NASDAQ false 0001832415 0001832415 2024-03-11 2024-03-11
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): March 11, 2024
BETTER THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
| Delaware |
|
001-39864 |
|
85-3472546 |
| (State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification No.) |
|
|
|
| 548 Market Street #49404 |
|
|
| San Francisco, California |
|
94104 |
| (Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: (415) 887-2311
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock |
|
BTTX |
|
Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 |
Regulation FD Disclosure. |
On March 11, 2024, Better Therapeutics, Inc. (the “Company”) posted an investor presentation to its website at https://investors.bettertx.com/news-events/events-presentations. A copy of the investor presentation is furnished herewith as Exhibit 99.1.
The information contained in Item 7.01 in this Current Report on Form 8-K and Exhibit 99.1 attached hereto is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing.
Forward-Looking Statements
Certain statements made in the investor presentation are “forward-looking statements” within the meaning of the safe harbor provisions under the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements are typically identified by words such as “plan,” “believe,” “expect,” “anticipate,” “intend,” “outlook,” “estimate,” “forecast,” “project,” “continue,” “could,” “may,” “might,” “possible,” “potential,” “predict,” “should,” “would” and other similar words and expressions, but the absence of these words does not mean that a statement is not forward-looking. These forward-looking statements involve a number of risks, uncertainties or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements including: risks related to commercial distribution, market acceptance and insurance reimbursement of AspyreRx; the Company’s ability to raise capital in the near term to fund its operations and continue as a going concern; the Company’s need to seek strategic alternatives in the event the company is unable to raise capital, including, without limitation, potential sale of its assets or business, assignment for the benefit of creditors or wind-down of the company; the Company’s ability to comply with ongoing covenants under its Hercules Capital debt facility (including the minimum cash covenant) and potential default and foreclosure under the debt facility; the outcome of the Company’s delisting hearing with Nasdaq appeals panel and potential delisting from the Nasdaq Capital Market, and other risks and uncertainties included under the header “Risk Factors” in the Company’s quarterly report on Form-10-Q for the fiscal quarter ended September 30, 2023 filed with the Securities and Exchange Commission (“SEC”) on November 09, 2023, and those that are included in any of the Company’s subsequent filings with the SEC.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
Better Therapeutics, Inc. |
|
|
|
|
| Dated: March 11, 2024 |
|
|
|
By: |
|
/s/ Frank Karbe |
|
|
|
|
Name: |
|
Frank Karbe |
|
|
|
|
Title: |
|
Chief Executive Officer |

Exhibit 99.1 Pioneering Prescription Digital Therapeutics for
Cardiometabolic Diseases March 2024 Property of Better Therapeutics, Inc. Not for distribution.

Disclaimer This presentation (“Presentation”) is for
informational purposes only. The information contained herein does not purport to be all-inclusive and neither Better Therapeutics, Inc. (“BetterTX” or the Company“) nor any of its respective affiliates nor any of its or their
control persons, officers, directors, employees or representatives makes any representation or warranty, express or implied, as to the accuracy, completeness or reliability of the information contained in this Presentation. You should consult your
own counsel and tax and financial advisors as to legal and related matters concerning the matters described herein, and, by accepting this Presentation, you confirm that you are not relying upon the information contained herein to make any decision.
The reader shall not rely upon any statement, representation or warranty made by any other person, firm or corporation in making its investment or decision to invest in the Company. Neither the Company nor any of its respective affiliates nor any of
its or their control persons, officers, directors, employees or representatives, shall be liable to the reader for any information set forth herein or any action taken or not taken by any reader, including any investment in shares of the Company.
Certain information contained in this Presentation relates to or is based on studies, publications, surveys and the Company’s own internal estimates and research. In addition, all of the market data included in this Presentation involves a
number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while the Company believes its internal research is reliable, such research has not been verified by any independent
source. This meeting and any information communicated at this meeting are strictly confidential and should not be discussed outside your organization. Forward-LookingStatements Certain statements in this Presentation may be considered
forward-looking statements, within the meaning of the safe harbor provisions under the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements are typically identified by words such as “plan,”
“believe,” “expect,” “anticipate,” “intend,” “outlook,” “estimate,” “forecast,” “project,” “continue,” “could,”
“may,” “might,” “possible,” “potential,” “predict,” “should,” “would” and other similar words and expressions, but the absence of these words does not mean that a
statement is not forward-looking. The forward-looking statements in this Presentation include, but are not limited to, statements regarding the delivery of cognitive behavioral therapy and/or prescription digital therapeutics by the Company to
address the root causes of type 2 diabetes and other cardio metabolic diseases, development of a proprietary platform and software-based solutions for treatment of type 2 diabetes, heart disease and other conditions, achievement of changes in neural
pathways of the brain and lasting changes in behavior through cognitive behavioral therapy delivered by the Company’s PDT, the capability of the Company to address the underlying causes of certain diseases and its related potential to improve
patient health while lowering healthcare costs, the results of the trial of BT-001 in patients with type 2 diabetes, the Company's plans regarding FDA submissions, plans and expectations regarding the commercialization of AspyreRx, expectations
related to the potential benefits of AspyreRx and CBT and their potential treatment applications, the Company's plans regarding the research and advancement of its product candidates for additional treatments, expectations related to the interest of
healthcare providers and payers in PDTs, including AspyreRx, the future financial stability, strength, or success of the Company, and legislative developments affecting PDTs and the outcome of such developments, the Company’s expectations
about potential business development and royalty deals, expectations and assumptions regarding the addressable market, covered lives, market penetration, prescription numbers and fill rates for AspyreRx, expectations regarding peak revenue, the
Company’s expectations about the gap in its current enterprise value and its commercial opportunity and the prospects for any near-term return, among others. These forward-looking statements are based on the current expectations of the
management of the Company and are inherently subject to uncertainties and changes in circumstances and their potential effects and speak only as of the date of such statement. There can be no assurance that future developments will be those that
have been anticipated. These forward-looking statements involve a number of risks, uncertainties or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking
statements including: risks related to the Company's business, such as the willingness of the FDA to authorize PDTs, for commercial distribution and insurance companies to reimburse their use, market acceptance of PDTs, including AspyreRx, the risk
that the results of previously conducted studies will not be repeated or observed in ongoing or future studies involving our product candidates; Better Therapeutics’ ability to raise capital in the near term to fund its operations and continue
as a going concern; Better Therapeutics’ need to seek strategic alternatives in the event the company is unable to raise capital, including, without limitation, potential sale of its assets or business, assignment for the benefit of creditors
or wind-down of the company; Better Therapeutics’ ability to comply with ongoing covenants under its Hercules Capital debt facility (including the minimum cash covenant) and potential default and foreclosure under the debt facility; the
outcome of Better Therapeutics’ delisting hearing with Nasdaq appeals panel and potential delisting from the Nasdaq Capital Market, and other risks and uncertainties included under the header “Risk Factors” in the Company’s
quarterly report on Form-10-Q for the fiscal quarter ended September 30, 2023 filed with the Securities and Exchange Commission (“SEC”) on November 9, 2023, and those that are included in any of the Company’s subsequent filings
with the SEC. 2

Our mission is to advance human health through the power of behavior
change. Many of the most common (and costly) chronic diseases share lifestyle behaviors that contribute to disease progression. Better Therapeutics combines medical, behavioral and data sciences to develop clinically proven software-based
therapeutics targeting behavior change at scale. We seek to make societies healthier and meaningfully reduce healthcare costs around the world.

