Filed Pursuant to Rule 253(g)(3)
Registration No. 024-12477
OFFERING CIRCULAR

22nd Century
Group, Inc.
321
Farmington Road
Mocksville, North
Carolina 27028
(716) 270-1523
37,500,000 Shares of Common Stock
By this offering circular (the
“Offering Circular”), 22nd Century Group, Inc., a Nevada corporation, is offering on a
“best-efforts” basis a maximum of 37,500,000 shares of its common stock, par value $0.0001 per share (the
“Offered Shares”), at a fixed price of $0.57 per share, pursuant to
Tier 2 of Regulation A of the United States Securities and Exchange Commission (the “SEC”). There is no minimum purchase
requirement for investors in this offering.
This offering is being conducted on a “best-efforts”
basis, which means that there is no minimum number of Offered Shares that must be sold by us for this offering to close; thus, we may
receive no or minimal proceeds from this offering. None of the proceeds received will be placed in an escrow or trust account. All proceeds
from this offering will become immediately available to us and may be used as they are accepted. Purchasers of the Offered Shares will
not be entitled to a refund and could lose their entire investments. Please see the “Risk Factors” section, beginning on
page 6, for a discussion of the risks associated with a purchase of the Offered Shares.
We estimate that this offering will commence
within two days of SEC qualification; this offering will terminate at the earliest of (a) the date on which the maximum offering
has been sold, (b) one year from the date of SEC qualification, or (c) the date on which this offering is earlier terminated
by us, in our sole discretion. (See “Plan of Distribution”).
| | |
Number of
Shares | | |
Price
to Public(1) | | |
Underwriting
Discounts and Commissions(2) | | |
Proceeds
to Company(3) | |
| Per Share | |
| - | | |
$ | 0.57 | | |
$ | - | | |
$ | 0.57 | |
| Total Minimum | |
| - | | |
$ | - | | |
$ | - | | |
$ | - | |
| Total Maximum | |
| 37,500,000 | | |
$ | 21,375,000 | | |
$ | - | | |
$ | 21,375,000 | |
| (1) |
We may also offer the Offered Shares through registered broker-dealers and we may pay finders. However, information as to any such broker-dealer or finder shall be disclosed in an amendment to this Offering Circular. |
| |
|
| (2) |
Does not account for the payment of expenses of this offering estimated at $42,500. See “Plan of Distribution.” |
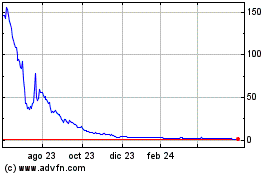
Our common stock is listed on The Nasdaq Capital
Market (“Nasdaq”), under the symbol “XXII.” On August 15, 2024, the last reported sale price of our common
stock was $0.5852 per share.
Investing in the Offered Shares is speculative
and involves substantial risks. You should purchase Offered Shares only if you can afford a complete loss of your investment. See “Risk
Factors”, beginning on page 6, for a discussion of certain risks that you should consider before purchasing any of the Offered
Shares.
THE UNITED STATES SECURITIES AND EXCHANGE
COMMISSION DOES NOT PASS UPON THE MERITS OF, OR GIVE ITS APPROVAL TO, ANY SECURITIES OFFERED OR THE TERMS OF THE OFFERING, NOR DOES IT
PASS UPON THE ACCURACY OR COMPLETENESS OF ANY OFFERING CIRCULAR OR OTHER SOLICITATION MATERIALS. THESE SECURITIES ARE OFFERED PURSUANT
TO AN EXEMPTION FROM REGISTRATION WITH THE COMMISSION; HOWEVER, THE COMMISSION HAS NOT MADE AN INDEPENDENT DETERMINATION THAT THE SECURITIES
OFFERED ARE EXEMPT FROM REGISTRATION.
The use of projections or forecasts in this
offering is prohibited. No person is permitted to make any oral or written predictions about the benefits you will receive from an investment
in Offered Shares.
No sale may be made to you in this
offering, if you do not satisfy the investor suitability standards described in this Offering Circular under “Plan of
Distribution—State Law Exemption and Offerings to “Qualified Purchasers” on page 23. Before making any
representation that you satisfy the established investor suitability standards, we encourage you to review
Rule 251(d)(2)(i)(C) of Regulation A. For general information on investing, we encourage you to refer to www.investor.gov.
This Offering Circular follows the disclosure
format of Form S-1, pursuant to the General Instructions of Part II(a)(1)(ii) of Form 1-A.
The date of this Offering Circular is August
16, 2024.
TABLE OF CONTENTS
CAUTIONARY STATEMENT REGARDING
FORWARD-LOOKING STATEMENTS
The information contained
in this Offering Circular includes some statements that are not historical and that are considered forward-looking statements. Such forward-looking
statements include, but are not limited to, statements regarding our development plans for our business; our strategies and business
outlook; anticipated development of our company; and various other matters (including contingent liabilities and obligations and changes
in accounting policies, standards and interpretations). These forward-looking statements express our expectations, hopes, beliefs and
intentions regarding the future. In addition, without limiting the foregoing, any statements that refer to projections, forecasts or
other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. The
words “anticipates,” “believes,” “continue,” “could,” “estimates,” “expects,”
“intends,” “may,” “might,” “plans,” “possible,” “potential,”
“predicts,” “projects,” “seeks,” “should,” “will,” “would” and
similar expressions and variations, or comparable terminology, or the negatives of any of the foregoing, may identify forward-looking
statements, but the absence of these words does not mean that a statement is not forward-looking.
The forward-looking statements
contained in this Offering Circular are based on current expectations and beliefs concerning future developments that are difficult to
predict. We cannot guarantee future performance, or that future developments affecting our company will be as currently anticipated.
These forward-looking statements involve a number of risks, uncertainties (some of which are beyond our control) or other assumptions
that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements.
All forward-looking statements
attributable to us are expressly qualified in their entirety by these risks and uncertainties. These risks and uncertainties, along with
others, are also described below in the section entitled “Risk Factors”. Should one or more of these risks or uncertainties
materialize, or should any of our assumptions prove incorrect, actual results may vary in material respects from those projected in these
forward-looking statements. You should not place undue reliance on any forward-looking statements and should not make an investment decision
based solely on these forward-looking statements. We undertake no obligation to update or revise any forward-looking statements, whether
as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
OFFERING CIRCULAR SUMMARY
The following summary highlights
material information contained in this Offering Circular. This summary does not contain all of the information you should consider before
purchasing our common stock. Before making an investment decision, you should read this Offering Circular carefully, including the section
entitled “Risk Factors” and the consolidated financial statements and the notes thereto. 22nd Century Group, Inc.
and its consolidated subsidiaries are referred to herein as “XXII,” “the Company,” “we,” “us”
and “our,” unless the context indicates otherwise.
Overview
22nd
Century Group, Inc. is a tobacco products company with sales and distribution of our own proprietary new reduced nicotine tobacco
products authorized as Modified Risk Tobacco Products by the FDA. Additionally, we provide contract manufacturing services for conventional
combustible tobacco products for third-party brands.
Our
mission in tobacco is dedicated to mitigating the harms of smoking through our proprietary reduced nicotine content (“RNC”)
tobacco plants and our Very Low Nicotine, VLN® combustible cigarette products. In December 2021, we secured the first and only
authorization from the FDA to market a combustible cigarette, our brand VLN® as a Modified Risk Tobacco Product (“MRTP”)
using certain reduced nicotine exposure claims. In April 2022, the inaugural launch of our proprietary VLN® cigarettes commenced
through a pilot program in select Circle K stores in and around Chicago, Illinois. Building on the success of the pilot, we initiated
a phased rollout strategy in 2023, progressing state by state and region by region to a store footprint spanning more than 5,000 stores
in 26 states. Our VLN® tobacco products are supported by a substantial intellectual property portfolio comprising issued patents
and patent applications related to tobacco plants, and in particular our reduced nicotine tobacco plants.
In
addition to continued focus on VLN®, we renewed our focus on utilizing our tobacco assets to attract additional tobacco business
to help fund the growth of VLN®. In addition to existing business relationships with multiple tobacco products companies, we will
continue to expand the number of brands in our contract manufacturing operations (“CMO”) portfolio in 2024.
Our Corporate Information
We are a Nevada corporation
and our corporate headquarters is located at 321 Farmington Road, Mocksville, North Carolina 27028. Our telephone number is (716) 270-1523.
Our internet address is www.xxiicentury.com. We do not incorporate the information on our website into this Offering Circular, and you
should not consider it to be a part of this Offering Circular. Our web site address is included as an inactive textual reference only.
Offering Summary
| Securities Offered |
37,500,000 shares of common stock, are being offered by the Company in a
“best-efforts” offering. |
| |
|
| Offering Price Per Share |
A price of $0.57 per
Offered Share |
| |
|
| Shares Outstanding Before This Offering* |
9,272,518 shares of common
stock issued and outstanding as of August 15, 2024. |
| |
|
| Shares Outstanding After This Offering* |
46,772,518 shares of common
stock issued and outstanding, assuming all of the Offered Shares are sold hereunder. The number of shares to be outstanding after
this offering is based on 9,272,518 shares outstanding as of August 15, 2024. |
| |
|
| Minimum Number of Shares to Be
Sold in This Offering |
None |
| |
|
| Investor Suitability Standards |
The Offered Shares are
being offered and sold to “qualified purchasers” (as defined in Regulation A under the Securities Act of 1933, as amended
(the “Securities Act”). “Qualified purchasers” include any person to whom securities are offered or sold
in a Tier 2 offering pursuant to Regulation A under the Securities Act. |
| |
|
| Market for our Common Stock |
Our common stock is listed
on Nasdaq under the symbol “XXII.” |
| |
|
| Termination of this Offering |
This offering will terminate
at the earliest of (a) the date on which all of the Offered Shares have been sold, (b) the date which is one year from
this offering being qualified by the SEC and (c) the date on which this offering is earlier terminated by us, in our sole discretion.
(See “Plan of Distribution”). |
| |
|
| Use of Proceeds |
We will use the proceeds
of this offering for marketing and advertising expenses and general corporate purposes, including working capital. See “Use
of Proceeds”. |
| |
|
| Continuing Reporting Requirements Under Regulation
A |
We are required to file periodic and
other reports with the SEC, pursuant to the requirements of Section 13(a) of the Exchange Act. Our continuing reporting
obligations under Regulation A are deemed to be satisfied as long as we comply with our Section 13(a) reporting requirements.
|
| Risk Factors |
An investment in the Offered Shares involves
a high degree of risk and should not be purchased by investors who cannot afford the loss of their entire investments. You should
carefully consider the information included in the Risk Factors section of this Offering Circular, as well as the other information
contained in this Offering Circular, prior to making an investment decision regarding the Offered Shares. |
*Shares of common stock outstanding as of August 15,
2024 excludes the following:
| · | 6,605
shares of common stock issuable upon the exercise of outstanding stock options with a weighted-average
exercise price of $358.81 per share: |
| · | 1,824
shares of common stock issuable upon vesting of restricted stock unit awards; |
| · | 5,707,584
shares of common stock reserved for future issuance under our 2021 Omnibus Incentive Plan; |
| · | 6,361,600
shares of common stock issuable upon exercise of warrants to purchase common stock with a
weighted-average exercise price of $2.70 per share; and |
| · | 1,150,000
shares of common stock issuable upon exercise of pre-funded warrants to purchase common stock. |
Continuing Reporting Requirements Under Regulation A
We are required to file periodic and other reports with the SEC, pursuant to the requirements of Section 13(a) of the Exchange Act. Our
continuing reporting obligations under Regulation A are deemed to be satisfied as long as we comply with our Section 13(a) reporting requirements.
RISK
FACTORS
An investment in the Offered
Shares involves substantial risks. You should carefully consider the following risk factors, in addition to the other information contained
in this Offering Circular, before purchasing any of the Offered Shares. The occurrence of any of the following risks might cause you
to lose a significant part of your investment. The risks and uncertainties discussed below are not the only ones we face, but do represent
those risks and uncertainties that we believe are most significant to our business, operating results, prospects and financial condition.
Some statements in this Offering Circular, including statements in the following risk factors, constitute forward-looking statements.
See “Cautionary Statement Regarding Forward-Looking Statements”.
Risks Related to
Our Business and Continued Operations
We have a history
of losses, and we expect to incur significant expenses and continuing losses for the foreseeable future and there is substantial doubt
regarding our ability to continue as a going concern.
We have incurred significant
losses and negative cash flows from operations since inception and expect to incur additional losses until such time that we can generate
significant revenue and profit in our tobacco business, which casts substantial doubt regarding our ability to continue as a going concern.
As of August 15, 2024, we had cash and cash equivalents of approximately $1.0 million.
Doubts about our ability
to continue as a going concern have and could continue to negatively impact our relationships with our commercial partners and our ability,
as part of our cost-cutting measures, to obtain, maintain, restructure and/or terminate agreements with them, or negatively impact our
negotiating leverage with such parties, which could have a material adverse effect on our business, financial condition and results of
operations or result in litigation. Furthermore, any loss of key personnel, employee attrition or material erosion of employee morale
arising out of doubts about our ability to operate as a going concern could have a material adverse effect on our ability to effectively
conduct our business, and could impair our ability to execute our business plan, thereby having a material adverse effect on our business,
financial condition and results of operations.
We need additional funding
to execute our business plan and to continue operations even with the anticipated proceeds from this offering. We continue to seek and
evaluate opportunities to raise additional funds through the issuance of our securities, asset sales, and through arrangements with strategic
partners. If capital is not available to us when, and in the amounts needed, we could be required to liquidate our inventory, cease or
curtail operations, or seek protection under applicable bankruptcy laws or similar state proceedings. There can be no assurance that
we will be able to raise the capital we need to continue our operations.
We may be unable
to comply with the covenants in our senior secured debentures.
Our
senior secured debentures contain customary representations, warranties and covenants including among other things and subject to certain
exceptions, covenants that restrict us from incurring additional indebtedness, creating or permitting liens on assets, making or holding
any investments, repaying outstanding indebtedness, paying dividends or distributions and entering into transactions with affiliates.
We are also required to maintain certain quarterly revenue targets.
As
a result of these covenants, our ability to respond to changes in business and economic conditions and engage in beneficial transactions,
including to obtain additional financing as needed, may be restricted. Furthermore, our failure to comply with the covenants could result
in a default under such agreements, which could permit the debt holders to accelerate our obligation to repay the debt. Although we recently
received a waiver with respect to our compliance with such covenants, there is no assurance that we will be able to secure a similar
waiver for the failure to comply with any future covenants. If any of our debt is accelerated, we likely would not have sufficient funds
available to repay it. Substantially all of our assets, including intellectual property, are collateralized under the debentures. If
such debt is accelerated, we could be required to liquidate our inventory, cease or curtail operations, or seek protection under applicable
bankruptcy laws or similar state proceedings.
Additionally,
the senior secured debentures may be converted into shares of the Company’s common stock. If the senior secured debentures are
converted into common stock in whole or in part, the existing stockholders could incur significant dilution in their relative percentage
ownership. The prospect of this possible dilution may also impact the price of our common stock.
We could continue
to incur restructuring and impairment charges as we continue to pursue a cost cutting initiative and pursue strategic alternatives.
We
continue to evaluate opportunities to optimize the cost structure of our operations in order to implement a cost savings initiative.
The actions driven from these opportunities could result in significant charges which could adversely affect our financial condition
and results of operations. Future actions could result in restructuring and related charges, including but not limited to impairments
and employee termination costs and costs associated with terminating contracts that could be significant. We have incurred significant
impairment charges for long-lived assets, including goodwill and intangible assets, which are subject to periodic impairment analysis
and review, and remain subject to the potential for additional charges. Identifying and assessing whether impairment indicators exist,
or if events or changes in circumstances have occurred, including market conditions, operating results, competition and general economic
conditions, requires significant judgment. Any of the above future actions could result in charges that could have an adverse effect
on our financial condition and results of operations. The cost-cutting initiatives have led, and may continue to lead, to legal claims
by service providers and other third-parties. Any resulting litigation could be costly and time consuming and an unfavorable outcome
could have a significant adverse effect on our business.
Our competitors generally have, and any
future competitors may have, greater financial resources and name recognition than we do, and they may therefore develop products or
other technologies similar or superior to ours, or otherwise compete more successfully than we do.
We are competing with large
tobacco companies and large pharmaceutical companies that have greater resources that us. The tobacco industry consists of major domestic
and international companies, most of which have existing relationships in the markets in which we plan to sell, as well as financial,
technical, research and development, marketing, sales, manufacturing, scaling capacity, distribution, lobbying and other resources and
name recognition substantially greater than ours. In addition, we expect new competitors will enter the markets for similar tobacco products
in the future and the nature and extent of this market entrance cannot be quantified at this time.
Potential customers may choose
to do business with more established competitors because of their perception that our competitors are more stable, can scale operations
more quickly, have greater manufacturing capacity, have robust marketing and sale programs and lend greater credibility to governmental
regulators and others. In addition, large companies have the ability to provide entry-level pricing for premium products in order make
us less competitive. If we are unable to compete successfully against larger companies with more financial resources and name recognition,
our business and prospects would be materially adversely affected.
Our
competitors may develop products that are less expensive, safer or otherwise more appealing, which may diminish or eliminate the commercial
success of our VLN® cigarettes or any other potential products that we may commercialize.
If our competitors develop
very low nicotine tobacco without infringing on our intellectual property or other products that are less expensive, safer or otherwise
more appealing than our RNC cigarettes or any of our other potential products, or that reach the market before ours, we may not achieve
commercial success. Currently, there are numerous companies developing products for which they may submit MRTPAs, working to develop
low nicotine tobacco and other tobacco alternative products to provide products that are potentially safer for human consumption or to
otherwise assist consumers to cease or begin to switch from smoking. If one of such competitors develops a cigarette that is safe for
human consumption, a safer alternative for nicotine that is widely accepted, superior low nicotine tobacco or otherwise develops a superior
quitting method, it could render our RNC tobacco and cigarettes obsolete, which would have a material adverse impact on our business
and operations and our ability to achieve profitability.
Our competitors may render
our technologies obsolete by advances in existing technological approaches or the development of new or different approaches, potentially
eliminating the advantages that we believe we derive from our research approach and proprietary technologies.
Our competitors may:
| · | develop
and market similar or new products that are less expensive, safer, or otherwise more appealing
than our products; |
| · | develop
similar or new technologies and products that render our products obsolete; |
| · | operate
larger research and development programs or have substantially greater financial resources
than we do; |
| · | have
greater success in recruiting skilled technical and scientific workers from the limited pool
of available talent; |
| · | more
effectively negotiate third-party licenses and strategic relationships; |
| · | commercialize
competing products before we or our partners can launch our products; |
| · | be
more effective in marketing and creating brand awareness of their products that we are; |
| · | develop
tobacco with superior traits to ours; |
| · | initiate
or withstand substantial price competition more successfully than we can; and/or |
| · | take
advantage of acquisition or other opportunities more readily than we can. |
Our research and development process may
not develop marketable products cost-effectively or at all, which would result in loss of our investment into such process.
We do not know whether our
research and development process will result in marketable products. Even if we develop marketable products, we may not be able to obtain
the necessary marketing authorizations for these potential products or our anticipated time of bringing these potential products to the
market may be substantially delayed. The development of new products is costly, time-consuming, and has no guarantee of success. Any
such delays or the inability to effectively develop new products in a cost-effective manner, or at all, would have a material adverse
effect on our business and a loss of our financial resources.
We have in the past invested in other companies
and may do so in the future, which may divert our management’s attention, result in additional dilution to our stockholders, and
consume resources that are necessary to sustain our business or result in losses.
We may acquire or invest
in complementary solutions, services, technologies, or businesses in the future. We may also enter into relationships with other businesses
to expand our intellectual property portfolio, which could involve preferred or exclusive licenses or investments in other companies.
Negotiating these transactions can be time-consuming, difficult and expensive, and our ability to complete these transactions may often
be subject to conditions or approvals that are beyond our control. Consequently, these transactions, even if undertaken and announced,
may not close or may not yield the benefits that we expect. Many of our acquisitions in the past have not yielded the results or synergies
that we anticipated. In addition, we may only be able to conduct limited due diligence on an acquired company’s operations. Following
an acquisition, we may be subject to liabilities arising from an acquired company’s past or present operations and these liabilities
may be greater than the warranty and indemnity limitations that we negotiate. Any liability that is greater than these warranty and indemnity
limitations could have a negative impact on our financial condition.
Acquisitions may also disrupt
our business, divert our resources, and require significant management attention that would otherwise be available for the development
of our business. Moreover, the anticipated benefits of any acquisition, investment, or business relationship may not be realized or we
may be exposed to unknown liabilities, including litigation against the companies that we may acquire.
The failure of our information systems
to function as intended or their penetration by outside parties with the intent to corrupt them could result in business disruption,
litigation and regulatory action, and loss of revenue, assets, or personal or confidential data (cybersecurity).
We use information systems
to help manage business processes, collect and interpret business data and communicate internally and externally with employees, suppliers,
customers and others. Some of these information systems are managed by third-party service providers. We have backup systems and business
continuity plans in place, and we take care to protect our systems and data from unauthorized access. However, a failure of our systems
to function as intended, or penetration of our systems by outside parties intent on extracting or corrupting information or otherwise
disrupting business processes, could interrupt our business and place us at a competitive disadvantage, result in a loss of revenue,
assets or personal or other sensitive data, litigation and regulatory action, cause damage to our reputation and that of our brands and
result in significant remediation and other costs. Any cybersecurity incident could cause substantial harm to our business and result
in regulatory action, fines, and/or substantial costs.
Business interruptions, whether caused
by natural disaster, terrorism, economic downturns, global pandemics or other events, could negatively impact our business.
A natural disaster (such
as an earthquake, hurricane, fire, or flood), pandemics, widespread power outage or internet failure or hack, or an act of terrorism
could cause substantial delays in our operations, damage or destroy our equipment or facilities, and cause us to incur additional expenses
and lose revenue. The insurance we maintain against natural disasters may not be adequate to cover our losses in any particular case,
which would require us to expend significant resources to replace any destroyed assets, thereby materially and adversely affecting our
financial condition and prospects. Other global incidents could have a similar effect of disrupting our business to the extent they reach
and impact the areas in which we operate, the availability of inventory we need, the customers we serve, the partners on whom we rely
for products or services or the employees who operate our businesses. For example, another pandemic or comparable heath concern could
disrupt our supply chain for tobacco, as well as negatively impact employee productivity, including affecting the availability of employees
reporting for work. Any business interruption caused by such unforeseen events could have a material adverse impact on our business and
operations.
Our prior operations in the hemp/cannabis
space could have a material adverse effect on our business, financial condition, and results of operations.
We previously operated in
the cannabis space. The hemp plant and the marijuana plant are both part of the same cannabis genus of plant, except that hemp, by definition,
has not more than 0.3% THC content and is legal under the federal 2018 Farm Bill and certain state laws, but the same plant with a higher
THC content is defined as marijuana, which is legal under certain state laws, is not legal under federal law. The similarities between
these plants can cause confusion, and our previous activities with legal hemp may be incorrectly perceived as us having been involved
in federally illegal marijuana. Also, despite growing support for the marijuana industry and legalization of marijuana in certain U.S.
states, many individuals and businesses remain opposed to the marijuana industry. Any negativity resulting from our prior cannabis operations
could result in a loss of current or future business. It could also adversely affect the public’s perception of us and lead to
reluctance by new parties to do business with us or to own our common stock. We cannot assure you that additional business partners,
including but not limited to financial institutions, banking institutions and customers, will not attempt to end or curtail their relationships
with us. Any such negative press or cessation of business could have a material adverse effect on our business, financial condition,
and results of operations.
Risks Related to the Tobacco Industry
We may be unsuccessful in our efforts to
commercialize our RNC tobacco using the reduced exposure claims authorized by the FDA.
While the FDA issued an exposure
modification order in connection with our MRTPA and we have been commercializing our VLN® cigarettes in select markets
across the United States, there are no guarantees regarding the commercial viability of our RNC tobacco cigarettes. To date, there has
never been a comparable product sold in the marketplace and we have only commercialized the cigarettes on a limited basis. We have obtained
an exposure modification order for our VLN® cigarettes, which enables us to make certain claims regarding the reduction
of nicotine within these products. Specifically, we are permitted to market the products with the claims “95% less nicotine,”
“helps reduce your nicotine consumption,” and “greatly reduces your nicotine consumption,” and we are required
to use the claim “helps you smoke less” in connection with the other authorized claims; we may not market our VLN cigarettes
for claims that have not been authorized pursuant to an FDA order. Although we believe these claims have the potential to increase our
product sales, these products may never achieve consumer acceptance at levels that make the product commercially viable for profitable
sales. In addition, the process of commercializing such product and creating consumer awareness could take longer and cost more than
we expect.
In addition, even if we believe
that certain legislative or regulatory changes may increase product demand, such as the proposals that FDA has historically made with
respect to requiring minimally or non-addictive levels of nicotine in all cigarettes sold in the U.S., there can be no assurance that
such regulations, if implemented, would increase or create demand for our RNC cigarettes.
The commercial success of our RNC tobacco cigarettes
will depend on a number of factors, including, but not limited to our ability to:
| · | achieve,
maintain and grow market identify of, acceptance of, and demand for, such products; |
| · | successfully
create consumer awareness of such products; |
| · | market
the product with the phrase “Helps You Smoke Less” and any other required
warnings or statements; |
| · | maintain,
manage or scale the necessary sales, marketing, manufacturing and other capabilities and
infrastructure that are required to successfully commercialize such products; |
| · | grow
or otherwise maintain an adequate supply of RNC tobacco; |
| · | maintain
and extend intellectual property protection for such products; |
| · | comply
with applicable legal and regulatory requirements, including FDA and MSA regulations or requirements
with respect to product advertising and our obligations in connection with our PMTAs and
MRTPs; |
| · | competitively
price our products; |
| · | compete
with other similar products or new technologies (if any); |
| · | obtain
cost-effective distribution outlets; and |
| · | effectively
sell our products into established markets where there is substantial market dominance by
large tobacco enterprises. |
If we are unsuccessful in
commercializing our RNC tobacco cigarettes, or such commercialization takes longer or costs more than we currently expect, our financial
results, business and future prospects would be materially adversely effected.
We have limited experience marketing and
selling Modified Exposure Cigarettes and our working capital and inventory estimates based on demand expectations may be incorrect, which
could harm our operating results and financial condition.
While members of management
and our board of directors are experienced in the selling of conventional cigarette and other consumer products, we have limited experience
in introducing a new low nicotine category for selling our VLN cigarettes pursuant to an exposure modification order. As we work to commercialize
one or more of our products for sale, including our VLN cigarettes, we base our working capital and inventory decisions on management’s
estimates of future demand. If demand for such potential new products does not increase as quickly as we have estimated, our inventory
costs, demands on working capital, expenses could increase, and our business and operating results could suffer. Alternatively, if we
experience sales that exceed our estimates, our working capital and inventory needs may be higher than those currently anticipated. Since
our RNC tobacco is not widely available and must be grown specifically for our potential products, any shortage in such tobacco could
prevent us from increasing sales to meet demand and any surplus could result in inventory obsolescence and become a total loss.
Our inability to incorrectly
estimate demand for future products could negatively harm our operating results and financial condition.
The manufacturing and sale of tobacco products
subjects us to significant governmental regulation and the failure to comply with such regulations could have a material adverse effect
on our business and subject us to substantial fines or other regulatory actions.
Companies that manufacture
and/or sell tobacco products face significant governmental regulation, especially in the United States pursuant to the Tobacco Control
Act, including but not limited to efforts aimed at reducing the incidence of tobacco use, restricting marketing and advertising, imposing
regulations on packaging, mandating warnings and disclosure of flavors or other ingredients, prohibiting the sale of tobacco products
with certain flavors or other characteristics, requiring compliance with certain environmental standards, limiting or prohibiting the
sale of tobacco products by certain retail establishments and the sale of tobacco products in certain packaging sizes, and seeking to
hold retailers and distributors responsible for the adverse health effects associated with both smoking and exposure to environmental
tobacco smoke.
The Tobacco Control Act requires
manufacturers of tobacco products to, among other things, provide the FDA with a list of ingredients added to tobacco products in the
manufacturing process and register any establishment engaged in the manufacture, preparation, or processing of a tobacco product. The
manufacture of products is subject to strict quality control, testing and record-keeping requirements, and continuing obligations regarding
the submission of safety reports and other post-market information. The Tobacco Control Act also authorizes the FDA to promulgate regulations
requiring that the methods used in, and the facilities and controls used for, the manufacture, preproduction design validation, packing,
and storage of a tobacco product conform to current good manufacturing practice (“CGMP”). On March 8, 2023, the FDA
issued a proposed rule to promulgate such CGMP regulations. The proposed rule, if finalized, would establish requirements for manufacturers
of finished and bulk tobacco products on the methods used in, and the facilities and controls used for, the manufacture, pre-production
design validation, packing, and storage of tobacco product.
We cannot guarantee that
our current manufacturing facility or any other manufacturing will successfully complete FDA inspections and/or similar inspections in
foreign, or that future CGMP regulations will not also negatively affect the cost or sustainability of our manufacturing facility. Our
failure to comply with applicable manufacturing regulations could result in sanctions being imposed on us, including fines, injunctions,
civil penalties, delays, suspension or withdrawal of marketing orders, seizures or recalls, operating restrictions and criminal prosecutions,
any of which could significantly and adversely affect our financial position. In addition, we and our customers for whom we manufacture
tobacco products also face significant governmental regulation, including efforts aimed at reducing the incidence of tobacco use. We
also cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative
action, either in the United States or abroad. Actions by the FDA and other foreign, federal, state or local governments or agencies
may impact the adult tobacco consumer acceptability of or access to tobacco products (for example, through product standards proposed
by the FDA for nicotine and flavors including menthol), delay or prevent the launch of new or modified tobacco products or products with
reduced exposure claims, require the recall or other removal of tobacco products from the marketplace, impose additional manufacturing,
labeling or packing requirements, interrupt manufacturing or otherwise significantly increase the cost of doing business. Any one or
more of these actions may have a material adverse impact on us or the business of our customers for whom we make tobacco products, which
could have a negative impact on our results of operations.
For example, the Tobacco
Control Act requires the FDA to issue new cigarette health warnings that would include a color graphic component depicting the negative
health consequences of smoking. In March 2020, the FDA published a final rule fulfilling this statutory requirement. The final
rule, entitled “Required Warnings for Cigarette Packages and Advertisements,” specifies the 11 new textual warning label
statements and accompanying color graphics that manufacturers would have to include with cigarette packaging and advertisements. On December 7,
2022, the U.S. District Court for the Eastern District of Texas vacated the final rule, and the case is currently pending before the
U.S. Court of Appeals for the Fifth Circuit.
It is possible that significant
regulatory developments will take place over the next few years across global markets, driven principally by the World Health Organization’s
Framework Convention on Tobacco Control (“FCTC”). The FCTC is the first international public health treaty on tobacco, and
its objective is to establish a global agenda for tobacco regulation with the purpose of reducing initiation of tobacco use and encouraging
cessation. In addition, the FCTC has led to increased efforts by tobacco control advocates and public health organizations to reduce
the appeal of tobacco products. Our operating results could be significantly affected by any significant increase in the cost of complying
with new regulatory requirements.
Compliance with current and
future regulations regarding tobacco could have a material impact on our business and operations and could result in fines, government
actions to restrict or prevent sales of products, as well as result in substantial costs and expenses.
We may become subject to litigation related
to cigarette smoking and/or exposure to environmental tobacco smoke, or ETS, which could severely impair our results of operations and
liquidity.
Although we are not currently
subject to legal proceedings related to cigarette smoking or ETS, we may become subject to litigation related to the sale of our Modified
Exposure Cigarettes or other tobacco products we sell or manufacture in the future. Legal proceedings covering a wide range of matters
related to tobacco use are pending or threatened in various U.S. and foreign jurisdictions. Various types of claims are raised in these
proceedings, including product liability, consumer protection, antitrust, tax, contraband shipments, patent infringement, employment
matters, claims for contribution, and claims of competitors and distributors.
Litigation is subject to
uncertainty, and it is possible that there could be adverse developments in pending cases. An unfavorable outcome or settlement of pending
tobacco related litigation could encourage the commencement of additional litigation. The variability in pleadings, together with the
actual experience of management in litigating claims, demonstrates that the monetary relief that may be specified in a lawsuit bears
little relevance to the ultimate outcome.
Damages claimed in some tobacco-related
litigations are significant and, in certain cases, range into the billions of dollars. We anticipate that new cases will continue to
be filed. The FCTC encourages litigation against tobacco product manufacturers. It is possible that our results of operations, cash flows,
or financial position could be materially affected by an unfavorable outcome or settlement of litigation.
Our production facility (NASCO) is integral
to our tobacco business and adverse changes or developments affecting our facility may have an adverse impact on our business.
Our production facility is
integral to our tobacco business. Adverse changes or developments affecting this facility, including, but not limited to, disease or
infestation of our raw materials, a fire, an explosion, a serious injury or fatality, a power failure, a natural disaster, an epidemic,
pandemic or other public health crisis, or a material failure of our security infrastructure, could reduce or require us to entirely
suspend operations.
A significant failure of
our site security measures and other facility requirements, including failure to comply with applicable regulatory requirements, could
have an impact on our ability to continue operating under our facility licenses and our prospects of renewing our licenses, and could
also result in a suspension or revocation of these licenses.
The loss of a significant customer for
whom we manufacture tobacco products could have an adverse impact on our results of operation.
Currently, a significant
portion of our revenues (and corresponding accounts receivable) from manufacturing tobacco products are derived from a small number of
large customers, and we do not have agreements with such customers requiring them to purchase a minimum amount of products from us or
guaranteeing any minimum future purchase amounts from us. Such customers may, at any time, delay or decrease their level of purchases
from us or cease doing business with us altogether. Since many of our manufacturing costs are fixed, if sales to such customers cease
or are reduced, we may not obtain sufficient purchase orders from other customers necessary to offset any such losses or reductions,
which could have a negative impact on our results of operations.
Product liability claims, product recalls,
or other claims could cause us to incur losses or damage our reputation.
The risk of product liability
claims, product recalls, and associated adverse publicity, is inherent in the development, manufacturing, marketing, and sale of tobacco
products. Any product recall or lawsuit seeking significant monetary damages may have a material adverse effect on our business and financial
condition. A successful product liability claim against us could require us to pay a substantial monetary award. Though we currently
have no pending product liability claims against us, we cannot assure you that such claims will not be made in the future and any such
claim could cause us to incur substantial losses or damage our reputation.
Cigarettes are subject to substantial taxes.
Significant increases in cigarette-related taxes have been proposed or enacted and are likely to continue to be proposed or enacted in
numerous jurisdictions. These tax increases may affect the sales of our potential products and our third-parties customers’ tobacco
products manufactured at our factory, which could result in decreased sales and profitability of our manufacturing business.
Tax regimes, including excise
taxes, sales taxes, and import duties, can disproportionately affect the retail price of manufactured cigarettes versus other tobacco
products, or disproportionately affect the relative retail price of our Modified Exposure Cigarettes versus lower-priced cigarette brands
manufactured by our competitors. Increases in cigarette taxes are expected to continue to have an adverse impact on sales of cigarettes
resulting in (i) lower consumption levels, (ii) a shift in sales from manufactured cigarettes to other tobacco products or
to lower-price cigarette categories, (iii) a shift from local sales to legal cross-border purchases of lower price products, and
(iv) illicit products such as contraband and counterfeit.
Government mandated prices or taxes, production
control programs, shifts in crops driven by economic conditions, climatic or adverse weather patterns may increase the cost or reduce
the quality and/or supply of the tobacco and other agricultural products used to manufacture our products.
We depend on a small number
of independent tobacco farmers to grow our specialty proprietary tobaccos with specific nicotine contents for our products. As with other
agricultural commodities, the price of tobacco leaf can be influenced by imbalances in supply and demand, and crop quality can be influenced
by variations in weather patterns, diseases, and pests. This risk is greater for us, as there would be no alternative supply of RNC tobacco
in the event that one of our growers experiences a material adverse event with respect to a particular RNC tobacco crop or the quantity
or quality was not as we anticipated, and we would not be able to supply leaf for our VLN® cigarettes.
We must also compete with
other tobacco companies for contract production with independent tobacco farmers. Tobacco production in certain countries is subject
to a variety of controls, including government mandated prices and production control programs. Changes in the patterns of demand for
agricultural products could cause farmers to plant less tobacco. Any significant change in tobacco leaf prices or taxes, quality and
quantity could affect our profitability and our business.
We distribute and sell our products outside
of the U.S., which subjects us to other regulatory risks.
In addition to the authorization
to market and sell our RNC tobacco cigarettes using modified risk claims in the U.S., we continue to seek governmental authorizations
required to market our RNC tobacco cigarettes and our other products in other countries. Marketing of our products is not permitted in
certain countries until we have obtained required authorizations or exemptions in these individual countries. The regulatory review process
varies from country to country, and authorization by foreign governmental authorities is unpredictable, uncertain, and generally expensive.
Our ability to market our potential products could be substantially limited due to delays in receipt of, or failure to receive, the necessary
authorizations or exemptions. We anticipate commencing the applications required in some or all of these countries in the future. Failure
to obtain necessary regulatory authorizations or exemptions could impair our ability to generate revenue from international sources.
We may become subject to governmental investigations
on a range of matters.
Tobacco companies are often
subject to investigations, including allegations of contraband shipments of cigarettes, allegations of unlawful pricing activities within
certain markets, allegations of underpayment of custom duties and/or excise taxes, and allegations of false and misleading usage of descriptors
such as “lights” and “ultra-lights.” We cannot predict the outcome of any investigations to which we may become
subject, but we may be materially affected by an unfavorable outcome of potential future investigations.
Our business model inherently loses customers.
Our
VLN® cigarette is designed to help people smoke less and eventually quit smoking
completely. If our product is successful, we will lose customers as a result. A significant loss in VLN®
customers, or our inability to add new VLN® customers
faster than we lose customers, could prevent our VLN® business from growing
and have a material negative impact on the results of our operations.
We may be unsuccessful in anticipating
changes in adult consumer preferences, responding to changes in consumer purchase behavior or managing through difficult competitive
and economic conditions, which could have an adverse effect on business.
In the tobacco industry,
we are subject to intense competition and changes in adult consumer preferences. To be successful, we must:
| · | anticipate
and respond to new and evolving adult consumer preferences; |
| · | develop,
manufacture, market and distribute new and innovative products that appeal to adult consumers
(including, where appropriate, through arrangements with, or investments in, third parties); |
| · | improve
productivity; and |
| · | protect
or enhance margins through cost savings and price increases. |
The willingness of adult
consumers to purchase premium consumer tobacco products, such as our RNC cigarettes, depends in part on economic conditions. In periods
of economic uncertainty, adult consumers may purchase more discount brands and/or, in the case of tobacco products, consider lower-priced
tobacco products, which could have a material adverse effect on the business and profitability.
We may be unsuccessful in developing and
commercializing adjacent products or processes, including innovative tobacco products that may reduce the health risks associated with
certain other tobacco products and that appeal to adult tobacco consumers.
Some innovative tobacco products
may reduce the health risks associated with certain other tobacco products, while continuing to offer adult tobacco consumers products
that meet their taste expectations and evolving preferences. Examples include tobacco-containing and nicotine-containing products that
reduce or eliminate exposure to cigarette smoke and/or constituents identified by public health authorities as harmful, such as electronically
heated tobacco products, oral nicotine pouches, and e-vapor products. We may not succeed in our efforts to develop and commercialize
any adjacent products.
Further, we cannot predict
whether regulators, including the FDA, will permit the marketing or sale of any particular innovative products (including products with
claims of reduced risk to adult consumers), the speed with which they may make such determinations or whether regulators will impose
an unduly burdensome regulatory framework on such products. In addition, the FDA could, for a variety of reasons, determine that innovative
products currently on the market, or those that have previously received authorization, including with a claim of reduced exposure, are
not appropriate for the public health and the FDA could require such products be taken off the market. We also cannot predict whether
any products will appeal to adult tobacco consumers or whether adult tobacco consumers’ purchasing decisions would be affected
by reduced-risk claims on such products if permitted. Adverse developments on any of these matters could negatively impact the commercial
viability of such products.
If we do not succeed in our
efforts to develop and commercialize innovative tobacco products or to obtain or maintain regulatory authorizations for the marketing
or sale of products, including for the use of claims of reduced exposure, but one or more of our competitors does succeed, we may be
at a competitive disadvantage, which could have an adverse effect on our ability to commercialize our products.
An extended disruption at a facility or
in service by a supplier, distributor or distribution chain service provider could have a material adverse effect on our business.
We face risks inherent in
reliance on one manufacturing facility and a small number of key suppliers, distributors and distribution chain service providers. A
pandemic (including COVID-19), natural or man-made disaster or other disruption that affects the manufacturing operations, the operations
of any key supplier, distributor or distribution chain service provider or any other disruption in the supply or distribution of goods
or services (including a key supplier’s inability to comply with government regulations or unwillingness to supply goods or services
to a tobacco company) could have a material adverse effect on our business.
The FDA could force the removal of our
products from the U.S. market.
The FDA has broad authority
over the regulation of tobacco products. The FDA could, among other things, force us to remove from the U.S. market our RNC tobacco cigarettes
even after the FDA authorization on December 17, 2019 of our PMTA for us to market our RNC tobacco cigarettes, or the authorization
of our MRTP application on December 23, 2021, to enable us to use certain modified exposure claims with respect to our VLN®
cigarettes. In addition, the exposure modification order that enables us to market our VLN® cigarettes as MRTPs was granted for a
period of five years, which is the maximum duration for a marketing granted order for such products under the Family Smoking Prevention &
Tobacco Control Act (PUBLIC LAW 111–31—JUNE 22, 2009). Consequently, we will need to reapply to FDA under a new MRTP application
to extend the FDA’s exposure modification order beyond December 23, 2026. The MRTP authorization process is a complex,
substantial and lengthy regulatory undertaking. The FDA may or may not grant continued authorization of these product claims, including
based on FDA's assessment of whether the product application(s) satisfy the statutory requirements for such an order, and whether
we have adequately complied with the conditions imposed on us in connection with the FDA’s exposure modification order, such as
requirements relating to recordkeeping, reporting and post-market studies. Any action by the FDA to remove our products from the U.S.
market, including the termination or non-renewal of the exposure modification orders for our VLN® cigarettes would have
a material adverse impact on our business.
A ban on menthol or flavored tobacco products could have a material
adverse impact on our business.
On April 27, 2022, the
FDA proposed new rules to prohibit menthol as a characterizing flavor in cigarettes and prohibit all characterizing flavors (other
than tobacco) in cigars. There has been increasing activity on the state and local levels with respect to scrutiny of menthol and flavored
tobacco products, including a recent law passed by the State of California prohibiting tobacco retailers from selling most flavored and
menthol tobacco products, including VLN® Menthol King. If these proposed rules are finalized and implemented, if new rules are
proposed or if additional states or governments pass laws similar to the State of California, we could be negatively impacted through
decreased sales, a requirement to remove non-compliant tobacco products from the marketplace, associated interruptions in manufacturing
or business disruptions. In addition, although we believe that our VLN® Menthol King reduced nicotine cigarettes will be exempted
from FDA’s menthol ban on cigarettes, there is no guarantee that they will be exempted by the FDA or any other state or local government.
Accordingly, the implementation of these proposed or new laws or rules may have a material adverse impact on our results of operations.
Risks Related to Intellectual Property
Certain of our proprietary rights have
expired or may expire or may not otherwise adequately protect our intellectual property, products and potential products, and if we cannot
obtain adequate protection of our intellectual property, products and potential products, we may not be able to successfully market our
products and potential products.
Our commercial success will
depend, in part, on obtaining and maintaining intellectual property protection for our technologies, products, and potential products.
We will only be able to protect our technologies, products, and potential products from unauthorized use by third parties to the extent
that valid and enforceable patents cover them, or to the extent that other market exclusionary rights apply.
The patent positions of life
sciences companies, like ours, can be highly uncertain and involve complex legal and factual questions for which important legal principles
remain unresolved. No consistent policy regarding the breadth of claims allowed in such companies’ patents has emerged to date
in the United States. The general patent environment outside the United States also involves significant uncertainty. Accordingly, we
cannot predict the breadth of claims that may be allowed or that the scope of these patent rights could provide a sufficient degree of
future protection that could permit us to gain or keep our competitive advantage with respect to these products and technology. Additionally,
life science companies like ours are often dependent on creating a pipeline of products. We may not be able to develop additional potential
products or proprietary technologies that produce commercially viable products or that are themselves patentable.
Our issued patents may be
subject to challenge and potential invalidation by third parties and our competitors may develop processes to achieve similar results
without infringing on our patents. Changes in either the patent laws or in the interpretations of patent laws in the United States, or
in other countries, may diminish the value of our intellectual property. In addition, others may independently develop similar or alternative
products and technologies that may be outside the scope of our intellectual property. Should third parties develop alternative methods
of regulating nicotine in tobacco or obtain patent rights to similar products or technology without infringing on our intellectual property
rights, this may have an adverse effect on our business.
The expiration of a portion
of the QPT patent family in 2018 may provide third parties with the freedom to target the QPT gene in the tobacco plant. This could result
in experiments to try to reduce nicotine levels in tobacco plants to levels that may satisfy the planned new nicotine reduction regulations
coming from the FDA. There can be no assurance about whether any third-parties will or will not be successful in such efforts, how long
or short in time such efforts will entail and/or if such efforts will or will not infringe other genes and other intellectual property
on which we have continuing patent protection that would need to be used, in combination with QPT, to result in RNC tobacco. If independent
researchers or our competitors are able to successfully reduce nicotine levels in tobacco plants without violating our patent protections,
our ability to license our technology would be negatively impacted and we would likely face increased competition.
We also rely on license agreements
and trade secrets to protect our technology, products, and potential products, especially where we do not believe patent protection is
appropriate or obtainable. Trade secrets, however, are difficult to protect. While we believe that we use reasonable efforts to protect
our trade secrets, our own, our licensees’ or our strategic partners’ employees, consultants, contractors or advisors may
unintentionally or willfully disclose our information to competitors. We seek to protect this information, in part, through the use of
non-disclosure and confidentiality agreements with employees, consultants, advisors, and others. These agreements may be breached, and
we may not have adequate remedies for a breach. In addition, we cannot ensure that those agreements will provide adequate protection
for our trade secrets, know-how, or other proprietary information, or prevent their unauthorized use or disclosure.
To the extent that consultants
or key employees apply technological information independently developed by them or by others to our products and potential products,
disputes may arise as to the proprietary rights of the information, which may not be resolved in our favor. Key employees are required
to assign all intellectual property rights in their discoveries to us. However, these key employees may terminate their relationship
with us, and we cannot preclude them indefinitely from dealing with our competitors. If our trade secrets become known to competitors
with greater experience and financial resources, the competitors may copy or use our trade secrets and other proprietary information
in the advancement of their products, methods, or technologies. If we were to prosecute a claim that a third party had illegally obtained
and was using our trade secrets, it could be expensive and time consuming and the outcome could be unpredictable. In addition, courts
outside the United States are sometimes less willing to protect trade secrets than courts in the United States. Moreover, if our competitors
independently develop equivalent knowledge, we would lack any contractual claim to this information, and our business could be harmed.
The ability to commercialize our existing
and potential products will depend on our ability to sell such products without infringing the patent or proprietary rights of third
parties. If we are sued for infringing intellectual property rights of third parties, such litigation could be costly and time consuming
and an unfavorable outcome could have a significant adverse effect on our business.
The ability to commercialize
our potential products will depend on our ability to sell such products without infringing the patents or other proprietary rights of
third parties. Third-party intellectual property rights in our field are complicated, and third-party intellectual property rights in
these fields are continuously evolving. While we have conducted searches for such third-party intellectual property rights, we have not
performed specific searches for third-party intellectual property rights that may raise freedom-to-operate issues, and we have not obtained
legal opinions regarding commercialization of our potential products. As such, there may be existing patents that may affect our ability
to commercialize our potential products.
In addition, because patent
applications are published up to 18 months after their filing, and because patent applications can take several years to issue,
there may be currently pending third-party patent applications and freedom-to-operate issues that are unknown to us, which may later
result in issued patents.
If a third-party claims that we infringe on its
patents or other proprietary rights, we could face a number of issues that could seriously harm our competitive position, including:
| · | infringement
claims that, with or without merit, can be costly and time consuming to litigate, can delay
regulatory authorization processes, and can divert management’s attention from our
core business strategy; |
| · | substantial
damages for past infringement which we may have to pay if a court determines that our products
or technologies infringe upon a competitor’s patent or other proprietary rights; |
| · | a
court order prohibiting us from commercializing our potential products or technologies unless
the holder licenses the patent or other proprietary rights to us, which such holder is not
required to do; |
| · | if
a license is available from a holder, we may have to pay substantial royalties or grant cross
licenses to our patents or other proprietary rights; and |
| · | redesigning
our process so that it does not infringe the third-party intellectual property, which may
not be possible, or which may require substantial time and expense including delays in bringing
our potential products to market. |
Such actions could harm our
competitive position and our ability to generate revenue and could result in increased costs.
Our patent applications may not result
in issued patents, which may have a material adverse effect on our ability to prevent others from commercially exploiting products similar
to ours.
We own or exclusively control
many issued patents and pending patent applications. We cannot be certain that these patent applications will issue, in whole or in part,
as patents. Patent applications in the United States are maintained in secrecy until the patents are published or are issued. Since publication
of discoveries in the scientific or patent literature tends to lag behind actual discoveries by several months, we cannot be certain
that we are the first creator of inventions covered by pending patent applications or the first to file patent applications on these
inventions. We also cannot be certain that our pending patent applications will result in issued patents or that any of our issued patents
will afford protection against a competitor. In addition, patent applications filed in foreign countries are subject to laws, rules and
procedures that differ from those of the United States, and thus we cannot be certain that foreign patent applications related to U.S.
patents will be issued. Furthermore, if these patent applications issue, some foreign countries provide significantly less effective
patent enforcement than in the United States.
The status of patents involves
complex legal and factual questions and the breadth of claims allowed is uncertain. Accordingly, we cannot be certain that the patent
applications that we or our licensors file will result in patents being issued, or that our patents and any patents that may be issued
to us in the near future will afford protection against competitors with similar technology. In addition, patents issued to us may be
infringed upon or designed around by others and others may obtain patents that we need to license or design around, either of which would
increase costs and may adversely affect our operations.
We license certain patent rights from third-party
owners. If such owners do not properly maintain or enforce the patents underlying such licenses, our competitive position and business
prospects could be harmed.
We license rights to third-party
intellectual property that is necessary or useful for our business, and we may enter into additional licensing agreements in the future.
Our success could depend in part on the ability of some of our licensors to obtain, maintain, and enforce patent protection for their
intellectual property, in particular, those patents to which we have secured exclusive rights. Our licensors may not successfully prosecute
the patent applications to which we are licensed and may in some instances retain rights to the intellectual property that allows them
to compete with us. Even if patents are issued with respect to these patent applications, our licensors may fail to maintain these patents,
may determine not to pursue litigation against other companies that are infringing these patents, or may pursue such litigation less
aggressively than we could. Without protection for the intellectual property we license, other companies might be able to offer substantially
identical products for sale, which could adversely affect our competitive business position and harm our business prospects.
Our worldwide exclusive licenses
relating to tobacco from NCSU involve multiple patent families and trade secrets. The exclusive rights under the NCSU agreements expire
on the date on which the last patent or registered plant variety covered by the subject license expires in the country or countries where
such patents or registered plant varieties are in effect. The NCSU licenses relate predominately to issued patents, and our exclusive
rights in the NCSU licenses are expected to expire in 2042.
If any of our license agreements
or other intellectual property agreements are not effective at preventing others from competing with us and/or using our intellectual
property, our business could be adversely affected.
Risks Relating to this Offering and Ownership
of Our Securities
If we are not able to comply with the applicable
continued listing requirements or standards of The NASDAQ Capital Market, The NASDAQ Capital Market could delist and adversely affect
the market price and liquidity of our common stock.
Our
common stock is currently traded on The NASDAQ Capital Market under the symbol “XXII”. We have in the past been, and may
in the future be, unable to comply with certain of the listing standards that we are required to meet to maintain the listing of our
common stock on The NASDAQ Capital Market. If we fail to meet any of the continued listing standards of The NASDAQ Capital Market, our
common stock will be delisted from The NASDAQ Capital Market.
These
continued listing standards include specifically enumerated criteria, such as a $1.00 minimum closing bid price and a requirement that
we maintain stockholders’ equity of at least $2,500,000. On April 4, 2024, we received a deficiency letter from the Nasdaq
Listing Qualifications Department indicating that we were not in compliance with Nasdaq's Listing Rule 5550(b)(1) because our
shareholders' equity for the year ended December 31, 2023 was below the minimum shareholders' equity requirement of $2,500,000 (the
"Shareholders' Equity Requirement"). We have until October 1, 2024 to regain compliance with the Shareholders’ Equity
Requirement.
On
July 16, 2024, we received a deficiency letter from the Nasdaq Listing Qualifications Department indicating that for the last 30
consecutive business days our common stock did not maintain a minimum closing bid price of $1.00 (“Minimum Bid Price Requirement”)
per share for continued listing. Under Nasdaq Listing Rule 5810(c)(3)(A), if during the 180 calendar days following the date of
the notification, or prior to January 13, 2025, the closing bid price of our common stock is at or above $1.00 for a minimum of
10 consecutive business days, we will regain compliance with the Minimum Bid Price Requirement. If we do not regain compliance with Rule 5550(a)(2) by
January 13, 2025, we may be afforded a second 180 calendar day period to regain compliance.
There
can be no assurance that we will be able to regain and maintain continued compliance the listing requirements of The NASDAQ Capital Market.
If our common stock were to be delisted from The NASDAQ Capital Market, trading of our common stock most likely will be conducted in
the over-the-counter market on an electronic bulletin board established for unlisted securities such as the OTC Markets or in the “pink
sheets.” Such a downgrading in our listing market may limit our ability to make a market in our common stock and which may impact
purchases or sales of our securities.
Purchasers in the offering may result in
dilution.
If you purchase Offered
Shares in this offering, the value of your shares based on our pro forma net tangible book value may immediately be less than the
offering price you paid. This reduction in the value of your equity is known as dilution. At a public offering price of
$0.57 per share, purchasers of common stock in this offering will
experience immediate dilution of approximately $0.26 per share, representing the difference between the public offering
price per share in this offering and our as adjusted net tangible book value per share as of June 30, 2024, after
giving effect to this offering, and after deducting estimated offering expenses,
including placement agent fees, payable by us. See “Dilution.”
You may experience future dilution as a
result of future equity issuances, offerings or acquisitions.
To raise additional capital,
we may in the future offer additional shares of our common stock or other securities convertible into or exchangeable for our common
stock at prices that may not be the same as the price per share in this offering. We may sell shares or other securities in any future
offering at a price per share that is less than the price per share paid by investors in this offering, and investors purchasing shares
or other securities in the future could have rights superior to existing stockholders. The price per share at which we sell additional
shares of our common stock, or securities convertible or exchangeable into our common stock, in future transactions or acquisitions may
be higher or lower than the price per share paid by investors in this offering.
In addition, we may engage
in one or more potential acquisitions in the future, which could involve issuing our common stock as some or all of the consideration
payable by us to complete such acquisitions. We may also settle our outstanding debt obligations with shares of common stock. If we issue
common stock or securities linked to our common stock, the newly issued securities may have a dilutive effect on the interests of the
holders of our common stock. Additionally, future sales of newly issued shares used to effect an acquisition could depress the market
price of our common stock.
This is a “best efforts” offering;
no minimum amount of Offered Shares is required to be sold, and we may not raise the amount of capital we believe is required for our
business.
There is no required minimum
number of Offered Shares that must be sold as a condition to completion of this offering. Because there is no minimum offering amount
required as a condition to the closing of this offering, the actual offering amount, and proceeds to us are not presently determinable
and may be substantially less than the maximum amounts set forth in this Offering Circular. We may sell fewer than all of the Offered
Shares offered hereby, which may significantly reduce the amount of proceeds received by us, and investors in this offering will not
receive a refund in the event that we do not sell an amount of Offered Shares sufficient to pursue the business goals outlined in this
Offering Circular. Thus, we may not raise the amount of capital we believe is required for our business and may need to raise additional
funds, which may not be available or available on terms acceptable to us. Despite this, any proceeds from the sale of the Offered Shares
offered by us will be available for our immediate use, and because there is no escrow account and no minimum offering amount in this
offering, investors could be in a position where they have invested in us, but we are unable to fulfill our objectives due to a lack
of interest in this offering.
Our management will have broad discretion
over the use of the net proceeds from this offering.
We currently intend to use
the net proceeds from the sale of Offered Shares under this offering for marketing and advertising expenses and general corporate purposes,
including working capital. We have not reserved or allocated specific amounts for any of these purposes and we cannot specify with certainty
how we will use the net proceeds. See “Use of Proceeds”. Accordingly, our management will have considerable discretion in
the application of the net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the
proceeds are being used appropriately. We may use the net proceeds for corporate purposes that do not increase our operating results
or market value.
We have not paid cash dividends in the
past and do not expect to pay dividends in the future. Any return on investment may be limited to the value of our common stock, which
may decrease in value.
We have never paid cash dividends
on our common stock and do not anticipate doing so in the foreseeable future. The payment of dividends on our common stock will depend
on earnings, financial condition and other business and economic factors affecting us at such time as our board of directors may consider
relevant. If we do not pay dividends, our common stock may be less valuable because a return on your investment will only occur if our
stock price appreciates.
Our issuance of
shares of preferred stock could adversely affect the market value of our common stock, dilute the voting power of common stockholders
and delay or prevent a change of control.
Our
board of directors has the authority to cause us to issue, without any further vote or action by the stockholders, shares of preferred
stock in one or more series, to designate the number of shares constituting any series, and to fix the rights, preferences, privileges
and restrictions thereof, including dividend rights, voting rights, rights and terms of redemption, redemption price or prices and liquidation
preferences of such series.
The
issuance of shares of preferred stock with dividend or conversion rights, liquidation preferences or other economic terms favorable to
the holders of preferred stock could adversely affect the market price for our common stock by making an investment in the common stock
less attractive. For example, investors in the common stock may not wish to purchase common stock at a price above the conversion price
of a series of convertible preferred stock because the holders of the preferred stock would effectively be entitled to purchase common
stock at the lower conversion price causing economic dilution to the holders of common stock.
Further,
the issuance of shares of preferred stock with voting rights may adversely affect the voting power of the holders of our other classes
of voting stock either by diluting the voting power of our other classes of voting stock if they vote together as a single class, or
by giving the holders of any such preferred stock the right to block an action on which they have a separate class vote even if the action
were approved by the holders of our other classes of voting stock. The issuance of shares of preferred stock may also have the effect
of delaying, deferring or preventing a change in control of our company without further action by the stockholders, even where stockholders
are offered a premium for their shares.
The market price
of our common stock has been, and may continue to be, subject to substantial volatility.
The
market price of our common stock may fluctuate significantly in response to numerous factors, many of which are beyond our control, including;
| |
· |
volatility in the trading
markets generally and in our particular market segment; |
| |
|
|
| |
· |
limited trading of our
common stock; |
| |
|
|
| |
· |
actual or anticipated fluctuations
in our results of operations; |
| |
|
|
| |
· |
our ability to remain listed
on Nasdaq; |
| |
|
|
| |
· |
announcements regarding
our business or the business of our customers or competitors; |
| |
|
|
| |
· |
changes in accounting standards,
policies, guidelines, interpretations, or principles; |
| |
|
|
| |
· |
actual or anticipated developments
in our business or our competitors’ businesses or the competitive landscape generally; |
| |
|
|
| |
· |
developments or disputes
concerning our intellectual property or our offerings, or third-party proprietary rights; |
| |
|
|
| |
· |
announced or completed
acquisitions of businesses or technologies by us or our competitors; |
| |
|
|
| |
· |
new laws or regulations
or new interpretations of existing laws or regulations applicable to our business; |
| |
|
|
| |
· |
any major change in our
board of directors or management; |
| |
|
|
| |
· |
sales of shares of our
common stock by us or by our stockholders; |
| |
|
|
| |
· |
lawsuits threatened or
filed against us; and |
| |
|
|
| |
· |
other events or factors,
including those resulting from war, incidents of terrorism, pandemics (such as the COVID-19 pandemic) or responses to these events. |
Statements
of, or changes in, opinions, ratings, or earnings estimates made by brokerage firms or industry analysts relating to the markets in which
we operate or expect to operate could have an adverse effect on the market price of our common stock. In addition, the stock market as
a whole, as well as our particular market segment, has from time-to-time experienced extreme price and volume fluctuations, which may
affect the market price for the securities of many companies, and which often have appeared unrelated to the operating performance of
such companies. Any of these factors could negatively affect our stockholders’ ability to sell their shares of common stock at
the time and price they desire.
A decline in the
price of our common stock could affect our ability to raise further working capital, which could adversely impact our ability to continue
operations.
A
prolonged decline in the price of our common stock could result in a reduction in the liquidity of our common stock and a reduction in
our ability to raise capital. We may attempt to acquire a significant portion of the funds we need in order to conduct our planned operations
through the sale of equity securities; thus, a decline in the price of our common stock could be detrimental to our liquidity and our
operations because the decline may adversely affect investors’ desire to invest in our securities. If we are unable to raise the
funds we require for all of our planned operations, we may be forced to reallocate funds from other planned uses and may suffer a significant
negative effect on our business plan and operations, including our ability to develop new products or services and continue our current
operations. As a result, our business may suffer, and we may be forced to reduce or discontinue operations. We also might not be able
to meet our financial obligations if we cannot raise enough funds through the sale of our common stock and we may be forced to reduce
or discontinue operations.
The elimination of monetary liability against our directors,
officers, and employees under Nevada law and the existence of indemnification rights for our obligations to our directors, officers,
and employees may result in substantial expenditures by us and may discourage lawsuits against our directors, officers, and employees.
Our
articles of incorporation and bylaws contain provisions permitting us to eliminate the personal liability of our directors and officers
to us and our stockholders for damages for the breach of a fiduciary duty as a director or officer to the extent provided by Nevada law.
In addition, we have entered into indemnification agreements with our directors and officers to provide such indemnification rights.
We may also have contractual indemnification obligations under any future employment agreements with our officers. The foregoing indemnification
obligations could result in us incurring substantial expenditures to cover the cost of settlement or damage awards against directors
and officers, which we may be unable to recoup. These provisions and the resulting costs may also discourage us from bringing a lawsuit
against directors and officers for breaches of their fiduciary duties and may similarly discourage the filing of derivative litigation
by our stockholders against our directors and officers even though such actions, if successful, might otherwise benefit us and our stockholders.
Anti-takeover
effects of certain provisions of Nevada state law could hinder a potential takeover of us.
Nevada
has a business combination law that prohibits certain business combinations between Nevada corporations and “interested stockholders”
for three years after an “interested stockholder” first becomes an “interested stockholder,” unless the corporation’s
board of directors approves the combination in advance. For purposes of Nevada law, an “interested stockholder” is any person
who is (i) the beneficial owner, directly or indirectly, of ten percent or more of the voting power of the outstanding voting shares
of the corporation or (ii) an affiliate or associate of the corporation and at any time within the three previous years was the
beneficial owner, directly or indirectly, of ten percent or more of the voting power of the then-outstanding shares of the corporation.
The definition of the term “business combination” is sufficiently broad to cover virtually any kind of transaction that would
allow a potential acquirer to use the corporation’s assets to finance the acquisition or otherwise to benefit its own interests
rather than the interests of the corporation and its other stockholders.
The
potential effect of Nevada’s business combination law is to discourage parties interested in taking control of us from doing so
if these parties cannot obtain the approval of our board of directors. Both of these provisions could limit the price investors would
be willing to pay in the future for shares of our common stock.
Our bylaws contain
an exclusive forum provision, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with
us or our directors, officers, employees or agents.
Our
bylaws provide that, unless we consent in writing to the selection of an alternative forum, the state and federal courts in the State
of Nevada shall be the exclusive forum for any litigation relating to our internal affairs, including, without limitation: (a) any
derivative action brought on behalf of us, (b) any action asserting a claim for breach of fiduciary duty to us or our stockholders
by any current or former officer, director, employee, or agent of us, or (c) any action against us or any current or former officer,
director, employee, or agent of us arising pursuant to any provision of the Nevada Revised Statutes, the articles of incorporation, or
the bylaws.
For
the avoidance of doubt, the exclusive forum provision described above does not apply to any claims arising under the Securities Act or
Exchange Act. Section 27 of the Exchange Act creates exclusive federal jurisdiction over all suits brought to enforce any duty or
liability created by the Exchange Act or the rules and regulations thereunder, and Section 22 of the Securities Act creates
concurrent jurisdiction for federal and state courts over all suits brought to enforce any duty or liability created by the Securities
Act or the rules and regulations thereunder.
The
choice of forum provision in our bylaws may limit our stockholders’ ability to bring a claim in a judicial forum that they find
favorable for disputes with us or our directors, officers, employees or agents, which may discourage such lawsuits against us and our
directors, officers, employees and agents even though an action, if successful, might benefit our stockholders. The applicable courts
may also reach different judgments or results than would other courts, including courts where a stockholder considering an action may
be located or would otherwise choose to bring the action, and such judgments or results may be more favorable to us than to our stockholders.
With respect to the provision making the state and federal courts in the State of Nevada the sole and exclusive forum for certain types
of actions, stockholders who do bring a claim in the state and federal courts in the State of Nevada could face additional litigation
costs in pursuing any such claim, particularly if they do not reside in or near Nevada. Finally, if a court were to find this provision
of our bylaws inapplicable to, or unenforceable in respect of, one or more of the specified types of actions or proceedings, we may incur
additional costs associated with resolving such matters in other jurisdictions, which could have a material adverse effect on us.
DILUTION
If you invest in our common
stock in this offering, your ownership interest will be diluted immediately to the extent of the difference between the public offering
price per share of our common stock and the as adjusted net tangible book value per share of our common stock after this offering.
