UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13a‑16 OR 15d‑16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
FOR THE MONTH OF JANUARY 2024
COMMISSION FILE NUMBER 001-39081
BioNTech SE
(Translation of registrant’s name into English)
An der Goldgrube 12
D-55131 Mainz
Germany
+49 6131-9084-0
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20‑F or Form 40‑F: Form 20‑F ☒ Form 40‑F ☐
Indicate by check mark if the registrant is submitting the Form 6‑K in paper as permitted by Regulation S‑T Rule 101(b)(1): ☐
Indicate by check mark if the registrant is submitting the Form 6‑K in paper as permitted by Regulation S‑T Rule 101(b)(7): ☐
DOCUMENTS INCLUDED AS PART OF THIS FORM 6-K
On January 9, 2024, BioNTech SE outlined its 2024 strategic priorities at the 42nd annual J.P. Morgan Healthcare Conference. The press release and presentation are attached as Exhibits 99.1 and 99.2, respectively.
SIGNATURE
Pursuant to the requirements of the Exchange Act, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | |
| BioNTech SE |
| | |
| | |
| By: | /s/ Dr. Sierk Poetting |
| | Name: Dr. Sierk Poetting |
| | Title: Chief Operating Officer |
Date: January 9, 2024
EXHIBIT INDEX
| | | | | |
| |
| Exhibit | Description of Exhibit |
| |
| 99.1 | |
| |
| 99.2 | |
| |
BioNTech Outlines 2024 Strategic Priorities at the
42nd Annual J.P. Morgan Healthcare Conference
•Plans to have ten or more potentially registrational trials by the end of 2024
•Preparing to be commercial-ready by the end of 2025
•Ended 2023 with approximately €17.5 billion (unaudited) in cash, cash equivalents and security investments
•Expects full year 2024 revenues of approximately €3 billion
•Presentation and webcast at the 42nd Annual J.P. Morgan Healthcare Conference on Tuesday, January 9, 2024, at 6:00 p.m. CET/ 12:00 p.m. ET
Mainz, Germany, January 9, 2024 (GLOBE NEWSWIRE) -- BioNTech SE (Nasdaq: BNTX, “BioNTech” or “the Company”) provided its full year 2024 revenue guidance as part of its outlined 2024 strategic priorities today at the 42nd Annual J.P. Morgan Healthcare Conference in San Francisco, California.
“At BioNTech, we are making important strides towards building a global immunotherapy company. In 2023, we continued our vaccine leadership in the fight against COVID-19 and significantly expanded our mid- and late-stage oncology pipeline. Currently, late-stage trials are ongoing in multiple oncology indications, and we plan to have ten or more potentially registrational trials in our pipeline by the end of 2024,” said Prof. Ugur Sahin, M.D., CEO and Co-Founder of BioNTech. “This year will be a year of significant execution at BioNTech as we continue to expand and develop our innovative pipeline towards our first oncology launches expected from 2026 onwards.”
Prof. Ugur Sahin, M.D., will present a corporate overview and update at the 42nd Annual J.P. Morgan Healthcare Conference on Tuesday, January 9, 2024, at 6:00 p.m. CET/ 12:00 p.m. ET. A live webcast of the presentation will be available on the “Events & Presentations” page in the Investor Relations section on the Company’s website. The replay of the webcast will be archived on the Company’s website for 30 days following the conference.
2024-2026 Financial Framework
BioNTech projects total company revenues of approximately €3 billion for the financial year 2024, mainly driven by the COVID-19 vaccine franchise which is expected to remain profitable given the Company’s cost sharing structure with its partner Pfizer Inc. (“Pfizer”). The Company plans to provide detailed full year 2024 financial guidance during its Full Year and Fourth Quarter 2023 Financial Results call on Wednesday, March 20, 2024.
BioNTech ended 2023 with approximately €17.5 billion (unaudited) in cash, cash equivalents and security investments. The Company plans to maintain a strong financial position and generate significant interest income in 2024. BioNTech expects to grow its topline again in 2025. In the outer years, the Company projects revenues derived from both oncology and respiratory combination vaccine launches, which are subject to successful development and regulatory approval.
As a science and innovation driven company, BioNTech will continue to focus investments on R&D and scaling the business for commercial readiness in oncology in multiple countries by the end of 2025 while continuing to be cost disciplined.
Summary of Selected Pipeline Updates and Expected Milestones
