false 0001817229 0001817229 2024-01-05 2024-01-05
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 5, 2024
Vor Biopharma Inc.
(Exact name of registrant as specified in its Charter)
|
|
|
|
|
| Delaware |
|
001-39979 |
|
81-1591163 |
| (State or Other Jurisdiction of Incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification No.) |
|
|
| 100 Cambridgepark Drive |
|
|
| Suite 101 |
|
|
| Cambridge, Massachusetts |
|
02140 |
| (Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: (617) 655-6580
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instructions A.2. below):
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, $0.0001 par value per share |
|
VOR |
|
Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
On January 5, 2024, Vor Biopharma Inc. (the “Company”) updated its investor presentation (the “Corporate Presentation”). The Corporate Presentation is available on the Company’s website and is filed as Exhibit 99.1 hereto and incorporated herein by reference.
| Item 9.01 |
Financial Statements and Exhibits |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
Vor Biopharma Inc. |
|
|
|
|
|
|
|
|
By: |
|
/s/ Robert Ang |
|
|
|
|
|
|
Robert Ang |
|
|
|
|
|
|
Chief Executive Officer |
| Date: January 5, 2024 |
|
|
|
|
|
|
January 2024 Ambition: Curing blood
cancers through cell and genome engineering Exhibit 99.1

This presentation (the
“Presentation”) contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 about Vor Biopharma Inc. (“Vor,” “Vor Bio” or the “Company”). The words
“aim,” “anticipate,” “believe,” “can,” “could,” “design,” “enable” “estimate,” “expect,” “intend,” “may,”
“ongoing,” “plan,” “potential,” “project,” “should,” “target,” “towards,” “will,” and similar expressions are intended to identify forward-looking
statements, although not all forward-looking statements contain these identifying words. Forward-looking statements in this Presentation include those regarding the feasibility of a trem-cel (formerly VOR33) transplant to be successfully
manufactured, to engraft normally, to maintain blood counts following treatment with Mylotarg following allogeneic hematopoietic cell transplant and to be well tolerated, the potential of VCAR33ALLO in combination with trem-cel as a Treatment
System, the potential of trem-cel to enable targeted therapies in the post-transplant setting including Mylotarg and CD33-targeted CAR-Ts, the potential of Vor Bio’s platform, Vor Bio’s plans, strategies, expectations and anticipated
milestones for its preclinical and clinical programs, the availability and timing of results from preclinical studies and clinical trials, the timing of regulatory filings, the expected safety profile of Vor Bio’s product candidates, cash
runway and expected capital requirements, and its plans and expectations related to the Company’s manufacturing and facilities. Vor Bio may not actually achieve the plans, intentions, or expectations disclosed in these forward-looking
statements, and you should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of
various factors, including: uncertainties inherent in the initiation, completion of, and availability and timing of results from, preclinical studies and clinical trials and clinical development of Vor Bio’s product candidates; whether
preclinical data or interim results from a clinical trial will be predictive of the final results of the trial or the results of future trials; the uncertainty of regulatory approvals to conduct trials or to market products; the success of Vor
Bio’s in-house manufacturing capabilities and efforts; and availability of funding sufficient for its foreseeable and unforeseeable operating expenses and capital expenditure requirements. The interim data for trem-cel presented in this
Presentation is based on five patients and future results for these patients or additional patients may not produce the same or consistent results. These and other risks are described in greater detail under the caption “Risk Factors”
included in Vor Bio’s most recent annual or quarterly report and in other reports it has filed or may file with the Securities and Exchange Commission. Any forward-looking statements contained in this Presentation speak only as of the date of
this Presentation, and Vor Bio expressly disclaims any obligation to update any forward-looking statements, whether because of new information, future events or otherwise, except as may be required by law. Certain information contained in this
Presentation relates to or is based on studies, publications, surveys and other data obtained from third party sources and Vor Bio’s own internal estimates and research. While we believe these third-party sources to be reliable as of the date