Our historical net tangible
book value as of June 30, 2024, was a deficit of $6.7 million, or ($0.72) per share of common stock based on 9,272,518 shares of common
stock outstanding as of June 30, 2024. Historical net tangible book value per share is calculated by subtracting our total liabilities
from our total tangible assets, which is total assets less intangible assets, and dividing this amount by the number of shares of common
stock outstanding as of such date.
After giving effect to the
assumed sale by us of the Offered Shares at a public offering price of $0.57 per share, and after deducting estimated offering expenses,
our as adjusted net tangible book value as of June 30, 2024 would have been approximately $14.7 million or $0.31 per share of common stock.
This represents an immediate increase in the net tangible book value of $1.03 per share to our existing stockholders and an immediate
and substantial dilution in net tangible book value of $0.26 per share to new investors. The following table illustrates this hypothetical
per share dilution:
| Assumed public offering price per share | |
$ | 0.57 | |
| Historical net tangible book value per share as of June 30, 2024 | |
$ | (0.72 | ) |
| Increase in historical net tangible book value per share attributable to this offering | |
$ | 1.03 | |
| As adjusted net tangible book value per share as of June 30, 2024 after giving effect to this offering | |
$ | 0.31 | |
| Dilution per share to purchasers of Offered Shares in this offering | |
$ | 0.26 | |
The as adjusted information discussed
above is illustrative only. Our net tangible book value following the completion of this offering is subject to adjustment based on the terms of this offering.
The number of shares of common stock
outstanding as of June 30, 2024, as shown above, is based on 9,272,518 shares of common stock issued and outstanding as of that date
and excludes:
| · |
6,605 shares of common stock issuable upon the exercise of outstanding stock options with a weighted-average exercise price of $358.81 per share: |
| · |
1,824 shares of common stock issuable upon vesting of restricted stock unit awards; |
| · |
5,707,584 shares of common stock reserved for future issuance under our 2021 Omnibus Incentive Plan; |
| · |
6,361,600 shares of common stock issuable upon exercise of warrants to purchase common stock with a weighted-average exercise price of $2.70 per share; and |
| · |
1,150,000 shares of common stock issuable upon exercise of pre-funded warrants to purchase common stock. |
USE OF PROCEEDS
The table below sets forth
the estimated proceeds we would derive from this offering, assuming the sale of 25%, 50%, 75% and 100% of the Offered Shares at a per share price of $0.57. There is, of course, no guaranty that we
will be successful in selling any of the Offered Shares in this offering.
| | |
Assumed Percentage of Offered Shares Sold in This Offering | |
| | |
25% | | |
50% | | |
75% | | |
100% | |
| Offered Shares sold | |
| 9,375,000 | | |
| 18,750,000 | | |
| 28,125,000 | | |
| 37,500,000 | |
| Gross proceeds | |
$ | 5,343,750 | | |
$ | 10,687,500 | | |
$ | 16,031,250 | | |
$ | 21,375,000 | |
| Offering expenses (1) | |
| (42,500 | ) | |
| (42,500 | ) | |
| (42,500 | ) | |
| (42,500 | ) |
| Net proceeds | |
$ | 5,301,250 | | |
$ | 10,645,000 | | |
$ | 15,988,750 | | |
$ | 21,332,500 | |
| (1) |
Represents legal and accounting
fees and expenses and miscellaneous out-of-pocket costs (See “Plan of Distribution”). |
The table below sets forth
the manner in which we intend to apply the net proceeds derived by us in this offering, assuming the sale of 25%, 50%, 75% and 100% of
the Offered Shares at a public per share offering price of $0.57. All amounts set forth below are estimates.
| | |
Use of Proceeds for Assumed Percentage
of Offered Shares Sold in This Offering | |
| | |
25% | | |
50% | | |
75% | | |
100% | |
| Marketing and Advertising | |
$ | 530,125 | | |
$ | 1,064,500 | | |
$ | 1,598,875 | | |
$ | 2,133,250 | |
| General Corporate Expenses, including Working Capital and potential repayment of debt | |
| 4,771,125 | | |
| 9,580,500 | | |
| 14,389,875 | | |
| 19,199,250 | |
| TOTAL | |
$ | 5,301,250 | | |
$ | 10,645,000 | | |
$ | 15,988,750 | | |
$ | 21,332,500 | |
A substantial portion of
the proceeds have not been allocated for a specific purpose. Accordingly, we reserve the right to change the foregoing use of proceeds,
should our management believe it to be in the best interest of our company. The allocations of the proceeds of this offering presented
above constitute the current estimates of our management and are based on our current plans, assumptions made with respect to the industry
in which we currently or, in the future, expect to operate, general economic conditions and our future revenue and expenditure estimates.
Investors are cautioned that
expenditures may vary substantially from the estimates presented above. Investors must rely on the judgment of our management, who will
have broad discretion regarding the application of the proceeds of this offering. The amounts and timing of our actual expenditures will
depend upon numerous factors, including market conditions, cash generated by our operations (if any), business developments and the rate
of our growth. We may find it necessary or advisable to use portions of the proceeds of this offering for other purposes.
In the event we do not obtain
the entire offering amount hereunder, we may attempt to obtain additional funds through private offerings of our securities or by borrowing
funds. Currently, we do not have any committed sources of financing.
PLAN OF DISTRIBUTION
In General
Our company is offering a
maximum of 37,500,000 Offered Shares on a “best-efforts” basis, at a fixed price of $0.57 per Offered Share. There is no
minimum purchase requirement for investors in this offering. This offering will terminate at the earliest of (a) the date on which
the maximum offering has been sold, (b) the date which is one year from this offering being qualified by the SEC or (c) the
date on which this offering is earlier terminated by us, in our sole discretion.
There is no minimum number
of Offered Shares that we are required to sell in this offering. All funds derived by us from this offering will be immediately available
for use by us, in accordance with the uses set forth in the section entitled “Use of Proceeds” of this Offering Circular.
No funds will be placed in an escrow account during the offering period and no funds will be returned once an investor’s subscription
agreement has been accepted by us.
We intend to sell the Offered
Shares in this offering through the efforts of our Chief Executive Officer, Lawrence D. Firestone. Mr. Firestone will not receive
any compensation for offering or selling the Offered Shares. We believe that Mr. Firestone is exempt from registration as a broker-dealer
under the provisions of Rule 3a4-1 promulgated under the Exchange Act. In particular, Mr. Firestone:
| |
· |
is not subject to a statutory
disqualification, as that term is defined in Section 3(a)(39) of the Securities Act; and |
| |
· |
is not to be compensated
in connection with his participation by the payment of commissions or other remuneration based either directly or indirectly on transactions
in securities; |
| |
· |
is not an associated person
of a broker or dealer; and |
| |
· |
meets the conditions of
the following: |
| |
· |
primarily performs, and
will perform at the end of this offering, substantial duties for us or on our behalf otherwise than in connection with transactions
in securities; |
| |
· |
was not a broker or dealer,
or an associated person of a broker or dealer, within the preceding 12 months; and |
| |
· |
did not participate in
selling an offering of securities for any issuer more than once every 12 months other than in reliance on paragraphs (a)(4)(i) or
(iii) of Rule 3a4-1 under the Exchange Act. |
As of the date of this Offering
Circular, we have not entered into any agreements with selling agents for the sale of the Offered Shares. However, we reserve the right
to engage FINRA-member broker-dealers. In the event we engage FINRA-member broker-dealers, we expect to pay sales commissions of up to
6.0% of the gross offering proceeds from their sales of the Offered Shares. In connection with our appointment of a selling broker-dealer,
we intend to enter into a standard selling agent agreement with the broker-dealer pursuant to which the broker-dealer would act as our
non-exclusive sales agent in consideration of our payment of commissions of up to 6.0% on the sale of Offered Shares effected by the
broker-dealer.
Procedures for Subscribing
If you are interested in
subscribing for Offered Shares in this offering, please submit a request for information by e-mail to investor relations at: InvestorRelations@xxiicentury.com;
all relevant information will be delivered to you by return e-mail. Thereafter, should you decide to subscribe for Offered Shares, you
are required to follow the procedures described in the subscription agreement included in the delivered information, which are:
| |
· |
Electronically execute and deliver to us a subscription
agreement; and |
| |
· |
Deliver funds directly by check or by wire or electronic funds
transfer via ACH to our specified bank account. |
Right to Reject Subscriptions
After we receive your complete,
executed subscription agreement and the funds required under the subscription agreement have been transferred to us, we have the right
to review and accept or reject your subscription in whole or in part, for any reason or for no reason. We will return all monies from
rejected subscriptions immediately to you, without interest or deduction.
Acceptance of Subscriptions
Conditioned upon our acceptance
of a subscription agreement, we will countersign the subscription agreement and issue the Offered Shares subscribed. Once you submit
the subscription agreement and it is accepted, you may not revoke or change your subscription or request your subscription funds. All
accepted subscription agreements are irrevocable.
This Offering Circular will
be furnished to prospective investors upon their request via electronic PDF format and will be available for viewing and download 24
hours per day, 7 days per week on our company’s page on the SEC’s website: www.sec.gov.
An investor will become a
shareholder of the Company and the Offered Shares will be issued, as of the date of settlement. Settlement will not occur until an investor’s
funds have cleared and we accept the investor as a shareholder.
By executing the subscription
agreement and paying the total purchase price for the Offered Shares subscribed, each investor agrees to accept the terms of the subscription
agreement and attests that the investor meets certain minimum financial standards.
An approved trustee must
process and forward to us subscriptions made through IRAs, Keogh plans and 401(k) plans. In the case of investments through IRAs,
Keogh plans and 401(k) plans, we will send the confirmation and notice of our acceptance to the trustee.
State Law Exemption and Offerings to “Qualified
Purchasers”
The Offered Shares are being
offered and sold to “qualified purchasers” (as defined in Regulation A under the Securities Act). As a Tier 2 offering pursuant
to Regulation A under the Securities Act, this offering will be exempt from state “Blue Sky” law review, subject to certain
state filing requirements and anti-fraud provisions, to the extent that the Offered Shares offered hereby are offered and sold only to
“qualified purchasers”.
“Qualified purchasers”
include any person to whom securities are offered or sold in a Tier 2 offering pursuant to Regulation A under the Securities Act. We
reserve the right to reject any investor’s subscription in whole or in part for any reason, including if we determine, in our sole
and absolute discretion, that such investor is not a “qualified purchaser” for purposes of Regulation A. We intend to offer
and sell the Offered Shares to qualified purchasers in every state of the United States.
Issuance of Offered Shares
Upon settlement, that is,
at such time as an investor’s funds have cleared and we have accepted an investor’s subscription agreement, we will either
issue such investor’s purchased Offered Shares in book-entry form or issue a certificate or certificates representing such investor’s
purchased Offered Shares.
Transferability of the Offered Shares
The Offered Shares will be
generally freely transferable, subject to any restrictions imposed by applicable securities laws or regulations.
Listing of Offered Shares
The Offered Shares will be
listed on The Nasdaq Capital Market under the symbol “XXII.”
DESCRIPTION OF SECURITIES
General
Our authorized capital stock
consists of 250,000,000 shares of common stock, $0.00001 par value per share, and 10,000,000 shares of preferred stock, $0.00001 par
value per share. As of August 15, 2024, 9,272,518 shares of common stock were issued and outstanding and no shares of preferred stock
were issued and outstanding.
On March 28, 2024 we
filed a Certificate of Change (the “Certificate”) authorizing a 1-for-16 reverse stock split of our issued and outstanding
shares of common stock, par value $0.00001 (the “Reverse Stock Split”). There was no change to our authorized shares. The
Reverse Stock Split became effective at 12:01 a.m. Eastern Time on April 2, 2024. Unless otherwise indicated, all share and
per share prices herein have been adjusted to retroactively reflect the Reverse Stock Split.
Common Stock
Our common stock is traded
on the Nasdaq Capital Market under the symbol “XXII.” Holders of our common stock are entitled to one vote for each share
held on all matters submitted to a vote of stockholders and do not have cumulative voting rights. Holders of common stock are entitled
to receive ratably such dividends, if any, as may be declared by the board of directors out of funds legally available therefore, subject
to a preferential dividend right of outstanding preferred stock. Upon the liquidation, dissolution or our winding up, the holders of
common stock are entitled to receive ratably our net assets available after the payment of all debts and other liabilities and subject
to the prior rights of any outstanding preferred stock. The rights, preferences and privileges of holders of our common stock are subject
to, and may be adversely affected by the rights of the holders any series of preferred stock that we may designate and issue in the future.
We have not previously and do not plan to declare or pay any dividends on our common stock. Our current policy is to retain all funds
and any earnings for use in the operation and expansion of our business. Payment of future dividends, if any, will be at the discretion
of our board of directors after taking into account various factors, including current financial condition, operating results and current
and anticipated cash needs.
Preferred Stock
Under the terms of our amended
and restated articles of incorporation, the board of directors is authorized, subject to any limitations prescribed by law, without stockholder
approval, to issue shares of preferred stock in one or more series. Each such series of preferred stock shall have such rights, preferences,
privileges and restrictions, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences,
as shall be determined by the board of directors.
The purpose of authorizing
the board of directors to issue preferred stock and determine its rights and preferences is to eliminate delays associated with a stockholder
vote on specific issuances. The issuance of preferred stock, while providing desirable flexibility in connection with possible acquisitions
and other corporate purposes, could have the effect of making it more difficult for a third part to acquire, or of discouraging a third
party from acquiring, a majority of our outstanding voting stock. We have no present plans to issue any additional shares of preferred
stock.
The effects of issuing
preferred stock could include one or more of the following:
| |
· |
decreasing the amount of
earnings and assets available for distribution to holders of common stock; |
| |
· |
restricting dividends on
the common stock; |
| |
· |
diluting the voting power
of the common stock; |
| |
· |
impairing the liquidation
rights of the common stock; or |
| |
· |
delaying, deferring or
preventing changes in our control or management. |
As of the date of this Offering
Circular, there were no shares of preferred stock outstanding.
Stock Options and Restricted Stock
As of August 15, 2024,
we had outstanding options to purchase a total of 6,605 shares of common stock at a weighted average exercise price of $358.81 per share
and 1,824 shares of unvested restricted stock or restricted stock units. As of August 15, 2024, an additional 707,584 shares of common
stock were available for future award grants under our stock incentive plan.
Outstanding Warrants
As of August 15, 2024, we had 7,511,600 shares
of common stock issuable upon the exercise of warrants as follows:
| |
|
Number of
Warrants |
|
|
Exercise
Price |
|
|
Expiration Date |
| July 2022 RDO warrants |
|
|
4,067 |
|
|
$ |
492.00 |
|
|
July 25, 2027 |
| Senior Secured Credit Facility - JGB |
|
|
20,645 |
|
|
$ |
205.248 |
|
|
September 3, 2028 |
| July 19, 2023 RDO warrants |
|
|
28,125 |
|
|
$ |
2.14 |
|
|
July 20, 2028 |
| October 2023 CMPO warrants |
|
|
168,750 |
|
|
$ |
2.14 |
|
|
October 19, 2028 |
| Inducement Warrants |
|
|
3,581,212 |
|
|
$ |
2.14 |
|
|
February 15, 2029 |
| April 2024 Warrants |
|
|
1,980,000 |
|
|
$ |
2.14 |
|
|
5 years following stockholder approval |
| April 2024 Placement Agent Warrants |
|
|
118,800 |
|
|
$ |
2.675 |
|
|
April 8, 2029 |
| Omnia Pre-Funded Warrants |
|
|
1,150,000 |
|
|
$ |
0.00001 |
|
|
May 1, 2029 |
| Omnia Purchase Warrants |
|
|
460,000 |
|
|
$ |
2.14 |
|
|
May 1, 2029 |
Convertible Debentures
As of August 15, 2024, there
was an aggregate of $8.3 million of outstanding convertible debentures that are convertible into shares of common stock at a conversion
price of $0.7458 per share.
Anti-Takeover Provisions Under Nevada Law
Combinations
with Interested Stockholder. Sections 78.411-78.444, inclusive, of the Nevada Revised Statutes (NRS) contain provisions
governing combinations with an interested stockholder. For purposes of the NRS, "combinations" include: (i) any merger
or consolidation of a Nevada corporation or any subsidiary of a Nevada corporation with the interested stockholder or any other entity,
whether or not itself is an interested stockholder of the Nevada corporation, which is, or after and as a result of the merger or consolidation
would be, an affiliate or associate of the interested stockholder; (ii) any sale, lease, exchange mortgage, pledge, transfer or
other disposition, in one transaction or a series of transactions, to or with the interested stockholder or any affiliate or associate
of the interested stockholder of assets of the Nevada corporation or any subsidiary of the Nevada corporation (x) having an aggregate
market value equal to more than 5% of the aggregate market value of all of the consolidated assets of the Nevada corporation, (y) having
an aggregate market value equal to more than 5% of the aggregate market value of all the outstanding voting shares of the Nevada corporation,
or (z) representing more than 10% of the earning power or net income of the Nevada corporation (determined on a consolidated basis);
(iii) the issuance or transfer by the Nevada corporation or any subsidiary of the Nevada corporation, in one transaction or a series
of transactions, of any shares of the Nevada corporation or any subsidiary of the Nevada corporation that have an aggregate market value
equal to 5% or more of the aggregate market value of all the outstanding voting shares of the Nevada corporation to the interested stockholder
or any affiliate or associate of the interested stockholder except under the exercise of warrants or rights to purchase shares offered,
or a dividend or distribution paid or made, pro rata to all stockholders of the Nevada corporation; (iv) the adoption of any plan
or proposal for the liquidation or dissolution of the Nevada corporation under any agreement, arrangement or understanding, whether or
not in writing, with the interested stockholder or affiliate or associate of the interested stockholder; (v) except for transactions
that would not constitute a combination pursuant to subsection (iii) above, any reclassification of securities (including share
splits, share dividend or other distribution of shares with respect to other shares, or any issuance of new shares in exchange for a
proportionately greater number of old shares), any recapitalization of the Nevada corporation, any merger or consolidation of the Nevada
corporation with any of its subsidiaries, or any other transaction, whether or not with or into or otherwise involving the interested
stockholder, under any agreement, arrangement or understanding, whether or not in writing, with the interested stockholder or any affiliate
or associate of the interested stockholder, which has the immediate and proximate effect of increasing the proportionate share of the
outstanding shares of any class or series of voting shares or securities convertible into voting shares of the Nevada corporation or
any subsidiary of the Nevada corporation which is beneficially owned by the interested stockholder or any affiliate or associate of the
interested stockholder, except as a result of immaterial changes because of adjustments of fractional shares; and (vi) any receipt
by the interested stockholder or any affiliate or associate of the interested stockholder of the benefit, directly or indirectly, except
proportionately as a stockholder of the Nevada corporation, of any loan, advance, guarantee, pledge or other financial assistance or
any tax credit or other tax advantage provided by or through the Nevada corporation.
For purposes of the NRS,
an "interested stockholder" is defined to include any person, other than the Nevada corporation or any subsidiary of the Nevada
corporation, that is: (a) a beneficial owner, directly or indirectly, of 10% or more of the voting power of the outstanding voting
shares of the Nevada corporation or (b) an affiliate or associate of the Nevada corporation and was, at any time within two years
immediately before the date in question, the beneficial owner, directly or indirectly, of 10% or more of the voting power of the then-outstanding
shares of the Nevada corporation.
Subject to certain exceptions,
the provisions of the NRS statute governing combinations with interested stockholders provide that a Nevada corporation may not engage
in a combination with an interested stockholder for two years after the date that the person first became an interested stockholder unless
(i) the combination or the transaction by which the person first became an interested stockholder is approved by the board of directors
before the person first became an interested stockholder or (ii) during the two-year period, the transaction is approved by the
board and by 60% of the disinterested stockholders at an annual or special meeting of the stockholders.
After such two-year period,
corporations subject to these statutes may not engage in specified business combinations and transactions unless: (i) the business
combination or transaction by which the person first became an interested stockholder is approved by the board of directors before the
stockholder became an interested stockholder; (ii) the business combination is approved by a majority of the outstanding voting
power (excluding the shares held by the interested stockholder or any affiliate or associate of the interested stockholder); or (iii) the
combination meets the requirements of 78.411 through 78.444 of the NRS, inclusive.
The NRS allows a corporation
to "opt out" of NRS 78.411 through 78.444, inclusive, by providing in such corporation's original articles of incorporation
or bylaws that such statutes do not apply to the corporation. Unless certain limited exceptions apply, corporations cannot opt out of
such statutes by amending their articles of incorporation or bylaws. We have not opted out of such statutes.
Control
Share Acquisitions. The NRS also contains a "control share acquisitions statute." If applicable to a Nevada
corporation, this statute restricts the voting rights of certain stockholders referred to as "acquiring persons," that acquire
or offer to acquire ownership of a "controlling interest" in the outstanding voting stock of an "issuing corporation."
For purposes of these provisions (i) a "controlling interest" means, with certain exceptions, the ownership of outstanding
voting stock sufficient to enable the acquiring person to exercise one-fifth or more but less than one-third, one-third or more but less
than a majority, or a majority or more of all voting power in the election of directors and (ii) an "issuing corporation"
means a Nevada corporation, as of any date, that has 200 or more stockholders of record, at least 100 of whom have addresses in Nevada
appearing on the stock ledger of the corporation at all times during the 90 days immediately preceding such date, and which does business
in Nevada directly or through an affiliated corporation. The voting rights of an acquiring person in the affected shares will be restored
only if such restoration is approved by the holders of a majority of the voting power of the corporation (excluding the shares held by
the acquiring person) at an annual or special meeting of the stockholders.
The NRS allows a corporation
to "opt out" of the control share acquisitions statute by providing in such corporation's articles of incorporation or bylaws,
in effect on the 10th day following the acquisition of a controlling interest by an acquiring person, that the control share acquisitions
statute does not apply to the corporation or to an acquisition of a controlling interest specifically by types of existing or future
stockholders, whether or not identified. We have not opted out of the control share acquisitions statute.
Liability and Indemnification of Directors and Officers
NRS Sections 78.7502 and
78.751 provide us with the power to indemnify any of our directors, officers, employees or agents, or any person who serves or served
at the corporation’s request as a director, officer, employee or agent of another corporation, partnership, joint venture, trust
or other enterprise (for purposes of this section, the “Indemnitee” or “Indemnitees”) against expenses, including
attorneys’ fees, actually and reasonably incurred related to any threatened, pending or completed action, suit or proceeding (whether
civil, criminal, administrative or investigative) arising by reason of an Indemnitee’s status as a director, officer employee or
agent of the corporation if: (i) the Indemnitee is not liable for breach of fiduciary duties to the corporation involving intentional
misconduct, fraud or knowing violation of law; (ii) the Indemnitee conducted himself or herself in good faith and reasonably believes
that his or her conduct was in, or not opposed to, our best interests; or (iii) in a criminal action, the Indemnitee must not have
had reasonable cause to believe that his or her conduct was unlawful. NRS Section 78.751 requires us to indemnify any Indemnitee
for any expenses referenced above if the Indemnitee has been successful on the merits or otherwise in defense of the foregoing actions,
suits or proceedings.
Under NRS Section 78.7502,
any discretionary indemnification, unless ordered by a court or advanced by the corporation in accordance with NRS Section 78.751(2),
can only occur if deemed proper by (i) the stockholders; (ii) a majority vote of a quorum consisting of disinterested directors;
or (iii) an independent counsel’s written legal opinion (if such an approach is approved by a majority vote of a quorum consisting
of disinterested directors or if a quorum consisting of disinterested directors cannot be obtained). Under NRS Section 78.751(2),
advances for expenses may be made by agreement if the Indemnitee affirms in writing that he or she believes that he or she has met the
statutory standards and will personally repay the expenses if a court of competent jurisdiction determines that such Indemnitee did not
meet the statutory standards.
Our amended and restated
bylaws include an indemnification provision under which we have the power to indemnify, to the extent permitted under Nevada law, our
current and former directors and officers, or any person who serves or served at our request for our benefit as a director or officer
of another corporation or our representative in a partnership, joint venture, trust or other enterprise, against all expenses, liability
and loss reasonably incurred by reason of being or having been a director, officer or representative of ours or any of our subsidiaries.
We may make advances for expenses upon receipt of an undertaking by or on behalf of the director or officer to repay the amount if it
is ultimately determined by a court of competent jurisdiction that he, she or it is not entitled to be indemnified by us.
Our amended and restated
articles of incorporation provides that we shall indemnify directors and officers to the fullest extent permitted by the NRS. Our amended
and restated articles of incorporation also provide a limitation of liability such that no director or officer shall be personally liable
to us or any of our stockholders to the fullest extent permitted by the NRS.
Insofar as indemnification
for liabilities arising under the Securities Act of 1933, as amended (the “Securities Act”) may be permitted to directors,
officers and controlling persons of ours under Nevada law or otherwise, we have been advised that the opinion of the SEC is that such
indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event a claim for
indemnification against such liabilities (other than payment by us for expenses incurred or paid by a director, officer or controlling
person of ours in successful defense of any action, suit, or proceeding) is asserted by a director, officer or controlling person in
connection with the securities being registered, we will, unless in the opinion of our legal counsel the matter has been settled by controlling
precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by our company is against public
policy in the Securities Act and will be governed by the final adjudication of such issue.
Nasdaq Capital Market Listing
Our common stock is listed on the Nasdaq Capital
Market under the symbol “XXII.”
Transfer Agent and
Registrar
The transfer agent and registrar for our common
stock is Continental Stock Transfer & Trust Company, One State Street, 30th Floor, New York, NY 10004-1561.
BUSINESS
Overview
22nd
Century Group, Inc. is a tobacco products company with sales and distribution of our own proprietary new reduced nicotine tobacco
products authorized as Modified Risk Tobacco Products by the FDA. Additionally, we provide contract manufacturing services for conventional
combustible tobacco products for third-party brands.
Our
mission in tobacco is dedicated to mitigating the harms of smoking through our proprietary reduced nicotine content (“RNC”)
tobacco plants and our Very Low Nicotine, VLN® combustible cigarette products.
In December 2021, we secured the first and only authorization from the FDA to market a combustible cigarette, our brand VLN®
as a Modified Risk Tobacco Product (“MRTP”) using certain reduced nicotine exposure
claims. In April 2022, the inaugural launch of our proprietary VLN® cigarettes
commenced through a pilot program in select Circle K stores in and around Chicago, Illinois. Building on the success of the pilot,
we initiated a phased rollout strategy in 2023, progressing state by state and region by region to a store footprint spanning more than
5,000 stores in 26 states. Our VLN® tobacco products are supported by a substantial
intellectual property portfolio comprising issued patents and patent applications related to tobacco plants, and in particular our reduced
nicotine tobacco plants.
In
addition to continued focus on VLN®, we renewed our focus on utilizing our
tobacco assets to attract additional tobacco business to help fund the growth of VLN®.
In addition to existing business relationships with multiple tobacco products companies, we will continue to expand the number of brands
in our contract manufacturing operations (“CMO”) portfolio in 2024.
GVB Divestiture
On
December 22, 2023, we completed the sale of substantially all of the GVB hemp/cannabis business (referred to as the “GVB Divestiture”).
As a result, we have classified the results of operations of the hemp/cannabis segment and disposal group as discontinued operations
in the Consolidated Statements of Operations for all periods presented. Additionally, the associated assets and liabilities linked to
the discontinued operations have been designated as held for sale in the Consolidated Balance Sheet as of December 31, 2023 and
2022, respectively. All results and information presented exclude the hemp/cannabis segment and disposal group unless otherwise noted.
For more detailed information regarding the divestiture, please refer to Note 2, titled “Discontinued Operations and Divestiture,”
in the Notes to Consolidated Financial Statements included herein.
Tobacco Overview
Our unwavering commitment
is centered around reducing the effects of nicotine from smoking and smoking cessation. We believe we can achieve this mission through
the commercialization of our proprietary RNC tobacco plants and cigarette products, prominently featured in our VLN®
brand. These products contain 95% less nicotine content compared to conventional tobacco
and cigarettes, which are intended to help users smoke less. The urgency of our mission is underscored by alarming statistics –
the FDA publicly acknowledged on July 28, 2017, that tobacco use remains the leading cause of preventable disease and death in the
United States. The repercussions include over 480,000 deaths annually and an economic toll of nearly $300 billion in lost productivity
and direct health care costs, as reported the U.S. Centers for Disease Control and Prevention (“CDC”).
Our
innovative approach involves utilizing both genetically modified organism (“GMO”) and non-GMO methods to modify and develop
proprietary bright, burley, and oriental RNC tobaccos, ensuring they grow with at least 95% less nicotine content. Our SPECTRUM®
research cigarettes, developed in collaboration with independent researchers, officials from the FDA, the National Institute on
Drug Abuse (“NIDA”), which is part of the National Institutes of Health (“NIH”), the National Cancer Institute
(“NCI”), and the CDC, have played and continue to play a crucial role in independent clinical studies, with more than 32.8
million variable nicotine research cigarettes provided since 2011. The extensive body of scientific evidenced derived from these studies,
published in peer-reviewed journals, including the New England Journal of Medicine and the Journal of the American Medical
Association, supports the potential impact of our RNC tobaccos. Smokers who opt for our RNC cigarettes in clinical studies experienced
reductions in smoking (measured in cigarettes per day), nicotine exposure, and dependence, coupled with minimal or no evidence of compensatory
smoking or withdrawal and without serious adverse events. A list of ongoing as well as completed and published clinical studies using
cigarettes made with our RNC tobaccos may be viewed at https://www.xxiicentury.com/vln-clinical-studies/published-clinical-studies-on-very-low-nicotine-content-vlnc-cigarettes.
These studies showed that smokers who used for RNC cigarettes increased their frequency of smoke-free days and doubled their efforts
to quit smoking. SPECTRUM® research cigarettes persist as a key component in various independent scientific studies, aimed
at substantiating the public health advantage acknowledged by the FDA and other entities. This advantage is associated with the FDA’s
proposal to establish a national product standard requiring that all cigarettes incorporate “minimally or nonaddictive” levels
of nicotine. Notably, our SPECTRUM® variable nicotine research cigarettes serve as the precursor to our innovative VLN®
cigarette products.
Our conviction in the significant
global market potential of our proprietary RNC cigarettes, marketed under the brand name VLN®, is rooted in substantial
data. As outlined in a 2021 report by the Foundation for a Smoke Free World, global full nicotine cigarette retail sales reached an estimated
84.1% or $612 billion of the $853 billion market for products that contain nicotine. The statistics from the CDC and the World Health
Organization (“WHO”) highlight a substantial market, with over 1 billion global adult smokers and 30 million in the U.S.
Despite the prevalence of
various nicotine delivery systems, including vaping, our belief is that smokers are actively seeking alternatives to traditional addictive
combustible cigarettes. Our confidence is reinforced by consumer perception studies, in which 60% of adult smokers expressed a likelihood
to adopt VLN® as their preferred choice. Importantly, VLN® is currently available in the market for sale,
positioning itself as a viable option for smokers seeking reduced harm alternatives.
Our VLN® cigarettes
are currently available in a large number of top U.S. markets and present a groundbreaking alternative with 95% less nicotine content
than conventional cigarettes. Maintaining a familiar combustible product format, VLN® replicates the conventional cigarette
smoking experience, encompassing sensory and experiential elements such as taste, scent, smell, and the familiar “hand-to-mouth”
behavior.
The tobacco in VLN®
cigarettes is meticulously crafted to contain a targeted 0.5 milligrams of nicotine per gram of tobacco, a threshold recognized
by the FDA, based on clinical studies, as “minimally or non-addictive.” We believe the reduced nicotine content of VLN®
can establish a dissociation between the act of smoking and the rapid introduction of nicotine to the bloodstream, which extensive
clinical data indicates helps smokers to smoke less and potentially quit.
The results of numerous completed
studies serve as an independent scientific foundation for the FDA’s advanced notice of proposed rule-making (“ANPRM”)
on July 28, 2017, which announced FDA’s intention to institute a new rule to require that all combustible cigarettes
sold in the United States contain only minimally or non-addictive levels of nicotine, also referred to as the Comprehensive Plan for
Tobacco and Nicotine Regulation. Although this proposal has not yet been finalized or adopted by the FDA, the announcement supported
our decision to submit and seek modified risk orders under MRTP applications (“MRTPAs”) for our VLN products. On December 23,
2021, we received authorization to market our VLN® cigarettes as a Modified Risk Tobacco Product (“MRTP”)
using certain modified exposure claims.
We initiated efforts to offer
our proprietary VLN® cigarettes for domestic sale after receiving the modified risk granted order. Furthermore, we continue
to plan to evaluate opportunities to make VLN® available for international sale or licensing by third parties.
Proposed Government Mandates Limiting the
Nicotine in Cigarettes.
In a press release dated
June 16, 2010, Dr. David Kessler, a former FDA Commissioner, advocated for swift action by the FDA to decrease nicotine levels
in cigarettes to non-addictive thresholds. Dr. Kessler emphasized that lowering the stimulus level would consequently diminish cravings,
deeming it the “ultimate harm reduction strategy.” Shortly thereafter, in a Washington Post article, Dr. Kessler
proposed reducing the nicotine content in cigarettes from approximately 10 milligrams to less than 1 milligram. Notably, VLN®
cigarettes contain between 0.3 to 0.7 mg/g.
In 2015, the WHO Study Group
on Tobacco Product Regulation issued an advisory note endorsing a global nicotine reduction strategy, urging limitations on the sale
of cigarettes to brands with nicotine content insufficient for addiction development or maintenance. Although the WHO did not specify
absolute threshold for addiction, it suggested a likely threshold equal to or possibly less than 0.4 mg/g of dry cigarette tobacco filler.
Our proprietary SPECTRUM® research cigarettes were cited in the WHO study as meeting this low nicotine level criterion
at 0.4 mg/g of cigarette tobacco filler.
The WHO report concluded
that establishing a maximum allowable nicotine content for all cigarettes could (i) reduce the initiation of smoking and progression
to addiction, (ii) decrease smoking prevalence among addicted smokers through behavioral extinction, and (iii) increase quit
rates while reducing relapse rates. Emphasizing population-wide benefits, the report highlighted the potential decrease in combusted
tobacco use among current cigarette smokers and the prevention of non-smokers, particularly young people, from developing addiction to
cigarettes.
On July 28, 2017, in
connection with the ANPRM then-FDA Commissioner Scott Gottlieb, M.D., announced the FDA’s intention to use its authority under
the Tobacco Control Act to require that all combustible cigarettes sold in the United States contain only minimally or non-addictive
levels of nicotine. We believe this announcement marked a significant step towards reducing the addictive nature of cigarettes.
Following this announcement,
on August 16, 2017, FDA Commissioner Scott Gottlieb, M.D., and Mitchell Zeller, J.D., the Director of the FDA’s Center for
Tobacco Products (“FDA/CTP”), authored and titled “A Nicotine-Focused Framework of Public Health,” published
in The New England Journal of Medicine. The article discussed the regulatory tool provided by the Tobacco Control Act, known as
a tobacco “product standard,” which could be employed to alter the addictiveness of combustible cigarettes. While the statute
prohibited reducing nicotine yields to zero, the FDA asserted its clear authority to otherwise reduce nicotine levels. The conclusion
drawn was that a nicotine-limiting standard could render cigarettes minimally or non-addictive, aiding current users in quitting and
preventing most future users from developing addiction. The FDA emphasized its commitment to being guided by scientific principles in
shaping health policy. This commitment was reiterated in the context of addressing nicotine levels in cigarettes, underlining the importance
of evidence-based decision-making.
We believe that recent political
changes and perceptions towards nicotine addiction have the potential to be favorable to our business prospects from a policy priority
and regulatory standpoint. Under the new leadership at the FDA and Center for Tobacco Products (“CTP”), we believe that the
FDA could refocus on implementing its ground-breaking Comprehensive Plan for Tobacco and Nicotine Regulation, and specifically could
renew efforts to cap the amount of nicotine in combustible cigarettes to a “minimally or non-addictive” level. We believe
that the MRTP authorization and the launch of our VLN® cigarettes could serve as a powerful catalyst supporting any such
policies.
For example, on January 27,
2022, the FDA posted an update on its FDA Voices site stating that it “remains on track” with its plans to prohibit menthol
in combustible tobacco products. The FDA published a proposed tobacco product standard to ban menthol as a characterizing flavor in cigarettes
in April 2022. The proposed FDA rule includes a process for firms to request an exemption from the standard for specific products
of certain types on a case-by-case basis, indicating “reduced nicotine” as an example of such an exemption. On August 1,
2022, we submitted public comments in support of a tobacco product standard for menthol in cigarettes.
In June 2022, the FDA
announced that the Biden-Harris Administration published plans for future regulatory action that includes the FDA’s plans to develop
a proposed product standard that would establish a maximum nicotine level to reduce the addictiveness of cigarettes and certain other
combusted tobacco products. On June 21, 2022, a proposed rule for a tobacco product standard for nicotine level of certain
tobacco products was published in the Spring 2022 Unified Agenda of Regulatory and Deregulatory Actions.
In late October 2022,
in coordination with the FDA, the National Institute on Drug Abuse (NIDA), and others, we received an order for 2.8 million variable
nicotine cigarettes. We believe our research cigarettes will continue to fuel numerous independent, scientific studies that could evaluate
the potential benefits suggested by the FDA and others of implementing a national standard requiring all cigarettes to contain minimally
or non-addictive levels of nicotine.
The FDA rule making
process continued to advance throughout 2023 on both the proposed menthol ban and the proposed reduced nicotine content standard, but
announcement of an FDA proposed rule was delayed multiple times for additional public comment and analysis.
We continue to advance on
our reduced nicotine technology as we believe that our next generation, non-GMO plant research is the key to commercializing our reduced
nicotine content tobacco and technology in international markets where non-GMO products are preferred or where GMO products are banned.
Our patented, non-GMO technology can introduce very low nicotine traits into virtually any variety of tobacco, including bright, burley,
and oriental. We have successfully applied our non-GMO technology to bright and burley varieties of tobacco and have initiated commercial
growing activities for our non-GMO bright and burley reduced nicotine varieties. We anticipate commercial production of our American
blend cigarettes featuring a mix of bright and burley VLN® tobacco varieties to begin in 2024.We believe that our RNC
tobacco technology and our production and delivery of millions of proprietary variable nicotine research cigarettes since 2011 demonstrates
the technical achievability of the FDA’s plan to dramatically reduce nicotine in cigarettes.
In the United States, we
are focused on working with the FDA on its efforts to reduce nicotine in cigarettes. Outside the United States, we will focus on working
with WHO-member countries that desire to utilize our proprietary RNC tobacco to implement the WHO recommendation of limiting the sale
of cigarettes to brands with a nicotine content that is not sufficient to lead to development and/or maintenance of addiction.
Modified Risk Tobacco Products (MRTP)
The Family Smoking Prevention
and Tobacco Control Act of 2009 (“Tobacco Control Act”) granted the FDA authority over the regulation of all tobacco products
in the United States. The Tobacco Control Act further establishes procedures for the FDA to regulate the labeling and marketing of so-called
MRTP, which includes, among other things tobacco products that may (i) reduce harm or the risk of tobacco-related disease or (ii) reduce
or eliminate exposure to a substance. The Tobacco Control Act also includes provisions allowing the submission and authorization of a
Premarket Tobacco Product Application (“PMTA”) for a new tobacco product, where the PMTA includes scientific data that demonstrates
the new tobacco product is appropriate for the protection of public health.
On December 5, 2018,
we submitted to the FDA a PMTA and on December 17, 2019, the FDA issued a marketing order in response to our PMTA. While the FDA’s
marketing order authorized us to market the products in the U.S., it did not allow us to make reduced exposure claims which would indicate
that the product contains 95% less nicotine. Marketing such reduced exposure claims requires the FDA to authorize an MRTPA.
Because of this, on December 27,
2018, we submitted to the FDA an MRTPA, seeking FDA authorization to market our reduced nicotine combustible cigarettes with certain
reduced exposure claims. In the MRTPA, we requested authorization from the FDA to market our reduced nicotine tobacco cigarettes with
certain product labeling claims under the brand name of VLN®.
On December 23, 2021,
we secured the first and only MRTP designation for a combustible cigarette for VLN® King and VLN® Menthol
King 95% reduced nicotine content cigarettes. The FDA authorized the marketing of VLN® with the following reduced exposure
claims: “95% less nicotine”, “Helps reduce your nicotine consumption”, and “Greatly reduces
your nicotine consumption,”. The FDA also required that any use of these claims be accompanied by the statement that the product
“Helps You Smoke Less,” which we consider an evidence-based claim supporting our products.
In previous years, we contracted
with farmers to grow considerable quantities of RNC tobacco in anticipation of FDA authorization of our MRTP and subsequent commercial
launch of VLN® cigarettes. In January 2022, at our manufacturing facility in North Carolina, we produced the first
cartons of our VLN® reduced nicotine cigarettes, destined for commercial sale. In April 2022, we launched VLN®
cigarettes in the U.S. market. We believe that the commercialization of VLN® cigarettes will create further opportunities
for us to license our proprietary technology tobaccos and the VLN® brand.
VLN® Commercialization Plan
In
April 2022, we initiated VLN® sales in more than 150 Circle K stores in the Chicago metro area through a pilot launch.
After the pilot concluded and given the positive results, we made the decision to further deepen our reach in the state of Illinois and
launch VLN® in Colorado to more than 3,000 potential locations across the state with our network of retailers and distribution
partners, including Eagle Rock Distributing Company and Creager Mercantile.
The pilot enabled us to refine
our VLN® rollout strategy and helped us develop our VLN® sales launch blueprint, an efficient, reproducible
sales plan that focuses our resources to achieve the greatest returns. In November 2022, we announced that we intend to expand our
VLN® launch to Arizona, New Mexico, and Utah, and announced plans to expand into up to 18 U.S. states over the following
12 months. In January 2023 we announced distribution partnerships with Core-Mark International and Eby-Brown Company, two of the
largest convenience store distributors in the U.S., providing access to retailers in virtually every key U.S. market.
By concentrating and going
deeper into select geographies and markets with high cigarette volume and large adult smoker populations, we believe we can capture greater
market share effectively. We also plan to target states where there is a tax exemption for MRTP. As of March 1, 2024, we have secured
regulatory authorizations to sell VLN® in 48 states and the District of Columbia. At year-end 2023, our
phased rollout strategy, progressing state by state and region by region, had placed VLN into a store footprint spanning more than 5,000
stores in 26 states.
Tobacco Master Settlement Agreement
In September 2013, we
entered into a Membership Interest Purchase Agreement (the “NASCO Acquisition”) to purchase all the issued and outstanding
membership interests of NASCO, a federally licensed tobacco product manufacturer and subsequent participating manufacturer under the
Master Settlement Agreement (“MSA”). The MSA is an accord reached in November 1998 between the State Attorneys General
of 46 states, five U.S. territories, the District of Columbia and the five largest tobacco companies in the United States concerning
the advertising, marketing and promotion of tobacco products. The MSA also set standards for, and imposes restrictions on, the sale and
marketing of cigarettes by participating cigarette manufacturers. On August 29, 2014, we entered into an Amended Adherence Agreement
with the 46 Settling States under the MSA pursuant to which the Company was approved to acquire NASCO and become a subsequent participating
manufacturer under the MSA. On that same date, we closed the NASCO Acquisition and became a subsequent participating manufacturer under
the MSA. NASCO has since been our wholly-owned subsidiary.
Tobacco Manufacturing
We lease our cigarette manufacturing
facility and warehouse located in Mocksville, North Carolina. In 2013, we purchased certain (i) cigarette manufacturing equipment,
and (ii) equipment parts, factory items, office furniture and fixtures, vehicles and computers from the bankruptcy estate of PTM
Technologies, Inc. for approximately $3.2 million.
The facility was primarily
in a pre-manufacturing stage during 2014 as we sought approval during that time for us to become a subsequent participating manufacturer
under the MSA. On August 29, 2014, we became a subsequent participating manufacturer under the MSA. Since 2015, we have manufactured
and sold our SPECTRUM® variable nicotine research cigarettes, as well as third-party filtered cigar brands and MSA-compliant
cigarette brands, at our factory in North Carolina.
The strategic acquisition
of our factory has allowed us to become vertically integrated so that we can control production priorities/timing and maintain the required
high quality of our products, including our SPECTRUM® research cigarettes and our MRTP-designated VLN®
brand cigarettes featuring 95% less nicotine than the top 100 leading brands sold in the United States. In January 2022, our cigarette
manufacturing facility began production of VLN® King and VLN® Menthol King cigarettes. With high-speed
manufacturing capabilities we continue to attract additional CMO business to absorb our manufacturing overhead and help keep our unit
cost profile low.
In 2023, we leased additional
warehouse space in Winston-Salem, North Carolina. This bonded and temperature conditioned space will further support VLN®
growth and provide additional distribution opportunities for customers.
Tobacco Sources of Raw Materials
We obtain our reduced nicotine
tobacco leaf from third party-growers, primarily in multiple states in the United States who are under direct contracts with us. These
contracts prohibit the transfer of our proprietary tobaccos, seeds and plant materials to any other party. We purchase conventional tobacco
destined for contract manufacturing operations through third parties.
Research & Development (R&D) &
Intellectual Property (IP)
Tobacco R&D
Since our inception, most
of our research and development (“R&D”) efforts have been outsourced to highly qualified groups in their respective fields.
Since 1998, we have had multiple R&D agreements with North Carolina State University (“NCSU”) and others resulting in
exclusive worldwide licenses to various patented technologies. We have utilized the same model employed by many public-sector research
organizations, which entails obtaining an exclusive option or license agreement to any invention arising out of our funded research.
In all such cases, we fund and control all patent filings as the exclusive licensee. This model of contracting with public-sector researchers
has enabled us to control R&D costs while achieving our desired results, including obtaining exclusive intellectual property rights
relating to our outsourced R&D.
On June 22, 2018, we
entered into an amendment to our existing license agreement with NCSU under which we exclusively licensed several bright and burley tobacco
plant lines with Very Low Nicotine Content that are not genetically modified (non-GMO) plants. The amendment provided for us to pay NCSU
a total exclusive license fee of $1.2 million. We will also pay running royalties to NCSU based on a portion of the net sales revenue
received by us from sales of products that contain any portions of the plant materials that have been received by us from NCSU.
On October 22, 2018,
we entered into a license agreement with the University of Kentucky (“UK”) to license on a non-exclusive basis a next-generation
very low nicotine content burley tobacco plant lines that are not genetically modified (non-GMO) plants. The UK license agreement provided
for us to pay UK a total license fee of $1.2 million. We will also pay running royalties to UK based on a portion of the net sales revenue
received from sales of products that contain any portions of the plant materials that have been received from UK.
On December 1, 2021,
we relocated our laboratory from Buffalo, New York to Rockville, Maryland, where we were conducting our own proprietary research and
development activities in tobacco. In February 2024, we relocated our laboratory activities to our Mocksville, NC manufacturing
facility. This reduces the fixed cost of our research and development activities, plus provides us an advantage with the proximity to
our factory and NCSU.
In 2022, our R&D collaboration
with NCSU delivered the proof of concept and field data for a new gene combination (non-GMO) to reduce nicotine below 95%. This unique
gene combination enables the production of a better-quality leaf and an increase in yield. In January 2022, a utility patent to
protect the new combination was filed. Our exclusive NCSU collaboration also yielded proof of concept and field data for Oriental lines
with a 90-95% nicotine reduction. These results will give us the option in the future to produce VLN cigarettes that comprise burley,
oriental, and bright tobacco thus improving overall quality. In addition, this year we extended our VLN production field trial to include
new burley and flue-cured VLN (non-GMO).
We are currently developing
new versions of our RNC cigarettes utilizing these non-GMO tobacco lines for future commercialization in the U.S. and globally.
Tobacco IP
Our intellectual property
enables us to alter the level of nicotine and other nicotinic alkaloids in tobacco plants through genetic engineering and modern plant
breeding. The basic techniques include, but are not limited to, those that are used in the production of genetically modified and gene-edited
varieties of other crops, which are also known as “biotech crops.”
We have extensive patent
protection and exclusive rights covering tobacco plants with altered nicotine content produced by modifying the expression of genes that
control the biosynthesis of nicotine in the tobacco plant. Our patent families related to nicotine biosynthesis are expected to expire
between 2026 and 2043, with certain extensions of terms in the U.S. applications resulting from patent term adjustments at the U.S. Patent
and Trademark Office (a “patent family” is a set of patent applications and patents, filed in various countries, that relate
to at least one common earlier application).
Plant variety protection
(“PVP”) certificates are issued in the United States by the U.S. Department of Agriculture. A PVP certificate prevents anyone
other than the owner/licensee from planting, propagating, selling, importing, or exporting a plant variety for twenty (20) years
in the U.S. and, generally, for twenty (20) years in other member countries of the International Union for the Protection of New
Varieties of Plants, known as UPOV, an international treaty concerning plant breeders’ rights. There are currently more than 70
countries that are members of UPOV. Our current RNC tobaccos are protected by our patent portfolio.
In addition to our patents,
patent applications, and PVP certificates, we own various registered trademarks in the United States and around the world. In November 2023,
we signed an additional reduced nicotine content technology license with NCSU, providing additional modes of efficiently producing reduced
nicotine content tobacco plants and extending our IP portfolio. This license will provide exclusive rights to the technology until 2042.
Government Regulation
The development, testing,
manufacturing, and marketing of our products and potential products are subject to extensive regulation by governmental authorities in
the United States and throughout the world.
FDA Regulation of Tobacco Products
The
Family Smoking Prevention and Tobacco Control Act (“Tobacco Control Act”) amended the Federal Food, Drug, and Cosmetic Act
(“FDCA”) to provide the FDA with broad authority to regulate the manufacture, quality control, advertising, promotion, labeling,
packaging, storage, distribution, recordkeeping, premarket authorization, post-authorization monitoring and post-authorization reporting
of tobacco products, including our tobacco products. Among its authorities, the FDA requires that manufacturers of tobacco products first
introduced or modified after February 15, 2007, undergo premarket review and obtain premarket authorization prior to commercialization.
While the Tobacco Control Act prohibits the FDA from banning cigarettes outright, or mandating that nicotine levels be reduced
to zero, it does allow the FDA to require the reduction of nicotine or other compounds in tobacco and cigarette smoke. The FDA has authority
to restrict marketing and advertising, impose regulations on packaging, mandate warnings and disclosure of flavors or other ingredients,
prohibit the sale of tobacco products with certain flavors or other characteristics, limit or prohibit the sale of tobacco products by
certain retail establishments and the sale of tobacco products in certain packaging sizes, and seek to hold retailers and distributors
responsible for the adverse health effects associated with both smoking and exposure to environmental tobacco smoke. In 2009, the Tobacco
Control Act also banned all sales in the United States of cigarettes with flavored tobacco (other than menthol). As of June 2010,
all cigarette companies were required to cease use of the terms “low tar,” “light” and “ultra light”
in describing cigarettes sold in the United States.
The Tobacco Control Act,
its implementing regulations and its 2016 deeming regulations establish broad FDA regulatory authority over all tobacco products and,
among other provisions:
| · | impose restrictions
on the advertising, promotion, sale and distribution of tobacco products; |
| · | establish pre-market
review pathways for new and modified tobacco products; |
| · | prohibit
any express or implied claims that a tobacco product is or may be less harmful than other
tobacco products without FDA authorization; |
| · | authorize the FDA
to impose tobacco product standards that are appropriate for the protection of the public
health; and |
| · | equip
the FDA with a variety of investigatory and enforcement tools, including the authority to
inspect product manufacturing and other facilities. |
The Tobacco Control Act requires
manufacturers of tobacco products to, among, other things, provide FDA with a list of ingredients added to tobacco products in the manufacturing
process and register any establishment engaged in the manufacture, preparation, or processing of a tobacco product. The manufacture of
products is subject to strict quality control, testing and record-keeping requirements, and continuing obligations regarding the submission
of safety reports and other post-market information. The FDA has several investigatory and enforcement tools available to it, including
document requests and other required information submissions, facility inspections, examinations and investigations, injunction proceedings,
monetary penalties, product withdrawal and recall orders, and product seizures.
The Tobacco Control Act also
authorizes FDA to promulgate regulations requiring that the methods used in, and the facilities and controls used for, the manufacture,
preproduction design validation, packing, and storage of a tobacco product conform to current good manufacturing practice (“CGMP”).
On March 8, 2023, FDA issued a proposed rule to promulgate such regulations. The proposed rule, if finalized, would establish
requirements for manufacturers of finished and bulk tobacco products on the methods used in, and the facilities and controls used for,
the manufacture, pre-production design validation, packing, and storage of tobacco product.
Regulation of Menthol Cigarettes
In April 2022, the FDA
announced proposed product standards to prohibit menthol as a characterizing flavor in cigarettes) and prohibit all characterizing flavors
(other than tobacco) in cigars. In January 2023, the Semi-Annual Agenda for Fall 2022 was released in the US. Here, the Department
of Health and Human Services (HHS), stated that it intended to issue a final rule on Menthol in Cigarettes. This product standard,
if enacted, would prohibit menthol as a characterizing flavor in cigarettes. Although this proposed rule was expected to be finalized
in August 2023, its implementation has been delayed. There has been increasing activity on the state and local levels with respect
to scrutiny of menthol and flavored tobacco products. For example, in 2022, the State of California banned tobacco retailers from selling
most flavored and menthol tobacco products, including VLN® Menthol King. The state of Massachusetts has similar laws prohibiting
the sale of flavored tobacco sales, including menthol cigarettes.
Premarket Tobacco Product Application (PMTA)
Certain of our products,
including our low nicotine cigarettes, are marketed in the United States pursuant to a PMTA. Under Section 910(b) of the FDCA,
a PMTA can be submitted for any new tobacco product seeking a marketing order to enable commercialization of a new tobacco product in
the United States. For FDA to grant such an order, the PMTA must enable the FDA to determine that: (1) permitting the marketing
of the new tobacco product would be appropriate for the protection of the public health; (2) the methods used in, or the facilities
and controls used for, the manufacture, processing, or packing of the product conform to the requirements of Section 906(e) of
the FD&C Act (21 U.S.C. 387f(e)); (3) the product labeling is not false or misleading in any particular; and (4) the product
complies with any applicable product standard in effect under section 907 of the FDCA or that there is adequate information to justify
a deviation from such standard. In determining whether to authorize a PMTA, FDA considers, among other things:
| · | risks and benefits
to the population as a whole, including people who would use the proposed new tobacco product
as well as nonusers; |
| · | whether people who
currently use any tobacco product would be more or less likely to stop using such products
if the proposed new tobacco product were available; |
| · | whether
people who currently do not use any tobacco products would be more or less likely to begin
using tobacco products if the new product were available; and |
| · | the methods, facilities,
and controls used to manufacture, process, and pack the new tobacco product. |
Once a PMTA is submitted
FDA conducts an initial acceptance review to determine whether the product falls under CTP jurisdiction and to confirm that the statutory
and regulatory requirements of an application are met based upon the criteria set forth in the Tobacco Control Act. The FDA endeavors
to complete its acceptance review within 21 to 60 days of receipt. If the application does not appear to contain the required information
(except for product samples), the FDA may refuse to accept the application for review, and in either case, will notify the applicant.
Once accepted for further review, the FDA makes a threshold determination of whether the application contains enough information to permit
a substantive review, referred to as “filing,” and may refuse to file any application that does not include sufficient information.
Once filed, the FDA intends to complete its review of a PMTA within 180 days of receipt, however the FDA’s review period may be
paused or even restarted in response to new information from the applicant, and as such, FDA’s review may take significantly longer
than expected. After the FDA completes its review of a PMTA, the FDA may issue a marketing denial order letter, or issue a marketing
granted order letter. A marketing granted order becomes effective on the date it is issued in response to a PMTA and permits the new
tobacco product to be legally marketed in the United States.
A marketing order may include
restrictions on the sale and distribution of the product, including restrictions on the access to, and the advertising and promotion
of, the tobacco product, and unique requirements for record-keeping and post market reporting, among other things. Holders of authorized
PMTAs are, among other things, required to submit detailed periodic and annual reports to the FDA within specified timelines, and are
further required to submit reports for serious and unexpected adverse events associated with the product. Once granted the FDA may suspend
or withdraw any marketing order on various grounds, such as a determination that the continued marketing of the tobacco product is no
longer appropriate for the protection of public health, or where the PMTA holder has failed to comply with applicable post-market requirements.
Modified Risk Tobacco Products (MRTP)
Certain of our products,
including our VLN® cigarettes, are marketed in the United States as MRTPs. MRTPs are tobacco products that are sold or distributed
for use to reduce harm, or the risk of tobacco-related disease associated with commercially marketed tobacco products. Before an MRTP
can be introduced or delivered into interstate commerce in the United States, the FDA must issue a either a “risk modification
order” or “exposure modification order” pursuant to the Tobacco Control Act. An order permitting the sale of an MRTP,
if granted by the FDA, enables the applicant to utilize certain claims with respect to a single, specific product, not an entire class
of tobacco products.
To obtain a risk modification
order under the FDCA, an applicant must demonstrate that the product, as it is actually used by consumers, will: (i) significantly
reduce harm and the risk of tobacco-related disease to individual tobacco users; and (ii) benefit the health of the population as
a whole; taking into account both users of tobacco products and persons who do not currently use tobacco products. To obtain an exposure
modification order under the FDCA, an applicant must demonstrate that:
| · | such an order would
be appropriate to promote the public health; |
| · | any
aspect of the label, labeling, and advertising for the product that would cause the product
to be a modified risk tobacco product is limited to an explicit or implicit representation
that the tobacco product or its smoke does not contain or is free of a substance or contains
a reduced level of a substance, or presents a reduced exposure to a substance in tobacco
smoke; |
| · | scientific
evidence is not available and, using the best available scientific methods, cannot be made
available without conducting long-term epidemiological studies for an application to meet
the standards for obtaining a risk modification order; and |
| · | the
scientific evidence that is available without conducting long-term epidemiological studies
demonstrates that a measurable and substantial reduction in morbidity or mortality among
individual tobacco users is reasonably likely in subsequent studies. |
Furthermore, for FDA to issue
an exposure modification order, FDA must find, among other things, that the applicant has demonstrated that the magnitude of overall
reductions in exposure to the substance specified in the application is substantial, that such substance is harmful, that the product
as actually used exposes consumers to the specified reduced level of the substance or substances, and will not expose them to higher
levels of other harmful substances similar marketed products, unless such increases are minimal and the reasonably likely overall impact
of use of the product remains a substantial and measurable reduction in overall morbidity and mortality among individual tobacco users.
Notably the FDA also requires the applicant to demonstrate, through testing of actual consumer perception, that consumers will not be
misled into believing that the product is or has been demonstrated to be less harmful or presents less of a risk of disease than other
commercially marketed tobacco products.
Similar to its review of
PMTAs, once an MRTPA is submitted FDA conducts an initial acceptance review to determine whether the product falls under CTP jurisdiction
and to confirm that the statutory and regulatory requirements of an application are met based upon the criteria set forth in the Tobacco
Control Act. If the application does not appear to contain the required information, the FDA may refuse to accept the application for
review. If and when the MRTP is accepted for further review, the FDA conducts a preliminary scientific review to ensure the application
contains the information required for MRTPAs under the FDCA, a process referred to as “filing,” and the FDA may refuse to
file any application that does not include the required information. Once filed, the FDA intends to complete its review of the PMTA within
360 days of receipt, however the FDA’s review may take significantly longer. As part of its substantive review, the FDA is required
to send the application to the Tobacco Products Scientific Advisory Committee (“TPSAC”) and ask the TPSAC to report its recommendations
on the application to the FDA within 60 days. After the FDA completes its review of the MRTPA, including the views expressed by the TPSAC,
the FDA may issue a modified risk order letter, or issue a no modified risk order letter. If the FDA grants a risk modification order,
the applicant must submit protocols for required post market surveillance for FDA concurrence within 30 days after receiving notice that
they are required to conduct such surveillance. If the FDA grants an exposure modification order, the applicant must agree to conduct
post market surveillance and studies in accordance with a protocol approved by the FDA. In either case, an FDA order permitting marketing
of an MRTP is valid only for the fixed time period specified in the order and is not permanent, and such period may not be longer than
five years. To continue marketing an MRTP after the set term, the company must submit a new MRTPA for FDA to determine that the product
still satisfies the requirements set forth in the Tobacco Control Act.
Environmental Regulations
We are subject to a variety
of federal, state and local environmental laws and regulations. We have developed specific programs across our business units for ensuring
high standards of environmental compliance, including, standard operating practices and procedures at our manufacturing facility as well
at our research and development centers. We believe that our manufacturing facility complies with all federal, state, and local environmental
regulations, including the Clean Air Act, the Clean Water Act, and the Resource Conservation and Recovery Act.
In addition, any new products
introduced by us are subject to a comprehensive environmental assessment by an independent third-party expert, including an assessment
of how such products may create environmental risks. For our PMTA product, the FDA prepared a programmatic environmental assessment (PEA),
based on our submitted data in accordance with the Council on Environmental Quality's regulations (40 CFR 1500-1508) implementing the
National Environmental Policy Act (NEPA) and FDA’s NEPA regulations (21 CFR 25.40). The PEA concluded that the marketing orders
would have no significant impact and that environmental impact statements would not be required.
Excise Taxes
Tobacco products are subject
to substantial excise taxes in the U.S. and other countries. Significant increases in tobacco-related taxes or fees have been proposed
or enacted and are likely to continue to be proposed or enacted at the federal, state and local levels within the U.S. and other countries.
The frequency and magnitude of excise tax increases can be influenced by various factors, including the composition of executive and
legislative bodies. Federal, state and local cigarette excise taxes have increased substantially over the past two decades. Tax increases
have an adverse impact on sales of tobacco products.
Competition
Although our products are
not approved as smoking cessation aids, we believe that our RNC tobacco cigarettes may compete with FDA-approved smoking cessation aids.
In the market for FDA-approved smoking cessation aids, principal competitors would include Pfizer Inc., GlaxoSmithKline plc, Perrigo
Company plc, Novartis International AG, and Niconovum AB, a subsidiary of Reynolds American Inc. The industry consists of major
domestic and international companies, most of which have existing relationships in the markets into which we plan to sell, as well as
financial, technical, marketing, sales, manufacturing, scaling capacity, distribution and other resources, and name recognition substantially
greater than ours. We are also aware that several domestic cigarette companies and other research groups are working to research and
grow reduced nicotine tobacco and have filed patent applications.
Cigarette and filtered cigar
companies compete primarily on the basis of product quality, brand recognition, brand loyalty, taste, innovation, packaging, service,
marketing, advertising, retail shelf space, and price. Cigarette sales can be significantly influenced by weak economic conditions, erosion
of consumer confidence, competitors’ introduction of low-price products or innovative products, higher taxes, higher absolute prices
and larger gaps between price categories, and product regulation that diminishes the ability to differentiate tobacco products. Domestic
cigarette competitors included Philip Morris USA Inc., Reynolds American Inc., ITG Brands, and Vector Group Ltd.
International competitors included Philip Morris International Inc., British American Tobacco, JT International SA, Imperial
Brands plc, and regional and local tobacco companies; and in some instances, government-owned tobacco enterprises such as the China National
Tobacco Corporation.
Human Capital Resources
As of December 31, 2023,
we had 64 employees. All employees are located in the United States. Our human capital resource objectives are designed to attract, and
retain, highly motivated and well-qualified employees. We believe that we offer a competitive compensation package and have also worked
diligently to provide a flexible and safe work environment.
Employees
As of the date of this Offering
Circular, we had 60 employees. All employees are located in the United States. Our human capital resource objectives are designed to attract,
and retain, highly motivated and well-qualified employees. We believe that we offer a competitive compensation package and have also worked
diligently to provide a flexible and safe work environment.
Properties
Our principal executive office
and headquarters is located in Mocksville, North Carolina, a leased facility. We previously held our principal executive office and headquarters
at leased office space in Buffalo, New York through the end of fiscal 2023.
As of the date of this Offering
Circular, we operated four tobacco facilities located in Mocksville, North Carolina and surrounding areas. These locations are comprised
of one manufacturing facility (which is also our principal executive office and headquarters) and three leased inventory storage facilities.
We believe the facilities we operate and their equipment are effectively utilized, well maintained, generally are in good condition,
and will be able to accommodate our capacity needs to meet current and growing levels of demand. We continuously review our anticipated
requirements for facilities and, on the basis of that review, may from time to time acquire additional facilities, expand or dispose
of existing facilities.
Legal Proceedings
The Company is subject to
litigation arising from time to time in the ordinary course of its business. The Company does not expect that the ultimate resolution
of any pending legal actions will have a material effect on its consolidated results of operations, financial position, or cash flows.
However, litigation is subject to inherent uncertainties. As such, there can be no assurance that any pending legal action, which the
Company currently believes to be immaterial, will not become material in the future. In accordance with applicable accounting guidance,
the Company establishes an accrued liability for litigation and regulatory matters when those matters present loss contingencies that
are both probable and estimable. In such cases, there may be an exposure to loss in excess of any amounts accrued. When a loss contingency
is not both probable and estimable, the Company does not establish an accrued liability. As a litigation or regulatory matter develops,
the Company, in conjunction with any outside counsel handling the matter, evaluates on an ongoing basis whether such matter presents
a loss contingency that is probable and estimable. If, at the time of evaluation, the loss contingency related to a litigation or regulatory
matter is not both probable and estimable, the matter will continue to be monitored for further developments that would make such loss
contingency both probable and estimable. When a loss contingency related to a litigation or regulatory matter is deemed to be both probable
and estimable, the Company will establish an accrued liability with respect to such loss contingency and record a corresponding amount
of related expenses. The Company will then continue to monitor the matter for further developments that could affect the amount of any
such accrued liability.
Class Action
On January 21, 2019, Matthew Jackson Bull, a resident of Denver, Colorado, filed a Complaint against the Company, the Company’s
then Chief Executive Officer, Henry Sicignano III, and the Company’s then Chief Financial Officer, John T. Brodfuehrer, in the United
States District Court for the Eastern District of New York entitled: Matthew Bull, Individually and on behalf of all others similarly
situated, v. 22nd Century Group, Inc., Henry Sicignano III, and John T. Brodfuehrer, Case No. 1:19 cv 00409.
