false 0001885522 0001885522 2024-01-08 2024-01-08
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 8, 2024
NEUMORA THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
| Delaware |
|
001-41802 |
|
84-4367680 |
| (State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification Number) |
490 Arsenal Way, Suite 200
Watertown, Massachusetts 02472
(Address of principal executive offices) (Zip Code)
(857) 760-0900
(Registrant’s telephone number, including area code)
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| |
☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| |
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| |
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| |
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, $0.0001 par value per share |
|
NMRA |
|
The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 |
Regulation FD Disclosure. |
On January 8, 2024, Neumora Therapeutics, Inc. (the “Company”) made available a corporate presentation, which it plans to use for meetings with investors and analysts at the 42nd Annual J.P. Morgan Healthcare Conference. A copy of the presentation is being furnished hereto as Exhibit 99.1 and is incorporated herein by reference.
The information in this Item 7.01 of this Current Report on Form 8-K and Exhibit 99.1 attached hereto shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that Section 11 and 12(a)(2) of the Securities Act of 1933, as amended. The information contained herein and in the accompanying exhibit shall not be incorporated by reference into any filing with the Securities and Exchange Commission made by the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
| Item 9.01 |
Financial Statements and Exhibits. |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
NEUMORA THERAPEUTICS, INC. |
|
|
|
|
| Date: January 8, 2024 |
|
|
|
By: |
|
/s/ Joshua Pinto |
|
|
|
|
|
|
Joshua Pinto |
|
|
|
|
|
|
Chief Financial Officer |

Exhibit 99.1 J.P. Morgan Healthcare Conference January 2024

Important Disclosures This presentation contains forward-looking
statements about Neumora Therapeutics, Inc. (the “Company,” “we,” “us,” or “our”) within the meaning of the federal securities laws, including statements related to: Neumora’s intention to
redefine neuroscience drug development by bringing forward the next generation of novel therapies that offer improved treatment outcomes and quality of life for patients suffering from brain diseases; the timing, progress and plans for its
therapeutic development programs, including the timing of initiation and data read outs for its programs and studies, as well as its clinical trial and development plans; timing and expectations related to regulatory filings and interactions;
expectations and projections regarding future operating results and financial performance, including the sufficiency of its cash resources and expectation of the timing of its cash runway; its ability to create significant value and; other
statements identified by words such as “could,” “expects,” “intends,” “may,” “plans,” “potential,” “should,” “will,” “would,” or similar
expressions and the negatives of those terms. Other than statements of historical facts, all statements contained in this presentation are forward-looking statements within the meaning of the safe harbor provisions of the Private Securities
Litigation Reform Act of 1995. These statements are subject to risks and uncertainties that could cause the actual results to be materially different from the information expressed or implied by these forward-looking statements, including, among
others: the risks related to the inherent uncertainty of clinical drug development and unpredictability and lengthy process for obtaining regulatory approvals; risks related to the timely initiation and enrollment in our clinical trials; risks
related to our reliance on third parties, including CROs; risks related to serious or undesirable side effects of our therapeutic candidates; risks related to our ability to utilize and protect our intellectual property rights; and other matters
that could affect sufficiency of capital resources to fund operations. For a detailed discussion of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks
relating to Neumora’s business in general, please refer to the risk factors identified in the Company’s filings with the Securities and Exchange Commission (SEC), including but not limited to its Registration Statement on Form S-1, as
amended (File No. 333-274229), filed with the SEC on September 11, 2023, and related Prospectus dated September 14, 2023 filed under 424(b)(4) of the Securities Act of 1933, as amended. Forward-looking statements speak only as of the date hereof,
and, except as required by law, Neumora undertakes no obligation to update or revise these forward-looking statements. 2

Our Mission We are focused on redefining neuroscience drug development
by bringing forward the next generation of novel therapies that offer improved treatment outcomes and quality of life for patients suffering from brain diseases 3 3
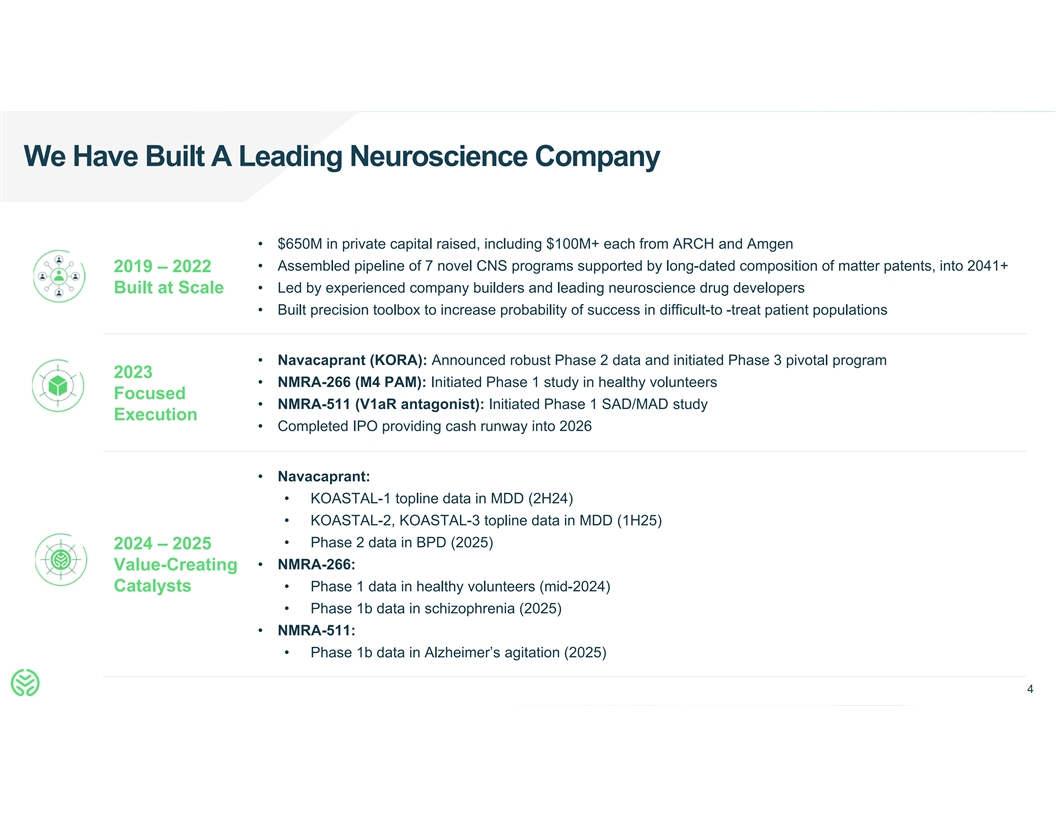
We Have Built A Leading Neuroscience Company • $650M in private
capital raised, including $100M+ each from ARCH and Amgen • Assembled pipeline of 7 novel CNS programs supported by long-dated composition of matter patents, into 2041+ 2019 – 2022 Built at Scale • Led by experienced company
builders and leading neuroscience drug developers • Built precision toolbox to increase probability of success in difficult-to -treat patient populations • Navacaprant (KORA): Announced robust Phase 2 data and initiated Phase 3 pivotal
program 2023 • NMRA-266 (M4 PAM): Initiated Phase 1 study in healthy volunteers Focused • NMRA-511 (V1aR antagonist): Initiated Phase 1 SAD/MAD study Execution • Completed IPO providing cash runway into 2026 • Navacaprant:
• KOASTAL-1 topline data in MDD (2H24) • KOASTAL-2, KOASTAL-3 topline data in MDD (1H25) • Phase 2 data in BPD (2025) 2024 – 2025 • NMRA-266: Value-Creating Catalysts • Phase 1 data in healthy volunteers
(mid-2024) • Phase 1b data in schizophrenia (2025) • NMRA-511: • Phase 1b data in Alzheimer’s agitation (2025) 4 4
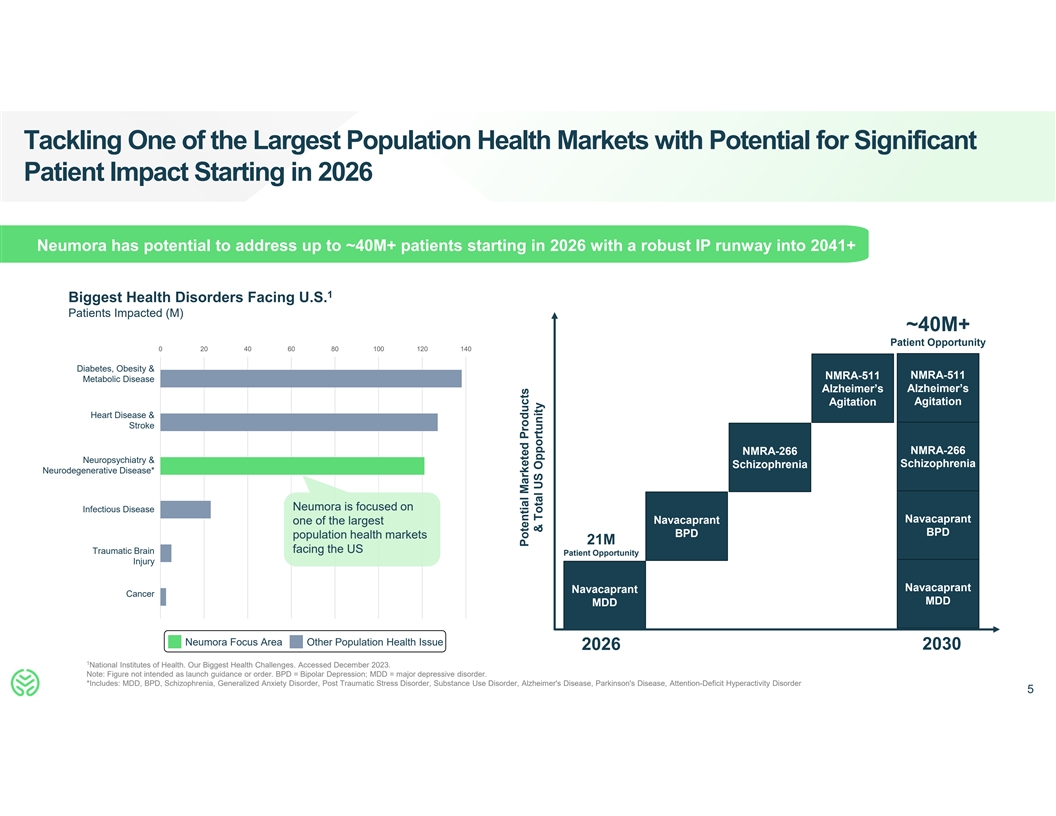
Tackling One of the Largest Population Health Markets with Potential for
Significant Patient Impact Starting in 2026 Neumora has potential to address up to ~40M+ patients starting in 2026 with a robust IP runway into 2041+ 1 Biggest Health Disorders Facing U.S. Patients Impacted (M) ~40M+ Patient Opportunity 0 20 40 60
80 100 120 140 Diabetes, Obesity & NMRA-511 NMRA-511 Metabolic Disease Alzheimer’s Alzheimer’s Agitation Agitation Heart Disease & Stroke NMRA-266 NMRA-266 Neuropsychiatry & Schizophrenia Schizophrenia Neurodegenerative
Disease* Neumora is focused on Infectious Disease Navacaprant Navacaprant one of the largest BPD BPD population health markets 21M facing the US Traumatic Brain Patient Opportunity Injury Navacaprant Navacaprant Cancer MDD MDD Neumora Focus Area
Other Population Health Issue 2030 2026 1 National Institutes of Health. Our Biggest Health Challenges. Accessed December 2023. Note: Figure not intended as launch guidance or order. BPD = Bipolar Depression; MDD = major depressive disorder.