COVID-19 & Other Infectious Diseases
BioNTech’s infectious disease portfolio seeks to address four key areas of high medical need: respiratory viruses, latent viruses, global health pathogens, and antimicrobials. The Company has
established a broad early-stage infectious disease vaccine candidate pipeline containing seven clinical programs leveraging its mRNA technology.
BNT162b2 + BNT161 is an mRNA-based combination vaccine program against COVID-19 and influenza being developed in collaboration with Pfizer. Topline data from the Phase 1/2 trial (NCT05596734) demonstrated robust immune responses to influenza A, influenza B, and SARS-CoV-2 strains and that the safety profile of the candidates was consistent with the companies’ COVID-19 vaccine.
Oncology
In 2023, BioNTech made significant progress in demonstrating the potential of its oncology programs as part of its in-house discovery and development efforts and added six new clinical assets, including next generation antibody-drug conjugate (ADC) candidates and antibody programs, to the Company’s oncology pipeline through internal and collaborative efforts. The Company’s pipeline continued to mature in 2023 with various programs advancing towards later stages of development. BioNTech’s pipeline currently contains 11 ongoing Phase 2 and 3 trials.
Selected later-stage programs:
BNT323/DB-1303 is an HER2-targeted antibody-drug conjugate candidate being developed in collaboration with Duality Biologics (Suzhou) Co. Ltd. (“DualityBio”). First-in-human data from an ongoing Phase 1/2 trial (NCT05150691) demonstrated anti-tumor activity in patients with heavily pretreated HER2-expressing solid tumors. In December 2023, the U.S. Food and Drug Administration (“FDA”) granted Breakthrough Designation for BNT323/DB-1303 for the treatment of advanced endometrial cancer in patients who progressed on or after treatment with immune checkpoint inhibitors. A pivotal Phase 3 trial (NCT06018337) in patients with Hormone Receptor-positive (“HR+”) and HER2-low metastatic breast cancer that have progressed on hormone and/or cyclin-dependent kinase 4/6 (“CDK4/6”) therapy is planned. Additional potentially registrational trials are planned to be initiated in 2024.
BNT316/ONC-392 (gotistobart) is a next-generation anti-CTLA-4 monoclonal antibody candidate jointly developed by BioNTech and OncoC4, Inc. (“OncoC4”). A pivotal Phase 3 trial (NCT05671510) evaluating BNT316/ONC-392 (gotistobart) in patients with immunotherapy-experienced non-small cell lung cancer (NSCLC) is ongoing.
BNT327/PM8002 (PD-L1xVEGF) is an anti-VEGF-A antibody candidate fused to a humanized anti-PD-L1 VHH being developed in collaboration with Biotheus Inc. (“Biotheus”). BNT327/PM8002 is currently being evaluated in several Phase 2/3 studies in China to assess the efficacy and safety of the candidate as a monotherapy or in combination with chemotherapy in various indications. Trial data are planned to be presented this year at a medical conference, and an Investigational New Drug application has been accepted by the FDA for further studies in the U.S. A potentially registrational trial is planned in 2024.
BNT311/GEN1046 (acasunlimab) is a potential first-in-class bispecific antibody candidate combining PD-L1 checkpoint inhibition with 4-1BB costimulatory activation being developed in collaboration with Genmab S/A (“Genmab”). Based on emerging clinical data, the companies have planned engagement with health authorities on the design of a Phase 3 trial for BNT311/GEN1046 (acasunlimab) in second line NSCLC. The companies intend to share the data on which this decision was based at a medical conference in 2024.
BNT312/GEN1042 is a potential first-in-class bispecific antibody candidate designed to induce conditional immune activation by crosslinking CD40 and 4-1BB positive cells, also being developed in collaboration with Genmab. Data required to determine next steps for this program are planned to be shared at a medical conference in 2024.
BNT122 (autogene cevumeran) is an mRNA cancer vaccine candidate based on an individualized neoantigen-specific immunotherapy (iNeST) approach being developed in collaboration with Genentech Inc. (“Genentech”), a member of the Roche Group. In October 2023, BioNTech announced the initiation of IMCODE003, a Phase 2 trial (NCT05968326) evaluating the efficacy and safety of autogene cevumeran in combination with the anti-PD-L1 immune checkpoint inhibitor atezolizumab and standard of care chemotherapy in patients with resected pancreatic ductal adenocarcinoma. This is the third indication for which autogene cevumeran is being evaluated in a Phase 2 trial, alongside other ongoing studies in first-line melanoma and adjuvant colorectal cancer. An additional Phase 2 trial is planned to be initiated as early as late 2024.
