Avicanna Subsidiary Completes Export of Aureus Branded CBG into Denmark
15 Agosto 2024 - 6:30AM

Avicanna Inc. (“
Avicanna” or the
“
Company) (TSX: AVCN) (OTCQX: AVCNF) (FSE: 0NN) a
biopharmaceutical company focused on the development, manufacturing
and commercialisation of plant-derived cannabinoid-based products
is pleased to announce that through its majority-owned subsidiary
Santa Marta Golden Hemp SAS, the Company has completed the
commercial export of Aureus branded products into Denmark.
The commercial export of Aureus branded purified
Cannabigerol (“CBG”), is a part of the Company’s portfolio of
cannabinoid active pharmaceutical ingredients, marks the 19th
international market for Aureus-branded products and also marks the
22nd market for all Avicanna products. This new market entry into
Denmark demonstrates the Company’s expertise in navigating complex
regulatory processes for its commercialization efforts
internationally.
Avicanna carries out its operations in
compliance with all applicable laws in the jurisdictions in
which it operates.
About Aureus™
Avicanna’s supply chain business unit is based
in Santa Marta, Colombia and provides a consistent source of
cannabinoid raw materials for the global marketplace. These include
active pharmaceutical ingredients and feminized seeds, for
Avicanna’s cosmetic, medical, and pharmaceutical products, in
addition to supplying the company’s partners around the world.
Aureus-branded products are cultivated,
extracted, and manufactured by Avicanna’s subsidiaries in Colombia
where they leverage optimal environmental conditions to produce
cannabinoid active pharmaceutical ingredients economically and
sustainably and include a range of extracts of CBD, THC and rare
cannabinoids such as CBG.
About Avicanna Inc.
Avicanna is a commercial-stage international
biopharmaceutical company focused on the advancement and
commercialization of cannabinoid-based products and formulations
for the global medical and pharmaceutical market segments. Avicanna
has an established scientific platform including R&D and
clinical development leading to the commercialization of more than
thirty proprietary, evidence-based finished products and supporting
four commercial stage business pillars.
- Medical Cannabis formulary
(RHO Phyto™): The formulary offers a diverse range of
proprietary products including oral, sublingual, topical, and
transdermal deliveries with varying ratios of cannabinoids,
supported by ongoing patient, and medical community education. RHO
Phyto is an established leading medical brand in Canada currently
available nationwide to patients across several medical channels
and continues to expand into new international markets.
- Medical cannabis care
platform (MyMedi.ca): MyMedi.ca is a medical cannabis care
platform formed with the aim to better serve medical cannabis
patients’ needs and enhance the patient journey. MyMedi.ca is
operated by Northern Green Canada Inc. and features a diverse
portfolio of products and bilingual pharmacist-led patient support
programs. MyMedi.ca also provides specialty services to distinct
patient groups such as veterans and collaborates with public and
private payers for adjudication and reimbursement. MyMedi.ca
provides educational resources to the medical community to
facilitate the incorporation of medical cannabis into health care
regimens.
- Pharmaceutical products
(Trunerox™) and pipeline: Leveraging Avicanna’s scientific
platform, vertical integration, and real-world evidence, Avicanna
has developed a pipeline of proprietary, indication-specific
pharmaceutical products that are in various stages of clinical
development and commercialization. These cannabinoid-based drug
candidates aim to address unmet medical needs in the areas of
dermatology, chronic pain, and various neurological disorders.
Avicanna’s first indication-specific pharmaceutical drug,
Trunerox™, was approved Q1 2024 by the Health Authority of Colombia
INVIMA as an adjuvant treatment for seizures associated with
Lennox-Gastaut Syndrome and Dravet Syndrome in Colombia. Trunerox™
has not been approved as a drug in Canada by Health Canada.
- Active pharmaceutical
ingredients (Aureus Santa Marta™): Active pharmaceutical
ingredients (“API”) supplied by the Company’s majority owned
subsidiary Santa Marta Golden Hemp SAS (“SMGH”) is a
commercial-stage business dedicated to providing a various forms
high-quality CBD, THC and CBG to the Company’s international
partners for use in the development and production of food,
cosmetics, medical, and pharmaceutical products. The business unit
also forms part of the Company’s supply chain and is a source of
reliable input products for its consumer retail, medical cannabis,
and pharmaceutical products for globally.
SOURCE Avicanna Inc. Stay
Connected
For more information about Avicanna, visit our
website, contact Ivana Maric by email at info@avicanna.com or
follow us on social media
on LinkedIn, Twitter, Facebook, or Instagram.
The Company posts updates through videos from
the Company YouTube channel.
Cautionary Note Regarding Forward-Looking Information
and Statements
This news release contains “forward-looking
information” within the meaning of applicable securities laws.
Forward-looking information contained in this news release may be
identified using words such as, “may”, “would”, “could”, “will”,
“likely”, “expect”, “anticipate”, “believe, “intend”, “plan”,
“forecast”, “project”, “estimate”, “outlook” and other similar
expressions. Although the Company believes that the expectations
and assumptions on which such forward looking information is based
are reasonable, undue reliance should not be placed on the
forward-looking information because the Company can give no
assurance that they will prove to be correct. Actual results and
developments may differ materially from those contemplated by these
statements. Forward-looking information is subject to a variety of
risks and uncertainties that could cause actual events or results
to differ materially from those projected in the forward-looking
information. Such risks and uncertainties include but are not
limited to current and future market conditions, including the
market price of the common shares of the Company, and the risk
factors set out in the Company’s annual information form dated
April 1, 2024 filed with the Canadian securities regulators and
available under the Company’s profile on SEDAR at www.sedar.com.
The statements in this news release are made as of the date of this
release. The Company disclaims any intent or obligation to update
any forward-looking information, whether as a result of new
information, future events or results or otherwise, other than as
required by applicable securities laws.
A photo accompanying this announcement is available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/80297f77-e0a9-4941-ac2d-81665deaac26
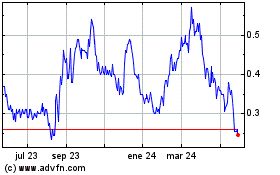
Avicanna (TSX:AVCN)
Gráfica de Acción Histórica
De Nov 2024 a Dic 2024
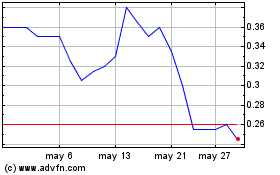
Avicanna (TSX:AVCN)
Gráfica de Acción Histórica
De Dic 2023 a Dic 2024