UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the month of November 2024
Commission File No. 001-41010
MAINZ BIOMED N.V.
(Translation of registrant’s name into English)
Robert Koch Strasse 50
55129 Mainz
Germany
(Address of principal executive office)
Indicate by check mark whether the registrant
files or will file annual reports under cover of Form 20-F or Form 40-F
Form 20-F ☒ Form
40-F ☐
Other Events
On November 6, 2024,
Mainz Biomed N.V. (the “Company”) entered into a Research Collaboration Agreement (the “Agreement”) by and between
the Company and Life Technologies Corporation, a Delaware corporation and subsidiary of Thermo Fisher Scientific (“Thermo Fisher”)
for a period of two years, extendable by mutual agreement. Pursuant to the Agreement, Thermo Fisher and the Company will establish a research
plan (the “Research Plan”) for the purpose of collaboratively researching and developing and potentially commercializing the
Company’s “Next Generation” colorectal cancer screening test for use in the detection of pre-cancerous lesions and colorectal
cancer. The collaboration will leverage each of Thermo Fisher’s and the Company’s combined capabilities to deliver testing
solutions being developed at Mainz Biomed’s laboratories in Mainz, Germany.
The Company issued a press release announcing
entry into the Agreement on November 12, 2024. A copy of the press release is attached hereto as Exhibits 99.1, and is incorporated by
reference into this this Current Report on Form 6-K.
This current report on Form 6-K and the exhibit hereto are hereby incorporated
by reference into our registration statement on Form F-3 (no. 333-269091) as well as our registration statement on Form S-8 (no. 333-273203).
SIGNATURE
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Date: November 12, 2024 |
By: |
/s/ William J. Caragol |
| |
Name: |
William J. Caragol |
| |
Title |
Chief Financial Officer |
2
Exhibit 99.1
Mainz Biomed and Thermo Fisher Scientific Sign
a Collaboration Agreement for the Development of Next Generation Colorectal Cancer Screening Product for Global Markets
BERKELEY, US and MAINZ, Germany – November 12, 2024 --
Mainz Biomed N.V. (NASDAQ:MYNZ) (“Mainz Biomed” or the “Company”), a molecular genetics diagnostic company specializing
in the early detection of cancer, today announced a collaborative agreement with Thermo Fisher Scientific Inc. (NYSE: TMO), through its
subsidiary Life Technologies Corporation (“Thermo Fisher”), a world leader in supplying life sciences solutions and services.
The collaboration agreement will enable Mainz Biomed and Thermo Fisher
to jointly develop and potentially commercialize Mainz Biomed’s Next Generation colorectal cancer screening test. The collaboration
will harness Thermo Fisher’s powerful technologies, instrumentation and information translation systems to enable Mainz Biomed to
develop the proprietary assays for its mRNA-based next-generation CRC screening tests which are redefining standards in early cancer detection.
Mainz Biomed’s flagship non-invasive test not only targets the early detection of colorectal cancer but also focuses on precancerous
lesions, particularly advanced adenomas, demonstrating significant clinical success in both US and European trials.
The collaboration will leverage combined capabilities to deliver testing
solutions being developed at Mainz Biomed’s laboratories in Mainz, Germany.
Guido Baechler, CEO of Mainz Biomed, said: “This collaboration
with Thermo Fisher will be instrumental to our goal to bring to market a home collection colorectal screening tool with highly effective
detection of adenomas. Our product development will be greatly enhanced by Thermo Fisher’s knowledge and scalable, class-leading
technologies, providing both partners with a means to accelerate the availability of an innovative new test for colorectal cancer screening
around the world.”
Peter Jacobs, Director, EMEA Clinical Business Development, Thermo
Fisher Scientific, said: “We are excited at the prospect of working with Mainz Biomed on their next generation screening test and
are confident that together we will be able to achieve rapid progress and deliver innovative new assays for the global clinical marketplace.”
