As filed with the Securities and Exchange Commission on September 18, 2023
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON DC 20549
FORM S-3
REGISTRATION STATEMENT
UNDER THE SECURITIES ACT OF 1933
Ampio Pharmaceuticals, Inc.
(Exact name of registrant as specified in its charter)
| |
Delaware
(State or other jurisdiction of incorporation
or organization)
|
|
|
26-0179592
(I.R.S. Employer Identification Number)
|
|
9800 Mount Pyramid Court, Suite 400
Englewood, Colorado 80112
(720) 437-6500
(Address, including zip code, and telephone number,
including area code, of registrant’s principal executive offices)
Copies to:
| |
Michael A. Martino
Chief Executive Officer
Ampio Pharmaceuticals, Inc.
9800 Mount Pyramid Court, Suite 400
Englewood, Colorado 80112
(720) 437-6500
(Address, including zip code, and telephone number
including area code, of agent for service)
|
|
|
April Hamlin, Esq.
Ballard Spahr LLP
2000 IDS Center
80 South 8th Street
Minneapolis, MN 55402
(612) 371-3211
|
|
Approximate date of commencement of proposed sale to the public: From time to time after the effective date of this registration statement.
If the only securities being registered on this Form are being offered pursuant to dividend or interest reinvestment plans, please check the following box. ☐
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, other than securities offered only in connection with dividend or interest reinvestment plans, check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a registration statement pursuant to General Instruction I.D. or a post-effective amendment thereto that shall become effective on filing with the Commission pursuant to Rule 462(e) under the Securities Act, check the following box. ☐
If this Form is a post-effective amendment to a registration statement filed pursuant to General Instruction I.D. filed to register additional securities or additional classes of securities pursuant to Rule 413(b) under the Securities Act, check the following box. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act:
| |
Large accelerated filer
☐
|
|
|
Accelerated filer
☐
|
|
| |
Non-accelerated filer
☒
|
|
|
Smaller reporting company
☒
|
|
| |
|
|
|
Emerging growth company
☐
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until this registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
EXPLANATORY NOTE
This registration statement of Ampio Pharmaceuticals, Inc. (“Ampio”) contains two prospectuses:
•
a base prospectus which covers the offering, issuance and sale by Ampio of up to $50,000,000 in the aggregate of the securities identified therein from time to time in one or more offerings; and
•
an at the market offering agreement prospectus covering the offering, issuance and sale of shares of Ampio’s common stock in an aggregate amount of up to $1,250,000 in an “at the market offering” pursuant to an At the Market Offering Agreement entered into between Ampio and H.C. Wainwright & Co., LLC, dated September 18, 2023 (the “ATM Agreement”), which amount is part of the up to $50,000,000 in the aggregate of the securities included in the base prospectus.
The base prospectus immediately follows this Explanatory Note. The specific terms of any securities to be offered pursuant to the base prospectus will be specified in a prospectus supplement to the base prospectus.
The at the market offering agreement prospectus immediately follows the base prospectus. The common stock that may be offered, issued and sold under the at the market offering agreement prospectus is included in the $50,000,000 of securities that may be offered, issued and sold by Ampio under the base prospectus. Upon termination of the ATM Agreement, any portion of Ampio’s common stock included in the relevant prospectus that is not sold pursuant to the ATM Agreement will be available for sale in other offerings by Ampio pursuant to the base prospectus and a corresponding prospectus supplement.
The information in this prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell nor does it seek an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED SEPTEMBER 18, 2023
PROSPECTUS
AMPIO PHARMACEUTICALS
$50,000,000
Common Stock
Preferred Stock
Warrants
Units
We may offer and sell up to $50,000,000 in the aggregate of the securities identified above from time to time in one or more offerings. This prospectus provides you with a general description of the securities we may offer.
Each time we sell securities, we will provide a supplement to this prospectus that will contain specific information about the terms of that offering and the amounts, prices and terms of the securities. The prospectus supplement may also add, update or change information contained in this prospectus. You should carefully read this prospectus and the applicable prospectus supplement before you invest in any of our securities.
We may sell the securities to or through one or more underwriters, dealers and agents, or directly to purchasers, or through a combination of these methods. Each applicable prospectus supplement will provide the terms of the plan of distribution relating to the specific offering. The applicable prospectus supplement also will include the names of any underwriters, dealers and agents involved in the sale of any securities and the purchase price of any securities, our net proceeds from the sale, and any underwriting discounts or commissions and other items constituting underwriters’ compensation.
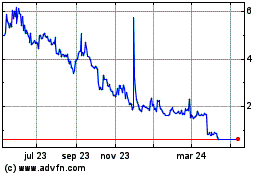
Our common stock is listed on the NYSE American under the symbol “AMPE.” On September 12, 2023, the last reported sale price of our common stock on the NYSE American was $4.17 per share.
As of September 11, 2023, the aggregate market value of the outstanding common stock held by non-affiliates was approximately $3,762,069, which was calculated based on 737,661 outstanding shares of common stock held by non-affiliates and a closing price per share of $5.10. Pursuant to General Instruction I.B.6 of Form S-3, in no event will we sell securities in a public primary offering with a value exceeding more than one-third of our public float in any 12-month period , so long as the aggregate market value of our common stock held by non-affiliates is less than $75.0 million. In the event that subsequent to the effective date of the registration statement of which this prospectus is a part, the aggregate market value of our outstanding common stock held by non-affiliates equals or exceeds $75.0 million, then the one-third limitation on sales shall not apply to additional sales made pursuant to that registration statement. We have not offered any securities pursuant to General Instruction I.B.6. of Form S-3 during the prior 12 calendar month period that ends on and includes the date hereof.
INVESTING IN OUR SECURITIES INVOLVES RISKS. SEE “RISK FACTORS” ON PAGE 4 OF THIS PROSPECTUS AND ANY SIMILAR SECTION CONTAINED IN THE APPLICABLE PROSPECTUS SUPPLEMENT CONCERNING FACTORS YOU SHOULD CONSIDER BEFORE INVESTING IN OUR SECURITIES.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is .
TABLE OF CONTENTS
| |
|
|
|
|
|
1 |
|
|
| |
|
|
|
|
|
2 |
|
|
| |
|
|
|
|
|
4 |
|
|
| |
|
|
|
|
|
4 |
|
|
| |
|
|
|
|
|
4 |
|
|
| |
|
|
|
|
|
4 |
|
|
| |
|
|
|
|
|
5 |
|
|
| |
|
|
|
|
|
9 |
|
|
| |
|
|
|
|
|
10 |
|
|
| |
|
|
|
|
|
11 |
|
|
| |
|
|
|
|
|
12 |
|
|
| |
|
|
|
|
|
12
|
|
|
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement that we filed with the U.S. Securities and Exchange Commission, or the SEC, using a “shelf” registration process. By using a shelf registration statement, we may sell securities from time to time and in one or more offerings up to a total dollar amount of $50,000,000 as further described in this prospectus.
Each time that we offer and sell securities, we will provide a prospectus supplement to this prospectus that contains specific information about the securities being offered and sold and the specific terms of that offering. We may also authorize one or more free writing prospectuses to be provided to you that may contain material information relating to these offerings. The prospectus supplement and any free writing prospectus that we may authorize to be provided to you may also add, update or change information contained in this prospectus with respect to that offering. If there is any inconsistency between the information in this prospectus and the applicable prospectus supplement, you should rely on the prospectus supplement.
This prospectus may not be used to consummate a sale of securities unless it is accompanied by a prospectus supplement.
Before purchasing any securities, you should carefully read both this prospectus, the applicable prospectus supplement and any free writing prospectus we have authorized for use in connection with a specific offering, together with the additional information described under the heading “Where You Can Find More Information; Incorporation by Reference.” We have not authorized any other person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. We will not make an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. You should assume that the information appearing in this prospectus, the applicable prospectus supplement to this prospectus and any free writing prospectus we have authorized for use in connection with a specific offering is accurate as of the date on its respective cover, and that any information incorporated by reference is accurate only as of the date of the document incorporated by reference, unless we indicate otherwise. Our business, financial condition, results of operations and prospects may have changed since those dates.
When we refer to “Ampio,” “we,” “our,” “us” and the “Company” in this prospectus, we mean Ampio Pharmaceuticals, Inc. and any consolidated subsidiaries, unless otherwise specified. When we refer to “you,” we mean the holders of the applicable securities.
WHERE YOU CAN FIND MORE INFORMATION; INCORPORATION BY REFERENCE
Available Information
We file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the public through the SEC’s website at www.sec.gov. Copies of certain information filed by us with the SEC are also available on our website, www.ampiopharma.com, by following the link under “Investors” to “Financial Filings.” Our website is not a part of this prospectus and is not incorporated by reference in this prospectus.
This prospectus and any prospectus supplement are part of a registration statement that we filed with the SEC and do not contain all of the information in the registration statement. The full registration statement may be obtained from the SEC through its website as provided above or from us, as provided below. Forms of the documents establishing the terms of the offered securities are or may be filed as exhibits to the registration statement. Statements in this prospectus or any prospectus supplement about these documents are summaries and each statement is qualified in all respects by reference to the document to which it refers. You should refer to the actual documents for a more complete description of the relevant matters.
Incorporation by Reference
The SEC’s rules allow us to “incorporate by reference” information into this prospectus, which means that we can disclose important information to you by referring you to another document filed separately with the SEC. The information incorporated by reference is deemed to be part of this prospectus, and subsequent information that we file with the SEC will automatically update and supersede that information. Any statement contained in a previously filed document incorporated by reference will be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained in this prospectus modifies or replaces that statement.
We incorporate by reference our documents listed below and any future filings made by us with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934, as amended, which we refer to as the “Exchange Act” in this prospectus, between the date of this prospectus and the termination of the offering of the securities described in this prospectus. We are not, however, incorporating by reference any documents or portions thereof, whether specifically listed below or filed in the future, that are not deemed “filed” with the SEC, including any Compensation Committee report or performance graph or any information furnished pursuant to Items 2.02 or 7.01 of Form 8-K or related exhibits furnished pursuant to Item 9.01 of Form 8-K.
This prospectus and any accompanying prospectus supplement incorporate by reference the documents set forth below that have previously been filed with the SEC:
•
The Annual Report on Form 10-K for the fiscal year ended December 31, 2022, filed on March 27, 2023 and Amendment No. 1 to Annual Report on Form 10-K filed on April 28, 2023;
•
The portions of the definitive proxy statement on Schedule 14A filed on June 14, 2023 and the definitive additional proxy materials filed on July 13, 2023 for the Company’s 2023 Annual Meeting of Stockholders held on July 27, 2023 that are incorporated by reference into the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022;
•
The Quarterly Report on Form 10-Q for the quarter ended March 31, 2023, filed on May 8, 2023 and the Quarterly Report on Form 10-Q for the quarter ended June 30, 2023, filed on August 8, 2023;
•
The Current Reports on Form 8-K filed (but not furnished) on January 17, 2023, March 7, 2023, March 13, 2023, April 18, 2023, May 26, 2023, July 5, 2023, August 1, 2023, and August 31, 2023; and
•
The description of the Company’s common stock contained in Exhibit 4.5 to the Company’s Annual Report on Form 10-K (No. 001-35182) for the fiscal year ended December 31, 2019, filed with the Commission on February 21, 2020, including any amendment or report filed for the purpose of updating such description.
All reports and other documents we subsequently file pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act prior to the termination of this offering, including all such documents we may file with
the SEC after the date of the initial registration statement and prior to the effectiveness of the registration statement, but excluding any information furnished to, rather than filed with, the SEC, will also be incorporated by reference into this prospectus and deemed to be part of this prospectus from the date of the filing of such reports and documents.
You may request a free copy of any of the documents incorporated by reference in this prospectus (other than exhibits, unless they are specifically incorporated by reference in the documents) by writing or telephoning us at the following address:
Ampio Pharmaceuticals, Inc.
9800 Mount Pyramid Court, Suite 400
Englewood, Colorado 80112
Attn: Corporate Secretary
(720) 437-6500
Exhibits to the filings will not be sent, however, unless those exhibits have specifically been incorporated by reference in this prospectus and any accompanying prospectus supplement.
THE COMPANY
About Ampio
Ampio Pharmaceuticals, Inc. (“Ampio” or the “Company”) is a pre-revenue stage biopharmaceutical company that until May 2022 was engaged with the development of Ampion and early development of AR-300, a synthetic version of Ampion and subsequently, a small molecule pre-clinical stage development asset which was designed to leverage the key attributes of Ampion, which is referred to as the OA-20X program. As part of the OA-20X program, we have been focusing our ongoing efforts toward optimizing two potential small molecule formulations to take forward into development. We intend to select one of these optimized formulations to move towards clinical development. Given that these formulations are unique, proprietary, and are neither Ampion nor AR-300 (or derivatives thereof), we now refer to development of these new formulations as the OA.201 program.
Corporate Information
Ampio Pharmaceuticals, Inc. is a Delaware corporation. Our predecessor, DMI Life Sciences, Inc. (“Life Sciences”), was incorporated in Delaware in December 2008. In March 2010, Life Sciences was merged with a subsidiary of Chay Enterprises, Inc., a Colorado corporation. As a result of this merger, Life Sciences stockholders became the controlling stockholders of Chay Enterprises, Inc. Following the merger, we reincorporated in Delaware as Ampio Pharmaceuticals, Inc. in March 2010 and changed our corporate name to Ampio Pharmaceuticals, Inc.
Our principal executive office is located at 9800 Mount Pyramid Court, Suite 400, Englewood, Colorado 80112. Our website address is www.ampiopharma.com. Information contained on our website is not a part of this prospectus, and the inclusion of our website address in this prospectus is an inactive textual reference only.
RISK FACTORS
Investment in any securities offered pursuant to this prospectus and the applicable prospectus supplement involves risks. You should carefully consider the risk factors incorporated by reference to our most recent Annual Report on Form 10-K and any subsequent Quarterly Reports on Form 10-Q or Current Reports on Form 8-K we file after the date of this prospectus, and all other information contained or incorporated by reference into this prospectus, as updated by our subsequent filings under the Exchange Act, and the risk factors and other information contained in the applicable prospectus supplement before acquiring any of such securities. The occurrence of any of these risks might cause you to lose all or part of your investment in the offered securities.
USE OF PROCEEDS
Unless otherwise set forth in an applicable prospectus supplement, we intend to use the net proceeds of any offering of securities sold by us to provide additional funding for general corporate purposes, which may include research and development expenses related to the OA.201 program. When particular securities are offered, the prospectus supplement relating to that offering will set forth our intended use of the net proceeds received from the sale of those securities.
DESCRIPTION OF SECURITIES
This prospectus contains summary descriptions of the common stock, preferred stock, warrants, and units that we may offer and sell from time to time. These summary descriptions are not meant to be complete descriptions of each security. The particular terms of any security will be described in the applicable prospectus supplement and/or other offering materials.
DESCRIPTION OF CAPITAL STOCK
The following description of our capital stock is not complete and may not contain all the information you should consider before investing in our capital stock. This description is summarized from, and qualified in its entirety by reference to, our articles of incorporation, which has been publicly filed with the SEC. See “Where You Can Find More Information; Incorporation by Reference.”
Our authorized capital stock consists of 300,000,000 shares of common stock, $0.0001 par value, and 10,000,000 shares of undesignated preferred stock, $0.0001 par value, of which no preferred shares are issued or outstanding. All shares shall be of one class and one series, except that the board of directors, by its action, may establish more than one class or series.
Common Stock
As of September 12, 2023, there were 755,088 shares of our common stock outstanding. Holders of our common stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders. There is no cumulative voting with respect to the election of directors, with the result that the holders of more than 50% of the shares voted for the election of directors can elect all of the directors. Holders of common stock will be entitled to one vote per share on matters to be voted on by stockholders and also will be entitled to receive such dividends, if any, as may be declared from time to time by our board of directors in its discretion out of funds legally available therefor. The payment of dividends, if ever, on the common stock will be subject to the prior payment of dividends on any outstanding preferred stock, of which there is currently none. Upon our liquidation or dissolution, the holders of common stock will be entitled to receive pro rata all assets remaining available for distribution to stockholders after payment of all liabilities and provision for the liquidation of any shares of preferred stock at the time outstanding. Our stockholders have no conversion, preemptive or other subscription rights and there are no sinking fund or redemption provisions applicable to the common stock.
Transfer Agent
The transfer agent and registrar for our common stock is Equiniti Trust Company.
Listing
Our common stock is listed on the NYSE American under the symbol “AMPE.”
Preferred Stock
Pursuant to our certificate of incorporation, our board of directors has the authority, without further action by the stockholders (unless such stockholder action is required by applicable law or stock exchange listing rules), to designate and issue up to 10,000,000 shares of preferred stock in one or more series, to establish from time to time the number of shares to be included in each such series, to fix the designations, powers, preferences, privileges and relative participating, optional or special rights and the qualifications, limitations or restrictions thereof, including dividend rights, conversion rights, voting rights, terms of redemption and liquidation preferences, any or all of which may be greater than the rights of the common stock, and to increase or decrease the number of shares of any such series, but not below the number of shares of such series then outstanding.
The board of directors, without stockholder approval, can issue preferred stock with voting, conversion or other rights that could adversely affect the voting power and other rights of the holders of common stock. Preferred stock could be issued quickly with terms designed to delay or prevent a change in control of our company or make removal of management more difficult. Additionally, the issuance of preferred stock may have the effect of decreasing the market price of the common stock and may adversely affect the voting power of holders of common stock and reduce the likelihood that common stockholders will receive dividend payments and payments upon liquidation.
Our board of directors will fix the designations, voting powers, preferences and rights of the each series, as well as the qualifications, limitations or restrictions thereof, of the preferred stock of each series that we offer under this prospectus and applicable prospectus supplements in the certificate of designation
relating to that series. We will file as an exhibit to the registration statement of which this prospectus is a part, or will incorporate by reference from reports that we file with the SEC, the form of any certificate of designation that describes the terms of the series of preferred stock we are offering before the issuance of that series of preferred stock. This description will include:
•
the title and stated value;
•
the number of shares we are offering;
•
the liquidation preference per share;
•
the purchase price per share;
•
the dividend rate per share, dividend period and payment dates and method of calculation for dividends;
•
whether dividends will be cumulative or non-cumulative and, if cumulative, the date from which dividends will accumulate;
•
our right, if any, to defer payment of dividends and the maximum length of any such deferral period;
•
the procedures for any auction and remarketing, if any;
•
the provisions for a sinking fund, if any;
•
the provisions for redemption or repurchase, if applicable, and any restrictions on our ability to exercise those redemption and repurchase rights;
•
any listing of the preferred stock on any securities exchange or market;
•
whether the preferred stock will be convertible into our common stock or other securities of ours, including depositary shares and warrants, and, if applicable, the conversion period, the conversion price, or how it will be calculated, and under what circumstances it may be adjusted;
•
whether the preferred stock will be exchangeable into debt securities, and, if applicable, the exchange period, the exchange price, or how it will be calculated, and under what circumstances it may be adjusted;
•
voting rights, if any, of the preferred stock;
•
preemption rights, if any;
•
restrictions on transfer, sale or other assignment, if any;
•
whether interests in the preferred stock will be represented by depositary shares;
•
a discussion of any material or special United States federal income tax considerations applicable to the preferred stock;
•
the relative ranking and preferences of the preferred stock as to dividend rights and rights if we liquidate, dissolve or wind up our affairs;
•
any limitations on issuances of any class or series of preferred stock ranking senior to or on a parity with the series of preferred stock being issued as to dividend rights and rights if we liquidate, dissolve or wind up our affairs; and
•
any other specific terms, rights, preferences, privileges, qualifications or restrictions of the preferred stock.
The General Corporation Law of the State of Delaware, the state of our incorporation, provides that the holders of preferred stock will have the right to vote separately as a class (or, in some cases, as a series) on an amendment to our certificate of incorporation if the amendment would change the par value or, unless the certificate of incorporation provided otherwise, the number of authorized shares of the class or change the powers, preferences or special rights of the class or series so as to adversely affect the class or series, as the case may be. This right is in addition to any voting rights that may be provided for in the applicable certificate of designation.
Delaware Anti-Takeover Law and Provisions of our Certificate of Incorporation and Bylaws
Delaware Anti-takeover law
As a Delaware corporation, we are governed by the provisions of Section 203 of the Delaware General Corporation Law (the “DGCL”), which generally has an anti-takeover effect for transactions not approved in advance by our board of directors. This may discourage takeover attempts that might result in payment of a premium over the market price for the shares of common stock held by stockholders. In general, Section 203 of the DGCL prohibits a publicly held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a three-year period following the time that such stockholder becomes an interested stockholder, unless the business combination is approved in a prescribed manner. A “business combination” includes, among other things, a merger, asset or stock sale or other transaction resulting in a financial benefit to the interested stockholder. An “interested stockholder” is a person who, together with affiliates and associates, owns, or did own within three years prior to the determination of interested stockholder status, 15% or more of the corporation’s voting stock.
Under Section 203 of the DGCL, a business combination between a corporation and an interested stockholder is prohibited unless it satisfies one of the following conditions:
•
before the stockholder became interested, the board of directors approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; or
•
upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder) shares owned by:
•
persons who are directors and also officers; and
•
employee stock plans, in some instances; or
•
at or after the time the stockholder became interested, the business combination was approved by the board of directors of the corporation and authorized at an annual or special meeting of the stockholders by the affirmative vote of at least two-thirds of the outstanding voting stock which is not owned by the interested stockholder.
Staggered board of directors
Our Delaware certificate of incorporation provides that our board of directors will be classified into three classes of directors of approximately equal size at a date selected by the board. Currently our board of directors is not classified. As a result, in most circumstances, a person can gain control of our board only by successfully engaging in a proxy contest at two or more annual meetings.
Advance notice requirements for stockholder proposals and director nominations
Our Delaware bylaws provide that stockholders seeking to bring business before our annual meeting of stockholders, or to nominate candidates for election as directors at our annual meeting of stockholders, must provide timely notice of their intent in writing. To be timely, a stockholder’s notice needs to be delivered to our principal executive offices not later than the close of business on the 90th day nor earlier than the close of business on the 120th day prior to the first anniversary of the preceding year’s annual meeting of stockholders. Our bylaws also specify certain requirements as to the form and content of a stockholders’ meeting. These provisions may preclude our stockholders from bringing matters before our annual meeting of stockholders or from making nominations for directors at our annual meeting of stockholders.
Authorized but unissued shares
Our authorized but unissued shares of common stock and preferred stock are available for future issuances without stockholder approval and could be utilized for a variety of corporate purposes, including
future offerings to raise additional capital, acquisitions and employee benefit plans. The existence of authorized but unissued and unreserved common stock and preferred stock could render more difficult or discourage an attempt to obtain control of us by means of a proxy contest, tender offer, merger or otherwise.
Limitation on liability and indemnification of directors and officers
Our Delaware certificate of incorporation and bylaws provide that our directors and officers will be indemnified by us to the fullest extent authorized by Delaware law as it now exists or may in the future be amended, against all expenses and liabilities reasonably incurred in connection with their service for or on our behalf. Our bylaws permit us to secure insurance on behalf of any officer, director or employee for any liability arising out of his or her actions, regardless of whether Delaware law would permit indemnification.
These provisions may discourage stockholders from bringing a lawsuit against our directors for breach of their fiduciary duty. These provisions also may have the effect of reducing the likelihood of derivative litigation against directors and officers, even though such an action, if successful, might otherwise benefit us and our stockholders. Furthermore, a stockholder’s investment may be adversely affected to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions. We believe that these provisions, insurance and the indemnity agreements are necessary to attract and retain talented and experienced directors and officers.
Other than the four putative class action lawsuits in the United States District Court for the District of Colorado, Kain v. Ampio Pharmaceuticals, Inc., et al., Case No. 22-cv-2105, Maresca v. Martino, et al., Case No. 22-cv-2646-KLM, Marquis v. Martino, et al., Case No. 22-cv-2803-KLM, and McCann v. Martino, et. al. Case No. 2023-cv-30287, and the October 12, 2022 Securities and Exchange Commission investigation into securities law violations, all of which are described in our Annual Report on Form 10-K filed with the SEC on March 27, 2023, there is no pending litigation or proceeding involving any of our directors or officers where indemnification by us would be required or permitted, nor are we aware of any threatened litigation or proceeding that might result in a claim for such indemnification. Insofar as indemnification for liabilities arising under the Securities Act of 1933, or the Act, may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable.
DESCRIPTION OF WARRANTS
We may issue warrants for the purchase of shares of our common stock, preferred stock or any combination thereof. We may issue warrants independently or together with other securities, and the warrants may be attached to or separate from any offered securities. Each series of warrants will be issued under a separate warrant agreement to be entered into between us and the investors or a warrant agent. The following summary of material provisions of the warrants and warrant agreements is subject to, and qualified in its entirety by reference to, all the provisions of the warrant agreement and warrant certificate applicable to a particular series of warrants. The terms of any warrants offered under a prospectus supplement may differ from the terms described below. We urge you to read the applicable prospectus supplement, as well as the complete warrant agreements and warrant certificates that contain the terms of the warrants.
The particular terms of any issue of warrants will be described in the prospectus supplement relating to the issue. Those terms may include:
•
the number or amount of securities purchasable upon the exercise of warrants and the price or prices at which such number of securities may be purchased upon such exercise;
•
the designation, amount and terms of the securities for which the warrants are exercisable;
•
the designation and terms of the other securities, if any, with which the warrants are to be issued and the number of warrants issued with each other security;
•
the date, if any, on and after which the warrants and the related securities will be separately transferable;
•
the terms of any rights to redeem or call the warrants;
•
the date on which the right to exercise the warrants will commence and the date on which the right will expire;
•
a discussion of certain United States federal income tax consequences applicable to the warrants; and
•
any additional terms of the warrants, including terms, procedures, and limitations relating to the exchange, exercise and settlement of the warrants.