*Includes: MDD, BPD, Schizophrenia, Generalized Anxiety Disorder, Post Traumatic Stress Disorder, Substance Use Disorder, Alzheimer's Disease, Parkinson's Disease, Attention-Deficit Hyperactivity Disorder 5 Potential Marketed Products & Total US
Opportunity
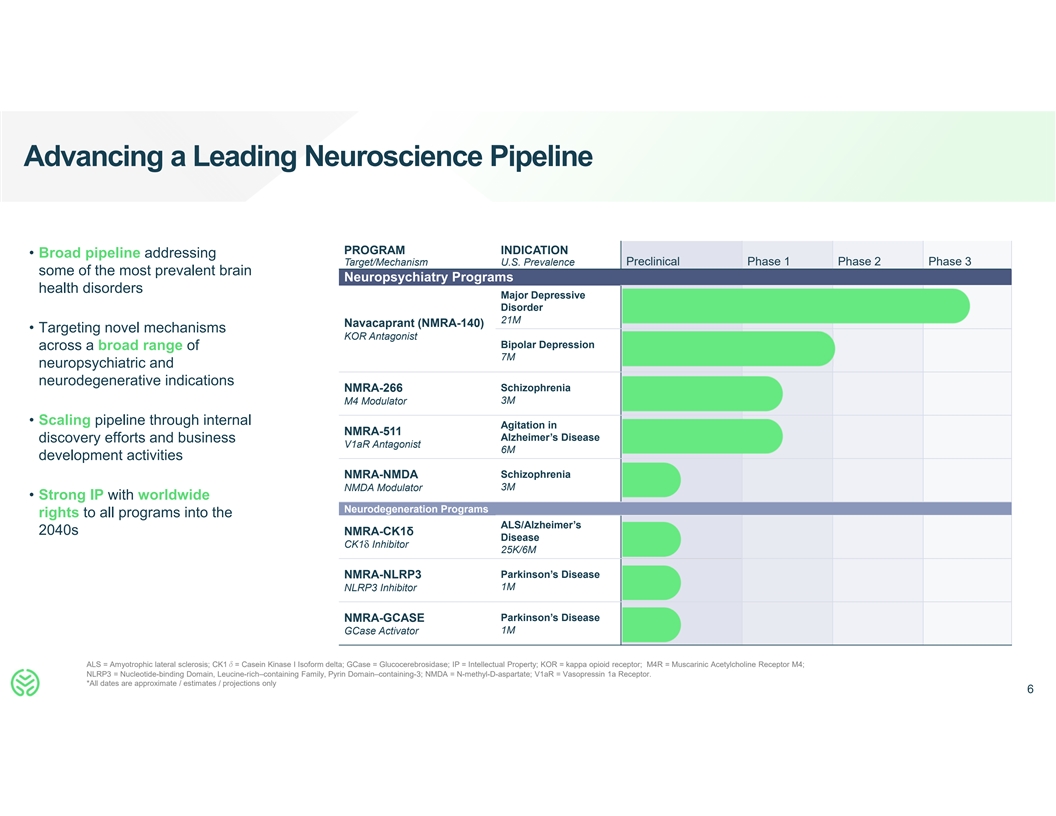
Advancing a Leading Neuroscience Pipeline PROGRAM INDICATION •
Broad pipeline addressing Target/Mechanism U.S. Prevalence Preclinical Phase 1 Phase 2 Phase 3 some of the most prevalent brain Neuropsychiatry Programs health disorders Major Depressive Disorder 21M Navacaprant (NMRA-140) • Targeting novel
mechanisms KOR Antagonist Bipolar Depression across a broad range of 7M neuropsychiatric and neurodegenerative indications NMRA-266 Schizophrenia 3M M4 Modulator • Scaling pipeline through internal Agitation in NMRA-511 Alzheimer’s
Disease discovery efforts and business V1aR Antagonist 6M development activities NMRA-NMDA Schizophrenia 3M NMDA Modulator • Strong IP with worldwide Neurodegeneration Programs rights to all programs into the ALS/Alzheimer’s 2040s
NMRA-CK1δ Disease CK1δ Inhibitor 25K/6M NMRA-NLRP3 Parkinson’s Disease 1M NLRP3 Inhibitor Parkinson’s Disease NMRA-GCASE 1M GCase Activator ALS = Amyotrophic lateral sclerosis; CK1δ= Casein Kinase I Isoform delta; GCase =
Glucocerebrosidase; IP = Intellectual Property; KOR = kappa opioid receptor; M4R = Muscarinic Acetylcholine Receptor M4; NLRP3 = Nucleotide-binding Domain, Leucine-rich–containing Family, Pyrin Domain–containing-3; NMDA =
N-methyl-D-aspartate; V1aR = Vasopressin 1a Receptor. *All dates are approximate / estimates / projections only 6
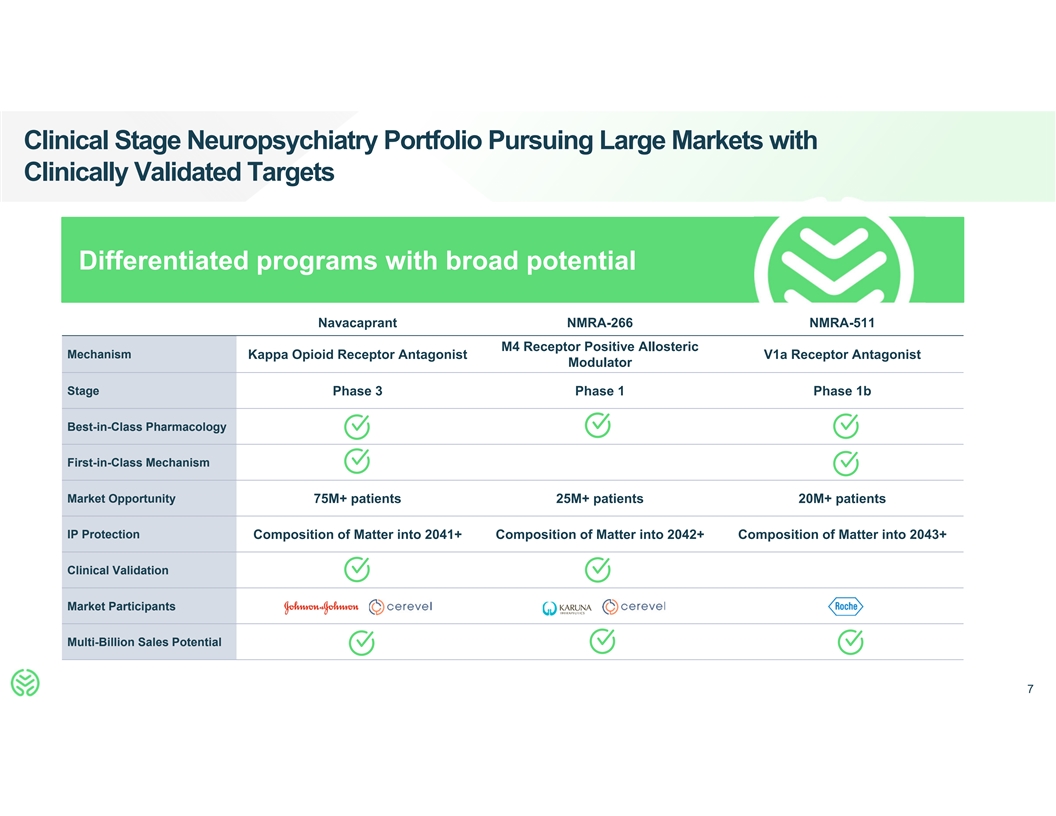
Clinical Stage Neuropsychiatry Portfolio Pursuing Large Markets with
Clinically Validated Targets Differentiated programs with broad potential Navacaprant NMRA-266 NMRA-511 M4 Receptor Positive Allosteric Mechanism Kappa Opioid Receptor Antagonist V1a Receptor Antagonist Modulator Stage Phase 3 Phase 1 Phase 1b
Best-in-Class Pharmacology First-in-Class Mechanism Market Opportunity 75M+ patients 25M+ patients 20M+ patients IP Protection Composition of Matter into 2041+ Composition of Matter into 2042+ Composition of Matter into 2043+ Clinical Validation
Market Participants Multi-Billion Sales Potential 7
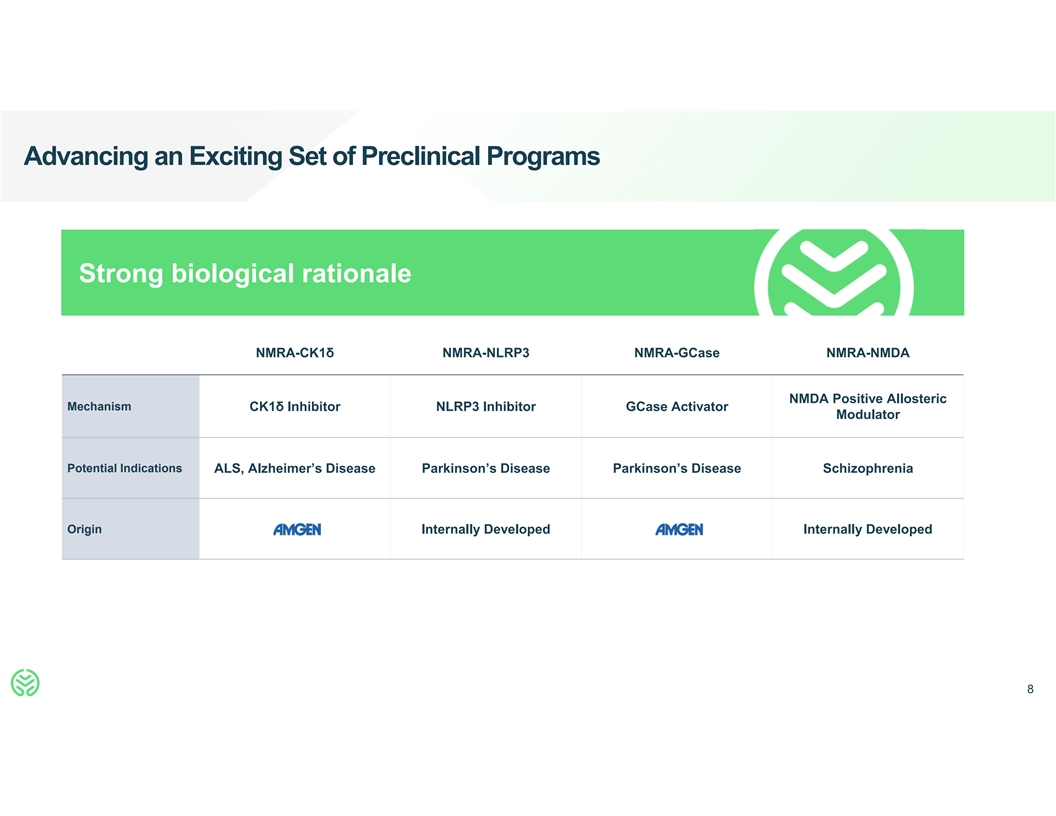
Advancing an Exciting Set of Preclinical Programs Strong biological
rationale NMRA-CK1δ NMRA-NLRP3 NMRA-GCase NMRA-NMDA NMDA Positive Allosteric Mechanism CK1δ Inhibitor NLRP3 Inhibitor GCase Activator Modulator Potential Indications ALS, Alzheimer’s Disease Parkinson’s Disease
Parkinson’s Disease Schizophrenia Origin Internally Developed Internally Developed 8
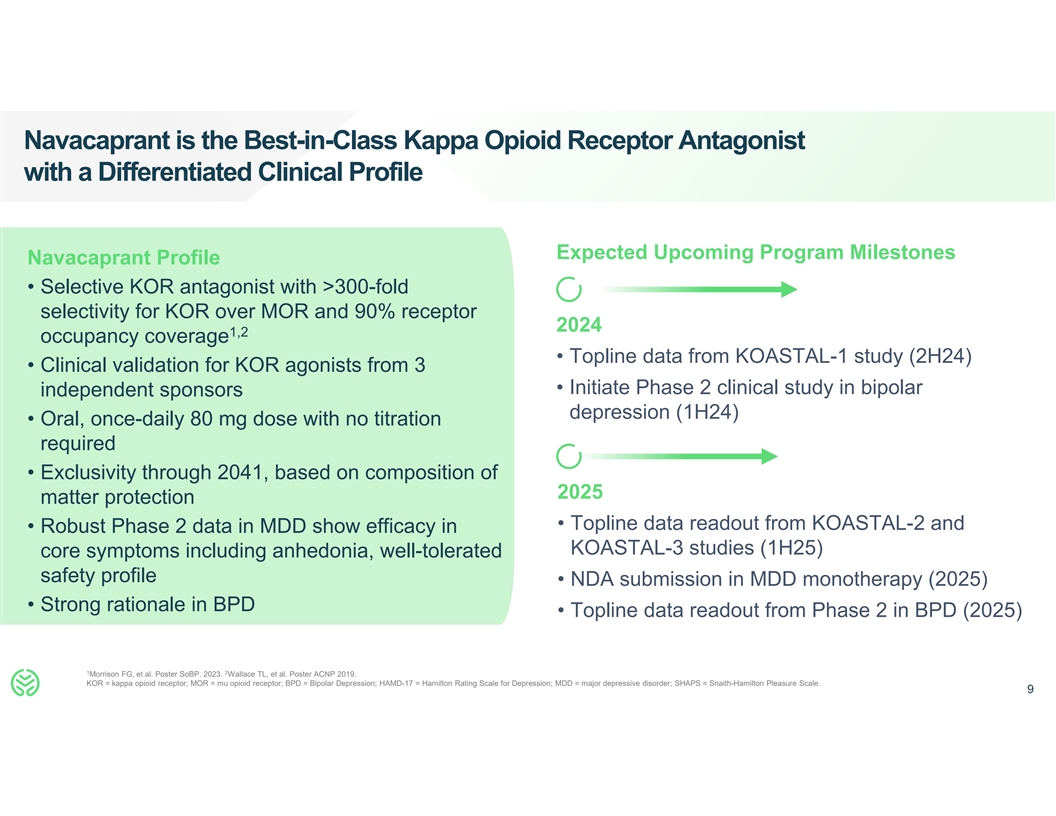
Navacaprant is the Best-in-Class Kappa Opioid Receptor Antagonist with a
Differentiated Clinical Profile Expected Upcoming Program Milestones Navacaprant Profile • Selective KOR antagonist with >300-fold selectivity for KOR over MOR and 90% receptor 2024 1,2 occupancy coverage • Topline data from KOASTAL-1
study (2H24) • Clinical validation for KOR agonists from 3 • Initiate Phase 2 clinical study in bipolar independent sponsors depression (1H24) • Oral, once-daily 80 mg dose with no titration required • Exclusivity through