The complaint alleges three counts: Count I sues the Company and Messrs. Sicignano and Brodfuehrer and alleges that the Company's quarterly
and annual reports, SEC filings, press releases and other public statements and documents contained false statements in violation of Section
10(b) of the Securities Exchange Act and Rule 10b-5; Count II sues Messrs. Sicignano and Brodfuehrer pursuant to Section 10(b) of the
Securities Exchange Act and Rule 10b5(a) and (c); and Count III sues Messrs. Sicignano and Brodfuehrer for the allegedly false statements
pursuant to Section 20(a) of the Securities Exchange Act. The Amended Complaint seeks to certify a class, and unspecified compensatory
and punitive damages, and attorney's fees and costs. Several other cases were subsequently filed and consolidated into the main action.
On April 25, 2023, the parties filed with the Court the Motion for Preliminary Approval of the Settlement, which includes the final terms
of the proposed settlement. The Court preliminarily approved the settlement on June 30, 2023, and scheduled a further settlement hearing
for October 3, 2023. The Court entered the Final Judgment and Order of Dismissal with Prejudice of the action on October 23, 2023. The
settlement amount that the defendants paid is $3,000 and is fully covered by the Company’s insurance, which has been funded by the
Company’s insurance carrier in an escrow account and anticipated to be disbursed in the third quarter of 2024.
Shareholder Derivative Cases
On February 6, 2019, Melvyn Klein, a resident of Nassau County New York, filed a shareholder derivative claim against the Company, the
Company’s then Chief Executive Officer, Henry Sicignano III, the Company’s Chief Financial Officer, John T. Brodfuehrer, and
each member of the Company’s Board of Directors in the United States District Court for the Eastern District of New York entitled:
Melvyn Klein, derivatively on behalf of 22nd Century Group v. Henry Sicignano, III, Richard M. Sanders, Joseph Alexander Dunn, Nora B.
Sullivan, James W. Cornell, John T. Brodfuehrer and 22nd Century Group, Inc., Case No. 1:19 cv 00748. Mr. Klein brings this action derivatively
alleging that (i) the director defendants supposedly breached their fiduciary duties for allegedly allowing the Company to make false
statements; (ii) the director defendants supposedly wasted corporate assets to defend this lawsuit and the other related lawsuits; (iii)
the defendants allegedly violated Section 10(b) of the Securities Exchange Act and Rule 10b 5 promulgated thereunder for allegedly approving
or allowing false statements regarding the Company to be made; and (iv) the director defendants allegedly violated Section 14(a) of the
Securities Exchange Act and Rule 14a 9 promulgated thereunder for allegedly approving or allowing false statements regarding the Company
to be made in the Company’s proxy statement. Numerous other shareholder derivative cases were subsequently filed and consolidated
into the main action.
On December 5, 2023, the parties entered
into a Memorandum of Settlement to fully resolve all claims pending the Court’s approval of a motion for preliminary approval
of settlement. The settlement amount is $768 related to plaintiffs attorney and legal fees and is fully covered by the Company’s insurance. The Company expects the settlement to be approved and funded in the fourth quarter
of 2024.
Insurance Litigation
In November 2022, there was a fire at the Company’s Grass Valley manufacturing facility in Oregon, which resulted in a total loss
of the facility. The Company submitted an insurance claim with Dorchester Insurance Company, Ltd. (“Dorchester”) for casualty
loss and business interruption coverage which was acknowledged on November 23, 2022. Dorchester funded $5,000 of casualty loss insurance
but has failed to issue any payments in connection with the Company’s business interruption claim.
On July 19, 2023, the Company filed a Complaint against Dorchester in the United States District Court for the District of Oregon, Pendleton
Division, Case No. 2:23-cv-01057-HL. The Company is alleging breach of contract and breach of duty of good faith and fair dealing. The
Company is seeking full recovery of its business interruption claim of approximately $9,000 under the policy plus direct and indirect
damages resulting from Dorchester’s continued delay in issuing coverage payments. Discovery is ongoing. The trial date is November
4, 2025.
KeyGene Dispute
On April 11, 2024 the Company received a Request for Arbitration from Keygene N.V. (“Keygene”) in connection with the Company’s
termination of various framework collaborative research agreements described below. On April 3, 2019, the Company entered into the Framework
Collaborative Research Agreement with KeyGene in the field of hemp/cannabis. On April 30, 2021, the Company and KeyGene entered into a
First Amended and Restated Framework Collaborative Research Agreement which extended the agreement term, from first quarter 2024 to first
quarter 2027. On March 30, 2022, the Company and KeyGene entered into a new Framework Collaborative Research Agreement for a term of three
years in the field related to the hops plant. On January 8, 2024, the Company formally terminated both Framework Collaborative Research
Agreements, as amended, related to hemp/cannabis and hops. KeyGene is seeking payment in the amount of $1,885 for current and future services
under the Framework Collaborative Research Agreements and has invoiced the Company $881 for services performed. The matter is being arbitrated
under the administration of the International Court of Arbitration.
The Company filed its Answer to Request for Arbitration with Defenses and Counterclaims on June 4, 2024. On July 25, 2024, an arbitrator
was formally appointed. Discovery has not yet commenced, and no arbitration date has been set. The Company believes it has substantial
defenses to KeyGene’s claims and intends to defend itself vigorously.
Maison Dispute
On January 23, 2024, the Company received a Notice of Intent to Arbitrate from Maison Placements Canada Inc. (“Maison”) in
connection with the Company’s March 2023 Senior Secured Credit Facility transaction. Maison claims it is owed fees for closure of
the Senior Secured Credit Facility transaction as a result of discussions with former Company personnel and a purported letter of engagement
dating from 2021. The parties have agreed on the selection of an arbitrator, but no arbitration date has been set. The Company believes
it has substantial defenses to Maison’s claims and intends to defend itself vigorously.
MANAGEMENT’S DISCUSSION
AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
For purposes of this MD&A,
references to the “Company,” “we,” “us” or “our” refer to the operations of 22nd Century
Group, Inc. and its direct and indirect subsidiaries for the periods described herein. In addition, dollars are in thousands, except
per share data or unless otherwise specified.
On March 28, 2024,
we implemented a 1-for-16 reverse stock split (the “Reverse Stock Split”) of our common stock. As a result of the Reverse
Stock Split, every sixteen (16) shares of our pre-Reverse Stock Split common stock were combined and reclassified into one share of our
Common Stock. The number of shares of common stock subject to outstanding options, warrants, and convertible securities were also reduced
by a factor of sixteen and the exercise price of such securities increased by a factor of sixteen, as of March 28, 2024. All historical
share and per-share amounts reflected throughout this section have been adjusted to reflect the Reverse Stock Split. The par value per
share of our common stock was not affected by the Reverse Stock Split.
The following discussion
and analysis should be read in conjunction with our consolidated financial statements and related notes, beginning on page F-1 of
this Offering Circular. Our actual results may differ materially from those anticipated in the following discussion, as a result of a
variety of risks and uncertainties, including those described herein under “Cautionary Statement Regarding Forward-Looking Statements”
and “Risk Factors.” We assume no obligation to update any of the forward-looking statements included herein.
Our Business
22nd Century Group, Inc. (NASDAQ: XXII) is an agricultural biotechnology company focused on tobacco harm reduction by offering tobacco
products with 95% less nicotine, designed to improve health and wellness by helping smokers smoke less. Backed by comprehensive and extensively
patented technologies that regulate nicotine biosynthesis activities in the tobacco plant, the Company has pioneered development of high-yield, proprietary reduced nicotine content (RNC) tobacco plants and clinically validated RNC cigarette
products. The Company received the first and only FDA Modified Risk Tobacco Product (MRTP) authorization for a combustible cigarette in
December 2021. The Company is a subsequent participating manufacturer under the Master Settlement Agreement ("MSA") and vertically integrated
for the production of its both own products and contract manufacturing operations ("CMO"), which consist primarily of branded filtered
cigars and conventional cigarettes.
Our Financial Results
Three and Six Months Ended
June 30, 2024 as Compared to the Three and Six Months Ended June 30, 2023
The following is a comparison
of our results of operations for the three months ended June 30, 2024 and 2023 (in thousands):
| | |
Three Months Ended | | |
| | |
| |
| | |
June 30 | | |
June 30 | | |
Change | |
| | |
2024 | | |
2023 | | |
$ | | |
% | |
| Revenues, net | |
$ | 7,947 | | |
$ | 8,050 | | |
| (103 | ) | |
| (1.3 | ) |
| Cost of goods sold | |
| 3,869 | | |
| 4,682 | | |
| (813 | ) | |
| (17.4 | ) |
| Excise taxes and fees on products | |
| 3,508 | | |
| 4,329 | | |
| (821 | ) | |
| (19.0 | ) |
| Gross (loss) profit | |
| 570 | | |
| (961 | ) | |
| 1,531 | | |
| (159.3 | ) |
| Gross (loss) profit as a % of revenues, net | |
| 7.2 | % | |
| (11.9 | )% | |
| | | |
| | |
| Operating expenses: | |
| | | |
| | | |
| | | |
| | |
| Sales, general and administrative ("SG&A") | |
| 2,360 | | |
| 10,283 | | |
| (7,923 | ) | |
| (77.0 | ) |
| SG&A as a % of revenues, net | |
| 29.7 | % | |
| 127.7 | % | |
| | | |
| | |
| Research and development ("R&D") | |
| 250 | | |
| 799 | | |
| (549 | ) | |
| (68.7 | ) |
| R&D as a % of revenues, net | |
| 3.2 | % | |
| 9.9 | % | |
| | | |
| | |
| Other operating expenses, net ("OOE") | |
| 7 | | |
| - | | |
| 7 | | |
| 100.0 | |
| Total operating expenses | |
| 2,617 | | |
| 11,082 | | |
| (8,465 | ) | |
| (76.4 | ) |
| Operating loss from continuing operations | |
| (2,047 | ) | |
| (12,043 | ) | |
| 9,996 | | |
| (83.0 | ) |
| Operating loss as a % of revenues, net | |
| (25.8 | )% | |
| (149.6 | )% | |
| | | |
| | |
| Other income (expense): | |
| | | |
| | | |
| | | |
| | |
| Other income (expense), net | |
| 339 | | |
| (613 | ) | |
| 952 | | |
| (155.3 | ) |
| Interest income, net | |
| 21 | | |
| 65 | | |
| (44 | ) | |
| (67.7 | ) |
| Interest expense | |
| (501 | ) | |
| (1,071 | ) | |
| 570 | | |
| (53.2 | ) |
| Total other expense | |
| (141 | ) | |
| (1,619 | ) | |
| 1,478 | | |
| (91.3 | ) |
| Loss before income taxes | |
| (2,188 | ) | |
| (13,662 | ) | |
| 11,474 | | |
| (84.0 | ) |
| Provision for income taxes | |
| 26 | | |
| 46 | | |
| (20 | ) | |
| (43.5 | ) |
| Net loss from continuing operations | |
$ | (2,214 | ) | |
$ | (13,708 | ) | |
| 11,494 | | |
| (83.8 | ) |
| Net loss as a % of revenues, net | |
| (27.9 | )% | |
| (170.3 | )% | |
| | | |
| | |
| Net loss per common share from continuing operations (basic and diluted)* | |
$ | (0.30 | ) | |
$ | (15.61 | ) | |
| 15.31 | | |
| (98.1 | ) |
*Giving retroactive effect to the 1-for-16 reverse stock split on
April 2, 2024 and the 1-for-15 reverse stock split on July 5, 2023.
The following is a comparison
of our results of operations for the six months ended June 30, 2024 and 2023 (in thousands):
| | |
Six Months Ended | | |
| | |
| |
| | |
June 30 | | |
June 30 | | |
Change | |
| | |
2024 | | |
2023 | | |
$ | | |
% | |
| Revenues, net | |
$ | 14,416 | | |
$ | 16,977 | | |
| (2,561 | ) | |
| (15.1 | ) |
| Cost of goods sold | |
| 8,082 | | |
| 9,407 | | |
| (1,325 | ) | |
| (14.1 | ) |
| Excise taxes and fees on products | |
| 6,893 | | |
| 8,514 | | |
| (1,621 | ) | |
| (19.0 | ) |
| Gross (loss) profit | |
| (559 | ) | |
| (944 | ) | |
| 385 | | |
| (40.8 | ) |
| Gross (loss) profit as a % of revenues, net | |
| (3.9 | )% | |
| (5.6 | )% | |
| | | |
| | |
| Operating expenses: | |
| | | |
| | | |
| | | |
| | |
| Sales, general and administrative ("SG&A") | |
| 5,266 | | |
| 20,119 | | |
| (14,853 | ) | |
| (73.8 | ) |
| SG&A as a % of revenues, net | |
| 36.5 | % | |
| 118.5 | % | |
| | | |
| | |
| Research and development ("R&D") | |
| 675 | | |
| 1,529 | | |
| (854 | ) | |
| (55.9 | ) |
| R&D as a % of revenues, net | |
| 4.7 | % | |
| 9.0 | % | |
| | | |
| | |
| Other operating expenses (income), net ("OOE") | |
| (19 | ) | |
| (146 | ) | |
| 127 | | |
| (87.0 | ) |
| Total operating expenses | |
| 5,922 | | |
| 21,502 | | |
| (15,580 | ) | |
| (72.5 | ) |
| Operating loss from continuing operations | |
| (6,481 | ) | |
| (22,446 | ) | |
| 15,965 | | |
| (71.1 | ) |
| Operating loss as a % of revenues, net | |
| (45.0 | )% | |
| (132.2 | )% | |
| | | |
| | |
| Other income (expense): | |
| | | |
| | | |
| | | |
| | |
| Other income (expense), net | |
| 339 | | |
| (768 | ) | |
| 1,107 | | |
| (144.1 | ) |
| Interest income, net | |
| 21 | | |
| 122 | | |
| (101 | ) | |
| (82.8 | ) |
| Interest expense | |
| (1,517 | ) | |
| (1,399 | ) | |
| (118 | ) | |
| 8.4 | |
| Total other expense | |
| (1,157 | ) | |
| (2,045 | ) | |
| 888 | | |
| (43.4 | ) |
| Loss before income taxes | |
| (7,638 | ) | |
| (24,491 | ) | |
| 16,853 | | |
| (68.8 | ) |
| Provision for income taxes | |
| 26 | | |
| 46 | | |
| (20 | ) | |
| (43.5 | ) |
| Net loss from continuing operations | |
| (7,664 | ) | |
| (24,537 | ) | |
| 16,873 | | |
| (68.8 | ) |
| Net loss as a % of revenues, net | |
| (53.2 | )% | |
| (144.5 | )% | |
| | | |
| | |
| Net loss per common share from continuing operations (basic and diluted)* | |
$ | (1.44 | ) | |
$ | (28.46 | ) | |
| 27.02 | | |
| (94.9 | ) |
Product line revenue, net
| | |
Three Months Ended | |
| | |
June 30, | | |
| | |
| |
| | |
2024 | | |
2023 | | |
Change | |
| | |
$ | | |
Cartons | | |
$ | | |
Cartons | | |
$ | | |
Cartons | |
| Contract Manufacturing | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Cigarettes | |
| 4,107 | | |
| 169 | | |
| 3,797 | | |
| 155 | | |
| 310 | | |
| 14 | |
| Filtered Cigars | |
| 3,303 | | |
| 459 | | |
| 3,931 | | |
| 612 | | |
| (628 | ) | |
| (153 | ) |
| Cigarillos | |
| 552 | | |
| 91 | | |
| - | | |
| - | | |
| 552 | | |
| 91 | |
| Total Contract Manufacturing | |
| 7,962 | | |
| 719 | | |
| 7,728 | | |
| 767 | | |
| 234 | | |
| (48 | ) |
| VLN®* | |
| (15 | ) | |
| 0 | | |
| 322 | | |
| 8 | | |
| (337 | ) | |
| (8 | ) |
| Total Product Line Revenues | |
| 7,947 | | |
| 719 | | |
| 8,050 | | |
| 774 | | |
| (103 | ) | |
| (56 | ) |
*VLN® sales for the three-month period ended June 30, 2024 were negligible, offset by promotion and rebate expenses accruals.
| | |
Six Months Ended | |
| | |
June 30, | | |
| | |
| |
| | |
2024 | | |
2023 | | |
Change | |
| | |
$ | | |
Cartons | | |
$ | | |
Cartons | | |
$ | | |
Cartons | |
| Contract Manufacturing | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Cigarettes | |
| 6,867 | | |
| 260 | | |
| 8,286 | | |
| 436 | | |
| (1,419 | ) | |
| (176 | ) |
| Filtered Cigars | |
| 6,927 | | |
| 995 | | |
| 8,326 | | |
| 1,316 | | |
| (1,399 | ) | |
| (321 | ) |
| Cigarillos | |
| 552 | | |
| 91 | | |
| - | | |
| - | | |
| 552 | | |
| 91 | |
| Total Contract Manufacturing | |
| 14,346 | | |
| 1,346 | | |
| 16,612 | | |
| 1,752 | | |
| (2,266 | ) | |
| (406 | ) |
| VLN® | |
| 70 | | |
| 2 | | |
| 365 | | |
| 9 | | |
| (295 | ) | |
| (7 | ) |
| Total Product Line Revenues | |
| 14,416 | | |
| 1,348 | | |
| 16,977 | | |
| 1,761 | | |
| (2,561 | ) | |
| (413 | ) |
| § | For the second quarter and first six months of 2024, cigarette net revenues, including export volume,
increased to $4,107 million or 8%, and decreased to $6,867 or (17%), respectively, compared to the prior year periods. For the second
quarter 2024, cigarette sales benefitted from strong summer seasonal demand with key customers, price increases that took effect in April
2024 and a one-time Spectrum® research cigarette order which provided
a $0.9 million boost, sequentially compared to the first quarter 2024. For the first six months of 2024, volume decreases were result
of prior year comparable period stocking orders of Pinnacle cigarettes launched in a top-five convenience store chain and a large one-time
order of export product. |
| § | For the second quarter and first six months of 2024, filtered cigars net revenues decreased to $3,303 and $6,927, respectively, compared
to $3,931 and $8,326 in the prior year comparable periods, reflecting lower volumes as the Company continues to transition away from low
or negative margin manufacturing agreements, in favor of higher margin cigarette manufacturing agreements. Additionally, price increases
for certain customers took effect in April 2024. |
| § | Cigarillo distribution net revenues for the second quarter and first six months of 2024 amounted to $552, reflective of the expanded
Pinnacle branded product offerings launched in April 2024 with a top-five national convenience store chain. |
| § | VLN® cigarette net revenues were negligible in the second quarter
2024, a decrease from the comparable prior year period which benefited from stocking orders with major c-stores. While the Company has
secured broad distribution of its VLN® products, the sell-through
has not yet materialized. The Company is currently making changes to rebrand and relaunch its VLN®
products. |
Gross (loss) profit
| | |
Three Months Ended | | |
Six Months Ended | |
| | |
June 30 | | |
June 30 | | |
June 30 | | |
June 30 | |
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| Gross (loss) profit | |
$ | 570 | | |
$ | (961 | ) | |
$ | (559 | ) | |
$ | (944 | ) |
| Percent of Revenues, net | |
| 7.2 | % | |
| (11.9 | )% | |
| (3.9 | )% | |
| (5.6 | )% |
The improvement in gross profit and gross profit
as a percent of revenues, net for the three and six-month periods ended June 30, 2024, compared to the prior year periods, was
primarily due to the benefit of ongoing cost cut initiatives, efficiency, and the shift in product mix.
Sales, general and administrative (“SG&A”)
expense
| | |
Changes From Prior Year Period | |
| | |
Three Months Ended | | |
Six Months Ended | |
| | |
June 30, 2024 | | |
June 30, 2024 | |
| Compensation and benefits (a) | |
$ | (3,890 | ) | |
$ | (6,382 | ) |
| Strategic consulting (b) | |
| (2,364 | ) | |
| (5,526 | ) |
| Sales and marketing (b) | |
| (858 | ) | |
| (1,259 | ) |
| Travel and entertainment (b) | |
| (263 | ) | |
| (489 | ) |
| Public company expenses (c) | |
| (214 | ) | |
| (640 | ) |
| Other (d) | |
| (334 | ) | |
| (557 | ) |
| Net decrease in SG&A expenses | |
$ | (7,923 | ) | |
$ | (14,853 | ) |
(a) Compensation and benefits and
equity compensation expense decreased for the three and six months ended June 30, 2024 compared to the prior year periods due
to a reduction of headcount as part of our cost cut initiatives.
(b) Decreases of strategic consulting,
sales and marketing and travel and entertainment for the three and six months ended June 30, 2024 compared to the prior year
periods were due to reduced spending as part of our cost cut initiatives.
(c) Decreases in public company
expenses for the three- and six-month periods ended June 30, 2024 compared to the prior year periods were mainly due to waived board of
director compensation fees in the current year periods and a decrease in professional services fees due to the restructuring of our business.
(d) Other expenses decreased for
the three and six months ended June 30, 2024 compared to the three and six month periods ended June 30, 2023 were mainly due to decreases
in insurance, technology and legal expenses.
Research and development (“R&D”) expense
| | |
Changes From Prior Year Period | |
| | |
Three Months Ended | | |
Six Months Ended | |
| | |
June 30, 2024 | | |
June 30, 2024 | |
| Compensation and benefits (a) | |
$ | (201 | ) | |
$ | (353 | ) |
| License, Royalty, and Contract costs (b) | |
| (180 | ) | |
| (180 | ) |
| IP costs (c) | |
| (118 | ) | |
| (221 | ) |
| Other (d) | |
| (50 | ) | |
| (100 | ) |
| Net decrease in R&D expenses | |
$ | (549 | ) | |
$ | (854 | ) |
| (a) | Decreased compensation and benefits for the three and six months
ended June 30, 2024 are mainly related to the decrease in headcount in the current year periods compared to the prior year
periods. |
| (b) | Decreases in licenses, royalty and contract costs for the three-
and six-month periods ended June 30, 2024 relate to growing and contract arrangements that occurred in the prior year periods. |
| (c) | Decreases in IP costs for the three- and six-month periods ended
June 30, 2024 compared to the prior year periods relate to a decrease in patent expenses and amortization from restructuring of our tobacco
IP portfolio to align to our current strategy. |
| (d) | Other expenses decreased for the three and six months ended June 30, 2024 compared to the prior year periods, were attributable to
a decrease in consulting and testing costs due to our cost cut initiatives. |
Other income (expense)
| | |
Changes From Prior Year Period | |
| | |
Three Months Ended | | |
Six Months Ended | |
| | |
June 30, 2024 | | |
June 30, 2024 | |
| Other income (expense), net (a) | |
$ | 952 | | |
$ | 1,107 | |
| Interest income, net | |
| (44 | ) | |
| (101 | ) |
| Interest expense (b) | |
| 570 | | |
| (118 | ) |
| Net decrease in other expense | |
$ | 1,478 | | |
$ | 888 | |
| (a) | Other income (expense), net decreased for the three months ended
June 30, 2024 compared to the same prior year period, due to a decrease of $44 of realized losses on short term investments and $908
gain resulting from change in fair value of warrant liabilities. |
Other income
(expense), net decreased for the six months ended June 30, 2024 compared to the same prior year period, due to a decrease of $60 of realized
losses on short term investments and $1,047 gain resulting from change in fair value of warrant liabilities.
| (b) | For the three months ended June 30, 2024 compared to the prior
year period, interest expense primarily decreased as a result of ongoing repayment and elimination of debt obligations on our balance
sheet. Cash interest decreased $169 and non-cash interest amortization decreased $287 recognized from the Senior Secured Credit Facility
(of these totals, interest that was allocated to discontinued operations decreased by $56), and additional decreases of $541 as a result
of change in fair value of conversion option derivative liability. Additionally, interest expense decreased $29 from the Subordinated
Note, which was extinguished prior to maturity in April 2024, and resulted in a loss on extinguishment of $400. |
For the six
months ended June 30, 2024 compared to the prior year period, interest expense partially increased as a result of higher PIK interest
accrual prior to extinguishment in April 2024 of $609, and $400 loss on extinguishment. This is offset by the ongoing repayment and elimination
of debt obligations on our balance sheet, resulting in a decrease of cash interest of $102 and a decrease of non-cash interest amortization
of $349 recognized from the Senior Secured Credit Facility (of these totals, interest that was allocated to discontinued operations decreased
by $18), and additional decreases of $459 as a result of change in fair value of conversion option derivative liability.
Fiscal Year Ended
December 31, 2023 Compared to Fiscal Year Ended December 31, 2022
The
following is a comparison of the results of our operations for the years ended December 31, 2023 and 2022 (in thousands):
| | |
Year Ended | | |
| | |
| |
| | |
December 31 | | |
December 31 | | |
Change | |
| | |
2023 | | |
2022 | | |
$ | | |
% | |
| Revenues, net | |
$ | 32,204 | | |
$ | 40,501 | | |
| (8,297 | ) | |
| (20.5 | ) |
| Cost of goods sold | |
| 40,900 | | |
| 38,654 | | |
| 2,246 | | |
| 5.8 | |
| Gross (loss) profit | |
| (8,696 | ) | |
| 1,847 | | |
| (10,543 | ) | |
| NM | |
| Gross (loss) profit as a % of revenues, net | |
| (27.0 | )% | |
| 4.6 | % | |
| | | |
| | |
| Operating expenses: | |
| | | |
| | | |
| | | |
| | |
| Sales, general and administrative ("SG&A") | |
| 31,064 | | |
| 32,231 | | |
| (1,167 | ) | |
| (3.6 | ) |
| SG&A as a % of revenues, net | |
| 96.5 | % | |
| 79.6 | % | |
| | | |
| | |
| Research and development ("R&D") | |
| 2,644 | | |
| 3,578 | | |
| (934 | ) | |
| (26.1 | ) |
| R&D as a % of revenues, net | |
| 8.2 | % | |
| 8.8 | % | |
| | | |
| | |
| Other operating expenses (income), net
("OOE") | |
| 2,527 | | |
| (327 | ) | |
| 2,854 | | |
| NM | |
| Total operating
expenses | |
| 36,235 | | |
| 35,482 | | |
| 753 | | |
| 2.1 | |
| Operating loss from continuing operations | |
| (44,931 | ) | |
| (33,635 | ) | |
| (11,296 | ) | |
| 33.6 | |
| Operating loss as a % of revenues, net | |
| (139.5 | )% | |
| (83.0 | )% | |
| | | |
| | |
| Other income (expense): | |
| | | |
| | | |
| | | |
| | |
| Other income (expense), net | |
| 334 | | |
| (366 | ) | |
| 700 | | |
| (191.3 | ) |
| Realized loss on Panacea investment | |
| - | | |
| (2,789 | ) | |
| 2,789 | | |
| NM | |
| Loss on transfer of promissory note | |
| (895 | ) | |
| - | | |
| (895 | ) | |
| NM | |
| Interest income, net | |
| 219 | | |
| 313 | | |
| (94 | ) | |
| (30.0 | ) |
| Interest expense | |
| (9,366 | ) | |
| (55 | ) | |
| (9,311 | ) | |
| NM | |
| Total other expense | |
| (9,708 | ) | |
| (2,897 | ) | |
| (6,811 | ) | |
| 235.1 | |
| Loss before income
taxes | |
| (54,639 | ) | |
| (36,532 | ) | |
| (18,107 | ) | |
| 49.6 | |
| Provision for income taxes | |
| 47 | | |
| 21 | | |
| 26 | | |
| NM | |
| Net loss from
continuing operations | |
| (54,686 | ) | |
| (36,553 | ) | |
| (18,133 | ) | |
| 49.6 | |
| Net loss as a % of revenues,
net | |
| (169.8 | )% | |
| (90.3 | )% | |
| | | |
| | |
| Net loss per common share from continuing
operations (basic and diluted)* | |
$ | (2.64 | ) | |
$ | (2.84 | ) | |
| 0.20 | | |
| (7.04 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| NM - calculated change not meaningful | |
| | | |
| | | |
| | | |
| | |
Revenue - Sale of products, net
| | |
Year Ended | |
| | |
December 31 | | |
December 31 | |
| | |
2023 | | |
2022 | |
| Revenues, net | |
$ | 32,204 | | |
$ | 40,501 | |
Tobacco revenue was $32,204,
a decrease of 20.5% from $40,501 in the prior year period, reflecting lower unit sales as a result of a planned reallocation in production
resources during 2023 at the Company’s NASCO facilities away from lower margin filtered cigars to higher margin VLN® and conventional
cigarette products. Full year 2023 cartons sold were of 3,459 compared to 5,782 in the comparable prior year period.
Gross profit
| | |
Year Ended | |
| | |
December 31 | | |
December 31 | |
| | |
2023 | | |
2022 | |
| Gross (loss) profit | |
$ | (8,696 | ) | |
$ | 1,847 | |
| Percent of Revenues, net | |
| (27.0 | )% | |
| 4.6 | % |
The
decrease in gross profit and gross profit as a percent of revenues, net for the year ended December 31, 2023, compared to the
year ended December 31, 2022, was primarily driven by lower volume due to an intentional shift during 2023 in product mix.
In connection with evaluation of strategic alternatives and tobacco focused restructuring efforts, during the fourth quarter of 2023,
the Company increased the reserve for excess, obsolete or expired leaf inventory by $7,720.
Sales, general and administrative expense
| | |
| Changes
From Prior Year | |
| Compensation and benefits (a) | |
$ | (2,239 | ) |
| Strategic consulting (b) | |
| (393 | ) |
| Sales and marketing (c) | |
| 986 | |
| Administrative, public company and other expenses (d) | |
| 274 | |
| Legal (e) | |
| 205 | |
| Net decrease in SG&A expenses | |
$ | (1,167 | ) |
| (a) | Decreases in compensation
and benefits primarily resulted from $3,200 benefit of lower equity based compensation expense
due to current year headcount reduction and forfeitures, and compared with prior year accelerated
vesting of an employee’s outstanding equity awards as part of a termination severance
agreement; $218 decrease in severance expenses offset by an increase of $1,179 in personnel
costs due to increased headcount during the year compared to the prior year period. |
| (b) | Decrease of strategic
consulting due to restructuring efforts and implementation of cost savings initiatives. |
| (c) | Increased
sales and marketing related to expansion of VLN®. |
| (d) | Other expenses increased
due to $291 of technology expenses, $579 in public company fees, $270 of facilities expense
offset by a decrease in insurance expenses of $469 and other of $397. |
| (e) | Increased legal expenses
due to regulatory compliance, business development, and contract matters. |
Research and development expense
| |
| |
Changes From Prior Year | |
| Compensation and benefits (a) | |
$ | (164 | ) |
| Royalty, license and contract costs (b) | |
| (376 | ) |
| Consulting and professional services (c) | |
| (478 | ) |
| Other | |
| 84 | |
| Net decrease in R&D expenses | |
$ | (934 | ) |
| (a) | Decreased compensation
and benefits primarily related to personnel bonus expense of $255 in the prior year period
as compared to $0 in the current year. |
| (b) | Decreased expenses
primarily due to a decrease in royalty fees due in the current year period. |
| (c) | Decreased consulting
due to an evaluation of strategic opportunities related to our tobacco patent portfolio that
occurred in the period year period. |
Other operating expenses (income), net
| | |
Year Ended | |
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Restructuring costs: | |
| | | |
| | |
| Impairment of intangible
assets | |
$ | 1,375 | | |
$ | 35 | |
| Impairment of fixed assets | |
| 56 | | |
| - | |
| Professional services | |
| 763 | | |
| - | |
| Severance | |
| 221 | | |
| - | |
| Total Restructuring costs (a) | |
| 2,415 | | |
| 35 | |
| | |
| | | |
| | |
| Acquisition and transaction costs (b) | |
| 223 | | |
| — | |
| Gain on sale or disposal of property,
plant and equipment (c) | |
| (111 | ) | |
| (362 | ) |
| Total other
operating expenses (income), net | |
$ | 2,527 | | |
$ | (327 | ) |
| | |
| | | |
| | |
| NM - calculated change not meaningful | |
| | | |
| | |
| (a) | During
the second half of 2023, the Company undertook various restructuring activities in an effort
to better align its internal organizational structure and costs with its strategy, as well
as preserve liquidity. As a result, the Company incurred $2,415 in restructuring costs for
the year ended December 31, 2023, which included costs related to employee termination,
professional services and consulting, and long-lived asset impairment. |
| (b) | Acquisition and transaction costs primarily
relate to professional fees incurred in connection with potential capital markets transactions. |
| (c) | Reflects gain on sale resulting from
sale of older manufacturing equipment. |
Other income (expense)
| |
| |
Changes From Prior Year | |
| Other income (expense): | |
| | |
| Realized loss on Panacea investment (a) | |
$ | (2,789 | ) |
| Other income (expense), net (b) | |
| (700 | ) |
| Loss on transfer of promissory note (c) | |
| 895 | |
| Interest income, net | |
| 94 | |
| Interest expense (d) | |
| 9,311 | |
| Net increase in
other expense | |
$ | 6,811 | |
| (a) | Realized
loss on PLSH investment reflects the change in fair value and write-off of our investment
in PLSH common stock during the year ended December 31, 2022 of $2,340 and extinguishment
of note receivable of $500 less adjusted discount of $51. |
| (b) | Other
income (expense), net includes a decrease of $336 of realized losses on short-terms investments
and $364 gain on change in fair value of warrant liability. |
| (c) | In
connection with the Senior Secured Credit Facility October Amendment, the Company assigned
$3,800 PLSH promissory note less unamortized discount of $305, and corresponding pay down
of indebtedness on outstanding principal of $600 and redemption of the related warrant liability
of $2,000 resulting in loss on sale of financial asset of $895. |
| (d) | Interest
expense increased in 2023, as compared to the prior year period, primarily due to the cash
interest of $1,104 and non-cash interest of $2,087 recognized from the Senior Secured Credit
Facility (of these totals, $366 of interest was allocated to discontinued operations), and
additional charges of $5,158 for extinguishment of debt and $557 of derivative liability
in connection with the December Amendment. Additionally, interest expense increased
as a result of PIK interest of $695 recognized from the Subordinated Note. |
Liquidity and Capital Resources
We have incurred significant
losses and negative cash flows from operations since inception and expect to incur additional losses until such time that we can generate
significant revenue and profit in our tobacco business. We had negative cash flow from operations of $6,970 for the six months ended
June 30, 2024 and an accumulated deficit of $385,558 as of June 30, 2024. As of June 30, 2024, we had cash
and cash equivalents of $1,279 and working capital deficit from continuing operations of ($2,249) (compared to working capital deficit
from continuing operations of ($6,826) at December 31, 2023). Given our projected operating requirements and existing cash and cash equivalents,
there is substantial doubt about our ability to continue as a going concern through one year following the date that the Condensed Consolidated
Financial Statements herein are issued.
In response to these
conditions, management is currently evaluating different strategies for reducing expenses, as well as pursuing financing strategies which
include raising additional funds through the issuance of securities, asset sales, and through arrangements with strategic partners. If
capital is not available to the Company when, and in the amounts needed, it could be required to liquidate inventory or assets, cease
or curtail operations, seek to negotiate new business deals with our business partners or seek protection under applicable bankruptcy
laws or similar state proceedings. There can be no assurance that the Company will be able to raise the capital it needs to continue operations.
Accordingly, there is substantial doubt regarding our ability to continue in operations. Management’s plans do not alleviate substantial
doubt about the Company’s ability to continue as a going concern through one year following the date that the Condensed Consolidated
Financial Statements are issued.
Our cash, and cash equivalents and working capital
from continuing operations as of June 30, 2024 and December 31, 2023 are set forth below:
| | |
June 30 | | |
December 31 | |
| | |
2024 | | |
2023 | |
| Cash and cash equivalents | |
$ | 1,279 | | |
$ | 2,058 | |
| Working capital | |
$ | (2,249 | ) | |
$ | (6,826 | ) |
Working Capital
As of June 30, 2024, we had working
capital deficit from continuing operations, excluding assets and liabilities held for sale, of approximately ($2,249) compared to working
capital deficit of approximately ($6,826) at December 31, 2023 an increase of $4,577. This increase in working capital was
primarily due to a $2,597 increase in net current assets and a decrease in net current liabilities of $7,174. Cash and cash equivalents
decreased by $779 and the remaining net current assets decreased by $1,818. As a result of the working capital balance, management has
taken a number of steps to improve liquidity. Refer below to “Cash demands on operations.”
Summary of Cash Flows
| | |
Six Months Ended | | |
| |
| | |
June 30, | | |
Change | |
| | |
2024 | | |
2023 | | |
$ | |
| Cash provided by (used in): | |
| | | |
| | | |
| | |
| Operating activities | |
$ | (6,970 | ) | |
$ | (37,332 | ) | |
| 30,362 | |
| Investing activities | |
| (49 | ) | |
| 18,923 | | |
| (18,972 | ) |
| Financing activities | |
| 6,240 | | |
| 27,322 | | |
| (21,082 | ) |
| Net change in cash, cash equivalents and restricted cash | |
$ | (779 | ) | |
$ | 8,913 | | |
| | |
Net cash used in operating activities
Cash used in operating activities decreased $30,362
from $37,332 in 2023 to $6,970 in 2024. The primary driver for this decrease was lower net loss of $31,870, a decrease of $5,830 related
to net adjustments to reconcile net loss to cash, and a decrease in cash used for working capital components related to operations in
the amount of $4,322 for the six months ended June 30, 2024, as compared to the six months ended June 30, 2023.
Net cash (used in) provided by investing activities
Cash used in investing activities amounted to $49
the six months ended June 30, 2024, as compared to cash provided by investing activities of $18,923 for the six months ended
June 30, 2023. The decrease in cash provided by investing activities of $18,972 was primarily the result of (i) a decrease in
net proceeds from short-term investments of $18,239; (ii) decrease of $3,500 in property, plant and equipment casualty loss insurance
proceeds collected in the prior year period (iii) decrease of $90 from the acquisition of RXP in the prior year period and (iv) a decrease
of $229 of proceeds from the sale of property, plant and equipment. These decreased cash inflows were partially offset by a decrease in
cash outflows of $3,086 related to the acquisitions of patents, trademarks and property, plant and equipment.
Net cash provided by financing activities
During the six months ended June 30, 2024,
cash provided by financing activities decreased by $21,082, from $27,322 in the prior year period, to $6,240, resulting from decreases
in (i) net proceeds of $16,048 from issuance of long-term debt, (ii) proceeds of $6,016 from issuance of detachable warrants, (iii) net
proceeds of $3,501 from the issuance of common stock (iv) proceeds from issuance of notes payable of $962 offset by an increase in net
proceeds from warrant exercise of $2,245. These cash inflows were offset by decreases in cash outflows of note payable payments of $3,030
and taxes paid related to net share settlement of RSUs of $419 and increases in payments of long-term debt of $249.
Cash demands on operations
As
of June 30, 2024, we had approximately $1,279 of cash and cash equivalents. Our principal sources of liquidity are our cash
and cash equivalents and cash generated from our tobacco contract manufacturing business and proceeds from debt and equity financing activities,
which cash flows provided by financing activities for the six months ended June 30, 2024 were $6,240.
Senior Secured Credit Facility
On
March 3, 2023, the Company entered into that certain Securities Purchase Agreement (the “SPA”)
with JGB Partners, LP (“JGB Partners”), JGB Capital, LP (“JGB Capital”)
and JGB Capital Offshore Ltd. (“JGB Offshore” and collectively with JGB Partners
and JGB Capital, the “Holders”) and JGB Collateral, LLC, as collateral agent
for the Holders (the “Agent”) which pursuant to the agreement, the Company
sold 5% original issuance discount senior secured debentures with an aggregate principal amount of $21,053. The Debentures bear interest
at a rate of 7% per annum, payable monthly in arrears as of the last trading day of each month and on the maturity date. The Debentures
mature on March 3, 2026. At the Company’s election, subject to certain conditions, interest can be paid in cash, shares of
the Company’s common stock, or a combination thereof. The Debentures are subject to an exit payment equal to 5% of the original
principal amount, or $1,053, payable on the maturity date or the date the Debentures are paid in full (the “Exit Payment”).
Any time after, March 3, 2024, the Company may irrevocably elect to redeem all of the then outstanding principal amount of the Debentures
for cash in an amount equal to the entire outstanding principal balance, including accrued and unpaid interest, the Exit Payment and
a prepayment premium in an amount equal to 3% of the outstanding principal balance as of the prepayment date (collectively, the “Prepayment
Amount”). Upon the entry into a definitive agreement that would effect a change in control (as defined in the Debentures) of the
Company, the Agent may require the Company to prepay the outstanding principal balance in an amount equal to the Prepayment Amount.
The
JGB Warrants are exercisable for five years from September 3, 2023, at an exercise price of $306.00 per share, a 50% premium to
the VWAP on the closing date, subject, with certain exceptions, to adjustments in the event of stock splits, dividends, subsequent dilutive
offerings and certain fundamental transactions. As a result of the June 19, 2023 offering, the Company’s outstanding JGB warrants
to purchase up to 20,834 shares of the Company’s common stock for an exercise price of $306.00 per share were automatically adjusted
to be $205.248 exercise price for up to 31,063 shares of common stock. There are no further anti-dilution adjustments on such warrants.
In connection with the JGB October Amendment, the Company and Holders agreed to exercise the outstanding put provision to redeem 10,418 Warrants
for an aggregate put price equal to $2,500.
On
April 8, 2024, the Company, the Holders and the Agent entered into that certain Letter Agreement to modify the terms of the Amendment
Agreement, the JGB SPA and the Debentures, as amended.
Under
the terms of the Letter Agreement, the Holders are permitted to convert their debt to common stock at anytime and the Conversion Price
(as defined in the Debentures) at which the Holders may convert the principal amount of their Debentures to the Company’s common
stock is reduced to $2.14 per share in accordance with applicable Nasdaq rules. The
principal amount of the Debentures converted shall be applied to the Monthly Allowance (as defined in the Debentures) for that month,
and any excess shall be applied to the Monthly Allowances for the succeeding months. The conversions will be a dollar for dollar reduction
of the remaining outstanding obligation owed to the Holders. The Agent and Holders have also agreed to daily limits on trading volume
and minimum conversion amounts. The Holders converted $428 of debt in exchange for 200,000 shares of common stock during the 20-day period.
The
provisions in Section 3(c)(i) of the Debentures requiring 20% of any equity issuances to be paid to the Holders was suspended
for 20 days.
On
May 10, 2024, the Company, the Holders and the Agent entered into that certain May 2024 Exchange Agreement and May 2024
Letter Agreement to modify the terms of the Amendment Agreement, the Securities Purchase Agreement and the Debentures, as amended.
Under
the terms of the May 2024 Letter Agreement, the Company and Holders have agreed the Company shall incur an aggregate amendment charge
to the undersigned holders equal to $275, which shall be added to the principal balance of the Debentures.
Under
the terms of the May 2024 Exchange Agreement, the Company and Holders exchanged an aggregate of $2,328 in principal, fees and expenses
owed under the Debentures for 395,000 shares of common stock and 895,000 immediately exercisable pre-funded warrants to purchase shares
of common stock at an exercise price of $.00001 (at an effective per share price of $1.69).
As
a result of the transaction, the exercise price on 5,876,887 of the Company’s outstanding warrants is reduced to $1.69 per share
in accordance with the adjustment provisions therein.
As
of June 30, 2024, the remaining principal balance of the Debentures is $8,325 of which $1,500 remains current with corresponding pledged
assets.
Omnia Subordinated Note
On
March 3, 2023, the Company executed a Subordinated Promissory Note (the “Subordinated Note”) with a principal amount
of $2,865 in favor of Omnia Ventures, LP (“Omnia”). The Subordinated Note refinanced the 12% Secured Promissory Note with
a principal amount of $1,000 dated as of October 29, 2021 payable to Omnia (the “October Note”) and the 12% Secured
Promissory Note with a principal amount of $1,500 dated as of January 14, 2022 payable to Omnia (the “January Note”,
and together with the October Note, the “Original Notes”), which were assumed by the Company in connection with the
acquisition of GVB Biopharma.
Under
the terms of the Subordinated Note, the Company is obligated to make interest payments in-kind (the “PIK Interest”). The
PIK Interest accrues at a rate of 26.5% per annum, payable monthly. The Company is not permitted to prepay all or any portion of the
outstanding balance on the Subordinated Note prior to maturity. The maturity date of the Subordinated Note was May 1, 2024.
In
connection with the Subordinated Note, the Company issued to Omnia, warrants to purchase up to 2,813 shares of the Company’s common
stock. The Omnia Warrants are exercisable for seven years from September 3, 2023, at an exercise price of $205.248 per share subject,
with certain exceptions, to adjustments in the event of stock splits, dividends, subsequent dilutive offerings and certain fundamental
transactions.
On April 29, 2024, the Company
entered into a General Release and Settlement Agreement (the “Omnia Agreement”) with Omnia Capital LP (“Omnia”).
The Omnia Agreement settles and extinguishes all outstanding debt and interest owed to Omnia under the outstanding Subordinated Promissory
Note dated March 3, 2023 (the “Old Note”) and the put provision contained the outstanding common stock purchase warrant dated
March 3, 2023 (the “Old Warrant”), amounting to a total of approximately $5,228, for (i) a cash payment of $249; (ii) 1,150,000
shares of common stock and 1,150,000 immediately exercisable pre-funded warrants to purchase shares of common stock at an exercise price
of $0.0001 that are exercisable until May 1, 2029 (at an effective per share price of $2.14) and (iii) 460,000 immediately exercisable
warrants to purchase an equal number of shares of common stock at an exercise price of $2.14 until May 1, 2029 (the “New Warrant”).
The New Warrant contains a put provision that permits the holder to require the Company to redeem the New Warrants, no earlier than May
1, 2025, for a purchase price equal to $2.675 per New Warrant, and had an initial fair value of $1,515. Subject to limited exceptions,
a holder of pre-funded warrants and New Warrants will not have the right to exercise any portion of its warrants if the holder, together
with its affiliates, would beneficially own in excess of 19.99% of the number of shares of our common stock outstanding immediately after
giving effect to such exercise. As part of the Omnia Agreement, the parties agreed to terminate and cancel the Old Note and the Old Warrant
and released all debts, claims or other obligations against each other occurring prior to the date of the Omnia Agreement. The total cash
and non-cash consideration amounted to $5,628, resulting in extinguishment charges of $400.
Warrant Inducement
Offering
On
November 28, 2023, the Company commenced a warrant inducement offering with the holders of the Company’s outstanding 1,986,229
warrants consisting of: (i) the common stock purchase warrants of the Company issued on or about June 22, 2023; (ii) the
common stock purchase warrants of the Company issued on or about July 10, 2023; (iii) the common stock purchase warrants of
the Company issued on or about July 21, 2023; and/or (iv) the common stock purchase warrants of the Company issued on or about
October 19, 2023 (collectively, the “Existing Warrants”), which Existing Warrants are exercisable for an equal number
of shares of common stock at an exercise price of $8.40. The Company agreed to issue new warrants (the “Inducement Warrants”)
to purchase up to a number of shares of common stock equal to 200% of the number of shares of common stock issued pursuant to the exercise
by the holders of the Existing Warrants during the inducement period, for cash, at a reduced exercise price equal to the Nasdaq Minimum
Price (as defined in Nasdaq Listing Rule 5635(d)).
For
the period from January 1, 2024 to February 15, 2024, the date of shareholder approval, the Company entered into warrant inducement
agreements with certain holders of the Existing Warrants to purchase an aggregate of 820,769 shares of common stock at a reduced weighted
average exercise price of approximately $2.9504 (which were subsequently reduced to $1.69 in connection with the May 2024 JGB debt
for equity exchange). Pursuant to the warrant inducement agreements, the exercising holders of the Existing Warrants received 1,641,535
Inducement Warrants and the Company received aggregate gross proceeds of approximately $2,421 from the exercise of the Existing Warrants.
Additionally, on the date of Shareholder Approval, the exercise price of the 3,581,213 outstanding Inducement Warrants, was reduced
to $2.8237 based on the lowest Nasdaq Minimum Price (as defined in Nasdaq Listing
Rule 5635(d)) during the inducement period.
April 2024 Registered
Direct Offering.
On April 8, 2024,
the Company and certain investors entered into a securities purchase agreement (“April SPA”) relating to the issuance
and sale of approximately $4,200 of shares and warrants, consisting of an aggregate of 1,855,000 shares of common stock, 125,000 pre-funded
warrants and 1,980,000 warrants to purchase an equal number of shares, at a purchase price of $2.14 per unit. The warrants are exercisable
immediately at an exercise price of $2.14 per share of common stock and expire five years after shareholder approval, as defined in the
April SPA (which were subsequently reduced to $1.69 in connection with the May 2024 JGB
debt for equity exchange). The net proceeds to the Company from the offering were approximately $3,913.
Outstanding Warrants
As
of August 15, 2024, we had the following warrants outstanding:
| | |
# of warrants
outstanding | | |
Exercise
price | | |
Expiration date |
| July 2022 RDO warrants | |
| 4,067 | | |
$ | 492.00 | | |
July 25, 2027 |
| Senior Secured Credit Facility - JGB | |
| 20,645 | | |
$ | 205.248 | | |
September 3, 2028 |
| July 19, 2023 RDO warrants | |
| 28,125 | | |
$ | 1.69 | | |
July 20, 2028 |
| October 2023 CMPO warrants | |
| 168,750 | | |
$ | 1.69 | | |
October 19, 2028 |
| Inducement warrants | |
| 3,581,213 | | |
$ | 1.69 | | |
February 15, 2029 |
| April 2024 RDO | |
| 1,980,000 | | |
$ | 1.69 | | |
June 28, 2029 |
| April 2024 RDO - Placement Agent | |
| 118,800 | | |
$ | 1.69 | | |
June 28, 2029 |
| Omnia Pre-Funded | |
| 1,150,000 | | |
$ | 0.00001 | | |
NA |
| Omnia 2024 warrants | |
| 460,000 | | |
$ | 2.14 | | |
May 1, 2029 |
| | |
| 7,511,600 | | |
| | | |
|
Impact of Recently Issued Accounting Standards
In
the normal course of business, we evaluate all new accounting pronouncements issued by the Financial Accounting Standards Board (“FASB”),
SEC, or other authoritative accounting bodies to determine the potential impact they may have on our Consolidated Financial Statements.
Refer to Note 1 “Summary of Significant Accounting Policies” of the Notes to Consolidated Financial Statements contained
in this Offering Circular for additional information about these recently issued accounting standards and their potential impact on our
financial condition or results of operations.
Critical Accounting Estimates
Management’s
discussion and analysis of financial condition and results of operations are based upon our consolidated financial statements, which
have been prepared in accordance with GAAP. We make estimates and assumptions in the preparation of our consolidated financial statements
that affect the reported amounts of assets and liabilities, revenue and expenses and related disclosures of contingent assets and liabilities.
We base our estimates and judgments upon historical experience and other factors that are believed to be reasonable under the circumstances.
Changes in estimates or assumptions could result in a material adjustment to the consolidated financial statements.
We
have identified several critical accounting estimates. An accounting estimate is considered critical if both: (a) the nature of
the estimates or assumptions is material due to the levels of subjectivity and judgment involved, and (b) the impact of changes
in the estimates and assumptions have had or are reasonably likely to have a material effect on the consolidated financial statements.
This listing is not a comprehensive list of all of our accounting policies. For further information regarding the application of these
and other accounting policies, see Note 1 “Summary of Significant Accounting Policies” of the Notes to Consolidated Financial
Statements included herein.
Inventories
Inventories
are measured on a first-in, first-out basis at the lower of cost or net realizable value. Net realizable value is the estimated selling
prices in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation. The valuation
of inventory requires us to estimate obsolete or excess inventory, as well as inventory that is not of saleable quality.
Historically,
our adjustments or write-off charges recorded against inventory have been adequate to cover our losses. However, variations in methods
or assumptions could have a material impact on our results. Additionally, if our demand forecasts for specific products is greater than
actual demand and we fail to reduce manufacturing output accordingly, we could be required to record additional inventory write-down
or expense a greater amount of overhead costs, which would negatively impact our gross profit and net income.
Valuation of Long-Lived Assets
We
make assumptions in establishing the carrying value, fair value and, if applicable, the estimated lives of our intangible and other long-lived
assets. Intangible assets determined to have an indefinite useful life are not amortized. Instead, these assets are evaluated for impairment
on an annual basis on December 1, the measurement date, and whenever events or business conditions change that could indicate that
the asset is impaired. Long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying
amount of an asset (asset group) may not be recoverable.
Evaluation of indefinite-lived
intangible assets for impairment
Our
indefinite-lived intangible assets include the MSA, cigarette brand predicate and trademarks. We perform an annual impairment review
of our indefinite-lived intangible assets on December 1, the measurement date, unless events occur that trigger the need for an
interim impairment review. We have the option to first assess qualitative factors in determining whether it is more-likely-than-not that
an indefinite-lived intangible asset is impaired. If we elect not to use this option, or we determine that it is more-likely-than-not
that the asset is impaired, we perform a quantitative assessment that requires us to estimate the fair value of each indefinite-lived
intangible asset and compare that amount to its carrying value. Impairment, if any, is based on the excess of the carrying value over
the fair value of these assets.
For our indefinite-lived
intangible assets, we performed a qualitative evaluation and considered factors such as current and future sales projections, strategic
objectives, future market and economic conditions, competition, and federal and state regulations. We determined as of December 1,
2023, it is more likely than not that that the assets are not impaired.
Evaluation of long-lived
assets for impairment
When
impairment indicators exist, we determine if the carrying value of the long-lived asset(s) including, but not limited to, PP&E,
right-of-use lease assets, and definite-lived intangible asset(s) exceeds the related undiscounted future cash flows. In cases where
the carrying value exceeds the undiscounted future cash flows, the carrying value is written down to fair value. Fair value is generally
determined using a discounted cash flow analysis. When it is determined that the useful life of an asset (asset group) is shorter than
the originally estimated life, and there are sufficient cash flows to support the carrying value of the asset (asset group), we accelerate
the rate of depreciation/amortization in order to fully depreciate/amortize the asset over its shorter useful life.
Estimation
of the cash flows and useful lives of long-lived assets and definite-lived intangible assets requires significant management judgment.
Events could occur that would materially affect our estimates and assumptions. Unforeseen changes, such as the loss of one or more significant
customers, technology obsolescence, or significant manufacturing disruption, among other factors, could substantially alter the assumptions
regarding the ability to realize the return of our investment in long-lived assets, definite-lived intangible assets or their estimated
useful lives.
For
our long-lived assets, we determined that impairment indicators occurred during the fourth quarter of 2023 in connection with ongoing
evaluation of our tobacco strategy and restructuring efforts and concluded that certain definite-lived intangible assets, including patents,
were impaired due to obsolescence or abandonment in the amount of $1,375. No other long-lived assets were concluded to be non-recoverable
based on undiscounted cash flow analysis performed.
Detachable Warrants
Warrants
issued pursuant to debt or equity offerings that the Company may be required to redeem through payment of cash or other assets outside
its control are classified as liabilities and therefore measured at fair value. The Company uses a Monte Carlo valuation model to estimate
fair value at each issuance and period-end date. The key assumptions used in the model are the expected future volatility in the price
of the Company’s shares and the expected life of the warrants.
Embedded Derivatives – Conversion Option
Our
December Amendment to the Senior Secured Credit Facility contained an embedded derivative conversion option. The Company evaluates
each debt agreement to determine whether any embedded features require bifurcation from the debt host in accordance with ASC 815, Derivatives
and Hedging ("ASC 815"). If the embedded feature requires bifurcation from its debt host, the Company
will account for it as either a derivative liability or as a derivative in equity. The Company uses valuation models to estimate the
fair value of the embedded derivatives. For the valuation to record the debt and embedded derivative related to the conversion option
at fair value, the Company uses a binomial lattice model at inception and on subsequent valuation dates. This model incorporates inputs
such as the stock price of the Company, risk-free interest rate, the effective debt yield and expected volatility. Certain inputs involve
unobservable inputs and are classified as level 3 of the fair value hierarchy (see Note 9, Fair Value Measurement to
our Consolidated Financial Statements included elsewhere in Item 15 of this Annual Report). The sensitivity of the fair value calculation
to these methods, assumptions, and estimates included could create materially different results under different conditions or using different
assumptions.
DIRECTORS, EXECUTIVE OFFICERS,
PROMOTERS AND CONTROL PERSONS
Directors and Executive Officers
Our directors and executive
officers, their ages as of the date of this Offering Circular, positions held, and duration of such, are as follows:
| Name |
|
Position
Held with Our Company |
| Lawrence D. Firestone |
|
Chairman of the Board, President, Chief Executive Officer |
| Daniel A. Otto |
|
Chief Financial Officer and Principal Accounting Officer |
| Jonathan Staffeldt |
|
General Counsel and Secretary |
| Andy Arno |
|
Lead Director |
| Lucille S. Salhany |
|
Director |
| Anthony Johnson |
|
Director |
Business Experience
The following is a brief
account of the education and business experience of directors and executive officers during at least the past five years, indicating
their principal occupation during the period, the name and principal business of the organization by which they were employed, and certain
of their other directorships:
Lawrence D. Firestone (66); Director Since
2023
Mr. Firestone
has served as Chief Executive Officer and Chairman of the Board since December 2023. Mr. Firestone brings over 40 years of
enterprise, operations, and financial management experience in both public and private companies, including tenures as CEO, CFO and COO
across multiple industry sectors. Mr. Firestone most recently served as Chief Financial Officer of Oakland Manager, a privately-held
purveyor of cannabis with both retail and wholesale market penetration, and as Chairman of FirePower Technology, a privately held manufacturer
of ATX power supplies for the IT and instrumentation markets. In the public company sector, Mr. Firestone has served as Chief Executive
Officer of Eastside Distilling, Inc. (NASDAQ: EAST), Chief Executive Officer of Qualstar Corporation (NASDAQ: QBAK), Chief Financial
Officer of Advanced Energy Industries (NASDAQ: AEIS), and Chief Financial Officer of Applied Films Corporation (NASDAQ: AFCO). He has
served on numerous boards, including those of Eastside Distilling, Qualstar, CVD Equipment Corporation (NASDAQ: CVD), Amtech Systems, Inc.
(NASDAQ: ASYS) and HyperSpace Communications, Inc. (NYSE: HYPR). Mr. Firestone received his Bachelor of Science in Business
Administration with a concentration in Accounting from Slippery Rock University of Pennsylvania. Mr. Firestone’s prior
public company experience led to the conclusion that he should serve as a director.
Daniel A. Otto (35)
Mr. Otto has served
as the Chief Financial Officer and Principal Accounting Officer of the Company since April 2024. Prior to that, Mr. Otto served
as the Company’s Corporate Controller since July 2022 where he was responsible for accounting, SEC external reporting, treasury,
tax and other finance management functions. Prior to joining the Company, Mr. Otto served as a Senior Manager at Deloitte &
Touche LLP providing audit and accounting advisory services to public companies, ranging from small to large cap issuers, for over ten
years. Mr. Otto is also a certified public accountant. Mr. Otto received his Master’s in Business Administration and
B.A. in Accounting from Niagara University.
Jonathan Staffeldt (42)
Mr. Staffeldt has served
as General Counsel and Secretary since April 2024, and prior to that as Deputy General Counsel of the Company since January 2023.
Prior to that, Mr. Staffeldt was Chief Legal Officer of GVB Biopharma, the former subsidiary of the Company that was acquired in
May 2022, and served in that role since September 2019. Mr. Staffeldt was previously in private practice with significant
experience in corporate, mergers and acquisitions, and litigation. Mr. Staffeldt received his B.S. in Accounting from the University
of Southern California and his Juris Doctorate from the University of California Los Angeles.
Andy Arno (64); Director Since 2023
Mr. Arno
previously served, until February 2023, as Vice Chairman of The Special Equities Group, a division of Dawson James Securities, Inc.,
plus other senior roles at investment banking firms. Prior, Mr. Arno served as Vice Chairman and Chief Marketing Officer of Unterberg
Capital, LLC, an investment advisory firm that he co-founded, and as Vice Chairman and Head of Equity Capital Markets of Merriman Capital
LLC, where he also served on the board of the parent company, Merriman Holdings, Inc. Mr. Arno was responsible for Capital
Markets for C.E. Unterberg, Towbin, a Vice President at Lehman Brothers and in the Individual Investors Services Division of L.F. Rothschild
Unterberg, Towbin in portfolio management for high-net-worth individuals. Mr. Arno is currently the Chairman of the Board of Oncocyte
Corporation and also serves on the boards of directors of Smith Micro Software, Inc., Independa Inc., and Comhear Inc. Mr. Arno
previously served as a director of Asterias Biotherapeutics, Inc. from August 2014 until it was acquired by Lineage Cell Therapeutics, Inc.
in March 2019. Mr. Arno received a BS degree from George Washington University. He is currently the Chair of the Audit Committee.
Mr. Arno’s capital markets experience led to the conclusion that he should serve as a director.
Lucille S. Salhany (77); Director since 2022
Ms. Salhany
is currently President and CEO of her own consulting company, JHMedia, which she founded in 1997. She was also one of the founding partners
of Echo Bridge Entertainment and CEO & President of LifeFX Networks, Inc. Prior to this, she served as Chairperson of the
Twentieth Television division of Fox, and was appointed the first woman in history to head a major television network when she accepted
the Chairmanship of Fox Broadcasting. After chairing Fox, Salhany accepted the post of Chief Executive Officer and President of United
Paramount Network (UPN), launching and growing UPN to become the fifth major broadcast network. She also served on the Board of Directors
for Echo Bridge Entertainment, Compaq / Hewlett-Packard, Fox, Inc., Avid Technologies, and American Media, Inc. Lucille was
also a trustee of Emerson College and Lasell College, where she received Honorary Doctorates. She is currently a member of the Compensation
and Corporate Governance & Nominating Committees of the Board. Ms. Salhany’s well-established track record
of success in growing businesses and strong background in and knowledge of the media industry led to the conclusion that she should serve
as a director.
Anthony Johnson (48); Director since 2021;
Mr. Johnson
is co-founder, President, and CEO of Kodikaz Therapeutic Solutions, a next-generation non-viral gene therapy company. He is also a founding
partner of Buffalo Biosciences in 2006 a life science strategic business management firm that supports the evaluation and commercialization
of bioscience technologies from concept to market. Previously he was President and CEO of Empire Genomics, an oncology molecular diagnostic
testing company, from 2006 until 2019. He also served as the business leader of the stem cell and regenerative medicine franchise for
Thermo Fisher (Invitrogen Corporation). Anthony has leveraged his business experience and numerous board positions to mentor technology
startups and entrepreneurs, spur state and local job creation, and introduce STEM curriculum into early childhood education. Mr. Johnson
is a founding board member of the Communities of Giving Legacy Initiative, which works to create positive change in the lives of low-income
youth of color. Additionally, he serves as Michigan Street African-American Heritage Corridor Commissioner and was an Opportunities Council
member for University of Buffalo. He holds an MBA from Manchester Business School, Manchester, UK, with an emphasis in international
strategy, and a BA in biology from Fisk University, Nashville, TN. He is the Chair of the Audit Committee and is a member of the Corporate
Governance & Nominating Committee. Mr. Johnson’s experience commercializing bioscience technologies and life
sciences experience led to the conclusion that he should serve as a director.
Commitment to Corporate Governance
Board of Directors
Our Board of Directors represents
the best interests of our stockholders by overseeing the business and affairs of the Company. Members of the Board participate in quarterly
Board and Committee meetings, engage with senior management of the Company, review, provide input, approve the Company’s strategic
plan and principal issues, and discuss feedback from stockholders and other stakeholders.
Under the Company’s
current Amended and Restated Articles of Incorporation, directors hold office for a term ending on the date of the third annual stockholders’
meeting following the Annual Meeting at which such director’s class was most recently elected until the earlier of their death,
resignation, removal or until their successors have been duly elected and qualified. If the Charter Amendment proposal (Proposal 1) is
approved, Directors will hold office for a term ending on the next annual stockholder meeting. Our Bylaws provide that the number of
members of our Board of Directors may be changed from time to time by resolutions adopted by the Board of Directors. Our Board of Directors
currently consists of six (6) members with no vacancies. Following the meeting, the Board will consist of four (4) members
with no vacancies, assuming the election of the director nominees.
Independent Directors
Our Board of Directors has
determined that Anthony Johnson, Lucille S. Salhany, and Andrew Arno are “independent” as defined by applicable Nasdaq
Stock Market listing standards. Each director serving on the Audit Committee and the Compensation Committee of our Board also meets the
more stringent independence requirements established by SEC and Nasdaq rules applicable to audit and compensation committees. Our
Board has determined that no director or nominee has a relationship that would interfere with the exercise of independent judgment in
carrying out their responsibilities as a director. There are no family relationships among our directors or executive officers. The Board
annually reviews all business and other relationships of directors and determines whether directors meet these categorical independence
tests.
Board Leadership Structure and the Role of the Board in Oversight of Risk Management
Our Board of Directors has
not adopted a policy requiring that the roles of Chief Executive Officer and chairperson of the Board be separate. Our Board reserves
the right to assign the responsibilities of the Chief Executive Officer and chair position as determined by our Board to be in the best
interest of our Company. In the circumstance where the responsibilities of the Chief Executive Officer and chair are vested in the same
individual or in other circumstances when deemed appropriate, the Board will designate a Lead Independent Director from among the independent
directors to preside at the meetings of non-employee director executive sessions.
Currently, Lawrence D. Firestone
serves as Board Chair and Chief Executive Officer, and Andrew Arno serves as the Lead Independent Director. Our Board reviews our leadership
structure annually and retains the authority to modify this structure to best address our Company’s unique circumstances as and
when appropriate.
Our Board is actively involved
in oversight of risks that could affect the Company. Our Board has assigned responsibility for addressing certain risks, and the steps
management has taken to monitor, control and report such risk, to our Audit Committee, including risks relating to execution of our growth
strategy, with appropriate reporting to the full Board. Our Board relies on our Compensation Committee to address significant risk exposures
facing our Company with respect to compensation. Our Board receives reports by each committee chair regarding the applicable committee’s
considerations and actions, as well as through regular reports directly from officers responsible for oversight of particular risks within
the Company.
Stockholder Communications with the Board
Stockholders wishing to communicate
with the Board of Directors or with an individual Board member concerning the Company may do so by writing to the Board or to the particular
Board member care of the Corporate Secretary, 22nd Century Group, Inc. The envelope or subject line should indicate that it contains
a stockholder communication.
Meetings of Board of Directors
Our Board held 40 meetings
throughout 2023. All directors attended at least 75% of meetings of the Board and Board committees on which they served in 2023.