Holders of equity warrants will not be entitled to:
•
vote, consent or receive dividends;
•
receive notice as stockholders with respect to any meeting of stockholders for the election of our directors or any other matter; or
•
exercise any rights as stockholders of Ampio.
Each warrant will entitle its holder to purchase the number or amount of securities at the exercise price or prices set forth in, or calculable as set forth in, the applicable prospectus supplement. Unless we otherwise specify in the applicable prospectus supplement, holders of the warrants may exercise the warrants at any time up to the specified time on the expiration date that we set forth in the applicable prospectus supplement. After the close of business on the expiration date, unexercised warrants will become void.
A holder of warrant certificates may exchange them for new warrant certificates of different denominations, present them for registration of transfer and exercise them at the corporate trust office of the warrant agent or any other office indicated in the applicable prospectus supplement. Until any warrants are exercised, the holders of the warrants will not have any rights of holders of the underlying securities, including in the case of shares of common stock or preferred stock, any rights to receive dividends or payments upon any liquidation, dissolution or winding up on the common stock or preferred stock.
DESCRIPTION OF UNITS
We may issue units consisting of any combination of the other types of securities offered under this prospectus in one or more series. We may evidence each series of units by unit certificates that we will issue under a separate agreement. We may enter into unit agreements with a unit agent. Each unit agent will be a bank or trust company that we select. We will indicate the name and address of the unit agent in the applicable prospectus supplement relating to a particular series of units.
If we offer any units, certain terms of that series of units will be described in the applicable prospectus supplement, including, without limitation, the following, as applicable:
•
the title of the series of units;
•
identification and description of the separate constituent securities comprising the units;
•
the price or prices at which the units will be issued;
•
the date, if any, on and after which the constituent securities comprising the units will be separately transferable;
•
a discussion of certain U.S. federal income tax considerations applicable to the units; and
•
any other terms of the units and their constituent securities.
The foregoing description, together with the additional information included in any applicable prospectus supplement, summarizes the general features of the units that we may offer under this prospectus. You should read any prospectus supplement related to the series of units being offered, as well as any unit agreements that contain the terms of the units. Specific unit agreements will contain additional important terms and provisions and we will file as an exhibit to the registration statement of which this prospectus is a part, or will incorporate by reference from another report that we file with the SEC, the form of each unit agreement, if any, relating to units offered under this prospectus.
PLAN OF DISTRIBUTION
The securities being offered by this prospectus may be sold by us to or through underwriters, through dealers (acting as agent or principal), through agents, directly by us to one or more purchasers, through a specific bidding or auction process or otherwise, through a combination of any such methods of sale, and/or through any other methods described in a prospectus supplement.
We may sell the securities from time to time in one or more transactions, including pursuant to underwritten public offerings, negotiated transactions, block trades, in “at the market offerings” within the meaning of Rule 415(a)(4) under the Securities Act, to or through market maker or into an existing trading market, on an exchange or otherwise, or a combination of these methods. We may issue the securities as a dividend or distribution to our existing stockholders. The securities may be sold at a fixed price or prices, which may be changed, at market prices prevailing at the time of sale, at prices related to such prevailing market prices, or at negotiated prices.
Each time that we sell securities covered by this prospectus, we will provide a prospectus supplement or supplements that will describe the method of distribution and set forth the terms and conditions of the offering of such securities, including the offering price of the securities and the proceeds to us, if applicable.
Any compensation paid to underwriters, dealers or agents in connection with the offering of the securities, and any discounts, concessions or commissions allowed by underwriters to participating dealers will be provided in the applicable prospectus supplement. Dealers and agents participating in the distribution of the securities may be deemed to be underwriters, and compensation received by them on resale of the securities may be deemed to be underwriting discounts. If such dealers or agents were deemed to be underwriters, they may be subject to statutory liabilities under the Securities Act.
Agents may from time to time solicit offers to purchase the securities. If required, we will name in the applicable prospectus supplement any agent involved in the offer or sale of the securities and set forth any compensation payable to the agent. Unless otherwise indicated in the prospectus supplement, any agent will be acting on a best efforts basis for the period of its appointment. Any agent selling the securities covered by this prospectus may be deemed to be an underwriter, as that term is defined in the Securities Act, of the securities.
If underwriters are used in a sale, securities will be acquired by the underwriters for their own account and may be resold from time to time in one or more transactions, including negotiated transactions, at a fixed public offering price or at varying prices determined at the time of sale, or under delayed delivery contracts or other contractual commitments. Securities may be offered to the public either through underwriting syndicates represented by one or more managing underwriters or directly by one or more firms acting as underwriters. If an underwriter or underwriters are used in the sale of securities, an underwriting agreement will be executed with the underwriter or underwriters at the time an agreement for the sale is reached. The applicable prospectus supplement will set forth the managing underwriter or underwriters, as well as any other underwriter or underwriters, with respect to a particular underwritten offering of securities, and will set forth the terms of the transactions, including compensation of the underwriters and dealers and the public offering price, if applicable. The prospectus and the applicable prospectus supplement will be used by the underwriters to resell the securities.
If a dealer is used in the sale of the securities, we or an underwriter will sell the securities to the dealer, as principal. The dealer may then resell the securities to the public at varying prices to be determined by the dealer at the time of resale. To the extent required, we will set forth in the prospectus supplement the name of the dealer and the terms of the transactions.
We may directly solicit offers to purchase the securities and we may make sales of securities directly to institutional investors or others. These persons may be deemed to be underwriters within the meaning of the Securities Act with respect to any resale of the securities. To the extent required, the prospectus supplement will describe the terms of any such sales, including the terms of any bidding or auction process, if used.
We may enter into derivative or hedging transactions with third parties, or sell securities not covered by this prospectus to third parties in privately negotiated transactions. In connection with such a transaction, the third parties may sell securities covered by and pursuant to this prospectus and an applicable prospectus
supplement or pricing supplement, as the case may be. If so, the third party may use securities borrowed from us or others to settle such sales and may use securities received from us to close out any related short positions. We may also loan or pledge securities covered by this prospectus and an applicable prospectus supplement to third parties, who may sell the loaned securities or, in an event of default in the case of a pledge, sell the pledged securities pursuant to this prospectus and the applicable prospectus supplement or pricing supplement, as the case may be.
Agents, underwriters and dealers may be entitled under agreements which may be entered into with us to indemnification by us against specified liabilities, including liabilities incurred under the Securities Act, or to contribution by us to payments they may be required to make in respect of such liabilities. If required, the prospectus supplement will describe the terms and conditions of such indemnification or contribution. Some of the agents, underwriters or dealers, or their affiliates may be customers of, engage in transactions with or perform services for us or our subsidiaries in the ordinary course of business.
Under the securities laws of some states, the securities offered by this prospectus may be sold in those states only through registered or licensed brokers or dealers.
Any person participating in the distribution of common stock registered under the registration statement that includes this prospectus will be subject to applicable provisions of the Exchange Act, and the applicable SEC rules and regulations, including, among others, Regulation M, which may limit the timing of purchases and sales of any of our common stock by any such person. Furthermore, Regulation M may restrict the ability of any person engaged in the distribution of our common stock to engage in market-making activities with respect to our common stock. These restrictions may affect the marketability of our common stock and the ability of any person or entity to engage in market-making activities with respect to our common stock.
Certain persons participating in an offering may engage in over-allotment, stabilizing transactions, short-covering transactions and penalty bids in accordance with Regulation M under the Exchange Act that stabilize, maintain or otherwise affect the price of the offered securities. If any such activities will occur, they will be described in the applicable prospectus supplement.
The specific terms of any lock-up provisions in respect of any given offering will be described in the applicable prospectus supplement.
LEGAL MATTERS
Ballard Spahr LLP, Minneapolis, Minnesota, will pass upon certain legal matters relating to the issuance and sale of the securities offered hereby on behalf of Ampio Pharmaceuticals, Inc. Additional legal matters may be passed upon for us or any underwriters, dealers or agents, by counsel that we will name in the applicable prospectus supplement.
EXPERTS
The financial statements of Ampio Pharmaceuticals, Inc. as of December 31, 2022 and 2021, and for the years then ended, incorporated in this prospectus by reference from our Annual Report on Form 10-K for the year ended December 31, 2022, have been audited by Moss Adams LLP, an independent registered public accounting firm, as stated in their report (which report expresses an unqualified opinion and includes an explanatory paragraph related to a going concern uncertainty), which is incorporated herein by reference. Such financial statements are incorporated by reference in reliance upon the report of such firm given their authority as experts in accounting and auditing.
AMPIO PHARMACEUTICALS, INC.
$50,000,000
Common Stock
Preferred Stock
Warrants
Units
PROSPECTUS
, 20
The information in this prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell nor does it seek an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED September 18, 2023
PROSPECTUS
AMPIO PHARMACEUTICALS
Up to $1,250,000 of Shares of Common Stock
We have entered into an at the market offering agreement (the “Offering Agreement”), with H.C. Wainwright & Co., LLC (“Wainwright”) relating to shares of our common stock, par value $0.0001 per share, offered by this prospectus and the accompanying base prospectus. In accordance with the terms of the Offering Agreement, from time to time we may offer and sell shares of our common stock having an aggregate gross sales price of up to $1,250,000 through Wainwright, acting as sales agent.
Our common stock is listed on the NYSE American (“NYSE American”) under the trading symbol “AMPE.” On September 12, 2023, the last reported sale price of our common stock on the NYSE American was $4.17 per share.
Sales of our common stock, if any, under this prospectus may be made in sales deemed to be “at the market offerings” as defined in Rule 415(a)(4) promulgated under the Securities Act of 1933, as amended (the “Securities Act”), including sales made directly on or through the NYSE American, or any other existing trading market in the United States for our common stock, sales made to or through a market maker other than on an exchange or otherwise, directly to Wainwright as principal, in negotiated transactions at market prices prevailing at the time of sale or at prices related to such prevailing market prices and/or in any other method permitted by law. If we and Wainwright agree on any method of distribution other than sales of shares of our common stock on or through the NYSE American or another existing trading market in the United States at market prices, we will file a further prospectus supplement providing all information about such offering as required by Rule 424(b) under the Securities Act. Wainwright is not required to sell any specific number or dollar amount of securities, but it will act as a sales agent using commercially reasonable efforts consistent with its normal trading and sales practices, on mutually agreed terms between Wainwright and us. There is no arrangement for funds to be received in any escrow, trust or similar arrangement.
Wainwright will be entitled to compensation at a fixed commission rate of 3.0% of the gross sales price per share sold. In connection with the sale of our common stock on our behalf, Wainwright will be deemed an “underwriter” within the meaning of the Securities Act and the compensation of Wainwright constitutes underwriting commissions or discounts. We have also agreed to provide indemnification and contributions to Wainwright against certain civil liabilities, including liabilities under the Securities Act.
As of September 11, 2023, the aggregate market value of the outstanding common stock held by non-affiliates was approximately $3,762,069, which was calculated based on 737,661 outstanding shares of common stock held by non-affiliates and a closing price per share of $5.10. Pursuant to General Instruction I.B.6 of Form S-3, in no event will we sell securities in a public primary offering with a value exceeding more than one-third of our public float in any 12-month period, so long as the aggregate market value of our common stock held by non-affiliates is less than $75.0 million. In the event that subsequent to the effective date of this registration statement, the aggregate market value of our outstanding common stock held by non-affiliates equals or exceeds $75.0 million, then the one-third limitation on sales shall not apply to additional sales made pursuant to this registration statement until the filing of our Annual Report on Form 10-K for the year ended December 31, 2023. We have not offered any securities pursuant to General Instruction I.B.6. of Form S-3 during the prior 12 calendar month period that ends on and includes the date hereof.
INVESTING IN OUR SECURITIES INVOLVES RISKS. SEE “RISK FACTORS” ON PAGE S-3 OF THIS PROSPECTUS CONCERNING FACTORS YOU SHOULD CONSIDER BEFORE INVESTING IN OUR COMMON STOCK.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
H.C. Wainwright & Co.
The date of this prospectus is , 2023.
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement that we filed with the U.S. Securities and Exchange Commission, or the SEC, using a “shelf” registration process. The $1,250,000 of common stock that may be offered, issued and sold under this prospectus is included in the $50,000,000 of securities that may be offered, issued and sold by us pursuant to the registration statement.
We provide information to you about this offering of shares of our common stock in this prospectus, which describes the details regarding this offering. If information in this prospectus is inconsistent with documents incorporated by reference in this prospectus filed prior to the date of this prospectus, you should rely on this prospectus. However, if any statement in one of these documents is inconsistent with a statement in another document having a later date (for example, a document incorporated by reference in this prospectus), the statement in the document having the later date modifies or supersedes the earlier statement as our business, financial condition, results of operations and prospects may have changed since the earlier dates.
You should rely only on the information contained in, or incorporated by reference into, this prospectus and in any free writing prospectus that we may authorize for use in connection with this offering. We have not, and Wainwright has not, authorized any other person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it.
We are not, and Wainwright is not, making an offer to sell or soliciting an offer to buy our securities in any jurisdiction in which an offer or solicitation is not authorized or in which the person making that offer or solicitation is not qualified to do so or to anyone to whom it is unlawful to make an offer or solicitation.
You should assume that the information appearing in this prospectus, the documents incorporated by reference into this prospectus, and in any free writing prospectus that we may authorize for use in connection with this offering, is accurate only as of the date of those respective documents. Our business, financial condition, results of operations and prospects may have changed since those dates. You should read this prospectus, the documents incorporated by reference into this prospectus, and any free writing prospectus that we may authorize for use in connection with this offering, in their entirety before making an investment decision. You should also read and consider the information in the documents to which we have referred you in the sections of this prospectus entitled “Where You Can Find Additional Information; Incorporation by Reference.”
We are offering to sell, and seeking offers to buy, shares of common stock only in jurisdictions where offers and sales are permitted. The distribution of this prospectus and the offering of the common stock in certain jurisdictions may be restricted by law. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the common stock and the distribution of this prospectus outside the United States. This prospectus does not constitute, and may not be used in connection with, an offer to sell, or a solicitation of an offer to buy, any securities offered by this prospectus by any person in any jurisdiction in which it is unlawful for such person to make such an offer or solicitation.
When we refer to “Ampio,” “we,” “our,” “us” and the “Company” in this prospectus, we mean Ampio Pharmaceuticals, Inc. and any consolidated subsidiaries, unless otherwise specified. When we refer to “you,” we mean the holders of the applicable securities.
PROSPECTUS SUMMARY
This summary highlights selected information contained elsewhere or incorporated by reference in this prospectus. This summary does not contain all the information you should consider before investing in our common stock. You should read and consider carefully the more detailed information in this prospectus, including the factors described under the heading “Risk Factors” in this prospectus and the financial statements, notes to financial statements, financial and other information incorporated by reference in this prospectus, as well as the information included in any free writing prospectus that we have authorized for use in connection with this offering, before making an investment decision.
About Ampio
Ampio Pharmaceuticals, Inc. (“Ampio” or the “Company”) is a pre-revenue stage biopharmaceutical company that until May 2022 was engaged with the development of Ampion and early development of AR-300, a synthetic version of Ampion and subsequently, a small molecule pre-clinical stage development asset which was designed to leverage the key attributes of Ampion, which is referred to as the OA-20X program. As part of the OA-20X program, we have been focusing our ongoing efforts toward optimizing two potential small molecule formulations to take forward into development. We intend to select one of these optimized formulations to move towards clinical development. Given that these formulations are unique, proprietary, and are neither Ampion nor AR-300 (or derivatives thereof), we now refer to development of these new formulations as the OA.201 program.
Corporate Information
Ampio Pharmaceuticals, Inc. is a Delaware corporation. Our predecessor, DMI Life Sciences, Inc. (“Life Sciences”), was incorporated in Delaware in December 2008. In March 2010, Life Sciences was merged with a subsidiary of Chay Enterprises, Inc., a Colorado corporation. As a result of this merger, Life Sciences stockholders became the controlling stockholders of Chay Enterprises, Inc. Following the merger, we reincorporated in Delaware as Ampio Pharmaceuticals, Inc. in March 2010 and changed our corporate name to Ampio Pharmaceuticals, Inc.
Our principal executive office is located at 9800 Mount Pyramid Court, Suite 400, Englewood, Colorado 80112. Our website address is www.ampiopharma.com. Information contained on our website is not a part of this prospectus, and the inclusion of our website address in this prospectus is an inactive textual reference only.
The Offering
Ampio Pharmaceuticals, Inc.
Common stock offered by us:
Shares of our common stock having an aggregate offering price of up to $1,250,000.
Common stock outstanding immediately after this
offering:
Up to 1,054,904 shares, assuming sales at a price of $4.17 per share, which was the closing price of our common stock on the NYSE American on September 12, 2023. The actual number of shares issued, if any, will vary depending on the sales price under this offering.
“At the market offering” that may be made from time to time through our sales agent, Wainwright. See “Plan of Distribution.”
We intend to use the net proceeds from this offering for general corporate purposes, which may include research and development expenses related to the OA.201 program. See “Use of Proceeds.”
Our common stock is currently listed on the NYSE American under the symbol “AMPE.”
You should read the “Risk Factors” section of this prospectus and in the documents incorporated by reference in this prospectus for a discussion of factors to consider before deciding to purchase shares of our common stock.
The number of our shares of common stock to be outstanding after this offering is based on 755,144 shares of common stock outstanding as of June 30, 2023 after giving effect to the 20-to-1 reverse stock split effective at 4:01 p.m., Eastern Time, on September 11, 2023, and excludes the following, all of which also were adjusted for the 20-to-1 reverse stock split:
•
53,257 shares issuable upon the exercise of outstanding common stock warrants at a weighted average exercise price of $318.80 per share;
•
12,719 shares issuable upon the exercise of outstanding stock options at a weighted average exercise price of $317.40 per share; and
•
1,200,000 additional shares reserved for future issuance under our 2023 Stock and Incentive Plan.
RISK FACTORS
Investing in our common stock involves a high degree of risk. You should carefully review the risks and uncertainties described below and under the section titled “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2022, as updated by our quarterly, annual and other reports and documents that we have filed or subsequently file that are incorporated by reference into this prospectus, before deciding whether to purchase any common stock in this offering. Each of the risk factors could adversely affect our business, operating results and financial condition, as well as adversely affect the value of an investment in our common stock, and the occurrence of any of these risks might cause you to lose all or part of your investment. Additional risks not presently known to us or that we currently believe are immaterial may also significantly impair our business operations.
Risks Related to this Offering
If you purchase shares of common stock sold in this offering, you may experience immediate and substantial dilution in the per share net tangible book value as a result of this offering.
Because the price per share of our common stock being offered may be higher than the net tangible book value per share of our common stock, you will experience dilution to the extent of the difference between the offering price per share of common stock you pay in this offering and the net tangible book value per share of our common stock immediately after this offering. Our net tangible book value as of June 30, 2023, was approximately $6.6 million, or $8.69 per share of common stock (after giving effect to the 20-to-1 reverse stock split effective on September 11, 2023).
Because the sales of the shares offered hereby will be made directly into the market, the prices at which we sell these shares will vary and these variations may be significant. Purchasers of the shares we sell, as well as our existing stockholders, will experience significant dilution if we sell shares at prices significantly below the price at which they invested.
If you purchase shares of our common stock in this offering, you may experience future dilution as a result of future equity offerings or other equity issuances.
In order to raise additional capital, we believe that we will offer and issue additional shares of our common stock or other securities convertible into or exchangeable for our common stock in the future. We cannot assure you that we will be able to sell shares or other securities in any other offering at a price per share that is equal to or greater than the price per share paid by purchasers in this offering, and investors purchasing other securities in the future could have rights superior to existing stockholders. The price per share at which we sell additional shares of our common stock or other securities convertible into or exchangeable for our common stock in future transactions may be higher or lower than the price per share in this offering.
In addition, we have a significant number of stock options and warrants outstanding. To the extent that outstanding stock options or warrants have been or may be exercised or other shares issued, you may experience further dilution. Further, we may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans.
Because we will have broad discretion and flexibility in how the net proceeds from this offering are used, we may use the net proceeds in ways in which you disagree.
We intend to use the net proceeds for general corporate purposes. See “Use of Proceeds” on page S-12 for additional information. General corporate purposes may include research and development expenses related to the OA.201 program. We have not allocated specific amounts of the net proceeds from this offering for any of the foregoing purposes. Accordingly, our management will have significant discretion and flexibility in applying the net proceeds of this offering. You will be relying on the judgment of our management with regard to the use of these net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the net proceeds are being used appropriately. It is possible that the net proceeds will be invested in a way that does not yield a favorable, or any, return for us. The failure of our management to use such funds effectively could have a material adverse effect on our business, financial condition, operating results and cash flow.
The actual number of shares we will issue under the Offering Agreement, at any one time or in total, is uncertain.
Subject to certain limitations in the Offering Agreement and compliance with applicable law, we have the discretion to deliver a placement notice to Wainwright at any time throughout the term of the Offering Agreement. The number of shares that are sold by Wainwright after delivering a placement notice will fluctuate based on the market price of the shares of common stock during the sales period and limits we set with Wainwright. Because the price per share of each share sold will fluctuate based on the market price of our common stock during the sales period, it is not possible at this stage to predict the number of shares that will be ultimately issued.
The common stock offered hereby will be sold in “at-the-market” offerings, and investors who buy shares at different times will likely pay different prices.
Investors who purchase shares in this offering at different times will likely pay different prices, and so may experience different outcomes in their investment results. We will have discretion, subject to market demand, to vary the timing, prices and numbers of shares sold, and there is no minimum or maximum sales price. Investors may experience a decline in the value of their shares as a result of share sales made at prices lower than the prices they paid.
If we are unable to maintain our listing on NYSE American, it will become more difficult to sell our common stock in the public market.
Our common stock was previously suspended from trading on the NYSE American. The staff of NYSE Regulation withdrew its delisting determination and on November 22, 2022, our common stock re-commenced trading on the NYSE American. For our common stock to continue trading on the NYSE American, we must comply with various listing standards, including that the Company maintain minimum stockholders’ equity of $6.0 million. As of June 30, 2023, our total stockholders’ equity was $6,560,000. If we are unable to continue to meet the NYSE American’s listing standards for any reason, our common stock could be delisted from the NYSE American. If delisted, we may seek to list our securities on a different stock exchange or, if one or more broker-dealer market makers comply with applicable requirements, the OTC. Listing on such other market or exchange likely would reduce the liquidity of our common stock. If our common stock were to trade in the OTC market, an investor would find it more difficult to dispose of, or to obtain accurate quotations for the price of, the common stock.
A delisting from the NYSE American and failure to obtain listing on another market or exchange would subject our common stock to so-called penny stock rules that impose additional sales practice and market-making requirements on broker-dealers who sell or make a market in such securities. Consequently, removal from the NYSE American and failure to obtain listing on another market or exchange could affect the ability or willingness of broker-dealers to sell or make a market in our common stock and the ability of purchasers of our common stock to sell their securities in the secondary market.
If we cannot continue to satisfy the NYSE American continued listing requirements and rules, our securities may be delisted, which could negatively impact the price of our securities.
Currently, our common stock is listed on the NYSE American. In order to maintain our listing on the NYSE American, we must continue to satisfy the applicable continued listing requirements and rules, including such rules and requirements relating to minimum share price, minimum stockholders’ equity and a minimum number of public stockholders.
The NYSE American may delist the securities of any issuer if, in its opinion, the issuer’s financial condition and/or operating results appear unsatisfactory; if it appears that the extent of public distribution or the aggregate market value of the security has become so reduced as to make continued listing on the NYSE American inadvisable; if the issuer sells or disposes of principal operating assets or ceases to be an operating company; if an issuer fails to comply with the NYSE American’s listing requirements; if an issuer’s common stock sells at what the NYSE American considers a “low selling price” (generally trading below $0.20 per share for an extended period of time); maintaining minimum stockholders’ equity at least $6.0 million; or if any other event occurs or any condition exists which makes continued listing on the
NYSE American, in its opinion, inadvisable. As of June 30, 2023, our total stockholders’ equity was $6,560,000. We are actively monitoring our stockholders’ equity, but our efforts to increase our stockholders’ equity by selling equity securities may be hampered by our depressed stock price, which may be further depressed by any future non-compliance with the minimum stockholders’ equity requirement.
If the NYSE American delists our securities, we could face significant consequences, including:
•
a limited availability for market quotations for our securities;
•
reduced liquidity with respect to our securities;
•
a determination that our common stock is a “penny stock,” which will require brokers trading in our common stock to adhere to more stringent rules and possibly result in reduced trading;
•
activity in the secondary trading market for our common stock;
•
reduced opportunities for strategic alternatives;
•
limited amount of news and analyst coverage; and
•
a decreased ability to issue additional securities or obtain additional financing in the future.
While we continue to monitor our compliance with the NYSE American continued listing requirements, there can be no assurance that we will be able to continue to comply with the NYSE American listing requirements.
Risks Related to Product Development, Strategic Alternatives and Our Business
We are dependent on the success of our technology and we cannot be certain that any preclinical data will support its further development.
As part of the OA-20X program, we have been focusing our ongoing efforts toward optimizing two potential small molecule formulations to take forward into development as a potential treatment of osteoarthritis. We intend to select one of these optimized formulations to move towards clinical development. Given that these formulations are unique, proprietary, and are neither Ampion nor AR-300 (or derivatives thereof), we now refer to development of these new formulations as the OA.201 program. These formulations have demonstrated promising anti-inflammatory properties in vitro and protection of cartilage in preclinical rat meniscal tear studies.
We expect to have some preclinical results in the third quarter of 2023. The future development of the OA.201 program will depend on the success and level of positive data from the current and near-term preclinical studies. At this time, OA.201 is our only development program and the two potential small molecule formulations are our only potential product in development. We do not have any products that are approved for commercial sale and may never be able to develop marketable products. We have generated no revenue from sales of any products or services.
Any future product candidate from the OA.201 program will require additional development, including further preclinical studies, as well as clinical trials, optimization of their formulation, and regulatory clearances, before they can be commercialized. Positive results obtained during early development do not necessarily mean later development will succeed. If formulations in the OA.201 program fail to demonstrate sufficiently positive data at any time or we determine there are other barriers to successful commercialization, we may abandon the OA.201 program.
