2024Q3FALSE--12-310001280776P3Yhttp://fasb.org/us-gaap/2024#NonoperatingIncomeExpensehttp://fasb.org/us-gaap/2024#NonoperatingIncomeExpensehttp://fasb.org/us-gaap/2024#OtherLiabilitiesCurrenthttp://fasb.org/us-gaap/2024#OtherLiabilitiesCurrentP3Yxbrli:sharesiso4217:USDiso4217:USDxbrli:sharesvtl:employeevtl:leasevtl:financialInstitutionxbrli:pureiso4217:EURutr:Dvtl:tranchevtl:numberOfVote00012807762024-01-012024-09-3000012807762024-10-3100012807762024-09-3000012807762023-12-3100012807762024-07-012024-09-3000012807762023-07-012023-09-3000012807762023-01-012023-09-300001280776us-gaap:CommonStockMember2023-12-310001280776us-gaap:AdditionalPaidInCapitalMember2023-12-310001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-12-310001280776us-gaap:RetainedEarningsMember2023-12-310001280776us-gaap:RetainedEarningsMember2024-01-012024-03-3100012807762024-01-012024-03-310001280776us-gaap:AdditionalPaidInCapitalMember2024-01-012024-03-310001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-01-012024-03-310001280776vtl:January2024PIPETransactionMember2024-01-012024-03-310001280776us-gaap:CommonStockMembervtl:January2024PIPETransactionMember2024-01-012024-03-310001280776us-gaap:AdditionalPaidInCapitalMembervtl:January2024PIPETransactionMember2024-01-012024-03-310001280776vtl:AtTheMarketSalesAgreementMember2024-01-012024-03-310001280776us-gaap:CommonStockMembervtl:AtTheMarketSalesAgreementMember2024-01-012024-03-310001280776us-gaap:AdditionalPaidInCapitalMembervtl:AtTheMarketSalesAgreementMember2024-01-012024-03-310001280776us-gaap:CommonStockMember2024-03-310001280776us-gaap:AdditionalPaidInCapitalMember2024-03-310001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-03-310001280776us-gaap:RetainedEarningsMember2024-03-3100012807762024-03-310001280776us-gaap:RetainedEarningsMember2024-04-012024-06-3000012807762024-04-012024-06-300001280776us-gaap:AdditionalPaidInCapitalMember2024-04-012024-06-300001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-04-012024-06-300001280776us-gaap:CommonStockMember2024-06-300001280776us-gaap:AdditionalPaidInCapitalMember2024-06-300001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-06-300001280776us-gaap:RetainedEarningsMember2024-06-3000012807762024-06-300001280776us-gaap:RetainedEarningsMember2024-07-012024-09-300001280776us-gaap:AdditionalPaidInCapitalMember2024-07-012024-09-300001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-07-012024-09-300001280776us-gaap:CommonStockMember2024-09-300001280776us-gaap:AdditionalPaidInCapitalMember2024-09-300001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-09-300001280776us-gaap:RetainedEarningsMember2024-09-300001280776us-gaap:CommonStockMember2022-12-310001280776us-gaap:AdditionalPaidInCapitalMember2022-12-310001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-12-310001280776us-gaap:RetainedEarningsMember2022-12-3100012807762022-12-310001280776us-gaap:RetainedEarningsMember2023-01-012023-03-3100012807762023-01-012023-03-310001280776us-gaap:AdditionalPaidInCapitalMember2023-01-012023-03-310001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-01-012023-03-310001280776us-gaap:CommonStockMember2023-01-012023-03-310001280776us-gaap:CommonStockMember2023-03-310001280776us-gaap:AdditionalPaidInCapitalMember2023-03-310001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-03-310001280776us-gaap:RetainedEarningsMember2023-03-3100012807762023-03-310001280776us-gaap:RetainedEarningsMember2023-04-012023-06-3000012807762023-04-012023-06-300001280776us-gaap:AdditionalPaidInCapitalMember2023-04-012023-06-300001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-04-012023-06-300001280776us-gaap:CommonStockMember2023-04-012023-06-300001280776us-gaap:CommonStockMember2023-06-300001280776us-gaap:AdditionalPaidInCapitalMember2023-06-300001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-06-300001280776us-gaap:RetainedEarningsMember2023-06-3000012807762023-06-300001280776us-gaap:RetainedEarningsMember2023-07-012023-09-300001280776us-gaap:AdditionalPaidInCapitalMember2023-07-012023-09-300001280776us-gaap:CommonStockMembervtl:AtTheMarketSalesAgreementMember2023-07-012023-09-300001280776us-gaap:AdditionalPaidInCapitalMembervtl:AtTheMarketSalesAgreementMember2023-07-012023-09-300001280776vtl:AtTheMarketSalesAgreementMember2023-07-012023-09-300001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-07-012023-09-300001280776us-gaap:CommonStockMember2023-09-300001280776us-gaap:AdditionalPaidInCapitalMember2023-09-300001280776us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-09-300001280776us-gaap:RetainedEarningsMember2023-09-3000012807762023-09-3000012807762024-08-0100012807762016-01-012024-09-300001280776country:US2024-09-300001280776country:DE2024-09-300001280776country:AU2024-09-300001280776country:USus-gaap:MoneyMarketFundsMember2024-09-300001280776country:DEus-gaap:MoneyMarketFundsMembersrt:MinimumMember2024-09-300001280776country:DEus-gaap:MoneyMarketFundsMembersrt:MaximumMember2024-09-300001280776srt:MinimumMember2024-09-300001280776srt:MaximumMember2024-09-300001280776country:AU2024-01-012024-09-300001280776vtl:PreFundedWarrantsMember2024-09-300001280776us-gaap:StockOptionMember2024-01-012024-09-300001280776us-gaap:StockOptionMember2023-01-012023-09-300001280776vtl:NewYorkCityMember2024-09-300001280776vtl:GrafelfingGermanyMember2024-09-300001280776vtl:NewYorkCityMember2024-01-012024-09-300001280776vtl:PlaneggGermanyMember2024-01-012024-09-300001280776vtl:PlaneggGermanyMember2024-09-300001280776us-gaap:FairValueMeasurementsRecurringMemberus-gaap:MoneyMarketFundsMember2024-09-300001280776us-gaap:FairValueInputsLevel1Memberus-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2024-09-300001280776us-gaap:FairValueInputsLevel2Memberus-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2024-09-300001280776us-gaap:FairValueInputsLevel3Memberus-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2024-09-300001280776us-gaap:FairValueMeasurementsRecurringMember2024-09-300001280776us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2024-09-300001280776us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2024-09-300001280776us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2024-09-300001280776us-gaap:FairValueMeasurementsRecurringMemberus-gaap:MoneyMarketFundsMember2023-12-310001280776us-gaap:FairValueInputsLevel1Memberus-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001280776us-gaap:FairValueInputsLevel2Memberus-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001280776us-gaap:FairValueInputsLevel3Memberus-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001280776us-gaap:FairValueMeasurementsRecurringMember2023-12-310001280776us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001280776us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-310001280776us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2023-12-3100012807762024-01-080001280776srt:MinimumMember2024-01-082024-01-080001280776srt:MaximumMember2024-01-082024-01-0800012807762024-01-082024-01-0800012807762024-03-040001280776srt:MinimumMember2024-03-042024-03-040001280776srt:MaximumMember2024-03-042024-03-0400012807762024-03-042024-03-040001280776vtl:December2020ATMMember2020-12-310001280776vtl:May2022ATMMember2022-05-3100012807762023-11-300001280776vtl:May2024ATMMember2024-05-310001280776vtl:May2024ATMMember2024-09-300001280776vtl:May2024ATMMember2024-01-012024-09-300001280776vtl:December2020ATMMember2024-01-012024-09-300001280776vtl:December2020ATMMember2024-09-300001280776vtl:December2020ATMMember2023-07-012023-09-300001280776vtl:December2020ATMMember2023-01-012023-09-300001280776vtl:December2020ATMMember2023-09-300001280776vtl:AccreditedInvestorsMemberus-gaap:PrivatePlacementMember2024-01-042024-01-040001280776us-gaap:CommonStockMemberus-gaap:PrivatePlacementMember2024-01-040001280776vtl:PreFundedWarrantsMemberus-gaap:PrivatePlacementMember2024-01-040001280776us-gaap:ShareBasedCompensationAwardTrancheOneMembervtl:AccreditedInvestorsMemberus-gaap:CommonStockMemberus-gaap:PrivatePlacementMember2024-01-082024-01-080001280776us-gaap:ShareBasedCompensationAwardTrancheOneMembervtl:AccreditedInvestorsMemberus-gaap:CommonStockMemberus-gaap:PrivatePlacementMember2024-01-080001280776us-gaap:ShareBasedCompensationAwardTrancheTwoMembervtl:AccreditedInvestorsMemberus-gaap:CommonStockMemberus-gaap:PrivatePlacementMember2024-01-042024-01-040001280776us-gaap:ShareBasedCompensationAwardTrancheTwoMembervtl:AccreditedInvestorsMemberus-gaap:CommonStockMemberus-gaap:PrivatePlacementMember2024-01-040001280776us-gaap:ShareBasedCompensationAwardTrancheThreeMembervtl:AccreditedInvestorsMemberus-gaap:CommonStockMemberus-gaap:PrivatePlacementMember2024-01-042024-01-040001280776us-gaap:ShareBasedCompensationAwardTrancheOneMemberus-gaap:CommonStockMemberus-gaap:PrivatePlacementMember2024-01-082024-01-080001280776us-gaap:ShareBasedCompensationAwardTrancheTwoMemberus-gaap:CommonStockMemberus-gaap:PrivatePlacementMember2024-01-042024-01-040001280776us-gaap:ShareBasedCompensationAwardTrancheThreeMemberus-gaap:CommonStockMemberus-gaap:PrivatePlacementMember2024-01-042024-01-040001280776us-gaap:CommonStockMemberus-gaap:CommonStockMember2024-01-080001280776vtl:CommonStockAndPreFundedWarrantsMembervtl:CommonStockAndPreFundedWarrantsMember2024-01-080001280776us-gaap:CommonStockMemberus-gaap:CommonStockMember2024-03-030001280776us-gaap:CommonStockMemberus-gaap:CommonStockMember2024-03-040001280776us-gaap:EquityMember2024-01-012024-09-300001280776us-gaap:OtherNonoperatingIncomeExpenseMember2024-01-012024-09-300001280776srt:MinimumMember2024-03-040001280776srt:MaximumMember2024-03-040001280776us-gaap:EmployeeStockOptionMembervtl:A2021EmployeeStockPurchasePlanMember2024-09-300001280776vtl:PreFundedWarrantsMember2024-09-300001280776us-gaap:EmployeeStockOptionMember2024-09-300001280776us-gaap:ShareBasedCompensationAwardTrancheTwoMember2024-09-300001280776us-gaap:ShareBasedCompensationAwardTrancheThreeMember2024-09-300001280776vtl:EmployeeStockOptionsforFutureGrantMembervtl:A2017InducementEquityIncentivePlanMember2024-09-300001280776vtl:EmployeeStockOptionsforFutureGrantMembervtl:TwoThousandNineteenOmnibusEquityIncentivePlanMember2024-09-300001280776vtl:A2021EmployeeStockPurchasePlanMember2021-04-252021-04-250001280776vtl:A2021EmployeeStockPurchasePlanMember2021-04-250001280776vtl:A2021EmployeeStockPurchasePlanMember2024-03-042024-03-040001280776vtl:A2021EmployeeStockPurchasePlanMember2024-03-040001280776vtl:A2021EmployeeStockPurchasePlanMember2021-08-012021-08-010001280776vtl:A2021EmployeeStockPurchasePlanMember2024-01-012024-09-300001280776vtl:A2021EmployeeStockPurchasePlanMember2024-07-012024-09-300001280776vtl:A2021EmployeeStockPurchasePlanMember2023-07-012023-09-300001280776vtl:A2021EmployeeStockPurchasePlanMember2023-01-012023-09-300001280776vtl:A2019OmnibusEquityIncentivePlanMember2019-07-310001280776vtl:TwoThousandNineteenOmnibusEquityIncentivePlanEvergreenProvisionMember2019-07-012019-07-310001280776vtl:TwoThousandNineteenOmnibusEquityIncentivePlanEvergreenProvisionMember2020-01-012023-12-310001280776vtl:TwoThousandNineteenOmnibusEquityIncentivePlanEvergreenProvisionMember2023-06-282023-06-280001280776vtl:TwoThousandNineteenOmnibusEquityIncentivePlanEvergreenProvisionMember2024-03-042024-03-040001280776vtl:A2019OmnibusEquityIncentivePlanMembersrt:MaximumMember2024-01-012024-09-300001280776vtl:IncentiveEmployeeStockOptionMembervtl:A2019OmnibusEquityIncentivePlanMember2024-01-012024-09-300001280776vtl:A2019OmnibusEquityIncentivePlanMembervtl:NonStatutoryEmployeeStockOptionMembersrt:MinimumMember2024-01-012024-09-300001280776vtl:A2019OmnibusEquityIncentivePlanMembervtl:NonStatutoryEmployeeStockOptionMembersrt:MaximumMember2024-01-012024-09-300001280776us-gaap:EmployeeStockOptionMembervtl:TwoThousandNineteenOmnibusEquityIncentivePlanMember2023-12-310001280776us-gaap:EmployeeStockOptionMembervtl:TwoThousandNineteenOmnibusEquityIncentivePlanMember2024-01-012024-09-300001280776us-gaap:EmployeeStockOptionMembervtl:TwoThousandNineteenOmnibusEquityIncentivePlanMember2024-09-300001280776us-gaap:EmployeeStockOptionMembervtl:TwoThousandNineteenOmnibusEquityIncentivePlanMember2022-12-310001280776us-gaap:EmployeeStockOptionMembervtl:TwoThousandNineteenOmnibusEquityIncentivePlanMember2023-01-012023-09-300001280776us-gaap:EmployeeStockOptionMembervtl:TwoThousandNineteenOmnibusEquityIncentivePlanMember2023-09-300001280776vtl:A2019OmnibusEquityIncentivePlanMember2024-01-012024-09-300001280776vtl:A2019OmnibusEquityIncentivePlanMember2023-01-012023-09-300001280776us-gaap:ResearchAndDevelopmentExpenseMembervtl:EmployeeMember2024-07-012024-09-300001280776us-gaap:ResearchAndDevelopmentExpenseMembervtl:EmployeeMember2023-07-012023-09-300001280776us-gaap:ResearchAndDevelopmentExpenseMembervtl:EmployeeMember2024-01-012024-09-300001280776us-gaap:ResearchAndDevelopmentExpenseMembervtl:EmployeeMember2023-01-012023-09-300001280776us-gaap:GeneralAndAdministrativeExpenseMembervtl:EmployeeMember2024-07-012024-09-300001280776us-gaap:GeneralAndAdministrativeExpenseMembervtl:EmployeeMember2023-07-012023-09-300001280776us-gaap:GeneralAndAdministrativeExpenseMembervtl:EmployeeMember2024-01-012024-09-300001280776us-gaap:GeneralAndAdministrativeExpenseMembervtl:EmployeeMember2023-01-012023-09-300001280776vtl:EmployeeMember2024-07-012024-09-300001280776vtl:EmployeeMember2023-07-012023-09-300001280776vtl:EmployeeMember2024-01-012024-09-300001280776vtl:EmployeeMember2023-01-012023-09-300001280776vtl:A2019OmnibusEquityIncentivePlanMember2024-09-300001280776vtl:TwoThousandFourteenEquityIncentivePlanMember2024-09-300001280776vtl:A2017InducementEquityIncentivePlanMember2017-09-300001280776vtl:A2017InducementEquityIncentivePlanMember2023-07-012023-09-300001280776vtl:A2017InducementEquityIncentivePlanMember2023-01-012023-09-300001280776vtl:A2017InducementEquityIncentivePlanMember2024-01-012024-09-300001280776vtl:A2017InducementEquityIncentivePlanMember2024-07-012024-09-300001280776srt:BoardOfDirectorsChairmanMembervtl:DuaneNashMDJDMBAMembervtl:ExecutiveChairmanAgreementMember2022-12-282023-12-310001280776srt:BoardOfDirectorsChairmanMembervtl:DuaneNashMDJDMBAMembervtl:ExecutiveChairmanAgreementMember2024-08-292024-08-290001280776srt:BoardOfDirectorsChairmanMembervtl:DuaneNashMDJDMBAMembervtl:ExecutiveChairmanAgreementMember2024-01-012024-08-28
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
(Mark One) | | | | | |
| ☒ | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended September 30, 2024
or | | | | | |
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 001-36201
Immunic, Inc.
(Exact name of registrant as specified in its charter)
| | | | | | | | |
| Delaware | | 56-2358443 |
| (State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| | |
| 1200 Avenue of the Americas | | |
| Suite 200 | | |
| New York, | NY | 10036 |
| (Address of principal executive offices) | | (Zip Code) |
(332) 255-9818
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | |
| Title of each class | Trading symbol(s) | Name of each exchange on which registered |
| Common Stock, $0.0001 par value | IMUX | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act. | | | | | | | | | | | |
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| | | |
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
| | | |
| | Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
On October 31, 2024, 90,079,016 shares of common stock, $0.0001 par value, were outstanding.
IMMUNIC, INC.
INDEX
| | | | | | | | |
| | | Page No. |
| |
| Item 1. | | |
| | |
| | |
| | |
| | |
| | |
| | |
| Item 2. | | |
| Item 3. | | |
| Item 4. | | |
| | |
| |
| Item 1. | | |
| Item 1A. | | |
| Item 2. | | |
| Item 3. | | |
| Item 4. | | |
| Item 5. | | |
| Item 6. | | |
IMMUNIC, INC.
Condensed Consolidated Balance Sheets
(In thousands, except share and per share amounts)
| | | | | | | | | | | |
| September 30, 2024 | | December 31, 2023 |
| (Unaudited) | | |
| Assets | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 59,071 | | | $ | 46,674 | |
| Other current assets and prepaid expenses | 4,195 | | | 5,860 | |
| Total current assets | 63,266 | | | 52,534 | |
| Property and equipment, net | 618 | | | 466 | |
| Right-of-use assets, net | 878 | | | 1,299 | |
| Total assets | $ | 64,762 | | | $ | 54,299 | |
| Liabilities and Stockholders’ Equity | | | |
| Current liabilities: | | | |
| Accounts payable | $ | 6,042 | | | $ | 5,099 | |
| Accrued expenses | 16,245 | | | 18,664 | |
| Other current liabilities | 1,070 | | | 966 | |
| Total current liabilities | 23,357 | | | 24,729 | |
| Long term liabilities | | | |
| Operating lease liabilities | 186 | | | 639 | |
| Total long-term liabilities | 186 | | | 639 | |
| Total liabilities | 23,543 | | | 25,368 | |
| Commitments and contingencies (Note 4) | | | |
| Stockholders’ equity: | | | |
Preferred stock, $0.0001 par value; 20,000,000 authorized and no shares issued or outstanding as of September 30, 2024 and December 31, 2023 | — | | | — | |
Common stock, $0.0001 par value; 500,000,000 and 130,000,000 shares authorized as of September 30, 2024 and December 31, 2023, respectively, and 90,079,016 and 45,177,730 shares issued and outstanding as of September 30, 2024 and December 31, 2023, respectively. | 8 | | | 4 | |
| Additional paid-in capital | 523,549 | | | 436,060 | |
| Accumulated other comprehensive income | 3,886 | | | 3,759 | |
| Accumulated deficit | (486,224) | | | (410,892) | |
| Total stockholders’ equity | 41,219 | | | 28,931 | |
| Total liabilities and stockholders’ equity | $ | 64,762 | | | $ | 54,299 | |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
IMMUNIC, INC.
Condensed Consolidated Statements of Operations
(In thousands, except share and per share amounts)
(Unaudited)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Three Months
Ended September 30, | | Nine Months
Ended September 30, |
| | | | | | 2024 | | 2023 | | 2024 | | 2023 |
| Operating expenses: | | | | | | | | | | | |
| Research and development | | | | | $ | 21,370 | | | $ | 19,796 | | | $ | 58,429 | | | $ | 63,931 | |
| General and administrative | | | | | 4,356 | | | 3,774 | | | 13,992 | | | 11,911 | |
| Total operating expenses | | | | | 25,726 | | | 23,570 | | | 72,421 | | | 75,842 | |
| Loss from operations | | | | | (25,726) | | | (23,570) | | | (72,421) | | | (75,842) | |
| Other income (expense): | | | | | | | | | | | |
| Interest income | | | | | 776 | | | 766 | | | 2,961 | | | 2,534 | |
| Change in fair value of the tranche rights | | | | | — | | | — | | | (4,796) | | | — | |
| Other income (expense), net | | | | | 582 | | | 35 | | | (1,076) | | | 1,268 | |
| Total other income (expense) | | | | | 1,358 | | | 801 | | | (2,911) | | | 3,802 | |
| Net loss | | | | | $ | (24,368) | | | $ | (22,769) | | | $ | (75,332) | | | $ | (72,040) | |
| | | | | | | | | | | |
| Net loss per share, basic and diluted | | | | | $ | (0.24) | | | $ | (0.51) | | | $ | (0.75) | | | $ | (1.63) | |
| | | | | | | | | | | |
| Weighted-average common shares outstanding, basic and diluted | | | | | 101,272,580 | | | 44,574,377 | | | 99,998,245 | | | 44,227,264 | |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
IMMUNIC, INC.
Condensed Consolidated Statements of Comprehensive Loss
(In thousands)
(Unaudited)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Three Months
Ended September 30, | | Nine Months
Ended September 30, |
| | | | | | 2024 | | 2023 | | 2024 | | 2023 |
| Net loss | | | | | $ | (24,368) | | | $ | (22,769) | | | $ | (75,332) | | | (72,040) | |
| Other comprehensive income (loss): | | | | | | | | | | | |
| Foreign currency translation | | | | | 104 | | | (77) | | | 127 | | | 870 | |
| | | | | | | | | | | |
| Total comprehensive loss | | | | | $ | (24,264) | | | $ | (22,846) | | | $ | (75,205) | | | $ | (71,170) | |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
IMMUNIC, INC.
Condensed Consolidated Statements of Stockholders’ Equity
(In thousands, except share amounts)
(Unaudited) | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Nine Months Ended September 30, 2024 |
| | Common Stock | | Additional
Paid-In
Capital | | Accumulated
Other
Comprehensive
Income (Loss) | | Accumulated
Deficit | | Total
Stockholders’
Equity |
| | Shares | | Amount | |
| Balance at January 1, 2024 | 45,177,730 | | | $ | 4 | | | $ | 436,060 | | | $ | 3,759 | | | $ | (410,892) | | | $ | 28,931 | |
| Net loss | — | | | — | | | — | | | — | | | (29,584) | | | (29,584) | |
| Stock-based compensation | — | | | — | | | 2,750 | | | — | | | — | | | 2,750 | |
| Foreign exchange translation adjustment | — | | | — | | | — | | | 528 | | | — | | | 528 | |
Issuance of common stock and pre-funded warrants - January 2024 Financing, net of issuance costs of $4,037 | 44,751,286 | | | 4 | | | 52,360 | | | — | | | — | | | 52,364 | |
| Conversion of tranche rights liability to equity | — | | | — | | | 28,396 | | | — | | | — | | | 28,396 | |
Issuance of common stock - At The Market Sales Agreement net of issuance costs of $6 | 150,000 | | | — | | | 191 | | | — | | | — | | | 191 | |
| Balance at March 31, 2024 | 90,079,016 | | | $ | 8 | | | $ | 519,757 | | | $ | 4,287 | | | $ | (440,476) | | | $ | 83,576 | |
| Net loss | — | | | — | | | — | | | — | | | (21,380) | | | (21,380) | |
| Stock-based compensation | — | | | — | | | 1,882 | | | — | | | — | | | 1,882 | |
| Foreign exchange translation adjustment | — | | | — | | | — | | | (505) | | | — | | | (505) | |
| Balance at June 30, 2024 | 90,079,016 | | | $ | 8 | | | $ | 521,639 | | | $ | 3,782 | | | $ | (461,856) | | | $ | 63,573 | |
| Net loss | — | | | — | | | — | | | — | | | (24,368) | | | (24,368) | |
| Stock-based compensation | — | | | — | | | 1,910 | | | — | | | — | | | 1,910 | |
| Foreign exchange translation adjustment | — | | | — | | | — | | | 104 | | | — | | | 104 | |
| Balance at September 30, 2024 | 90,079,016 | | | $ | 8 | | | $ | 523,549 | | | $ | 3,886 | | | $ | (486,224) | | | $ | 41,219 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Nine Months Ended September 30, 2023 |
| Common Stock | | Additional
Paid-In
Capital | | Accumulated
Other
Comprehensive
Income (Loss) | | Accumulated
Deficit | | Total
Stockholders’
Equity |
| Shares | | Amount | |
| Balance at January 1, 2023 | 39,307,286 | | | $ | 4 | | | $ | 427,925 | | | $ | 3,035 | | | $ | (317,280) | | | $ | 113,684 | |
| Net loss | — | | | — | | | — | | | — | | | (25,272) | | | (25,272) | |
| Stock-based compensation | — | | | — | | | 1,979 | | | — | | | — | | | 1,979 | |
| Foreign exchange translation adjustment | — | | | — | | | — | | | 776 | | | — | | | 776 | |
| Shares issued from exercise of pre-funded warrants | 5,096,552 | | | — | | | 51 | | | — | | | — | | | 51 | |
| Balance at March 31, 2023 | 44,403,838 | | | $ | 4 | | | $ | 429,955 | | | $ | 3,811 | | | $ | (342,552) | | | $ | 91,218 | |
| Net loss | — | | | — | | | — | | | — | | | (23,999) | | | (23,999) | |
| Stock-based compensation | — | | | — | | | 1,798 | | | — | | | — | | | 1,798 | |
| Foreign exchange translation adjustment | — | | | — | | | — | | | 171 | | | — | | | 171 | |
| Shares issued in connection with the Company's Employee Stock Purchase Plan | 84,533 | | | — | | | 96 | | | — | | | — | | | 96 | |
| Balance at June 30, 2023 | 44,488,371 | | | $ | 4 | | | $ | 431,849 | | | $ | 3,982 | | | $ | (366,551) | | | $ | 69,284 | |
| Net loss | — | | | — | | | — | | | — | | | (22,769) | | | (22,769) | |
| Stock-based compensation | — | | | — | | | 1,687 | | | — | | | — | | | 1,687 | |
| Issuance of common stock - At The Market Sales Agreement net of issuance costs of $9 | 107,012 | | | — | | | 282 | | | — | | | — | | | 282 | |
| Foreign exchange translation adjustment | — | | | — | | | — | | | (77) | | | — | | | (77) | |
| Balance at September 30, 2023 | 44,595,383 | | | $ | 4 | | | $ | 433,818 | | | $ | 3,905 | | | $ | (389,320) | | | $ | 48,407 | |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
IMMUNIC, INC.
Condensed Consolidated Statements of Cash Flows
(In thousands)
(Unaudited)
| | | | | | | | | | | |
| | Nine Months
Ended September 30, |
| | 2024 | | 2023 |
| Cash flows from operating activities: | | | |
| Net loss | $ | (75,332) | | | $ | (72,040) | |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | |
| Depreciation and amortization | 99 | | | 88 | |
| Unrealized foreign currency (gain) loss | (91) | | | 712 | |
| | | |
| Stock-based compensation | 6,542 | | | 5,464 | |
| Change in fair value of tranche rights | 4,796 | | | — | |
| Fees expensed as part of January 2024 Financing | 1,690 | | | — | |
| Changes in operating assets and liabilities: | | | |
| Other current assets and prepaid expenses | 1,460 | | | 3,881 | |
| Accounts payable | 871 | | | (951) | |
| Accrued expenses | (1,900) | | | 5,961 | |
| Other liabilities | 62 | | | 84 | |
| Net cash used in operating activities | (61,803) | | | (56,801) | |
| Cash flows from investing activities: | | | |
| | | |
| | | |
| | | |
| Purchases of property and equipment | (261) | | | (169) | |
| Sale of investments - Other | — | | | 9,796 | |
| Net cash provided by (used in) investing activities | (261) | | | 9,627 | |
| Cash flows from financing activities: | | | |
| | | |
| Proceeds from public offering of common stock through At The Market Sales Agreement, net | 191 | | | 282 | |
| Proceeds from January 2024 Financing, net of issuance costs | 74,273 | | | — | |
| Proceeds from the exercise of pre-funded warrants | — | | | 51 | |
| Proceeds from shares issued in connection with the Company's Employee Stock Purchase Plan | — | | | 96 | |
| | | |
| Net cash provided by financing activities | 74,464 | | | 429 | |
| Effect of exchange rate changes on cash and cash equivalents | (3) | | | (311) | |
| Net change in cash and cash equivalents | 12,397 | | | (47,056) | |
| Cash and cash equivalents, beginning of period | 46,674 | | | 106,745 | |
| Cash and cash equivalents, end of period | $ | 59,071 | | | $ | 59,689 | |
| | | |
| | | |
| Supplemental disclosure of noncash investing and financing activities: | | | |
| Conversion of tranche rights liability to equity upon increase in authorized shares | $ | 28,396 | | | $ | — | |
| Operating lease right-of use asset obtained in exchange for lease obligation | $ | — | | | $ | 544 | |
| | | |
| | | |
| | | |
| | | |
| | | |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
IMMUNIC, INC.
Notes to Condensed Consolidated Financial Statements
(Unaudited)
1. Description of Business and Basis of Financial Statements
Description of Business
Immunic, Inc. ("Immunic" or the "Company") is a biotechnology company developing a clinical pipeline of selective oral immunology therapies focused on treating chronic inflammatory and autoimmune diseases. The Company is headquartered in New York City with its main operations in Gräfelfing near Munich, Germany. The Company had approximately 85 employees as of November 1, 2024.
The Company is pursuing clinical development of orally administered, small molecule programs, each of which has unique features intended to directly address the unmet needs of patients with serious chronic inflammatory and autoimmune diseases. These include the vidofludimus calcium (IMU-838) program, which is in Phase 3 and Phase 2 clinical development for patients with relapsing and progressive multiple sclerosis (“MS”), respectively, and which has shown therapeutic activity in Phase 2 clinical trials in patients suffering from relapsing-remitting MS, progressive MS and moderate-to-severe ulcerative colitis (“UC”); the IMU-856 program, which is targeted to regenerate bowel epithelium and restore intestinal barrier function, which could potentially be applicable in numerous gastrointestinal diseases, such as celiac disease, inflammatory bowel disease and Graft-versus-Host-Disease ("GvHD"); and the IMU-381 program, which is a next generation molecule being developed to specifically address the needs of gastrointestinal diseases.
The Company’s business, operating results, financial condition and growth prospects are subject to significant risks and uncertainties, including the failure of its clinical trials to meet their endpoints, failure to obtain regulatory approval and needing additional funding to complete the development and commercialization of the Company's three development programs.
Liquidity and Financial Condition
Immunic has no products approved for commercial sale and has not generated any revenue from product sales. It has never been profitable and has incurred operating losses in each year since inception in 2016. The Company has an accumulated deficit of approximately $486.2 million as of September 30, 2024 and $410.9 million as of December 31, 2023. Substantially all of Immunic's operating losses resulted from expenses incurred in connection with its research and development programs and from general and administrative costs associated with its operations.
Immunic expects to incur significant expenses and increasing operating losses for the foreseeable future as it initiates and continues the development of its product candidates and adds personnel necessary to advance its pipeline of product candidates. Immunic expects that its operating losses will fluctuate significantly from quarter-to-quarter and year-to-year due to timing of development programs.
From inception through September 30, 2024, Immunic has raised net cash of approximately $430.9 million from private and public offerings of preferred stock, common stock, pre-funded warrants and tranche rights. As of September 30, 2024, the Company had cash and cash equivalents of approximately $59.1 million. With these funds, the Company does not have adequate liquidity to fund its operations for at least twelve months from the issuance of these consolidated financial statements without raising additional capital, but such actions are not solely within the control of the Company. If the Company is unable to obtain additional capital, it would have a material adverse effect on the operations of the Company, its clinical development program, and the Company may have to cease operations altogether. These factors raise substantial doubt about the Company’s ability to continue as a going concern.
Basis of Presentation and Consolidation
The accompanying condensed consolidated financial statements have been prepared in conformity with United States generally accepted accounting principles ("U.S. GAAP") and include the accounts of Immunic and its wholly-owned subsidiaries, Immunic AG and Immunic Australia Pty Ltd. All intercompany accounts and transactions have been eliminated in consolidation. Immunic manages its operations as a single reportable segment for the purposes of assessing performance and making operating decisions.
Unaudited Interim Financial Information
Immunic has prepared the accompanying interim unaudited condensed consolidated financial statements in accordance with United States generally accepted accounting principles, (“US GAAP”), for interim financial information and with the instructions to Form 10-Q and Regulation S-X of the SEC. Accordingly, they do not include all of the information and footnotes required by U.S. GAAP for complete financial statements. These interim unaudited condensed consolidated financial statements reflect all adjustments consisting of normal recurring accruals which, in the opinion of management, are necessary to present fairly Immunic’s consolidated financial position, consolidated results of operations, consolidated statement of stockholders’ equity and consolidated cash flows for the periods and as of the dates presented. The Company’s fiscal year ends on December 31. The condensed consolidated balance sheet as of December 31, 2023 was derived from audited consolidated financial statements but does not include all disclosures required by U.S. GAAP. These condensed consolidated financial statements should be read in conjunction with the annual consolidated financial statements and the notes thereto included on the Company's Annual Report on Form 10-K filed on February 22, 2024. The nature of Immunic’s business is such that the results of any interim period may not be indicative of the results to be expected for the entire year or for corresponding interim periods in any subsequent year.
2. Summary of Significant Accounting Policies
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires the Company to make certain estimates and assumptions that affect the reported amounts of assets, liabilities, expenses and the disclosure of contingent assets and liabilities in the Company’s consolidated financial statements. The most significant estimates in the Company’s financial statements and accompanying notes relate to clinical trial expenses and stock-based compensation. Management believes its estimates to be reasonable under the circumstances. Actual results could differ materially from those estimates and assumptions.
Foreign Currency Translation and Presentation
The Company’s reporting currency is United States (“U.S.”) dollars. Immunic AG is located in Germany with the Euro being its functional currency. Immunic Australia Pty Ltd.’s functional currency is the Australian dollar. All amounts in the financial statements where the functional currency is not the U.S. dollar are translated into U.S. dollar equivalents at exchange rates as follows:
• assets and liabilities at reporting period-end rates;
• income statement accounts at average exchange rates for the reporting period; and
• components of equity at historical rates.
Gains and losses from translation of the financial statements into U.S. dollars are recorded in stockholders’ equity as a component of accumulated other comprehensive income (loss). Realized and unrealized gains and losses resulting from foreign currency transactions denominated in currencies other than the functional currency are reflected as general and administrative expenses in the Consolidated Statements of Operations. Foreign currency transaction gains and losses related to long-term intercompany loans that are payable in the foreseeable future are recorded in Other Income (Expense). The Consolidated Statements of Cash Flows were prepared by using the average exchange rate in effect during the reporting period which reasonably approximates the timing of the cash flows.
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of three months or less to be cash equivalents.
Cash and cash equivalents consist of cash on hand and deposits in banks located in the U.S. of approximately $40.6 million, Germany of approximately $15.6 million and Australia of approximately $2.9 million as of September 30, 2024. The Company maintains cash and cash equivalent balances denominated in Euro and U.S. dollars with major financial institutions in the U.S. and Germany in excess of the deposit limits insured by the government. Management periodically reviews the credit standing of these financial institutions. The Company currently deposits its cash and cash equivalents with two large financial institutions. Cash and cash equivalents in the U.S. are held at JP Morgan and are primarily held in a U.S. Government money
market fund account earning interest at a rate of 4.8% as of September 30, 2024. Cash and cash equivalents in Germany are earning interest at a rate of 3.25% to 3.75% as of September 30, 2024.
Fair Value Measurement
Fair value is defined as the price that would be received to sell an asset or be paid to transfer a liability in an orderly transaction between market participants on the measurement date. Accounting guidance establishes a fair value hierarchy that requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. The standard describes three levels of inputs that may be used to measure fair value:
Level 1—Quoted prices in active markets for identical assets or liabilities. Level 1 assets consisted of money market funds for the periods presented. The Company had no Level 1 liabilities for the periods presented.
Level 2—Inputs other than observable quoted prices for the asset or liability, either directly or indirectly; these include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active. The Company had no Level 2 assets or liabilities for the periods presented.
Level 3—Unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of assets or liabilities. The Company had no Level 3 assets or liabilities for the periods presented. The Company did have tranche rights that were at level 3 during the first quarter of 2024.
The carrying value of cash and cash equivalents, other current assets and prepaid expenses, accounts payable, accrued expenses, and other current liabilities approximates fair value due to the short period of time to maturity.
Property and Equipment
Property and equipment is stated at cost. Depreciation is computed using the straight-line method based on the estimated service lives of the assets, which range from three to thirteen years. Depreciation expense was $42,000 and $34,000 for the three months ended September 30, 2024 and 2023, respectively. Depreciation expense was $99,000 and $88,000 for the nine months ended September 30, 2024 and 2023, respectively.
Impairment of Long-Lived Assets
The Company records impairment losses on long-lived assets used in operations when indicators of impairment are present and the undiscounted cash flows estimated to be generated by those assets are less than the assets’ carrying amount. Impaired assets are then recorded at their estimated fair value. There were no impairment losses during the three and nine months ended September 30, 2024 and 2023.
Research and Development Expenses
These costs primarily include external development expenses and internal personnel expenses for its development programs, vidofludimus calcium and IMU-856. Immunic has spent the majority of its research and development resources on vidofludimus calcium, the Company's lead development program, for clinical trials in MS and UC.
Research and development expenses consist of expenses incurred in research and development activities, which include clinical trials, contract research services, certain milestone payments, salaries and related employee benefits, allocated facility costs and other outsourced services. Research and development expenses are charged to operations as incurred.
The Company enters into agreements with contract research organizations (“CROs”) to provide clinical trial services for individual studies and projects by executing individual work orders governed by a Master Service Arrangement (“MSA”). The MSAs and associated work orders provide for regular recurrent payments and payments upon the completion of certain milestones. The Company regularly assesses the timing of payments against actual costs incurred to ensure a proper accrual of related expenses in the appropriate accounting period.
Collaboration Arrangements
Certain collaboration and license agreements may include payments to or from the Company of one or more of the following: non-refundable or partially refundable upfront or license fees; development, regulatory and commercial milestone payments; payment for manufacturing supply services; partial or complete reimbursement of research and development costs; and royalties on net sales of licensed products. The Company assesses whether such contracts are within the scope of Financial Accounting Standards Board (FASB) Accounting Standards Update (“ASU”) 2014-09 “Revenue from Contracts with Customers” and ASU No. 2018-18, “Collaborative Arrangements” ("ASU 2018-18"). ASU 2018-18, clarifies that certain elements of collaborative arrangements could qualify as transactions with customers in the scope of ASC 606.
In October 2018, the Company entered into an option and license agreement (the "Daiichi Sankyo Agreement") with Daiichi Sankyo Co., Ltd. ("Daiichi Sankyo") which granted the Company the right to license a group of compounds, designated by the Company as IMU-856, as a potential new oral treatment option for gastrointestinal diseases such as celiac disease, inflammatory bowel disease, irritable bowel syndrome with diarrhea and other barrier function associated diseases. During the option period, the Company performed agreed upon research and development activities for which it was reimbursed by Daiichi Sankyo up to a maximum agreed-upon limit. Such reimbursement was recorded as other income. There are no additional research and development reimbursements expected under this agreement.
On January 5, 2020, the Company exercised its option to obtain the exclusive worldwide right to commercialization of IMU-856. Among other things, the option exercise grants Immunic AG the rights to Daiichi Sankyo’s patent application related to IMU-856, for which the Company received a notice of allowance from the U.S. Patent & Trademark Office in August 2022. In connection with the option exercise, the Company paid a one-time upfront licensing fee to Daiichi Sankyo. Under the Daiichi Sankyo Agreement, Daiichi Sankyo is also eligible to receive future development, regulatory and sales milestone payments, as well as royalties related to IMU-856.
Government assistance
Government assistance relating to research and development performed by Immunic Australia is recorded as a component of other (income) expense. This government assistance is recognized at a rate of 43.5% of the qualified research and development expenditures which are incurred. We also receive government assistance from the German Government for reimbursement of research and development expenses up to 3.5 million Euros per year. We recognized $0.7 million and $0.7 million of other income related to research activities performed during the three and nine months ended September 30, 2024, respectively and $0.2 million and $2.3 million related to research activities performed during the three and nine months ended September 30, 2023, respectively.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and related costs for personnel in executive, finance, business development and other support functions. Other general and administrative expenses include, but are not limited to, stock-based compensation, insurance costs, professional fees for legal, accounting and tax services, consulting, related facility costs and travel.
Stock-Based Compensation
The Company measures the cost of employee and non-employee services received in exchange for equity awards based on the grant-date fair value of the award recognized generally as an expense (i) on a straight-line basis over the requisite service period for those awards whose vesting is based upon a service condition, and (ii) on an accelerated method for awards whose vesting is based upon a performance condition, but only to the extent it is probable that the performance condition will be met. Stock-based compensation is (i) estimated at the date of grant based on the award’s fair value for equity classified awards and (ii) final measurement date for liability classified awards. Forfeitures are recorded in the period in which they occur.
The Company estimates the fair value of stock options using the Black-Scholes-Merton option-pricing model ("BSM"), which requires the use of estimates and subjective assumptions, including the risk-free interest rate, the fair value of the underlying common stock, the expected dividend yield of the Company’s common stock, the expected volatility of the price of the Company’s common stock, and the expected term of the option. These estimates involve inherent uncertainties and the application of management’s judgment. If factors change and different assumptions are used, the Company’s stock-based compensation expense could be materially different in the future.
Leases
The Company leases office space and office equipment. The underlying lease agreements have lease terms of less than 12 months and up to 60 months. Leases with terms of 12 months or less at inception are not included in the operating lease right of use asset and operating lease liability.
The Company has three existing leases for office and laboratory space. At inception of a lease agreement, the Company determines whether an agreement represents a lease and at commencement each lease agreement is assessed as to classification as an operating or financing lease. The Company's leases have been classified as operating leases and an operating lease right-of-use asset and an operating lease liability have been recorded on the Company’s balance sheet. A right-of-use lease asset represents the Company’s right to use the underlying asset for the lease term and the lease obligation represents its commitment to make the lease payments arising from the lease. Right-of-use lease assets and obligations are recognized at the commencement date based on the present value of remaining lease payments over the lease term. As the Company’s leases do not provide an implicit rate, the Company has used an estimated incremental borrowing rate based on the information available at the commencement date in determining the present value of lease payments. The right-of-use lease asset includes any lease payments made prior to commencement and excludes any lease incentives. The lease term used in estimating future lease payments may include options to extend when it is reasonably certain that the Company will exercise that option. Operating lease expense is recognized on a straight-line basis over the lease term, subject to any changes in the lease or changes in expectations regarding the lease term. Variable lease costs such as common area costs and property taxes are expensed as incurred. Leases with an initial term of twelve months or less are not recorded on the balance sheet.
Comprehensive Income (Loss)
Comprehensive income (loss) is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources. Accumulated other comprehensive income (loss) has been reflected as a separate component of stockholders’ equity in the accompanying Consolidated Balance Sheets and consists of foreign currency translation adjustments.
Income Taxes
The Company is subject to corporate income tax laws and regulations in the U.S., Germany and Australia. Tax regulations within each jurisdiction are subject to the interpretation of the related tax laws and regulations and require significant judgment in their application.
The Company utilizes the asset and liability method of accounting for income taxes which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the consolidated financial statements. Deferred income tax assets and liabilities are determined based on the differences between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. The effect of changes in tax rates on deferred tax assets and liabilities is recognized in operations in the period that includes the enactment date. Deferred taxes are reduced by a valuation allowance when, in the opinion of management, it is more likely than not some portion or the entire deferred tax asset will not be realized. As of September 30, 2024 and 2023, respectively, the Company maintained a full valuation allowance against the balance of deferred tax assets.
It is the Company’s policy to provide for uncertain tax positions and the related interest and penalties based upon management’s assessment of whether a tax benefit is more likely than not to be sustained upon examination by tax authorities. The Company recognizes interest and penalties accrued on any unrecognized tax benefits as a component of income tax expense. The Company is subject to U.S. federal, New York, California, Texas, German and Australian income taxes. The Company is subject to U.S. federal or state income tax examination by tax authorities for tax returns filed for the years 2003 and forward due to the carryforward of NOLs. Tax years 2019 through 2023 are subject to audit by German and Australian tax authorities. The Company is not currently under examination by any tax jurisdictions.
Warrants and Tranche Rights
The Company accounts for issued financial instruments either as a liability or equity in accordance with ASC 480-10, Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity (“ASC 480-10”) or ASC 815-40, Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock (“ASC 815-40”). If financial instruments do not meet liability classification under ASC 480-10, the Company considers the
requirements of ASC 815-40 to determine whether the financial instruments should be classified as a liability or as equity. Liability-classified financial instruments are measured at fair value on the issuance date and at the end of each reporting period. Any change in the fair value of the financial instruments after the issuance date is recorded in the consolidated statements of operations as a gain or loss. If financial instruments do not require liability classification under ASC 815-40, the instrument is classified in permanent equity. Equity-classified financial instruments are accounted for at fair value on the issuance date with no changes in fair value recognized after the issuance date.
Net Loss Per Share
Basic net loss per share attributable to common stockholders is calculated by dividing the net loss by the weighted-average number of common shares outstanding for the period, without consideration for common stock equivalents. Diluted net loss per share attributable to common stockholders is computed by dividing the net loss by the weighted-average number of common shares and, if dilutive, common stock equivalents outstanding for the period determined using the treasury-stock method. For all periods presented, there is no difference in the number of shares used to calculate basic and diluted shares outstanding due to the Company’s net loss position. The weighted average shares outstanding calculation for basic and diluted earnings per share for the three and nine months ended September 30, 2024 includes 11,193,564 pre-funded warrants that remain unexercised as of September 30, 2024.
Potentially dilutive securities, not included in the calculation of diluted net loss per share attributable to common stockholders because to do so would be anti-dilutive, are as follows: | | | | | | | | | | | |
| As of September 30, |
| 2024 | | 2023 |
| Options to purchase common stock | 11,546,138 | | | 6,263,910 | |
| | | |
Recently Issued and/or Adopted Accounting Standards
There are no recently issued accounting standards that would have a significant impact on the company's consolidated financial statements.
3. Balance Sheet Details
Other Current Assets and Prepaid Expenses
Other Current Assets and Prepaid Expenses consist of (in thousands): | | | | | | | | | | | |
| | | |
| September 30, 2024 | | December 31, 2023 |
| Prepaid clinical and related costs | $ | 2,066 | | | $ | 2,314 | |
| VAT receivable | 780 | | | 703 | |
| Australian research and development tax incentive | 39 | | | 670 | |
| Research grant | — | | | 1,104 | |
| Prepaid Insurance | 553 | | | 198 | |
| Other | 757 | | | 871 | |
| Total | $ | 4,195 | | | $ | 5,860 | |
Accounts Payable
Accounts Payable consist of (in thousands): | | | | | | | | | | | |
| | | |
| September 30, 2024 | | December 31, 2023 |
| Clinical costs | $ | 5,789 | | | $ | 4,726 | |
| Legal and audit costs | 90 | | | 160 | |
| | | |
| Other | 163 | | | 213 | |
| Total | $ | 6,042 | | | $ | 5,099 | |
Accrued Expenses
Accrued expenses consist of (in thousands): | | | | | | | | | | | |
| | | |
| September 30, 2024 | | December 31, 2023 |
| Accrued clinical and related costs | $ | 14,386 | | | $ | 16,863 | |
| Accrued legal and audit costs | 136 | | | 216 | |
| Accrued compensation | 1,509 | | | 1,460 | |
| Accrued other | 214 | | | 125 | |
| Total | $ | 16,245 | | | $ | 18,664 | |
Other Current Liabilities
Other Current Liabilities consist of (in thousands): | | | | | | | | | | | | | | |
| | | | |
| September 30, 2024 | | December 31, 2023 | |
| Lease liabilities | $ | 713 | | | $ | 695 | | |
| Other | 357 | | | 271 | | |
| Total | 1,070 | | | 966 | | |
4. Commitments and Contingencies
Operating Leases
The Company leases certain office space under non-cancelable operating leases. The leases terminate on July 31, 2025 for the New York City office, June 30, 2025 for the Gräfelfing, Germany office and November 30, 2028 for the research laboratory in Planegg, Germany. These agreements include both lease (e.g., fixed rent) and non-lease components (e.g., common-area and other maintenance costs). The non-lease components are deemed to be executory costs and are therefore excluded from the minimum lease payments used to determine the present value of the operating lease obligation and related right-of-use asset. The New York City lease was extended on December 22, 2022 for an additional 27 months resulting in the new lease termination date of July 31, 2025. The New York City lease has a renewal option, but this was not included in calculating the right of use asset and liabilities. On April 7, 2020, the Company signed a five year lease for its facility in Gräfelfing, Germany. On March 1, 2021 and August 1, 2022 the Company added additional lease space at the Gräfelfing, Germany office. Renewal options were not included in calculating the right of use asset and liabilities for this facility. In February 2023, the Company leased space in Germany for a research laboratory. The leases do not have concessions, leasehold improvement incentives or other build-out clauses. Further, the leases do not contain contingent rent provisions. The New York City lease had a six month
rent holiday at the beginning of the lease as well as a three month rent holiday upon the 27 month extension starting May 2023. There were net additions of $544,000 related to the addition of new laboratory space in Planegg, Germany in February 2023.
The leases do not provide an implicit rate and, due to the lack of a commercially salable product, the Company is generally considered unable to obtain commercial credit. Therefore, the Company estimated its incremental interest rate to be 6% for the original leases and 8% for the New York City extension and German laboratory, considering the quoted rates for the lowest investment-grade debt and the interest rates implicit in recent financing leases. Immunic used its estimated incremental borrowing rate and other information available at the lease commencement date in determining the present value of the lease payments.
Immunic’s operating lease costs and variable lease costs were $260,000 and $209,000 for the three months ended September 30, 2024 and 2023, respectively and $787,000 and $642,000 for the nine months ended September 30, 2024 and 2023, respectively. Variable lease costs consist primarily of common area maintenance costs, insurance and taxes which are paid based upon actual costs incurred by the lessor.
Maturities of the operating lease obligation are as follows as of September 30, 2024 (in thousands): | | | | | | | | |
| 2024 | $ | 275 | |
| 2025 | | 433 | |
| 2026 | | 78 | |
| 2027 | | 82 | |
| 2028 | | 79 | |
| Thereafter | | — | |
| Total | | 947 | |
| Interest | | (48) | |
| Present Value of obligation | $ | 899 | |
Contractual Obligations
As of September 30, 2024, the Company has non-cancelable contractual obligations under certain agreements related to its development programs for vidofludimus calcium and IMU-856 totaling approximately $3.2 million, all of which is expected to be paid in 2024 and 2025.
Other Commitments and Obligations
Daiichi Sankyo Agreement
On January 5, 2020, the Company exercised its option to obtain the exclusive worldwide right to commercialization of IMU-856. Among other things, the option exercise grants Immunic AG the rights to Daiichi Sankyo’s patent application related to IMU-856, for which the Company received a notice of allowance from the U.S. Patent & Trademark Office in August 2022. In connection with the option exercise, the Company paid a one-time upfront licensing fee to Daiichi Sankyo. Under the Daiichi Sankyo Agreement, Daiichi Sankyo is also eligible to receive future development, regulatory and sales milestone payments, as well as royalties related to IMU-856.
Legal Proceedings
The Company is not currently a party to any litigation, nor is it aware of any pending or threatened litigation, that it believes would materially affect its business, operating results, financial condition or cash flows. However, its industry is characterized by frequent claims and litigation including securities litigation, claims regarding patent and other intellectual property rights and claims for product liability. As a result, in the future, the Company may be involved in various legal proceedings from time to time.
5. Fair Value
The following fair value hierarchy tables present information about each major category of the Company’s financial assets and liabilities measured at fair value on a recurring basis (in thousands): | | | | | | | | | | | | | | | | | | | | | | | |
| | Fair Value Measurement at September 30, 2024 |
| | Fair Value | | Level 1 | | Level 2 | | Level 3 |
| Assets | | | | | | | |
| Money market funds | $ | 40,359 | | | $ | 40,359 | | | $ | — | | | $ | — | |
| Total assets at fair value | $ | 40,359 | | | $ | 40,359 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | |
| Fair Value Measurement at December 31, 2023 |
| Fair Value | | Level 1 | | Level 2 | | Level 3 |
| Assets | | | | | | | |
| Money market funds | $ | 34,087 | | | $ | 34,087 | | | $ | — | | | $ | — | |
| Total assets | $ | 34,087 | | | $ | 34,087 | | | $ | — | | | $ | — | |
There were no transfers between Level 1, Level 2 or Level 3 assets during the periods presented.
For the Company’s money market funds which are included as a component of cash and cash equivalents on the consolidated balance sheet, realized gains and losses are included in interest income on the consolidated statements of operations.
Our money market fund account is held in our bank in the U.S. and was earning interest at a rate of 4.8% in a U.S. Government money market fund.
The Company has cash balances in banks in excess of the maximum amount insured by the FDIC and other international agencies as of September 30, 2024. The Company has not historically experienced any credit losses with balances in excess of FDIC limits.
The Company recorded tranche rights of $23.6 million at January 8, 2024 as a result of the January 2024 Financing (see note 6). The fair value measurement of the tranche rights associated with the January 2024 Financing was classified as Level 3 under the fair value hierarchy. The fair value of the tranche rights was determined using a Black Scholes Option Pricing Model. The inputs to this model included a risk-free rate range of 3.93%-4.36%, a stock price volatility range of 105-115%, an expected dividend rate of —% and remaining term of 1.81-4.81 years. This liability was revalued on March 4, 2024, upon stockholder approval to increase its authorized shares of common stock from 130 million to 500 million, which resulted in the reclassification of the tranche rights from a liability to equity. This revaluation resulted in an increase in the tranche rights liability of $4.8 million using a Black Scholes Option Pricing Model. The inputs to this model as of the date of the reclassification included a risk-free rate range of 4.16%-4.63%, a stock price volatility range of 90-105%, an expected dividend rate of —% and remaining term of 1.66-4.66 years. The inputs used in the determination of fair value of the liability are level 3 inputs. A rollforward of the fair value of the tranche rights is as follows (in thousands):
| | | | | |
| December 31, 2023 | $ | — | |
| Fair value as of January 8, 2024 | $ | 23,600 | |
| Change in fair value through March 4, 2024 | $ | 4,796 | |
| Reclassification to equity | $ | (28,396) | |
| March 31, 2024 | $ | — | |
The carrying amounts of other current assets and prepaid expenses, accounts payable, accrued expenses, and other current liabilities approximate their fair values due to their short-term nature. The fair value and book value of the money market funds presented in the table above are the same.
6. Common Stock
In December 2020, the Company filed a Prospectus Supplement to the shelf registration statement on Form S-3 filed on November 13, 2020 and declared effective on November 24, 2020 (the "2020 Shelf Registration Statement") for the offering, issuance and sale of up to a maximum aggregate offering price of $50.0 million of common stock that may be issued and sold under an at-the-market sales agreement with SVB Leerink LLC (now Leerink Partners LLC) as agent ("December 2020 ATM"). The Company used the net proceeds from the December 2020 ATM to fund the ongoing clinical development of our product candidates and for other general corporate purposes, including funding existing and potential new clinical programs and product candidates. The December 2020 ATM terminated in May 2024.
In May 2022, the Company filed a Prospectus Supplement to the 2020 Shelf Registration Statement for the offering, issuance and sale of up to a maximum aggregate offering price of $80.0 million of common stock to be issued and sold under another at-the-market sales agreement ("May 2022 ATM") with Leerink Partners LLC (formerly SVB Leerink LLC) as agent. The 2020 Shelf Registration Statement expired in November 2023.
In November 2023, we filed a shelf registration statement on Form S-3 (the "2023 Shelf Registration Statement"). The 2023 Shelf Registration Statement permits the offering, issuance and sale of up to $250.0 million of common stock, preferred stock, warrants, debt securities, and/or units in one or more offerings and in any combination of the foregoing. This registration statement was declared effective on May 31, 2024. Unsold securities from the expired 2020 shelf registration statement can continue to be sold under the 2023 Shelf Registration Statement resulting in a total S-3 shelf availability of $412.3 million as of September 30, 2024.
In May 2024, we filed a Prospectus Supplement to the 2023 Shelf Registration Statement for the offering, issuance and sale of up to a maximum aggregate offering price of $80.0 million of common stock that may be issued and sold under an at-the-market sales agreement with Leerink Partners LLC as agent ("May 2024 ATM"), which rolls over the $80.0 million of unsold common stock from the May 2022 ATM. We intend to use the net proceeds from the May 2024 ATM to continue to fund the ongoing clinical development of our product candidates and for other general corporate purposes, including funding existing and potential new clinical programs and product candidates. The May 2024 ATM will terminate upon the earlier of (i) the issuance and sale of all of the shares through Leerink Partners LLC on the terms and subject to the conditions set forth in the May 2024 ATM or (ii) termination of the May 2024 ATM as otherwise permitted thereby. The May 2024 ATM may be terminated at any time by either party upon ten days’ prior notice, or by Leerink Partners LLC at any time in certain circumstances, including the occurrence of a material adverse effect on us. As of September 30, 2024, $80.0 million in capacity remains under the May 2024 ATM.
The Company has agreed to pay Leerink Partners LLC a commission equal to 3.0% of the gross proceeds from the sales of common stock pursuant to the May 2024 ATM and has agreed to provide Leerink Partners LLC with customary indemnification and contribution rights.
For the nine months ended September 30, 2024, the Company raised gross proceeds of $0.2 million pursuant to the December 2020 ATM through the sale of 150,000 shares of common stock at a weighted average price of $1.31 per share. The net proceeds from the December 2020 ATM were $0.2 million after deducting sales agent commissions of $6,000. The Company did not have any ATM activity for the three months ended September 30, 2024.
In the three and nine months ended September 30, 2023, the Company raised gross proceeds of $0.3 million pursuant to the December 2020 ATM through the sale of 107,012 shares of common stock at a weighted average price of $2.72 per share. The net proceeds from the December 2020 ATM were $0.3 million after deducting underwriter commissions of $9,000.
Equity Offerings
Private Placement of up to $240 million (the "January 2024 Financing")
On January 4, 2024, Immunic entered into a Securities Purchase Agreement with select accredited investors, pursuant to which the Company agreed to issue and sell to the Investors in a three-tranche private placement shares of the Company’s common stock, $0.0001 par value per share, or in lieu thereof, pre-funded warrants to purchase shares of Common Stock. The Pre-Funded Warrants are exercisable immediately for $0.0001 per share and until exercised in full.
•The first tranche, which closed on January 8, 2024, resulted in the purchase by the Investors of an aggregate of $80 million of Common Stock (or pre-funded warrants) from the Company at a price of $1.43 per share;
•The second tranche is a conditional mandatory purchase by the Investors of an additional $80 million of Common Stock (or pre-funded warrants) from the Company at a price of $1.716 per share, equal to 120% of the price paid in the first tranche and is subject to the satisfaction of three conditions:
◦release by the Company of topline data from its Phase 2b clinical trial of vidofludimus calcium (IMU-838) in progressive multiple sclerosis, which data is currently expected in or around April 2025;
◦the 10-day volume-weighted average price of the Common Stock is at least $8.00 per share during the 6 months following the data release; and
◦aggregate trading volume during the same 10-day period is at least $100 million.
•The third tranche must occur no later than three years after the second tranche and is conditioned on the same volume-weighted average share price and minimum trading volumes as the second tranche. The third tranche provides for the issuance of $80 million of shares of common stock (or pre-funded warrants) at the same price per share as the second tranche, but permits investors to fund their purchase obligations on a “cashless” or net settlement basis, which would reduce the cash proceeds to be raised by the Company in the January 2024 Financing.
Any of the conditions in the second or third tranches can be waived by holders of a majority of the outstanding securities (including the lead investor). The fair value methodology used by the Company assumed the conditions will be waived if the trading price of the stock exceeds the purchase price.
The January 2024 Financing resulted in gross proceeds to the Company of approximately $80 million in the first tranche, and an additional $80 million if and when the second tranche occurs. If the second tranche is completed and conditions for the third tranche are satisfied or waived, the Company could receive up to an additional $80 million in the third tranche.
As of the closing date of the transaction of January 8, 2024, the Company did not have enough authorized shares to be able to issue the potential shares for tranche 2 and tranche 3 (collectively referred to hereafter as "the tranche rights"). Therefore, the Company recorded the value associated to the tranche rights as a liability of $23.6 million and allocated the remainder of the $80 million received (or $56.4 million) with the common stock and pre-funded warrants to equity. On March 4, 2024, the stockholders voted to increase the Company's authorized common stock from 130 million to 500 million shares. As a result of the ability to issue shares in satisfaction of the tranche rights, the instrument was reclassified to stockholders' equity. The Company allocated the transaction costs across the instruments on a relative fair value basis at the grant date. As a result $4.0 million was netted against the equity proceeds and $1.7 million was recorded in other expense in the Consolidated Statements of Operations for the nine months ended September 30, 2024.
The Company registered for resale by the investors in the January 2024 Financing up to 55,944,850 shares of common stock issued (or issuable upon exercise of pre-funded warrants) in the first tranche. The Company will not receive any proceeds from the sale of these shares of common stock. These shares are registered on a registration statement on Form S-3 (registration No. 333-277040).
Common Stock
On March 4, 2024, the stockholders of the Company voted to increase the authorized shares of the Company from 130,000,000 shares of common stock to 500,000,000 shares of common stock, par value of $0.0001 per share. The voting, dividend and liquidation rights of the holders of the Company’s common stock are subject to and qualified by the rights, powers and preferences of any holders of preferred stock.
Each share of common stock entitles the holder to one vote on all matters submitted to a vote of the Company’s stockholders. Common stockholders are entitled to receive dividends, as may be declared by the Board of Directors, if any. Through September 30, 2024, no cash dividends had been declared or paid.
Pre-funded Warrants
The Company issued 11,193,564 pre-funded warrants in connection with the January 2024 Financing, which all remain outstanding as of September 30, 2024.
Preferred Stock
The Company’s certificate of incorporation, as amended and restated, authorizes the Company to issue 20 million shares of $0.0001 par value preferred stock, with such voting powers (if any), designations, powers, preferences, and relative, participating, optional or other rights, if any, and any qualifications, limitations or restrictions thereof, as shall be set by the Board of Directors. No preferred shares were issued or outstanding as of September 30, 2024.
Stock Reserved for Future Issuance
Shares reserved for future issuance at September 30, 2024 are as follows: | | | | | |
| Number of
Shares |
| Common stock reserved for issuance for: | |
| 2021 Employee Stock Purchase Plan | 1,000,011 | |
| Pre-funded stock warrants | 11,193,564 | |
| Outstanding stock options | 11,546,138 | |
| Common shares reserved for tranche 2 rights | 46,620,046 | |
| Maximum common shares reserved for tranche 3 rights | 46,620,046 | |
| Common stock options available for future grant: | |
| 2017 Inducement Equity Incentive Plan | 46,250 | |
| 2019 Omnibus Equity Incentive Plan | 7,903,691 | |
| Total common shares reserved for future issuance | 124,929,746 | |
7. Stock-Based Compensation Plans
2021 Employee Stock Purchase Plan
On April 25, 2021, the Company adopted the 2021 Employee Stock Purchase Plan ("ESPP"), which was approved by stockholder vote at the 2021 Annual Meeting of Stockholders held on June 10, 2021. The ESPP provides eligible employees of the Company with an opportunity to purchase common stock of the Company through accumulated payroll deductions, which are included in other current liabilities until they are used to purchase Company shares. Eligible employees participating in the bi-annual offering period can choose to have up to the lesser of 15% of their annual base earnings or the IRS annual share purchase limit of $25,000 in aggregate market value to purchase shares of the Company’s common stock. The purchase price of the stock is the lesser of (i) 85% of the closing market price on the date of purchase and (ii) the closing market price at the beginning of the bi-annual offering period. The maximum number of shares initially reserved for delivery under the plan was 200,000 shares. An increase of 1 million shares to 1.2 million shares was approved by stockholders of the Company at the Company's Special Meeting of stockholders held on March 4, 2024.
The first enrollment period under the plan commenced on August 1, 2021 and the Company has issued 199,989 shares life-to-date under the ESPP. The Company recognized $24,000 of expense related to the plan during the three and nine months ended September 30, 2024. The Company recognized $19,000 and $102,000 of expense related to the plan during the three and nine months ended September 30, 2023, respectively.
Stock Option Programs
In July 2019, the Company’s stockholders approved the 2019 Omnibus Equity Incentive Plan, (as amended, the “2019 Plan”), which was adopted by the Board of Directors (the "Board") with an effective date of June 14, 2019. The 2019 Plan allows for the grant of equity awards to employees, consultants and non-employee directors. An initial maximum of 1,500,000 shares of the Company’s common stock were available for grant under the 2019 Plan. The 2019 Plan included an evergreen provision that allowed for the annual addition of up to 4% of the Company’s fully-diluted outstanding stock, with a maximum allowable increase of 4,900,000 shares over the term of the 2019 Plan. In accordance with this provision, the shares available for grant were increased in 2020 through 2023 by a total of 4,408,871 shares. At the Company's Annual Stockholders meeting on June 28, 2023, stockholders voted to increase the allowable shares under the 2019 plan by 4,440,000 shares as well as to eliminate the evergreen provision. On March 4, 2024, the stockholders voted at the Company's Special Meeting to increase the allowable shares under the 2019 plan by 9,100,000. The 2019 Plan (as amended on June 28, 2023 and March 4, 2024) is currently administered by the Board, or, at the discretion of the Board, by a committee of the Board, which determines the exercise prices, vesting schedules and other restrictions of awards under the 2019 Plan at its discretion. Options to purchase stock may not have an exercise price that is less than the fair market value of underlying shares on the date of grant, and may not have a term greater than ten years. Incentive stock options granted to employees typically vest over four years. Non-statutory options granted to employees, officers, members of the Board, advisors, and consultants of the Company typically vest over three or four years.
Shares that are expired, terminated, surrendered or canceled under the 2019 Plan without having been fully exercised will be available for future awards.
Stock Option Repricing
On March 4, 2024, the Company's stockholders voted to approve the repricing of outstanding stock options having an exercise price above $3.00 per share to $1.72 per share. All other terms of the grant remained the same. There were 3,317,596 stock options that were repriced to $1.72 per share. The repricing will result in $1.2 million of stock compensation being recognized by the Company over the remaining term of the repriced grants and $86,000 and $1.0 million of this amount was recognized in the three months and nine months ended September 30, 2024, respectively.
Movements during the year
The following table summarizes stock option activity for the nine months ended September 30, 2024 and 2023, respectively, for the 2019 Plan: | | | | | | | | | | | | | | | | | | | | | | | |
| Options | | Weighted-
Average
Exercise
Price | | Weighted-
Average
Remaining
Contractual
Term (Years) | | Aggregate
Intrinsic
Value |
| Outstanding as of January 1, 2024 | 6,196,140 | | | $ | 7.15 | | | | | |
| Granted | 5,715,349 | | | $ | 1.23 | | | | | |
| Exercised | — | | | $ | — | | | — | | | |
| Repricing modification | — | | | $ | 9.55 | | | | | |
| Forfeited or expired | (365,351) | | | $ | 2.08 | | | | | |
| Outstanding as of September 30, 2024 | 11,546,138 | | | $ | 1.63 | | | 8.33 | | $ | 2,794,286 | |
| Options vested and expected to vest as of September 30, 2024 | 11,546,138 | | | $ | 1.63 | | | 8.33 | | $ | 2,794,286 | |
| Options exercisable as of September 30, 2024 | 4,171,969 | | | $ | 2.09 | | | 7.07 | | $ | 329,830 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| Options | | Weighted-
Average
Exercise
Price | | Weighted-
Average
Remaining
Contractual
Term (Years) | | Aggregate
Intrinsic
Value |
| Outstanding as of January 1, 2023 | 3,791,688 | | | $ | 11.33 | | | | | |
| Granted | 2,740,564 | | | $ | 1.71 | | | | | |
