellectual property licensed
from third parties, including the license to use the SEE-Tx® platform; |
| · | our ability to identify, recruit and retain key
personnel; |
| · | our ability to accurately estimate anticipated
operating losses, expenses, future revenues, capital requirements, including our anticipated cash runway; |
| · | developments or projections relating to our competitors
or our industry; |
| · | the impact of laws and regulations; |
| · | our expectations regarding government and third-party
payor coverage and reimbursement; |
| · | our expectations regarding the time during which
we will be an emerging growth company under the JOBS Act; |
| · | the impact of worsening macroeconomic conditions,
including heightened global inflation, actions taken by central banks to counter inflation, liquidity concerns at and failures of banks
and other financial institutions, capital market instability, exchange rate fluctuations, supply chain disruptions and increases in commodity,
energy and fuel prices; |
| · | the impacts of pandemics or endemics including
on our operations, access to capital, research and development and clinical trials and potential disruption in the operations and business
of third-party manufacturers, contract research organizations, other service providers, and collaborators with whom we conduct business; |
| · | the impact of other global events, including
political instability, natural disaster, events of terrorism and wars, including the war between Ukraine and Russia, and the corresponding
tensions created from such conflict between Russia, the United States and countries in Europe as well as other countries such as China;
and the conflict between Hamas and Israel; and |
| · | other factors discussed under the section
“Risk Factors” in this prospectus and in our most recent Annual Report on Form 10-K for the fiscal year ended
December 31, 2022 filed with the SEC on March 23, 2023, and our Quarterly Reports on Form 10-Q for the quarterly periods ended March
31, 2023, June 30, 2023 and September 30, 2023, as well as any amendments thereto reflected in subsequent reports we will file from
time to time with the SEC. |
The foregoing does not represent an
exhaustive list of matters that may be covered by the forward-looking statements contained herein or risk factors that we are faced
with that may cause our actual results to differ from those anticipated in such forward-looking statements. The events and
circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially
from those projected in the forward-looking statements. You should review the factors and risks and other information we describe in
our most recent Annual Report on Form 10-K for the fiscal year ended December 31, 2022 filed with the SEC on March 23, 2023, and our
Quarterly Reports on Form 10-Q for the quarterly periods ended March 31, 2023, June 30, 2023 and September 30, 2023, as well as any
amendments thereto reflected in subsequent reports we will file from time to time with the SEC.
All forward-looking statements are expressly qualified in their entirety
by this cautionary note. You are cautioned to not place undue reliance on any forward-looking statements, which speak only as of the date
of this prospectus or the date of the document incorporated by reference herein. You should read this prospectus and the documents that
we incorporate by reference and have filed as exhibits to the registration statement, of which this prospectus is a part, completely and
with the understanding that our actual future results may be materially different from what we expect. In light of the significant uncertainties
in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person
that we will achieve our objectives and plans in any specified time frame, or at all. We have no obligation, and expressly disclaim any
obligation, to update, revise or correct any of the forward-looking statements, whether as a result of new information, future events
or otherwise. We have expressed our expectations, beliefs and projections in good faith and believe they have a reasonable basis. However,
we cannot assure you that our expectations, beliefs or projections will result or be achieved or accomplished.
USE OF PROCEEDS
We will not receive any proceeds from the sale
of Common Stock offered by the selling stockholder under this prospectus. However, we will receive the proceeds of any cash exercise of
the Warrants. If all of the Warrants were exercised for cash, we would receive aggregate proceeds of approximately $6.8 million.
We intend to use the net proceeds from any cash exercise of the Warrants for working capital and general corporate purposes.
This expected use of the net proceeds from this
resale represents our intentions based upon our current plans and business conditions, and our management will retain broad discretion
as to the ultimate allocation of the proceeds. We may temporarily invest funds that we do not immediately need for these purposes in investment
securities or use them to make payments on our borrowings.
SELLING STOCKHOLDER
This prospectus covers the resale or other disposition
by the selling stockholder identified in the table below of up to an aggregate of (i) 744,026 Private
Shares, (ii) 1,756,062 shares of our Common Stock issuable upon the exercise of the Pre-Funded Warrants, and (iii) 2,500,088 shares of
our Common Stock issuable upon the exercise of the Private Warrants.
The selling stockholder acquired his securities
in the Private Placement transaction described above under the heading “Prospectus Summary - Recent Developments – Private
Placement Offering.”
The table below sets forth, as of December 18,
2023, the following information regarding the selling stockholder:
| · | the name of the selling stockholder; |
| · | the number of shares of Common Stock owned by
the selling stockholder prior to this offering, without regard to any beneficial ownership limitations contained in the Warrants; |
| · | the number of shares of Common Stock to be offered
by the selling stockholder in this offering; |
| · | the number of shares of Common Stock to be owned
by the selling stockholder assuming the sale of all of the shares of Common Stock covered by this prospectus; and |
| · | the percentage of our issued and outstanding
shares of Common Stock to be owned by the selling stockholder assuming the sale of all of the shares of Common Stock covered by this prospectus
based on the number of shares of Common Stock issued and outstanding as of December 18, 2023. |
Except as described above, the number of shares
of Common Stock beneficially owned by the selling stockholder has been determined in accordance with Rule 13d-3 under the Exchange
Act and includes, for such purpose, shares of Common Stock that the selling stockholder has the right to acquire within 60 days of
December 18, 2023.
All information with respect to the Common
Stock ownership of the selling stockholder has been furnished by or on behalf of the selling stockholder. We believe, based on
information supplied by the selling stockholder, that except as may otherwise be indicated in the footnotes to the table below, the
selling stockholder has sole voting and dispositive power with respect to the shares of Common Stock reported as beneficially owned
by the selling stockholder. Because the selling stockholder identified in the table may sell some or all of the shares of Common
Stock beneficially owned by him and covered by this prospectus, and because there are currently no agreements, arrangements or
understandings with respect to the sale of any of the shares of Common Stock, no estimate can be given as to the number of shares of
Common Stock available for resale hereby that will be held by the selling stockholder upon termination of this offering. We cannot
advise you as to whether the selling stockholder will in fact sell any or all of such Common Stock. In addition, the selling
stockholder may have sold, transferred or otherwise disposed of, or may sell, transfer or otherwise dispose of, at any time and from
time to time, the shares of Common Stock he beneficially owns in transactions exempt from the registration requirements of
the Securities Act after the date on which he provided the information set forth in the table below. We have, therefore,
assumed for the purposes of the following table, that the selling stockholder will sell all of the shares of Common Stock owned
beneficially by him that are covered by this prospectus, but will not sell any other shares of Common Stock that he presently owns.
The selling stockholder has not held any position or office, or has otherwise had a material relationship, with us or any of our
subsidiaries within the past three years other than as a result of the ownership of our shares of Common Stock or other securities.
The selling stockholder may sell all, some or none of his shares in this offering. See “Plan of Distribution.” in this prospectus for more information.
| Name of Selling Stockholder | |
Shares
Owned
prior to
Offering | | |
Shares
Offered
by this
Prospectus | | |
Shares
Owned
after
Offering | | |
Percentage of
Shares
Beneficially
Owned after
Offering (1) | |
| Andrew Schwartzberg (2) | |
| 5,000,176 | | |
| 5,000,176 | | |
| 0 | | |
| 4.99 | % |
| (1) | Percentage is based on 20,455,518 shares of Common Stock outstanding as of December 18, 2023, assuming the exercise of all of the
Warrants and the resale of all of the shares of Common Stock covered by this prospectus. |
| (2) | Consists of (i) 744,026 shares of Common Stock, (ii) 1,756,062
shares of Common Stock issuable upon exercise of the Pre-Funded Warrants and (iii) 2,500,088 shares of Common Stock issuable upon exercise
of the Private Warrants. The exercise of the Pre-Funded Warrants and the Private Warrants are subject to a 4.99% (or 9.99% at election
of the selling stockholder) beneficial ownership limitation which prohibits the holder from exercising any portion of those warrants
to the extent that, following such exercise, the holder would hold a number of our shares of Common Stock exceeding the applicable beneficial
ownership limitation. The number of shares listed in the second column is based on the number of shares of Common Stock and warrants
held by the selling stockholder, assuming exercise in full of the Private Warrants and the Pre-Funded Warrants without regard to any
limitations on exercise, but the percentage set forth in the fifth column is limited by the 4.99% (or 9.99% at the election of the selling
stockholder) beneficial ownership blocker in the Pre-Funded Warrants and the Private Warrants. The securities are directly held by Andrew
Schwartzberg. The address of Andrew Schwartzberg is 11810 Grand Park Ave, Suite 625, N. Bethesda M.D. 20852. |
DESCRIPTION OF SECURITIES
General
We are authorized to issue 50,000,000 shares of
Common Stock, par value $0.0001 per share, of which 16,199,368 shares were issued and outstanding as of December 18, 2023, and 10,000,000
shares of preferred stock, par value $0.0001 per share no shares of which are outstanding as of December 18, 2023. Unless our board of
directors determines otherwise, we will issue all shares of our capital stock in uncertificated form.