Better Therapeutics is poised to create the first, multi-billion dollar
Prescription Digital Therapeutics company • Type 2 Diabetes is the most expensive chronic condition in the US ($400B+ annually) and its cost and prevalence continue to increase; despite advances in drug treatments, approximately
50%ofdiabetespatientsremainuncontrolled TM • AspyreRx is the first and only cognitive behavioral therapy (CBT) mobile app to receive FDAauthorizationtotreattherootcauseoftype2diabetes • Digital Only” treatment enables unique value
proposition & rapid deployment to large populationswithguaranteedROI • AspyreRx proven to reduce A1c – a universally accepted, easily measurable endpoint in type 2 diabetes, based on a large randomized controlled trial that also
demonstrated weight loss, BP reduction,reduceduseofconcomitantmedicationsandlowerAE'svs.controlarm • AspyreRx could save insurers money by improving patient outcomes and by reducing or delayingtheadditionofmoreexpensivemedicationssuchasGLP-1
• Ourteamiscomposedofseniorindustryveteranswithexperiencelaunchingnovelproducts • AspyreRx launched in Q4 2023; we have the potential to rapidly drive commercial traction and demonstrate $1 bn+ commercial potential with a go-to-market
strategy that builds on our core advantageofscalabilityandbroadaccesstoofferahighimpactpopulationhealthsolution 4

LEADERSHIP TEAM Frank Karbe David Perry Mark Berman Jessica Meng Kristin
Wynholds Chief Executive Co-Founder & Chief Medical Officer Chief Commercial Officer Chief Product Officer Officer Executive Chairman 5

Empowering people with type 2 diabetes Clinically validated via a
randomized controlled trial against established TM AspyreRx is the first cognitive clinical endpoint (A1c) behavioral therapy mobile app “Digital Only” treatment enables unique to receive FDA authorization to value proposition &
rapid deployment to large populations with guaranteed ROI treat type 2 diabetes in adults Effective, broadly accessible & affordable treatment intervention Widely available in EHR to prescribe 6
Watch an AspyreRx product demo video at www.AspyreRx.com

The Problem Better Therapeutics Approach How our Product Works Contents
Clinical Evidence Go-to-Market Plan NASDAQ Compliance 9

% % T 60 86 $ 4.1 of adults in the US of healthcare dollars are spent on
US National Health 2 2 have a chronic disease 1 chronic disease maintenance Expenditure in 2020 Currently available drugs treat symptoms but do not impact the behaviors that contribute to disease progression. Most patients get worse over time
despite being on multiple prescription drugs 10 Sources: 1. Centers for Medicare and Medicaid Services; 2. Centers for Disease Control and Prevention

Diabetes is the most expensive chronic condition in the US and
prevalence 1,2,3 continues to rapidly increase despite advances in pharmacotherapy Total US Healthcare Expenditures ($ in trillions) Americans Diagnosed with Diabetes (millions) 40 $4 40 Prevalence projected to increase 30 30 $3 % % 25 of total US
healthcare 54 20 $2 20 expenditure attributed between 2015 and 1,2 to diabetes 2030 to more than 3 55M patients 10 $1 10 0 1965 1975 1985 1995 2005 2015 1) 2) Sources: American Diabetes Association. Economic costs of diabetes in the US in 2017.
Diabetes Care. 2018;41(5):917–928. Dieleman JL, Baral R, Birger M, et al. US spending on 2) 3) personal health care and public health, 1996–2013. JAMA. 2016;316(24):2627–2646. Centers for Disease Control and Prevention Rowley WR,
Bezold C, Arikan Y, Byrne E, Krohe S. 11 Diabetes 2030: Insights from Yesterday, Today, and Future Trends. Popul Health Manag. 2017 Feb;20(1):6-12. doi: 10.1089/pop.2015.0181. Epub 2016 Apr 28. PMID: 27124621; PMCID: PMC5278808.

The vast majority of patients diagnosed with cardiometabolic diseases
progress in their disease, leading to more costly complications and interventions over time Example: Type 2 Diabetes Typical Disease Progression Additional Advanced Insulin Non-Insulin Pre-Diabetes Diagnosis Non-Insulin Comorbidities Treatment
Treatment Treatments Incremental cost $2,000 $10,000 $19,000 $35,000+ per patient per year LIFESTYLE DUAL THERAPY TRIPLE THERAPY STEP UP TO INSULIN FIRST LINE CHANGES TREATMENT Lifestyle Changes Lifestyle Changes Lifestyle Changes Changes to
Lifestyle Changes + Metformin + Metformin + Metformin exercise and diet + Metformin + Sulfonylurea + GLP-1 + GLP-1 + SGLT2 + SGLT2 + Insulin 12

Current clinical guidelines highlight behavior change as the foundation
of treatment in type 2 diabetes CBT is considered the Gold Standard to help patients make lasting behavior changes by changing neural pathways in the brain Traditional CBT is also proven to be effective at addressing the behavioral root causes of
many cardiometabolic diseases But traditional CBT has many limitations as it is neither scalable, broadly accessible, standardized or affordable Other approaches targeting behavior change rely on care provider intervention as an integral part of
treatment and lack efficient scalability Current approaches generally lack solid clinical validation and FDA oversight to give healthcare providers confidence to implement 13

The Problem Better Therapeutics Approach How our Product Works Contents
Clinical Evidence Go-to-Market Plan NASDAQ Compliance 14
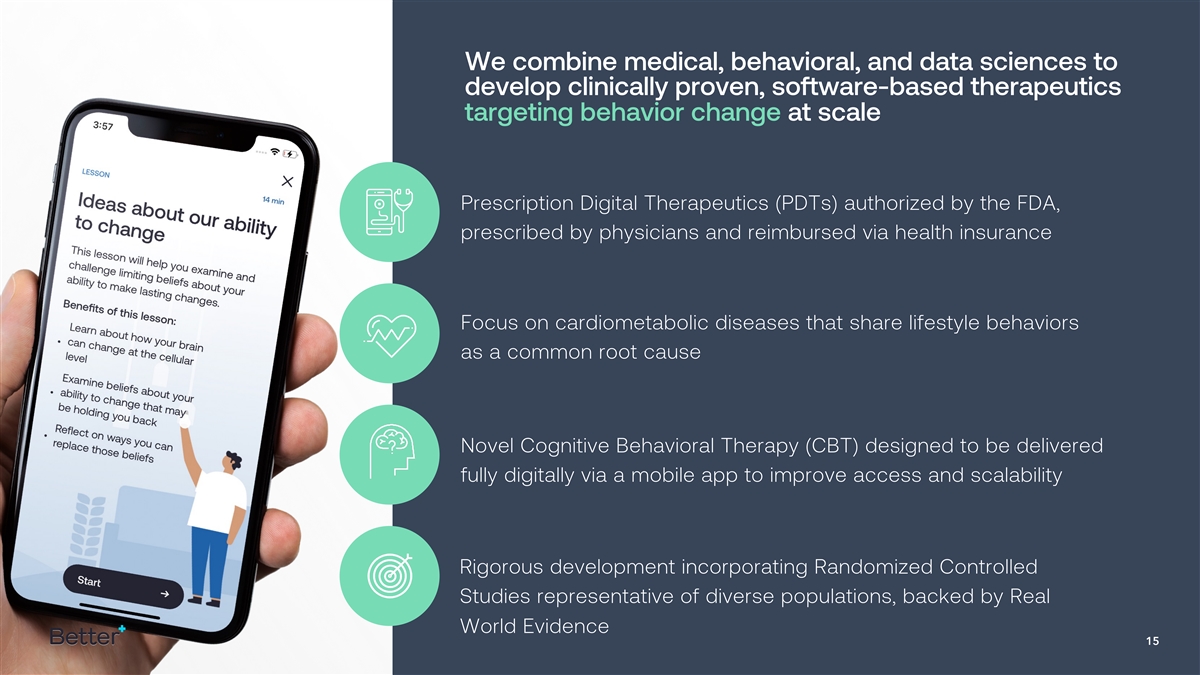
We combine medical, behavioral, and data sciences to develop clinically
proven, software-based therapeutics targeting behavior change at scale Prescription Digital Therapeutics (PDTs) authorized by the FDA, prescribed by physicians and reimbursed via health insurance Focus on cardiometabolic diseases that share
lifestyle behaviors as a common root cause Novel Cognitive Behavioral Therapy (CBT) designed to be delivered fully digitally via a mobile app to improve access and scalability Rigorous development incorporating Randomized Controlled Studies
representative of diverse populations, backed by Real World Evidence 15