| Exercised | — | | | $ | — | | | | | |
| Forfeited or expired | (268,342) | | | $ | 7.80 | | | | | |
| Outstanding as of September 30, 2023 | 6,263,910 | | | $ | 7.27 | | | 8.27 | | $ | 119,691 | |
| Options vested and expected to vest as of September 30, 2023 | 6,263,910 | | | $ | 7.27 | | | 8.27 | | $ | 119,691 | |
| Options exercisable as of September 30, 2023 | 2,393,294 | | | $ | 11.67 | | | 7.12 | | $ | 6,160 | |
Measurement
The weighted-average assumptions used in the BSM option pricing model to determine the fair value of the employee and non-employee stock option grants relating to the 2019 Plan were as follows:
Risk-Free Interest Rate
The risk-free rate assumption is based on U.S. Treasury instruments with maturities similar to the expected term of the stock options.
Expected Dividend Yield
The Company has not issued any dividends and does not expect to issue dividends over the life of the options. As a result, the Company has estimated the dividend yield to be zero.
Expected Volatility
Due to the Company’s limited operating history and a lack of company specific historical and implied volatility data, the Company estimates expected volatility based on the historical volatility of its own stock combined with a group of comparable companies that are publicly traded. The historical volatility data was computed using the daily closing prices for the selected companies’ shares during the equivalent period of the calculated expected term of the stock-based awards.
Expected Term
The expected term of options is estimated considering the vesting period at the grant date, the life of the option and the average length of time similar grants have remained outstanding in the past.
The weighted-average grant date fair value of stock options granted under the 2019 Plan during the nine months ended September 30, 2024 and 2023 was $0.99 and $1.32, respectively. The following are the underlying assumptions used in the Black-Scholes-Merton option pricing model to determine the fair value of stock options granted to employees and to non-employees under this stock plan:
| | | | | | | | |
| Nine Months Ended September 30, |
| 2024 | 2023 |
| Risk-free interest rate | 4.11% | 4.00% |
| Expected dividend yield | 0% | 0% |
| Expected volatility | 100.0% | 96.0% |
| Expected term of options (years) | 5.95 | 6.01 |
Stock-Based Compensation Expense
Total stock-based compensation expense for all stock awards recognized in the accompanying unaudited condensed consolidated statements of operations is as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Three Months
Ended September 30, | | Nine Months
Ended September 30, |
| | | | | | 2024 | | 2023 | | 2024 | | 2023 |
| Research and development | | | | | $ | 811,000 | | | $ | 815,000 | | | $ | 2,816,000 | | | $ | 2,607,000 | |
| General and administrative | | | | | 1,099,000 | | | 872,000 | | | 3,726,000 | | | 2,857,000 | |
| Total | | | | | $ | 1,910,000 | | | $ | 1,687,000 | | | $ | 6,542,000 | | | $ | 5,464,000 | |
As of September 30, 2024, there was $10.8 million in total unrecognized compensation expense relating to the 2019 Plan to be recognized over a weighted average period of 2.82 years.
Summary of Equity Incentive Plans Assumed from Vital
Upon completion of the Transaction with Vital Therapies ("Vital") on April 12, 2019, Vital’s 2012 Stock Option Plan (the “2012 Plan”), Vital’s 2014 Equity Incentive Plan (the “2014 Plan”) and Vital’s 2017 Inducement Equity Incentive Plan (the “Inducement Plan”), were assumed by the Company. All awards granted under these plans have either been forfeited or expired.
There are no longer any shares available for grant under the 2014 Plan as of September 30, 2024.
On September 2017, Vital’s board of directors approved the Inducement Plan, which was amended and restated in November 2017. Under the Inducement Plan 46,250 shares of Vital’s common stock were reserved to be used exclusively for non-qualified grants to individuals who were not previously employees or directors as an inducement material to a grantee's entry into employment within the meaning of Rule 5635(c)(4) of the Nasdaq Listing Rules.
No expense was recorded for the plans assumed from Vital during the three and nine months ended September 30, 2024 and 2023, respectively.
8. Related Party Transactions
Executive Chairman Agreement with Duane Nash
On April 15, 2020, the compensation committee of the Board of Directors of the Company independently reviewed and approved entering into an employment agreement with the Executive Chairman of the Board, Duane Nash, MD, JD, MBA (the “Executive Chairman Agreement”) and pursuant to such approval, on April 17, 2020, the Company and Dr. Nash entered into the Executive Chairman Agreement. The Executive Chairman Agreement establishes an “at will” employment relationship. On December 28, 2022, the Company and Dr. Nash entered into Addendum No. Four, which extended the term of employment from December 31, 2022 to December 31, 2023 with a base salary of $30,250 per month. On October 17, 2023, Immunic, Inc. and Dr. Duane Nash entered into Addendum Number 5 to the Executive Chairman Agreement to extend the term of Dr. Nash’s employment as Executive Chairman of the Board of Directors of the Company to December 31, 2024. In connection with Addendum Number 5, the Company increased Dr. Nash’s monthly base salary to $32,368 from $30,250 (which includes the cash retainer payable for serving on the Company’s Board or for acting as the Chairman of the Board). On August 29, 2024, Immunic, Inc. and Dr. Duane Nash entered into Addendum Number 6 to the Executive Chairman Agreement to extend the term of Dr. Nash’s employment as Executive Chairman of the Board of Directors of the Company to December 31, 2025. In connection with Addendum Number 6, the Company increased Dr. Nash’s monthly base salary to $33,986 from $32,368 (which includes the cash retainer payable for serving on the Company’s Board or for acting as the Chairman of the Board) All other terms of the Executive Chairman Agreement remain the same.
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis of financial condition and results of operations should be read in conjunction with our unaudited interim condensed consolidated financial statements and notes thereto included in Item 1 “Financial Statements” in this Quarterly Report and audited Consolidated Financial Statements for the years ended December 31, 2023 and 2022 of Immunic, Inc. filed with the Securities and Exchange Commission ("SEC"), in our Annual Report on Form 10-K on February 22, 2024. As used in this report, unless the context suggests otherwise, “we,” “us,” “our,” “the Company” or “Immunic” refer to Immunic, Inc. and its subsidiaries.
Forward-Looking Statements
In addition to historical information, this Quarterly Report includes forward-looking statements within the meaning of federal securities laws. Forward-looking statements are subject to certain risks and uncertainties, many of which are beyond our control. Such statements include, but are not limited to, statements preceded by, followed by or that otherwise include the words, “believe,” “may,” “might,” “can,” “could,” “will,” “would,” “should,” “estimate,” “continue,” “anticipate,” “intend,” “seek,” “plan,” “project,” “expect,” “potential,” “predicts,” or similar expressions and the negatives of those terms.
Forward-looking statements discuss matters that are not historical facts. Our forward-looking statements involve assumptions that, if they ever materialize or prove correct, could cause our results to differ materially from those expressed or implied by such forward-looking statements. In this Quarterly Report, for example, we make forward-looking statements, among others, regarding potential strategic options; financial estimates and projections; and the sufficiency of our capital resources to fund our operations.
The inclusion of any forward-looking statements in this Quarterly Report should not be regarded as a representation that any of our plans will be achieved. Our actual results may differ from those anticipated in our forward-looking statements as a result of various factors, including those noted below under the caption “Part II, Item 1A-Risk Factors,” and the differences may be material. These risk factors include, but are not limited to statements relating to our two development programs and the targeted diseases; the potential for vidofludimus calcium and IMU-856 to safely and effectively target diseases; the nature, strategy and focus of the Company; expectations regarding our capitalization and financial resources; the development, timing and commercial potential of any product candidates of the Company; and our ability to attract and retain certain personnel
important to our ongoing operations and to maintain effective internal control over financial reporting.
Although our forward-looking statements reflect the good faith judgment of our management, these statements are based only on facts and factors currently known by us. As a result, investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, and we undertake no obligation to revise or update such statements to reflect events or circumstances after the date hereof, except as required by law.
Overview
Immunic, Inc. ("Immunic," “we,” “us,” “our” or the "Company") is a biotechnology company developing a clinical pipeline of selective oral immunology therapies focused on treating chronic inflammatory and autoimmune diseases. We are headquartered in New York City with our main operations in Gräfelfing near Munich, Germany. We had approximately 85 employees as of November 1, 2024.
We are pursuing clinical development of orally administered, small molecule programs, each of which has unique features intended to directly address the unmet needs of patients with serious chronic inflammatory and autoimmune diseases. These include the vidofludimus calcium (IMU-838) program, which is in Phase 3 and Phase 2 clinical development for patients with relapsing and progressive multiple sclerosis (“MS”), respectively, and which has shown therapeutic activity in Phase 2 clinical trials in patients suffering from relapsing-remitting MS, progressive MS and moderate-to-severe ulcerative colitis (“UC”); the IMU-856 program, which is targeted to regenerate bowel epithelium and restore intestinal barrier function, which could potentially be applicable in numerous gastrointestinal diseases, such as celiac disease, inflammatory bowel disease (“IBD”) and Graft-versus-Host-Disease ("GvHD"); and the IMU-381 program, which is a next generation molecule being developed to specifically address the needs of gastrointestinal diseases.
The following table summarizes the potential indications, clinical targets and clinical development status of our three product candidates:
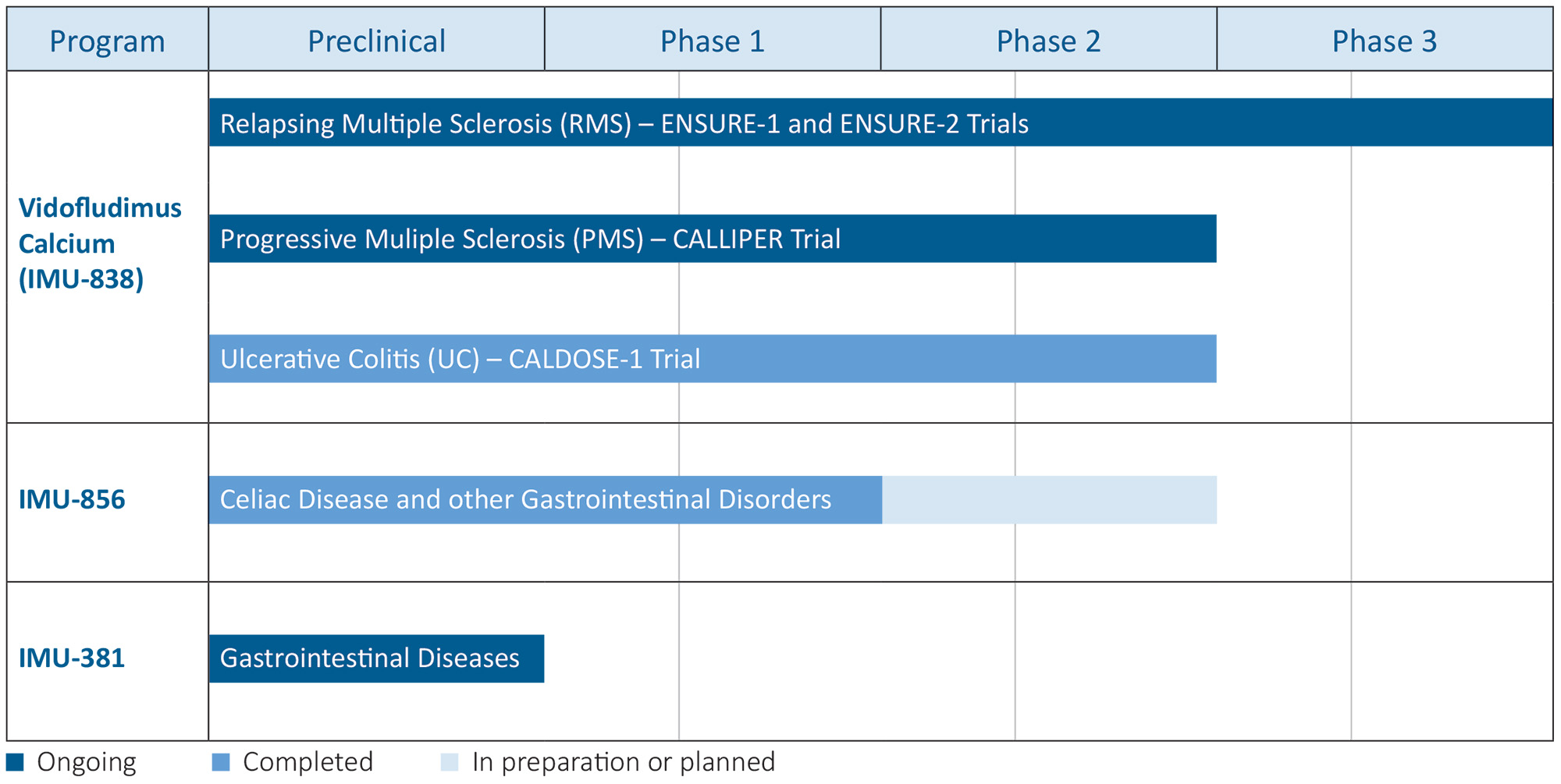
Our most advanced drug candidate, vidofludimus calcium (IMU-838), is being tested in several ongoing MS trials as part of its overall clinical program in order to support a potential approval for patients with MS in major markets. The Phase 3 ENSURE program of vidofludimus calcium in relapsing multiple sclerosis (“RMS”), comprising twin studies evaluating efficacy, safety, and tolerability of vidofludimus calcium versus placebo, and the Phase 2 CALLIPER trial of vidofludimus calcium in progressive multiple sclerosis (“PMS”), designed to corroborate vidofludimus calcium’s neuroprotective potential, are ongoing. On October 9, 2023, we announced positive interim data from the CALLIPER trial, showing biomarker evidence that vidofludimus calcium’s activity extends beyond the previously observed anti-inflammatory effects, thereby further reinforcing its neuroprotective potential. Top-line data from the CALLIPER trial, for which the recruitment of in total 467 patients was completed in August 2023, is expected to be available in April 2025. Moreover, on October 22, 2024, we
announced a positive outcome of an interim analysis of the ENSURE program, with an unblinded Independent Data Monitoring Committee ("IDMC") confirming that the predetermined futility criteria have not been met and recommending that both ENSURE trials should continue without changes, including no need for a potential upsizing of the sample size. Completion of the first of the ENSURE trials is currently anticipated in the second quarter of 2026; and the second ENSURE trial in the second half of 2026. Although we currently believe that each of these goals is achievable, they are each dependent on numerous factors, most of which are not under our direct control and can be difficult to predict. We plan to periodically review this assessment and provide updates of material changes as appropriate.
If approved, we believe that vidofludimus calcium, with combined neuroprotective, anti-inflammatory, and antiviral effects, has the potential to be a unique treatment option targeted to the complex pathophysiology of MS. Preclinical data showed that vidofludimus calcium activates the neuroprotective transcription factor nuclear receptor related 1 (“Nurr1”), which is associated with direct neuroprotective properties and may enhance the potential benefit for patients. Additionally, vidofludimus calcium is a known inhibitor of the enzyme dihydroorotate dehydrogenase (“DHODH”), which is a key enzyme in the metabolism of overactive immune cells and virus-infected cells. This mechanism is associated with the anti-inflammatory and antiviral effects of vidofludimus calcium. We believe that the combined mechanisms of vidofludimus calcium are unique in the MS space and support the therapeutic performance shown in our Phase 2 EMPhASIS trial in relapsing-remitting MS patients, in particular, via data illustrating the potential to reduce magnetic resonance imaging (“MRI”) lesions, prevent relapses, reduce the rate of disability progression, and reduce levels of serum neurofilament light chain (“NfL”), an important biomarker of neuronal damage. Vidofludimus calcium has shown in clinical trials reported to date a consistent pharmacokinetic, safety and tolerability profile and has already been exposed to more than 1,800 human subjects and patients in either of the drug’s formulations.
IMU-856 is an orally available and systemically acting small molecule modulator that targets Sirtuin 6 (“SIRT6”), a protein which serves as a transcriptional regulator of intestinal barrier function and regeneration of bowel epithelium. Based on preclinical data, we believe this compound may represent a unique treatment approach, as the mechanism of action targets the restoration of the intestinal barrier function and bowel wall architecture in patients suffering from gastrointestinal diseases such as celiac disease, IBD, GvHD and other intestinal barrier function associated diseases. Based on preclinical investigations demonstrating no suppression of immune cells, IMU-856 may have the potential to maintain immune surveillance for patients during therapy, which would be an important advantage versus immunosuppressive medications and may allow the potential for combination with available treatments in gastroenterological diseases.
Data from a Phase 1b clinical trial in celiac disease patients during periods of gluten-free diet and gluten challenge demonstrated positive effects for IMU-856 over placebo in four key dimensions of celiac disease pathophysiology: protection of the gut architecture, improvement of patients’ symptoms, biomarker response, and enhancement of nutrient absorption. IMU-856 was also observed to be safe and well-tolerated in this trial. We are currently preparing clinical Phase 2 testing of IMU-856 in patients with ongoing active celiac disease (“OACD”) despite gluten-free diet, while also considering further potential clinical applications in other gastrointestinal disorders.
Immunic has selected IMU-381 as a development candidate to specifically address the needs of gastrointestinal diseases. IMU-381 is a next generation molecule with improved overall properties, supported by a series of chemical derivatives. IMU-381 is currently in preclinical testing.
Additional research and development activities remain ongoing through preclinical research examining the potential to treat a broad set of neuroinflammatory, autoimmune and viral diseases with new molecules leveraging our chemical and pharmacological research platform as well as generated intellectual property in these areas.
We expect to continue to lead most of our research and development activities from our Gräfelfing, Germany location, where dedicated scientific, regulatory, clinical and medical teams conduct their activities. Due to these teams' key relationships with local and international service providers, we anticipate that this should result in more timely and cost-effective execution of our development programs. In addition, we are using our subsidiary in Melbourne, Australia to perform research and development activities in the Australasia region. We also conduct preclinical work in Halle/Saale, Germany through a collaboration with the Fraunhofer Institute.
Our business, operating results, financial condition and growth prospects are subject to significant risks and uncertainties, including delays in clinical trials, the failure of our clinical trials to meet their endpoints, failure to obtain regulatory approval and failure to obtain needed additional funding on acceptable terms, if at all, to complete the development and commercialization of our three development programs.
Strategy
We are focused on the development of new molecules that maximize the therapeutic benefits for patients by uniquely addressing biologically relevant immunological targets. We take advantage of our established research and development
infrastructure and operations in Germany and Australia to more efficiently develop our product candidates in indications of high unmet need and where the product candidates have the potential to elevate the standard of care for the benefit of patients. Given the mechanisms of action and the data generated for our product candidates, to date, we continue to execute on the clinical development of our programs for established indications as well as explore additional indications where patients could potentially benefit from the unique profiles of each product candidate.
We are currently focused on maximizing the potential of our development programs through the following strategic initiatives:
•Executing the ongoing Phase 3 ENSURE and Phase 2 CALLIPER clinical trial programs of vidofludimus calcium in RMS and PMS, respectively.
•Executing the IMU-856 development program, including preparation of a Phase 2 clinical trial.
•Continuing preclinical research to complement the existing clinical activities, explore additional indications for future development and generating additional molecules for potential future development.
•Facilitating readiness for potential commercial launch of our product candidates through targeted and stage-appropriate pre-commercial activities.
•Evaluating potential strategic collaborations for each product candidate in order to complement our existing research and development capabilities and to facilitate potential commercialization of these product candidates by taking advantage of the resources and capabilities of strategic collaborators in order to enhance the potential and value of each product candidate.
Financial Condition, Liquidity and Going Concern
We have no products approved for commercial sale and have not generated any revenue from product sales. We have never been profitable and have incurred operating losses in each year since inception in 2016. We have an accumulated deficit of approximately $486.2 million as of September 30, 2024 and $410.9 million as of December 31, 2023. Substantially all of our operating losses resulted from expenses incurred in connection with our research and development programs and from general and administrative costs associated with our operations.
We expect to incur significant expenses and increasing operating losses for the foreseeable future as we initiate and continue the development of our product candidates and add personnel necessary to advance our pipeline of product candidates. We expect that our operating losses will fluctuate significantly from quarter-to-quarter and year-to-year due to timing of development programs.
From inception through September 30, 2024, we have raised net cash of approximately $430.9 million from private and public offerings of preferred stock, common stock, pre-funded warrants and tranche rights. As of September 30, 2024, we had cash and cash equivalents of approximately $59.1 million. With these funds, the Company does not have adequate liquidity to fund its operations for at least twelve months from the issuance of these consolidated financial statements without raising additional capital but, such actions are not solely within the control of the Company. If the Company is unable to obtain additional capital, it would have a material adverse effect on the operations of the Company, its clinical development program, and the Company may have to cease operations altogether. These factors raise substantial doubt about the Company’s ability to continue as a going concern.
Key Status Updates
Private Placement of up to $240 Million (the "January 2024 Financing")
On January 4, 2024, Immunic entered into a Securities Purchase Agreement with select accredited investors, pursuant to which the Company agreed to issue and sell to the Investors in a three-tranche private placement shares of the Company’s common stock, $0.0001 par value per share or in lieu thereof, pre-funded warrants to purchase shares of Common Stock. The Pre-Funded Warrants are exercisable immediately for $0.0001 per share until exercised in full.
•The first tranche, which closed on January 8, 2024, resulted in the purchase by the Investors of an aggregate of $80 million of Common Stock (or pre-funded warrants) from the Company at a price of $1.43 per share;
•The second tranche is a conditional mandatory purchase by the Investors of an additional $80 million of Common Stock (or pre-funded warrants) from the Company at a price of $1.716 per share, equal to 120% of the price paid in the first tranche and is subject to the satisfaction of three conditions:
◦release by the Company of topline data from its Phase 2b clinical trial of vidofludimus calcium (IMU-838) in progressive multiple sclerosis, which data is currently expected in or around April 2025;
◦the 10-day volume-weighted average price of the Common Stock is at least $8.00 per share during the 6 months following the data release; and
◦aggregate trading volume during the same 10-day period is at least $100 million.
•The third tranche must occur no later than three years after the second tranche and is conditioned on the same volume-weighted average share price and minimum trading volumes as the second tranche. The third tranche provides for the issuance of $80 million of shares of common stock (or pre-funded warrants) at the same price per share as the second tranche, but permits investors to fund their purchase obligations on a “cashless” or net settlement basis, which would reduce the cash proceeds to be raised by the Company in the January 2024 Financing.
Any of the conditions in the second or third tranches can be waived by holders of a majority of the outstanding securities (including the lead investor). The fair value methodology used by the Company assumed the conditions will be waived if the trading price of the stock exceeds the purchase price.
The January 2024 Financing resulted in gross proceeds to the Company of approximately $80 million in the first tranche, and an additional $80 million if and when the second tranche occurs. If the second tranche is completed and conditions for the third tranche are satisfied or waived, the Company could receive up to an additional $80 million in the third tranche. However, the amount of cash received in the third tranche would depend on the extent to which the Investors elect to fund the third tranche through a “cashless” or net settlement basis. Therefore, total gross proceeds from the offering to the Company could actually be between $80 million and $240 million. Gross proceeds to the Company will be reduced by fees paid to the placement agents, capital markets advisors and payments of transaction expenses. The Company intends to use the net proceeds from the January 2024 Financing to fund the ongoing clinical development of its three lead product candidates, vidofludimus calcium (IMU-838), IMU-856 and IMU-381, and for other general corporate purposes.
As of the closing date of the transaction of January 8, 2024, the Company did not have enough authorized shares to be able to issue the potential shares for tranche 2 and tranche 3 (collectively referred to hereafter as "the tranche rights"). Therefore, the Company recorded the value associated to the tranche rights as a liability of $23.6 million and allocated the remainder of the $80 million received (or $56.4 million) from the common stock and pre-funded warrants to equity. On March 4, 2024, the stockholders voted to increase the Company's authorized common shares from 130 million to 500 million shares. As a result of the ability to issue shares in satisfaction of the tranche rights, the instrument was reclassified to stockholders' equity. The Company allocated the transaction cost across the instruments on a relative fair value basis. As a result $4.0 million was netted against the equity proceeds and $1.7 million was recorded in other expense in the Consolidated Statements of Operation for the nine months ended September 30, 2024.
The financing was led by BVF Partners L.P., and included participation from new and existing investors, including Avidity Partners, Janus Henderson Investors, Soleus Capital, RTW Investments and Adage Capital Partners LP. Leerink Partners acted as the lead placement agent and Ladenburg Thalmann acted as a placement agent in connection with the offering. Piper Sandler, B. Riley Securities and Brookline Capital Markets, a division of Arcadia Securities, LLC, acted as capital markets advisors to the Company.
The Company registered for resale by the investors in the January 2024 Financing up to 55,944,850 shares of common stock issued (or issuable upon exercise of pre-funded warrants) in the first tranche. The Company will not receive any proceeds from the sale of these shares of common stock. These shares are registered on a registration statement on Form S-3 (registration No. 333-277040), which was declared effective by the SEC on April 30, 2024.
Notice of Allowance for Composition-of-Matter Patent of a Specific Polymorph of Vidofludimus Calcium in the United States
On March 20, 2024, we announced Notice of Allowance from the United States Patent and Trademark Office (“USPTO”) for patent application 16/981,122, entitled, “Calcium salt polymorphs as anti-inflammatory, immunomodulatory and anti-proliferative agents,” covering the composition-of-matter of a specific polymorph of vidofludimus calcium and a related method of production of the material. The claims are expected to provide protection into 2039, unless extended further. The patent was previously granted to the company in Australia, Canada, Indonesia, Japan and Mexico.
Publication of Extended Data From Phase 2 EMPhASIS Trial of Vidofludimus Calcium in Relapsing-Remitting Multiple Sclerosis in the Peer Reviewed Journal, Neurology® Neuroimmunology & Neuroinflammation
On April 30, 2024, we announced that data from our Phase 2 EMPhASIS trial of vidofludimus calcium in patients with relapsing-remitting MS has been published online on April 25, 2024 in Neurology® Neuroimmunology & Neuroinflammation, an official journal of the American Academy of Neurology. The paper, lead authored by coordinating investigator, Robert J. Fox, M.D., Staff Neurologist, Mellen Center for Multiple Sclerosis, Vice-Chair for Research, Neurological Institute, Cleveland Clinic, Cleveland, Ohio, is entitled, “Safety and Dose-Response of Vidofludimus Calcium in Relapsing Multiple Sclerosis: Extended Results of a Placebo-Controlled Phase 2 Trial.”
Appointment of Jason Tardio as Chief Operating Officer and President and Promotion of Werner Gladdines to Chief Development Officer
On July 9, 2024, we announced that seasoned biopharmaceutical executive, Jason Tardio, will be joining the company as Chief Operating Officer and President, effective July 12, 2024. In the newly created role, Mr. Tardio leads internal efforts to prepare for the potential launch of vidofludimus calcium. Jason also works closely with Patrick Walsh, Chief Business Officer, to prepare the Company for a range of potential partnership outcomes for vidofludimus calcium, as well as our other drug candidates.
On July 9, 2024, we also reported that Werner Gladdines, former Vice President, Program Management & Clinical Development Operations, has been promoted to Chief Development Officer. In his new role, Mr. Gladdines takes over additional strategic and operational responsibility for our overall clinical operations functions.
Appointment of Simona Skerjanec to Board of Directors
On July 24, 2024, we announced the appointment of Simona Skerjanec, M.Pharm, MBA, a thought-leader in brain health with decades of experience in drug development and commercialization, as a member of our Board of Directors, effective as of July 22, 2024. As a Class I director, Ms. Skerjanec’s term lasts until the Company’s 2027 annual meeting of stockholders.
First Patient Enrolled in Investigator-Sponsored Phase 2 Clinical Trial of Vidofludimus Calcium in Patients with Post COVID Syndrome
On September 4, 2024, we announced enrollment of the first patient in an investigator-sponsored Phase 2 clinical trial of vidofludimus calcium, entitled, “Randomized Adaptive Assessment of Post COVID Syndrome Treatments_Reducing Inflammatory Activity in Patients with Post COVID Syndrome (RAPID_REVIVE).” The Phase 2 RAPID_REVIVE trial, for which Immunic is providing study medication, is a randomized, placebo-controlled, double-blind, parallel group trial sponsored by the Goethe University Frankfurt, which received trial funding via a grant from the German Federal Ministry of Education and Research.
Hosted Two Multiple Sclerosis R&D Days in New York City and San Francisco
At two MS R&D Days, on September 10, 2024 in New York City and on April 9, 2024 in San Francisco, management discussed the latest developments in the MS landscape, along with recent mode of action, preclinical and clinical data supporting the combined neuroprotective, anti-inflammatory and antiviral profile of vidofludimus calcium.
Presented Key Vidofludimus Calcium Data at the 40th Congress of ECTRIMS, Highlighting Its Therapeutic Potential in Multiple Sclerosis
On September 18, 2024, we announced the presentation of key data at the 40th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (“ECTRIMS”), highlighting vidofludimus calcium’s therapeutic potential in MS. The data was presented in an oral poster presentation and three ePosters at this conference in Copenhagen, Denmark.
Positive Outcome of Interim Analysis of Phase 3 ENSURE Program of Vidofludimus Calcium in Relapsing Multiple Sclerosis
On October 22, 2024, we announced a positive outcome of an interim analysis of our Phase 3 ENSURE program, investigating vidofludimus calcium for the treatment of RMS. An unblinded IDMC confirmed that the predetermined futility criteria have not been met and recommended that both ENSURE trials should continue without changes. The IDMC considered
if a sample size adjustment would be appropriate for either of the two trials based on factors that include statistical analysis and ultimately recommended that the sample sizes for both trials remain unchanged.
Components of Results of Operations
Revenue
To date, we have not generated any revenue from product sales and do not expect to generate any revenue from the sale of products in the foreseeable future. If our development efforts for our product candidates are successful and result in regulatory approval, we may generate revenue in the future from product sales. We cannot predict if, when, or to what extent we will generate revenue from the commercialization and sale of our product candidates. We may never succeed in obtaining regulatory approval for any of our product candidates or achieving market acceptance and commercial success for any product that does receive regulatory approval.
Research and Development Expenses
Research and development expenses consist of costs associated with our research activities, including our product discovery efforts and the development of our product candidates. Our research and development expenses include:
•external research and development expenses and milestone payments incurred under arrangements with third parties, such as CROs, contract manufacturing organizations, collaborations with partners, consultants, and our scientific advisors; and
•internal personnel expenses.
We expense research and development costs as incurred. Non-refundable advance payments for goods and services that will be used in future research and development activities are capitalized as prepaid expenses and expensed when the service has been performed or when the goods have been received.
Since our inception in March 2016, we have spent a total of approximately $356.5 million in research and development expenses through September 30, 2024.
These costs primarily include external development expenses and internal personnel expenses for the three development programs, vidofludimus calcium, IMU-856 and IMU-381. We have spent the majority of our research and development resources on vidofludimus calcium, our lead development program, for clinical trials in MS and UC.
In August 2019, Immunic AG received a grant of up to approximately $726,000 from the German Federal Ministry of Education and Research, in support of the InnoMuNiCH (Innovations through Munich-Nippon Cooperation in Healthcare) project. The grant funds have been used to fund a three-year research project relating to autoimmune diseases by us and our three project partners. Since the inception of the grant, we have recorded $726,000 of income in total which was classified in Other Income in the accompanying consolidated statement of operations. No income was recorded for the periods ended September 30, 2024 and 2023. The funding of this grant is now completed.
Our research and development expenses are expected to increase in the foreseeable future as we continue to conduct ongoing research and development activities, initiate new preclinical and clinical trials and build our pipeline of product candidates. Our research and development expenses may also increase in the foreseeable future due to the current inflationary environment as well as supply chain shortages, which result in increased costs. The process of conducting clinical trials and preclinical studies necessary to obtain regulatory approval is costly and time consuming. We may never succeed in achieving regulatory approval for any of our product candidates.
Successful development of product candidates is highly uncertain and may not result in approved products. Completion dates and completion costs can vary significantly for each product candidate and are difficult to predict. We anticipate that we will make determinations as to which programs to pursue and how much funding to direct to each program on an ongoing basis in response to the development and regulatory success of each product candidate, and ongoing assessments as to each product candidate’s commercial potential.
General and Administrative Expenses
General and administrative expenses consist primarily of personnel expenses, professional fees for legal, accounting, tax and business consulting services, insurance premiums and stock-based compensation.
Other Income (Expense), Net
Interest Income
Interest income consists of interest earned on our money market funds and bank accounts which are a portion of our cash and cash equivalents balance. Our interest income is expected to decrease as our money market funds balance has decreased and U.S. interest rates have started to and are expected to continue to decrease in the near future.
Change in the Fair Value of the Tranche Rights
The change in fair value of the tranche rights is a non-cash charge related to the change in fair value of the tranche 2 and tranche 3 rights associated with the January 2024 Financing from January 8, 2024 until March 4, 2024.
Other Income (Expense), Net
Other income (expense) consists of (i) deal costs from the January 2024 Financing related to tranche rights that were established at the time of the deal closing (ii) a research and development tax incentive related to clinical trials performed in Australia, (iii) a German Government research and development grant and (iv) foreign currency transaction gains and losses.
Results of Operations
Comparison of the Three Months Ended September 30, 2024 and 2023
The following table summarizes our operating expenses for the three months ended September 30, 2024 and 2023: | | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months Ended September 30, | | Change |
| | 2024 | | 2023 | | $ | | % |
| (dollars in thousands) | (unaudited) |
| Operating expenses: | | | |
| Research and development | $ | 21,370 | | | $ | 19,796 | | | $ | 1,574 | | | 8 | % |
| General and administrative | 4,356 | | | 3,774 | | | 582 | | | 15 | % |
| | | | | | | |
| Total operating expenses | 25,726 | | | 23,570 | | | 2,156 | | | 9 | % |
| Loss from operations | (25,726) | | | (23,570) | | | (2,156) | | | 9 | % |
| Other income: | | | | | | | |
| Interest income | 776 | | | 766 | | | $ | 10 | | | 1 | % |
| | | | | | | |
| Other income, net | 582 | | | 35 | | | $ | 547 | | | 1,563 | % |
| Total other income | 1,358 | | | 801 | | | 557 | | | 70 | % |
| Net loss | $ | (24,368) | | | $ | (22,769) | | | $ | (1,599) | | | 7 | % |
Research and development expenses increased by $1.6 million during the three months ended September 30, 2024, as compared to the three months ended September 30, 2023. The increase reflects (i) a $1.4 million increase in external development costs related to the vidofludimus calcium trials, (ii) a $0.3 million increase in external development costs related to IMU-856, (iii) $0.3 million increase in personnel costs due to an increase in headcount and (iv) a $0.3 million increase related costs across numerous categories. The increases were offset by a decrease of $0.7 million from deprioritizing the izumerogant program in psoriasis and castration-resistant prostate cancer.
General and administrative expenses increased by $0.6 million during the three months ended September 30, 2024, as compared to the three months ended September 30, 2023. The increase was primarily due to a $0.6 million increase in personnel expense in general and administrative, $0.2 million of which is related to non-cash stock compensation expense and the remainder of which is related to an increase in headcount.
Interest income remains unchanged at $0.8 million during the three months ended September 30, 2024, as compared to the three months ended September 30, 2023.
Other income increased by $0.5 million during the three months ended September 30, 2024, as compared to the three months ended September 30, 2023. The increase was primarily attributable to a $0.6 million increase in research and development tax incentives for clinical trials in Australia.
Comparison of the Nine Months Ended September 30, 2024 and 2023
The following table summarizes our operating expenses for the nine months ended September 30, 2024 and 2023: | | | | | | | | | | | | | | | | | | | | | | | |
| | Nine Months Ended September 30, | | Change |
| | 2024 | | 2023 | | $ | | % |
| (dollars in thousands) | (unaudited) |
| Operating expenses: | | | |
| Research and development | $ | 58,429 | | | $ | 63,931 | | | $ | (5,502) | | | (9) | % |
| General and administrative | 13,992 | | | 11,911 | | | 2,081 | | | 17 | % |
| | | | | | | |
| Total operating expenses | $ | 72,421 | | | $ | 75,842 | | | $ | (3,421) | | | (5) | % |
| Loss from operations | (72,421) | | | (75,842) | | | 3,421 | | | (5) | % |
| Other income (expense): | | | | | | | |
| Interest income | 2,961 | | | 2,534 | | | $ | 427 | | | 17 | % |
| Change in fair value of the tranche rights | (4,796) | | | — | | | $ | (4,796) | | | N/A |
| Other income (expense), net | (1,076) | | | 1,268 | | | $ | (2,344) | | | (185) | % |
| Total other income (expense) | (2,911) | | | 3,802 | | | (6,713) | | | (177) | % |
| Net loss | (75,332) | | | $ | (72,040) | | | $ | (3,292) | | | 5 | % |
Research and development expenses decreased by $5.5 million during the nine months ended September 30, 2024, as compared to the nine months ended September 30, 2023. The decrease reflects (i) a decrease of $4.1 million from deprioritizing the izumerogant program in psoriasis and castration-resistant prostate cancer, (ii) a $2.6 million decrease in external development costs related to IMU-856 due to the completion of the phase 1 clinical trial in celiac disease and (iii) a $0.5 million decrease related costs across numerous categories. The decreases were offset by (i) a $1.2 million increase in personnel costs, $0.2 million of which is related to non-cash stock compensation and the remainder of which is due to an increase in headcount and (ii) a $0.5 million increase in external development costs related to the vidofludimus calcium program.
General and administrative expenses increased by $2.1 million during the nine months ended September 30, 2024, as compared to the nine months ended September 30, 2023. The increase was primarily due to (i) a $1.7 million increase in personnel expense in general and administrative, $0.9 million of which is related to non-cash stock compensation expense and the remainder of which is related to an increase in headcount, (ii) $0.3 million in legal and consultancy expenses and (iii) a $0.1 million increase related to costs across numerous categories.
Interest income increased by $0.4 million during the nine months ended September 30, 2024, as compared to the nine months ended September 30, 2023 due to higher interest rates.
The change in fair value of the tranche rights of $4.8 million was a non-cash charge related to the change in value of the tranche rights associated with the January 2024 Financing from January 8, 2024 until March 4, 2024. These tranches were initially classified as a liability but were reclassified to equity on March 4, 2024, when stockholders approved the increase in our authorized shares from 130 million to 500 million shares of common stock and therefore the tranche 2 and tranche 3 rights needed to be revalued to fair value upon the reclass to equity.
Other income (expense) decreased by $2.3 million during the nine months ended September 30, 2024, as compared to the nine months ended September 30, 2023. The decrease was primarily attributable to (i) a $1.7 million expense related to the portion of deal costs from the January 2024 Financing related to the tranche rights that were established at the time of the deal closing, (ii) the German Federal Ministry of Finance grant of $1.1 million being recognized in the fourth quarter of 2023 which was one quarter earlier than in the prior year that was recognized in the first quarter of 2023 and (iii) a $0.4 million decrease in other grants which were received in 2023. The decrease was offset by a $0.9 million increase in foreign exchange gains.
Liquidity and Capital Resources
Financial Condition, Liquidity and Going Concern
We have no products approved for commercial sale and have not generated any revenue from product sales. We have never been profitable and have incurred operating losses in each year since inception in 2016. We have an accumulated deficit of approximately $486.2 million as of September 30, 2024 and $410.9 million as of December 31, 2023. Substantially all of our operating losses resulted from expenses incurred in connection with our research and development programs and from general and administrative costs associated with our operations.
We expect to continue to incur significant expenses and increasing operating losses for the foreseeable future as we initiate and continue the preclinical and clinical development of our product candidates and add personnel necessary to operate as a company with an advanced clinical pipeline of product candidates. To the extent additional funds are necessary to meet long-term liquidity needs as we continue to execute our business strategy, we anticipate that they will be obtained through the incurrence of indebtedness, additional equity financings or a combination of these potential sources of funds, although we can provide no assurance that these sources of funding will be available on reasonable terms, if at all.
From inception through September 30, 2024, we have raised net cash of approximately $430.9 million from private and public offerings of preferred stock, common stock, pre-funded warrants and tranche rights. As of September 30, 2024, we had cash and cash equivalents of approximately $59.1 million. With these funds, the Company does not have adequate liquidity to fund its operations for at least twelve months from the issuance of these consolidated financial statements without raising additional capital and such actions are not solely within the control of the Company. If the Company is unable to obtain additional capital, it would have a material adverse effect on the operations of the Company, its clinical development program, and the Company may have to cease operations altogether. These factors raise substantial doubt about the Company’s ability to continue as a going concern.
In December 2020, the Company filed a Prospectus Supplement to the shelf registration statement on Form S-3 filed on November 13, 2020 and declared effective on November 24, 2020 (the "2020 Shelf Registration Statement") for the offering, issuance and sale of up to a maximum aggregate offering price of $50.0 million of common stock that may be issued and sold under an at-the-market sales agreement with SVB Leerink LLC (now Leerink Partners LLC) as agent ("December 2020 ATM"). The Company used the net proceeds from the December 2020 ATM to fund the ongoing clinical development of our product candidates and for other general corporate purposes, including funding existing and potential new clinical programs and product candidates. The December 2020 ATM terminated in May 2024.
In May 2022, the Company filed a Prospectus Supplement to the 2020 Shelf Registration Statement for the offering, issuance and sale of up to a maximum aggregate offering price of $80.0 million of common stock to be issued and sold under another at-the-market sales agreement ("May 2022 ATM") with Leerink Partners LLC (formerly SVB Leerink LLC) as agent. The 2020 Shelf Registration Statement expired in November 2023.
In November 2023, we filed a shelf registration statement on Form S-3 (the "2023 Shelf Registration Statement"). The 2023 Shelf Registration Statement permits the offering, issuance and sale of up to $250.0 million of common stock, preferred stock, warrants, debt securities, and/or units in one or more offerings and in any combination of the foregoing. The 2023 Registration Statement was declared effective on May 31, 2024. Unsold securities from the expired 2020 Shelf Registration Statement can continue to be sold under the 2023 Shelf Registration Statement resulting in a total S-3 shelf availability of $412.3 million as of September 30, 2024.
In May 2024, we filed a Prospectus Supplement to the 2023 Shelf Registration Statement for the offering, issuance and sale of up to a maximum aggregate offering price of $80.0 million of common stock that may be issued and sold under an at-the-market sales agreement with Leerink Partners LLC as agent ("May 2024 ATM"), which rolls over the $80.0 million of unsold common stock from the May 2022 ATM. We intend to use the net proceeds from the May 2024 ATM to continue to fund the ongoing clinical development of our product candidates and for other general corporate purposes, including funding existing and potential new clinical programs and product candidates. The May 2024 ATM will terminate upon the earlier of (i) the issuance and sale of all of the shares through Leerink Partners LLC on the terms and subject to the conditions set forth in the May 2024 ATM or (ii) termination of the May 2024 ATM as otherwise permitted thereby. The May 2024 ATM may be terminated at any time by either party upon ten days’ prior notice, or by Leerink Partners LLC at any time in certain circumstances, including the occurrence of a material adverse effect on us. As of September 30, 2024, $80.0 million in capacity remains under the May 2024 ATM.
We agreed to pay Leerink Partners LLC a commission equal to 3.0% of the gross proceeds from the sales of common shares pursuant to the May 2024 ATM and have agreed to provide Leerink Partners LLC with customary indemnification and contribution rights.
For the nine months ended September 30, 2024, we raised gross proceeds of $0.2 million pursuant to the December 2020 ATM through the sale of 150,000 shares of common stock at a weighted average price of $1.31 per share. The net proceeds from the December 2020 ATM were $0.2 million after deducting sales agent commissions of $6,000. We did not have any ATM activity for the three months ended September 30, 2024.
In the three and nine months ended September 30, 2023, we raised gross proceeds of $0.3 million pursuant to the December 2020 ATM through the sale of 107,012 shares of common stock at a weighted average price of $2.72 per share. The net proceeds from the December 2020 ATM were $0.3 million after deducting underwriter commissions of $9,000.
Equity Offerings
Private Placement of up to $240 Million
On January 4, 2024, Immunic entered into a Securities Purchase Agreement with select accredited investors, pursuant to which the Company agreed to issue and sell to the Investors in a three-tranche private placement shares of the Company’s common stock, $0.0001 par value per share or in lieu thereof, pre-funded warrants to purchase shares of Common Stock. The pre-funded warrants are exercisable immediately for $0.0001 per share and until exercised in full.
The first tranche, which closed on January 8, 2024, resulted in the purchase by the Investors of an aggregate of $80 million of Common Stock (or pre-funded warrants) from the Company at a price of $1.43 per share; The second tranche is a conditional mandatory purchase by the Investors of an additional $80 million of Common Stock (or pre-funded warrants) from the Company at a price of $1.716 per share, equal to 120% of the price paid in the first tranche and is subject to the satisfaction of three conditions:
•release by the Company of topline data from its Phase 2b clinical trial of vidofludimus calcium (IMU-838) in progressive multiple sclerosis, which data is currently expected in or around April 2025;
•the 10-day volume-weighted average price of the Common Stock is at least $8.00 per share during the 6 months following the data release; and
•aggregate trading volume during the same 10-day period is at least $100 million.
The third tranche must occur no later than three years after the second tranche and is conditioned on the same volume-weighted average share price and minimum trading volumes as the second tranche. The third tranche provides for the issuance of $80 million of shares of common stock (or pre-funded warrants) at the same price per share as the second tranche, but permits investors to fund their purchase obligations on a “cashless” or net settlement basis, which would reduce the cash proceeds to be raised by the Company in the January 2024 Financing.
Any of the conditions in the second or third tranches can be waived by holders of a majority of the outstanding securities (including the lead Investor). The fair value methodology used by the Company assumed the conditions will be waived if the trading price of the stock exceeds the purchase price.
The January 2024 Financing resulted in gross proceeds to the Company of approximately $80 million in the first tranche, and an additional $80 million if and when the second tranche occurs. If the second tranche is completed and conditions for the third tranche are satisfied or waived, the Company could receive up to an additional $80 million in the third tranche. However, the amount of cash received in the third tranche would depend on the extent to which the Investors elect to fund the third tranche through a “cashless” or net settlement basis. Therefore, total gross proceeds from the offering to the Company could actually be between $80 million and $240 million. Gross proceeds to the Company will be reduced by fees paid to the placement agents, capital markets advisors and payments of transaction expenses. The Company intends to use the net proceeds from the January 2024 Financing to fund the ongoing clinical development of its three lead product candidates, vidofludimus calcium (IMU-838), IMU-856 and IMU-381, and for other general corporate purposes.
Future Capital Requirements
As noted above, we have not generated any revenue from product sales and we do not know when, or if, we will generate any revenue from product sales. We will not be able to generate any revenue from product sales unless and until we obtain regulatory approval for and commercialize any of our product candidates. We expect our expenses to continue to increase as we
continue the ongoing research, development, manufacture and clinical trials of, and seek regulatory approval for, our product candidates. We also incur additional costs associated with operating as a public company. In addition, subject to obtaining regulatory approval of any of our product candidates, we anticipate that we will need substantial additional funding in connection with our continuing operations.
Our future expenses and capital requirements are difficult to forecast and will depend on many factors, including, but not limited to:
•the timing and structure of any strategic options and transactions, if any;
•personnel-related expenses, including salaries, benefits, stock-based compensation expense and other compensation expenses related to retention and termination of personnel;
•the scope, progress, duration, results and costs of research and development and ongoing clinical trials;
•the cost and timing of future regulatory submissions;
•the cost and timing of developing and validating the manufacturing processes for any potential product candidates;
•the cost and timing of any commercialization activities, including reimbursement, marketing, sales and distribution costs;
•our ability to establish new collaborations, licensing or other arrangements and the financial terms of such agreements;
•the number and characteristics of any future product candidates we pursue;
•the costs involved with being a public company;
•the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patents, including litigation costs and the outcome of such litigation;
•the cost, timing and outcome of any future litigation; and
•the timing, receipt and amount from the sales of, or royalties on, any future products.
Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through a combination of stock offerings, debt financings, strategic alliances, collaborations and licensing arrangements. We do not expect to achieve revenue from product sales prior to the use of all the net proceeds from our public and private offerings to date. We do not have any committed external source of funds. Additional funds may not be available on acceptable terms, if at all. To the extent that we raise additional capital through the sale of equity securities, the ownership interest of our stockholders will be diluted and it may be on terms that are not favorable to us or our stockholders. Sales of equity securities will also be more difficult for at least the foreseeable future because of general volatility in the equity markets for companies like us. Debt financing, if available, may involve covenants restricting our operations or our ability to incur additional debt or other terms that are not favorable to us or our stockholders. Also, the cost of debt financing has increased due to the rise in interest rates over the past few years. If we raise additional funds through collaborations and licensing arrangements with third parties, we would expect to relinquish substantial rights to our technologies or our future products, or grant licenses on terms that may not be favorable to us. If we were to complete a merger, or other business combination, we may relinquish all control over the organization and could experience detrimental tax effects. If we are unable to raise adequate funds, we may have to curtail our product development programs and liquidate some or all of our assets. Any of these factors could harm our operating results and could result in substantial declines in the trading price of our common stock.
As of September 30, 2024, we had cash and cash equivalents of approximately $59.1 million.
Cash Flows
The following table shows a summary of our cash flows for the nine months ended September 30, 2024 and 2023: | | | | | | | | | | | |
| | Nine Months Ended September 30, |
| | 2024 | | 2023 |
| (in thousands) | (unaudited) |
| Cash (used in) provided by: | | | |
| Operating activities | $ | (61,803) | | | $ | (56,801) | |
| Investing activities | (261) | | | 9,627 | |
| Financing activities | 74,464 | | | 429 | |
Operating activities
During the nine months ended September 30, 2024, operating activities used $61.8 million of cash. The use of cash primarily resulted from (i) our net loss of $75.3 million adjusted for non-cash charges of $6.6 million related to stock-based compensation and depreciation and amortization, $4.8 million related to a change in the fair value of the tranche rights, $1.7 million for fees expensed as part of the January 2024 financing and (ii) a net increase in operating assets and liabilities of $0.5 million. Changes in our operating assets and liabilities during the nine months ended September 30, 2024 consisted primarily of (i) a $1.5 million increase in our other current assets and prepaid expenses and (ii) a decrease of $1.0 million in our other current liabilities.
During the nine months ended September 30, 2023, operating activities used $56.8 million of cash. The use of cash primarily resulted from (i) our net loss of $72.0 million adjusted for non-cash charges of $6.3 million related to $0.7 million for an unrealized foreign currency loss and $5.6 million related to stock-based compensation and depreciation and amortization and a $9.0 million net increase in operating assets and liabilities. Changes in our operating assets and liabilities during the nine months ended September 30, 2023 consisted primarily of (i) a $3.9 million increase in our other current assets and prepaid expenses and (ii) an increase of $5.1 million in our other current liabilities.
Investing activities
During the nine months ended September 30, 2024, net investing activities used $0.3 million due to the purchase of property and equipment.
During the nine months ended September 30, 2023, net investing activities provided $9.6 million of cash, primarily due to the sale of $9.8 million of time deposits partially offset by the purchase of $169,000 of property and equipment.
Financing Activities
Net cash provided by financing activities was $74.4 million during the nine months ended September 30, 2024 primarily
consisting of net cash proceeds from the January 2024 Financing and $0.2 million of net proceeds from sale of common stock through our At The Market Sales Agreement.
Net cash provided by financing activities was $0.4 million during the nine months ended September 30, 2023 consisting primarily of net cash proceeds from the sale of common stock under our 2020 ATM facility.
Off-Balance Sheet Arrangements
Through September 30, 2024, we have not entered into and did not have any relationships with unconsolidated entities or financial collaborations, such as entities often referred to as structured finance or special purpose entities, established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purpose.
Contractual Obligations
Maturities of the operating lease obligation are as follows as of September 30, 2024:
| | | | | | | | |
| 2024 | $ | 275,000 | |
| 2025 | | 433,000 | |
| 2026 | | 78,000 | |
| 2027 | | 82,000 | |
| 2028 | | 79,000 | |
| Thereafter | | — | |
| Total | | 947,000 | |
| Interest | | (48,000) | |
| Present Value of obligation | $ | 899,000 | |
As of September 30, 2024, we have non-cancelable contractual obligations under certain agreements related to our development programs IMU-838, IMU-935 and IMU-856 totaling approximately $3.2 million, all of which is expected to be paid in 2024 and 2025.
Critical Accounting Policies and Estimates
Our unaudited condensed consolidated financial statements are prepared in conformity with U.S. GAAP. The preparation of our unaudited condensed consolidated financial statements and related disclosures requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, costs and expenses, and the disclosure of contingent assets and liabilities in our financial statements. We base our estimates on historical experience, known trends and events and various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. We evaluate our estimates and assumptions on an ongoing basis. Our actual results may differ from these estimates under different assumptions or conditions. We have reviewed these critical accounting policies and related disclosures with the Audit Committee of our Board.
During the first three months of 2024, we updated our warrants and tranche rights accounting policy which is described along with our other significant accounting policies in more detail in (i) Note 2 to our unaudited condensed consolidated financial statements included elsewhere in this Quarterly Report and (ii) Note 2 to our audited consolidated financial statements for the years ended December 31, 2023 and 2022 filed in our Annual Report on Form 10-K on February 22, 2024.
Recently Issued Accounting Standards
There are no recently issued accounting standards that would have a significant impact on the company's consolidated financial statements.
Item 3. Quantitative and Qualitative Disclosures about Market Risk
Interest Rate Sensitivity
We had cash and cash equivalents of $59.1 million as of September 30, 2024, which were held for working capital purposes. We do not enter into investments for trading or speculative purposes. We do not believe that we have any material exposure to changes in the fair value of these investments as a result of changes in interest rates due to their short-term nature. Decreases or increases in interest rates, however, will reduce or increase future investment income, respectively, to the extent we have funds available for investment.
Foreign Currency Exchange Risk
Our primary research and development operations are conducted in our facilities in Germany. We have entered into and may continue to enter into international agreements, primarily related to our clinical studies. Accordingly, we have exposure to foreign currency exchange rates and fluctuations between the U.S. dollar and foreign currencies, primarily the Euro and the Australian dollar, which could adversely affect our financial results, including income and losses as well as assets and liabilities. To date, we have not entered into, and do not have any current plans to enter into, any foreign currency hedging transactions or derivative financial transactions. Our exposure to foreign currency risk will fluctuate in future periods as our research and clinical development activities in Europe and Australia change. We currently maintain a significant amount of our assets outside of the U.S.
The functional currencies of our foreign subsidiaries are the applicable local currencies. Accordingly, the effects of exchange rate fluctuations on the net assets of these operations are accounted for as translation gains or losses in accumulated other comprehensive income (loss) within stockholders’ equity. Foreign currency transaction gains and losses related to long-term intercompany loans that are payable in the foreseeable future are recorded in Other Income (Expense). Our German subsidiary is currently a significant portion of our business and, accordingly, a change of 10% in the currency exchange rates, primarily the Euro, could have a material impact on our financial position or results of operations.
Although operating in local currencies may limit the impact of currency rate fluctuations on the results of operations of our German and Australian subsidiaries, rate fluctuations may impact the consolidated financial position as the assets and liabilities of our foreign operations are translated into U.S. dollars in preparing our condensed consolidated balance sheets. As of September 30, 2024, our German and Australian subsidiaries had net current liabilities (defined as current assets less current liabilities), subject to foreign currency translation risk, of $0.5 million. An increase of approximately $50,000 in net current liabilities would result as of September 30, 2024, from a hypothetical 10% adverse change in quoted foreign currency exchange rates, primarily due to the Euro. In addition, a 10% change in the foreign currency exchange rates for the nine months ended September 30, 2024 would have impacted our net loss by approximately $5.8 million, primarily due to the Euro.
Effects of Inflation
We have experienced a general increase in costs as a result of global inflation, however, we do not believe that inflation and changing prices had a material impact on our results of operations for any periods presented herein.
Item 4. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
An evaluation was carried out, under the supervision of and with the participation of our management, including our Chief Executive Officer and our Principal Financial Officer, of the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15 (e)) under the Securities Exchange Act of 1934, as amended ("the Exchange Act"), as of the end of the period covered by this report. Based on such evaluation, our Chief Executive Officer and our Principal Financial Officer have concluded that our disclosure controls and procedures are effective to ensure that the information required to be disclosed by us in the reports we file or submit under the Exchange Act was recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms.
Changes in Internal Control over Financial Reporting
There was no change in our internal control over financial reporting that occurred during the nine months ended September 30, 2024 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Part II - OTHER INFORMATION
Item 1. Legal Proceedings
We are not currently a party to any litigation, nor are we aware of any pending or threatened litigation against us, that we believe would materially affect our business, operating results, financial condition or cash flows. Our industry is characterized by frequent claims and litigation including securities litigation, claims regarding patent and other intellectual property rights and claims for product liability. As a result, in the future, we may be involved in various legal proceedings from time to time.
Item 1A. Risk Factors
You should carefully consider the risk factors included in Item 1A. of our Annual Report on Form 10-K for the year ended December 31, 2023 filed with the SEC on February 22, 2024 and the other information in this Report, including the section of this report titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes. If any of the events described in our Annual Report, and the following risk factor and the risks described in our Form 10-K and elsewhere in this Report occur, our business, operating results and financial condition could be seriously harmed. This Report also contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in the forward-looking statements as a result of factors that are described in our Annual Report and elsewhere in this Report.
Clinical failure can occur at any stage of clinical development. because the results of earlier clinical trials are not necessarily predictive of future results, any product candidate we advance through clinical trials may not have favorable results in later clinical trials or receive marketing approval.
Clinical failure can occur at any stage of clinical development. The results of preclinical studies and early clinical trials of our product candidates may not be predictive of the results of later-stage clinical trials. Product candidates in later stages of clinical trials may fail to show the desired safety and efficacy traits despite having progressed through preclinical studies and initial clinical trials.
A number of pharmaceutical companies have suffered significant setbacks in advanced clinical trials due to lack of efficacy or adverse safety profiles, notwithstanding promising results in earlier trials. Clinical trials may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical or preclinical testing. For example, we announced in October 2022 the analysis of interim group-level data of our Phase 1b clinical trial of IMU-935 in patients with moderate-to-severe psoriasis did not separate from placebo. Following this announcement, our stock price declined significantly, which caused us to record a full impairment of our goodwill in the quarter ended December 31, 2022. Data obtained from trials are susceptible to varying interpretations, and regulators may not interpret our data as favorably as we do, which may delay, limit or prevent marketing approval of our product candidates. In addition, the design of a clinical trial can determine whether its results will support approval of a product, or approval of a product for desired indications, and flaws or shortcomings in the design of a clinical trial may not become apparent until the clinical trial is well advanced. We have limited experience in designing clinical trials and may be unable to properly design and execute a clinical trial to support marketing approval for our desired indications. Further, clinical trials of product candidates often reveal that it is not practical or feasible to continue development efforts. If one of our product candidates is found to be unsafe or lack efficacy, we will not be able to obtain marketing approval for such product candidate and our business would be harmed. If the results of our clinical trials of our product candidates do not achieve pre-specified endpoints, we are unable to provide primary or secondary endpoint measurements deemed acceptable by the FDA or comparable foreign regulators, or we are unable to demonstrate an acceptable level of safety relative to the efficacy associated with our proposed indications, the prospects for approval of our product candidates would be materially and adversely affected. A number of companies in the pharmaceutical industry, including those with greater resources and experience than we, have suffered significant setbacks in Phase 2 and Phase 3 clinical trials, even after seeing promising results in earlier clinical trials.
In October 2024, an independent data monitoring committee, or IDMC, conducted a non-binding, interim futility analysis of our phase IMU-838 3 ENSURE program for the treatment of RMS and reported that the trials are not futile and should continue as planned. In addition, the IDMC recommended that we continue the trials without any adjustment to the sample sizes of each trial. There can be no assurances that the observations made at the interim regarding futility will be consistent with the final study results for various reasons, including that the final study results based upon the full sample size may not be consistent with the results achieved by the limited sample size used to conduct the interim analysis and the current sample size of approximately 1,050 adult patients with active RMS patients for each trial may not be sufficiently large to demonstrate efficacy.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
None.
Item 3. Defaults Upon Senior Securities
Not applicable.
Item 4. Mine Safety Disclosures
Not applicable.
Item 5. Other Information
(a) Not applicable.
(b) Not applicable.
(c) Trading Plans.
During the quarter ended September 30, 2024, no director or Section 16 officer adopted or terminated any Rule 10b5-1 trading arrangements or non-Rule 10b5-1 trading arrangements (in each case, as defined in Item 408(a) of Regulation S-K promulgated by the SEC).
Item 6. Exhibits
EXHIBITS
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | Incorporated by Reference |
Exhibit Number | | Exhibit Title | | Form | | Exhibit | | Filing Date |
| 3.1 | | | | 8-K | | 3.1 | | | July 17, 2019 |
| 3.2 | | | | 8-K | | 3.1 | | | July 17, 2019 |
| 3.3 | | | | 8-K | | 3.1 | | | March 8, 2024 |
| 4.1 | | | | S-8 | | 4.2 | | | August 21, 2023 |
| 4.2+ | | | | S-8 | | 10.3 | | | July 28, 2021 |
| 4.4 | | | | 8-K | | 4.1 | | January 4, 2024 |
| 10.1 | | | | 8-K | | 10.1 | | September 3, 2024 |
| 10.2 | | | | 8-K | | 10.3 | | December 18, 2023 |
| 10.3 | | | | 8-K | | 10.1 | | December 18, 2023 |
| 10.4 | | | | 8-K | | 10.2 | | December 18, 2023 |
| 10.5 | | | | 8-K | | 10.4 | | December 18, 2023 |
| 10.6 | | | | 8-K | | 10.1 | | January 4, 2024 |
| 10.7 | | | | 8-K | | 10.4 | | July 17, 2019 |
| 10.8 | | | | 10-K | | 10.5 | | February 23, 2023 |
| 10.9 | | | | 10-K | | 10.6 | | February 23, 2023 |
| 10.10 | | | | 8-K | | 10.5 | | July 17, 2019 |
| 10.11 | | | | 8-K | | 99.3 | | September 5, 2019 |
| 10.12 | | | | 8-K | | 10.2 | | April 20, 2020 |
| 10.13 | | | | 8-K | | 10.3 | | June 10, 2021 |
| 10.14 | | | | 8-K | | 10.4 | | June 10, 2021 |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| 10.15 | | | | 8-K | | 10.1 | | October 14, 2021 |
| 10.16*+ | | | | | | | | |
| 24.1 | | | | | | | | |
| 31.1* | | | | | | | | |
| 31.2* | | | | | | | | |
| 32.1** | | | | | | | | |
| 32.2** | | | | | | | | |
| 101.INS* | | XBRL Instance Document | | | | | | |
| 101.SCH* | | XBRL Taxonomy Extension Schema Document. | | | | | | |
| 101.CAL* | | XBRL Taxonomy Extension Calculation Linkbase Document. | | | | | | |
| 101.DEF* | | XBRL Taxonomy Extension Definition Linkbase Database. | | | | | | |
| 101.LAB* | | XBRL Taxonomy Extension Label Linkbase Document. | | | | | | |
| 101.PRE* | | XBRL Taxonomy Extension Presentation Linkbase Document. | | | | | | |
| 104* | | Cover Page Interactive Data File | | | | | | |
| | | | | |
| + | Indicates a management contract or compensatory plan or arrangement. |
| * | Filed herewith |
| ** | In accordance with Item 601(b)(32)(ii) of Regulation S-K and SEC Release No. 33-8238 and 34-47986, Final Rule: Management’s Reports on Internal Control Over Financial Reporting and Certification of Disclosure in Exchange Act Periodic Reports, the certifications furnished in Exhibits 32.1 and 32.2 hereto are deemed to accompany this Form 10-Q and will not be deemed “filed” for purposes of Section 18 of the Exchange Act. Such certifications will not be deemed to be incorporated by reference into any filings under the Securities Act or the Exchange Act, except to the extent that the registrant specifically incorporates it by reference. |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
IMMUNIC, INC.
Date: November 7, 2024 By: /s/ Daniel Vitt
Daniel Vitt
Chief Executive Officer and President