We believe that sufficiently positive pre-clinical data in the OA.201 program is a condition to future capital raising to fund development work relating to a formulation. If our available cash resources are insufficient to fund our expenses (including relating to legal proceedings) and the development of a formulation and/or completion of a strategic transaction, we may implement further cost reduction and other cash-focused measures to manage liquidity and we may pursue a plan of liquidation or dissolution of Ampio or seek bankruptcy protection. If we decided to cease operations and dissolve and liquidate our assets, it is unclear to what extent we would be able to pay our obligations. In such a circumstance and in light of the Company’s current liquidity position and pending legal matters, it is unlikely that cash would be available for distributions to stockholders.
We may explore strategic alternatives but there can be no assurance that we will be successful in identifying or completing any strategic alternative or that any such strategic alternative will yield value for our stockholders.
Given the risks associated with preclinical drug development, we continue to opportunistically identify and evaluate strategic opportunities to acquire or license later stage assets and/or merge with companies that have those assets. To-date, we have evaluated more than a dozen such opportunities. Finding attractive and affordable assets and/or merger partners has been challenging due to competition from the high number of companies with failed clinical trials that are pursuing the same strategy; in addition to our circumstances regarding our cash balance, the uncertainty around our continued listing on a major exchange, and the potential risks associated with ongoing legal and regulatory matters.
The process of exploring strategic alternatives is time consuming, and our board of directors has not set a timetable for the conclusion of its review of strategic alternatives. Our review of strategic options and alternatives could result in, among other things, a sale, merger, reverse merger, consolidation or business combination, asset divestiture, partnering, licensing or other collaboration agreements, or potential acquisitions, recapitalizations or restructurings, or in one or more transactions. There can be no assurance that the exploration of strategic alternatives is the correct strategy to pursue or that it will result in the identification or consummation of any transaction. Certain potential strategic transaction alternatives, if available and achieved, could result in substantial dilution to existing stockholders and have a material adverse effect on the market price of Ampio’s common stock.
Additionally, in light of our current stock price and ongoing legal matters, there can be no assurance that we will have sufficient capital resources to fund any strategic transaction, if available. If we raise additional funds through the issuance of equity securities, including as part of a strategic transaction, it could result in substantial dilution to our existing stockholders, increased fixed payment obligations, and any issued securities may have rights senior to those of the Company’s shares of common stock.
We also cannot assure that any potential transaction or other strategic alternative, if identified, evaluated and consummated, will provide greater value to our stockholders or otherwise successfully address the challenges associated with our dependence upon a single preclinical asset for our business. Any potential transaction would be dependent upon a number of factors that may be beyond our control, including, among other factors, market conditions, industry trends, the interest of third parties in our business or preclinical development progress, and the availability of financing to potential buyers on reasonable terms.
We rely on third parties for critical resources, including for development of the OA.201 program, and we may not be able to manage these third parties to provide timely, high quality, and cost-effective services to us.
As of September 12, 2023, we had five full-time employees. These employees are focused on project management, accounting and finance, IT, and corporate governance. As part of our development strategy, we have determined to outsource and contract with independent organizations, advisors and consultants to provide specific services, such as orthopedic expertise to assist with designing and implementing preclinical, clinical and regulatory development plans for the OA.201 program. We have also determined to contract with third parties for other business-related functions such as finance and accounting and administrative support. We believe that we will be able to obtain support and relevant expertise from the third party resources at an overall lower cost profile than hiring our own employees as well as benefit from greater range of expertise from third party resources than may be found in any number of employees. However, there can be no assurance that our strategy of using third parties will result in these intended benefits.
We currently rely, and for the foreseeable future will continue to rely, in substantial part on third parties to provide critical services to us. We cannot assure you that the services of these third parties will continue to be available to us on a timely basis when needed, or that we can find qualified replacements. If the cost of these services increase for any reason, or if these third parties are unable or unwilling provide services to us, we may have to find another third party to provide these services which could result in interruptions, increased costs, delays, in other challenges in the development of formulations in the OA.201 program, in the execution of strategic alternatives or strategic transactions, in our ability to fulfill our SEC reporting obligation or comply with the continued listing requirements of NYSE American, or in the proper functioning of other business functions. In addition, if we are unable to effectively manage our outsourced activities or if the quality or accuracy of the services provided by third-party service providers is compromised
for any reason, we may similarly suffer from interruptions, increased costs, delays and from the other challenges described above. We cannot assure you that we will be able to manage our existing third-party service providers or find other competent outside contractors and consultants on economically reasonable terms, or at all.
Risks Related to Our Financial Position and Capital Requirements
Our history of losses and our cash resources available to execute our business plan over the next twelve months raise substantial doubt about our ability to continue as a going concern.
In 2022, we experienced net losses of $16.3 million, had no revenue other than interest income, and used $21.1 million in cash to fund our operations. Due to the current level of liquidity at December 31, 2022 and the projected shortfall to cover operating expenses requiring cash for a period of 12 months from the report date of the annual report, management has expressed substantial doubt as to our ability to continue as a going concern.
As of June 30, 2023, our source of liquidity consisted of approximately $7.0 million of cash and cash equivalents and approximately $1.4 million of an insurance recovery receivable. While we have implemented cost reductions, our finite cash resources available to execute our business plan present the risk that we will not have sufficient cash available in the amount or at the time we need it to fund our ongoing operations and execute our business plan involving the development of formulations in the OA.201 program and strategic alternatives over the next twelve months.
Our capital needs are based upon management estimates as to future expense and potential future capital raising activity, which involve significant judgment particularly given that we are pursuing a strategic alternatives process and cannot predict the duration or expense associated with this process. Additionally, the expense associated with and outcome of any legal proceeding is not possible to determine at this time. We cannot assure you that additional financing will be available in the amount or at the time we need it, or that it will be available on acceptable terms or at all. We believe that positive pre-clinical data formulations in the OA.201 program is a condition to future capital raising to fund further OA.201 program development and an identifiable, attractive strategic transaction is a condition to future capital raising to fund that strategic transaction.
If our available cash resources are insufficient to fund our expenses (including relating to legal proceedings) and OA.201 program development and/or completion of a strategic transaction, we may implement further cost reduction and other cash-focused measures to manage liquidity and we may pursue a plan of liquidation or dissolution of Ampio or seek bankruptcy protection. If we decided to cease operations and dissolve and liquidate our assets, it is unclear to what extent we would be able to pay our obligations. In such a circumstance and in light of the Company’s current liquidity position and pending legal matters, it is unlikely that cash would be available for distributions to stockholders. If we decided to cease operations and dissolve and liquidate our assets, we instead may seek bankruptcy protection, which could cause the value of any investment in the shares of common stock of the Company to decline to zero.
We are involved in legal proceedings that likely will adversely affect our financial position and our pursuit of strategic alternatives.
We are involved in and may in the future be involved in legal proceedings. Regardless of whether any claims against us are valid or whether we are liable, litigation claims or regulatory proceedings have been and will be expensive and time consuming to defend against, require us to advance substantial amounts to director and officer defendants for their defense of the claims, and result in the diversion of management attention and resources from our business and strategic goals. Insurance may not be available at all or in sufficient amounts to cover any liabilities with respect to these or other matters. The outcome of any legal proceeding is not possible to determine at this time.
If we are liable in any legal proceeding, such proceeding could result in injunctions or other equitable relief, settlements, penalties, fines or damages that could materially adversely affect our results of operations, cash position and the conduct of our business and pursuit of strategic alternatives. The uncertainty relating to any legal proceedings may also impair our ability to raise capital. Given our limited cash resources,
significant liabilities resulting from legal proceedings could force us to implement further cost reduction and other cash-focused measures to manage liquidity, including potential termination of our strategic alternatives process, and the Company may pursue a plan of liquidation or dissolution of the Company or seek bankruptcy protection, any of which could cause the value of any investment in the Company to decline to zero. If we decided to cease operations and dissolve and liquidate our assets, it is unclear to what extent we would be able to pay our obligations. In such a circumstance and in light of the Company’s current liquidity position and pending legal matters, it is unlikely that cash would be available for distributions to stockholders.
We will need additional capital to fund our future operations, the OA.201 program, and any strategic transaction, as well as to assure compliance with the NYSE American minimum stockholders’ equity requirement.
As of June 30, 2023, we had $7.0 million of cash and cash equivalents and an insurance recovery receivable of $1.4 million which we expect can fund our operations through the first quarter of 2024. Our future capital requirements will depend on, and could increase significantly as a result of, many factors including:
•
progress in and the costs of preclinical studies and any future clinical trials and research and development relating to formulations as part of the OA.201 program;
•
costs relating to the exploration of strategic alternatives and costs associated with pursuit of any strategic transaction, including any consideration we may pay to acquire or license later stage assets and/or merge with companies that have those assets or other transaction or series of transactions;
•
the costs of defending lawsuits and other claims, such as the four currently pending cases (a securities fraud class action and three derivative actions) and the pending SEC investigation, and any amounts paid to resolve those legal matters;
•
the costs involved in filing, prosecuting, enforcing, and defending patent claims and other intellectual property rights;
•
efforts to cure any future non-compliance with the $6.0 million minimum stockholders’ equity or other requirement of the NYSE American; and
•
the costs of sustaining our corporate overhead requirements, including D&O insurance, and hiring and retaining necessary personnel or third parties.
Our capital needs are based upon management estimates as to future expense and potential future capital raising activity, which involve significant judgment. In particular, the expense associated with, and outcome of, any legal proceeding is not possible to determine at this time. Insurance may not be available at all or in sufficient amounts to cover any liabilities with respect to the pending legal proceedings.
We cannot assure you that additional financing will be available in the amount or at the time we need it, or that it will be available on acceptable terms or at all. We believe that positive pre-clinical data for a formulation in the OA.201 program is a condition to pursue future capital raising to fund the OA.201 program and an identifiable, attractive strategic transaction is a condition to pursue future capital raising to fund that strategic transaction.
As of June 30, 2023, our total stockholders’ equity was $6,560,000 as compared to $6.0 million minimum stockholders’ equity required by NYSE American. We are actively monitoring our stockholders’ equity, but our efforts to increase our stockholders’ equity by selling equity securities may be hampered by our depressed stock price, which may be further depressed by any future non-compliance with the minimum stockholders’ equity requirement.
We may obtain future additional financing by incurring indebtedness or from an offering of our equity securities or any of these. If we raise equity financing, our stockholders may experience significant dilution of their ownership interests and the value of shares of our common stock could decline. Our efforts to raise additional funds from the sale of equity may be hampered by the currently depressed trading price of our common stock, by pending legal matters, and by our prior non-compliance or any future non-compliance with the continued listing requirements of the NYSE American. If we raise additional equity financing, new investors may demand rights, preferences or privileges senior to those of existing holders of common stock. Our efforts to raise funds by incurring indebtedness may be hampered by our limited assets to secure
debt and the absence of any revenue to support debt service payments. Any financing would likely have covenants that would affect the manner in which we conduct our business, including by restricting our ability to incur indebtedness or sell additional equity securities.
If we cannot timely raise any needed funds, we may implement further cost reduction and other cash-focused measures to manage liquidity and we may pursue a plan of liquidation or dissolution of Ampio or seek bankruptcy protection. If we decided to cease operations and dissolve and liquidate our assets, it is unclear to what extent we would be able to pay our obligations. In such a circumstance and in light of the Company’s current liquidity position and pending legal matters, it is unlikely that cash would be available for distributions to stockholders.
Risks Related to Our Intellectual Property
We are dependent on adequate protection of our patent and proprietary rights.
We rely on patents, trade secrets, trademarks, copyrights, know-how, and contractual provisions to establish and protect our intellectual property rights. As part of the OA.201 program, we own a number of United States provisional patent applications covering proprietary small molecule pharmaceutical formulations, as well as their uses, formulations, and manufacturing processes. We anticipate filing additional patent applications in the future, covering new discoveries, formulations and/or research advancements in or relating to formulations, as needs arise. If we do not diligently pursue our intellectual property rights or they are invalidated or circumvented, our development of the OA.201 program and any future commercialization of any formulation of the OA.201 program will be adversely affected. We must successfully defend these rights against third-party challenges.
However, these legal means afford us only limited protection and may not adequately protect our rights or remedies to gain or keep any advantages we may have over other companies seeking to commercialize product candidates similar or identical to formulations from the OA.201 program. If the scope of any patent protection we obtain is not sufficiently broad, or if we lose any of our patent or other legal protections of our intellectual property rights, our ability to prevent our competitors from commercializing product candidates similar or identical to formulations from the OA.201 program would be adversely affected.
Additionally, competitors, many of which have substantial resources and may make substantial investments in competing products and product candidates, may apply for and obtain patents that will prevent, limit, or interfere with our ability to develop, manufacture or market any product relating to the OA.201 program. Further, while we do not believe that our claimed intellectual property interferes with the rights of others, third parties may nonetheless assert patent infringement claims against us in the future.
Costly litigation may be necessary to enforce patents issued or licensed to us, to protect trade secrets or “know-how” we own, to defend us against claimed infringement of the rights of others or to determine the ownership, scope, or validity of our proprietary rights and the rights of others.
Any claim of infringement against us may involve significant liabilities to third parties, could require us to seek licenses from third parties, and could prevent us from manufacturing, selling, or using any products that we may develop. The occurrence of this litigation or the effect of an adverse determination in any of this type of litigation could have a material adverse effect on our business and financial condition
Risks Related to Our Common Stock
The price of our stock has been extremely volatile and may continue to be volatile and fluctuate substantially, which could result in substantial losses for purchasers of our common stock.
The price of our common stock has been extremely volatile and may continue to be so, particularly as we confront and attempt to address the risks relating to the OA.201 program, pending legal proceedings, our strategic alternatives process, our capital resources, and the other risk factors described in this section. Additionally, the stock market in general and the market for pre-revenue stage biopharmaceutical companies have experienced extreme volatility that has often been unrelated to the operating performance of a
particular company. The following factors, in addition to the other risk factors described in this section, may also have a significant impact on the market price of our common stock:
•
any actual or perceived adverse developments in the OA.201 program, including timing and status of studies and study results;
•
uncertainties relating to the strategic alternatives or any strategic transaction, including actual or perceived adverse developments in this process or the announcement or pendency of any transaction;
•
any announcements of developments with, or comments by, the FDA or other regulatory authorities that may impact Ampio or the potential regulatory path for a formulation from the OA.201 program;
•
developments in any legal proceeding in which we are or may become involved;
•
any announcements concerning our retention or loss of key employees;
•
our continued compliance with NYSE American listing requirements and any action taken by the NYSE American relating to our common stock;
•
announcements of patent issuances or denials, infringement claims or other intellectual property related developments;
•
announcements of the introduction of new competitive products by other companies;
•
future issuances of common stock or other securities;
•
sales of stock by our stockholders holding a significant position in the Company;
•
economic and other external factors beyond our control; and
•
public confidence in the securities markets and regulation by or of the securities market.
A significant drop in the price of our stock could expose us to the risk of securities class action lawsuits, which could result in substantial costs and divert management’s attention and resources, which could adversely affect our business.
The market for the Company’s common stock may be thinly traded and stockholders may be unable to sell at or near ask prices or at all.
The Company’s common stock may be thinly traded on the NYSE American, meaning that the number of persons interested in purchasing the Company’s shares at or near ask prices at any given time may be relatively small or non-existent. Consequently, there may be periods of several days or more when trading activity in the Company’s shares is minimal or non-existent. The Company cannot assure investors that a broader or more active public trading market for the Company’s common stock will develop or be sustained or that current trading levels will be maintained.
Anti-takeover provisions in our charter and bylaws and in Delaware law could prevent or delay a change in control of Ampio.
Provisions of our certificate of incorporation and bylaws may discourage, delay, or prevent a merger or acquisition that stockholders may consider favorable, including transactions in which stockholders might otherwise receive a premium for their shares. These provisions include:
•
requiring supermajority stockholder voting to effect certain amendments to our certificate of incorporation and bylaws;
•
restricting the ability of stockholders to call special meetings of stockholders; and
•
establishing advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted on by stockholders at stockholder meetings.
If securities or industry analysts do not publish research or reports or publish unfavorable research or reports about our business, our stock price and trading volume could decline.
The trading market for our common stock depends in part on the research and reports that securities or industry analysts publish about us, our business, our market or our competitors. Coverage by securities and industry analysts and initiation of coverage in the future is uncertain at this time. If securities or industry analysts do not cover our company, the trading price for our stock could continue to be negatively impacted. Additionally, if any analyst downgrades our stock, our stock price would likely decline.
USE OF PROCEEDS
We intend to use the net proceeds of from the sale of our securities under this prospectus to provide additional funding for general corporate purposes, which may include research and development expenses related to the OA.201 program. Because there is no minimum offering amount required as a condition of this offering, the actual total public offering amount, commissions, and proceeds to us, if any, are not determinable at this time. There can be no assurance that we will sell any shares under or fully utilize the Offering Agreement with Wainwright as a source of financing. We will retain broad discretion in determining how we will allocate the net proceeds from the sale of securities under this prospectus.
DESCRIPTION OF CAPITAL STOCK
The following description of our capital stock is not complete and may not contain all the information you should consider before investing in our capital stock. This description is summarized from, and qualified in its entirety by reference to, our Certificate of Incorporation, as amended, which has been publicly filed with the SEC. See “Where You Can Find More Information; Incorporation by Reference.”
Our authorized capital stock consists of 300,000,000 shares of common stock, $0.0001 par value, and 10,000,000 shares of undesignated preferred stock, $0.0001 par value, of which no preferred shares are issued or outstanding. All shares shall be of one class and one series, except that the board of directors, by its action, may establish more than one class or series.
Common Stock
As of September 12, 2023, there were 755,088 shares of our common stock outstanding. Holders of our common stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders. There is no cumulative voting with respect to the election of directors, with the result that the holders of more than 50% of the shares voted for the election of directors can elect all of the directors. Holders of common stock will be entitled to one vote per share on matters to be voted on by stockholders and also will be entitled to receive such dividends, if any, as may be declared from time to time by our board of directors in its discretion out of funds legally available therefor. The payment of dividends, if ever, on the common stock will be subject to the prior payment of dividends on any outstanding preferred stock, of which there is currently none. Upon our liquidation or dissolution, the holders of common stock will be entitled to receive pro rata all assets remaining available for distribution to stockholders after payment of all liabilities and provision for the liquidation of any shares of preferred stock at the time outstanding. Our stockholders have no conversion, preemptive or other subscription rights and there are no sinking fund or redemption provisions applicable to the common stock.
Transfer Agent
The transfer agent and registrar for our common stock is Equiniti Trust Company.
Listing
Our common stock is listed on the NYSE American under the symbol “AMPE.”
Preferred Stock
Pursuant to our certificate of incorporation, our board of directors has the authority, without further action by the stockholders (unless such stockholder action is required by applicable law or stock exchange listing rules), to designate and issue up to 10,000,000 shares of preferred stock in one or more series, to establish from time to time the number of shares to be included in each such series, to fix the designations, powers, preferences, privileges and relative participating, optional or special rights and the qualifications, limitations or restrictions thereof, including dividend rights, conversion rights, voting rights, terms of redemption and liquidation preferences, any or all of which may be greater than the rights of the common stock, and to increase or decrease the number of shares of any such series, but not below the number of shares of such series then outstanding.
The board of directors, without stockholder approval, can issue preferred stock with voting, conversion or other rights that could adversely affect the voting power and other rights of the holders of common stock. Preferred stock could be issued quickly with terms designed to delay or prevent a change in control of our company or make removal of management more difficult. Additionally, the issuance of preferred stock may have the effect of decreasing the market price of the common stock and may adversely affect the voting power of holders of common stock and reduce the likelihood that common stockholders will receive dividend payments and payments upon liquidation.
Our board of directors will fix the designations, voting powers, preferences and rights of the each series, as well as the qualifications, limitations or restrictions thereof, of the preferred stock of each series in the certificate of designation relating to that series, including:
•
the title and stated value;
•
the number of shares we are offering;
•
the liquidation preference per share;
•
the purchase price per share;
•
the dividend rate per share, dividend period and payment dates and method of calculation for dividends;
•
whether dividends will be cumulative or non-cumulative and, if cumulative, the date from which dividends will accumulate;
•
our right, if any, to defer payment of dividends and the maximum length of any such deferral period;
•
the procedures for any auction and remarketing, if any;
•
the provisions for a sinking fund, if any;
•
the provisions for redemption or repurchase, if applicable, and any restrictions on our ability to exercise those redemption and repurchase rights;
•
any listing of the preferred stock on any securities exchange or market;
•
whether the preferred stock will be convertible into our common stock or other securities of ours, including depositary shares and warrants, and, if applicable, the conversion period, the conversion price, or how it will be calculated, and under what circumstances it may be adjusted;
•
whether the preferred stock will be exchangeable into debt securities, and, if applicable, the exchange period, the exchange price, or how it will be calculated, and under what circumstances it may be adjusted;
•
voting rights, if any, of the preferred stock;
•
preemption rights, if any;
•
restrictions on transfer, sale or other assignment, if any;
•
whether interests in the preferred stock will be represented by depositary shares;
•
a discussion of any material or special United States federal income tax considerations applicable to the preferred stock;
•
the relative ranking and preferences of the preferred stock as to dividend rights and rights if we liquidate, dissolve or wind up our affairs;
•
any limitations on issuances of any class or series of preferred stock ranking senior to or on a parity with the series of preferred stock being issued as to dividend rights and rights if we liquidate, dissolve or wind up our affairs; and
•
any other specific terms, rights, preferences, privileges, qualifications or restrictions of the preferred stock.
The General Corporation Law of the State of Delaware, the state of our incorporation, provides that the holders of preferred stock will have the right to vote separately as a class (or, in some cases, as a series) on an amendment to our certificate of incorporation if the amendment would change the par value or, unless the certificate of incorporation provided otherwise, the number of authorized shares of the class or change the powers, preferences or special rights of the class or series so as to adversely affect the class or series, as the case may be. This right is in addition to any voting rights that may be provided for in the applicable certificate of designation.
Delaware Anti-Takeover Law and Provisions of our Certificate of Incorporation and Bylaws
Delaware Anti-takeover law
As a Delaware corporation, we are governed by the provisions of Section 203 of the Delaware General Corporation Law (the “DGCL”), which generally has an anti-takeover effect for transactions not approved in advance by our board of directors. This may discourage takeover attempts that might result in payment of a premium over the market price for the shares of common stock held by stockholders. In general, Section 203 of the DGCL prohibits a publicly held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a three-year period following the time that such stockholder becomes an interested stockholder, unless the business combination is approved in a prescribed manner. A “business combination” includes, among other things, a merger, asset or stock sale or other transaction resulting in a financial benefit to the interested stockholder. An “interested stockholder” is a person who, together with affiliates and associates, owns, or did own within three years prior to the determination of interested stockholder status, 15% or more of the corporation’s voting stock.
Under Section 203 of the DGCL, a business combination between a corporation and an interested stockholder is prohibited unless it satisfies one of the following conditions:
•
before the stockholder became interested, the board of directors approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; or
•
upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder) shares owned by:
•
persons who are directors and also officers; and
•
employee stock plans, in some instances; or
•
at or after the time the stockholder became interested, the business combination was approved by the board of directors of the corporation and authorized at an annual or special meeting of the stockholders by the affirmative vote of at least two-thirds of the outstanding voting stock which is not owned by the interested stockholder.
Staggered board of directors
Our Delaware certificate of incorporation provides that our board of directors will be classified into three classes of directors of approximately equal size at a date selected by the board. Currently our board of directors is not classified. As a result, in most circumstances, a person can gain control of our board only by successfully engaging in a proxy contest at two or more annual meetings.
Advance notice requirements for stockholder proposals and director nominations
Our Delaware bylaws provide that stockholders seeking to bring business before our annual meeting of stockholders, or to nominate candidates for election as directors at our annual meeting of stockholders, must provide timely notice of their intent in writing. To be timely, a stockholder’s notice needs to be delivered to our principal executive offices not later than the close of business on the 90th day nor earlier than the close of business on the 120th day prior to the first anniversary of the preceding year’s annual meeting of
stockholders. Our bylaws also specify certain requirements as to the form and content of a stockholders’ meeting. These provisions may preclude our stockholders from bringing matters before our annual meeting of stockholders or from making nominations for directors at our annual meeting of stockholders.
Authorized but unissued shares
Our authorized but unissued shares of common stock and preferred stock are available for future issuances without stockholder approval and could be utilized for a variety of corporate purposes, including future offerings to raise additional capital, acquisitions and employee benefit plans. The existence of authorized but unissued and unreserved common stock and preferred stock could render more difficult or discourage an attempt to obtain control of us by means of a proxy contest, tender offer, merger or otherwise.
Limitation on liability and indemnification of directors and officers
Our Delaware certificate of incorporation and bylaws provide that our directors and officers will be indemnified by us to the fullest extent authorized by Delaware law as it now exists or may in the future be amended, against all expenses and liabilities reasonably incurred in connection with their service for or on our behalf. Our bylaws permit us to secure insurance on behalf of any officer, director or employee for any liability arising out of his or her actions, regardless of whether Delaware law would permit indemnification.
These provisions may discourage stockholders from bringing a lawsuit against our directors for breach of their fiduciary duty. These provisions also may have the effect of reducing the likelihood of derivative litigation against directors and officers, even though such an action, if successful, might otherwise benefit us and our stockholders. Furthermore, a stockholder’s investment may be adversely affected to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions. We believe that these provisions, insurance and the indemnity agreements are necessary to attract and retain talented and experienced directors and officers.