We do not have a formal policy
requiring directors to attend Annual Meetings of stockholders. However, all of our continuing directors who were members of the Board
at the time of our 2023 Annual Meeting attended the meeting virtually, and we anticipate that all continuing directors will attend the
2024 Annual Meeting.
Executive Sessions of Independent Directors
The independent directors
hold regularly scheduled executive sessions of the Board and its committees no management directors or employees present. The independent
directors met in executive session at most of the regularly scheduled Board and committee meetings held in 2023.
Corporate Governance Guidelines
Our Board has adopted a set
of Corporate Governance Guidelines, which describe the Board’s responsibility for oversight of the business and affairs of the
Company as well as guidelines for determining director independence and consideration of potential nominees to the Board. The Board,
directly and through its Corporate Governance and Nominating Committee, regularly reviews developments in corporate governance and best
practices and annually reviews its Corporate Governance Guidelines, committee charters, and other key governance documents, policies,
and practices. Our Corporate Governance Guidelines provide:
Limits
on Board Service. We do not allow “over-boarding,” or a director serving on an excessive number of public company
boards. Excessive board commitments can lead to a director being unable to appropriately fulfill his or her duties to the Company and
its stockholders. Our Corporate Governance Guidelines have long limited our directors to no more than two (2) other public company
boards.
Board
Self-Assessment and Evaluation. We conduct an annual self-evaluation and assessment of our individual Board performance to
help ensure that the Board and its committees function effectively and in the best interest of our stockholders. This process promotes
governance in accordance with current best practices and helps set expectations about the relationship and interaction of and between
the Board and management.
Standing Committees
Our Board of Directors currently
has three (3) standing committees: (i) an Audit Committee, (ii) a Compensation Committee, and (iii) a Corporate Governance
and Nominating Committee. Members of these committees are elected annually by the Board. The charters of each committee are each available
on the investor relations section of our website at www.xxiicentury.com.
The Audit Committee oversees
the Company’s financial reporting process and system of internal accounting controls, as well as appointment and oversight of the
independent public accountants engaged to audit the Company’s financial statements. The Audit Committee also assists the Board
in monitoring compliance with legal and regulatory requirements and oversees the Company’s policies with respect to risk assessment
and management, including but not limited to cybersecurity risks.
The Audit Committee is comprised
solely of non−employee directors who satisfy current Nasdaq standards with respect to independence, financial expertise and experience.
During 2023, the Audit Committee was comprised of Mr. Johnson, Mr. Sanders, Dr. Koganov and Nora B. Sullivan. On August 24,
2023, Mr. Arno was appointed to the committee. Our Board of Directors has determined that Mr. Arno meets the SEC’s definition
of “audit committee financial expert,” and that all members of the Audit Committee meet the financial literacy requirements
of the Nasdaq Stock Market. No members of the Audit Committee serve on the audit committees of more than three public companies. The
Audit Committee held 4 meetings during 2023. To ensure independence, the Audit Committee also meets separately with our independent public
accountants apart from meetings with members of management. At the time of the 2024 Annual Meeting, the Audit Committee will consist
of Mr. Johnson, Ms. Salhany and Mr. Arno as Chair.
The Compensation Committee
is comprised solely of directors who meet the current Nasdaq requirements for independence. During 2023, the Compensation Committee was
comprised of Ms. Salhany, Mr. Sanders, Ms. Sullivan and Dr. Koganov. On August 24, 2023, Mr. Arno was appointed
to the committee. The Compensation Committee establishes and regularly reviews our compensation and benefits philosophy and program in
a manner consistent with corporate financial goals and objectives. The Compensation Committee also approves compensation arrangements
for senior management, including annual incentive and long-term compensation; administers grants under our equity incentive plans; annually
evaluates the performance of our Chief Executive Officer; and reviews leadership development and succession planning. The Compensation
Committee held 8 meetings in 2023. At the time of the 2024 Annual Meeting, the Compensation Committee will consist of Mr. Arno,
Mr. Johnson and Ms. Salhany as Chair.
The Corporate Governance
and Nominating Committee is comprised solely of independent directors and during 2023 was comprised of Mr. Johnson, Ms. Salhany
and Ms. Sullivan. The Corporate Governance and Nominating Committee develops and recommends to the Board corporate governance guidelines
applicable to the Company; identifies, evaluates and recommends candidates for election to the Board; leads the Board in its annual review
of the Board’s performance; and recommends Board members to serve on each committee of the Board. The Corporate Governance and
Nominating Committee held 7 meetings in 2023. At the time of the 2024 Annual Meeting, the Corporate Governance and Nominating Committee
will consist of Mr. Arno, Ms. Salhany Mr. Johnson as Chair.
The guidelines and procedures
for identifying and evaluating nominees for election to the Board are set forth in the charter of the Corporate Governance and Nominating
Committee. In general, persons considered for nomination to the Board must have demonstrated outstanding achievement, integrity and judgment
and such other skills and experience as will enhance the Board’s ability to serve the long−term interests of the Company
and its stockholders. Candidates must also be willing and able to devote the necessary time for Board service. The Corporate Governance
and Nominating Committee also considers diversity in terms of gender, ethnicity, age, and other attributes that could contribute to Board
effectiveness, and assesses diversity in the course of the Committee’s annual evaluation of Board structure and composition. The
Corporate Governance and Nominating Committee considers potential candidates recommended by current directors, company officers, employees
and others, and will consider candidates recommended by stockholders for consideration as director nominees. Nominations of persons for
election to the Board at the Annual Meeting may be made by any stockholder entitled to vote for the election of directors at the meeting
who complies with the notice procedures set forth in our Bylaws. Such nominations by any stockholder shall be made pursuant to timely
notice in writing to the Secretary. To be timely, a stockholder’s notice shall be delivered to the Secretary at our principal executive
offices not later than the close of business on the ninetieth (90th) day nor earlier than the close of business on the one hundred twentieth
(120th) day prior to the first anniversary of the preceding year’s Annual Meeting; provided, however, that in the event that the
date of the Annual Meeting is more than thirty (30) days before or more than seventy (70) days after such anniversary date, notice by
the stockholder must be so delivered not earlier than the close of business on the one hundred twentieth (120th) day prior to such Annual
Meeting and not later than the close of business on the later of the ninetieth (90th) day prior to such Annual Meeting or the tenth (10th)
day following the day on which public announcement of the date of such meeting is first made.
Code of Business Conduct and Corporate Ethics
Our Board of Directors has
long maintained a Code of Ethics that applies to all our directors, officers, and employees. A copy of our Code of Ethics is available
on our website at http://www.xxiicentury.com.
Insider Trading Policy
Our directors, executive
officers, and employees are required to comply with the 22nd Century Group, Inc. Insider Trading Policy and may not engage in any
transaction (such as short-selling) to hedge against the potential decline in value of any of our securities. Our Insider Trading policy
also clearly sets forth the prohibition on trading based on material non-public information.
DISCLOSURE OF COMMISSION
POSITION ON INDEMNIFICATION
FOR SECURITIES ACT LIABILITIES
NRS Sections 78.7502 and
78.751 provide us with the power to indemnify any of our directors, officers, employees or agents, or any person who serves or served
at the corporation’s request as a director, officer, employee or agent of another corporation, partnership, joint venture, trust
or other enterprise (for purposes of this section, the “Indemnitee” or “Indemnitees”) against expenses, including
attorneys’ fees, actually and reasonably incurred related to any threatened, pending or completed action, suit or proceeding (whether
civil, criminal, administrative or investigative) arising by reason of an Indemnitee’s status as a director, officer employee or
agent of the corporation if: (i) the Indemnitee is not liable for breach of fiduciary duties to the corporation involving intentional
misconduct, fraud or knowing violation of law; (ii) the Indemnitee conducted himself or herself in good faith and reasonably believes
that his or her conduct was in, or not opposed to, our best interests; or (iii) in a criminal action, the Indemnitee must not have
had reasonable cause to believe that his or her conduct was unlawful. NRS Section 78.751 requires us to indemnify any Indemnitee
for any expenses referenced above if the Indemnitee has been successful on the merits or otherwise in defense of the foregoing actions,
suits or proceedings.
Under NRS Section 78.7502,
any discretionary indemnification, unless ordered by a court or advanced by the corporation in accordance with NRS Section 78.751(2),
can only occur if deemed proper by (i) the stockholders; (ii) a majority vote of a quorum consisting of disinterested directors;
or (iii) an independent counsel’s written legal opinion (if such an approach is approved by a majority vote of a quorum consisting
of disinterested directors or if a quorum consisting of disinterested directors cannot be obtained). Under NRS Section 78.751(2),
advances for expenses may be made by agreement if the Indemnitee affirms in writing that he or she believes that he or she has met the
statutory standards and will personally repay the expenses if a court of competent jurisdiction determines that such Indemnitee did not
meet the statutory standards.
Our amended and restated
bylaws include an indemnification provision under which we have the power to indemnify, to the extent permitted under Nevada law, our
current and former directors and officers, or any person who serves or served at our request for our benefit as a director or officer
of another corporation or our representative in a partnership, joint venture, trust or other enterprise, against all expenses, liability
and loss reasonably incurred by reason of being or having been a director, officer or representative of ours or any of our subsidiaries.
We may make advances for expenses upon receipt of an undertaking by or on behalf of the director or officer to repay the amount if it
is ultimately determined by a court of competent jurisdiction that he, she or it is not entitled to be indemnified by us.
Our amended and restated
articles of incorporation provides that we shall indemnify directors and officers to the fullest extent permitted by the NRS. Our amended
and restated articles of incorporation also provide a limitation of liability such that no director or officer shall be personally liable
to us or any of our stockholders to the fullest extent permitted by the NRS.
Insofar as indemnification
for liabilities arising under the Securities Act of 1933, as amended (the “Securities Act”) may be permitted to directors,
officers and controlling persons of ours under Nevada law or otherwise, we have been advised that the opinion of the SEC is that such
indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event a claim for
indemnification against such liabilities (other than payment by us for expenses incurred or paid by a director, officer or controlling
person of ours in successful defense of any action, suit, or proceeding) is asserted by a director, officer or controlling person in
connection with the securities being registered, we will, unless in the opinion of our legal counsel the matter has been settled by controlling
precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by our company is against public
policy in the Securities Act and will be governed by the final adjudication of such issue.
Insofar
as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling the
Company pursuant to the foregoing provisions, the Company has been informed that in the opinion of the SEC such indemnification is against
public policy as expressed in the Securities Act and is therefore unenforceable.
EXECUTIVE
COMPENSATION
Unless otherwise
specified, all dollar amounts in this section are in thousands except per share amounts and par values. All historical share and per-share
amounts reflected throughout this section have been adjusted to reflect the 1-for-16 reverse stock split effective on April 2, 2024.
Executive Summary and Overview of 2023 Compensation
Our Company’s long-term success depends
on our ability to fulfill the expectations of our customers and clients in a competitive environment and deliver value to stockholders.
To achieve these goals, it is critical that we are able to attract, motivate, and retain highly talented individuals at all levels of
the organization that are committed to our values and objectives.
We strive to provide compensation that is (a) linked
to stockholder value creation, (b) reflective of the overall performance of the Company and each individual executive, and (c) considerate
of the competitive market levels of compensation needed to recruit, retain and motivate top executive talent, while remaining consistent
with the other objectives.
Compensation Philosophy and Objectives
The Company’s executive compensation program
is based on the same principles that guide us in establishing all of the Company’s compensation programs:
| · | Compensation
fosters the long-term focus required for the Company’s success. In general, the compensation
of Company executives includes longer-term incentives because they are in a greater position
to influence longer-term results. |
| · | Compensation
reflects the level of job responsibility, individual performance, and Company performance.
As employees progress to higher levels in the organization, an increasing proportion of their
pay should be linked to Company performance and stockholder returns because those employees
are more able to affect the Company’s results. |
| · | Compensation
reflects the value of the job in the marketplace. To attract and retain a highly skilled
work force, we must remain competitive with the pay of other premier employers who compete
with us for talent. |
| · | While
compensation programs and individual pay levels will always reflect differences in job responsibilities,
geographies and marketplace considerations, the overall structure of the compensation and
benefit programs should be broadly similar and equitable across the organization. |
Overview of Executive Compensation
The Compensation Committee
Our Compensation Committee has primary responsibility
for, among other things, determining our compensation philosophy, evaluating the performance of our executive officers, setting the compensation
and other benefits of our executive officers, and considering the outcome of the advisory votes of stockholders on executive compensation.
To ensure alignment of compensation programs
with the Company’s needs and goals, the Compensation Committee is informed by and responsive to the overall mission and strategies
of the Company as determined by the full Board of Directors. As the strategic focus of the Company evolves, as for example toward commercialization
of its products and profitability, the Compensation Committee has and will continue to adapt the Company’s compensation programs
to meet these evolving needs.
The Compensation Committee also evaluates risks
and rewards associated with the Company’s overall compensation philosophy and structure. To the extent our compensation programs
provide for incentive−based compensation, the Compensation Committee evaluates whether these programs are designed to pay for performance,
and thus encourage only appropriate risk-taking. These programs are also subject to oversight of the Compensation Committee and various
functional departments of the Company to ensure that our employees, including our executive officers, are not encouraged to take excessive
or unnecessary risks in managing our business.
Role of Executive Officers in Compensation
Discussions
The Compensation Committee meets with our Chief
Executive Officer in order to obtain recommendations with respect to the Company’s compensation programs and practices for executives
and other employees. Management discusses with the Compensation Committee the practices that have been put in place to identify and mitigate,
as necessary, potential risks. The Chief Executive Officer annually reviews the performance of each executive officer, other than himself.
The Chief Executive Officer’s performance is reviewed annually by the Compensation Committee.
With support from market compensation data, performance
reviews and other information, management makes recommendations to the Compensation Committee on the base salaries, bonus targets and
equity compensation for the executive officers and other employees. The Compensation Committee takes management’s recommendations
into consideration, but is not bound by management’s recommendations with respect to executive compensation.
While management attends certain meetings of
the Compensation Committee, the Compensation Committee also holds executive sessions not attended by any members of management or by
non−independent directors. The Compensation Committee annually reviews and recommends for approval to the full Board all elements
of compensation of the Chief Executive Officer, and reviews, counsels, and makes recommendations regarding the compensation elements
of other senior executives. The Compensation Committee also approves equity awards to all employees and directors of the Company.
Benchmarking Against Peer Companies
In February 2022, our Compensation Committee
engaged the governance consulting firm of Morrow Sodali to assist with say-on-pay and the election of new directors.
In August 2022, our Compensation Committee
engaged the compensation consulting firm of Pay Governance to assist the Committee in developing a peer group to benchmark executive
compensation against other biotechnology, pharmaceutical, and life science companies with similar revenues and market capitalizations.
Pay Governance then reviewed our current executive compensation program and made recommendations with respect to the design of our compensation
program and future compensation decisions.
During 2023, Morrow Sodali and Pay Governance
continued to provide assistance and recommendations to the Compensation Committee for determination of 2022 incentive awards earned,
2023 peer group benchmarking, and in transitioning to quantitative, finance-based metrics for future awards of incentive compensation.
In addition, the Committee considered the Company’s most recent ISS Proxy Analysis Report and publicly available advisory materials
relating to executive compensation benchmarking.
Elements of Executive Compensation
For 2023, the principal components of compensation
for named executive officers were: (1) Base Salary, (2) Performance-Based Incentive Compensation, (3) Long-Term Equity
Incentive Compensation, (4) Personal Benefits, and (5) Other Compensation. In determining the amount and relative allocation
among each component of compensation for each named executive officer, the Compensation Committee considered, among other factors, each
executive officer’s experience level and historical performance, compensation paid by companies comparable in size, data obtained
from management’s recruitment activities, historical rates of executive compensation, Company revenues and financial outlook, and
alignment with the Company’s overall compensation philosophy.
In connection with Mr. Firestone’s
appointment as our Chief Executive Officer on November 28, 2023, the Compensation Committee recommended, and the Board approved,
a cash target bonus of 75% of his base salary, or $319,000, subject to performance conditions to be determined by the Compensation Committee
in its sole discretion. No performance-based or long-term incentive compensation was awarded to Mr. Firestone for the fiscal year
ending December 31, 2023.
Base Salary
Base salaries are set at levels that the Compensation
Committee deems to be sufficient to attract and retain highly talented executive officers capable of fulfilling the Company’s key
objectives. Base salaries are also set with the goal of rewarding executive officers on a day−to−day basis for their time
and services while encouraging them to strive for performance−based and long−term incentives.
Performance-Based Incentive Compensation
Historically, the Compensation Committee has
considered and in some cases established incentive bonus plans for other executive officers that align pay with performance. Because
the Company has not in the Committee’s judgment achieved sufficient levels of revenues or profits, the Committee did not approve
performance-based incentive compensation awards for named executive officers in 2023.
Long-Term Equity Incentive Program Compensation
Our 2021 Omnibus Incentive Plan, as amended and
restated, authorizes the Company to grant various types of equity awards, including stock options and restricted stock units, as
incentives for management to increase stockholder value. Equity awards are granted to executive officers as long-term incentives in order
to align executives’ performance with the interests of the Company’s stockholders. In addition, the multi-year nature of
the vesting periods of such awards encourages executive retention.
Our Compensation Committee has authority to determine
eligible participants, the types of awards, and the terms and conditions of awards.
Personal Benefits
As employees, the executives were eligible to
participate in health and welfare benefits, as offered to our general workforce, designed to attract and retain a skilled workforce in
a competitive marketplace. These benefits help ensure that the Company has a healthy and focused workforce through reliable and competitive
health and other personal benefits. We do not maintain any pension or non-qualified deferred compensation plans, but we do sponsor a
401(k)-plan pursuant to which we make a safe harbor non-elective contribution of 3% of the employee’s annual compensation, subject
to certain wage maximums, to provide employees with the opportunity to save for retirement on a tax deferred basis. These benefits were
considered in relation to total compensation packages, but did not materially impact decisions regarding other elements of executive
officer compensation.
Other Compensation
On August 20, 2023, the Company entered
into a Retention Agreement with R. Hugh Kinsman, the Company’s Chief Financial Officer, under which Mr. Kinsman would be entitled
to certain cash payments if he remained an employee in Good Standing (as defined in Retention Agreement) through each applicable payment
date. Pursuant to this agreement, the Company paid Mr. Kinsman two retention bonuses of $92,500 each on September 29 and October 31,
2023. Mr. Kinsman and the Company were also parties to an Employment Agreement entered into on June 15, 2022, which provided
that if Mr. Kinsman’s employment were terminated by the Company without Cause (as defined in the Employment Agreement) he
would be entitled to a severance benefit in the form of a continuation of his then-base salary for a period of 12 months (plus continuing
health care coverage during such period). Both the Retention and Employment Agreements between Mr. Kinsman and the Company were
mutually terminated on January 23, 2024 with no remaining obligations under either agreement.
On August 23, 2023, the Company entered
into a Retention Agreement with John Miller, the Company’s President of the Tobacco Business and at that time its Interim Chief
Executive Officer, under which Mr. Miller would be entitled to certain cash payments if he remained an employee in Good Standing
(as defined in Retention Agreement) through each applicable payment date. Pursuant to this agreement, the Company paid Mr. Miller
two retention bonuses of $92,500 each on September 29 and October 31, 2023. Upon the appointment of Lawrence D. Firestone as
the Company’s Chief Executive Officer on November 28, 2023, Mr. Miller resumed his position of President of the Tobacco
Business. Mr. Miller and the Company were also parties to an Employment Agreement entered into on November 11, 2022, which
provided that if Mr. Miller’s employment were terminated by the Company without Cause (as defined in the Employment Agreement)
he would be entitled to a severance benefit in the form of a continuation of his then-base salary for a period of 12 months (plus
continuing health care coverage during such period), as well as accelerated vesting of previously awarded restricted stock units.
Both the Retention and Employment Agreements between Mr. Miller and the Company were mutually terminated on January 23, 2024
with no remaining obligations under either agreement.
Policy on Hedging Transactions
We prohibit our officers and directors from engaging
in hedging transactions or arrangements designed to lock in the value of their company securities. This prevents our officers and directors
from continuing to own company securities without having the full risks and rewards of ownership.
Recoupment/Clawback Policies
The Sarbanes-Oxley Act of 2002 subjects incentive
compensation and stock sale profits of our CEO and CFO to forfeiture in the event of an accounting restatement resulting from any non-compliance,
as a result of misconduct, with any financial reporting requirement under GAAP and SEC rules. We acknowledge the SEC’s new Rule 10D-1
regarding clawback policies and NASDAQ’s Listing Rule 5608(a) that went into effect on December 1, 2023. In anticipation
of Listing Rule 5608(a), on June 22, 2023 the Board voted to adopt the 22nd Century Group, Inc. Compensation Recovery
Policy in full compliance with Listing Rule 5608(a).
Summary Compensation Table
The following table summarizes
the compensation of our NEOs for 2023. The amounts reported for stock awards may not represent the amounts that the NEOs will actually
realize from the awards. Whether, and to what extent, a named executive officer realizes value will depend on our performance, stock
price and continued employment.
| Name
and Principal Position | |
Year | |
Salary | |
Bonus
(1) | |
Option Awards | |
Restricted Stock
Unit Awards
(2) | |
Non-Equity Incentive Compensation | |
All
Other Compensation (3) |
| |
Total |
| Lawrence D. Firestone
(4) | |
2023 | |
$ | 32,692 | |
$ | — | |
$ | — | |
$ | — | |
$ | — | |
$ | — |
| $ |
32,692 |
| Chief Executive Officer | |
| |
| | |
| | |
| | |
| | |
| | |
| |
| |
|
| R. Hugh Kinsman (5) | |
2023 | |
$ | 358,600 | |
$ | 185,000 | |
$ | — | |
$ | 228,555 | |
$ | — | |
$ | 19,432 |
| $ |
791,497 |
| Former Chief Financial Officer | |
2022 | |
$ | 160,033 | |
$ | — | |
$ | — | |
$ | — | |
$ | 90,575 | |
$ | 4,646 |
| $ |
255,254 |
| John
J. Miller (6) | |
2023 | |
$ | 422,515 | |
$ | 185,000 | |
$ | — | |
$ | — | |
$ | — | |
$ | 24,016 |
| $ |
631,531 |
| President of Tobacco | |
2022 | |
$ | 277,836 | |
$ | — | |
$ | — | |
$ | 982,500 | |
$ | 160,000 | |
$ | 19,625 |
| $ |
1,439,961 |
| James A. Mish (7) | |
2023 | |
$ | 320,281 | |
$ | — | |
$ | — | |
$ | 357,904 | |
$ | — | |
$ | 28,593 |
| $ |
706,778 |
| Former Chief Executive Officer | |
2022 | |
$ | 470,976 | |
$ | — | |
$ | — | |
$ | 3,025,989 | |
$ | 481,950 | |
$ | 32,179 |
| $ |
4,011,094 |
| | |
2021 | |
$ | 452,763 | |
$ | — | |
$ | — | |
$ | 1,440,000 | |
$ | 608,000 | |
$ | 33,721 |
| $ |
2,534,484 |
| Peter Ferola (8) | |
2023 | |
$ | 388,140 | |
$ | 250,000 | |
$ | — | |
$ | 256,149 | |
$ | — | |
$ | 25,647 |
| $ |
919,936 |
| Former Chief Legal Officer | |
| |
| | |
| | |
| | |
| | |
| | |
| |
| |
|
| (1) | Bonus amounts relate to amounts paid under retention agreements
entered during August 2023 with respective NEO’s. |
| (2) | The fair value of each restricted stock unit is based on the stock
price of the Company’s common stock on the grant date of the award. |
| (3) | All Other Compensation consists of the following: |
| | |
| | |
| | |
| | |
Employer | | |
| |
| | |
| | |
| | |
| | |
Contributions | | |
| |
| | |
| | |
| | |
| | |
to Company | | |
All Other | |
| | |
| | |
Fringe | | |
| | |
401(k) | | |
Compensation | |
| Name | |
Year | | |
Benefits * | | |
Severance | | |
Plan | | |
Total | |
| Lawrence D. Firestone | |
| 2023 | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | — | |
| R. Hugh Kinsman | |
| 2023 | | |
$ | 9,038 | | |
$ | — | | |
$ | 10,304 | | |
$ | 19,342 | |
| John J. Miller | |
| 2023 | | |
$ | 16,349 | | |
$ | — | | |
$ | 7,667 | | |
$ | 24,016 | |
| James A. Mish | |
| 2023 | | |
$ | 18,455 | | |
$ | — | | |
$ | 10,138 | | |
$ | 28,593 | |
| Peter Ferola | |
| 2023 | | |
$ | 16,882 | | |
$ | — | | |
$ | 8,765 | | |
$ | 25,647 | |
| * | Includes Company paid premiums for health insurance, dental insurance,
group-term life insurance, and long-term disability insurance. |
| (4) | Mr. Firestone joined the Company in December 2023. |
| (5) | Mr. Kinsman joined the Company in May 2022. |
| (6) | Mr. Miller joined the Company in May 2022. |
| (7) | Mr. Mish was granted 1,288 shares at a grant date fair value
of $199.04; all shares were forfeited upon resignation in 2023. |
| (8) | Mr. Ferola was granted 1,799 shares at a grant date fair value
of $199.04; all shares were forfeited upon termination in 2023. |
Grants of Plan-Based Awards
As described above in the
Compensation Discussion and Analysis, we granted restricted stock units to our NEOs in 2023. The following table sets forth information
regarding all such awards:
| | |
| | |
| | |
|
|
| | |
| | |
Grant Date | |
| | |
| | |
| | |
Restricted |
|
Stock | | |
| | |
Fair Value | |
| | |
| | |
| | |
Stock Unit |
|
Option | | |
Exercise | | |
Restricted | |
| | |
| | |
| | |
Awards: |
|
Awards: | | |
Price of | | |
Stock Units, | |
| | |
| | |
| | |
Number of |
|
Number | | |
Option | | |
Stock Awards | |
| | |
| | |
Date of Board | | |
Shares of |
|
of Shares | | |
Awards | | |
and Option | |
| Name | |
Grant Date | | |
Action | | |
Stock (#)(1) |
|
(#) | | |
($) | | |
Awards
($) (3) | |
| Lawrence D. Firestone | |
| — | | |
| — | | |
| — |
|
| — | | |
| — | | |
$ | — | |
| R. Hugh Kinsman | |
| 3/14/2023 | | |
| 3/14/2023 | | |
| 1,149 |
(2) |
| — | | |
| — | | |
$ | 228,555 | |
| John J. Miller | |
| — | | |
| — | | |
| — |
|
| — | | |
| — | | |
$ | — | |
| James A. Mish | |
| 3/14/2023 | | |
| 3/14/2023 | | |
| 1,799 |
(2)(4) |
| — | | |
| — | | |
$ | 357,904 | |
| Peter Ferola | |
| 3/14/2023 | | |
| 3/14/2023 | | |
| 1,288 |
(2)(5) |
| — | | |
| — | | |
$ | 256,149 | |
| (1) | Number
of shares is adjusted to reflect 1:15 reverse stock split that occurred July 5, 2023
and 1:16 reverse stock split that occurred on April 2, 2024 |
| (2) | Represents RSUs which vest in equal increments over three years
on March 14, 2024, 2025 and 2026, subject to continued service. |
| (3) | The fair value of each restricted stock unit is based on the stock
price of the Company’s common stock on the grant date of the award. |
| (4) | Mr. Mish was granted 1,799 shares at a grant date fair value
of $199.04; all shares were forfeited upon resignation in 2023. |
| (5) | Mr. Ferola was granted 1,288 shares at a grant date fair value
of $199.04; all shares were forfeited upon termination in 2023. |
Outstanding Equity Awards
The following table sets forth information about
outstanding equity awards held on December 31, 2023 by our NEOs.
| | |
| | |
| | |
| | |
| | |
| | |
Equity Incentive |
| | |
| | |
| | |
| | |
| | |
| | |
Plan Awards: |
| | |
| | |
| | |
| | |
| | |
| | |
Market or |
| | |
| | |
| | |
| | |
| | |
| | |
Payout Value of |
| | |
Number of | | |
Number of | | |
| | |
| | |
| | |
Unearned |
| | |
Securities | | |
Securities | | |
| | |
| | |
Equity Incentive Plan | | |
Shares, |
| | |
Underlying | | |
Underlying | | |
| | |
| | |
Awards: Number of | | |
Restricted Stock |
| | |
Unexercised | | |
Unexercised | | |
| | |
| | |
Unearned Shares, | | |
Units or Other |
| | |
Options | | |
Options | | |
Option | | |
Option | | |
Restricted Stock Units or | | |
Rights That |
| | |
Exercisable | | |
Unexercisable | | |
Exercise | | |
Expiration | | |
Other Rights That Have | | |
Have Not Vested |
| Name | |
(#) | | |
(#) | | |
Price | | |
Date | | |
Not Vested (#) | | |
($)(3) |
| Lawrence D. Firestone | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | $ |
— |
| R. Hugh Kinsman | |
| — | | |
| — | | |
| — | | |
| — | | |
| 1,149 | (1) | $ |
3,419 |
| John J. Miller | |
| — | | |
| — | | |
| — | | |
| — | | |
| 2,500 | (2) | $ |
7,440 |
| James A. Mish | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | $ |
— |
| Peter Ferola | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | $ |
— |
| (1) | Represents RSUs which vest in equal increments over three years
on March 14, 2024, 2025 and 2026 subject to continued service. |
| (2) | Represents performance shares, with one-half of such shares vesting
on each of May 1, 2024 and 2025 provided that our tobacco business plan revenue objectives
and other performance requirements are satisfied at such times. |
| (3) | The amounts in this column are based on the closing stock price
of the Company’s common stock on December 31, 2023. These amounts do not reflect
the actual amounts that may be realized. |
Option Exercises and Stock Vested in 2023
| | |
Option Awards | | |
Stock Awards | |
| | |
Number of | | |
| | |
Number of | | |
| |
| | |
Shares | | |
Value | | |
Shares | | |
Value | |
| | |
Acquired on | | |
Realized on | | |
Acquired on | | |
Realized on | |
| | |
Exercise | | |
Exercise | | |
Vesting | | |
Vesting | |
| Name | |
(#) | | |
($) | | |
(#) | | |
($)(1) | |
| Lawrence D. Firestone | |
| — | | |
$ | — | | |
| — | | |
$ | — | |
| R. Hugh Kinsman | |
| — | | |
$ | — | | |
| — | | |
$ | — | |
| John J. Miller | |
| — | | |
$ | — | | |
| 625 | | |
$ | 124,350 | |
| James A. Mish | |
| — | | |
$ | — | | |
| 3,204 | | |
$ | 670,494 | |
| Peter Ferola | |
| — | | |
$ | — | | |
| — | | |
$ | — | |
| (1) | The value realized on vesting is based
on the closing stock price of the Company’s common stock on the date of vesting or
date of exercise. The amount does not reflect the actual amount that may be realized. |
2021 Omnibus Incentive Plan
Our
board of directors approved our 2021 Omnibus Incentive Plan, or “Incentive Plan,” and on May 20, 2021, our stockholders
approved and adopted the Incentive Plan. The material terms of the Incentive Plan are summarized below.
Our
board of directors approved an additional 3,500,000 shares of our common stock to be authorized for awards granted under the Incentive
Plan, and on June 16, 2023, our stockholders approved the additional 3,500,000 shares of our common stock to be authorized for awards
granted under the Incentive Plan (subsequently adjusted for 1-for-15 reverse stock split effective July 5, 2023).
Our
board of directors approved an additional 5,000,000 shares of common stock to be authorized under the Incentive Plan, and on June 28,
2024, our stockholders approved the additional 5,000,000 shares of our common stock to be authorized for awards granted under the Incentive
Plan.
Summary of the Terms
of the Plan
The following is a summary
of the material provisions of the Plan. This summary is qualified in its entirety by reference to the full and complete text of the Plan.
Any inconsistencies between this summary and the text of the Plan will be governed by the text of the Plan.
Purpose and Effective
Date
The two complementary purposes
of the Plan are (1) to attract, retain, focus and motivate our executives and other selected employees, directors, consultants and
advisors; and (2) to increase stockholder value. Our Board amended and restated the Plan on April 19, 2024, contingent on approval
by the Company’s stockholders. The Plan as amended and restated will become effective upon receiving stockholder approval at the
2024 Annual Meeting.
Administration and
Eligibility
The Compensation Committee
of our Board, or any successor committee with similar authority that the Board may appoint, which in either case consists of not less
than two members of the Board who meet the “non-employee director” requirements of Rule 16b-3(b)(3) under the Securities
Exchange Act of 1934 (the “Exchange Act”) (either referred to as the “Committee”) will administer the Plan and
all awards (the “Administrator”). The Plan authorizes the Committee to interpret the provisions of the Plan and awards; prescribe,
amend and rescind rules and regulations relating to the Plan; correct any defect, supply any omission, or reconcile any inconsistency
in the Plan, any award or any agreement covering an award; and make all other determinations necessary or advisable for the administration
of the Plan, in each case in its sole discretion. The Board may also administer the Plan to the extent it retains authority and responsibility
as administrator of the Plan.
To the extent applicable
law permits, the Board may delegate to another committee of the Board, or the Compensation Committee may delegate to one or more of our
officers, any or all of their respective authority and responsibility as an administrator of the Plan. However, no such delegation is
permitted with respect to stock-based awards made to any participant who is subject to the reporting requirements of Section 16(a) of
the Exchange Act or the liability provisions of Section 16(b) of the Exchange Act at the time any such delegated authority
or responsibility is exercised unless the delegation is to another committee of the Board consisting entirely of non-employee directors.
The Administrator may designate
any of the following as a participant from time to time, to the extent of the Administrator’s authority: any officer or other employee
of our Company or its affiliates; any individual who we or one of our affiliates has engaged to become an officer or employee; any consultant
or advisor who provides services to the Company or its affiliates; or any director, including a non-employee director. Currently the
persons eligible to participate in the Plan consist of approximately 60 employees and 6 non-employee directors.
Types of Awards
The Plan permits the grant
of stock options (including incentive stock options), stock appreciation rights, restricted stock, restricted stock units, performance
shares, performance units, annual cash incentives, long-term cash incentives, dividend equivalent units and other types of stock-based
awards. These award types are described in further detail below.
Stock Subject to
the Plan and Award Limits
Prior to the currently proposed
amendment and restatement, the Plan provided that 707,584 shares of our common stock were reserved for issuance under the Plan. Other
award types authorized by the Plan include nonqualified stock options, stock appreciation rights, restricted stock, restricted stock
units, performance shares, performance units, annual cash incentives, long-term cash incentives, dividend equivalent units and other
types of stock-based awards.
The Plan as amended and restated
provides that an aggregate of 5,000,000 shares of our common stock will be reserved for issuance under the Plan, and that we may issue
an aggregate of 5,000,000 shares upon the exercise of incentive stock options.
The number of shares reserved
under the Plan will be depleted by the maximum number of shares, if any, that may be issuable under an award at the time of grant. In
general, if an award granted under the Plan lapses, expires, terminates or is cancelled without the issuance of shares under the award,
if it is determined during or at the conclusion of the term of an award that all or some portion of the shares under the award will not
be issuable on the basis that the conditions for such issuance will not be satisfied, if shares are forfeited under an award or if shares
are issued under any award and we reacquire them pursuant to rights reserved upon the issuance of the shares, then such shares will again
be available for issuance under the Plan, except that shares reacquired pursuant to reserved rights may not be issued pursuant to incentive
stock options. Shares not issued or delivered as a result of the net settlement of an outstanding option or stock appreciation right,
shares tendered in payment of the exercise price of an option, shares withheld to satisfy tax withholding obligations and shares purchased
by us using proceeds from option exercises may not be re-credited to the reserve.
The Plan also provides that
any shares subject to awards granted under the 2014 Omnibus Incentive Plan (the “Prior Plan”) that would be re-credited to
the Prior Plan’s reserve if the Prior Plan was still in effect (but applying the provisions concerning share recycling of the Plan
and the Prior Plan’s limits on re-crediting), then those shares will be available for the purpose of granting awards under the
Plan, thereby increasing the reserve.
Subject to the Plan’s
adjustment provisions, the maximum number of shares that may be subject to awards granted during any fiscal year to any non-employee
director shall not exceed that number of shares that equates to a grant date fair value of, when added to any cash compensation received
by such non-employee director, $600,000 (the “Director Limit”). However, the Board of Directors may make exceptions to these
limits in extraordinary circumstances as the Board may determine in its discretion, so long as the non-employee director receive such
additional compensation may not participation in the decision to award such compensation.
Options
The Administrator will generally
determine all terms and conditions of each option. However, the grant date may not be any day prior to the date that the Administrator
approves the grant, the exercise price may not be less than the fair market value of the shares subject to the option as determined on
the date of grant (other than in the case of an option that is not an incentive stock option and that complies with Section 409A
of the Internal Revenue Code of 1986, as amended (the “Code”)) and the option must terminate no later than ten years after
the date of grant. Unless restricted by the Administrator, and subject to such procedures as the Administrator may specify, the payment
of the exercise price of options may be made (1) by delivery of cash or other of our shares or other securities having a then fair
market value equal to the purchase price of such shares; (2) by delivery to us or our designated agent of an executed irrevocable
option exercise form together with irrevocable instructions to a broker-dealer to sell or margin a sufficient portion of the shares and
deliver the sale or margin loan proceeds directly to us to pay for the exercise price; (3) by surrendering the right to receive
shares otherwise deliverable to the participant upon exercise of the award having a fair market value at the time of exercise equal to
the total exercise price; or (4) by any combination of (1), (2) and/or (3). Except to the extent otherwise set forth in an
award agreement, a participant will have no rights as a holder of our common stock as a result of the grant of an option until the option
is exercised, the exercise price and applicable withholding taxes are paid and the shares subject to the option are issued thereunder.
Stock Appreciation
Rights
The Administrator will generally
determine all terms and conditions of each stock appreciation right. A stock appreciation right is the right of a participant to receive
cash in an amount, and/or common stock with a fair market value, equal to the appreciation of the fair market value of a share of our
common stock during a specified period of time. However, the grant date may not be any day prior to the date that the Administrator approves
the grant, the grant price may not be less than the fair market value of the shares subject to the stock appreciation right as determined
on the date of grant (unless the stock appreciation right complies with Code Section 409A) and the stock appreciation right must
terminate no later than ten years after the date of grant.
Performance and Stock
Awards
The Administrator will generally
determine all terms and conditions of each award of shares, restricted stock, restricted stock units, performance shares or performance
units. Restricted stock means shares of our common stock that are subject to a risk of forfeiture, restrictions on transfer or both a
risk of forfeiture and restrictions on transfer. Restricted stock unit means the right to receive a payment equal to the fair market
value of one share of our common stock. Performance share means the right to receive shares of our common stock, including restricted
stock, to the extent performance goals are achieved. Performance unit means the right to receive a payment valued in relation to a unit
that has a designated dollar value or the value of which is equal to the fair market value of one or more shares of our common stock,
to the extent performance goals are achieved. The terms and conditions that the Administrator will determine include the length of the
vesting and/or performance period.
Incentive Awards
The Administrator has the
authority to grant annual and long-term incentive awards. An incentive award is the right to receive a cash payment to the extent performance
goals are achieved. The Administrator will determine all of the terms and conditions of each incentive award, including the performance
goals, the performance period, the potential amount payable and the timing of payment, provided that the Administrator must require that
payment of all or any portion of the amount subject to the award is contingent on the achievement of one or more performance goals during
the period the Administrator specifies, although the Administrator may specify that all or a portion of the goals are deemed achieved
upon a participant’s death, disability or retirement, or such other circumstances as the Administrator may specify. For long-term
incentive awards, the performance period must relate to a period of more than one fiscal year.
Dividend Equivalents
and Dividends
In no event may dividends
or dividend equivalent units be awarded with respect to options, stock appreciation rights or any other stock-based award that is not
a grant of performance shares, performance units, restricted stock, restricted stock units or shares. For the avoidance of doubt, the
Plan expressly prohibits the payment of dividends or dividend equivalent units on unvested awards for all equity award types.
If cash dividends are paid
while shares of restricted stock are unvested, then such dividends will either, at the discretion of the Administrator, be (1) automatically
reinvested as additional shares of restricted stock that are subject to the same terms and conditions, including the risk of forfeiture,
as the original grant of restricted stock, or (2) paid in cash at the same time and the same extent that the restricted stock vests.
For clarity, in no event will dividends be distributed to a participant unless, until and to the same extent as the underlying shares
of restricted stock vest. The Administrator may grant dividend equivalent units only in tandem with restricted stock units, performance
shares or performance units. For clarity, in no event will a participant receive payment with respect to a dividend equivalent unit unless,
until and to the same extent as the tandem award vests and is paid.
Other Stock-Based
Awards
The Administrator may grant
to participants other types of awards that may be denominated or payable in, valued in whole or in part by reference to, or otherwise
based on, shares of our common stock, either alone or in addition to or in conjunction with other awards, and payable in shares or cash.
Subject to the limits of the Plan, an award may include the issuance of shares of unrestricted common stock, which may be awarded in
payment of director fees, in lieu of cash compensation, in exchange for cancellation of a compensation right, as a bonus, or upon the
attainment of performance goals or otherwise, or rights to acquire our common stock from us. The Administrator will generally determine
all terms and conditions of the award, except that any award that provides for purchase rights must be priced at 100% of fair market
value on the date of the award.
Minimum Vesting and
Discretion to Accelerate
All awards granted under
the Plan that may be settled in shares must have a minimum vesting period of one year from the date of grant, although that minimum vesting
period will not apply to awards with respect to up to 5% of the total number of shares reserved under the Plan. For purposes of awards
granted to non-employee directors, “one year” may mean the period of time from one annual stockholders meeting to the next
annual stockholders meeting as long as the period of time is not less than 50 weeks. The Administrator may accelerate the vesting of
an award or deem an award to be earned, in whole or in part, in the event of a participant’s death, disability, retirement, or
termination without cause, as provided in the Plan’s provisions concerning a change of control or upon any other event as determined
by the Administrator in its sole and absolute discretion.
Performance Goals
For purposes of the Plan,
performance goals means any goals the Administrator establishes. Performance goals may, without limitation, relate to one or more of
the following with respect to us or any one or more of our subsidiaries, affiliates or other business units: net sales; earnings before
interest and taxes; earnings before interest, taxes, depreciation and amortization; fair market value of shares; basic earnings per share;
diluted earnings per share; return on stockholder equity; return on average equity; return on average total capital employed; return
on net assets employed before interest and taxes; economic value added; return on year-end equity; capital; cost of capital; cost of
equity; cost of debt; taxes; market share; operating ratios; productivity measurements; revenue to budget compliance; net income to budget
compliance; return on total awards reinvestment; net free cash flow; new sales; divisional profitability; customer satisfaction measurements;
production quotas; project criteria or a combination of the foregoing.
As to each performance goal,
unless otherwise determined by the Administrator at any time, the relevant measurement of performance will be computed in accordance
with generally accepted accounting principles to the extent applicable. The Administrator may, at the time of establishing the performance
goals, exclude the effects of (1) extraordinary, unusual and/or non-recurring items of gain or loss, (2) gains or losses on
the disposition of a business, (3) changes in tax or accounting regulations or laws, or (4) the effect of a merger or acquisition.
Performance goals may be expressed in terms of attaining a specified level of the particular criterion or the attainment of an increase
or decrease (expressed as absolute numbers, averages and/or percentages) in the particular criterion or achievement in relation to a
peer group or other index. The performance goals also may include a threshold level of performance below which no payment will be made
(or no vesting will occur), levels of performance at which specified payments will be paid (or specified vesting will occur), and a maximum
level of performance above which no additional payment will be made (or at which full vesting will occur). In addition, the Administrator
may establish other performance goals and provide for other exclusions or adjustments not listed in the Plan.
Effect of Termination of Employment or Service on Awards
The Administrator will have
the discretion to determine, at the time an award is made to a participant or any time thereafter, the effect of the participant’s
termination of employment or service with us or our affiliates on the award.
Transferability of Awards
Awards under the Plan generally
will be nontransferable except (a) as otherwise determined by the Compensation Committee; (b) by will or the laws of descent
and distribution; and (c) to the spouse, children, or grandchildren of a participant, or to trusts or partnerships for their benefit,
under certain circumstances.
Adjustments
Under the terms of the Plan,
if any of the following occurs:
| · | We
are involved in a merger or other transaction in which our common stock is changed or exchanged; |
| · | We
subdivide or combine our common stock or declare a dividend payable in our common stock,
other securities or other property; |
| · | We
effect a cash dividend, the amount of which, on a per share basis, exceeds 10% of the fair
market value of a share of our common stock at the time the dividend is declared, or we effect
any other dividend or other distribution on our common stock in the form of cash, or a repurchase
of shares of our common stock, that our Board of Directors determines is special or extraordinary
in nature or that is in connection with a transaction that we characterize publicly as a
recapitalization or reorganization involving our common stock; or |
| · | Any
other event occurs, which, in the judgment of our Board of Directors or Compensation Committee
necessitates an adjustment to prevent an increase or decrease in the benefits or potential
benefits intended to be made available under the Plan; |
then the Administrator will,
in a manner it deems equitable to prevent an increase or decrease in the benefits or potential benefits intended to be made available
under the Plan and subject to certain provisions of the Code, adjust the number and type of shares of our common stock subject to the
Plan and which may, after the event, be made the subject of awards; the number and type of shares of our common stock subject to outstanding
awards; the grant, purchase or exercise price with respect to any award; and performance goals of an award.
No such adjustments may
be authorized in the case of incentive stock options to the extent that such authority would cause the Plan to violate Code Section 422(b).
Without limitation, if there
is a reorganization, merger, consolidation, combination or other similar corporate transaction or event, whether or not constituting
a change of control (other than any such transaction in which we are the continuing corporation and in which the outstanding shares are
not being converted into or exchanged for different securities, cash or other property, or any combination thereof), the Administrator
may substitute for each share then subject to an award and the shares subject to the Plan the number and kind of shares of stock, other
securities, cash or other property to which holders of our common stock will be entitled in respect of each share pursuant to the transaction.
In the case of a stock dividend
(other than a stock dividend declared in lieu of an ordinary cash dividend) or subdivision or combination of the shares (including a
reverse stock split), if no action is taken by the Administrator, the adjustments described above will automatically be made.
In connection with any merger,
consolidation, acquisition of property or stock, or reorganization, the Administrator may authorize the issuance or assumption of awards
under the Plan.
Change of Control
Unless otherwise provided
in an applicable employment, retention, change of control, severance, award or similar agreement, in the event of a change of control,
the successor or purchaser in the change of control transaction may assume an award or provide a substitute award with similar terms
and conditions and preserving the same benefits as the award it is replacing. If the awards are not so assumed or replaced, then unless
otherwise determined by the board of directors prior to the date of the change of control, immediately prior to the date of the change
of control:
| · | each
stock option or stock appreciation right that is then held by a participant who is employed
by or in the service of us or one of our affiliates will become fully vested, and all stock
options and stock appreciation rights will be cancelled in exchange for a cash payment equal
to the excess of the change of control price (as determined by the administrator) of the
shares of common stock covered by the stock option or stock appreciation right over the purchase
or grant price of such shares of common stock under the award; |
| · | restricted
stock, restricted stock units and share awards that are not vested will vest; |
| · | each
holder of a performance share and/or performance unit that has been earned but not yet paid
will receive cash equal to the value of the performance share and/or performance unit, and
each performance share and/or performance unit for which the performance period has not expired
will be cancelled in exchange for a cash payment equal to the value of the performance shares
and/or performance units that would have been earned if the performance goals (as measured
at the time of the change of control) were to continue to be achieved at the same rate through
the end of the performance period, or if higher, assuming the target performance goals had
been met at the time of such change of control, multiplied by a percentage based on the portion
of the performance period that has elapsed as of the date of the change of control; |
| · | all
incentive awards that are earned but not yet paid will be paid, and all incentive awards
that are not yet earned will be cancelled in exchange for a cash payment equal to the amount
that would have been due if the performance goals (measured at the time of the change of
control) continued to be achieved through the end of the performance period at the higher
of the then-current trend or target, multiplied by a percentage based on the portion of the
performance period that has elapsed as of the date of the change of control; |
| · | all
dividend equivalent units that are not vested will vest and be paid in cash; and |
| · | all
other awards that are not vested will vest, and if an amount is payable under such vested
award, then such amount will be paid in cash based on the value of the award. |
The terms of any awards
that are subject to Code Section 409A will govern the treatment of such awards upon a change of control to the extent required for
such awards to remain compliant with Code Section 409A, as applicable.
“Change of control”
under the Plan means the occurrence of any one of the following:
| · | Any
person (other than an employee benefit plan of our Company or of any subsidiary and fiduciaries
and certain other parties related to any of these plans) becomes the beneficial owner of
our securities representing 35% or more of the combined voting power of our then outstanding
securities; |
| · | We
are merged or consolidated with any other corporation or other entity, other than a merger
or consolidation which would result in our voting securities outstanding immediately prior
thereto continuing to represent more than 65% of the combined voting power of our voting
securities or the voting securities of such surviving entity outstanding immediately after
such merger or consolidation, or we engage in a merger or consolidation effected to implement
a recapitalization of our Company (or similar transaction) in which no person acquires 35%
or more of the combined voting power of our then outstanding securities. Notwithstanding
the foregoing, a merger or consolidation involving our Company will not be considered a change
of control if we are the surviving corporation and shares are not converted into or exchanged
for stock or securities of any other corporation, cash or any other thing of value, unless
persons who beneficially owned shares outstanding immediately prior to such transaction own
beneficially less than a majority of our outstanding voting securities immediately following
the merger or consolidation; |
| · | We
or any of our affiliates sell, assign or otherwise transfer assets in a transaction or series
of related transactions, if the aggregate market value of the assets so transferred exceeds
35% of our consolidated book value, determined by us in accordance with generally accepted
accounting principles, measured at the time at which such transaction occurs or the first
of such series of related transactions occurs; provided, however, that such a transfer effected
pursuant to a spin-off or split-up where our stockholders retain ownership of the transferred
assets proportionate to their pro rata ownership interest in our Company will not be a change
of control; |
| · | We
dissolve and liquidate substantially all of our assets; or |
| · | At
any time when the “continuing directors” cease to constitute a majority of our
Board of Directors. For this purpose, a “continuing director” means the individuals
who, at the effective date of the Plan, constitute the Board and any new directors (other
than directors designated by a person who has entered into an agreement with us to effect
a change of control transaction) whose appointment to the Board or nomination for election
by our stockholders was approved by a vote of at least two-thirds of the then-serving continuing
directors. |
If an award is considered
deferred compensation subject to the provisions of Code Section 409A, then the Administrator may include an amended definition of
“change of control” in the award agreement issued with respect to such award as necessary to comply with, or as necessary
to permit a deferral under, Code Section 409A.
The Plan does not provide
for a “gross-up” for any excise taxes imposed on golden parachute payments under Code Section 4999. Rather, except to
the extent the participant has in effect an employment or similar agreement with us or any affiliate or is subject to a policy that provides
for a more favorable result to the participant, if any payments or benefits paid by us pursuant to the Plan would cause some or all of
such payments or benefits in conjunction with any other payments or benefits in connection with a change of control to be subject to
the tax imposed by Code Section 4999, then these payments will either be cut back to a level below the amount triggering the tax
or be delivered in full, whichever will provide the greater after-tax benefit to the participant.
Termination and Amendment
The Plan will expire on
the tenth anniversary of the most recent stockholder approval, subject to the Board’s right to terminate the Plan at any time.
If approved, this proposal constitutes stockholder approval. In addition, the Board or the Administrator may amend the Plan at any time,
except:
| · | Our
Board of Directors must approve any amendment to the Plan if we determine such approval is
required by prior action of the Board, applicable corporate law or any other applicable law; |
| · | Stockholders
must approve any amendment to the Plan if we determine that such approval is required by
Section 16 of the Exchange Act, the listing requirements of any principal securities
exchange or market on which our common stock is then traded, or any other applicable law;
and |
Stockholders must approve
any further amendment to the Plan that materially increases the number of shares of common stock reserved under the Plan, that would
materially expand the group of individuals eligible to become participants or that diminishes the provisions prohibiting repricing or
backdating stock options and stock appreciation rights.
The Administrator generally
may modify, amend or cancel any award or waive any restrictions or conditions applicable to any award or the exercise of the award. Any
modification or amendment that materially diminishes the rights of the participant or any other person who may have an interest in the
award, or that cancels any award, will be effective only if agreed to by that participant or other person. The Administrator does not
need to obtain participant or other interested party consent, however, for the adjustment or cancellation of an award pursuant to the
adjustment provisions of the Plan or the modification of an award to the extent deemed necessary to comply with any applicable law or
the listing requirements of any principal securities exchange or market on which our common stock is then traded, to the extent the Administrator
deems necessary to preserve favorable accounting or tax treatment of any award for us, or to the extent the Administrator determines
that the action does not materially and adversely affect the value of an award or that such action is in the best interest of the affected
participant or any other person(s) with an interest in the award.
The authority of the Administrator
to terminate or modify the Plan or awards will extend beyond the termination date of the Plan. In addition, termination of the Plan will
not affect the rights of participants with respect to awards previously granted to them, and all unexpired awards will continue in force
after termination of the Plan except as they may lapse or be terminated by their own terms and conditions.
Cancellation, Disgorgement and Recoupment of Awards
The Compensation Committee
may cancel an award or require a participant to return to us any compensation received under an award in certain circumstances, such
as if the participant is terminated for cause or breaches any restrictive covenants, such as a non-compete, with us. In addition, all
awards will be subject to any recoupment or clawback policy that we adopt from time to time.
Repricing Prohibited
Neither the Administrator
nor any other person may: (1) amend the terms of outstanding stock options or stock appreciation rights to reduce the exercise price
of such outstanding stock options or stock appreciation rights; (2) cancel outstanding stock options or stock appreciation rights
in exchange for stock options or stock appreciation rights with an exercise price that is less than the exercise price of the original
stock options or stock appreciation rights; or (3) cancel outstanding stock options or stock appreciation rights with an exercise
price above the current share price in exchange for cash or other securities.
Backdating Prohibited
The Administrator may not
grant a stock option or stock appreciation right with a grant date that is effective prior to the date the Administrator takes action
to approve such award.
Foreign Participation
To assure the viability
of awards granted to participants employed or residing in foreign countries, the Administrator may provide for such special terms as
it may consider necessary or appropriate to accommodate differences in local law, tax policy, accounting or custom. Moreover, the Administrator
may approve such supplements to, or amendments, restatements or alternative versions of, the Plan as it determines is necessary or appropriate
for such purposes. Any such amendment, restatement or alternative versions that the Administrator approves for purposes of using the
Plan in a foreign country will not affect the terms of the Plan for any other country.
Certain Federal Income Tax Consequences
The following summarizes
certain federal income tax consequences relating to the Plan. The summary is based upon the laws and regulations in effect as of the
date of this proxy statement and does not purport to be a complete statement of the law in this area. Furthermore, the discussion below
does not address the tax consequences of the receipt or exercise of awards under foreign, state or local tax laws, and such tax laws
may not correspond to the federal income tax treatment described herein. The exact federal income tax treatment of transactions under
the Plan will vary depending upon the specific facts and circumstances involved and participants are advised to consult their personal
tax advisors with regard to all consequences arising from the grant or exercise of awards and the disposition of any acquired shares.
Stock Options
The grant of a stock option
under the Plan will create no income tax consequences to us or to the recipient. A participant who is granted a non-qualified stock option
will generally recognize ordinary compensation income at the time of exercise in an amount equal to the excess of the fair market value
of our common stock at such time over the exercise price. We will generally be entitled to a deduction in the same amount and at the
same time as the participant recognizes ordinary income. Upon the participant’s subsequent disposition of the shares of our common
stock received with respect to such stock option, the participant will recognize a capital gain or loss (long-term or short-term, depending
on the holding period) to the extent the amount realized from the sale differs from the tax basis (i.e., the fair market value of our
common stock on the exercise date).
In general, a participant
will recognize no income or gain as a result of the exercise of an incentive stock option, except that the alternative minimum tax may
apply. Except as described below, the participant will recognize a long-term capital gain or loss on the disposition of our common stock
acquired pursuant to the exercise of an incentive stock option and we will not be allowed a deduction. If the participant fails to hold
the shares of our common stock acquired pursuant to the exercise of an incentive stock option for at least two years from the grant date
of the incentive stock option and one year from the exercise date, then the participant will recognize ordinary compensation income at
the time of the disposition equal to the lesser of the gain realized on the disposition and the excess of the fair market value of the
shares of our common stock on the exercise date over the exercise price. We will generally be entitled to a deduction in the same amount
and at the same time as the participant recognizes ordinary income. Any additional gain realized by the participant over the fair market
value at the time of exercise will be treated as a capital gain.
Stock Appreciation Rights
The grant of a stock appreciation
right under the Plan will create no income tax consequences to us or to the recipient. A participant who is granted a stock appreciation
right will generally recognize ordinary compensation income at the time of exercise in an amount equal to the excess of the fair market
value of our common stock at such time over the grant price. We will generally be entitled to a deduction in the same amount and at the
same time as the participant recognizes ordinary income. If the stock appreciation right is settled in shares of our common stock, upon
the participant’s subsequent disposition of such shares, the participant will recognize a capital gain or loss (long-term or short-term,
depending on the holding period) to the extent the amount realized from the sale differs from the tax basis (i.e., the fair market value
of our common stock on the exercise date).
Restricted Stock
Generally, a participant
will not recognize income and we will not be entitled to a deduction at the time an award of restricted stock is made under the Plan,
unless the participant makes the election described below. A participant who has not made such an election will recognize ordinary income
at the time the restrictions on the stock lapse in an amount equal to the fair market value of the restricted stock at such time. We
will generally be entitled to a corresponding deduction in the same amount and at the same time as the participant recognizes income.
Any otherwise taxable disposition of the restricted stock after the time the restrictions lapse will result in a capital gain or loss
(long-term or short-term, depending on the holding period) to the extent the amount realized from the sale differs from the tax basis
(i.e., the fair market value of our common stock on the date the restrictions lapse). Dividends paid in cash and received by a participant
prior to the time the restrictions lapse will constitute ordinary income to the participant in the year paid and we will generally be
entitled to a corresponding deduction for such dividends. Any dividends paid in stock will be treated as an award of additional restricted
stock subject to the tax treatment described herein.
A participant may, within
30 days after the date of the award of restricted stock, elect to recognize ordinary income as of the date of the award in an amount
equal to the fair market value of such restricted stock on the date of the award (less the amount, if any, the participant paid for such
restricted stock). If the participant makes such an election, then we will generally be entitled to a corresponding deduction in the
same amount and at the same time as the participant recognizes income. If the participant makes the election, then any cash dividends
the participant receives with respect to the restricted stock will be treated as dividend income to the participant in the year of payment
and will not be deductible by us. Any otherwise taxable disposition of the restricted stock (other than by forfeiture) will result in
a capital gain or loss. If the participant who has made an election subsequently forfeits the restricted stock, then the participant
will not be entitled to claim a credit for the tax previously paid. In addition, we would then be required to include as ordinary income
the amount of any deduction it originally claimed with respect to such shares.
Restricted Stock Units
A participant will not recognize
income and we will not be entitled to a deduction at the time an award of a restricted stock unit is made under the Plan. Upon the participant’s
receipt of shares (or cash) at the end of the restriction period, the participant will recognize ordinary income equal to the amount
of cash and/or the fair market value of the shares received, and we will be entitled to a corresponding deduction in the same amount
and at the same time. If the restricted stock units are settled in whole or in part in shares, upon the participant’s subsequent
disposition of the shares the participant will recognize a capital gain or loss (long-term or short-term, depending on the holding period)
to the extent the amount realized upon disposition differs from the shares’ tax basis (i.e., the fair market value of the shares
on the date the participant received the shares).
Performance Shares
The grant of performance
shares will create no income tax consequences for us or the participant. Upon the participant’s receipt of shares at the end of
the applicable performance period, the participant will recognize ordinary income equal to the fair market value of the shares received,
except that if the participant receives shares of restricted stock in payment of performance shares, recognition of income may be deferred
in accordance with the rules applicable to restricted stock as described above. In addition, the participant will recognize ordinary
compensation income equal to the dividend equivalents paid on performance shares prior to or at the end of the performance period. We
will generally be entitled to a deduction in the same amount and at the same time as the participant recognizes income. Upon the participant’s
subsequent disposition of the shares, the participant will recognize a capital gain or loss (long-term or short-term depending on the
holding period) to the extent the amount realized from the disposition differs from the shares’ tax basis (i.e., the fair market
value of the shares on the date the participant received the shares).
Performance Units
The grant of a performance
unit will create no income tax consequences to us or the participant. Upon the participant’s receipt of cash and/or shares at the
end of the applicable performance period, the participant will recognize ordinary income equal to the amount of cash and/or the fair
market value of the shares received, and we will be entitled to a corresponding deduction in the same amount and at the same time. If
performance units are settled in whole or in part in shares, upon the participant’s subsequent disposition of the shares the participant
will recognize a capital gain or loss (long-term or short-term, depending on the holding period) to the extent the amount realized upon
disposition differs from the shares’ tax basis (i.e., the fair market value of the shares on the date the participant received
the shares).
Incentive Awards
A participant who is paid
an incentive award will recognize ordinary income equal to the amount of cash paid, and we will generally be entitled to a corresponding
income tax deduction.
Dividend Equivalent Units
A participant who is paid
a dividend equivalent with respect to an award will recognize ordinary income equal to the value of cash or common stock paid, and we
will be entitled to a corresponding deduction in the same amount and at the same time.
Section 162(m) Limit on Deductibility of Compensation
Section 162(m) of
the Code limits the deduction we can take for compensation, including compensation arising from awards under the Plan, paid to covered
employees to $1,000,000 per person per year. The covered employees for any fiscal year generally include any employee (i) who served
as our chief executive officer or chief financial officer at any point during the fiscal year, (ii) whose compensation was otherwise
required to be included in our proxy statement by reason of being among our three highest compensated officers for the fiscal year, or
(iii) who was a covered employee for any preceding fiscal year beginning after December 31, 2016. The American Rescue Plan
Act of 2021 will, for taxable years beginning after December 31, 2026, include as covered employees an additional five employees
who are among the most highly compensated.
Code Sections 409A and 280G
Awards under the Plan may
constitute, or provide for, a deferral of compensation under Section 409A of the Code. If the requirements of Code Section 409A
are not complied with, then holders of such awards may be taxed earlier than would otherwise be the case (e.g., at the time of vesting
instead of the time of payment) and may be subject to an additional 20% penalty tax and, potentially, interest and penalties. The Plan
is intended to permit compliance with Code Section 409A and the Department of Treasury regulations and other interpretive guidance
that may be issued pursuant to Code Section 409A. To the extent that we determine that any award granted under the Plan is subject
to Code Section 409A, the award agreement evidencing such award is expected generally to incorporate the terms and conditions required
by Code Section 409A. The Plan and any applicable awards may be modified to exempt the awards from Code Section 409A or comply
with the requirements of Code Section 409A.
Code Sections 280G and 4999
may limit our income tax deduction and impose an excise tax on golden parachute payments to participants in the event there is a change
of control of our Company. The Plan does not provide for a “gross-up” for any excise taxes imposed on golden parachute payments
under Code Section 4999. Rather, except to the extent the participant has in effect an employment or similar agreement with us or
any affiliate or is subject to a policy that provides for a more favorable result to the participant, if any payments or benefits paid
by us pursuant to the Plan would cause some or all of such payments or benefits in conjunction with any other payments or benefits in
connection with a change of control to be subject to the tax imposed by Code Section 4999, then these payments will either be cut
back to a level below the amount triggering the tax or be delivered in full, whichever will provide the greater after-tax benefit to
the participant. Accordingly, some or all of the amount which would otherwise be deductible may not be deductible with respect to benefits
under the Plan that are contingent on or otherwise provided in connection with a change of control of our Company.
New Plan Benefits
The awards that may be granted
under the Plan in the future to the executive officers or non-employee directors named in this proxy statement or to other officers,
non-employee directors, employees, or other persons cannot be determined at this time. Our Board of Directors, along with management,
will make such determinations from time to time.
Severance or Change of Control Arrangements
We have no agreements that
provide for payments to our directors or executive officers at, following, or in connection with the resignation, retirement, or other
termination of our directors or executive officers, or a change of control of the Company.
Director Compensation
Non-employee directors are compensated for their
service on our Board as shown below. Directors who are employees of the Company receive no additional compensation for serving as directors.
The Compensation Committee periodically reviews the compensation of our non-employee directors and considers market practices.
During
2022 and 2023, the Compensation Committee received advice and recommendations from Pay Governance LLC regarding compensation benchmarking
as compared to a peer group of companies, including compensation of non-employee directors (see below, “Compensation Discussion
and Analysis – Benchmarking Against Peer Companies”). Upon review, the Compensation Committee recommended, and the
Board approved, maintaining the 2022 level of non-employee director compensation for 2023 which is set forth below.
| 2023
Director Compensation (non-employee) ¹ |
| Annual
cash retainer: |
$75,000 |
| Additional
Board Fees: |
|
| Chair
of the Board |
$50,000 |
| Chair
of a Board Committee |
$20,000 |
| Member
of a Board Committee |
$10,000 |
| Annual
RSU award value: |
$135,000 |
Given the recent performance of the Company and
the resulting cash constraints, the Board approved a significant reduction in the cash compensation for non-employee directors in 2024
along with the removal of the equity component, as set forth below:
| 2024
Director Compensation (non-employee) ² |
| Annual
cash retainer: |
$20,000 |
| Chair
of the Board or Lead Independent Director |
$20,000 |
| Chair
of Audit Committee |
$10,000 |
| Chair
of Compensation or Corporate Governance & Nominating Committee |
$5,000 |
| Member
of a Board Committee |
$5,000 |
| Annual
RSU award value: |
$0 |
¹The
Directors voted to forfeit payment of their cash fees for the fourth quarter of 2023, thus the cash actually paid is lower than reflected
in the table.
²The
Board voted to forgo any director compensation until after the 2024 Annual Stockholder Meeting, thus the director compensation paid in
2024 will be lower than reflected in the table.
