Filed Pursuant to Rule 424(b)(3)
Registration No. 333-275229
PROSPECTUS SUPPLEMENT
(to Prospectus dated November 7, 2023)

Portage Biotech Inc.
9,631,580 Ordinary Shares underlying Warrants
This prospectus supplement is being filed to update and supplement the information
contained in the prospectus dated November 7, 2023 (the “Prospectus”), which forms a part of our Registration Statement on
Form F-1 (Registration No. 333-275229), with the information contained in our Notification of Late Filing on Form 12b-25 with
respect to our Annual Report on Form 20-F for the year ended March 31, 2024, which such Notification of Late Filing on Form 12b-25
was filed with the Securities and Exchange Commission on August 1, 2024 (the “August 1, 2024 Form 12b-25”). Accordingly, we
have attached the August 1, 2024 Form 12b-25 to this prospectus supplement.
This prospectus supplement updates and supplements the information in the
Prospectus and is not complete without, and may not be delivered or utilized except in combination with, the Prospectus, including any
amendments or supplements thereto. This prospectus supplement should be read in conjunction with the Prospectus and if there is any inconsistency
between the information in the Prospectus and this prospectus supplement, you should rely on the information in this prospectus supplement.
Our Ordinary Shares are listed on The Nasdaq Capital Market (“Nasdaq”)
under the symbol “PRTG”. On July 31, 2024, the closing sale price of our Ordinary Shares as reported on Nasdaq was $0.176.
___________________________
Investing in the securities offered in the Prospectus involves a high
degree of risk. Before making any investment in these securities, you should consider carefully the risks and uncertainties in the section
entitled “Risk Factors” beginning on page 9 of the Prospectus, and in the other documents that are incorporated by reference
into the Prospectus.
Neither the Securities and Exchange Commission nor any state or non-U.S.
regulatory body has approved or disapproved of the securities offered in the Prospectus or passed upon the accuracy or adequacy of the
Prospectus or this prospectus supplement. Any representation to the contrary is a criminal offense.
___________________________
The date of this prospectus supplement is August 1, 2024
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 12b-25
NOTIFICATION OF LATE FILING
SEC FILE NUMBER: 001-40086
CUSIP NUMBER: G7185A128
| (Check one): |
|
☐ Form 10-K ☒ Form 20-F ☐ Form 11-K
☐ Form 10-Q ☐ Form 10-D ☐ Form N-CEN ☐ Form N-CSR |
| |
|
| |
|
For Period Ended: March 31, 2024 |
| |
|
| |
|
☐ Transition Report on Form 10-K |
| |
|
| |
|
☐ Transition Report on Form 20-F |
| |
|
| |
|
☐ Transition Report on Form 11-K |
| |
|
| |
|
☐ Transition Report on Form 10-Q |
| |
|
| |
|
For the Transition Period Ended: _____________________ |
| |
|
Nothing in this form shall be construed to imply that the Commission
has verified any information contained herein.
|
| |
|
If the notification relates to a portion of the filing checked above, identify the Item(s) to
which the notification relates:
|
PART I — REGISTRANT INFORMATION
Portage Biotech Inc.
(Full Name of Registrant)
N/A
(Former Name if Applicable)
Clarence Thomas Building, P.O. Box 4649
(Address of Principal Executive Office (Street and Number))
Road Town, Tortola
British Virgin Islands, VG1110
(City, State and Zip Code)
PART II — RULES 12b-25(b) AND (c)
If the subject report could not be filed without unreasonable effort or expense and the registrant
seeks relief pursuant to Rule 12b-25(b), the following should be completed. (Check box if appropriate)
| |
|
|
|
|
| |
|
(a) |
|
The reason described in reasonable detail in Part III of this form could not be eliminated without unreasonable effort or expense; |
| |
|
|
| ☒ |
|
(b) |
|
The subject annual report, semi-annual report, transition report on Form 10-K, Form 20-F, Form 11-K, Form N-CEN or Form N-CSR, or portion thereof, will be filed on or before the fifteenth calendar day following the prescribed due date; or the subject quarterly report or transition report on Form 10-Q or subject distribution report on Form 10-D, or portion thereof, will be filed on or before the fifth calendar day following the prescribed due date; and |
| |
|
|
| |
|
(c) |
|
The accountant’s statement or other exhibit required by Rule 12b-25(c) has been attached if applicable. |
PART III — NARRATIVE
State below in reasonable detail why forms 10-K, 20-F, 11-K, 10-Q, 10-D, N-CEN, N-CSR, or
the transition report or portion thereof, could not be filed within the prescribed time period.