of this Presentation, we have not independently verified, and make no representation as to the adequacy, fairness, accuracy or completeness of any information obtained from third party sources. In addition, there can be no guarantee as to the
accuracy or reliability of any assumptions or limitations that may be included in such third-party information. While we believe our own internal research is reliable, such research has not been verified by any independent source. Disclaimer
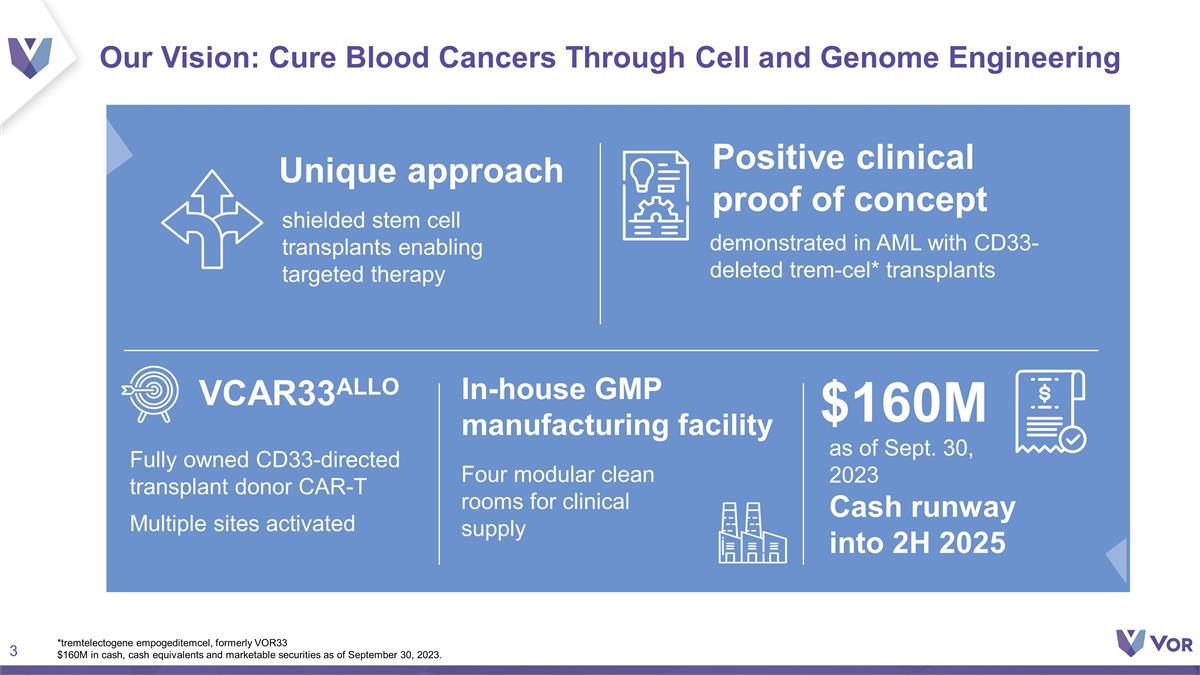
Our Vision: Cure Blood Cancers Through
Cell and Genome Engineering Unique approach Positive clinical proof of concept VCAR33ALLO as of Sept. 30, 2023 Cash runway into 2H 2025 $160M In-house GMP manufacturing facility *tremtelectogene empogeditemcel, formerly VOR33 $160M in cash,
cash equivalents and marketable securities as of September 30, 2023. Fully owned CD33-directed transplant donor CAR-T Multiple sites activated Four modular clean rooms for clinical supply shielded stem cell transplants enabling targeted therapy
demonstrated in AML with CD33-deleted trem-cel* transplants
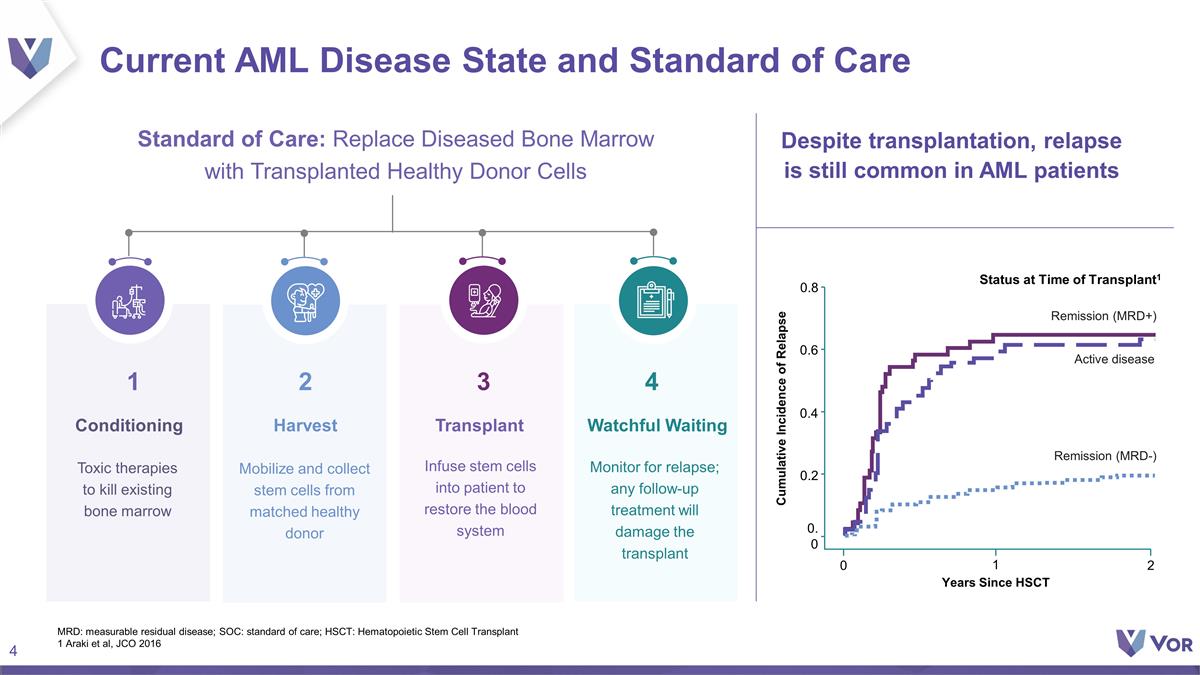
Current AML Disease State and Standard
of Care Conditioning Mobilize and collect stem cells from matched healthy donor Transplant Remission (MRD-) 2 0.8 Years Since HSCT Active disease Remission (MRD+) 0.0 0.2 0.4 0.6 0 Cumulative Incidence of Relapse 1 Status at Time of Transplant1
Despite transplantation, relapse is still common in AML patients 1 MRD: measurable residual disease; SOC: standard of care; HSCT: Hematopoietic Stem Cell Transplant 1 Araki et al, JCO 2016 4 Watchful Waiting 3 2 3 3 Standard of Care: Replace
Diseased Bone Marrow with Transplanted Healthy Donor Cells Toxic therapies to kill existing bone marrow Harvest Infuse stem cells into patient to restore the blood system Monitor for relapse; any follow-up treatment will damage the
transplant
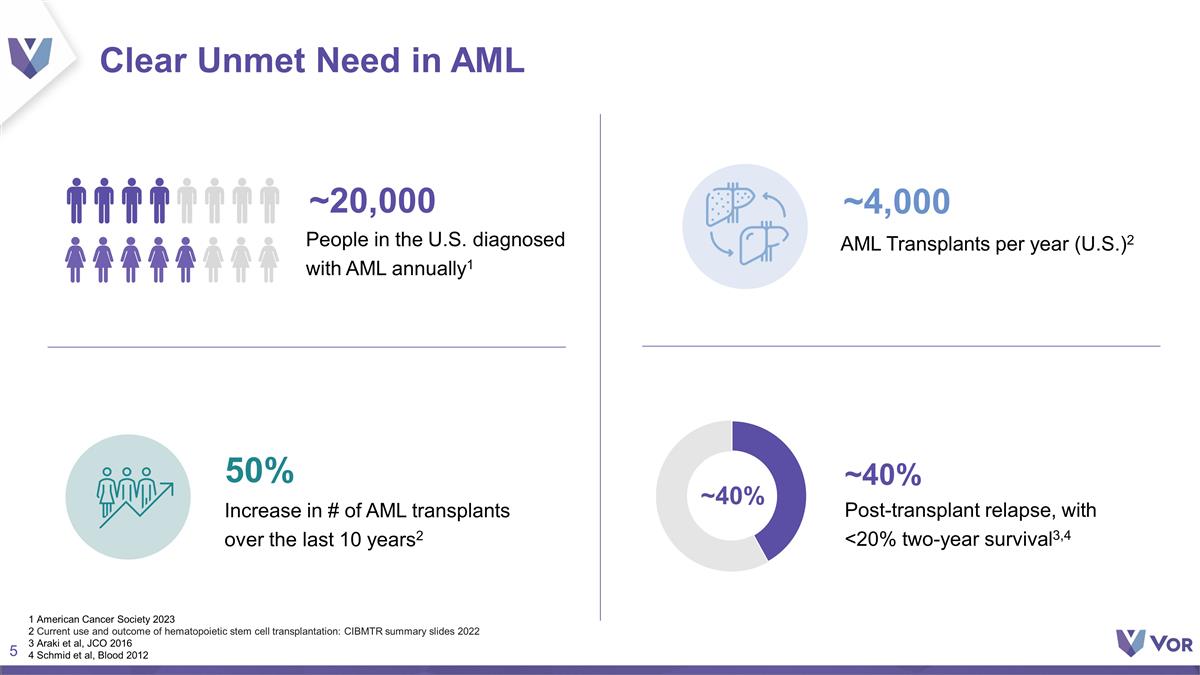
Clear Unmet Need in AML ~200K
Post-transplant relapse, with <20% two-year survival3,4 50% Increase in # of AML transplants over the last 10 years2 ~20,000 People in the U.S. diagnosed with AML annually1 AML Transplants per year (U.S.)2 ~4,000 ~40% ~40% 1 American Cancer
Society 2023 2 Current use and outcome of hematopoietic stem cell transplantation: CIBMTR summary slides 2022 3 Araki et al, JCO 2016 4 Schmid et al, Blood 2012
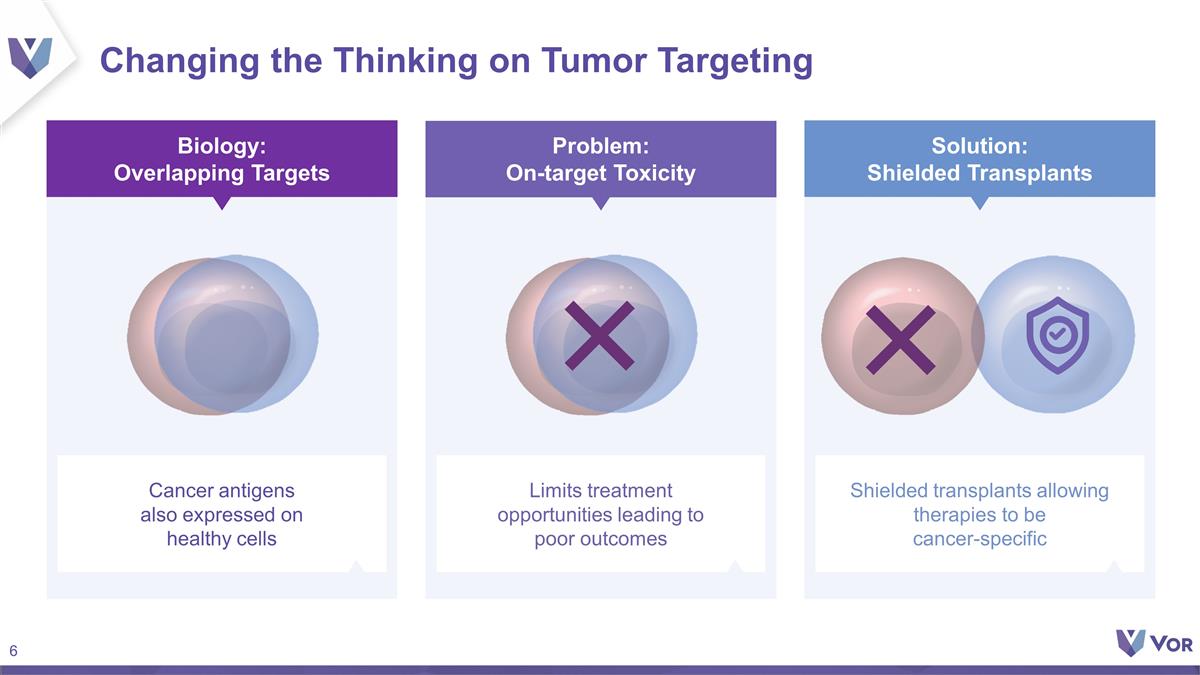
Changing the Thinking on Tumor
Targeting Cancer antigens also expressed on healthy cells Biology: Overlapping Targets Limits treatment opportunities leading to poor outcomes Problem: On-target Toxicity Shielded transplants allowing therapies to be cancer-specific Solution:
Shielded Transplants
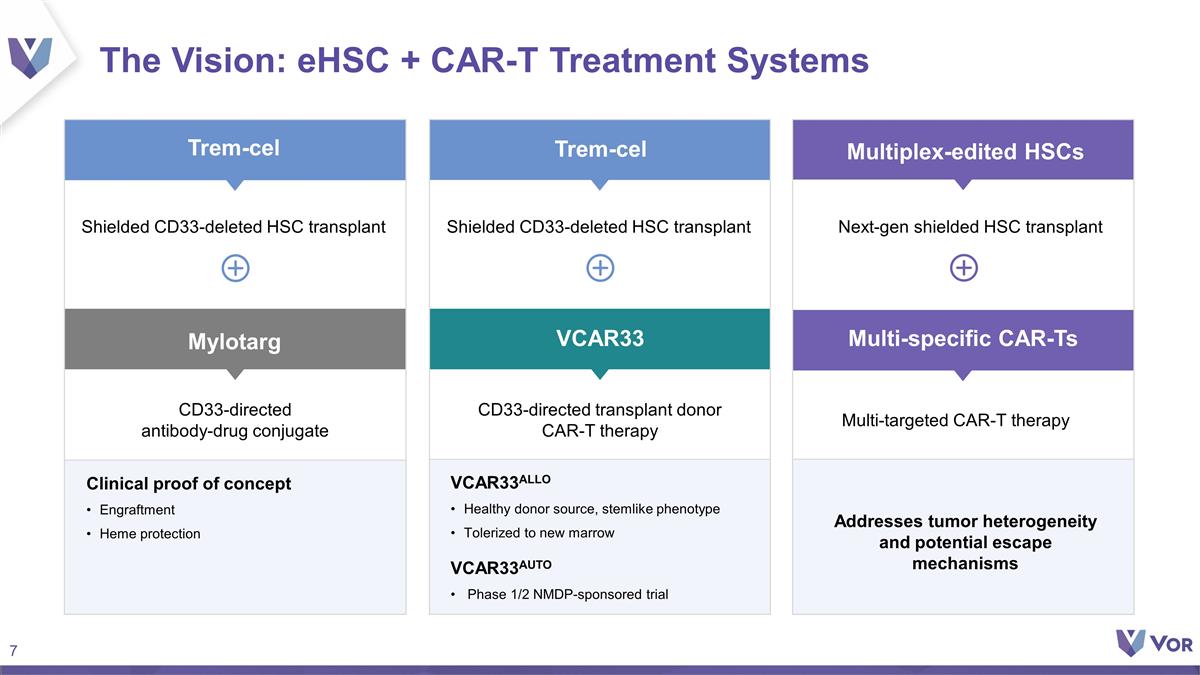
The Vision: eHSC + CAR-T Treatment
Systems CD33-directed antibody-drug conjugate CD33-directed transplant donor CAR-T therapy Clinical proof of concept Engraftment Heme protection VCAR33ALLO Healthy donor source, stemlike phenotype Tolerized to new marrow VCAR33AUTO Phase 1/2
NMDP-sponsored trial Addresses tumor heterogeneity and potential escape mechanisms Next-gen shielded HSC transplant Mylotarg Multi-targeted CAR-T therapy Trem-cel Trem-cel VCAR33 Multiplex-edited HSCs Multi-specific CAR-Ts Shielded CD33-deleted HSC
transplant Shielded CD33-deleted HSC transplant
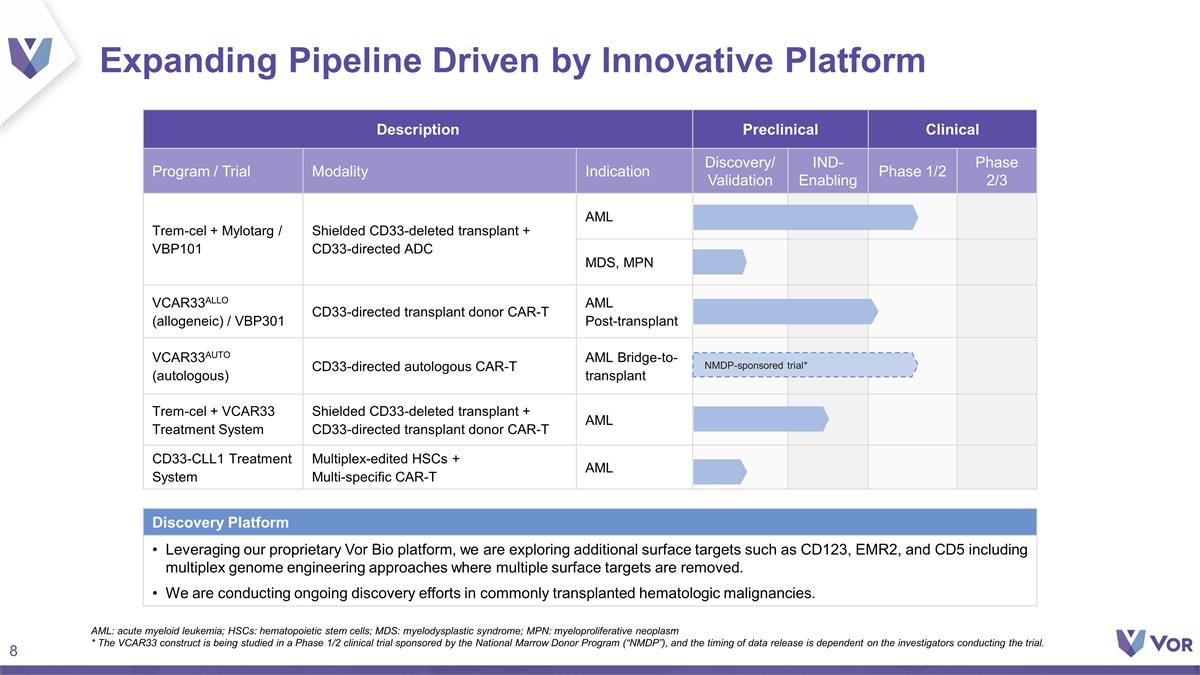
Description Preclinical Clinical
Program / Trial Modality Indication Discovery/ Validation IND- Enabling Phase 1/2 Phase 2/3 Trem-cel + Mylotarg / VBP101 Shielded CD33-deleted transplant + CD33-directed ADC AML MDS, MPN MDS, MPN VCAR33ALLO (allogeneic) / VBP301 CD33-directed
transplant donor CAR-T AML Post-transplant VCAR33AUTO (autologous) CD33-directed autologous CAR-T AML Bridge-to-transplant Trem-cel + VCAR33 Treatment System Shielded CD33-deleted transplant + CD33-directed transplant donor CAR-T AML CD33-CLL1
Treatment System Multiplex-edited HSCs + Multi-specific CAR-T AML Expanding Pipeline Driven by Innovative Platform AML: acute myeloid leukemia; HSCs: hematopoietic stem cells; MDS: myelodysplastic syndrome; MPN: myeloproliferative neoplasm * The
VCAR33 construct is being studied in a Phase 1/2 clinical trial sponsored by the National Marrow Donor Program (“NMDP”), and the timing of data release is dependent on the investigators conducting the trial. NMDP-sponsored trial*
Discovery Platform Leveraging our proprietary Vor Bio platform, we are exploring additional surface targets such as CD123, EMR2, and CD5 including multiplex genome engineering approaches where multiple surface targets are removed. We are conducting
ongoing discovery efforts in commonly transplanted hematologic malignancies.