The following description summarizes selected
information regarding the Common Stock, as well as relevant provisions of: (i) the Company’s amended and restated certificate of
incorporation, as currently in effect (“Amended Charter”), (ii) the Company’s amended and restated bylaws, as
currently in effect (the “Bylaws”) and (iii) the Delaware General Corporation Law (the “DGCL”).
The following summary description of the Common Stock is qualified in its entirety by, and should be read in conjunction with, the Amended
Charter and the Bylaws, copies of which have been filed as exhibits to the Company’s periodic reports under the Exchange Act, and
the applicable provisions of the DGCL.
Common Stock
The following description of certain rights of
our Common Stock does not purport to be complete and is qualified in its entirety by reference to our Amended Charter and our Bylaws.
Voting Rights. Holders of shares of our
Common Stock are entitled to one vote for each share held of record on all matters on which stockholders are entitled to vote generally,
including the election or removal of directors elected by our stockholders generally. The holders of our Common Stock do not have cumulative
voting rights in the election of directors.
Dividends and Liquidation Rights. Holders
of shares of our Common Stock are entitled to receive dividends when, as and if declared by our board of directors out of funds legally
available therefor, subject to any statutory or contractual restrictions on the payment of dividends and to any restrictions on the payment
of dividends imposed by the terms of any outstanding preferred stock. Upon our liquidation, dissolution or winding up and after payment
in full of all amounts required to be paid to creditors and to the holders of preferred stock having liquidation preferences, if any,
the holders of shares of our Common Stock will be entitled to receive our remaining assets available for distribution on a pro rata basis.
Miscellaneous. All shares of our Common
Stock that will be outstanding are fully paid and non-assessable. The Common Stock will not be subject to further calls or assessments
by us. Holders of shares of our Common Stock do not have preemptive, subscription, redemption or conversion rights. There will be no redemption
or sinking fund provisions applicable to the Common Stock. The rights powers, preferences and privileges of our Common Stock will be subject
to those of the holders of any shares of our preferred stock or any other series or class of stock we may authorize and issue in the future.
Listing. Our Common Stock is listed on Nasdaq under the symbol
“GANX.”
Transfer Agent and Registrar. The transfer
agent and registrar for our Common Stock is Pacific Stock Transfer Company.
Preferred Stock
Voting Rights. No shares of preferred stock
are issued or outstanding as of December 18, 2023. Our Amended Charter authorizes our board of directors to establish one or more series
of preferred stock (including convertible preferred stock). Unless required by law or any stock exchange, the authorized shares of preferred
stock will be available for issuance without further action by the holders of our Common Stock. Our board of directors is able to determine,
with respect to any series of preferred stock, the powers (including voting powers), preferences and relative, participating, optional
or other special rights, and the qualifications, limitations or restrictions thereof, including, without limitation:
| · | the
designation of the series; |
| · | the
number of shares of the series, which our board of directors may, except where otherwise provided in the preferred stock designation,
increase (but not above the total number of authorized shares of the class) or decrease (but not below the number of shares then outstanding); |
| · | whether
dividends, if any, will be cumulative or non-cumulative and the dividend rate of the series; |
| · | the
dates at which dividends, if any, will be payable; |
| · | the
redemption or repurchase rights and price or prices, if any, for shares of the series; |
| · | the
terms and amounts of any sinking fund provided for the purchase or redemption of shares of the series; |
| · | the
amounts payable on shares of the series in the event of any voluntary or involuntary liquidation, dissolution or winding-up of our affairs; |
| · | whether
the shares of the series will be convertible into shares of any other class or series, or any other security, of us or any other entity,
and, if so, the specification of the other class or series or other security, the conversion price or prices or rate or rates, any rate
adjustments, the date or dates as of which the shares will be convertible and all other terms and conditions upon which the conversion
may be made; |
| · | restrictions
on the issuance of shares of the same series or of any other class or series; and |
| · | the
voting rights, if any, of the holders of the series. |
We could issue a series of preferred stock that
could, depending on the terms of the series, impede or discourage an acquisition attempt or other transaction that some, or a majority,
of the holders of our Common Stock might believe to be in their best interests or in which the holders of our Common Stock might receive
a premium over the market price of the shares of our Common Stock. Additionally, the issuance of preferred stock may adversely affect
the rights of holders of our Common Stock by restricting dividends on the Common Stock, diluting the voting power of the Common Stock
or subordinating the liquidation rights of the Common Stock. As a result of these or other factors, the issuance of preferred stock could
have an adverse impact on the market price of our Common Stock.
Anti-Takeover Effects of Our Amended
Charter and Our Bylaws and Certain Provisions of Delaware Law
Our Amended Charter, Bylaws and the DGCL contains
provisions that are intended to enhance the likelihood of continuity and stability in the composition of our board of directors. These
provisions are intended to avoid costly takeover battles, reduce our vulnerability to a hostile or abusive change of control and enhance
the ability of our board of directors to maximize stockholder value in connection with any unsolicited offer to acquire us. However, these
provisions may have an anti-takeover effect and may delay, deter or prevent a merger or acquisition of our company by means of a tender
offer, a proxy contest or other takeover attempt that a stockholder might consider in its best interest, including those attempts that
might result in a premium over the prevailing market price for the shares of Common Stock held by stockholders.
Authorized but Unissued Capital Stock
The authorized but unissued shares of Common Stock
and preferred stock are available for future issuance without stockholder approval, subject to any limitations imposed by the listing
standards of Nasdaq. These additional shares may be used for a variety of corporate finance transactions, acquisitions and employee benefit
plans. The existence of authorized but unissued and unreserved Common Stock and preferred stock could make more difficult or discourage
an attempt to obtain control of us by means of a proxy contest, tender offer, merger or otherwise.
Business Combinations
We are subject to Section 203 of the DGCL,
which prohibits persons deemed to be “interested stockholders” from engaging in a “business combination”
with a publicly held Delaware corporation for three years following the date these persons become interested stockholders unless the
business combination is, or the transaction in which the person became an interested stockholder was, approved in a prescribed
manner or another prescribed exception applies. Generally, an “interested stockholder” is a person who, together with
affiliates and associates, owns, or within three years prior to the determination of interested stockholder status did own, 15% or
more of a corporation’s voting stock. Generally, a “business combination” includes a merger, asset or stock sale,
or other transaction resulting in a financial benefit to the interested stockholder. The existence of this provision may have an
anti-takeover effect with respect to transactions not approved in advance by the board of directors.
No Cumulative Voting
Under Delaware law, the right to vote cumulatively
does not exist unless the certificate of incorporation specifically authorizes cumulative voting. Our Amended Charter does not authorize
cumulative voting. Therefore, stockholders holding a majority of the shares of our capital stock entitled to vote generally in the election
of directors will be able to elect all of our directors.
Special Stockholder Meetings
Our Amended Charter provides that special meetings
of our stockholders may be called at any time only by or at the direction of the board of directors or the chairman of the board of directors.
Our Bylaws prohibit the conduct of any business at a special meeting other than as specified in the notice for such meeting. These provisions
may have the effect of deferring, delaying, or discouraging hostile takeovers, or changes in control or management of our company.
Director Nominations and Stockholder Proposals
Our Bylaws establish advance notice procedures
with respect to stockholder proposals and the nomination of candidates for election as directors, other than nominations made by or at
the direction of the board of directors or a committee of the board of directors. In order for any matter to be “properly brought”
before a meeting, a stockholder will have to comply with advance notice requirements and provide us with certain information. Generally,
to be timely, a stockholder’s notice must be received at our principal executive offices not less than 90 days nor more than 120
days prior to the first anniversary date of the immediately preceding annual meeting of stockholders. Our Bylaws also specify requirements
as to the form and content of a stockholder’s notice. Our Bylaws allow the chairman of the meeting at a meeting of the stockholders
to adopt rules and regulations for the conduct of meetings that may have the effect of precluding the conduct of certain business at a
meeting if the rules and regulations are not followed. These provisions may also defer, delay, or discourage a potential acquirer from
conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to influence or obtain
control of the company.
Stockholder Action by Written Consent
Pursuant to Section 228 of the DGCL, any action
required to be taken at any annual or special meeting of the stockholders may be taken without a meeting, without prior notice, and without
a vote if a consent or consents in writing, setting forth the action so taken, is or are signed by the holders of outstanding stock having
not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting at which all shares of
our stock entitled to vote thereon were present and voted, unless our Amended Charter will provide otherwise. Our Amended Charter precludes
stockholder action by written consent at any time.