BNT211 consists of two investigational medicinal products: a CAR-T cell product candidate targeting Claudin-6 (CLDN6)-positive solid tumors, in combination with a CAR-T cell-amplifying RNA vaccine (CARVac) encoding CLDN6. BioNTech plans to initiate a pivotal Phase 2 trial in relapsed/refractory germ cell tumors in 2024.
In 2024, BioNTech intends to accelerate the development of its portfolio of next-generation investigational medicines both as monotherapies and in combination with immunotherapy agents and other targeted therapies across a wide range of tumor types. BioNTech believes it is well positioned to have ten or more potentially registrational trials in areas of unmet medical need by the end of 2024 in advance of launching its first oncology products from 2026 onwards.
Upcoming Investor and Analyst Events
•Full Year and Fourth Quarter 2023 Financial Results: March 20, 2024
•Annual General Meeting: May 17, 2024
About BioNTech
Biopharmaceutical New Technologies (BioNTech) is a next generation immunotherapy company pioneering novel therapies for cancer and other serious diseases. The Company exploits a wide array of computational discovery and therapeutic drug platforms for the rapid development of novel biopharmaceuticals. Its broad portfolio of oncology product candidates includes individualized and off-the-shelf mRNA-based therapies, innovative chimeric antigen receptor T cells, bispecific immune checkpoint modulators, targeted cancer antibodies and small molecules. Based on its deep expertise in mRNA vaccine development and in-house manufacturing capabilities, BioNTech is developing multiple mRNA vaccine candidates for evaluation for a range of infectious diseases alongside its diverse oncology pipeline, either on its own or together with collaborators. BioNTech has established a broad set of relationships with multiple global pharmaceutical collaborators, including DualityBio, Fosun Pharma, Genentech, a member of the Roche Group, Genevant, Genmab, OncoC4, Regeneron and Pfizer.
For more information, please visit www.BioNTech.com
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, but not limited to, statements concerning: planned next steps in BioNTech’s pipeline programs, including, but not limited to, statements regarding timing or plans for initiation of clinical trials, enrollment or submission for, and receipt of product approvals with respect to BioNTech’s product candidates; BioNTech's estimates of certain financial information, including financial guidance for full year 2024 revenue, which includes expected revenues related to sales of BioNTech's COVID-19 vaccine (referred to as COMIRNATY where approved for use under full or conditional marketing authorization) in territories controlled by BioNTech's collaboration partners, particularly for those figures that are derived from preliminary estimates provided by BioNTech's partners; the rate and degree of market acceptance of BioNTech's COVID-19 vaccine and, if approved, BioNTech's investigational medicines; expectations regarding
anticipated changes in COVID-19 vaccine demand, including changes to the ordering environment and expected regulatory recommendations to adapt vaccines to address new variants or sublineages; the registrational potential of any trials BioNTech may initiate; the initiation, timing, progress, results, and cost of BioNTech's research and development programs, including statements regarding the timing of initiation and completion of studies or trials and related preparatory work, the availability of results, and characterization and timing of clinical data; BioNTech’s targeted timing for a potential oncology product launch, subject to approval, including expectations regarding the timing of commercial readiness activities; the potential safety and efficacy of BioNTech’s product candidates; BioNTech’s expectations with respect to its intellectual property; and BioNTech’s ongoing relationships with Pfizer, Inc.; Duality Biologics (Suzhou) Co. Ltd.; OncoC4, Inc.; Biotheus