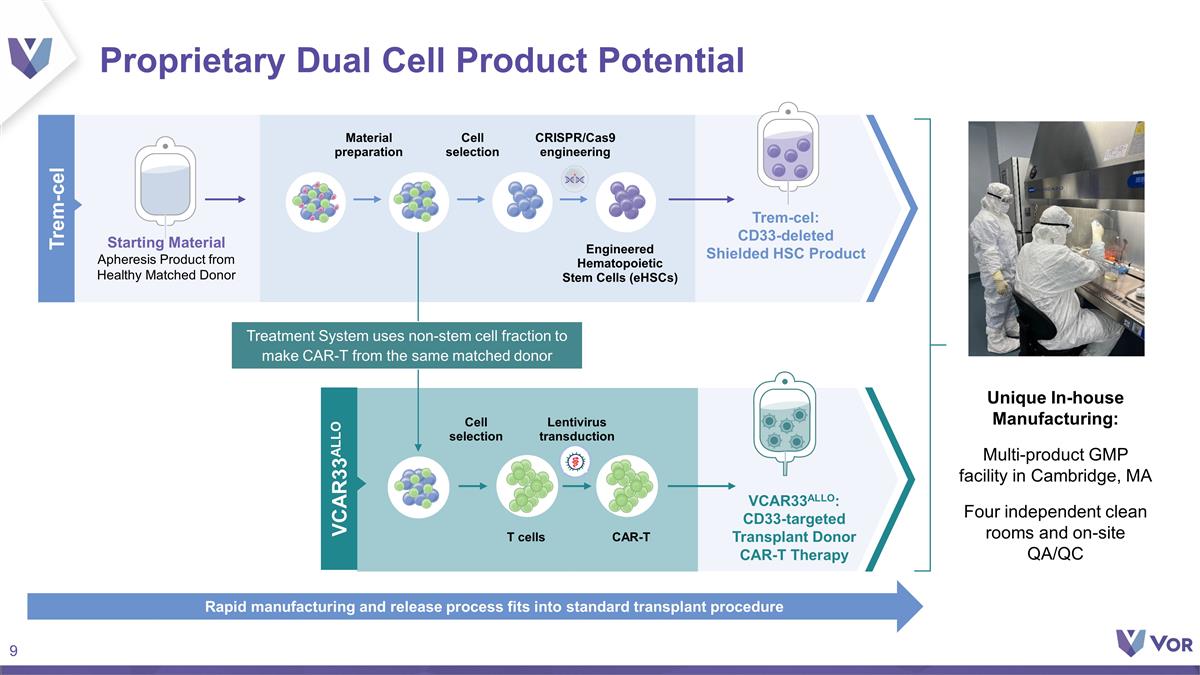
Proprietary Dual Cell Product
Potential Trem-cel Starting Material Apheresis Product from Healthy Matched Donor Trem-cel: CD33-deleted Shielded HSC Product VCAR33ALLO: CD33-targeted Transplant Donor CAR-T Therapy Cell selection Lentivirus transduction T cells CAR-T VCAR33ALLO
Material preparation Cell selection CRISPR/Cas9 engineering Engineered Hematopoietic Stem Cells (eHSCs) Treatment System uses non-stem cell fraction to make CAR-T from the same matched donor Unique In-house Manufacturing: Multi-product GMP facility
in Cambridge, MA Four independent clean rooms and on-site QA/QC Rapid manufacturing and release process fits into standard transplant procedure
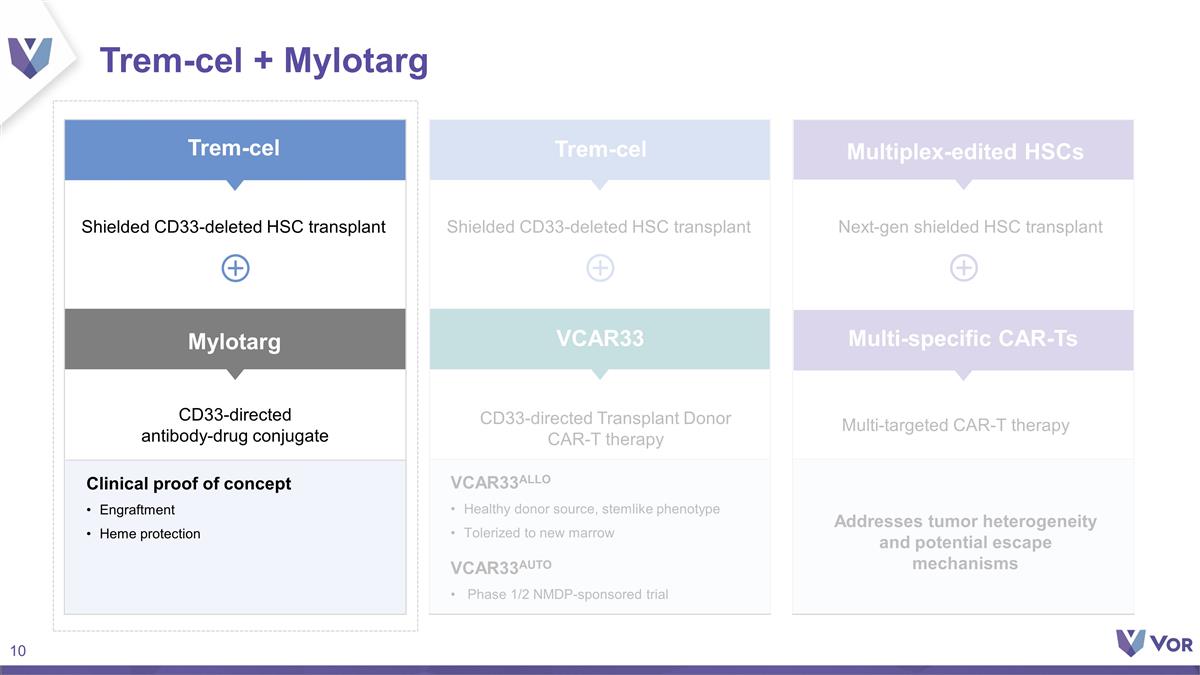
Trem-cel + Mylotarg CD33-directed
antibody-drug conjugate CD33-directed Transplant Donor CAR-T therapy Clinical proof of concept Engraftment Heme protection VCAR33ALLO Healthy donor source, stemlike phenotype Tolerized to new marrow VCAR33AUTO Phase 1/2 NMDP-sponsored trial
Addresses tumor heterogeneity and potential escape mechanisms Next-gen shielded HSC transplant Mylotarg Multi-targeted CAR-T therapy Trem-cel Trem-cel VCAR33 Multiplex-edited HSCs Multi-specific CAR-Ts Shielded CD33-deleted HSC transplant Shielded
CD33-deleted HSC transplant
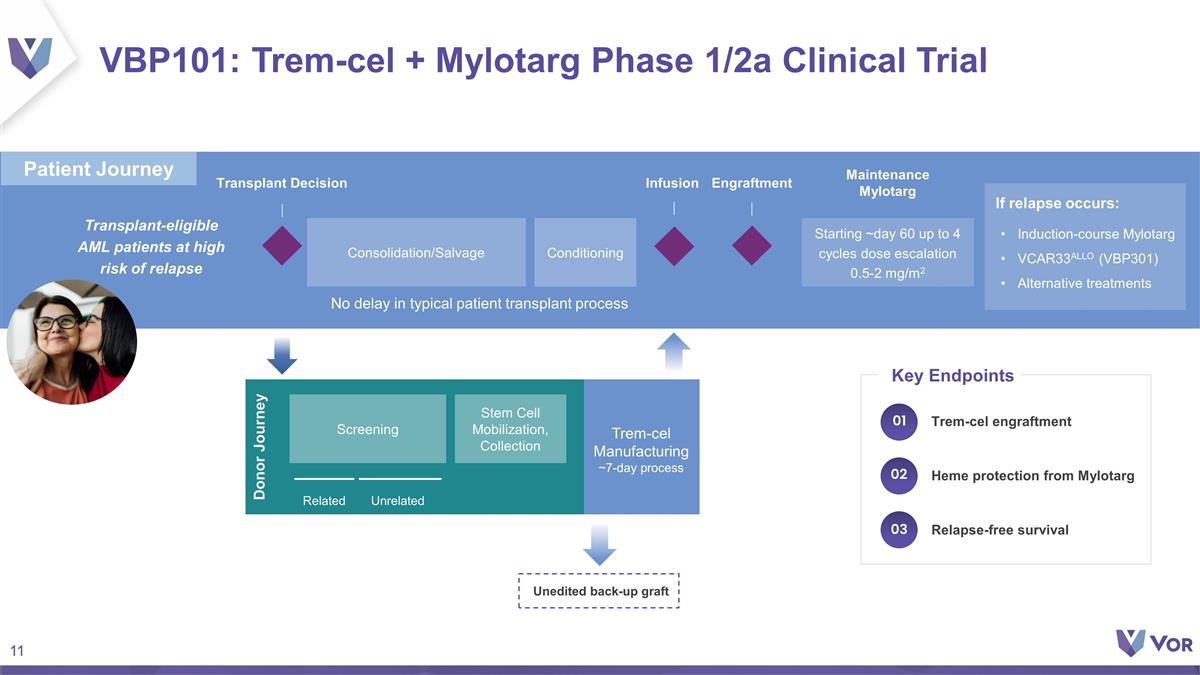
VBP101: Trem-cel + Mylotarg Phase
1/2a Clinical Trial Transplant Decision Infusion Donor Journey Related Unrelated Maintenance Mylotarg Manufacturing vein-to-vein 7-10 days Unedited back-up graft Transplant-eligible AML patients at high risk of relapse Trem-cel Manufacturing
~7-day process Engraftment No delay in typical patient transplant process If relapse occurs: Induction-course Mylotarg VCAR33ALLO (VBP301) Alternative treatments Starting ~day 60 up to 4 cycles dose escalation 0.5-2 mg/m2 Conditioning
Consolidation/Salvage Stem Cell Mobilization, Collection Screening Relapse-free survival Heme protection from Mylotarg Trem-cel engraftment Key Endpoints 01 02 03 Patient Journey
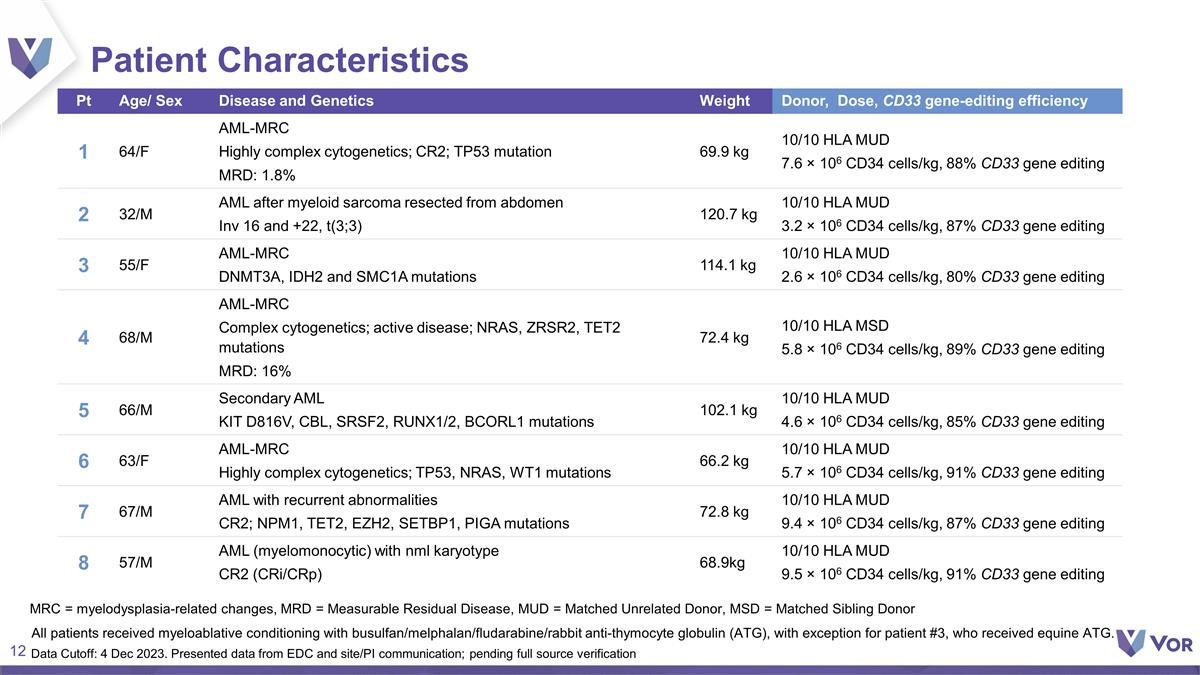
Patient Characteristics Pt Age/ Sex
Disease and Genetics Weight Donor, Dose, CD33 gene-editing efficiency 1 64/F AML-MRC Highly complex cytogenetics; CR2; TP53 mutation MRD: 1.8% 69.9 kg 10/10 HLA MUD 7.6 × 106 CD34 cells/kg, 88% CD33 gene editing 2 32/M AML after myeloid
sarcoma resected from abdomen Inv 16 and +22, t(3;3) 120.7 kg 10/10 HLA MUD 3.2 × 106 CD34 cells/kg, 87% CD33 gene editing 3 55/F AML-MRC DNMT3A, IDH2 and SMC1A mutations 114.1 kg 10/10 HLA MUD 2.6 × 106 CD34 cells/kg, 80% CD33
gene editing 4 68/M AML-MRC Complex cytogenetics; active disease; NRAS, ZRSR2, TET2 mutations MRD: 16% 72.4 kg 10/10 HLA MSD 5.8 × 106 CD34 cells/kg, 89% CD33 gene editing 5 66/M Secondary AML KIT D816V, CBL, SRSF2, RUNX1/2, BCORL1 mutations