Amendment of our Amended Charter or Bylaws
The DGCL provides generally that the affirmative
vote of a majority of the shares entitled to vote on any matter is required to amend a corporation’s certificate of incorporation
or bylaws, unless a corporation’s certificate of incorporation or bylaws, as the case may be, requires a greater percentage. Our
Bylaws may be amended or repealed by a majority vote of our board of directors or by the affirmative vote of the holders of at least 66
⅔% of the votes which all our stockholders would be entitled to cast in any annual election of directors. In addition, the affirmative
vote of the holders of at least 66 ⅔% of the votes which all our stockholders would be entitled to cast in any election of directors
will be required to amend or repeal or to adopt any provisions inconsistent with any of the provisions of our Amended Charter described
above.
The foregoing provisions of our Amended
Charter and Bylaws could discourage potential acquisition proposals and could delay or prevent a change in control. These
provisions are intended to enhance the likelihood of continuity and stability in the composition of our board of directors and in
the policies formulated by our board of directors and to discourage certain types of transactions that may involve an actual or
threatened change of control. These provisions are designed to reduce our vulnerability to an unsolicited acquisition proposal. The
provisions also are intended to discourage certain tactics that may be used in proxy fights. However, such provisions could have the
effect of discouraging others from making tender offers for our shares and, as a consequence, they also may inhibit fluctuations in
the market price of our shares of Common Stock that could result from actual or rumored takeover attempts. Such provisions also may
have the effect of preventing changes in our management or delaying or preventing a transaction that might benefit you or other
minority stockholders.
Dissenters’ Rights of Appraisal and Payment
Under the DGCL, with certain exceptions, our stockholders
will have appraisal rights in connection with a merger or consolidation of us. Pursuant to the DGCL, stockholders who properly request
and perfect appraisal rights in connection with such merger or consolidation will have the right to receive payment of the fair value
of their shares as determined by the Delaware Court of Chancery.
Stockholders’ Derivative Actions
Under the DGCL, any of our stockholders may bring
an action in our name to procure a judgment in our favor, also known as a derivative action, provided that the stockholder bringing the
action is a holder of our shares at the time of the transaction to which the action relates or such stockholder’s stock thereafter
devolved by operation of law.
Exclusive Forum
Our Amended Charter provides that, unless
we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware will, to the fullest extent
permitted by applicable law, be the sole and exclusive forum for: (1) any derivative action or proceeding brought on our behalf; (2)
any action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers or other employees or stockholders
to us or our stockholders; (3) any action asserting a claim against us, any director or our officers and employees arising pursuant to
any provision of the DGCL, our Amended Charter or our Bylaws, or as to which the DGCL confers exclusive jurisdiction on the Court of
Chancery; or (4) any action asserting a claim against us, any director or our officers or employees that is governed by the internal
affairs doctrine; provided that the exclusive forum provisions will not apply to suits brought to enforce any liability or duty created
by the Exchange Act, or to any claim for which the federal courts have exclusive jurisdiction. Our Amended Charter further provides that,
unless we consent in writing to the selections of an alternative forum, the federal district courts are the sole and exclusive forum
for the resolution of any complaint asserting a cause of action arising under the Securities Act, subject to a final adjudication in
the State of Delaware of the enforceability of such exclusive forum provision. We note that investors cannot waive compliance with the
federal securities laws and the rules and regulations thereunder. Although we believe the provision benefits us by providing increased
consistency in the application of Delaware law in the types of lawsuits to which it applies, the provision may have the effect of discouraging
lawsuits against our directors and officers.
Officers and Directors
The DGCL authorizes corporations to limit or eliminate
the personal liability of directors to corporations and their stockholders for monetary damages for breaches of directors’ fiduciary
duties, subject to certain exceptions. Our Amended Charter includes a provision that eliminates the personal liability of directors for
monetary damages to the corporation or its stockholders for any breach of fiduciary duty as a director, except to the extent such exemption
from liability or limitation thereof is not permitted under the DGCL. The effect of these provisions is to eliminate the rights of us
and our stockholders, through stockholders’ derivative suits on our behalf, to recover monetary damages from a director for breach
of fiduciary duty as a director, including breaches resulting from grossly negligent behavior. However, exculpation does not apply to
any breaches of the director’s duty of loyalty, any acts or omissions not in good faith or that involve intentional misconduct or
knowing violation of law, any authorization of dividends or stock redemptions or repurchases paid or made in violation of the DGCL, or
for any transaction from which the director derived an improper personal benefit.
Our Bylaws generally provide that we must
indemnify and advance expenses to our directors and officers to the fullest extent authorized by the DGCL. We also are expressly
authorized to carry directors’ and officers’ liability insurance providing indemnification for our directors, officers
and certain employees for some liabilities. We believe that these indemnification and advancement provisions and insurance are
useful to attract and retain qualified directors and executive officers.
The limitation of liability, indemnification and
advancement provisions in our Amended Charter and Bylaws may discourage stockholders from bringing a lawsuit against directors for breach
of their fiduciary duty. These provisions also may have the effect of reducing the likelihood of derivative litigation against directors
and officers, even though such an action, if successful, might otherwise benefit us and our stockholders. In addition, your investment
may be adversely affected to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these
indemnification provisions.
There is currently no pending material litigation
or proceeding involving any of our directors, officers or employees for which indemnification is sought.
Indemnification Agreements
We entered into an indemnification agreement with
each of our directors and executive officers. Insofar as indemnification for liabilities arising under the Securities Act may be permitted
to directors or executive officers, we have been informed that in the opinion of the SEC such indemnification is against public policy
and is therefore unenforceable.
Private Warrants
The Private Warrants were issued on November 21,
2023 in the Private Placement that was completed simultaneously with the Public Offering.
Exercisability. The Private Warrants
are exercisable beginning on May 24, 2024 and expire on November 24, 2028. The Private Warrants are exercisable, at the option of each
holder, in whole or in part by delivering to us a duly executed exercise notice and, at any time a registration statement registering
the issuance of the Private Warrant Shares under the Securities Act is effective and available for the issuance of such shares,
or an exemption from registration under the Securities Act is available for the issuance of such shares, by payment in full
in immediately available funds for the number of Common Stock purchased upon such exercise. If at the time of exercise there is no effective
registration statement registering, or the prospectus contained therein is not available for the issuance of the Common Stock underlying
the Private Warrants, then the Private Warrants may also be exercised, in whole or in part, at such time by means of a cashless exercise,
in which case the holder would receive upon such exercise the net number of common shares determined according to the formula set forth
in the Private Warrant.
Exercise Limitation. A holder will
not have the right to exercise any portion of the Private Warrant if the holder (together with its affiliates) would beneficially own
in excess of 4.99% (or 9.99% upon the request of the investor) of the number of Common Stock outstanding immediately after giving effect
to the exercise, as such percentage ownership is determined in accordance with the terms of the Private Warrants. However, any holder
may increase or decrease such percentage, provided that any increase will not be effective until the 61st day after such
election.
Exercise Price. The Private Warrants
have an exercise price of $2.75 per share. The exercise price is subject to appropriate adjustment in the event of certain stock dividends
and distributions, stock splits, stock combinations, reclassifications or similar events affecting our common shares and also upon any
distributions of assets, including cash, stock or other property to our stockholders.
Exchange Listing. There is no established
public trading market for the Private Warrants and we do not expect a market to develop. We do not intend to apply for listing of the
Private Warrants on any securities exchange or other nationally recognized trading system. Without an active trading market, the liquidity
of the Private Warrants will be limited.