2041, based on composition of 2025 matter protection • Topline data readout from KOASTAL-2 and • Robust Phase 2 data in MDD show efficacy in KOASTAL-3 studies (1H25) core symptoms including anhedonia, well-tolerated safety profile
• NDA submission in MDD monotherapy (2025) • Strong rationale in BPD • Topline data readout from Phase 2 in BPD (2025) 1 2 Morrison FG, et al. Poster SoBP. 2023. Wallace TL, et al. Poster ACNP 2019. KOR = kappa opioid receptor; MOR
= mu opioid receptor; BPD = Bipolar Depression; HAMD-17 = Hamilton Rating Scale for Depression; MDD = major depressive disorder; SHAPS = Snaith-Hamilton Pleasure Scale. 9
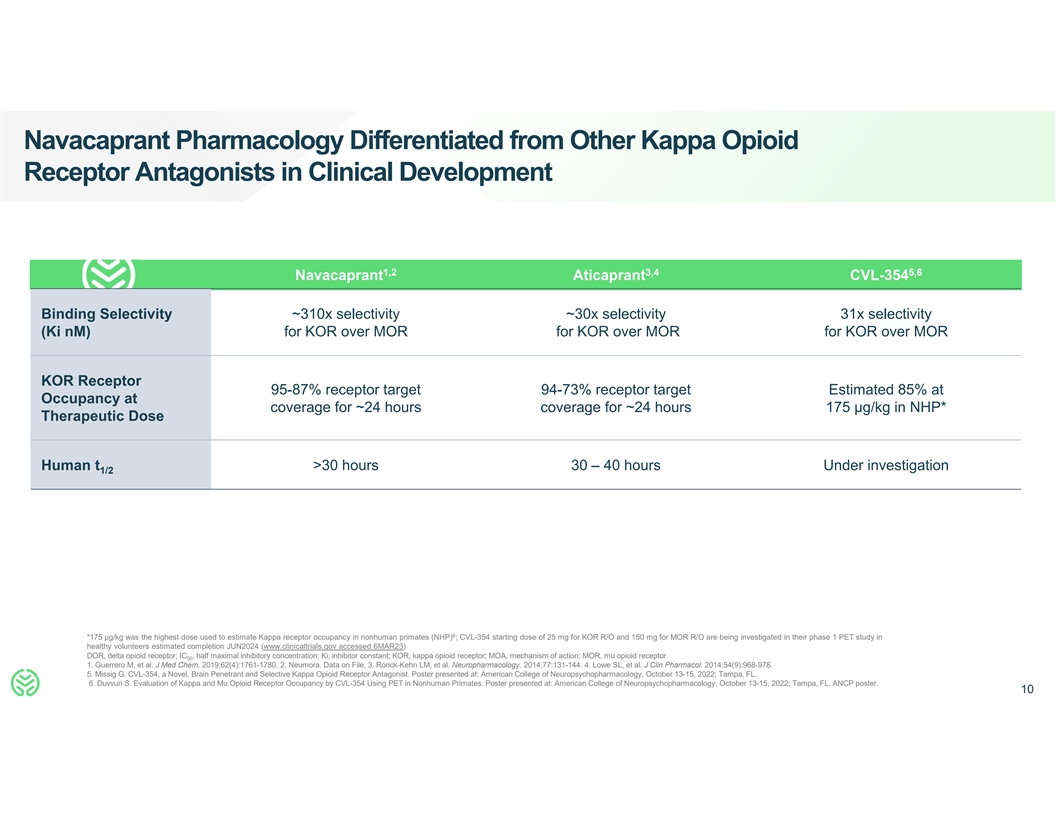
Navacaprant Pharmacology Differentiated from Other Kappa Opioid
Receptor Antagonists in Clinical Development 1,2 3,4 5,6 Navacaprant Aticaprant CVL-354 Binding Selectivity ~310x selectivity ~30x selectivity 31x selectivity (Ki nM) for KOR over MOR for KOR over MOR for KOR over MOR KOR Receptor 95-87% receptor
target 94-73% receptor target Estimated 85% at Occupancy at coverage for ~24 hours coverage for ~24 hours 175 µg/kg in NHP* Therapeutic Dose Human t >30 hours 30 – 40 hours Under investigation 1/2 6 *175 µg/kg was the highest dose
used to estimate Kappa receptor occupancy in nonhuman primates (NHP) ; CVL-354 starting dose of 25 mg for KOR R/O and 150 mg for MOR R/O are being investigated in their phase 1 PET study in healthy volunteers estimated completion JUN2024
(www.clinicaltrials.gov accessed 6MAR23) DOR, delta opioid receptor; IC , half maximal inhibitory concentration; Ki, inhibitor constant; KOR, kappa opioid receptor; MOA, mechanism of action; MOR, mu opioid receptor. 50 1. Guerrero M, et al. J Med
Chem. 2019;62(4):1761-1780. 2. Neumora. Data on File. 3. Rorick-Kehn LM, et al. Neuropharmacology. 2014;77:131-144. 4. Lowe SL, et al. J Clin Pharmacol. 2014;54(9):968-978. 5. Missig G. CVL-354, a Novel, Brain Penetrant and Selective Kappa Opioid
Receptor Antagonist. Poster presented at: American College of Neuropsychopharmacology, October 13-15, 2022; Tampa, FL. 6. Duvvuri S. Evaluation of Kappa and Mu Opioid Receptor Occupancy by CVL-354 Using PET in Nonhuman Primates. Poster presented at:
American College of Neuropsychopharmacology, October 13-15, 2022; Tampa, FL. ANCP poster. 10

First Program to Demonstrate Efficacy in Symptoms of Depression and
Anhedonia HAMD-17 Week 0 Week 4 Week 8 Safety profile with significant advantages 0 over existing treatment options Robust Phase 2 Data -2 • Study included 40 sites in the U.S. -4 TEAEs Incidence (≥2% in either treatment group) •
204 patients enrolled in the study, 100 -5.5 -6 patients included in pre-specified -7.1 Placebo Navacaprant moderate-to-severe population -8 n=102 n=102 -8.5 • Statistically significant results seen on -9.9 -10 Preferred Terms n (%) n (%) both
depression and anhedonia in ΔLSM = -3.0 ΔLSM = -2.8 moderate-to-severe patients with MDD -12 P = 0.015 P = 0.037 Headache 5 (4.9) 5 (4.9) • Efficacy demonstrated across additional SHAPS outcome measures in the moderate-to- COVID-19 3
(2.9) 4 (3.9) Week 0 Week 4 Week 8 severe MDD population, including 0 HAMD-17 response and remission rates, Nausea 1 (1.0) 5 (4.9) HAMD-6 and CGI-S -2 -4.0 • Navacaprant was well tolerated and was Diarrhea 3 (2.9) 2 (2.0) -4 -4.4 not