Other than the four lawsuits in the United States District Court for the District of Colorado, Kain v. Ampio Pharmaceuticals, Inc., et al., Case No. 22-cv-2105, Maresca v. Martino, et al., Case No. 22-cv-2646-KLM, Marquis v. Martino, et al., Case No. 22-cv-2803-KLM, and McCann v. Martino, et. al., Case No. 2023-cv-30287, and the October 12, 2022 Securities and Exchange Commission investigation into securities law violations, all of which are described in our Annual Report on Form 10-K filed with the SEC on March 27, 2023 (and in subsequent reports we file with the SEC), there is no pending litigation or proceeding involving any of our directors or officers where indemnification by us would be required or permitted, nor are we aware of any threatened litigation or proceeding that might result in a claim for such indemnification. Insofar as indemnification for liabilities arising under the Securities Act of 1933, or the Act, may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable.
PLAN OF DISTRIBUTION
We have entered into the Offering Agreement with Wainwright, pursuant to which we may issue and sell from time to time shares of our common stock having an aggregate offering price of up to $1,250,000 through Wainwright as our sales agent pursuant to this prospectus and the accompanying base prospectus. Sales of the shares of common stock, if any, will be made by any method permitted by law deemed to be an “at the market offering” as defined in Rule 415 promulgated under the Securities Act. If we and Wainwright agree on any method of distribution other than sales of shares on or through the NYSE American or another existing trading market in the United States at market prices, we will file a further prospectus supplement providing all information about such offering as required by Rule 424(b) under the Securities Act.
Wainwright will offer shares of our common stock at prevailing market prices subject to the terms and conditions of the Offering Agreement as agreed upon by us and Wainwright. We will designate the number of shares which we desire to sell, the time period during which sales are requested to be made, any limitation on the number of shares that may be sold in one day and any minimum price below which sales may not be made. Subject to the terms and conditions of the Offering Agreement, Wainwright will use its commercially reasonable efforts consistent with its normal trading and sales practices to sell on our behalf all of the shares requested to be sold by us. We or Wainwright may suspend the offering of the shares of Common Stock being made through Wainwright under the Offering Agreement upon proper notice to the other party.
Settlement for sales of common stock will occur on the second trading day or such shorter settlement cycle as may be in effect under Exchange Act Rule 15c6-1 from time to time, following the date on which any sales are made, or on some other date that is agreed upon by us and Wainwright in connection with a particular transaction, in return for payment of the net proceeds to us. Sales of our shares of our common stock as contemplated in this prospectus will be settled through the facilities of The Depository Trust Company or by such other means as we and Wainwright may agree upon. There is no arrangement for funds to be received in an escrow, trust or similar arrangement.
We will pay Wainwright in cash, upon each sale of shares of our common stock pursuant to the Offering Agreement, a commission of 3.0% of the gross proceeds from each sale of shares. Because there is no minimum offering amount required as a condition to this offering, the actual total public offering amount, commissions and proceeds to us, if any, are not determinable at this time. Pursuant to the terms of the Offering Agreement, we agreed to reimburse Wainwright for the documented fees and costs of its legal counsel reasonably incurred in connection with entering into the transactions contemplated by the Offering Agreement in an amount not to exceed $50,000 in the aggregate, in addition to the reimbursement of up to $2,500 per due diligence update session for the fees of counsel to Wainwright. We will report at least quarterly the number of shares of our Common Stock sold through Wainwright under the Offering Agreement, the net proceeds to us and the compensation paid by us to Wainwright in connection with the sales of shares of our Common Stock.
In connection with the sales of shares of our Common Stock on our behalf, Wainwright will be deemed an “underwriter” within the meaning of the Securities Act, and the compensation paid to Wainwright constitutes underwriting commissions or discounts. We have agreed in the Offering Agreement to provide indemnification and contribution to Wainwright against certain liabilities, including liabilities under the Securities Act.
The offering of our shares of our Common Stock pursuant to the Offering Agreement will terminate upon the earlier of the sale of all of the shares of our Common Stock provided for in this prospectus or termination of the Offering Agreement as permitted therein.
To the extent required by Regulation M, Wainwright will not engage in any market making activities involving our Common Stock while the offering is ongoing under this prospectus.
From time to time, Wainwright may provide in the future various advisory, investment and commercial banking and other services to us in the ordinary course of business, for which they may receive customary fees and commissions. However, we have no present arrangements with Wainwright for any further services.
LEGAL MATTERS
The validity of the common stock offered by this prospectus will be passed upon for us by Ballard Spahr LLP, Minneapolis, Minnesota. Wainwright is being represented in connection with this offering by Ellenoff Grossman & Schole LLP, New York, New York.
EXPERTS
The financial statements of Ampio Pharmaceuticals, Inc. as of December 31, 2022 and 2021, and for the years then ended, incorporated in this prospectus by reference from our Annual Report on Form 10-K for the year ended December 31, 2022, have been audited by Moss Adams LLP, an independent registered public accounting firm, as stated in their report (which report expresses an unqualified opinion and includes an explanatory paragraph related to a going concern uncertainty), which is incorporated herein by reference. Such financial statements are incorporated by reference in reliance upon the report of such firm given their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION; INCORPORATION BY REFERENCE
Available Information
We file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the public through the SEC’s website at www.sec.gov. Copies of certain information filed by us with the SEC are also available on our website, www.ampiopharma.com, by following the link under “Investors” to “Financial Filings.” Our website is not a part of this prospectus and is not incorporated by reference in this prospectus.
This prospectus does not contain all of the information in the registration statement. The full registration statement may be obtained from the SEC through its website as provided above or from us, as provided below. Forms of the documents establishing the terms of the offered securities are or may be filed as exhibits to the registration statement. Statements in this prospectus about these documents are summaries and each statement is qualified in all respects by reference to the document to which it refers. You should refer to the actual documents for a more complete description of the relevant matters.
Incorporation by Reference
The SEC’s rules allow us to “incorporate by reference” information into this prospectus, which means that we can disclose important information to you by referring you to another document filed separately with the SEC. The information incorporated by reference is deemed to be part of this prospectus, and subsequent information that we file with the SEC will automatically update and supersede that information. Any statement contained in a previously filed document incorporated by reference will be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained in this prospectus modifies or replaces that statement.
We incorporate by reference our documents listed below and any future filings made by us with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act in this prospectus, between the date of this prospectus and the termination of the offering of the securities described in this prospectus. We are not, however, incorporating by reference any documents or portions thereof, whether specifically listed below or filed in the future, that are not deemed “filed” with the SEC, including any Compensation Committee report or performance graph or any information furnished pursuant to Items 2.02 or 7.01 of Form 8-K or related exhibits furnished pursuant to Item 9.01 of Form 8-K.
This prospectus incorporates by reference the documents set forth below that have previously been filed with the SEC:
•
The Annual Report on Form 10-K for the fiscal year ended December 31, 2022, filed on March 27, 2023 and Amendment No. 1 to Annual Report on Form 10-K for the fiscal year ended December 31, 2022, filed on April 28, 2023;
•
The portions of the definitive proxy statement on Schedule 14A filed on June 14, 2023 and the definitive additional proxy materials filed on July 13, 2023 for the Company’s 2023 Annual Meeting of Stockholders held on July 27, 2023 that are incorporated by reference into the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022;
•
The Quarterly Report on Form 10-Q for the quarter ended March 31, 2023, filed on May 8, 2023 and the Quarterly Report on Form 10-Q for the quarter ended June 30, 2023, filed on August 8, 2023;
•
The Current Reports on Form 8-K filed (but not furnished) on January 17, 2023, March 7, 2023, March 13, 2023, April 18, 2023, May 26, 2023, July 5, 2023, August 1, 2023, and August 31, 2023; and
•
The description of the Company’s common stock contained in Exhibit 4.5 to the Company’s Annual Report on Form 10-K (No. 001-35182) for the fiscal year ended December 31, 2019, filed with the Commission on February 21, 2020, including any amendment or report filed for the purpose of updating such description.
All reports and other documents we subsequently file pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act prior to the termination of this offering, including all such documents we may file with
the SEC after the date of the initial registration statement and prior to the effectiveness of the registration statement, but excluding any information furnished to, rather than filed with, the SEC, will also be incorporated by reference into this prospectus and deemed to be part of this prospectus from the date of the filing of such reports and documents.
You may request a free copy of any of the documents incorporated by reference in this prospectus (other than exhibits, unless they are specifically incorporated by reference in the documents) by writing or telephoning us at the following address:
Ampio Pharmaceuticals, Inc.
9800 Mount Pyramid Court, Suite 400
Englewood, Colorado 80112
Attn: Corporate Secretary
(720) 437-6500
Exhibits to the filings will not be sent, however, unless those exhibits have specifically been incorporated by reference in this prospectus.
Up to $1,250,000 of Shares of
Common Stock
of
Ampio Pharmaceuticals, Inc.
PROSPECTUS
, 2023
H.C. Wainwright & Co.
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 14. Other Expenses of Issuance and Distribution
The following table sets forth the expenses payable by the registrant in connection with the securities being registered hereby:
| |
SEC registration fee
|
|
|
|
$ |
5,510.00 |
|
|
| |
Legal fees and expenses
|
|
|
|
|
*
|
|
|
| |
Accounting fees and expenses
|
|
|
|
|
*
|
|
|
| |
Printing and engraving expenses
|
|
|
|
|
*
|
|
|
| |
Miscellaneous
|
|
|
|
|
*
|
|
|
| |
Total
|
|
|
|
$ |
5,510.00* |
|
|
*
These fees are calculated based on the securities offered and the number of issuances and accordingly cannot be estimated at this time.
Item 15. Indemnification of Directors and Officers
The Company is a corporate organized under the laws of the State of Delaware and is subject to the Delaware General Corporation Law (“DGCL”).
Article IX of the Company’s Certificate of Incorporation provides that, to the fullest extent permitted by the DGCL, a director of the Company shall not be liable to the Company or its stockholders for monetary damages for breach of fiduciary duty as a director.
Section 145 of the DGCL permits a corporation to indemnify any director, officer, employee or agent of the corporation against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with any action, suit or proceeding brought by reason of the fact that such person is or was a director or officer of the corporation, if such person acted in good faith and in a manner that he or she reasonably believed to be in, or not opposed to, the best interests of the corporation, and, with respect to any criminal action or proceeding, if he or she had no reason to believe his or her conduct was unlawful. A similar standard is applicable in the case of actions brought by or on behalf of the corporation (i.e., derivative actions), except that indemnification only extends to expenses (including attorneys’ fees) actually and reasonably incurred in connection with the defense or settlement of such action, and the statute requires court approval before there can be any indemnification where the person seeking indemnification has been found liable to the corporation. The statute provides that it is not exclusive of other indemnification that may be granted by a corporation’s certificate of incorporation, bylaws, disinterested director vote, stockholder vote, agreement or otherwise.
Article IX of the Company’s Certificate of Incorporation requires the Company to indemnify, in the manner and to the fullest extent permitted by the DGCL, any person (or the estate of any person) who is or was a party to, or is threatened to be made a party to, any threatened, pending or completed action, suit or proceeding, by reason of the fact that such person is or was a director or officer of the Company, or is or was serving at the request of the Corporation as a director or officer of another corporation, partnership, joint venture, trust or other enterprise.
Further, Article XI of the Company’s bylaws requires the Company to indemnify its present and former directors and executive officers (as that term is defined in Rule 3b-7 of the Securities and Exchange Act of 1934, as amended) for such expenses and liabilities, in such manner, under such circumstances, and to the fullest extent, as required or permitted by the DGCL, as in effect from time to time. The Company has the right to modify the indemnification provided for in the Company’s bylaws by individual contracts with its directors and executive officers. Additionally, the Company’s bylaws provide that the Company shall not be required to indemnify any director or executive officer in connection with any proceeding (or part thereof) initiated by such person unless (i) such indemnification is expressly required to be made by law, (ii) the
proceeding was authorized by the Board of Directors of the Company, (iii) such indemnification is provided by the Company, in its sole discretion, pursuant to the powers vested in the Company under the DGCL or any other applicable law or (iv) such indemnification is required to be made pursuant to the enforcement provisions of the Company’s bylaws.
The Company’s bylaws also authorize the Board, in its discretion, to pay the expenses of any such action in advance of the final disposition of such action upon a written undertaking by such indemnitee to repay such amounts if it shall ultimately be determined that he or she is not entitled to indemnification under the standard set by the DGCL and the Company’s bylaws.
The Company has entered into indemnification agreements with each of its directors and executive officers. The indemnification agreements provide indemnification to each director or executive officer, or the Indemnitee, against all expenses, judgments, penalties, fines and amounts paid in settlement actually and reasonably incurred by the Indemnitee, or on his or her behalf if the Indemnitee is, or is threatened to be made, a party to or participant in any proceeding related to his or her status as a director and/or executive officer of the Company, as long as the Indemnitee acted in good faith and in a manner the Indemnitee reasonably believed to be in or not opposed to the best interests of the Company, and with respect to any criminal proceeding, had no reasonable cause to believe the Indemnitee’s conduct was unlawful. For proceedings by or in the right of the Company, indemnification is provided as set forth above; provided, however, if applicable law so provides, no indemnification against such expenses will be made in respect of any claim, issue or matter in such proceeding as to which Indemnitee shall have been adjudged to be liable to the Registrant unless and to the extent that the Court of Chancery of the State of Delaware shall determine that such indemnification may be made.
The Company also maintains a director and officer liability insurance policy.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, in the opinion of the Commission, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Item 16. Exhibits
| |
Exhibit
Number
|
|
|
Description
|
|
| |
1.1
|
|
|
Form of Underwriting Agreement.*
|
|
| |
1.2
|
|
|
|
|
| |
3.1
|
|
|
Certificate of Incorporation of Chay Enterprises, Inc. (n/k/a Ampio Pharmaceuticals, Inc.) (incorporated by reference to Exhibit 3.3 to the Current Report on Form 8-K filed March 30, 2010).
|
|
| |
3.2
|
|
|
Certificate of Amendment to Certificate of Incorporation of Ampio Pharmaceuticals, Inc. (f/k/a Chay Enterprises, Inc.) (incorporated by reference to Exhibit 3.4 to the Current Report on Form 8-K filed March 30, 2010).
|
|
| |
3.3
|
|
|
Certificate of Amendment to Certificate of Incorporation of Ampio Pharmaceuticals, Inc. (incorporated by reference to Exhibit 3.1 to the Current Report on Form 8-K filed December 18, 2019).
|
|
| |
3.4
|
|
|
Certificate of Amendment to Certificate of Incorporation of Ampio Pharmaceuticals, Inc. dated November 8, 2022 (incorporated by reference to Exhibit 3.1 to the Current Report on Form 8-K filed November 9, 2022).
|
|
| |
3.5
|
|
|
Certificate of Amendment to Certificate of Incorporation of Ampio Pharmaceuticals, Inc. filed August 30, 2023 (incorporated by reference to Exhibit 3.1 to the Current Report on Form 8-K filed on August 31, 2023).
|
|
*
To be filed by amendment or as an exhibit to a document to be incorporated by reference, if applicable.
Item 17. Undertakings
(a)
The undersigned registrant hereby undertakes:
(1)
To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i)
To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
(ii)
To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii)
To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
provided, however, that paragraphs (a)(1)(i), (a)(1)(ii) and (a)(1)(iii) above do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the registrant pursuant to section 13 or section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration statement, or is contained in a form of prospectus filed pursuant to Rule 424(b) that is part of the registration statement.
(2)
That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3)
To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(5)
That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser:
(A)
Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and
(B)
Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5) or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information required by section 10(a) of the Securities Act of 1933 shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date.
(6)
That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i)
Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii)
Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii)
The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv)
Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(b)
The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to Section 13(a) or Section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to Section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(h)
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form S-3 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Englewood, State of Colorado, on September 18, 2023.
AMPIO PHARMACEUTICALS, INC.
By:
/s/ Michael A. Martino
Michael A. Martino
Chief Executive Officer
POWER OF ATTORNEY
KNOW ALL MEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Michael A. Martino and Daniel G. Stokely, or either of them, as his or her true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him and in his name, place and stead, in any and all capacities, to sign any and all amendments to this Registration Statement on Form S-3 or other applicable form, with all exhibits thereto, or any and all amendments (including pre-effective and post-effective amendments) and supplements to a registration statement, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or their substitute or substitutes may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed below by the following persons on behalf of the registrant in the capacities indicated on September 18, 2023.
| |
/s/ Michael A. Martino
Michael A. Martino
|
|
|
Chief Executive Officer (principal executive officer) and Director
|
|
| |
/s/ Daniel G. Stokely
Daniel G. Stokely
|
|
|
Chief Financial Officer (principal financial and accounting officer)
|
|
| |
/s/ David R. Stevens
David R. Stevens
|
|
|
Director
|
|
| |
/s/ J. Kevin Buchi
J. Kevin Buchi
|
|
|
Director
|
|
| |
/s/ Elizabeth Varki Jobes
Elizabeth Varki Jobes
|
|
|
Director
|
|
Exhibit 1.2
AT THE MARKET OFFERING AGREEMENT
September 18, 2023
H.C. Wainwright & Co., LLC
430 Park Avenue
New York, NY 10022
Ladies and Gentlemen:
Ampio Pharmaceuticals, Inc.,
a corporation organized under the laws of Delaware (the “Company”), confirms its agreement (this “Agreement”)
with H.C. Wainwright & Co., LLC (the “Manager”) as follows:
1. Definitions.
The terms that follow, when used in this Agreement and any Terms Agreement, shall have the meanings indicated.
“Accountants” shall
have the meaning ascribed to such term in Section 4(m).
“Act”
shall mean the Securities Act of 1933, as amended, and the rules and regulations of the Commission promulgated thereunder.
“Action”
shall have the meaning ascribed to such term in Section 3(p).
“Affiliate”
shall have the meaning ascribed to such term in Section 3(o).
“Applicable
Time” shall mean, with respect to any Shares, the time of sale of such Shares pursuant to this Agreement or any relevant Terms
Agreement.
“Base Prospectus”
shall mean the base prospectus contained in the Registration Statement at the Effective Time.
“Board”
shall have the meaning ascribed to such term in Section 2(b)(iii).
“Broker
Fee” shall have the meaning ascribed to such term in Section 2(b)(v).
“Business
Day” shall mean any day other than Saturday, Sunday or other day on which commercial banks in The City of New York are authorized
or required by law to remain closed; provided, however, that, for purposes of clarity, commercial banks shall not be deemed
to be authorized or required by law to remain closed due to “stay at home”, “shelter-in-place”, “non-essential
employee” or any other similar orders or restrictions or the closure of any physical branch locations at the direction of
any governmental authority so long as the electronic funds transfer systems (including for wire transfers) of commercial banks in The
City of New York generally are open for use by customers on such day.
“Commission”
shall mean the United States Securities and Exchange Commission.
“Common
Stock” shall have the meaning ascribed to such term in Section 2.
“Common
Stock Equivalents” shall have the meaning ascribed to such term in Section 3(g).
“Company
Counsel” shall have the meaning ascribed to such term in Section 4(l).
“DTC”
shall have the meaning ascribed to such term in Section 2(b)(vii).
“Effective
Date” shall mean each date and time that the Registration Statement and any post-effective amendment or amendments thereto became
or becomes effective.
“Effective
Time” shall mean the first date and time that the Registration Statement becomes effective.
“Exchange
Act” shall mean the Securities Exchange Act of 1934, as amended, and the rules and regulations of the Commission promulgated
thereunder.
“Execution
Time” shall mean the date and time that this Agreement is executed and delivered by the parties hereto.
“Free Writing
Prospectus” shall mean a free writing prospectus, as defined in Rule 405.
“GAAP”
shall have the meaning ascribed to such term in Section 3(m).
“Incorporated
Documents” shall mean the documents or portions thereof filed with the Commission on or prior to the Effective Date that are
incorporated by reference in the Registration Statement or the Prospectus and any documents or portions thereof filed with the Commission
after the Effective Date that are deemed to be incorporated by reference in the Registration Statement or the Prospectus.
“Intellectual
Property Rights” shall have the meaning ascribed to such term in Section 3(v).
“Issuer
Free Writing Prospectus” shall mean an issuer free writing prospectus, as defined in Rule 433.
“Losses”
shall have the meaning ascribed to such term in Section 7(d).
“Material
Adverse Effect” shall have the meaning ascribed to such term in Section 3(b).
“Material
Permits” shall have the meaning ascribed to such term in Section 3(t).
“Net Proceeds”
shall have the meaning ascribed to such term in Section 2(b)(v).
“Permitted
Free Writing Prospectus” shall have the meaning ascribed to such term in Section 4(g).
“Placement”
shall have the meaning ascribed to such term in Section 2(c).
“Proceeding”
shall have the meaning ascribed to such term in Section 3(b).
“Prospectus”
shall mean the Base Prospectus, as supplemented by the Prospectus Supplement in the Registration Statement at the Effective Time and any
subsequently filed Prospectus Supplement.
“Prospectus
Supplement” shall mean the prospectus supplement relating to the Shares contained in the Registration Statement at the Effective
Time and any other prospectus supplement relating to the Shares prepared and filed pursuant to Rule 424(b) from time to time.
“Registration
Statement” shall mean the shelf registration statement on Form S-3 registering $50,000,000 of securities of the Company
to be filed on or immediately following the Execution Time, including exhibits and financial statements and any prospectus supplement
relating to the Shares that is filed with the Commission pursuant to Rule 424(b) and deemed part of such registration statement
pursuant to Rule 430B, as amended on each Effective Date and, in the event any post-effective amendment thereto becomes effective,
shall also mean such registration statement as so amended.
“Representation
Date” shall have the meaning ascribed to such term in Section 4(k).
“Required
Approvals” shall have the meaning ascribed to such term in Section 3(e).
“Rule 158”,
“Rule 164”, “Rule 172”, “Rule 173”, “Rule 405”,
“Rule 415”, “Rule 424”, “Rule 430B” and “Rule 433”
refer to such rules under the Act.
“Sales
Notice” shall have the meaning ascribed to such term in Section 2(b)(i).
“SEC Reports”
shall have the meaning ascribed to such term in Section 3(m).
“Settlement
Date” shall have the meaning ascribed to such term in Section 2(b)(vii).
“Subsidiary”
shall have the meaning ascribed to such term in Section 3(a).
“Terms
Agreement” shall have the meaning ascribed to such term in Section 2(a).
“Time of
Delivery” shall have the meaning ascribed to such term in Section 2(c).
“Trading
Day” means a day on which the Trading Market is open for trading.
“Trading
Market” means The NYSE American.
2. Sale
and Delivery of Shares. The Company proposes to issue and sell through or to the Manager, as sales agent and/or principal, from time
to time during the term of this Agreement and on the terms set forth herein, up to the lesser of such number of shares (the “Shares”)
of the Company’s common stock, $0.0001 par value per share (“Common Stock”), that does not exceed (a) the
number or dollar amount of shares of Common Stock registered on the Registration Statement, pursuant to which the offering is being made,
(b) the number of authorized but unissued shares of Common Stock (less the number of shares of Common Stock issuable upon exercise,
conversion or exchange of any outstanding securities of the Company or otherwise reserved from the Company’s authorized capital
stock), or (c) the number or dollar amount of shares of Common Stock that would cause the Company or the offering of the Shares to
not satisfy the eligibility and transaction requirements for use of Form S-3, including, if applicable, General Instruction I.B.6
of Registration Statement on Form S-3 (the lesser of (a), (b) and (c), the “Maximum Amount”). Notwithstanding
anything to the contrary contained herein, the parties hereto agree that compliance with the limitations set forth in this Section 2
on the number and aggregate sales price of Shares issued and sold under this Agreement shall be the sole responsibility of the Company
and that the Manager shall have no obligation in connection with such compliance.
(a) Appointment
of Manager as Selling Agent; Terms Agreement. For purposes of selling the Shares through the Manager, the Company hereby appoints
the Manager as exclusive agent of the Company for the purpose of selling the Shares of the Company pursuant to this Agreement and the
Manager agrees to use its commercially reasonable efforts to sell the Shares on the terms and subject to the conditions stated herein.
The Company agrees that, whenever it determines to sell the Shares directly to the Manager as principal, it will enter into a separate
agreement (each, a “Terms Agreement”) in substantially the form of Annex I hereto, relating to such sale in
accordance with Section 2 of this Agreement.
(b) Agent
Sales. Subject to the terms and conditions and in reliance upon the representations and warranties herein set forth, following the
effectiveness of the Registration Statement, the Company will issue and agrees to sell Shares from time to time through the Manager, acting
as sales agent, and the Manager agrees to use its commercially reasonable efforts to sell, as sales agent for the Company, on the following
terms:
(i) The
Shares are to be sold on a daily basis or otherwise as shall be agreed to by the Company and the Manager on any day that (A) is a
Trading Day, (B) the Company has instructed the Manager by telephone (confirmed promptly by electronic mail) to make such sales (“Sales
Notice”) and (C) the Company has satisfied its obligations under Section 6 of this Agreement. The Company will designate
the maximum amount of the Shares to be sold by the Manager daily (subject to the limitations set forth in Section 2(d)) and the minimum
price per Share at which such Shares may be sold. Subject to the terms and conditions hereof, the Manager shall use its commercially reasonable
efforts to sell on a particular day all of the Shares designated for the sale by the Company on such day. The gross sales price of the
Shares sold under this Section 2(b) shall be the market price for the shares of Common Stock sold by the Manager under this
Section 2(b) on the Trading Market at the time of sale of such Shares.
(ii) The
Company acknowledges and agrees that (A) there can be no assurance that the Manager will be successful in selling the Shares, (B) the
Manager will incur no liability or obligation to the Company or any other person or entity if it does not sell the Shares for any reason
other than a failure by the Manager to use its commercially reasonable efforts consistent with its normal trading and sales practices
and applicable law and regulations to sell such Shares as required under this Agreement, and (C) the Manager shall be under no obligation
to purchase Shares on a principal basis pursuant to this Agreement, except as otherwise specifically agreed by the Manager and the Company
pursuant to a Terms Agreement.