Fundamental Transactions. In the
event of any fundamental transaction, as described in the Private Warrants and generally including any merger with or into another
entity, sale of all or substantially all of our assets, tender offer or exchange offer, or reclassification of our Common Stock,
then upon any subsequent exercise of the Private Warrants, the holder will have the right to receive as alternative consideration,
for each share of Common Stock that would have been issuable upon such exercise immediately prior to the occurrence of such
fundamental transaction, the number of shares of Common Stock of the successor or acquiring corporation or of our company, if it is
the surviving corporation, and any additional consideration receivable upon or as a result of such transaction by a holder of the
number of Common Stock for which the Warrant is exercisable immediately prior to such event. If holders of Common Stock are given
any choice as to the securities, cash or property to be received in a Fundamental Transaction, then the holder shall be given the
same choice as to the alternate consideration it receives upon any exercise of the Private Warrant following the Fundamental
Transaction. Notwithstanding anything to the contrary, in the event of a Fundamental Transaction (other than (x) any stock split or
reverse stock split, (y) any transaction effected solely for the purpose of changing the jurisdiction of incorporation of the
Company, or (z) any holding company reorganization or parent-subsidiary merger not requiring stockholder approval), the Company or
any successor entity will, at the holder’s option, exercisable at any time concurrently with, or within 30 days after, the
consummation of the Fundamental Transaction (or, if later, the date of the public announcement of the applicable Fundamental
Transaction), purchase the Private Warrant from the holder by paying to the holder an amount of cash equal to the Black Scholes
Value (as defined in the Private Warrants) of the remaining unexercised portion of the Private Warrant on the date of the
consummation of the Fundamental Transaction; provided, however, that, if the Fundamental Transaction
is not within the Company's control, including not approved by the Company's Board of Directors, the holder will only be entitled to
receive from the Company or any successor entity, as of the date of consummation of the Fundamental Transaction, the same type or
form of consideration (and in the same proportion), at the Black Scholes Value of the unexercised portion of the Private Warrant,
that is being offered and paid to the holders of Common Stock of the Company in connection with the Fundamental Transaction, whether
that consideration be in the form of cash, stock or any combination thereof, or whether the holders of Common Stock are given the
choice to receive from among alternative forms of consideration in connection with the Fundamental
Transaction; provided, further, that if holders of Common Stock of the Company are not offered or paid any
consideration in such Fundamental Transaction, such holders of Common Stock will be deemed to have received common stock of the
successor entity (which entity may be the Company following such Fundamental Transaction) in such Fundamental Transaction.
Rights as a Stockholder. Except as otherwise
provided in the Private Warrants or by virtue of such holder’s ownership of our common shares, the holder of a Warrant does not
have the rights or privileges of a holder of our common shares, including any voting rights, until the holder exercises the Warrant.
Registration Rights. We are filing this registration statement
on Form S-1 with the SEC that includes this prospectus to register for resale under the Securities Act the common shares issuable
upon exercise of the Private Warrants to satisfy our obligations in connection with the Private Placement. We will use commercially reasonable
efforts to keep the registration statement effective at all times until the selling stockholder no longer owns any Warrants or shares
issuable upon exercise thereof.
Pre-Funded Warrants
The Pre-Funded Warrants were issued on November
24, 2023 in the Private Placement that was completed simultaneously with the Public Offering. The Pre-Funded Warrants have substantially
the same terms as the Private Warrants except as follows:
Exercisability. The Pre-Funded Warrants
are exercisable immediately and the holder of the Pre-Funded Warrant is entitled to exercise at any time until the Pre-Funded Warrant
is exercised in full. The aggregate exercise price of each Pre-Funded Warrant, except for a nominal exercise price of $0.0001 per Pre-Funded
Warrant Share, was pre-funded to the Company on or prior to November 24, 2023 and consequently, no additional consideration (other than
the nominal exercise price of $0.0001 per Pre-Funded Warrant Share) shall be required to be paid by the holder of the Pre-Funded Warrant
to any person to effect any exercise of the Pre-Funded Warrant. The holder of the Pre-Funded Warrant is not entitled to return or refund
all, or any portion of such prepaid aggregate exercise price under any circumstance for any reason whatsoever. The remaining unpaid exercise
price per share of Common Stock under the Pre-Funded Warrant is $0.0001, subject to adjustment in accordance with the terms of the Pre-Funded
Warrants.
Exercise Limitation. A holder
will not have the right to exercise any portion of the Pre-Funded Warrant if the holder (together with its affiliates) would
beneficially own in excess of 4.99% (or 9.99% upon the request of the investor) of the number of Common Stock outstanding
immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the
Pre-Funded Warrants. However, any holder may increase or decrease such percentage, provided that any increase will not be effective
until the 61st day after such election.
Mechanics of Exercise. With respect
to any Notice(s) of Exercise delivered on or prior to 12:00 p.m. (New York City time) on November 24, 2023, which may be delivered
at any time after the time of execution of the Securities Purchase Agreement, the Company delivered the Pre-Funded Warrant Shares
subject to such notice(s) by 4:00 p.m. (New York City time) on November 24, 2023 and such date shall be considered the
“Warrant Share Delivery Date” for purposes the Pre-Funded Warrants, provided that payment of the aggregate exercise
price (other than in the case of a cashless exercise) is received by such “Warrant Share Delivery Date.”
Amendment. Except as otherwise provided
by the Pre-Funded Warrants, the provisions of the Pre-Funded Warrants may be amended and the Company may take any action to do so, only
if the Company has obtained the written consent of the holder of the Pre-Funded Warrant (or, if after the initial issuance date of the
Pre-Funded Warrant, the Pre-Funded Warrant is subdivided into multiple Pre-Funded Warrants, the holders of the replacement or substitute
Pre-Funded Warrants representing at least a majority of the shares of Common Stock underlying such Pre-Funded Warrants then outstanding).
Any such amendment shall apply to all Pre-Funded Warrants outstanding and be binding upon all holders of such Pre-Funded Warrants.
PLAN OF DISTRIBUTION
The selling stockholder, which as used herein
includes donees, pledgees, transferees or other successors-in-interest selling the Private Shares and the Warrant Shares, or interests
in the shares of Common Stock received after the date of this prospectus from the selling stockholder as a gift, pledge, partnership distribution
or other transfer, may, from time to time, sell, transfer or otherwise dispose of any or all of his shares of Common Stock or interests
in the shares of Common Stock on any stock exchange, market or trading facility on which the shares are traded or in private transactions.
The selling stockholder may sell all or a portion of the shares of Common Stock held by him and offered hereby from time to time directly
or through one or more underwriters, broker-dealers or agents. If the shares of Common Stock are sold through underwriters or broker-dealers,
the selling stockholder will be responsible for underwriting discounts or commissions or agent’s commissions. The shares of Common
Stock may be sold in one or more transactions at fixed prices, at prevailing market prices at the time of the sale, at varying prices
determined at the time of sale or at negotiated prices. These sales may be effected in transactions, which may involve crosses or block
transactions, pursuant to one or more of the following methods:
| · | on any national securities exchange or quotation
service on which the securities may be listed or quoted at the time of sale; |
| · | in the over-the-counter market; |
| · | in transactions otherwise than on these exchanges
or systems or in the over-the-counter market; |
| · | through the writing or settlement of options
or other hedging transactions, whether such options are listed on an options exchange or otherwise; |
| · | ordinary brokerage transactions and transactions
in which the broker-dealer solicits purchasers; |
| · | block trades in which the broker-dealer will
attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| · | purchases by a broker-dealer as principal and
resale by the broker-dealer for its account; |
| · | an exchange distribution in accordance with the
rules of the applicable exchange; |
| · | privately negotiated transactions; |
| · | short sales effected after the date the registration
statement of which this prospectus is a part was declared effective by the SEC; |
| · | broker-dealers may agree with the selling stockholder
to sell a specified number of such shares at a stipulated price per share; |
| · | a combination of any such methods of sale; and |
| · | any other method permitted pursuant to applicable
law. |
The aggregate proceeds to the selling stockholder from the sale
of the shares of Common Stock offered by him will be the purchase price of the shares of Common Stock less discounts or commissions,
if any. The selling stockholder reserves the right to accept and, together with their agents from time to time, to reject, in whole
or in part, any proposed purchase of shares of Common Stock to be made directly or through agents. We will not receive any of the
proceeds from sales of shares by the selling stockholder.
The selling stockholder may also sell shares of Common Stock under
Rule 144 promulgated under the Securities Act, if available, rather than under this prospectus. In addition, the selling
stockholder may transfer the shares of Common Stock by other means not described in this prospectus. If the selling stockholder effects
such transactions by selling shares of Common Stock to or through underwriters, broker-dealers or agents, such underwriters, broker-dealers
or agents may receive commissions in the form of discounts, concessions or commissions from the selling stockholder or commissions from
purchasers of the shares of Common Stock for whom they may act as agent or to whom they may sell as principal (which discounts, concessions
or commissions as to particular underwriters, broker-dealers or agents may be in excess of those customary in the types of transactions
involved but, except as set forth in a supplement to this prospectus to the extent required, in the case of an agency transaction will
not be in excess of a customary brokerage commission in compliance with FINRA Rule 5110).