High Strength of Evidence Rx Behavioral Therapeutic COMPETITIVE
LANDSCAPE TM AspyreRx is the first and only Regulatory Threshold CBT mobile app to receive FDA authorization to treat a metabolic disease Glucose Monitoring / Insulin Management It is also the only T2D treatment option delivered entirely digitally,
unlocking a unique Services Products value proposition, as well as scalability and accessibility Consumer Apps for Tracking and Monitoring Diabetes Management No other product can claim to be a fully scalable, FDA-backed therapy that delivers the
gold- standard cognitive behavioral DTC therapy for type 2 diabetes Behavioral Therapy +360,000 health and wellness apps Low Strength 1 6 of Evidence

Our platform is grounded in behavioral theory and developed by
experienced designers and engineers in collaboration with clinical experts • 8 years of design, testing, and iteration 6,244 • 6,244 patients participated in cohort studies, usability studies, and RCTs • Product evolution from
human to software-led intervention, informed by 5 FDA interactions Patients BT-001 Initial Usability (T2D) 199 3,062 BT-001 Pivotal Trial (T2D) 327 a Usability Study (T2D, HTN, HLD, NAFLD) 229 BT-001 Usability Diabetes Cohort Validation Study a 238
Study (T2D) (T2D) 473 448 BT-001 Payer Employee 23 95 a RWE Studies 885 Cohort Study BT-002 Metabolic Health (T2D) (T2D, HTN, HLD) a “Hyper” LivVita Cohort Study Study (Obesity) 265 a Cohort Study (NASH/NAFLD) Payer Cohort (HTN, HLD,
T2D) a Study (T2D) 2016 2017 2018 2019 2020 2021 2022 2023 Mobile-App V2 V4 V6 V7 Web-App V3 V5 V1 a Study used a combination of virtual health coaching/behavioral counseling + software. 17 Definitions: T2D: Type 2 Diabetes; HTN: Hypertension; HLD:
Hyperlipidemia; NAFLD: Nonalcoholic Fatty Liver Disease

Hypertension (High blood pressure) Our DTx platform is designed to be
highly 70 million Type 2 Diabetes people suffering and rapidly scalable (High blood sugar) across large disease $30 billion 35 million people suffering Rx drug spending states because most cardiometabolic $52 billion Root Causes diseases are caused
Rx drug spending • Poor diet and driven by the • Sedentary lifestyle • Stress same behaviors • Poor sleep Hyperlipidemia • Alcohol, Tobacco (High cholesterol) With moderate platform NASH / NAFLD (Non-alcoholic fatty
liver disease) modifications, we can pursue a 40 million people suffering broad range of potential 64 million indications people suffering $28 billion Rx drug spending $100 billion 18 Direct Healthcare Costs
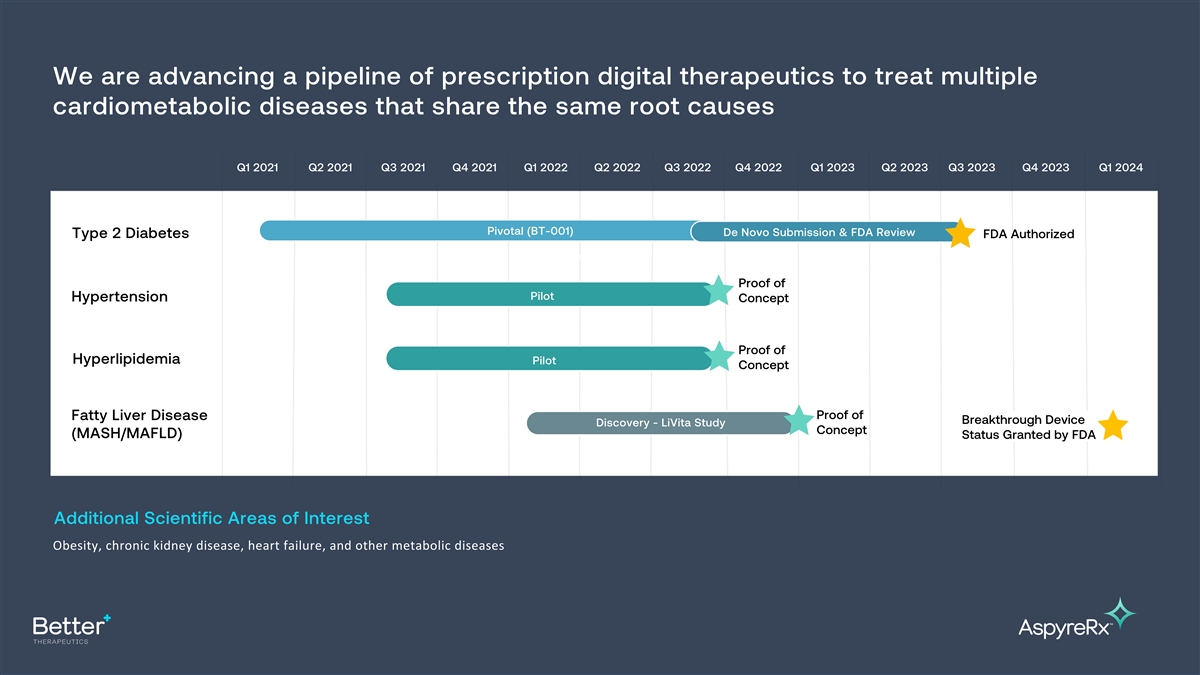
We are advancing a pipeline of prescription digital therapeutics to
treat multiple cardiometabolic diseases that share the same root causes Q1 2021 Q2 2021 Q3 2021 Q4 2021 Q1 2022 Q2 2022 Q3 2022 Q4 2022 Q1 2023 Q2 2023 Q3 2023 Q4 2023 Q1 2024 Pivotal (BT-001) De Novo Submission & FDA Review Type 2 Diabetes FDA
Authorized Pilot Proof of Pilot Hypertension Concept Proof of Hyperlipidemia Pilot Concept Proof of Fatty Liver Disease Breakthrough Device Discovery - LiVita Study Concept (MASH/MAFLD) Status Granted by FDA Additional Scientific Areas of Interest
Obesity, chronic kidney disease, heart failure, and other metabolic diseases