Inc.; Genmab S/A; Genentech Inc., a member of the Roche Group; and others. In some cases, forward-looking statements can be identified by terminology such as “will,” “may,” “should,” “expects,” “intends,” “plans,” “aims,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. The forward-looking statements in this press release are neither promises nor guarantees, and you should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond BioNTech’s control and which could cause actual results to differ materially from those expressed or implied by these forward-looking statements. These risks and uncertainties include, but are not limited to: the uncertainties inherent in research and development, including the ability to meet anticipated clinical endpoints, commencement and/or completion dates for clinical trials, regulatory submission dates, regulatory approval dates and/or launch dates, as well as risks associated with preclinical and clinical data, including the data discussed in this release, and including the possibility of unfavorable new preclinical, clinical or safety data and further analyses of existing preclinical, clinical or safety data; the nature of the clinical data, which is subject to ongoing peer review, regulatory review and market interpretation; discussions with regulatory agencies regarding timing and requirements for additional clinical trials; the ability to produce comparable clinical results in future clinical trials; the timing of and BioNTech's ability to obtain and maintain regulatory approval for BioNTech's product candidates; the ability of BioNTech’s mRNA technology to demonstrate clinical efficacy outside of BioNTech’s infectious disease platform; BioNTech's pricing and coverage negotiations with governmental authorities, private health insurers and other third-party payors after BioNTech's initial sales to national governments; the future commercial demand and medical need for initial or booster doses of a COVID-19 vaccine; competition from other COVID-19 vaccines or related to BioNTech's other product candidates, including those with different mechanisms of action and different manufacturing and distribution constraints, on the basis of, among other things, efficacy, cost, convenience of storage and distribution, breadth of approved use, side-effect profile and durability of immune response; the ability of BioNTech’s COVID-19 vaccines to prevent COVID-19 caused by emerging virus variants; BioNTech's and its counterparties’ ability to manage and source necessary energy resources; BioNTech's ability to identify research opportunities and discover and develop investigational medicines; the ability and willingness of BioNTech's third-party collaborators to continue research and development activities relating to BioNTech's development candidates and investigational medicines; unforeseen safety issues and potential claims that are alleged to arise from the use of BioNTech's COVID-19 vaccine and other products and product candidates developed or manufactured by BioNTech; BioNTech's and its collaborators’ ability to commercialize and market BioNTech's COVID-19 vaccine and, if approved, its product candidates; BioNTech's ability to manage its development and expansion; regulatory developments in the United States and other countries; BioNTech's ability to effectively scale BioNTech's production capabilities and manufacture BioNTech's products, including BioNTech's target COVID-19 vaccine production levels, and BioNTech's product candidates; risks relating to the global financial system and markets; and other factors not known to BioNTech at this time.
You should review the risks and uncertainties described under the heading “Risk Factors” in BioNTech's Report on Form 6-K for the period ended September 30, 2023, and in subsequent filings made by BioNTech with the SEC, which are available on the SEC’s website at www.sec.gov. Except as required by law, BioNTech disclaims any intention or responsibility for updating or revising any
forward-looking statements contained in this press release in the event of new information, future developments or otherwise. These forward-looking statements are based on BioNTech’s current expectations and speak only as of the date hereof.
CONTACTS
Investor Relations
Victoria Meissner, M.D.
+1 617 528 8293
Investors@biontech.de
Media Relations
Jasmina Alatovic
+49 (0)6131 9084 1513
Media@biontech.de