In connection with sales of the shares of Common
Stock or otherwise, the selling stockholder may enter into hedging transactions with broker-dealers or other financial institutions, which
may in turn engage in short sales of the shares of Common Stock in the course of hedging in positions it assumes. The selling stockholder
may also sell shares of Common Stock short and deliver shares of Common Stock covered by this prospectus to close out short positions
and to return borrowed shares in connection with such short sale. The selling stockholder may also loan or pledge shares of Common Stock
to broker-dealers that in turn may sell such shares. The selling stockholder may also enter into option or other transactions with broker-dealers
or other financial institutions or the creation of one or more derivative securities which require the delivery to such broker-dealer
or other financial institution of shares of Common Stock offered by this prospectus, which shares of Common Stock such broker-dealer or
other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The selling stockholder may pledge or grant a
security interest in some or all of the shares of Common Stock owned by him and, if he defaults in the performance of his secured obligations,
the pledgees or secured parties may offer and sell the shares of Common Stock from time to time pursuant to this prospectus or any amendment
to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act amending, if necessary, the list
including the selling stockholder to include the pledgee, transferee or other successors in interest as selling stockholders under this
prospectus. The selling stockholder also may transfer and donate the shares of Common Stock in other circumstances as permitted by applicable
law, in which case the transferees, donees, pledgees or other successors in interest will be the selling beneficial owners for purposes
of this prospectus.
To the extent required by the Securities
Act and the rules and regulations thereunder, the selling stockholder and any broker-dealer participating in the distribution of
the shares of Common Stock may be deemed to be “underwriters” within the meaning of the Securities Act. In such event,
any commission paid, or any discounts or concessions allowed to, any such broker-dealer may be deemed to be underwriting commissions or
discounts under the Securities Act. Selling stockholders who are deemed to be “underwriters” under the Securities
Act (if any) will be subject to the prospectus delivery requirements of the Securities Act and may be subject to certain
statutory liabilities of, including but not limited to, Sections 11, 12 and 17 of the Securities Act and Rule 10b-5 under the Exchange
Act.
The selling stockholder has informed us that he
is not a registered broker-dealer and does not have any written or oral agreement or understanding, directly or indirectly, with any person
to engage in a distribution of the shares of Common Stock. Upon us being notified in writing by the selling stockholder that any material
arrangement has been entered into with a broker-dealer for the distribution of shares of Common Stock, a prospectus supplement, if required,
will be distributed, which will set forth the aggregate amount of shares of Common Stock being distributed and the terms of the offering,
including the name or names of any broker-dealers or agents, any discounts, commissions and other terms constituting compensation from
the selling stockholder and any discounts, commissions or concessions allowed or re-allowed or paid to broker-dealers.
Under the securities laws of some states, the
shares of Common Stock may be sold in such states only through registered or licensed brokers or dealers. In addition, in some states
the shares of Common Stock may not be sold unless such shares have been registered or qualified for sale in such state or an exemption
from registration or qualification is available and is complied with.
The selling stockholder may sell all, some or
none of the Shares of Common Stock registered pursuant to the registration statement on Form S-1 of which this prospectus forms a part.
If sold under the registration statement on Form S-1 of which this prospectus forms a part, the shares of Common Stock registered hereunder
will be freely tradable in the hands of persons other than our affiliates that acquire such shares.
We have advised the selling stockholder that the
anti-manipulation rules of Regulation M under the Exchange Act may apply to sales of shares of Common Stock in the
market and to the activities of the selling stockholder and his affiliates. In addition, to the extent applicable, we will make copies
of this prospectus (as it may be supplemented or amended from time to time) available to the selling stockholder for the purpose of satisfying
the prospectus delivery requirements of the Securities Act. The selling stockholder may indemnify any broker-dealer that participates
in transactions involving the sale of the shares of Common Stock against certain liabilities, including liabilities arising under the Securities
Act.
LEGAL MATTERS
The validity of the shares of Common Stock offered
hereby will be passed upon for us by Lowenstein Sandler LLP, New York, New York.
EXPERTS
The consolidated financial statements of Gain Therapeutics, Inc. appearing in Gain Therapeutics, Inc.’s Annual Report on Form 10-K for the years ended December 31, 2022 and 2021 have been audited by Ernst & Young AG, independent registered public accounting firm, as
set forth in their report thereon, included therein, and incorporated herein by reference. Such consolidated financial statements are
incorporated herein by reference in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
INFORMATION INCORPORATED BY REFERENCE
The SEC allows us to “incorporate by reference”
information that we file with it into this prospectus, which means that we can disclose important information to you by referring you
to those documents. The information incorporated by reference is an important part of this prospectus. The information incorporated by
reference is considered to be a part of this prospectus, and information that we file later with the SEC will automatically update and
supersede information contained in this prospectus.
We incorporate by reference the documents listed below that we have
previously filed with the SEC:
| · | our Quarterly Reports on Form 10-Q for the quarters ended March 31, 2023,
June 30, 2023 and September 30, 2023, as filed with the SEC on May 12, 2023, August 10, 2023 and November 14, 2023, respectively; |
All reports and other documents that we file with
the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of this prospectus but prior to the termination
of the offering of the securities hereunder will also be considered to be incorporated by reference into this prospectus from the date
of the filing of these reports and documents, and will supersede the information herein; provided, however, that all reports,
exhibits and other information that we “furnish” to the SEC will not be considered incorporated by reference into this prospectus.
Any statement contained in a document incorporated by reference in this prospectus shall be deemed to be modified or superseded to the
extent that a statement contained herein, therein or in any other subsequently filed document that also is incorporated by reference herein
or therein modifies or supersedes such statement. Any statement so modified or superseded shall not be deemed, except as so modified or
superseded, to constitute a part of this prospectus or the registration statement.
We will provide you without charge, upon your
oral or written request, with a copy of any or all reports, proxy statements and other documents we file with the SEC, as well as any
or all of the documents incorporated by reference in this prospectus or the registration statement (other than exhibits to such documents
unless such exhibits are specifically incorporated by reference into such documents). Requests for such copies should be directed to:
Gain Therapeutics, Inc.
Attn: C. Evan Ballantyne
Chief Financial Officer
4800 Montgomery Lane, Suite 220
Bethesda, Maryland 20814
Telephone: 301-500-1556
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement
on Form S-1 under the Securities Act with respect to the shares of Common Stock offered by this prospectus. This prospectus, which
is part of the registration statement, omits certain information, exhibits, schedules and undertakings set forth in the registration statement.
For further information pertaining to us and our securities, reference is made to our SEC filings and the registration statement and the
exhibits and schedules to the registration statement. Statements contained in this prospectus as to the contents or provisions of any
documents referred to in this prospectus are not necessarily complete, and in each instance where a copy of the document has been filed
as an exhibit to the registration statement, reference is made to the exhibit for a more complete description of the matters involved.
In addition, registration statements and certain
other filings made with the SEC electronically are publicly available through the SEC’s web site at http://www.sec.gov. The
registration statement, including all exhibits and amendments to the registration statement, has been filed electronically with the SEC.
We are subject to the information and periodic
reporting requirements of the Exchange Act, and, in accordance with such requirements, will file periodic reports, proxy statements,
and other information with the SEC. These periodic reports, proxy statements, and other information will be available for inspection
and copying at the web site of the SEC referred to above. We also maintain a website at https://www.gaintherapeutics.com, at which
you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished
to, the SEC. The information contained in, or that can be accessed through, our website is not part of, and is not incorporated into,
this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.

744,026 Shares of Common Stock
4,256,150 Shares of Common Stock Issuable Upon
Exercise of Outstanding Warrants
PRELIMINARY PROSPECTUS
, 2023
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
| Item 13. |
Other Expenses of Issuance and Distribution. |
The following table sets forth the costs and expenses, other than placement
agent fees, paid or payable by Gain Therapeutics, Inc., or the Registrant, in connection with the sale and distribution of the securities
being registered. All amounts are estimated except the SEC registration fee.
| Item | |
Amount | |
| SEC registration fee | |
$ | 1,605.32 | |
| Legal fees and expenses | |
| 33,000.00 | |
| Accounting fees and expenses | |
| 20,000.00 | |
| Printing and engraving expenses | |
| 3,000.00 | |
| Transfer agent and registrar fees and expenses | |
| 1,000.00 | |
| Miscellaneous fees and expenses | |
| 1,394.68 | |
| Total | |
$ | 60,000.00 | |
| Item 14. |
Indemnification of Directors and Officers. |
Section
102(b)(7) of the DGCL permits a corporation to provide in its certificate of incorporation that a director of the corporation shall not
be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director, except for
liability (i) for any breach of the director’s duty of loyalty to the corporation or its stockholders, (ii) for acts or
omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) for unlawful payment of
dividends or unlawful stock purchases or redemptions, or (iv) for any transaction from which the director derived an improper personal
benefit. Our amended and restated certificate of incorporation will contain such a provision.