The Problem Better Therapeutics Approach How our Product Works Contents
Clinical Evidence Go-to-Market Plan NASDAQ Compliance 20
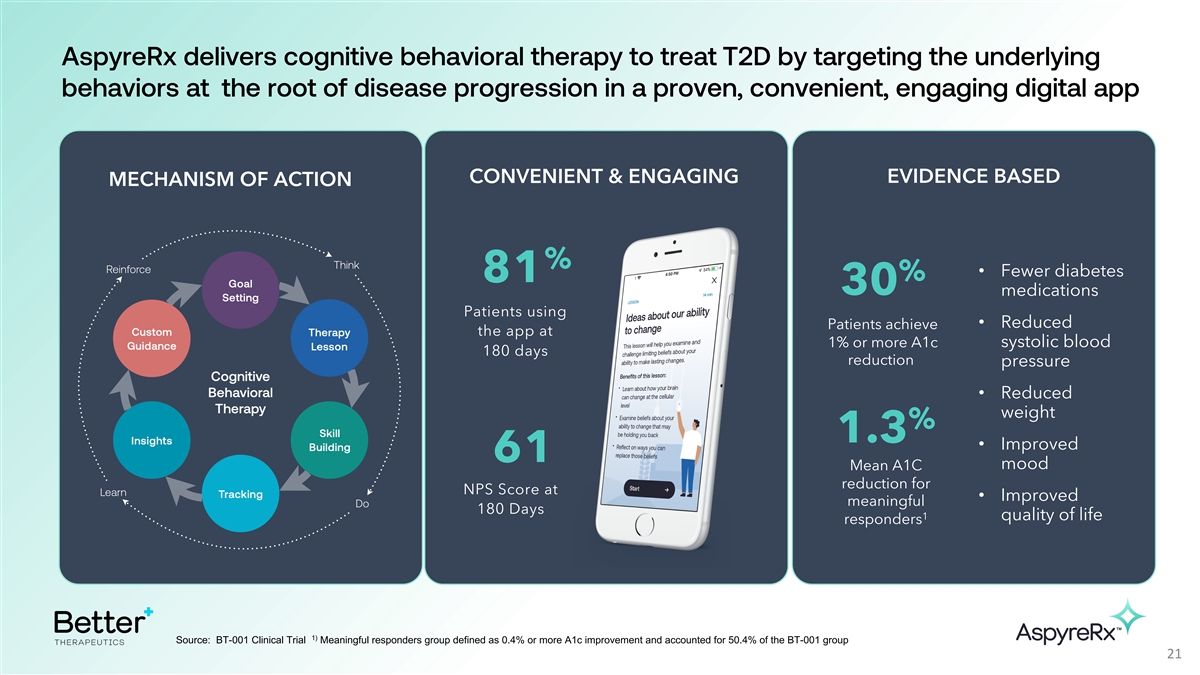
AspyreRx delivers cognitive behavioral therapy to treat T2D by
targeting the underlying behaviors at the root of disease progression in a proven, convenient, engaging digital app EVIDENCE BASED CONVENIENT & ENGAGING MECHANISM OF ACTION % • Fewer diabetes % 81 30 medications Patients using •
Reduced Patients achieve the app at 1% or more A1c systolic blood 180 days reduction pressure • Reduced weight % 1.3 • Improved 61 mood Mean A1C reduction for NPS Score at • Improved meaningful 180 Days 1 quality of life responders
1) Source: BT-001 Clinical Trial Meaningful responders group defined as 0.4% or more A1c improvement and accounted for 50.4% of the BT-001 group 21

AspyreRx leverages CBT principles to target thoughts and emotions that
underlie a complex set of diabetes self-care behaviors Therapy Lessons help patients identify existing thoughts Solve Identify Helpful Therapy Problems and beliefs about their current diabetes self-care Ideas / Cope with Lessons behaviors and
introduces adaptive thoughts that can / Beliefs Emotions lead to positive behavioral changes Skills help patients by improving their capacity to solve Skill problems, and cope with interfering thoughts and Building emotions Goal Setting Goal Setting
instructs patients to set goals for key daily behaviors, biometric tracking, and completion of core Build CBT components, such as lessons Self-Efficacy 22

Supportive features enhance Tracking & Progress the CBT experience
Product Support • Tracking of key type 2 diabetes biometrics to show purposeful progress Therapy • Biometric notifications are in place for Lessons safety and tells patients when they need to talk to their doctor Skill • Nudges
remind and encourage patients to Biometric continue their journey Building Notifications • Rewards and acknowledgement of Goal progress keep patients motivated and Setting engaged • Automated, customized product support is there to help
patients if they have any Rewards issues Nudges 23

The Problem Better Therapeutics Approach How our Product Works Contents
Clinical Evidence Go-to-Market Plan NASDAQ Compliance 24
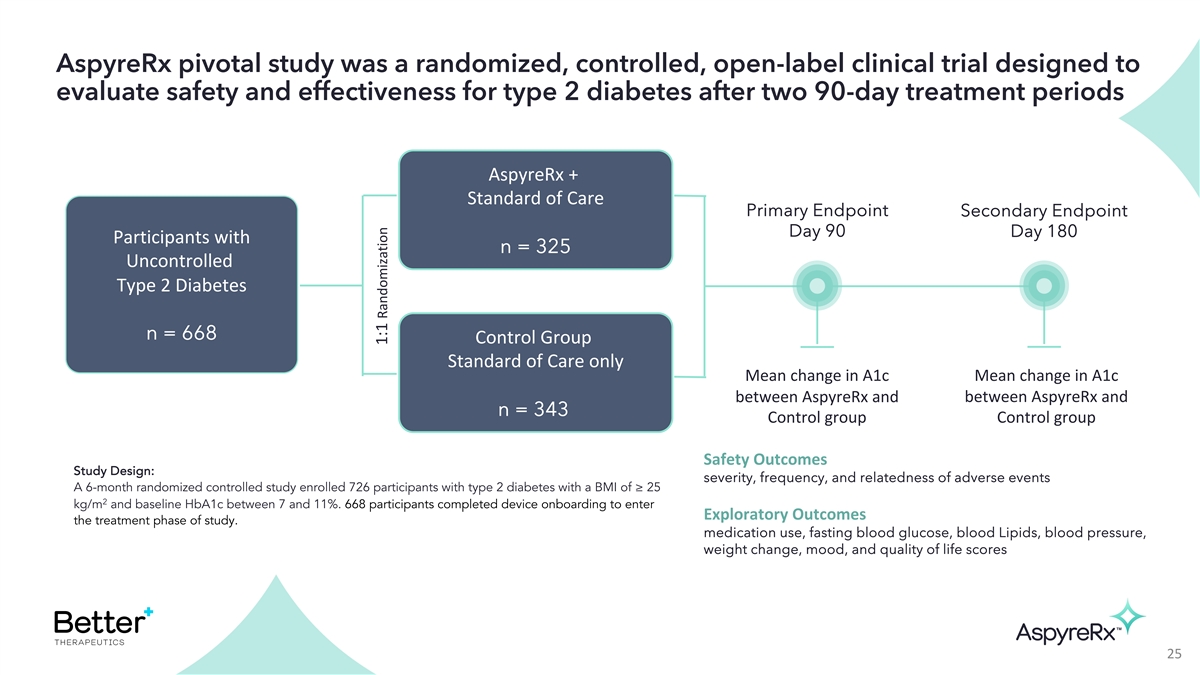
AspyreRx pivotal study was a randomized, controlled, open-label
clinical trial designed to evaluate safety and effectiveness for type 2 diabetes after two 90-day treatment periods AspyreRx + Standard of Care Primary Endpoint Secondary Endpoint Day 90 Day 180 Participants with n = 325 Uncontrolled Type 2 Diabetes
n = 668 Control Group Standard of Care only Mean change in A1c Mean change in A1c between AspyreRx and between AspyreRx and n = 343 Control group Control group Safety Outcomes Study Design: severity, frequency, and relatedness of adverse events A
6-month randomized controlled study enrolled 726 participants with type 2 diabetes with a BMI of ≥ 25 2 kg/m and baseline HbA1c between 7 and 11%. 668 participants completed device onboarding to enter Exploratory Outcomes the treatment phase
of study. medication use, fasting blood glucose, blood Lipids, blood pressure, weight change, mood, and quality of life scores 25 1:1 Randomization

Broad eligibility criteria and decentralized recruitment ensured a
nationally representative, diverse population enrolled across 6 U.S. States ~ 40% Zip Codes Represented Non-White 44% 56% Men Women ~ 30% Black 588 ~ 17% Latinx Without Median Household 98% Income College Degree Lived in low-income or medium-income
~ 40% ~ 68K neighborhoods 26

Control Intervention The AspyreRx pivotal study was No Glycemic
Equipoise designed to test a real-world, difficult- to-treat population ++ ++ Key attributes allowing for imbalance of medication use: • Open-label A1c review at Day 90 and Day 180 Medications (+): Fixed throughout study • Study
requirement to adjust medications per standard of care guidelines • Poorly controlled T2D at baseline Glycemic Equipoise Key attributes favoring real-world conditions: Control Intervention • Robust background therapy allowed ++++ ++
• Patients with multiple comorbidities and advanced disease included TM • No study requirement to use the CBT within AspyreRx Medications (+): Not fixed, therefore control can respond to higher A1c by increasing meds Property of Better
Therapeutics, Inc. Not for distribution. 27