102.1 kg 10/10 HLA MUD 4.6 × 106 CD34 cells/kg, 85% CD33 gene editing 6 63/F AML-MRC Highly complex cytogenetics; TP53, NRAS, WT1 mutations 66.2 kg 10/10 HLA MUD 5.7 × 106 CD34 cells/kg, 91% CD33 gene editing 7 67/M AML with recurrent
abnormalities CR2; NPM1, TET2, EZH2, SETBP1, PIGA mutations 72.8 kg 10/10 HLA MUD 9.4 × 106 CD34 cells/kg, 87% CD33 gene editing 8 57/M AML (myelomonocytic) with nml karyotype CR2 (CRi/CRp) 68.9kg 10/10 HLA MUD 9.5 × 106 CD34 cells/kg, 91%
CD33 gene editing MRC = myelodysplasia-related changes, MRD = Measurable Residual Disease, MUD = Matched Unrelated Donor, MSD = Matched Sibling Donor All patients received myeloablative conditioning with busulfan/melphalan/fludarabine/rabbit
anti-thymocyte globulin (ATG), with exception for patient #3, who received equine ATG. Data Cutoff: 4 Dec 2023. Presented data from EDC and site/PI communication; pending full source verification
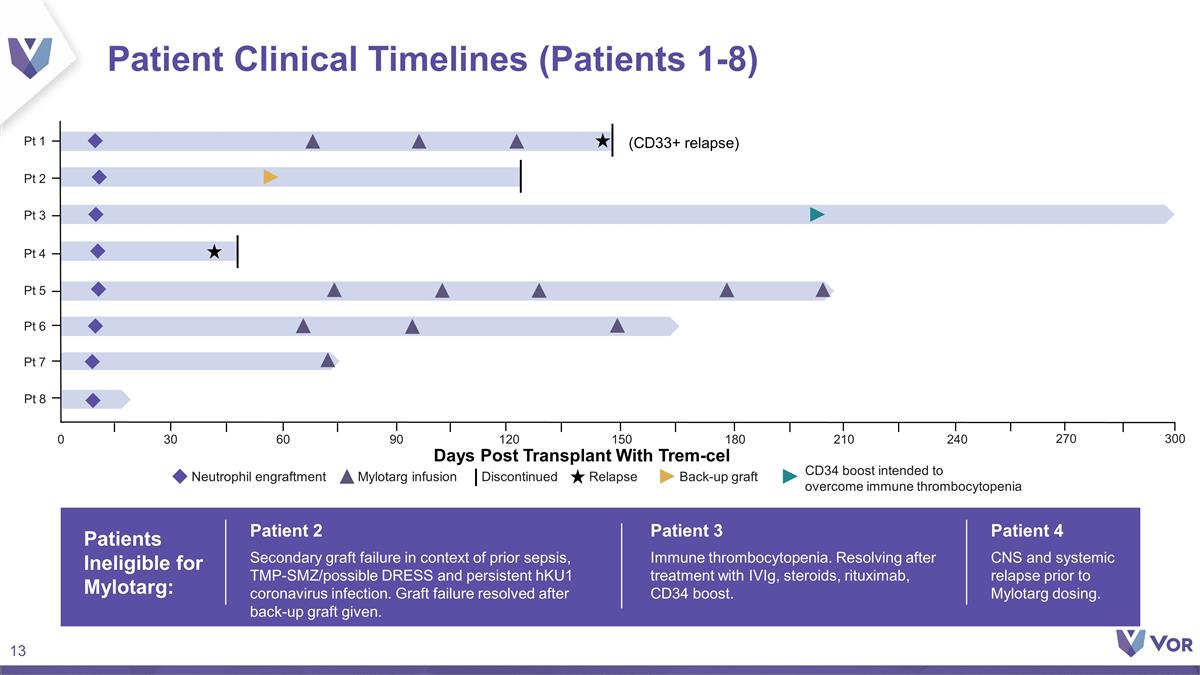
Patient Clinical Timelines
(Patients 1-8) Patients Ineligible for Mylotarg: Patient 2 Secondary graft failure in context of prior sepsis, TMP-SMZ/possible DRESS and persistent hKU1 coronavirus infection. Graft failure resolved after back-up graft given. Patient 3 Immune
thrombocytopenia. Resolving after treatment with IVIg, steroids, rituximab, CD34 boost. Patient 4 CNS and systemic relapse prior to Mylotarg dosing. Days Post Transplant With Trem-cel Neutrophil engraftment Mylotarg infusion Discontinued Back-up
graft Relapse CD34 boost intended to overcome immune thrombocytopenia 0 30 60 90 120 180 210 240 150 270 300 (CD33+ relapse) Pt 3 Pt 5 Pt 2 Pt 4 Pt 1 Pt 6 Pt 7 Pt 8
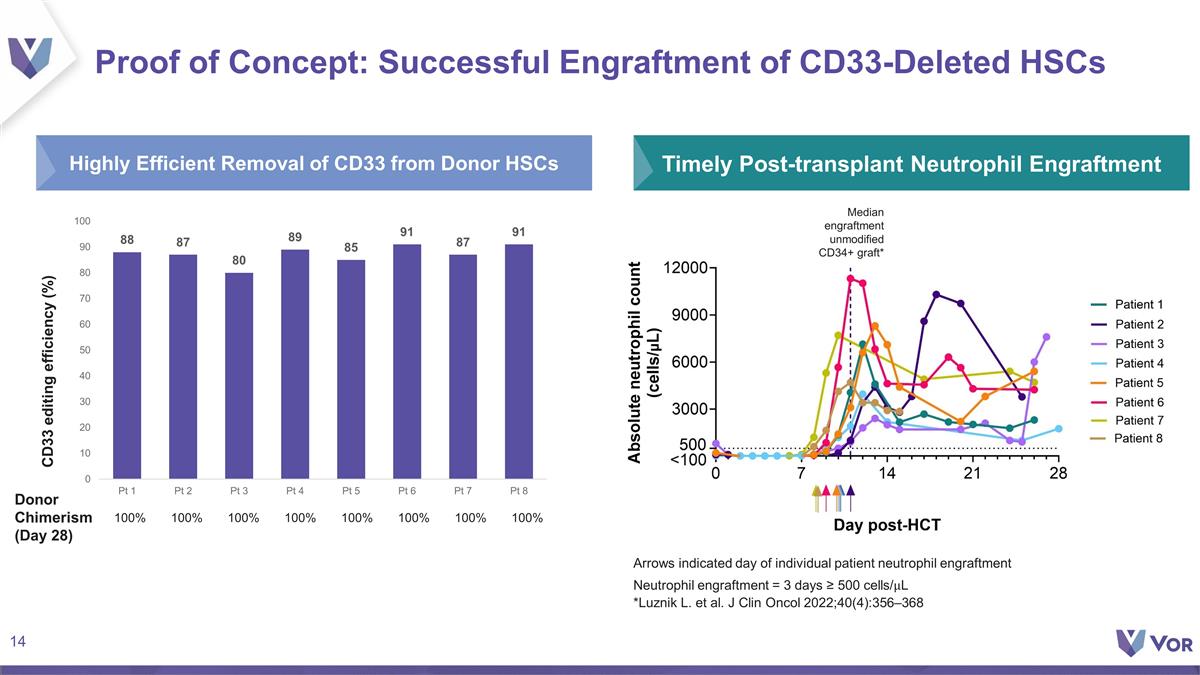
Highly Efficient Removal of CD33
from Donor HSCs Proof of Concept: Successful Engraftment of CD33-Deleted HSCs CD33 editing efficiency (%) Arrows indicated day of individual patient neutrophil engraftment Neutrophil engraftment = 3 days ≥ 500 cells/L *Luznik L. et al. J Clin
Oncol 2022;40(4):356–368 Timely Post-transplant Neutrophil Engraftment Donor Chimerism (Day 28) 100% 100% 100% 100% 100% 100% 100% Median engraftment unmodified CD34+ graft* 100%
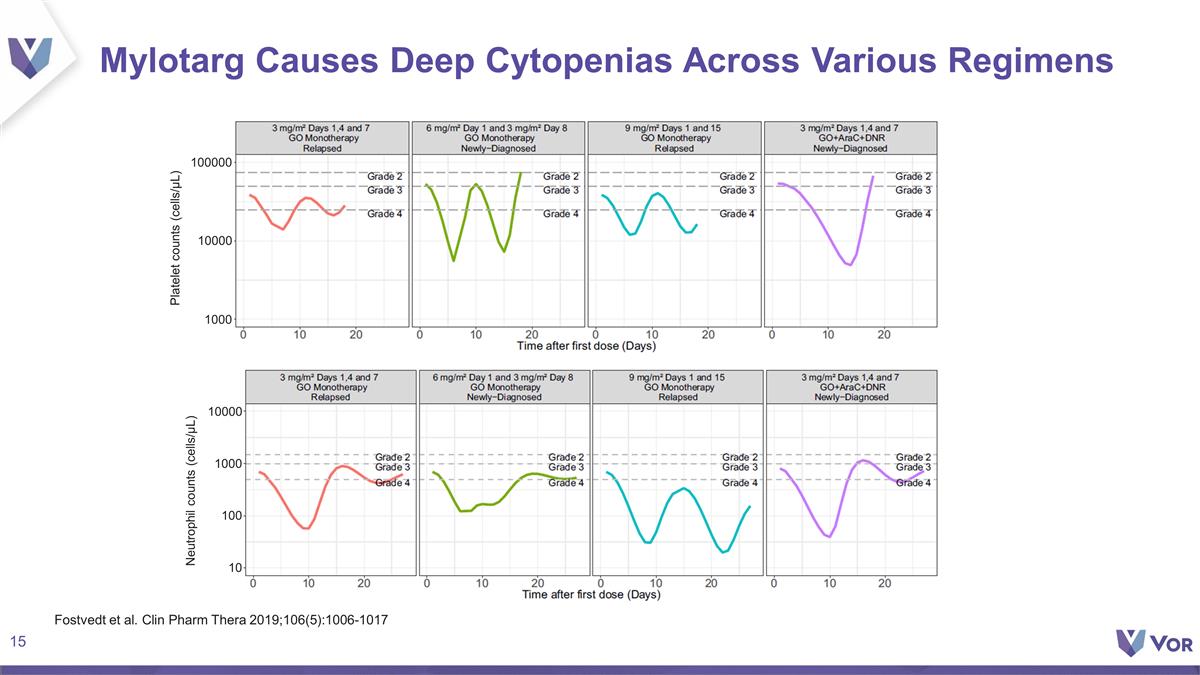
Mylotarg Causes Deep Cytopenias
Across Various Regimens Fostvedt et al. Clin Pharm Thera 2019;106(5):1006-1017 Platelet counts (cells/µL) 1000 10000 100000 10000 1000 100 10 Neutrophil counts (cells/µL)
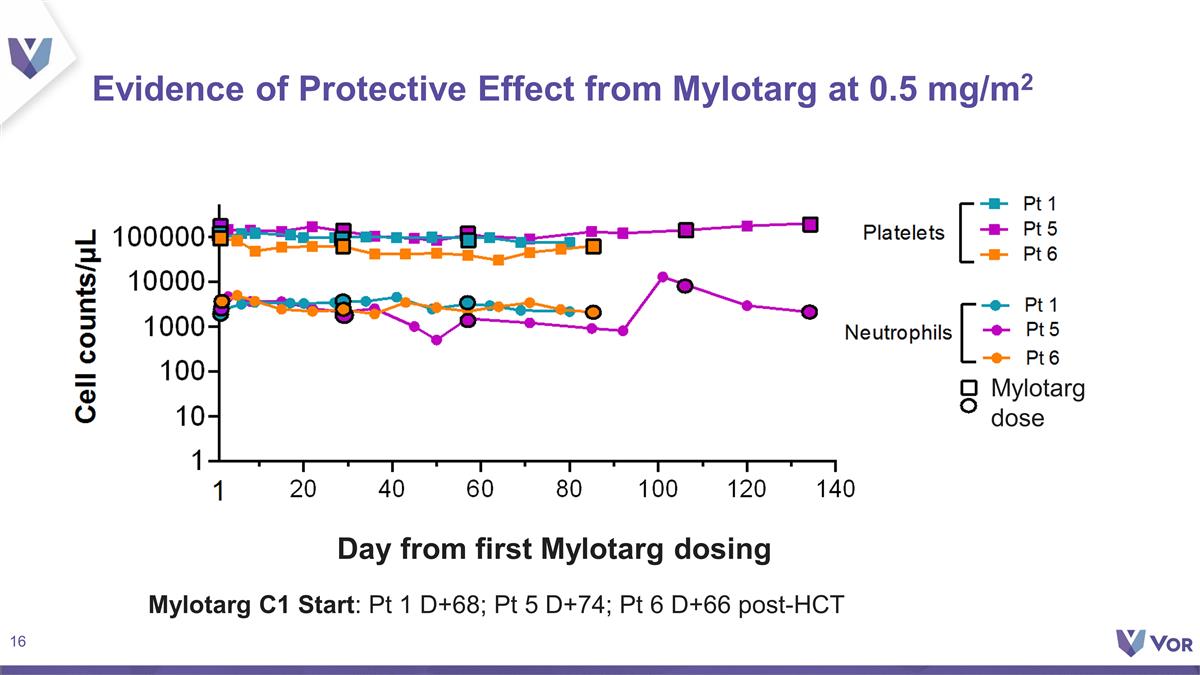
Evidence of Protective Effect from
Mylotarg at 0.5 mg/m2 Mylotarg C1 Start: Pt 1 D+68; Pt 5 D+74; Pt 6 D+66 post-HCT Day from first Mylotarg dosing Mylotarg dose
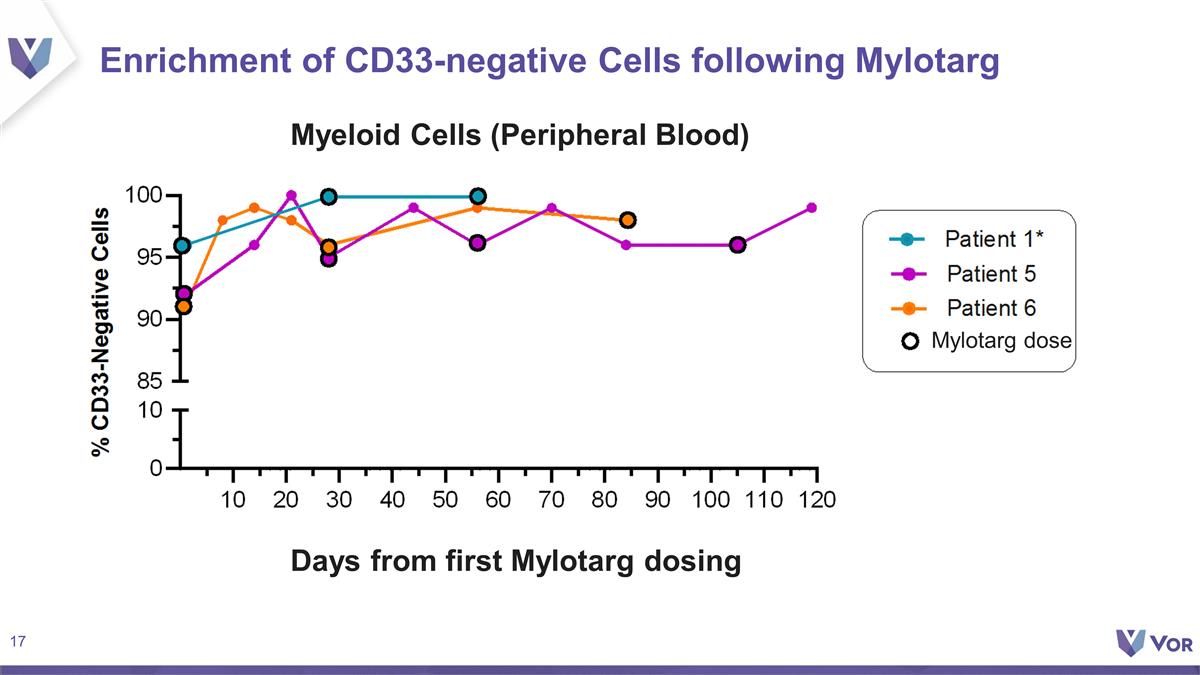
Enrichment of CD33-negative Cells
following Mylotarg Myeloid Cells (Peripheral Blood) Days from first Mylotarg dosing Mylotarg dose