Section
145 of the DGCL provides that a corporation may indemnify directors and officers as well as other employees and individuals against expenses
(including attorneys’ fees), judgments, fines, and amounts paid in settlement in connection with specified actions, suits, or proceedings,
whether civil, criminal, administrative, or investigative (other than an action by or in the right of the corporation—a “derivative
action”), if they acted in good faith and in a manner they reasonably believed to be in or not opposed to the best interests of
the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe their conduct was unlawful.
A similar standard is applicable in the case of derivative actions, except that indemnification only extends to expenses (including attorneys’
fees) incurred in connection with defense or settlement of such action, and the statute requires court approval before there can be any
indemnification where the person seeking indemnification has been found liable to the corporation. Our Amended Charter and Bylaws contain
such a provision.
We
have in effect a directors and officers liability insurance policy indemnifying our directors and officers for certain liabilities incurred
by them, including liabilities under the Securities Act and the Exchange Act. We pay the entire premium of this policy.
We
have entered into indemnification agreements with each of our directors and officers that provide the maximum indemnity allowed to directors
and officers by Section 145 of the DGCL and which allow for certain additional procedural protections.
These
indemnification provisions and the indemnification agreements may be sufficiently broad to permit indemnification of our officers and
directors for liabilities (including reimbursement of expenses incurred) arising under the Securities Act.
| Item 15. |
Recent Sales of Unregistered Securities. |
In the three years preceding the filing of this registration statement,
the Company made sales of the following unregistered securities:
Equity Awards
From
March 31, 2021 to June 30, 2021, we have granted option awards to employees, directors and consultants, covering an aggregate of
88,200 shares of Common Stock with a threshold amount of $10.03, in connection with services provided to us by such parties.
From
January 1, 2021 to March 31, 2021, we have granted option awards to directors and consultants, covering an aggregate of 88,080
shares of Common Stock with a threshold amount of $3.38, in connection with services provided to us by such parties.
Unless otherwise stated, the issuances of the above securities were
deemed to be exempt from registration under the Securities Act in reliance upon Section 4(a)(2) of the Securities
Act or Regulation D promulgated thereunder, or Rule 701 promulgated under Section 3(b) of the Securities Act
as transactions by an issuer not involving any public offering or pursuant to benefit plans and contracts relating to compensation as
provided under Rule 701. Individuals who purchased securities as described above represented their intention to acquire the securities
for investment only and not with a view to or for sale in connection with any distribution thereof, and appropriate legends were affixed
to the share certificates issued in such transactions.
Original Issuances of Stock
On
March 17, 2021, we completed our initial public offering, or IPO, and issued and sold an aggregate of 4,181,818 shares of our Common
Stock at a public offering price of $11.00 per share, including approximately 0.5 million shares in connection with the full exercise
of the underwriters’ option to purchase an additional 545,454 shares of Common Stock, resulting in net proceeds of approximately
$40.5 million, after deducting the underwriting discounts and commissions and offering expenses. Upon the closing of the IPO, series A
convertible preferred stock and series B convertible preferred stock were converted into shares of Common Stock at ratio of 1-for-1.
No
expenses incurred by us in connection with our IPO were made directly or indirectly to (i) any of our officers or directors or their associates,
(ii) any persons owning 10% or more of any class of our equity securities, or (iii) any of our affiliates. There has been no material
change in the planned use of proceeds from our IPO from that described in the final prospectus filed by us with the SEC pursuant to Rule
424(b) on March 17, 2021.
November 2023 Private Placement
On November 21, 2023, in
the Private Placement that was completed simultaneously with the Public Offering, we entered into that certain securities purchase agreement
(the “Securities Purchase Agreement”) with a certain private purchaser whereby we issued the Private Shares, the Pre-Funded
Warrants and the Private Warrants, for aggregate gross proceeds of $5.0 million. The Private Warrants are exercisable at an exercise
price of $2.75 per share, will be exercisable six months after issuance and expire five years from the date of issuance. The Pre-Funded
Warrants are each exercisable for one share of Common Stock at an exercise price of $0.0001 per share and will expire when exercised
in full. The offering price for each of the Private Shares and each of the accompanying Private Warrants to purchase one of the Private
Warrant Shares was $2.00. The net proceeds from the Private Placement, after deducting placement agent fees and other estimated offering
expenses payable by us, was approximately $4.65 million.
Pursuant to a letter agreement
dated as of November 14, 2023, we engaged Newbridge to act as our exclusive placement agent in connection with the Private Placement.
We paid Newbridge a cash fee equal to 7.0% of the aggregate gross proceeds of the Private Placement, excluding the proceeds, if any, from
the exercise of the Private Warrant Shares. In addition, we issued to Newbridge or its designees, warrants to purchase up to 175,006 shares
of our Common Stock. The warrants will be exercisable on May 24, 2023 and will expire on November 24, 2028.
The Private Placement closed
on November 24, 2023.
Securities Act Exemptions
We deemed the offers, sales and issuances of the
securities described above under “Equity Awards,” “Original Issuances of Stock,” and “November
2023 Private Placement” to be exempt from registration under the Securities Act in reliance on Section 4(2) of the
Securities Act, including Regulation D and Rule 506 promulgated thereunder, relative to transactions by an issuer not involving
a public offering. All purchasers of securities in transactions exempt from registration pursuant to Regulation D represented
to us that they were accredited investors and were acquiring the shares for investment purposes only and not with a view to, or for sale
in connection with, any distribution thereof and that they could bear the risks of the investment and could hold the securities for an
indefinite period of time. The purchasers received written disclosures that the securities had not been registered under the Securities
Act and that any resale must be made pursuant to a registration statement or an available exemption from such registration.
We deemed the grants of stock options and issuances
of Common Stock upon exercise of such options described above under “Equity Awards” to be exempt from registration
under the Securities Act in reliance on Rule 701 of the Securities Act as offers and sales of securities
under compensatory benefit plans and contracts relating to compensation in compliance with Rule 701. Each of the recipients of securities
in any transaction exempt from registration either received or had adequate access, through employment, business or other relationships,
to information about us.
| Item 16. |
Exhibits and Financial Statement Schedules. |
See the Exhibit Index attached to this Registration Statement, which
is incorporated by reference herein.
| |
(b) |
Financial Statement Schedules |
No financial statement schedules are provided because the information
called for is not required or is shown either in the financial statements or the notes thereto.
The undersigned registrant hereby undertakes:
1. To file, during any period in which offers
or sales are being made, a post-effective amendment to this registration statement:
| |
i. |
To include any prospectus required by section 10(a)(3) of the Securities Act of 1933; |
| |
ii. |
To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Securities and Exchange Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement. |
| |
iii. |
To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement, provided, however, that paragraphs (1)(i), (1)(ii) and (1)(iii) do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the registrant pursuant to Section 13 or 15(d) of the Exchange Act that are incorporated by reference in the registration statement. |
2. That, for the purpose of determining any liability
under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating
to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering
thereof.
3. To remove from registration by means of a post-effective
amendment any of the securities being registered which remain unsold at the termination of the offering.
4. That, for the purpose of determining liability
under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement
relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule
430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided,
however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document
incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement
will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the
registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such
date of first use.
5. That, for the purpose of determining liability
of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities, regardless
of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means
of any of the following communications, the registrant will be a seller to the purchaser and will be considered to offer or sell such
securities to such purchaser:
| (i) | any preliminary prospectus or prospectus of the registrant relating
to the offering filed pursuant to Rule 424; |
| (ii) | any free writing prospectus relating to the offering prepared
by or on behalf of the registrant or used or referred to by the registrant; |
| (iii) | the portion of any other free writing prospectus relating to
the offering containing material information about the registrant or its securities provided by or on behalf of the registrant; and |
| (iv) | any other communication that is an offer in the offering made
by the registrant to the purchaser. |
6. That, for purposes of determining any liability
under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to Section 13(a) or 15(d) of the Securities
Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to Section 15(d) of
the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration
statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial
bona fide offering thereof.