US_ACTIVE127388755V-1 EMPLOYMENT AGREEMENT This Employment Agreement (the “Agreement”) is dated July 22, 2024 and effective as of July 9, 2024 (the “Effective Date”), by and between IMMUNIC, INC., a Delaware corporation (the “Company”), and Jason Tardio (the “Employee”). WHEREAS, the Company has appointed the Employee to be an officer of the Company effective as of July 9, 2024; WHEREAS, the Employee will begin to serve pursuant to the terms of this Agreement on July 12, 2024; WHEREAS, the Company desires that the Employee joins the Company to serve in the capacity of Chief Operating Officer and President of the Company, and the Employee has agreed to serve in such position in accordance with the terms and conditions of this Agreement; NOW, THEREFORE, in consideration of the premises and mutual covenants contained herein, and for other valuable consideration, the Company and the Employee hereby agree as follows: 1. Certain Definitions. The following terms, as used herein, have the following meanings: (a) “Cause” means one or more of the following: (i) the Employee’s willful failure to perform his duties hereunder or the lawful directives of the Company’s Board of Directors or nominees thereof (other than as a result of illness or injury), (ii) the conviction of, or plea of nolo contendere by, the Employee to, a felony or a crime involving moral turpitude, (iii) the Employee’s commission of any willful acts of personal dishonesty in connection with his responsibilities as an employee of the Company that could reasonably be expected to materially impair or damage the property, goodwill, reputation, business or finances of the Company, (iv) the Employee’s willful and material violation of the Company’s policies regarding ethics or conduct (including sexual harassment and other similar policies) that could reasonably be expected to impair or damage the property, goodwill, reputation, business or finances of the Company or its affiliates or (v) the Employee’s breach of his obligations under the Confidentiality Agreement. (b) “Change of Control” means the occurrence of any of the following events: (i) a change in the ownership of the Company which occurs on the date that any one person or entity, or more than one person or entity acting as a group (collectively, a “Person” for purposes of this definition), acquires ownership of the stock of the Company that, together with the stock held by such Person, constitutes more than fifty percent (50%) of the total voting power of the stock of the Company; (ii) a change in the effective control of the Company which occurs on the date that a majority of members of the Company’s Board of Directors is replaced during any twelve (12) month period by Directors whose appointment or election is not endorsed by a majority of the members of the Company’s Board of Directors prior to the date of the appointment or election; or (iii) change in the ownership of a substantial portion of the Company’s assets which occurs on the date that any Person acquires (or has acquired during the twelve (12) month period ending on the date of the most recent acquisition by such Person or Persons) assets from the Company that have a total gross fair market value equal to or more than fifty percent (50%) of the total gross fair market value of all of the assets of the Company