AspyreRx demonstrated clinically meaningful and statistically
significant reductions in A1c of 0.4% vs. Control at 90 days 0.2 1.5x more SOC patients 0.1 increased medications prior AspyreRx continued 0.1 to Day 180 to provide statistically 0 significant reductions -0.1 in A1c of 0.3% vs. Primary Endpoint -0.1
Control at 180 days 0.4% Delta p < 0.001 -0.2 Secondary Endpoint 0.3% Delta p = 0.01 -0.3 Control -0.3 -0.4 -0.4 AspyreRx Baseline Day 90 Day 180 28 % Mean Change in HbA1c
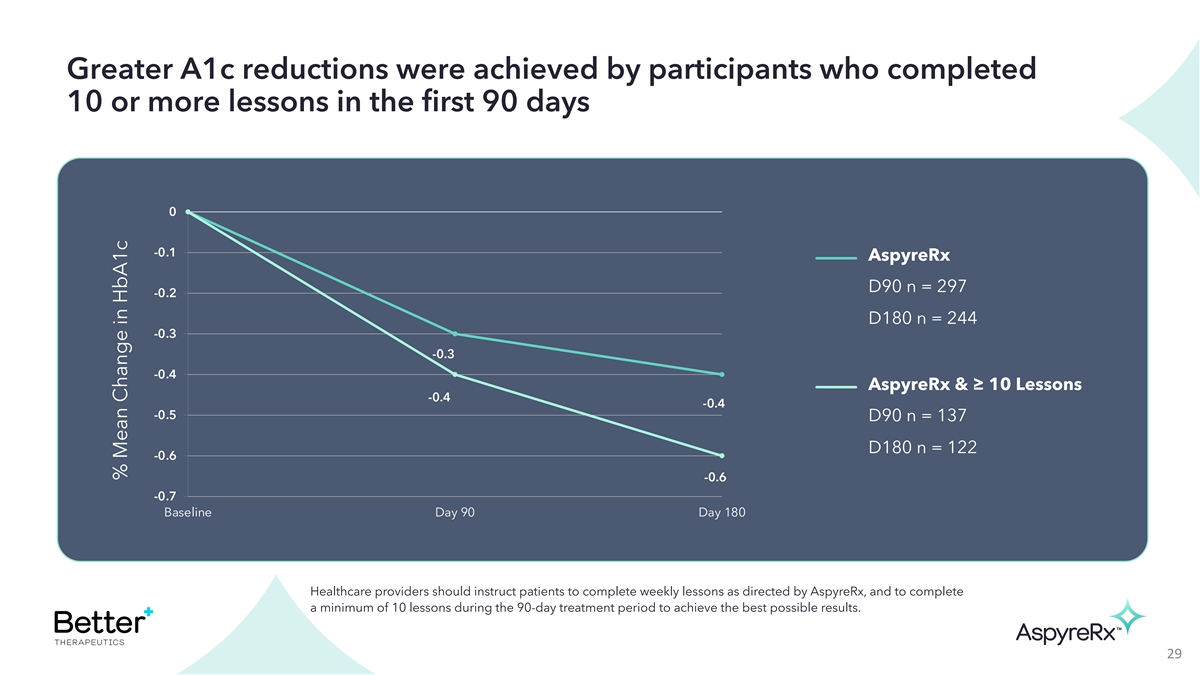
Greater A1c reductions were achieved by participants who completed 10
or more lessons in the first 90 days 0 -0.1 AspyreRx D90 n = 297 -0.2 D180 n = 244 -0.3 -0.3 -0.4 AspyreRx & ≥ 10 Lessons -0.4 -0.4 -0.5 D90 n = 137 D180 n = 122 -0.6 -0.6 -0.7 Baseline Day 90 Day 180 Healthcare providers should instruct
patients to complete weekly lessons as directed by AspyreRx, and to complete a minimum of 10 lessons during the 90-day treatment period to achieve the best possible results. 29 % Mean Change in HbA1c

Meaningful Responders saw a mean decrease of -1.3% HbA1c over 180 days*
Meaningful Responders defined as > 0.4% A1c improvement and accounted for 50.4% of the AspyreRx group 14 12 MEDIAN MEAN -1.1% -1.3% 10 8 6 4 2 0 -0.4 -0.5 -0.6 -0.7 -0.8 -0.9 -1 -1.1 -1.2 -1.3 -1.4 -1.5 -1.6 -1.7 -1.8 -1.9 -2 -2.2 -2.3 -2.7 -2.8
-2.9 -3.1 < -3.3 D180 A1c Change From Baseline (% HbA1c) *Data on file: Better Therapeutics 30 % of AspyreRx patients
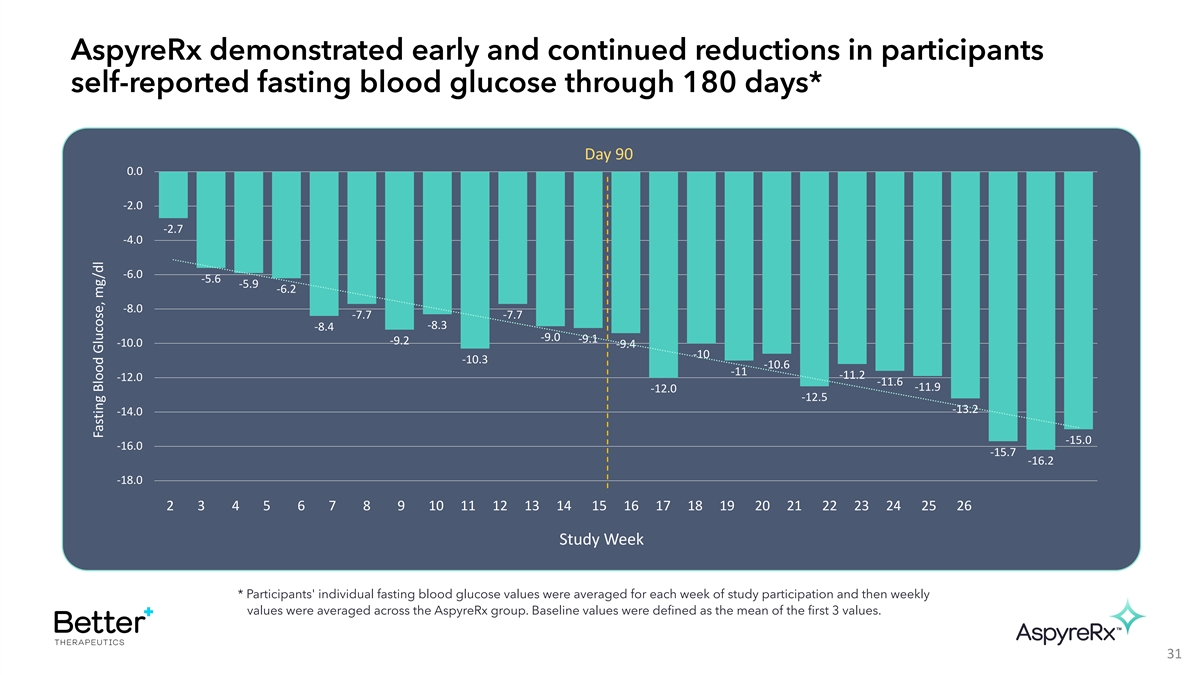
AspyreRx demonstrated early and continued reductions in participants
self-reported fasting blood glucose through 180 days* Day 90 0.0 -2.0 -2.7 -4.0 -6.0 -5.6 -5.9 -6.2 -8.0 -7.7 -7.7 -8.3 -8.4 -9.0 -9.1 -9.2 -10.0 -9.4 -10 -10.3 -10.6 -11 -11.2 -12.0 -11.6 -11.9 -12.0 -12.5 -13.2 -14.0 -15.0 -16.0 -15.7 -16.2 -18.0
2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 Study Week * Participants' individual fasting blood glucose values were averaged for each week of study participation and then weekly values were averaged across the AspyreRx group.
Baseline values were defined as the mean of the first 3 values. 31 Fasting Blood Glucose, mg/dl