7. Insofar as indemnification for liabilities
arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant
to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission
such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for
indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer
or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer
or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the
matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification
by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
SIGNATURES
Pursuant to the requirements of the Securities
Act of 1933, as amended, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for
filing on Form S-1 and has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized,
in the City of Bethesda, State of Maryland on December 19, 2023.
| GAIN THERAPEUTICS, INC. |
|
| |
|
|
| By: |
/s/ Matthias Alder |
|
| |
|
|
| |
Matthias Alder |
|
| |
Chief Executive Officer and Director |
|
POWER OF ATTORNEY AND SIGNATURES
Each person whose signature appears below constitutes
and appoints Matthias Alder and C. Evan Ballantyne and each of them singly, his or her true and lawful attorneys-in-fact and agents, with
full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign
any and all amendments (including, without limitation, post-effective amendments) to this registration statement and any and all additional
registration statements pursuant to Rule 462(b) of the Securities Act and to file the same, with all exhibits thereto
and all other documents in connection therewith, with the SEC, granting unto each said attorney-in-fact and agent full power and authority
to do and perform each and every act in person, hereby ratifying and confirming all that said attorneys-in-fact and agents or either of
them or their, his or her substitute or substitutes may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities
Act of 1933, as amended, this Registration Statement has been signed by the following persons on behalf of the registrant in the capacities
and on the dates indicated.
| Signature |
|
Title |
|
Date |
| |
|
|
|
|
| /s/ Matthias Alder |
|
Chief Executive Officer and Director (Principal Executive Officer) |
|
December 19, 2023 |
| Matthias Alder |
|
|
|
|
| |
|
|
|
|
| /s/ C. Evan Ballantyne |
|
Chief Financial Officer (Principal Financial Officer) |
|
December 19, 2023 |
| C. Evan Ballantyne |
|
|
|
|
| |
|
|
|
|
| /s/ Gianluca Fuggetta |
|
Senior Director, Corporate Reporting (Principal Accounting Officer) |
|
December 19, 2023 |
| Gianluca Fuggetta |
|
|
|
|
| |
|
|
|
|
| /s/ Khalid
Islam |
|
Chairman of the Board of Directors |
|
December 19, 2023 |
| Khalid Islam |
|
|
|
|
| |
|
|
|
|
| /s/ Dov Goldstein |
|
Director |
|
December 19, 2023 |
| Dov Goldstein |
|
|
|
|
| |
|
|
|
|
| /s/ Hans Peter Hasler |
|
Director |
|
December 19, 2023 |
| Hans Peter Hasler |
|
|
|
|
| |
|
|
|
|
| /s/ Gwen Melincoff |
|
Director |
|
December 19, 2023 |
| Gwen Melincoff |
|
|
|
|
| |
|
|
|
|
| /s/ Claude Nicaise |
|
Director |
|
December 19, 2023 |
| Claude Nicaise |
|
|
|
|
| |
|
|
|
|
| /s/ Eric I. Richman |
|
Director |
|
December 19, 2023 |
| Eric I. Richman |
|
|
|
|
| |
|
|
|
|
| /s/ Jeffrey Riley |
|
Director |
|
December 19, 2023 |
| Jeffrey Riley |
|
|
|
|
EXHIBIT INDEX
Exhibit
No. |
|
Description of Document |
| |
|
|
| 3.1** |
|
Amended and Restated Certificate of Incorporation of Gain Therapeutics, Inc. (incorporated by reference to Exhibit 3.1 of the Company’s Current Report on Form 8-K filed with the SEC on March 17, 2021) |
| 3.2** |
|
Amended and Restated Bylaws of Gain Therapeutics, Inc. (incorporated by reference to Exhibit 3.2 of the Company’s Current Report on Form 8-K filed with the SEC on March 17, 2021) |
| 4.1** |
|
Investors’ Rights Agreement, dated as of July 20, 2020, by and among Gain Therapeutics, Inc. and certain holders of its capital stock. (incorporated by reference to Exhibit 4.2 of the Company’s Registration Statement on Form S-1 filed with the SEC on February 19, 2021) |
| 4.2** |
|
Form of Public Warrant issued in the 2023 Public Offering (incorporated by reference to Exhibit 4.1 of the Company’s Current Report on Form 8-K filed with the SEC on November 22, 2023) |
| 4.3** |
|
Form of Private Warrant issued in the 2023 Private Placement (incorporated by reference to Exhibit 4.2 of the Company’s Current Report on Form 8-K filed with the SEC on November 22, 2023) |
| 4.4** |
|
Form of Underwriter’s Warrant issued in the 2023 Public Offering (incorporated by reference to Exhibit 4.3 of the Company’s Current Report on Form 8-K filed with the SEC on November 22, 2023) |
| 4.5** |
|
Form of Pre-Funded Warrant issued in the 2023 Private Placement (incorporated by reference to Exhibit 4.4 of the Company’s Current Report on Form 8-K filed with the SEC on November 22, 2023) |
| 4.6** |
|
Warrant Agency Agreement by and between Gain Therapeutics, Inc. and Pacific Stock Transfer Company (incorporated by reference to Exhibit 4.5 of the Company’s Current Report on Form 8-K filed with the SEC on November 22, 2023) |
| 4.7** |
|
Form of Placement Agent Warrant issued in the 2023 Private Placement (incorporated by reference to Exhibit 4.6 of the Company’s Current Report on Form 8-K filed with the SEC on November 22, 2023) |
| 5.1* |
|
Opinion of Lowenstein Sandler LLP |
| 10.1**† |
|
Form of Indemnification Agreement for Officers and Directors. (incorporated by reference to Exhibit 10.3 of the Company’s Registration Statement on Form S-1 filed with the SEC on March 10, 2021) |
| 10.2**† |
|
2020 Omnibus Incentive Plan. (incorporated by reference to Exhibit 10.2 of the Company’s Registration Statement on Form S-1 filed with the SEC on March 10, 2021) |
| 10.3**† |
|
Gain Therapeutics, Inc. 2021 Inducement Equity Incentive Plan (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the SEC on December 28, 2021) |
| 10.4**† |
|
Form of Stock Option Agreement under the 2021 Inducement Plan. (incorporated by reference to Exhibit 10.2 of the Company’s Current Report on Form 8-K filed with the SEC on February 19, 2021) |
| 10.5** |
|
Minoryx Agreement between Minoryx Therapeutics, S.L. and GT Gain Therapeutics SA (incorporated by reference to Exhibit 10.3 of the Company’s Registration Statement on Form S-1 filed with the SEC on March 10, 2021) |
| 10.6**† |
|
Executive Employment Agreement, effective as of July 20, 2020, between Gain Therapeutics, Inc. and Eric I. Richman (incorporated by reference to Exhibit 10.4 of the Company’s Current Report on Form 8-K filed with the SEC on February 19, 2021) |
| 10.7**† |
|
Executive Employment Agreement, effective as of October 15, 2021, between Gain Therapeutics, Inc. and Matthias Alder (incorporated by reference to Exhibit 10.8 of the Company’s Annual Report on Form 10-K filed with the SEC on March 25, 2022). |
| 10.8** |
|
Form of Exchange Agreement (incorporated by reference to Exhibit 10.10 of the Company’s Registration Statement on Form S-1 filed with the SEC on February 19, 2021). |
| 10.9** |
|
Form of Placement Agent Warrant (incorporated by reference to Exhibit 10.11 of the Company’s Registration Statement on Form S-1 filed with the SEC on February 19, 2021). |
| 10.10**† |
|
Gain Therapeutics Inc. 2021 Inducement Equity Incentive Plan Restricted Stock Unit Award Agreement (incorporated by reference to Exhibit 4.5 of the Company’s Registration Statement on Form S-8 filed with the SEC on July 15, 2022). |
| 10.11**† |
|
Gain Therapeutics Inc. 2022 Equity Incentive Plan (incorporated by reference to Exhibit 4.6 of the Company’s Registration Statement on Form S-8 filed with the SEC on July 15, 2022). |
| 10.12**† |
|
Gain Therapeutics Inc. RSU Award Grant Notice and Award Agreement (2022 Equity Incentive Plan) (incorporated by reference to Exhibit 4.7 of the Company’s Registration Statement on Form S-8 filed with the SEC on July 15, 2022). |
| 10.13**† |
|
Gain Therapeutics Inc. Stock Option Grant Notice and Award Agreement (2022 Equity Incentive Plan) (incorporated by reference to Exhibit 4.8 of the Company’s Registration Statement on Form S-8 filed with the SEC on July 15, 2022). |
| 10.14**† |
|
Transition Agreement, between Gain Therapeutics, Inc. and Eric Richman, dated September 19, 2022 (incorporated by reference to Exhibit 10.1 on the Company’s Current Report on Form 8-K filed with the SEC September 19, 2022) |
| 10.15**† |
|
Consulting Agreement, between Gain Therapeutics, Inc. and Eric Richman, dated September 20, 2022 (included as Exhibit A to the Transition Agreement) (incorporated by reference to Exhibit 10.2 on the Company’s Current Report on Form 8-K filed with the SEC September 19, 2022). |
| 10.16**† |
|
Amended and Restated Employment Agreement, between Gain Therapeutics, Inc. and Matthias Alder, dated September 20, 2022 (incorporated by reference to Exhibit 10.3 on the Company’s Current Report on Form 8-K filed with the SEC September 19, 2022). |
| 10.17** |
|
Employment Agreement, by and between the Company and Evan Ballantyne, dated April 10, 2023 (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the SEC on April 12, 2023) |
| 10.18**† |
|
Separation Agreement and Release, by and between GT Gain Therapeutics SA and Salvatore Calabrese, dated April 27, 2023 (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the SEC on April 28, 2023). |
| 10.19**† |
|
Form of Indemnification Agreement for Officers and Directors (incorporated by reference to Exhibit 10.2 of the Company’s Quarterly Report on Form 10-Q filed with the SEC on November 14, 2023) |
| 10.20** |
|
Form of Securities Purchase Agreement (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the SEC on November 22, 2023) |
| 23.1* |
|
Consent of Independent Registered Public Accounting Firm |
| 23.2* |
|
Consent of Lowenstein Sandler LLP (included as part of Exhibit 5.1). |
| 24.1* |
|
Power of Attorney. |
| 107* |
|
Filing Fee Table. |
___________________
| # |
Portions of this exhibit (indicated by asterisks) are omitted in accordance with the rules of the SEC. |
| * |
Filed herewith. |
| ** |
Previously Filed. |
| † |
Indicates a management contract or compensation plan, contract or arrangement. |
EXHIBIT 5.1

December 19, 2023
Gain Therapeutics, Inc.