(iii) The
Company shall not authorize the issuance and sale of, and the Manager shall not be obligated to use its commercially reasonable efforts
to sell, any Share at a price lower than the minimum price therefor designated from time to time by the Company’s Board of Directors
(the “Board”), or a duly authorized committee thereof, or such duly authorized officers of the Company, and notified
to the Manager in writing. The Company or the Manager may, upon notice to the other party hereto by telephone (confirmed promptly by electronic
mail), suspend the offering of the Shares for any reason and at any time; provided, however, that such suspension or termination
shall not affect or impair the parties’ respective obligations with respect to the Shares sold hereunder prior to the giving of
such notice.
(iv) The
Manager may sell Shares by any method permitted by law deemed to be an “at the market offering” as defined in Rule 415
under the Act, including without limitation sales made directly on the Trading Market, on any other existing trading market for the Common
Stock or to or through a market maker. The Manager may also sell Shares in privately negotiated transactions, provided that the Manager
receives the Company’s prior written approval for any sales in privately negotiated transactions and if so provided in the “Plan
of Distribution” section of the Prospectus Supplement or a supplement to the Prospectus Supplement or a new Prospectus Supplement
disclosing the terms of such privately negotiated transaction.
(v) The
compensation to the Manager for sales of the Shares under this Section 2(b) shall be a placement fee of 3.0% of the gross sales
price of the Shares sold pursuant to this Section 2(b) (“Broker Fee”). The foregoing rate of compensation
shall not apply when the Manager acts as principal, in which case the Company may sell Shares to the Manager as principal at a price agreed
upon at the relevant Applicable Time pursuant to a Terms Agreement. The remaining proceeds, after deduction of the Broker Fee and deduction
of any transaction fees imposed by any clearing firm, execution broker, or governmental or self-regulatory organization in respect of
such sales, shall constitute the net proceeds to the Company for such Shares (the “Net Proceeds”).
(vi) The
Manager shall provide written confirmation (which may be by electronic mail) to the Company following the close of trading on the Trading
Market each day in which the Shares are sold under this Section 2(b) setting forth the number of the Shares sold on such day,
the aggregate gross sales proceeds and the Net Proceeds to the Company, and the compensation payable by the Company to the Manager with
respect to such sales.
(vii) Unless
otherwise agreed between the Company and the Manager, settlement for sales of the Shares will occur at 10:00 a.m. (New York City
time) on the second (2nd) Trading Day (and on and after May 28, 2024, on the first (1st) Trading Day, or
any such shorter settlement cycle as may be in effect pursuant to Rule 15c6-1 under the Exchange Act from time to time) following
the date on which such sales are made (each, a “Settlement Date”). On or before the Trading Day prior to each Settlement
Date, the Company will, or will cause its transfer agent to, electronically transfer the Shares being sold by crediting the Manager’s
or its designee’s account (provided that the Manager shall have given the Company written notice of such designee at least one Trading
Day prior to the Settlement Date) at The Depository Trust Company (“DTC”) through its Deposit and Withdrawal at Custodian
System or by such other means of delivery as may be mutually agreed upon by the parties hereto which Shares in all cases shall be freely
tradable, transferable, registered shares in good deliverable form. On each Settlement Date, the Manager will deliver the related Net
Proceeds in same day funds to an account designated by the Company. The Company agrees that, if the Company, or its transfer agent (if
applicable), defaults in its obligation to deliver duly authorized Shares on a Settlement Date, in addition to and in no way limiting
the rights and obligations set forth in Section 7 hereto, the Company will (i) hold the Manager harmless against any loss, claim,
damage, or reasonable, documented expense (including reasonable and documented legal fees and expenses), as incurred, arising out of or
in connection with such default by the Company, and (ii) pay to the Manager any commission, discount or other compensation to which
the Manager would otherwise have been entitled absent such default.
(viii) At
each Applicable Time, Settlement Date, and Representation Date, the Company shall be deemed to have affirmed each representation and warranty
contained in this Agreement as if such representation and warranty were made as of such date, modified as necessary to relate to the Registration
Statement and the Prospectus as amended as of such date. Any obligation of the Manager to use its commercially reasonable efforts to sell
the Shares on behalf of the Company shall be subject to the continuing accuracy of the representations and warranties of the Company herein,
to the performance by the Company of its obligations hereunder and to the continuing satisfaction of the additional conditions specified
in Section 6 of this Agreement.
(ix) Notwithstanding
any other provision of this Agreement, during any period in which the Company is in possession of material non-public information, the
Company and the Manager agree that (i) no sales of Shares will take place, (ii) the Company shall not request the sale of any
Shares, and (iii) the Manager shall not be obligated to sell or offer to sell any Shares.
(x) If
the Company shall declare or make any dividend or other distribution of its assets (or rights to acquire its assets) to holders of shares
of Common Stock, by way of return of capital or otherwise (including, without limitation, any distribution of cash, stock or other securities,
property or options by way of a dividend, spin off, reclassification, corporate rearrangement, scheme of arrangement or other similar
transaction) (a “Distribution” and the record date for the determination of stockholders entitled to receive the Distribution,
the “Record Date”), the Company hereby covenants that, in connection with any sales of Shares pursuant to a Sales Notice
on the Record Date, the Company shall issue and deliver such Shares to the Manager on the Record Date and the Record Date shall be the
Settlement Date and the Company shall cover any additional costs of the Manager in connection with the delivery of Shares on the Record
Date.
(c) Term
Sales. If the Company wishes to sell the Shares pursuant to this Agreement in a manner other than as set forth in Section 2(b) of
this Agreement (each, a “Placement”), the Company will notify the Manager of the proposed terms of such Placement.
If the Manager, acting as principal, wishes to accept such proposed terms (which it may decline to do for any reason in its sole discretion)
or, following discussions with the Company wishes to accept amended terms, the Manager and the Company will enter into a Terms Agreement
setting forth the terms of such Placement. The terms set forth in a Terms Agreement will not be binding on the Company or the Manager
unless and until the Company and the Manager have each executed such Terms Agreement accepting all of the terms of such Terms Agreement.
In the event of a conflict between the terms of this Agreement and the terms of a Terms Agreement, the terms of such Terms Agreement will
control. A Terms Agreement may also specify certain provisions relating to the reoffering of such Shares by the Manager. The commitment
of the Manager to purchase the Shares pursuant to any Terms Agreement shall be deemed to have been made on the basis of the representations
and warranties of the Company herein contained and shall be subject to the terms and conditions herein set forth. Each Terms Agreement
shall specify the number of the Shares to be purchased by the Manager pursuant thereto, the price to be paid to the Company for such Shares,
any provisions relating to rights of, and default by, underwriters acting together with the Manager in the reoffering of the Shares, and
the time and date (each such time and date being referred to herein as a “Time of Delivery”) and place of delivery
of and payment for such Shares. Such Terms Agreement shall also specify any requirements for opinions of counsel, accountants’ letters
and officers’ certificates pursuant to Section 6 of this Agreement and any other information or documents required by the Manager.
(d) Maximum
Number of Shares. Under no circumstances shall the Company cause or request the offer or sale of any Shares if, after giving effect
to the sale of such Shares, the aggregate amount of Shares sold pursuant to this Agreement would exceed the lesser of (A) together
with all sales of Shares under this Agreement, the Maximum Amount, (B) the amount available for offer and sale under the currently
effective Registration Statement and (C) the amount authorized from time to time to be issued and sold under this Agreement by the
Board, a duly authorized committee thereof or a duly authorized executive committee, and notified to the Manager in writing. Under no
circumstances shall the Company cause or request the offer or sale of any Shares pursuant to this Agreement at a price lower than the
minimum price authorized from time to time by the Board, a duly authorized committee thereof or a duly authorized executive officer, and
notified to the Manager in writing. Further, under no circumstances shall the Company cause or permit the aggregate offering amount of
Shares sold pursuant to this Agreement to exceed the Maximum Amount.
(e) Regulation
M Notice. Unless the exceptive provisions set forth in Rule 101(c)(1) of Regulation M under the Exchange Act are satisfied
with respect to the Shares, the Company shall give the Manager at least one (1) Business Day’s prior notice of its intent to
sell any Shares in order to allow the Manager time to comply with Regulation M.
3. Representations
and Warranties. The Company represents and warrants to, and agrees with, the Manager at the Execution Time and the Effective Time
and on each such time that the following representations and warranties are repeated or deemed to be made pursuant to this Agreement,
as set forth below, except as set forth in the Registration Statement, the Prospectus or the Incorporated Documents.
(a) Subsidiaries.
All of the direct and indirect subsidiaries (individually, a “Subsidiary”) of the Company are set forth on Exhibit 21.1
to the Company’s most recent Annual Report on Form 10-K filed with the Commission. The Company owns, directly or indirectly,
all of the capital stock or other equity interests of each Subsidiary free and clear of any “Liens” (which for purposes
of this Agreement shall mean a lien, charge, security interest, encumbrance, right of first refusal, preemptive right or other restriction),
and all of the issued and outstanding shares of capital stock of each Subsidiary are validly issued and are fully paid, non-assessable
and free of preemptive and similar rights to subscribe for or purchase securities.
(b) Organization
and Qualification. The Company and each of the Subsidiaries is an entity duly incorporated or otherwise organized, validly existing
and in good standing under the laws of the jurisdiction of its incorporation or organization, with the requisite power and authority to
own and use its properties and assets and to carry on its business as currently conducted. Neither the Company nor any Subsidiary is in
violation or in default of any of the provisions of its respective certificate or articles of incorporation, bylaws or other organizational
or charter documents. Each of the Company and the Subsidiaries is duly qualified to conduct business and is in good standing as a foreign
corporation or other entity in each jurisdiction in which the nature of the business conducted or property owned by it makes such qualification
necessary, except where the failure to be so qualified or in good standing, as the case may be, could not have or reasonably be expected
to result in: (i) a material adverse effect on the legality, validity or enforceability of this Agreement, (ii) a material adverse
effect on the results of operations, assets, business, prospects or condition (financial or otherwise) of the Company and the Subsidiaries,
taken as a whole, from that set forth in the Registration Statement, the Base Prospectus, any Prospectus Supplement, the Prospectus or
the Incorporated Documents, or (iii) a material adverse effect on the Company’s ability to perform in any material respect
on a timely basis its obligations under this Agreement (any of (i), (ii) or (iii), a “Material Adverse Effect”)
and no “Proceeding” (which for purposes of this Agreement shall mean any action, claim, suit, investigation or proceeding
(including, without limitation, an informal investigation or partial proceeding, such as a deposition), whether commenced or threatened)
has been instituted in any such jurisdiction revoking, limiting or curtailing or seeking to revoke, limit or curtail such power and authority
or qualification.
(c) Authorization
and Enforcement. The Company has the requisite corporate power and authority to enter into and to consummate the transactions contemplated
by this Agreement and otherwise to carry out its obligations hereunder. The execution and delivery of this Agreement by the Company and
the consummation by it of the transactions contemplated hereby have been duly authorized by all necessary action on the part of the Company
and no further action is required by the Company, the Board or the Company’s stockholders in connection herewith other than in connection
with the Required Approvals. This Agreement has been duly executed and delivered by the Company and, when delivered in accordance with
the terms hereof, will constitute the valid and binding obligation of the Company enforceable against the Company in accordance with its
terms, except (i) as limited by general equitable principles and applicable bankruptcy, insolvency, reorganization, moratorium and
other laws of general application affecting enforcement of creditors’ rights generally, (ii) as limited by laws relating to
the availability of specific performance, injunctive relief or other equitable remedies and (iii) insofar as indemnification and
contribution provisions may be limited by applicable law.
(d) No
Conflicts. The execution, delivery and performance by the Company of this Agreement, the issuance and sale of the Shares and the consummation
by it of the transactions contemplated hereby do not and will not (i) conflict with or violate any provision of the Company’s
or any Subsidiary’s certificate or articles of incorporation, bylaws or other organizational or charter documents, or (ii) conflict
with, or constitute a default (or an event that with notice or lapse of time or both would become a default) under, result in the creation
of any Lien upon any of the properties or assets of the Company or any Subsidiary, or give to others any rights of termination, amendment,
anti-dilution or similar adjustments, acceleration or cancellation (with or without notice, lapse of time or both) of, any agreement,
credit facility, debt or other instrument (evidencing a Company or Subsidiary debt or otherwise) or other understanding to which the Company
or any Subsidiary is a party or by which any property or asset of the Company or any Subsidiary is bound or affected, or (iii) subject
to the Required Approvals, conflict with or result in a violation of any law, rule, regulation, order, judgment, injunction, decree or
other restriction of any court or governmental authority to which the Company or a Subsidiary is subject (including federal and state
securities laws and regulations), or by which any property or asset of the Company or a Subsidiary is bound or affected; except in the
case of each of clauses (ii) and (iii), such as could not, individually or in the aggregate, have or reasonably be expected to result
in a Material Adverse Effect.
(e) Filings,
Consents and Approvals. The Company is not required to obtain any consent, waiver, authorization or order of, give any notice to,
or make any filing or registration with, any court or other federal, state, local or other governmental authority or other “Person”
(defined as an individual or corporation, partnership, trust, incorporated or unincorporated association, joint venture, limited liability
company, joint stock company, government (or an agency or subdivision thereof) or other entity of any kind, including the Trading Market)
in connection with the execution, delivery and performance by the Company of this Agreement, other than (i) the filings required
by this Agreement, (ii) the filing with the Commission of the Prospectus Supplement, (iii) the filing of application(s) to
and approval by the Trading Market for the listing of the Shares for trading thereon in the time and manner required thereby, and (iv) such
filings as are required to be made under applicable state securities laws and the rules and regulations of the Financial Industry
Regulatory Authority, Inc. (“FINRA”) (collectively, the “Required Approvals”).
(f) Issuance
of Shares. The Shares are duly authorized and, when issued and paid for in accordance with this Agreement, will be duly and validly
issued, fully paid and nonassessable, free and clear of all Liens imposed by the Company. The Company has reserved from its duly authorized
capital stock the maximum number of shares of Common Stock issuable pursuant to this Agreement. On and after the Effective Time, the issuance
by the Company of the Shares has been registered under the Act and all of the Shares are freely transferable and tradable by the purchasers
thereof without restriction (other than any restrictions arising solely from an act or omission of such a purchaser). The Shares are being
issued pursuant to the Registration Statement and, on and after the Effective Time, the issuance of the Shares has been registered by
the Company under the Act. The “Plan of Distribution” section within the Registration Statement permits the issuance
and sale of the Shares as contemplated by this Agreement. Upon receipt of the Shares, the purchasers of such Shares will have good and
marketable title to such Shares and the Shares will be freely tradable on the Trading Market.
(g) Capitalization.
The capitalization of the Company is as set forth in the SEC Reports. Except as disclosed in the SEC Reports, the Company has not issued
any capital stock since its most recently filed periodic report under the Exchange Act, other than pursuant to the exercise of stock options
or other awards issued under the Company’s stock incentive plans pursuant to the conversion and/or exercise of securities exercisable,
exchangeable or convertible into Common Stock (“Common Stock Equivalents”) outstanding as of the date of the most recently
filed periodic report under the Exchange Act. No Person has any right of first refusal, preemptive right, right of participation, or any
similar right to participate in the transactions contemplated by this Agreement. Except as set forth in the SEC Reports, there are no
outstanding options, warrants, scrip rights to subscribe to, calls or commitments of any character whatsoever relating to, or securities,
rights or obligations convertible into or exercisable or exchangeable for, or giving any Person any right to subscribe for or acquire,
any shares of Common Stock or the capital stock of any Subsidiary, or contracts, commitments, understandings or arrangements by which
the Company or any Subsidiary is or may become bound to issue additional shares of Common Stock or Common Stock Equivalents or capital
stock of any Subsidiary. The issuance and sale of the Shares will not obligate the Company or any Subsidiary to issue shares of Common
Stock or other securities to any Person. There are no outstanding securities or instruments of the Company or any Subsidiary with any
provision that adjusts the exercise, conversion, exchange or reset price of such security or instrument upon an issuance of securities
by the Company or any Subsidiary. There are no outstanding securities or instruments of the Company or any Subsidiary that contain any
redemption or similar provisions, and there are no contracts, commitments, understandings or arrangements by which the Company or any
Subsidiary is or may become bound to redeem a security of the Company or such Subsidiary. The Company does not have any stock appreciation
rights or “phantom stock” plans or agreements or any similar plan or agreement. All of the outstanding shares of capital stock
of the Company are duly authorized, validly issued, fully paid and nonassessable, have been issued in compliance with all federal and
state securities laws, and none of such outstanding shares was issued in violation of any preemptive rights or similar rights to subscribe
for or purchase securities. No further approval or authorization of any stockholder, the Board or others is required for the issuance
and sale of the Shares. There are no stockholders agreements, voting agreements or other similar agreements with respect to the Company’s
capital stock to which the Company is a party or, to the knowledge of the Company, between or among any of the Company’s stockholders.
(h) Registration
Statement. The Company meets the requirements for use of Form S-3 under the Act and has prepared and will file, on or immediately
following the Execution Time, with the Commission the Registration Statement, including a related Base Prospectus, for registration under
the Act of the offering and sale of the Shares. After the Effective Time, such Registration Statement will be effective and available
for the offer and sale of the Shares as of the date hereof. As filed, the Base Prospectus contains all information required by the Act
and the rules thereunder, and, except to the extent the Manager shall agree in writing to a modification, shall be in all substantive
respects in the form furnished to the Manager prior to the Execution Time or prior to any such time this representation is repeated or
deemed to be made. The Registration Statement, at the Execution Time, each such time this representation is repeated or deemed to be made,
and at all times during which a prospectus is required by the Act to be delivered (whether physically or through compliance with Rule 172,
173 or any similar rule) in connection with any offer or sale of the Shares, meets the requirements set forth in Rule 415(a)(1)(x).
The Company meets the transaction requirements as set forth in General Instruction I.B.1 of Form S-3 or, if applicable, as set forth
in General Instruction I.B.6 of Form S-3 with respect to the aggregate market value of securities being sold pursuant to this offering
and during the twelve (12) months prior to this offering.
(i) Accuracy
of Incorporated Documents. The Incorporated Documents, when they were filed with the Commission, conformed in all material respects
to the requirements of the Exchange Act and the rules thereunder, and none of the Incorporated Documents, when they were filed with
the Commission, contained any untrue statement of a material fact or omitted to state a material fact necessary to make the statements
therein, in light of the circumstances under which they were made not misleading; and any further documents so filed and incorporated
by reference in the Registration Statement, the Base Prospectus, the Prospectus Supplement or the Prospectus, when such documents are
filed with the Commission, will conform in all material respects to the requirements of the Exchange Act and the rules thereunder,
as applicable, and will not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements
therein, in light of the circumstances under which they were made, not misleading.
(j) Ineligible
Issuer. (i) At the earliest time after the filing of the Registration Statement that the Company or another offering participant
made a bona fide offer (within the meaning of Rule 164(h)(2)) of the Shares and (ii) as of the Execution Time and on each such
time this representation is repeated or deemed to be made (with such date being used as the determination date for purposes of this clause (ii)),
the Company was not and is not an Ineligible Issuer (as defined in Rule 405), without taking account of any determination by the
Commission pursuant to Rule 405 that it is not necessary that the Company be considered an Ineligible Issuer.
(k) Free
Writing Prospectus. The Company is eligible to use Issuer Free Writing Prospectuses. Each Issuer Free Writing Prospectus does not
include any information the substance of which conflicts with the information contained in the Registration Statement, including any Incorporated
Documents and any prospectus supplement deemed to be a part thereof that has not been superseded or modified; and each Issuer Free Writing
Prospectus does not contain any untrue statement of a material fact or omit to state any material fact necessary in order to make the
statements therein, in the light of the circumstances under which they were made, not misleading. The foregoing sentence does not apply
to statements in or omissions from any Issuer Free Writing Prospectus based upon and in conformity with written information furnished
to the Company by the Manager specifically for use therein. Any Issuer Free Writing Prospectus that the Company is required to file pursuant
to Rule 433(d) has been, or will be, filed with the Commission in accordance with the requirements of the Act and the rules thereunder.
Each Issuer Free Writing Prospectus that the Company has filed, or is required to file, pursuant to Rule 433(d) or that was
prepared by or behalf of or used by the Company complies or will comply in all material respects with the requirements of the Act and
the rules thereunder. The Company will not, without the prior consent of the Manager, prepare, use or refer to, any Issuer Free Writing
Prospectuses.
(l) Proceedings
Related to Registration Statement. The Registration Statement is not the subject of a pending proceeding or examination under Section 8(d) or
8(e) of the Act, and the Company is not the subject of a pending proceeding under Section 8A of the Act in connection with the
offering of the Shares. The Company has not received any notice that the Commission has issued or intends to issue a stop-order with respect
to the Registration Statement or that the Commission otherwise has suspended or withdrawn the effectiveness of the Registration Statement,
either temporarily or permanently, or intends or has threatened in writing to do so.
(m) SEC
Reports. The Company has filed all reports, schedules, forms, statements and other documents required to be filed by the Company under
the Act and the Exchange Act, including pursuant to Section 13(a) or 15(d) thereof, for the two years preceding the date
hereof (or such shorter period as the Company was required by law or regulation to file such material) (the foregoing materials, including
the exhibits thereto and documents incorporated by reference therein, together with the Prospectus and the Prospectus Supplement, being
collectively referred to herein as the “SEC Reports”) on a timely basis or has received a valid extension of such time
of filing and has filed any such SEC Reports prior to the expiration of any such extension. As of their respective dates, the SEC Reports
complied in all material respects with the requirements of the Act and the Exchange Act, as applicable, and, since December 31, 2022
(including, without limitation, the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022),
none of the SEC Reports, when filed, contained any untrue statement of a material fact or omitted to state a material fact required to
be stated therein or necessary in order to make the statements therein, in the light of the circumstances under which they were made,
not misleading. The financial statements of the Company included in the SEC Reports comply in all material respects with applicable accounting
requirements and the rules and regulations of the Commission with respect thereto as in effect at the time of filing. Such financial
statements have been prepared in accordance with United States generally accepted accounting principles applied on a consistent basis
during the periods involved (“GAAP”), except as may be otherwise specified in such financial statements or the notes
thereto and except that unaudited financial statements may not contain all footnotes required by GAAP, and fairly present in all material
respects the financial position of the Company and its consolidated Subsidiaries as of and for the dates thereof and the results of operations
and cash flows for the periods then ended, subject, in the case of unaudited statements, to normal, immaterial, year-end audit adjustments.
(n) [RESERVED]
(o) Material
Changes; Undisclosed Events, Liabilities or Developments. Since the date of the latest audited financial statements included within
the SEC Reports, except as specifically disclosed in a subsequent SEC Report filed prior to the date on which this representation is being
made, (i) there has been no event, occurrence or development that has had or that could reasonably be expected to result in a Material
Adverse Effect, (ii) the Company has not incurred any liabilities (contingent or otherwise) other than (A) trade payables and
accrued expenses incurred in the ordinary course of business consistent with past practice and (B) liabilities not required to be
reflected in the Company’s financial statements pursuant to GAAP or disclosed in filings made with the Commission, (iii) the
Company has not altered its method of accounting, (iv) the Company has not declared or made any dividend or distribution of cash
or other property to its stockholders or purchased, redeemed or made any agreements to purchase or redeem any shares of its capital stock,
(v) the Company has not issued any equity securities to any officer, director or “Affiliate” (defined as any Person
that, directly or indirectly through one or more intermediaries, controls or is controlled by or is under common control with a Person,
as such terms are used in and construed under Rule 144 under the Act), except pursuant to existing Company stock incentive plans,
and (vi) no executive officer of the Company or member of the Board has resigned from any position with the Company. The Company
does not have pending before the Commission any request for confidential treatment of information. Except for the issuance of the Shares
contemplated by this Agreement, no event, liability, fact, circumstance, occurrence or development has occurred or exists or is reasonably
expected to occur or exist with respect to the Company or its Subsidiaries or their respective businesses, prospects, properties, operations,
assets or financial condition that would be required to be disclosed by the Company under applicable securities laws at the time this
representation is made or deemed made that has not been publicly disclosed at least one (1) Trading Day prior to the date that this
representation is made.
(p) Litigation.
Except as set forth in the SEC Reports, there is no action, suit, inquiry, notice of violation, proceeding or investigation pending or,
to the knowledge of the Company, threatened against or affecting the Company, any Subsidiary or any of their respective properties before
or by any court, arbitrator, governmental or administrative agency or regulatory authority (federal, state, county, local or foreign)
(collectively, an “Action”). Except as set forth in the SEC Reports, none of the Actions set forth in the SEC Reports,
(i) adversely affects or challenges the legality, validity or enforceability of this Agreement or the Shares or (ii) could,
if there were an unfavorable decision, have or reasonably be expected to result in a Material Adverse Effect. Except as set forth in the
SEC Reports, neither the Company nor any Subsidiary, nor any director or officer thereof, is or has been the subject of any Action involving
a claim of violation of or liability under federal or state securities laws or a claim of breach of fiduciary duty. Except as set forth
in the SEC Reports, there has not been, and to the knowledge of the Company, there is not pending or contemplated, any investigation by
the Commission involving the Company or any current or former director or officer of the Company. The Commission has not issued any stop
order or other order suspending the effectiveness of any registration statement filed by the Company or any Subsidiary under the Exchange
Act or the Act.
(q) Labor
Relations. No labor dispute exists or, to the knowledge of the Company, is imminent with respect to any of the employees of the Company,
which could reasonably be expected to result in a Material Adverse Effect. None of the Company’s or its Subsidiaries’ employees
is a member of a union that relates to such employee’s relationship with the Company or such Subsidiary, and neither the Company
nor any of its Subsidiaries is a party to a collective bargaining agreement, and the Company and its Subsidiaries believe that their relationships
with their employees are good. To the knowledge of the Company, no executive officer of the Company or any Subsidiary, is, or is now expected
to be, in violation of any material term of any employment contract, confidentiality, disclosure or proprietary information agreement
or non-competition agreement, or any other contract or agreement or any restrictive covenant in favor of any third party, and the continued
employment of each such executive officer does not subject the Company or any of its Subsidiaries to any liability with respect to any
of the foregoing matters. The Company and its Subsidiaries are in compliance with all applicable U.S. federal, state, local and foreign
laws and regulations relating to employment and employment practices, terms and conditions of employment and wages and hours, except where
the failure to be in compliance could not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect.
