Medidata and Thermo Fisher Scientific’s PPD Clinical Research Business Collaborate to Accelerate Clinical Trials Innovation
28 Febrero 2024 - 7:00AM
Business Wire
Agreement spans wide range of Medidata’s
solutions to enhance clinical trial efficiency
Medidata, a Dassault Systèmes company and leading provider of
clinical trial solutions to the life sciences industry, today
announced it has renewed its relationship with the PPD clinical
research business of Thermo Fisher Scientific Inc.
The agreement features the Medidata Platform, as well as
Medidata Adjudicate and additional Medidata Rave product offerings
that support the PPD clinical research business in advancing its
customers’ drug development programs.
A key element of this relationship is the evolution of PPD
TrueCast, a business solution powered by Medidata AI that shortens
study timelines by combining patient recruitment and site
performance data to deliver powerful predictive models and advanced
analytics.
“Over the past 15 years, we have built a valued connection with
the PPD clinical research business to enhance visibility and
decision-making during clinical trials,” said Janet Butler,
executive vice president and head of global sales, Medidata. “We
look forward to working together to complete studies faster, boost
drug development productivity, and ultimately bring life-changing
treatments to patients sooner.”
Medidata has collaborated with the PPD clinical research
business since 2009 on more than 1,000 clinical studies for
biopharmaceutical and medical device companies across a broad range
of therapeutic areas from oncology to vaccines.
About Medidata Medidata is powering smarter treatments
and healthier people through digital solutions to support clinical
trials. Celebrating 25 years of ground-breaking technological
innovation across more than 32,000 trials and 9 million patients,
Medidata offers industry-leading expertise, analytics-powered
insights, and the largest patient-level historical clinical trial
data set in the world. More than 1 million registered users across
2,200+ customers trust Medidata’s seamless, end-to-end platform to
improve patient experiences, accelerate clinical breakthroughs, and
bring therapies to market faster. A Dassault Systèmes company
(Euronext Paris: FR0014003TT8, DSY.PA), Medidata is headquartered
in New York City and has been recognized as a Leader by Everest
Group and IDC. Discover more at www.medidata.com and follow us
@Medidata.
About Dassault Systèmes Dassault Systèmes, the
3DEXPERIENCE® Company, is a catalyst for human progress. We provide
business and people with collaborative virtual environments to
imagine sustainable innovations. By creating virtual twin
experiences of the real world with our 3DEXPERIENCE platform and
applications, our customers can redefine the creation, production
and life-cycle-management processes of their offer and thus have a
meaningful impact to make the world more sustainable. The beauty of
the Experience Economy is that it is a human-centered economy for
the benefit of all–consumers, patients, and citizens. Dassault
Systèmes brings value to more than 300,000 customers of all sizes,
in all industries, in more than 150 countries. For more
information, visit www.3ds.com.
© Dassault Systèmes. All rights reserved. 3DEXPERIENCE, the 3DS
logo, the Compass icon, IFWE, 3DEXCITE, 3DVIA, BIOVIA, CATIA,
CENTRIC PLM, DELMIA, ENOVIA, GEOVIA, MEDIDATA, NETVIBES, OUTSCALE,
SIMULIA and SOLIDWORKS are commercial trademarks or registered
trademarks of Dassault Systèmes, a European company (Societas
Europaea) incorporated under French law, and registered with the
Versailles trade and companies registry under number 322 306 440,
or its subsidiaries in the United States and/or other
countries.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20240228873753/en/
Medidata PR Medidata.PR@3ds.com
Analyst Relations Medidata.AR@3ds.com
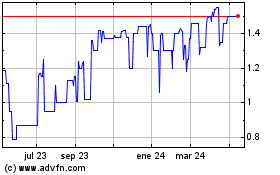
Destiny Media Technologies (TSXV:DSY)
Gráfica de Acción Histórica
De Dic 2024 a Ene 2025
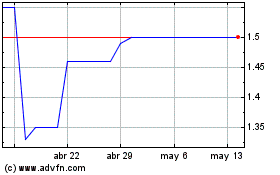
Destiny Media Technologies (TSXV:DSY)
Gráfica de Acción Histórica
De Ene 2024 a Ene 2025