4800 Montgomery Lane, Suite 220
Bethesda, M.D. 20814
Ladies and Gentlemen:
We have acted as counsel for Gain Therapeutics,
Inc., a Delaware corporation (the “Company”), in connection with the preparation and filing of a Registration Statement on
Form S-1 (the “Registration Statement”), including a related prospectus filed with the Registration Statement (the “Prospectus”),
with the Securities and Exchange Commission (the “Commission”) pursuant to the Securities Act of 1933, as amended (the “Securities
Act”). The Registration Statement relates to the offer and sale by the selling stockholder(s) identified therein of up to 5,000,176
shares of the Company’s common stock, par value $0.0001 per share (the “Common Stock”). Such shares of Common Stock
consist of (i) 744,026 shares (the “Private Shares”) of Common Stock that were issued in connection with a private placement
(the “Private Placement”), (ii) 1,756,062 shares of Common Stock issuable upon the exercise of pre-funded warrants (the “Pre-Funded
Warrants” and the shares of Common Stock issuable upon the exercise thereof, the “Pre-Funded Warrant Shares”) issued
in the Private Placement, and (iii) 2,500,088 shares (the “Private Warrant Shares” and together with the Pre-Funded Warrant
Shares, the “Warrant Shares”) of Common Stock issuable upon the exercise of warrants issued in the Private Placement (the
“Private Warrants” and together with the Pre-Funded Warrants, the “Warrants”) described in the Registration Statement,
which are currently issued and outstanding.
In connection with rendering this opinion, we
have examined the Amended and Restated Certificate of Incorporation, as amended, and the Amended and Restated Bylaws of the Company, as
amended, the forms of the Warrants and such other corporate records, agreements, documents and instruments, and such certificates or comparable
documents of public officials and of officers and representatives of the Company, and we have made such inquiries of such officers and
representatives, as we have deemed necessary or appropriate for the purposes of this opinion.
In such examination, we have assumed the genuineness
of all signatures, the legal capacity of all natural persons, the authenticity of all documents submitted to us as originals, the conformity
to original documents of all documents submitted to us as certified, conformed or photostatic copies, and the authenticity of the originals
of such latter documents. As to certain questions of fact material to this opinion, we have relied upon certificates or comparable documents
of officers and representatives of the Company and have not sought to independently verify such facts.
Based on the foregoing, and subject to the qualifications
stated herein, we are of the opinion that, the Private Shares are validly issued, fully paid and non-assessable, and when issued
in accordance with the terms of the respective Warrants, the Warrant Shares will be duly authorized, validly issued, fully paid and non-assessable.
The opinion expressed herein is limited to the
General Corporation Law of the State of Delaware (including reported judicial decisions interpreting the General Corporation Law of the
State of Delaware), and we express no opinion as to the effect on the matters covered by this letter of the laws of any other jurisdiction.
We hereby consent to the filing of this letter
as an exhibit to the Registration Statement and to the reference to our firm under the caption “Legal Matters” in the Prospectus
which is a part of the Registration Statement. In giving such consents, we do not thereby admit that we are in the category of persons
whose consent is required under Section 7 of the Securities Act or the Rules and Regulations of the Commission promulgated thereunder.
| |
Very truly yours, |
|
| |
|
|
| |
|
/s/ Lowenstein Sandler LLP |
| |
|
Lowenstein Sandler LLP |
Exhibit
23.1
Consent
of Independent Registered Public Accounting Firm
We consent to the reference
to our firm under the caption “Experts” in the Registration Statement (Form S-1) and related Prospectus of Gain Therapeutics,
Inc. and to the incorporation by reference therein of our report dated March 23, 2023, with respect to the consolidated financial statements
of Gain Therapeutics, Inc. included in its Annual Report (Form 10-K) for the year ended December 31, 2022, filed with the Securities and
Exchange Commission.
| /s/ Ernst & Young AG |
|
| Ernst & Young AG |
|
| Lugano, Switzerland |
|
December 19, 2023
EXHIBIT 107
Calculation of Filing Fee Tables
Form S-1
(Form Type)
Gain Therapeutics, Inc.
(Exact Name of Registrant as Specified in its Charter)
| |
|
Security
Type |
|
Security
Class
Title |
|
Fee
Calculation
or Carry
Forward
Rule |
|
Amount
Registered (1) |
|
|
Proposed
Maximum
Offering
Price Per
Unit(2) |
|
|
Maximum
Aggregate
Offering
Price |
|
|
Fee Rate |
|
|
Amount of
Registration
Fee |
|
Fees to Be
Paid |
|
Equity |
|
Common Stock, par value $0.0001 per share |
|
Rule 457(c) |
|
|
744,026 |
|
|
$ |
2.445 |
|
|
$ |
1,819,180.77 |
|
|
|
0.00014760 |
|
|
$ |
268.51 |
|
| Fees to Be Paid |
|
Equity |
|
Common Stock, par value $0.0001 per share, issuable upon exercise of Pre-funded Warrants (3) |
|
Rule 457(c) |
|
|
1,756,062 |
|
|
$ |
2.445 |
|
|
$ |
4,763,318.18 |
|
|
|
0.00014760 |
|
|
|
703.07 |
|
Fees to Be
Paid |
|
Equity |
|
Common Stock, par value $0.0001 per share, issuable upon the exercise of Warrants (4) |
|
Rule 457(c) |
|
|
2,500,088 |
|
|
$ |
2.445 |
|
|
$ |
4,293,659.39 |
|
|
|
0.00014760 |
|
|
|
633.74 |
|
| Fees Previously Paid |
|
N/A |
|
N/A |
|
N/A |
|
|
N/A |
|
|
|
N/A |
|
|
|
N/A |
|
|
|
N/A |
|
|
|
|
|
| Carry Forward Securities |
|
N/A |
|
N/A |
|
N/A |
|
|
N/A |
|
|
|
N/A |
|
|
|
N/A |
|
|
|
N/A |
|
|
|
|
|
| |
|
Total Offering Amounts |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
1,605.32 |
|
| |
|
Total Fees Previously Paid |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
0 |
|
| |
|
Net Fee Due |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
1,605.32 |
|
| (1) |
Pursuant to Rule 416(a) under the Securities Act of 1933, as amended (the “Securities Act”), there is also being registered hereby such indeterminate number of additional common stock, par value $0.0001 per share (the “Common Stock”) of Gain Therapeutics, Inc. (the “Registrant”) as may be issued or issuable because of stock splits, stock dividends stock distributions, and similar transactions. |
| (2) |
Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(c) under
the Securities Act based on a per share price of the average of the high $2.55 and low $2.34 reported sales prices of the
Registrant’s Common Stock on the Nasdaq Stock Market LLC on December 18,
2023, which date is within five (5) business days of the filing of the registration statement by the Registrant for the registration
of the securities listed in the table above. |
| (3) |
Represents shares of Common Stock issuable upon the exercise of Pre-Funded Warrants to purchase Common Stock at a nominal exercise price of $0.0001 per share, subject to adjustment in accordance with the terms of the Pre-Funded Warrant issued by the Registrant on November 23, 2023. |
| (4) |
Represents shares of Common Stock issuable upon the exercise of warrants to purchase Common Stock at an exercise price of $2.75 per share issued by the Registrant on November 23, 2023. |
Gain Therapeutics (NASDAQ:GANX)
Gráfica de Acción Histórica
De Abr 2024 a May 2024

Gain Therapeutics (NASDAQ:GANX)
Gráfica de Acción Histórica
De May 2023 a May 2024
