AVITA Medical Receives Best Product Award at the American Association for the Surgery of Trauma
25 Septiembre 2023 - 7:00AM

AVITA Medical, Inc. (NASDAQ: RCEL, ASX: AVH), a regenerative
medicine company leading the development and commercialization of
first-in-class devices and autologous cellular therapies for skin
restoration, is pleased to announce that the RECELL® System won
Best Product for the second year in a row at the Annual Meeting of
the American Association for the Surgery of Trauma (AAST). In its
82nd year, the annual meeting attracts over 700 physicians and
scientists focused on the investigation and treatment of various
forms of trauma and the application of surgical critical care.
Dr. Carmen Flores, Assistant Professor of Surgery at the Kirk
Kerkorian School of Medicine at University of Nevada, Las Vegas,
presented “RECELL® Spray-On™ Skin Cells: A New Option for Treating
Full-Thickness Defects” in a Product Theater symposium on the first
day of the conference, with the room filled to capacity.
In addition to the well-attended Product Theater and Best
Product award, the results of AVITA Medical’s pivotal trial for
full-thickness skin defects were presented by Dr. Sharon Henry,
Professor of Surgery and Director, Division of Wound Healing and
Metabolism at the R Adams Cowley Shock Trauma Center at University
of Maryland Medical Center. Results of the pivotal trial were
recently accepted for publication in AAST’s Journal of Trauma and
Acute Care Surgery. To view the abstract and download a copy of the
publication, click here.
In coordination with the AAST Diversity, Equity and Inclusion
Committee, Dr. Henry also led a workshop for local high school
students. The workshop is intended to provide high school students
with exposure to trauma surgery as a career path. Along with three
other companies, AVITA Medical proudly supported this workshop with
personnel and supplies, including RECELL.
About AVITA Medical, Inc.AVITA Medical® is a
regenerative medicine company leading the development and
commercialization of devices and autologous cellular therapies for
skin restoration. The RECELL® System technology platform, approved
by the Food and Drug Administration for the treatment of thermal
burn wounds and full-thickness skin defects and for repigmentation
of stable depigmented vitiligo lesions, harnesses the regenerative
properties of a patient’s own skin to create Spray-On Skin™ cells.
Delivered at the point-of-care, RECELL enables improved clinical
outcomes. RECELL is the catalyst of a new treatment paradigm and
AVITA Medical is leveraging its proven and differentiated
capabilities to develop first-in-class cellular therapies for
multiple indications.
In international markets, our products are approved under the
RECELL System brand to promote skin healing in a wide range of
applications including burns, soft tissue repair, vitiligo, and
aesthetics. The RECELL System is TGA-registered in Australia,
received CE-mark approval in Europe and has PMDA approval in
Japan.
To learn more, visit www.avitamedical.com.
Investor & Media Contact:Jessica
EkebergPhone +1-661-904-9269
investor@avitamedical.commedia@avitamedical.com
Authorized for release by the Chief Financial Officer of AVITA
Medical, Inc.
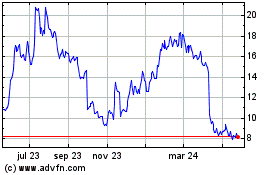
Avita Medical (NASDAQ:RCEL)
Gráfica de Acción Histórica
De Dic 2024 a Ene 2025
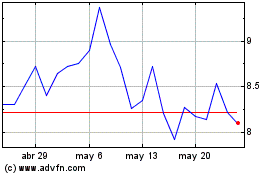
Avita Medical (NASDAQ:RCEL)
Gráfica de Acción Histórica
De Ene 2024 a Ene 2025