The
table below summarizes the compensation paid to our non-employee directors for the fiscal year ended December 31, 2023 (in thousands):
| Name | |
Fees
earned
or paid in
cash(1) | | |
Option
Awards | | |
Restricted
Stock Unit
Awards(2) | | |
All Other
Compensation | | |
Total | |
| Clifford
B. Fleet(6) | |
$ | 37,500 | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 37,500 | |
| Anthony Johnson | |
$ | 81,250 | | |
$ | — | | |
$ | 106,406 | | |
$ | — | | |
$ | 187,656 | |
| Michael Koganov | |
$ | 81,250 | | |
$ | — | | |
$ | 106,406 | | |
$ | — | | |
$ | 187,656 | |
| Roger
D. O’Brien(6) | |
$ | 47,500 | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 47,500 | |
| Richard M. Sanders | |
$ | 83,750 | | |
$ | — | | |
$ | 106,406 | | |
$ | — | | |
$ | 190,156 | |
| Lucille S. Salhany | |
$ | 68,750 | | |
$ | — | | |
$ | 106,406 | | |
$ | — | | |
$ | 175,156 | |
| Nora
B. Sullivan(3) (6) | |
$ | 388,750 | | |
$ | — | | |
$ | 106,406 | | |
$ | — | | |
$ | 495,156 | |
| James
Mish(4) (6) | |
$ | 7,083 | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 7,083 | |
| Andrew
Arno(5) | |
$ | 19,103 | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 19,103 | |
| (1) | On
February 9, 2024, the Board of Directors voted to waive any cash compensation for non-employee
directors for the fourth quarter of 2023 and the first quarter of 2024. All amounts reflected
as fees earned or paid in cash exclude the cash portion waived in the aggregate amount of
$193,750. |
| (2) | The
fair value of each restricted stock unit is based on the stock price of the Company’s
common stock on the grant date of the award. |
| (3) | Ms. Sullivan
assumed a leadership role in the second half of 2023 overseeing all non-tobacco functions,
including human resources, research and development, finance, legal and investor relations,
receiving additional fees of $260,000. |
| (4) | Mr. Mish
resigned from employment as Chief Executive Officer on July 21, 2023 and therefore received
pro rata compensation for service as a director subsequent to this date during the third
quarter of 2023. |
| (5) | Mr. Arno
was appointed as a director on July 19, 2023 and received pro rata compensation for
service during 2023. |
| (6) | Former director. |
MARKET PRICE OF AND DIVIDENDS
ON THE COMPANY’S COMMON STOCK
AND RELATED STOCKHOLDER MATTERS
Market Information
Our common stock trades on
the Nasdaq Capital Market under the symbol “XXII.”
Holders
As of August 15, 2024, there
were approximately 135 holders of record of our common stock based on the records of our transfer agent. However, because many of our
shares of common stock are held by brokers and other institutions on behalf of shareholders, we believe there are considerably more beneficial
holders of our common stock than record holders.
Dividends
We have never declared or
paid dividends. We do not intend to pay cash dividends on our common stock for the foreseeable future, but currently intend to retain
any future earnings to fund the development and growth of our business. The payment of dividends, if any, on our common stock will rest
solely within the discretion of our board of directors and will depend, among other things, upon our earnings, capital requirements,
financial condition, and other relevant factors.
SECURITY OWNERSHIP OF CERTAIN
BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth
information regarding the beneficial ownership of our common stock as of August 15, 2024, by (i) each person who, to our knowledge,
owns more than 5% of our common stock, (ii) each of our current directors and executive officers, and (iii) all our current
directors and executive officers as a group. To our knowledge, no person owns more than 5% of our common stock. Derivative securities
exercisable or convertible into shares of our common stock within sixty (60) days of August 15, 2024 are deemed to be beneficially
owned and outstanding for computing the share ownership and percentage of the person holding securities but are not deemed outstanding
for computing the percentage of any other person. Beneficial ownership representing less than 1% is denoted with an asterisk (*). The
address of named beneficial owners that are officers and/or directors of the Company is: c/o 22nd Century Group, Inc., 321 Farmington
Road, Mocksville, North Carolina. 27028. The following table is based upon information supplied by officers and directors, and with respect
to 5% or greater stockholders who are not officers or directors, information filed with the SEC.
| | |
Number of | | |
| |
| | |
Shares | | |
Percentage | |
| | |
Beneficially | | |
Beneficially | |
| Name of Beneficial
Owner | |
Owned | | |
Owned (1) | |
| Management and Directors: | |
| | | |
| | |
| Lawrence D. Firestone | |
| 15,250 | | |
| * | |
| Daniel
Otto(2) | |
| 129 | | |
| * | |
| Jonathan
Staffeldt(3) | |
| 817 | | |
| * | |
| Andrew Arno | |
| 2,387 | | |
| * | |
| Anthony Johnson | |
| 918 | | |
| * | |
| Lucille S. Salhany | |
| 650 | | |
| * | |
| All
directors and executive officers as a group (6 persons) (2) - (3) | |
| 20,151 | | |
| * | |
| |
(1) |
Based on 9,272,518 shares of common stock issued and outstanding as of August 15, 2024. |
| |
(2) |
298 restricted stock units are not included in the number of beneficially owned shares because they do not vest within 60 days of August 15, 2024. |
| |
(3) |
74 restricted stock units are not included in the number of beneficially owned shares because they do not vest within 60 days of August 15, 2024. |
RELATED PARTY TRANSACTIONS
Our policy is to enter into transactions with
related persons on terms that, on the whole, are no less favorable to us than those available from unaffiliated third parties. Our Board
of Directors has adopted written policies and procedures regarding related person transactions. For purposes of these policies and procedures:
| · | A
“related person” means any of our directors, executive officers, nominees for
director, holder of 5% or more of our common stock or any of their immediate family members;
and |
| · | A
“related person transaction” generally is a transaction (including any indebtedness
or a guarantee of indebtedness) in which we were or are to be a participant and the amount
involved exceeds $120,000 and in which a related person had or will have a direct or indirect
material interest. |
Each of our executive officers, directors or
nominees for director is required to disclose to our Audit Committee certain information relating to related person transactions for
review, approval or ratification by our Audit Committee. In making a determination about approval or ratification of a related person
transaction, our Audit Committee will consider the information provided regarding the related person transaction and whether consummation
of the transaction is believed by the Audit Committee to be in our best interests. Our Audit Committee may take into account the effect
of a director’s related person transaction on the director’s status as in independent member of our Board of Directors and
eligibility to serve on committees of our Board under SEC rules and the listing standards of the Nasdaq Stock Market. Any related
person transaction must be disclosed to our full Board of Directors. There were no related party transactions during 2023 and 2022.
EXPERTS
The consolidated balance sheets
of the Company as of December 31, 2023, the related consolidated statements of operations and comprehensive loss, stockholders’
equity and cash flows for the year ended December 31, 2023 and the related notes, have been audited by Freed Maxick CPAs, P.C., an independent
registered public accounting firm, as stated in their report, which is included herein. Such consolidated financial statements have been
included herein, in reliance upon the report of such firm (which report expresses an unqualified and includes an explanatory paragraph
relating to substantial doubt about the ability to continue as a going concern) given upon their authority as experts in accounting and
auditing.
LEGAL MATTERS
Certain legal matters with
respect to the Offered Shares offered by this Offering Circular will be passed upon for us by Foley & Lardner LLP, Jacksonville,
Florida
WHERE YOU CAN FIND MORE
INFORMATION
We have filed an offering
statement on Form 1-A with the SEC under the Securities Act with respect to the common stock offered by this Offering Circular.
This Offering Circular, which constitutes a part of the offering statement, does not contain all of the information set forth in the
offering statement or the exhibits and schedules filed therewith. For further information with respect to us and our common stock, please
see the offering statement and the exhibits and schedules filed with the offering statement. Statements contained in this Offering Circular
regarding the contents of any contract or any other document that is filed as an exhibit to the offering statement are not necessarily
complete, and each such statement is qualified in all respects by reference to the full text of such contract or other document filed
as an exhibit to the offering statement. The offering statement, including its exhibits and schedules, may be accessed at the SEC’s
website http://www.sec.gov. These filings will be available as soon as reasonably practicable after we electronically file such material
with, or furnish it to, the SEC.
22nd CENTURY GROUP, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
(Unaudited)
(amounts in thousands, except share and per-share
data)
| | |
June 30, | | |
December 31, | |
| | |
2024 | | |
2023 | |
| ASSETS | |
| | | |
| | |
| Current assets: | |
| | | |
| | |
| Cash and cash equivalents | |
$ | 1,279 | | |
$ | 2,058 | |
| Accounts receivable, net | |
| 2,448 | | |
| 1,671 | |
| Inventories | |
| 2,664 | | |
| 4,346 | |
| Insurance recoveries | |
| 3,768 | | |
| 3,768 | |
| GVB promissory note | |
| 500 | | |
| 2,000 | |
| Prepaid expenses and other current assets | |
| 1,767 | | |
| 1,180 | |
| Current assets of discontinued operations held for sale | |
| 1,058 | | |
| 1,254 | |
| Total current assets | |
| 13,484 | | |
| 16,277 | |
| Property, plant and equipment, net | |
| 3,093 | | |
| 3,393 | |
| Operating lease right-of-use assets, net | |
| 1,769 | | |
| 1,894 | |
| Intangible assets, net | |
| 5,728 | | |
| 5,924 | |
| Other assets | |
| 15 | | |
| 15 | |
| Total assets | |
$ | 24,089 | | |
$ | 27,503 | |
| | |
| | | |
| | |
| LIABILITIES AND SHAREHOLDERS' DEFICIT | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | |
| Notes and loans payable - current | |
$ | 877 | | |
$ | 543 | |
| Current portion of long-term debt | |
| 1,500 | | |
| 5,848 | |
| Operating lease obligations | |
| 245 | | |
| 231 | |
| Accounts payable | |
| 3,893 | | |
| 4,445 | |
| Accrued expenses | |
| 884 | | |
| 1,322 | |
| Accrued litigation | |
| 3,768 | | |
| 3,768 | |
| Accrued payroll | |
| 300 | | |
| 883 | |
| Accrued excise taxes and fees | |
| 2,510 | | |
| 2,234 | |
| Deferred income | |
| 389 | | |
| 726 | |
| Other current liabilities | |
| 309 | | |
| 1,849 | |
| Current liabilities of discontinued operations held for sale | |
| 1,397 | | |
| 3,185 | |
| Total current liabilities | |
| 16,072 | | |
| 25,034 | |
| Long-term liabilities: | |
| | | |
| | |
| Operating lease obligations | |
| 1,571 | | |
| 1,698 | |
| Long-term debt | |
| 6,046 | | |
| 8,058 | |
| Other long-term liabilities | |
| 1,355 | | |
| 1,123 | |
| Total liabilities | |
| 25,044 | | |
| 35,914 | |
| Commitments and contingencies (Note 12) | |
| | | |
| | |
| Shareholders' equity (deficit) | |
| | | |
| | |
| Preferred stock, $.00001 par value, 10,000,000 shares authorized | |
| | | |
| | |
| Common stock, $.00001 par value, 250,000,000 shares authorized | |
| | | |
| | |
| Capital stock issued and outstanding: | |
| | | |
| | |
| 9,272,518 common shares (2,720,437 at December 31, 2023) | |
| | | |
| | |
| Common stock, par value | |
| — | | |
| — | |
| Capital in excess of par value | |
| 384,603 | | |
| 370,297 | |
| Accumulated deficit | |
| (385,558 | ) | |
| (378,707 | ) |
| Total shareholders' deficit | |
| (955 | ) | |
| (8,410 | ) |
| Total liabilities and shareholders’ deficit | |
$ | 24,089 | | |
$ | 27,503 | |
See accompanying notes to Condensed Consolidated
Financial Statements.
22nd CENTURY GROUP, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
AND COMPREHENSIVE LOSS
(Unaudited)
(amounts in thousands, except share and per-share
data)
| | |
Three Months Ended | | |
Six Months Ended | |
| | |
June 30, | | |
June 30, | |
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| Revenues, net | |
$ | 7,947 | | |
$ | 8,050 | | |
$ | 14,416 | | |
$ | 16,977 | |
| Cost of goods sold | |
| 3,869 | | |
| 4,682 | | |
| 8,082 | | |
| 9,407 | |
| Excise taxes and fees on products | |
| 3,508 | | |
| 4,329 | | |
| 6,893 | | |
| 8,514 | |
| Gross (loss) profit | |
| 570 | | |
| (961 | ) | |
| (559 | ) | |
| (944 | ) |
| Operating expenses: | |
| | | |
| | | |
| | | |
| | |
| Sales, general and administrative | |
| 2,360 | | |
| 10,283 | | |
| 5,266 | | |
| 20,119 | |
| Research and development | |
| 250 | | |
| 799 | | |
| 675 | | |
| 1,529 | |
| Other operating expense (income), net | |
| 7 | | |
| — | | |
| (19 | ) | |
| (146 | ) |
| Total operating expenses | |
| 2,617 | | |
| 11,082 | | |
| 5,922 | | |
| 21,502 | |
| Operating loss from continuing operations | |
| (2,047 | ) | |
| (12,043 | ) | |
| (6,481 | ) | |
| (22,446 | ) |
| Other income (expense): | |
| | | |
| | | |
| | | |
| | |
| Other income (expense), net | |
| 339 | | |
| (613 | ) | |
| 339 | | |
| (768 | ) |
| Interest income, net | |
| 21 | | |
| 65 | | |
| 21 | | |
| 122 | |
| Interest expense | |
| (501 | ) | |
| (1,071 | ) | |
| (1,517 | ) | |
| (1,399 | ) |
| Total other expense | |
| (141 | ) | |
| (1,619 | ) | |
| (1,157 | ) | |
| (2,045 | ) |
| Loss from continuing operations before income taxes | |
| (2,188 | ) | |
| (13,662 | ) | |
| (7,638 | ) | |
| (24,491 | ) |
| Provision for income taxes | |
| 26 | | |
| 46 | | |
| 26 | | |
| 46 | |
| Net loss from continuing operations | |
$ | (2,214 | ) | |
$ | (13,708 | ) | |
$ | (7,664 | ) | |
$ | (24,537 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Discontinued operations: | |
| | | |
| | | |
| | | |
| | |
| Income (loss) from discontinued operations before income taxes | |
$ | 1,102 | | |
$ | (6,831 | ) | |
$ | 813 | | |
$ | (14,184 | ) |
| Provision (benefit) for income taxes | |
| — | | |
| — | | |
| — | | |
| — | |
| Net income (loss) from discontinued operations | |
$ | 1,102 | | |
$ | (6,831 | ) | |
$ | 813 | | |
$ | (14,184 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Net loss | |
$ | (1,112 | ) | |
$ | (20,539 | ) | |
$ | (6,851 | ) | |
$ | (38,721 | ) |
| Deemed dividends | |
| (445 | ) | |
| (367 | ) | |
| (4,034 | ) | |
| (367 | ) |
| Net loss available to common shareholders | |
$ | (1,557 | ) | |
$ | (20,906 | ) | |
$ | (10,885 | ) | |
$ | (39,088 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Basic and diluted loss per common share from continuing operations | |
$ | (0.30 | ) | |
$ | (15.61 | ) | |
$ | (1.44 | ) | |
$ | (28.46 | ) |
| Basic and diluted loss per common share from discontinued operations | |
$ | 0.15 | | |
$ | (7.78 | ) | |
$ | 0.15 | | |
$ | (16.45 | ) |
| Basic and diluted loss per common share from deemed dividends | |
$ | (0.06 | ) | |
$ | (0.42 | ) | |
$ | (0.76 | ) | |
$ | (0.43 | ) |
| Basic and diluted loss per common share | |
$ | (0.21 | ) | |
$ | (23.81 | ) | |
$ | (2.05 | ) | |
$ | (45.34 | ) |
| Weighted average common shares outstanding - basic and diluted | |
| 7,449,706 | | |
| 878,171 | | |
| 5,307,471 | | |
| 862,177 | |
| | |
| | | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | |
| Net loss | |
$ | (1,112 | ) | |
$ | (20,539 | ) | |
$ | (6,851 | ) | |
$ | (38,721 | ) |
| Other comprehensive income: | |
| | | |
| | | |
| | | |
| | |
| Unrealized gain on short-term investment securities | |
| — | | |
| 10 | | |
| — | | |
| 71 | |
| Foreign currency translation | |
| — | | |
| 42 | | |
| — | | |
| 38 | |
| Reclassification of realized losses to net loss | |
| — | | |
| 28 | | |
| — | | |
| 41 | |
| Other comprehensive income | |
| — | | |
| 80 | | |
| — | | |
| 150 | |
| Comprehensive loss | |
$ | (1,112 | ) | |
$ | (20,459 | ) | |
$ | (6,851 | ) | |
$ | (38,571 | ) |
See accompanying notes to Condensed Consolidated
Financial Statements.
22nd CENTURY GROUP, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CHANGES
IN SHAREHOLDERS’ EQUITY (DEFICIT)
(Unaudited)
(amounts in thousands, except share data)
| | |
Six Months Ended June 30, 2024 | |
| | |
| | |
| | |
| | |
| | |
| |
| | |
Common | | |
Par Value | | |
Capital in | | |
| | |
Total | |
| | |
Shares | | |
of Common | | |
Excess of | | |
Accumulated | | |
Shareholders’ | |
| | |
Outstanding* | | |
Shares* | | |
Par Value* | | |
Deficit | | |
Deficit | |
| Balance at January 1, 2024 | |
| 2,720,437 | | |
$ | — | | |
$ | 370,297 | | |
$ | (378,707 | ) | |
$ | (8,410 | ) |
| Stock issued in connection with RSU vesting, net of 405 shares withheld for taxes | |
| 3,810 | | |
| — | | |
| (1 | ) | |
| — | | |
| (1 | ) |
| Stock issued in connection with licensing arrangement | |
| 11,480 | | |
| — | | |
| 100 | | |
| — | | |
| 100 | |
| Stock issued in connection with warrant exercises, net of fees of $176 | |
| 747,001 | | |
| — | | |
| 2,245 | | |
| — | | |
| 2,245 | |
| Equity-based compensation | |
| — | | |
| — | | |
| 181 | | |
| — | | |
| 181 | |
| Fractional shares issued for reverse stock split | |
| 118,207 | | |
| — | | |
| — | | |
| — | | |
| — | |
| Net loss | |
| — | | |
| — | | |
| — | | |
| (5,739 | ) | |
| (5,739 | ) |
| Balance at March 31, 2024 | |
| 3,600,935 | | |
$ | — | | |
$ | 372,822 | | |
$ | (384,446 | ) | |
$ | (11,624 | ) |
| Stock issued in connection with warrant exercises | |
| 265,625 | | |
| — | | |
| — | | |
| — | | |
| — | |
| Stock issued for extinguishment of Subordinated Note | |
| 1,150,000 | | |
| — | | |
| 3,864 | | |
| — | | |
| 3,864 | |
| Stock issued in connection with capital raise, net of issuance costs of $324 1 | |
| 1,980,000 | | |
| — | | |
| 3,913 | | |
| — | | |
| 3,913 | |
| Stock issued upon conversion of Senior Secured Credit Facility 2 | |
| 1,575,000 | | |
| — | | |
| 2,756 | | |
| — | | |
| 2,756 | |
| Stock issued in connection with settled indebtedness | |
| 700,958 | | |
| — | | |
| 1,192 | | |
| — | | |
| 1,192 | |
| Equity-based compensation | |
| — | | |
| — | | |
| 56 | | |
| — | | |
| 56 | |
| Net loss | |
| — | | |
| — | | |
| — | | |
| (1,112 | ) | |
| (1,112 | ) |
| Balance at June 30, 2024 | |
| 9,272,518 | | |
$ | — | | |
$ | 384,603 | | |
$ | (385,558 | ) | |
$ | (955 | ) |
*Giving retroactive effect to the 1-for-15 reverse stock split on July 5, 2023 and subsequent 1-for-16 reverse stock split on April 2, 2024.
1 Includes exercises of 125,000 shares of prefunded warrants during the period ended June 30, 2024.
2 Includes exercises of 980,000 shares of prefunded warrants during the period ended June 30, 2024.
| | |
Six Months Ended June 30, 2023 | |
| | |
| | |
| | |
| | |
Accumulated | | |
| | |
| |
| | |
Common | | |
Par Value | | |
Capital in | | |
Other | | |
| | |
Total | |
| | |
Shares | | |
of Common | | |
Excess of | | |
Comprehensive | | |
Accumulated | | |
Shareholders’ | |
| | |
Outstanding* | | |
Shares* | | |
Par Value* | | |
Income (Loss) | | |
Deficit | | |
Equity | |
| Balance at January 1, 2023 | |
| 843,731 | | |
$ | — | | |
$ | 333,900 | | |
$ | (111 | ) | |
$ | (237,814 | ) | |
$ | 95,975 | |
| Stock issued in connection with RSU vesting, net of 1,976 shares withheld for taxes | |
| 5,644 | | |
| — | | |
| (414 | ) | |
| — | | |
| — | | |
| (414 | ) |
| Stock issued in connection with acquisition | |
| 1,941 | | |
| — | | |
| 503 | | |
| — | | |
| — | | |
| 503 | |
| Equity-based compensation | |
| — | | |
| — | | |
| 1,175 | | |
| — | | |
| — | | |
| 1,175 | |
| Adoption of ASU 2016-13 | |
| — | | |
| — | | |
| — | | |
| — | | |
| (118 | ) | |
| (118 | ) |
| Equity detachable warrants | |
| — | | |
| — | | |
| 1,577 | | |
| — | | |
| — | | |
| 1,577 | |
| Other comprehensive income | |
| — | | |
| — | | |
| — | | |
| 70 | | |
| — | | |
| 70 | |
| Net loss | |
| — | | |
| — | | |
| — | | |
| — | | |
| (18,182 | ) | |
| (18,182 | ) |
| Balance at March 31, 2023 | |
| 851,316 | | |
$ | — | | |
$ | 336,741 | | |
$ | (41 | ) | |
$ | (256,114 | ) | |
$ | 80,586 | |
| Stock issued in connection with RSU vesting, net of shares withheld for taxes | |
| 1,534 | | |
| — | | |
| (5 | ) | |
| — | | |
| — | | |
| (5 | ) |
| Stock issued in connection with ATM, net of fees of $178 | |
| 17,783 | | |
| — | | |
| 2,563 | | |
| — | | |
| — | | |
| 2,563 | |
| Stock issued in connection with capital raise, net of issuance costs of $422 | |
| 46,753 | | |
| — | | |
| 4,851 | | |
| — | | |
| — | | |
| 4,851 | |
| Stock issued in connection with licensing arrangement | |
| 20,834 | | |
| — | | |
| 3,570 | | |
| — | | |
| — | | |
| 3,570 | |
| Equity-based compensation | |
| — | | |
| — | | |
| 1,486 | | |
| — | | |
| — | | |
| 1,486 | |
| Other comprehensive loss | |
| — | | |
| — | | |
| — | | |
| 80 | | |
| — | | |
| 80 | |
| Net loss | |
| — | | |
| — | | |
| — | | |
| — | | |
| (20,539 | ) | |
| (20,539 | ) |
| Fractional shares issued for reverse stock split | |
| 4,128 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Balance at June 30, 2023 | |
| 942,348 | | |
$ | — | | |
$ | 349,206 | | |
$ | 39 | | |
$ | (276,653 | ) | |
$ | 72,592 | |
*Giving retroactive effect to the 1-for-15 reverse stock split on July 5, 2023 and subsequent 1-for-16 reverse stock split on April 2, 2024.
See accompanying notes to Condensed Consolidated
Financial Statements.
22nd CENTURY GROUP, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(Unaudited)
(amounts in thousands)
| | |
Six Months Ended | |
| | |
June 30, | |
| | |
2024 | | |
2023 | |
| Cash flows from operating activities: | |
| | | |
| | |
| Net loss | |
$ | (6,851 | ) | |
$ | (38,721 | ) |
| Adjustments to reconcile net loss to cash used in operating activities: | |
| | | |
| | |
| Amortization and depreciation | |
| 514 | | |
| 2,093 | |
| Amortization of right-of-use asset | |
| 125 | | |
| 524 | |
| Other non-cash losses | |
| (947 | ) | |
| 18 | |
| Provision for credit losses | |
| 7 | | |
| 154 | |
| Loss on the sale of machinery and equipment | |
| 65 | | |
| 75 | |
| Debt related charges included in interest expense | |
| 1,674 | | |
| 1,100 | |
| Equity-based employee compensation expense | |
| 237 | | |
| 2,661 | |
| Gain on change of contingent consideration | |
| — | | |
| (195 | ) |
| Change in fair value of warrant liabilities | |
| (324 | ) | |
| 723 | |
| Change in fair value of derivative liability | |
| (459 | ) | |
| — | |
| Increase in inventory reserves | |
| 431 | | |
| — | |
| Changes in operating assets and liabilities, net of acquisition: | |
| | | |
| | |
| Accounts receivable | |
| (784 | ) | |
| (3,322 | ) |
| Inventories | |
| 1,251 | | |
| (4,285 | ) |
| Prepaid expenses and other assets | |
| (548 | ) | |
| (2,178 | ) |
| Accounts payable | |
| 24 | | |
| 2,257 | |
| Accrued expenses | |
| (437 | ) | |
| 2,066 | |
| Accrued payroll | |
| (583 | ) | |
| (774 | ) |
| Accrued excise taxes and fees | |
| 276 | | |
| 1,280 | |
| Other liabilities | |
| (641 | ) | |
| (808 | ) |
| Net cash used in operating activities | |
| (6,970 | ) | |
| (37,332 | ) |
| Cash flows from investing activities: | |
| | | |
| | |
| Acquisition of patents, trademarks, and licenses | |
| — | | |
| (398 | ) |
| Acquisition of property, plant and equipment | |
| (71 | ) | |
| (2,759 | ) |
| Proceeds from the sale of property, plant and equipment | |
| 22 | | |
| 251 | |
| Acquisition, net of cash acquired | |
| — | | |
| 90 | |
| Property, plant and equipment insurance proceeds | |
| — | | |
| 3,500 | |
| Sales and maturities of short-term investment securities | |
| — | | |
| 21,714 | |
| Purchase of short-term investment securities | |
| — | | |
| (3,475 | ) |
| Net cash (used in) provided by investing activities | |
| (49 | ) | |
| 18,923 | |
| Cash flows from financing activities: | |
| | | |
| | |
| Payments on notes payable | |
| (924 | ) | |
| (3,954 | ) |
| Proceeds from issuance of notes payable | |
| 1,256 | | |
| 2,218 | |
| Payments of long-term debt | |
| (249 | ) | |
| — | |
| Proceeds from issuance of long-term debt | |
| — | | |
| 16,849 | |
| Payment of debt issuance costs | |
| — | | |
| (801 | ) |
| Proceeds from issuance of detachable warrants | |
| — | | |
| 6,016 | |
| Net proceeds from warrant exercise | |
| 2,245 | | |
| — | |
| Proceeds from issuance of common stock related to the ATM | |
| — | | |
| 2,741 | |
| Payment of common stock issuance costs related to the ATM | |
| — | | |
| (178 | ) |
| Proceeds from issuance of common stock | |
| 4,237 | | |
| 5,273 | |
| Payment of common stock issuance costs | |
| (324 | ) | |
| (422 | ) |
| Taxes paid related to net share settlement of RSUs | |
| (1 | ) | |
| (420 | ) |
| Net cash provided by financing activities | |
| 6,240 | | |
| 27,322 | |
| Net (decrease) increase in cash, cash equivalents and restricted cash | |
| (779 | ) | |
| 8,913 | |
| Cash, cash equivalents and restricted cash - beginning of period | |
| 2,058 | | |
| 3,020 | |
| Cash, cash equivalents and restricted cash - end of period | |
$ | 1,279 | | |
$ | 11,933 | |
| | |
| | | |
| | |
| Reconciliation of cash and cash equivalents and restricted cash | |
| | | |
| | |
| Cash and cash equivalents at beginning of period | |
$ | 2,058 | | |
$ | 3,020 | |
| Restricted cash at beginning of period | |
| — | | |
| — | |
| Cash, cash equivalents and restricted cash at beginning of period | |
$ | 2,058 | | |
$ | 3,020 | |
| | |
| | | |
| | |
| Cash and cash equivalents at end of period | |
$ | 1,279 | | |
$ | 4,433 | |
| Restricted cash at end of period | |
| — | | |
| 7,500 | |
| Cash, cash equivalents and restricted cash at end of period | |
$ | 1,279 | | |
$ | 11,933 | |
| | |
| | | |
| | |
| Supplemental disclosures of cash flow information: | |
| | | |
| | |
| Non-cash transactions: | |
| | | |
| | |
| Capital expenditures incurred but not yet paid | |
$ | 25 | | |
$ | 64 | |
| Right-of-use assets and corresponding operating lease obligations | |
$ | — | | |
$ | 4,803 | |
| Deemed dividends | |
$ | 4,034 | | |
$ | 367 | |
| Stock issued in connection with settled indebtedness | |
$ | 1,192 | | |
$ | — | |
| Non-cash consideration RXP acquisition | |
$ | — | | |
$ | 1,926 | |
| Non-cash licensing arrangement | |
$ | — | | |
$ | 3,500 | |
| Payment for extinguishment of Subordinated Note | |
$ | 3,864 | | |
$ | — | |
| Payment of GVB Promissory Note | |
$ | 1,500 | | |
$ | — | |
| Payment of debt issuance costs | |
$ | 275 | | |
$ | — | |
| Equity conversion of Senior Secured Credit Facility | |
$ | 2,481 | | |
$ | — | |
See
accompanying notes to Condensed Consolidated Financial Statements.
22nd CENTURY GROUP, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL
STATEMENTS
June 30, 2024
(Unaudited)
Amounts in thousands, except for share and per-share
data
NOTE 1. - NATURE OF BUSINESS AND SUMMARY OF SIGNIFICANT
ACCOUNTING POLICIES
Basis
of Presentation – 22nd Century Group, Inc. (together with its consolidated subsidiaries, “22nd Century
Group” or the “Company”) is a Nevada corporation publicly traded on the NASDAQ Capital Market under the symbol “XXII.”
22nd Century Group is a tobacco products company with sales and distribution of the Company’s own proprietary new reduced nicotine
tobacco products authorized as Modified Risk Tobacco Products by the FDA. Additionally, the Company provides contract manufacturing services
for conventional combustible tobacco products for third-party brands.
The accompanying Condensed
Consolidated Financial Statements are presented in accordance with the rules and regulations of the United States ("U.S.")
Securities and Exchange Commission ("SEC") and do not include all of the disclosures required by U.S. generally accepted
accounting principles (“U.S. GAAP”) as contained in the Company’s Annual Report on Form 10-K. Accordingly, these
Condensed Consolidated Financial Statements should be read in conjunction with the Consolidated Financial Statements and notes thereto
included in the Company’s most recent Annual Report on Form 10-K for the fiscal year ended December 31, 2023.
In the opinion of management, the Condensed Consolidated
Financial Statements reflect all adjustments (consisting of normal recurring adjustments) considered necessary for a fair presentation
of the results of the Company for the periods presented. The results for interim periods are not necessarily indicative of results or
trends that may be expected for the fiscal year as a whole. The Condensed Consolidated Financial Statements were prepared using U.S.
GAAP, which require management to make estimates and assumptions that affect the reported amounts of assets, liabilities, certain
components of equity, sales, expenses, and related disclosures at the date of the financial statements and during the reporting period.
Actual results could differ materially from these estimates.
Liquidity
and Capital Resources – These Condensed Consolidated Financial Statements have been prepared in accordance with generally
accepted accounting principles applicable to a going concern, which contemplates the realization of assets and the satisfaction of liabilities
in the normal course of business.
The Company has incurred significant losses and
negative cash flows from operations since inception and expects to incur additional losses until such time that it can generate significant
revenue and profit in its tobacco business. The Company had negative cash flow from operations of $6,970 and $37,332 for the six months
ended June 30, 2024 and 2023, respectively, and an accumulated deficit of $385,558 and $378,707 as of June 30, 2024
and December 31, 2023, respectively. As of June 30, 2024, the Company had cash and cash equivalents of $1,279.
Given the Company’s projected operating requirements
and its existing cash and cash equivalents, there is substantial doubt about the Company’s ability to continue as a going concern
through one year following the date that the Condensed Consolidated Financial Statements are issued.
In response to these conditions, management is
currently evaluating different strategies for reducing expenses, as well as pursuing financing strategies which include raising additional
funds through the issuance of securities, asset sales, and through arrangements with strategic partners. If capital is not available to
the Company when, and in the amounts needed, it could be required to liquidate inventory, cease or curtail operations, or seek protection
under applicable bankruptcy laws or similar state proceedings. There can be no assurance that the Company will be able to raise the capital
it needs to continue operations. Management’s plans do not alleviate substantial doubt about the Company’s ability to continue
as a going concern through one year following the date that the Condensed Consolidated Financial Statements are issued.
The Condensed Consolidated Financial Statements
do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification
of liabilities that might result from the outcome of this uncertainty.
Other
Significant Risks and Uncertainties - The Company is subject to a number of risks, including, but not limited to, the lack
of available capital; the possible delisting of our common stock from Nasdaq; future covenant non-compliance with respect to the Company’s
Senior Secured Credit Facility giving rise to an event of default; unsuccessful commercialization strategy and launch plans for the Company’s
products or market acceptance of the Company’s products; risks inherent in litigation, including purported class actions; and protection
of proprietary technology.
Reclassifications
– The Company has revised the presentation and classification of Excise taxes on products, net which was previously recorded in
Cost of goods sold in the Condensed Consolidated Statement of Operations and Comprehensive Loss.
Reverse
Stock Split – On April 2, 2024, the Company effected a 1-for-16 reverse stock split of its common stock in order
to regain compliance with Nasdaq's continued listing requirements. Fractional shares resulting from the reverse stock split were rounded
up to the nearest whole share, which resulted in the issuance of a total of 118,207 shares of common stock to implement the reverse stock
split. All share and per share amounts, and exercise prices of stock options, and warrants in the Condensed Consolidated Financial Statements
and notes thereto have been retroactively adjusted for all periods presented to give effect to this reverse stock split.
Warrants
- The Company accounts for stock warrants as either equity instruments, derivative liabilities, or liabilities in accordance with ASC
480, Distinguishing Liabilities from Equity (ASC 480) and ASC 815, Derivatives and Hedging (ASC 815) depending on the specific
terms of the warrant agreement. The assessment considers whether the warrants are freestanding financial instruments pursuant to ASC 480,
meet the definition of a liability pursuant to ASC 480, and whether the warrants meet all of the requirements for equity classification
under ASC 815, including whether the warrants are indexed to the Company’s own ordinary shares and whether the warrant holders could
potentially require “net cash settlement” in a circumstance outside of the Company’s control, among other conditions
for equity classification. This assessment, which requires the use of professional judgment, is conducted at the time of warrant issuance
and as of each subsequent quarterly period end date while the warrants are outstanding.
Warrants that the Company may be required to redeem
through payment of cash or other assets outside its control are classified as liabilities pursuant to ASC 480 and are initially and subsequently
measured at their estimated fair values. For issued or modified warrants that meet all of the criteria for equity classification, the
warrants are required to be recorded as a component of Capital in excess of par value at the time of issuance. For additional discussion
on warrants, see Note 5 and Note 10.
Deemed
dividends associated with anti-dilution or down round provisions (commonly referred to as “ratchets”) represent the economic
transfer of value to holders of equity-classified freestanding financial instruments when these provisions are triggered. These deemed
dividends are presented as a reduction in net income or an increase in net loss available to common shareholders and a corresponding increase
to Capital in excess of par value resulting in no change to shareholders’ equity (deficit). During the three and six months ended
June 30, 2024, total deemed dividends were $445 and $4,034, compared to the three and six months ended June 30, 2023
of $367 and $367, respectively, resulting from equity offerings (see Note 10).
Debt
Issued with Detachable Warrants - The Company considers guidance within ASC 470-20, Debt (ASC 470), ASC 480,
and ASC 815 when accounting for the issuance of debt with detachable warrants. As described above under the caption “Warrants”,
the Company classifies stock warrants as either equity instruments, derivative liabilities, or liabilities depending on the specific terms
of the warrant agreement. In circumstances in which debt is issued with detachable warrants, the proceeds from the issuance of the debt
are first allocated to the warrants at their full estimated fair value with a corresponding debt discount. The remaining proceeds, as
further reduced by discounts (including those created by the bifurcation of embedded derivatives), is allocated to the debt. The Company
accounts for debt as liabilities measured at amortized cost and amortizes the resulting debt discount from the allocation of proceeds,
to interest expense using the effective interest method over the expected term of the debt instrument pursuant to ASC 835, Interest (ASC
835).
Embedded
Derivatives – The Company considers whether there are any embedded features in debt instruments that require bifurcation
and separate accounting as derivative financial instruments pursuant to ASC 815. Embedded derivatives are initially and subsequently measured
at fair value. With the exception of the bifurcated embedded conversion option as described in Note 6 “Debt”, the embedded
derivatives associated with the Company’s Senior Secured Credit Facility and Subordinated Note are not material.
Debt
Issuance Costs and Discounts - Debt issuance costs and discounts associated with the issuance of debt by the Company are
deferred and amortized over the term of the related debt. Debt issuance costs and discounts related to the Company’s Senior Secured
Credit Facility and Subordinated Note are recorded as a reduction of the carrying value of the related debt and are amortized to Interest
expense using the effective interest method over the period from the date of issuance to the maturity date, whichever is earlier. The
amortization of debt issuance costs and discounts are included in Debt related charges included in interest expense in the Condensed Consolidated
Statements of Cash Flows. Note 6 “Debt” contains additional information on the Company’s debt issuance costs and discounts.
Impairment
of Long-Lived Assets - The Company reviews all long-lived assets to be held and used for recoverability, when events or
changes in circumstances occur that indicate that the carrying value of the asset may not be recoverable. The assessment of possible impairment
is based on the ability to recover the carrying value of the assets from the expected future cash flows (undiscounted and without interest
expense) of the related operations. If these cash flows are less than the carrying value of such assets, an impairment loss for the difference
between the estimated fair value and carrying value is recorded. The Company determined that there were no impairment indicators during
the three and six months ended June 30, 2024.
Gain
and Loss Contingencies – The Company establishes an accrued liability for litigation and regulatory matters
when those matters present loss contingencies that are both probable and estimable. In such cases, there may be an exposure to loss in
excess of any amounts accrued. When a loss contingency is not both probable and estimable, the Company does not establish an accrued liability.
As a litigation or regulatory matter develops, the Company, in conjunction with any outside counsel handling the matter, evaluates on
an ongoing basis whether such matter presents a loss contingency that is probable and estimable. If, at the time of evaluation, the loss
contingency related to a litigation or regulatory matter is not both probable and estimable, the matter will continue to be monitored
for further developments that would make such loss contingency both probable and estimable. When a loss contingency related to a litigation
or regulatory matter is deemed to be both probable and estimable, the Company will establish an accrued liability with respect to such
loss contingency and record a corresponding amount of related expenses. The Company will then continue to monitor the matter for further
developments that could affect the amount of any such accrued liability.
In accordance with ASC 450-30, Gain
Contingencies, gain contingencies are recognized when earned and realized, which typically will occur at the time of final settlement
or when cash is received. Insurance recoveries may be realized earlier than cash receipt if a claim and amount of reimbursement is
acknowledged by the insurance company that payment is due and collection is probable.
The Company maintains
general liability insurance policies for its facilities. Under the terms of our insurance policies, in the case of loss to a property,
the Company follows the guidance in ASC 610-30, Other Income —Gains and Losses on Involuntary Conversions, for
the conversion of nonmonetary assets (the properties) to monetary assets (insurance recoveries). Under ASC 610-30, once the recovery
is deemed probable the Company recognizes an asset for the insurance recovery receivable in the Condensed Consolidated Balance Sheets,
with corresponding income that is offsetting to the casualty losses recorded in the Condensed Consolidated Statements of Operations and
Comprehensive Loss. If the insurance recovery is less than the amount of the casualty charges recognized, the Company will recognize a
loss whereas if the insurance recovery is greater than the amount of casualty loss recognized, the Company will only recognize a recovery
up to the amount of the casualty loss and will account for the excess as a gain contingency. Business interruption insurance is treated
as a gain contingency.
Refer to further discussion
of all commitments and contingencies in Note 12.
Severance
charges - From time to time, the Company evaluates its resources and optimizes its business plan to align to changing needs
of executing on its strategy. These actions may result in voluntary or involuntary employee termination benefits. Voluntary termination
benefits are accrued when an employee accepts the related offer. Involuntary termination benefits are accrued upon the commitment to a
termination plan and when the benefit arrangement is communicated to affected employees, or when liabilities are determined to be probable
and estimable, depending on the existence of a substantive plan for severance or termination.
The following table summarizes
the change in accrued severance liabilities, presented within Other current liabilities on the Condensed Consolidated Balance Sheets:
| Balance at January 1, 2024 | |
$ | 386 | |
| Cash payments | |
| (64 | ) |
| Balance at March 31, 2024 | |
| 322 | |
| Cash payments | |
| (73 | ) |
| Balance at June 30, 2024 | |
$ | 249 | |
The following table summarizes
the classification of severance charges on the Condensed Consolidated Statements of Operations and Comprehensive Loss:
| | |
Three Months Ended | | |
Six Months Ended | |
| | |
June 30, | | |
June 30, | |
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| Sales, general, and administrative | |
$ | — | | |
$ | 569 | | |
$ | — | | |
$ | 569 | |
| Total severance charges | |
$ | — | | |
$ | 569 | | |
$ | — | | |
$ | 569 | |
Income
Taxes - For interim income tax reporting, due to a full valuation allowance on net deferred tax assets, no income tax
expense or benefit is recorded unless it is related to certain state, local, or franchise taxes, or an unusual or infrequently occurring
items. The tax effects of unusual or infrequently occurring items, including changes in judgment about valuation allowances and effects
of changes in tax laws or rates, are reported in the interim period in which they occur.
Recent Accounting Pronouncements –
Adoption of Accounting Standards Codification Topic 326
The Company adopted ASU 2016-13, or ASC 326 Financial
Instruments-Credit Losses, effective January 1, 2023 under a modified retrospective approach. Under the current expected credit losses
(“CECL”) model, the Company immediately recognizes an estimate of credit losses expected to occur over the life of the financial
asset at the time the financial asset is originated or acquired. Estimated credit losses are determined by taking into consideration historical
loss conditions, current conditions and reasonable and supportable forecasts. Changes to the expected lifetime credit losses are recognized
each period. The new guidance applies to the Company’s trade receivables and contract asset balances. Due to the nature of business
operations and contracts with customers, the Company has historically not experienced significant bad debt expense or write-offs and as
a result, the adoption of ASC 326 did not have a material impact to the Company’s Condensed Consolidated Financial Statements. In
connection with the adoption of ASC 326, the Company recorded a provision for credit losses of $118 with an offsetting cumulative-effect
adjustment to the opening balance of accumulated deficit as of January 1, 2023.
Accounting
Guidance Not Yet Elected or Adopted
In December 2023, the FASB issued ASU 2023-09, Income
Taxes (Topic 740)-Improvements to Income Tax Disclosures. The ASU requires additional quantitative and qualitative income tax disclosures
to allow readers of the consolidated financial statements to assess how the Company’s operations, related tax risks and tax planning
affect its tax rate and prospects for future cash flows. For public business entities, the ASU is effective for annual periods beginning
after December 15, 2024. The Company is currently evaluating the impact that the adoption of this ASU will have on its consolidated
financial statements.
We consider the applicability and impact of all
ASUs. If the ASU is not listed above, it was determined that the ASU was either not applicable or would have an immaterial impact on our
financial statements and related disclosures.
NOTE 2. DISCONTINUED OPERATIONS AND DIVESTITURES
As of June 30, 2024, all assets and liabilities
of the former hemp/cannabis disposal group are presented as current in the Condensed Consolidated Balance Sheets. The carrying amounts
of the former hemp/cannabis disposal group assets and liabilities that were classified as assets and liabilities of discontinued operations
held for sale were as follows:
| | |
June 30, | | |
December 31, | |
| | |
2024 | | |
2023 | |
| Prepaid expenses and other current assets | |
$ | 7 | | |
$ | 9 | |
| Property, plant and equipment, net | |
| 1,051 | | |
| 1,207 | |
| Other assets | |
| — | | |
| 38 | |
| Current assets of discontinued operations held for sale | |
$ | 1,058 | | |
$ | 1,254 | |
| | |
| | | |
| | |
| Notes and loans payable - current | |
$ | — | | |
$ | 2 | |
| Operating lease obligations | |
| — | | |
| 1,083 | |
| Accounts payable | |
| 1,279 | | |
| 2,013 | |
| Accrued expenses | |
| 75 | | |
| 79 | |
| Deferred income | |
| — | | |
| 8 | |
| Other current liabilities | |
| 43 | | |
| — | |
| Current liabilities of discontinued operations held for sale | |
$ | 1,397 | | |
$ | 3,185 | |
| | |
| | | |
| | |
| Net liabilities | |
$ | (339 | ) | |
$ | (1,931 | ) |
Net loss
from discontinued operations for the six months ended June 30, 2024 and 2023 was as follows:
| | |
Three Months Ended | | |
Six Months Ended | |
| | |
June 30, | | |
June 30, | |
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| Revenues, net | |
$ | — | | |
$ | 15,377 | | |
$ | — | | |
$ | 28,412 | |
| Cost of goods sold | |
| — | | |
| 16,761 | | |
| — | | |
| 30,990 | |
| Gross loss | |
| — | | |
| (1,384 | ) | |
| — | | |
| (2,578 | ) |
| Operating expenses: | |
| | | |
| | | |
| | | |
| | |
| Sales, general and administrative | |
| (387 | ) | |
| 4,257 | | |
| (320 | ) | |
| 8,652 | |
| Research and development | |
| 84 | | |
| 994 | | |
| 132 | | |
| 1,781 | |
| Other operating expense, net | |
| (865 | ) | |
| 91 | | |
| (766 | ) | |
| 996 | |
| Total operating (income) expense | |
| (1,168 | ) | |
| 5,342 | | |
| (954 | ) | |
| 11,429 | |
| Operating income (loss) from discontinued operations | |
| 1,168 | | |
| (6,726 | ) | |
| 954 | | |
| (14,007 | ) |
| Other income (expense): | |
| | | |
| | | |
| | | |
| | |
| Other income, net | |
| — | | |
| 17 | | |
| — | | |
| 38 | |
| Interest expense | |
| (66 | ) | |
| (122 | ) | |
| (141 | ) | |
| (215 | ) |
| Total other expense | |
| (66 | ) | |
| (105 | ) | |
| (141 | ) | |
| (177 | ) |
| Income (loss) from discontinued operations before income taxes | |
| 1,102 | | |
| (6,831 | ) | |
| 813 | | |
| (14,184 | ) |
| Provision (benefit) for income taxes | |
| — | | |
| — | | |
| — | | |
| — | |
| Net income (loss) from discontinued operations | |
$ | 1,102 | | |
$ | (6,831 | ) | |
$ | 813 | | |
$ | (14,184 | ) |
During the three- and six-month periods ended June 30,
2024, the Company settled outstanding obligations which resulted in reversals of previously accrued liabilities of $1,399 and $1,551,
respectively.
Cash flow information from discontinued operations
for the three and six months ended June 30, 2024 and 2023 was as follows:
| | |
Six Months Ended | |
| | |
June 30, | |
| | |
2024 | | |
2023 | |
| Cash used in operating activities | |
$ | 867 | | |
$ | 49,882 | |
| Cash provided by investing activities | |
$ | 22 | | |
$ | 1,194 | |
| | |
| | | |
| | |
| Depreciation and amortization | |
$ | - | | |
$ | 1,367 | |
| Capital expenditures | |
$ | - | | |
$ | 2,277 | |
NOTE 3. – INVENTORIES
Inventories at June 30, 2024 and December 31, 2023
consisted of the following:
| | |
June 30, | | |
December 31, | |
| | |
2024 | | |
2023 | |
| Raw materials | |
$ | 1,975 | | |
$ | 3,580 | |
| Work in process | |
| — | | |
| — | |
| Finished goods | |
| 689 | | |
| 766 | |
| | |
$ | 2,664 | | |
$ | 4,346 | |
NOTE 4. – INTANGIBLE ASSETS, NET
Intangible Assets, Net
Our intangible assets, net at June 30, 2024 and December 31,
2023 consisted of the following:
| | |
Gross | | |
Accumulated | | |
Net Carrying | |
| June 30, 2024 | |
Carrying Amount | | |
Amortization | | |
Amount | |
| Definite-lived: | |
| | | |
| | | |
| | |
| Patent | |
$ | 2,922 | | |
$ | (2,185 | ) | |
$ | 737 | |
| License fees | |
| 4,165 | | |
| (1,860 | ) | |
| 2,305 | |
| Total amortizing intangible assets | |
$ | 7,087 | | |
$ | (4,045 | ) | |
$ | 3,042 | |
| Indefinite-lived: | |
| | | |
| | | |
| | |
| Trademarks | |
| | | |
| | | |
$ | 134 | |
| MSA signatory costs | |
| | | |
| | | |
| 2,202 | |
| License fee for predicate cigarette brand | |
| | | |
| | | |
| 350 | |
| Total indefinite-lived intangible assets | |
| | | |
| | | |
$ | 2,686 | |
| Total intangible assets, net | |
| | | |
| | | |
$ | 5,728 | |
| | |
Gross | | |
Accumulated | | |
| | |
Net Carrying | |
| December 31, 2023 | |
Carrying Amount | | |
Amortization | | |
Impairment | | |
Amount | |
| Definite-lived: | |
| | | |
| | | |
| | | |
| | |
| Patent | |
$ | 2,913 | | |
$ | (1,622 | ) | |
$ | (487 | ) | |
$ | 804 | |
| License fees | |
| 4,165 | | |
| (1,666 | ) | |
| (65 | ) | |
| 2,434 | |
| Total amortizing intangible assets | |
$ | 7,078 | | |
$ | (3,288 | ) | |
$ | (552 | ) | |
$ | 3,238 | |
| Indefinite-lived: | |
| | | |
| | | |
| | | |
| | |
| Trademarks | |
| | | |
| | | |
| | | |
$ | 134 | |
| MSA signatory costs | |
| | | |
| | | |
| | | |
| 2,202 | |
| License fee for predicate cigarette brand | |
| | | |
| | | |
| | | |
| 350 | |
| Total indefinite-lived intangible assets | |
| | | |
| | | |
| | | |
$ | 2,686 | |
| Total intangible assets, net | |
| | | |
| | | |
| | | |
$ | 5,924 | |
Aggregate intangible asset amortization expense
comprises of the following:
| | |
Three Months Ended | | |
Six Months Ended | |
| | |
June 30, | | |
June 30, | |
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| Cost of goods sold | |
$ | 2 | | |
$ | 3 | | |
$ | 5 | | |
$ | 6 | |
| Research and development | |
| 102 | | |
| 162 | | |
| 202 | | |
| 320 | |
| Total amortization expense | |
$ | 104 | | |
$ | 165 | | |
$ | 207 | | |
$ | 326 | |
Estimated future intangible asset amortization
expense based on the carrying value as of June 30, 2024 is as follows:
| | |
Remainder of 2024 | | |
2025 | | |
2026 | | |
2027 | | |
2028 | | |
Thereafter | |
| Amortization expense | |
$ | 209 | | |
$ | 417 | | |
$ | 377 | | |
$ | 368 | | |
$ | 296 | | |
$ | 1,375 | |
NOTE 5. – FAIR VALUE MEASUREMENTS AND SHORT-TERM
INVESTMENTS
Assets and Liabilities Measured at Fair
Value on a Recurring Basis
Fair value measurement
standards apply to certain financial assets and liabilities that are measured at fair value on a recurring basis (each reporting period).
For the Company, these financial assets and liabilities include equity investments. The Company does not have any nonfinancial assets
or liabilities that are measured at fair value on a recurring basis.
The following table presents
information about our liabilities measured at fair value as of June 30, 2024 and December 31, 2023, and indicates
the fair value hierarchy of the valuation techniques the Company utilized to determine such fair value:
| | |
Fair Value | |
| | |
June 30, 2024 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | | |
Total | |
| Liabilities | |
| | | |
| | | |
| | | |
| | |
| Omnia 2024 warrants | |
$ | — | | |
$ | — | | |
$ | 1,191 | | |
$ | 1,191 | |
| Derivative liability | |
| — | | |
| — | | |
| 98 | | |
| 98 | |
| Total liabilities | |
$ | — | | |
$ | — | | |
$ | 1,289 | | |
$ | 1,289 | |
| | |
Fair Value | |
| | |
December 31, 2023 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | | |
Total | |
| Liabilities | |
| | | |
| | | |
| | | |
| | |
| Omnia 2023 warrants | |
$ | — | | |
$ | — | | |
$ | 1,350 | | |
$ | 1,350 | |
| Derivative liability | |
| — | | |
| — | | |
| 557 | | |
| 557 | |
| Total liabilities | |
$ | — | | |
$ | — | | |
$ | 1,907 | | |
$ | 1,907 | |
Warrants
The following table sets
forth a summary of the changes in fair value of the Company’s common stock warrants accounted for as liabilities (Level 3) for the
period ended June 30, 2024:
| Fair value measurement at January 1, 2024 | |
$ | 1,350 | |
| Fair value measurement adjustment | |
| — | |
| Fair value measurement at March 31, 2024 | |
$ | 1,350 | |
| Settlement and release (See Note 6) | |
| (1,350 | ) |
| Initial measurement (See Note 6) | |
| 1,515 | |
| Fair value measurement adjustment | |
| (324 | ) |
| Fair value measurement at June 30, 2024 | |
$ | 1,191 | |
The Omnia warrants were
measured at June 30, 2024 and December 31, 2023 using a Monte Carlo valuation model with the following assumptions:
| | |
June 30, | | |
December 31, | |
| | |
2024 | | |
2023 | |
| | |
Omnia 2024 warrants | | |
Omnia 2023 warrants | |
| Risk-free interest rate per year | |
| 4.3 | % | |
| 4.6 | % |
| Expected volatility per year | |
| 113.1 | % | |
| 90.9 | % |
| Expected dividend yield | |
| — | % | |
| — | % |
| Contractual expiration | |
| 4.8 years | | |
| 6.6 years | |
| Exercise price | |
$ | 2.14 | | |
$ | 205.248 | |
| Stock price | |
$ | 0.75 | | |
$ | 3.04 | |
The warrants are measured
at fair value using certain estimated factors which are classified within Level 3 of the valuation hierarchy. Significant unobservable
inputs that are used in the fair value measurement of the Company’s detachable warrants include the volatility factor, anti-dilution
provisions, and contingent put option. Significant increases or decreases in the volatility factor would have resulted in a significantly
higher or lower fair value measurement. Additionally, a change in probability regarding the anti-dilution provision or put option would
have resulted in a significantly higher or lower fair value measurement. The Omnia 2023 warrants were extinguished and the Omnia 2024
warrants were issued in April 2024. See Note 6 for further details.
Derivative Liability
The following table sets
forth a summary of the changes in fair value of the Company’s derivative liability accounted for as liabilities (Level 3) as of
June 30, 2024:
| Fair value measurement at January 1, 2024 | |
$ | 557 | |
| Fair value measurement adjustment | |
| 82 | |
| Fair value measurement at March 31, 2024 | |
$ | 639 | |
| Fair value measurement adjustment | |
| (541 | ) |
| Fair value measurement at June 30, 2024 | |
$ | 98 | |
The derivative liability related to the debentures
and embedded conversion option was measured at June 30, 2024 and December 31, 2023 using a binomial lattice valuation model
under a “with and without” approach and contained the following assumptions:
| | |
June 30, | | |
December 31, | |
| | |
2024 | | |
2023 | |
| Stock price volatility | |
| 137.3 | % | |
| 104.1 | % |
| Expected term | |
| 1.6 years | | |
| 2.2 years | |
| Stock price | |
$ | 0.75 | | |
$ | 3.04 | |
| Risk-free rate | |
| 4.8 | % | |
| 4.3 | % |
| Credit rating | |
| CCC | | |
| CCC | |
| Market yield (credit risk) | |
| 19.8 | % | |
| 13.8 | % |
The debentures and derivative
liability are measured at fair value using certain estimated factors which are classified within Level 3 of the valuation hierarchy. Significant
unobservable inputs that are used in the fair value measurement of the Company’s derivative liability include a decrease/increase
in our stock price, stock price volatility, credit rating, and simulated stock price upon conversion could significantly change the fair
value measurement as either an increase or decrease.
Assets and Liabilities Measured at Fair
Value on a Nonrecurring Basis
During the three and six
months ended June 30, 2024 and 2023 respectively, the Company did not have any financial assets or liabilities measured at fair
value on a nonrecurring basis.
NOTE 6. DEBT
The Company has a senior secured credit facility
(the “Senior Secured Credit Facility”), which consists of Debentures (as defined below) and previously, a subordinated promissory
note (the “Subordinated Note). The Debentures were issued at a 5% original issuance discount and are subject to a 5% exit payment.
The Subordinated Note was extinguished in April 2024, as described below.
Debt related to the Senior Secured Credit Facility
and Subordinate Note as of June 30, 2024 and December 31, 2023 consists of the following:
| | |
June 30, | | |
December 31, | |
| | |
2024 | | |
2023 | |
| Senior Secured Credit Facility | |
$ | 8,325 | | |
$ | 11,805 | |
| Subordinated Note | |
| — | | |
| 3,554 | |
| Unamortized discount on loan and deferred debt issuance costs | |
| (779 | ) | |
| (1,453 | ) |
| Total debt | |
$ | 7,546 | | |
$ | 13,906 | |
| Current portion of long-term debt | |
| (1,500 | ) | |
| (5,848 | ) |
| Total long-term debt | |
$ | 6,046 | | |
$ | 8,058 | |
Debentures
On
March 3, 2023, the Company entered into a Securities Purchase Agreement with each of the purchasers party thereto (collectively,
the “Purchasers”) and JGB Collateral, LLC, as collateral agent for the Purchasers (the “Agent”) which pursuant
to the agreement, the Company sold 5% original issuance discount senior secured debentures with an aggregate principal amount of $21,053.
The Debentures bear interest at a rate of 7% per annum, payable monthly in arrears as of the last trading day of each month and on the
maturity date. The Debentures mature on March 3, 2026. At the Company’s election, subject to certain conditions, interest can
be paid in cash, shares of the Company’s common stock, or a combination thereof. The Debentures are subject to an exit payment equal
to 5% of the original principal amount, or $1,053, payable on the maturity date or the date the Debentures are paid in full (the “Exit
Payment”). Any time after, March 3, 2024, the Company may irrevocably elect to redeem all of the then outstanding principal
amount of the Debentures for cash in an amount equal to the entire outstanding principal balance, including accrued and unpaid interest,
the Exit Payment and a prepayment premium in an amount equal to 3% of the outstanding principal balance as of the prepayment date (collectively,
the “Prepayment Amount”). Upon the entry into a definitive agreement that would effect a change in control (as defined in
the Debentures) of the Company, the Agent may require the Company to prepay the outstanding principal balance in an amount equal to the
Prepayment Amount. Commencing on March 3, 2024, at its option, the holder of a Debenture may require the Company to redeem 2% of
the original principal amount of the Debentures per calendar month which amount may at the Company’s election, subject to certain
exceptions, be paid in cash, shares of the Company’s common stock, or a combination thereof.
The
Company’s obligations under the Debentures can be accelerated upon the occurrence of certain customary events of default. In the
event of a default and acceleration of the Company’s obligations, the Company would be required to pay the Prepayment Amount, liquidated
damages and other amounts owing in respect thereof through the date of acceleration.
The
Debentures contain customary representations, warranties and covenants including among other things and subject to certain exceptions,
covenants that restrict the Company from incurring additional indebtedness, creating or permitting liens on assets, making or holding
any investments, repaying outstanding indebtedness, paying dividends or distributions and entering into transactions with affiliates.
Substantially all of the company’s assets, including intellectual property, are collateralized and at risk if Debenture obligation
is not satisfied. In addition, the Company was required to maintain at least $7,500 on its balance sheet as restricted cash in a separate
account and has financial covenants to maintain certain quarterly revenue targets.
In connection with the sale of the Debentures,
the Company issued warrants to purchase up to 20,835 shares of common stock for an exercise price of $306.00 per share (the “JGB
Warrants”), which had an initial fair value of $4,475 net of issuance costs of $139. On June 22, 2023, as a result of the June 19,
2023 offering, the Company’s outstanding JGB warrants to purchase up to 31,060 shares of the Company’s common stock for an
exercise price of $306.00 per share were automatically adjusted to be $205.248 exercise price for up to 31,060 shares of common stock.
There are no further anti-dilution adjustments on such warrants.
On
October 16, 2023, the Company entered into a Waiver and Amendment Agreement (the “October Amendment”) with each
of the subsidiaries of the Company executing the Debentures, the Holders and the Agent, pursuant to which, among other things, (a) the
Holders waived an event of default under Section 7(d) of the Debentures which required the Company to achieve revenue of at
least $18,500 for the quarter ended September 30, 2023 (the “waiver”), (b) the parties agreed to amend Schedule
E of the Debentures to reduce the Revenue Target (as such term is defined in the Debentures), for the quarter ended December 31,
2023, to $15,500, and (c) the Company agreed to release to the Purchasers the $7,500 that the Company was required to maintain in
a separate account (the “Escrow Funds”) which Escrow Funds were applied to, and reduce, the outstanding principal amount of
the Debentures on a dollar-for-dollar basis.
As
additional consideration for the waiver, the Company agreed to assign, transfer and convey to the Agent, the Company’s entire right,
title and interest in and to (i) the Promissory Note made by J&N Real Estate Company, L.L.C. (“J&N”) payable
to the Company in the principal amount of $3,800 and (ii) the Deed of Trust, Assignment of Rents, Security Agreement and Fixture
Filing dated June 30, 2021, between J&N, as borrower, for the benefit of the Company, as lender (collectively, the “Pledged
Indebtedness”). Upon assignment of the Pledged Indebtedness, the Company recognized the $2,600 of consideration in exchange to be
applied as a $2,000 reduction of the Put Price (as defined below), $600 reduction of the outstanding principal amount of Debentures and
$895 loss on sale of financial asset.
In
connection with the waiver, the Company and Holders agreed to exercise the outstanding put provision to redeem 10,418 Warrants for an
aggregate put price equal to $2,500 (the “Put Price”), which was concurrently reduced by $2,000, as described above, with
the remaining $500 payable by the Company on the Maturity Date recorded as Other long-term liabilities on the Condensed Consolidated Balance
Sheets. No cash was exchanged as a result of executing the October 2023 Amendment.
Subsequently,
on December 22, 2023, the Company, the Holders and the Agent entered into an Amendment Agreement (the “December 2023 Amendment”)
pursuant to which the Holders and the Agent consented to the Purchase Agreement, as amended by the GVB Amendment (see Note 2 “Discontinued
Operations and Divestitures”). In consideration of the Holders and the Agents’ consent, the Company agreed to (i) pay
to the Agent, a cash payment of $2,200 to reduce the outstanding principal of the Debentures (which includes the cash portion of the New
Purchase Price paid directly to Agent by Buyer which consists of a cash payment of $1,100 and an additional $1,100 paid by the Company),
(ii) a 12% secured promissory note issued to the Company’s senior lender, on behalf of and at the direction of the Company,
in an aggregate principal amount of $2,000 (the “GVB Promissory Note”), (iii) assign the GVB Insurance Proceeds to the
Agent until the outstanding aggregate principal amount of the Debentures, plus accrued and unpaid interest, has been repaid in full; provided
that the first $1,000 of Insurance Proceeds in excess of $5,000 shall be applied as stated above, and (iv) post-closing enter into
a deed in lieu of foreclosure agreement with respect to 224 acres of real property in Delta County, Colorado commonly known as Needle
Rock Farms, resulting in a non-monetary exchange yielding additional debt reduction of $1,000.
Effective
June 24, 2024, GVB Biopharma (“GVB”), the Company’s former subsidiary, made a scheduled principal and interest
payment against the Company’s outstanding indebtedness to JGB, reducing the Company’s total outstanding principal indebtedness
with JGB by $1,500. The remaining $500 payable by GVB under the note has been extended to December 31, 2024.
As
of June 30, 2024, the $500 remaining GVB Promissory Note and $1,000 real estate farm asset are pledged to the senior lender
for principal reduction and accordingly $1,500 of the Senior Secured Credit Facility is recorded as Current portion of long-term debt
on the Condensed Consolidated Balance Sheets.
Additionally,
the Company, the Holders and the Agent agreed to amend the Debentures to (i) allow the Holders to voluntarily convert the Debentures,
in whole or in part, into shares of the Company’s common stock (“Voluntary Conversion Option”) on the earlier of (i) June 30,
2024 and (ii) the public announcement of a Fundamental Transaction at a conversion price equal to the lower of (x) $1.00 per
share and (y) the closing sale price of the Company’s common stock on June 29, 2024 (the “Conversion Price”),
and (ii) include a mandatory prepayment of the outstanding principal of the Debentures in an amount equal to 20% of the net cash
proceeds of any issuance by the Company of any of its stock, or other Equity Interests (as defined in the Debentures) or the incurrence
or issuance of any indebtedness. The Voluntary Conversion Option remains subject to the approval of the Company’s shareholders and
the Company is required pursuant to the December 2023 Amendment to use its commercially reasonable efforts to obtain such approval.
Additional
terms of the December 2023 Amendment include a financial covenant holiday through the third quarter of 2024 and revised certain covenants
thereafter to reflect the sale of the Purchased Interests, including lowering the Company’s quarterly revenue targets.
On
April 8, 2024, the Company, the Holders and the Agent entered into that certain Letter Agreement to modify the terms of the Amendment
Agreement, the JGB SPA and the Debentures, as amended (“April 2024 Amendment”).
Under
the terms of the Letter Agreement, the Holders are permitted to convert their debt to common stock at anytime and the Conversion Price
(as defined in the Debentures) at which the Holders may convert the principal amount of their Debentures to the Company’s common
stock is reduced to $2.14 per share in accordance with applicable Nasdaq rules. The
principal amount of the Debentures converted shall be applied to the Monthly Allowance (as defined in the Debentures) for that month,
and any excess shall be applied to the Monthly Allowances for the succeeding months. The conversions will be a dollar for dollar reduction
of the remaining outstanding obligation owed to the Holders. The Agent and Holders have also agreed to daily limits on trading volume
and minimum conversion amounts. The Holders converted $428 of debt in exchange for 200,000 shares of common stock during the quarter-ended
June 30, 2024.
