Notification Filed by National Security Exchange to Report the Removal From Listing and Registration of Matured, Redeemed or Retired Securities Initial Filing Amendments (25-nse)
15 Octubre 2021 - 9:15AM
Edgar (US Regulatory)
NOTIFICATION OF REMOVAL FROM LISTING AND/OR REGISTRATION UNDER SECTION 12(b)
OF THE SECURITIES EXCHANGE ACT OF 1934.
| |
|
Commission File Number
001-06571 |
|
| Issuer: | Merck & Co., Inc. |
| Exchange: | NEW YORK STOCK EXCHANGE LLC |
|
(Exact name of Issuer as specified in its charter, and name of
Exchange where security is listed and/or registered)
|
|
| Address: | 2000 Galloping Hill Road
Kenilworth,
NEW JERSEY
07033 |
| Telephone number: | (908) 740-4000 |
|
(Address, including zip code, and telephone number, including area code, of Issuer's
principal executive offices)
|
|
| 1.125% Notes due 2021 |
|
(Description of class of securities)
|
|
|
Please place an X in the box to designate the rule provision relied upon to strike
the class of securities from listing and registration:
|
| o 17 CFR 240.12d2-2(a)(1) |
| x 17 CFR 240.12d2-2(a)(2) |
| o 17 CFR 240.12d2-2(a)(3) |
| o 17 CFR 240.12d2-2(a)(4) |
| o
Pursuant to 17 CFR 240.12d2-2(b), the Exchange has complied with its rules to strike the class of securities from listing and/or withdraw registration on the Exchange.
1 |
| o
Pursuant to 17 CFR 240.12d2-2(c), the Issuer has complied with its rules of the Exchange and the requirements of 17 CFR 240.12d-2(c) governing the voluntary withdrawal of the class of securities from listing and registration on the Exchange.
|
|
|
Pursuant to the requirements for the Securities Exchange Act of 1934,
NEW YORK STOCK EXCHANGE LLC
certifies that it has reasonable grounds to believe that it
meets all of the requirements for filing the Form 25 and has caused this notification to be
signed on its behalf by the undersigned duly authorized person.
|
|
| 2021-10-15 | By | Lauren Frawley | | Regulation Analyst |
|
Date
| | Name | | Title |
|
| 1 |
Form 25 and attached Notice will be considered compliance with the provisions of
17 CFR 240.19d-1 as applicable. See General Instructions.
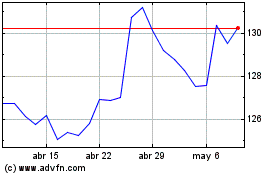
Merck (NYSE:MRK) Gráfica de Acción Histórica De Mar 2024 a Abr 2024
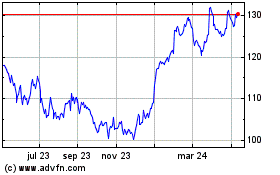
Merck (NYSE:MRK) Gráfica de Acción Histórica De Abr 2023 a Abr 2024
U.S. Stocks Climb Well Off Worst Levels But Close Mostly Lower
Jueves 25 de Abril 2024 (8 horas hace) • IH Market News |
Futures Pointing To Sharply Lower Open On Wall Street
Jueves 25 de Abril 2024 (16 horas hace) • IH Market News |
Southwest Shares Tumble 9.6% Post $231 Million 1Q Loss, AstraZeneca Surges on 19% Annual Increase, and More on Earnings
Jueves 25 de Abril 2024 (17 horas hace) • IH Market News |
Merck Announces First-Quarter 2024 Financial Results
Jueves 25 de Abril 2024 (18 horas hace) • Business Wire |
Health Canada Approves KEYTRUDA® as a first-line treatment for adult patients with locally advanced unresectable or metastatic HER2-negative gastric or gastroesophageal junction (GEJ) adenocarcinoma in combination with fluoropyrimidine- and platinum-conta
Viernes 19 de Abril 2024 (7 días hace) • PR Newswire (Canada) |
Delta Generates US$37 Million Profit in Q1, Google and Intel Unveil Cutting-Edge AI Chips, and More News
Miércoles 10 de Abril 2024 (2 semanas hace) • IH Market News |
Merck Initiates Phase 3 Clinical Trial of MK-1084, an Investigational Oral KRAS G12C Inhibitor, in Combination with KEYTRUDA® (pembrolizumab) for First-Line Treatment of Certain Patients With Metastatic Non-Small Cell Lung Cancer
Jueves 4 de Abril 2024 (3 semanas hace) • Business Wire |
REJOICE-Ovarian01 Phase 2/3 Trial of Raludotatug Deruxtecan Initiated in Patients with Platinum-Resistant Ovarian Cancer
Miércoles 3 de Abril 2024 (3 semanas hace) • Business Wire |
Merck to Hold First-Quarter 2024 Sales and Earnings Conference Call April 25
Lunes 1 de Abril 2024 (4 semanas hace) • Business Wire |
European Commission Approves Merck’s KEYTRUDA® (pembrolizumab) Plus Chemotherapy as Neoadjuvant Treatment, Then Continued as Monotherapy as Adjuvant Treatment, for Resectable Non-Small Cell Lung Cancer (NSCLC) at High Risk of Recurrence in Adults
Jueves 28 de Marzo 2024 (4 semanas hace) • Business Wire |
GameStop Shares Tumble 20% in Pre-Market Trading Amid Revenue Decline, Direct Digital Plummets 42%, and More News
Miércoles 27 de Marzo 2024 (4 semanas hace) • IH Market News |
FDA Approves Merck’s WINREVAIR™ (sotatercept-csrk), a First-in-Class Treatment for Adults with Pulmonary Arterial Hypertension (PAH, WHO* Group 1)
Martes 26 de Marzo 2024 (1 mes hace) • Business Wire |
Más de Merck & Co., Inc. Artículos de Noticias

Parece que no se ha autenticado. Haga click al botón de abajo para iniciar la sesión y ver los símbolos recientemente consultados.
|