42nd J.P. Morgan Healthcare Conference Prof. Ugur Sahin, M.D. CEO & Co-founder 9 January 2024 – 9:40 AM PST Exhibit 99.2

This Slide Presentation Includes Forward-Looking Statements 2 This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, but not limited to, statements concerning: planned next steps in BioNTech’s pipeline programs, including, but not limited to, statements regarding timing or plans for initiation of clinical trials, enrollment or submission for, and receipt of product approvals with respect to BioNTech’s product candidates; BioNTech's estimates of certain financial information, including financial guidance for full year 2024 revenue, which includes expected revenues related to sales of BioNTech's COVID-19 vaccine (referred to as COMIRNATY where approved for use under full or conditional marketing authorization) in territories controlled by BioNTech's collaboration partners, particularly for those figures that are derived from preliminary estimates provided by BioNTech's partners; the rate and degree of market acceptance of BioNTech's COVID-19 vaccine and, if approved, BioNTech's investigational medicines; expectations regarding anticipated changes in COVID-19 vaccine demand, including changes to the ordering environment and expected regulatory recommendations to adapt vaccines to address new variants or sublineages; the registrational potential of any trials BioNTech may initiate; the initiation, timing, progress, results, and cost of BioNTech's research and development programs, including statements regarding the timing of initiation and completion of studies or trials and related preparatory work, the availability of results, and characterization and timing of clinical data; BioNTech’s targeted timing for a potential oncology product launch, subject to approval, including expectations regarding the timing of commercial readiness activities; the potential safety and efficacy of BioNTech’s product candidates; BioNTech’s expectations with respect to its intellectual property; and BioNTech’s ongoing relationships with Pfizer, Inc.; Duality Biologics (Suzhou) Co. Ltd.; OncoC4, Inc.; Biotheus Inc.; Genmab S/A; Genentech Inc., a member of the Roche Group; and others. In some cases, forward-looking statements can be identified by terminology such as “will,” “may,” “should,” “expects,” “intends,” “plans,” “aims,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. The forward-looking statements in this presentation are neither promises nor guarantees, and you should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond BioNTech’s control and which could cause actual results to differ materially from those expressed or implied by these forward-looking statements. These risks and uncertainties include, but are not limited to: the uncertainties inherent in research and development, including the ability to meet anticipated clinical endpoints, commencement and/or completion dates for clinical trials, regulatory submission dates, regulatory approval dates and/or launch dates, as well as risks associated with preclinical and clinical data, including the data discussed in this release, and including the possibility of unfavorable new preclinical, clinical or safety data and further analyses of existing preclinical, clinical or safety data; the nature of the clinical data, which is subject to ongoing peer review, regulatory review and market interpretation; discussions with regulatory agencies regarding timing and requirements for additional clinical trials; the ability to produce comparable clinical results in future clinical trials; the timing of and BioNTech's ability to obtain and maintain regulatory approval for BioNTech's product candidates; the ability of BioNTech’s mRNA technology to demonstrate clinical efficacy outside of BioNTech’s infectious disease platform; BioNTech's pricing and coverage negotiations with governmental authorities, private health insurers and other third-party payors after BioNTech's initial sales to national governments; the future commercial demand and medical need for initial or booster doses of a COVID-19 vaccine; competition from other COVID-19 vaccines or related to BioNTech's other product candidates, including those with different mechanisms of action and different manufacturing and distribution constraints, on the basis of, among other things, efficacy, cost, convenience of storage and distribution, breadth of approved use, side-effect profile and durability of immune response; the ability of BioNTech’s COVID-19 vaccines to prevent COVID-19 caused by emerging virus variants; BioNTech's and its counterparties’ ability to manage and source necessary energy resources; BioNTech's ability to identify research opportunities and discover and develop investigational medicines; the ability and willingness of BioNTech's third-party collaborators to continue research and development activities relating to BioNTech's development candidates and investigational medicines; unforeseen safety issues and potential claims that are alleged to arise from the use of BioNTech's COVID-19 vaccine and other products and product candidates developed or manufactured by BioNTech; BioNTech's and its collaborators’ ability to commercialize and market BioNTech's COVID-19 vaccine and, if approved, its product candidates; BioNTech's ability to manage its development and expansion; regulatory developments in the United States and other countries; BioNTech's ability to effectively scale BioNTech's production capabilities and manufacture BioNTech's products, including BioNTech's target COVID-19 vaccine production levels, and BioNTech's product candidates; risks relating to the global financial system and markets; and other factors not known to BioNTech at this time. You should review the risks and uncertainties described under the heading “Risk Factors” in BioNTech's Report on Form 6-K for the period ended September 30, 2023 and in subsequent filings made by BioNTech with the SEC, which are available on the SEC’s website at www.sec.gov. Except as required by law, BioNTech disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this presentation in the event of new information, future developments or otherwise. These forward-looking statements are based on BioNTech’s current expectations and speak only as of the date hereof. Furthermore, certain statements contained in this presentation relate to or are based on studies, publications, surveys and other data obtained from third-party sources and BioNTech’s own internal estimates and research. While BioNTech believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, any market data included in this presentation involves assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. While BioNTech believes its own internal research is reliable, such research has not been verified by any independent source. In addition, BioNTech is the owner of various trademarks, trade names and service marks that may appear in this presentation. Certain other trademarks, trade names and service marks appearing in this presentation are the property of third parties. Solely for convenience, the trademarks and trade names in this presentation may be referred to without the ® and TM symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto.