associated with weight gain or Upper respiratory tract -6 -6.4 sexual dysfunction 1 (1.0) 3 (2.9) infection -8 • No evidence of suicidal behavior was -9.2 identified as assessed the Columbia -10 Overall discontinuation rates were higher on
Suicide Severity Rating Scale ΔLSM = -2.4 ΔLSM = -4.8 placebo compared to navacaprant (29% -12 P = 0.071 P = 0.0006 navacaprant and 37% placebo) Navacaprant Placebo Note: Graphs depict prespecified statistical sensitivity analysis for
moderate-to-severe patients (n=100; baseline HAMD-17 ≥ 22). HAMD-17 = 17-Item Hamilton Rating Scale for Depression; MDD = Major Depressive Disorder; SHAPS= Snaith-Hamilton Pleasure Scale; HAMD-17 response = a reduction of ≥50% on HAMD-17
score; HAMD-17 remission = HAMD-17 score ≤7 ; CGI-S = Clinical Global Impression Scale-Severity. 11 Change From Baseline Change From Baseline SHAPS, LS Mean (SE) HAMD-17, LS Mean (SE) Improvement Improvement
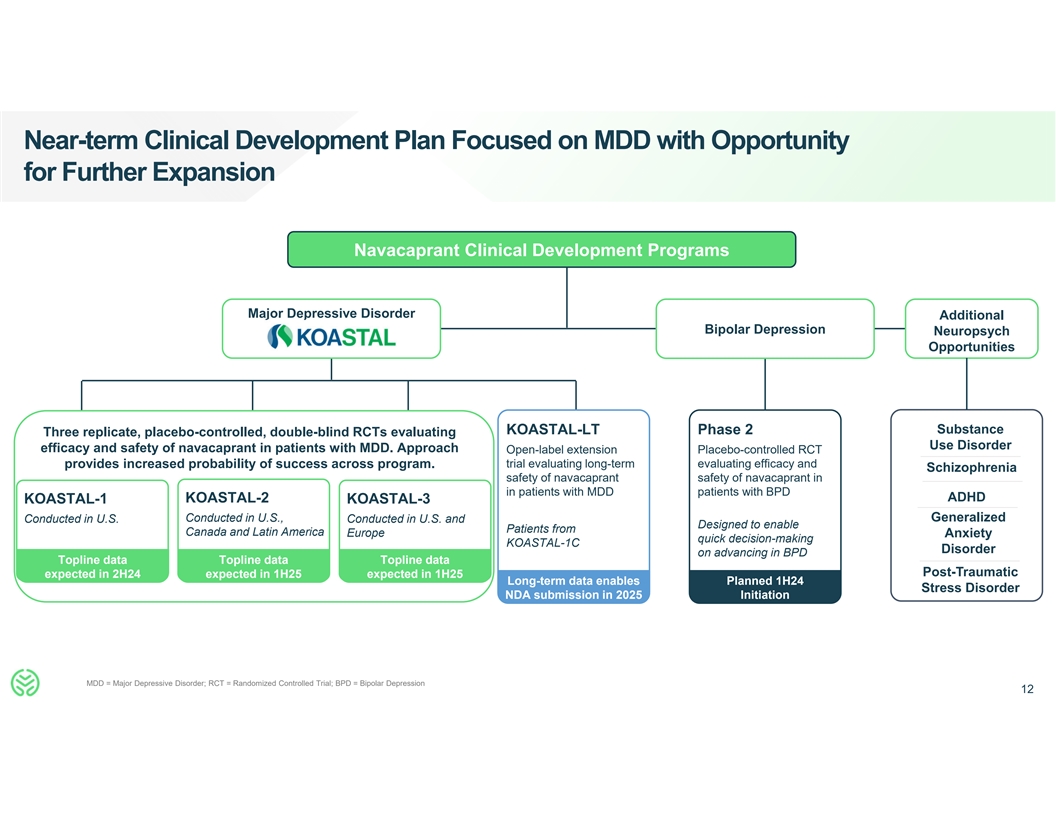
Near-term Clinical Development Plan Focused on MDD with Opportunity for
Further Expansion Navacaprant Clinical Development Programs Major Depressive Disorder Additional Bipolar Depression Neuropsych Opportunities Substance KOASTAL-LT Phase 2 Three replicate, placebo-controlled, double-blind RCTs evaluating Use Disorder
efficacy and safety of navacaprant in patients with MDD. Approach Open-label extension Placebo-controlled RCT provides increased probability of success across program. trial evaluating long-term evaluating efficacy and Schizophrenia safety of
navacaprant safety of navacaprant in in patients with MDD patients with BPD ADHD KOASTAL-2 KOASTAL-1 KOASTAL-3 Generalized Conducted in U.S. Conducted in U.S., Conducted in U.S. and Designed to enable Patients from Canada and Latin America Europe
Anxiety quick decision-making KOASTAL-1C Disorder on advancing in BPD Topline data Topline data Topline data Post-Traumatic expected in 2H24 expected in 1H25 expected in 1H25 Long-term data enables Planned 1H24 Stress Disorder NDA submission in 2025
Initiation MDD = Major Depressive Disorder; RCT = Randomized Controlled Trial; BPD = Bipolar Depression 12

������������
���� �� KOASTAL Pivotal Study Design Well Suited for Navacaprant Pharmacology KOASTAL Pivotal Efficacy Studies Robust placebo Navacaprant 80 mg QD Opportunity minimization R to enroll in strategies include
1:1 KOASTAL-LT stringent site Placebo QD selection, substantial internal medical Randomized, double-blind treatment monitoring, use of central raters and placebo-control Baseline WK WK WK WK 1 2 4 6 reminder scripts Key Efficacy Assessments
KOASTAL-1, KOASTAL-2, KOASTAL-3 Summary • Adults ages 18 – 65 diagnosed with MDD • from baseline to each timepoint in CGI-S and CGI-I Inclusion Criteria: • MADRS ≥ 25 at baseline • from baseline to each timepoint
in PHQ-9 Other Secondary Endpoints Include: • from baseline to each timepoint in HAM-A Primary Endpoint: • from baseline to Week 6 in MADRS total score • from baseline to each timepoint in SDS Key Exploratory • from baseline
to each timepoint in the EQ-5D 5L Key Secondary Endpoint: • from baseline to Week 6 in SHAPS total score Endpoints*: • from baseline to each timepoint in the WPAI-GH *Safety Assessments include Change in Sexual Functioning Questionnaire