(r) Compliance.
Except as set forth in the SEC Reports, neither the Company nor any Subsidiary: (i) is in default under or in violation of (and no
event has occurred that has not been waived that, with notice or lapse of time or both, would result in a default by the Company or any
Subsidiary under), nor has the Company or any Subsidiary received notice of a claim that it is in default under or that it is in violation
of, any indenture, loan or credit agreement or any other agreement or instrument to which it is a party or by which it or any of its properties
is bound (whether or not such default or violation has been waived), (ii) is in violation of any judgment, decree or order of any
court, arbitrator or other governmental authority or (iii) is or has been in violation of any statute, rule, ordinance or regulation
of any governmental authority, including without limitation all foreign, federal, state and local laws relating to taxes, environmental
protection, occupational health and safety, product quality and safety and employment and labor matters, except in each case as could
not have or reasonably be expected to result in a Material Adverse Effect.
(s) Environmental
Laws. The Company and its Subsidiaries (i) are in compliance
with all federal, state, local and foreign laws relating to pollution or protection of human health or the environment (including ambient
air, surface water, groundwater, land surface or subsurface strata), including laws relating to emissions, discharges, releases or threatened
releases of chemicals, pollutants, contaminants, or toxic or hazardous substances or wastes (collectively, “Hazardous Materials”)
into the environment, or otherwise relating to the manufacture, processing, distribution, use, treatment, storage, disposal, transport
or handling of Hazardous Materials, as well as all authorizations, codes, decrees, demands, or demand letters, injunctions, judgments,
licenses, notices or notice letters, orders, permits, plans or regulations, issued, entered, promulgated or approved thereunder (“Environmental
Laws”); (ii) have received all permits licenses or other approvals required of them under applicable Environmental Laws
to conduct their respective businesses; and (iii) are in compliance with all terms and conditions of any such permit, license or
approval where in each clause (i), (ii) and (iii), the failure to so comply could be reasonably expected to have, individually or
in the aggregate, a Material Adverse Effect.
(t) Regulatory
Permits. The Company and the Subsidiaries possess all certificates, authorizations and permits issued by the appropriate federal,
state, local or foreign regulatory authorities necessary to conduct their respective businesses as described in the SEC Reports, except
where the failure to possess such permits could not reasonably be expected to result in a Material Adverse Effect (“Material
Permits”), and neither the Company nor any Subsidiary has received any notice of proceedings relating to the revocation or modification
of any Material Permit.
(u) Title
to Assets. The Company and the Subsidiaries have good and marketable title in fee simple to all real property owned by them and good
and marketable title in all personal property owned by them that is material to the business of the Company and the Subsidiaries, in each
case free and clear of all Liens, except for (i) Liens as do not materially affect the value of such property and do not materially
interfere with the use made and proposed to be made of such property by the Company and the Subsidiaries and (ii) Liens for the payment
of federal, state or other taxes, for which appropriate reserves have been made therefor in accordance with GAAP and, the payment of which
is neither delinquent nor subject to penalties. Any real property and facilities held under lease by the Company and the Subsidiaries
are held by them under valid, subsisting and enforceable leases with which the Company and the Subsidiaries are in compliance.
(v) Intellectual
Property. The Company and the Subsidiaries own, or have rights to use, all patents, patent applications, trademarks, trademark applications,
service marks, trade names, trade secrets, inventions, copyrights, licenses and/or other intellectual property rights necessary or required
for use in connection with their respective businesses as described in the SEC Reports since December 31, 2022 (including, without
limitation, the Company’s annual report on Form 10-K for the fiscal year ended December 31, 2022) and which the failure
to do so have could have a Material Adverse Effect (collectively, the “Intellectual Property Rights”). None of, and
neither the Company nor any Subsidiary has received a notice (written or otherwise) that any of the Intellectual Property Rights has expired,
terminated or been abandoned, or is expected to expire or terminate or be abandoned, nor are any Intellectual Property Rights expected
to expire or terminate or be abandoned within two (2) years from the date of this Agreement except if the Company shall maintain
such Intellectual Property Rights in substantially the same, or similar, form. Neither the Company nor any Subsidiary has received, since
the date of the latest audited financial statements included within the SEC Reports, a written notice of a claim or otherwise has any
knowledge that the Intellectual Property Rights violate or infringe upon the rights of any Person, except as could not have or reasonably
be expected to not have a Material Adverse Effect. To the knowledge of the Company, all such Intellectual Property Rights are enforceable
and there is no existing infringement by another Person of any of the Intellectual Property Rights. The Company and its Subsidiaries have
taken reasonable security measures to protect the secrecy, confidentiality and value of all of their Intellectual Property Rights, except
where failure to do so could not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect.
(w) Insurance.
The Company and the Subsidiaries are insured by insurers of recognized financial responsibility against such losses and risks and in such
amounts as are prudent and customary in the businesses in which the Company and the Subsidiaries are engaged, including, but not limited
to, directors and officers insurance coverage. Neither the Company nor any Subsidiary has any reason to believe that it will not be able
to renew its existing insurance coverage as and when such coverage expires or to obtain similar coverage from similar insurers as may
be necessary to continue its business.
(x) Affiliate
Transactions. Except as set forth in the SEC Reports, none of the officers or directors of the Company or any Subsidiary and, to the
knowledge of the Company, none of the employees of the Company or any Subsidiary is presently a party to any transaction with the Company
or any Subsidiary (other than for services as employees, officers and directors), including any contract, agreement or other arrangement
providing for the furnishing of services to or by, providing for rental of real or personal property to or from, providing for the borrowing
of money from or lending of money to or otherwise requiring payments to or from any officer, director or such employee or, to the knowledge
of the Company, any entity in which any officer, director, or any such employee has a substantial interest or is an officer, director,
trustee, stockholder, member or partner, in each case in excess of $120,000 other than for (i) payment of salary or consulting or
director fees for services rendered, (ii) reimbursement for expenses incurred on behalf of the Company, (iii) indemnification
and advancement of expenses in connection with Actions that are disclosed in the SEC Reports, and (iv) other employee benefits, including
equity awards under any stock incentive plan of the Company.
(y) Sarbanes
Oxley Compliance. The Company and the Subsidiaries are in compliance with any and all applicable requirements of the Sarbanes-Oxley
Act of 2002, as amended, that are effective as of the date hereof, and any and all applicable rules and regulations promulgated by
the Commission thereunder that are effective as of the date hereof. The Company and the Subsidiaries maintain a system of internal accounting
controls sufficient to provide reasonable assurance that: (i) transactions are executed in accordance with management’s general
or specific authorizations, (ii) transactions are recorded as necessary to permit preparation of financial statements in conformity
with GAAP and to maintain asset accountability, (iii) access to assets is permitted only in accordance with management’s general
or specific authorization, and (iv) the recorded accountability for assets is compared with the existing assets at reasonable intervals
and appropriate action is taken with respect to any differences. The Company and the Subsidiaries have established disclosure controls
and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) for the Company and the Subsidiaries and designed
such disclosure controls and procedures to ensure that information required to be disclosed by the Company in the reports it files or
submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the Commission’s
rules and forms. The Company’s certifying officers have evaluated the effectiveness of the disclosure controls and procedures
of the Company and the Subsidiaries as of the end of the period covered by the most recently filed periodic report under the Exchange
Act (such date, the “Evaluation Date”). The Company presented in its most recently filed periodic report under the
Exchange Act the conclusions of the certifying officers about the effectiveness of the disclosure controls and procedures based on their
evaluations as of the Evaluation Date. Since the Evaluation Date, there have been no changes in the internal control over financial reporting
(as such term is defined in the Exchange Act) of the Company and its Subsidiaries that have materially affected, or is reasonably likely
to materially affect, the internal control over financial reporting of the Company and its Subsidiaries.
(z) Certain
Fees. Other than payments to be made to the Manager, no brokerage or finder’s fees or commissions are or will be payable by
the Company or any Subsidiary to any broker, financial advisor or consultant, finder, placement agent, investment banker, bank or other
Person with respect to the transactions contemplated by this Agreement. The Manager shall have no obligation with respect to any fees
or with respect to any claims made by or on behalf of other Persons for fees of a type contemplated in this Section that may be due
in connection with the transactions contemplated by this Agreement.
(aa) No
Other Sales Agency Agreement. The Company has not entered into any other sales agency agreements or other similar arrangements with
any agent or any other representative in respect of at the market offerings of the Shares.
(bb) [RESERVED]
(cc) Listing
and Maintenance Requirements. The Common Stock is listed on the Trading Market and the issuance of the Shares as contemplated by this
Agreement does not contravene the rules and regulations of the Trading Market. The Common Stock is registered pursuant to Section 12(b) or
12(g) of the Exchange Act, and the Company has taken no action designed to, or which to its knowledge is likely to have the effect
of, terminating the registration of the Common Stock under the Exchange Act nor has the Company received any notification that the Commission
is contemplating terminating such registration. Except as set forth in the SEC Reports, the Company has not, in the 12 months preceding
the date hereof, received notice from any Trading Market on which the Common Stock is or has been listed or quoted to the effect that
the Company is not in compliance with the listing or maintenance requirements of such Trading Market. Except as set forth in the SEC Reports,
the Company is, and has no reason to believe that it will not in the foreseeable future continue to be, in compliance with all such listing
and maintenance requirements. The Common Stock is currently eligible for electronic transfer through the Depository Trust Company or another
established clearing corporation and the Company is current in payment of the fees to the Depository Trust Company (or such other established
clearing corporation) in connection with such electronic transfer.
(dd) [RESERVED]
(ee) Solvency.
Except as set forth in the SEC Reports, based on the consolidated financial condition of the Company as of the date hereof, (i) the
fair saleable value of the Company’s assets exceeds the amount that will be required to be paid on or in respect of the Company’s
existing debts and other liabilities (including known contingent liabilities) as they mature, (ii) the Company’s assets do
not constitute unreasonably small capital to carry on its business as now conducted and as proposed to be conducted including its capital
needs taking into account the particular capital requirements of the business conducted by the Company, consolidated and projected capital
requirements and capital availability thereof, and (iii) the current cash flow of the Company, together with the proceeds the Company
would receive, were it to liquidate all of its assets, after taking into account all anticipated uses of the cash, would be sufficient
to pay all amounts on or in respect of its liabilities when such amounts are required to be paid. Except as set forth in the SEC Reports,
the Company does not intend to incur debts beyond its ability to pay such debts as they mature (taking into account the timing and amounts
of cash to be payable on or in respect of its debt) within one year from the date hereof. Except as set forth in the SEC Reports, the
Company has no knowledge of any facts or circumstances which lead it to believe that it will file for reorganization or liquidation under
the bankruptcy or reorganization laws of any jurisdiction within one year from the date hereof. The SEC Reports set forth as of the date
hereof all outstanding secured and unsecured Indebtedness of the Company or any Subsidiary, or for which the Company or any Subsidiary
has commitments. For the purposes of this Agreement, “Indebtedness” means (x) any liabilities for borrowed money
or amounts owed in excess of $50,000 (other than trade accounts payable incurred in the ordinary course of business), (y) all guaranties,
endorsements and other contingent obligations in respect of indebtedness of others, whether or not the same are or should be reflected
in the Company’s consolidated balance sheet (or the notes thereto), except guaranties by endorsement of negotiable instruments for
deposit or collection or similar transactions in the ordinary course of business; and (z) the present value of any lease payments
in excess of $50,000 due under leases required to be capitalized in accordance with GAAP. Neither the Company nor any Subsidiary is in
default with respect to any Indebtedness.
(ff) Tax
Status. Except for matters that would not, individually or in the aggregate, have or reasonably be expected to result in a Material
Adverse Effect, the Company and its Subsidiaries each (i) has made or filed all United States federal, state and local income and
all foreign income and franchise tax returns, reports and declarations required by any jurisdiction to which it is subject, (ii) has
paid all taxes and other governmental assessments and charges that are material in amount, shown or determined to be due on such returns,
reports and declarations and (iii) has set aside on its books provision reasonably adequate for the payment of all material taxes
for periods subsequent to the periods to which such returns, reports or declarations apply. There are no unpaid taxes in any material
amount claimed to be due by the taxing authority of any jurisdiction, and the officers of the Company or of any Subsidiary know of no
basis for any such claim.
(gg) Foreign
Corrupt Practices. Neither the Company nor any Subsidiary, nor to the knowledge of the Company or any Subsidiary, any agent or other
person acting on behalf of the Company or any Subsidiary, has (i) directly or indirectly, used any funds for unlawful contributions,
gifts, entertainment or other unlawful expenses related to foreign or domestic political activity, (ii) made any unlawful payment
to foreign or domestic government officials or employees or to any foreign or domestic political parties or campaigns from corporate funds,
(iii) failed to disclose fully any contribution made by the Company or any Subsidiary (or made by any person acting on its behalf
of which the Company is aware) which is in violation of law, or (iv) violated in any material respect any provision of the Foreign
Corrupt Practices Act of 1977, as amended.
(hh) Accountants.
The Company’s accounting firm is set forth in the SEC Reports. To the knowledge and belief of the Company, such accounting firm
(i) is a registered public accounting firm as required by the Exchange Act and (ii) shall express its opinion with respect to
the financial statements to be included in the Company’s Annual Report for the fiscal year ending December 31, 2023.
(ii) Regulation
M Compliance. The Company has not, and to its knowledge no one acting on its behalf has, (i) taken, directly or indirectly,
any action designed to cause or to result in the stabilization or manipulation of the price of any security of the Company to facilitate
the sale or resale of any of the Shares, (ii) sold, bid for, purchased, or, paid any compensation for soliciting purchases of, any
of the Shares, or (iii) paid or agreed to pay to any Person any compensation for soliciting another to purchase any other securities
of the Company, other than, in the case of clauses (ii) and (iii), compensation paid to the Manager in connection with the Shares.
(jj) FDA.
As to each product subject to the jurisdiction of the U.S. Food and Drug Administration (“FDA”) under the Federal Food,
Drug and Cosmetic Act, as amended, and the regulations thereunder (“FDCA”) that is manufactured, packaged, labeled,
tested, distributed, sold, and/or marketed by the Company or any of its Subsidiaries (each such product, a “Pharmaceutical Product”),
such Pharmaceutical Product is being manufactured, packaged, labeled, tested, distributed, sold and/or marketed by the Company in compliance
with all applicable requirements under FDCA and similar laws, rules and regulations relating to registration, investigational use,
premarket clearance, licensure, or application approval, good manufacturing practices, good laboratory practices, good clinical practices,
product listing, quotas, labeling, advertising, record keeping and filing of reports, except where the failure to be in compliance would
not have a Material Adverse Effect. There is no pending, completed or, to the Company's knowledge, threatened, action (including any lawsuit,
arbitration, or legal or administrative or regulatory proceeding, charge, complaint, or investigation) against the Company or any of its
Subsidiaries, and none of the Company or any of its Subsidiaries has received any notice, warning letter or other communication from the
FDA or any other governmental entity, which (i) contests the premarket clearance, licensure, registration, or approval of, the uses
of, the distribution of, the manufacturing or packaging of, the testing of, the sale of, or the labeling and promotion of any Pharmaceutical
Product, (ii) withdraws its approval of, requests the recall, suspension, or seizure of, or withdraws or orders the withdrawal of
advertising or sales promotional materials relating to, any Pharmaceutical Product, (iii) imposes a clinical hold on any clinical
investigation by the Company or any of its Subsidiaries, (iv) enjoins production at any facility of the Company or any of its Subsidiaries,
(v) enters or proposes to enter into a consent decree of permanent injunction with the Company or any of its Subsidiaries, or (vi) otherwise
alleges any violation of any laws, rules or regulations by the Company or any of its Subsidiaries, and which, either individually
or in the aggregate, would have a Material Adverse Effect. The properties, business and operations of the Company have been and are being
conducted in all material respects in accordance with all applicable laws, rules and regulations of the FDA. The Company has
not been informed by the FDA that the FDA will prohibit the marketing, sale, license or use in the United States of any product proposed
to be developed, produced or marketed by the Company nor has the FDA expressed any concern as to approving or clearing for marketing any
product being developed or proposed to be developed by the Company.
(kk) Stock
Plans. Each stock option granted by the Company under the Company’s stock incentive plan was granted (i) in accordance
with the terms of such stock incentive plan and (ii) with an exercise price at least equal to the fair market value of the Common
Stock on the date such stock option would be considered granted under GAAP and applicable law. No stock option granted under the Company’s
stock incentive plan has been backdated. The Company has not knowingly granted, and there is no and has been no Company policy or practice
to knowingly grant, stock options prior to, or otherwise knowingly coordinate the grant of stock options with, the release or other public
announcement of material information regarding the Company or its Subsidiaries or their financial results or prospects.
(ll) Cybersecurity.
(i)(x) There has been no security breach or other compromise of or relating to any of the Company’s or any Subsidiary’s
information technology and computer systems, networks, hardware, software, data (including the data of its respective customers, employees,
suppliers, vendors and any third party data maintained by or on behalf of it), equipment or technology (collectively, “IT Systems
and Data”), except as would not, individually or in the aggregate, have a Material Adverse Effect, and (y) the Company
and the Subsidiaries have not been notified of any security breach or other compromise to its IT Systems and Data; (ii) the Company
and the Subsidiaries are presently in compliance with all applicable laws or statutes and all judgments, orders, rules and regulations
of any court or arbitrator or governmental or regulatory authority, internal policies and contractual obligations relating to the privacy
and security of IT Systems and Data and to the protection of such IT Systems and Data from unauthorized use, access, misappropriation
or modification, except as would not, individually or in the aggregate, have a Material Adverse Effect; (iii) the Company and the
Subsidiaries use commercially reasonable efforts to maintain and protect its material confidential information and to ensure the integrity,
operation, redundancy and security of the IT Systems and Data that is material to the Company; and (iv) the Company and the Subsidiaries
have implemented commercially reasonable backup and disaster recovery technology plans in all material respects.
(mm) Office
of Foreign Assets Control. Neither the Company nor any Subsidiary nor, to the Company's knowledge, any director, officer, agent, employee
or Affiliate of the Company or any Subsidiary is currently subject to any U.S. sanctions administered by the Office of Foreign Assets
Control of the U.S. Treasury Department.
(nn) U.S.
Real Property Holding Corporation. The Company is not and has never been a U.S. real property holding corporation within the meaning
of Section 897 of the Internal Revenue Code of 1986, as amended, and the Company shall so certify upon the Manager’s request.
(oo) Bank
Holding Company Act. Neither the Company nor any of its Subsidiaries nor, to the knowledge of the Company, any of its Affiliates
is subject to the Bank Holding Company Act of 1956, as amended (the “BHCA”) and to regulation by the Board of Governors
of the Federal Reserve System (the “Federal Reserve”). Neither the Company nor any of its Subsidiaries nor, to the
knowledge of the Company, any of its Affiliates owns or controls, directly or indirectly, five percent (5%) or more of the outstanding
shares of any class of voting securities or twenty-five percent (25%) or more of the total equity of a bank or any entity that is subject
to the BHCA and to regulation by the Federal Reserve. Neither the Company nor any of its Subsidiaries nor, to the knowledge of the Company,
any of its Affiliates exercises a controlling influence over the management or policies of a bank or any entity that is subject to the
BHCA and to regulation by the Federal Reserve.
(pp) Money
Laundering. The operations of the Company and its Subsidiaries are and have been conducted at all times in compliance with applicable
financial record-keeping and reporting requirements of the Currency and Foreign Transactions Reporting Act of 1970, as amended, applicable
money laundering statutes and applicable rules and regulations thereunder (collectively, the “Money Laundering Laws”),
and no Action or Proceeding by or before any court or governmental agency, authority or body or any arbitrator involving the Company or
any Subsidiary with respect to the Money Laundering Laws is pending or, to the knowledge of the Company or any Subsidiary, threatened.
(qq) FINRA
Member Shareholders. There are no affiliations with any FINRA member firm among the Company’s officers, directors or, to the
knowledge of the Company, any five percent (5%) or greater stockholder of the Company, except as set forth in the Registration Statement,
the Base Prospectus, any Prospectus Supplement or the Prospectus.
4. Agreements.
The Company agrees with the Manager that:
(a) Right
to Review Amendments and Supplements to Registration Statement and Prospectus. During any period when the delivery of a prospectus
relating to the Shares is required (including in circumstances where such requirement may be satisfied pursuant to Rule 172, 173
or any similar rule) to be delivered under the Act in connection with the offering or the sale of Shares, the Company will not file any
amendment to the Registration Statement or supplement (including any Prospectus Supplement) to the Base Prospectus unless the Company
has furnished to the Manager a copy for its review prior to filing and will not file any such proposed amendment or supplement to which
the Manager reasonably objects (provided, however, that the Company will have no obligation to provide the Manager any advance copy of
such filing or to provide the Manager an opportunity to object to such filing if the filing does not name the Manager and does not relate
to the transactions under this Agreement). The Company will cause any supplement to the Prospectus filed after the Effective Time to be
properly completed, in a form approved by the Manager, and will file such supplement with the Commission pursuant to the applicable paragraph
of Rule 424(b) within the time period prescribed thereby and will provide evidence reasonably satisfactory to the Manager of
such timely filing. The Company will promptly advise the Manager (i) when the Prospectus, and any supplement thereto, shall have
been filed (if required) with the Commission pursuant to Rule 424(b), (ii) when, during any period when the delivery of a prospectus
(whether physically or through compliance with Rule 172, 173 or any similar rule) is required under the Act in connection with the
offering or sale of the Shares, any amendment to the Registration Statement shall have been filed or become effective (other than any
annual report of the Company filed pursuant to Section 13(a) or 15(d) of the Exchange Act), (iii) of any request by
the Commission or its staff for any amendment of the Registration Statement, or for any supplement to the Prospectus or for any additional
information, (iv) of the issuance by the Commission of any stop order suspending the effectiveness of the Registration Statement
or of any notice objecting to its use or the institution or threatening of any proceeding for that purpose and (v) of the receipt
by the Company of any notification with respect to the suspension of the qualification of the Shares for sale in any jurisdiction or the
institution or threatening of any proceeding for such purpose. The Company will use its reasonable efforts to prevent the issuance of
any such stop order or the occurrence of any such suspension or objection to the use of the Registration Statement and, upon such issuance,
occurrence or notice of objection, to obtain as soon as possible the withdrawal of such stop order or relief from such occurrence or objection,
including, if necessary, by filing an amendment to the Registration Statement or a new registration statement and using its reasonable
efforts to have such amendment or new registration statement declared effective as soon as practicable.
(b) Subsequent
Events. If, at any time on or after an Applicable Time but prior to the related Settlement Date, any event occurs as a result of which
the Registration Statement or Prospectus would include any untrue statement of a material fact or omit to state any material fact necessary
to make the statements therein in the light of the circumstances under which they were made or the circumstances then prevailing not misleading,
the Company will (i) notify promptly the Manager so that any use of the Registration Statement or Prospectus may cease until such
are amended or supplemented; (ii) amend or supplement the Registration Statement or Prospectus to correct such statement or omission;
and (iii) supply any such amendment or supplement to the Manager in such quantities as the Manager may reasonably request.
(c) Notification
of Subsequent Filings. During any period when the delivery of a prospectus relating to the Shares is required (including in circumstances
where such requirement may be satisfied pursuant to Rule 172, 173 or any similar rule) to be delivered under the Act, any event occurs
as a result of which the Prospectus as then supplemented would include any untrue statement of a material fact or omit to state any material
fact necessary to make the statements therein in the light of the circumstances under which they were made not misleading, or if it shall
be necessary to amend the Registration Statement, file a new registration statement or supplement the Prospectus to comply with the Act
or the Exchange Act or the respective rules thereunder, including in connection with use or delivery of the Prospectus, the Company
promptly will (i) notify the Manager of any such event, (ii) subject to Section 4(a), prepare and file with the Commission
an amendment or supplement or new registration statement which will correct such statement or omission or effect such compliance, (iii) use
its reasonable efforts to have any amendment to the Registration Statement or new registration statement declared effective as soon as
practicable in order to avoid any disruption in use of the Prospectus and (iv) supply any supplemented Prospectus to the Manager
in such quantities as the Manager may reasonably request.
(d) Earnings
Statements. As soon as practicable, the Company will make generally available to its security holders and to the Manager an earnings
statement or statements of the Company and its Subsidiaries which will satisfy the provisions of Section 11(a) of the Act and
Rule 158. For the avoidance of doubt, the Company’s compliance with the reporting requirements of the Exchange Act shall be
deemed to satisfy the requirements of this Section 4(d).
(e) Delivery
of Registration Statement. Upon the request of the Manager, the Company will furnish to the Manager and counsel for the Manager, without
charge, signed copies of the Registration Statement (including exhibits thereto) and, so long as delivery of a prospectus by the Manager
or dealer may be required by the Act (including in circumstances where such requirement may be satisfied pursuant to Rule 172, 173
or any similar rule), as many copies of the Prospectus and each Issuer Free Writing Prospectus and any supplement thereto as the Manager
may reasonably request. The Company will pay the expenses of printing or other production of all documents relating to the offering.
(f) Qualification
of Shares. The Company will arrange, if necessary, for the qualification of the Shares for sale under the laws of such jurisdictions
as the Manager may designate and will maintain such qualifications in effect so long as required for the distribution of the Shares; provided
that in no event shall the Company be obligated to qualify to do business in any jurisdiction where it is not now so qualified or to take
any action that would subject it to service of process in suits, other than those arising out of the offering or sale of the Shares, in
any jurisdiction where it is not now so subject.