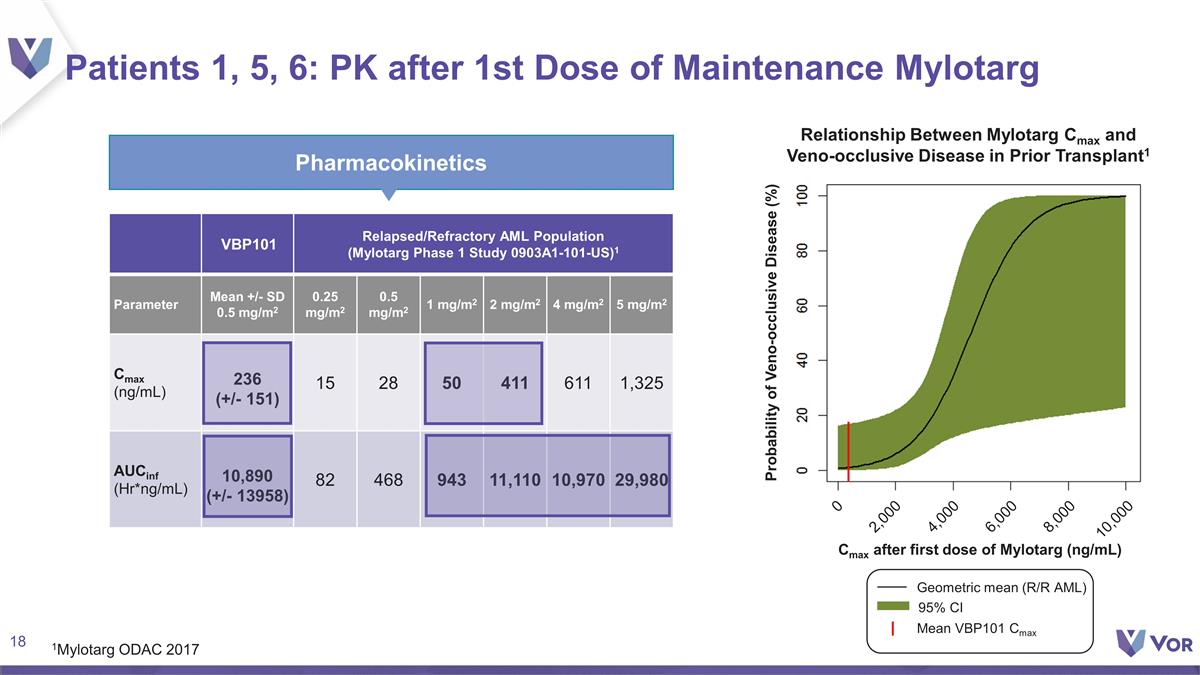
Patients 1, 5, 6: PK after 1st Dose
of Maintenance Mylotarg Pharmacokinetics VBP101 Relapsed/Refractory AML Population (Mylotarg Phase 1 Study 0903A1-101-US)1 Parameter Mean +/- SD 0.5 mg/m2 0.25 mg/m2 0.5 mg/m2 1 mg/m2 2 mg/m2 4 mg/m2 5 mg/m2 Cmax (ng/mL) 236 (+/- 151) 15 28 50
411 611 1,325 AUCinf (Hr*ng/mL) 10,890 (+/- 13958) 82 468 943 11,110 10,970 29,980 1Mylotarg ODAC 2017 Relationship Between Mylotarg Cmax and Veno-occlusive Disease in Prior Transplant1 Probability of Veno-occlusive Disease (%) Cmax after first dose
of Mylotarg (ng/mL) 2,000 4,000 6,000 8,000 10,000 0 95% CI Geometric mean (R/R AML) Mean VBP101 Cmax
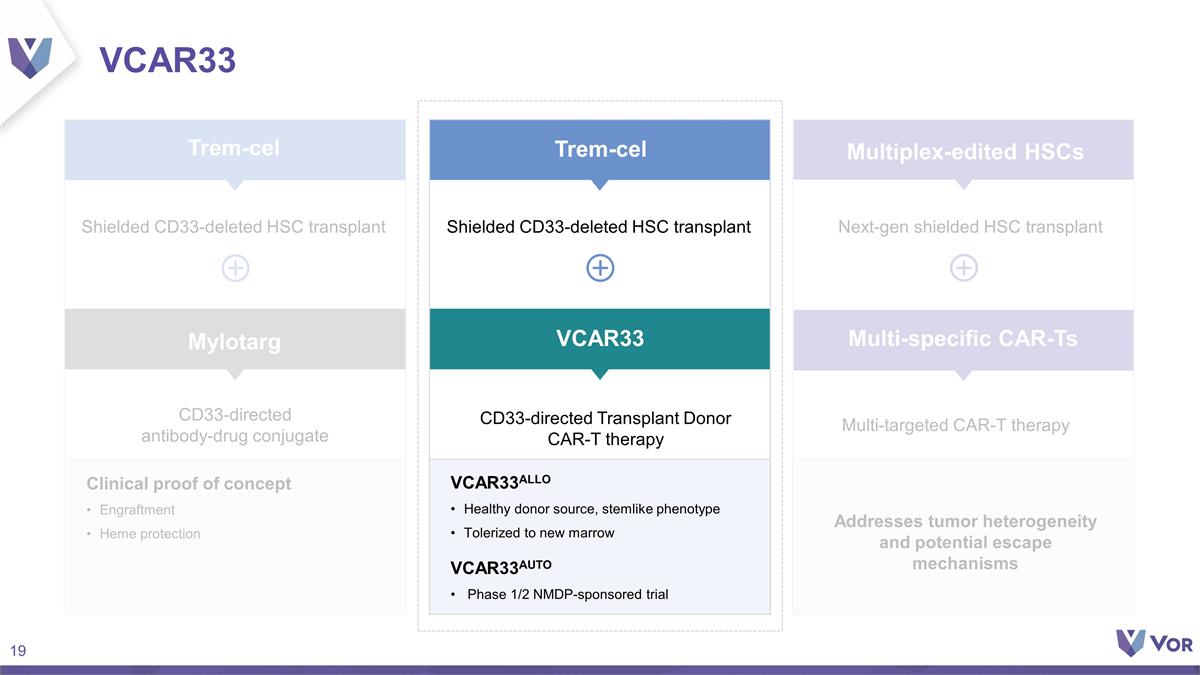
VCAR33 CD33-directed antibody-drug
conjugate CD33-directed Transplant Donor CAR-T therapy Clinical proof of concept Engraftment Heme protection VCAR33ALLO Healthy donor source, stemlike phenotype Tolerized to new marrow VCAR33AUTO Phase 1/2 NMDP-sponsored trial Addresses tumor
heterogeneity and potential escape mechanisms Next-gen shielded HSC transplant Mylotarg Multi-targeted CAR-T therapy Trem-cel Trem-cel VCAR33 Multiplex-edited HSCs Multi-specific CAR-Ts Shielded CD33-deleted HSC transplant Shielded CD33-deleted HSC
transplant
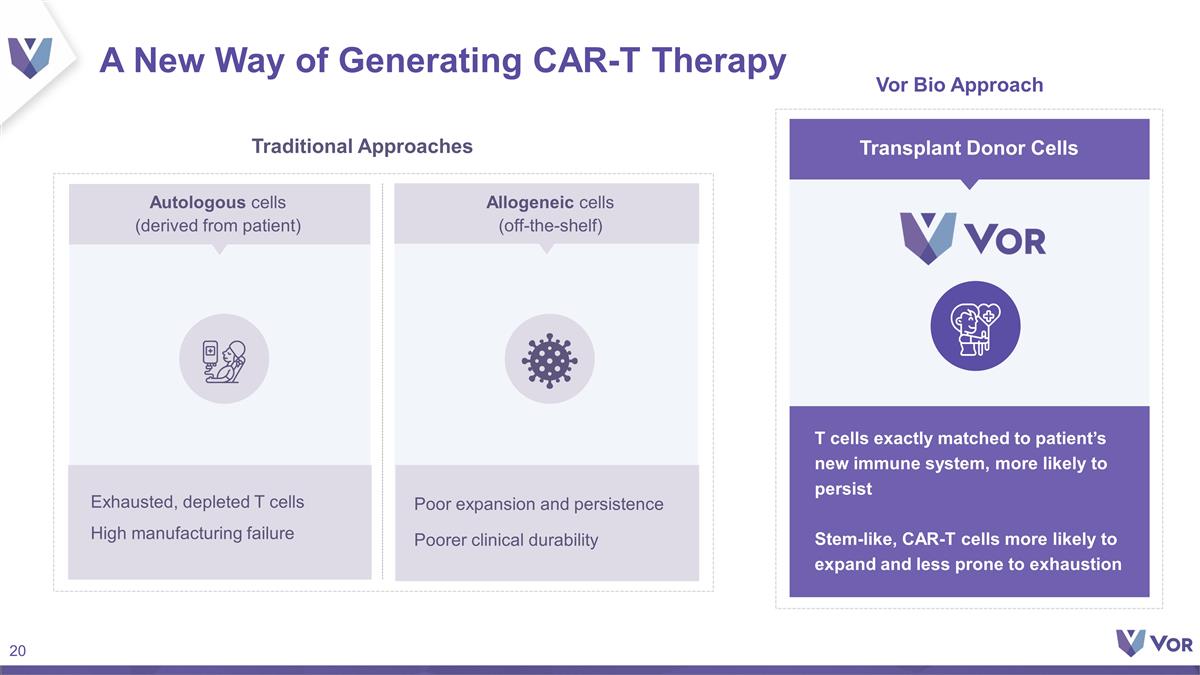
A New Way of Generating CAR-T
Therapy T cells exactly matched to patient’s new immune system, more likely to persist Stem-like, CAR-T cells more likely to expand and less prone to exhaustion Poor expansion and persistence Poorer clinical durability Exhausted, depleted T
cells High manufacturing failure Traditional Approaches Vor Bio Approach Autologous cells (derived from patient) Allogeneic cells (off-the-shelf) Transplant Donor Cells
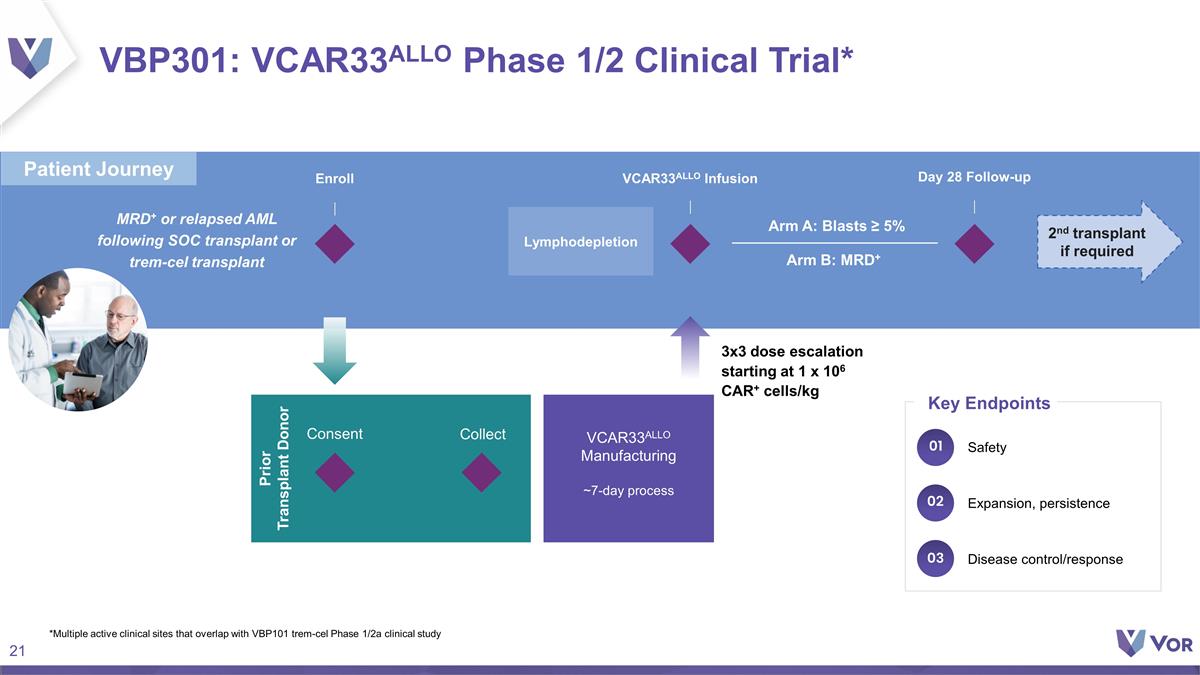
VBP301: VCAR33ALLO Phase 1/2
Clinical Trial* Enroll VCAR33ALLO Infusion Prior Transplant Donor MRD+ or relapsed AML following SOC transplant or trem-cel transplant VCAR33ALLO Manufacturing ~7-day process Day 28 Follow-up Consent Collect 2nd transplant if required Arm B: MRD+
Arm A: Blasts ≥ 5% 3x3 dose escalation starting at 1 x 106 CAR+ cells/kg *Multiple active clinical sites that overlap with VBP101 trem-cel Phase 1/2a clinical study Lymphodepletion Disease control/response Expansion, persistence Safety Key
Endpoints 01 02 03 Patient Journey
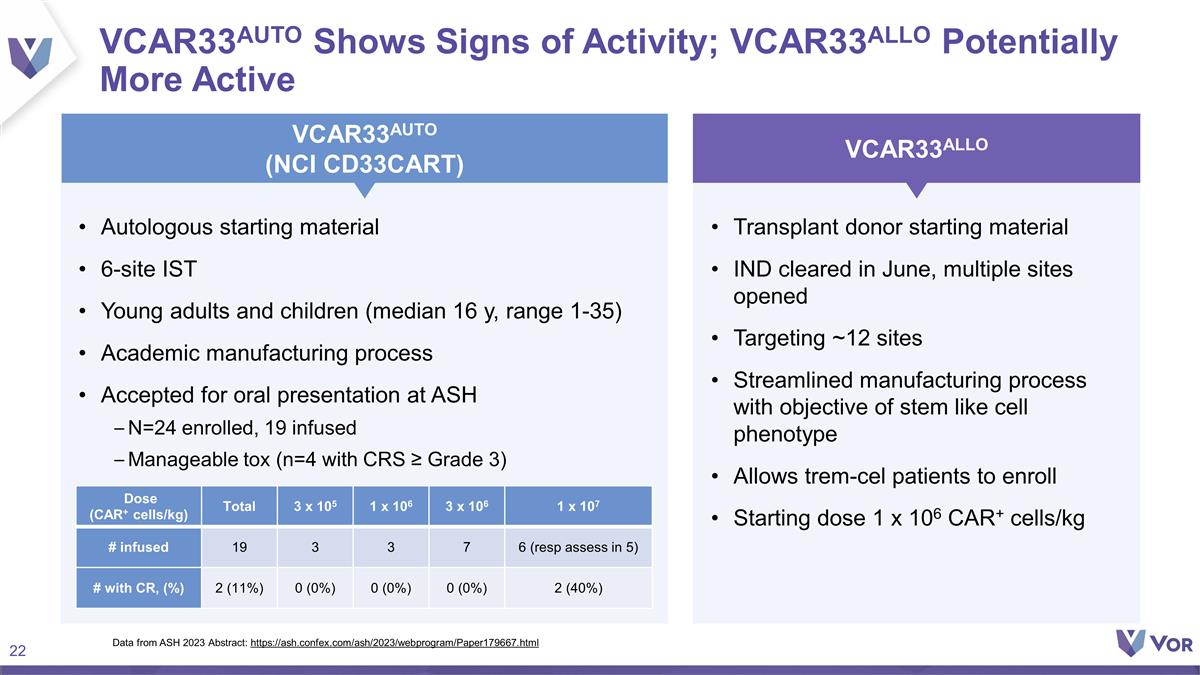
VCAR33AUTO Shows Signs of Activity;
VCAR33ALLO Potentially More Active Autologous starting material 6-site IST Young adults and children (median 16 y, range 1-35) Academic manufacturing process Accepted for oral presentation at ASH N=24 enrolled, 19 infused Manageable tox (n=4 with