Our Vision: Harnessing the Power of the Immune System to Fight Human Disease 3 Diversified innovative precision medicine pipeline targeting multiple product approvals per year Multi-product immunotherapy pioneer addressing medical need worldwide BioNTech’s Vision Powered by breakthrough science, disruptive technologies & AI Sustainable respiratory vaccine business AI = artificial intelligence.

BioNTech Today Execution 4 Innovative pipeline with 27 clinical programs in oncology and infectious diseases Long-term sustainable seasonal respiratory vaccine business Building a commercial organization behind a growing late-stage pipeline with 6 ongoing potentially registrational trials mRNA pioneer with a multi-platform innovation engine 1 2 3 4

BioNTech Today Execution 5 Innovative pipeline with 27 clinical programs in oncology and infectious diseases Long-term sustainable seasonal respiratory vaccine business Building a commercial organization behind a growing late-stage pipeline with 6 ongoing potentially registrational trials mRNA pioneer with a multi-platform innovation engine 1 2 3 4

2023 accomplishments Franchise highlights Building and Expanding a Long-term and Successful COVID-19 Franchise1 6 1. Partnered with Pfizer, 2. Cumulative doses shipped in the years 2021-2023; 3. COVID-19 Excess Mortality Collaborators: Lancet. 2022. 4. January to December 3, 2023. 5. September to December, as of January 8, 2024. 6. Company assessment as of December 3, 2023. >400 million total vaccine doses distributed in 20234 >190 million XBB.1.5-adapted monovalent vaccine doses distributed in 20235 Introduced single-dose vials and never-frozen prefilled syringes in the U.S. Maintained market leadership in the U.S. (54%), EU (90%), and Japan (85%)6 First approved mRNA vaccine >4.5 billion doses shipped to >180 countries and territories2 Millions of deaths averted3

BioNTech Today Execution 7 Innovative pipeline with 27 clinical programs in oncology and infectious diseases Long-term sustainable seasonal respiratory vaccine business Building a commercial organization behind a growing late-stage pipeline with 6 ongoing potentially registrational trials mRNA pioneer with a multi-platform innovation engine 1 2 3 4

Multi-Technology Innovation Engine 8 1. mRNA encoded cancer-targeting antibodies and cytokines. CAR = chimeric antigen receptor; TCR = T cell receptor. Individualized and/or targeted therapies ANTIBODY DRUG CONJUGATES TARGETED CANCER THERAPIES RIBOLYSIN mRNA-encoded lysins OFF-THE- SHELF mRNA CANCER VACCINES INFECTIOUS DISEASES VACCINES INDIVIDUA- LIZED mRNA CANCER VACCINES INDIVIDUALIZED TCR-THERAPY INDIVIDUALIZED EX VIVO T CELL THERAPY SOLID TUMOR CAR-T MULTI- TARGET TCR CARVac mRNA vaccine boosted CAR-T-cells NEXT-GEN IMMUNO- MODULATORS RIBO- LOGICALS1 RiboCytokines RiboMabs mRNA ENCODED HUMABODIES PROTEIN- BASED THERAPIES CELL & GENE THERAPIES mRNA TECHNOLOGY SMALL MOLECULES Fundamental science- driven multi-technology- approach Each technology platform is able to deliver multiple product candidates Synergistic combinations Enable individualization of treatment Leverage AI Core principles of our technology strategy Drug class Program Artificial Intelligence IMMUNE AGONISTS

mRNA: A Broad Technology Toolbox 9 Holtkamp et al. Blood 2006; Kuhn et al. Gene Therapy 2010; Sahin et al. Nat Drug Discovery 2014; Vogel et al. Mol Therapy 2018; Beissert et al. Mol Therapy 2020. mRNA formats Local Tissue-specific Systemic Lipoplex nanoparticles Lipid nanoparticles Polymer nanoparticles Uridine mRNA Pseudo-uridine mRNA Self-amplifying mRNA Trans-amplifying mRNA Nanoparticles Vaccines (e.g. cancer, inf. disease, autoimmunity) Antibodies (e.g. receptor blockade) Signaling molecules (e.g. cytokine) Enzymes (e.g. endonuclease) Transcription factors (e.g. Yamanaka factors) Encoded drugs A30-L-A70Cap UTRORFUTR A30-L-A70Cap UTRORFUTR vUTR A30-L-A70Cap AntigenSGPORFvUTR A30-L-A70Cap UTRReplicaseUTR vUTR A30-L-A70Cap ORFvUTR Circular RNA, chemically synthesized mRNA Multimodal optimization of mRNA potency and performance over decades (>10,000x)