(CSFQ-14) = Change; CGI-I = Clinical Global Impression-Improvement scale; CGI-S = Clinical Global Impression-Severity scale; EQ-5D 5L = EuroQol-5D 5L; HAM-A = Hamilton Anxiety Rating Scale; MADRS = Montgomery-Åsberg Depression Rating Scale; MDD
= Major Depressive Disorder; PHQ-9 = Patient Health Questionnaire-9; QD = once daily; SDS = Sheehan Disability Scale; SHAPS = Snaith-Hamilton Pleasure Scale; wk = week; WPAI-GH = Work Productivity and Activity Impairment Questionnaire –
General Health. 13
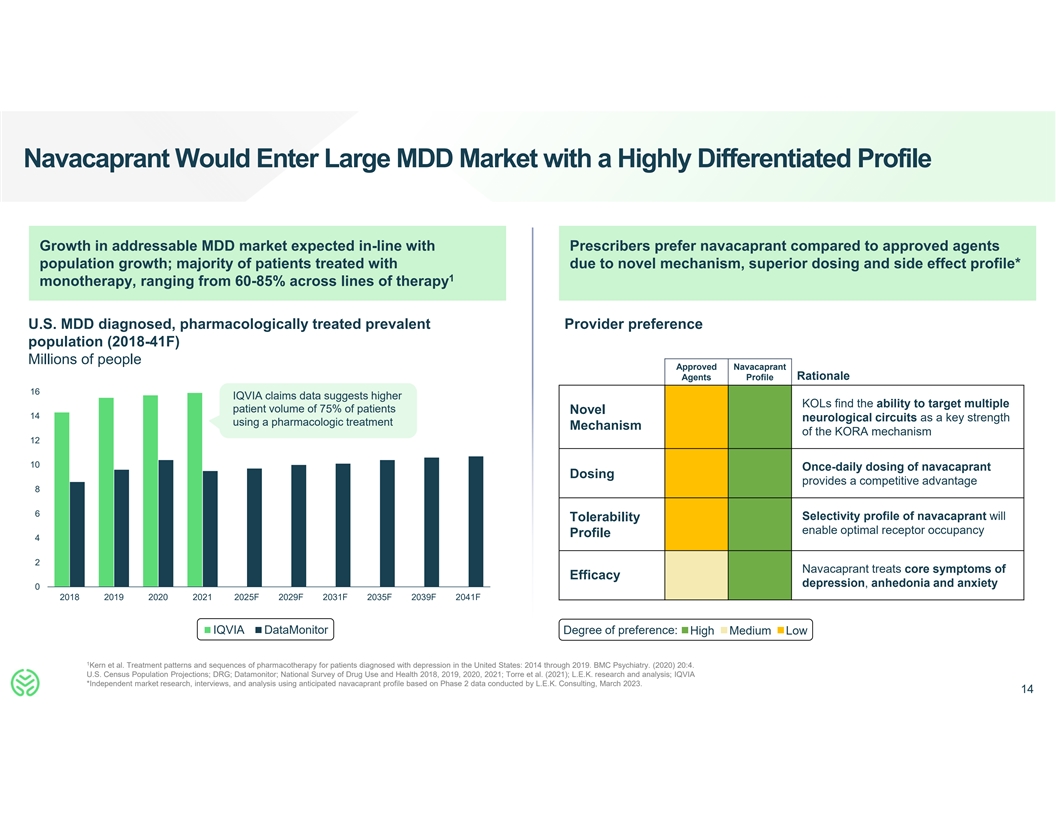
Navacaprant Would Enter Large MDD Market with a Highly Differentiated
Profile Growth in addressable MDD market expected in-line with Prescribers prefer navacaprant compared to approved agents population growth; majority of patients treated with due to novel mechanism, superior dosing and side effect profile* 1
monotherapy, ranging from 60-85% across lines of therapy U.S. MDD diagnosed, pharmacologically treated prevalent Provider preference population (2018-41F) Millions of people Approved Navacaprant Agents Profile Rationale 16 IQVIA claims data suggests
higher KOLs find the ability to target multiple patient volume of 75% of patients Novel 14 neurological circuits as a key strength using a pharmacologic treatment Mechanism of the KORA mechanism 12 10 Once-daily dosing of navacaprant Dosing provides
a competitive advantage 8 6 Selectivity profile of navacaprant will Tolerability enable optimal receptor occupancy Profile 4 2 Navacaprant treats core symptoms of Efficacy depression, anhedonia and anxiety 0 2018 2019 2020 2021 2025F 2029F 2031F
2035F 2039F 2041F IQVIA DataMonitor Degree of preference: High Medium Low 1 Kern et al. Treatment patterns and sequences of pharmacotherapy for patients diagnosed with depression in the United States: 2014 through 2019. BMC Psychiatry. (2020) 20:4.
U.S. Census Population Projections; DRG; Datamonitor; National Survey of Drug Use and Health 2018, 2019, 2020, 2021; Torre et al. (2021); L.E.K. research and analysis; IQVIA *Independent market research, interviews, and analysis using anticipated
navacaprant profile based on Phase 2 data conducted by L.E.K. Consulting, March 2023. 14

NMRA-266 is a Potentially Differentiated M4 Receptor PAM for
Schizophrenia 2 3 NMRA-266 Emraclidine Pharmacology Designed as a highly selective M4 muscarinic M EC (cAMP) 32 nM 12 nM 4 50 receptor PAM for antipsychotic-like efficacy with the potential for improved safety profile Human t Pending Phase 1 Study
9-12 h 1/2 Indication Schizophrenia [1] Brain: Plasma ratio 1:1 1:1 Epidemiology Selectivity at other M > 10 µM, M 6.8 µM M > 10 µM, M 5.8 µM Estimated 3 million patients in 1,3,5 2 1,3,5 2 M subtypes (EC ) 50 1 the U.S.
with schizophrenia Bioavailability 67% (predicted) Unknown Drug Profile Oral, once-daily Expected Upcoming Program Milestones Strong IP Protection Expect exclusivity through 2042+, based on composition of matter protection and 2024 2025 •
Phase 1 healthy volunteer data readout (mid-2024) • Topline data readout from Phase 1b study in estimated patent term extension • Initiate Phase 1b study in schizophrenia (2H24) schizophrenia (2025) 1 2 3 Wander, C. Am J Manag Care.