2 US_ACTIVE127388755V-1 immediately prior to such acquisition or acquisitions; provided, however, that for purposes of this Section 1(b)(iii), the following will not constitute a change in the ownership of a substantial portion of the Company’s assets: (A) a transfer to an entity that is controlled by the Company’s stockholders immediately after the transfer, or (B) a transfer of assets by the Company to: (1) a stockholder of the Company (immediately before the asset transfer) in exchange for or with respect to the Company’s stock, or (2) an entity, fifty percent (50%) or more of the total value or voting power of which is owned, directly or indirectly, by the Company. For purposes of this Section 1(b)(iii), gross fair market value means the value of the assets of the Company, or the value of the assets being disposed of, determined without regard to any liabilities associated with such assets. For purposes of this definition, Persons will be considered to be acting as a group if they are owners of a corporation that enters into a merger, consolidation, purchase or acquisition of stock, or similar business transaction with the Company. Notwithstanding the foregoing, a transaction will not be deemed a Change of Control unless the transaction qualifies as a change in control event within the meaning of Code Section 409A, as it has been and may be amended from time to time, and any proposed or final Treasury Regulations and Internal Revenue Service guidance that has been promulgated or may be promulgated thereunder from time to time. Further and for the avoidance of doubt, a transaction will not constitute a Change of Control if: (i) its sole purpose is to change the state of the Company’s incorporation, or (ii) its sole purpose is to create a holding company that will be owned in substantially the same proportions by the persons who held the Company’s securities immediately before such transaction. (c) “Change of Control Period” means the twelve (12) month period following a Change of Control. (d) “Date of Termination” means the date specified in a written notice of termination delivered pursuant to Section 6, or the Employee’s last date as an active employee of the Company before a termination of employment due to his death or Non-Renewal. (e) “Disabled” or “Disability” means a mental or physical condition that renders the Employee substantially incapable of performing his duties and obligations under this Agreement, after taking into account provisions for reasonable accommodation, as determined by a medical doctor (such doctor to be mutually determined in good faith by the parties) for four (4) or more consecutive months or for a total of four (4) months during any twelve (12) consecutive months. (f) “Good Reason” means, unless the Employee has consented in writing thereto, the occurrence of any of the following: (i) the assignment to the Employee of any duties materially inconsistent with the Employee’s position, including any change in status, title, authority, duties or responsibilities or any other action which results in a material diminution in such status, title, authority, duties or responsibilities, (ii) a material reduction in the Employee’s Base Salary by the Company or (iii) the relocation of the Employee’s office to a location more than fifty (50) miles from his current residence in Massachusetts. 2. Term of Employment. The terms and conditions set forth in this Agreement will comence on the Effective Date and end on the earlier of: (a) December 31, 2024 (subject to extension as provided in the following sentence) and (b) the Employee’s Date of Termination (such period, including any extension as provided below, shall be referred to as the “Term of Employment”). This Agreement and the Term of Employment shall be automatically extended for successive additional one (1)-year terms, unless either party provides written notice of non- renewal at least ninety (90) days before the end of then-current Term of Employment. The