Please visit Mainz Biomed’s official website for investors
at mainzbiomed.com/investors/ for more information
Please follow us to stay up to date:
LinkedIn
X (Previously Twitter)
Facebook
About
Thermo Fisher Scientific
Thermo Fisher Scientific Inc. (NYSE: TMO) is the world leader in serving science, with annual revenue
over $40 billion. Our Mission is to enable our customers to make the world healthier, cleaner and safer. Whether our customers are accelerating
life sciences research, solving complex analytical challenges, increasing productivity in their laboratories, improving patient health
through diagnostics or the development and manufacture of life-changing therapies, we are here to support them. Our global team delivers
an unrivaled combination of innovative technologies, purchasing convenience and pharmaceutical services through our industry-leading
brands, including Thermo Scientific, Applied Biosystems, Invitrogen, Fisher Scientific, Unity Lab Services, Patheon and PPD. For more
information, please visit www.thermofisher.com.
About
Mainz Biomed NV
Mainz Biomed
develops market-ready molecular genetic diagnostic solutions for life-threatening conditions. The Company’s flagship product is
ColoAlert®, an accurate, non-invasive and easy-to-use, early-detection diagnostic test for colorectal cancer. ColoAlert® is marketed
across Europe. The Company is currently running a pivotal FDA clinical study for US regulatory approval. Mainz Biomed’s product
candidate portfolio also includes PancAlert, an early-stage pancreatic cancer screening test based on real-time Polymerase Chain Reaction-based
(PCR) multiplex detection of molecular-genetic biomarkers in stool samples. To learn more, visit mainzbiomed.com or follow us
on LinkedIn, Twitter and Facebook.
For media inquiries
MC Services AG
Anne Hennecke/Caroline Bergmann
+49 211 529252 20
mainzbiomed@mc-services.eu
For investor inquiries, please contact info@mainzbiomed.com
Forward-Looking Statements
Certain statements made in this press release are “forward-looking
statements” within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995.
Forward-looking statements may be identified by the use of words such as “anticipate”, “believe”, “expect”,
“estimate”, “plan”, “outlook”, and “project” and other similar expressions that predict
or indicate future events or trends or that are not statements of historical matters. These forward-looking statements reflect the current
analysis of existing information and are subject to various risks and uncertainties. As a result, caution must be exercised in relying
on forward-looking statements. Due to known and unknown risks, actual results may differ materially from the Company’s expectations
or projections. The following factors, among others, could cause actual results to differ materially from those described in these forward-looking
statements: (i) the failure to meet projected development and related targets; (ii) changes in applicable laws or regulations; (iii) the
effect of the COVID-19 pandemic on the Company and its current or intended markets; and (iv) other risks and uncertainties described herein,
as well as those risks and uncertainties discussed from time to time in other reports and other public filings with the Securities and
Exchange Commission (the “SEC”) by the Company. Additional information concerning these and other factors that may impact
the Company’s expectations and projections can be found in its initial filings with the SEC, including its annual report on Form
20-F filed on April 9, 2024. The Company’s SEC filings are available publicly on the SEC’s website at www.sec.gov. Any forward-looking
statement made by us in this press release is based only on information currently available to Mainz Biomed and speaks only as of the
date on which it is made. Mainz Biomed undertakes no obligation to publicly update any forward-looking statement, whether written or oral,
that may be made from time to time, whether as a result of new information, future developments or otherwise, except as required by law.
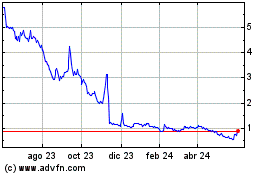
Mainz BioMed NV (NASDAQ:MYNZ)
Gráfica de Acción Histórica
De Nov 2024 a Dic 2024
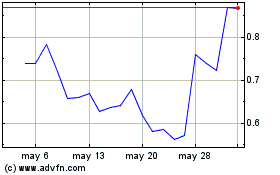
Mainz BioMed NV (NASDAQ:MYNZ)
Gráfica de Acción Histórica
De Dic 2023 a Dic 2024