2020;26:S62-S68. NMRA data on file; CERE Company data. Note: Data on this slide is presented for illustrative purposes only and the data for emraclidine were not derived from Neumora clinical trials or preclinical studies. PAM = positive allosteric
modulator 15
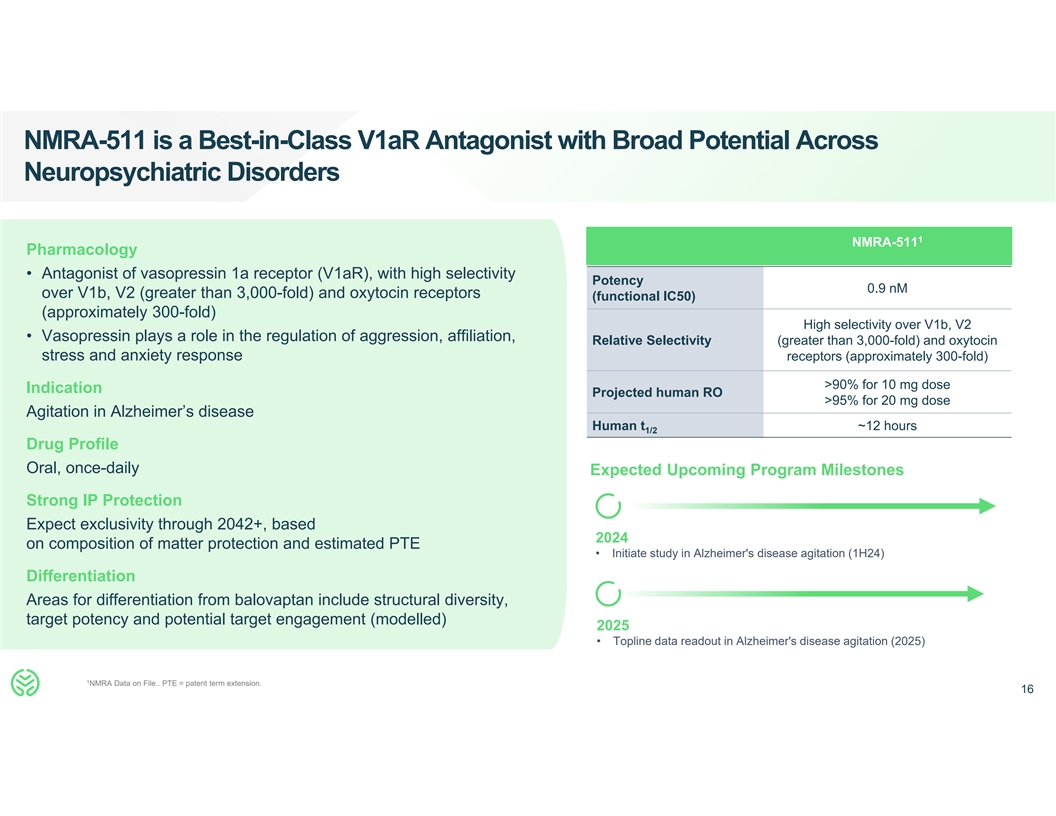
NMRA-511 is a Best-in-Class V1aR Antagonist with Broad Potential Across
Neuropsychiatric Disorders 1 NMRA-511 Pharmacology • Antagonist of vasopressin 1a receptor (V1aR), with high selectivity Potency 0.9 nM over V1b, V2 (greater than 3,000-fold) and oxytocin receptors (functional IC50) (approximately 300-fold)
High selectivity over V1b, V2 • Vasopressin plays a role in the regulation of aggression, affiliation, Relative Selectivity (greater than 3,000-fold) and oxytocin stress and anxiety response receptors (approximately 300-fold) >90% for 10 mg
dose Indication Projected human RO >95% for 20 mg dose Agitation in Alzheimer’s disease Human t ~12 hours 1/2 Drug Profile Oral, once-daily Expected Upcoming Program Milestones Strong IP Protection Expect exclusivity through 2042+, based
2024 on composition of matter protection and estimated PTE • Initiate study in Alzheimer's disease agitation (1H24) Differentiation Areas for differentiation from balovaptan include structural diversity, target potency and potential target
engagement (modelled) 2025 • Topline data readout in Alzheimer's disease agitation (2025) 1 NMRA Data on File.. PTE = patent term extension. 16
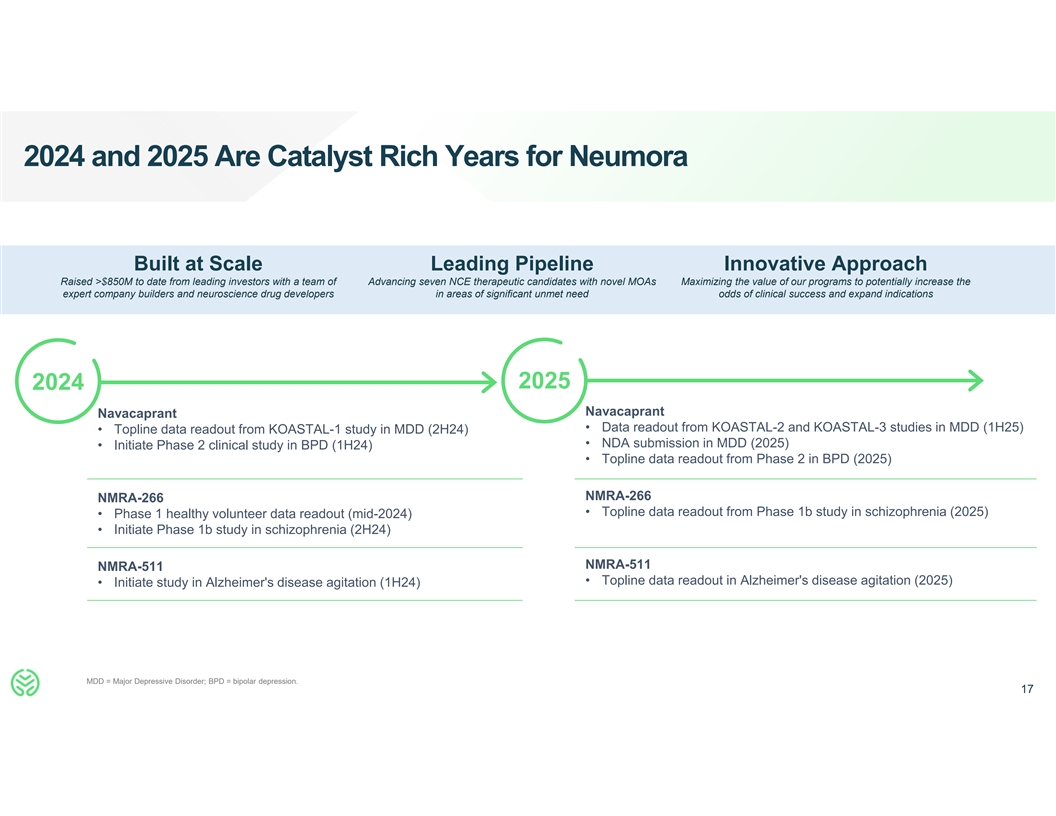
2024 and 2025 Are Catalyst Rich Years for Neumora Built at Scale
Leading Pipeline Innovative Approach Raised >$850M to date from leading investors with a team of Advancing seven NCE therapeutic candidates with novel MOAs Maximizing the value of our programs to potentially increase the expert company builders
and neuroscience drug developers in areas of significant unmet need odds of clinical success and expand indications 2025 2024 Navacaprant Navacaprant • Data readout from KOASTAL-2 and KOASTAL-3 studies in MDD (1H25) • Topline data
readout from KOASTAL-1 study in MDD (2H24) • NDA submission in MDD (2025) • Initiate Phase 2 clinical study in BPD (1H24) • Topline data readout from Phase 2 in BPD (2025) NMRA-266 NMRA-266 • Topline data readout from Phase
1b study in schizophrenia (2025) • Phase 1 healthy volunteer data readout (mid-2024) • Initiate Phase 1b study in schizophrenia (2H24) NMRA-511 NMRA-511 • Topline data readout in Alzheimer's disease agitation (2025) •
Initiate study in Alzheimer's disease agitation (1H24) MDD = Major Depressive Disorder; BPD = bipolar depression. 17
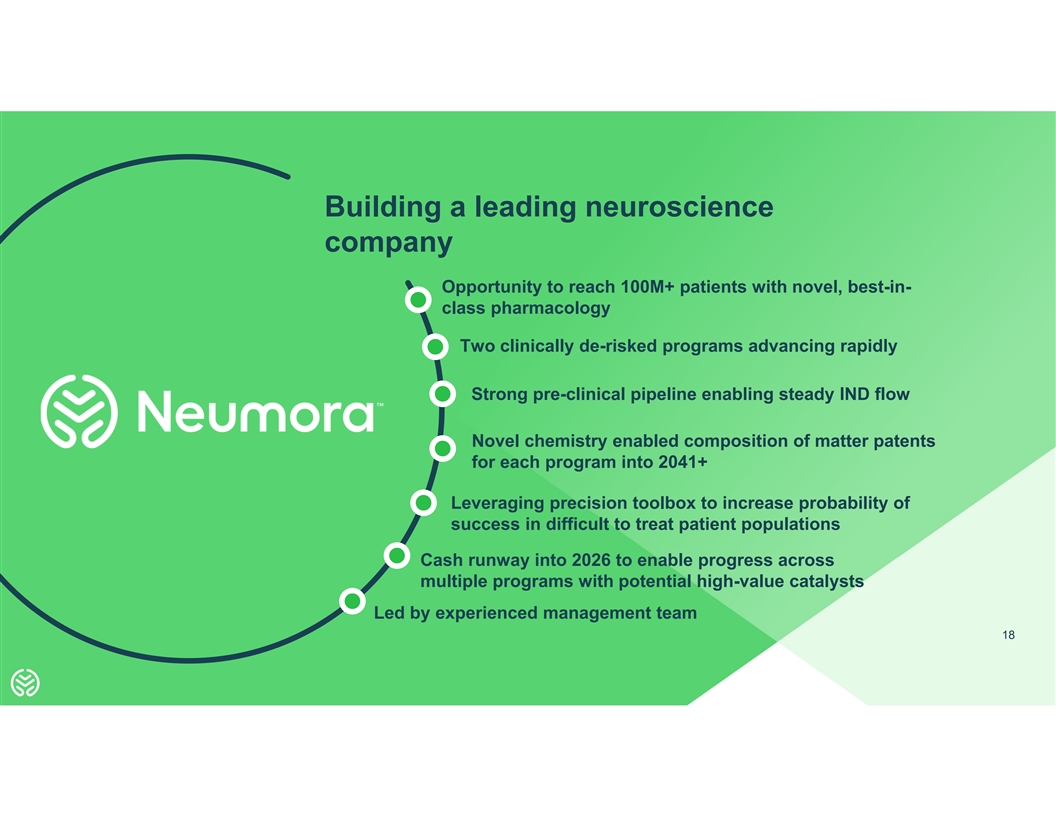
Building a leading neuroscience company Opportunity to reach 100M+
patients with novel, best-in- class pharmacology Two clinically de-risked programs advancing rapidly Strong pre-clinical pipeline enabling steady IND flow Novel chemistry enabled composition of matter patents for each program into 2041+ Leveraging
precision toolbox to increase probability of success in difficult to treat patient populations Cash runway into 2026 to enable progress across multiple programs with potential high-value catalysts Led by experienced management team 18

Appendix 19

Led by Experienced Company Builders and Leading Neuroscience Drug
Developers Leadership Board of Directors Paul L. Berns Henry Gosebruch Carol Suh Paul L. Berns Co-Founder and Executive Chairman Chief Executive Officer Chief Operating Officer and Co-Founder Co-Founder, Executive Chair Henry Gosebruch President