3 US_ACTIVE127388755V-1 Employee agrees to sign all documentation evidencing the foregoing as may be presented to the Employee for signature by the Company. 3. Employee’s Duties and Obligations. (a) Duties. The Employee shall serve as the Chief Operating Officer and President. The Employee shall be responsible for all duties customarily associated with the Chief Operating Officer and President of a publicly-traded company. The Employee shall report to the Chief Executive Officer of the Company and shall be subject to reasonable policies established by such Chief Executive Officer. (b) Location of Employment. It is understood that Employee will be working predominantly out of his home office in Massachsusetts unless the Employee and the Company agree to an alternative arrangement. In addition, the Employee acknowledges and agrees that the performance by the Employee of the Employee’s duties shall require travel including, without limitation, overseas travel from time to time. (c) Confidential Information, Assignment of Rights, Non-Solicitation and Non-Competition Agreement. In consideration of the covenants contained herein, the Employee has executed and agrees to be bound by the Confidential Information, Assignment of Rights, Non-Solicitation and Non-Competition Agreement (the “Confidentiality Agreement”) attached to this Agreement as Exhibit A. The Employee shall comply at all times with the covenants (including covenants not to compete or solicit employees, consultants and independent contractors) and other terms and conditions of the Confidentiality Agreement and all other reasonable policies of the Company governing its confidential and proprietary information. The Employee’s obligations under the Confidentiality Agreement shall survive the Term of Employment. 4. Devotion of Time to the Company’s Business. During the Term of Employment, the Employee shall devote substantially all of his business time, attention and effort to the affairs of the Company, excluding any periods of disability, vacation, or sick leave to which the Employee is entitled, and shall use his reasonable best efforts to perform the duties properly assigned to him hereunder and to promote the interests of the Company. 5. Compensation and Benefits. (a) Signing Bonus. The Company shall pay to the Employee a cash bonusof sixty-thousand dollars ($60,000) within one month of the Effective Date. In addition, and provided that the Employee is an employee of the Company in good standing on January 12, 2025, the Company shall pay to the Employee an additional cash bonusof sixty-thousand dollars ($60,000) after January 12, 2025. (b) Base Salary. The Company shall pay to the Employee in accordance with its normal payroll practices (but not less frequently than monthly) an annual salary at a rate of five hundred thousand dollars ($500,000) per annum (“Base Salary”). The Employee’s Base Salary shall be reviewed annually for the purpose of determining increases, if any, based on the Employee’s performance, the performance of the Company, then prevailing salary scales for comparable positions, inflation and other relevant factors. Effective as of the date of any increase in the Employee’s Base Salary, Base Salary as so increased shall be considered the new Base Salary for all purposes of this Agreement and may not thereafter be reduced. Any

4 US_ACTIVE127388755V-1 increase in Base Salary shall not limit or reduce any other obligation of the Company to the Employee under this Agreement. (c) Annual Bonus. During the Term of Employment, the Employee shall be eligible to receive an annual cash incentive award (“Annual Bonus”) pursuant to the bonus plan then in effect for Employee employees of the Company (the “Bonus Plan”). All Annual Bonuses are subject to the terms and conditions of then-current Bonus Plan adopted by the Company. If the Employee achieves his target performance goals for a fiscal year, which goals shall be determined by the Compensation Committee of the Company’s Board of Directors (the“Compensation Committee”) on an annual or more frequent basis, the Annual Bonus shall be not less than forty percent (40%) of the Employee’s Base Salary. To be eligible to receive an Annual Bonus, or any portion thereof, the Employee must be actively employed by the Company at the time the Annual Bonus, if any, is paid, except as otherwise provided below. (d) Equity Awards. The Employee has been granted stock option equity awards under the Immunic, Inc. 2019 Omnibus Equity Incentive Plan (the “Equity Plan”). From time to time, the Employee may receive additional equity incentive awards under the Equity Plan (or under any other equity incentive plan adopted by the Company to supplement or succeed the Equity Plan). All such awards are referred to herein as the “Equity Awards.” (e) Benefits. During the Term of Employment, the Employee shall be entitled to participate in all employee benefit plans, programs and arrangements made available generally to the Company’s senior employees or to other full-time employees on substantially the same basis that such benefits are provided to such senior Employees of a similar level or to other full-time employees. (f) Vacations. During the Term of Employment, the Employee shall be entitled to twenty (20) days paid vacation per year, or such greater amount as may be earned under the Company’s standard vacation policy. (g) Reimbursement of Expenses. During the Term of Employment, the Employee shall be entitled to receive prompt reimbursement for all reasonable business-related or employment-related expenses incurred by the Employee upon the receipt by the Company of reasonable documentation in accordance with standard practices, policies and procedures applicable to other senior Employees of the Company. 6. Termination of Employment. The Term of Employment shall be automatically terminated upon the first to occur of the following: (a) Death. The Employee’s employment shall terminate immediately upon the Employee’s death. (b) Disability. If the Employee is Disabled, either party may terminate the Employee’s employment due to such Disability upon delivery of written notice to the other party. The effective date of such termination of employment will be the Date of Termination set forth in such written notice or immediately upon delivery of such written notice if no effective date is specified in the written notice. For avoidance of doubt, if the Employee’s employment is terminated pursuant to this Section 6(b), his employment will not constitute a termination of employment by the Company without Cause or by the Employee for Good Reason.

5 US_ACTIVE127388755V-1 (c) Termination by the Employee Without Good Reason. The Employee may terminate his employment for any reason other than Good Reason upon his delivery of written notice to the Company at least thirty (30) days prior to his Date of Termination. (d) Termination by the Employee for Good Reason. The Employee may terminate his employment for Good Reason if (i) not later than ninety (90) days after the occurrence of any act or omission that constitutes Good Reason, the Employee provides the Company with a written notice setting forth in reasonable detail the acts or omissions that constitute Good Reason, (ii) the Company fails to correct or cure the acts or omissions within thirty (30) days after it receives such written notice, and (iii) the Employee terminates his employment with the Company after the expiration of such cure period but not later than thirty (30) days after the expiration of such cure period. (e) Termination by the Company Without Cause. The Company may terminate the Employee’s employment without Cause upon delivery of written notice to the Employee at least thirty (30) days prior to his Date of Termination. (f) Termination Upon Non-Renewal. Unless otherwise agreed to by the parties, the Employee’s employment shall terminate on the last day of then-current Term of Employment if either the Company or the Employee provides the other party with a written notice of non-renewal of this Agreement in accordance with Section 2 and the parties do not enter into a new employment agreement prior to the expiration of this Agreement (“Non- Renewal”). (g) Termination by the Company for Cause. Upon the occurrence of any act or omission that constitutes Cause, the Company may terminate the Employee’s employment upon delivery of written notice to the Employee at least fifteen (15) days prior to his Date of Termination, unless the Employee cures, if curable, such acts or omissions constituting Cause to the satisfaction of the Company prior to the expiration of such period. 7. Compensation and Benefits Payable Upon of Termination of Employment Unrelated to a Change of Control. (a) Payment of Accrued But Unpaid Compensation and Benefits. Upon the Employee’s termination of employment for any reason outside of the Change of Control Period, the Employee (or his Beneficiary following the Employee’s death) shall receive (i) a lump sum payment on the Date of Termination in an amount equal to the sum of the Employee’s earned but unpaid Base Salary through his Date of Termination plus his accrued but unused vacation days at the Employee’s Base Salary in effect as of his Date of Termination; plus (ii) any other benefits or rights the Employee has accrued or earned through his Date of Termination in accordance with the terms of the applicable fringe or employee benefit plans and programs of the Company. Except as provided in Section 7(b) or Section 7(c) below or as expressly provided pursuant to the terms of any employee benefit plan, the Employee will not be entitled to earn or accrue any additional compensation or benefits for any period following his Date of Termination. (b) Termination of Employment Due to Death or Disability. In addition to the compensation and benefits payable under Section 7(a) above, if the Employee’s employment is terminated due to his death or Disability outside of the Change of Control Period, the Employee (or his Beneficiary following the Employee’s death) shall receive:

6 US_ACTIVE127388755V-1 (i) the Employee’s accrued but unpaid Annual Bonus, if any, for the fiscal year ended prior to his Date of Termination payable at the same time annual bonuses for such fiscal year are paid to other key Employees of the Company pursuant to the terms of the Bonus Plan; (ii) fifty percent (50%) of the Employee’s outstanding unvested Equity Awards as of the Date of Termination will be fully vested and exercisable; and (iii) reimbursement of the COBRA premiums, if any, paid by the Employee’s spouse and dependents for continuation coverage for the Employee’s spouse and dependents under the Company’s group health, dental and vision plans for a six (6) month period from the Date of Termination. (c) Termination of Employment by the Company Without Cause, by the Employee for Good Reason or Upon Non-Renewal by the Company. In addition to the compensation and benefits payable under Section 7(a) above, if the Employee’s employment is terminated by the Company without Cause, by the Employee for Good Reason or upon Non- Renewal where it is the Company that provided written notice of non-renewal of this Agreement in accordance with Section 2, and such termination occurs outside of the Change of Control Period, and the Employee returns an executed Release to the Company, which becomes final, binding and irrevocable within sixty (60) days following the Employee’s Date of Termination in accordance with Section 10, the Employee (or his Beneficiary following the Employee’s death) shall receive: (i) the Employee’s accrued but unpaid Annual Bonus, if any, for the fiscal year ended prior to his Date of Termination payable at the same time annual bonuses for such fiscal year are paid to other key Employees of the Company pursuant to the terms of the Bonus Plan; (ii) fifty percent (50%) of the Employee’s outstanding unvested Equity Awards as of the Date of Termination will be fully vested and exercisable; (iii) a severance payment payable in a single lump sum within five (5) business days after the Employee’s Release becomes final, binding and irrevocable in accordance with Section 10, in an amount equal to twelve (12) months of Base Salary; and (iv) reimbursement of the COBRA premiums, if any, paid by the Employee for continuation coverage for the Employee, his spouse and dependents under the Company’s group health, dental and vision plans for a twelve (12) month period from the Date of Termination. Notwithstanding the foregoing, if the Employee materially breaches this Agreement or the Employee’s Confidentiality Agreement, then the Company’s continuing obligations under this Section 7(c) shall cease as of the date of the breach and the Employee shall be entitled to no further payments hereunder. 8. Termination of Employment by the Company Without Cause, by the Employee for Good Reason or Upon Non-Renewal by the Company in Connection with a Change of Control. In addition to the compensation and benefits payable under Section 7(a) above, if the Employee’s employment is terminated by the Company without Cause, by the Employee for Good Reason or upon Non-Renewal where it is the Company that provided written notice of

7 US_ACTIVE127388755V-1 non-renewal of this Agreement in accordance with Section 2, and such termination occurs during the Change of Control Period, and the Employee returns an executed Release to the Company, which becomes final, binding and irrevocable within sixty (60) days following the Employee’s Date of Termination in accordance with Section 10, the Employee (or his Beneficiary following the Employee’s death) shall receive: (a) a single lump sum within five (5) business days after the Employee’s Release becomes final, binding and irrevocable in accordance with Section 10, equal to the Employee’s accrued but unpaid Annual Bonus, if any, for the fiscal year ended prior to his Date of Termination; (b) a single lump sum within five (5) business days after the Employee’s Release becomes final, binding and irrevocable in accordance with Section 10, equal one hundred percent (100%) of Employee’s target bonus as in effect for the fiscal year in which Employee’s termination of employment occurs; provided that, for avoidance of doubt, the amount paid to Employee pursuant to this Section 8(b) will not be prorated based on the actual amount of time Employee is employed by the Company during the fiscal year (or the relevant performance period if something different than a fiscal year) during which the termination occurs; (c) one hundred percent (100%) of the Employee’s outstanding unvested Equity Awards as of the Date of Termination will be fully vested and exercisable; (d) a severance payment payable in a single lump sum within five (5) business days after the Employee’s Release becomes final, binding and irrevocable in accordance with Section 10, in an amount equal to twelve (12) months of Base Salary; and (e) reimbursement of the COBRA premiums, if any, paid by the Employee for continuation coverage for the Employee, his spouse and dependents under the Company’s group health, dental and vision plans for a twelve (12) month period from the Date of Termination. 9. Terminations Within Sixty (60) Days Prior to a Change of Control. If (a) the Employee incurred a termination prior to a Change of Control that qualifies Employee for severance payments under Section 7(c) and (b) a Change of Control occurs within sixty (60) days following Employee’s termination of employment, then upon the Change of Control, the Employee shall be entitled to a lump-sum payment of the amount calculated under this Section 8, less amounts already paid under Section 7(c), subject to compliance with Section 10. 10. Release. As a condition of receiving the compensation and benefits described in Section 7(c) or Section 8, the Employee must execute a release of any and all claims arising out of the Employee’s employment with the Company or the Employee’s separation from such employment (including, without limitation, claims relating to age, disability, sex or race discrimination to the extent permitted by law), excepting (i) claims for benefits under any employee benefit plan in accordance with the terms of such employee benefit plan, (ii) any right to exercise Equity Awards that are vested on the Date of Termination pursuant to the terms of such Equity Awards (as modified by the Employment Agreement), (iii) claims based on breach of the Company’s obligations to pay the compensation and benefits described in Section 5 and Section 7(a), Section 7(c) or Section 8 of this Employment Agreement, (iv) claims arising under the Age Discrimination in Employment Act after the date the Employee signs such release, and (v) any right to indemnification by the Company or to coverage under

8 US_ACTIVE127388755V-1 directors and officers liability insurance to which the Employee is otherwise entitled in accordance with this Agreement and the Company’s articles of incorporation or by laws or other agreement between the Employee and the Company (the “Release”). Such Release shall be in a form tendered to the Employee by the Company within five (5) business days following the termination of the Employee’s employment by the Company without Cause or by the Employee for Good Reason, which shall comply with any applicable legislation or judicial requirements, including, but not limited to, the Older Workers Benefit Protection Act, and shall be substantially in the form of release attached as Exhibit B. The compensation and benefits described in Section 7(c) or Section 8 will not be paid to the Employee if the Employee fails to execute the Release within the time frame specified in such Release, if the Employee revokes the Release within the applicable revocation period set forth in such Release or if the revocation period expires more than sixty (60) days following the Employee’s Date of Termination. 11. Excess Parachute Excise Tax. (a) Anything in this Agreement to the contrary notwithstanding, in the event it shall be determined that any payment, award, benefit or distribution (including any acceleration) by the Company or any entity which effectuates a transaction described in Section 280G(b)(2)(A)(i) of the Internal Revenue Code of 1986, as amended and the regulations promulgated thereunder (the “Code”) to or for the benefit of the Employee (whether pursuant to the terms of this Agreement or otherwise, but determined before application of any reductions required pursuant to this Section 11) (a “Payment”) would be subject to the excise tax imposed by Section 4999 of the Code or any interest or penalties are incurred with respect to such excise tax by the Employee (such excise tax, together with any such interest and penalties, are hereinafter collectively referred to as the “Excise Tax”), the Company will automatically reduce such Payments to the extent, but only to the extent, necessary so that no portion of the remaining Payments will be subject to the Excise Tax, unless the amount of such Payments that the Employee would retain after payment of the Excise Tax and all applicable Federal, state and local income taxes without such reduction would exceed the amount of such Payments that the Employee would retain after payment of all applicable Federal, state and local taxes after applying such reduction. Unless otherwise elected by the Employee, to the extent permitted under Code Section 409A, such reduction shall first be applied to any severance payments payable to the Employee under this Agreement, then to the accelerated vesting on any Equity Awards. (b) All determinations required to be made under this Section 11, including the assumptions to be utilized in arriving at such determination, shall be made by the Company’s independent auditors or such other certified public accounting firm of national standing reasonably acceptable to the Employee as may be designated by the Company (the “Accounting Firm”) which shall provide detailed supporting calculations both to the Company and the Employee within fifteen (15) business days of the receipt of notice from the Employee that there has been a Payment, or such earlier time as is requested by either the Company or the Employee. All fees and expenses of the Accounting Firm shall be borne solely by the Company. If the Accounting Firm determines that no Excise Tax is payable by the Employee, it shall furnish the Employee with a written opinion to such effect. Any determination by the Accounting Firm shall be binding upon the Company and the Employee. 12. Legal Fees. Each party shall be responsible for its own legal fees and expenses in connection with any claim or dispute relating to this Agreement.

9 US_ACTIVE127388755V-1 13. Beneficiary. If the Employee dies prior to receiving all of the amounts payable to him in accordance with the terms of this Agreement, such amounts shall be paid to one or more beneficiaries (each, a “Beneficiary”) designated by the Employee in writing to the Company during his lifetime, or if no such Beneficiary is designated, to the Employee’s estate. Such payments shall be made in accordance with the terms of this Agreement. The Employee, without the consent of any prior Beneficiary, may change his designation of Beneficiary or Beneficiaries at any time or from time to time by a submitting to the Company a new designation in writing. 14. Notices. All notices, requests, demands and other communications hereunder shall be in writing and shall be deemed to have been duly given if delivered by hand, email or mailed within the continental United States by first class certified mail, return receipt requested, postage prepaid, addressed as follows: If to the Company: Immunic, Inc. c/o Immunic AG Lochhamer Schlag 21 82166 Gräfelfing, Germany Attn: Chief Executive Officer Email: daniel.vitt@imux.com If to the Employee: To the address on file with the records of the Company. Addresses may be changed by written notice sent to the other party at the last recorded address of that party. 15. Withholding. The Company shall be entitled to withhold from payments due hereunder any required federal, state or local withholding or other taxes. 16. Arbitration. (a) If the parties are unable to resolve any dispute or claim relating directly or indirectly to this agreement or any dispute or claim between the Employee and the Company or its officers, directors, agents, or employees (a “Dispute”), then either party may require the matter to be settled by final and binding arbitration by sending written notice of such election to the other party clearly marked “Arbitration Demand.” Thereupon such Dispute shall be arbitrated in accordance with the terms and conditions of this Section 16. Notwithstanding the foregoing, either party may apply to a court of competent jurisdiction for a temporary restraining order, a preliminary injunction, or other equitable relief to preserve the status quo or prevent irreparable harm or to enforce the terms of the Confidentiality Agreement. (b) The Dispute shall be resolved by a single arbitrator in an arbitration administered by the American Arbitration Association in accordance with its Employment Arbitration Rules and judgment upon the award rendered by the arbitrator may be entered in any court having jurisdiction thereof. The decision of the arbitrator shall be final and binding on the parties, and specific performance giving effect to the decision of the arbitrator may be ordered by any court of competent jurisdiction.

10 US_ACTIVE127388755V-1 (c) Nothing contained herein shall operate to prevent either party from asserting counterclaim(s) in any arbitration commenced in accordance with this Agreement, and any such party need not comply with the procedural provisions of this Section 16 in order to assert such counterclaim(s). (d) The arbitration shall be filed with the office of the American Arbitration Association (“AAA”) located in New York or such other AAA office as the parties may agree upon (without any obligation to so agree). The arbitration shall be conducted pursuant to the Employment Arbitration Rules of the AAA as in effect at the time of the arbitration hearing, such arbitration to be completed in a sixty (60)-day period. In addition, the following rules and procedures shall apply to the arbitration: (e) The arbitrator shall have the sole authority to decide whether or not any Dispute between the parties is arbitrable and whether the party presenting the issues to be arbitrated has satisfied the conditions precedent to such party’s right to commence arbitration as required by this Section 16. (f) The decision of the arbitrator, which shall be in writing and state the findings, the facts and conclusions of law upon which the decision is based, shall be final and binding upon the parties, who shall forthwith comply after receipt thereof. Judgment upon the award rendered by the arbitrator may be entered by any competent court. Each party submits itself to the jurisdiction of any such court, but only for the entry and enforcement to judgment with respect to the decision of the arbitrator hereunder. (g) The arbitrator shall have the power to grant all legal and equitable remedies (including, without limitation, specific performance) and award compensatory and punitive damages if authorized by applicable law. (h) Except as otherwise provided in Section 12 or by law, the parties shall bear their own costs in preparing for and participating in the resolution of any Dispute pursuant to this Section 16, and the costs of the arbitrator(s) shall be equally divided between the parties. (i) Except as provided in the last sentence of Section 16(a), the provisions of this Section 16 shall be a complete defense to any suit, action or proceeding instituted in any federal, state or local court or before any administrative tribunal with respect to any Dispute arising in connection with this Agreement. Any party commencing a lawsuit in violation of this Section 16 shall pay the costs of the other party, including, without limitation, reasonable attorney’s fees and defense costs. 17. Recoupment. (a) Policy. Any incentive-based compensation received by the Employee including Annual Bonus and Equity Awards, whether pursuant to this Agreement or otherwise, that is granted, earned or vested based in any part on attainment of a financial reporting measure, shall be subject to the terms and conditions of the Company’s Claw Back Compensation Policy (the “Recoupment Policy”), and any other policy of recoupment of compensation as shall be adopted from time to time by the Company’s Board of Directors or the Compensation Committee as it deems necessary or appropriate to comply with the requirements of Section 954 of the Dodd-Frank Wall Street Reform and Consumer Protection Act, Section 304 of the Sarbanes-Oxley Act of 2002, and any implementing rules and regulations of the U.S. Securities and Exchange Commission and applicable listing standards

11 US_ACTIVE127388755V-1 of a national securities exchange adopted in accordance with any of the foregoing. The terms and conditions of the Recoupment Policy, including any changes to the Recoupment Policy adopted from time to time by the Company, are hereby incorporated by reference into this Agreement. (b) Non-Indemnification and Advancement for Recoupment. The Company shall not be obligated to indemnify or advance funds to the Employee for any payment or reimbursement by the Employee to the Company of any bonus or other incentive- based or equity-based compensation previously received by the Employee or payment of any profits realized by the Employee from the sale of securities of the Company, as required in each case under the Securities Exchange Act of 1934 or under the rules of the stock exchange on which the common stock of the Company is listed (including any such payments or reimbursements under Section 304 and 306 of the Sarbanes-Oxley Act of 2002, or pursuant to Section 954 of the Dodd-Frank Wall Street Reform and Consumer Protection Act and any implementing rules and regulations of the U.S. Securities and Exchange Commission and applicable listing standards of a national securities exchange adopted in accordance with any of the foregoing). 18. Miscellaneous (a) Governing Law. This Agreement shall be interpreted, construed, governed and enforced according to the laws of the State of New York without regard to the application of choice of law rules. (b) Entire Agreement. This Agreement contains the entire agreement between the parties with respect to the subject matter hereof and supersedes any and all other prior agreements, promises, understandings and representations regarding the Employee’s employment, compensation, severance or other payments contingent upon the Employee’s termination of employment, whether written or otherwise. (c) Amendments. No amendment or modification of the terms or conditions of this Agreement shall be valid unless in writing and signed by the parties hereto. (d) Severability. If one or more provisions of this Agreement are held to be invalid or unenforceable under applicable law, such provisions shall be construed, if possible, so as to be enforceable under applicable law, or such provisions shall be excluded from this Agreement and the balance of the Agreement shall be interpreted as if such provision were so excluded and shall be enforceable in accordance with its terms. (e) Binding Effect. This Agreement shall be binding upon and inure to the benefit of the beneficiaries, heirs and representatives of the Employee (including the Beneficiary) and the successors and assigns of the Company. The Company shall require any successor (whether direct or indirect, by purchase, merger, reorganization, consolidation, acquisition of property or stock, liquidation, or otherwise) to all or substantially all of its assets, by agreement in form and substance satisfactory to the Employee, expressly to assume and agree to perform this Agreement in the same manner and to the same extent that the Company would be required to perform this Agreement if no such succession had taken place. Regardless whether such agreement is executed, this Agreement shall be binding upon any successor of the Company in accordance with the operation of law and such successor shall be deemed the Company for purposes of this Agreement.

12 US_ACTIVE127388755V-1 (f) Successors and Assigns; Non-alienation of Benefits. Except as provided in Section 18(e) in the case of the Company, or to the Beneficiary in the case of the death of the Employee, this Agreement is not assignable by any party. Compensation and benefits payable to the Employee under this Agreement shall not be subject in any manner to anticipation, alienation, sale, transfer, assignment, pledge, encumbrance, charge, garnishment, execution or levy of any kind, either voluntary or involuntary, prior to actually being received by the Employee or a Beneficiary, as applicable, and any such attempt to dispose of any right to benefits payable hereunder shall be void and no payment to be made hereunder shall be subject to anticipation, alienation, sale, transfer, assignment, pledge, encumbrance or other charge. (g) Remedies Cumulative; No Waiver. No remedy conferred upon either party by this Agreement is intended to be exclusive of any other remedy, and each and every such remedy shall be cumulative and shall be in addition to any other remedy given hereunder or now or hereafter existing at law or in equity. No delay or omission by either party in exercising any right, remedy or power hereunder or existing at law or in equity shall be construed as a waiver thereof, and any such right, remedy or power may be exercised by such party from time to time and as often as may be deemed expedient or necessary by such party in such party’s sole discretion. (h) Survivorship. Notwithstanding anything in this Agreement to the contrary, all terms and provisions of this Agreement that by their nature extend beyond the Date of Termination shall survive termination of this Agreement. (i) Counterparts. This Agreement may be executed in two or more counterparts, each of which shall constitute an original, but all of which, when taken together, shall constitute one document. 19. No Contract of Employment. Nothing contained in this Agreement will be construed as a right of the Employee to be continued in the employment of the Company, or as a limitation of the right of the Company to discharge the Employee with or without Cause. 20. Section 409A of the Code. (a) The intent of the parties is that payments and benefits under this Agreement comply with, or be exempt from, Section 409A of the Code and, accordingly, to the maximum extent permitted, this Agreement shall be construed and interpreted in accordance with such intent. The Employee’s termination of employment (or words to similar effect) shall not be deemed to have occurred for purposes of this Agreement unless such termination of employment constitutes a “separation from service” within the meaning of Code Section 409A and the regulations and other guidance promulgated thereunder. (b) Notwithstanding any provision in this Agreement to the contrary, if the Employee is deemed on the date of the Employee’s separation from service to be a “specified employee” within the meaning of that term under Code Section 409A(a)(2)(B) and using the identification methodology selected by the Company from time to time, or if none, the default methodology set forth in Code Section 409A, then with regard to any payment or the providing of any benefit that constitutes “non-qualified deferred compensation” pursuant to Code Section 409A and the regulations issued thereunder that is payable due to the Employee’s separation from service, to the extent required to be delayed in compliance with Code Section 409A(a)(2)(B), such payment or benefit shall not be made or provided to the Employee prior

13 US_ACTIVE127388755V-1 to the earlier of (i) the expiration of the six (6)-month period measured from the date of the Employee’s separation from service, and (ii) the date of the Employee’s death. On the first day of the seventh (7th) month following the date of the Employee’s separation from service or, if earlier, on the date of the Employee’s death, all payments delayed pursuant to this Section 20 shall be paid or reimbursed to the Employee in a lump sum, and any remaining payments and benefits due to the Employee under this Agreement shall be paid or provided in accordance with the normal payment dates specified for them herein. (c) To the extent any reimbursement of costs and expenses (including reimbursement of COBRA premiums pursuant to Section 7(c)(iv)) provided for under this Agreement constitutes taxable income to the Employee for Federal income tax purposes, such reimbursements shall be made as soon as practicable after the Employee provides proper documentation supporting reimbursement but in no event later than December 31 of the calendar year next following the calendar year in which the expenses to be reimbursed are incurred. With regard to any provision herein that provides for reimbursement of expenses or in-kind benefits, except as permitted by Code Section 409A, (i) the right to reimbursement or in-kind benefits is not subject to liquidation or exchange for another benefit, and (ii) the amount of expenses eligible for reimbursement, or in-kind benefits, provided during any taxable year shall not affect the expenses eligible for reimbursement, or in-kind benefits to be provided, in any other taxable year. (d) If under this Agreement, any amount is to be paid in two (2) or more installments, each such installment shall be treated as a separate payment for purposes of Section 409A. 21. Employee Acknowledgement. The Employee hereby acknowledges that the Employee has read and understands the provisions of this Agreement, that the Employee has been given the opportunity for the Employee’s legal counsel to review this Agreement, that the provisions of this Agreement are reasonable and that the Employee has received a copy of this Agreement. [SIGNATURE PAGE FOLLOWS]

US_ACTIVE127388755V-1 IN WITNESS WHEREOF, the parties hereto have caused this Employment Agreement to be executed as of the 22nd day of July 20244. IMMUNIC, INC. By: /s/ Daniel Vitt Name: Daniel Vitt Title: Chief Executive Officer EMPLOYEE /s/ Jason Tardio Jason Tardio

US_ACTIVE127388755V-1 EXHIBIT A CONFIDENTIAL INFORMATION, ASSIGNMENT OF RIGHTS, NON-SOLICITATION AND NON-COMPETITION AGREEMENT [SEE ATTACHED]

US_ACTIVE127388755V-1 EXHIBIT B WAIVER AND RELEASE This is a Waiver and Release (“Release”) between Jason Tardio (“Employee”) and Immunic, Inc. (the “Company”). The Company and the Employee agree that they have entered into this Release voluntarily, and that it is intended to be a legally binding commitment between them. In consideration for and contingent upon the Employee’s right to receive the benefits described in the Employment Agreement between the Company and the Employee (the “Employment Agreement”) and this Release, the Employee hereby agrees as follows: (a) General Waiver and Release. Except as provided in Paragraph (e) below, the Employee and any person acting through or under the Employee hereby release, waive and forever discharge the Company, its past and present subsidiaries and affiliates, and their respective successors and assigns, and their respective past and present officers, trustees, directors, shareholders, Employees and agents of each of them, from any and all claims, demands, actions, liabilities and other claims for relief and remuneration whatsoever (including without limitation attorneys’ fees and expenses), whether known or unknown, absolute, contingent or otherwise (each, a “Claim”), arising or which could have arisen up to and including the date of his execution of this Release, including without limitation those arising out of or relating to the Employee’s employment or cessation and termination of employment, or any other written or oral agreement, any change in the Employee’s employment status, any benefits or compensation, any tortious injury, breach of contract, wrongful discharge (including any Claim for constructive discharge), infliction of emotional distress, slander, libel or defamation of character, and any Claims arising under Title VII of the Civil Rights Act of 1964 (as amended by the Civil Rights Act of 1991), the Americans With Disabilities Act, the Rehabilitation Act of 1973, the Equal Pay Act, the Older Workers Benefits Protection Act, the Age Discrimination in Employment Act, the Employee Retirement Income Security Act of 1974, or any other federal, state or local statute, law, ordinance, regulation, rule or Employee order, any tort or contract claims, and any of the claims, matters and issues which could have been asserted by the Employee against the Company or its subsidiaries and affiliates in any legal, administrative or other proceeding. the Employee agrees that if any action is brought in his name before any court or administrative body, the Employee will not accept any payment of monies in connection therewith. (b) Miscellaneous. the Employee agrees that Section 7(c) of the Employment Agreement (which is specifically incorporated herein by reference) specifies payments from the Company to himself, the total of which meets or exceeds any and all funds due him by the Company, and that he will not seek to obtain any additional funds from the Company with the exception of non-reimbursed business expenses. (This covenant does not preclude the Employee from seeking workers’ compensation, unemployment compensation, or benefit payments from the Company’s insurance carriers that could be due him.) (c) Non-Solicitation, Confidentiality and Non-Solicitation Covenants. the Employee warrants that the Employee has, and will comply fully with Section 3(c) of the Employment Agreement and the provisions of the Confidential Information, Assignment of Rights, Non-Solicitation and Non-Competition Agreement by and between the Company and the Employee.

