Y-mAbs Therapeutics New Drug Application Gets FDA Clearance
17 Octubre 2023 - 5:01PM
Noticias Dow Jones
By Denny Jacob
Y-mAbs Therapeutics' investigational new drug application for
CD38-SADA was cleared by the Food and Drug Administration.
The commercial-stage biopharmaceutical company said CD38-SADA is
its second program within its self-assembly disassembly pre-target
radioimmunotherapy theranostic platform. The Phase 1 trial is
investigating the safety and tolerability of the CD38-SADA: (177)
Lu-DOTA Drug Complex in patients with relapsed or refractory
non-Hodgkin lymphoma.
Interim Chief Executive Thomas Gad said the FDA's clearance
marks the second program utilizing its SADA technology platform to
enter clinic development within just 15 months.
Y-mAbs said it expects to dose the first patient in its Phase 1
trial in 2024.
Write to Denny Jacob at denny.jacob@wsj.com
(END) Dow Jones Newswires
October 17, 2023 17:46 ET (21:46 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
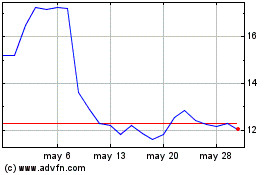
Y mAbs Therapeutics (NASDAQ:YMAB)
Gráfica de Acción Histórica
De May 2024 a Jun 2024
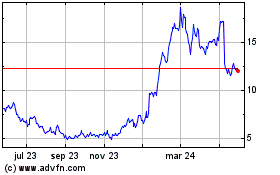
Y mAbs Therapeutics (NASDAQ:YMAB)
Gráfica de Acción Histórica
De Jun 2023 a Jun 2024