& Chief Executive Officer Rob Lenz, MD, Ph.D. Kristina Burrow Joshua Pinto, Ph.D. Bill Aurora, Pharm.D. Head of Research & Development Managing Director, ARCH Venture Chief Financial Officer Chief Strategy Officer Partners Matthew K. Fust
Biotechnology Advisor Alaa Halawa Nick Brandon, Ph.D. Mary Chamberlain-Tharp, Ph.D. Michael Gold, MD Executive Director, Mubadala Capital Chief Medical Officer Chief Scientific Officer Chief Business Officer Maykin Ho, Ph.D. Retired Partner, Goldman
Sachs David Piacquad Raj Manchanda, Ph.D. Biotechnology Advisor Jason Duncan Lori Houle Chief Technical Operations Officer Chief Legal Officer Chief Quality Officer Amy Sullivan SVP, Human Resources 20

Navacaprant: Demonstrated Efficacy Across Broad Range of Treatment
Outcome Measures in Moderate-to-Severe Population Week 4 Difference Week 8 Difference (p-value) (p-value) Depressive Symptom Improvement -3.0 -2.8 HAMD-17 Total Score Change from Baseline (0.015) (0.037) 21.4% 25.9% HAMD-17 Response Rate %
≥50% Reduction in HAMD-17 from Baseline (0.010) (0.007) Remission 14.9% 20.3% HAMD-17 Score ≤7 (0.014) (0.005) -2.4 -1.9 HAMD-6 Score (Core Symptoms) Change from Baseline in HAMD-6 (<0.001) (0.013) CGI-I 12.4% 19.0% % of Patients with
Very Much / Much Improvement (0.178) (0.056) -0.5 CGI-S NA Change from Baseline (0.041) Anhedonia Symptom Improvement SHAPS Total Score -2.4 -4.8 Change from Baseline (0.071) (<0.001) Anxiety Symptom Improvement HAM-A Total Score -2.4 -1.6 Change
from Baseline (0.035) (0.197) Functional Improvement SDS Total Score -2.5 -4.0 Change from Baseline (0.146) (0.013) Note: Prespecified statistical sensitivity analysis for moderate-to-severe patients (HAMD-17 > 22) 21
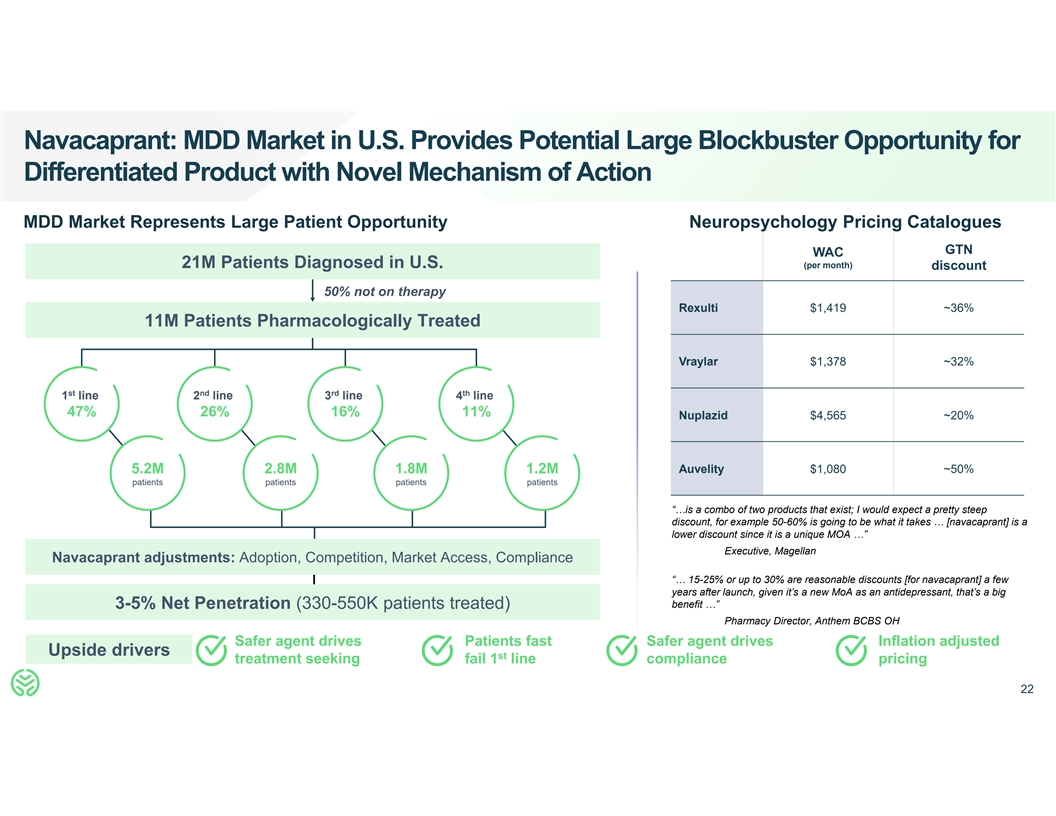
Navacaprant: MDD Market in U.S. Provides Potential Large Blockbuster
Opportunity for Differentiated Product with Novel Mechanism of Action MDD Market Represents Large Patient Opportunity Neuropsychology Pricing Catalogues GTN WAC 21M Patients Diagnosed in U.S. (per month) discount 50% not on therapy Rexulti $1,419
~36% 11M Patients Pharmacologically Treated Vraylar $1,378 ~32% st nd rd th 1 line 2 line 3 line 4 line 47% 26% 16% 11% Nuplazid $4,565 ~20% 5.2M 2.8M 1.8M 1.2M Auvelity $1,080 ~50% patients patients patients patients “…is a combo of two
products that exist; I would expect a pretty steep discount, for example 50-60% is going to be what it takes … [navacaprant] is a lower discount since it is a unique MOA …” Executive, Magellan Navacaprant adjustments: Adoption,
Competition, Market Access, Compliance “… 15-25% or up to 30% are reasonable discounts [for navacaprant] a few years after launch, given it’s a new MoA as an antidepressant, that’s a big benefit …” 3-5% Net
Penetration (330-550K patients treated) Pharmacy Director, Anthem BCBS OH Safer agent drives Patients fast Safer agent drives Inflation adjusted Upside drivers st treatment seeking fail 1 line compliance pricing 22

Strong Rationale for Efficacy in Bipolar Depression Navacaprant in
Bipolar Depression (BPD): • Evidence from the navacaprant Phase 2 and NIMH’s FASTMAS demonstrate utility of KOR pharmacology for depression and 1 anhedonia • Anhedonia is a highly prevalent and clinically relevant symptom in BPD
• A growing body of research supports the pathophysiologic 2 underpinnings of anhedonia in BPD • Given that navacaprant studies have demonstrated meaningful improvements in anhedonia symptoms in patients with MDD, it may also be
effective in treating anhedonia related to BPD • The primary endpoint for evaluating efficacy in BPD is MADRS • Currently approved therapies (e.g., atypical antipsychotics) have significant limitations 1 2 Krystal, AD., et al. 2020.
Whitton AE., et al. 2023.