US_ACTIVE127388755V-1 (d) THE COMPANY AND THE EMPLOYEE AGREE THAT THE BENEFITS DESCRIBED IN SECTION 7(C) OF THE EMPLOYMENT AGREEMENT AS SUBJECT TO EMPLOYEE’S COMPLIANCE WITH SECTION 9 THEREOF ARE CONTINGENT UPON THE EMPLOYEE SIGNING THIS RELEASE. THE EMPLOYEE FURTHER UNDERSTANDS AND AGREES THAT IN SIGNING THIS RELEASE, EMPLOYEE IS RELEASING POTENTIAL LEGAL CLAIMS AGAINST THE COMPANY. THE EMPLOYEE UNDERSTANDS AND AGREES THAT IF HE DECIDES NOT TO SIGN THIS RELEASE, OR IF HE REVOKES THIS RELEASE, THAT HE WILL IMMEDIATELY REFUND TO THE COMPANY ANY AND ALL SEVERANCE PAYMENTS AND OTHER BENEFITS HE MAY HAVE ALREADY RECEIVED. (e) The waiver contained in Paragraph (a) and (b) above does not apply to: (i) Any claims for benefits under employee benefit plans in accordance with the terms of the applicable employee benefit plan, including the Employee’s right to elect continuation coverage under the Company’s group health, dental and/or visions plans pursuant to the Consolidated Omnibus Budget Reconciliation Act of 1985, as amended (COBRA), (ii) Any right to exercise stock options or stock appreciation rights that were vested and exercisable on the Date of Termination in accordance with the terms thereof (as modified by the Employment Agreement); (iii) Any Claim under or based on a breach of the Company’s obligations to pay the compensation and benefits described in Sections 5 or 7(a) or (c) of the Employment Agreement, (iv) Rights or Claims that may arise under the Age Discrimination in Employment Act after the date that the Employee signs this Release, and (v) Any right to indemnification by the Company or to coverage under directors and officers liability insurance to which the Employee is otherwise entitled in accordance with the Employment Agreement or the Company’s articles of incorporation or by-laws or other agreement between the Employee and the Company. (f) EMPLOYEE ACKNOWLEDGES THAT HE HAS READ AND IS VOLUNTARILY SIGNING THIS RELEASE. EMPLOYEE ALSO ACKNOWLEDGES THAT HE IS HEREBY ADVISED TO CONSULT WITH AN ATTORNEY, HE HAS BEEN GIVEN AT LEAST [21][45] DAYS TO CONSIDER THIS RELEASE BEFORE THE DEADLINE FOR SIGNING IT; [HE HAS RECEIVED A RECEIVED A WRITTEN DESCRIPTION OF THE JOB TITLES AND AGES ALL INDIVIDUALS SELECTED FOR THIS JOB ELIMINATION PROGRAM AND THE AGES OF ANY INDIVIDUALS IN THE SAME JOB CLASSIFICATIONS WHO ARE NOT SELECTED FOR THIS JOB ELIMINATION PROGRAM AS PROVIDED BY THE ADEA (SUCH DESCRIPTION ATTACHED AS EXHIBIT A HERETO)]; AND HE UNDERSTANDS THAT HE MAY REVOKE THE RELEASE WITHIN SEVEN (7) DAYS AFTER SIGNING IT. IF NOT REVOKED WITHIN SUCH PERIOD, THIS RELEASE WILL BECOME EFFECTIVE ON THE EIGHTH (8) DAY AFTER IT IS SIGNED BY EMPLOYEE.