In addition to A1c reductions, AspyreRx also demonstrated improvements
in systolic blood pressure and weight Mean Change in Systolic Blood Pressure (mmHg) Mean Change in Body Weight (lbs) a b from Baseline to Day 180 from Baseline to Day 180 0 All Patients Baseline SBP > 139 mmHg -1 0 -2 -1.8 -1.9 -5 -3 -4.7 -3.6
lbs difference (p=0.005) -2.9 mmHg -4 - difference - -10 (P=0.042) -5 -6 -5.5 -15 AspyreRx Control -15.7 -20 -7.2 mmHg difference Weight Change at AspyreRx Control (n=310) (P=0.009) Day 180 (n=290) - -22.9 -25 5%or more, n (%) 50 (17.2) 30 (9.7)
AspyreRx Control 7% or more, n (%) 24(8.3) 14 (4.5) a: Mean baseline systolic blood pressure was 127 mmHg and 126 mmHg for the AspyreRx and control groups, respectively. P-values were based on LS means. b Mean baseline weight was 217 lbs and 222 lbs
for the AspyreRx and control groups, respectively. P-values were based on LS Means. Key: HbA1c – hemoglobin A1c; mmHg – millimeters of mercury; lbs – pounds. 32

Statistically significant improvements in mood and quality of life
(QoL) were also observed Improvements QoL Improvements in Mood PHQ-9 Change from Baseline at Day 180 SF-12 Physical Component Change from Baseline at Day 180 -0.2 0 0.2 0.4 0.6 0.8 0 0.5 1 1.5 2 2.5 3 3.5 4 AspyreRx -0.1 AspyreRx 3.7 Control 0.7
Control 1.3 Lower score is indicative of less depression Higher score is indicative of better quality of life Treatment Group Difference, tested by regression analysis Treatment Group Difference, tested by regression analysis LS mean(95% CI): -0.8
(-1.5, -0.2), p=0.009 LS mean(95% CI): 1.8 (0.4, 3.1), p=0.009 33
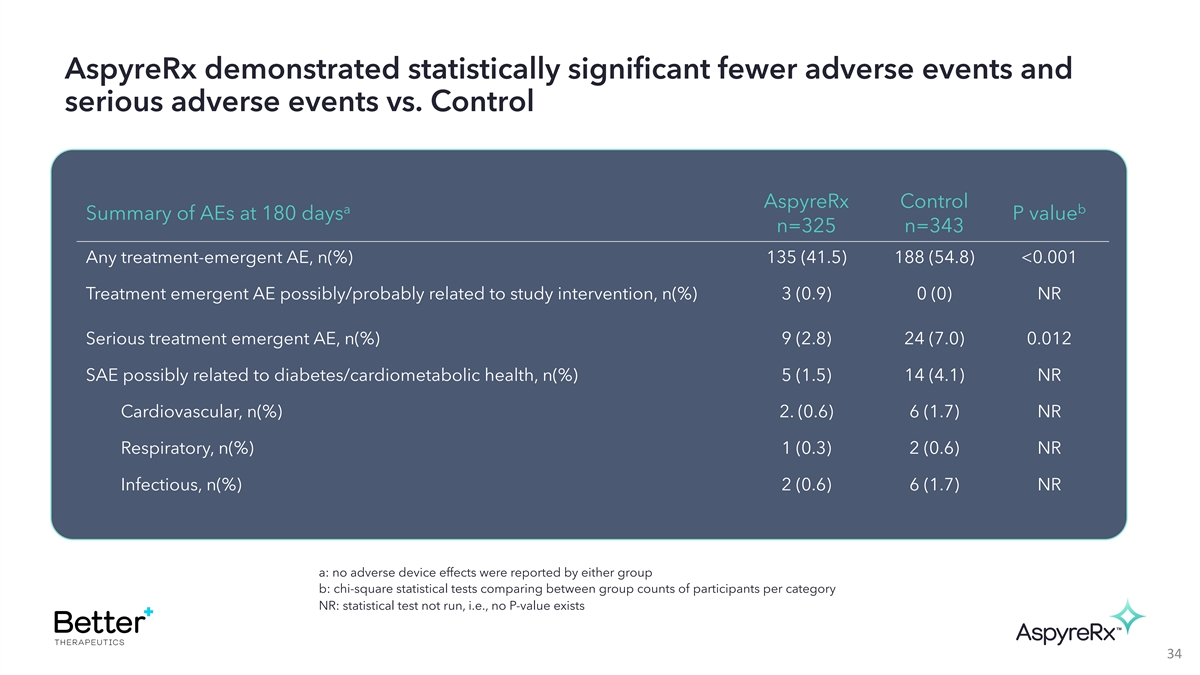
AspyreRx demonstrated statistically significant fewer adverse events
and serious adverse events vs. Control AspyreRx Control a b Summary of AEs at 180 days P value n=325 n=343 Any treatment-emergent AE, n(%) 135 (41.5) 188 (54.8) <0.001 Treatment emergent AE possibly/probably related to study intervention, n(%) 3
(0.9) 0 (0) NR Serious treatment emergent AE, n(%) 9 (2.8) 24 (7.0) 0.012 SAE possibly related to diabetes/cardiometabolic health, n(%) 5 (1.5) 14 (4.1) NR Cardiovascular, n(%) 2. (0.6) 6 (1.7) NR Respiratory, n(%) 1 (0.3) 2 (0.6) NR Infectious,
n(%) 2 (0.6) 6 (1.7) NR a: no adverse device effects were reported by either group b: chi-square statistical tests comparing between group counts of participants per category NR: statistical test not run, i.e., no P-value exists 34
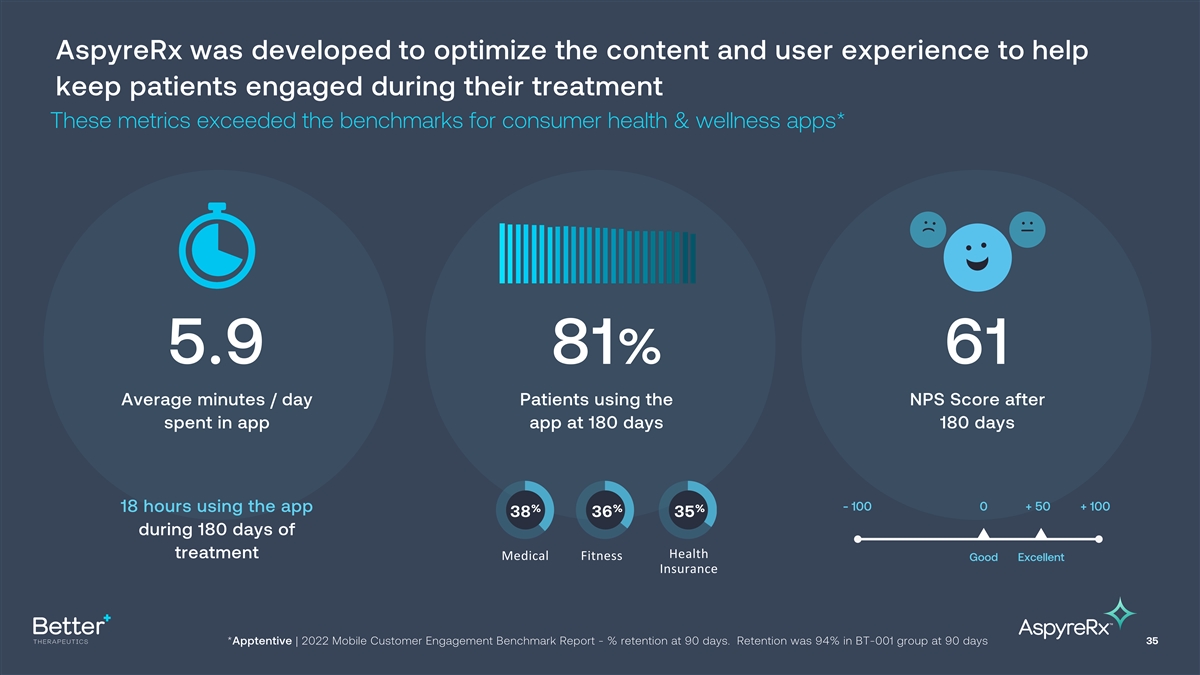
AspyreRx was developed to optimize the content and user experience to
help keep patients engaged during their treatment These metrics exceeded the benchmarks for consumer health & wellness apps* 5.9 81% 61 Average minutes / day Patients using the NPS Score after spent in app app at 180 days 180 days - 100 0 + 50 +
100 18 hours using the app % % % 38 36 35 during 180 days of treatment Health Medical Fitness Good Excellent Insurance *Apptentive | 2022 Mobile Customer Engagement Benchmark Report - % retention at 90 days. Retention was 94% in BT-001 group at 90
days 35