CRS ≥ Grade 3) Transplant donor starting material IND cleared in June, multiple sites opened Targeting ~12 sites Streamlined manufacturing process with objective of stem like cell phenotype Allows trem-cel patients to enroll Starting dose 1 x
106 CAR+ cells/kg VCAR33AUTO (NCI CD33CART) VCAR33ALLO Dose (CAR+ cells/kg) Total 3 x 105 1 x 106 3 x 106 1 x 107 # infused 19 3 3 7 6 (resp assess in 5) # with CR, (%) 2 (11%) 0 (0%) 0 (0%) 0 (0%) 2 (40%) Data from ASH 2023 Abstract:
https://ash.confex.com/ash/2023/webprogram/Paper179667.html
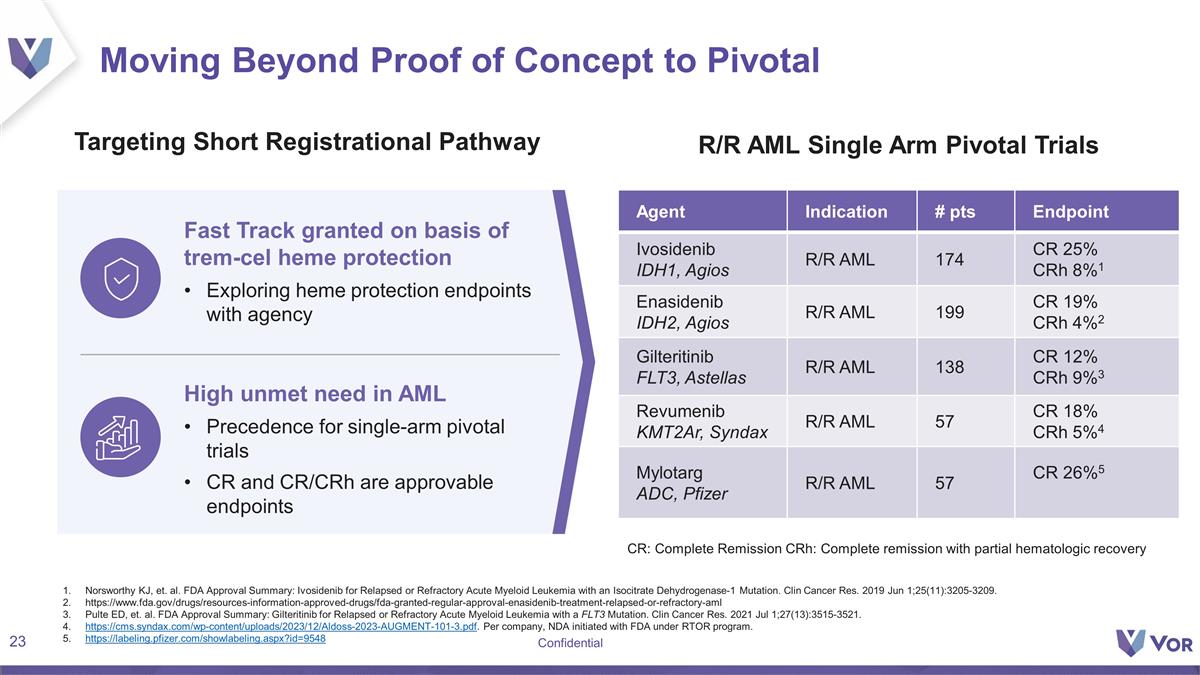
Moving Beyond Proof of Concept to
Pivotal Targeting Short Registrational Pathway R/R AML Single Arm Pivotal Trials Fast Track granted on basis of trem-cel heme protection Exploring heme protection endpoints with agency Agent Indication # pts Endpoint Ivosidenib IDH1, Agios R/R AML
174 CR 25% CRh 8%1 Enasidenib IDH2, Agios R/R AML 199 CR 19% CRh 4%2 Gilteritinib FLT3, Astellas R/R AML 138 CR 12% CRh 9%3 Revumenib KMT2Ar, Syndax R/R AML 57 CR 18% CRh 5%4 Mylotarg ADC, Pfizer R/R AML 57 CR 26%5 High unmet need in AML Precedence
for single-arm pivotal trials CR and CR/CRh are approvable endpoints Norsworthy KJ, et. al. FDA Approval Summary: Ivosidenib for Relapsed or Refractory Acute Myeloid Leukemia with an Isocitrate Dehydrogenase-1 Mutation. Clin Cancer Res. 2019 Jun
1;25(11):3205-3209. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-granted-regular-approval-enasidenib-treatment-relapsed-or-refractory-aml Pulte ED, et. al. FDA Approval Summary: Gilteritinib for Relapsed or Refractory Acute
Myeloid Leukemia with a FLT3 Mutation. Clin Cancer Res. 2021 Jul 1;27(13):3515-3521. https://cms.syndax.com/wp-content/uploads/2023/12/Aldoss-2023-AUGMENT-101-3.pdf. Per company, NDA initiated with FDA under RTOR program.
https://labeling.pfizer.com/showlabeling.aspx?id=9548 CR: Complete Remission CRh: Complete remission with partial hematologic recovery
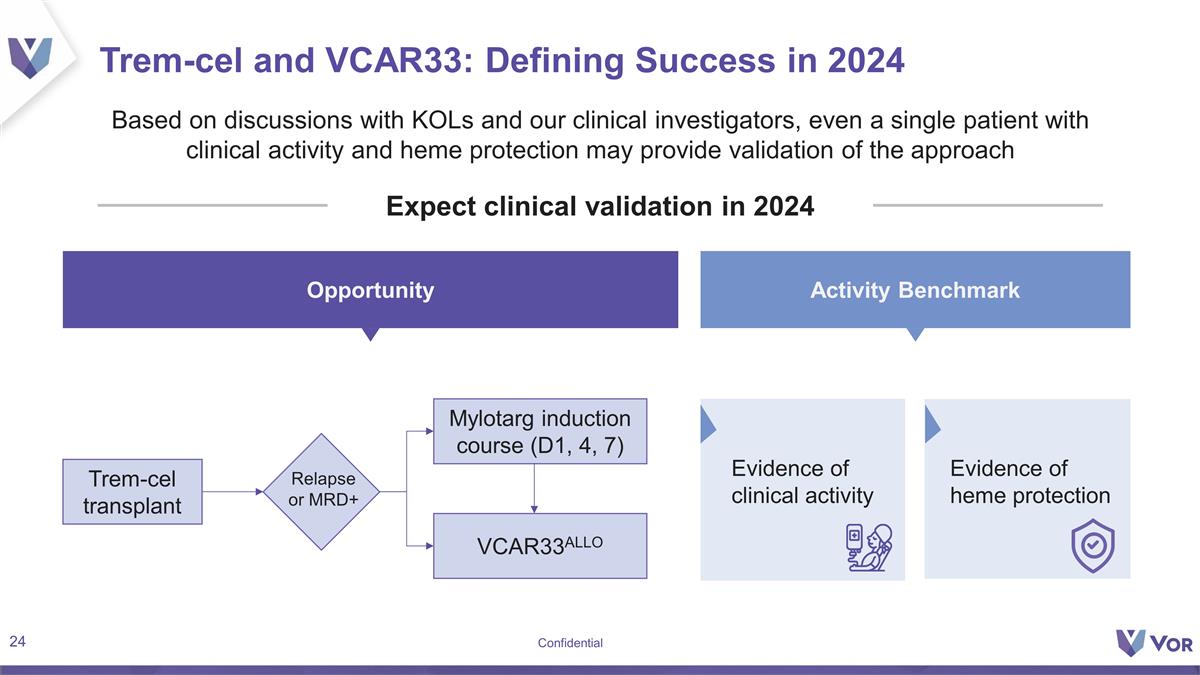
Trem-cel and VCAR33: Defining
Success in 2024 Based on discussions with KOLs and our clinical investigators, even a single patient with clinical activity and heme protection may provide validation of the approach Expect clinical validation in 2024 Opportunity Activity Benchmark
Evidence of clinical activity Trem-cel transplant Relapse or MRD+ Mylotarg induction course (D1, 4, 7) VCAR33ALLO Evidence of heme protection
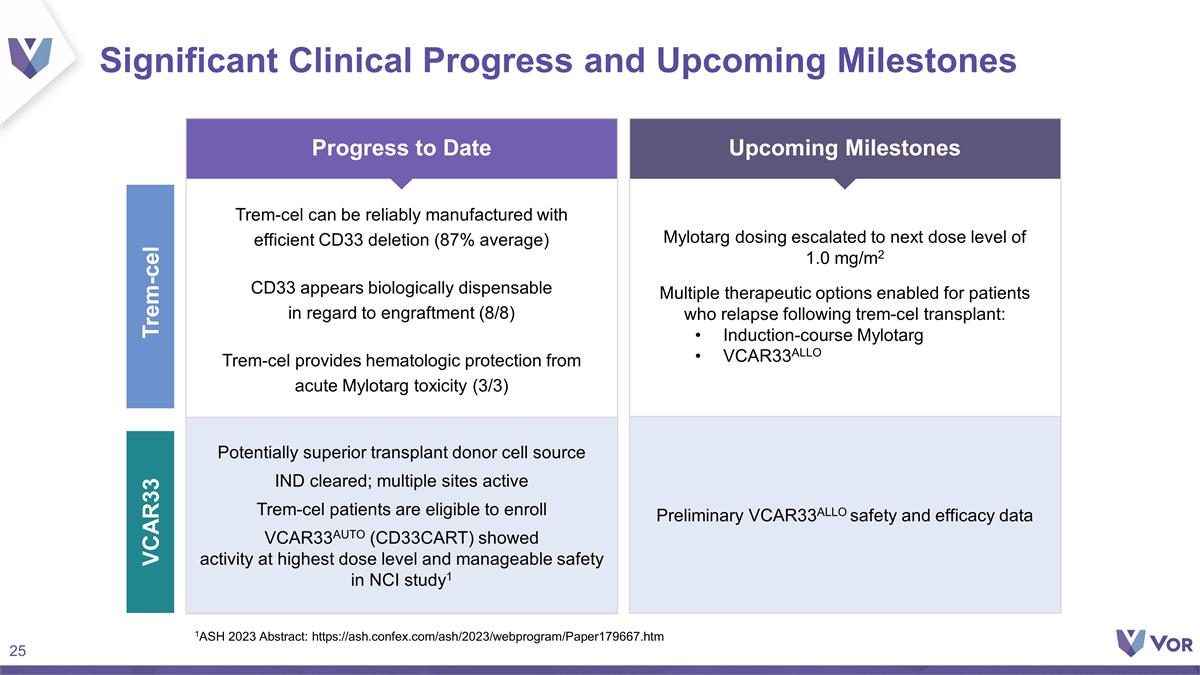
Mylotarg dosing escalated to next
dose level of 1.0 mg/m2 Multiple therapeutic options enabled for patients who relapse following trem-cel transplant: Induction-course Mylotarg VCAR33ALLO Upcoming Milestones Significant Clinical Progress and Upcoming Milestones Trem-cel Potentially