(g) Free
Writing Prospectus. The Company agrees that, unless it has or shall have obtained the prior written consent of the Manager, and the
Manager agrees with the Company that, unless it has or shall have obtained, as the case may be, the prior written consent of the Company,
it has not made and will not make any offer relating to the Shares that would constitute an Issuer Free Writing Prospectus or that would
otherwise constitute a “free writing prospectus” (as defined in Rule 405) required to be filed by the Company with the
Commission or retained by the Company under Rule 433. Any such free writing prospectus consented to by the Manager or the Company
is hereinafter referred to as a “Permitted Free Writing Prospectus.” The Company agrees that (i) it has treated
and will treat, as the case may be, each Permitted Free Writing Prospectus as an Issuer Free Writing Prospectus and (ii) it has complied
and will comply, as the case may be, with the requirements of Rules 164 and 433 applicable to any Permitted Free Writing Prospectus,
including in respect of timely filing with the Commission, legending and record keeping.
(h) Subsequent
Equity Issuances. The Company shall not deliver any Sales Notice hereunder (and any Sales Notice previously delivered shall not apply
during such two Trading Days) for at least two (2) Trading Days prior to any date on which the Company or any Subsidiary offers,
sells, issues, contracts to sell, contracts to issue or otherwise disposes of, directly or indirectly, any other shares of Common Stock
or any Common Stock Equivalents (other than the Shares), subject to Manager’s right to waive this obligation, provided that, without
compliance with the foregoing obligation, the Company may issue and sell Common Stock pursuant to any employee equity plan, stock ownership
plan or dividend reinvestment plan of the Company in effect at the Execution Time and the Company may issue Common Stock issuable upon
the conversion or exercise of Common Stock Equivalents outstanding at the Execution Time.
(i) Market
Manipulation. Until the termination of this Agreement, the Company will not take, directly or indirectly, any action designed to or
that would constitute or that might reasonably be expected to cause or result in, under the Exchange Act or otherwise, stabilization or
manipulation in violation of the Act, Exchange Act or the rules and regulations thereunder of the price of any security of the Company
to facilitate the sale or resale of the Shares or otherwise violate any provision of Regulation M under the Exchange Act.
(j) Notification
of Incorrect Certificate. The Company will, at any time during the term of this Agreement, as supplemented from time to time, promptly
advise the Manager after it shall have received notice or obtained knowledge thereof, of any information or fact that would cause any
opinion, certificate, letter and other document provided to the Manager pursuant to Section 6 herein to be untrue or inaccurate in
any material respect.
(k) Certification
of Accuracy of Disclosure. Upon commencement of the offering of the Shares under this Agreement (and upon the recommencement of the
offering of the Shares under this Agreement following the termination of a suspension of sales hereunder lasting more than 30 Trading
Days), and each time that (i) the Registration Statement or Prospectus shall be amended or supplemented, other than by means of Incorporated
Documents, (ii) the Company files its Annual Report on Form 10-K under the Exchange Act, (iii) the Company files its quarterly
reports on Form 10-Q under the Exchange Act, (iv) the Company files a Current Report on Form 8-K containing amended financial
information (other than information that is furnished and not filed), if the Manager reasonably determines that the information in such
Form 8-K is material, or (v) the Shares are delivered to the Manager as principal at the Time of Delivery pursuant to a Terms
Agreement (such commencement or recommencement date and each such date referred to in (i), (ii), (iii), (iv) and (v) above,
a “Representation Date”), unless waived by the Manager, the Company shall furnish or cause to be furnished to the Manager
forthwith a certificate dated and delivered on the Representation Date, in form reasonably satisfactory to the Manager to the effect that
the statements contained in the certificate referred to in Section 6 of this Agreement which were last furnished to the Manager are
true and correct at the Representation Date, as though made at and as of such date (except that such statements shall be deemed to relate
to the Registration Statement and the Prospectus as amended and supplemented to such date) or, in lieu of such certificate, a certificate
of the same tenor as the certificate referred to in said Section 6, modified as necessary to relate to the Registration Statement
and the Prospectus as amended and supplemented to the date of delivery of such certificate. Notwithstanding the foregoing, the requirement
to provide a certificate under this Section 4(k) shall be waived for any Representation Date occurring at a time at which no
Sales Notice is pending or a suspension is in effect, which waiver shall continue until the earlier to occur of the date the Company delivers
a Sales Notice hereunder (which for such calendar quarter shall be considered a Representation Date) and the next occurring Representation
Date on which the Company files its annual report on Form 10-K. Notwithstanding the foregoing, (i) upon the delivery of the
first Sales Notice hereunder and (ii) if the Company subsequently decides to sell Shares following a Representation Date when the
Company relied on such waiver and did not provide the Manager with a certificate under this Section 4(k), then before the Manager
sells any Shares, the Company shall provide the Manager with such certificate dated the date of the Sales Notice.
(l) Bring
Down Opinions; Negative Assurance. Within five (5) Trading Days of each Representation Date, unless waived by the Manager, the
Company shall furnish or cause to be furnished forthwith to the Manager and to counsel to the Manager a written opinion of counsel to
the Company (“Company Counsel”) addressed to the Manager and dated and delivered within five (5) Trading Days
of such Representation Date, in form and substance reasonably satisfactory to the Manager, including a negative assurance representation.
The requirement to furnish or cause to be furnished an opinion (but not with respect to a negative assurance representation) under this
Section 4(l) shall be waived for any Representation Date other than a Representation Date on which a material amendment to
the Registration Statement or Prospectus is made or the Company files its Annual Report on Form 10-K or a material amendment thereto
under the Exchange Act, unless the Manager reasonably requests such deliverable required this Section 4(l) in connection with
a Representation Date, upon which request such deliverable shall be deliverable hereunder. Notwithstanding the foregoing, the requirement
to provide an opinion or negative assurance representation under this Section 4(l) shall be waived for any Representation Date
occurring at a time at which no Sales Notice is pending or a suspension is in effect, which waiver shall continue until the earlier to
occur of the date the Company delivers a Sales Notice hereunder (which for such calendar quarter shall be considered a Representation
Date) and the next occurring Representation Date on which the Company files its annual report on Form 10-K. Notwithstanding the
foregoing, (i) upon the delivery of the first Sales Notice hereunder and (ii) if the Company subsequently decides to sell Shares
following a Representation Date when the Company relied on such waiver and did not provide the Manager with an opinion or negative assurance
representation under this Section 4(l), then before the Manager sells any Shares, the Company shall provide the Manager with such
opinion or negative assurance representation dated the date of the Sales Notice.
(m) Auditor
Bring Down “Comfort” Letter. Within five (5) Trading Days of each Representation Date, unless waived by the Manager,
the Company shall cause (1) the Company’s auditors (the “Accountants”), or other independent accountants
satisfactory to the Manager forthwith to furnish the Manager a letter, and (2) the Chief Financial Officer of the Company forthwith
to furnish the Manager a certificate, in each case dated within five (5) Trading Days of such Representation Date, in form and substance
reasonably satisfactory to the Manager, of the same tenor as the letters and certificate referred to in Section 6 of this Agreement
but modified to relate to the Registration Statement and the Prospectus, as amended and supplemented to the date of such letters and certificate.
The requirement to furnish or cause to be furnished a “comfort” letter under this Section 4(m) shall be waived for
any Representation Date other than a Representation Date on which a material amendment to the Registration Statement or Prospectus is
made or the Company files its Annual Report on Form 10-K or a material amendment thereto under the Exchange Act, unless the Manager
reasonably requests the deliverables required by this Section 4(m) in connection with a Representation Date, upon which request
such deliverable shall be deliverable hereunder. Notwithstanding the foregoing, the requirement to deliver or cause to be delivered one
or more letters or certificates under this Section 4(m) shall at the request of the Company be waived for any Representation
Date occurring at a time at which no Sales Notice is pending or a suspension is in effect, which waiver shall continue until the earlier
to occur of the date the Company delivers a Sales Notice hereunder (which for such calendar quarter shall be considered a Representation
Date) and the next occurring Representation Date on which the Company files its annual report on Form 10-K. Notwithstanding the foregoing,
(i) upon the delivery of the first Sales Notice hereunder and (ii) if the Company subsequently decides to sell Shares following
a Representation Date when the Company relied on such waiver and did not provide the Manager with a letter or certificate under this Section 4(m),
then before the Manager sells any Shares, the Company shall provide the Manager with such letter or certificate dated the date of the
Sales Notice.
(n) Due
Diligence Session. Upon commencement of the offering of the Shares under this Agreement (and upon the recommencement of the offering
of the Shares under this Agreement following the termination of a suspension of sales hereunder lasting more than 30 Trading Days), and
at each Representation Date, the Company will conduct a due diligence session, in form and substance, reasonably satisfactory to the Manager,
which shall include representatives of management and Accountants. The Company shall cooperate timely with any reasonable due diligence
request from or review conducted by the Manager or its agents from time to time in connection with the transactions contemplated by this
Agreement, including, without limitation, providing information and available documents and access to appropriate corporate officers and
the Company’s agents during regular business hours, and timely furnishing or causing to be furnished such certificates, letters
and opinions from the Company, its officers and its agents, as the Manager may reasonably request. The Company shall reimburse the Manager
for Manager’s counsel’s fees in each such due diligence update session, up to a maximum of $2,500 per update.
(o) Acknowledgment
of Trading. The Company consents to the Manager trading in the Common Stock for the Manager’s own account and for the account
of its clients at the same time as sales of the Shares occur pursuant to this Agreement or pursuant to a Terms Agreement.
(p) Disclosure
of Shares Sold. The Company will disclose in its Annual Reports on Form 10-K and Quarterly Reports on Form 10-Q, as applicable,
the number of Shares sold through the Manager under this Agreement, the Net Proceeds to the Company and the compensation paid by the Company
with respect to sales of Shares pursuant to this Agreement during the relevant quarter; and, if required by any subsequent change in Commission
policy or request, more frequently by means of a Current Report on Form 8-K or a further Prospectus Supplement.
(q) Rescission
Right. If, at any time on or after the time that a person has agreed to purchase Shares from the Company as the result of an offer
to purchase solicited by the Manager pursuant to a Sales Notice but prior to the related Settlement Date, the Company or the Manager becomes
aware that the Registration Statement or the Prospectus included at the date of such sale any untrue statement of a material fact or an
omission to state any material fact necessary to make the statements therein, in the light of the circumstances under which they were
made, not misleading, the Company will, prior to the related Settlement Date for such Shares, offer to such person the right to refuse
to purchase and pay for such Shares.
(r) Bring
Down of Representations and Warranties. Each acceptance by the Company of an offer to purchase the Shares hereunder, and each execution
and delivery by the Company of a Terms Agreement, shall be deemed to be an affirmation to the Manager that the representations and warranties
of the Company contained in or made pursuant to this Agreement are true and correct as of the date of such acceptance or of such Terms
Agreement as though made at and as of such date, and an undertaking that such representations and warranties will be true and correct
as of the Settlement Date for the Shares relating to such acceptance or as of the Time of Delivery relating to such sale, as the case
may be, as though made at and as of such date (except that such representations and warranties shall be deemed to relate to the Registration
Statement and the Prospectus as amended and supplemented relating to such Shares).
(s) Reservation
of Shares. The Company shall ensure that there are at all times sufficient shares of Common Stock to provide for the issuance, free
of any preemptive rights, out of its authorized but unissued shares of Common Stock or shares of Common Stock held in treasury, of the
maximum aggregate number of Shares authorized for issuance by the Board pursuant to the terms of this Agreement. The Company will use
its commercially reasonable efforts to cause the Shares to be listed for trading on the Trading Market and to maintain such listing.
(t) Obligation
Under Exchange Act. During any period when the delivery of a prospectus relating to the Shares is required (including in circumstances
where such requirement may be satisfied pursuant to Rule 172, 173 or any similar rule) to be delivered under the Act, the Company
will file all documents required to be filed with the Commission pursuant to the Exchange Act within the time periods required by the
Exchange Act and the regulations thereunder.
(u) DTC
Facility. The Company shall cooperate with the Manager and use its reasonable efforts to permit the Shares to be eligible for clearance
and settlement through the facilities of DTC.
(v) Use
of Proceeds. The Company will apply the Net Proceeds from the sale of the Shares in the manner set forth in the Prospectus.
(w) Filing
of Prospectus Supplement. If any sales are made pursuant to this Agreement which are not made in “at the market” offerings
as defined in Rule 415, including, without limitation, any Placement pursuant to a Terms Agreement, the Company shall file a Prospectus
Supplement describing the terms of such transaction, the amount of Shares sold, the price thereof, the Manager’s compensation, and
such other information as may be required pursuant to Rule 424 and Rule 430B, as applicable, within the time required by Rule 424.
(x) Additional
Registration Statement. To the extent that the Registration Statement is not available for the sales of the Shares as contemplated
by this Agreement, the Company may file a new registration statement with respect to any additional shares of Common Stock necessary to
complete such sales of the Shares and, upon the filing of such Registration Statement, shall cause such registration statement to become
effective as promptly as practicable. After the effectiveness of any such registration statement, all references to “Registration
Statement” included in this Agreement shall be deemed to include such new registration statement, including all documents incorporated
by reference therein pursuant to Item 12 of Form S-3, and all references to “Base Prospectus” included in
this Agreement shall be deemed to include the final form of prospectus, including all documents incorporated therein by reference, included
in any such registration statement at the time such registration statement became effective.
5. Payment
of Expenses. The Company agrees to pay the costs and expenses incident to the performance of its obligations under this Agreement,
whether or not the transactions contemplated hereby are consummated, including without limitation: (i) the preparation, printing
or reproduction and filing with the Commission of the Registration Statement (including financial statements and exhibits thereto), the
Prospectus and each Issuer Free Writing Prospectus, and each amendment or supplement to any of them; (ii) the printing (or reproduction)
and delivery (including postage, air freight charges and charges for counting and packaging) of such copies of the Registration Statement,
the Prospectus, and each Issuer Free Writing Prospectus, and all amendments or supplements to any of them, as may, in each case, be reasonably
requested for use in connection with the offering and sale of the Shares; (iii) the preparation, printing, authentication, issuance
and delivery of certificates for the Shares, including any stamp or transfer taxes in connection with the original issuance and sale of
the Shares; (iv) the printing (or reproduction) and delivery of this Agreement, any blue sky memorandum and all other agreements
or documents printed (or reproduced) and delivered in connection with the offering of the Shares; (v) the registration of the Shares
under the Exchange Act, if applicable, and the listing of the Shares on the Trading Market; (vi) any registration or qualification
of the Shares for offer and sale under the securities or blue sky laws of the several states (including filing fees); (vii) the fees
and expenses of the Company’s accountants and the fees and expenses of counsel (including local and special counsel) for the Company;
(viii) the filing fee under FINRA Rule 5110; (ix) the reasonable fees and expenses of the Manager’s counsel, not
to exceed $50,000 (excluding any periodic due diligence fees provided for under Section 4(n)), which shall be paid upon the Effective
Time; and (x) all other costs and expenses incident to the performance by the Company of its obligations hereunder.
6. Conditions
to the Obligations of the Manager. The obligations of the Manager under this Agreement and any Terms Agreement shall be subject to
(i) the accuracy of the representations and warranties on the part of the Company contained herein as of the Execution Time, each
Representation Date, and as of each Applicable Time, Settlement Date and Time of Delivery, (ii) the performance by the Company of
its obligations hereunder and (iii) the following additional conditions:
(a) Effectiveness
of the Registration Statement; Filing of Prospectus Supplement. The Registration Statement shall have been declared effective by the
Commission and the Prospectus, and any supplement thereto, required by Rule 424 to be filed with the Commission shall have been filed
in the manner and within the time period required by Rule 424(b) with respect to any sale of Shares; each Prospectus Supplement
shall have been filed in the manner required by Rule 424(b) within the time period required hereunder and under the Act; any
other material required to be filed by the Company pursuant to Rule 433(d) under the Act, shall have been filed with the Commission
within the applicable time periods prescribed for such filings by Rule 433; and no stop order suspending the effectiveness of the
Registration Statement or any notice objecting to its use shall have been issued and, to the knowledge of the Company, no proceedings
for that purpose shall have been instituted or threatened.
(b) Delivery
of Opinion. The Company shall have caused the Company Counsel to furnish to the Manager its opinion and negative assurance statement,
dated as of such date and addressed to the Manager in form and substance reasonably acceptable to the Manager, which opinions and negative
assurance statement shall be substantively similar to those required under Section 4(l) of this Agreement.
(c) Delivery
of Officer’s Certificate. The Company shall have furnished or caused to be furnished to the Manager a certificate of the Company
signed by the Chief Executive Officer or the President and the principal financial or accounting officer of the Company, dated as of such
date, to the effect that the signers of such certificate have carefully examined the Registration Statement, the Prospectus, any Prospectus
Supplement and any documents incorporated by reference therein and any supplements or amendments thereto and this Agreement and that:
(i) the
representations and warranties of the Company in this Agreement are true and correct on and as of such date with the same effect as if
made on such date and the Company has complied with all the agreements and satisfied all the conditions on its part to be performed or
satisfied at or prior to such date;
(ii) no
stop order suspending the effectiveness of the Registration Statement or any notice objecting to its use has been issued and no proceedings
for that purpose have been instituted or, to the Company’s knowledge, threatened; and
(iii) since
the date of the most recent financial statements included in the Registration Statement, the Prospectus and the Incorporated Documents,
there has been no Material Adverse Effect, except as set forth in or contemplated in the Registration Statement and the Prospectus.
(d) Delivery
of Accountants’ “Comfort” Letter. The Company shall have requested and caused the Accountants to have furnished
to the Manager letters (which may refer to letters previously delivered to the Manager), dated as of such date, in form and substance
satisfactory to the Manager, confirming that they are independent accountants within the meaning of the Act and the Exchange Act and the
respective applicable rules and regulations adopted by the Commission thereunder and that they have performed a review of any unaudited
interim financial information of the Company included or incorporated by reference in the Registration Statement and the Prospectus and
provide customary “comfort” as to such review in form and substance satisfactory to the Manager.
(e) No
Material Adverse Event. Since the respective dates as of which information is disclosed in the Registration Statement, the Prospectus
and the Incorporated Documents, except as otherwise stated therein, there shall not have been (i) any change or decrease in previously
reported results specified in the letter or letters referred to in paragraph (d) of this Section 6 or (ii) any change,
or any development involving a prospective change, in or affecting the condition (financial or otherwise), earnings, business or properties
of the Company and its subsidiaries taken as a whole, whether or not arising from transactions in the ordinary course of business, except
as set forth in or contemplated in the Registration Statement, the Prospectus and the Incorporated Documents (exclusive of any amendment
or supplement thereto) the effect of which, in any case referred to in clause (i) or (ii) above, is, in the sole judgment
of the Manager, so material and adverse as to make it impractical or inadvisable to proceed with the offering or delivery of the Shares
as contemplated by the Registration Statement (exclusive of any amendment thereof), the Incorporated Documents and the Prospectus (exclusive
of any amendment or supplement thereto).
(f) Payment
of All Fees. The Company shall have paid the required Commission filing fees relating to the Shares within the time period required
by Rule 456(b)(1)(i) of the Act without regard to the proviso therein and otherwise in accordance with Rules 456(b) and
457(r) of the Act and, if applicable, shall have updated the “Calculation of Registration Fee” table in accordance with
Rule 456(b)(1)(ii) either in a post-effective amendment to the Registration Statement or on the cover page of a prospectus
filed pursuant to Rule 424(b).
(g) No
FINRA Objections. FINRA shall not have raised any objection with respect to the fairness and reasonableness of the terms and arrangements
under this Agreement.
(h) Shares
Listed on Trading Market. The Shares shall have been listed and admitted and authorized for trading on the Trading Market, and satisfactory
evidence of such actions shall have been provided to the Manager.
(i) Other
Assurances. Prior to each Settlement Date and Time of Delivery, as applicable, the Company shall have furnished to the Manager such
further information, certificates and documents as the Manager may reasonably request.
If any of the conditions specified
in this Section 6 shall not have been fulfilled when and as provided in this Agreement, or if any of the opinions and certificates
mentioned above or elsewhere in this Agreement shall not be reasonably satisfactory in form and substance to the Manager and counsel for
the Manager, this Agreement and all obligations of the Manager hereunder may be canceled at, or at any time prior to, any Settlement Date
or Time of Delivery, as applicable, by the Manager. Notice of such cancellation shall be given to the Company in writing or by telephone
and confirmed in writing by electronic mail.
The
documents required to be delivered by this Section 6 shall be delivered to the office of Ellenoff Grossman & Schole LLP,
counsel for the Manager, at 1345 Avenue of the Americas, New York, New York 10105, email: capmkts@egsllp.com, on each such date
as provided in this Agreement.
7. Indemnification
and Contribution.
(a) Indemnification
by Company. The Company agrees to indemnify and hold harmless the Manager, the directors, officers, employees and agents of the Manager
and each person who controls the Manager within the meaning of either the Act or the Exchange Act against any and all losses, claims,
damages or liabilities, joint or several, to which they or any of them may become subject under the Act, the Exchange Act or other Federal
or state statutory law or regulation, at common law or otherwise, insofar as such losses, claims, damages or liabilities (or actions in
respect thereof) arise out of or are based upon any untrue statement or alleged untrue statement of a material fact contained in the Registration
Statement for the registration of the Shares as originally filed or in any amendment thereof, or in the Base Prospectus, any Prospectus
Supplement, the Prospectus, any Issuer Free Writing Prospectus, or in any amendment thereof or supplement thereto, or arise out of or
are based upon the omission or alleged omission to state therein a material fact required to be stated therein or necessary to make the
statements therein not misleading or result from or relate to any breach of any of the representations, warranties, covenants or agreements
made by the Company in this Agreement, and agrees to reimburse each such indemnified party for any legal or other expenses reasonably
incurred by them in connection with investigating or defending any such loss, claim, damage, liability or action; provided, however,
that the Company will not be liable in any such case to the extent that any such loss, claim, damage or liability arises out of or is
based upon any such untrue statement or alleged untrue statement or omission or alleged omission made therein in reliance upon and in
conformity with written information furnished to the Company by the Manager specifically for inclusion therein. This indemnity agreement
will be in addition to any liability that the Company may otherwise have.
(b) Indemnification
by Manager. The Manager agrees to indemnify and hold harmless the Company, each of its directors, each of its officers who signs the
Registration Statement, and each person who controls the Company within the meaning of either the Act or the Exchange Act, to the same
extent as the foregoing indemnity from the Company to the Manager, but only with reference to written information relating to the Manager
furnished to the Company by the Manager specifically for inclusion in the documents referred to in the foregoing indemnity; provided,
however, that in no case shall the Manager be responsible for any amount in excess of the Broker Fee applicable to the Shares and
paid hereunder. This indemnity agreement will be in addition to any liability which the Manager may otherwise have.
(c) Indemnification
Procedures. Promptly after receipt by an indemnified party under this Section 7 of notice of the commencement of any action,
such indemnified party will, if a claim in respect thereof is to be made against the indemnifying party under this Section 7, notify
the indemnifying party in writing of the commencement thereof; but the failure so to notify the indemnifying party (i) will not relieve
it from liability under paragraph (a) or (b) above unless and to the extent it did not otherwise learn of such action
and such failure results in the forfeiture by the indemnifying party of substantial rights and defenses and (ii) will not, in any
event, relieve the indemnifying party from any obligations to any indemnified party other than the indemnification obligation provided
in paragraph (a) or (b) above. The indemnifying party shall be entitled to appoint counsel of the indemnifying party’s
choice at the indemnifying party’s expense to represent the indemnified party in any action for which indemnification is sought
(in which case the indemnifying party shall not thereafter be responsible for the fees and expenses of any separate counsel retained by
the indemnified party or parties except as set forth below); provided, however, that such counsel shall be reasonably satisfactory
to the indemnified party. Notwithstanding the indemnifying party’s election to appoint counsel to represent the indemnified party
in an action, the indemnified party shall have the right to employ separate counsel (including local counsel), and the indemnifying party
shall bear the reasonable fees, costs and expenses of such separate counsel if (i) the use of counsel chosen by the indemnifying
party to represent the indemnified party would present such counsel with a conflict of interest, (ii) the actual or potential defendants
in, or targets of, any such action include both the indemnified party and the indemnifying party and the indemnified party shall have
reasonably concluded that there may be legal defenses available to it and/or other indemnified parties which are different from or additional
to those available to the indemnifying party, (iii) the indemnifying party shall not have employed counsel reasonably satisfactory
to the indemnified party to represent the indemnified party within a reasonable time after notice of the institution of such action or
(iv) the indemnifying party shall authorize the indemnified party to employ separate counsel at the expense of the indemnifying party.
An indemnifying party will not, without the prior written consent of the indemnified parties, settle or compromise or consent to the entry
of any judgment with respect to any pending or threatened claim, action, suit or proceeding in respect of which indemnification or contribution
may be sought hereunder (whether or not the indemnified parties are actual or potential parties to such claim or action) unless such settlement,
compromise or consent includes an unconditional release of each indemnified party from all liability arising out of such claim, action,
suit or proceeding.
(d) Contribution.
In the event that the indemnity provided in paragraph (a), (b) or (c) of this Section 7 is unavailable to or insufficient
to hold harmless an indemnified party for any reason, the Company and the Manager agree to contribute to the aggregate losses, claims,
damages and liabilities (including legal or other expenses reasonably incurred in connection with investigating or defending the same)
(collectively “Losses”) to which the Company and the Manager may be subject in such proportion as is appropriate to
reflect the relative benefits received by the Company on the one hand and by the Manager on the other from the offering of the Shares;
provided, however, that in no case shall the Manager be responsible for any amount in excess of the Broker Fee applicable
to the Shares and paid hereunder. If the allocation provided by the immediately preceding sentence is unavailable for any reason, the
Company and the Manager severally shall contribute in such proportion as is appropriate to reflect not only such relative benefits but
also the relative fault of the Company on the one hand and of the Manager on the other in connection with the statements or omissions
which resulted in such Losses as well as any other relevant equitable considerations. Benefits received by the Company shall be deemed
to be equal to the total net proceeds from the offering (before deducting expenses) received by it, and benefits received by the Manager
shall be deemed to be equal to the Broker Fee applicable to the Shares and paid hereunder as determined by this Agreement. Relative fault
shall be determined by reference to, among other things, whether any untrue or any alleged untrue statement of a material fact or the
omission or alleged omission to state a material fact relates to information provided by the Company on the one hand or the Manager on
the other, the intent of the parties and their relative knowledge, access to information and opportunity to correct or prevent such untrue
statement or omission. The Company and the Manager agree that it would not be just and equitable if contribution were determined by pro
rata allocation or any other method of allocation which does not take account of the equitable considerations referred to above. Notwithstanding
the provisions of this paragraph (d), no person guilty of fraudulent misrepresentation (within the meaning of Section 11(f) of
the Act) shall be entitled to contribution from any person who was not guilty of such fraudulent misrepresentation. For purposes of this
Section 7, each person who controls the Manager within the meaning of either the Act or the Exchange Act and each director, officer,
employee and agent of the Manager shall have the same rights to contribution as the Manager, and each person who controls the Company
within the meaning of either the Act or the Exchange Act, each officer of the Company who shall have signed the Registration Statement
and each director of the Company shall have the same rights to contribution as the Company, subject in each case to the applicable terms
and conditions of this paragraph (d).