On
May 10, 2024, the Company, the Holders and the Agent entered into that certain May 2024 Exchange Agreement and May 2024
Letter Agreement to modify the terms of the Amendment Agreement, the Securities Purchase Agreement and the Debentures, as amended (“May 2024
Amendment”).
Under
the terms of the May 2024 Letter Agreement, the Company and Holders have agreed the Company shall incur an aggregate amendment charge
to the undersigned holders equal to $275, which shall be added to the principal balance of the Debentures. Under the terms of the May 2024
Exchange Agreement, the Company and Holders exchanged an aggregate of $2,328 in principal, fees and expenses owed under the Debentures
for 395,000 shares of common stock and 895,000 immediately exercisable pre-funded warrants to purchase shares of common stock at an exercise
price of $.00001 (at an effective per share price of $1.69). All pre-funded warrants were subsequently exercised during the quarter-ended
June 30, 2024.
As
a result of the May 2024 Amendment, the exercise price on 5,876,887 of the Company’s outstanding warrants is reduced to $1.69
per share in accordance with the adjustment provisions therein (see Note 10).
In
accordance with ASC 470-60 Troubled Debt Restructurings by Debtors and ASC 470-50, Debt Modifications and Extinguishment, the Company
performed an assessment of whether the transaction was deemed to be a troubled debt restructuring, and if no, whether the transaction
was deemed modification of existing debt, or an extinguishment of existing debt and new debt.
The
October 2023 Amendment, April 2024 Amendment, and May 2024 Amendment were concluded to be a modification, and not an extinguishment,
based on an analysis of the present value of future cash flows. A new effective interest rate was determined, and the debt continued to
be amortized. The December 2023 Amendment was concluded to be an extinguishment, due to the addition of a substantive conversion
option. As a result, the pre-amended debt carrying value was extinguished and the new debt was recorded at fair value, which is subsequently
amortized using the effective interest method. Extinguishment charges were $5,158 and recorded in Interest expense on the Consolidated
Statements of Operations and Comprehensive Loss for the quarter ended December 31, 2023.
The
Company analyzed the conversion feature of the December 2023 Amendment for derivative accounting consideration under ASC 815-15 and
determined that the embedded conversion features should be classified as a bifurcated derivative because the exercise price of these convertible
notes are subject to a variable conversion rate. The Company has determined that the conversion feature is not considered to be solely
indexed to the Company’s own stock and is therefore not afforded equity treatment. In accordance with ASC 815, the Company has bifurcated
the conversion feature of the note and recorded a derivative liability at fair value in the amount of $557 as of December 31, 2023
as a component of Other Long-Term Liabilities on the Condensed Consolidated Balance Sheets. As of June 30, 2024, the fair value
of the derivative liability was $98. See Note 5 “Fair Value Measurement” for additional information related to measurement
of the debentures and derivative liability.
Subordinated
Note
On
March 3, 2023, the Company executed a Subordinated Promissory Note (the “Subordinated Note”) with a principal amount
of $2,865 in favor of Omnia Ventures, LP (“Omnia”). The Subordinated Note refinanced the 12% Secured Promissory Note with
a principal amount of $1,000 dated as of October 29, 2021 payable to Omnia (the “October Note”) and the 12% Secured
Promissory Note with a principal amount of $1,500 dated as of January 14, 2022 payable to Omnia (the “January Note”,
and together with the October Note, the “Original Notes”), which were assumed by the Company in connection with the acquisition
of GVB Biopharma.
Under
the terms of the Subordinated Note, the Company is obligated to make interest payments in-kind (the “PIK Interest”). The PIK
Interest accrues monthly at a compounding rate of 26.5% per annum, payable monthly. The Company is not permitted to prepay all or any
portion of the outstanding balance on the Subordinated Note prior to maturity. The maturity date of the Subordinated Note was May 1,
2024. The Subordinated Note was terminated and extinguished in April 2024.
In
connection with the Subordinated Note, the Company issued to Omnia, warrants to purchase up to 2,813 shares of the Company’s common
stock (the “2023 Omnia Warrants”). The 2023 Omnia Warrants were exercisable for seven years from September 3, 2023, at
an exercise price of $205.248 per share, subject, with certain exceptions, to adjustments in the event of stock splits, dividends, subsequent
dilutive offerings and certain fundamental transactions.
On
April 29, 2024, the Company entered into a General Release and Settlement Agreement (the “Omnia Agreement”) with Omnia
Capital LP (“Omnia”). The Omnia Agreement settles and extinguishes all outstanding debt and interest owed to Omnia under the
outstanding Subordinated Promissory Note dated March 3, 2023 (the “Old Note”) and the put provision contained in the
2023 Omnia Warrants, amounting to a total of approximately $5,228, for (i) a cash payment of $249; (ii) 1,150,000 shares of
common stock and 1,150,000 immediately exercisable pre-funded warrants to purchase shares of common stock at an exercise price of $0.0001
that are exercisable until May 1, 2029 (at an effective per share price of $2.14) and (iii) 460,000 immediately exercisable
warrants to purchase an equal number of shares of common stock at an exercise price of $2.14 until May 1, 2029 (the “2024 Omnia
Warrants”). The 2024 Omnia Warrants contain a put provision that permits the holder to require the Company to redeem the 2024 Omnia
Warrants, no earlier than May 1, 2025, for a purchase price equal to $2.675 per warrant, and had an initial fair value of $1,515
(see Note 5). Subject to limited exceptions, a holder of pre-funded warrants and 2024 Omnia Warrants will not have the right to exercise
any portion of its warrants if the holder, together with its affiliates, would beneficially own in excess of 19.99% of the number of shares
of our common stock outstanding immediately after giving effect to such exercise. As part of the Omnia Agreement, the parties agreed to
terminate and cancel the Old Note and the 2023 Omnia Warrants and released all debts, claims or other obligations against each other occurring
prior to the date of the Omnia Agreement. The total cash and non-cash consideration amounted to $5,628, resulting in extinguishment charges
of $400 for the three months ended June 30, 2024, recorded in Interest expense in the Statement of Operations and Comprehensive Loss.
Contractual Maturities
The Company has $1,500 pledged against outstanding
indebtedness under the Senior Secured Credit Facility comprised of (i) $500 GVB promissory note and (ii) $1,000 assignment of
Needle Rock Farms to be applied as principal reduction in 2024. As of June 30, 2024, contractual maturities under the Senior
Secured Credit Facility for the remainder of 2024 and through maturity, excluding any discounts or premiums, were to be paid in 2024 of
$1,500 and 2026 of $6,046.
Additionally, commencing August 2024, at its
option, JGB may require the Company to redeem 2% of the original principal amount of the Debentures, or $428, per calendar month which
amount may at the Company’s election, subject to certain exceptions, be paid in cash, shares of the Company’s common stock,
or a combination thereof.
Debt Issuance Costs
The fair values of the warrants at issuance of
$5,791, together with the Debentures original issuance discount of $1,053, Debentures exit payment of $1,053, and third-party debt issuance
costs of $801, are being amortized using the effective interest method over the term of the respective debt instrument, recorded as Interest
expense in the Condensed Consolidated Statement of Operations and Comprehensive Loss. The components and activity of unamortized discount
and deferred debt issuance costs related to the Senior Secured Credit Facility and Subordinated Note is as follows:
| | |
Total | |
| January 1, 2023 | |
$ | - | |
| Issuance | |
| 8,698 | |
| Amortization during the year | |
| (2,087 | ) |
| Debt extinguishment charges | |
| (5,158 | ) |
| December 31, 2023 | |
| 1,453 | |
| Amortization during the period | |
| (567 | ) |
| March 31, 2024 | |
$ | 886 | |
| Amortization during the period | |
| (107 | ) |
| June 30, 2024 | |
$ | 779 | |
NOTE 7. – NOTES & LOANS PAYABLE
The table below outlines our notes and loans payable
balances as of June 30, 2024 and December 31, 2023:
| | |
June 30, | | |
December 31, | |
| | |
2024 | | |
2023 | |
| Insurance loans payable | |
$ | 877 | | |
$ | 543 | |
| Total current notes and loans payable | |
$ | 877 | | |
$ | 543 | |
Insurance loans payable
During the second quarter
of 2024, the Company renewed its Director and Officer (“D&O”) insurance for a one-year policy premium totaling
$866. The Company paid $147 as a premium down payment and financed the remaining $719 of policy premiums over ten months at
a 8.3% annual percentage rate.
During the second quarter
of 2023, the Company renewed its Director and Officer (“D&O”) insurance for a one-year policy premium totaling
$1,626. The Company paid $285 as a premium down payment and financed the remaining $1,341 of policy premiums over ten months at
a 7.88% annual percentage rate. Additionally, during the third quarter of 2023, the Company expanded its D&O coverage, resulting
in additional financing of $143, at 9.38% annual percentage rate over six months.
The Company also has other
insurance loans payable related to property and general liability across the Company.
Estimated
future principal payments to be made under the above notes and loans payable as of June 30, 2024 are as follows:
| 2024 | |
$ | 622 | |
| 2025 | |
| 255 | |
| Total | |
$ | 877 | |
NOTE 8. – REVENUE RECOGNITION
The Company’s revenues are derived primarily
from contract manufacturing organization (“CMO”) customer contracts that consist of obligations to manufacture the customers’
branded filtered cigars and cigarettes. Additional revenues are generated from sale of the Company’s proprietary low nicotine content
cigarettes, sold under the brand name VLN®, or research cigarettes sold under the brand name SPECTRUM®.
The Company recognizes revenue when it satisfies
a performance obligation by transferring control of the product to a customer. For certain CMO contracts, the performance obligation is
satisfied over time as the Company determines, due to contract restrictions, it does not have an alternative use of the product and it
has an enforceable right to payment as the product is manufactured. The Company recognizes revenue under those contracts at the unit price
stated in the contract based on the units to customers and is recognized net of cash discounts, sales returns and allowances. There was
no allowance for discounts or returns at June 30, 2024 and December 31, 2023.
Disaggregation of Revenue
The Company’s net revenue is derived from
customers located primarily in the United States and is disaggregated by the timing of revenue. Revenue recognized from Tobacco products
transferred to customers over time represented 66% and 63%, respectively, of total Tobacco revenue for the three and six months ended
June 30, 2024, compared to 67% and 66%, respectively, for the three and six months ended June 30, 2023.
The following table presents net revenue by product
line:
| |
|
Three Months Ended |
|
|
Six Months Ended |
| |
|
June 30, |
|
|
June 30, |
| |
|
2024 |
|
2023 |
|
|
2024 |
|
2023 |
| Contract Manufacturing |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cigarettes |
|
$ |
4,107 |
|
$ |
3,797 |
|
|
$ |
6,867 |
|
$ |
8,286 |
| Filtered Cigars |
|
|
3,303 |
|
|
3,931 |
|
|
|
6,927 |
|
|
8,326 |
| Cigarillos |
|
|
552 |
|
|
- |
|
|
|
552 |
|
|
- |
| Total Contract Manufacturing |
|
|
7,962 |
|
|
7,728 |
|
|
|
14,346 |
|
|
16,612 |
| VLN® |
|
|
(15) |
|
|
322 |
|
|
|
70 |
|
|
365 |
| Total Product Line Revenues |
|
$ |
7,947 |
|
$ |
8,050 |
|
|
$ |
14,416 |
|
$ |
16,977 |
The following tables present net revenues by significant
customers, which are defined as any customer who individually represents 10% or more of disaggregated product line net revenues:
| | |
Three Months Ended | |
| | |
June 30, | |
| | |
2024 | | |
2023 | |
| Customer A | |
| 34.37 | % | |
| 29.42 | % |
| Customer B | |
| 21.37 | % | |
| 14.34 | % |
| Customer C | |
| 7.46 | % | |
| 27.26 | % |
| Customer D | |
| 20.93 | % | |
| 17.92 | % |
| All other customers | |
| 15.87 | % | |
| 11.06 | % |
| | |
Six Months Ended | |
| | |
June 30, | |
| | |
2024 | | |
2023 | |
| Customer A | |
| 36.25 | % | |
| 27.68 | % |
| Customer B | |
| 22.87 | % | |
| 16.46 | % |
| Customer C | |
| 14.16 | % | |
| 27.15 | % |
| Customer D | |
| 13.95 | % | |
| 14.75 | % |
| All other customers | |
| 12.77 | % | |
| 13.96 | % |
Contract Assets and Liabilities
Unbilled receivables (contract assets) represent
revenues recognized for performance obligations that have been satisfied but have not been billed. These receivables are included as Accounts
receivable, net on the Condensed Consolidated Balance Sheets. Customer payment terms vary depending on the terms of each customer contract,
but payment is generally due prior to product shipment or within credit terms up to 30 days after shipment. Deferred income (contract
liabilities) relates to down payments received from customers in advance of satisfying a performance obligation and is included as Deferred
income on the Condensed Consolidated Balance Sheets.
Total contract assets and contract liabilities are as follows:
| | |
June 30, | | |
December 31, | |
| | |
2024 | | |
2023 | |
| Unbilled receivables | |
$ | 1,135 | | |
$ | 1,053 | |
| Deferred income | |
| (389 | ) | |
| (726 | ) |
| Net contract assets | |
$ | 746 | | |
$ | 327 | |
During the six months ended June 30, 2024,
the Company recognized $726 of revenue that was included in the contract liability balance as of December 31, 2023. During the six
months ended June 30, 2023, the Company recognized $688 of revenue that was included in the contract asset balance as of December 31,
2022.
NOTE 9 – EQUITY- BASED COMPENSATION
The Company maintains certain stock-based compensation
plans that were approved by the Company’s shareholders and are administered by the Compensation Committee of the Company’s
Board of Directors. The stock-based compensation plans provide for the granting of stock options, time and performance based restricted
stock units (RSU’s), among other awards to employees, non-employee directors, consultants, and service providers. The 2021 Omnibus
Incentive Plan was amended on June 28, 2024, increasing the authorized shares by an additional 5,000,000. As of June 30, 2024,
the Company had available 5,707,584 shares remaining for future awards under its Omnibus Incentive Plans.
Compensation
Expense – The Company recognized the following compensation costs, net of actual forfeitures, related to restricted stock
units (“RSUs”) and stock options:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Three Months Ended |
|
Six Months Ended |
|
| |
|
June 30, |
|
June 30, |
|
| |
|
2024 |
|
2023 |
|
2024 |
|
2023 |
|
| Sales, general, and administrative |
|
$ |
36 |
|
$ |
1,389 |
|
$ |
176 |
|
$ |
2,435 |
|
| Research and development |
|
|
20 |
|
|
67 |
|
|
61 |
|
|
117 |
|
| Total equity based compensation - continuing operations |
|
|
56 |
|
|
1,456 |
|
|
237 |
|
|
2,552 |
|
| Total equity based compensation - discontinued operations |
|
|
— |
|
|
30 |
|
|
— |
|
|
109 |
|
| Total equity based compensation |
|
$ |
56 |
|
$ |
1,486 |
|
$ |
237 |
|
$ |
2,661 |
|
Restricted
Stock Units – We typically grant RSUs to employees and non-employee directors. The following table summarizes the changes
in unvested RSUs from January 1, 2024 through June 30, 2024.
| |
|
Unvested RSUs |
| |
|
|
|
Weighted |
| |
|
|
|
Average |
| |
|
Number of |
|
Grant-date |
| |
|
Shares |
|
Fair Value |
| |
|
|
|
$ per share |
| Unvested at January 1, 2024 |
|
9,681 |
|
$ |
251.12 |
| Vested |
|
(4,234) |
|
|
233.09 |
| Forfeited |
|
(354) |
|
|
274.16 |
| Unvested at March 31, 2024 |
|
5,093 |
|
$ |
264.45 |
| Forfeited |
|
(3,269) |
|
|
287.31 |
| Unvested at June 30, 2024 |
|
1,824 |
|
$ |
221.93 |
The fair value of RSUs that vested during the six
months ended June 30, 2024 was approximately $9 based on the stock price at the time of vesting. As of June 30, 2024,
unrecognized compensation expense for RSUs amounted to $335 which is expected to be recognized over a weighted average period of approximately
1.6 years.
Stock
Options – Our outstanding stock options were valued using the Black-Scholes option-pricing model on the date of the award.
There was no stock option grant activity during the six months ended June 30, 2024. A summary of the status of stock options
activity since January 1, 2024 and at June 30, 2024 is as follows:
| | |
| | |
| | |
Weighted | |
| |
| | |
| | |
Weighted | | |
Average | |
| |
| | |
| | |
Average | | |
Remaining | |
Aggregate | |
| | |
Number of | | |
Exercise | | |
Contractual | |
Intrinsic | |
| | |
Options | | |
Price | | |
Term | |
Value | |
| | |
| | |
$ per share | | |
|
|
| |
| Outstanding at January 1, 2024 | |
| 13,729 | | |
$ | 421.51 | | |
| |
|
| | |
| Expired | |
| (2,778 | ) | |
| 330.74 | | |
| |
|
| | |
| Forfeited | |
| (417 | ) | |
| 621.60 | | |
| |
|
| | |
| Outstanding at March 31, 2024 | |
| 10,534 | | |
$ | 437.52 | | |
| 1.4 years |
|
$ | — | |
| Expired | |
| (3,929 | ) | |
| 570.27 | | |
| |
|
| | |
| Outstanding at June 30, 2024 | |
| 6,605 | | |
$ | 358.81 | | |
| 0.4 years |
|
$ | — | |
| Exercisable at June 30, 2024 | |
| 6,605 | | |
$ | 358.81 | | |
| 0.4 years |
|
$ | — | |
The intrinsic value of a stock option is the amount
by which the current market value or the market value upon exercise of the underlying stock exceeds the exercise price of the option.
NOTE 10. – CAPITAL RAISES AND WARRANTS FOR
COMMON STOCK
The following tables summarize the Company’s
warrant activity:
| Warrants outstanding at January 1, 2024 | |
| 2,984,847 | |
| Issued | |
| 1,641,535 | |
| Exercised | |
| (820,769 | ) |
| Warrants outstanding at March 31, 2024 | |
| 3,805,613 | |
| Issued | |
| 4,813,800 | |
| Abandoned | |
| (2,813 | ) |
| Exercised | |
| (1,105,000 | ) |
| Warrants outstanding at June 30, 2024 | |
| 7,511,600 | |
The following tables summarizes the Company’s
outstanding warrants as of June 30, 2024:
| | |
# of warrants outstanding | | |
Exercise price | | |
Expiration date |
| July 2022 RDO warrants | |
| 4,067 | | |
$ | 492.00 | | |
July 25, 2027 |
| Senior Secured Credit Facility - JGB | |
| 20,645 | | |
$ | 205.248 | | |
September 3, 2028 |
| July 19, 2023 RDO warrants | |
| 28,125 | | |
$ | 1.69 | | |
July 20, 2028 |
| October 2023 CMPO warrants | |
| 168,750 | | |
$ | 1.69 | | |
October 19, 2028 |
| Inducement warrants | |
| 3,581,213 | | |
$ | 1.69 | | |
February 15, 2029 |
| April 2024 RDO | |
| 1,980,000 | | |
$ | 1.69 | | |
June 28, 2029 |
| April 2024 RDO - Placement Agent | |
| 118,800 | | |
$ | 1.69 | | |
June 28, 2029 |
| Omnia Pre-Funded | |
| 1,150,000 | | |
$ | 0.00001 | | |
NA |
| Omnia 2024 warrants | |
| 460,000 | | |
$ | 2.14 | | |
May 1, 2029 |
| | |
| 7,511,600 | | |
| | | |
|
Warrant Inducement
Offering
On November 28,
2023, the Company commenced a warrant inducement offering with the holders of the Company’s outstanding 1,986,229 warrants consisting
of: (i) the common stock purchase warrants of the Company issued on or about June 22, 2023; (ii) the common stock purchase
warrants of the Company issued on or about July 10, 2023; (iii) the common stock purchase warrants of the Company issued on
or about July 21, 2023; and/or (iv) the common stock purchase warrants of the Company issued on or about October 19, 2023
(collectively, the “Existing Warrants”), which Existing Warrants were exercisable for an equal number of shares of common
stock at an exercise price of $8.40. The Company agreed to issue new warrants (the “Inducement Warrants”) to purchase up to
a number of shares of common stock equal to 200% of the number of shares of common stock issued pursuant to the exercise by the holders
of the Existing Warrants during the inducement period, for cash, at a reduced exercise price equal to the Nasdaq Minimum Price (as defined in Nasdaq Listing Rule 5635(d)).
For
the period from January 1, 2024 to February 15, 2024, the date of shareholder approval, the Company entered into warrant inducement
agreements with certain holders of the Existing Warrants to purchase an aggregate of 820,769 shares of common stock at a reduced weighted
average exercise price of approximately $2.9504. Pursuant to the warrant inducement agreements, the exercising holders of the Existing
Warrants received 1,641,535 Inducement Warrants and the Company received aggregate gross proceeds of approximately $2,421 from the exercise
of the Existing Warrants. Additionally, on the date of Shareholder Approval, the exercise price of the 3,581,213 outstanding Inducement
Warrants, was reduced to $2.8237 based on the lowest Nasdaq Minimum Price (as defined in Nasdaq Listing Rule 5635(d)) during the inducement period. The exercise price
was further reduced to $1.69 in May 2024. As a result of the inducement and subsequent exercise, the Company determined the incremental
fair value provided to the holders using Black Scholes and Monte Carlo models as (i) $148 increase in fair value due to the adjustment
in exercise price of Existing Warrants attributable to down round pricing protection (ii) $3,441 fair value of Inducement Warrants
issued to the holders that exercised Existing Warrants. The incremental fair value is recorded as non-cash deemed dividend. The proceeds
of the warrant inducement and issuance of common stock are recorded as Capital in excess of par value.
As a result
of subsequent offerings, the exercise price on 3,581,213 warrants was automatically adjusted triggering non-cash deemed
dividends as a result of the down-round adjustments. In April 2024, the exercise price was adjusted to $2.14 and further in
May 2024 was adjusted to $1.69.
April 2024 Registered Direct Offering
On April 8, 2024,
the Company and certain investors (the “Investors”) entered into a securities purchase agreement (the “Securities Purchase
Agreement”) relating to the issuance and sale of shares of common stock (or pre-funded warrants in lieu of common stock) pursuant
to a registered direct offering and a private placement of warrants to purchase shares of common stock (collectively, the “Offering”).
The Investors purchased approximately $4,237 of shares and warrants, consisting of an aggregate of 1,855,000 shares of common stock, pre-funded
warrants to purchase 125,000 shares of common stock and warrants to purchase 1,980,000 shares of common stock, at a purchase price of
$2.14 per share and accompanying warrant. The warrants are exercisable after the Shareholder Approval Date (as defined in the Securities
Purchase Agreement) at an exercise price of $2.14 per share of common stock, expire on the date that is five (5) years after the
Shareholder Approval Date and are subject to adjustment in certain circumstances, including upon any subsequent equity sales at a price
per share lower than the then effective exercise price of such warrants, then such exercise price shall be lowered to such price at which
the shares were offered. The pre-funded warrants are exercisable immediately upon issuance at an exercise price of $0.00001. The Offering
closed on April 9, 2024.
The
Company agreed to pay the Placement Agent a cash fee of 6.0% of the gross proceeds from the Offering, an additional 6.0% cash fee of any
cash exercise of the warrants and to reimburse the Placement Agent for its expenses, including the reimbursement of legal fees up to an
aggregate of $50,000. In addition, the Company issued an aggregate of 118,800 placement agent warrants to the Placement Agent and its
designees with substantially the same terms as the warrants to the Investors, except that the placement agent warrants will terminate
five years following the commencement of sales of the Offering and have an exercise price of $2.675.
The
net proceeds to the Company from the Offering, after deducting placement agent fees and the Company’s estimated offering expenses,
were approximately $3,913.
As a result
of subsequent equity issuance in May 2024, the exercise price on 2,098,800 warrants was automatically adjusted to
$1.69 triggering non-cash deemed dividends as a result of the down-round adjustments.
Other
Agreements
On
April 29, 2024, the Company settled an aggregate of $1,192 of outstanding indebtedness under various commercial agreements for an
aggregate of 700,958 shares of common stock at an effective price per share of $2.14.
NOTE 11. – LOSS PER COMMON SHARE
The following table sets forth the computation
of basic and diluted loss per common share for the three and six months ended June 30, 2024 and 2023, respectively. Outstanding
warrants, options and RSUs were excluded from the calculation of diluted EPS as the effect was antidilutive to consolidated net loss.
| | |
Three Months Ended | | |
Six Months Ended | |
| | |
June 30, | | |
June 30, | |
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| | |
(in thousands, except for per-share data) | |
| Net loss from continuing operations | |
$ | (2,214 | ) | |
$ | (13,708 | ) | |
$ | (7,664 | ) | |
$ | (24,537 | ) |
| Net (income) loss from discontinued operations | |
| 1,102 | | |
| (6,831 | ) | |
| 813 | | |
| (14,184 | ) |
| Net loss | |
| (1,112 | ) | |
| (20,539 | ) | |
| (6,851 | ) | |
| (38,721 | ) |
| Deemed dividends | |
| (445 | ) | |
| (367 | ) | |
| (4,034 | ) | |
| (367 | ) |
| Net loss available to common shareholders | |
$ | (1,557 | ) | |
$ | (20,906 | ) | |
$ | (10,885 | ) | |
$ | (39,088 | ) |
| Weighted average common shares outstanding - basic and diluted | |
| 7,449,706 | | |
| 878,171 | | |
| 5,307,471 | | |
| 862,177 | |
| | |
| | | |
| | | |
| | | |
| | |
| Basic and diluted loss per common share from continuing operations | |
$ | (0.30 | ) | |
$ | (15.61 | ) | |
$ | (1.44 | ) | |
$ | (28.46 | ) |
| Basic and diluted loss per common share from discontinued operations | |
| 0.15 | | |
| (7.78 | ) | |
| 0.15 | | |
| (16.45 | ) |
| Basic and diluted loss per common share from deemed dividends | |
| (0.06 | ) | |
| (0.42 | ) | |
| (0.76 | ) | |
| (0.43 | ) |
| Basic and diluted loss per common share | |
$ | (0.21 | ) | |
$ | (23.81 | ) | |
$ | (2.05 | ) | |
$ | (45.34 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Anti-dilutive shares are as follows as of June 30: | |
| | | |
| | | |
| | | |
| | |
| Warrants | |
| 7,511,600 | | |
| 151,775 | | |
| 7,511,600 | | |
| 151,775 | |
| Options | |
| 6,605 | | |
| 20,036 | | |
| 6,605 | | |
| 20,036 | |
| Restricted stock units | |
| 1,824 | | |
| 24,434 | | |
| 1,824 | | |
| 24,434 | |
| | |
| 7,520,029 | | |
| 196,245 | | |
| 7,520,029 | | |
| 196,245 | |
NOTE 12. - COMMITMENTS AND CONTINGENCIES
License
agreements and sponsored research – The Company has entered into various consulting, license and tobacco
growing agreements (the “Agreements”) with various counter parties in connection with the Company’s plant biotechnology
business relating to tobacco. The schedule below summarizes the Company’s commitments, both financial and other, associated with
each Agreement. Costs incurred under the Agreements are generally recorded as research and development expenses on the Company’s
Condensed Consolidated Statements of Operations and Comprehensive Loss.
| | |
| |
| |
Future
Commitments | |
| Commitment | |
Counter
Party | |
Commitment
Type | |
2024 | | |
2025 | | |
2026 | | |
2027 | | |
2028 &
After | | |
Total | |
| License
Agreement | |
NCSU | |
Minimum
annual royalty | |
$ | 100 | | |
$ | 100 | | |
$ | 100 | | |
$ | 100 | | |
$ | 3,575 | | |
$ | 3,975 | (1) |
| License
Agreement | |
NCSU | |
Contract
fee | |
| 150 | | |
| 250 | | |
| 250 | | |
| — | | |
| — | | |
| 650 | (2) |
| Consulting
Agreements | |
Various | |
Contract
fee | |
| 928 | | |
| 373 | | |
| 146 | | |
| — | | |
| — | | |
| 1,447 | (3) |
| Growing
Agreements | |
Various | |
Contract
fee | |
| 149 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 149 | (4) |
| | |
| |
| |
$ | 1,327 | | |
$ | 723 | | |
$ | 496 | | |
$ | 100 | | |
$ | 3,575 | | |
$ | 6,221 | |
| (1) | The minimum annual royalty fee is credited against running royalties on sales of licensed products. The Company is also responsible
for reimbursing NCSU for actual third-party patent costs incurred, including capitalized patent costs and patent maintenance costs. These
costs vary from year to year and the Company has certain rights to direct the activities that result in these costs. |
| (2) | On November 1, 2023, the Company entered into a license agreement with NCSU for an exclusive sublicensable right and license
under specific patent rights and plant variety rights for the field of use in specific licensed territories. Additional milestone fees
could be required pending achievement of events pursuant to the agreement. |
| (3) | As a requirement for a modified risk tobacco product and condition of the marketing authorization by the FDA, the Company engaged
various consulting firms to conduct post-market studies and research. |
| (4) | Various R&D growing agreements for tobacco. |
Litigation
- The Company is subject to litigation arising from time to time in the ordinary course of its business. The Company does
not expect that the ultimate resolution of any pending legal actions will have a material effect on its consolidated results of operations,
financial position, or cash flows. However, litigation is subject to inherent uncertainties. As such, there can be no assurance that any
pending legal action, which the Company currently believes to be immaterial, will not become material in the future. In accordance with
applicable accounting guidance, the Company establishes an accrued liability for litigation and regulatory matters when those matters
present loss contingencies that are both probable and estimable. In such cases, there may be an exposure to loss in excess of any amounts
accrued. When a loss contingency is not both probable and estimable, the Company does not establish an accrued liability. As a litigation
or regulatory matter develops, the Company, in conjunction with any outside counsel handling the matter, evaluates on an ongoing basis
whether such matter presents a loss contingency that is probable and estimable. If, at the time of evaluation, the loss contingency related
to a litigation or regulatory matter is not both probable and estimable, the matter will continue to be monitored for further developments
that would make such loss contingency both probable and estimable. When a loss contingency related to a litigation or regulatory matter
is deemed to be both probable and estimable, the Company will establish an accrued liability with respect to such loss contingency and
record a corresponding amount of related expenses. The Company will then continue to monitor the matter for further developments that
could affect the amount of any such accrued liability.
In connection with ongoing restructuring efforts
and the hemp/cannabis disposal group (see Note 2 “Divestitures and discontinued operations”) the Company has received unasserted
claims related to disputed contracts, which could result in accrual of an additional amount up to $1,314 on the Condensed Consolidated
Balance Sheets. The Company is vigorously defending its position against these claims.
Class Action
On January 21, 2019, Matthew Jackson Bull,
a resident of Denver, Colorado, filed a Complaint against the Company, the Company’s then Chief Executive Officer, Henry Sicignano
III, and the Company’s then Chief Financial Officer, John T. Brodfuehrer, in the United States District Court for the Eastern District
of New York entitled: Matthew Bull, Individually and on behalf of all others similarly situated, v. 22nd Century Group, Inc.,
Henry Sicignano III, and John T. Brodfuehrer, Case No. 1:19 cv 00409.
The complaint alleges three counts: Count I sues
the Company and Messrs. Sicignano and Brodfuehrer and alleges that the Company's quarterly and annual reports, SEC filings, press
releases and other public statements and documents contained false statements in violation of Section 10(b) of the Securities
Exchange Act and Rule 10b-5; Count II sues Messrs. Sicignano and Brodfuehrer pursuant to Section 10(b) of the Securities
Exchange Act and Rule 10b5(a) and (c); and Count III sues Messrs. Sicignano and Brodfuehrer for the allegedly false statements
pursuant to Section 20(a) of the Securities Exchange Act. The Amended Complaint seeks to certify a class, and unspecified compensatory
and punitive damages, and attorney's fees and costs. Several other cases were subsequently filed and consolidated into the main action.
On April 25, 2023, the parties filed with
the Court the Motion for Preliminary Approval of the Settlement, which includes the final terms of the proposed settlement. The Court
preliminarily approved the settlement on June 30, 2023, and scheduled a further settlement hearing for October 3, 2023. The
Court entered the Final Judgment and Order of Dismissal with Prejudice of the action on October 23, 2023. The settlement amount that
the defendants paid is $3,000 and is fully covered by the Company’s insurance, which has been funded by the Company’s insurance
carrier in an escrow account and anticipated to be disbursed in the third quarter of 2024. Accordingly, the Company has recorded an accrual
for litigation settlement and corresponding indemnification receivable on the Condensed Consolidated Balance Sheets as of June 30, 2024
and December 31, 2023.
Shareholder Derivative Cases
On February 6, 2019, Melvyn Klein, a resident
of Nassau County New York, filed a shareholder derivative claim against the Company, the Company’s then Chief Executive Officer,
Henry Sicignano III, the Company’s Chief Financial Officer, John T. Brodfuehrer, and each member of the Company’s Board of
Directors in the United States District Court for the Eastern District of New York entitled: Melvyn Klein, derivatively on behalf of 22nd
Century Group v. Henry Sicignano, III, Richard M. Sanders, Joseph Alexander Dunn, Nora B. Sullivan, James W. Cornell, John T. Brodfuehrer
and 22nd Century Group, Inc., Case No. 1:19 cv 00748. Mr. Klein brings this action derivatively alleging that (i) the
director defendants supposedly breached their fiduciary duties for allegedly allowing the Company to make false statements; (ii) the
director defendants supposedly wasted corporate assets to defend this lawsuit and the other related lawsuits; (iii) the defendants
allegedly violated Section 10(b) of the Securities Exchange Act and Rule 10b 5 promulgated thereunder for allegedly approving
or allowing false statements regarding the Company to be made; and (iv) the director defendants allegedly violated Section 14(a) of
the Securities Exchange Act and Rule 14a 9 promulgated thereunder for allegedly approving or allowing false statements regarding
the Company to be made in the Company’s proxy statement. Numerous other shareholder derivative cases were subsequently filed and
consolidated into the main action.
On December 5, 2023,
the parties entered into a Memorandum of Settlement to fully resolve all claims pending the Court’s approval of a motion for preliminary
approval of settlement. The settlement amount is $768 related to plaintiffs attorney and legal fees and is fully covered by the Company’s
insurance. The Company expects the settlement to be approved and funded in the fourth quarter of 2024. Accordingly, the Company has recorded
an accrual for litigation settlement and corresponding indemnification receivable on the Consolidated Balance Sheets as of June 30,
2024 and December 31, 2023.
Insurance Litigation
In November 2022,
there was a fire at the Company’s Grass Valley manufacturing facility in Oregon, which resulted in a total loss of the facility.
The Company submitted an insurance claim with Dorchester Insurance Company, Ltd. (“Dorchester”) for casualty loss and
business interruption coverage which was acknowledged on November 23, 2022. Dorchester funded $5,000 of casualty loss insurance but
has failed to issue any payments in connection with the Company’s business interruption claim.
On
July 19, 2023, the Company filed a Complaint against Dorchester in the United States District Court for the District of Oregon, Pendleton
Division, Case No. 2:23-cv-01057-HL. The Company is alleging breach of contract and breach of duty of good faith and fair dealing.
The Company is seeking full recovery of its business interruption claim of approximately $9,000 under the policy plus direct and indirect
damages resulting from Dorchester’s continued delay in issuing coverage payments. Discovery is ongoing. The trial date is November 4,
2025.
KeyGene Dispute
On April 11, 2024 the Company received a Request
for Arbitration from Keygene N.V. (“Keygene”) in connection with the Company’s termination of various framework collaborative
research agreements described below. On April 3, 2019, the Company entered into the Framework Collaborative Research Agreement with
KeyGene in the field of hemp/cannabis. On April 30, 2021, the Company and KeyGene entered into a First Amended and Restated Framework
Collaborative Research Agreement which extended the agreement term, from first quarter 2024 to first quarter 2027. On March 30, 2022,
the Company and KeyGene entered into a new Framework Collaborative Research Agreement for a term of three years in the field related to
the hops plant. On January 8, 2024, the Company formally terminated both Framework Collaborative Research Agreements, as amended,
related to hemp/cannabis and hops. KeyGene is seeking payment in the amount of $1,885 for current and future services under the Framework
Collaborative Research Agreements and has invoiced the Company $881 for services performed. The matter is being arbitrated under the administration
of the International Court of Arbitration.
The Company filed its Answer to Request for Arbitration
with Defenses and Counterclaims on June 4, 2024. On July 25, 2024, an arbitrator was formally appointed. Discovery has not yet
commenced, and no arbitration date has been set. The Company believes it has substantial defenses to KeyGene’s claims and intends
to defend itself vigorously.
Maison Dispute
On
January 23, 2024, the Company received a Notice of Intent to Arbitrate from Maison Placements Canada Inc. (“Maison”)
in connection with the Company’s March 2023 Senior Secured Credit Facility transaction. Maison claims it is owed fees for closure
of the Senior Secured Credit Facility transaction as a result of discussions with former Company personnel and a purported letter of engagement
dating from 2021. The parties have agreed on the selection of an arbitrator, but no arbitration date has been set. The Company
believes it has substantial defenses to Maison’s claims and intends to defend itself vigorously.
INDEX TO FINANCIAL STATEMENTS
22nd CENTURY GROUP, INC.
Audited Financial Statements for the Years
Ended December 31, 2023 and 2022
REPORT OF INDEPENDENT REGISTERED
PUBLIC ACCOUNTING FIRM
To the Shareholders and the Board of Directors of 22nd
Century Group, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated
balance sheets of 22nd Century Group, Inc. and subsidiaries (the Company) as of December 31, 2023 and 2022, the
related consolidated statements of operations and comprehensive loss, changes in shareholders' equity and cash flows for each of the
two years in the period ended December 31, 2023, and the related notes to the consolidated financial statements (collectively, the
financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the
Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for the years then ended, in conformity
with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying financial
statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements,
the Company has incurred significant losses and negative cash flows from operations since inception and expects to incur additional losses
until such time that it can generate significant revenue and profit in its tobacco business. Further, the Company has negative working
capital and a shareholders’ deficit as of December 31, 2023. This raises substantial doubt about the Company's ability to
continue as a going concern. Management's plans in regard to these matters also are described in Note 1. The financial statements do
not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility
of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our
audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are
required to be independent with respect to the Company in accordance with U.S. federal securities laws and the applicable rules and
regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the
standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial
statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged
to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding
of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s
internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to
assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that
respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial
statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well
as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below
are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to
the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved
our especially challenging, subjective or complex judgments. The communication of critical audit matters does not alter in any way our
opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate
opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Debt related accounting, classification, and
valuation
Critical Audit Matter description
As discussed in Note 1 and 13 of the consolidated
financial statements, during the year ended December 31, 2023, prior to the amendments described in Note 13, the Company entered
into a senior secured credit facility, which consisted of a three-year debenture with a principal amount of $21,053 (as defined in Note
13) and a $2,865 subordinated promissory note (the “Subordinated Note). The Debentures were issued at a 5% original issuance discount
and are subject to a 5% exit payment. In connection with the issuance with the Debentures and the Subordinated Note, the Company issued
warrants to purchase common stock (“Detachable Warrants")
The Debenture, Subordinated Note, and Detachable
Warrants included various terms that required evaluation at the issuance date. Further, a portion of the Detachable Warrants met the
criteria for equity classification (Note 10), while a portion are treated as liabilities (Note 9) due to holder put features applicable
to only a portion of the warrants issued. The fair value of both classes of warrants was determined at the date of issuance and recorded
as a debt discount. The liability classified warrants were subsequently adjusted to fair value at the end of each reporting period.
We identified the accounting for the terms of
the Debentures (prior to amendments), Subordinated Note, and Detachable Warrants as well as the valuation and classification of the same
as a critical audit matter. Auditing the accounting for these was especially challenging due to the inherent complexity of the agreements
and the related valuation models. Auditing these elements required an increased level of audit effort, including the involvement of professionals
with specialized skill and knowledge.
How the Critical Audit Matter was addressed
in the Audit
Addressing the matter involved performing subjective
procedures and evaluating audit evidence in connection with forming our overall opinion on the financial statements. The primary procedures
we performed include: Inspecting the underlying agreements and ensuring appropriate application of the relevant accounting literature
to the terms of the Debentures and Subordinated Note; evaluating the appropriateness of the fair value and classification of the Detachable
Warrants; and utilizing personnel with specialized skill and knowledge in valuation to assist in assessing the fair value determined.
Debt extinguishment, conversion option and
fair value measurement
Critical Audit Matter description
During the year ended December 31, 2023,
the Company amended the Debentures (as defined in Note 13) with its lenders. The terms of the amendments are described in Note 13. For
each amendment, the Company was required to evaluate troubled debt restructuring applicability and debt modification versus extinguishment
analysis. Neither qualified as a troubled debt restructuring and the first was a modification while the second was extinguishment, primarily
resulting from the addition of conversion feature. Further, the conversion feature did require bifurcation from the debt host. Therefore,
both the conversion feature (Note 9) and the Debentures post-extinguishment were subject to fair market measurement. The derivative liability
is subsequently adjusted to fair value at the end of each reporting period.
We identified the accounting for the amended
terms as well as the valuations related to the conversion option and Debentures post extinguishment as a critical audit matter. Auditing
the accounting for these items was especially challenging due to the inherent complexity of the agreements and the related valuation
models. Auditing these elements required an increased level of audit effort, including the involvement of professionals with specialized
skill and knowledge.
How the Critical Audit Matter was addressed
in the Audit
Addressing the matter involved performing subjective
procedures and evaluating audit evidence in connection with forming our overall opinion on the financial statements. The primary procedures
we performed include: Inspecting the underlying agreements and ensuring appropriate application of the relevant accounting literature
to the terms of the amended Debentures; evaluating the appropriateness of the fair value for the bifurcated conversion option derivative
and the amended Debentures post-extinguishment utilizing personnel with specialized skill and knowledge in valuation to assist in assessing
the fair values determined.
Discontinued Operations
Critical Audit Matter description
As discussed in Notes 1, and 2 of the consolidated
financial statements, during the year ended December 31, 2023, the Company divested substantially all of its assets in the GVB hemp/cannabis
business and recorded impairment charges. As a result of the agreement management determined the hemp/cannabis disposal group has met
the requirements to be presented as held for sale and discontinued operations for all periods presented. The Company has not segregated
the statement of cash flows.
We identified the reporting of discontinued operations
and the impairment charges as a critical audit matter, which required extensive effort and a higher degree of auditor judgement.
How the Critical Audit Matter was addressed
in the audit
Addressing
the matter involved performing subjective procedures and evaluating audit evidence in connection with forming our overall opinion
on the financial statements. The primary procedures we performed include: assessed management’s conclusion regarding discontinued
operations treatment, obtained and read the related agreements and compared the terms of that agreement
to the identification of the assets and liabilities included in the disposal group, reviewed management assumptions and judgment for
determining historical numbers related to discontinued operations, reviewed and recomputed the loss on disposal of discontinued operations
and related income tax benefit, and assessed the completeness and accuracy of the presentation and disclosures.
| /s/ Freed Maxick, CPAs, P.C. |
|
| |
|
| We have served as the Company’s auditor since
2011. |
|
| |
|
| Buffalo, New York |
|
| March 28, 2024 |
|
| 22nd CENTURY
GROUP, INC. AND SUBSIDIARIES |
| CONSOLIDATED
BALANCE SHEETS |
(amounts
in thousands, except share and per-share data)
|
| | |
December 31, | | |
December 31, | |
| | |
2023 | | |
2022 | |
| ASSETS | |
| | | |
| | |
| Current assets: | |
| | | |
| | |
| Cash and cash equivalents | |
$ | 2,058 | | |
$ | 2,205 | |
| Short-term investment securities | |
| — | | |
| 18,193 | |
| Accounts receivable, net | |
| 1,671 | | |
| 1,363 | |
| Inventories | |
| 4,346 | | |
| 7,270 | |
| Insurance recoveries | |
| 3,768 | | |
| — | |
| GVB promissory note | |
| 2,000 | | |
| — | |
| Prepaid expenses and other current assets | |
| 1,180 | | |
| 1,928 | |
| Current assets of
discontinued operations held for sale | |
| 1,254 | | |
| 13,646 | |
| Total current assets | |
| 16,277 | | |
| 44,605 | |
| Property, plant and equipment, net | |
| 3,393 | | |
| 3,692 | |
| Operating lease right-of-use assets, net | |
| 1,894 | | |
| 943 | |
| Intangible assets, net | |
| 5,924 | | |
| 7,212 | |
| Other assets | |
| 15 | | |
| 3,417 | |
| Noncurrent assets
of discontinued operations held for sale | |
| — | | |
| 54,782 | |
| Total assets | |
$ | 27,503 | | |
$ | 114,651 | |
| | |
| | | |
| | |
| LIABILITIES AND SHAREHOLDERS' EQUITY | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | |
| Notes and loans payable - current | |
$ | 543 | | |
$ | 689 | |
| Current portion of long-term debt | |
| 5,848 | | |
| — | |
| Operating lease obligations | |
| 231 | | |
| 252 | |
| Accounts payable | |
| 4,445 | | |
| 2,051 | |
| Accrued expenses | |
| 1,322 | | |
| 766 | |
| Accrued litigation | |
| 3,768 | | |
| — | |
| Accrued payroll | |
| 883 | | |
| 2,662 | |
| Accrued excise taxes and fees | |
| 2,234 | | |
| 1,423 | |
| Deferred income | |
| 726 | | |
| 688 | |
| Other current liabilities | |
| 1,849 | | |
| 349 | |
| Current liabilities
of discontinued operations held for sale | |
| 3,185 | | |
| 4,138 | |
| Total current liabilities | |
| 25,034 | | |
| 13,018 | |
| Long-term liabilities: | |
| | | |
| | |
| Operating lease obligations | |
| 1,698 | | |
| 711 | |
| Long-term debt | |
| 8,058 | | |
| — | |
| Other long-term liabilities | |
| 1,123 | | |
| 344 | |
| Noncurrent liabilities
of discontinued operations held for sale | |
| — | | |
| 4,603 | |
| Total liabilities | |
| 35,914 | | |
| 18,676 | |
| Commitments and contingencies (Note 12) | |
| | | |
| | |
| Shareholders' equity (deficit) | |
| | | |
| | |
| Preferred stock, $.00001 par value, 10,000,000 shares
authorized | |
| | | |
| | |
| Common stock, $.00001 par value, 66,666,667 shares authorized | |
| | | |
| | |
| Capital stock issued and outstanding: | |
| | | |
| | |
| 43,525,862 common shares (14,349,275 at December 31, 2022) | |
| | | |
| | |
| Common stock, par value | |
| — | | |
| — | |
| Capital in excess of par value | |
| 370,297 | | |
| 333,900 | |
| Accumulated other comprehensive loss | |
| — | | |
| (111 | ) |
| Accumulated deficit | |
| (378,707 | ) | |
| (237,814 | ) |
| Total shareholders' equity (deficit) | |
| (8,410 | ) | |
| 95,975 | |
| Total liabilities and shareholders’
equity (deficit) | |
$ | 27,503 | | |
$ | 114,651 | |
See accompanying notes to consolidated financial
statements.
| 22nd CENTURY
GROUP, INC. AND SUBSIDIARIES |
CONSOLIDATED STATEMENTS
OF OPERATIONS AND COMPREHENSIVE LOSS
(amounts in thousands, except per-share
data) |
| |
| | |
Year Ended | |
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Revenues, net | |
$ | 32,204 | | |
$ | 40,501 | |
| Cost of goods sold | |
| 40,900 | | |
| 38,654 | |
| Gross (loss) profit | |
| (8,696 | ) | |
| 1,847 | |
| Operating expenses: | |
| | | |
| | |
| Sales, general and administrative | |
| 31,064 | | |
| 32,231 | |
| Research and development | |
| 2,644 | | |
| 3,578 | |
| Other operating expense
(income), net | |
| 2,527 | | |
| (327 | ) |
| Total operating expenses | |
| 36,235 | | |
| 35,482 | |
| Operating loss from continuing operations | |
| (44,931 | ) | |
| (33,635 | ) |
| Other income (expense): | |
| | | |
| | |
| Realized loss on Panacea investment | |
| — | | |
| (2,789 | ) |
| Other income (expense), net | |
| 334 | | |
| (366 | ) |
| Loss on transfer of promissory note | |
| (895 | ) | |
| — | |
| Interest income, net | |
| 219 | | |
| 313 | |
| Interest expense | |
| (9,366 | ) | |
| (55 | ) |
| Total other expense | |
| (9,708 | ) | |
| (2,897 | ) |
| Loss from continuing operations before
income taxes | |
| (54,639 | ) | |
| (36,532 | ) |
| Provision for income taxes | |
| 47 | | |
| 21 | |
| Net loss from continuing operations | |
$ | (54,686 | ) | |
$ | (36,553 | ) |
| | |
| | | |
| | |
| Discontinued operations: | |
| | | |
| | |
| Loss from discontinued operations before income taxes | |
| (85,634 | ) | |
| (23,703 | ) |
| Provision (benefit) for income taxes | |
| 455 | | |
| (455 | ) |
| Net loss from discontinued operations | |
$ | (86,089 | ) | |
$ | (23,248 | ) |
| | |
| | | |
| | |
| Net loss | |
$ | (140,775 | ) | |
$ | (59,801 | ) |
| Deemed dividends | |
| (9,992 | ) | |
| — | |
| Net loss available to common shareholders | |
$ | (150,767 | ) | |
$ | (59,801 | ) |
| | |
| | | |
| | |
| Basic and diluted loss per common share
from continuing operations | |
$ | (2.64 | ) | |
$ | (2.84 | ) |
| Basic and diluted loss per common share
from discontinued operations | |
$ | (4.16 | ) | |
$ | (1.81 | ) |
| Basic and diluted loss per common share
from deemed dividends | |
$ | (0.48 | ) | |
$ | — | |
| Basic and diluted loss per common share | |
$ | (7.28 | ) | |
$ | (4.65 | ) |
| Weighted average common shares outstanding
- basic and diluted | |
| 20,711 | | |
| 12,856 | |
| | |
| | | |
| | |
| Net loss | |
$ | (140,775 | ) | |
$ | (59,801 | ) |
| Other comprehensive income: | |
| | | |
| | |
| Unrealized gain (loss) on short-term investment
securities | |
| 71 | | |
| (316 | ) |
| Foreign currency translation | |
| (1 | ) | |
| 1 | |
| Reclassification of
realized losses to net loss | |
| 41 | | |
| 366 | |
| Other comprehensive
income | |
| 111 | | |
| 51 | |
| Comprehensive loss | |
$ | (140,664 | ) | |
$ | (59,750 | ) |
See accompanying notes to consolidated financial
statements.
| 22nd CENTURY
GROUP, INC. AND SUBSIDIARIES |
| CONSOLIDATED
STATEMENTS OF CHANGES IN SHAREHOLDERS’ EQUITY |
(amounts
in thousands, except share amounts)
|
| | |
Years
Ended December 31, 2023 and 2022 | |
| | |
Common
| | |
Par Value
| | |
Capital in
| | |
Other
| | |
| | |
| |
| | |
Shares
| | |
of Common
| | |
Excess of
| | |
Comprehensive
| | |
Accumulated
| | |
Shareholders'
| |
| | |
Outstanding | | |
Shares | | |
Par Value | | |
Income
(Loss) | | |
Deficit | | |
Equity | |
| Balance at January 1, 2022 | |
| 10,858,237 | | |
$ | — | | |
$ | 244,249 | | |
$ | (162 | ) | |
$ | (178,013 | ) | |
$ | 66,074 | |
| Stock issued in connection with
option exercises | |
| 10,001 | | |
| — | | |
| 174 | | |
| — | | |
| — | | |
| 174 | |
| Stock issued in connection with
RSU vesting, net of shares withheld for taxes | |
| 149,482 | | |
| — | | |
| (149 | ) | |
| — | | |
| — | | |
| (149 | ) |
| Stock issued in connection with
acquisition | |
| 2,193,334 | | |
| — | | |
| 51,653 | | |
| — | | |
| — | | |
| 51,653 | |
| Stock issued in connection with
capital raise, net of issuance costs of 2,516 | |
| 1,138,221 | | |
| — | | |
| 32,484 | | |
| — | | |
| — | | |
| 32,484 | |
| Equity-based compensation | |
| — | | |
| — | | |
| 5,489 | | |
| — | | |
| — | | |
| 5,489 | |
| Other comprehensive income | |
| — | | |
| — | | |
| — | | |
| 51 | | |
| — | | |
| 51 | |
| Net loss | |
| — | | |
| — | | |
| — | | |
| — | | |
| (59,801 | ) | |
| (59,801 | ) |
| Balance at December 31, 2022 | |
| 14,349,275 | | |
$ | — | | |
$ | 333,900 | | |
$ | (111 | ) | |
$ | (237,814 | ) | |
$ | 95,975 | |
| Stock issued in connection with
RSU vesting, net of shares withheld for taxes | |
| 114,786 | | |
| — | | |
| (419 | ) | |
| — | | |
| — | | |
| (419 | ) |
| Stock issued in connection with
acquisition | |
| 31,056 | | |
| — | | |
| 503 | | |
| — | | |
| — | | |
| 503 | |
| Stock issued in connection with
ATM, net of fees of $178 | |
| 284,343 | | |
| — | | |
| 2,563 | | |
| — | | |
| — | | |
| 2,563 | |
| Stock issued in connection with
licensing arrangement | |
| 333,334 | | |
| — | | |
| 3,570 | | |
| — | | |
| — | | |
| 3,570 | |
| Stock issued in connection with
capital raises, net of issuance costs of 2,279 | |
| 13,499,827 | | |
| — | | |
| 22,880 | | |
| — | | |
| — | | |
| 22,880 | |
| Stock issued in connection
with warrant exercises, net of fees of $292 | |
| 14,847,206 | | |
| — | | |
| 3,044 | | |
| — | | |
| — | | |
| 3,044 | |
| Equity detachable warrants | |
| — | | |
| — | | |
| 1,577 | | |
| — | | |
| — | | |
| 1,577 | |
| Adoption of ASU 2016-13 | |
| — | | |
| — | | |
| — | | |
| — | | |
| (118 | ) | |
| (118 | ) |
| Fractional shares issued for
reverse stock split | |
| 66,035 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Equity-based compensation | |
| — | | |
| — | | |
| 2,679 | | |
| — | | |
| — | | |
| 2,679 | |
| Other comprehensive income | |
| — | | |
| — | | |
| — | | |
| 111 | | |
| — | | |
| 111 | |
| Net loss | |
| — | | |
| — | | |
| — | | |
| — | | |
| (140,775 | ) | |
| (140,775 | ) |
| Balance at December 31, 2023 | |
| 43,525,862 | | |
$ | — | | |
$ | 370,297 | | |
$ | — | | |
$ | (378,707 | ) | |
$ | (8,410 | ) |
See accompanying notes to consolidated financial
statements.
| 22nd CENTURY
GROUP, INC. AND SUBSIDIARIES |
| CONSOLIDATED
STATEMENTS OF CASH FLOWS |
| (amounts in thousands) |
| |
| | |
Year Ended | |
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Cash flows from operating activities: | |
| | | |
| | |
| Net loss | |
$ | (140,775 | ) | |
$ | (59,801 | ) |
| Adjustments to reconcile net loss to cash
used in operating activities: | |
| | | |
| | |
| Impairment of long-lived assets | |
| 3,297 | | |
| 1,488 | |
| Amortization and depreciation | |
| 3,951 | | |
| 2,858 | |
| Amortization of right-of-use asset | |
| 908 | | |
| 733 | |
| Amortization of inventory step-up | |
| — | | |
| 978 | |
| Unrealized loss on investment | |
| — | | |
| 5 | |
| GVB fire write-offs | |
| — | | |
| 4,549 | |
| Other non-cash (gains) and losses | |
| (15 | ) | |
| 563 | |
| Provision for credit losses | |
| 1,024 | | |
| 770 | |
| (Gain) loss on the sale of machinery and
equipment | |
| 73 | | |
| (368 | ) |
| Realized loss (gain) on Panacea investment | |
| — | | |
| 2,789 | |
| Inventory write-off | |
| — | | |
| 237 | |
| Debt related charges included in interest
expense | |
| 8,006 | | |
| — | |
| Equity-based employee compensation expense | |
| 2,679 | | |
| 5,489 | |
| Gain on change of contingent consideration | |
| (1,138 | ) | |
| — | |
| Change in fair value of warrant liabilities | |
| (364 | ) | |
| — | |
| Change in fair value of derivative liability | |
| 557 | | |
| — | |
| Loss on disposal of discontinued operations | |
| 58,521 | | |
| — | |
| Loss on transfer of promissory note | |
| 895 | | |
| — | |
| Deferred income taxes | |
| 434 | | |
| (434 | ) |
| Increase in inventory reserves | |
| 8,695 | | |
| — | |
| Changes in operating assets and liabilities,
net of acquisition: | |
| | | |
| | |
| Accounts receivable | |
| (18 | ) | |
| (2,881 | ) |
| Inventory | |
| (5,925 | ) | |
| (8,789 | ) |
| Prepaid expenses and other assets | |
| 451 | | |
| (920 | ) |
| Accounts payable | |
| 4,752 | | |
| 416 | |
| Accrued expenses | |
| 681 | | |
| (582 | ) |
| Accrued payroll | |
| (2,153 | ) | |
| 748 | |
| Accrued excise taxes and fees | |
| 811 | | |
| 153 | |
| Other liabilities | |
| (334 | ) | |
| 285 | |
| Net cash used in operating activities | |
| (54,987 | ) | |
| (51,714 | ) |
| Cash flows from investing activities: | |
| | | |
| | |
| Acquisition of patents, trademarks, and
licenses | |
| (961 | ) | |
| (772 | ) |
| Acquisition of property, plant and equipment | |
| (4,656 | ) | |
| (3,657 | ) |
| Proceeds from the sale of property, plant
and equipment | |
| 283 | | |
| 409 | |
| Acquisition, net of cash acquired | |
| (254 | ) | |
| (1,297 | ) |
| Proceeds from sale of discontinued operations | |
| 665 | | |
| — | |
| Investment in Change Agronomy Ltd. | |
| — | | |
| (682 | ) |
| Property, plant and equipment insurance
proceeds | |
| 3,500 | | |
| — | |
| Sales and maturities of short-term investment
securities | |
| 21,714 | | |
| 101,990 | |
| Purchase of short-term
investment securities | |
| (3,475 | ) | |
| (73,413 | ) |
| Net cash provided by investing activities | |
| 16,816 | | |
| 22,578 | |
| Cash flows from financing activities: | |
| | | |
| | |
| Payments on notes payable | |
| (5,581 | ) | |
| (3,822 | ) |
| Proceeds from issuance of notes payable | |
| 2,360 | | |
| 2,162 | |
| Other financing activities | |
| — | | |
| (29 | ) |
| Payments of long-term debt | |
| (9,700 | ) | |
| — | |
| Proceeds from issuance of long-term debt | |
| 16,849 | | |
| — | |
| Payment of debt issuance costs | |
| (801 | ) | |
| — | |
| Proceeds from issuance of detachable warrants | |
| 6,016 | | |
| — | |
| Net proceeds from option exercise | |
| — | | |
| 174 | |
| Net proceeds from warrant exercise | |
| 3,044 | | |
| — | |
| Proceeds from issuance of common stock
related to the ATM | |
| 2,741 | | |
| — | |
| Payment of common stock issuance costs
related to the ATM | |
| (178 | ) | |
| — | |
| Proceeds from issuance of common stock | |
| 25,158 | | |
| 35,000 | |
| Payment of common stock issuance costs | |
| (2,279 | ) | |
| (2,516 | ) |
| Taxes paid related
to net share settlement of RSUs | |
| (420 | ) | |
| (149 | ) |
| Net cash provided
by financing activities | |
| 37,209 | | |
| 30,820 | |
| Net (decrease) increase in cash and cash equivalents | |
| (962 | ) | |
| 1,684 | |
| Cash and cash equivalents -
beginning of period | |
| 3,020 | | |
| 1,336 | |
| Cash and cash equivalents - end
of period | |
$ | 2,058 | | |
$ | 3,020 | |
| | |
| | | |
| | |
| Supplemental disclosures of cash flow information: | |
| | | |
| | |
| Net cash paid for: | |
| | | |
| | |
| Cash paid during the period for interest | |
$ | 1,313 | | |
$ | 34 | |
| Cash paid during the period for income
taxes | |
$ | 40 | | |
$ | 14 | |
| Non-cash transactions: | |
| | | |
| | |
| Capital expenditures
incurred but not yet paid | |
$ | 118 | | |
$ | 94 | |
| Right-of-use assets
and corresponding operating lease obligations | |
$ | 5,166 | | |
$ | — | |
| Non-cash assignment
of PLSH Promissory Note | |
$ | 2,600 | | |
$ | — | |
| Insurance/litigation
gross up | |
$ | 3,768 | | |
$ | — | |
| Non-cash proceeds
from sale of discontinued operations | |
$ | 2,000 | | |
$ | — | |
| Deemed dividends | |
$ | 9,801 | | |
$ | — | |
| Stock issued in
connection with acquisition | |
$ | — | | |
$ | 51,653 | |
| Non-cash consideration
RXP acquisition | |
$ | 1,641 | | |
$ | — | |
| Non-cash licensing
arrangement | |
$ | 3,500 | | |
$ | — | |
See
accompanying notes to consolidated financial statements.
22nd CENTURY GROUP, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED
FINANCIAL STATEMENTS
December 31, 2023
Amounts in thousands, except for share and
per share data
NOTE 1. – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
22nd Century Group, Inc.
(together with its consolidated subsidiaries, “22nd Century Group” or the “Company”) is a publicly
traded Nevada corporation on the NASDAQ Capital Market under the symbol “XXII.” 22nd Century Group is a
tobacco products company with sales and distribution of the Company’s own proprietary new reduced nicotine tobacco products authorized
as Modified Risk Tobacco Products by the FDA. Additionally, the Company provides contract manufacturing services for conventional combustible
tobacco products for third-party brands.
Basis
of Presentation and Principles of Consolidation – The consolidated financial statements have been prepared
in conformity with accounting principles generally accepted in the United States of America (“GAAP”) and include the accounts
of 22nd Century Group and its wholly owned subsidiaries. All intercompany balances and transactions have been eliminated in
consolidation.
As described in Note 2, on
December 22, 2023, the Company divested substantially all of the assets of GVB Biopharma’s (“GVB”) business within
its former hemp/cannabis segment.
As
a result of the divestiture of GVB and strategic shift away from hemp/cannabis, the Company has realigned its corporate and management
reporting structure to focus solely on its tobacco business. As a result, during the fourth quarter of 2023, the Company reorganized
its business to become a single reportable segment: (1) tobacco. This segment structure reflects the financial information
and reports used by the Company’s management, specifically its Chief Operating Decision Maker (“CODM”), to make decisions
regarding the Company’s business, including resource allocations and performance assessments. All assets and continuing operations
of the Company are physically located or domiciled in the United States.
The results of operations
of the former hemp/cannabis segment are reported as discontinued operations in the Consolidated Statements of Operations and Comprehensive
Loss for all periods presented and the related assets and liabilities associated with the discontinued operations are classified as held
for sale in the Consolidated Balance Sheets as of December 31, 2023, and 2022, respectively. The Consolidated Statements of Cash
Flows includes cash flows related to the discontinued operations due to 22nd Century’s (parent) centralized treasury
and cash management processes, and, accordingly, cash flow amounts for discontinued operations are disclosed in Note 2 “Discontinued
Operations and Divestitures.” All results and information in the Consolidated Financial Statements are presented as continuing
operations and exclude the former hemp/cannabis segment unless otherwise noted specifically as discontinued operations.
Use
of Estimates – The preparation of financial statements in conformity with GAAP requires management to make
estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities
at the date of the financial statements and the reported amounts of income and expenses during the reporting period. Actual results could
differ from those estimates.
Liquidity
and Capital Resources – These Consolidated Financial Statements
have been prepared in accordance with generally accepted accounting principles applicable to a going concern, which contemplates the
realization of assets and the satisfaction of liabilities in the normal course of business.
The
Company has incurred significant losses and negative cash flows from operations since inception and expects to incur additional losses
until such time that it can generate significant revenue and profit in its tobacco business. The Company had negative cash flow from
operations of $54,987 and $51,714 for the years ended December 31, 2023 and 2022, respectively, and an accumulated
deficit of $378,707 and $237,814 as of December 31, 2023 and December 31, 2022, respectively. As of December 31, 2023,
the Company had cash and cash equivalents of $2,058. Subsequent to December 31, 2023, the Company completed a warrant inducement
offering with gross proceeds to the Company of approximately $2,421, before deducting the placement agent fees of $165 (see
Note 21 “Subsequent Events”).
Given
the Company’s projected operating requirements and its existing cash and cash equivalents, there is substantial doubt about
the Company’s ability to continue as a going concern through one year following the date that the Consolidated Financial Statements
are issued.
In response to these conditions,
management is currently evaluating different strategies for reducing expenses, as well as pursuing financing strategies which include
raising additional funds through the issuance of securities, asset sales, and through arrangements with strategic partners. If capital
is not available to the Company when, and in the amounts needed, it could be required to liquidate inventory or assets, cease or curtail
operations, or seek protection under applicable bankruptcy laws or similar state proceedings. There can be no assurance that the Company
will be able to raise the capital it needs to continue operations. Management’s plans do not alleviate substantial doubt about
the Company’s ability to continue as a going concern through one year following the date that the Consolidated Financial Statements
are issued.
The Consolidated Financial
Statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts
and classification of liabilities that might result from the outcome of this uncertainty.
Other
Significant Risks and Uncertainties - The Company is subject to a number of risks, including, but not limited to,
the lack of available capital; the possible delisting of its common stock from Nasdaq; future covenant non-compliance with respect to
the Company’s Senior Secured Credit Facility giving rise to an event of default; inability to identify or consummate any strategic
initiatives and transactions; unsuccessful commercialization strategy and launch plans for the Company’s products or market acceptance
of the Company’s products; risks inherent in litigation, including purported class actions; and protection of proprietary technology.