US_ACTIVE127388755V-1 BY SIGNING BELOW, BOTH THE COMPANY AND EMPLOYEE AGREE THAT THEY UNDERSTAND AND ACCEPT EACH PART OF THIS RELEASE. Jason Tardio (Date Signed) ACCEPTED AND DATED AS OF ___________________ IMMUNIC, INC. By: Name: Title:
Exhibit 31.1
CERTIFICATIONS
I, Daniel Vitt, certify that:
1. I have reviewed this Quarterly Report on Form 10-Q of Immunic, Inc.;
2. Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4. The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a) Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b) Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c)Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d) Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5. The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a) All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b) Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
| | | | | | | | |
| Date: November 7, 2024 | By: | /s/ Daniel Vitt |
| | Daniel Vitt |
| | Chief Executive Officer |
| | (Principal Executive Officer) |
Exhibit 31.2
CERTIFICATIONS
I, Glenn Whaley, certify that:
1. I have reviewed this Quarterly Report on Form 10-Q of Immunic, Inc.;
2. Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of and for the periods presented in this report;
4. The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a) Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b) Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c) Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d) Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5. The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a) All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b) Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
| | | | | | | | |
| Date: November 7, 2024 | By: | /s/ Glenn Whaley |
| | Glenn Whaley |
| | Chief Financial Officer |
| | (Principal Financial Officer) |
Exhibit 32.1
CERTIFICATION PURSUANT TO 18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Quarterly Report on Form 10-Q of Immunic, Inc. (the “Company”) for the period ended September 30, 2024, as filed with the Securities and Exchange Commission on the date hereof (the “Report”), the undersigned, Daniel Vitt, as Chief Executive Officer of the Company, hereby certifies, pursuant to 18 U.S.C. Section 1350, that to my knowledge:
(1) the Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
(2) the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
| | | | | | | | |
| Date: November 7, 2024 | By: | /s/ Daniel Vitt |
| | Daniel Vitt |
| | Chief Executive Officer |
| | (Principal Executive Officer) |
Exhibit 32.2
CERTIFICATION PURSUANT TO 18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Quarterly Report on Form 10-K of Immunic, Inc. (the “Company”) for the period ended September 30, 2024, as filed with the Securities and Exchange Commission on the date hereof (the “Report”), the undersigned, Glenn Whaley as Chief Financial Officer of the Company, hereby certifies, pursuant to 18 U.S.C. Section 1350, that to my knowledge::
(1) the Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
(2) the information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
| | | | | | | | |
| Date: November 7, 2024 | By: | /s/ Glenn Whaley |
| | Glenn Whaley |
| | Chief Financial Officer |
| | (Principal Financial Officer) |
v3.24.3
Cover Page - shares
|
9 Months Ended |
|
Sep. 30, 2024 |
Oct. 31, 2024 |
| Cover [Abstract] |
|
|
| Document Type |
10-Q
|
|
| Document Quarterly Report |
true
|
|
| Document Period End Date |
Sep. 30, 2024
|
|
| Document Transition Report |
false
|
|
| Entity File Number |
001-36201
|
|
| Entity Registrant Name |
Immunic, Inc.
|
|
| Entity Incorporation, State or Country Code |
DE
|
|
| Entity Tax Identification Number |
56-2358443
|
|
| Entity Address, Address Line One |
1200 Avenue of the Americas
|
|
| Entity Address, Address Line Two |
Suite 200
|
|
| Entity Address, City or Town |
New York,
|
|
| Entity Address, State or Province |
NY
|
|
| Entity Address, Postal Zip Code |
10036
|
|
| City Area Code |
332
|
|
| Local Phone Number |
255-9818
|
|
| Title of 12(b) Security |
Common Stock, $0.0001 par value
|
|
| Trading Symbol |
IMUX
|
|
| Security Exchange Name |
NASDAQ
|
|
| Entity Current Reporting Status |
Yes
|
|
| Entity Interactive Data Current |
Yes
|
|
| Entity Filer Category |
Non-accelerated Filer
|
|
| Entity Small Business |
true
|
|
| Entity Emerging Growth Company |
false
|
|
| Entity Shell Company |
false
|
|
| Entity Common Stock, Shares Outstanding |
|
90,079,016
|
| Document Fiscal Year Focus |
2024
|
|
| Document Fiscal Period Focus |
Q3
|
|
| Amendment Flag |
false
|
|
| Current Fiscal Year End Date |
--12-31
|
|
| Entity Central Index Key |
0001280776
|
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEnd date of current fiscal year in the format --MM-DD.
| Name: |
dei_CurrentFiscalYearEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gMonthDayItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFiscal period values are FY, Q1, Q2, and Q3. 1st, 2nd and 3rd quarter 10-Q or 10-QT statements have value Q1, Q2, and Q3 respectively, with 10-K, 10-KT or other fiscal year statements having FY.
| Name: |
dei_DocumentFiscalPeriodFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fiscalPeriodItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThis is focus fiscal year of the document report in YYYY format. For a 2006 annual report, which may also provide financial information from prior periods, fiscal 2006 should be given as the fiscal year focus. Example: 2006.
| Name: |
dei_DocumentFiscalYearFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gYearItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as an quarterly report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-Q
-Number 240
-Section 308
-Subsection a
| Name: |
dei_DocumentQuarterlyReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as a transition report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Forms 10-K, 10-Q, 20-F
-Number 240
-Section 13
-Subsection a-1
| Name: |
dei_DocumentTransitionReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate number of shares or other units outstanding of each of registrant's classes of capital or common stock or other ownership interests, if and as stated on cover of related periodic report. Where multiple classes or units exist define each class/interest by adding class of stock items such as Common Class A [Member], Common Class B [Member] or Partnership Interest [Member] onto the Instrument [Domain] of the Entity Listings, Instrument.
| Name: |
dei_EntityCommonStockSharesOutstanding |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionIndicate 'Yes' or 'No' whether registrants (1) have filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that registrants were required to file such reports), and (2) have been subject to such filing requirements for the past 90 days. This information should be based on the registrant's current or most recent filing containing the related disclosure.
| Name: |
dei_EntityCurrentReportingStatus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityFilerCategory |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:filerCategoryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-T
-Number 232
-Section 405
| Name: |
dei_EntityInteractiveDataCurrent |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant is a shell company as defined in Rule 12b-2 of the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityShellCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates that the company is a Smaller Reporting Company (SRC). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntitySmallBusiness |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Condensed Consolidated Balance Sheets - USD ($)
$ in Thousands |
Sep. 30, 2024 |
Dec. 31, 2023 |
| Current assets: |
|
|
| Cash and cash equivalents |
$ 59,071
|
$ 46,674
|
| Other current assets and prepaid expenses |
4,195
|
5,860
|
| Total current assets |
63,266
|
52,534
|
| Property and equipment, net |
618
|
466
|
| Right-of-use assets, net |
878
|
1,299
|
| Total assets |
64,762
|
54,299
|
| Current liabilities: |
|
|
| Accounts payable |
6,042
|
5,099
|
| Accrued expenses |
16,245
|
18,664
|
| Other current liabilities |
1,070
|
966
|
| Total current liabilities |
23,357
|
24,729
|
| Long term liabilities |
|
|
| Operating lease liabilities |
186
|
639
|
| Total long-term liabilities |
186
|
639
|
| Total liabilities |
23,543
|
25,368
|
| Commitments and contingencies (Note 4) |
|
|
| Stockholders’ equity: |
|
|
| Preferred stock, $0.0001 par value; 20,000,000 authorized and no shares issued or outstanding as of September 30, 2024 and December 31, 2023 |
0
|
0
|
| Common stock, $0.0001 par value; 500,000,000 and 130,000,000 shares authorized as of September 30, 2024 and December 31, 2023, respectively, and 90,079,016 and 45,177,730 shares issued and outstanding as of September 30, 2024 and December 31, 2023, respectively. |
8
|
4
|
| Additional paid-in capital |
523,549
|
436,060
|
| Accumulated other comprehensive income |
3,886
|
3,759
|
| Accumulated deficit |
(486,224)
|
(410,892)
|
| Total stockholders’ equity |
41,219
|
28,931
|
| Total liabilities and stockholders’ equity |
$ 64,762
|
$ 54,299
|
| X |
- DefinitionCarrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
| Name: |
us-gaap_AccountsPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred and payable, pertaining to costs that are statutory in nature, are incurred on contractual obligations, or accumulate over time and for which invoices have not yet been received or will not be rendered. Examples include taxes, interest, rent and utilities. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after tax, of accumulated increase (decrease) in equity from transaction and other event and circumstance from nonowner source. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14A
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-14A
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-14
| Name: |
us-gaap_AccumulatedOtherComprehensiveIncomeLossNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of excess of issue price over par or stated value of stock and from other transaction involving stock or stockholder. Includes, but is not limited to, additional paid-in capital (APIC) for common and preferred stock. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AdditionalPaidInCapital |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset recognized for present right to economic benefit. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 49
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-49
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 5: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 12: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 30: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset recognized for present right to economic benefit, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
| Name: |
us-gaap_AssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the caption on the face of the balance sheet to indicate that the entity has entered into (1) purchase or supply arrangements that will require expending a portion of its resources to meet the terms thereof, and (2) is exposed to potential losses or, less frequently, gains, arising from (a) possible claims against a company's resources due to future performance under contract terms, and (b) possible losses or likely gains from uncertainties that will ultimately be resolved when one or more future events that are deemed likely to occur do occur or fail to occur. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(15))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_CommitmentsAndContingencies |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_CommonStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liability recognized for present obligation requiring transfer or otherwise providing economic benefit to others. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(24))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(26))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(21))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 15: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(14))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 28: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
| Name: |
us-gaap_Liabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(32))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesAndStockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal obligations incurred as part of normal operations that are expected to be paid during the following twelve months or within one business cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(21))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-5
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 21: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
| Name: |
us-gaap_LiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of obligation due after one year or beyond the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(24))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(26))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 22: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
| Name: |
us-gaap_LiabilitiesNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesNoncurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities classified as other, due within one year or the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
| Name: |
us-gaap_OtherLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable preferred shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(21))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_PreferredStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits in future periods, and amount of other assets that are expected to be realized or consumed within one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PrepaidExpenseAndOtherAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-7A
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478451/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480990/946-20-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 12: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Condensed Consolidated Balance Sheets (Parenthetical) - $ / shares
|
Sep. 30, 2024 |
Dec. 31, 2023 |
| Statement of Financial Position [Abstract] |
|
|
| Preferred stock, par value (in USD per share) |
$ 0.0001
|
$ 0.0001
|
| Preferred stock, shares authorized (in shares) |
20,000,000
|
20,000,000
|
| Preferred stock, shares issued (in shares) |
0
|
0
|
| Preferred stock, shares outstanding (in shares) |
0
|
0
|
| Common stock, par value (in USD per share) |
$ 0.0001
|
$ 0.0001
|
| Common stock, shares authorized (in shares) |
500,000,000
|
130,000,000
|
| Common stock, shares issued (in shares) |
90,079,016
|
45,177,730
|
| Common stock, shares outstanding (in shares) |
90,079,016
|
45,177,730
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionFace amount or stated value per share of preferred stock nonredeemable or redeemable solely at the option of the issuer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
| Name: |
us-gaap_PreferredStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_PreferredStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares issued for nonredeemable preferred shares and preferred shares redeemable solely at option of issuer. Includes, but is not limited to, preferred shares issued, repurchased, and held as treasury shares. Excludes preferred shares classified as debt. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
| Name: |
us-gaap_PreferredStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate share number for all nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer) held by stockholders. Does not include preferred shares that have been repurchased. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
| Name: |
us-gaap_PreferredStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StatementOfFinancialPositionAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Condensed Consolidated Statements of Operations - USD ($)
$ in Thousands |
3 Months Ended |
9 Months Ended |
Sep. 30, 2024 |
Sep. 30, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
| Operating expenses: |
|
|
|
|
| Research and development |
$ 21,370
|
$ 19,796
|
$ 58,429
|
$ 63,931
|
| General and administrative |
4,356
|
3,774
|
13,992
|
11,911
|
| Total operating expenses |
25,726
|
23,570
|
72,421
|
75,842
|
| Loss from operations |
(25,726)
|
(23,570)
|
(72,421)
|
(75,842)
|
| Other income (expense): |
|
|
|
|
| Interest income |
776
|
766
|
2,961
|
2,534
|
| Change in fair value of the tranche rights |
0
|
0
|
(4,796)
|
0
|
| Other income (expense), net |
582
|
35
|
(1,076)
|
1,268
|
| Total other income (expense) |
1,358
|
801
|
(2,911)
|
3,802
|
| Net loss |
$ (24,368)
|
$ (22,769)
|
$ (75,332)
|
$ (72,040)
|
| Net loss per share, basic (in USD per share) |
$ (0.24)
|
$ (0.51)
|
$ (0.75)
|
$ (1.63)
|
| Net loss per share, diluted (in USD per share) |
$ (0.24)
|
$ (0.51)
|
$ (0.75)
|
$ (1.63)
|
| Weighted-average common shares outstanding, basic (in shares) |
101,272,580
|
44,574,377
|
99,998,245
|
44,227,264
|
| Weighted-average common shares outstanding, diluted (in shares) |
101,272,580
|
44,574,377
|
99,998,245
|
44,227,264
|
| X |
- DefinitionThe amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-52
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-15
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-7
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-10
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-52
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-15
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-7
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total of expenses of managing and administering the affairs of an entity, including affiliates of the reporting entity, which are not directly or indirectly associated with the manufacture, sale or creation of a product or product line. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
| Name: |
us-gaap_GeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount before accretion (amortization) of purchase discount (premium) of interest income on nonoperating securities. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(7)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
| Name: |
us-gaap_InvestmentIncomeInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate amount of income or expense from ancillary business-related activities (that is to say, excluding major activities considered part of the normal operations of the business). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_NonoperatingIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NonoperatingIncomeExpenseAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGenerally recurring costs associated with normal operations except for the portion of these expenses which can be clearly related to production and included in cost of sales or services. Includes selling, general and administrative expense.
| Name: |
us-gaap_OperatingExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OperatingExpensesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe net result for the period of deducting operating expenses from operating revenues. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-31
| Name: |
us-gaap_OperatingIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of income (expense) related to nonoperating activities, classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_OtherNonoperatingIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for research and development. Includes, but is not limited to, cost for computer software product to be sold, leased, or otherwise marketed and writeoff of research and development assets acquired in transaction other than business combination or joint venture formation or both. Excludes write-down of intangible asset acquired in business combination or from joint venture formation or both, used in research and development activity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482916/730-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479532/912-730-25-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-16
| Name: |
us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-10
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFair Value, Change in Fair Value of Tranche Rights, Transaction Costs
| Name: |
vtl_FairValueChangeInFairValueOfTrancheRightsTransactionCosts |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.24.3
Condensed Consolidated Statements of Comprehensive Loss - USD ($)
$ in Thousands |
3 Months Ended |
9 Months Ended |
Sep. 30, 2024 |
Sep. 30, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
| Statement of Comprehensive Income [Abstract] |
|
|
|
|
| Net loss |
$ (24,368)
|
$ (22,769)
|
$ (75,332)
|
$ (72,040)
|
| Other comprehensive income (loss): |
|
|
|
|
| Foreign currency translation |
104
|
(77)
|
127
|
870
|
| Total comprehensive loss |
$ (24,264)
|
$ (22,846)
|
$ (75,205)
|
$ (71,170)
|
| X |
- DefinitionAmount after tax of increase (decrease) in equity from transactions and other events and circumstances from net income and other comprehensive income, attributable to parent entity. Excludes changes in equity resulting from investments by owners and distributions to owners. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(24))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(26))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-5
| Name: |
us-gaap_ComprehensiveIncomeNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax and reclassification adjustments of gain (loss) on foreign currency translation adjustments, foreign currency transactions designated and effective as economic hedges of a net investment in a foreign entity and intra-entity foreign currency transactions that are of a long-term-investment nature, attributable to parent entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-19
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-20
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(3)
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_OtherComprehensiveIncomeForeignCurrencyTransactionAndTranslationAdjustmentNetOfTaxPortionAttributableToParent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OtherComprehensiveIncomeLossNetOfTaxPortionAttributableToParentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_StatementOfIncomeAndComprehensiveIncomeAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Condensed Consolidated Statements of Stockholders’ Equity - USD ($)
$ in Thousands |
Total |
January2024 P I P E Transaction |
At The Market Sales Agreement |
Common Stock |
Common Stock
January2024 P I P E Transaction
|
Common Stock
At The Market Sales Agreement
|
Additional Paid-In Capital |
Additional Paid-In Capital
January2024 P I P E Transaction
|
Additional Paid-In Capital
At The Market Sales Agreement
|
Accumulated Other Comprehensive Income (Loss) |
Accumulated Deficit |
| Beginning balance (in shares) at Dec. 31, 2022 |
|
|
|
39,307,286
|
|
|
|
|
|
|
|
| Beginning balance at Dec. 31, 2022 |
$ 113,684
|
|
|
$ 4
|
|
|
$ 427,925
|
|
|
$ 3,035
|
$ (317,280)
|
| Increase (Decrease) in Stockholders' Equity [Roll Forward] |
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
(25,272)
|
|
|
|
|
|
|
|
|
|
(25,272)
|
| Stock-based compensation |
1,979
|
|
|
|
|
|
1,979
|
|
|
|
|
| Foreign currency translation |
776
|
|
|
|
|
|
|
|
|
776
|
|
| Shares issued from exercise of pre-funded warrants (in shares) |
|
|
|
5,096,552
|
|
|
|
|
|
|
|
| Shares issued from exercise of pre-funded warrants |
51
|
|
|
|
|
|
51
|
|
|
|
|
| Ending balance (in shares) at Mar. 31, 2023 |
|
|
|
44,403,838
|
|
|
|
|
|
|
|
| Ending balance at Mar. 31, 2023 |
91,218
|
|
|
$ 4
|
|
|
429,955
|
|
|
3,811
|
(342,552)
|
| Beginning balance (in shares) at Dec. 31, 2022 |
|
|
|
39,307,286
|
|
|
|
|
|
|
|
| Beginning balance at Dec. 31, 2022 |
113,684
|
|
|
$ 4
|
|
|
427,925
|
|
|
3,035
|
(317,280)
|
| Increase (Decrease) in Stockholders' Equity [Roll Forward] |
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
(72,040)
|
|
|
|
|
|
|
|
|
|
|
| Foreign currency translation |
870
|
|
|
|
|
|
|
|
|
|
|
| Ending balance (in shares) at Sep. 30, 2023 |
|
|
|
44,595,383
|
|
|
|
|
|
|
|
| Ending balance at Sep. 30, 2023 |
48,407
|
|
|
$ 4
|
|
|
433,818
|
|
|
3,905
|
(389,320)
|
| Beginning balance (in shares) at Mar. 31, 2023 |
|
|
|
44,403,838
|
|
|
|
|
|
|
|
| Beginning balance at Mar. 31, 2023 |
91,218
|
|
|
$ 4
|
|
|
429,955
|
|
|
3,811
|
(342,552)
|
| Increase (Decrease) in Stockholders' Equity [Roll Forward] |
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
(23,999)
|
|
|
|
|
|
|
|
|
|
(23,999)
|
| Stock-based compensation |
1,798
|
|
|
|
|
|
1,798
|
|
|
|
|
| Foreign currency translation |
171
|
|
|
|
|
|
|
|
|
171
|
|
| Shares issued in connection with the Company's Employee Stock Purchase Plan (in shares) |
|
|
|
84,533
|
|
|
|
|
|
|
|
| Shares issued in connection with the Company's Employee Stock Purchase Plan |
96
|
|
|
|
|
|
96
|
|
|
|
|
| Ending balance (in shares) at Jun. 30, 2023 |
|
|
|
44,488,371
|
|
|
|
|
|
|
|
| Ending balance at Jun. 30, 2023 |
69,284
|
|
|
$ 4
|
|
|
431,849
|
|
|
3,982
|
(366,551)
|
| Increase (Decrease) in Stockholders' Equity [Roll Forward] |
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
(22,769)
|
|
|
|
|
|
|
|
|
|
(22,769)
|
| Stock-based compensation |
1,687
|
|
|
|
|
|
1,687
|
|
|
|
|
| Foreign currency translation |
(77)
|
|
|
|
|
|
|
|
|
(77)
|
|
| Issuance of common stock (in shares) |
|
|
|
|
|
107,012
|
|
|
|
|
|
| Issuance of common stock |
|
|
$ 282
|
|
|
|
|
|
$ 282
|
|
|
| Ending balance (in shares) at Sep. 30, 2023 |
|
|
|
44,595,383
|
|
|
|
|
|
|
|
| Ending balance at Sep. 30, 2023 |
$ 48,407
|
|
|
$ 4
|
|
|
433,818
|
|
|
3,905
|
(389,320)
|
| Beginning balance (in shares) at Dec. 31, 2023 |
45,177,730
|
|
|
45,177,730
|
|
|
|
|
|
|
|
| Beginning balance at Dec. 31, 2023 |
$ 28,931
|
|
|
$ 4
|
|
|
436,060
|
|
|
3,759
|
(410,892)
|
| Increase (Decrease) in Stockholders' Equity [Roll Forward] |
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
(29,584)
|
|
|
|
|
|
|
|
|
|
(29,584)
|
| Stock-based compensation |
2,750
|
|
|
|
|
|
2,750
|
|
|
|
|
| Foreign currency translation |
528
|
|
|
|
|
|
|
|
|
528
|
|
| Issuance of common stock (in shares) |
|
|
|
|
44,751,286
|
150,000
|
|
|
|
|
|
| Issuance of common stock |
|
$ 52,364
|
$ 191
|
|
$ 4
|
|
|
$ 52,360
|
$ 191
|
|
|
| Conversion of tranche rights liability to equity |
28,396
|
|
|
|
|
|
28,396
|
|
|
|
|
| Ending balance (in shares) at Mar. 31, 2024 |
|
|
|
90,079,016
|
|
|
|
|
|
|
|
| Ending balance at Mar. 31, 2024 |
$ 83,576
|
|
|
$ 8
|
|
|
519,757
|
|
|
4,287
|
(440,476)
|
| Beginning balance (in shares) at Dec. 31, 2023 |
45,177,730
|
|
|
45,177,730
|
|
|
|
|
|
|
|
| Beginning balance at Dec. 31, 2023 |
$ 28,931
|
|
|
$ 4
|
|
|
436,060
|
|
|
3,759
|
(410,892)
|
| Increase (Decrease) in Stockholders' Equity [Roll Forward] |
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
(75,332)
|
|
|
|
|
|
|
|
|
|
|
| Foreign currency translation |
$ 127
|
|
|
|
|
|
|
|
|
|
|
| Ending balance (in shares) at Sep. 30, 2024 |
90,079,016
|
|
|
90,079,016
|
|
|
|
|
|
|
|
| Ending balance at Sep. 30, 2024 |
$ 41,219
|
|
|
$ 8
|
|
|
523,549
|
|
|
3,886
|
(486,224)
|
| Beginning balance (in shares) at Mar. 31, 2024 |
|
|
|
90,079,016
|
|
|
|
|
|
|
|
| Beginning balance at Mar. 31, 2024 |
83,576
|
|
|
$ 8
|
|
|
519,757
|
|
|
4,287
|
(440,476)
|
| Increase (Decrease) in Stockholders' Equity [Roll Forward] |
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
(21,380)
|
|
|
|
|
|
|
|
|
|
(21,380)
|
| Stock-based compensation |
1,882
|
|
|
|
|
|
1,882
|
|
|
|
|
| Foreign currency translation |
(505)
|
|
|
|
|
|
|
|
|
(505)
|
|
| Ending balance (in shares) at Jun. 30, 2024 |
|
|
|
90,079,016
|
|
|
|
|
|
|
|
| Ending balance at Jun. 30, 2024 |
63,573
|
|
|
$ 8
|
|
|
521,639
|
|
|
3,782
|
(461,856)
|
| Increase (Decrease) in Stockholders' Equity [Roll Forward] |
|
|
|
|
|
|
|
|
|
|
|
| Net loss |
(24,368)
|
|
|
|
|
|
|
|
|
|
(24,368)
|
| Stock-based compensation |
1,910
|
|
|
|
|
|
1,910
|
|
|
|
|
| Foreign currency translation |
$ 104
|
|
|
|
|
|
|
|
|
104
|
|
| Ending balance (in shares) at Sep. 30, 2024 |
90,079,016
|
|
|
90,079,016
|
|
|
|
|
|
|
|
| Ending balance at Sep. 30, 2024 |
$ 41,219
|
|
|
$ 8
|
|
|
$ 523,549
|
|
|
$ 3,886
|
$ (486,224)
|
| X |
- DefinitionAmount of increase to additional paid-in capital (APIC) for recognition of cost for award under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480483/718-10-35-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 20
-Section 55
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481089/718-20-55-13
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 20
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481089/718-20-55-12
| Name: |
us-gaap_AdjustmentsToAdditionalPaidInCapitalSharebasedCompensationRequisiteServicePeriodRecognitionValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionA roll forward is a reconciliation of a concept from the beginning of a period to the end of a period.
| Name: |
us-gaap_IncreaseDecreaseInStockholdersEquityRollForward |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax and reclassification adjustments of gain (loss) on foreign currency translation adjustments, foreign currency transactions designated and effective as economic hedges of a net investment in a foreign entity and intra-entity foreign currency transactions that are of a long-term-investment nature, attributable to parent entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-19
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-20
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(3)
-SubTopic 10
-Topic 810
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_OtherComprehensiveIncomeForeignCurrencyTransactionAndTranslationAdjustmentNetOfTaxPortionAttributableToParent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued during the period as a result of the conversion of convertible securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1E
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-3
| Name: |
us-gaap_StockIssuedDuringPeriodSharesConversionOfConvertibleSecurities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued during the period as a result of an employee stock purchase plan. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesEmployeeStockPurchasePlans |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe gross value of stock issued during the period upon the conversion of convertible securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueConversionOfConvertibleSecurities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate change in value for stock issued during the period as a result of employee stock purchase plan. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueEmployeeStockPurchasePlan |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of new stock issued during the period. Includes shares issued in an initial public offering or a secondary public offering. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478009/946-205-45-4
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 12: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAdjustments To Additional Paid In Capital Conversion Of Tranche Rights Liability To Equity
| Name: |
vtl_AdjustmentsToAdditionalPaidInCapitalConversionOfTrancheRightsLiabilityToEquity |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.24.3
| X |
- DefinitionAmount of decrease in additional paid in capital (APIC) resulting from direct costs associated with issuing stock. Includes, but is not limited to, legal and accounting fees and direct costs associated with stock issues under a shelf registration. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_AdjustmentsToAdditionalPaidInCapitalStockIssuedIssuanceCosts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=vtl_January2024PIPETransactionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=vtl_AtTheMarketSalesAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
Condensed Consolidated Statement of Cash Flows - USD ($)
$ in Thousands |
9 Months Ended |
Sep. 30, 2024 |
Sep. 30, 2023 |
| Cash flows from operating activities: |
|
|
| Net loss |
$ (75,332)
|
$ (72,040)
|
| Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
| Depreciation and amortization |
99
|
88
|
| Unrealized foreign currency (gain) loss |
(91)
|
712
|
| Stock-based compensation |
6,542
|
5,464
|
| Change in fair value of tranche rights |
4,796
|
0
|
| Fees expensed as part of January 2024 Financing |
1,690
|
0
|
| Changes in operating assets and liabilities: |
|
|
| Other current assets and prepaid expenses |
1,460
|
3,881
|
| Accounts payable |
871
|
(951)
|
| Accrued expenses |
(1,900)
|
5,961
|
| Other liabilities |
62
|
84
|
| Net cash used in operating activities |
(61,803)
|
(56,801)
|
| Cash flows from investing activities: |
|
|
| Purchases of property and equipment |
(261)
|
(169)
|
| Sale of investments - Other |
0
|
9,796
|
| Net cash provided by (used in) investing activities |
(261)
|
9,627
|
| Cash flows from financing activities: |
|
|
| Proceeds from public offering of common stock through At The Market Sales Agreement, net |
191
|
282
|
| Proceeds from January 2024 Financing, net of issuance costs |
74,273
|
0
|
| Proceeds from the exercise of pre-funded warrants |
0
|
51
|
| Proceeds from shares issued in connection with the Company's Employee Stock Purchase Plan |
0
|
96
|
| Net cash provided by financing activities |
74,464
|
429
|
| Effect of exchange rate changes on cash and cash equivalents |
(3)
|
(311)
|
| Net change in cash and cash equivalents |
12,397
|
(47,056)
|
| Cash and cash equivalents, beginning of period |
46,674
|
106,745
|
| Cash and cash equivalents, end of period |
59,071
|
59,689
|
| Supplemental disclosure of noncash investing and financing activities: |
|
|
| Conversion of tranche rights liability to equity upon increase in authorized shares |
28,396
|
0
|
| Operating lease right-of use asset obtained in exchange for lease obligation |
$ 0
|
$ 544
|
| X |
- References
+ Details
| Name: |
us-gaap_AdjustmentsNoncashItemsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage. Excludes amount for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in cash, cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including effect from exchange rate change. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 230
-Topic 830
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477401/830-230-45-1
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate expense recognized in the current period that allocates the cost of tangible assets, intangible assets, or depleting assets to periods that benefit from use of the assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 49
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-49
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
| Name: |
us-gaap_DepreciationDepletionAndAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) from effect of exchange rate changes on cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; held in foreign currencies. Excludes amounts for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 230
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477401/830-230-45-1
| Name: |
us-gaap_EffectOfExchangeRateOnCashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, before tax, of unrealized gain (loss) from foreign currency transaction. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 6
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-6
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(7)(c))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481956/830-20-45-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481926/830-20-50-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ForeignCurrencyTransactionGainLossUnrealized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate amount of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate amount of expenses incurred but not yet paid. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccruedLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncreaseDecreaseInOperatingCapitalAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in operating liabilities classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInOtherOperatingLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in prepaid expenses, and assets classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInPrepaidDeferredExpenseAndOtherAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for investment management fee, including, but not limited to, expense in connection with research, selection, supervision, and custody of investment. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 850
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483326/850-10-50-1
| Name: |
us-gaap_ManagementFeeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from financing activities, including discontinued operations. Financing activity cash flows include obtaining resources from owners and providing them with a return on, and a return of, their investment; borrowing money and repaying amounts borrowed, or settling the obligation; and obtaining and paying for other resources obtained from creditors on long-term credit. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivitiesContinuingOperationsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from investing activities, including discontinued operations. Investing activity cash flows include making and collecting loans and acquiring and disposing of debt or equity instruments and property, plant, and equipment and other productive assets. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivitiesContinuingOperationsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivitiesContinuingOperationsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NoncashInvestingAndFinancingItemsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe net cash paid (received) associated with the acquisition or disposal of all investments, including securities and other assets.
| Name: |
us-gaap_PaymentsForProceedsFromInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow associated with the acquisition of long-lived, physical assets that are used in the normal conduct of business to produce goods and services and not intended for resale; includes cash outflows to pay for construction of self-constructed assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquirePropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from additional borrowings, net of cash paid to third parties in connection with debt origination. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromDebtNetOfIssuanceCosts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the additional capital contribution to the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow from issuance of shares under share-based payment arrangement. Excludes option exercised. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2A
-Subparagraph (a)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2A
| Name: |
us-gaap_ProceedsFromIssuanceOfSharesUnderIncentiveAndShareBasedCompensationPlans |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the amount received from holders exercising their stock warrants. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromWarrantExercises |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase in right-of-use asset obtained in exchange for operating lease liability. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_RightOfUseAssetObtainedInExchangeForOperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionConversion Of Tranche Rights Liability To Equity Upon Increase In Authorized Shares
| Name: |
vtl_ConversionOfTrancheRightsLiabilityToEquityUponIncreaseInAuthorizedShares |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionGain (Loss) From Change In Fair Value Of Tranche Rights
| Name: |
vtl_GainLossFromChangeInFairValueOfTrancheRights |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.24.3
Description of Business and Basis of Financial Statements
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Accounting Policies [Abstract] |
|
| Description of Business and Basis of Financial Statements |
Description of Business and Basis of Financial Statements Description of Business Immunic, Inc. ("Immunic" or the "Company") is a biotechnology company developing a clinical pipeline of selective oral immunology therapies focused on treating chronic inflammatory and autoimmune diseases. The Company is headquartered in New York City with its main operations in Gräfelfing near Munich, Germany. The Company had approximately 85 employees as of November 1, 2024. The Company is pursuing clinical development of orally administered, small molecule programs, each of which has unique features intended to directly address the unmet needs of patients with serious chronic inflammatory and autoimmune diseases. These include the vidofludimus calcium (IMU-838) program, which is in Phase 3 and Phase 2 clinical development for patients with relapsing and progressive multiple sclerosis (“MS”), respectively, and which has shown therapeutic activity in Phase 2 clinical trials in patients suffering from relapsing-remitting MS, progressive MS and moderate-to-severe ulcerative colitis (“UC”); the IMU-856 program, which is targeted to regenerate bowel epithelium and restore intestinal barrier function, which could potentially be applicable in numerous gastrointestinal diseases, such as celiac disease, inflammatory bowel disease and Graft-versus-Host-Disease ("GvHD"); and the IMU-381 program, which is a next generation molecule being developed to specifically address the needs of gastrointestinal diseases. The Company’s business, operating results, financial condition and growth prospects are subject to significant risks and uncertainties, including the failure of its clinical trials to meet their endpoints, failure to obtain regulatory approval and needing additional funding to complete the development and commercialization of the Company's three development programs. Liquidity and Financial Condition Immunic has no products approved for commercial sale and has not generated any revenue from product sales. It has never been profitable and has incurred operating losses in each year since inception in 2016. The Company has an accumulated deficit of approximately $486.2 million as of September 30, 2024 and $410.9 million as of December 31, 2023. Substantially all of Immunic's operating losses resulted from expenses incurred in connection with its research and development programs and from general and administrative costs associated with its operations. Immunic expects to incur significant expenses and increasing operating losses for the foreseeable future as it initiates and continues the development of its product candidates and adds personnel necessary to advance its pipeline of product candidates. Immunic expects that its operating losses will fluctuate significantly from quarter-to-quarter and year-to-year due to timing of development programs. From inception through September 30, 2024, Immunic has raised net cash of approximately $430.9 million from private and public offerings of preferred stock, common stock, pre-funded warrants and tranche rights. As of September 30, 2024, the Company had cash and cash equivalents of approximately $59.1 million. With these funds, the Company does not have adequate liquidity to fund its operations for at least twelve months from the issuance of these consolidated financial statements without raising additional capital, but such actions are not solely within the control of the Company. If the Company is unable to obtain additional capital, it would have a material adverse effect on the operations of the Company, its clinical development program, and the Company may have to cease operations altogether. These factors raise substantial doubt about the Company’s ability to continue as a going concern. Basis of Presentation and Consolidation The accompanying condensed consolidated financial statements have been prepared in conformity with United States generally accepted accounting principles ("U.S. GAAP") and include the accounts of Immunic and its wholly-owned subsidiaries, Immunic AG and Immunic Australia Pty Ltd. All intercompany accounts and transactions have been eliminated in consolidation. Immunic manages its operations as a single reportable segment for the purposes of assessing performance and making operating decisions. Unaudited Interim Financial Information Immunic has prepared the accompanying interim unaudited condensed consolidated financial statements in accordance with United States generally accepted accounting principles, (“US GAAP”), for interim financial information and with the instructions to Form 10-Q and Regulation S-X of the SEC. Accordingly, they do not include all of the information and footnotes required by U.S. GAAP for complete financial statements. These interim unaudited condensed consolidated financial statements reflect all adjustments consisting of normal recurring accruals which, in the opinion of management, are necessary to present fairly Immunic’s consolidated financial position, consolidated results of operations, consolidated statement of stockholders’ equity and consolidated cash flows for the periods and as of the dates presented. The Company’s fiscal year ends on December 31. The condensed consolidated balance sheet as of December 31, 2023 was derived from audited consolidated financial statements but does not include all disclosures required by U.S. GAAP. These condensed consolidated financial statements should be read in conjunction with the annual consolidated financial statements and the notes thereto included on the Company's Annual Report on Form 10-K filed on February 22, 2024. The nature of Immunic’s business is such that the results of any interim period may not be indicative of the results to be expected for the entire year or for corresponding interim periods in any subsequent year.
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for the business description and basis of presentation concepts. Business description describes the nature and type of organization including but not limited to organizational structure as may be applicable to holding companies, parent and subsidiary relationships, business divisions, business units, business segments, affiliates and information about significant ownership of the reporting entity. Basis of presentation describes the underlying basis used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/235/tableOfContent
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 275
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/275/tableOfContent
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 205
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/205/tableOfContent
| Name: |
us-gaap_BusinessDescriptionAndBasisOfPresentationTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Summary of Significant Accounting Policies
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Accounting Policies [Abstract] |
|
| Summary of Significant Accounting Policies |
Summary of Significant Accounting Policies Use of Estimates The preparation of financial statements in conformity with U.S. GAAP requires the Company to make certain estimates and assumptions that affect the reported amounts of assets, liabilities, expenses and the disclosure of contingent assets and liabilities in the Company’s consolidated financial statements. The most significant estimates in the Company’s financial statements and accompanying notes relate to clinical trial expenses and stock-based compensation. Management believes its estimates to be reasonable under the circumstances. Actual results could differ materially from those estimates and assumptions. Foreign Currency Translation and Presentation The Company’s reporting currency is United States (“U.S.”) dollars. Immunic AG is located in Germany with the Euro being its functional currency. Immunic Australia Pty Ltd.’s functional currency is the Australian dollar. All amounts in the financial statements where the functional currency is not the U.S. dollar are translated into U.S. dollar equivalents at exchange rates as follows: • assets and liabilities at reporting period-end rates; • income statement accounts at average exchange rates for the reporting period; and • components of equity at historical rates. Gains and losses from translation of the financial statements into U.S. dollars are recorded in stockholders’ equity as a component of accumulated other comprehensive income (loss). Realized and unrealized gains and losses resulting from foreign currency transactions denominated in currencies other than the functional currency are reflected as general and administrative expenses in the Consolidated Statements of Operations. Foreign currency transaction gains and losses related to long-term intercompany loans that are payable in the foreseeable future are recorded in Other Income (Expense). The Consolidated Statements of Cash Flows were prepared by using the average exchange rate in effect during the reporting period which reasonably approximates the timing of the cash flows. Cash and Cash Equivalents The Company considers all highly liquid investments with an original maturity of three months or less to be cash equivalents. Cash and cash equivalents consist of cash on hand and deposits in banks located in the U.S. of approximately $40.6 million, Germany of approximately $15.6 million and Australia of approximately $2.9 million as of September 30, 2024. The Company maintains cash and cash equivalent balances denominated in Euro and U.S. dollars with major financial institutions in the U.S. and Germany in excess of the deposit limits insured by the government. Management periodically reviews the credit standing of these financial institutions. The Company currently deposits its cash and cash equivalents with two large financial institutions. Cash and cash equivalents in the U.S. are held at JP Morgan and are primarily held in a U.S. Government money market fund account earning interest at a rate of 4.8% as of September 30, 2024. Cash and cash equivalents in Germany are earning interest at a rate of 3.25% to 3.75% as of September 30, 2024. Fair Value Measurement Fair value is defined as the price that would be received to sell an asset or be paid to transfer a liability in an orderly transaction between market participants on the measurement date. Accounting guidance establishes a fair value hierarchy that requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. The standard describes three levels of inputs that may be used to measure fair value: Level 1—Quoted prices in active markets for identical assets or liabilities. Level 1 assets consisted of money market funds for the periods presented. The Company had no Level 1 liabilities for the periods presented. Level 2—Inputs other than observable quoted prices for the asset or liability, either directly or indirectly; these include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active. The Company had no Level 2 assets or liabilities for the periods presented. Level 3—Unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of assets or liabilities. The Company had no Level 3 assets or liabilities for the periods presented. The Company did have tranche rights that were at level 3 during the first quarter of 2024. The carrying value of cash and cash equivalents, other current assets and prepaid expenses, accounts payable, accrued expenses, and other current liabilities approximates fair value due to the short period of time to maturity. Property and Equipment Property and equipment is stated at cost. Depreciation is computed using the straight-line method based on the estimated service lives of the assets, which range from three to thirteen years. Depreciation expense was $42,000 and $34,000 for the three months ended September 30, 2024 and 2023, respectively. Depreciation expense was $99,000 and $88,000 for the nine months ended September 30, 2024 and 2023, respectively. Impairment of Long-Lived Assets The Company records impairment losses on long-lived assets used in operations when indicators of impairment are present and the undiscounted cash flows estimated to be generated by those assets are less than the assets’ carrying amount. Impaired assets are then recorded at their estimated fair value. There were no impairment losses during the three and nine months ended September 30, 2024 and 2023. Research and Development Expenses These costs primarily include external development expenses and internal personnel expenses for its development programs, vidofludimus calcium and IMU-856. Immunic has spent the majority of its research and development resources on vidofludimus calcium, the Company's lead development program, for clinical trials in MS and UC. Research and development expenses consist of expenses incurred in research and development activities, which include clinical trials, contract research services, certain milestone payments, salaries and related employee benefits, allocated facility costs and other outsourced services. Research and development expenses are charged to operations as incurred. The Company enters into agreements with contract research organizations (“CROs”) to provide clinical trial services for individual studies and projects by executing individual work orders governed by a Master Service Arrangement (“MSA”). The MSAs and associated work orders provide for regular recurrent payments and payments upon the completion of certain milestones. The Company regularly assesses the timing of payments against actual costs incurred to ensure a proper accrual of related expenses in the appropriate accounting period. Collaboration Arrangements Certain collaboration and license agreements may include payments to or from the Company of one or more of the following: non-refundable or partially refundable upfront or license fees; development, regulatory and commercial milestone payments; payment for manufacturing supply services; partial or complete reimbursement of research and development costs; and royalties on net sales of licensed products. The Company assesses whether such contracts are within the scope of Financial Accounting Standards Board (FASB) Accounting Standards Update (“ASU”) 2014-09 “Revenue from Contracts with Customers” and ASU No. 2018-18, “Collaborative Arrangements” ("ASU 2018-18"). ASU 2018-18, clarifies that certain elements of collaborative arrangements could qualify as transactions with customers in the scope of ASC 606. In October 2018, the Company entered into an option and license agreement (the "Daiichi Sankyo Agreement") with Daiichi Sankyo Co., Ltd. ("Daiichi Sankyo") which granted the Company the right to license a group of compounds, designated by the Company as IMU-856, as a potential new oral treatment option for gastrointestinal diseases such as celiac disease, inflammatory bowel disease, irritable bowel syndrome with diarrhea and other barrier function associated diseases. During the option period, the Company performed agreed upon research and development activities for which it was reimbursed by Daiichi Sankyo up to a maximum agreed-upon limit. Such reimbursement was recorded as other income. There are no additional research and development reimbursements expected under this agreement. On January 5, 2020, the Company exercised its option to obtain the exclusive worldwide right to commercialization of IMU-856. Among other things, the option exercise grants Immunic AG the rights to Daiichi Sankyo’s patent application related to IMU-856, for which the Company received a notice of allowance from the U.S. Patent & Trademark Office in August 2022. In connection with the option exercise, the Company paid a one-time upfront licensing fee to Daiichi Sankyo. Under the Daiichi Sankyo Agreement, Daiichi Sankyo is also eligible to receive future development, regulatory and sales milestone payments, as well as royalties related to IMU-856. Government assistance Government assistance relating to research and development performed by Immunic Australia is recorded as a component of other (income) expense. This government assistance is recognized at a rate of 43.5% of the qualified research and development expenditures which are incurred. We also receive government assistance from the German Government for reimbursement of research and development expenses up to 3.5 million Euros per year. We recognized $0.7 million and $0.7 million of other income related to research activities performed during the three and nine months ended September 30, 2024, respectively and $0.2 million and $2.3 million related to research activities performed during the three and nine months ended September 30, 2023, respectively. General and Administrative Expenses General and administrative expenses consist primarily of salaries and related costs for personnel in executive, finance, business development and other support functions. Other general and administrative expenses include, but are not limited to, stock-based compensation, insurance costs, professional fees for legal, accounting and tax services, consulting, related facility costs and travel. Stock-Based Compensation The Company measures the cost of employee and non-employee services received in exchange for equity awards based on the grant-date fair value of the award recognized generally as an expense (i) on a straight-line basis over the requisite service period for those awards whose vesting is based upon a service condition, and (ii) on an accelerated method for awards whose vesting is based upon a performance condition, but only to the extent it is probable that the performance condition will be met. Stock-based compensation is (i) estimated at the date of grant based on the award’s fair value for equity classified awards and (ii) final measurement date for liability classified awards. Forfeitures are recorded in the period in which they occur. The Company estimates the fair value of stock options using the Black-Scholes-Merton option-pricing model ("BSM"), which requires the use of estimates and subjective assumptions, including the risk-free interest rate, the fair value of the underlying common stock, the expected dividend yield of the Company’s common stock, the expected volatility of the price of the Company’s common stock, and the expected term of the option. These estimates involve inherent uncertainties and the application of management’s judgment. If factors change and different assumptions are used, the Company’s stock-based compensation expense could be materially different in the future. Leases The Company leases office space and office equipment. The underlying lease agreements have lease terms of less than 12 months and up to 60 months. Leases with terms of 12 months or less at inception are not included in the operating lease right of use asset and operating lease liability. The Company has three existing leases for office and laboratory space. At inception of a lease agreement, the Company determines whether an agreement represents a lease and at commencement each lease agreement is assessed as to classification as an operating or financing lease. The Company's leases have been classified as operating leases and an operating lease right-of-use asset and an operating lease liability have been recorded on the Company’s balance sheet. A right-of-use lease asset represents the Company’s right to use the underlying asset for the lease term and the lease obligation represents its commitment to make the lease payments arising from the lease. Right-of-use lease assets and obligations are recognized at the commencement date based on the present value of remaining lease payments over the lease term. As the Company’s leases do not provide an implicit rate, the Company has used an estimated incremental borrowing rate based on the information available at the commencement date in determining the present value of lease payments. The right-of-use lease asset includes any lease payments made prior to commencement and excludes any lease incentives. The lease term used in estimating future lease payments may include options to extend when it is reasonably certain that the Company will exercise that option. Operating lease expense is recognized on a straight-line basis over the lease term, subject to any changes in the lease or changes in expectations regarding the lease term. Variable lease costs such as common area costs and property taxes are expensed as incurred. Leases with an initial term of twelve months or less are not recorded on the balance sheet. Comprehensive Income (Loss) Comprehensive income (loss) is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources. Accumulated other comprehensive income (loss) has been reflected as a separate component of stockholders’ equity in the accompanying Consolidated Balance Sheets and consists of foreign currency translation adjustments. Income Taxes The Company is subject to corporate income tax laws and regulations in the U.S., Germany and Australia. Tax regulations within each jurisdiction are subject to the interpretation of the related tax laws and regulations and require significant judgment in their application. The Company utilizes the asset and liability method of accounting for income taxes which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the consolidated financial statements. Deferred income tax assets and liabilities are determined based on the differences between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. The effect of changes in tax rates on deferred tax assets and liabilities is recognized in operations in the period that includes the enactment date. Deferred taxes are reduced by a valuation allowance when, in the opinion of management, it is more likely than not some portion or the entire deferred tax asset will not be realized. As of September 30, 2024 and 2023, respectively, the Company maintained a full valuation allowance against the balance of deferred tax assets. It is the Company’s policy to provide for uncertain tax positions and the related interest and penalties based upon management’s assessment of whether a tax benefit is more likely than not to be sustained upon examination by tax authorities. The Company recognizes interest and penalties accrued on any unrecognized tax benefits as a component of income tax expense. The Company is subject to U.S. federal, New York, California, Texas, German and Australian income taxes. The Company is subject to U.S. federal or state income tax examination by tax authorities for tax returns filed for the years 2003 and forward due to the carryforward of NOLs. Tax years 2019 through 2023 are subject to audit by German and Australian tax authorities. The Company is not currently under examination by any tax jurisdictions. Warrants and Tranche Rights The Company accounts for issued financial instruments either as a liability or equity in accordance with ASC 480-10, Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity (“ASC 480-10”) or ASC 815-40, Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock (“ASC 815-40”). If financial instruments do not meet liability classification under ASC 480-10, the Company considers the requirements of ASC 815-40 to determine whether the financial instruments should be classified as a liability or as equity. Liability-classified financial instruments are measured at fair value on the issuance date and at the end of each reporting period. Any change in the fair value of the financial instruments after the issuance date is recorded in the consolidated statements of operations as a gain or loss. If financial instruments do not require liability classification under ASC 815-40, the instrument is classified in permanent equity. Equity-classified financial instruments are accounted for at fair value on the issuance date with no changes in fair value recognized after the issuance date. Net Loss Per Share Basic net loss per share attributable to common stockholders is calculated by dividing the net loss by the weighted-average number of common shares outstanding for the period, without consideration for common stock equivalents. Diluted net loss per share attributable to common stockholders is computed by dividing the net loss by the weighted-average number of common shares and, if dilutive, common stock equivalents outstanding for the period determined using the treasury-stock method. For all periods presented, there is no difference in the number of shares used to calculate basic and diluted shares outstanding due to the Company’s net loss position. The weighted average shares outstanding calculation for basic and diluted earnings per share for the three and nine months ended September 30, 2024 includes 11,193,564 pre-funded warrants that remain unexercised as of September 30, 2024. Potentially dilutive securities, not included in the calculation of diluted net loss per share attributable to common stockholders because to do so would be anti-dilutive, are as follows: | | | | | | | | | | | | | As of September 30, | | 2024 | | 2023 | | Options to purchase common stock | 11,546,138 | | | 6,263,910 | | | | | |
Recently Issued and/or Adopted Accounting Standards There are no recently issued accounting standards that would have a significant impact on the company's consolidated financial statements.
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for all significant accounting policies of the reporting entity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483426/235-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/235/tableOfContent
| Name: |
us-gaap_SignificantAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Balance Sheet Details
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
| Balance Sheet Details |
Balance Sheet Details Other Current Assets and Prepaid Expenses Other Current Assets and Prepaid Expenses consist of (in thousands): | | | | | | | | | | | | | | | | | September 30, 2024 | | December 31, 2023 | | Prepaid clinical and related costs | $ | 2,066 | | | $ | 2,314 | | | VAT receivable | 780 | | | 703 | | | Australian research and development tax incentive | 39 | | | 670 | | | Research grant | — | | | 1,104 | | | Prepaid Insurance | 553 | | | 198 | | | Other | 757 | | | 871 | | | Total | $ | 4,195 | | | $ | 5,860 | |
Accounts Payable Accounts Payable consist of (in thousands): | | | | | | | | | | | | | | | | | September 30, 2024 | | December 31, 2023 | | Clinical costs | $ | 5,789 | | | $ | 4,726 | | | Legal and audit costs | 90 | | | 160 | | | | | | | Other | 163 | | | 213 | | | Total | $ | 6,042 | | | $ | 5,099 | |
Accrued Expenses Accrued expenses consist of (in thousands): | | | | | | | | | | | | | | | | | September 30, 2024 | | December 31, 2023 | | Accrued clinical and related costs | $ | 14,386 | | | $ | 16,863 | | | Accrued legal and audit costs | 136 | | | 216 | | | Accrued compensation | 1,509 | | | 1,460 | | | Accrued other | 214 | | | 125 | | | Total | $ | 16,245 | | | $ | 18,664 | |
Other Current Liabilities Other Current Liabilities consist of (in thousands): | | | | | | | | | | | | | | | | | | | | | September 30, 2024 | | December 31, 2023 | | | Lease liabilities | $ | 713 | | | $ | 695 | | | | Other | 357 | | | 271 | | | | Total | 1,070 | | | 966 | | |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for supplemental balance sheet disclosures, including descriptions and amounts for assets, liabilities, and equity. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/210/tableOfContent
| Name: |
us-gaap_SupplementalBalanceSheetDisclosuresTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Commitments and Contingencies
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Commitments and Contingencies Disclosure [Abstract] |
|
| Commitments and Contingencies |
Commitments and Contingencies Operating Leases The Company leases certain office space under non-cancelable operating leases. The leases terminate on July 31, 2025 for the New York City office, June 30, 2025 for the Gräfelfing, Germany office and November 30, 2028 for the research laboratory in Planegg, Germany. These agreements include both lease (e.g., fixed rent) and non-lease components (e.g., common-area and other maintenance costs). The non-lease components are deemed to be executory costs and are therefore excluded from the minimum lease payments used to determine the present value of the operating lease obligation and related right-of-use asset. The New York City lease was extended on December 22, 2022 for an additional 27 months resulting in the new lease termination date of July 31, 2025. The New York City lease has a renewal option, but this was not included in calculating the right of use asset and liabilities. On April 7, 2020, the Company signed a five year lease for its facility in Gräfelfing, Germany. On March 1, 2021 and August 1, 2022 the Company added additional lease space at the Gräfelfing, Germany office. Renewal options were not included in calculating the right of use asset and liabilities for this facility. In February 2023, the Company leased space in Germany for a research laboratory. The leases do not have concessions, leasehold improvement incentives or other build-out clauses. Further, the leases do not contain contingent rent provisions. The New York City lease had a six month rent holiday at the beginning of the lease as well as a three month rent holiday upon the 27 month extension starting May 2023. There were net additions of $544,000 related to the addition of new laboratory space in Planegg, Germany in February 2023. The leases do not provide an implicit rate and, due to the lack of a commercially salable product, the Company is generally considered unable to obtain commercial credit. Therefore, the Company estimated its incremental interest rate to be 6% for the original leases and 8% for the New York City extension and German laboratory, considering the quoted rates for the lowest investment-grade debt and the interest rates implicit in recent financing leases. Immunic used its estimated incremental borrowing rate and other information available at the lease commencement date in determining the present value of the lease payments. Immunic’s operating lease costs and variable lease costs were $260,000 and $209,000 for the three months ended September 30, 2024 and 2023, respectively and $787,000 and $642,000 for the nine months ended September 30, 2024 and 2023, respectively. Variable lease costs consist primarily of common area maintenance costs, insurance and taxes which are paid based upon actual costs incurred by the lessor. Maturities of the operating lease obligation are as follows as of September 30, 2024 (in thousands): | | | | | | | | | | 2024 | $ | 275 | | | 2025 | | 433 | | | 2026 | | 78 | | | 2027 | | 82 | | | 2028 | | 79 | | | Thereafter | | — | | | Total | | 947 | | | Interest | | (48) | | | Present Value of obligation | $ | 899 | |
Contractual Obligations As of September 30, 2024, the Company has non-cancelable contractual obligations under certain agreements related to its development programs for vidofludimus calcium and IMU-856 totaling approximately $3.2 million, all of which is expected to be paid in 2024 and 2025. Other Commitments and Obligations Daiichi Sankyo Agreement On January 5, 2020, the Company exercised its option to obtain the exclusive worldwide right to commercialization of IMU-856. Among other things, the option exercise grants Immunic AG the rights to Daiichi Sankyo’s patent application related to IMU-856, for which the Company received a notice of allowance from the U.S. Patent & Trademark Office in August 2022. In connection with the option exercise, the Company paid a one-time upfront licensing fee to Daiichi Sankyo. Under the Daiichi Sankyo Agreement, Daiichi Sankyo is also eligible to receive future development, regulatory and sales milestone payments, as well as royalties related to IMU-856. Legal Proceedings The Company is not currently a party to any litigation, nor is it aware of any pending or threatened litigation, that it believes would materially affect its business, operating results, financial condition or cash flows. However, its industry is characterized by frequent claims and litigation including securities litigation, claims regarding patent and other intellectual property rights and claims for product liability. As a result, in the future, the Company may be involved in various legal proceedings from time to time.
|
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for commitments and contingencies. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 405
-SubTopic 30
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/405-30/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482648/440-10-50-4
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/450/tableOfContent
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 954
-SubTopic 440
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478522/954-440-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482648/440-10-50-4
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 440
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/440/tableOfContent
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Fair Value
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Fair Value Disclosures [Abstract] |
|
| Fair Value |
Fair ValueThe following fair value hierarchy tables present information about each major category of the Company’s financial assets and liabilities measured at fair value on a recurring basis (in thousands): | | | | | | | | | | | | | | | | | | | | | | | | | | Fair Value Measurement at September 30, 2024 | | | Fair Value | | Level 1 | | Level 2 | | Level 3 | | Assets | | | | | | | | | Money market funds | $ | 40,359 | | | $ | 40,359 | | | $ | — | | | $ | — | | | Total assets at fair value | $ | 40,359 | | | $ | 40,359 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | Fair Value Measurement at December 31, 2023 | | Fair Value | | Level 1 | | Level 2 | | Level 3 | | Assets | | | | | | | | | Money market funds | $ | 34,087 | | | $ | 34,087 | | | $ | — | | | $ | — | | | Total assets | $ | 34,087 | | | $ | 34,087 | | | $ | — | | | $ | — | |
There were no transfers between Level 1, Level 2 or Level 3 assets during the periods presented. For the Company’s money market funds which are included as a component of cash and cash equivalents on the consolidated balance sheet, realized gains and losses are included in interest income on the consolidated statements of operations. Our money market fund account is held in our bank in the U.S. and was earning interest at a rate of 4.8% in a U.S. Government money market fund. The Company has cash balances in banks in excess of the maximum amount insured by the FDIC and other international agencies as of September 30, 2024. The Company has not historically experienced any credit losses with balances in excess of FDIC limits. The Company recorded tranche rights of $23.6 million at January 8, 2024 as a result of the January 2024 Financing (see note 6). The fair value measurement of the tranche rights associated with the January 2024 Financing was classified as Level 3 under the fair value hierarchy. The fair value of the tranche rights was determined using a Black Scholes Option Pricing Model. The inputs to this model included a risk-free rate range of 3.93%-4.36%, a stock price volatility range of 105-115%, an expected dividend rate of —% and remaining term of 1.81-4.81 years. This liability was revalued on March 4, 2024, upon stockholder approval to increase its authorized shares of common stock from 130 million to 500 million, which resulted in the reclassification of the tranche rights from a liability to equity. This revaluation resulted in an increase in the tranche rights liability of $4.8 million using a Black Scholes Option Pricing Model. The inputs to this model as of the date of the reclassification included a risk-free rate range of 4.16%-4.63%, a stock price volatility range of 90-105%, an expected dividend rate of —% and remaining term of 1.66-4.66 years. The inputs used in the determination of fair value of the liability are level 3 inputs. A rollforward of the fair value of the tranche rights is as follows (in thousands): | | | | | | | December 31, 2023 | $ | — | | | Fair value as of January 8, 2024 | $ | 23,600 | | | Change in fair value through March 4, 2024 | $ | 4,796 | | | Reclassification to equity | $ | (28,396) | | | March 31, 2024 | $ | — | |
|
| X |
- References
+ Details
| Name: |
us-gaap_FairValueDisclosuresAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for the fair value of financial instruments (as defined), including financial assets and financial liabilities (collectively, as defined), and the measurements of those instruments as well as disclosures related to the fair value of non-financial assets and liabilities. Such disclosures about the financial instruments, assets, and liabilities would include: (1) the fair value of the required items together with their carrying amounts (as appropriate); (2) for items for which it is not practicable to estimate fair value, disclosure would include: (a) information pertinent to estimating fair value (including, carrying amount, effective interest rate, and maturity, and (b) the reasons why it is not practicable to estimate fair value; (3) significant concentrations of credit risk including: (a) information about the activity, region, or economic characteristics identifying a concentration, (b) the maximum amount of loss the entity is exposed to based on the gross fair value of the related item, (c) policy for requiring collateral or other security and information as to accessing such collateral or security, and (d) the nature and brief description of such collateral or security; (4) quantitative information about market risks and how such risks are managed; (5) for items measured on both a recurring and nonrecurring basis information regarding the inputs used to develop the fair value measurement; and (6) for items presented in the financial statement for which fair value measurement is elected: (a) information necessary to understand the reasons for the election, (b) discussion of the effect of fair value changes on earnings, (c) a description of [similar groups] items for which the election is made and the relation thereof to the balance sheet, the aggregate carrying value of items included in the balance sheet that are not eligible for the election; (7) all other required (as defined) and desired information. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 107
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482078/820-10-55-107
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 100
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482078/820-10-55-100
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2E
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2E
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 940
-SubTopic 820
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478119/940-820-50-1
| Name: |
us-gaap_FairValueDisclosuresTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Common Stock
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Equity [Abstract] |
|
| Common Stock |
Common Stock In December 2020, the Company filed a Prospectus Supplement to the shelf registration statement on Form S-3 filed on November 13, 2020 and declared effective on November 24, 2020 (the "2020 Shelf Registration Statement") for the offering, issuance and sale of up to a maximum aggregate offering price of $50.0 million of common stock that may be issued and sold under an at-the-market sales agreement with SVB Leerink LLC (now Leerink Partners LLC) as agent ("December 2020 ATM"). The Company used the net proceeds from the December 2020 ATM to fund the ongoing clinical development of our product candidates and for other general corporate purposes, including funding existing and potential new clinical programs and product candidates. The December 2020 ATM terminated in May 2024.
In May 2022, the Company filed a Prospectus Supplement to the 2020 Shelf Registration Statement for the offering, issuance and sale of up to a maximum aggregate offering price of $80.0 million of common stock to be issued and sold under another at-the-market sales agreement ("May 2022 ATM") with Leerink Partners LLC (formerly SVB Leerink LLC) as agent. The 2020 Shelf Registration Statement expired in November 2023. In November 2023, we filed a shelf registration statement on Form S-3 (the "2023 Shelf Registration Statement"). The 2023 Shelf Registration Statement permits the offering, issuance and sale of up to $250.0 million of common stock, preferred stock, warrants, debt securities, and/or units in one or more offerings and in any combination of the foregoing. This registration statement was declared effective on May 31, 2024. Unsold securities from the expired 2020 shelf registration statement can continue to be sold under the 2023 Shelf Registration Statement resulting in a total S-3 shelf availability of $412.3 million as of September 30, 2024. In May 2024, we filed a Prospectus Supplement to the 2023 Shelf Registration Statement for the offering, issuance and sale of up to a maximum aggregate offering price of $80.0 million of common stock that may be issued and sold under an at-the-market sales agreement with Leerink Partners LLC as agent ("May 2024 ATM"), which rolls over the $80.0 million of unsold common stock from the May 2022 ATM. We intend to use the net proceeds from the May 2024 ATM to continue to fund the ongoing clinical development of our product candidates and for other general corporate purposes, including funding existing and potential new clinical programs and product candidates. The May 2024 ATM will terminate upon the earlier of (i) the issuance and sale of all of the shares through Leerink Partners LLC on the terms and subject to the conditions set forth in the May 2024 ATM or (ii) termination of the May 2024 ATM as otherwise permitted thereby. The May 2024 ATM may be terminated at any time by either party upon ten days’ prior notice, or by Leerink Partners LLC at any time in certain circumstances, including the occurrence of a material adverse effect on us. As of September 30, 2024, $80.0 million in capacity remains under the May 2024 ATM. The Company has agreed to pay Leerink Partners LLC a commission equal to 3.0% of the gross proceeds from the sales of common stock pursuant to the May 2024 ATM and has agreed to provide Leerink Partners LLC with customary indemnification and contribution rights. For the nine months ended September 30, 2024, the Company raised gross proceeds of $0.2 million pursuant to the December 2020 ATM through the sale of 150,000 shares of common stock at a weighted average price of $1.31 per share. The net proceeds from the December 2020 ATM were $0.2 million after deducting sales agent commissions of $6,000. The Company did not have any ATM activity for the three months ended September 30, 2024. In the three and nine months ended September 30, 2023, the Company raised gross proceeds of $0.3 million pursuant to the December 2020 ATM through the sale of 107,012 shares of common stock at a weighted average price of $2.72 per share. The net proceeds from the December 2020 ATM were $0.3 million after deducting underwriter commissions of $9,000. Equity Offerings Private Placement of up to $240 million (the "January 2024 Financing")
On January 4, 2024, Immunic entered into a Securities Purchase Agreement with select accredited investors, pursuant to which the Company agreed to issue and sell to the Investors in a three-tranche private placement shares of the Company’s common stock, $0.0001 par value per share, or in lieu thereof, pre-funded warrants to purchase shares of Common Stock. The Pre-Funded Warrants are exercisable immediately for $0.0001 per share and until exercised in full.
•The first tranche, which closed on January 8, 2024, resulted in the purchase by the Investors of an aggregate of $80 million of Common Stock (or pre-funded warrants) from the Company at a price of $1.43 per share; •The second tranche is a conditional mandatory purchase by the Investors of an additional $80 million of Common Stock (or pre-funded warrants) from the Company at a price of $1.716 per share, equal to 120% of the price paid in the first tranche and is subject to the satisfaction of three conditions: ◦release by the Company of topline data from its Phase 2b clinical trial of vidofludimus calcium (IMU-838) in progressive multiple sclerosis, which data is currently expected in or around April 2025; ◦the 10-day volume-weighted average price of the Common Stock is at least $8.00 per share during the 6 months following the data release; and ◦aggregate trading volume during the same 10-day period is at least $100 million. •The third tranche must occur no later than three years after the second tranche and is conditioned on the same volume-weighted average share price and minimum trading volumes as the second tranche. The third tranche provides for the issuance of $80 million of shares of common stock (or pre-funded warrants) at the same price per share as the second tranche, but permits investors to fund their purchase obligations on a “cashless” or net settlement basis, which would reduce the cash proceeds to be raised by the Company in the January 2024 Financing. Any of the conditions in the second or third tranches can be waived by holders of a majority of the outstanding securities (including the lead investor). The fair value methodology used by the Company assumed the conditions will be waived if the trading price of the stock exceeds the purchase price.
The January 2024 Financing resulted in gross proceeds to the Company of approximately $80 million in the first tranche, and an additional $80 million if and when the second tranche occurs. If the second tranche is completed and conditions for the third tranche are satisfied or waived, the Company could receive up to an additional $80 million in the third tranche.
As of the closing date of the transaction of January 8, 2024, the Company did not have enough authorized shares to be able to issue the potential shares for tranche 2 and tranche 3 (collectively referred to hereafter as "the tranche rights"). Therefore, the Company recorded the value associated to the tranche rights as a liability of $23.6 million and allocated the remainder of the $80 million received (or $56.4 million) with the common stock and pre-funded warrants to equity. On March 4, 2024, the stockholders voted to increase the Company's authorized common stock from 130 million to 500 million shares. As a result of the ability to issue shares in satisfaction of the tranche rights, the instrument was reclassified to stockholders' equity. The Company allocated the transaction costs across the instruments on a relative fair value basis at the grant date. As a result $4.0 million was netted against the equity proceeds and $1.7 million was recorded in other expense in the Consolidated Statements of Operations for the nine months ended September 30, 2024.
The Company registered for resale by the investors in the January 2024 Financing up to 55,944,850 shares of common stock issued (or issuable upon exercise of pre-funded warrants) in the first tranche. The Company will not receive any proceeds from the sale of these shares of common stock. These shares are registered on a registration statement on Form S-3 (registration No. 333-277040). Common Stock On March 4, 2024, the stockholders of the Company voted to increase the authorized shares of the Company from 130,000,000 shares of common stock to 500,000,000 shares of common stock, par value of $0.0001 per share. The voting, dividend and liquidation rights of the holders of the Company’s common stock are subject to and qualified by the rights, powers and preferences of any holders of preferred stock. Each share of common stock entitles the holder to one vote on all matters submitted to a vote of the Company’s stockholders. Common stockholders are entitled to receive dividends, as may be declared by the Board of Directors, if any. Through September 30, 2024, no cash dividends had been declared or paid. Pre-funded Warrants The Company issued 11,193,564 pre-funded warrants in connection with the January 2024 Financing, which all remain outstanding as of September 30, 2024. Preferred Stock The Company’s certificate of incorporation, as amended and restated, authorizes the Company to issue 20 million shares of $0.0001 par value preferred stock, with such voting powers (if any), designations, powers, preferences, and relative, participating, optional or other rights, if any, and any qualifications, limitations or restrictions thereof, as shall be set by the Board of Directors. No preferred shares were issued or outstanding as of September 30, 2024. Stock Reserved for Future Issuance Shares reserved for future issuance at September 30, 2024 are as follows: | | | | | | | Number of
Shares | | Common stock reserved for issuance for: | | | 2021 Employee Stock Purchase Plan | 1,000,011 | | | Pre-funded stock warrants | 11,193,564 | | | Outstanding stock options | 11,546,138 | | | Common shares reserved for tranche 2 rights | 46,620,046 | | | Maximum common shares reserved for tranche 3 rights | 46,620,046 | | | Common stock options available for future grant: | | | 2017 Inducement Equity Incentive Plan | 46,250 | | | 2019 Omnibus Equity Incentive Plan | 7,903,691 | | | Total common shares reserved for future issuance | 124,929,746 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-14
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477968/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477968/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-6
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480237/815-40-50-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(e)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 10: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/505/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-14
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-14
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 16
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-16
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-18
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-18
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-18
| Name: |
us-gaap_StockholdersEquityNoteDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Stock-Based Compensation Plans
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Share-Based Payment Arrangement [Abstract] |
|
| Stock-Based Compensation Plans |
Stock-Based Compensation Plans 2021 Employee Stock Purchase Plan
On April 25, 2021, the Company adopted the 2021 Employee Stock Purchase Plan ("ESPP"), which was approved by stockholder vote at the 2021 Annual Meeting of Stockholders held on June 10, 2021. The ESPP provides eligible employees of the Company with an opportunity to purchase common stock of the Company through accumulated payroll deductions, which are included in other current liabilities until they are used to purchase Company shares. Eligible employees participating in the bi-annual offering period can choose to have up to the lesser of 15% of their annual base earnings or the IRS annual share purchase limit of $25,000 in aggregate market value to purchase shares of the Company’s common stock. The purchase price of the stock is the lesser of (i) 85% of the closing market price on the date of purchase and (ii) the closing market price at the beginning of the bi-annual offering period. The maximum number of shares initially reserved for delivery under the plan was 200,000 shares. An increase of 1 million shares to 1.2 million shares was approved by stockholders of the Company at the Company's Special Meeting of stockholders held on March 4, 2024.
The first enrollment period under the plan commenced on August 1, 2021 and the Company has issued 199,989 shares life-to-date under the ESPP. The Company recognized $24,000 of expense related to the plan during the three and nine months ended September 30, 2024. The Company recognized $19,000 and $102,000 of expense related to the plan during the three and nine months ended September 30, 2023, respectively.
Stock Option Programs
In July 2019, the Company’s stockholders approved the 2019 Omnibus Equity Incentive Plan, (as amended, the “2019 Plan”), which was adopted by the Board of Directors (the "Board") with an effective date of June 14, 2019. The 2019 Plan allows for the grant of equity awards to employees, consultants and non-employee directors. An initial maximum of 1,500,000 shares of the Company’s common stock were available for grant under the 2019 Plan. The 2019 Plan included an evergreen provision that allowed for the annual addition of up to 4% of the Company’s fully-diluted outstanding stock, with a maximum allowable increase of 4,900,000 shares over the term of the 2019 Plan. In accordance with this provision, the shares available for grant were increased in 2020 through 2023 by a total of 4,408,871 shares. At the Company's Annual Stockholders meeting on June 28, 2023, stockholders voted to increase the allowable shares under the 2019 plan by 4,440,000 shares as well as to eliminate the evergreen provision. On March 4, 2024, the stockholders voted at the Company's Special Meeting to increase the allowable shares under the 2019 plan by 9,100,000. The 2019 Plan (as amended on June 28, 2023 and March 4, 2024) is currently administered by the Board, or, at the discretion of the Board, by a committee of the Board, which determines the exercise prices, vesting schedules and other restrictions of awards under the 2019 Plan at its discretion. Options to purchase stock may not have an exercise price that is less than the fair market value of underlying shares on the date of grant, and may not have a term greater than ten years. Incentive stock options granted to employees typically vest over four years. Non-statutory options granted to employees, officers, members of the Board, advisors, and consultants of the Company typically vest over three or four years. Shares that are expired, terminated, surrendered or canceled under the 2019 Plan without having been fully exercised will be available for future awards. Stock Option Repricing On March 4, 2024, the Company's stockholders voted to approve the repricing of outstanding stock options having an exercise price above $3.00 per share to $1.72 per share. All other terms of the grant remained the same. There were 3,317,596 stock options that were repriced to $1.72 per share. The repricing will result in $1.2 million of stock compensation being recognized by the Company over the remaining term of the repriced grants and $86,000 and $1.0 million of this amount was recognized in the three months and nine months ended September 30, 2024, respectively. Movements during the year The following table summarizes stock option activity for the nine months ended September 30, 2024 and 2023, respectively, for the 2019 Plan: | | | | | | | | | | | | | | | | | | | | | | | | | Options | | Weighted-
Average
Exercise
Price | | Weighted-
Average
Remaining
Contractual
Term (Years) | | Aggregate
Intrinsic
Value | | Outstanding as of January 1, 2024 | 6,196,140 | | | $ | 7.15 | | | | | | | Granted | 5,715,349 | | | $ | 1.23 | | | | | | | Exercised | — | | | $ | — | | | — | | | | | Repricing modification | — | | | $ | 9.55 | | | | | | | Forfeited or expired | (365,351) | | | $ | 2.08 | | | | | | | Outstanding as of September 30, 2024 | 11,546,138 | | | $ | 1.63 | | | 8.33 | | $ | 2,794,286 | | | Options vested and expected to vest as of September 30, 2024 | 11,546,138 | | | $ | 1.63 | | | 8.33 | | $ | 2,794,286 | | | Options exercisable as of September 30, 2024 | 4,171,969 | | | $ | 2.09 | | | 7.07 | | $ | 329,830 | |
| | | | | | | | | | | | | | | | | | | | | | | | | Options | | Weighted-
Average
Exercise
Price | | Weighted-
Average
Remaining
Contractual
Term (Years) | | Aggregate
Intrinsic
Value | | Outstanding as of January 1, 2023 | 3,791,688 | | | $ | 11.33 | | | | | | | Granted | 2,740,564 | | | $ | 1.71 | | | | | | | Exercised | — | | | $ | — | | | | | | | Forfeited or expired | (268,342) | | | $ | 7.80 | | | | | | | Outstanding as of September 30, 2023 | 6,263,910 | | | $ | 7.27 | | | 8.27 | | $ | 119,691 | | | Options vested and expected to vest as of September 30, 2023 | 6,263,910 | | | $ | 7.27 | | | 8.27 | | $ | 119,691 | | | Options exercisable as of September 30, 2023 | 2,393,294 | | | $ | 11.67 | | | 7.12 | | $ | 6,160 | |
Measurement The weighted-average assumptions used in the BSM option pricing model to determine the fair value of the employee and non-employee stock option grants relating to the 2019 Plan were as follows: Risk-Free Interest Rate The risk-free rate assumption is based on U.S. Treasury instruments with maturities similar to the expected term of the stock options. Expected Dividend Yield The Company has not issued any dividends and does not expect to issue dividends over the life of the options. As a result, the Company has estimated the dividend yield to be zero. Expected Volatility Due to the Company’s limited operating history and a lack of company specific historical and implied volatility data, the Company estimates expected volatility based on the historical volatility of its own stock combined with a group of comparable companies that are publicly traded. The historical volatility data was computed using the daily closing prices for the selected companies’ shares during the equivalent period of the calculated expected term of the stock-based awards. Expected Term The expected term of options is estimated considering the vesting period at the grant date, the life of the option and the average length of time similar grants have remained outstanding in the past. The weighted-average grant date fair value of stock options granted under the 2019 Plan during the nine months ended September 30, 2024 and 2023 was $0.99 and $1.32, respectively. The following are the underlying assumptions used in the Black-Scholes-Merton option pricing model to determine the fair value of stock options granted to employees and to non-employees under this stock plan: | | | | | | | | | | Nine Months Ended September 30, | | 2024 | 2023 | | Risk-free interest rate | 4.11% | 4.00% | | Expected dividend yield | 0% | 0% | | Expected volatility | 100.0% | 96.0% | | Expected term of options (years) | 5.95 | 6.01 |
Stock-Based Compensation Expense Total stock-based compensation expense for all stock awards recognized in the accompanying unaudited condensed consolidated statements of operations is as follows: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Three Months
Ended September 30, | | Nine Months
Ended September 30, | | | | | | | 2024 | | 2023 | | 2024 | | 2023 | | Research and development | | | | | $ | 811,000 | | | $ | 815,000 | | | $ | 2,816,000 | | | $ | 2,607,000 | | | General and administrative | | | | | 1,099,000 | | | 872,000 | | | 3,726,000 | | | 2,857,000 | | | Total | | | | | $ | 1,910,000 | | | $ | 1,687,000 | | | $ | 6,542,000 | | | $ | 5,464,000 | |
As of September 30, 2024, there was $10.8 million in total unrecognized compensation expense relating to the 2019 Plan to be recognized over a weighted average period of 2.82 years. Summary of Equity Incentive Plans Assumed from Vital Upon completion of the Transaction with Vital Therapies ("Vital") on April 12, 2019, Vital’s 2012 Stock Option Plan (the “2012 Plan”), Vital’s 2014 Equity Incentive Plan (the “2014 Plan”) and Vital’s 2017 Inducement Equity Incentive Plan (the “Inducement Plan”), were assumed by the Company. All awards granted under these plans have either been forfeited or expired. There are no longer any shares available for grant under the 2014 Plan as of September 30, 2024. On September 2017, Vital’s board of directors approved the Inducement Plan, which was amended and restated in November 2017. Under the Inducement Plan 46,250 shares of Vital’s common stock were reserved to be used exclusively for non-qualified grants to individuals who were not previously employees or directors as an inducement material to a grantee's entry into employment within the meaning of Rule 5635(c)(4) of the Nasdaq Listing Rules. No expense was recorded for the plans assumed from Vital during the three and nine months ended September 30, 2024 and 2023, respectively.
|
| X |
- DefinitionThe entire disclosure for share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/718/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (l)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_DisclosureOfCompensationRelatedCostsShareBasedPaymentsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Related Party Transactions
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Related Party Transactions [Abstract] |
|
| Related Party Transactions |
Related Party Transactions Executive Chairman Agreement with Duane Nash
On April 15, 2020, the compensation committee of the Board of Directors of the Company independently reviewed and approved entering into an employment agreement with the Executive Chairman of the Board, Duane Nash, MD, JD, MBA (the “Executive Chairman Agreement”) and pursuant to such approval, on April 17, 2020, the Company and Dr. Nash entered into the Executive Chairman Agreement. The Executive Chairman Agreement establishes an “at will” employment relationship. On December 28, 2022, the Company and Dr. Nash entered into Addendum No. Four, which extended the term of employment from December 31, 2022 to December 31, 2023 with a base salary of $30,250 per month. On October 17, 2023, Immunic, Inc. and Dr. Duane Nash entered into Addendum Number 5 to the Executive Chairman Agreement to extend the term of Dr. Nash’s employment as Executive Chairman of the Board of Directors of the Company to December 31, 2024. In connection with Addendum Number 5, the Company increased Dr. Nash’s monthly base salary to $32,368 from $30,250 (which includes the cash retainer payable for serving on the Company’s Board or for acting as the Chairman of the Board). On August 29, 2024, Immunic, Inc. and Dr. Duane Nash entered into Addendum Number 6 to the Executive Chairman Agreement to extend the term of Dr. Nash’s employment as Executive Chairman of the Board of Directors of the Company to December 31, 2025. In connection with Addendum Number 6, the Company increased Dr. Nash’s monthly base salary to $33,986 from $32,368 (which includes the cash retainer payable for serving on the Company’s Board or for acting as the Chairman of the Board) All other terms of the Executive Chairman Agreement remain the same.
|
| X |
- DefinitionThe entire disclosure for related party transactions. Examples of related party transactions include transactions between (a) a parent company and its subsidiary; (b) subsidiaries of a common parent; (c) and entity and its principal owners; and (d) affiliates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480990/946-20-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480990/946-20-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480990/946-20-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477968/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477968/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483326/850-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(g)(3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(c))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(e))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/850/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483326/850-10-50-6
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483326/850-10-50-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483326/850-10-50-1
| Name: |
us-gaap_RelatedPartyTransactionsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Pay vs Performance Disclosure - USD ($)
$ in Thousands |
3 Months Ended |
9 Months Ended |
Sep. 30, 2024 |
Jun. 30, 2024 |
Mar. 31, 2024 |
Sep. 30, 2023 |
Jun. 30, 2023 |
Mar. 31, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
| Pay vs Performance Disclosure |
|
|
|
|
|
|
|
|
| Net loss |
$ (24,368)
|
$ (21,380)
|
$ (29,584)
|
$ (22,769)
|
$ (23,999)
|
$ (25,272)
|
$ (75,332)
|
$ (72,040)
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection v
-Paragraph 1
| Name: |
ecd_PvpTable |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.24.3