superior transplant donor cell source IND cleared; multiple sites active Trem-cel patients are eligible to enroll VCAR33AUTO (CD33CART) showed activity at highest dose level and manageable safety in NCI study1 Trem-cel can be reliably manufactured
with efficient CD33 deletion (87% average) CD33 appears biologically dispensable in regard to engraftment (8/8) Trem-cel provides hematologic protection from acute Mylotarg toxicity (3/3) Progress to Date Preliminary VCAR33ALLO safety and efficacy
data VCAR33 1ASH 2023 Abstract: https://ash.confex.com/ash/2023/webprogram/Paper179667.htm
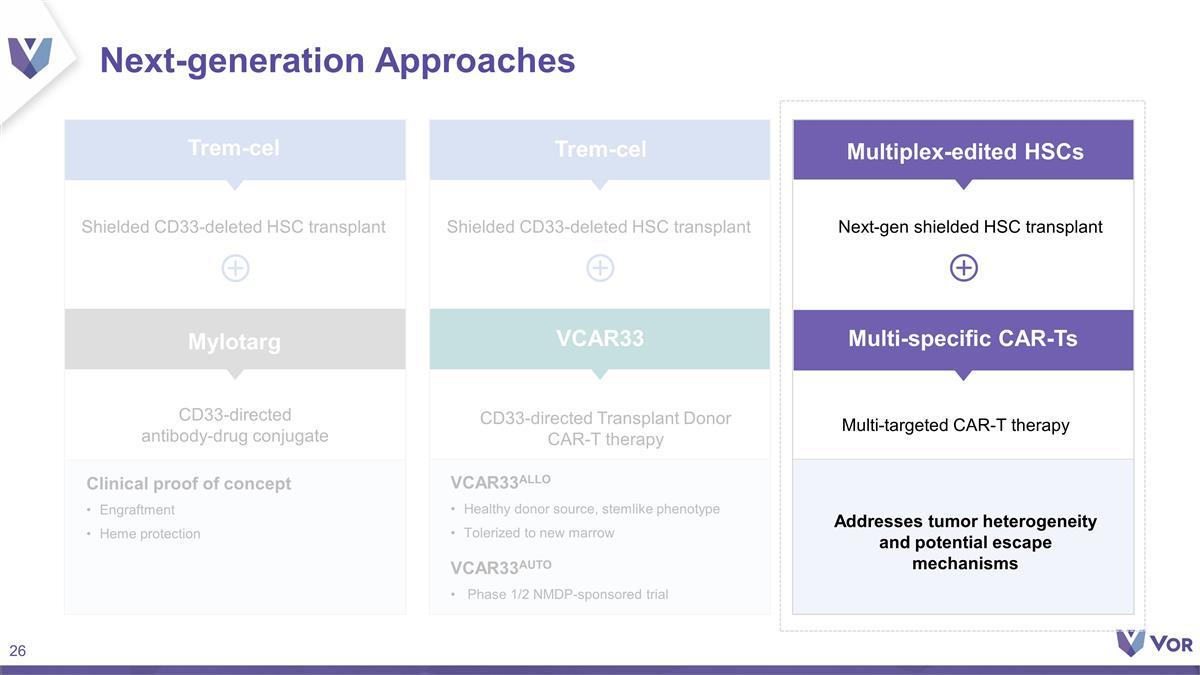
Next-generation Approaches
CD33-directed antibody-drug conjugate CD33-directed Transplant Donor CAR-T therapy Clinical proof of concept Engraftment Heme protection VCAR33ALLO Healthy donor source, stemlike phenotype Tolerized to new marrow VCAR33AUTO Phase 1/2 NMDP-sponsored
trial Addresses tumor heterogeneity and potential escape mechanisms Next-gen shielded HSC transplant Mylotarg Multi-targeted CAR-T therapy Trem-cel Trem-cel VCAR33 Multiplex-edited HSCs Multi-specific CAR-Ts Shielded CD33-deleted HSC transplant
Shielded CD33-deleted HSC transplant
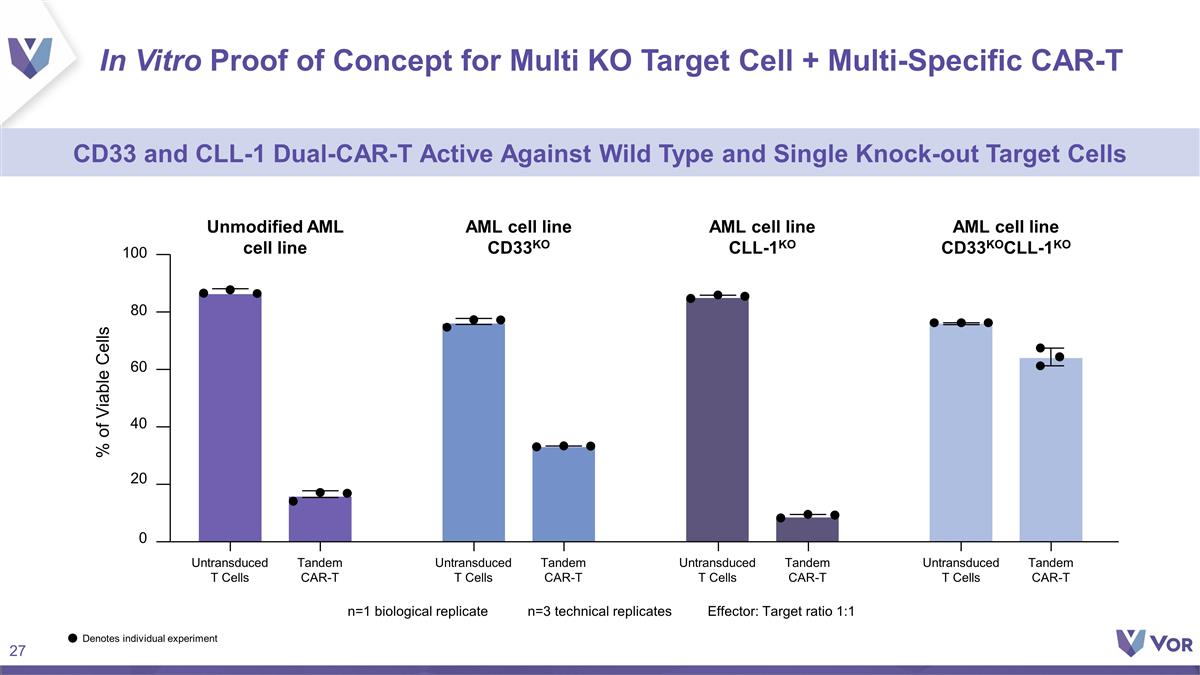
In Vitro Proof of Concept for Multi
KO Target Cell + Multi-Specific CAR-T CD33 and CLL-1 Dual-CAR-T Active Against Wild Type and Single Knock-out Target Cells 0 40 80 100 20 60 % of Viable Cells Tandem CAR-T Untransduced T Cells Tandem CAR-T Untransduced T Cells Tandem CAR-T
Untransduced T Cells Tandem CAR-T Untransduced T Cells Unmodified AML cell line AML cell line CD33KO AML cell line CLL-1KO AML cell line CD33KOCLL-1KO n=1 biological replicaten=3 technical replicatesEffector: Target ratio 1:1 Denotes individual
experiment
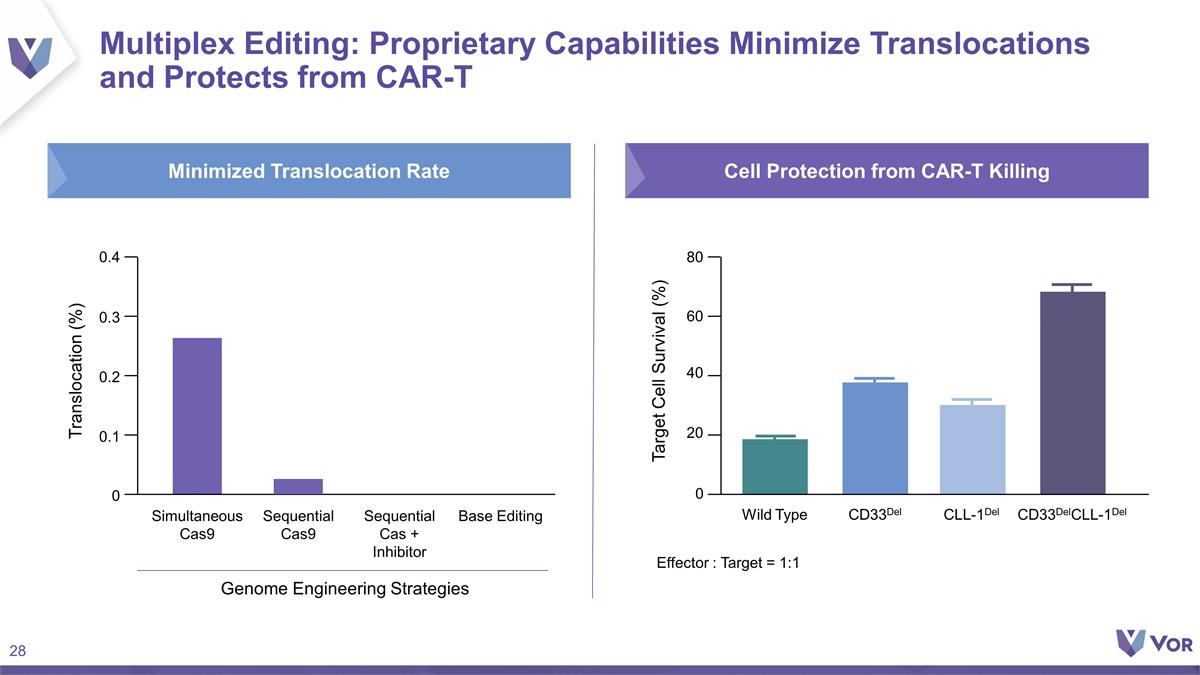
Multiplex Editing: Proprietary
Capabilities Minimize Translocations and Protects from CAR-T Effector : Target = 1:1 Target Cell Survival (%) Wild Type CD33Del 0 40 60 80 20 CLL-1Del CD33DelCLL-1Del Translocation (%) Simultaneous Cas9 0.3 0.4 0.2 0.1 0 Sequential Cas9 Sequential
Cas + Inhibitor Base Editing Genome Engineering Strategies Minimized Translocation Rate Cell Protection from CAR-T Killing
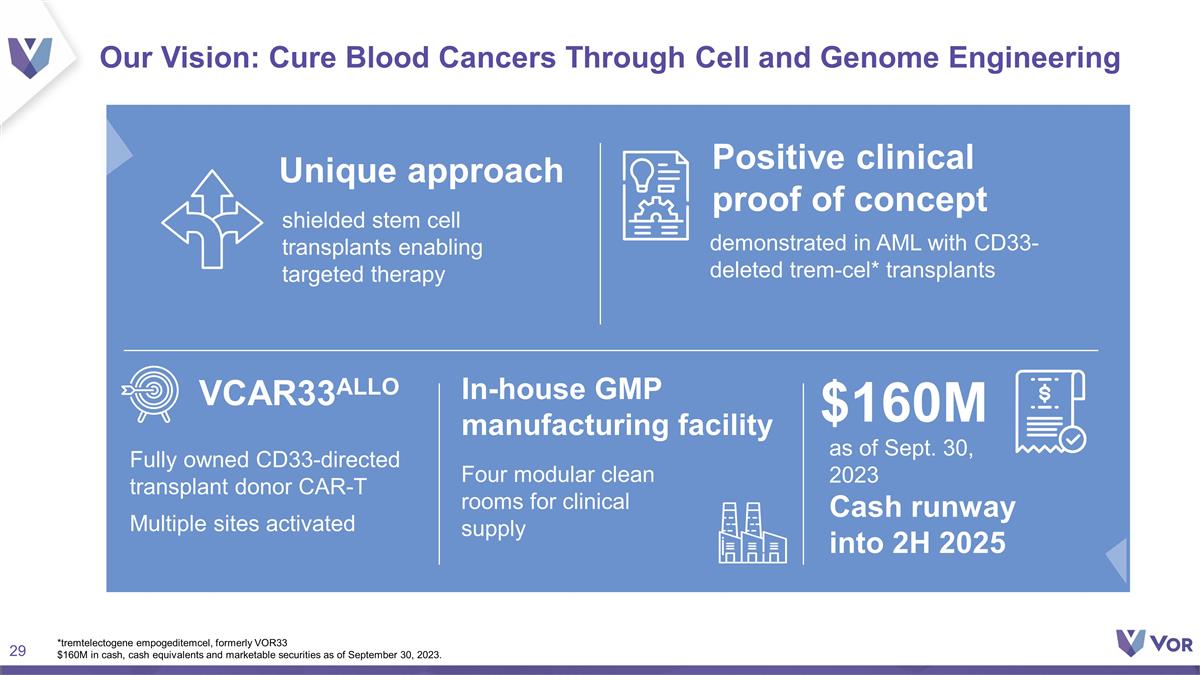
Our Vision: Cure Blood Cancers
Through Cell and Genome Engineering Unique approach Positive clinical proof of concept VCAR33ALLO as of Sept. 30, 2023 Cash runway into 2H 2025 $160M In-house GMP manufacturing facility *tremtelectogene empogeditemcel, formerly VOR33 $160M in
cash, cash equivalents and marketable securities as of September 30, 2023. Fully owned CD33-directed transplant donor CAR-T Multiple sites activated Four modular clean rooms for clinical supply shielded stem cell transplants enabling targeted
therapy demonstrated in AML with CD33-deleted trem-cel* transplants


Experienced and Passionate
