AVITA Medical Announces FDA Approval of RECELL GO mini, Optimizing Treatment for Smaller Wounds
23 Diciembre 2024 - 3:02PM

AVITA Medical, Inc. (NASDAQ: RCEL, ASX: AVH), a commercial-stage
regenerative medicine company focused on first-in-class devices for
wound care management and skin restoration, today announced that
the U.S. Food and Drug Administration (FDA) has approved its
premarket approval (PMA) supplement for RECELL GO® mini. As a line
extension of the RECELL GO system, the RECELL GO mini disposable
cartridge is designed specifically to treat smaller wounds up to
480 square centimeters, compared to the standard RECELL GO
disposable cartridge, which treats an area of 1,920 square
centimeters.
RECELL GO mini addresses a critical need in the full-thickness
skin defect market, which includes a high volume of smaller wounds.
As part of the RECELL GO platform, RECELL GO mini uses the same
multi-use processing device as the standard disposable cartridge
but features a modified cartridge optimized for smaller skin
samples that reduces resource use and minimizes waste. This design
provides an entry point for clinicians who may not have previously
used the RECELL GO platform for smaller wounds, enabling broader
accessibility and use in trauma and burn centers.
“The FDA approval of RECELL GO mini strengthens our ability to
provide clinicians with fit-for-purpose solutions that meet the
diverse needs of patients with full-thickness wounds,” said Jim
Corbett, Chief Executive Officer of AVITA Medical. “By introducing
a treatment option specifically for smaller wounds, we are
expanding the accessibility of RECELL to a wider range of patients.
We believe this addition will drive greater adoption across trauma
centers, where smaller wounds are common, and support our broader
growth strategy.”
The company expects RECELL GO mini to serve as a growth driver
within the broader RECELL GO platform, further advancing AVITA
Medical’s strategy to expand its impact on patient care. Rollout
will begin with trauma and burn centers that currently treat
smaller wounds during the first quarter of 2025.
The PMA supplement follows the original PMA of RECELL Autologous
Cell Harvesting Device and subsequent PMA supplements.
About AVITA Medical, Inc.AVITA Medical is a
commercial-stage regenerative medicine company transforming the
standard of care in wound care management and skin restoration with
innovative devices. At the forefront of our platform is the RECELL
System, approved by the FDA for the treatment of thermal burn
wounds and full-thickness skin defects, and for repigmentation of
stable depigmented vitiligo lesions. RECELL harnesses the
regenerative properties of a patient’s own skin to create Spray-On
Skin™ Cells, delivering a transformative solution at the
point-of-care. This breakthrough technology serves as the catalyst
for a new treatment paradigm enabling improved clinical outcomes.
In the United States, AVITA Medical also holds the exclusive rights
to market, sell, and distribute PermeaDerm®, a biosynthetic wound
matrix, and Cohealyx, an AVITA Medical-branded collagen-based
dermal matrix.
In international markets, the RECELL System is approved to
promote skin healing in a wide range of applications including
burns, full-thickness skin defects, and vitiligo. The RECELL
System, excluding RECELL GO™, is TGA-registered in Australia, has
received CE mark approval in Europe, and has PMDA approval in
Japan.
To learn more, visit www.avitamedical.com.
Forward-Looking StatementsThis press release
may contain forward-looking statements within the meaning of the
Private Securities Litigation Reform Act of 1995. Such
forward-looking statements are subject to significant risks and
uncertainties that could cause actual results to differ materially
from those expressed or implied by such statements. Forward-looking
statements generally may be identified by the use of words such as
“anticipate,” “expect,” “intend,” “could,” “would,” “may,” “will,”
“believe,” “continue,” “estimate,” “look forward,” “forecast,”
“goal,” “target,” “project,” “outlook,” “guidance,” “future,” and
similar words or expressions, and the use of future dates.
Forward-looking statements include, but are not limited to,
statements relating to the timing and realization of regulatory
approvals of our products; physician acceptance, endorsement, and
use of our products; anticipated market share growth and revenue
generation from certain products; failure to achieve the
anticipated benefits from approval of our products; the effect of
regulatory actions; product liability claims; risks associated with
international operations and expansion; and other business effects,
including the effects of industry, as well as other economic or
political conditions outside of the Company’s control. These
statements are made as of the date of this release, and the Company
undertakes no obligation to publicly update or revise any of these
statements, except as required by law. For additional information
and other important factors that may cause actual results to differ
materially from forward-looking statements, please see the “Risk
Factors” section of the Company’s latest Annual Report on Form 10-K
and other publicly available filings for a discussion of these and
other risks and uncertainties.
Authorized for release by the Chief Financial Officer of AVITA
Medical, Inc.
A photo accompanying this announcement is available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/b6b7df71-e67c-4a6e-847c-bdcca54fad27
Investor & Media Contact:
Jessica Ekeberg
Phone +1-661-904-9269
investor@avitamedical.com
media@avitamedical.com
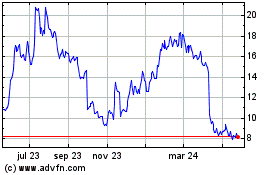
Avita Medical (NASDAQ:RCEL)
Gráfica de Acción Histórica
De Dic 2024 a Ene 2025
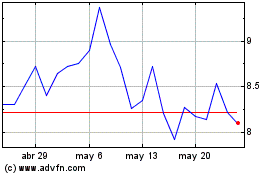
Avita Medical (NASDAQ:RCEL)
Gráfica de Acción Histórica
De Ene 2024 a Ene 2025