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_NonRule10b51ArrAdoptedFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_NonRule10b51ArrTrmntdFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_Rule10b51ArrAdoptedFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 1
| Name: |
ecd_Rule10b51ArrTrmntdFlag |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 408
-Subsection a
-Paragraph 2
-Subparagraph A
| Name: |
ecd_TradingArrByIndTable |
| Namespace Prefix: |
ecd_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Summary of Significant Accounting Policies (Policies)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Accounting Policies [Abstract] |
|
| Basis of Presentation and Consolidation |
Basis of Presentation and Consolidation The accompanying condensed consolidated financial statements have been prepared in conformity with United States generally accepted accounting principles ("U.S. GAAP") and include the accounts of Immunic and its wholly-owned subsidiaries, Immunic AG and Immunic Australia Pty Ltd. All intercompany accounts and transactions have been eliminated in consolidation. Immunic manages its operations as a single reportable segment for the purposes of assessing performance and making operating decisions. Unaudited Interim Financial Information Immunic has prepared the accompanying interim unaudited condensed consolidated financial statements in accordance with United States generally accepted accounting principles, (“US GAAP”), for interim financial information and with the instructions to Form 10-Q and Regulation S-X of the SEC. Accordingly, they do not include all of the information and footnotes required by U.S. GAAP for complete financial statements. These interim unaudited condensed consolidated financial statements reflect all adjustments consisting of normal recurring accruals which, in the opinion of management, are necessary to present fairly Immunic’s consolidated financial position, consolidated results of operations, consolidated statement of stockholders’ equity and consolidated cash flows for the periods and as of the dates presented. The Company’s fiscal year ends on December 31. The condensed consolidated balance sheet as of December 31, 2023 was derived from audited consolidated financial statements but does not include all disclosures required by U.S. GAAP. These condensed consolidated financial statements should be read in conjunction with the annual consolidated financial statements and the notes thereto included on the Company's Annual Report on Form 10-K filed on February 22, 2024. The nature of Immunic’s business is such that the results of any interim period may not be indicative of the results to be expected for the entire year or for corresponding interim periods in any subsequent year.
|
| Use of Estimates |
Use of Estimates The preparation of financial statements in conformity with U.S. GAAP requires the Company to make certain estimates and assumptions that affect the reported amounts of assets, liabilities, expenses and the disclosure of contingent assets and liabilities in the Company’s consolidated financial statements. The most significant estimates in the Company’s financial statements and accompanying notes relate to clinical trial expenses and stock-based compensation. Management believes its estimates to be reasonable under the circumstances. Actual results could differ materially from those estimates and assumptions.
|
| Foreign Currency Translation and Presentation |
Foreign Currency Translation and Presentation The Company’s reporting currency is United States (“U.S.”) dollars. Immunic AG is located in Germany with the Euro being its functional currency. Immunic Australia Pty Ltd.’s functional currency is the Australian dollar. All amounts in the financial statements where the functional currency is not the U.S. dollar are translated into U.S. dollar equivalents at exchange rates as follows: • assets and liabilities at reporting period-end rates; • income statement accounts at average exchange rates for the reporting period; and • components of equity at historical rates. Gains and losses from translation of the financial statements into U.S. dollars are recorded in stockholders’ equity as a component of accumulated other comprehensive income (loss). Realized and unrealized gains and losses resulting from foreign currency transactions denominated in currencies other than the functional currency are reflected as general and administrative expenses in the Consolidated Statements of Operations. Foreign currency transaction gains and losses related to long-term intercompany loans that are payable in the foreseeable future are recorded in Other Income (Expense). The Consolidated Statements of Cash Flows were prepared by using the average exchange rate in effect during the reporting period which reasonably approximates the timing of the cash flows.
|
| Cash and Cash Equivalents |
Cash and Cash Equivalents The Company considers all highly liquid investments with an original maturity of three months or less to be cash equivalents. Cash and cash equivalents consist of cash on hand and deposits in banks located in the U.S. of approximately $40.6 million, Germany of approximately $15.6 million and Australia of approximately $2.9 million as of September 30, 2024. The Company maintains cash and cash equivalent balances denominated in Euro and U.S. dollars with major financial institutions in the U.S. and Germany in excess of the deposit limits insured by the government. Management periodically reviews the credit standing of these financial institutions. The Company currently deposits its cash and cash equivalents with two large financial institutions. Cash and cash equivalents in the U.S. are held at JP Morgan and are primarily held in a U.S. Government money market fund account earning interest at a rate of 4.8% as of September 30, 2024. Cash and cash equivalents in Germany are earning interest at a rate of 3.25% to 3.75% as of September 30, 2024.
|
| Fair Value Measurement |
Fair Value Measurement Fair value is defined as the price that would be received to sell an asset or be paid to transfer a liability in an orderly transaction between market participants on the measurement date. Accounting guidance establishes a fair value hierarchy that requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. The standard describes three levels of inputs that may be used to measure fair value: Level 1—Quoted prices in active markets for identical assets or liabilities. Level 1 assets consisted of money market funds for the periods presented. The Company had no Level 1 liabilities for the periods presented. Level 2—Inputs other than observable quoted prices for the asset or liability, either directly or indirectly; these include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active. The Company had no Level 2 assets or liabilities for the periods presented. Level 3—Unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of assets or liabilities. The Company had no Level 3 assets or liabilities for the periods presented. The Company did have tranche rights that were at level 3 during the first quarter of 2024. The carrying value of cash and cash equivalents, other current assets and prepaid expenses, accounts payable, accrued expenses, and other current liabilities approximates fair value due to the short period of time to maturity.
|
| Property and Equipment |
Property and Equipment Property and equipment is stated at cost. Depreciation is computed using the straight-line method based on the estimated service lives of the assets, which range from three to thirteen years.
|
| Impairment of Long-Lived Assets |
Impairment of Long-Lived Assets The Company records impairment losses on long-lived assets used in operations when indicators of impairment are present and the undiscounted cash flows estimated to be generated by those assets are less than the assets’ carrying amount. Impaired assets are then recorded at their estimated fair value.
|
| Research and Development Expenses |
Research and Development Expenses These costs primarily include external development expenses and internal personnel expenses for its development programs, vidofludimus calcium and IMU-856. Immunic has spent the majority of its research and development resources on vidofludimus calcium, the Company's lead development program, for clinical trials in MS and UC. Research and development expenses consist of expenses incurred in research and development activities, which include clinical trials, contract research services, certain milestone payments, salaries and related employee benefits, allocated facility costs and other outsourced services. Research and development expenses are charged to operations as incurred. The Company enters into agreements with contract research organizations (“CROs”) to provide clinical trial services for individual studies and projects by executing individual work orders governed by a Master Service Arrangement (“MSA”). The MSAs and associated work orders provide for regular recurrent payments and payments upon the completion of certain milestones. The Company regularly assesses the timing of payments against actual costs incurred to ensure a proper accrual of related expenses in the appropriate accounting period.
|
| Collaboration Arrangements |
Collaboration Arrangements Certain collaboration and license agreements may include payments to or from the Company of one or more of the following: non-refundable or partially refundable upfront or license fees; development, regulatory and commercial milestone payments; payment for manufacturing supply services; partial or complete reimbursement of research and development costs; and royalties on net sales of licensed products. The Company assesses whether such contracts are within the scope of Financial Accounting Standards Board (FASB) Accounting Standards Update (“ASU”) 2014-09 “Revenue from Contracts with Customers” and ASU No. 2018-18, “Collaborative Arrangements” ("ASU 2018-18"). ASU 2018-18, clarifies that certain elements of collaborative arrangements could qualify as transactions with customers in the scope of ASC 606. In October 2018, the Company entered into an option and license agreement (the "Daiichi Sankyo Agreement") with Daiichi Sankyo Co., Ltd. ("Daiichi Sankyo") which granted the Company the right to license a group of compounds, designated by the Company as IMU-856, as a potential new oral treatment option for gastrointestinal diseases such as celiac disease, inflammatory bowel disease, irritable bowel syndrome with diarrhea and other barrier function associated diseases. During the option period, the Company performed agreed upon research and development activities for which it was reimbursed by Daiichi Sankyo up to a maximum agreed-upon limit. Such reimbursement was recorded as other income. There are no additional research and development reimbursements expected under this agreement. On January 5, 2020, the Company exercised its option to obtain the exclusive worldwide right to commercialization of IMU-856. Among other things, the option exercise grants Immunic AG the rights to Daiichi Sankyo’s patent application related to IMU-856, for which the Company received a notice of allowance from the U.S. Patent & Trademark Office in August 2022. In connection with the option exercise, the Company paid a one-time upfront licensing fee to Daiichi Sankyo. Under the Daiichi Sankyo Agreement, Daiichi Sankyo is also eligible to receive future development, regulatory and sales milestone payments, as well as royalties related to IMU-856.
|
| Government assistance |
Government assistance Government assistance relating to research and development performed by Immunic Australia is recorded as a component of other (income) expense. This government assistance is recognized at a rate of 43.5% of the qualified research and development expenditures which are incurred. We also receive government assistance from the German Government for reimbursement of research and development expenses up to 3.5 million Euros per year. We recognized $0.7 million and $0.7 million of other income related to research activities performed during the three and nine months ended September 30, 2024, respectively and $0.2 million and $2.3 million related to research activities performed during the three and nine months ended September 30, 2023, respectively.
|
| General and Administrative Expenses |
General and Administrative Expenses General and administrative expenses consist primarily of salaries and related costs for personnel in executive, finance, business development and other support functions. Other general and administrative expenses include, but are not limited to, stock-based compensation, insurance costs, professional fees for legal, accounting and tax services, consulting, related facility costs and travel.
|
| Stock-Based Compensation |
Stock-Based Compensation The Company measures the cost of employee and non-employee services received in exchange for equity awards based on the grant-date fair value of the award recognized generally as an expense (i) on a straight-line basis over the requisite service period for those awards whose vesting is based upon a service condition, and (ii) on an accelerated method for awards whose vesting is based upon a performance condition, but only to the extent it is probable that the performance condition will be met. Stock-based compensation is (i) estimated at the date of grant based on the award’s fair value for equity classified awards and (ii) final measurement date for liability classified awards. Forfeitures are recorded in the period in which they occur. The Company estimates the fair value of stock options using the Black-Scholes-Merton option-pricing model ("BSM"), which requires the use of estimates and subjective assumptions, including the risk-free interest rate, the fair value of the underlying common stock, the expected dividend yield of the Company’s common stock, the expected volatility of the price of the Company’s common stock, and the expected term of the option. These estimates involve inherent uncertainties and the application of management’s judgment. If factors change and different assumptions are used, the Company’s stock-based compensation expense could be materially different in the future.
|
| Leases |
Leases The Company leases office space and office equipment. The underlying lease agreements have lease terms of less than 12 months and up to 60 months. Leases with terms of 12 months or less at inception are not included in the operating lease right of use asset and operating lease liability. The Company has three existing leases for office and laboratory space. At inception of a lease agreement, the Company determines whether an agreement represents a lease and at commencement each lease agreement is assessed as to classification as an operating or financing lease. The Company's leases have been classified as operating leases and an operating lease right-of-use asset and an operating lease liability have been recorded on the Company’s balance sheet. A right-of-use lease asset represents the Company’s right to use the underlying asset for the lease term and the lease obligation represents its commitment to make the lease payments arising from the lease. Right-of-use lease assets and obligations are recognized at the commencement date based on the present value of remaining lease payments over the lease term. As the Company’s leases do not provide an implicit rate, the Company has used an estimated incremental borrowing rate based on the information available at the commencement date in determining the present value of lease payments. The right-of-use lease asset includes any lease payments made prior to commencement and excludes any lease incentives. The lease term used in estimating future lease payments may include options to extend when it is reasonably certain that the Company will exercise that option. Operating lease expense is recognized on a straight-line basis over the lease term, subject to any changes in the lease or changes in expectations regarding the lease term. Variable lease costs such as common area costs and property taxes are expensed as incurred. Leases with an initial term of twelve months or less are not recorded on the balance sheet.
|
| Comprehensive Income (Loss) |
Comprehensive Income (Loss) Comprehensive income (loss) is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources. Accumulated other comprehensive income (loss) has been reflected as a separate component of stockholders’ equity in the accompanying Consolidated Balance Sheets and consists of foreign currency translation adjustments.
|
| Income Taxes |
Income Taxes The Company is subject to corporate income tax laws and regulations in the U.S., Germany and Australia. Tax regulations within each jurisdiction are subject to the interpretation of the related tax laws and regulations and require significant judgment in their application. The Company utilizes the asset and liability method of accounting for income taxes which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the consolidated financial statements. Deferred income tax assets and liabilities are determined based on the differences between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. The effect of changes in tax rates on deferred tax assets and liabilities is recognized in operations in the period that includes the enactment date. Deferred taxes are reduced by a valuation allowance when, in the opinion of management, it is more likely than not some portion or the entire deferred tax asset will not be realized. As of September 30, 2024 and 2023, respectively, the Company maintained a full valuation allowance against the balance of deferred tax assets. It is the Company’s policy to provide for uncertain tax positions and the related interest and penalties based upon management’s assessment of whether a tax benefit is more likely than not to be sustained upon examination by tax authorities. The Company recognizes interest and penalties accrued on any unrecognized tax benefits as a component of income tax expense. The Company is subject to U.S. federal, New York, California, Texas, German and Australian income taxes. The Company is subject to U.S. federal or state income tax examination by tax authorities for tax returns filed for the years 2003 and forward due to the carryforward of NOLs. Tax years 2019 through 2023 are subject to audit by German and Australian tax authorities. The Company is not currently under examination by any tax jurisdictions.
|
| Warrants and Tranche Rights |
Warrants and Tranche Rights The Company accounts for issued financial instruments either as a liability or equity in accordance with ASC 480-10, Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity (“ASC 480-10”) or ASC 815-40, Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock (“ASC 815-40”). If financial instruments do not meet liability classification under ASC 480-10, the Company considers the requirements of ASC 815-40 to determine whether the financial instruments should be classified as a liability or as equity. Liability-classified financial instruments are measured at fair value on the issuance date and at the end of each reporting period. Any change in the fair value of the financial instruments after the issuance date is recorded in the consolidated statements of operations as a gain or loss. If financial instruments do not require liability classification under ASC 815-40, the instrument is classified in permanent equity. Equity-classified financial instruments are accounted for at fair value on the issuance date with no changes in fair value recognized after the issuance date.
|
| Net Loss Per Share |
Net Loss Per Share Basic net loss per share attributable to common stockholders is calculated by dividing the net loss by the weighted-average number of common shares outstanding for the period, without consideration for common stock equivalents. Diluted net loss per share attributable to common stockholders is computed by dividing the net loss by the weighted-average number of common shares and, if dilutive, common stock equivalents outstanding for the period determined using the treasury-stock method. For all periods presented, there is no difference in the number of shares used to calculate basic and diluted shares outstanding due to the Company’s net loss position. The weighted average shares outstanding calculation for basic and diluted earnings per share for the three and nine months ended September 30, 2024 includes 11,193,564 pre-funded warrants that remain unexercised as of September 30, 2024.
|
| Recently Issued and/or Adopted Accounting Standards |
Recently Issued and/or Adopted Accounting Standards There are no recently issued accounting standards that would have a significant impact on the company's consolidated financial statements.
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for basis of accounting, or basis of presentation, used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS).
| Name: |
us-gaap_BasisOfAccountingPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for collaborative arrangements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-SubTopic 10
-Topic 808
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479402/808-10-50-1
| Name: |
us-gaap_CollaborativeArrangementAccountingPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for comprehensive income.
| Name: |
us-gaap_ComprehensiveIncomePolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for computing basic and diluted earnings or loss per share for each class of common stock and participating security. Addresses all significant policy factors, including any antidilutive items that have been excluded from the computation and takes into account stock dividends, splits and reverse splits that occur after the balance sheet date of the latest reporting period but before the issuance of the financial statements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-2
| Name: |
us-gaap_EarningsPerSharePolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for determining the fair value of financial instruments. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 825
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-1
| Name: |
us-gaap_FairValueOfFinancialInstrumentsPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for (1) transactions denominated in a currency other than the reporting enterprise's functional currency, (2) translating foreign currency financial statements that are incorporated into the financial statements of the reporting enterprise by consolidation, combination, or the equity method of accounting, and (3) remeasurement of the financial statements of a foreign reporting enterprise in a hyperinflationary economy. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/830/tableOfContent
| Name: |
us-gaap_ForeignCurrencyTransactionsAndTranslationsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for government assistance. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 832
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483507/832-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 832
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483507/832-10-50-5
| Name: |
us-gaap_GovernmentAssistancePolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for recognizing and measuring the impairment of long-lived assets. An entity also may disclose its accounting policy for long-lived assets to be sold. This policy excludes goodwill and intangible assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.CC)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480091/360-10-S99-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 05
-Paragraph 4
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482338/360-10-05-4
| Name: |
us-gaap_ImpairmentOrDisposalOfLongLivedAssetsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for income taxes, which may include its accounting policies for recognizing and measuring deferred tax assets and liabilities and related valuation allowances, recognizing investment tax credits, operating loss carryforwards, tax credit carryforwards, and other carryforwards, methodologies for determining its effective income tax rate and the characterization of interest and penalties in the financial statements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-20
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-19
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482525/740-10-45-25
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(h)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-17
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-9
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482525/740-10-45-28
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-1
| Name: |
us-gaap_IncomeTaxPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for leasing arrangement entered into by lessee. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-1
| Name: |
us-gaap_LesseeLeasesPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy pertaining to new accounting pronouncements that may impact the entity's financial reporting. Includes, but is not limited to, quantification of the expected or actual impact.
| Name: |
us-gaap_NewAccountingPronouncementsPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for long-lived, physical asset used in normal conduct of business and not intended for resale. Includes, but is not limited to, work of art, historical treasure, and similar asset classified as collections. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477798/958-360-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477798/958-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for costs it has incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483044/730-10-05-1
| Name: |
us-gaap_ResearchAndDevelopmentExpensePolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for inclusion of significant items in the selling, general and administrative (or similar) expense report caption. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 720
-SubTopic 35
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483406/720-35-50-1
| Name: |
us-gaap_SellingGeneralAndAdministrativeExpensesPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for award under share-based payment arrangement. Includes, but is not limited to, methodology and assumption used in measuring cost. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.C.Q3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479830/718-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.D.1.Q5)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479830/718-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.D.3.Q2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479830/718-10-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.D.2.Q6)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479830/718-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/718/tableOfContent
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationOptionAndIncentivePlansPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for its capital stock transactions, including dividends and accumulated other comprehensive income. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477968/946-235-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-1
| Name: |
us-gaap_StockholdersEquityPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for the use of estimates in the preparation of financial statements in conformity with generally accepted accounting principles. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-9
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-12
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-8
| Name: |
us-gaap_UseOfEstimates |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCash And Cash Equivalents And Other Investments
| Name: |
vtl_CashAndCashEquivalentsAndOtherInvestmentsPolicyTextBlock |
| Namespace Prefix: |
vtl_ |
| Data Type: |
dtr-types1:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of securities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) in the future that were not included in the computation of diluted EPS because to do so would increase EPS amounts or decrease loss per share amounts for the period presented, by antidilutive securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
| Name: |
us-gaap_ScheduleOfAntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Balance Sheet Details (Tables)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Organization, Consolidation and Presentation of Financial Statements [Abstract] |
|
| Schedule of Other Current Assets and Prepaid Expenses |
Other Current Assets and Prepaid Expenses consist of (in thousands): | | | | | | | | | | | | | | | | | September 30, 2024 | | December 31, 2023 | | Prepaid clinical and related costs | $ | 2,066 | | | $ | 2,314 | | | VAT receivable | 780 | | | 703 | | | Australian research and development tax incentive | 39 | | | 670 | | | Research grant | — | | | 1,104 | | | Prepaid Insurance | 553 | | | 198 | | | Other | 757 | | | 871 | | | Total | $ | 4,195 | | | $ | 5,860 | |
|
| Schedule of Accounts Payable and Accrued Expenses |
Accounts Payable consist of (in thousands): | | | | | | | | | | | | | | | | | September 30, 2024 | | December 31, 2023 | | Clinical costs | $ | 5,789 | | | $ | 4,726 | | | Legal and audit costs | 90 | | | 160 | | | | | | | Other | 163 | | | 213 | | | Total | $ | 6,042 | | | $ | 5,099 | |
Accrued expenses consist of (in thousands): | | | | | | | | | | | | | | | | | September 30, 2024 | | December 31, 2023 | | Accrued clinical and related costs | $ | 14,386 | | | $ | 16,863 | | | Accrued legal and audit costs | 136 | | | 216 | | | Accrued compensation | 1,509 | | | 1,460 | | | Accrued other | 214 | | | 125 | | | Total | $ | 16,245 | | | $ | 18,664 | |
|
| Schedule of Other Current Liabilities |
Other Current Liabilities consist of (in thousands): | | | | | | | | | | | | | | | | | | | | | September 30, 2024 | | December 31, 2023 | | | Lease liabilities | $ | 713 | | | $ | 695 | | | | Other | 357 | | | 271 | | | | Total | 1,070 | | | 966 | | |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of other current liabilities.
| Name: |
us-gaap_OtherCurrentLiabilitiesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the (a) carrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business (accounts payable); (b) other payables; and (c) accrued liabilities. Examples include taxes, interest, rent and utilities. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). An alternative caption includes accrued expenses.
| Name: |
us-gaap_ScheduleOfAccountsPayableAndAccruedLiabilitiesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the carrying amounts of other current assets.
| Name: |
us-gaap_ScheduleOfOtherCurrentAssetsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of undiscounted cash flows of lessee's operating lease liability. Includes, but is not limited to, reconciliation of undiscounted cash flows to operating lease liability recognized in statement of financial position. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityMaturityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Fair Value (Tables)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Fair Value Disclosures [Abstract] |
|
| Schedule of Financial Assets and Liabilities Measured at Fair Value on a Recurring Basis |
The following fair value hierarchy tables present information about each major category of the Company’s financial assets and liabilities measured at fair value on a recurring basis (in thousands): | | | | | | | | | | | | | | | | | | | | | | | | | | Fair Value Measurement at September 30, 2024 | | | Fair Value | | Level 1 | | Level 2 | | Level 3 | | Assets | | | | | | | | | Money market funds | $ | 40,359 | | | $ | 40,359 | | | $ | — | | | $ | — | | | Total assets at fair value | $ | 40,359 | | | $ | 40,359 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | Fair Value Measurement at December 31, 2023 | | Fair Value | | Level 1 | | Level 2 | | Level 3 | | Assets | | | | | | | | | Money market funds | $ | 34,087 | | | $ | 34,087 | | | $ | — | | | $ | — | | | Total assets | $ | 34,087 | | | $ | 34,087 | | | $ | — | | | $ | — | |
|
| Schedule of Fair Value of Tranche Rights |
A rollforward of the fair value of the tranche rights is as follows (in thousands): | | | | | | | December 31, 2023 | $ | — | | | Fair value as of January 8, 2024 | $ | 23,600 | | | Change in fair value through March 4, 2024 | $ | 4,796 | | | Reclassification to equity | $ | (28,396) | | | March 31, 2024 | $ | — | |
|
| X |
- References
+ Details
| Name: |
us-gaap_FairValueDisclosuresAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the fair value measurement of liabilities using significant unobservable inputs (Level 3), a reconciliation of the beginning and ending balances, separately presenting changes attributable to the following: (1) total gains or losses for the period (realized and unrealized), segregating those gains or losses included in earnings (or changes in net assets), and gains or losses recognized in other comprehensive income (loss) and a description of where those gains or losses included in earnings (or changes in net assets) are reported in the statement of income (or activities); (2) purchases, sales, issues, and settlements (each type disclosed separately); and (3) transfers in and transfers out of Level 3 (for example, transfers due to changes in the observability of significant inputs) by class of liability. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
| Name: |
us-gaap_FairValueLiabilitiesMeasuredOnRecurringBasisUnobservableInputReconciliationTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of assets and liabilities, including [financial] instruments measured at fair value that are classified in stockholders' equity, if any, that are measured at fair value on a recurring basis. The disclosures contemplated herein include the fair value measurements at the reporting date by the level within the fair value hierarchy in which the fair value measurements in their entirety fall, segregating fair value measurements using quoted prices in active markets for identical assets (Level 1), significant other observable inputs (Level 2), and significant unobservable inputs (Level 3). Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
| Name: |
us-gaap_ScheduleOfFairValueAssetsAndLiabilitiesMeasuredOnRecurringBasisTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Common Stock (Tables)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Equity [Abstract] |
|
| Schedule of Shares Reserved for Future Issuance |
Shares reserved for future issuance at September 30, 2024 are as follows: | | | | | | | Number of
Shares | | Common stock reserved for issuance for: | | | 2021 Employee Stock Purchase Plan | 1,000,011 | | | Pre-funded stock warrants | 11,193,564 | | | Outstanding stock options | 11,546,138 | | | Common shares reserved for tranche 2 rights | 46,620,046 | | | Maximum common shares reserved for tranche 3 rights | 46,620,046 | | | Common stock options available for future grant: | | | 2017 Inducement Equity Incentive Plan | 46,250 | | | 2019 Omnibus Equity Incentive Plan | 7,903,691 | | | Total common shares reserved for future issuance | 124,929,746 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionSchedule Of Common Stock Reserved For Future Issuance [Table Text Block]
| Name: |
vtl_ScheduleOfCommonStockReservedForFutureIssuanceTableTextBlock |
| Namespace Prefix: |
vtl_ |
| Data Type: |
dtr-types1:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Stock-Based Compensation Plans (Tables)
|
9 Months Ended |
Sep. 30, 2024 |
|---|
| Share-Based Payment Arrangement [Abstract] |
|
| Schedule of Stock Option Activity |
The following table summarizes stock option activity for the nine months ended September 30, 2024 and 2023, respectively, for the 2019 Plan: | | | | | | | | | | | | | | | | | | | | | | | | | Options | | Weighted-
Average
Exercise
Price | | Weighted-
Average
Remaining
Contractual
Term (Years) | | Aggregate
Intrinsic
Value | | Outstanding as of January 1, 2024 | 6,196,140 | | | $ | 7.15 | | | | | | | Granted | 5,715,349 | | | $ | 1.23 | | | | | | | Exercised | — | | | $ | — | | | — | | | | | Repricing modification | — | | | $ | 9.55 | | | | | | | Forfeited or expired | (365,351) | | | $ | 2.08 | | | | | | | Outstanding as of September 30, 2024 | 11,546,138 | | | $ | 1.63 | | | 8.33 | | $ | 2,794,286 | | | Options vested and expected to vest as of September 30, 2024 | 11,546,138 | | | $ | 1.63 | | | 8.33 | | $ | 2,794,286 | | | Options exercisable as of September 30, 2024 | 4,171,969 | | | $ | 2.09 | | | 7.07 | | $ | 329,830 | |
| | | | | | | | | | | | | | | | | | | | | | | | | Options | | Weighted-
Average
Exercise
Price | | Weighted-
Average
Remaining
Contractual
Term (Years) | | Aggregate
Intrinsic
Value | | Outstanding as of January 1, 2023 | 3,791,688 | | | $ | 11.33 | | | | | | | Granted | 2,740,564 | | | $ | 1.71 | | | | | | | Exercised | — | | | $ | — | | | | | | | Forfeited or expired | (268,342) | | | $ | 7.80 | | | | | | | Outstanding as of September 30, 2023 | 6,263,910 | | | $ | 7.27 | | | 8.27 | | $ | 119,691 | | | Options vested and expected to vest as of September 30, 2023 | 6,263,910 | | | $ | 7.27 | | | 8.27 | | $ | 119,691 | | | Options exercisable as of September 30, 2023 | 2,393,294 | | | $ | 11.67 | | | 7.12 | | $ | 6,160 | |
|
| Schedule of Valuation Assumptions Used |
The following are the underlying assumptions used in the Black-Scholes-Merton option pricing model to determine the fair value of stock options granted to employees and to non-employees under this stock plan: | | | | | | | | | | Nine Months Ended September 30, | | 2024 | 2023 | | Risk-free interest rate | 4.11% | 4.00% | | Expected dividend yield | 0% | 0% | | Expected volatility | 100.0% | 96.0% | | Expected term of options (years) | 5.95 | 6.01 |
|
| Schedule of Stock-based Compensation Expense for Stock Awards Recognized |
Total stock-based compensation expense for all stock awards recognized in the accompanying unaudited condensed consolidated statements of operations is as follows: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | Three Months
Ended September 30, | | Nine Months
Ended September 30, | | | | | | | 2024 | | 2023 | | 2024 | | 2023 | | Research and development | | | | | $ | 811,000 | | | $ | 815,000 | | | $ | 2,816,000 | | | $ | 2,607,000 | | | General and administrative | | | | | 1,099,000 | | | 872,000 | | | 3,726,000 | | | 2,857,000 | | | Total | | | | | $ | 1,910,000 | | | $ | 1,687,000 | | | $ | 6,542,000 | | | $ | 5,464,000 | |
|
| X |
- DefinitionTabular disclosure of allocation of amount expensed and capitalized for award under share-based payment arrangement to statement of income or comprehensive income and statement of financial position. Includes, but is not limited to, corresponding line item in financial statement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (h)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfEmployeeServiceShareBasedCompensationAllocationOfRecognizedPeriodCostsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure for stock option plans. Includes, but is not limited to, outstanding awards at beginning and end of year, grants, exercises, forfeitures, and weighted-average grant date fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedCompensationStockOptionsActivityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the significant assumptions used during the year to estimate the fair value of stock options, including, but not limited to: (a) expected term of share options and similar instruments, (b) expected volatility of the entity's shares, (c) expected dividends, (d) risk-free rate(s), and (e) discount for post-vesting restrictions. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (f)(2)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedPaymentAwardStockOptionsValuationAssumptionsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.3
Description of Business and Basis of Financial Statements (Details)
$ in Thousands |
105 Months Ended |
|
|
|
|
|
Sep. 30, 2024
USD ($)
lease
|
Aug. 01, 2024
employee
|
Dec. 31, 2023
USD ($)
|
Sep. 30, 2023
USD ($)
|
Dec. 31, 2022
USD ($)
|
| Accounting Policies [Abstract] |
|
|
|
|
|
| Number of employees | employee |
|
85
|
|
|
|
| Number of development programs | lease |
3
|
|
|
|
|
| Accumulated deficit |
$ (486,224)
|
|
$ (410,892)
|
|
|
| Proceeds from issuance of private placement |
430,900
|
|
|
|
|
| Cash, cash equivalents and restricted cash |
$ 59,071
|
|
$ 46,674
|
$ 59,689
|
$ 106,745
|
| X |
- DefinitionNumber of persons employed by the Entity
| Name: |
dei_EntityNumberOfEmployees |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:decimalItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage. Excludes amount for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe cash inflow associated with the amount received from entity's raising of capital via private rather than public placement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfPrivatePlacement |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480990/946-20-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionNumber Of Development Programs
| Name: |
vtl_NumberOfDevelopmentPrograms |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
v3.24.3
Summary of Significant Accounting Policies - Additional Information (Details)
€ in Millions |
3 Months Ended |
9 Months Ended |
|
|
|
Sep. 30, 2024
USD ($)
lease
financialInstitution
shares
|
Sep. 30, 2023
USD ($)
|
Sep. 30, 2024
USD ($)
lease
financialInstitution
shares
|
Sep. 30, 2023
USD ($)
|
Sep. 30, 2024
EUR (€)
lease
financialInstitution
shares
|
Dec. 31, 2023
USD ($)
|
| Property, Plant and Equipment [Line Items] |
|
|
|
|
|
|
| Cash and cash equivalents |
$ 59,071,000
|
|
$ 59,071,000
|
|
|
$ 46,674,000
|
| Number of financial institutions used for cash deposits | financialInstitution |
2
|
|
2
|
|
2
|
|
| Depreciation expense |
$ 42,000
|
$ 34,000
|
$ 99,000
|
$ 88,000
|
|
|
| Impairment losses |
0
|
0
|
0
|
0
|
|
|
| Australian research and development tax incentive |
39,000
|
|
39,000
|
|
|
$ 670,000
|
| Government assistance, amount |
$ 700,000
|
$ 200,000
|
$ 700,000
|
$ 2,300,000
|
|
|
| Government Assistance, Statement of Income or Comprehensive Income [Extensible Enumeration] |
|
|
Nonoperating Income (Expense)
|
Nonoperating Income (Expense)
|
|
|
| Number of existing leases | lease |
3
|
|
3
|
|
3
|
|
| Pre-Funded Warrants |
|
|
|
|
|
|
| Property, Plant and Equipment [Line Items] |
|
|
|
|
|
|
| Warrant exercised (in shares) | shares |
11,193,564
|
|
11,193,564
|
|
11,193,564
|
|
| Minimum |
|
|
|
|
|
|
| Property, Plant and Equipment [Line Items] |
|
|
|
|
|
|
| Estimated useful life (in years) |
3 years
|
|
3 years
|
|
3 years
|
|
| Lease term (in months) |
12 months
|
|
12 months
|
|
12 months
|
|
| Maximum |
|
|
|
|
|
|
| Property, Plant and Equipment [Line Items] |
|
|
|
|
|
|
| Estimated useful life (in years) |
13 years
|
|
13 years
|
|
13 years
|
|
| Lease term (in months) |
60 months
|
|
60 months
|
|
60 months
|
|
| United States |
|
|
|
|
|
|
| Property, Plant and Equipment [Line Items] |
|
|
|
|
|
|
| Cash and cash equivalents |
$ 40,600,000
|
|
$ 40,600,000
|
|
|
|
| Investment interest rate (as percent) |
4.80%
|
|
4.80%
|
|
4.80%
|
|
| United States | Money market funds |
|
|
|
|
|
|
| Property, Plant and Equipment [Line Items] |
|
|
|
|
|
|
| Investment interest rate (as percent) |
4.80%
|
|
4.80%
|
|
4.80%
|
|
| Germany |
|
|
|
|
|
|
| Property, Plant and Equipment [Line Items] |
|
|
|
|
|
|
| Cash and cash equivalents |
$ 15,600,000
|
|
$ 15,600,000
|
|
|
|
| Australian research and development tax incentive | € |
|
|
|
|
€ 3.5
|
|
| Germany | Money market funds | Minimum |
|
|
|
|
|
|
| Property, Plant and Equipment [Line Items] |
|
|
|
|
|
|
| Investment interest rate (as percent) |
3.25%
|
|
3.25%
|
|
3.25%
|
|
| Germany | Money market funds | Maximum |
|
|
|
|
|
|
| Property, Plant and Equipment [Line Items] |
|
|
|
|
|
|
| Investment interest rate (as percent) |
3.75%
|
|
3.75%
|
|
3.75%
|
|
| Australia |
|
|
|
|
|
|
| Property, Plant and Equipment [Line Items] |
|
|
|
|
|
|
| Cash and cash equivalents |
$ 2,900,000
|
|
$ 2,900,000
|
|
|
|
| Government R&D assistance rate |
|
|
43.50%
|
|
|
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of securities into which the class of warrant or right may be converted. For example, but not limited to, 500,000 warrants may be converted into 1,000,000 shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightNumberOfSecuritiesCalledByWarrantsOrRights |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of expense recognized in the current period that reflects the allocation of the cost of tangible assets over the assets' useful lives. Includes production and non-production related depreciation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-1
| Name: |
us-gaap_Depreciation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in income from government assistance. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 832
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483507/832-10-50-3
| Name: |
us-gaap_GovernmentAssistanceAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates line item in statement of income or comprehensive income that includes increase (decrease) in income from government assistance. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 832
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483507/832-10-50-3
| Name: |
us-gaap_GovernmentAssistanceStatementOfIncomeOrComprehensiveIncomeExtensibleEnumeration |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
enum2:enumerationSetItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionRate of interest on investment. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 55
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477439/946-210-55-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481800/320-10-50-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481800/320-10-50-5
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.12-12A(Column A)(Footnote 2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.12-12A(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column A)(Footnote 2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column A)(Footnote 4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 4)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-6
| Name: |
us-gaap_InvestmentInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTerm of lessee's operating lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-3
| Name: |
us-gaap_LesseeOperatingLeaseTermOfContract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-7A
| Name: |
us-gaap_PropertyPlantAndEquipmentLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionUseful life of long lived, physical assets used in the normal conduct of business and not intended for resale, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Examples include, but not limited to, land, buildings, machinery and equipment, office equipment, furniture and fixtures, and computer equipment.
| Name: |
us-gaap_PropertyPlantAndEquipmentUsefulLife |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe charge against earnings resulting from the aggregate write down of tangible assets from their carrying value to their fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482099/360-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-1
| Name: |
us-gaap_TangibleAssetImpairmentCharges |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionGovernment Assistance, Qualified Research and Development Expenditures, Maximum Amount
| Name: |
vtl_GovernmentAssistanceQualifiedResearchAndDevelopmentExpendituresMaximumAmount |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionGovernment Assistance, Qualified Research and Development Expenditures, Percent
| Name: |
vtl_GovernmentAssistanceQualifiedResearchAndDevelopmentExpendituresPercent |
| Namespace Prefix: |
vtl_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLessee, Operating Leases, Number Of Existing Leases
| Name: |
vtl_LesseeOperatingLeasesNumberOfExistingLeases |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of Financial Institutions Used For Cash Deposit
| Name: |
vtl_NumberOfFinancialInstitutionsUsedForCashDeposit |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=vtl_PreFundedWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_StatementGeographicalAxis=country_US |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_CashAndCashEquivalentsAxis=us-gaap_MoneyMarketFundsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_StatementGeographicalAxis=country_DE |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_StatementGeographicalAxis=country_AU |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
| X |
- DefinitionSecurities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) or earnings per unit (EPU) in the future that were not included in the computation of diluted EPS or EPU because to do so would increase EPS or EPU amounts or decrease loss per share or unit amounts for the period presented. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=us-gaap_StockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of current assets classified as other. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits in future periods, and amount of other assets that are expected to be realized or consumed within one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PrepaidExpenseAndOtherAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for insurance that provides economic benefits within a future period of one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (g)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483032/340-10-45-1
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 5
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482955/340-10-05-5
| Name: |
us-gaap_PrepaidInsurance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying amount as of the balance sheet date of value added taxes due either from customers arising from sales on credit terms, or as previously overpaid to tax authorities. For classified balance sheets, represents the current amount receivable, that is amounts expected to be collected within one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(3)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_ValueAddedTaxReceivableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionGovernment Assistance, Qualified Research and Development Expenditures, Maximum Amount
| Name: |
vtl_GovernmentAssistanceQualifiedResearchAndDevelopmentExpendituresMaximumAmount |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionResearch and Development Grant
| Name: |
vtl_ResearchAndDevelopmentGrant |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.24.3
| X |
- DefinitionCarrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
| Name: |
us-gaap_AccountsPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of obligations incurred classified as other, payable within one year or the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableOtherCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionClinical Costs Payable, Current
| Name: |
vtl_ClinicalCostsPayableCurrent |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionLegal And Audit Costs Payable, Current
| Name: |
vtl_LegalAndAuditCostsPayableCurrent |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.24.3
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred and payable, pertaining to costs that are statutory in nature, are incurred on contractual obligations, or accumulate over time and for which invoices have not yet been received or will not be rendered. Examples include taxes, interest, rent and utilities. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expenses incurred but not yet paid classified as other, due within one year or the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_OtherAccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAccrued Clinical Costs, Current
| Name: |
vtl_AccruedClinicalCostsCurrent |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAccrued Compensation, Current
| Name: |
vtl_AccruedCompensationCurrent |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAccrued Legal And Audit Costs, Current
| Name: |
vtl_AccruedLegalAndAuditCostsCurrent |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.24.3
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionIndicates line item in statement of financial position that includes current operating lease liability. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-2
| Name: |
us-gaap_OperatingLeaseLiabilityCurrentStatementOfFinancialPositionExtensibleList |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
enum2:enumerationSetItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of liabilities classified as other, due within one year or the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
| Name: |
us-gaap_OtherLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionObligations not otherwise itemized or categorized in the footnotes to the financial statements that are due within one year or operating cycle, if longer, from the balance sheet date. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 470
-SubTopic 10
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481573/470-10-45-10
| Name: |
us-gaap_OtherSundryLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.24.3
Commitments and Contingencies - Additional Information (Details) - USD ($)
$ in Thousands |
3 Months Ended |
9 Months Ended |
Sep. 30, 2024 |
Sep. 30, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
| Loss Contingencies [Line Items] |
|
|
|
|
| Operating and variable lease costs |
$ 260
|
$ 209
|
$ 787
|
$ 642
|
| Contractual obligation |
$ 3,200
|
|
$ 3,200
|
|
| New York City |
|
|
|
|
| Loss Contingencies [Line Items] |
|
|
|
|
| Renewal option period (in months) |
27 months
|
|
27 months
|
|
| Rent holiday period (in months) |
|
|
6 months
|
|
| Lessee, operating lease, extended rent holiday period (in months) |
|
|
3 months
|
|
| Incremental borrowing rate on operating leases |
6.00%
|
|
6.00%
|
|
| Grafelfing, Germany |
|
|
|
|
| Loss Contingencies [Line Items] |
|
|
|
|
| Lease term (in months) |
5 years
|
|
5 years
|
|
| Planegg, Germany |
|
|
|
|
| Loss Contingencies [Line Items] |
|
|
|
|
| Additions to right-of-use assets |
|
|
$ 544
|
|
| Incremental borrowing rate on operating leases |
8.00%
|
|
8.00%
|
|
| X |
- DefinitionAmount of contractual obligation, including, but not limited to, long-term debt, lease obligation, purchase obligation, and other commitments. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-3
| Name: |
us-gaap_ContractualObligation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTerm of lessee's operating lease renewal, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-3
| Name: |
us-gaap_LesseeOperatingLeaseRenewalTerm |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTerm of lessee's operating lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-3
| Name: |
us-gaap_LesseeOperatingLeaseTermOfContract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483076/450-20-50-4
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483076/450-20-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 720
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483359/720-20-50-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 460
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 27
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482395/460-10-55-27
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 450
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483076/450-20-50-9
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 450
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483076/450-20-50-4
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 460
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482425/460-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 450
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483076/450-20-50-1
| Name: |
us-gaap_LossContingenciesLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIncrease (Decrease) In Operating Lease, Right-Of-Use Asset
| Name: |
vtl_IncreaseDecreaseInOperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionLessee, Operating Lease, Beginning of Lease, Rent Holiday Period
| Name: |
vtl_LesseeOperatingLeaseBeginningOfLeaseRentHolidayPeriod |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLessee, Operating Lease, Extension of Lease, Rent Holiday Period
| Name: |
vtl_LesseeOperatingLeaseExtensionOfLeaseRentHolidayPeriod |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionOperating And Variable Leases, Cost
| Name: |
vtl_OperatingAndVariableLeasesCost |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionOperating Lease, Incremental Borrowing Rate
| Name: |
vtl_OperatingLeaseIncrementalBorrowingRate |
| Namespace Prefix: |
vtl_ |
| Data Type: |
dtr-types1:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_StatementGeographicalAxis=vtl_NewYorkCityMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_StatementGeographicalAxis=vtl_GrafelfingGermanyMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_StatementGeographicalAxis=vtl_PlaneggGermanyMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in next fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueNextTwelveMonths |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in fourth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearFour |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in third fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearThree |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in second fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearTwo |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease having initial or remaining lease term in excess of one year to be paid in remainder of current fiscal year. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsRemainderOfFiscalYear |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payments in excess of discounted obligation for lease payments for operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityUndiscountedExcessAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionLessee Operating Lease Liability Payments Due After Year Four
| Name: |
vtl_LesseeOperatingLeaseLiabilityPaymentsDueAfterYearFour |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.24.3
Fair Value - Schedule of Financial Assets and Liabilities Measured at Fair Value on Recurring Basis (Details) - Fair Value, Measurements, Recurring - USD ($)
$ in Thousands |
Sep. 30, 2024 |
Dec. 31, 2023 |
| Assets |
|
|
| Total assets at fair value |
$ 40,359
|
$ 34,087
|
| Level 1 |
|
|
| Assets |
|
|
| Total assets at fair value |
40,359
|
34,087
|
| Level 2 |
|
|
| Assets |
|
|
| Total assets at fair value |
0
|
0
|
| Level 3 |
|
|
| Assets |
|
|
| Total assets at fair value |
0
|
0
|
| Money market funds |
|
|
| Assets |
|
|
| Total assets at fair value |
40,359
|
34,087
|
| Money market funds | Level 1 |
|
|
| Assets |
|
|
| Total assets at fair value |
40,359
|
34,087
|
| Money market funds | Level 2 |
|
|
| Assets |
|
|
| Total assets at fair value |
0
|
0
|
| Money market funds | Level 3 |
|
|
| Assets |
|
|
| Total assets at fair value |
$ 0
|
$ 0
|
| X |
- DefinitionFair value portion of asset recognized for present right to economic benefit. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 100
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482078/820-10-55-100
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
| Name: |
us-gaap_AssetsFairValueDisclosure |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsFairValueDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_FairValueByMeasurementFrequencyAxis=us-gaap_FairValueMeasurementsRecurringMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_InvestmentTypeAxis=us-gaap_MoneyMarketFundsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
Fair Value - Additional Information (Detail) - USD ($)
$ in Millions |
|
|
9 Months Ended |
|
Mar. 04, 2024 |
Jan. 08, 2024 |
Sep. 30, 2024 |
Dec. 31, 2023 |
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
|
|
| Tranche right, fair value |
|
$ 23.6
|
|
|
| Expected dividend yield (as percent) |
0.00%
|
0.00%
|
0.00%
|
|
| Common stock, shares authorized (in shares) |
500,000,000
|
130,000,000
|
500,000,000
|
130,000,000
|
| Reclassification of future tranche right liability upon settlement |
$ 4.8
|
|
|
|
| Minimum |
|
|
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
|
|
| Risk-free interest rate (as percent) |
4.16%
|
3.93%
|
|
|
| Expected volatility (as percent) |
90.00%
|
105.00%
|
|
|
| Expected term of options (years) |
1 year 7 months 28 days
|
1 year 9 months 21 days
|
|
|
| Common stock, shares authorized (in shares) |
130,000,000
|
|
|
|
| Maximum |
|
|
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
|
|
| Risk-free interest rate (as percent) |
4.63%
|
4.36%
|
|
|
| Expected volatility (as percent) |
105.00%
|
115.00%
|
|
|
| Expected term of options (years) |
4 years 7 months 28 days
|
4 years 9 months 21 days
|
|
|
| Common stock, shares authorized (in shares) |
500,000,000
|
|
|
|
| United States |
|
|
|
|
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] |
|
|
|
|
| Investment interest rate (as percent) |
|
|
4.80%
|
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 100
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482078/820-10-55-100
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-3
| Name: |
us-gaap_FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionRate of interest on investment. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 55
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477439/946-210-55-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481800/320-10-50-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481800/320-10-50-5
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.12-12A(Column A)(Footnote 2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.12-12A(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column A)(Footnote 2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column A)(Footnote 4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 4)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477271/946-320-S99-6
| Name: |
us-gaap_InvestmentInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe estimated dividend rate (a percentage of the share price) to be paid (expected dividends) to holders of the underlying shares over the option's term. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedDividendRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated measure of the percentage by which a share price is expected to fluctuate during a period. Volatility also may be defined as a probability-weighted measure of the dispersion of returns about the mean. The volatility of a share price is the standard deviation of the continuously compounded rates of return on the share over a specified period. That is the same as the standard deviation of the differences in the natural logarithms of the stock prices plus dividends, if any, over the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe risk-free interest rate assumption that is used in valuing an option on its own shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExpected term of award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFair Value, Reclassification of Future Tranche Right Liability Upon Settlement
| Name: |
vtl_FairValueReclassificationOfFutureTrancheRightLiabilityUponSettlement |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTranche Right, Fair Value Disclosure
| Name: |
vtl_TrancheRightFairValueDisclosure |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_StatementGeographicalAxis=country_US |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
Fair Value - Schedule of Fair Value of Tranche Rights (Details)
|
3 Months Ended |
|
Mar. 31, 2024
USD ($)
|
|---|
| Fair Value, Liabilities Measured on Recurring Basis, Unobservable Input Reconciliation, Calculation [Roll Forward] |
|
| December 31, 2023 |
$ 0
|
| Fair value as of January 8, 2024 |
23,600
|
| Change in fair value through March 4, 2024 |
4,796
|
| Reclassification to equity |
(28,396)
|
| March 31, 2024 |
$ 0
|
| X |
- DefinitionThe increase (decrease) during the period in the carrying value of derivative instruments reported as liabilities that are due to be disposed of within one year (or the normal operating cycle, if longer). Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInDerivativeLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionReclassification to Equity
| Name: |
vtl_ReclassificationToEquity |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.24.3
Common Stock - Shelf Registration Statement (Details)
$ / shares in Units, $ in Thousands |
3 Months Ended |
9 Months Ended |
|
|
|
|
|
Sep. 30, 2023
USD ($)
$ / shares
shares
|
Sep. 30, 2024
USD ($)
d
$ / shares
shares
|
Sep. 30, 2023
USD ($)
$ / shares
shares
|
May 31, 2024
USD ($)
|
Nov. 30, 2023
USD ($)
|
May 31, 2022
USD ($)
|
Dec. 31, 2020
USD ($)
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
| Maximum aggregate offering price of common stock, preferred stock, warrants, debt securities, and/or units |
|
|
|
|
$ 250,000
|
|
|
| Unsold Securities To Be Sold Under Shelf Registration |
|
$ 412,300
|
|
|
|
|
|
| Proceeds from issuance of common stock at premium |
|
191
|
$ 282
|
|
|
|
|
| December 2020 ATM |
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
| Maximum aggregate offering price of common stock, preferred stock, warrants, debt securities, and/or units |
|
|
|
|
|
|
$ 50,000
|
| Shares issued |
$ 300
|
$ 200
|
$ 300
|
|
|
|
|
| Stock issued (in shares) | shares |
107,012
|
150,000
|
107,012
|
|
|
|
|
| Issuance of stock (in USD per share) | $ / shares |
$ 2.72
|
$ 1.31
|
$ 2.72
|
|
|
|
|
| Proceeds from issuance of common stock at premium |
$ 300
|
$ 200
|
$ 300
|
|
|
|
|
| Underwriter commissions |
$ 9
|
$ 6
|
$ 9
|
|
|
|
|
| May 2022 ATM |
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
| Maximum aggregate offering price of common stock, preferred stock, warrants, debt securities, and/or units |
|
|
|
|
|
$ 80,000
|
|
| May 2024 ATM |
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
| Maximum aggregate offering price of common stock, preferred stock, warrants, debt securities, and/or units |
|
|
|
$ 80,000
|
|
|
|
| Shelf registration, termination, prior written notice, number of days | d |
|
10
|
|
|
|
|
|
| Sale of stock, remaining capacity |
|
$ 80,000
|
|
|
|
|
|
| Commission, percent of gross proceeds from sale of common stock |
|
3.00%
|
|
|
|
|
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/recommendedDisclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483014/272-10-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482987/272-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-14
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-18
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(27)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ClassOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of cash paid for commissions during the current period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (g)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-25
| Name: |
us-gaap_PaymentsForCommissions |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the additional capital contribution to the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionPer share or per unit amount of equity securities issued.
| Name: |
us-gaap_SharesIssuedPricePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of new stock issued during the period. Includes shares issued in an initial public offering or a secondary public offering. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478009/946-205-45-4
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478448/946-505-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionMaximum Aggregate Offering Price Of Securities Under Shelf Registration
| Name: |
vtl_MaximumAggregateOfferingPriceOfSecuritiesUnderShelfRegistration |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSale Of Stock, Remaining Capacity
| Name: |
vtl_SaleOfStockRemainingCapacity |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSales of Stock, Commissions, Percent Of Gross Proceeds
| Name: |
vtl_SalesOfStockCommissionsPercentOfGrossProceeds |
| Namespace Prefix: |
vtl_ |
| Data Type: |
dtr-types1:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionShelf Registration, Termination, Prior Written Notice, Number of Days
| Name: |
vtl_ShelfRegistrationTerminationPriorWrittenNoticeNumberOfDays |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionUnsold Securities Rolled Over To Be Sold Under New Shelf Registration
| Name: |
vtl_UnsoldSecuritiesRolledOverToBeSoldUnderNewShelfRegistration |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=vtl_December2020ATMMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=vtl_May2022ATMMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=vtl_May2024ATMMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
Common Stock - Equity Offering (Details)
$ / shares in Units, $ in Thousands |
|
|
3 Months Ended |
9 Months Ended |
105 Months Ended |
|
|
|
|
Jan. 08, 2024
USD ($)
$ / shares
shares
|
Jan. 04, 2024
USD ($)
tranche
$ / shares
|
Sep. 30, 2024
USD ($)
shares
|
Sep. 30, 2023
USD ($)
|
Sep. 30, 2024
USD ($)
shares
|
Sep. 30, 2023
USD ($)
|
Sep. 30, 2024
USD ($)
shares
|
Mar. 04, 2024
USD ($)
shares
|
Mar. 03, 2024
shares
|
Dec. 31, 2023
shares
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Proceeds from issuance of private placement |
|
|
|
|
|
|
$ 430,900
|
|
|
|
| Reclassification of future tranche right liability upon settlement |
|
|
|
|
|
|
|
$ 4,800
|
|
|
| Common stock, shares authorized (in shares) | shares |
130,000,000
|
|
500,000,000
|
|
500,000,000
|
|
500,000,000
|
500,000,000
|
|
130,000,000
|
| Change in fair value of the tranche rights |
|
|
$ 0
|
$ 0
|
$ 4,796
|
$ 0
|
|
|
|
|
| Other Nonoperating Income (Expense) |
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Change in fair value of the tranche rights |
|
|
|
|
1,700
|
|
|
|
|
|
| Equity |
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Change in fair value of the tranche rights |
|
|
|
|
$ 4,000
|
|
|
|
|
|
| Minimum |
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Common stock, shares authorized (in shares) | shares |
|
|
|
|
|
|
|
130,000,000
|
|
|
| Maximum |
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Common stock, shares authorized (in shares) | shares |
|
|
|
|
|
|
|
500,000,000
|
|
|
| Common Stock | Common Stock |
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Reclassification of future tranche right liability upon settlement |
$ 23,600
|
|
|
|
|
|
|
|
|
|
| Common stock, shares authorized (in shares) | shares |
|
|
|
|
|
|
|
500,000,000
|
130,000,000
|
|
| Private Placement | Common Stock |
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Private placement, number of tranche | tranche |
|
3
|
|
|
|
|
|
|
|
|
| Sale of stock, price per share (in dollars per share) | $ / shares |
|
$ 0.0001
|
|
|
|
|
|
|
|
|
| Private Placement | Common Stock | Tranche 1 |
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Proceeds from issuance of private placement |
$ 80,000
|
|
|
|
|
|
|
|
|
|
| Private Placement | Common Stock | Tranche 2 |
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Proceeds from issuance of private placement |
|
$ 80,000
|
|
|
|
|
|
|
|
|
| Private Placement | Common Stock | Tranche 3 |
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Proceeds from issuance of private placement |
|
$ 80,000
|
|
|
|
|
|
|
|
|
| Private Placement | Pre-Funded Warrants |
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Sale of stock, price per share (in dollars per share) | $ / shares |
|
$ 0.0001
|
|
|
|
|
|
|
|
|
| Private Placement | Accredited Investors |
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Proceeds from issuance of private placement |
|
$ 240,000
|
|
|
|
|
|
|
|
|
| Private Placement | Accredited Investors | Common Stock | Tranche 1 |
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Sale of stock, price per share (in dollars per share) | $ / shares |
$ 1.43
|
|
|
|
|
|
|
|
|
|
| Consideration received on transaction |
$ 80,000
|
|
|
|
|
|
|
|
|
|
| Number of shares registered for resale | shares |
55,944,850
|
|
|
|
|
|
|
|
|
|
| Private Placement | Accredited Investors | Common Stock | Tranche 2 |
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Sale of stock, price per share (in dollars per share) | $ / shares |
|
$ 1.716
|
|
|
|
|
|
|
|
|
| Consideration received on transaction |
|
$ 80,000
|
|
|
|
|
|
|
|
|
| Sale of stock, consideration received, percentage |
|
120.00%
|
|
|
|
|
|
|
|
|
| Number of tranche subjected to condition | tranche |
|
3
|
|
|
|
|
|
|
|
|
| Threshold trading days for weighted average price calculation |
|
10 days
|
|
|
|
|
|
|
|
|
| Weighted average price (in USD per share) | $ / shares |
|
$ 8.00
|
|
|
|
|
|
|
|
|
| Threshold trading days for aggregate trading volume calculation |
|
10 days
|
|
|
|
|
|
|
|
|
| Aggregate trading volume of last 10 days |
|
$ 100,000
|
|
|
|
|
|
|
|
|
| Private Placement | Accredited Investors | Common Stock | Tranche 3 |
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Consideration received on transaction |
|
$ 80,000
|
|
|
|
|
|
|
|
|
| Third tranche close period after second tranche (in years) |
|
3 years
|
|
|
|
|
|
|
|
|
| Common Stock and Pre-funded Warrants | Common Stock and Pre-funded Warrants |
|
|
|
|
|
|
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Tranche rights as a liability, remainder amount |
$ 80,000
|
|
|
|
|
|
|
|
|
|
| Tranche rights as a liability, received amount |
$ 56,400
|
|
|
|
|
|
|
|
|
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/recommendedDisclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483014/272-10-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482987/272-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-14
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-18
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(27)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ClassOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe cash inflow associated with the amount received from entity's raising of capital via private rather than public placement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfPrivatePlacement |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionCash received on stock transaction after deduction of issuance costs.
| Name: |
us-gaap_SaleOfStockConsiderationReceivedOnTransaction |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionPer share amount received by subsidiary or equity investee for each share of common stock issued or sold in the stock transaction.
| Name: |
us-gaap_SaleOfStockPricePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionCommon Stock, Number Of Shares Registered For Resale
| Name: |
vtl_CommonStockNumberOfSharesRegisteredForResale |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionFair Value, Change in Fair Value of Tranche Rights, Transaction Costs
| Name: |
vtl_FairValueChangeInFairValueOfTrancheRightsTransactionCosts |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionFair Value, Reclassification of Future Tranche Right Liability Upon Settlement
| Name: |
vtl_FairValueReclassificationOfFutureTrancheRightLiabilityUponSettlement |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSale of Stock, Aggregate Trading Volume of Last 10 Days
| Name: |
vtl_SaleOfStockAggregateTradingVolumeOfLast10Days |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionSale of Stock, Consideration Received, Percentage
| Name: |
vtl_SaleOfStockConsiderationReceivedPercentage |
| Namespace Prefix: |
vtl_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionSale of Stock, Equity Offerings, Period
| Name: |
vtl_SaleOfStockEquityOfferingsPeriod |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionSale of Stock, Number of Tranche Conditions
| Name: |
vtl_SaleOfStockNumberOfTrancheConditions |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionSale of Stock, Private Placement, Number of Tranche
| Name: |
vtl_SaleOfStockPrivatePlacementNumberOfTranche |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThreshold Trading Days for Aggregate Trading Volume Calculation
| Name: |
vtl_ThresholdTradingDaysForAggregateTradingVolumeCalculation |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThreshold Trading Days for Weighted Average Price Calculation
| Name: |
vtl_ThresholdTradingDaysForWeightedAveragePriceCalculation |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTranche Rights as a Liability, Received Amount
| Name: |
vtl_TrancheRightsAsALiabilityReceivedAmount |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionTranche Rights as a Liability, Remainder Amount
| Name: |
vtl_TrancheRightsAsALiabilityRemainderAmount |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted Average Price of Last 10 Days
| Name: |
vtl_WeightedAveragePriceOfLast10Days |
| Namespace Prefix: |
vtl_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementOfFinancialPositionLocationActivityCapitalizationAxis=us-gaap_OtherNonoperatingIncomeExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementOfFinancialPositionLocationActivityCapitalizationAxis=us-gaap_EquityMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=us-gaap_CommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=us-gaap_PrivatePlacementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_VestingAxis=us-gaap_ShareBasedCompensationAwardTrancheOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_VestingAxis=us-gaap_ShareBasedCompensationAwardTrancheTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_VestingAxis=us-gaap_ShareBasedCompensationAwardTrancheThreeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=vtl_PreFundedWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=vtl_AccreditedInvestorsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=vtl_CommonStockAndPreFundedWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=vtl_CommonStockAndPreFundedWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
Common Stock - Common Stock (Details)
|
9 Months Ended |
|
|
|
|
Sep. 30, 2024
numberOfVote
$ / shares
shares
|
Mar. 04, 2024
$ / shares
shares
|
Jan. 08, 2024
shares
|
Dec. 31, 2023
$ / shares
shares
|
| Class of Stock [Line Items] |
|
|
|
|
| Common stock, shares authorized (in shares) | shares |
500,000,000
|
500,000,000
|
130,000,000
|
130,000,000
|
| Common stock, par value (in USD per share) | $ / shares |
$ 0.0001
|
$ 0.0001
|
|
$ 0.0001
|
| Number of votes per each share of common stock | numberOfVote |
1
|
|
|
|
| Common stock, cash dividends declared (in USD per share) | $ / shares |
$ 0
|
|
|
|
| Common stock, cash dividends paid (in USD per share) | $ / shares |
$ 0
|
|
|
|
| Minimum |
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
| Common stock, shares authorized (in shares) | shares |
|
130,000,000
|
|
|
| Maximum |
|
|
|
|
| Class of Stock [Line Items] |
|
|
|
|
| Common stock, shares authorized (in shares) | shares |
|
500,000,000
|
|
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/recommendedDisclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483014/272-10-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482987/272-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-14
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-18
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(27)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ClassOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate dividends paid during the period for each share of common stock outstanding. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_CommonStockDividendsPerShareCashPaid |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate dividends declared during the period for each share of common stock outstanding. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_CommonStockDividendsPerShareDeclared |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of Votes Per Each Share of Common Stock
| Name: |
vtl_NumberOfVotesPerEachShareOfCommonStock |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/recommendedDisclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483014/272-10-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482987/272-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-14
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-18
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(27)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ClassOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of warrants or rights outstanding.
| Name: |
us-gaap_ClassOfWarrantOrRightOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=vtl_PreFundedWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
| X |
- References
+ Details
| Name: |
us-gaap_EquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFace amount or stated value per share of preferred stock nonredeemable or redeemable solely at the option of the issuer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
| Name: |
us-gaap_PreferredStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_PreferredStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate share number for all nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer) held by stockholders. Does not include preferred shares that have been repurchased. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
| Name: |
us-gaap_PreferredStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
v3.24.3
Common Stock - Schedule of Shares Reserved for Future Issuance (Details)
|
Sep. 30, 2024
shares
|
| Class of Stock [Line Items] |
|
| Total common shares reserved for future issuance (in shares) |
124,929,746
|
| Tranche 2 |
|
| Class of Stock [Line Items] |
|
| Total common shares reserved for future issuance (in shares) |
46,620,046
|
| Tranche 3 |
|
| Class of Stock [Line Items] |
|
| Total common shares reserved for future issuance (in shares) |
46,620,046
|
| Outstanding stock options |
|
| Class of Stock [Line Items] |
|
| Total common shares reserved for future issuance (in shares) |
11,546,138
|
| Outstanding stock options | 2021 Employee Stock Purchase Plan |
|
| Class of Stock [Line Items] |
|
| Total common shares reserved for future issuance (in shares) |
1,000,011
|
| Pre-Funded Warrants |
|
| Class of Stock [Line Items] |
|
| Total common shares reserved for future issuance (in shares) |
11,193,564
|
| Common stock options available for future grant: | 2017 Inducement Equity Incentive Plan |
|
| Class of Stock [Line Items] |
|
| Total common shares reserved for future issuance (in shares) |
46,250
|
| Common stock options available for future grant: | 2019 Omnibus Equity Incentive Plan |
|
| Class of Stock [Line Items] |
|
| Total common shares reserved for future issuance (in shares) |
7,903,691
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/recommendedDisclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483014/272-10-45-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 272
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482987/272-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-14
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-18
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(27)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(2)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ClassOfStockLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAggregate number of common shares reserved for future issuance. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockCapitalSharesReservedForFutureIssuance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_VestingAxis=us-gaap_ShareBasedCompensationAwardTrancheTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_VestingAxis=us-gaap_ShareBasedCompensationAwardTrancheThreeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_EmployeeStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=vtl_A2021EmployeeStockPurchasePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=vtl_PreFundedWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=vtl_EmployeeStockOptionsforFutureGrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=vtl_A2017InducementEquityIncentivePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=vtl_TwoThousandNineteenOmnibusEquityIncentivePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
Stock-Based Compensation Plans - Additional Information (Details) - USD ($)
|
|
|
|
|
|
1 Months Ended |
3 Months Ended |
9 Months Ended |
48 Months Ended |
|
Mar. 04, 2024 |
Jan. 08, 2024 |
Jun. 28, 2023 |
Aug. 01, 2021 |
Apr. 25, 2021 |
Jul. 31, 2019 |
Sep. 30, 2024 |
Sep. 30, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
Dec. 31, 2023 |
Sep. 30, 2017 |
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Options repriced (in shares) |
3,317,596
|
|
|
|
|
|
|
|
|
|
|
|
| Stock-based compensation, exercise price (in dollars per share) |
$ 1.72
|
|
|
|
|
|
|
|
|
|
|
|
| Repriced cost recognized over remaining term |
$ 1,200,000
|
|
|
|
|
|
|
|
|
|
|
|
| Repricing cost during the period |
|
|
|
|
|
|
$ 86,000
|
|
$ 1,000,000
|
|
|
|
| Expected dividend yield (as percent) |
0.00%
|
0.00%
|
|
|
|
|
|
|
0.00%
|
|
|
|
| Period over which compensation cost will be recognized (in years) |
|
|
|
|
|
|
|
|
2 years 9 months 25 days
|
|
|
|
| Maximum |
|
|
|
|
|
|
|
|
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Expected term of options (years) |
4 years 7 months 28 days
|
4 years 9 months 21 days
|
|
|
|
|
|
|
|
|
|
|
| Exercise price of warrants (in USD per share) |
$ 3.00
|
|
|
|
|
|
|
|
|
|
|
|
| Minimum |
|
|
|
|
|
|
|
|
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Expected term of options (years) |
1 year 7 months 28 days
|
1 year 9 months 21 days
|
|
|
|
|
|
|
|
|
|
|
| Exercise price of warrants (in USD per share) |
$ 1.72
|
|
|
|
|
|
|
|
|
|
|
|
| 2021 Employee Stock Purchase Plan |
|
|
|
|
|
|
|
|
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Maximum annual contributions per employee (as a percent) |
|
|
|
|
15.00%
|
|
|
|
|
|
|
|
| Purchase price of the stock, at discount from market price at purchase date (as a percent) |
|
|
|
|
85.00%
|
|
|
|
|
|
|
|
| Shares available for grant (in shares) |
1,200,000
|
|
|
|
200,000
|
|
|
|
|
|
|
|
| Additional shares authorized (in shares) |
1,000,000
|
|
|
|
|
|
|
|
|
|
|
|
| Shares issued under the ESPP (in shares) |
|
|
|
199,989
|
|
|
|
|
|
|
|
|
| Share-based compensation expense |
|
|
|
|
|
|
24,000
|
$ 19,000
|
$ 24,000
|
$ 102,000
|
|
|
| 2019 Omnibus Equity Incentive Plan |
|
|
|
|
|
|
|
|
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Shares available for grant (in shares) |
|
|
|
|
|
1,500,000
|
|
|
|
|
|
|
| Expected term of options (years) |
|
|
|
|
|
|
|
|
5 years 11 months 12 days
|
6 years 3 days
|
|
|
| Expected dividend yield (as percent) |
|
|
|
|
|
|
|
|
0.00%
|
0.00%
|
|
|
| Options granted in period, weighted-average grant date fair value (in USD per share) |
|
|
|
|
|
|
|
|
$ 0.99
|
$ 1.32
|
|
|
| Total unrecognized compensation expense |
|
|
|
|
|
|
$ 10,800,000
|
|
$ 10,800,000
|
|
|
|
| 2019 Omnibus Equity Incentive Plan | Incentive stock options |
|
|
|
|
|
|
|
|
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Vesting period (in years) |
|
|
|
|
|
|
|
|
4 years
|
|
|
|
| 2019 Omnibus Equity Incentive Plan | Maximum |
|
|
|
|
|
|
|
|
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Expected term of options (years) |
|
|
|
|
|
|
|
|
10 years
|
|
|
|
| 2019 Omnibus Equity Incentive Plan | Maximum | Non-statutory options |
|
|
|
|
|
|
|
|
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Vesting period (in years) |
|
|
|
|
|
|
|
|
4 years
|
|
|
|
| 2019 Omnibus Equity Incentive Plan | Minimum | Non-statutory options |
|
|
|
|
|
|
|
|
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Vesting period (in years) |
|
|
|
|
|
|
|
|
3 years
|
|
|
|
| 2019 Omnibus Equity Incentive Plan, Evergreen Provision |
|
|
|
|
|
|
|
|
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Additional shares authorized (in shares) |
|
|
|
|
|
4,900,000
|
|
|
|
|
4,408,871
|
|
| Additional shares authorized, percent |
|
|
|
|
|
4.00%
|
|
|
|
|
|
|
| Increase in other share (in shares) |
9,100,000
|
|
4,440,000
|
|
|
|
|
|
|
|
|
|
| 2014 Equity Incentive Plan |
|
|
|
|
|
|
|
|
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Shares available for grant (in shares) |
|
|
|
|
|
|
0
|
|
0
|
|
|
|
| 2017 Inducement Equity Incentive Plan |
|
|
|
|
|
|
|
|
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] |
|
|
|
|
|
|
|
|
|
|
|
|
| Shares available for grant (in shares) |
|
|
|
|
|
|
|
|
|
|
|
46,250
|
| Share-based compensation expense |
|
|
|
|
|
|
$ 0
|
$ 0
|
$ 0
|
$ 0
|
|
|
| X |
- DefinitionAmount of expense for award under share-based payment arrangement. Excludes amount capitalized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.F)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479830/718-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_AllocatedShareBasedCompensationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionMaximum percentage of employee gross pay the employee may contribute to a defined contribution plan.
| Name: |
us-gaap_DefinedContributionPlanMaximumAnnualContributionsPerEmployeePercent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cost not yet recognized for nonvested award under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted-average period over which cost not yet recognized is expected to be recognized for award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedPeriodForRecognition1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPeriod over which grantee's right to exercise award under share-based payment arrangement is no longer contingent on satisfaction of service or performance condition, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Includes, but is not limited to, combination of market, performance or service condition. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardAwardVestingPeriod1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDiscount rate from fair value on purchase date that participants pay for shares. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardDiscountFromMarketPricePurchaseDate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of grants made during the period on other than stock (or unit) option plans (for example, phantom stock or unit plan, stock or unit appreciation rights plan, performance target plan). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsGrantsInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated dividend rate (a percentage of the share price) to be paid (expected dividends) to holders of the underlying shares over the option's term. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedDividendRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of additional shares authorized for issuance under share-based payment arrangement.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfAdditionalSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe difference between the maximum number of shares (or other type of equity) authorized for issuance under the plan (including the effects of amendments and adjustments), and the sum of: 1) the number of shares (or other type of equity) already issued upon exercise of options or other equity-based awards under the plan; and 2) shares (or other type of equity) reserved for issuance on granting of outstanding awards, net of cancellations and forfeitures, if applicable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfSharesAvailableForGrant |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe weighted average grant-date fair value of options granted during the reporting period as calculated by applying the disclosed option pricing methodology. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionOther than shares newly issued, the number of additional shares issued (for example, a stock split) or canceled (for example, to correct a share issuance), during the period under the plan. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOtherShareIncreaseDecrease |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAn excess of the fair value of the modified award over the fair value of the award immediately before the modification. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardPlanModificationIncrementalCompensationCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average price at which option holders acquired shares when converting their stock options into shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsExercisesInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExpected term of award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionShare-based Compensation Arrangement By Share-Based Payment Award, Additional Shares Authorized, Percent
| Name: |
vtl_ShareBasedCompensationArrangementByShareBasedPaymentAwardAdditionalSharesAuthorizedPercent |
| Namespace Prefix: |
vtl_ |
| Data Type: |
dtr-types1:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionShare-Based Compensation By Share-Based Payment Award, Options, Repriced, Amortization Cost over Remaining Term
| Name: |
vtl_ShareBasedCompensationByShareBasedPaymentAwardOptionsRepricedAmortizationCostOverRemainingTerm |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionShare-Based Compensation By Share-Based Payment Award, Options, Repriced, Number Of Shares
| Name: |
vtl_ShareBasedCompensationByShareBasedPaymentAwardOptionsRepricedNumberOfShares |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionShare-based Payment Arrangement, Plan Modification, Option, Exercise Price
| Name: |
vtl_ShareBasedPaymentArrangementPlanModificationOptionExercisePrice |
| Namespace Prefix: |
vtl_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=vtl_A2021EmployeeStockPurchasePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=vtl_A2019OmnibusEquityIncentivePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=vtl_IncentiveEmployeeStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=vtl_NonStatutoryEmployeeStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=vtl_TwoThousandNineteenOmnibusEquityIncentivePlanEvergreenProvisionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=vtl_TwoThousandFourteenEquityIncentivePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=vtl_A2017InducementEquityIncentivePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
Stock-Based Compensation Plans - Schedule of Stock Option Activity (Details) - Outstanding stock options - 2019 Omnibus Equity Incentive Plan - USD ($)
|
9 Months Ended |
Sep. 30, 2024 |
Sep. 30, 2023 |
| Options |
|
|
| Outstanding, beginning balance (in shares) |
6,196,140
|
3,791,688
|
| Granted (in shares) |
5,715,349
|
2,740,564
|
| Exercised (in shares) |
0
|
0
|
| Repricing Modification (in shares) |
0
|
|
| Forfeited or expired (in shares) |
(365,351)
|
(268,342)
|
| Outstanding, ending balance (in shares) |
11,546,138
|
6,263,910
|
| Options vested and expected to vest, Ending balance (in shares) |
11,546,138
|
6,263,910
|
| Options exercisable, Ending balance (in shares) |
4,171,969
|
2,393,294
|
| Weighted- Average Exercise Price |
|
|
| Outstanding, beginning balance (usd per share) |
$ 7.15
|
$ 11.33
|
| Granted (usd per share) |
1.23
|
1.71
|
| Exercised (usd per share) |
0
|
0
|
| Repricing Modifications (usd per share) |
9.55
|
|
| Forfeited or expired (usd per share) |
2.08
|
7.80
|
| Outstanding, ending balance (usd per share) |
1.63
|
7.27
|
| Options vested and expected to vest, Weighted-Average Exercise Price, Ending balance (usd per share) |
1.63
|
7.27
|
| Options exercisable, Weighted-Average Exercise Price, Ending balance (usd per share) |
$ 2.09
|
$ 11.67
|
| Other Disclosures |
|
|
| Outstanding, Weighted-Average Remaining Contractual Term, Ending balance |
8 years 3 months 29 days
|
8 years 3 months 7 days
|
| Options vested and expected to vest , Weighted-Average Remaining Contractual Term, Ending balance |
8 years 3 months 29 days
|
8 years 3 months 7 days
|
| Options exercisable, Weighted-Average Remaining Contractual Term, Ending balance |
7 years 25 days
|
7 years 1 month 13 days
|
| Outstanding, Aggregate Intrinsic Value, Ending balance |
$ 2,794,286
|
$ 119,691
|
| Options vested and expected to vest , Aggregate Intrinsic Value, Ending balance |
2,794,286
|
119,691
|
| Options exercisable, Aggregate Intrinsic Value, Ending balance |
$ 329,830
|
$ 6,160
|
| X |
- References
+ Details
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsAdditionalDisclosuresAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares into which fully or partially vested stock options outstanding as of the balance sheet date can be currently converted under the option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe weighted-average price as of the balance sheet date at which grantees can acquire the shares reserved for issuance on vested portions of options outstanding and currently exercisable under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionFor presentations that combine terminations, the number of shares under options that were cancelled during the reporting period as a result of occurrence of a terminating event specified in contractual agreements pertaining to the stock option plan or that expired. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresAndExpirationsInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average price of options that were either forfeited or expired. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresAndExpirationsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount by which the current fair value of the underlying stock exceeds the exercise price of options outstanding. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingIntrinsicValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionA roll forward is a reconciliation of a concept from the beginning of a period to the end of a period.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingRollForward |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePriceRollforward |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount by which current fair value of underlying stock exceeds exercise price of fully vested and expected to vest options outstanding. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestOutstandingAggregateIntrinsicValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of fully vested and expected to vest options outstanding that can be converted into shares under option plan. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted-average exercise price, at which grantee can acquire shares reserved for issuance, for fully vested and expected to vest options outstanding. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which option holders acquired shares when converting their stock options into shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsExercisesInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average per share amount at which grantees can acquire shares of common stock by exercise of options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of difference between fair value of the underlying shares reserved for issuance and exercise price of vested portions of options outstanding and currently exercisable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsExercisableIntrinsicValue1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average remaining contractual term for vested portions of options outstanding and currently exercisable or convertible, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsExercisableWeightedAverageRemainingContractualTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average remaining contractual term for option awards outstanding, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (e)(1)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsOutstandingWeightedAverageRemainingContractualTerm2 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average remaining contractual term for fully vested and expected to vest exercisable or convertible options, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsVestedAndExpectedToVestExercisableWeightedAverageRemainingContractualTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of share options (or share units) exercised during the current period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionShare-based Compensation Arrangement by Share-based Payment Award, Options, Repricing Modification in Period
| Name: |
vtl_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsRepricingModificationInPeriod |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionShare-based Compensation Arrangements by Share-based Payment Award, Options, Exercises in Period, Weighted Average Repricing Modifications Price
| Name: |
vtl_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsExercisesInPeriodWeightedAverageRepricingModificationsPrice |
| Namespace Prefix: |
vtl_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_EmployeeStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=vtl_TwoThousandNineteenOmnibusEquityIncentivePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
| X |
- DefinitionThe estimated dividend rate (a percentage of the share price) to be paid (expected dividends) to holders of the underlying shares over the option's term. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedDividendRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated measure of the percentage by which a share price is expected to fluctuate during a period. Volatility also may be defined as a probability-weighted measure of the dispersion of returns about the mean. The volatility of a share price is the standard deviation of the continuously compounded rates of return on the share over a specified period. That is the same as the standard deviation of the differences in the natural logarithms of the stock prices plus dividends, if any, over the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe risk-free interest rate assumption that is used in valuing an option on its own shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExpected term of award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=vtl_A2019OmnibusEquityIncentivePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
Stock-Based Compensation Plans - Schedule of Stock-based Compensation Expense for Stock Awards Recognized (Details) - USD ($)
$ in Thousands |
3 Months Ended |
9 Months Ended |
Sep. 30, 2024 |
Sep. 30, 2023 |
Sep. 30, 2024 |
Sep. 30, 2023 |
| Share-based Compensation Arrangement by Share-based Payment Award, Compensation Cost [Line Items] |
|
|
|
|
| Total |
|
|
$ 6,542
|
$ 5,464
|
| Employees |
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Compensation Cost [Line Items] |
|
|
|
|
| Total |
$ 1,910,000
|
$ 1,687,000
|
6,542,000
|
5,464,000
|
| Employees | Research and development |
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Compensation Cost [Line Items] |
|
|
|
|
| Total |
811,000
|
815,000
|
2,816,000
|
2,607,000
|
| Employees | General and administrative |
|
|
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Compensation Cost [Line Items] |
|
|
|
|
| Total |
$ 1,099,000
|
$ 872,000
|
$ 3,726,000
|
$ 2,857,000
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationAllocationOfRecognizedPeriodCostsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=vtl_EmployeeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_ResearchAndDevelopmentExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_GeneralAndAdministrativeExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.3
Related Party Transactions (Details) - USD ($)
|
|
8 Months Ended |
12 Months Ended |
Aug. 29, 2024 |
Aug. 28, 2024 |
Dec. 31, 2023 |
| Duane Nash, MD, JD, MBA | Executive Chairman Agreement | Board of Directors Chairman |
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
| Salary and Wage, Officer, Monthly Base Salary |
$ 32,368
|
$ 33,986
|
$ 30,250
|
| X |
- DefinitionSalary and Wage, Officer, Monthly Base Salary
| Name: |
vtl_SalaryAndWageOfficerMonthlyBaseSalary |
| Namespace Prefix: |
vtl_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_CounterpartyNameAxis=vtl_DuaneNashMDJDMBAMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Immunic (NASDAQ:IMUX)
Gráfica de Acción Histórica
De Nov 2024 a Dic 2024

Immunic (NASDAQ:IMUX)
Gráfica de Acción Histórica
De Dic 2023 a Dic 2024