Leadership Team Deep Cell & Gene Therapy Expertise Robert Ang, MBBS, MBA President and CEO Tirtha Chakraborty, PhD Chief Scientific Officer Eyal Attar, MD Chief Medical Officer Tania Philipp Chief People Officer John King, MBA Chief Commercial
Officer & Head of Business Development Nathan Jorgensen, PhD MBA Chief Financial Officer Robert Pietrusko, PharmD Chief Regulatory & Quality Officer David Phillips, MBA Senior Vice President, Head of Quality Samir Vattompadam, MS Senior Vice
President, Portfolio Strategy and Program Management
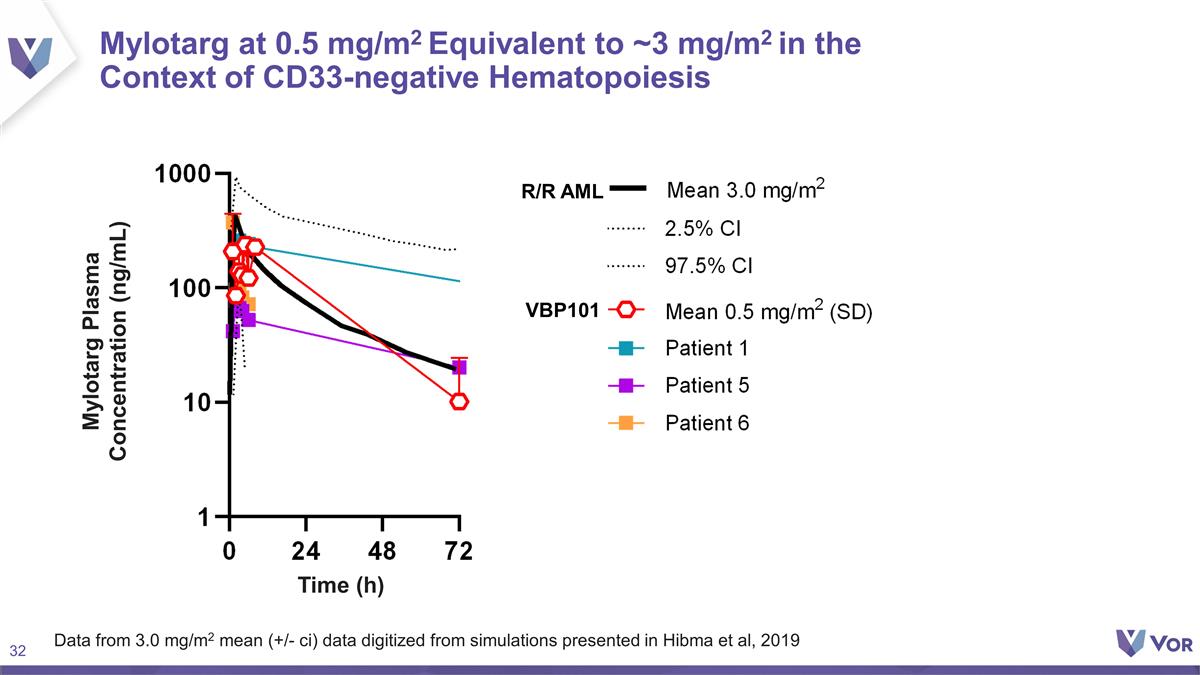
Data from 3.0 mg/m2 mean (+/- ci)
data digitized from simulations presented in Hibma et al, 2019 VBP101 R/R AML Mylotarg at 0.5 mg/m2 Equivalent to ~3 mg/m2 in the Context of CD33-negative Hematopoiesis Mylotarg Plasma Concentration (ng/mL) Time (h)
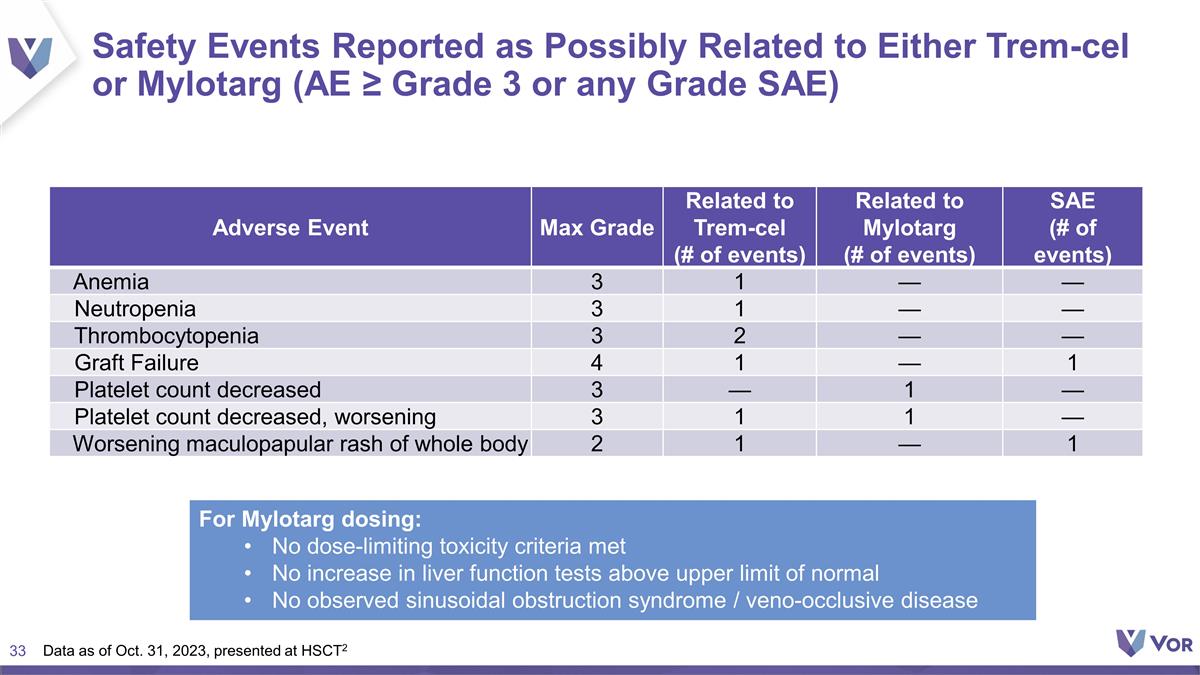
Safety Events Reported as Possibly
Related to Either Trem-cel or Mylotarg (AE ≥ Grade 3 or any Grade SAE) Adverse Event Max Grade Related to Trem-cel (# of events) Related to Mylotarg (# of events) SAE (# of events) Anemia 3 1 — — Neutropenia 3 1 — —
Thrombocytopenia 3 2 — — Graft Failure 4 1 — 1 Platelet count decreased 3 — 1 — Platelet count decreased, worsening 3 1 1 — Worsening maculopapular rash of whole body 2 1 — 1 For Mylotarg dosing: No
dose-limiting toxicity criteria met No increase in liver function tests above upper limit of normal No observed sinusoidal obstruction syndrome / veno-occlusive disease Data as of Oct. 31, 2023, presented at HSCT2
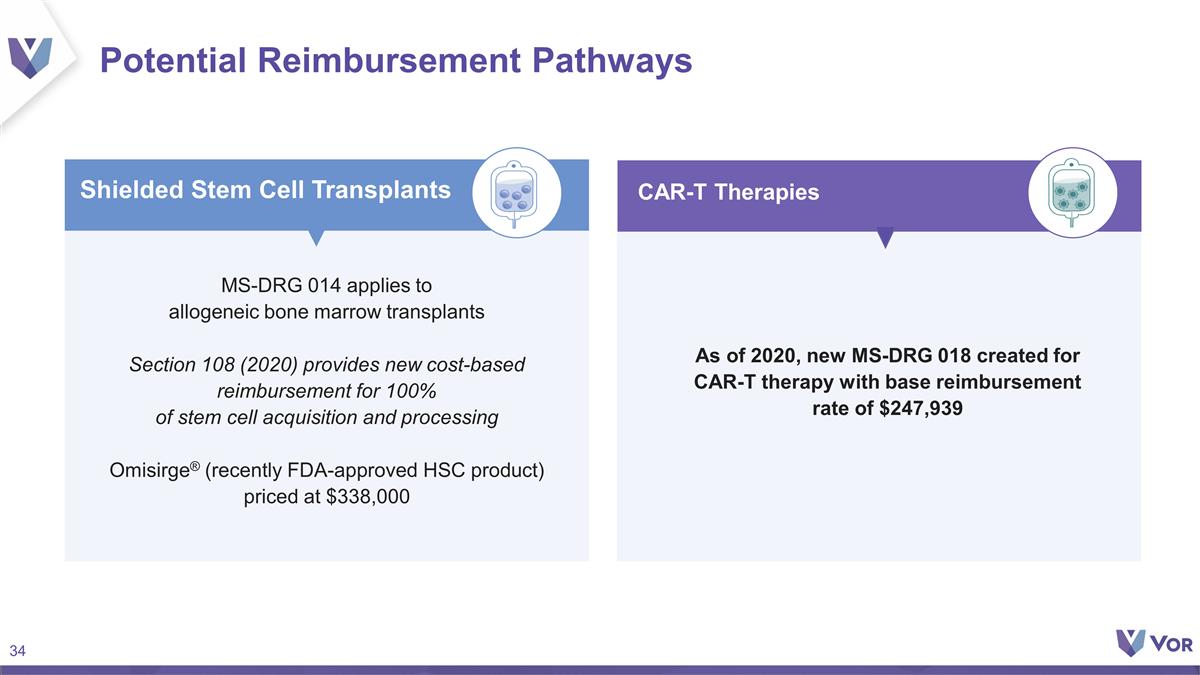
Potential Reimbursement Pathways
MS-DRG 014 applies to allogeneic bone marrow transplants Section 108 (2020) provides new cost-based reimbursement for 100% of stem cell acquisition and processing Omisirge® (recently FDA-approved HSC product) priced at $338,000 Shielded Stem
Cell Transplants As of 2020, new MS-DRG 018 created for CAR-T therapy with base reimbursement rate of $247,939 CAR-T Therapies
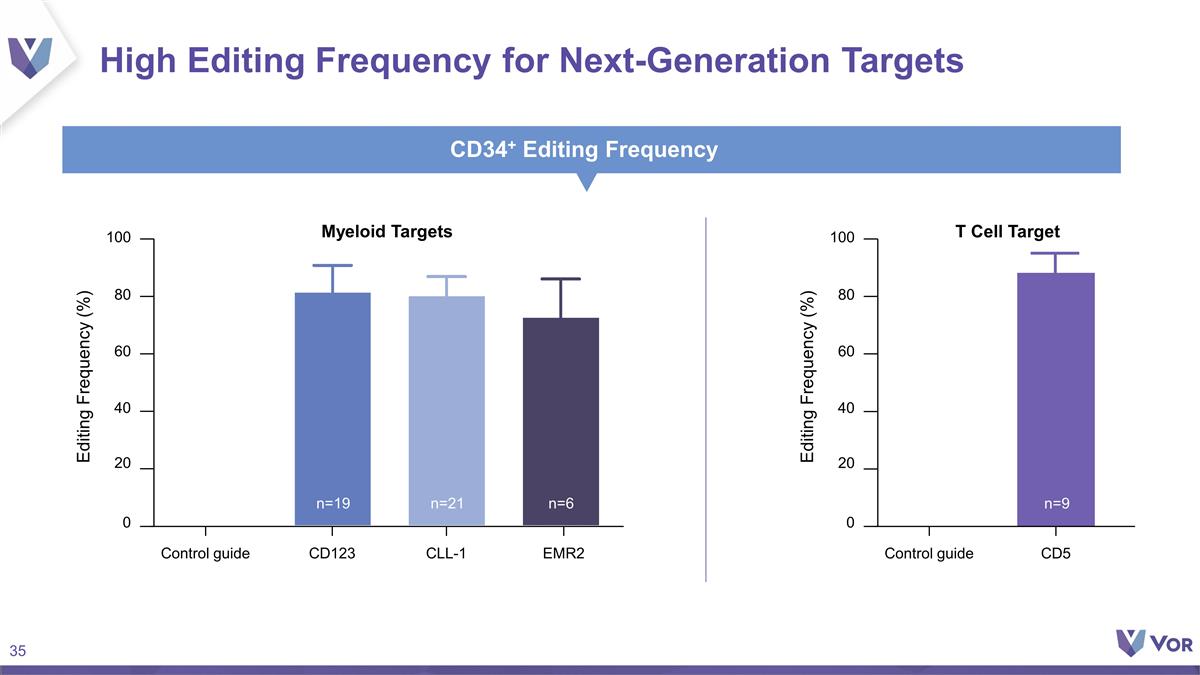
High Editing Frequency for
Next-Generation Targets CD34+ Editing Frequency CD5 0 40 80 100 20 60 Editing Frequency (%) Control guide T Cell Target n=9 CD123 0 40 80 100 20 60 Editing Frequency (%) Control guide Myeloid Targets n=19 n=21 n=6 CLL-1 EMR2
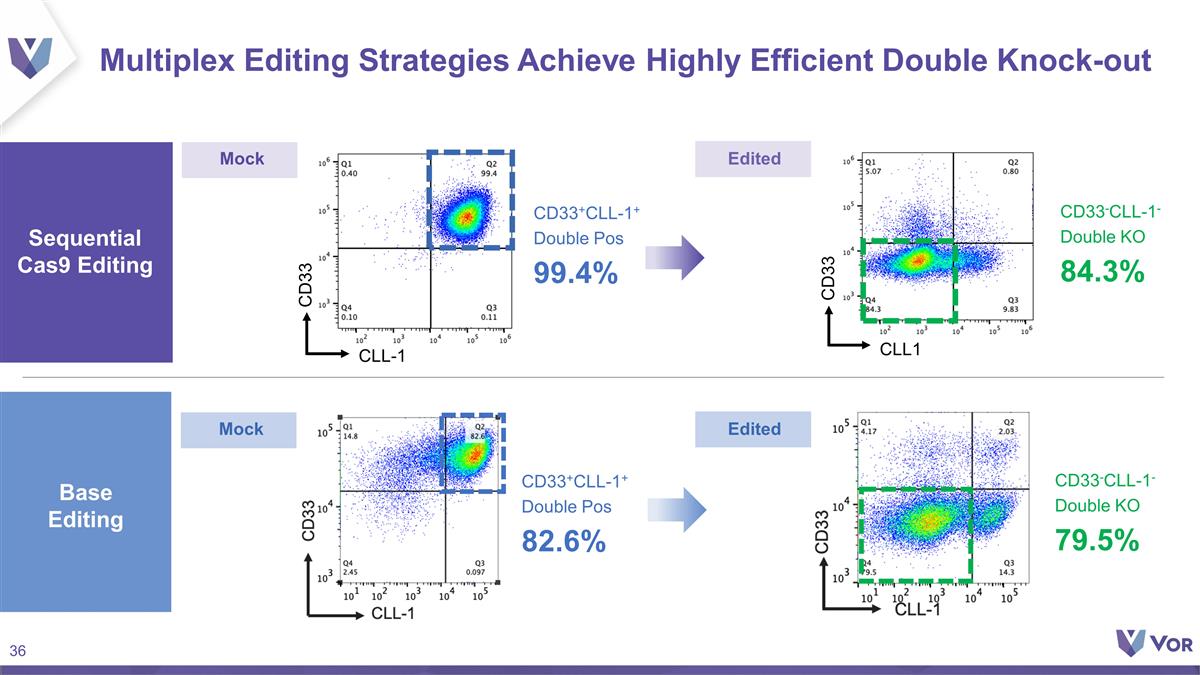
Multiplex Editing Strategies
Achieve Highly Efficient Double Knock-out CLL1 CD33 CLL-1 CD33 CD33-CLL-1- Double KO 79.5% CD33+CLL-1+ Double Pos 82.6% Sequential Cas9 Editing Base Editing Mock Mock Edited Edited CD33+CLL-1+ Double Pos 99.4% CD33-CLL-1- Double KO 84.3%
v3.23.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Vor Biopharma (NASDAQ:VOR)
Gráfica de Acción Histórica
De Dic 2024 a Ene 2025

Vor Biopharma (NASDAQ:VOR)
Gráfica de Acción Histórica
De Ene 2024 a Ene 2025