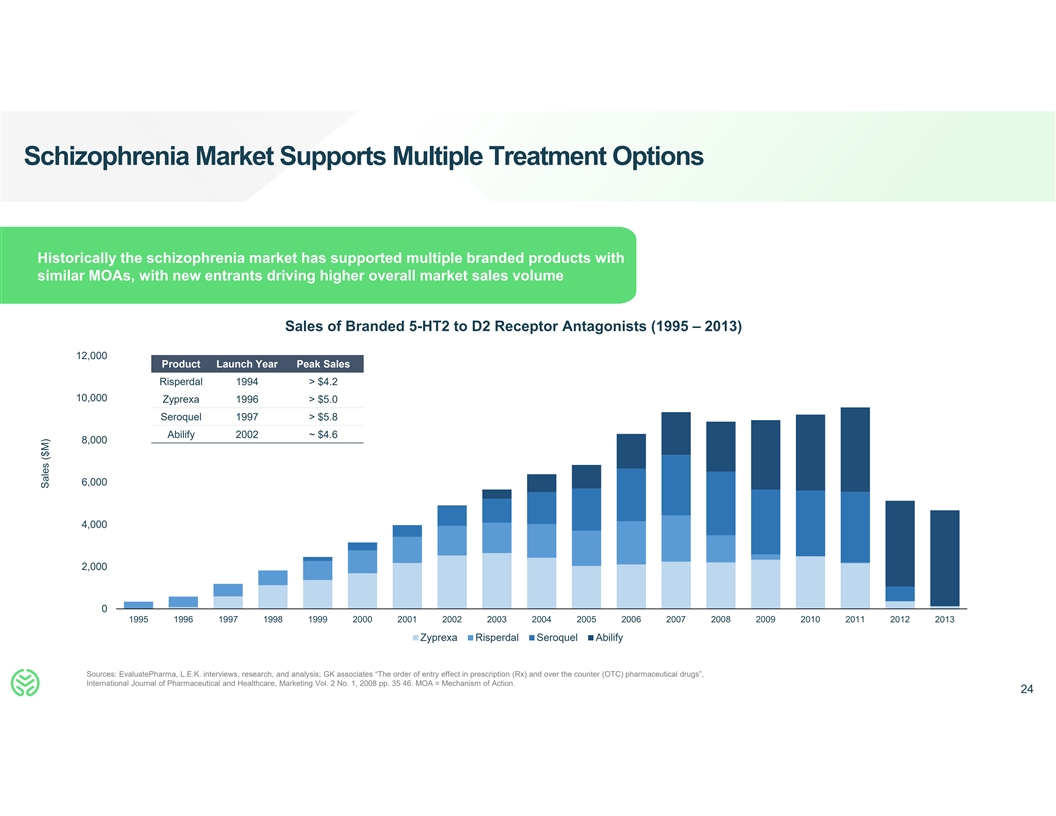
Schizophrenia Market Supports Multiple Treatment Options Historically
the schizophrenia market has supported multiple branded products with similar MOAs, with new entrants driving higher overall market sales volume Sales of Branded 5-HT2 to D2 Receptor Antagonists (1995 – 2013) 12,000 Product Launch Year Peak
Sales Risperdal 1994 > $4.2 10,000 Zyprexa 1996 > $5.0 Seroquel 1997 > $5.8 Abilify 2002 ~ $4.6 8,000 6,000 4,000 2,000 0 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 Zyprexa Risperdal
Seroquel Abilify Sources: EvaluatePharma, L.E.K. interviews, research, and analysis; GK associates “The order of entry effect in prescription (Rx) and over the counter (OTC) pharmaceutical drugs”, International Journal of Pharmaceutical
and Healthcare, Marketing Vol. 2 No. 1, 2008 pp. 35 46. MOA = Mechanism of Action. 24 Sales ($M)

NMRA-511 Reduces Threat Behaviors in Marmosets and Alters EEG Power
Spectra Similarly in Marmosets and Humans In a human threat test (HTT) study completed in marmosets, NMRA-511 demonstrated activity in brain circuits that regulate mood and anxiety. An additional EEG study demonstrated that the pharmacodynamic
effects of NMRA-511 translated to humans. NMRA-511 (10 and 30 mg/kg) and chlordiazepoxide (CDP, Analysis of qEEG collected in the frontal region following 2mg/kg, SC) significantly reduced anxiety-related behaviors dosing of NMRA-511 to marmosets
(10 mg/kg; n=6) and in marmosets (n=8) as measured by a decrease in the healthy human subjects (15 mg; placebo n=11; NMRA-511 number of threat-elicited postures observed in the HTT n=6) increased relative power in the theta and alpha bands without
affecting locomotor activity or causing sedation under physiological/resting state (eyes open) conditions Wallace TL. et al, NMRA-511, a novel vasopressin 1a (V1a) receptor antagonist reduces threat behaviors in marmosets and alters EEG power
spectra similarly in marmosets and humans. Presented at the American College of Neuropsychopharmacology 2022 Annual Congress. 25
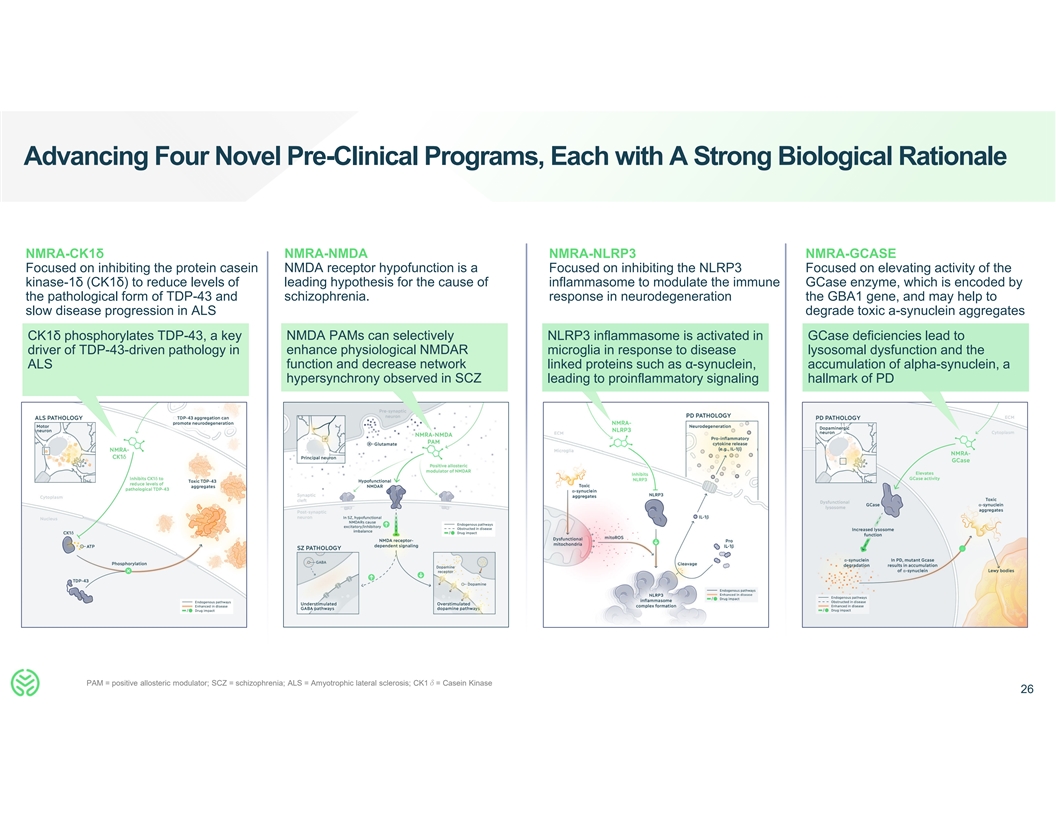
Advancing Four Novel Pre-Clinical Programs, Each with A Strong
Biological Rationale NMRA-CK1δ NMRA-NMDA NMRA-NLRP3 NMRA-GCASE Focused on inhibiting the protein casein NMDA receptor hypofunction is a Focused on inhibiting the NLRP3 Focused on elevating activity of the kinase-1δ (CK1δ) to reduce
levels of leading hypothesis for the cause of inflammasome to modulate the immune GCase enzyme, which is encoded by the pathological form of TDP-43 and schizophrenia. response in neurodegeneration the GBA1 gene, and may help to slow disease
progression in ALS degrade toxic a-synuclein aggregates CK1δ phosphorylates TDP-43, a key NMDA PAMs can selectively NLRP3 inflammasome is activated in GCase deficiencies lead to driver of TDP-43-driven pathology in enhance physiological NMDAR
microglia in response to disease lysosomal dysfunction and the function and decrease network ALS linked proteins such as α-synuclein, accumulation of alpha-synuclein, a hypersynchrony observed in SCZ leading to proinflammatory signaling
hallmark of PD PAM = positive allosteric modulator; SCZ = schizophrenia; ALS = Amyotrophic lateral sclerosis; CK1δ= Casein Kinase 26

Neumora’s Precision Medicine Approach Can Be Leveraged to
Maximize the Value of Our Programs Challenge: Match Right Neumora’s Maximize Value: Improve Drug to the Right Patient Precision Toolbox Probability of Success & Proprietary analytical capabilities with Expand Indications one petabyte of
data onboarded • How do we gain further • Gain confidence in target and/or Molecular, Translational, confidence in a selected target? indication and Clinical Tools • How do we identify indications for • Characterize more
homogeneous, (e.g., genomics, proteomics, EEG, a given target? targeted patient populations Imaging, Digital, Clinical measures) • How do we identify likely • Inform inclusion / exclusion criteria responders / treatment non- Multimodal
Methods • Increase indication expansion responders? (e.g., AI/ML, analytic capabilities) opportunities • Identify placebo responders Longitudinal, Multi-modal patient • Identify biomarkers datasets (includes multiple disorders)
Exclusive partnership with deCODE Genetics (through Amgen relationship) Neumora’s precision toolbox provides a key competitive advantage in our development approach 27

28 28
v3.23.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Neumora Therapeutics (NASDAQ:NMRA)
Gráfica de Acción Histórica
De Oct 2024 a Nov 2024

Neumora Therapeutics (NASDAQ:NMRA)
Gráfica de Acción Histórica
De Nov 2023 a Nov 2024