8. Termination.
(a) The
Company shall have the right, by giving written notice as hereinafter specified, to terminate the provisions of this Agreement relating
to the solicitation of offers to purchase the Shares in its sole discretion at any time upon seven (7) Business Days’ prior
written notice. Any such termination shall be without liability of any party to any other party except that (i) with respect to any
pending sale, through the Manager for the Company, the obligations of the Company, including in respect of compensation of the Manager,
shall remain in full force and effect notwithstanding the termination and (ii) the provisions of Sections 5, 6, 7, 8, 9, 10,
12, 14 and 15 of this Agreement shall remain in full force and effect notwithstanding such termination.
(b) The
Manager shall have the right, by giving written notice as hereinafter specified, to terminate the provisions of this Agreement relating
to the solicitation of offers to purchase the Shares in its sole discretion at any time. Any such termination shall be without liability
of any party to any other party except that the provisions of Sections 5, 6, 7, 8, 9, 10, 12, 14 and 15 of this Agreement shall remain
in full force and effect notwithstanding such termination.
(c) This
Agreement shall remain in full force and effect until such date that this Agreement is terminated pursuant to Sections 8(a) or
(b) above or otherwise by mutual agreement of the parties, provided that any such termination by mutual agreement shall in all cases
be deemed to provide that Sections 5, 6, 7, 8, 9, 10, 12, 14 and 15 of this Agreement shall remain in full force and effect.
(d) Any
termination of this Agreement shall be effective on the date specified in such notice of termination, provided that such termination shall
not be effective until the close of business on the date of receipt of such notice by the Manager or the Company, as the case may be.
If such termination shall occur prior to the Settlement Date or Time of Delivery for any sale of the Shares, such sale of the Shares shall
settle in accordance with the provisions of Section 2(b) of this Agreement.
(e) In
the case of any purchase of Shares by the Manager pursuant to a Terms Agreement, the obligations of the Manager pursuant to such Terms
Agreement shall be subject to termination, in the absolute discretion of the Manager, by prompt oral notice given to the Company prior
to the Time of Delivery relating to such Shares, if any, and confirmed promptly by electronic mail, if since the time of execution of
the Terms Agreement and prior to such delivery and payment, (i) trading in the Common Stock shall have been suspended by the Commission
or the Trading Market or trading in securities generally on the Trading Market shall have been suspended or limited or minimum prices
shall have been established on such exchange, (ii) a banking moratorium shall have been declared either by Federal or New York State
authorities or (iii) there shall have occurred any outbreak or escalation of hostilities, declaration by the United States of a national
emergency or war, or other calamity or crisis the effect of which on financial markets is such as to make it, in the sole judgment of
the Manager, impractical or inadvisable to proceed with the offering or delivery of the Shares as contemplated by the Prospectus (exclusive
of any amendment or supplement thereto).
9. Representations
and Indemnities to Survive. The respective agreements, representations, warranties, indemnities and other statements of the Company
or its officers and of the Manager set forth in or made pursuant to this Agreement will remain in full force and effect, regardless of
any investigation made by the Manager or the Company or any of the officers, directors, employees, agents or controlling persons referred
to in Section 7, and will survive delivery of and payment for the Shares.
10. Notices.
All communications hereunder will be in writing and effective only on receipt, and will be mailed, delivered, or e-mailed to the addresses
of the Company and the Manager, respectively, set forth on the signature page hereto.
11. Successors.
This Agreement will inure to the benefit of and be binding upon the parties hereto and their respective successors and the officers, directors,
employees, agents and controlling persons referred to in Section 7, and no other person will have any right or obligation hereunder.
12. No
Fiduciary Duty. The Company hereby acknowledges that (a) the purchase and sale of the Shares pursuant to this Agreement is an
arm’s-length commercial transaction between the Company, on the one hand, and the Manager and any Affiliate through which it may
be acting, on the other, (b) the Manager is acting solely as sales agent and/or principal in connection with the purchase and sale
of the Company’s securities and not as a fiduciary of the Company and (c) the Company’s engagement of the Manager in
connection with the offering and the process leading up to the offering is as independent contractors and not in any other capacity. Furthermore,
the Company agrees that it is solely responsible for making its own judgments in connection with the offering (irrespective of whether
the Manager has advised or is currently advising the Company on related or other matters). The Company agrees that it will not claim that
the Manager has rendered advisory services of any nature or respect, or owe an agency, fiduciary or similar duty to the Company, in connection
with such transaction or the process leading thereto.
13. Integration.
This Agreement and any Terms Agreement supersede all prior agreements and understandings (whether written or oral) between the Company
and the Manager with respect to the subject matter hereof.
14. Amendments;
Waivers. No provision of this Agreement may be waived, modified, supplemented or amended except in a written instrument signed, in
the case of an amendment, by the Company and the Manager. No waiver of any default with respect to any provision, condition or requirement
of this Agreement shall be deemed to be a continuing waiver in the future or a waiver of any subsequent default or a waiver of any other
provision, condition or requirement hereof, nor shall any delay or omission of any party to exercise any right hereunder in any manner
impair the exercise of any such right.
15. Applicable
Law. This Agreement and any Terms Agreement will be governed by and construed in accordance with the laws of the State of New York
applicable to contracts made and to be performed within the State of New York. Each of the Company and the Manager: (i) agrees
that any legal suit, action or proceeding arising out of or relating to this Agreement shall be instituted exclusively in New York Supreme
Court, County of New York, or in the United States District Court for the Southern District of New York, (ii) waives any objection
which it may have or hereafter to the venue of any such suit, action or proceeding, and (iii) irrevocably consents to the exclusive
jurisdiction of the New York Supreme Court, County of New York, and the United States District Court for the Southern District of New
York in any such suit, action or proceeding. Each of the Company and the Manager further agrees to accept and acknowledge service of any
and all process which may be served in any such suit, action or proceeding in the New York Supreme Court, County of New York, or in the
United States District Court for the Southern District of New York and agrees that service of process upon the Company mailed by certified
mail to the Company’s address shall be deemed in every respect effective service of process upon the Company, in any such suit,
action or proceeding, and service of process upon the Manager mailed by certified mail to the Manager’s address shall be deemed
in every respect effective service process upon the Manager, in any such suit, action or proceeding. If either party shall commence an
action or proceeding to enforce any provision of this Agreement, then the prevailing party in such action or proceeding shall be reimbursed
by the other party for its reasonable attorney’s fees and other costs and expenses incurred with the investigation, preparation
and prosecution of such action or proceeding.
16. Waiver
of Jury Trial. The Company hereby irrevocably waives, to the fullest extent permitted by applicable law, any and all right to trial
by jury in any legal proceeding arising out of or relating to this Agreement, any Terms Agreement or the transactions contemplated hereby
or thereby.
17. Counterparts.
This Agreement and any Terms Agreement may be signed in one or more counterparts, each of which shall constitute an original and all of
which together shall constitute one and the same agreement, which may be delivered in .pdf file via e-mail.
***************************
18. Headings.
The section headings used in this Agreement and any Terms Agreement are for convenience only and shall not affect the construction hereof.
If the foregoing is in accordance
with your understanding of our agreement, please sign and return to us the enclosed duplicate hereof, whereupon this letter and your acceptance
shall represent a binding agreement among the Company and the Manager.
Very truly yours,
| Ampio
Pharmaceuticals, Inc. |
|
| |
|
| By: |
/s/ Michael A. Martino |
|
| Name: Michael A. Martino |
|
| Title: Chief Executive Officer |
|
Address
for Notice:
9800 Mount Pyramid Court, Suite 400
Englewood, Colorado 80112
Attention: Michael A. Martino
E-mail: [redacted]
The foregoing Agreement is hereby confirmed and accepted as of the
date first written above.
| H.C.
Wainwright & Co., LLC |
|
| |
|
| |
|
| By: |
/s/ Edward D. Silvera |
|
| Name: Edward D. Silvera |
|
| Title: Chief Operating Officer |
|
Address
for Notice:
430 Park Avenue
New York, New York 10022
Attention: Chief Executive Officer
E-mail: notices@hcwco.com
Form of Terms Agreement
ANNEX I
Ampio
Pharmaceuticals, Inc.
TERMS AGREEMENT
Dear Sirs:
Ampio Pharmaceuticals, Inc.
(the “Company”) proposes, subject to the terms and conditions stated herein and in the At The Market Offering Agreement,
dated September 18, 2023 (the “At The Market Offering Agreement”), between the Company and H.C. Wainwright &
Co., LLC (“Manager”), to issue and sell to Manager the securities specified in the Schedule I hereto (the
“Purchased Shares”).
Each of the provisions
of the At The Market Offering Agreement not specifically related to the solicitation by the Manager, as agent of the Company, of offers
to purchase securities is incorporated herein by reference in its entirety, and shall be deemed to be part of this Terms Agreement to
the same extent as if such provisions had been set forth in full herein. Each of the representations and warranties set forth therein
shall be deemed to have been made at and as of the date of this Terms Agreement and the Time of Delivery, except that each representation
and warranty in Section 3 of the At The Market Offering Agreement which makes reference to the Prospectus (as therein defined) shall
be deemed to be a representation and warranty as of the date of the At The Market Offering Agreement in relation to the Prospectus, and
also a representation and warranty as of the date of this Terms Agreement and the Time of Delivery in relation to the Prospectus as amended
and supplemented to relate to the Purchased Shares.
An amendment to
the Registration Statement (as defined in the At The Market Offering Agreement), or a supplement to the Prospectus, as the case may be,
relating to the Purchased Shares, in the form heretofore delivered to the Manager is now proposed to be filed with the Securities and
Exchange Commission.
Subject to the terms
and conditions set forth herein and in the At The Market Offering Agreement which are incorporated herein by reference, the Company agrees
to issue and sell to the Manager and the latter agrees to purchase from the Company the number of shares of the Purchased Shares at the
time and place and at the purchase price set forth in the Schedule I hereto.
If the foregoing is in accordance
with your understanding, please sign and return to us a counterpart hereof, whereupon this Terms Agreement, including those provisions
of the At The Market Offering Agreement incorporated herein by reference, shall constitute a binding agreement between the Manager and
the Company.
| AMPIO PHARMACEUTICALS, INC. |
|
| |
|
| |
|
| By: |
|
|
| |
Name: |
|
| |
Title: |
|
ACCEPTED as of the date first written above.
| H.C. Wainwright &
Co., LLC |
|
| |
|
| |
|
| By: |
|
|
| |
Name: |
|
| |
Title: |
|
EXHIBIT 5.1
|
|
2000 IDS Center
80 South 8th Street
Minneapolis, MN 55402-2119
Tel: 612.371.3211
Fax: 612.271.3207
www.ballardspahr.com |
|
|
September 18, 2023
Ampio Pharmaceuticals, Inc.
9800 Mount Pyramid Court, Suite 400
Englewood, Colorado 80112
RE: Ampio Pharmaceuticals, Inc.
Ladies and Gentlemen:
We have acted as counsel to Ampio Pharmaceuticals, Inc.,
a Delaware corporation (the “Company”), in connection with a Registration Statement on Form S-3 (the “Registration
Statement”) filed on the date hereof with the Securities and Exchange Commission (the “Commission”) under
the Securities Act of 1933, as amended (the “Securities Act”).
The
Registration Statement provides for the offer and sale of up to $50,000,000 in aggregate offering price of the following securities: (i) shares
of common stock, par value $0.0001 per share, of the Company (the “Common Stock”), (ii) shares of one or more
series of the Company’s preferred stock, par value $0.0001 per share (the “Preferred Stock”), (iii) warrants
to purchase Common Stock, Preferred Stock, or any combination of the foregoing, either individually or as units comprised of two or more
securities (the “Warrants”), and (iv) units consisting of Common Stock, Preferred Stock, and/or Warrants, which
may or may not be separable from one another (the “Units”). The Common Stock, Preferred Stock, Warrants and Units are
each a “Security” and are collectively referred to as the “Securities.”
The
Registration Statement includes two prospectuses: (i) a base prospectus (the “Base Prospectus”) covering the offer,
issuance and sale of up to $50,000,000 of Securities and (ii) an at-the-market offering agreement prospectus (the “Offering
Agreement Prospectus”), covering the offering, issuance and sale of up to $1,250,000 of Common Stock (the “Offering
Agreement Shares”) that may be issued and sold under the At the Market Offering Agreement, dated as of September 18, 2023,
by and between the Company and H.C. Wainwright & Co., LLC, as sales agent (such agreement, the “Offering Agreement,”
and H.C. Wainwright & Co., LLC, the “Sales Agent”). The $1,250,000 of Common Stock that may be offered, issued
and sold under the Offering Agreement Prospectus is included in the $50,000,000 of Securities that may be offered, issued and sold under
the Base Prospectus.
Ampio Pharmaceuticals, Inc.
September 18, 2023
Page 2
The
Securities may be offered and sold from time to time pursuant to Rule 415 promulgated under the Securities Act, in amounts, at prices
and on terms to be determined at the time of the offering thereof (and, in the case of the Offering Agreement Shares, in accordance
with the terms of the Offering Agreement).
The Base Prospectus provides that it will be supplemented in the future
by one or more supplements to the Base Prospectus describing the Securities offered thereby and the terms of the offering (each, a “Prospectus
Supplement”).
The Preferred Stock may be exchangeable and/or convertible into other
Securities. The Warrants may be issued under one or more warrant agreements (each, a “Warrant Agreement”) between the
Company and a third party to be identified therein as warrant agent or directly issued by the Company to the purchasers of such Warrants.
The Units may be issued under one or more unit agreements (each, a “Unit Agreement”) between the Company and a third
party to be identified therein as unit agent or directly issued by the Company to the purchasers of such Units. The Warrants and the Units
are herein collectively referred to as the “Covered Securities.” The Warrant Agreements and the Unit Agreements are
herein collectively referred to as the “Agreements.”
We have examined such matters of fact and questions of law as we have
considered appropriate for purposes of this letter. We have relied upon the certificate of incorporation and bylaws of the Company in
effect on the date hereof, as and in the forms certified to us by the Company. We have relied upon certificates and other assurances of
officers of the Company and others as to factual matters without having independently verified such factual matters. We have assumed that
the proceedings to be taken by the Company after the date hereof in connection with the authorization of the Agreements and the authorization,
issuance and sale of the Securities, and the terms of each issuance, will be in compliance with law. In our examination, we have assumed
the genuineness of all signatures, the authenticity of all documents submitted to us as originals and the conformity to authentic original
documents of all documents submitted to us as copies.
With
respect to the Offering Agreement Shares, we have assumed that no more than 1,250,000 Offering Agreement Shares will be sold. We
have further assumed that each Offering Agreement Share will be sold at a price that is not less than the par value per share of the Common
Stock. We express no opinion with respect to the issuance of shares of Common Stock (including Offering Agreement Shares) or Preferred
Stock to the extent that such issuance would exceed the number of shares then authorized and available for such issuance.
The
opinions expressed below are limited to the Delaware General Corporation Law, and, with respect to the opinions set forth in paragraphs
3, 4, and 5 below, the internal laws of the State of New York.
Subject to the foregoing and the other matters set forth herein, it
is our opinion that, as of the date hereof:
1. When
an issuance of shares of Common Stock (other than Offering Agreement Shares) has been duly authorized by all necessary corporate
action of the Company, upon issuance, delivery and payment of lawful consideration therefor in an amount not less than the par value thereof
in the manner contemplated by the Registration Statement, the Base Prospectus and the applicable Prospectus Supplement(s), by such corporate
action and by the terms of any underwriting, purchase or other agreement, instrument or Security relating to such issuance, such shares
of Common Stock will be validly issued, fully paid and nonassessable.
Ampio Pharmaceuticals, Inc.
September 18, 2023
Page 2
2. When
a series of Preferred Stock has been duly authorized and established in accordance with the Delaware General Corporation Law and the terms
of the Company’s Certificate of Incorporation, and an issuance of shares of such series of Preferred Stock has been duly authorized
by all necessary corporate action of the Company, upon issuance, delivery and payment of lawful consideration therefor in an amount not
less than the par value thereof in the manner contemplated by the Registration Statement, the Base Prospectus and the applicable Prospectus
Supplement(s), by such corporate action and by the terms of any underwriting, purchase or other agreement, instrument or Security relating
to such issuance, such shares of Preferred Stock will be validly issued, fully paid and nonassessable.
3. When
a Warrant Agreement, if applicable, has been duly authorized by all necessary corporate action of the Company and duly executed and delivered,
and when the specific terms of a particular issuance of Warrants have been duly established in accordance with any such Warrant Agreement
and authorized by all necessary corporate action of the Company, and the Warrants have been duly executed, authenticated, issued and delivered
against payment therefor in accordance with any such Warrant Agreement and in the manner contemplated by the Registration Statement, the
Base Prospectus and the applicable Prospectus Supplement(s), by such corporate action and by the terms of any underwriting, purchase or
other agreement, instrument or Security relating to such issuance, the Warrants will be the legally valid and binding obligations of the
Company, enforceable against the Company in accordance with their terms.
4. When
a Unit Agreement, if applicable, has been duly authorized by all necessary corporate action of the Company and duly executed and delivered,
and when the specific terms of a particular issuance of Units have been duly established in accordance with any such Unit Agreement and
authorized by all necessary corporate action of the Company, and the Units have been duly executed, authenticated, issued and delivered
against payment therefor in accordance with any such Unit Agreement and in the manner contemplated by the Registration Statement, the
Base Prospectus and the applicable Prospectus Supplement(s), by such corporate action and by the terms of any underwriting, purchase or
other agreement, instrument or Security relating to such issuance, the Units will be the legally valid and binding obligations of the
Company, enforceable against the Company in accordance with their terms.
5. When
issued and paid for in accordance with the terms and conditions of the Offering Agreement in the manner contemplated by the Registration
Statement and the Offering Agreement Prospectus, the Offering Agreement Shares will be validly issued, fully paid and nonassessable.
Our
opinions are subject to the qualification that we express no opinion regarding the applicability of, compliance with or effect of (i) any
bankruptcy, insolvency, reorganization, preference, fraudulent conveyance, fraudulent transfer, moratorium or other similar laws relating
to or affecting the rights and remedies of creditors; (ii) general principles of equity, whether considered in a proceeding in equity
or at law (including the possible unavailability of specific performance or injunctive relief), concepts of materiality, reasonableness,
good faith and fair dealing, and the discretion of the court before which a proceeding is brought; (iii) the invalidity under certain
circumstances under law or court decisions of provisions providing for the indemnification of or contribution to a party with respect
to a liability where such indemnification or contribution is contrary to public policy or (iv) public policy considerations that
may limit the rights of parties to obtain certain remedies. We express no opinion as to (a) any provision for liquidated damages,
monetary penalties, or other economic remedies to the extent such provisions are deemed to constitute a penalty, (b) consents to,
or restrictions upon, governing law, jurisdiction, forum, venue (including waivers of forum non conveniens), arbitration, remedies or
judicial relief, (c) any provision requiring the payment of attorneys’ fees, where such payment is contrary to law or public
policy, (d) advance waivers of claims, defenses, rights granted by law, or notice, opportunity for hearing, evidentiary requirements,
statutes of limitation, trial by jury or at law, or other procedural rights, (e) waivers of broadly or vaguely stated rights, (f) provisions
for exclusivity, election or cumulation of rights or remedies, (g) provisions authorizing or validating conclusive or discretionary
determinations, (h) proxies, powers and trusts, (i) provisions prohibiting, restricting or requiring consent to assignment or
transfer of any right or property, or (j) the severability, if invalid, of provisions to the foregoing effect.
Ampio Pharmaceuticals, Inc.
September 18, 2023
Page 4
We
have assumed that (i) each of the Covered Securities and the respective Agreements governing such Covered Securities will be governed
by the internal laws of the State of New York, (ii) the Sales Agents is, and each of the parties to any Agreement other than the
Company will be, duly organized, validly existing and in good standing under the laws of its jurisdiction of organization and qualified
to perform its obligations under the Offering Agreement or such Agreement, as applicable, (iii) the Offering Agreement has
been, and the Covered Securities and the Agreements will be, duly authorized, executed and delivered by the parties thereto other than
the Company, (iv) the Offering Agreement constitutes, and the Covered Securities and the Agreements will constitute, legally valid
and binding obligations of the parties thereto other than the Company, enforceable against each of them in accordance with their respective
terms, and (v) the status of the Offering Agreement, the Covered Securities and the Agreements as legally valid and binding obligations
of the parties will not be affected by any (a) breaches of, or defaults under, agreements or instruments, (b) violations of
statutes, rules, regulations or court or governmental orders, or (c) failures to obtain required consents, approvals or authorizations
from, or to make required registrations, declarations or filings with, governmental authorities.
This opinion is to be relied upon only in connection with the offer
and sale of the Securities while the Registration Statement and any and all required post-effective amendments thereto are effective.
This
opinion is being delivered solely for the benefit of the Company and such other persons as are entitled to rely upon it pursuant to applicable
provisions of the Securities Act. This opinion may not be used, quoted, relied upon or referred to for any other purpose, nor may this
opinion be used, quoted, relied upon or referred to by any other person, for any purpose, in each case without our prior written consent.
We assume no obligation to advise you of any changes in the foregoing subsequent to the effective date of the Registration Statement.
We hereby consent to the filing of this opinion
as an exhibit to the Registration Statement and to reference to us under the caption “Legal Matters” in the prospectus forming
a part of the Registration Statement. In giving such consent, we do not hereby admit that we are acting within the category of persons
whose consent is required under Section 7 of the Securities Act or the rules or regulations of the Commission thereunder.
Sincerely,
/s/ Ballard Spahr LLP
Exhibit 23.2
Consent of Independent Registered
Public Accounting Firm
We consent to the incorporation by reference in this Registration Statement
on Form S-3 of Ampio Pharmaceuticals, Inc. of our report dated March 27, 2023, relating to the financial statements of Ampio
Pharmaceuticals, Inc. (the “Company”) (which report expresses an unqualified opinion and includes an explanatory paragraph
relating to a going concern uncertainty), appearing in the Annual Report on Form 10-K of the Company for the year ended December
31, 2022, filed with the Securities and Exchange Commission. We also consent to the reference to us under the heading “Experts”
in such Registration Statement.
/s/ Moss Adams LLP
Denver, Colorado
September 18, 2023
Exhibit 107.1
Calculation of Filing Fee Tables
Form
S-3
(Form Type)
Ampio Pharmaceuticals, Inc.
(Exact Name of Registrant as Specified in its Charter)
Table 1: Newly Registered and Carry Forward
Securities
| |
Security Type |
Security Class Title |
Fee Calculation or Carry
Forward Rule |
Amount
Registered |
Proposed
Maximum
Offering Price
Per
Unit |
Maximum
Aggregate
Offering Price |
Fee Rate |
Amount of
Registration Fee |
| Newly Registered Securities |
| Fees to Be Paid |
Equity |
Common Stock,
$0.0001 par value
per share |
457(o) |
(1) |
(1) |
(1) |
|
|
| Equity |
Preferred Stock,
$0.0001 par value
per share |
457(o) |
(1) |
(1) |
(1) |
|
|
| |
Other |
Warrants |
457(o) |
(1) |
(1) |
(1) |
|
|
| |
Other |
Units |
457(o) |
(1) |
(1) |
(1) |
|
|
| |
Unallocated
(Universal)
Shelf |
|
457(o) |
(1) |
(1) |
$50,000,000 |
.00011020 |
$5,510(2) |
| |
Total Offering Amounts |
|
$50,000,000 |
|
$5,510.00 |
| |
Total Fees Previously Paid |
|
|
|
$0.00 |
| |
Total Fee Offsets |
|
|
|
$0.00 |
| |
Net Fee Due |
|
|
|
$5,510.00 |
| (1) | Omitted pursuant to General Instruction II.D of Form S-3 under the Securities Act of 1933, as amended (the "Securities Act").
There is being registered hereunder an indeterminate number or amount, as the case may be, of the securities of each identified class
as may from time to time be offered and sold at indeterminate prices, which together shall have a maximum aggregate offering price not
to exceed $50,000,000. Any securities registered hereunder may be sold separately or in combination with the other securities registered
hereunder. The securities registered hereunder also include an indeterminate number or amount, as the case may be, of securities registered
hereunder as may be issued upon conversion, redemption, exchange, exercise or settlement, as applicable of any other securities registered
hereunder that provide for such conversion, redemption, exchange, exercise or settlement, including such securities as may be issued pursuant
to anti-dilution adjustments pursuant to the anti-dilution provisions of any such securities or as a result of stock splits, stock dividends
or similar transactions, pursuant to Rule 416 under the Securities Act. |
| (2) | Calculated in accordance with Rule 457(o) under the Securities Act. |
Ampio Pharmaceuticals (AMEX:AMPE)
Gráfica de Acción Histórica
De Abr 2024 a May 2024
Ampio Pharmaceuticals (AMEX:AMPE)
Gráfica de Acción Histórica
De May 2023 a May 2024