Charles River Introduces Global Biotech Incubator Program
05 Diciembre 2024 - 7:00AM
Business Wire
Program offers biotechnology developers access
to extensive scientific expertise and a wide ecosystem of
discovery, development and manufacturing capabilities
Charles River Laboratories International, Inc. (NYSE: CRL) today
announced the launch of the Charles River Incubator Program (CIP),
specializing in supporting early-stage biotechnology companies in
the discovery, development and phase-appropriate manufacturing of
advanced therapies.
“The Charles River Incubator Program launch, through our Global
Innovation Center of Excellence, demonstrates a continued
commitment to the global biotechnology industry by offering
knowledge, connectivity, and priority access to the Charles River
portfolio,” said Kerstin Dolph, Corporate Senior Vice President,
Global Manufacturing, Charles River.
Building on the success of the Company’s established global Cell
and Gene Therapy (CGT) Accelerator Program (CAP), the CIP focuses
on nurturing innovative start-up and early-stage biotechnology
companies. The objective of the CIP is to form a strong foundation
for commercial viability as its participants gain momentum with the
goal of imparting cost-effective, consultative regulatory and
quality expertise, personnel training initiatives, and enabling
access to laboratory space and equipment. Developers are invited to
apply to be part of the next cohort today:
- CIP – Incubates biotechnology developers in the
discovery phase, 24+ months from Investigational New Drug (IND) or
Clinical Trial Application (CTA) submission.
- CAP – Accelerates advanced therapy developers 18-24
months from IND or CTA submission.
Leveraging a Concept to Cure Portfolio
The CIP and CAP are reinforced by the Company’s Global
Innovation Center of Excellence (CoE), strategically positioned and
fully integrated at the heart of Charles River’s extensive virtual
and physical network. The Global Innovation CoE amalgamates a
cross-functional team of scientific, regulatory and manufacturing
experts in:
- Drug Discovery
- Safety Assessment
- Research Models
- Clinical to Commercial Contract Development and
Manufacturing
- Microbial Solutions
Based out of the Company’s contract development and
manufacturing organization (CDMO) CoE located in the Alderley Park
campus, the UK’s largest, single-site life science ecosystem, the
Global Innovation CoE is geared to foster and accelerate life
sciences innovation, with enhanced access to life sciences and
biotechnology experts.
In alignment with the Company’s strategic areas of interest and
commitment to driving innovation, the applicant selection process
remains at the discretion of Charles River and the next cohort
shortlist will be identified in conjunction with the upcoming Cell
and Gene Therapy Summit, March 4, 2025, in San Francisco, CA.
Our Experts: An Extension of Your Team
Bolstering its concept to cure capabilities, Charles River is
proud to showcase a world-class team of scientific experts across
the drug development continuum, providing broad experience and
consultative advice.
With distinguished operational, quality, and regulatory experts
including previous FDA, EMA, and MHRA advisors, the integrated
network of Charles River professionals has supported thousands of
IND and CTA submissions, reducing overall risk and setting programs
up for success.
Meet with a Charles River expert at Advanced
Therapies Week, January 20-23, 2025, in Dallas, TX to discuss the
Global Innovation CoE, CIP, and how our cross-functional team and
fit-for purpose facilities can support your program from concept to
cure. Schedule a meeting: https://bit.ly/3ZjKKla
About Charles River
Charles River provides essential products and services to help
pharmaceutical and biotechnology companies, government agencies and
leading academic institutions around the globe accelerate their
research and drug development efforts. Our dedicated employees are
focused on providing clients with exactly what they need to improve
and expedite the discovery, early-stage development and safe
manufacture of new therapies for the patients who need them. To
learn more about our unique portfolio and breadth of services,
visit www.criver.com.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20241205936401/en/
Charles River Investor: Todd Spencer Corporate Vice
President, Investor Relations 781.222.6455 todd.spencer@crl.com
Charles River Media: Amy Cianciaruso Corporate Vice
President, Chief Communications Officer 781.222.6168
amy.cianciaruso@crl.com
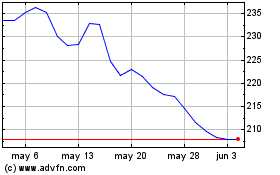
Charles River Laboratories (NYSE:CRL)
Gráfica de Acción Histórica
De Nov 2024 a Dic 2024
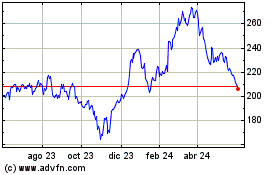
Charles River Laboratories (NYSE:CRL)
Gráfica de Acción Histórica
De Dic 2023 a Dic 2024