Reverse
Stock Split – On July 5, 2023, the Company effected a 1-for-15 reverse stock split
of its common stock in order to regain compliance with Nasdaq's continued listing requirements. Fractional shares resulting from the
reverse stock split were rounded up to the nearest whole share, which resulted in the issuance of a total of 66,035 shares
of common stock to implement the reverse stock split. All share and per share amounts, and exercise prices of stock options, and warrants
in the Consolidated Financial Statements and notes thereto have been retroactively adjusted for all periods presented to give effect
to this reverse stock split.
Preferred
stock authorized – The Company is authorized to issue “blank check” preferred stock, which could
be issued with voting, liquidation, dividend and other rights superior to our common stock.
Concentration
of Credit Risk – Financial instruments that potentially subject the Company to concentration of credit risk
consist of cash accounts in financial institutions. Although the cash accounts exceed the federally insured deposit amount, management
does not anticipate nonperformance by the financial institutions. Management reviews the financial viability of these institutions on
a periodic basis.
Cash
and cash equivalents – The Company considers all highly liquid investments with maturities of three months
or less at the date of acquisition to be cash equivalents. However, the Company has elected to classify money market mutual funds related
to its short-term investment portfolio as short-term investment securities. There are no restrictions on the Company’s cash and
cash equivalents.
Short-term
investment securities – The Company’s short-term investment securities are classified as available-for-sale
securities and consist of money market funds, corporate bonds, U.S. government agency bonds, U.S. treasury securities, and commercial
paper with maturities that may extend beyond three months at the time of acquisition. The Company’s short-term investment
securities are carried at fair value within current assets on the Company’s Consolidated Balance Sheets. The Company views its
available-for-sale securities as available for use in current operations regardless of the stated maturity date of the security. The
Company’s investment policy states that all investment securities must have a maximum maturity of twenty-four (24) months
or less and the maximum weighted maturity of the investment securities must not exceed twelve (12) months. Some of the Company’s
short-term investment securities are fixed-income debt instruments, and accordingly, unrealized gains and losses incurred on the short-term
investment securities (the adjustment to fair value) are recorded in other comprehensive income or loss on the Company’s Consolidated
Statements of Operations and Comprehensive Loss. Realized gains and losses on short-term investment securities are recorded in the other
income (expense) portion of the Company’s Consolidated Statements of Operations and Comprehensive Loss. Interest income is recorded
on the accrual basis and presented net of investment related fees.
Trade
Accounts Receivable and Provision for Current Expected Credit Losses – The Company provides credit, in the normal
course of business, to its tobacco customers in the form of trade receivables. Credit is extended based on evaluation of a customer’s
financial condition and collateral is not required. The Company maintains a provision for those trade receivables that it does not expect
to collect. In accordance with Accounting Standards Codification (“ASC”) Topic 326, the Company accrues its estimated losses
from uncollectable accounts receivable to the provision based upon recent historical experience, the length of time the receivable has
been outstanding, other specific information as it becomes available, and reasonable and supportable forecasts not already reflected
in the historical loss information. Provisions for current expected credit losses are charged to current operating expenses. Actual losses
are charged against the provision when incurred. As of December 31, 2023, and 2022, the Company recorded a provision for credit
losses of $8 and $0, respectively.
Inventories
– Inventories are valued at the lower of historical cost or net realizable value. Cost is determined using an average
cost method for tobacco leaf inventory and raw materials inventory. Standard cost is primarily used for finished goods inventory. Inventories
are evaluated to determine whether any amounts are not recoverable based on slow moving or obsolete condition and are written off or
reserved as appropriate.
Property,
plant and equipment – Plant and equipment are recorded at their acquisition cost and depreciated on
a straight-line basis over their estimated useful lives. Leasehold improvements are depreciated on a straight-line basis over the term
of the lease or the estimate useful life of the asset, whichever is shorter. Depreciation commences when the asset is placed in service.
The following table shows estimated useful lives of property, plant and equipment:
| |
|
|
| Classification |
|
Estimated
Useful Lives |
| Leasehold improvements |
|
shorter of 20 years or
lease term |
| Manufacturing equipment |
|
5 to 15 years |
| Office furniture, fixtures
and equipment |
|
3 to 10 years |
Acquisitions
- The Company accounts for acquisitions under the acquisition method of accounting for business combinations. Results
of operations of acquired companies are included in the Company’s results of operations as of the respective acquisition dates.
The purchase price of each acquisition is allocated to the net assets acquired based on estimates of their fair values at the date of
the acquisition. Any purchase price in excess of these net assets is recorded as goodwill. The allocation of purchase price in certain
cases may be subject to revision based on the final determination of fair values during the measurement period, which may be up to one
year from the acquisition date.
Discontinued
Operations - In determining whether a group of assets which has been disposed of (or is to be disposed of) should be presented
as a discontinued operation, the Company analyzes whether the group of assets being disposed of represented a component of the entity;
that is, whether it had historic operations and cash flows that were clearly distinguished (both operationally and for financial reporting
purposes). In addition, the Company considers whether the disposal represents a strategic shift that has or will have a major effect
on the Company’s operations and financial results.
The
assets and liabilities of a discontinued operation held for sale, other than goodwill, are measured at the lower of carrying amount or
fair value less cost to sell. The Company allocates interest to discontinued operations if the interest is directly attributable to the
discontinued operations or is interest on debt that is required to be repaid as a result of the disposal transaction.
Contingent
Consideration - Contingent consideration arising from a business acquisition is included as part of the purchase
price and is recorded at fair value as of the acquisition date. Subsequent to the acquisition date, the Company remeasures contingent
consideration arrangements at fair value at each reporting period until the contingency is resolved. The changes in fair value are recognized
within Other operating expenses (income), net in the Company’s Consolidated Statement of Operations and Comprehensive Loss. Changes
in fair values reflect new information about the likelihood of the payment of the contingent consideration and the passage of time. See
Note 3 “Business Acquisitions” for the contingent consideration arising from the acquisition of RX Pharmatech Ltd.
Goodwill
- Goodwill represents the excess of cost over the fair value of identifiable net assets of a business acquired and is
assigned to one or more reporting units. The Company tests its reporting unit’s goodwill for impairment at least annually as of
the measurement date year and between annual tests if an event occurs or circumstances change that would more-likely-than-not reduce
the fair value of a reporting unit below its carrying amount. The Company concluded an interim impairment trigger event occurred and
tested its goodwill for impairment during the quarter ended September 30, 2023 and concluded that goodwill impairment existed. No
goodwill remained as of December 31, 2023. See Note 2 “Discontinued Operations and Divestitures” for additional information.
Intangible
Assets – Definite lived intangible assets are recorded at cost and consist primarily of (1) expenditures incurred
with third-parties related to the processing of patent claims and trademarks with government authorities, as well as costs to acquire
patent rights from third-parties, (2) license fees paid for third-party intellectual property. The amounts capitalized relate to
intellectual property that the Company owns or to which it has rights to use. The Company’s capitalized intellectual property costs
are amortized using the straight-line method over the remaining statutory life of the patent assets in each of the Company’s patent
families, which have estimated expiration dates ranging from 2026 to 2043. Periodic maintenance or renewal fees are expensed as incurred.
Annual minimum license fees are charged to expense. License fees paid for third-party intellectual property are amortized on a straight-line
basis over the last to expire patents, which have expected expiration dates from 2028 through 2043.
The
Company believes that costs associated with becoming a signatory to the master settlement agreement “MSA”, costs related
to the acquisition of a predicate cigarette brand, and tobacco brand related trademarks have indefinite lives. At each reporting period,
the Company evaluates whether the nature and use of the asset continue to support the indefinite-lived classification.
Impairment
of Long-Lived Assets – The Company reviews the carrying value of its long-lived assets at each reporting period
to determine if impairment indicators are present in accordance with ASC 360-Property, plant, and equipment or ASC 350- Intangibles,
Goodwill, and Other.
Definite
lived intangible assets subject to amortization are reviewed for strategic importance and commercialization opportunity prior to expiration.
If it is determined that the asset no longer supports the Company’s strategic objectives and/or will not be commercially viable
prior to expiration, the asset is impaired. In addition, the Company will assess the expected future undiscounted cash flows for its
intellectual property based on consideration of future market and economic conditions, competition, federal and state regulations, and
licensing opportunities. If the carrying value of such assets are not recoverable, the carrying value will be reduced to fair value and
the difference is recorded as impairment.
Indefinite-lived
intangible asset carrying values are reviewed at least annually or more frequently if events or changes in circumstances indicate that
it is more likely than not that an impairment exists. The Company first performs a qualitative assessment and considers its current strategic
objectives, future market and economic conditions, competition, and federal and state regulations to determine if an impairment is more
likely than not. If it is determined that an impairment is more likely than not, a quantitative assessment is performed to compare the
asset carrying value to fair value.
Leases
– The Company determines if an arrangement is, or contains, a lease at inception and classifies it as operating
or finance. The Company has operating and finance leases for office and manufacturing facilities, machinery and vehicles. Finance lease
assets and corresponding liabilities are not material to the Consolidated Financial Statements.
Any
operating lease having a lease term greater than twelve months will be recognized on the Consolidated Balance Sheets as a right-of-use
(ROU) asset with an associated lease obligation—all other leases are considered short-term in nature and will be expensed on a
month-to-month basis. The ROU assets and lease obligations are recognized as of the commencement date at the net present value of the
fixed minimum lease payments for the lease term. The lease term is determined based on the contractual conditions, including whether
renewal options are reasonably certain to be exercised. The discount rate used is the interest rate implicit in the lease, if available,
or the Company’s incremental borrowing rate which is determined using a base line rate plus an applicable spread.
Refer
to Note 6 “Right-of-use Assets, Lease Obligations, and Other Leases” for additional information.
Fair
Value of Financial Instruments – FASB ASC 820 - Fair Value Measurements and Disclosures
establishes a valuation hierarchy for disclosure of the inputs to valuation used to measure fair value. This hierarchy prioritizes
the inputs into three broad levels as follows:
| · | Level
1 inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities; |
| · | Level
2 inputs are quoted prices for similar assets and liabilities in active markets or inputs
that are observable for the asset or liability, either directly or indirectly through market
corroboration, for substantially the full term of the financial instrument; and |
| · | Level
3 inputs are unobservable inputs based on the Company’s own assumptions used to measure
assets and liabilities at fair value. |
A financial asset’s
or a financial liability’s classification within the hierarchy is determined based on the lowest level input that is significant
to the fair value measurement. The Company estimates that the carrying amounts reported on the Consolidated Balance Sheets for cash and
cash equivalents, accounts receivable, contract assets, promissory note receivable, accounts payable and accrued expenses, and notes
and loans payable approximate their fair value due to the short-term nature of these items. Note 9 “Fair Value Measurements”
contains additional information on assets and liabilities recorded at fair value in the Consolidated Financial Statements.
Warrants
- The Company accounts for stock warrants as either equity instruments, derivative liabilities, or liabilities in accordance
with ASC 480, Distinguishing Liabilities from Equity (ASC 480) and ASC 815, Derivatives and Hedging (ASC
815) depending on the specific terms of the warrant agreement. The assessment considers whether the warrants are freestanding financial
instruments pursuant to ASC 480, meet the definition of a liability pursuant to ASC 480, and whether the warrants meet all of the requirements
for equity classification under ASC 815, including whether the warrants are indexed to the Company’s own ordinary shares and whether
the warrant holders could potentially require “net cash settlement” in a circumstance outside of the Company’s control,
among other conditions for equity classification. This assessment, which requires the use of professional judgment, is conducted at the
time of warrant issuance and as of each subsequent quarterly period end date while the warrants are outstanding.
Warrants
that the Company may be required to redeem through payment of cash or other assets outside its control are classified as liabilities
pursuant to ASC 480 and are initially and subsequently measured at their estimated fair values. Changes in subsequent measurement
fair value are recorded in Other income (expense), net of the Company’s Consolidated Statements of Operations and Comprehensive
Loss. For issued or modified warrants that meet all of the criteria for equity classification, the warrants are required to be recorded
as a component of additional paid-in capital at the time of issuance. For additional discussion on warrants, see Note 9 “Fair
Value Measurements” and Note 10 “Capital Raise and Warrants for Common Stock”.
Deemed
dividends associated with anti-dilution or down round provisions (commonly referred to as “ratchets”) represent the economic
transfer of value to holders of equity-classified freestanding financial instruments when these provisions are triggered. These deemed
dividends are presented as a reduction in net income or an increase in net loss available to common stockholders and a corresponding
increase to additional paid-in-capital resulting in no change to stockholders’ equity/deficit. The incremental value of modifications
to warrants as a result of the trigger of down round provisions in connection with equity financings was $3,029, the incremental value
of replacement warrants was $6,596, and the incremental value of modifications to warrants as a result of the trigger of anti-dilution
provisions of the JGB warrants was $367. Such amounts were determined using Monte-Carlo valuation models and are recorded as Deemed dividends
for the year ended December 31, 2023 on the Consolidated Statement of Operations and Comprehensive Loss.
Debt
Issued with Detachable Warrants - The Company considers guidance within ASC 470-20, Debt (ASC 470), ASC
480, and ASC 815 when accounting for the issuance of debt with detachable warrants. As described above under the caption “Warrants”,
the Company classifies stock warrants as either equity instruments, derivative liabilities, or liabilities depending on the specific
terms of the warrant agreement. In circumstances in which debt is issued with detachable warrants, the proceeds from the issuance of
the debt are first allocated to the warrants at their full estimated fair value with a corresponding debt discount. The remaining proceeds,
as further reduced by discounts (including those created by the bifurcation of embedded derivatives), is allocated to the debt. The Company
accounts for debt as liabilities measured at amortized cost and amortizes the resulting debt discount from the allocation of proceeds,
to interest expense using the effective interest method over the expected term of the debt instrument pursuant to ASC 835, Interest (ASC
835).
Embedded
Derivatives - The Company considers whether there are any embedded features in debt instruments that require
bifurcation and separate accounting as derivative financial instruments pursuant to ASC 815. Embedded derivatives are initially and subsequently
measured at fair value. With the exception of the bifurcated embedded conversion option as described in Note 13 “Debt”, the
embedded derivatives associated with the Company’s Senior Secured Credit Facility and Subordinated Note are not material.
Debt
Issuance Costs and Discounts - Debt issuance costs and discounts associated with the issuance of debt by the Company are
deferred and amortized over the term of the related debt. Debt issuance costs and discounts related to the Company’s Senior Secured
Credit Facility and Subordinated Note are recorded as a reduction of the carrying value of the related debt and are amortized to Interest
expense using the effective interest method over the period from the date of issuance to the maturity date, whichever is earlier. The
amortization of debt issuance costs and discounts are included in Debt related charges included in interest expense in the Consolidated
Statements of Cash Flows. Note 13 “Debt” contains additional information on the Company’s debt issuance costs and discounts.
Transfers
of Financial Assets – The Company accounts for transfers of financial assets as sales when it has surrendered control over
the related assets. Whether control has been relinquished requires, among other things, an evaluation of relevant legal considerations
and an assessment of the nature and extent of the Company’s continuing involvement with the assets transferred. Gains and losses
resulting from transfers reported as sales are included as a component of Other income (expense) in the Consolidated Statement of Operations
and Comprehensive Loss.
Gain/Loss
on Debt Extinguishment – Gain or loss on debt extinguishment is generally recorded upon an extinguishment of a debt instrument.
Gain or loss on extinguishment of debt is calculated as the difference between the reacquisition price and net carrying amount of the
debt, which includes unamortized debt issuance costs. Gains and losses on debt extinguishment are included as a component of Interest
expense in the Consolidated Statement of Operations and Comprehensive Loss.
Revenue
Recognition – The Company recognizes revenue when it satisfies a performance obligation by transferring control
of the product to a customer. For additional discussion on revenue recognition, refer to Note 17 “Revenue Recognition”.
Research
and Development – Research and development costs are expensed as incurred.
Stock
Based Compensation – The Company’s Omnibus Incentive Plan allows for various types of equity-based incentive
awards. Stock based compensation expense is based on awards that are expected to vest over the requisite service periods and are based
on the fair value of the award measured on the grant date. Vesting requirements vary for directors, officers, and employees. In general,
time-based awards fully vest after one year for directors and vest in equal annual installments over a three-year period for officers
and employees. Performance-based awards vest upon achievement of certain milestones. Forfeitures are accounted for when they occur.
Income
Taxes – The Company recognizes deferred tax assets and liabilities for any basis differences in its assets
and liabilities between tax and U.S. GAAP reporting, and for operating loss and credit carry-forwards.
As
a result of the Company’s history of cumulative net operating losses and the uncertainty of their future utilization, the Company
has established a valuation allowance to fully offset its net deferred tax assets as of December 31, 2023, and December 31, 2022.
The
Company’s federal and state tax returns for the years ended December 31, 2020 through December 31, 2022 are currently
open to audit under the statutes of limitations. There are no pending audits as of December 31, 2023.
Loss
Per Common Share – Basic loss per common share is computed using the weighted-average number of common shares
outstanding. Diluted loss per share is computed assuming conversion of all potentially dilutive securities. Potential common shares outstanding
are excluded from the computation if their effect is anti-dilutive. Refer to Note 16 “Loss Per Common Share” for additional
information.
Gain
and Loss Contingencies – The Company establishes an accrued liability for litigation and regulatory matters when
those matters present loss contingencies that are both probable and estimable. In such cases, there may be an exposure to loss in excess
of any amounts accrued. When a loss contingency is not both probable and estimable, the Company does not establish an accrued liability.
As a litigation or regulatory matter develops, the Company, in conjunction with any outside counsel handling the matter, evaluates on
an ongoing basis whether such matter presents a loss contingency that is probable and estimable. If, at the time of evaluation, the loss
contingency related to a litigation or regulatory matter is not both probable and estimable, the matter will continue to be monitored
for further developments that would make such loss contingency both probable and estimable. When a loss contingency related to a litigation
or regulatory matter is deemed to be both probable and estimable, the Company will establish an accrued liability with respect to such
loss contingency and record a corresponding amount of related expenses. The Company will then continue to monitor the matter for further
developments that could affect the amount of any such accrued liability.
The
Company maintains general liability insurance policies for its facilities. Under the terms of our insurance policies, in the case of
loss to a property, the Company follows the guidance in ASC 610-30, Other Income —Gains and Losses on Involuntary Conversions, for
the conversion of nonmonetary assets (the properties) to monetary assets (insurance recoveries). Under ASC 610-30, once the recovery
is deemed probable the Company recognizes an asset for the insurance recovery receivable in the Consolidated Balance Sheets, with corresponding
income that is offsetting to the casualty losses recorded in the Consolidated Statements of Operations and Comprehensive Loss. If the
insurance recovery is less than the amount of the casualty charges recognized, the Company will recognize a loss whereas if the insurance
recovery is greater than the amount of casualty loss recognized, the Company will only recognize a recovery up to the amount of the casualty
loss and will account for the excess as a gain contingency in accordance with ASC 450-30, Gain Contingencies. Business interruption
insurance is treated as a gain contingency. Gain contingencies are recognized when earned and realized, which typically will occur at
the time of final settlement or when cash is received.
Refer
to Note 12 “Commitments and Contingencies”.
Severance
charges - From time to time, the Company evaluates its resources and optimizes its business plan to align to changing
needs of executing on its strategy. These actions may result in voluntary or involuntary employee termination benefits. Voluntary termination
benefits are accrued when an employee accepts the related offer. Involuntary termination benefits are accrued upon the commitment to
a termination plan and the benefit arrangement is communicated to affected employees, or when liabilities are determined to be probable
and estimable, depending on the existence of a substantive plan for severance or termination. The following table summarizes the change
in accrued liabilities, presented within Other current liabilities and Other long-term liabilities Consolidated Balance Sheets:
| Balance at January 1, 2022 | |
$ | 238 | |
| Accruals | |
| 692 | |
| Cash payments | |
| (296 | ) |
| Balance at December 31, 2022 | |
| 634 | |
| Accruals | |
| 790 | |
| Reversal from settlement | |
| (168 | ) |
| Cash payments | |
| (870 | ) |
| Balance at December 31, 2023 | |
$ | 386 | |
| | |
December 31, | | |
December 31, | |
| | |
2023 | | |
2022 | |
| Current | |
$ | 386 | | |
$ | 349 | |
| Noncurrent | |
| — | | |
| 285 | |
| Total severance
liability | |
$ | 386 | | |
$ | 634 | |
| | |
Year Ended | |
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Sales, general, and administrative | |
$ | 401 | | |
$ | 692 | |
| Other operating expense, net | |
| 221 | | |
| — | |
| Total severance
charges | |
$ | 622 | | |
$ | 692 | |
Recent
Accounting Pronouncement(s) –
The
Company adopted ASU 2016-13, or ASC 326 Financial Instruments-Credit Losses, effective January 1, 2023 under a modified
retrospective approach. Under the current expected credit losses (“CECL”) model, the Company immediately recognizes an estimate
of credit losses expected to occur over the life of the financial asset at the time the financial asset is originated or acquired. Estimated
credit losses are determined by taking into consideration historical loss conditions, current conditions and reasonable and supportable
forecasts. Changes to the expected lifetime credit losses are recognized each period. The new guidance applies to the Company’s
trade receivables and contract asset balances. Due to the nature of business operations and contracts with customers, the Company has
historically not experienced significant bad debt expense or write-offs and as a result, the adoption of ASC 326 did not have a material
impact to the Company’s Consolidated Financial Statements. In connection with the adoption of ASC 326, the Company recorded a provision
for credit losses of $118 with an offsetting cumulative-effect adjustment to the opening balance of retained earnings as of January 1,
2023.
Accounting Guidance Not Yet
Elected or Adopted
In
November 2023, the FASB issued ASU 2023-07, Segment Reporting (Topic 280)-Improvements to Reportable Segment Disclosures.
The ASU enhances disclosure of significant segment expenses by requiring disclosure of significant segment expenses regularly provided
to the chief operating decision maker, extend certain annual disclosures to interim periods, and permits more than one measure of segment
profit or loss to be reported under certain conditions. The amendments are effective for the Company in years beginning after December 15,
2023, and interim periods within years beginning after December 15, 2024. Early adoption of the ASU is permitted, including adoption
in any interim period for which financial statements have not been issued. The Company is currently evaluating the impact that the adoption
of this ASU will have on its consolidated financial statements.
In
December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740)-Improvements to Income Tax Disclosures. The
ASU requires additional quantitative and qualitative income tax disclosures to allow readers of the consolidated financial statements
to assess how the Company’s operations, related tax risks and tax planning affect its tax rate and prospects for future cash flows.
For public business entities, the ASU is effective for annual periods beginning after December 15, 2024. The Company is currently
evaluating the impact that the adoption of this ASU will have on its consolidated financial statements.
We
consider the applicability and impact of all ASUs. If the ASU is not listed above, it was determined that the ASU was either not applicable
or would have an immaterial impact on our financial statements and related disclosures.
NOTE 2. – DISCONTINUED OPERATIONS AND DIVESTITURES
Provision for Impairment of GVB Hemp/Cannabis Business
During
the third quarter of 2023, the Company identified certain events and circumstances that could potentially be an impairment triggering
event for both the tobacco and hemp/cannabis reporting units in connection with (1) the announcement of initiating a process to
evaluate strategic alternatives for the Company’s assets, and (2) announcement of cost cut initiatives intended to yield significant
cash savings on an annual basis. The initiation of these two processes was in response to the sustained decline in the Company's market
capitalization, operating losses and negative cash flows from operations, and current liquidity position, and is intended to monetize
the value or more effectively expand the market reach of our products.
Accordingly,
the Company evaluated the impact on each of its reporting units to assess whether there was an impairment triggering event requiring
it to perform a goodwill impairment test. The Company had no recorded goodwill in its tobacco reporting unit. For the hemp/cannabis reporting
unit, as part of this impairment test, the Company considered certain qualitative factors, such as the Company’s performance, business
forecasts, and strategic plans. It reviewed key assumptions, including projected cash flows and future revenues. After reviewing the
qualitative assessment, the Company determined a quantitative assessment was required to be performed.
Using
the income approach, with the discount rate selected considering and capturing the related risk associated with the forecast, the Company
compared the fair value of the reporting unit to carrying value. Based on the results, the carrying value of the hemp/cannabis reporting
unit exceeded its fair value and the goodwill was determined to be impaired and $33,360, representing the full amount of goodwill recorded
to the hemp/cannabis reporting unit, was written off as impaired during the quarter ended September 30, 2023.
The
impairment charge is the result of the Company's Step-1 goodwill impairment test for the former hemp/cannabis reporting unit, which reflected
a decrease in the future expected cash flows related to bulk ingredient and CDMO+D product sales, along with increases in discount rates
to reflect the uncertainty of future cash flows. Estimating the fair value of goodwill requires the use of estimates and significant
judgments that are based on a number of factors, including unobservable level 3 inputs. These estimates and judgments may not be within
the control of the Company and accordingly it is reasonably possible that the judgments and estimates could change in future periods.
The
Company also evaluated the recoverability of its hemp/cannabis segment other intangible assets, net and long-lived assets to determine
whether any assets or asset groups were impaired. The Company determined that the carrying value of certain tradenames, patents and license
intangible assets, net were greater than their fair value, as these intangible assets related to hemp/cannabis operations. Therefore,
the Company recorded additional provision for impairment in the amount of $10,879, the Cookies license acquired in the second quarter
of 2023 was written-off and fully impaired in the amount of $3,037, and a loss on equity investments of $682. Additionally, through a
similar analysis, the Company recorded provision for impairment of $7,418 for property, plant and equipment and $5,038 for
operating lease right-of-use assets related to manufacturing and lab facilities. The undiscounted cash flow analysis and fair value determination
requires the use of estimates and significant judgments that are based on a number of factors, including unobservable level 3 inputs.
For the year ended December 31, 2023, total impairment charges for other intangibles and long-lived assets is $25,189.
Discontinued Operations and Divestiture of GVB Hemp/Cannabis
Business
On
November 20, 2023, the Company entered into an Equity Purchase Agreement (the “Purchase Agreement”) with Specialty Acquisition
Corporation, a Nevada corporation (the “Buyer”) pursuant to which the Company agreed to sell substantially all of its equity
interests in its GVB hemp/cannabis business (the “Purchased Interests”) for a purchase price of $2,250 (the “Purchase
Price”).
On
December 22, 2023, the Company and the Buyer entered into an Amendment to Equity Purchase Agreement (the “GVB Amendment”)
pursuant to which the Company and the Buyer increased the Purchase Price to $3,100 (the “New Purchase Price”) which consisted
of (i) a cash payment of $1,100 to the Company’s senior lender, on behalf of and at the direction of the Company and (ii) a
12% secured promissory note issued by the Buyer to the Company’s senior lender, on behalf of and at the direction of the Company,
in an aggregate principal amount of $2,000 (the “GVB Note”). Until repaid to the senior lender, the GVB Note is recorded
as a current asset and corresponding amount is pledged as Current portion of long term debt on the Consolidated Balance Sheet as of December 31,
2023.
The
parties previously agreed that the Company would retain any insurance proceeds received in connection with the fire at the Grass Valley
manufacturing facility, if any (the “Insurance Proceeds”) and up to the first $2,000 of the Insurance Proceeds would be used
to offset the Buyer’s portion of certain shared liabilities. Pursuant to the terms of the GVB Amendment, the Buyer will be entitled
to offset its portion of certain shared contingent liabilities up to $1,000; provided that, the Insurance Proceeds exceed $5,000.
In
connection with the closing of the transaction on December 22, 2023, but prior to any adjustments for Insurance Proceeds and certain
shared liabilities, after selling expenses of $434, the Company recognized a loss on disposal of discontinued operations of $58,521 during
the year ended December 31, 2023, which includes the third quarter impairment charges described above.
For
disposal transactions, a component of an entity that is anticipated to be sold in the future is reported in discontinued operations after
it meets the criteria for held-for-sale classification, and if the disposition represents a strategic shift that has (or will have) a
major effect on the entity's operations and financial results. The Company evaluated the quantitative and qualitative factors related
to the expected sale of the GVB hemp/cannabis business and exit from the hemp/cannabis space, and concluded that it met the held-for-sale
criteria and that all other conditions for discontinued operations presentation were not met until November 30, 2023. Property,
plant and equipment are not depreciated, and intangibles assets are not amortized once classified as held-for-sale.
As
a result, the operating results of the hemp/cannabis disposal group have been classified as discontinued operations in the Consolidated
Statements of Operations and Comprehensive Loss for all periods presented and the assets and liabilities of the hemp/cannabis disposal
group have been classified as assets and liabilities of discontinued operations in the Consolidated Balance Sheets at December 31,
2023 and 2022, respectively. See additional information in Note 3, “Business Acquisitions” related to GVB And RXP, including
the date of transactions and periods that operating results of the acquired business are included in the Consolidated Financial Statements.
The
assets and liabilities of a discontinued operation held for sale, other than goodwill, are measured at the lower of carrying amount or
fair value less cost to sell. Following the provision for impairment charges recorded during the third quarter of 2023 as described above,
the Company concluded the carrying value of assets and liabilities of the GVB hemp/cannabis business approximated fair value when deemed
held for sale based on the purchase price consideration of $3,100.
As
of December 31, 2023, all assets and liabilities of the hemp/cannabis disposal group are presented as current in the Consolidated
Balance Sheet as management believes the remaining disposal and exit from hemp/cannabis is deemed probable and will occur within one
year. The carrying amounts of the hemp/cannabis disposal group assets and liabilities that were classified as assets and liabilities
of discontinued operations held for sale were as follows:
| | |
December 31, | | |
December 31, | |
| | |
2023 | | |
2022 | |
| Cash and cash equivalents | |
$ | — | | |
$ | 815 | |
| Accounts receivable, net | |
| — | | |
| 4,278 | |
| Inventories | |
| — | | |
| 2,738 | |
| Insurance recoveries | |
| — | | |
| 5,000 | |
| Prepaid expenses and other current assets | |
| 9 | | |
| 815 | |
| Property, plant and equipment, net - current | |
| 1,207 | | |
| — | |
| Other current assets | |
| 38 | | |
| — | |
| Current assets of discontinued operations
held for sale | |
$ | 1,254 | | |
$ | 13,646 | |
| Property, plant and equipment, net | |
| — | | |
| 9,401 | |
| Operating lease right-of-use assets, net | |
| — | | |
| 1,732 | |
| Goodwill | |
| — | | |
| 33,160 | |
| Intangible assets, net | |
| — | | |
| 9,641 | |
| Investments | |
| — | | |
| 682 | |
| Other assets | |
| — | | |
| 166 | |
| Noncurrent assets of discontinued operations
held for sale | |
$ | — | | |
$ | 54,782 | |
| | |
| | | |
| | |
| Notes and loans payable - current | |
$ | 2 | | |
$ | 219 | |
| Operating lease obligations | |
| 1,083 | | |
| 429 | |
| Accounts payable | |
| 2,013 | | |
| 2,117 | |
| Accrued expenses | |
| 79 | | |
| 662 | |
| Accrued payroll | |
| — | | |
| 537 | |
| Deferred income | |
| 8 | | |
| 143 | |
| Other current liabilities | |
| — | | |
| 31 | |
| Current liabilities of discontinued operations
held for sale | |
$ | 3,185 | | |
$ | 4,138 | |
| Notes and loans payable | |
| — | | |
| 3,001 | |
| Operating lease obligations | |
| — | | |
| 1,430 | |
| Other long-term liabilities | |
| — | | |
| 172 | |
| Noncurrent liabilities of discontinued
operations held for sale | |
$ | — | | |
$ | 4,603 | |
| | |
| | | |
| | |
| Net (liabilities)
assets | |
$ | (1,931 | ) | |
$ | 59,687 | |
Net
loss from discontinued operations for year ended December 31, 2023 and 2022 was as follows:
| | |
Year Ended | |
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Revenues, net | |
$ | 42,113 | | |
$ | 21,610 | |
| Cost of goods sold | |
| 49,185 | | |
| 22,283 | |
| Gross loss | |
| (7,072 | ) | |
| (673 | ) |
| Operating expenses: | |
| | | |
| | |
| Sales, general and administrative | |
| 16,540 | | |
| 12,286 | |
| Research and development | |
| 3,010 | | |
| 2,983 | |
| Other
operating expense, net (1) | |
| 118 | | |
| 7,529 | |
| Loss on disposal of
discontinued operations | |
| 58,521 | | |
| — | |
| Total operating expenses | |
| 78,189 | | |
| 22,798 | |
| Operating loss from discontinued operations | |
| (85,261 | ) | |
| (23,471 | ) |
| Other income (expense): | |
| | | |
| | |
| Other income, net | |
| 65 | | |
| 66 | |
| Interest
expense (2) | |
| (438 | ) | |
| (298 | ) |
| Total other expense | |
| (373 | ) | |
| (232 | ) |
| Loss from discontinued operations before
income taxes | |
| (85,634 | ) | |
| (23,703 | ) |
| Provision (benefit) for income taxes | |
| 455 | | |
| (455 | ) |
| Net loss from discontinued operations | |
$ | (86,089 | ) | |
$ | (23,248 | ) |
(1) The Company recorded $25,189
of impairment charges in Other operating expenses, net and recorded $33,360 of Goodwill impairment from discontinued operations during
the three months ended September 30, 2023, which were reclassified to Loss on disposal of discontinued operations during the three
months ended December 31, 2023.
(2) The Company allocates interest
to discontinued operations if the interest is directly attributable to the discontinued operations or is interest on debt that is required
to be repaid as a result of the disposal transaction. Interest expense included in discontinued operations reflects an estimate of interest
expense related to the $3,100 principal balance of debt that is required to be repaid with the proceeds from the sale of the GVB hemp/cannabis
business.
The
components of discontinued operations “Other operating expenses, net” were as follows:
| | |
Year Ended | |
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Grass Valley fire: | |
| | | |
| | |
| Fixed asset write-offs | |
$ | — | | |
$ | 5,550 | |
| Inventory charges | |
| — | | |
| 3,998 | |
| Lease obligations | |
| — | | |
| 20 | |
| Professional services | |
| 407 | | |
| 36 | |
| Compensation & benefits | |
| — | | |
| 195 | |
| Insurance recoveries | |
| — | | |
| (5,000 | ) |
| Total Grass Valley fire | |
| 407 | | |
| 4,799 | |
| Severance | |
| 13 | | |
| — | |
| Impairment of intangible assets | |
| — | | |
| 1,453 | |
| Gain on change in contingent consideration | |
| (1,138 | ) | |
| — | |
| Needlerock Farms settlement | |
| 769 | | |
| — | |
| Impairment of inventory | |
| — | | |
| 237 | |
| Gain on sale or disposal of property, plant and equipment | |
| (64 | ) | |
| (6 | ) |
| Acquisition costs | |
| 131 | | |
| 1,046 | |
| Total other operating expenses, net | |
$ | 118 | | |
$ | 7,529 | |
Grass Valley fire
In
November 2022, there was a fire at our Grass Valley manufacturing facility in Oregon, which manufactures bulk ingredients, primarily
CBD isolate and distillate. The Company has incurred continuous expenses throughout 2023 related to consulting, legal and demolition
at this facility.
Cash
flow information from discontinued operations for years ended December 31, 2023 and 2022 was as follows:
| | |
Year Ended | |
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Cash used in operating activities | |
$ | 21,281 | | |
$ | 17,274 | |
| Cash used in investing activities | |
$ | 799 | | |
$ | 3,665 | |
| | |
| | | |
| | |
| Depreciation and amortization | |
$ | 2,443 | | |
$ | 1,566 | |
| Capital expenditures | |
$ | 3,752 | | |
$ | 2,752 | |
NOTE 3. – BUSINESS ACQUISITIONS
The
following acquisitions occurring during the years ended December 31, 2023, and 2022, respectively, are included in the Company’s
former hemp/cannabis reportable segment. Accordingly, the results of operations are reported as discontinued operations in the Consolidated
Statements of Operations and Comprehensive Loss for all periods presented. See Note 2 “Discontinued Operations and Divestitures”
for additional information.
RX Pharmatech, Ltd.
On
January 19, 2023, the Company acquired RX Pharmatech Ltd (“RXP”) pursuant to a share purchase agreement ("SPA”)
a privately held distributor of cannabinoids with 1,276 novel food applications with the U.K. Food Standards Agency (“FSA”).
RXP’s products include CBD isolate and numerous variations of finished products like gummies, oils, drops, candies, tinctures,
sprays, capsules and others.
The
initial consideration paid to acquire RXP included $200 in cash and $503 in common stock (consisting of 31,056 unregistered shares of
common stock), and an initial estimate of target working capital true-up of $286. The fair value of the Company’s common stock
issued as part of the consideration was determined based upon the opening stock price of the Company’s shares as of the acquisition
date. Additionally, the contingent consideration in the transaction represents the estimated fair value of the Company’s obligation,
under the share purchase agreement, to make additional equity based payments of up to $1,550 over the next three years based on specified
conditions being met, which has an initial fair value of contingent consideration of $1,138. The fair value of the aggregate consideration
in the transaction is $2,127.
Based
on the preliminary purchase price allocation, the assets acquired and liabilities assumed principally comprise $1,744 of intangible assets,
and other immaterial working capital items representing a net asset of $93 (net of cash acquired of $290). There was no excess purchase
price and therefore no goodwill recorded as part of the business combination. The determination of estimated fair value required management
to make significant estimates and assumptions based on information that was available at the time the Consolidated Financial Statements
were prepared.
Intangible
assets include the intellectual property associated with the 1,276 novel food applications with the FSA, which is determined to be indefinite
lived. The preliminary fair value was determined by utilizing the cost approach and considered market data to evaluate the replacement
cost per application. The intellectual property is included in the former hemp/cannabis reportable segment.
The
Company utilizes third-party valuation experts to assist in estimating the fair value of the contingent consideration and develops estimates
by considering weighted-average probabilities of likely outcomes and discounted cash flow analysis. These estimates require the Company
to make various assumptions about forecasted revenues and discount rates, which are unobservable and considered Level 3 inputs in the
fair value hierarchy. A change in these inputs to a different amount might result in a significantly higher or lower fair value measurement
at the reporting date.
The
following table provides quantitative information associated with the initial fair value measurement of the Company’s liabilities
for contingent consideration as of January 19, 2023:
| | |
Maximum Payout | | |
| | |
| |
Weighted Average | |
| Contingency Type | |
(undiscounted) | | |
Fair Value | | |
Unobservable Inputs | |
or Range | |
| Revenue-based payments | |
$ | 1,550 | | |
$ | 1,138 | | |
Discount rate | |
| 16% | |
| | |
| | | |
| | | |
Projected year(s) of payment | |
| 2024-2026 | |
During
the third quarter of 2023, the Company finalized amounts recorded as purchase price allocation and recorded measurement period adjustments
of $53, resulting from an increase of the working capital true-up amount based on final payment made to the sellers.
On
December 22, 2023, concurrent with the GVB divestiture (as described in Note 2) which included RXP, the Company entered into a binding
letter agreement to terminate its’ remaining contingent consideration obligation payable in shares under the SPA with the sellers
of RXP. Accordingly, for the year-ended December 31, 2023, the Company recognized within discontinued operations a gain of $1,138
in Other operating expenses, net in connection with the change in fair value of the contingent consideration.
GVB Biopharma
On
May 13, 2022, the Company entered into and closed the transactions contemplated by the Reorganization and Acquisition Agreement
(the “Reorganization Agreement”) with GVB. Under the terms of the Reorganization Agreement, the Company acquired substantially
all of the assets of GVB’s business dedicated to hemp-based cannabinoid extraction, refinement, contract manufacturing and product
development (the “Transaction”).
The
aggregate consideration for the Transaction consisted of (i) the assumption of approximately $4,637 of debt, (ii) the assumption
and direct payment of certain third-party transaction costs incurred by GVB in connection with the Transaction totaling approximately
$1,753 and (iii) the issuance to GVB of 2,193,334 unregistered shares of common stock of the Company (the “Shares”)
with a fair value of $51,653. The fair value of the Company’s common stock issued as part of the consideration was determined based
upon the opening stock price of the Company’s shares as of the acquisition date.
The
Transaction was structured as a tax-free re-organization pursuant to Internal Revenue Code Section 368(a)(1)(c). Accordingly, the
tax basis of net assets acquired retain their carry over tax basis and holding period in purchase accounting.
The
Company recorded provisional estimated fair values for the assets purchased, liabilities assumed and purchase consideration as of the
date of the acquisition during the second quarter of 2022, resulting in goodwill of $44,200. The determination of estimated fair value
required management to make significant estimates and assumptions based on information that was available at the time the Consolidated
Financial Statements were prepared.
Following
the initial acquisition accounting, the Company recorded final measurement period adjustments, in which the preliminary fair values of
the assets acquired and liabilities assumed as of May 13, 2022 were adjusted to reflect the ongoing acquisition valuation analysis
procedures of property and equipment, intangible assets, deferred taxes, and working capital adjustments. These adjustments resulted
in a combined reduction to goodwill of $10,840. The impact of depreciation and amortization to Operating loss recorded in the third quarter
of 2022 as a result of completing valuation procedures for property and equipment and intangible assets, that would have been recorded
in the prior period since the date of acquisition was $70.
The
following table presents management’s purchase price allocation:
| Cash | |
$ | 456 | |
| Accounts receivable | |
| 2,944 | |
| Inventory | |
| 3,551 | |
| Other assets | |
| 519 | |
| Property, plant & equipment | |
| 11,189 | |
| Operating leases right-of-use assets, net | |
| 1,231 | |
| Goodwill | |
| 33,360 | |
| Tradename | |
| 4,600 | |
| Customer relationships | |
| 5,800 | |
| Accounts payable and accrued expenses | |
| (2,777 | ) |
| Other current liabilities | |
| (944 | ) |
| Lease liabilities | |
| (1,259 | ) |
| Auto loans | |
| (387 | ) |
| Deferred tax liability | |
| (627 | ) |
| Bridge loan | |
| (4,250 | ) |
| Fair value of net assets acquired | |
$ | 53,406 | |
The
fair values of the assets acquired were determined using one of three valuation approaches: market, income or cost. The selection of
a particular method for a given asset depended on the reliability of available data and the nature of the asset, among other considerations.
The
market approach estimates the value for a subject asset based on available market pricing for comparable assets. The income approach
estimates the value for a subject asset based on the present value of cash flows projected to be generated by the asset. The projected
cash flows were discounted at a required rate of return that reflects the relative risk of the asset and the time value of money. The
projected cash flows for each asset considered multiple factors from the perspective of a marketplace participant including revenue projections
from existing customers, attrition trends, tradename life-cycle assumptions, marginal tax rates and expected profit margins giving consideration
to historical and expected margins. The cost approach estimates the value for a subject asset based on the cost to replace the asset
and reflects the estimated reproduction or replacement cost for the asset, less an allowance for loss in value due to depreciation or
obsolescence, with specific consideration given to economic obsolescence if indicated. These fair value measurement approaches are based
on significant unobservable inputs, including management estimates and assumptions.
Current Assets and Liabilities
The
fair value of current assets and liabilities, excluding inventory, was assumed to approximate their carrying value as of the acquisition
date due to the short-term nature of these assets and liabilities.
The
fair value of in-process and finished goods inventory acquired was estimated by applying a version of the income approach called the
comparable sales method. This approach estimates the fair value of the assets by calculating the potential revenue generated from selling
the inventory and subtracting from it the costs related to the completion and sale of that inventory and a reasonable profit allowance
for these remaining efforts. Based upon this methodology, the Company recorded the inventory acquired at fair value resulting in an increase
in inventory of $978, which was fully amortized in the three month period ended June 30, 2022 in the Consolidated Statement of Operations
and Comprehensive Loss.
Property, Plant and Equipment
The
fair value of PP&E acquired was estimated by applying the cost approach for personal property and leasehold improvements. The cost
approach was applied by developing a replacement cost and adjusting for economic depreciation and obsolescence.
Leases
The
Company recognized operating lease liabilities and operating lease right-of-use assets for office and manufacturing facilities in (i) Las
Vegas, Nevada (ii) Grass Valley, Oregon (iii) Prineville, Oregon, and (iv) Tygh Valley, Oregon, accordance with ASC 842,
Leases. All facilities were subsequently divested as part of the GVB sale discussed in Note 2 “Discontinued Operations and
Divestitures.”
The
following table summarizes the Company’s discount rate and remaining lease terms as of the acquisition date:
| Weighted average remaining lease term in years | |
| 3.8 | |
| Weighted average discount rate | |
| 8.3 | % |
The
Company concluded there were no off-market lease intangibles on the date of acquisition based on an evaluation of market rents per square
foot, geographic location and nature of use of the underlying asset, among other considerations.
Intangible assets
The
purchase price was allocated to intangible assets as follows:
| | |
| | |
Weighted Average | | |
| |
| | |
Fair Value | | |
Amortization Period | | |
Weighted Average | |
| Definite-lived Intangible
Assets | |
Assigned | | |
(Years) | | |
Discount
Rate | |
| Customer relationships | |
$ | 5,800 | | |
| 10 | | |
| 23.50 | % |
| Tradename | |
$ | 4,600 | | |
| Indefinite | | |
| 23.50 | % |
Customer Relationships
Customer
relationships represent the estimated fair value of contractual and non-contractual customer relationships GVB had as of the acquisition
date. These relationships were valued separately from goodwill at the amount that an independent third party would be willing to pay
for these relationships. The fair value of customer relationships was determined using the multi-period excess-earnings method, a form
of the income approach. The estimated useful life of the existing customer base was based upon the historical customer annual attrition
rate of 20%, as well as management’s understanding of the industry and product life cycles.
Tradename
Tradename
represents the estimated fair value of GVB’s corporate and product names. The acquired tradename was valued separately from goodwill
at the amount that an independent third party would be willing to pay for use of these names. The fair value of the tradename was determined
by utilizing the relief from royalty method, a form of the income approach, with a royalty rate of 1.0%. The GVB tradename was assumed
to have an indefinite useful life based upon long-term management expectations and future operating plans.
Deferred Taxes
The
Company determined the deferred tax position to be recorded at the time of the GVB acquisition in accordance with ASC Topic 740, Income
Taxes, resulting in recognition of deferred tax liabilities for future reversing of taxable temporary differences primarily
for intangible assets and property, plant and equipment. This resulted in a preliminary net deferred tax liability of $627, which includes
the carryover basis of historical recognized deferred tax assets, liabilities and valuation allowance.
The
net deferred tax liabilities recorded as a result of the acquisition of GVB was determined by the Company to also provide future taxable
temporary differences that allow for the Company to utilize certain previously fully reserved deferred tax assets. Accordingly, the Company
recognized a reduction to its valuation allowance resulting in a net tax benefit of approximately $434 for the year ended December 31,
2022.
Goodwill
The
excess of the purchase price over the fair value of net tangible and intangible assets acquired and liabilities assumed was allocated
to goodwill. A variety of factors contributed to the goodwill recognized, including the value of GVB’s assembled work force, the
incremental value resulting from GVB’s capabilities in hemp/cannabis, operational synergies across the plant science platform,
and the expected revenue growth over time that is attributable to increased market share from future products and customers. Goodwill
recorded in the transaction will be non-deductible.
Acquisition costs
During
the year ended December 31, 2023, direct costs incurred as a result of the acquisition of RXP were $130, compared to direct
costs incurred as a result of the acquisition of GVB of $1,046 during the year ended December 31, 2022. Acquisition costs are expensed
as incurred and included in Other operating expenses, net in the Consolidated Statements of Operations and Comprehensive Loss.
NOTE 4. – INVENTORIES
Inventories
at December 31, 2023 and 2022 consisted of the following:
| | |
December 31, | | |
December 31, | |
| | |
2023 | | |
2022 | |
| Raw materials | |
$ | 3,580 | | |
$ | 7,090 | |
| Work in process | |
| — | | |
| 3 | |
| Finished goods | |
| 766 | | |
| 177 | |
| | |
$ | 4,346 | | |
$ | 7,270 | |
During the year ended December 31, 2023,
the Company reserved certain leaf inventory totaling $7,720 resulting from restructuring initiatives implemented, as described in Note
18 “Other Operating Expenses, Net”. Inventory charges are included within Cost of goods sold on the Company’s Consolidated
Statement of Operations and Comprehensive Loss.
NOTE 5. – PROPERTY, PLANT AND EQUIPMENT, NET
Property,
plant and equipment, net at December 31, 2023 and 2022 consisted of the following:
| | |
December 31, | | |
December 31, | |
| | |
2023 | | |
2022 | |
| Leasehold improvements | |
$ | 262 | | |
$ | 232 | |
| Manufacturing equipment | |
| 7,254 | | |
| 6,780 | |
| Office furniture, fixtures and equipment | |
| 254 | | |
| 414 | |
| | |
| 7,770 | | |
| 7,426 | |
| Less: accumulated depreciation | |
| (4,377 | ) | |
| (3,734 | ) |
| Property, plant
and equipment, net | |
$ | 3,393 | | |
$ | 3,692 | |
Depreciation
expense was $852 and $673 for the year ended December 31, 2023 and 2022, respectively.
NOTE 6. – RIGHT-OF-USE ASSETS, LEASE OBLIGATIONS,
AND OTHER LEASES
The
Company leases a manufacturing facility in Mocksville, North Carolina and an inventory storage facility in Winston-Salem, North Carolina.
On
January 1, 2023, the Company signed the lease agreement for the inventory storage facility. The lease has an initial monthly base
rent of $15 (escalating 3.0% annually after the first year), an initial term of 36 months – with two twenty-four-month optional
renewal options at the Company’s discretion.
On
March 31, 2023, the Company extended the lease terms for its manufacturing facility. As a result of this lease modification, the
Company re-measured the lease liability and adjusted the ROU asset on the modification dates.
The
following table summarizes the Company’s discount rate and remaining lease terms as of December 31, 2023:
| Weighted average remaining lease term in years | |
| 5.9 | |
| Weighted average discount rate | |
| 9.0 | % |
Future
minimum lease payments as of December 31, 2023 are as follows:
| 2024 | |
$ | 396 | |
| 2025 | |
| 403 | |
| 2026 | |
| 422 | |
| 2027 | |
| 430 | |
| 2028 | |
| 449 | |
| Thereafter | |
| 414 | |
| Total lease payments | |
| 2,514 | |
| Less: imputed interest | |
| (585 | ) |
| Present value of lease liabilities | |
| 1,929 | |
| Less: current portion of lease liabilities | |
| (231 | ) |
| Total long-term
lease liabilities | |
$ | 1,698 | |
Operating
lease costs for the year ended December 31, 2023 and 2022, were $475 and $288, respectively.
Supplemental
cash flow information for leases for fiscal years 2023 and 2022 are comprised of the following:
| | |
December 31, | | |
December 31, | |
| | |
2023 | | |
2022 | |
| Cash paid for operating leases | |
$ | 436 | | |
$ | 276 | |
| Assets acquired under operating leases | |
$ | 1,602 | | |
$ | — | |
NOTE 7. – INTANGIBLE ASSETS, NET
Our intangible assets at December 31, 2023 and 2022 consisted
of the following:
| | |
Gross | | |
Accumulated | | |
| | |
Net Carrying | |
| December 31, 2023 | |
Carrying
Amount | | |
Amortization | | |
Impairment | | |
Amount | |
| Definite-lived: | |
| | | |
| | | |
| | | |
| | |
| Patent | |
$ | 2,913 | | |
$ | (1,622 | ) | |
$ | (487 | ) | |
$ | 804 | |
| License fees | |
| 4,165 | | |
| (1,666 | ) | |
| (65 | ) | |
| 2,434 | |
| Total amortizing
intangible assets | |
$ | 7,078 | | |
$ | (3,288 | ) | |
$ | (552 | ) | |
$ | 3,238 | |
| Indefinite-lived: | |
| | | |
| | | |
| | | |
| | |
| Trademarks | |
$ | 134 | | |
| NA | | |
$ | - | | |
$ | 134 | |
| MSA signatory costs | |
| 2,202 | | |
| NA | | |
| - | | |
| 2,202 | |
| License fee for predicate
cigarette brand | |
| 350 | | |
| NA | | |
| - | | |
| 350 | |
| Total indefinite-lived
intangible assets | |
$ | 2,686 | | |
| NA | | |
$ | - | | |
$ | 2,686 | |
| Total intangible
assets, net | |
$ | 9,764 | | |
$ | (3,288 | ) | |
$ | (552 | ) | |
$ | 5,924 | |
| | |
Gross | | |
Accumulated | | |
Net Carrying | |
| December 31, 2022 | |
Carrying
Amount | | |
Amortization | | |
Amount | |
| Definite-lived: | |
| | | |
| | | |
| | |
| Patent | |
$ | 5,723 | | |
$ | (3,588 | ) | |
$ | 2,135 | |
| License fees | |
| 3,801 | | |
| (1,417 | ) | |
| 2,384 | |
| Total amortizing
intangible assets | |
$ | 9,524 | | |
$ | (5,005 | ) | |
$ | 4,519 | |
| Indefinite-lived: | |
| | | |
| | | |
| | |
| Trademarks | |
| | | |
| | | |
$ | 141 | |
| MSA signatory costs | |
| | | |
| | | |
| 2,202 | |
| License fee for predicate
cigarette brand | |
| | | |
| | | |
| 350 | |
| Total indefinite-lived
intangible assets | |
| | | |
| | | |
$ | 2,693 | |
| Total intangible
assets, net | |
| | | |
| | | |
$ | 7,212 | |
Aggregate
intangible asset amortization expense comprises of the following:
| | |
Year Ended | |
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Cost of goods sold | |
$ | 11 | | |
$ | 10 | |
| Research and development | |
| 644 | | |
| 609 | |
| Total amortization
expense | |
$ | 655 | | |
$ | 619 | |
During
the years ended December 31, 2023 and 2022, the Company incurred impairment charges of $1,375 and $35, respectively, related to
write-downs and disposals of patents, licenses and trademarks as a result of a shift in strategy related to the nature and use of the
related assets. Impairment charges during the year-ended December 31, 2023 consisted of $552 for patents and trademarks the Company
continues to hold but does not align with its current strategy, $772 was related to disposals of patents abandoned from future maintenance
and renewal and $51 was related to disposals of trademarks abandoned. The Company also disposed of $1,501 of patents that had a net book
value of $0.
The
impairment charges are included in Other operating expenses, net on the Company’s Consolidated Statements of Operations and Comprehensive
Loss.
Estimated
future intangible asset amortization expense based on the carrying value as of December 31, 2023 is as follows:
| | |
2024 | | |
2025 | | |
2026 | | |
2027 | | |
2028 | | |
Thereafter | |
| Amortization expense | |
$ | 422 | | |
$ | 410 | | |
$ | 351 | | |
$ | 365 | | |
$ | 321 | | |
$ | 1,369 | |
NOTE 8. – INVESTMENTS & OTHER ASSETS
Panacea Investment – Promissory Note:
On
June 30, 2021, the Company entered into a Promissory Note Exchange Agreement with Panacea Life Sciences Holdings, Inc. (“PLSH”)
as a component of various investment transactions with PLSH. The promissory note was issued in the amount of $4,300 (the “Promissory
note receivable”) with a maturity date of June 30, 2026 and a 0% interest rate. The Promissory note receivable is with J&N
Real Estate Company, L.L.C., a related party of Panacea and is fully secured by a first priority lien on Panacea’s headquarters
located in Golden, Colorado.
The
Promissory note receivable was originally valued at $3,684 ($4,300 face value less $616 discount) and is included within the Consolidated
Balance Sheets as “Other Assets.” Subsequently, on December 31, 2022 the Company and PLSH entered into a settlement
agreement in which the Company agreed to a reduction to the face value of the Promissory note receivable of $500, in exchange for resolution
to all contractual requirements surrounding the investment and business relationship. Accordingly, the Company recognized an extinguishment
charge of note receivable of $500 less adjusted discount of $51 during the year-ended December 31, 2022.
As
of October 16, 2023, the $3,800 Promissory note receivable was fully assigned in connection with the Senior Secured Credit Facility
Amendment and Waiver. The remaining discount of $305 was extinguished and after recognizing consideration of $2,600, resulted in a loss
on transfer of financial asset of $895 recorded as a component of Other income (expense) on the Consolidated Statement of Operations
and Comprehensive Loss. Refer to Note 13 “Debt.” Through the date of assignment, the Company intended to hold the remaining
outstanding Promissory note receivable to maturity and the associated discount will be amortized into interest income over the term of
the note.
NOTE 9. – FAIR VALUE MEASUREMENTS
Assets and Liabilities Measured at Fair Value on a Recurring Basis
Fair
value measurement standards apply to certain financial assets and liabilities that are measured at fair value on a recurring basis (each
reporting period). For the Company, these financial assets and liabilities include its short-term investment securities and equity investments.
The Company does not have any nonfinancial assets or liabilities that are measured at fair value on a recurring basis.
The
following table presents information about our assets and liabilities measured at fair value at December 31, 2023 and 2022,
and indicates the fair value hierarchy of the valuation techniques the Company utilized to determine such fair value:
| | |
Fair Value | |
| | |
December 31, 2023 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | | |
Total | |
| Liabilities | |
| | |
| | |
| | |
| |
| Detachable warrants | |
$ | — | | |
$ | — | | |
$ | 1,350 | | |
$ | 1,350 | |
| Derivative liability | |
| — | | |
| — | | |
| 557 | | |
| 557 | |
| Total liabilities | |
$ | — | | |
$ | — | | |
$ | 1,907 | | |
$ | 1,907 | |
| | |
Fair Value | |
| | |
December 31, 2022 | |
| | |
Level 1 | | |
Level 2 | | |
Level 3 | | |
Total | |
| Assets | |
| | |
| | |
| | |
| |
| Money market funds | |
$ | 10,163 | | |
$ | — | | |
$ | — | | |
$ | 10,163 | |
| Corporate bonds | |
| — | | |
| 7,031 | | |
| — | | |
| 7,031 | |
| U.S. treasury securities | |
| — | | |
| 999 | | |
| — | | |
| 999 | |
| Total assets | |
$ | 10,163 | | |
$ | 8,030 | | |
$ | — | | |
$ | 18,193 | |
Money
market mutual funds are valued at their daily closing price as reported by the fund. Money market mutual funds held by the Company are
open-end mutual funds that are registered with the SEC that generally transact at a stable $1.00 Net Asset Value (“NAV”)
representing its estimated fair value. On a daily basis the fund’s NAV is determined by the fund based on the amortized cost of
the funds underlying investments. The Company classifies its money market funds within Level 1 because it uses quoted market prices to
determine their fair value. The Company classifies its commercial paper, corporate notes, certificates of deposit, and U.S. government
bonds within Level 2 because it uses quoted prices for similar assets or liabilities in active markets and each has a specified term
and all level 2 inputs are observable for substantially the full term of each instrument.
Corporate
bonds are valued using pricing models maximizing the use of observable inputs for similar securities.
The
following tables set forth a summary of the Company’s available-for-sale debt securities from amortized cost basis to fair value
as of December 31, 2022:
| | |
Available for Sale Debt Securities | |
| | |
December 31, 2022 | |
| | |
Amortized | | |
Gross | | |
Gross | | |
| |
| | |
Cost | | |
Unrealized | | |
Unrealized | | |
Fair | |
| | |
Basis | | |
Gains | | |
Losses | | |
Value | |
| Corporate bonds | |
$ | 7,143 | | |
$ | — | | |
$ | (112 | ) | |
$ | 7,031 | |
The
following table sets forth a summary of the Company’s available-for-sale debt securities at amortized cost basis and fair value
by contractual maturity as of December 31, 2022:
| | |
December 31, 2022 | |
| | |
Amortized | | |
| |
| | |
Cost Basis | | |
Fair Value | |
| Due in one year or less | |
$ | 7,143 | | |
$ | 7,031 | |
The
Company recognized interest income on short-term investment securities recorded in Interest income, net on the Consolidated Statement
of Operations and Comprehensive Loss during the years ended December 31, 2023 and 2022 of $52 and $546, respectively.
Detachable Warrants
The
following table sets forth a summary of the changes in fair value of the Company’s stock warrants accounted for as liabilities
(Level 3 asset) for the period ended December 31, 2023:
| Fair value measurement at January 1, 2023 | |
$ |
— | |
| Initial measurement (see Note 1 and 10) | |
| 4,214 | |
| Fair value measurement adjustment | |
| (364 | ) |
| JGB redemption of 166,667 warrants | |
| (2,500 | ) |
| Fair value measurement at December 31, 2023 | |
$ | 1,350 | |
The
Omnia detachable warrants were measured at December 31, 2023 using a Monte Carlo valuation model with the following assumptions:
| Risk-free interest rate per year | |
| 4.6 |
% |
| Expected volatility per year | |
| 90.9 |
% |
| Expected dividend yield | |
| — |
% |
| Contractual expiration | |
| 6.6
years |
|
| Exercise price | |
$ | 12.828 |
|
| Stock price | |
$ | 0.19 |
|
The
detachable warrants are measured at fair value using certain estimated factors which are classified within Level 3 of the valuation hierarchy.
Significant unobservable inputs that are used in the fair value measurement of the Company’s detachable warrants include the volatility
factor, anti-dilution provisions, and contingent put option. Significant increases or decreases in the volatility factor would have resulted
in a significantly higher or lower fair value measurement. Additionally, a change in probability regarding the anti-dilution provision
or put option would have resulted in a significantly higher or lower fair value measurement.
Derivative Liability
The
derivative liability related to the debentures and embedded conversion option using was measured at December 31, 2023 using a binomial
lattice valuation model under a “with and without” approach and contained the following assumptions:
| Stock price volatility | |
| 104.1 |
% |
| Expected term | |
| 2.2
years |
|
| Stock price as of measurement date (per share) | |
$ | 0.19 |
|
| Risk-free rate | |
| 4.3 |
% |
| Credit rating | |
| CCC |
|
| Market yield (credit risk) | |
| 13.8 |
% |
The
debentures and derivative liability are measured at fair value using certain estimated factors which are classified within Level 3 of
the valuation hierarchy. Significant unobservable inputs that are used in the fair value measurement of the Company’s derivative
liability include a decrease/increase in our stock price, stock price volatility, credit rating, and simulated stock price upon conversion
could significantly change the fair value measurement as either an increase or decrease.
Assets and Liabilities
Measured at Fair Value on a Nonrecurring Basis
Fair
value standards also apply to certain assets and liabilities that are measured at fair value on a nonrecurring basis. During the years
ended December 31, 2023 and 2022, the Company did not have any financial assets or liabilities
measured at fair value on a nonrecurring basis.
NOTE 10. – CAPITAL RAISES AND WARRANTS FOR COMMON
STOCK
The
following tables summarize the Company’s warrant activity:
| Warrants outstanding at January 1, 2022 | |
— | |
| Exercised | |
— | |
| Issued | |
| 1,138,212 | |
| Warrants outstanding at December 31, 2022 | |
| 1,138,212 | |
| Exercised | |
| (18,084,052 | ) |
| Abandoned | |
| (325,205 | ) |
| Issued | |
| 65,028,421 | |
| Warrants outstanding at December 31, 2023 | |
| 47,757,376 | |
| | |
# of warrants
outstanding | | |
Exercise
price | | |
Expiration date |
| July 2022 RDO warrants | |
| 65,042 | | |
$ | 30.75 | | |
July 25, 2027 |
| Senior Secured Credit Facility - JGB | |
| 330,294 | | |
$ | 12.828 | | |
September 3, 2028 |
| Subordinated Note - Omnia | |
| 45,000 | | |
$ | 12.828 | | |
September 3, 2030 |
| July 6, 2023 RDO warrants | |
| 1,557,268 | | |
$ | 0.2042 | | |
January 10, 2029 |
| July 19, 2023 RDO warrants | |
| 1,225,000 | | |
$ | 0.2042 | | |
July 20, 2028 |
| October 2023 CMPO warrants | |
| 13,500,000 | | |
$ | 0.2042 | | |
October 19, 2028 |
| Inducement warrants | |
| 31,034,772 | | |
$ | 0.2042 | | |
February 15, 2029 |
| | |
| 47,757,376 | | |
| | | |
|
2022 Registered Direct Offering & Warrant Repricing
On
July 21, 2022, the Company and certain institutional investors (the “July 2022 Investors”) entered into a securities
purchase agreement (the “July 2022 Securities Purchase Agreement”) relating to the issuance and sale of shares of common
stock pursuant to a registered direct offering (the “July 2022 Registered Offering” and, together with the July 2022
Private Placement (as defined below), the “July 2022 Offerings”). The July 2022 Investors purchased approximately
$35,000 of shares, consisting of an aggregate of 1,138,221 shares of common stock at a purchase price of $30.75 per
share, subject to certain restrictions. The net proceeds to the Company from the July 2022 Offerings, after deducting the fees and
the Company’s offering expenses, were $32,484.
Pursuant
to the July 2022 Securities Purchase Agreement, in a concurrent private placement, the Company issued and sold to the July 2022
Investors warrants (the “July 2022 Warrants”) to purchase up to 1,138,221 shares of common stock (the “July 2022
Private Placement”). The July 2022 Warrants were exercisable immediately upon issuance at an exercise price of $30.75 per
share of common stock, subject to adjustment in certain circumstances, and expire on July 25, 2027.
As
a result of the June 19, 2023 offering described below, certain of the July 2022 Investors and the Company entered a warrant
reprice letter (the “Warrant Repricing”) and agreed to reduce the exercise price on the previously issued 747,974 warrants
owned by the investors participating in the June 19, 2023 offering from $30.75 to $7.05 and to add a provision in the warrants that
upon any subsequent equity sales at a price per share lower than the then effective exercise price of such warrants, such exercise price
shall be lowered to such price at which the shares were offered. The Warrant Repricing is accounted for as a modification of a freestanding
equity-classified written call option, and therefore resulted in an immediate and incremental increase of approximately $2,025 in the
estimated fair value of the related 747,974 warrants, recorded as a component of Capital in excess of par value, with an offsetting equal
amount recorded as equity issuance costs.
As
a result of subsequent offerings, the exercise price on 747,974 warrants was automatically adjusted triggering non-cash deemed dividends
as a result of the down-round adjustments. In July 2023 the exercise price was adjusted to $3.80 and $2.42, respectively, and further
in October 2023 was adjusted to $0.525. All of the outstanding warrants were subsequently exercised in connection with the Warrant
Inducement Offering.