Portage Biotech Inc. (the “Company”) is unable to file, without unreasonable effort
or expense and within the prescribed time period, its Annual Report on Form 20-F for the fiscal year ended March 31, 2024 (the “Form
20-F”), due to the completion of fair value assessments of certain of its assets and liabilities.
The Company expects to file the Form 20-F within the extension period
of 15 calendar days as provided under Rule 12b-25 under the U.S. Securities Exchange Act of 1934, as amended.
PART IV — OTHER INFORMATION
| (1) |
|
|
|
Name and telephone number of person to contact in regard to this notification |
| |
|
|
|
|
|
|
| |
|
|
|
Allan Shaw |
|
|
|
917 |
|
|
|
741-1856 |
| |
|
|
|
(Name) |
|
|
|
(Area Code) |
|
|
|
(Telephone Number) |
| |
|
|
| (2) |
|
|
|
Have all other periodic reports required under Section 13 or 15(d) of the Securities Exchange
Act of 1934 or Section 30 of the Investment Company Act of 1940 during the preceding 12 months or for such shorter period that the registrant
was required to file such report(s) been filed? If answer is no, identify report(s).
Yes ☒ No ☐ |
| |
|
|
| |
|
|
| (3) |
|
|
|
Is it anticipated that any significant change in results of operations from the corresponding
period for the last fiscal year will be reflected by the earnings statements to be included in the subject report or portion thereof?
Yes ☒ No ☐
The Company anticipates a full impairment of in-process
research and development (“IPR&D”) resulting in charges totaling approximately $81.5 million for the year ended
March 31, 2024, which is attributable to the full impairment of the invariant Natural Killer T-cells (iNKT cells) platform and adenosine
receptor antagonist platform related to iOx Therapeutics Ltd. and Tarus Therapeutics,
LLC (subsidiaries of the Company) IPR&D, respectively. There was approximately $107.8 million goodwill and IPR&D impairment
charge for the prior year ended March 31, 2023. The decrease in year-over-year impairment charges is primarily attributable to the decrease
in the expected net loss for the year ended March 31, 2024. This estimate of IPR&D impairment charge for the year ended March 31,
2024 is preliminary and subject to completion. As a result, this unaudited preliminary financial information reflects the Company’s
preliminary estimate with respect to such information, based on information currently available to management, and may vary from the Company’s
actual financial results as of March 31, 2024. The unaudited preliminary IPR&D impairment charge included herein has been prepared
by, and is the responsibility of, management. |
| |
|
|
| |
Forward-Looking Statements
This Form 12b-25 contains forward-looking statements. These statements are statements
that are not historical facts and are based on management’s current view and estimates of future circumstances, conditions, performance
and results. The words “anticipates,” “believes,” “estimates,” “expects,” “plans”
and similar expressions are intended to identify forward-looking statements. There is no guarantee that the expected events or results
will actually occur. The statements are based on many assumptions and factors, including the risk that the completion and filing of the
Form 20-F will take longer than expected. The Registrant undertakes no
commitment to update or revise forward-looking statements except as required by law.
Portage Biotech Inc.
(Name of Registrant as Specified in Charter)
has caused this notification to be signed on its behalf by the undersigned hereunto duly authorized.
| |
|
|
|
| Date: July 31, 2024 |
By |
|
/s/ Ian Walters, MD, MBA |
| |
|
|
Name: Ian Walters, MD, MBA |
| |
|
|
Title: Chief Executive Officer |
| |
ATTENTION |
|
| Intentional misstatements or omissions of fact constitute Federal Criminal Violations (See 18 U.S.C. 1001). |
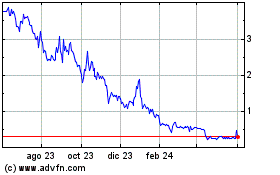
Portage Biotech (NASDAQ:PRTG)
Gráfica de Acción Histórica
De Jul 2024 a Ago 2024
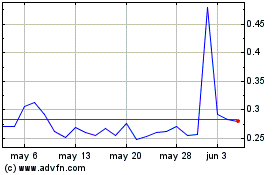
Portage Biotech (NASDAQ:PRTG)
Gráfica de Acción Histórica
De Ago 2023 a Ago 2024