Pivotal Trial: Patient Feedback “It works! It has kept me on
track and aware. There “I have learned so much about myself and my poor is great information that is given. I love the lessons. eating habits. I am learning to love the new way of life Just love the app.” and want everyone suffering from
diabetes to try this awesome journey…” Female | NY | 65 | -1% A1c | 11 years with T2D Female | CA | 60 | -0.7% A1c | 2 years with T2D “This app really helps me. It motivates me to live a healthier life by teaching me skills, giving
me “It is helping me see what is really going on in my recipes, and let's me know its ok when I make life. I love the lessons and skills. I can really make a mistakes. It teaches me that just because I have plan for positive changes.”
diabetes I can still live, enjoy food and be happy.” Female | TX | 58 | -0.7% A1c | 2 years with T2D Female | GA | 40 | -1.2% A1c | 16 years with T2D “Yes - life changer. If you stay with it, it becomes “It has helped me to better
understand and who you are [even] after the program…” control my diabetes.” Male | CA | 67 | -0.4% A1c | 12 years with T2D Male | FL | 63 | -1.4% A1c | 8 years with T2D 36

• Significant & growing unmet medical need • In-line
with existing treatment guidelines We envision AspyreRx becoming part of the • Valuable at any stage in T2D disease progression standard of care for adults with T2D • Broadly accessible to anyone with a smartphone • Potential cost
savings for payers & health systems 37

We also received Breakthrough Device Designation from the FDA in
February for our platform with the intent to treat advanced liver disease (MASH) STATISTICALLY SIGNIFICANT REDUCTION IN LIVER FAT REDUCTION IN MARKERS OF LIVER DAMAGE Change in ALT from Baseline Percent Change in MRI-PDFF Intent-to-Treat Population
Intent-to-Treat Population 38 40 18 1 Elevated Baseline ALT: No Yes -4 -5 REDUCTION IN RISK OF DISEASE PROGRESSION TO MASH - -11 -12 -12 -15 11 -16 -17 -18 Change in FAST Score -26 Intent-to-Treat Population -36 -48 -50 -64 -70 Baseline FAST Score:
Low Risk Intermediate High Risk 38 Percent Change in MRI-PDFF Change in FAST Score Change in ALT (IU/L)
The Problem Better Therapeutics Approach How our Product Works Contents
Clinical Evidence Go-to-Market Plan NASDAQ Compliance 39

Our commercialization approach builds on our core advantage of
scalability and broad access to offer a high impact population health solution INITIAL FOCUS PAYERS POPULATION HEALTH PROVIDERS PATIENTS Gain coverage by showing Drive large scale adoption Position AspyreRx as Drive immediate engagement at better
patient outcomes through high publicity health effective treatment that the point of Rx and deliver and cost savings as a equities initiatives and extends HCP’s reach and positive experience throughout complement or a step to revenue
generating addresses the root cause to maximize treatment success other, more costly Tx institutional partnerships of the disease and enable word of mouth options 40

Population Health: Our initial commercial priority will be to create
public & private population health partnerships at scale The “digital-only” aspect of our treatment approach enables rapid implementation, scalability and performance-based contracting opportunities that are unique to us. We expect
this to drive broad product and adoption with little incremental cost Incentives are fully aligned • T2D is the #1 population health challenge in the U.S. • Public and private healthcare delivery organizations are charged with delivering
quality clinical care at an affordable cost Why • They regularly evaluate and pay for solutions that can be implemented for their patient population at scale • Early market feedback: strong early buying signals from heads of population
health to implement at their institutions Negotiate and implement institutional partnerships for product fit and long-term traction • Charge for outcomes to drive product trial at scale, with minimal variable cost to Better Therapeutics
• Show ease of integration into existing care pathways and institutional workflow How • Demonstrate willingness to pay: health benefit to employees/members, buy-n-bill or pass-through to patients • Establish reference customers,
advocates, and real-world ROI Engage C-Suite/Population Health decision makers on quickly build and implement innovative partnerships • ACLM partnership to provide 1M Rx to FQHC clinics nationwide: Drives national product awareness among HCPs,
Announced proves scalability, delivers transformative population health benefit with no cannibalization of commercial patient opportunity Examples Examples 41
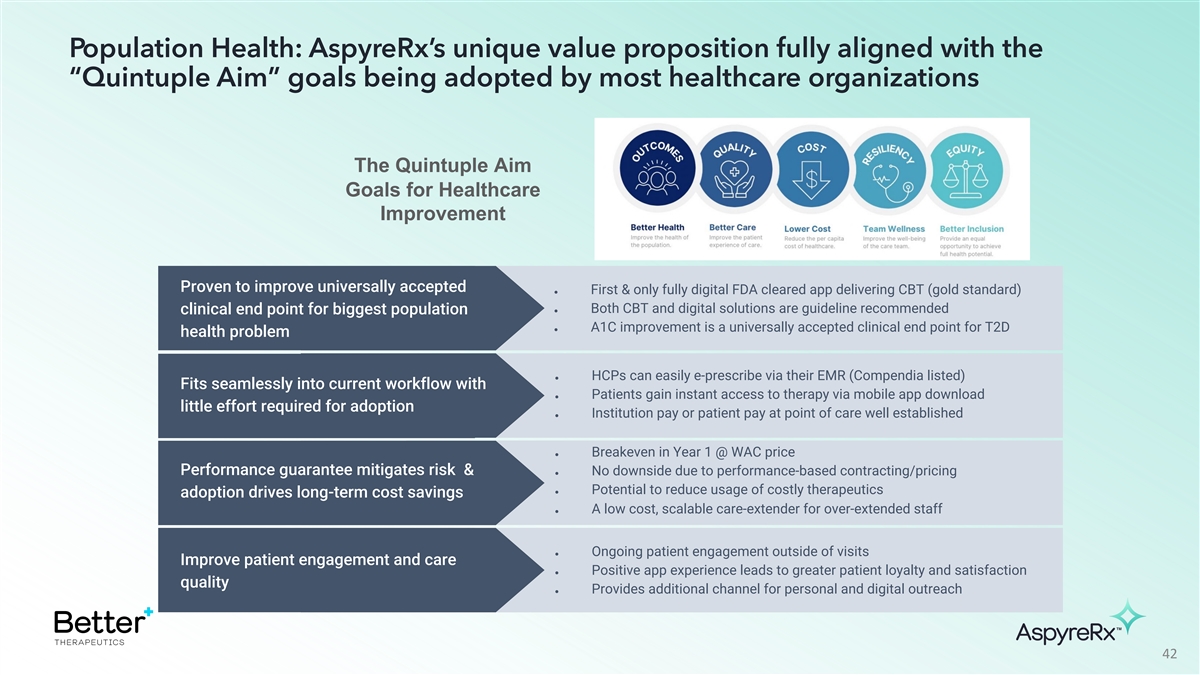
Population Health: AspyreRx’s unique value proposition fully
aligned with the “Quintuple Aim” goals being adopted by most healthcare organizations The Quintuple Aim Goals for Healthcare Improvement Proven to improve universally accepted ● First & only fully digital FDA cleared app
delivering CBT (gold standard) ● Both CBT and digital solutions are guideline recommended clinical end point for biggest population ● A1C improvement is a universally accepted clinical end point for T2D health problem ● HCPs can
easily e-prescribe via their EMR (Compendia listed) Fits seamlessly into current workflow with ● Patients gain instant access to therapy via mobile app download little effort required for adoption ● Institution pay or patient pay at
point of care well established ● Breakeven in Year 1 @ WAC price Performance guarantee mitigates risk & ● No downside due to performance-based contracting/pricing ● Potential to reduce usage of costly therapeutics adoption
drives long-term cost savings ● A low cost, scalable care-extender for over-extended staff ● Ongoing patient engagement outside of visits Improve patient engagement and care ● Positive app experience leads to greater patient
loyalty and satisfaction quality ● Provides additional channel for personal and digital outreach 42