The
remaining 390,247 previously issued July 2022 Warrants were not repriced and on December 7, 2023, the Company was provided
notice of irrevocable abandonment of 325,205 warrants. Accordingly, the Company has 65,042 remaining July 2022 Warrants with an
exercise price of $30.75 and an expiration date of July 25, 2027.
June 19, 2023
Registered Direct Offering
On
June 19, 2023, the Company and certain investors entered into a securities purchase agreement relating to the issuance and sale
of shares of approximately $5,300 of shares and warrants, consisting of an aggregate of 747,974 shares of common stock and 747,974 warrants
to purchase an equal number of shares, at a purchase price of $7.05 per unit. The net proceeds to the Company from the offering were
approximately $4,800.
The
warrants were exercisable immediately upon issuance at an exercise price of $7.05 per share of common stock, expire on June 22,
2028 and are subject to adjustment in certain circumstances, including upon any subsequent equity sales at a price per share lower than
the then effective exercise price of such warrants, then such exercise price shall be lowered to such price at which the shares were
offered.
As
a result of the subsequent offerings, the exercise price on the 747,974 warrants was automatically adjusted triggering non-cash
deemed dividends as a result of the down-round adjustments. In July 2023, the exercise price was adjusted to $3.80 and $2.42, respectively,
and further in October 2023 was adjusted to $0.525. All of the outstanding warrants were subsequently exercised in connection with
the Warrant Inducement Offering and no warrants issued in the June 2023 registered direct offering remain outstanding as of December 31,
2023.
July 6, 2023
Registered Direct Offering.
On July 6, 2023, the
Company and certain investors entered into a securities purchase agreement relating to the issuance and sale of approximately $3,000
of shares and warrants, consisting of an aggregate of 778,634 shares of common stock and 1,557,268 warrants to purchase an equal number
of shares, at a purchase price of $3.80 per unit. The warrants became exercisable six months after issuance at an exercise price of $3.80
per share of common stock and expire on January 10, 2029. The net proceeds to the Company from the offering were approximately $2,722.
As
a result of subsequent offerings, the exercise price on 1,557,268 warrants was automatically adjusted triggering non-cash deemed
dividends as a result of the down-round adjustments. In July 2023, the exercise price was adjusted to $2.42 and further in October 2023
was adjusted to $0.525. 1,557,368 warrants that remained outstanding as of December 31, 2023 were subsequently exercised in connection
with the Warrant Inducement Offering in January 2024 (see Note 21 “Subsequent Events.”)
July 19, 2023
Registered Direct Offering.
On July 19, 2023, the
Company and certain investors entered into a securities purchase agreement relating to the issuance and sale of approximately $11,700
of shares and warrants, consisting of an aggregate of 4,373,219 shares of common stock and 8,746,438 warrants to purchase an equal number
of shares, at a purchase price of $2.67 per unit. The warrants were exercisable immediately at an exercise price of $2.42 per share of
common stock and expire five years after issuance. The net proceeds to the Company from the offering were approximately $10,742.
As
a result of a subsequent offering, the exercise price on 8,746,438 warrants was automatically adjusted triggering non-cash deemed
dividends as a result of the down-round adjustment. In October 2023 the exercise price was adjusted to $0.525. 7,521,438 of the
warrants were subsequently exercised in connection with the Warrant Inducement Offering through December 31, 2023. 1,225,000 warrants
remained outstanding with an exercise price of $0.1765 and an expiration date of July 19, 2028, of which 775,000 were subsequently
exercised in January 2024 (see Note 21 “Subsequent Events.”).
October 2023-
Public Equity Offering
On
October 17, 2023, the Company entered into a securities purchase agreement with certain investors, pursuant to which the Company
agreed to sell and issue, in a registered public offering, (i) an aggregate of 7,600,000 shares of the Company’s
common stock, par value $0.00001 per share, (ii) warrants to purchase 20,000,000 shares of common stock (the “October Warrants”)
and (iii) pre-funded warrants to purchase 2,400,000 shares of common stock (the “Pre-Funded Warrants”). The
Common Warrants had an exercise price of $0.525, are immediately exercisable and have a term of exercise equal to five years following
the original issuance date. The Pre-Funded Warrants have an exercise price of $0.0001, are immediately exercisable and will be able to
be exercised at any time after their original issuance until such Pre-Funded Warrants are exercised in full. The shares were offered
at a combined public offering price of $0.525 per share and two accompanying October Warrants. The Pre-Funded Warrants were
offered at a combined public offering price of $0.5249 per Pre-Funded Warrant and two accompanying October Warrants.
In
addition, the Company issued the placement agent warrants to purchase up to 1,000,000 shares of common stock (equal to 10%
of the aggregate number of shares and Pre-Funded Warrants sold in the offering) at an exercise price of $0.65625, which represents 125%
of the public offering price per share and accompanying October Warrant. The placement agent agreed not to exercise the such warrants
until the Company subsequently increases its authorized shares of common stock.
The
offering closed on October 19, 2023 with gross proceeds to the Company of approximately $5,250, before deducting the placement agent
fees of $367 and other offering expenses payable by the Company of approximately $288. As a result of the offering, the exercise
price on 11,799,654 previously outstanding warrants were automatically adjusted from $2.42 per share to $0.525 per
share.
The
Pre-Funded Warrants were subsequently exercised on a cashless basis in October 2023, resulting in issuance of 2,399,512 shares
of common stock. 3,800,000 of the warrants were subsequently exercised in connection with the Warrant Inducement Offering through
December 31, 2023. 13,500,000 warrants remained outstanding with an exercise price of $0.1765 and an expiration date of October 19,
2028, of which 10,800,000 were subsequently exercised in January 2024 (see Note 21 “Subsequent Events.”).
Warrant Inducement
Offering
On
November 28, 2023, the Company commenced a warrant inducement offering with the holders of the Company’s outstanding 31,779,654
warrants consisting of: (i) the common stock purchase warrants of the Company issued on or about June 22, 2023; (ii) the
common stock purchase warrants of the Company issued on or about July 10, 2023; (iii) the common stock purchase warrants of
the Company issued on or about July 21, 2023; and/or (iv) the common stock purchase warrants of the Company issued on or about
October 19, 2023 (collectively, the “Existing Warrants”), which Existing Warrants were exercisable for an equal number
of shares of common stock at an exercise price of $0.525. The Company agreed to issue new warrants (the “Inducement Warrants”)
to purchase up to a number of shares of common stock equal to 200% of the number of shares of common stock issued pursuant to the exercise
by the holders of the Existing Warrants during the inducement period, for cash, at a reduced exercise price equal to the Nasdaq Minimum
Price (as defined in Nasdaq Listing Rule 5635(d)).
For
the period from November 28, 2023 to December 31, 2023, the Company entered into warrant inducement agreements with certain
holders of the Existing Warrants to purchase an aggregate of 15,517,386 shares of common stock at a reduced exercise price of $0.215.
Pursuant to the warrant inducement agreements, the exercising holders of the Existing Warrants received 31,034,772 Inducement Warrants
and the Company received aggregate gross proceeds of approximately $3,336 from the exercise of the Existing Warrants before deducting
the placement agent fees of $234 and other offering expenses payable by the Company of approximately $58.
As a result of the inducement and subsequent exercise, the Company determined the incremental fair value provided to the holders using
Black Scholes and Monte Carlo models as (i) $883 increase in fair value due to the adjustment in exercise price of Existing Warrants
attributable to down round pricing protection (ii) $6,596 fair value of Inducement Warrants issued to the holders that exercised
Existing Warrants. The incremental fair value is recorded as non-cash deemed dividend. The proceeds of the warrant inducement and issuance
of common stock are recorded as Capital in excess of par value. Refer to Note 21 “Subsequent Events.”
March 2023 JGB
Warrants
In connection with the sale
of the Debentures as described in Note 13 “Debt”, the Company issued the JGB Warrants to purchase up to 333,334 shares of
common stock for an exercise price of $19.125 per share. The JGB Warrants are exercisable for five years from September 3, 2023,
at an exercise price of $19.125 per share, determined as a 50% premium to the VWAP on the closing date, subject, with certain exceptions,
to adjustments in the event of stock splits, dividends, subsequent dilutive offerings and certain fundamental transactions. The JGB warrants
initial fair value of $4,475 net of issuance costs of $139 (see Note 9 “Fair Value Measurements”), of which half of the warrants
meet the criteria for liability classification due to a contingent put option which allows the holder to require that the Company redeem
the warrants in cash for a purchase price equal to $15.00 upon certain conditional events such as change in control or event of default.
Accordingly, at issuance half of the warrants with the put provision are classified as Other long-term liabilities on the Consolidated
Balance Sheets in the amount of $2,898 whereas the remainder of the warrants without the put provision are equity classified and recorded
as a component of Capital in excess of par value in the amount of $1,577. The valuation assumptions of the warrants at issuance, as detailed
below, are the same for all warrants except for the put provision which derives a greater fair value.
As
a result of the June 19, 2023 offering, the Company’s outstanding JGB warrants to purchase up to 333,334 shares of the Company’s
common stock for an exercise price of $19.125 per share were automatically adjusted to be $12.828 exercise price for up to 496,960 shares
of common stock. As a result of the anti-dilution provision being triggered, the Company recognized a non-cash deemed dividend of $367
in connection with these adjustments, recorded on the Consolidated Statement of Operations and Comprehensive Loss and within Capital
in excess of par value (as the Company has an accumulated deficit and therefore the deemed dividend is treated as paid out of Capital
in excess of par value). There are no further anti-dilution adjustments on such warrants.
In
connection with the Senior Secured Credit Facility Amendment and Waiver, the Company redeemed 166,667 of such warrants for an aggregate
put price equal to $2,500. See Note 13 “Debt.”
The
JGB detachable warrants were valued at the closing dates of the Senior Secured Credit Facility using a Monte Carlo valuation model with
the following assumptions:
| Risk-free interest rate per year | |
| 4.2 |
% |
| Expected volatility per year | |
| 88.1 |
% |
| Expected dividend yield | |
| — |
% |
| Contractual expiration | |
| 5.5
years |
|
| Exercise price | |
$ | 19.125 |
|
| Stock price | |
$ | 13.65 |
|
March 2023 Omnia
Warrants
In connection with the Subordinated
Note as described in Note 13 “Debt”, the Company issued to Omnia, the Omnia Warrants to purchase up to 45,000 shares of the
Company’s common stock (the “Omnia Warrants”). The Omnia Warrants are exercisable for seven years from September 3,
2023, at an exercise price of $12.828 per share, subject, with certain exceptions, to adjustments in the event of stock splits, dividends,
subsequent dilutive offerings and certain fundamental transactions. The Omnia warrants initial fair value was $1,316 (see Note 9 “Fair
Value Measurements”), and meet the criteria for liability classification due to contingent put option which allows the holder to
require that the Company redeem the warrants in cash for a purchase price equal to $30.00 upon certain conditional events such as change
in control or event of default. The Omnia warrants are classified as Other long-term liabilities on the Consolidated Balance Sheets.
The
Omnia detachable warrants were valued at the closing dates of the Subordinated Note using a Monte Carlo valuation model with the following
assumptions:
| Risk-free interest rate per year | |
| 4.1 | % |
| Expected volatility per year | |
| 83.8 | % |
| Expected dividend yield | |
| — | % |
| Contractual expiration | |
| 7.5
years | |
| Exercise price | |
$ | 12.828 | |
| Stock price | |
$ | 13.65 | |
ATM Offering
On
March 31, 2023, the Company established an at-the-market common equity offering program (“ATM Program”), through which
it may, through which it had the ability to offer and sell shares of common stock having an aggregate gross sales price of up to $50,000.
The Company paid a 3.00% sales commission based on the gross proceeds of the sales price per share of common stock sold. On June 19,
2023, the Company terminated the ATM Program in connection with the June 2023 Capital Raise. The following table shows the number
of shares sold under the ATM Program prior to its termination:
| | |
Year Ended | |
| | |
December 31, | |
| (in thousands, except for per-share data) | |
2023 | |
| Number of common shares issued | |
| 284 | |
| Weighted average sale price per share | |
$ | 9.65 | |
| Gross proceeds | |
$ | 2,741 | |
| Net proceeds | |
$ | 2,563 | |
NOTE 11. – RETIREMENT PLAN
The Company sponsors a defined
contribution plan under IRC Section 401(k). The plan covers all employees who meet the minimum eligibility requirements. Under the
401(k) plan eligible employees are allowed to make voluntary deferred salary contribution to the plan, subject to statutory limits.
The Company has elected to make Safe Harbor Non-Elective Contributions to the plan for eligible employees in the amount of three percent
(3%) of the employee’s compensation. Total employer contributions to the plan for the years ended December 31, 2023 and
2022 amounted to $231 and $198, respectively.
NOTE 12. – COMMITMENTS AND CONTINGENCIES
License
and growing agreements – The Company has entered into various license and tobacco growing agreements
(the “Agreements”) with various counter parties in connection with the Company’s plant biotechnology business relating
to tobacco. The schedule below summarizes the Company’s commitments, both financial and other, associated with each Agreement.
Costs incurred under the Agreements are generally recorded as research and development expenses on the Company’s Consolidated Statements
of Operations and Comprehensive Loss.
| | |
| |
| |
| Future
Commitments | |
| Commitment | |
Counter
Party | |
Commitment
Type | |
| 2024 | | |
| 2025 | | |
| 2026 | | |
| 2027 | | |
| 2028 &
After | | |
| Total | |
| License Agreement | |
NCSU | |
Minimum annual royalty | |
$ | 100 | | |
$ | 100 | | |
$ | 100 | | |
$ | 100 | | |
$ | 3,575 | | |
$ | 3,975 | (1) |
| License Agreement | |
NCSU | |
Contract fee | |
| 150 | | |
| 250 | | |
| 250 | | |
| — | | |
| — | | |
| 650 | (2) |
| Consulting Agreements | |
Various | |
Contract fee | |
| 214 | | |
| 24 | | |
| — | | |
| — | | |
| — | | |
| 238 | (3) |
| Growing Agreements | |
Various | |
Contract fee | |
| 225 | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 225 | (4) |
| | |
| |
| |
$ | 689 | | |
$ | 374 | | |
$ | 350 | | |
$ | 100 | | |
$ | 3,575 | | |
$ | 5,088 | |
| (1) | The minimum annual royalty fee is credited
against running royalties on sales of licensed products. The Company is also responsible
for reimbursing NCSU for actual third-party patent costs incurred, including capitalized
patent costs and patent maintenance costs. These costs vary from year to year and the Company
has certain rights to direct the activities that result in these costs. |
| (2) | On November 1, 2023, the Company
entered into a license agreement with NCSU for an exclusive sublicensable right and license
under specific patent rights and plant variety rights for the field of use in specific licensed
territories. Additional milestone fees could be required pending achievement of events pursuant
to the agreement. |
| (3) | As a requirement for a modified risk
tobacco product and a condition of the marketing authorization by the FDA, the Company engaged
various consultants to conduct post-market studies and research. |
| (4) | Various R&D tobacco growing agreements. |
Litigation -
The Company is subject to litigation arising from time to time in the ordinary course of its business. The Company does not expect that
the ultimate resolution of any pending legal actions will have a material effect on its consolidated results of operations, financial
position, or cash flows. However, litigation is subject to inherent uncertainties. As such, there can be no assurance that any pending
legal action, which the Company currently believes to be immaterial, will not become material in the future. In accordance with applicable
accounting guidance, the Company establishes an accrued liability for litigation and regulatory matters when those matters present loss
contingencies that are both probable and estimable. In such cases, there may be an exposure to loss in excess of any amounts accrued.
When a loss contingency is not both probable and estimable, the Company does not establish an accrued liability. As a litigation or regulatory
matter develops, the Company, in conjunction with any outside counsel handling the matter, evaluates on an ongoing basis whether such
matter presents a loss contingency that is probable and estimable. If, at the time of evaluation, the loss contingency related to a litigation
or regulatory matter is not both probable and estimable, the matter will continue to be monitored for further developments that would
make such loss contingency both probable and estimable. When a loss contingency related to a litigation or regulatory matter is deemed
to be both probable and estimable, the Company will establish an accrued liability with respect to such loss contingency and record a
corresponding amount of related expenses. The Company will then continue to monitor the matter for further developments that could affect
the amount of any such accrued liability.
In connection with ongoing
restructuring efforts and the hemp/cannabis disposal group (see Note 2 “Divestitures and discontinued operations,” the Company
has received unasserted claims related to disputed contracts, which could result in accrual of an additional amount up to $1,314 on the
Consolidated Balance Sheet. The Company is vigorously defending its position against these claims.
Class Action
On January 21, 2019,
Matthew Jackson Bull, a resident of Denver, Colorado, filed a Complaint against the Company, the Company’s then Chief Executive
Officer, Henry Sicignano III, and the Company’s then Chief Financial Officer, John T. Brodfuehrer, in the United States District
Court for the Eastern District of New York entitled: Matthew Bull, Individually and on behalf of all others similarly situated,
v. 22nd Century Group, Inc., Henry Sicignano III, and John T. Brodfuehrer, Case No. 1:19 cv 00409.
On January 29, 2019, Ian
M. Fitch, a resident of Essex County Massachusetts, filed a Complaint against the Company, the Company’s then Chief Executive Officer,
Henry Sicignano III, and the Company’s then Chief Financial Officer, John T. Brodfuehrer, in the United States District Court for
the Eastern District of New York entitled: Ian Fitch, Individually and on behalf of all others similarly situated, v. 22nd Century
Group, Inc., Henry Sicignano III, and John T. Brodfuehrer, Case No. 2:19 cv 00553.
On May 28, 2019, the
plaintiff in the Fitch case voluntarily dismissed that action. On August 1, 2019, the Court in the Bull case issued an order designating
Joseph Noto, Garden State Tire Corp, and Stephens Johnson as lead plaintiffs.
On September 16, 2019,
pursuant to a joint motion by the parties, the Court in the Bull case transferred the class action to federal district court in the Western
District of New York, where it remains pending as Case No. 1:19-cv-01285.
Plaintiffs in the Bull case
filed an Amended Complaint on November 19, 2019 that alleges three counts: Count I sues the Company and Messrs. Sicignano and
Brodfuehrer and alleges that the Company's quarterly and annual reports, SEC filings, press releases and other public statements and
documents contained false statements in violation of Section 10(b) of the Securities Exchange Act and Rule 10b-5; Count
II sues Messrs. Sicignano and Brodfuehrer pursuant to Section 10(b) of the Securities Exchange Act and Rule 10b5(a) and
(c); and Count III sues Messrs. Sicignano and Brodfuehrer for the allegedly false statements pursuant to Section 20(a) of
the Securities Exchange Act. The Amended Complaint seeks to certify a class, and unspecified compensatory and punitive damages, and attorney's
fees and costs.
On January 29, 2020,
the Company and Messrs. Sicignano and Brodfuehrer filed a Motion to Dismiss the Amended Complaint. On January 14, 2021, the
Court granted the motion, dismissing all claims with prejudice. The Plaintiffs filed a notice of appeal on February 12, 2021 to
the Second Circuit Court of Appeals. On May 24, 2022, after briefing and oral argument, the Second Circuit issued an order affirming
in part, and reversing in part, the District Court’s dismissal order. The Second Circuit affirmed the District Court’s dismissal
of the claims relating to the non-disclosure of stock promotion articles, but reversed the District Court’s dismissal order of
the claims alleging the non-disclosure of an SEC investigation. The Second Circuit noted in its opinion, however, that the District Court
had not addressed certain arguments raised by the Company and Messrs. Sicignano and Brodfuehrer in the Motion to Dismiss the Amended
Complaint as to these remaining claims, and remanded the case to the District Court to address these arguments for the dismissal of the
remaining claims. On August 8, 2022, the Company and Messrs. Sicignano and Brodfuehrer filed a renewed motion to dismiss the
remaining claims in the Amended Complaint to address the arguments not previously addressed by the District Court. On September 22,
2022, Plaintiffs filed a brief in opposition to the motion. On October 12, 2022, the Company and Messrs. Sicignano and Brodfuehrer
filed a reply brief in further support of the motion. On January 6, 2023, the District Court denied the motion to dismiss.
The parties participated
in a mediation on March 21, 2023 and reached an initial memorandum of understanding for settlement in principle to resolve the litigation
and release all claims against the Company. On April 25, 2023, the parties filed with the Court the Motion for Preliminary Approval
of the Settlement, which includes the final terms of the proposed settlement. The Court preliminarily approved the settlement on June 30,
2023, and scheduled a further settlement hearing for October 3, 2023. The Court entered the Final Judgment and Order of Dismissal
with Prejudice of the action on October 23, 2023. The settlement amount that the defendants paid is $3,000 and is fully covered
by the Company’s insurance, which has been funded by the Company’s insurance carrier in an escrow account and anticipated
to be disbursed in the first or second quarter of 2024. Accordingly, the Company has recorded an accrual for litigation settlement and
corresponding indemnification receivable on the Consolidated Balance Sheets as of December 31, 2023.
Shareholder Derivative Cases
On February 6, 2019,
Melvyn Klein, a resident of Nassau County New York, filed a shareholder derivative claim against the Company, the Company’s then
Chief Executive Officer, Henry Sicignano III, the Company’s Chief Financial Officer, John T. Brodfuehrer, and each member of the
Company’s Board of Directors in the United States District Court for the Eastern District of New York entitled: Melvyn Klein, derivatively
on behalf of 22nd Century Group v. Henry Sicignano, III, Richard M. Sanders, Joseph Alexander Dunn, Nora B. Sullivan, James W. Cornell,
John T. Brodfuehrer and 22nd Century Group, Inc., Case No. 1:19 cv 00748. Mr. Klein brings this action derivatively alleging
that (i) the director defendants supposedly breached their fiduciary duties for allegedly allowing the Company to make false statements;
(ii) the director defendants supposedly wasted corporate assets to defend this lawsuit and the other related lawsuits; (iii) the
defendants allegedly violated Section 10(b) of the Securities Exchange Act and Rule 10b 5 promulgated thereunder for allegedly
approving or allowing false statements regarding the Company to be made; and (iv) the director defendants allegedly violated Section 14(a) of
the Securities Exchange Act and Rule 14a 9 promulgated thereunder for allegedly approving or allowing false statements regarding
the Company to be made in the Company’s proxy statement.
On February 11, 2019,
Stephen Mathew filed a shareholder derivative claim against the Company, the Company’s then Chief Executive Officer, Henry Sicignano
III, the Company’s Chief Financial Officer, John T. Brodfuehrer, and each member of the Company’s Board of Directors in the
Supreme Court of the State of New York, County of Erie, entitled: Stephen Mathew, derivatively on behalf of 22nd Century Group, Inc.
v. Henry Sicignano, III, John T. Brodfuehrer, Richard M. Sanders, Joseph Alexander Dunn, James W. Cornell, Nora B. Sullivan and
22nd Century Group, Inc., Index No. 801786/2019. Mr. Mathew brings this action derivatively generally alleging the
same allegations as in the Klein case. The Complaint seeks declaratory relief, unspecified monetary damages, corrective corporate governance
actions, and attorney’s fees and costs.
On August 15, 2019,
the Court consolidated the Mathew and Klein actions pursuant to a stipulation by the parties (Western District of New York, Case No. 1-19-cv-0513).
On May 3, 2019, the Court ordered the Mathew case stayed. This stay was applied to the Consolidated Action pursuant to the
Court’s August 15, 2019 Order Consolidated Related Shareholder Derivative Actions and Establishing a Leadership Structure.
As a result of the Court’s denial of the renewed Motion to Dismiss the Amended Complaint, the May 3, 2019 stay will be lifted.
No trial date has been set. We believe that the claims are frivolous, meritless and that the Company and the individual defendants have
substantial legal and factual defenses to the claims. We intend to vigorously defend the Company and the individual defendants against
such claims.
On June 10, 2019, Judy
Rowley filed a shareholder derivative claim against the Company, the Company’s then Chief Executive Officer, Henry Sicignano III,
the Company’s Chief Financial Officer, John T. Brodfuehrer, and each member of the Company’s Board of Directors in the Supreme
Court of the State of New York, County of Erie, entitled: Judy Rowley, derivatively on behalf of 22nd Century Group, Inc. v. Henry
Sicignano, III, Richard M. Sanders, Joseph Alexander Dunn, Nora B. Sullivan, James W. Cornell, John T. Brodfuehrer, and 22nd Century
Group, Inc., Index No. 807214/2019. Ms. Rowley brought the action derivatively alleging that the director defendants
supposedly breached their fiduciary duties by allegedly allowing the Company to make false statements. The Complaint sought declaratory
relief, unspecified monetary damages, corrective corporate governance actions, and attorney’s fees and costs. We believe that the
claims are frivolous, meritless and that the Company and the individual defendants have substantial legal and factual defenses to the
claims. We intend to vigorously defend the Company and the individual defendants against such claims. On September 13, 2019, the
Court ordered the litigation stayed pursuant to a joint stipulation by the parties. On August 3, 2022, Plaintiff dismissed the case
with prejudice by filing a stipulation of discontinuance with the Court. This dismissal was not pursuant to a settlement.
On January 15, 2020,
Kevin Broccuto filed a shareholder derivative claim against the Company, the Company's then Chief Executive Officer, Henry Sicignano
III, the Company's Chief Financial Officer, John T. Brodfuehrer, and certain members of the Company's prior Board of Directors in the
District Court of the State of Nevada, County of Clark, entitled: Kevin Broccuto, derivatively on behalf of 22nd Century Group, Inc.
v. James W. Cornell, Richard M. Sanders, Nora B. Sullivan, Henry Sicignano, III, and John T. Brodfuehrer, Case No. A-20-808599.
Mr. Broccuto brings this action derivatively alleging three counts: Count I alleges that the defendants breached their fiduciary
duties; Count II alleges they committed corporate waste; and Count III that they were unjustly enriched, by allegedly allowing the Company
to make false statements.
On February 11, 2020,
Jerry Wayne filed a shareholder derivative claim against the Company, the Company's then Chief Executive Officer, Henry Sicignano III,
the Company's Chief Financial Officer, John T. Brodfuehrer, and certain members of the Company's prior Board of Directors in the District
Court of the State of Nevada, County of Clark, entitled: Jerry Wayne, derivatively on behalf of 22nd Century Group, Inc. v. James
W. Cornell, Richard M. Sanders, Nora B. Sullivan, Henry Sicignano, III, and John T. Brodfuehrer, Case No. A-20-808599. Mr. Wayne
brings this action derivatively alleging generally the same allegations as the Broccuto case. The Complaint seeks unspecified monetary
damages, corrective corporate governance actions, disgorgement of alleged profits and imposition of constructive trusts, and attorney's
fees and costs. The Complaint also seeks to declare as unenforceable the Company's Bylaw requiring derivative lawsuits to be filed in
Erie County, New York, where the Company is headquartered.
On March 25, 2020, the
Court ordered the Broccuto and Wayne cases consolidated and stayed pursuant to a joint stipulation from the parties. On June 27,
2022, the Court ordered that the stay continue until thirty (30) days after the District Court rules on the renewed Motion to Dismiss
the Amended Complaint in the Noto Class Action case. As a result of the Court’s denial of the Motion to Dismiss the Amended
Complaint, the June 27, 2022 stay will be lifted if the case is not resolved. No trial date has been set.
The
parties participated in a mediation on March 21, 2023, and a subsequent mediation on October 17, 2023. On December 5,
2023, the parties entered into a Memorandum of Settlement to fully resolve all claims pending the Court’s approval of a motion
for preliminary approval of settlement. The settlement amount is $768 related to plaintiffs attorney and legal fees and is fully
covered by the Company’s insurance. Accordingly, the Company has recorded an accrual for litigation settlement and corresponding
indemnification receivable on the Consolidated Balance Sheets as of December 31, 2023.
On September 1, 2023,
Kenneth Troup filed a shareholder derivative claim against the Company, the Company's then Chief Executive Officer, Henry Sicignano III,
the Company's Chief Financial Officer, John T. Brodfuehrer, and certain members of the Company's Board of Directors in the United States
District Court for the Western District of New York entitled: Kenneth Troup, derivatively on behalf of 22nd Century Group v. Nora Sullivan,
James Mish, Michael Koganov, Anthony Johnson, Richard Sanders, Lucille Salhany, Andy Arno, James W. Cornell, Henry Sicignano, III,
and John T. Brodfuehrer, and 22nd Century Group, Inc., Case No. 1:23-cv-00916. Mr. Troup brings this action derivatively
generally alleging the same allegations as in the Klein case. The Complaint seeks declaratory relief, unspecified monetary damages, corrective
corporate governance actions, and attorney’s fees and costs. On February 9, 2024, defendants filed an unopposed Motion to
Consolidate the Troup action with the consolidated derivative cases, which would include the Troup case in the preliminary settlement
described above.
We believe that the claims
are frivolous, meritless and that the Company and the individual defendants have substantial legal and factual defenses to the claims.
We intend to vigorously defend the Company and the individual defendants against such claims.
Insurance Litigation
In
November 2022, there was a fire at the Company’s Grass Valley manufacturing facility in Oregon, which resulted in a total
loss of the facility. The Company submitted an insurance claim with Dorchester Insurance Company, Ltd. (“Dorchester”)
for casualty loss and business interruption coverage which was acknowledged on November 23, 2022. Dorchester funded $5,000 of
casualty loss insurance but has failed to issue any payments in connection with the Company’s business interruption claim.
On
July 19, 2023, the Company filed a Complaint against Dorchester in the United States District Court for the District of Oregon,
Pendleton Division, Case No. 2:23-cv-01057-HL. The Company is alleging breach of contract, breach of duty of good faith and fair
dealing and negligence per se. The Company is seeking full recovery of its business interruption claim under the policy plus direct,
indirect and consequential damages resulting from Dorchester’s continued delay in issuing coverage payments. Discovery is ongoing.
No trial date has been set.
Needle Rock Farms – Settlement Agreement
During March 2023, the
Company negotiated and entered into a settlement agreement related to water rights dispute with the adjacent property owner for Needle
Rock Farms in which the Company agreed to pay $250 in cash upon execution of the settlement, transferred certain farm equipment with
net book value of $272, and accrued an additional payment of $225 that is contingent on either the sale of the farm or will be paid within
one year. The total charges of $747 recorded in connection with the settlement agreement is included in discontinued operations
within Other operating expenses, net on the Consolidated Statements of Operations and Comprehensive Loss.
KeyGene Dispute
On April 3, 2019, the
Company entered into the Framework Collaborative Research Agreement with KeyGene in the field of hemp/cannabis. On April 30, 2021,
the Company and KeyGene entered into a First Amended and Restated Framework Collaborative Research Agreement which extended the agreement
term, from first quarter 2024 to first quarter 2027, and preserves the Company’s option for an additional 2-year extension, through
first quarter of 2029. On March 30, 2022, the Company and KeyGene entered into a new Framework Collaborative Research Agreement
for a term of three years in the field related to the hops plant. On January 8, 2024, the Company formally terminated the new Framework
Collaborative Agreement, as amended, related to hemp/cannabis and hops. KeyGene is seeking payment in the amount of $1,885 for current
and future services under the Framework Collaborative Agreement and has invoiced the Company $881 for services performed. The parties
anticipate mediating the dispute although no mediation date has been set.
Maison Dispute
On January 23, 2024,
the Company received a Notice of Intent to Arbitrate from Maison Placements Canada Inc. (“Maison”) in connection with the
Company’s March 2023 Senior Secured Credit Facility transaction (infra). Maison claims it is owed fees for closure
of the Senior Secured Credit Facility transaction as a result of discussions with former Company personnel and a purported letter of
engagement dating from 2021. The Company believes it has substantial defenses to Maison’s claims and intends to defend itself
vigorously.
NOTE 13. – DEBT
The Company has a senior
secured credit facility (the “Senior Secured Credit Facility”), which consists of three-year $21,053 Debentures (as
defined below) and $2,865 subordinated promissory note (the “Subordinated Note). The Debentures were issued at a 5% original
issuance discount and are subject to a 5% exit payment.
Debt related to the Senior Secured Credit Facility
and Subordinate Note as of December 31, 2023, consists of the following:
| | |
December 31, | | |
December 31, | |
| | |
2023 | | |
2022 | |
| Senior Secured Credit Facility | |
$ | 11,805 | | |
$ | — | |
| Subordinated Note | |
| 3,554 | | |
| — | |
| Unamortized discount on loan and deferred
debt issuance costs | |
| (1,453 | ) | |
| — | |
| Total debt | |
$ | 13,906 | | |
$ | — | |
| Current portion of long-term debt | |
| (5,848 | ) | |
| — | |
| Total long-term
debt | |
$ | 8,058 | | |
$ | — | |
Debentures
On
March 3, 2023, the Company entered into a Securities Purchase Agreement (the “SPA”)
with JGB Partners, LP (“JGB Partners”), JGB Capital, LP (“JGB Capital”)
and JGB Capital Offshore Ltd. (“JGB Offshore” and collectively with JGB Partners
and JGB Capital, the “Holders”) and JGB Collateral, LLC, as collateral agent
for the Holders (the “Agent”) which pursuant to the agreement, the Company
sold 5% original issuance discount senior secured debentures with an aggregate principal amount of $21,053. The Debentures bear interest
at a rate of 7% per annum, payable monthly in arrears as of the last trading day of each month and on the maturity date. The Debentures
mature on March 3, 2026. At the Company’s election, subject to certain conditions, interest can be paid in cash, shares of
the Company’s common stock, or a combination thereof. The Debentures are subject to an exit payment equal to 5% of the original
principal amount, or $1,053, payable on the maturity date or the date the Debentures are paid in full (the “Exit Payment”).
Any time after, March 3, 2024, the Company may irrevocably elect to redeem all of the then outstanding principal amount of the Debentures
for cash in an amount equal to the entire outstanding principal balance, including accrued and unpaid interest, the Exit Payment and
a prepayment premium in an amount equal to 3% of the outstanding principal balance as of the prepayment date (collectively, the “Prepayment
Amount”). Upon the entry into a definitive agreement that would effect a change in control (as defined in the Debentures) of the
Company, the Agent may require the Company to prepay the outstanding principal balance in an amount equal to the Prepayment Amount. Commencing
on May 1, 2024, at its option, the holder of a Debenture may require the Company to redeem 2% of the original principal amount of
the Debentures per calendar month which amount may at the Company’s election, subject to certain exceptions, be paid in cash, shares
of the Company’s common stock, or a combination thereof.
The
Company’s obligations under the Debentures can be accelerated upon the occurrence of certain customary events of default. In the
event of a default and acceleration of the Company’s obligations, the Company would be required to pay the Prepayment Amount, liquidated
damages and other amounts owing in respect thereof through the date of acceleration.
The
Debentures contain customary representations, warranties and covenants including among other things and subject to certain exceptions,
covenants that restrict the Company from incurring additional indebtedness, creating or permitting liens on assets, making or holding
any investments, repaying outstanding indebtedness, paying dividends or distributions and entering into transactions with affiliates.
Substantially all of the company’s assets, including intellectual property, are collateralized and at risk if Debenture
obligation is not satisfied. In addition, the Company is required to maintain at least $7,500 on its balance sheet as restricted cash
in a separate account and has financial covenants to maintain certain quarterly revenue targets.
In connection with the sale
of the Debentures, the Company issued warrants to purchase up to 333,334 shares of common stock for an exercise price of $19.125 per
share (the “JGB Warrants”), which had an initial fair value of $4,475 net of issuance costs of $139 (see Note 9 “Fair
Value Measurements” and Note 10 “Capital Raise and Warrants for Common Stock”). On June 22, 2023, as a result
of the June 19, 2023 offering, the Company’s outstanding JGB warrants to purchase up to 333,334 shares of the Company’s
common stock for an exercise price of $19.125 per share were automatically adjusted to be $12.828 exercise price for up to 496,960 shares
of common stock. There are no further anti-dilution adjustments on such warrants.
On October 16, 2023,
the Company entered into a Waiver and Amendment Agreement (the “October Amendment”) with each of the subsidiaries of
the Company executing the Debentures, the Holders and the Agent, pursuant to which, among other things, (a) the Holders waived an
event of default under Section 7(d) of the Debentures which required the Company to achieve revenue of at least $18,500 for
the quarter ended September 30, 2023 (the “waiver”), (b) the parties agreed to amend Schedule E of the Debentures
to reduce the Revenue Target (as such term is defined in the Debentures), for the quarter ended December 31, 2023, to $15,500, and
(c) the Company agreed to release to the Purchasers the $7,500 that the Company was required to maintain in a separate account
(the “Escrow Funds”) which Escrow Funds were be applied to, and reduce, the outstanding principal amount of the Debentures
on a dollar-for-dollar basis.
As additional consideration
for the waiver, the Company agreed to assign, transfer and convey to the Agent, the Company’s entire right, title and interest
in and to (i) the Promissory Note made by J&N Real Estate Company, L.L.C. (“J&N”) payable to the Company in
the principal amount of $3,800 and (ii) the Deed of Trust, Assignment of Rents, Security Agreement and Fixture Filing dated
June 30, 2021, between J&N, as borrower, for the benefit of the Company, as lender (collectively, the “Pledged Indebtedness”).
Upon assignment of the Pledged Indebtedness, the Company recognized the $2,600 of consideration in exchange to be applied as a $2,000
reduction of the Put Price (as defined below), $600 reduction of the outstanding principal amount of Debentures and $895 loss on
sale of financial asset.
In connection with the waiver,
the Company and Holders agreed to exercise the outstanding put provision to redeem 166,667 Warrants for an aggregate put price
equal to $2,500 (the “Put Price”), which was concurrently reduced by $2,000, as described above, with the remaining
$500 payable by the Company on the Maturity Date recorded as Other long-term liabilities on the Consolidated Balance Sheet. No cash
was exchanged as a result of executing the October Amendment.
Subsequently,
on December 22, 2023, the Company, the Holders and the Agent entered into an Amendment Agreement (the “December Amendment”)
pursuant to which the Holders and the Agent consented to the Purchase Agreement, as amended by the GVB Amendment (see Note 2 “Discontinued
Operations and Divestitures”). In consideration of the Holders and the Agents’ consent, the Company agreed to (i) pay
to the Agent, a cash payment of $2,200 to reduce the outstanding principal of the Debentures (which includes the cash portion of the
New Purchase Price paid directly to Agent by Buyer which consists of a cash payment of $1,100 and
an additional $1,100 paid by the Company), (ii) a 12% secured promissory note issued
to the Company’s senior lender, on behalf of and at the direction of the Company, in an aggregate principal amount of $2,000 (the
“GVB Promissory Note”), (iii) assign the GVB Insurance Proceeds to the Agent until the outstanding aggregate
principal amount of the Debentures, plus accrued and unpaid interest, has been repaid in full; provided that the first $1,000
of Insurance Proceeds in excess of $5,000 shall be applied as stated above, and (iv) post-closing enter into a deed in lieu of foreclosure
agreement with respect to 224 acres of real property in Delta County, Colorado commonly known as Needle Rock Farms, resulting in a non-monetary
exchange yielding additional debt reduction of $1,000. As of December 31, 2023, the $2,000 GVB Promissory Note and $1,000 real estate
farm asset are pledged to the senior lender for principal reduction and accordingly $3,000 of the Senior Secured Credit Facility is recorded
as Current portion of long-term debt on the Consolidated Balance Sheets.
Additionally,
the Company, the Holders and the Agent agreed to amend the Debentures to (i) allow the Holders to voluntarily convert the Debentures,
in whole or in part, into shares of the Company’s common stock (“Voluntary Conversion Option”) on the earlier of (i) June 30,
2024 and (ii) the public announcement of a Fundamental Transaction at a conversion price equal to the lower of (x) $1.00 per
share and (y) the closing sale price of the Company’s common stock on June 29, 2024 (the “Conversion Price”),
and (ii) include a mandatory prepayment of the outstanding principal of the Debentures in an amount equal to 20% of the net cash
proceeds of any issuance by the Company of any of its stock, or other Equity Interests (as defined in the Debentures) or the incurrence
or issuance of any indebtedness. The Voluntary Conversion Option remains subject to the approval of the Company’s stockholders
and the Company is required pursuant to the December Amendment to use its commercially reasonable efforts to obtain such approval.
Additional
terms of the December Amendment include a financial covenant holiday through the third quarter of 2024 and revised certain covenants
thereafter to reflect the sale of the Purchased Interests, including lowering the Company’s quarterly revenue targets.
In accordance with ASC 470-60 Troubled
Debt Restructurings by Debtors and ASC 470-50, Debt Modifications and Extinguishment, the Company performed an assessment of
whether the transaction was deemed to be a troubled debt restructuring, and if no, whether the transaction was deemed modification of
existing debt, or an extinguishment of existing debt and new debt.
The October Amendment
was concluded to be a modification, and not an extinguishment, based on an analysis of the present value of future cash flows. A new
effective interest rate was determined, and the debt continued to be amortized. The December Amendment was concluded to be an extinguishment,
due to the addition of a substantive conversion option. As a result, the pre-amended debt carrying value was extinguished and the new
debt was recorded at fair value, which is subsequently amortized using the effective interest method. Extinguishment charges were $5,158
and recorded in Interest expense on the Consolidated Statements of Operations and Comprehensive Loss.
The
Company analyzed the conversion feature of the December Amendment for derivative accounting consideration under ASC 815-15 and determined
that the embedded conversion features should be classified as a bifurcated derivative because the exercise price of these convertible
notes are subject to a variable conversion rate. The Company has determined that the conversion feature is not considered to be solely
indexed to the Company’s own stock and is therefore not afforded equity treatment. In accordance with AC 815, the Company has bifurcated
the conversion feature of the note and recorded a derivative liability at fair value in the amount of $557 as a component of Other
Long-Term Liabilities on the Consolidated Balance Sheet. See Note 9 “Fair Value Measurement” for additional information related
to measurement of the debentures and derivative liability.
Subordinated
Note
On
March 3, 2023, the Company executed a Subordinated Promissory Note (the “Subordinated Note”) with a principal amount
of $2,865 in favor of Omnia Ventures, LP (“Omnia”). The Subordinated Note refinanced the 12% Secured Promissory Note with
a principal amount of $1,000 dated as of October 29, 2021 payable to Omnia (the “October Note”) and the 12% Secured
Promissory Note with a principal amount of $1,500 dated as of January 14, 2022 payable to Omnia (the “January Note”,
and together with the October Note, the “Original Notes”), which were assumed by the Company in connection with the
acquisition of GVB Biopharma (see Note 3 “Business Acquisitions”). The accrued PIK interest refinanced from the Original
Notes was $365.
Under
the terms of the Subordinated Note, the Company is obligated to make interest payments in-kind (the “PIK Interest”). The
PIK Interest accrues monthly at a compounding rate of 26.5% per annum. For the year ended December 31, 2023 the PIK Interest accrual
amounts were $695. The Company is not permitted to prepay all or any portion of the outstanding balance on the Subordinated Note prior
to maturity. The maturity date of the Subordinated Note is May 1, 2024. The Subordinated Note includes customary event of default
provisions. The Subordinated Note is subordinated to the Debenture pursuant to a Subordination Agreement between the Company, the Agent
and Omnia.
In
connection with the Subordinated Note, the Company issued to Omnia, warrants to purchase up to 45,000 shares of the Company’s common
stock (the “Omnia Warrants”). The Omnia Warrants are exercisable for seven years from September 3, 2023, at an exercise
price of $12.828 per share, subject, with certain exceptions, to adjustments in the event of stock splits, dividends, subsequent dilutive
offerings and certain fundamental transactions, as more fully described in the Omnia Warrants. The Omnia warrants initial fair value
was $1,316 (see Note 9 “Fair Value Measurements” and 10 “Capital Raise and Warrants for Common Stock”).
Contractual maturities under
the Senior Secured Credit Facility and Subordinate Note through maturity, excluding any discounts or premiums, as of December 31, 2023
is as follows:
| | |
2024 | | |
2025 | | |
2026 | | |
2027 | | |
2028 | | |
Thereafter | |
| Future minimum principal payments | |
$ | 5,848 | | |
$ | — | | |
$ | 8,058 | | |
$ | — | | |
$ | — | | |
$ | — | |
The fair values of the warrants
at issuance of $5,791, together with the Debentures original issuance discount of $1,053, Debentures exit payment of $1,053, and third-party
debt issuance costs of $801, are being amortized using the effective interest method over the term of the respective debt instrument,
recorded as Interest expense in the Consolidated Statement of Operations and Comprehensive Loss. The components and activity of unamortized
discount and deferred debt issuance costs related to the Senior Secured Credit Facility and Subordinated Note is as follows:
| | |
Total | |
| Issuance | |
$ | 8,698 | |
| Amortization during the year | |
| (2,087 | ) |
| Debt extinguishment
charges | |
| (5,158 | ) |
| December 31, 2023 | |
$ | 1,453 | |
NOTE 14. – NOTES AND LOANS PAYABLE
The table below outlines our notes payable balances
as of December 31, 2023 and 2022:
| | |
December 31, | | |
December 31, | |
| | |
2023 | | |
2022 | |
| Insurance loans payable | |
$ | 543 | | |
$ | 689 | |
| Total current notes
and loans payable | |
$ | 543 | | |
$ | 689 | |
Insurance loans payable
During the second quarter
of 2023, the Company renewed its Director and Officer (“D&O”) insurance for a one-year policy premium totaling $1,626.
The Company paid $285 as a premium down payment and financed the remaining $1,341 of policy premiums over ten months at a 7.88% annual
percentage rate. Additionally, during the third quarter of 2023, the Company expanded its D&O coverage, resulting in additional financing
of $143, at 9.38% annual percentage rate over six months.
During the second quarter
of 2022, the Company renewed its Director and Officer (“D&O”) insurance for a one-year policy premium totaling $2,394.
The Company paid $400 as a premium down payment and financed the remaining $1,994 of policy premiums over ten months at a 3.25% annual
percentage rate. Additionally, during the third quarter of 2022, the Company expanded its D&O coverage as a result of the acquisition
of GVB, resulting in an additional premium down payment of $90 and financing of $168, under the same terms as the original one-year policy.
The Company also has other
insurance loans payables related to pollution, property, and general liability across the Company.
As of December 31, 2023,
all estimated future principal payments to be made under the above notes and loans payable will be paid in 2024.
NOTE 15. – EQUITY BASED COMPENSATION
Stock Compensation Plan
On May 20, 2021, the
stockholders of 22nd Century Group, Inc. (the “Company”) approved the 22nd Century Group, Inc. 2021 Omnibus Incentive
Plan (the “2021 Plan”). The 2021 Plan allows for the granting of equity awards to eligible individuals over the life of the
2021 Plan, including the issuance of up to 333,334 shares of the Company’s common stock, in addition to any remaining shares under
the Company’s 2014 Omnibus Incentive Plan pursuant to awards under the 2021 Plan. The 2021 Omnibus Incentive Plan was amended on
June 16, 2023, increasing the authorized shares by 233,334. The 2021 Plan has a term of ten years and is administered by the Compensation
Committee of the Company’s Board of Directors to determine the various types of incentive awards that may be granted to recipients
under the 2021 Plan and the number of shares of common stock to underlie each such award under the 2021 Plan. As of December 31, 2023,
the Company had available 606,406 shares remaining for future awards under the 2021 Plan.
Compensation Expense
The Company recognized the following compensation
costs, net of actual forfeitures, related to RSUs and stock options:
| | |
Year Ended | |
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Sales, general, and administrative | |
$ | 2,052 | | |
$ | 5,252 | |
| Research and development | |
| 179 | | |
| 182 | |
| Total equity based compensation - continuing
operations | |
| 2,231 | | |
| 5,434 | |
| Total equity based compensation - discontinued
operations | |
| 448 | | |
| 55 | |
| Total equity based
compensation | |
$ | 2,679 | | |
$ | 5,489 | |
During the years ended December 31,
2023, and 2022, equity-based compensation expense reversals due to employee termination forfeitures amounted to $1,960 and $84, respectively.
Additionally, the Company recorded $523 and $1,237 of accelerated equity compensation expense, respectively, in connection with the vesting
of an employees’ outstanding equity awards as part of termination severance agreements. Amounts are recorded as Selling, general
and administrative in the Consolidated Statements of Operations and Comprehensive Loss.
Restricted
Stock Units (“RSUs”). We typically grant RSUs to employees and non-employee directors. The following table summarizes
the changes in unvested RSUs from January 1, 2022 through December 31, 2023.
| | |
| Unvested
RSUs | |
| | |
| | | |
| Weighted | |
| | |
| | | |
| Average | |
| | |
| Number
of | | |
| Grant-date | |
| | |
| Shares | | |
| Fair
Value | |
| | |
| in
thousands | | |
| $
per share | |
| Unvested at January 1, 2022 | |
| 211 | | |
$ | 37.50 | |
| Granted | |
| 236 | | |
| 29.40 | |
| Vested | |
| (154 | ) | |
| 31.65 | |
| Forfeited | |
| (24 | ) | |
| 35.85 | |
| Unvested at December 31, 2022 | |
| 269 | | |
$ | 31.88 | |
| Granted | |
| 293 | | |
| 12.44 | |
| Vested | |
| (147 | ) | |
| 29.67 | |
| Forfeited | |
| (260 | ) | |
| 20.86 | |
| Unvested at December 31, 2023 | |
| 155 | | |
$ | 15.69 | |
The fair value of RSUs that
vested during the years ended December 31, 2023 and 2022 was approximately $1,838 and $4,505, respectively, based on the stock
price at the time of vesting. As of December 31, 2023, unrecognized compensation expense for RSUs amounted to $823 which is expected
to be recognized over a weighted average period of approximately 1.7 years. In addition, there is approximately $786 of unrecognized
compensation expense that requires the achievement of certain milestones which are not yet probable.
Stock
Options. Our outstanding stock options were valued using the Black-Scholes option-pricing model on the date of the award.
There was no stock option grant activity during 2023 and 2022. A summary of all stock option activity since January 1, 2022 is as
follows:
| | |
| | |
Weighted | | |
Weighted
Average | | |
| |
| | |
| | |
Average | | |
Remaining | | |
Aggregate | |
| | |
Number of | | |
Exercise | | |
Contractual | | |
Intrinsic | |
| | |
Options | | |
Price | | |
Term | | |
Value | |
| | |
| in
thousands | | |
| $
per share | | |
| | | |
| | |
| Outstanding at January 1, 2022 | |
| 345 | | |
$ | 24.75 | | |
| | | |
| | |
| Exercised | |
| (10 | ) | |
| 17.40 | | |
| | | |
| | |
| Forfeited | |
| (7 | ) | |
| 20.85 | | |
| | | |
| | |
| Expired | |
| (1 | ) | |
| 41.40 | | |
| | | |
| | |
| Outstanding at December 31, 2022 | |
| 327 | | |
| 24.82 | | |
| | | |
| | |
| Forfeited | |
| (101 | ) | |
| 21.29 | | |
| | | |
| | |
| Expired | |
| (7 | ) | |
| 41.40 | | |
| | | |
| | |
| Outstanding at December 31, 2023 | |
| 219 | | |
$ | 26.34 | | |
| 1.9
years | | |
$ | — | |
| Exercisable at December 31, 2023 | |
| 213 | | |
$ | 25.95 | | |
| 1.8
years | | |
$ | — | |
The intrinsic value of a
stock option is the amount by which the current market value or the market value upon exercise of the underlying stock exceeds the exercise
price of the option. In addition, there is approximately $190 of unrecognized compensation expense for stock options that requires the
achievement of certain milestones which are not yet probable.
NOTE 16. – LOSS PER COMMON SHARE
The following table sets
forth the computation of basic and diluted loss per common share for the years ended December 31, 2023 and 2022, respectively. Outstanding
warrants, options, and restricted stock units were excluded from the calculation of diluted EPS as the effect was antidilutive.
| | |
Year Ended | |
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Net loss from continuing operations | |
$ | (54,686 | ) | |
$ | (36,553 | ) |
| Net loss from discontinued operations | |
| (86,089 | ) | |
| (23,248 | ) |
| Net loss | |
$ | (140,775 | ) | |
$ | (59,801 | ) |
| Deemed dividends | |
| (9,992 | ) | |
| — | |
| Net loss available to common shareholders | |
$ | (150,767 | ) | |
$ | (59,801 | ) |
| Weighted average common shares outstanding - basic and diluted | |
| 20,711 | | |
| 12,856 | |
| | |
| | | |
| | |
| Basic and diluted loss per common share from continuing operations | |
$ | (2.64 | ) | |
$ | (2.84 | ) |
| Basic and diluted loss per common share from discontinued
operations | |
| (4.16 | ) | |
| (1.81 | ) |
| Basic and diluted loss per common share
from deemed dividends | |
| (0.48 | ) | |
| — | |
| Basic and diluted loss per common share | |
$ | (7.28 | ) | |
$ | (4.65 | ) |
| | |
| | | |
| | |
| Anti-dilutive shares are as follows as of December 31 (in thousands): | |
| | | |
| | |
| Warrants | |
| 47,757 | | |
| 1,138 | |
| Options | |
| 219 | | |
| 327 | |
| Restricted stock units | |
| 155 | | |
| 269 | |
| | |
| 48,131 | | |
| 1,734 | |
NOTE 17. – REVENUE RECOGNITION
The
Company’s revenues are derived primarily from contract manufacturing organization (“CMO”) customer contracts that consist
of obligations to manufacture the customers’ branded filtered cigars and cigarettes. Additional revenues are generated from sale
of the Company’s proprietary low nicotine content cigarettes, sold under the brand name VLN®, or research
cigarettes sold under the brand name SPECTRUM®.
The Company recognizes revenue
when it satisfies a performance obligation by transferring control of the product to a customer. For certain CMO contracts, the performance
obligation is satisfied over time as the Company determines, due to contract restrictions, it does not have an alternative use of the
product and it has an enforceable right to payment as the product is manufactured. The Company recognizes revenue under those contracts
at the unit price stated in the contract based on the units manufactured. Revenue from the sale of the Company’s products, which
include excise taxes and shipping and handling charges billed to customers, is recognized net of cash discounts, sales returns and allowances.
There was no allowance for discounts or returns and allowances at December 31, 2023 and December 31, 2022. Excise
taxes recorded in Cost of Goods Sold on the Consolidated Statement of Operations and Comprehensive Loss for the years ended December 31, 2023
and 2022 was $10,413 and $12,619, respectively.
Disaggregation of Revenue
The Company’s net revenue
is derived from customers located primarily in the United States and is disaggregated by the timing of revenue. Revenue recognized from
Tobacco products transferred to customers over time represented 63% and 74%, for the year ended December 31, 2023 and 2022, respectively.
The following table presents
net revenues by significant customers, which are defined as any customer who individually represents 10% or more of disaggregated product
line net revenues:
| | |
Year Ended | |
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Customer A | |
| 31.49 | % | |
| 23.61 | % |
| Customer B | |
| 23.92 | % | |
| 23.22 | % |
| Customer C | |
| 21.70 | % | |
| 35.20 | % |
| All other customers | |
| 22.89 | % | |
| 17.97 | % |
Contract Assets and Liabilities
Unbilled receivables (contract
assets) represent revenues recognized for performance obligations that have been satisfied but have not been billed. These receivables
are included as Accounts receivable, net on the Consolidated Balance Sheets. Customer payment terms vary depending on the terms of each
customer contract, but payment is generally due prior to product shipment or within extended credit terms up to twenty-one (21) days
after shipment. Deferred Revenue (contract liabilities) relate to down payments received from customers in advance of satisfying a performance
obligation. This deferred revenue is included as Deferred income on the Consolidated Balance Sheets.
Total contract assets and contract liabilities
are as follows:
| | |
December 31, | | |
December 31, | | |
December 31, | |
| | |
2023 | | |
2022 | | |
2021 | |
| Unbilled receivables | |
$ | 1,053 | | |
$ | 354 | | |
$ | 178 | |
| Deferred income | |
| (726 | ) | |
| (688 | ) | |
| (119 | ) |
| Net contract assets
(liabilities) | |
$ | 327 | | |
$ | (334 | ) | |
$ | 59 | |
During the years ended December 31,
2023 and 2022, the Company recognized $688 and $119 of revenue that was included in the contract asset balance as of December 31,
2022 and 2021 respectively.
NOTE 18. – OTHER OPERATING EXPENSES (INCOME), NET
The components of “Other
operating expenses (income), net” were as follows:
| | |
Year Ended | |
| | |
December 31, | |
| | |
2023 | | |
2022 | |
| Restructuring costs: | |
| | | |
| | |
| Impairment of intangible assets
(see Note 7) | |
$ | 1,375 | | |
$ | 35 | |
| Impairment of fixed assets | |
| 56 | | |
| — | |
| Professional services | |
| 763 | | |
| — | |
| Severance (see Note
1) | |
| 221 | | |
| — | |
| Total Restructuring costs | |
| 2,415 | | |
| 35 | |
| | |
| | | |
| | |
| Acquisition and transaction costs | |
| 223 | | |
| - | |
| Gain on sale or disposal of property,
plant and equipment | |
| (111 | ) | |
| (362 | ) |
| Total other operating expenses (income),
net | |
$ | 2,527 | | |
$ | (327 | ) |
Restructuring costs
During
the third quarter of 2023, the Company undertook various restructuring activities in an effort to better align its internal organizational
structure and costs with its strategy, as well as preserve liquidity. As a component of the restructuring, the Company has initiated
a process to evaluate strategic alternatives with respect to the Company’s tobacco assets. The process will include consideration
of a range of strategic, operational and financial transactions and alternatives, such as business combinations, asset sales, licensing
agreements, alternate financing strategies and other options.
As
a result, the Company incurred $2,415 in restructuring costs for the year ended December 31, 2023, which included costs related
to employee termination, professional services and consulting, and long-lived asset impairment.
NOTE 19. – INCOME TAXES
The following is a summary
of the components giving rise to the (benefit) provision for income taxes from continuing operations for the years ended December 31,
2023 and 2022:
| | |
2023 | | |
2022 | |
| Current: | |
| | | |
| | |
| Federal | |
$ | — | | |
$ | — | |
| State | |
| 40 | | |
| 14 | |
| Foreign | |
| — | | |
| — | |
| Total current provision | |
$ | 40 | | |
$ | 14 | |
| | |
| | | |
| | |
| Deferred: | |
| | | |
| | |
| Federal | |
| (11,351 | ) | |
| (6,610 | ) |
| State | |
| (736 | ) | |
| (4,404 | ) |
| Foreign | |
| — | | |
| — | |
| Total deferred benefit | |
| (12,087 | ) | |
| (11,014 | ) |
| Change in valuation
allowance | |
| 12,094 | | |
| 11,021 | |
| Total income tax provision | |
$ | 47 | | |
$ | 21 | |
The (benefit) provision for
income tax from continuing operations varies from that which would be expected based on applying the statutory federal rate to pre-tax
book loss, including the effect of the change in the U.S. corporate income tax rates, as follows:
| | |
2023 | | |
2022 | |
| Statutory federal rate | |
| 21.0 | % | |
| 21.0 | % |
| Other items | |
| 0.2 | | |
| (0.8 | ) |
| Stock based compensation | |
| (0.8 | ) | |
| (1.3 | ) |
| Research and development credit carryforward | |
| 0.4 | | |
| — | |
| State tax, net of federal benefit | |
| 1.3 | | |
| 12.0 | |
| 162(m) limitation | |
| (0.2 | ) | |
| (0.9 | ) |
| Valuation allowance | |
| (22.0 | ) | |
| (30.1 | ) |
| Effective tax rate | |
| (0.1 | )% | |
| (0.1 | )% |
Individual components of deferred taxes consist
of the following as of December 31:
| | |
2023 | | |
2022 | |
| Deferred tax assets: | |
| | | |
| | |
| Net operating loss carry-forward | |
$ | 54,453 | | |
$ | 34,029 | |
| Inventory | |
| 2,020 | | |
| 220 | |
| Stock-based compensation | |
| 862 | | |
| 1,144 | |
| Start-up expenditures | |
| 155 | | |
| 175 | |
| Research and development credit carryforward | |
| 1,424 | | |
| 1,205 | |
| Accrued bonus | |
| 133 | | |
| 458 | |
| Severance liability | |
| 95 | | |
| 151 | |
| Credit loss reserves | |
| 2 | | |
| — | |
| Research and development costs | |
| 1,617 | | |
| 813 | |
| Operating lease obligations | |
| 476 | | |
| 229 | |
| Capital loss on investment | |
| 2,449 | | |
| 2,209 | |
| Note payable and warrant liability | |
| 581 | | |
| — | |
| Other | |
| 1,758 | | |
| 50 | |
| | |
$ | 66,025 | | |
$ | 40,683 | |
| Deferred tax liabilities: | |
| | | |
| | |
| Machinery and equipment | |
| (283 | ) | |
| (221 | ) |
| Patents and trademarks | |
| (193 | ) | |
| (203 | ) |
| Operating lease right-of-use assets | |
| (467 | ) | |
| (225 | ) |
| Other intangible assets | |
| (385 | ) | |
| (334 | ) |
| | |
| (1,328 | ) | |
| (983 | ) |
| Valuation allowance | |
| (64,763 | ) | |
| (39,759 | ) |
| Net deferred taxes | |
$ | (66 | ) | |
$ | (59 | ) |
The Company has net operating
loss (“NOL”) carryforwards of approximately $193,322 as of December 31, 2023 that do not expire. The Company had accumulated
an NOL carryforward of approximately $46,920 through December 31, 2017 and this NOL carryforward begins to expire in 2030. As of
December 31, 2023, the Company has a research and development credit carryforward of approximately $1,424 that begins to expire
in 2030. The Company generated a capital loss carryover of approximately $9,932 as of December 31, 2023, that begins to expire in
2026. Utilization of these NOL carryforwards may be subject to an annual limitation in the case of equity ownership changes, as defined
by law. Due to the uncertainty of the Company’s ability to generate sufficient taxable income in the future, the Company has recorded
a valuation allowance to reduce the net deferred tax asset to zero. These carryforwards are included in the net deferred tax asset that
has been fully offset by the valuation allowance. The valuation allowance increased for continuing operations by $12,094 and $11,021
for the years ended December 31, 2023, and 2022, respectively, and increased an additional $12,910 due to tax attributes that were
generated as a part of discontinued operations but remain on a prospective basis with continuing operations due to the Company filing
a consolidated US Federal return for the year ended December 31, 2023.
ASC 740 provides guidance
on the financial statement recognition and measurement for uncertain income tax positions that are taken or expected to be taken in a
company’s income tax return. The Company has evaluated its tax positions and believes there are no uncertain tax positions as of
December 31, 2023.
NOTE 20. – QUARTERLY REVENUE AND EARNINGS DATA – UNAUDITED
| | |
Three
Months Ended | |
| | |
December 31, | | |
September 30, | | |
June 30, | | |
March 31, | |
| | |
2023 | | |
2023 | | |
2023 | | |
2023 | |
| Revenues, net | |
$ | 7,357 | | |
$ | 7,871 | | |
$ | 8,050 | | |
$ | 8,926 | |
| Gross profit (loss) | |
$ | (7,829 | ) | |
$ | 77 | | |
$ | (961 | ) | |
$ | 17 | |
| Net loss from continuing operations (2) | |
$ | (22,068 | ) | |
$ | (8,081 | ) | |
$ | (13,707 | ) | |
$ | (10,830 | ) |
| Basic and diluted loss per common share from continuing operations
(1) | |
$ | (0.66 | ) | |
$ | (0.41 | ) | |
$ | (0.92 | ) | |
$ | (0.75 | ) |
| | |
Three
Months Ended | |
| | |
December 31, | | |
September 30, | | |
June 30, | | |
March 31, | |
| | |
2022 | | |
2022 | | |
2022 | | |
2022 | |
| Revenues, net | |
$ | 9,951 | | |
$ | 11,535 | | |
$ | 9,970 | | |
$ | 9,045 | |
| Gross profit | |
$ | (44 | ) | |
$ | 636 | | |
$ | 928 | | |
$ | 328 | |
| Net loss from continuing operations (3) | |
$ | (11,114 | ) | |
$ | (10,490 | ) | |
$ | (7,699 | ) | |
$ | (7,250 | ) |
| Basic and diluted loss per common share from continuing operations
(1) | |
$ | (0.77 | ) | |
$ | (0.75 | ) | |
$ | (0.63 | ) | |
$ | (0.67 | ) |
| (1) | The quarterly per share data in this table has been rounded and therefore
may not sum to total year-to-date EPS. |
| (2) | For the quarter ended December 31,
2023, net loss from continuing operations increased from the previous current year quarters,
mainly due to an inventory leaf reserve charge of $7,720 and loss on extinguishment of debt
in the amount of $5,158. |
| (3) | For the quarter ended December 31,
2022, net loss from continuing operations increased from the previous current year quarters,
mainly due to higher personnel and strategic consulting costs. |
NOTE 21. – SUBSEQUENT EVENTS
Increase in Authorized Shares
On
February 15, 2024, our stockholders approved an amendment (the “Articles Amendment”) to our Articles of Incorporation,
as amended, to increase the number of authorized shares of common stock sixty-six million, six hundred sixty-six thousand six hundred
and sixty-seven (66,666,667) to two hundred fifty million (250,000,000), which Articles Amendment was filed and effective with the Secretary
of the State of Nevada on February 15, 2024.
Warrant Inducement
For
the period from January 1, 2024 to February 15, 2024, the date of Stockholder Approval, the Company entered into warrant inducement
agreements with certain holders of the Existing Warrants to purchase an aggregate of 13,132,268 shares of common stock at a reduced weighted
average exercise price of approximately $0.1844. Pursuant to the warrant inducement agreements, the exercising holders of the Existing
Warrants received 26,264,536 Inducement Warrants and the Company received aggregate gross proceeds of approximately $2,421 from the exercise
of the Existing Warrants. Additionally, on the date of Stockholder Approval, the exercise price of the 57,299,308 outstanding
Inducement Warrants, was reduced to $0.1765 based on the lowest Nasdaq Minimum Price (as defined in Nasdaq Listing Rule 5635(d)) during the inducement period.
22nd Century (NASDAQ:XXII)
Gráfica de Acción Histórica
De Nov 2024 a Dic 2024
22nd Century (NASDAQ:XXII)
Gráfica de Acción Histórica
De Dic 2023 a Dic 2024