Reinforcement learning & large language models Supporting R&D efforts and biology-focused generative AI Building a Leading Biotechnology and AI Company at Scale 10 AI = artificial intelligence; ML = machine learning; GPU = Graphics Processing Unit; RL = reinforcement learning; LLM = large language models. Capabilities to leverage the power of computational medicine & AI • AI drug design to develop next-generation products with a more efficacious or safer profile • Speed up workflows to develop novel therapeutic & vaccine product candidates • Capability scale up with fully digitalized automation throughout the whole drug discovery and development process AI researchers, ML engineers and ML Operations experts Fully managed 500 Petaflop High Performance GPU Cluster in UK*2. Quantum computing R&D with multiple academic & commercial partnerships Physically realistic representations of complex environments, optimized for speed 300+ AI Experts Frontier RL & LLMs Supercomputing Assets & Quantum Machine Learning Simulation Expertise
BioNTech Today Execution 11 Innovative pipeline with 27 clinical programs in oncology and infectious diseases Long-term sustainable seasonal respiratory vaccine business Building a commercial organization behind a growing late-stage pipeline with 6 ongoing potentially registrational trials mRNA pioneer with a multi-platform innovation engine 1 2 3 4

O nc ol og y In fe ct io us D is ea se 7 clinical trials started Growing clinical stage pipeline 6 clinical assets in-licensed 3 first-in-human trials started 11 Phase 2 & 3 trials ongoing Developing an Innovative Pipeline Focused on Oncology and Infectious Disease 12 1. Partnered with Pfizer; 2. In collaboration with Bill & Melinda Gates Foundation 3. Partnered with CEPI = Coalition for Epidemic Preparedness Innovations. Tuberculosis2 Shingles1 Mpox3 20 clinical stage programs 7 clinical stage programs Rigorous pipeline prioritization guided by clinical data and medical need BioNTech’s pipeline Clinical and scientific execution in 2023

BioNTech Today Execution 13 Innovative pipeline with 27 clinical programs in oncology and infectious diseases Long-term sustainable seasonal respiratory vaccine business Building a commercial organization behind a growing late-stage pipeline with 6 ongoing potentially registrational trials mRNA pioneer with a multi-platform innovation engine 1 2 3 4

Progressing Innovation to Address a Broad Range of Unmet Needs 14 1. Partnered with OncoC4; 2. Partnered with DualityBio; 3. Partnered with Genentech, member of Roche Group. NSCLC = non-small cell lung cancer; HPV = human papillomavirus; PDAC = pancreatic ductal adenocarcinoma; CRC = colorectal cancer, HNSCC = head and neck squamous cell carcinoma. Plan to have 10+ potentially registrational trials in 2024 and beyond Additional product candidates advancing to late-stage development BNT316/ONC-392 (gotistobart)1 BNT323/DB-13032 BNT323/DB-13032 autogene cevumeran/BNT1223 autogene cevumeran/BNT1223 BNT113 NSCLC Endometrial cancer Breast cancer PDAC CRC HPV+ HNSCC Ongoing mid- & late stage trials

Grew team and expanded global presence on 5 continents >1,600 new employees joined in 2023 Corporate Execution in 2023 1. Preliminary, unaudited figure; consists of cash, cash equivalents and security investments, as of December 31, 2023. 15 Strengthened balance sheet with strong financial performance* Acquired InstaDeep and in-licensed 6 new clinical stage candidates ~€ 17.5 bn Total cash plus security investments1 Building a multi-product, AI-powered, patient-centric company embedded in the biotech ecosystem * As of Dec. 31, 2023

Infectious Disease Overview

Variant-adapted vaccines Designed to be effective against multiple variants of concern5 Long-term health consequences Accumulating evidence demonstrates that COVID-19 vaccination reduces long- COVID4 Continuous evolution Ongoing antigenic evolution of SARS-CoV-21,2 Risk remains high For severe COVID-19 in vulnerable populations3 Long-Term Need for Annually Adapted Vaccines Anticipated 17 1. World Health Organization Tracking SARS-CoV-2 variant www.who.int/en/activities/tracking-SARS-CoV-2-variants accessed 30 October 2023; 2. Global Initiative on Sharing All Influenza Data https://gisaid.org/ accessed 30 October 2023; 3. FDA Briefing Document Vaccines and Related Biological Products Advisory Committee Meeting June 15, 2023; 4. Brannock et al. Nature Comm. 2023; 5. Stankov M. V. et al. medRxiv pre-print. 2023. Annual and/or SEASONAL VACCINATION Variant-adapted COVID-19 & respiratory pathogen combination vaccines in foreseeable future Combination vaccines have the potential to provide optimized protection against multiple pathogens in at-risk population