2026 Path to Growth Drive Broad Adoption • Convert institutional
adoption to payer pay • Begin national HCP targeting to pull-through broad payer 2025 coverage Grow Traction • Launch targeted digital DTC to convert “ready to change” patients actively looking for solutions online •
Continue to grow Population Health Partnerships; entrench product into 2024 workflow and care pathways Initial Go-To-Market • Continue to grow payer coverage • Begin targeted HCP sales & promo to pull-through population health and
• Population Health Partnerships to payer wins demonstrate product fit and scalability • Seek regional payer coverage in areas of high T2D prevalence 43 Payer Coverage Ramp
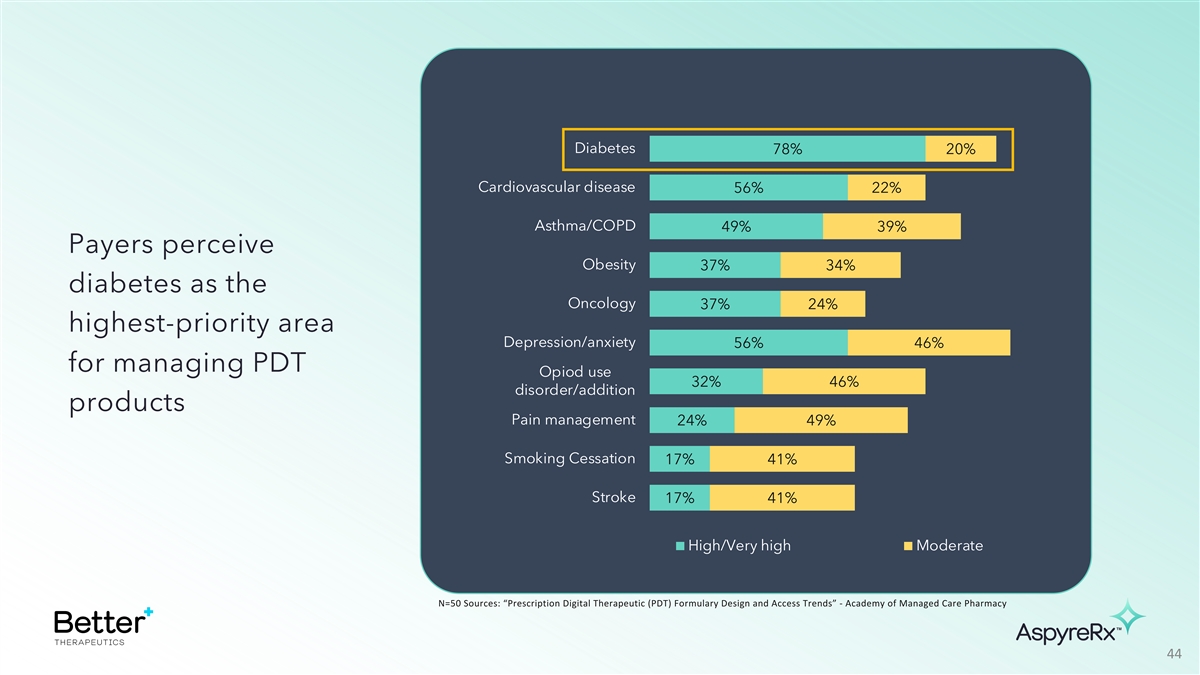
Diabetes 78% 20% Cardiovascular disease 56% 22% Asthma/COPD 49% 39%
Payers perceive Obesity 37% 34% diabetes as the Oncology 37% 24% highest-priority area Depression/anxiety 56% 46% for managing PDT Opiod use 32% 46% disorder/addition products Pain management 24% 49% Smoking Cessation 17% 41% Stroke 17% 41%
High/Very high Moderate N=50 Sources: “Prescription Digital Therapeutic (PDT) Formulary Design and Access Trends” - Academy of Managed Care Pharmacy 44

Based on our cost effectiveness analysis of our pivotal study data,
AspyreRx leads to improvements for patients and may be less expensive for payers Cost Effectiveness Plane Initial Results: AspyreRx* Cost Cost Increasing Increasing New intervention Standard care more costly and dominates more effective New
intervention is more costly and Use ICER to help decide less effective on whether to invest Less More Less More Effectiveness Effective Effective Effective Effective New intervention New intervention BT-001 dominates less costly and less effective
New intervention less Use ICER to help decide costly and more effective on whether to invest than standard care Cost Saving Cost Saving Through its impact on HbA1c and other variables, we expect BT- 001 to demonstrate greater Life Years, greater
Quality-Adjusted Life Years, and fewer costs over the modeled time horizon. *Results have not been peer-reviewed or published and are based on internal analyses 45
Glooko Partnerships further CGMs accelerates AspyreRx adoption
Pharmaceutical Drugs • Access to Glooko’s endocrinologist customers in 4,000+ clinics in the US • AspyreRx integration into Glooko platform increases awareness and educates HCPs on how to prescribe Other Connected •
Glooko’s Precision Engagement tools Devices enable selection of patients for whom AspyreRx may be a good fit • Most digital therapeutics will be used Diabetes alongside other treatment modalities. Education Glooko platform enables
integration of multiple data sources into AspyreRx Insulin Pumps 46

List Price for AspyreRx & Pricing Model to Ensure ROI •
Expect most patients to be prescribed AspyreRx with one $750 refill for minimum 6-month treatment period WAC • AspyreRx has a predefined duration, offering cost predictability for payers per 90-day script • AspyreRx is competitively
priced when compared to chronic drug costs Pricing based on Patient Engagement • “Engagement-Based Pricing” guarantees ROI to payers • Limited-time cash pay option to drive adoption through Engagement Drives affordable access
while gaining broad insurance coverage Outcomes Outcomes Drive ROI WAC = Wholesale Acquisition Cost 47

The Problem Better Therapeutics Approach How our Product Works Contents
Clinical Evidence Go-to-Market Plan Nasdaq Compliance 48

NASDAQ Compliance •
WereceivedadelistingnoticefromNASDAQonDecember13,2023 • WerequestedanappealwithaNASDAQpanelonDecember21,2023. Therequestforanappealwillstaythesuspensionanddelisting ofourcommonstockpendingthedecisionofthePanel •
TheNASDAQhearingiscurrentlyscheduledforMarch14,2024 • WehaveengagedspecialcounseltohelpusnavigateourNASDAQappealprocessandregaincompliance 49
v3.24.0.1
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Better Therapeutics (NASDAQ:BTTX)
Gráfica de Acción Histórica
De Dic 2024 a Ene 2025

Better Therapeutics (NASDAQ:BTTX)
Gráfica de Acción Histórica
De Ene 2024 a Ene 2025