Collaborative work with Pfizer to develop combination vaccines for various respiratory diseases* Seasonal Covid-Flu Combination Vaccine Could Address Dual Disease Burden In Overlapping Populations 18 1. Investigator’s Brochure Version 5.0. BNT162/PF-07302048. Available at: https://www.tga.gov.au/sites/default/files/foi-2183-09.pdf; 2. Kirchdoerfer R, et al. Nature. 2016; 3. Verbeke R, et al. J Control Release 2021; 4. Vogel AB, et al. Nature. 2021; 5. Sahin U, et al. Nature. 2021; 6. Chaudhary N, et al. Nat Rev Drug Discov. 2021; 7. Vogel AB, et al. Nature. 2021; 8. Pfizer. Press release. Available at: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and- biontech-provide-update-omicron-variant; 9. Lewis LM, et al. J Pharm Sci. 2023; 10. Financial Times. Available at: https://www.ft.com/content/26f396c2-3dfd-4b57-8fe7-aa6784a2abd9. *The activities relate to the development of respiratory combination vaccine candidates utilizing the companies’ COVID-19 vaccine in various combinations with further approved and investigational respiratory vaccines. Target population: Healthy individuals 18-64 years old Trial design: Single-dose mRNA influenza and COVID-19 vaccine candidates vs. SoC influenza and COVID-19 vaccines Primary endpoints: Safety, tolerability and immunogenicity Induction of neutralizing antibodies and T-cell responses4,5 Easily adaptable and rapid production time6-9 Effective multi-antigen targeting10 Intrinsic adjuvanticity and generation of high & robust antigen-specific adaptive immune responses1-3 COVID- 19 FluPhase 3 mRNA technology is optimally suited for combinations vaccines

COVID-19 Franchise1: Adaptable Approach in the Face of Dynamic Virus Evolution for Continued Success 19 1 Partnered with Pfizer. 2023 2024 2025 Launch of seasonal adapted vaccine If approved, earliest potential introduction of combination respiratory vaccines Expect continued shift to single dose vials and pre-filled syringes Improve Comirnaty properties, e.g., extend shelf half-life Shift to commercialization model in key markets

20 HSV Malaria Tuberculosis Mpox 3.7 billion people under age 50 globally infected with HSV-2 ~491 million people aged 15-49 infected with HSV-1 worldwide ~249 million cases in 2022 608,000 deaths in 2022 in 85 countries Children under 5 accounted for 80% of all malaria deaths 10.6 million cases globally in 2022 1.3 million deaths globally in 2022 2nd leading infectious killer after COVID-19 91,000 cases during 22/23 outbreak2 WHO warning about risk of international spread of current outbreak in DRC Infectious Diseases: Important Growth Area Addressing High Medical and Global Health Needs1 1. All figures are from World Health Organization fact sheets unless otherwise referenced https://www.who.int/news-room/fact-sheets (accessed January 04 2024); 2. WHO 2022-23 Mpox outbreak: global trends. 2023. accessed October 19, 2023 . https://worldhealthorg.shinyapps.io/mpx_global 3. Pan CX, et al. Ther Adv Vaccines Immunother. 2022; 4. Piot P. et al. Nature. 2019. WHO = World Health Organization; HSV = Herpes Simplex Virus; DRC = Democratic Republic of the Congo. Individuals who live to 85 years old have ~50% risk of developing shingles3 Incidence and severity of shingles rise with age, with a marked increase after age 504 Shingles Additional preclinical programs to start clinical trials in 2024 / 2025

Healthcare and Social Responsibility 21 1. Tuberculosis program run in collaboration with the Bill & Melinda Gates Foundation, Mpox partnered with the Coalition for Epidemic Preparedness Innovations (CEPI), Malaria wholly owned program; 2. Partnered with Pfizer, 3. As of December, 2023. Recently inaugurated manufacturing facility in Kigali, Rwanda which could become the first commercial- scale mRNA manufacturing facility in Africa Advanced mRNA-based vaccine candidates into the clinic to address global health threats1 35% of doses of COVID-19 vaccine delivered to low- and middle-income countries in 20232,3 Contributing to democratizing access to novel medicines around the globe

Oncology Overview
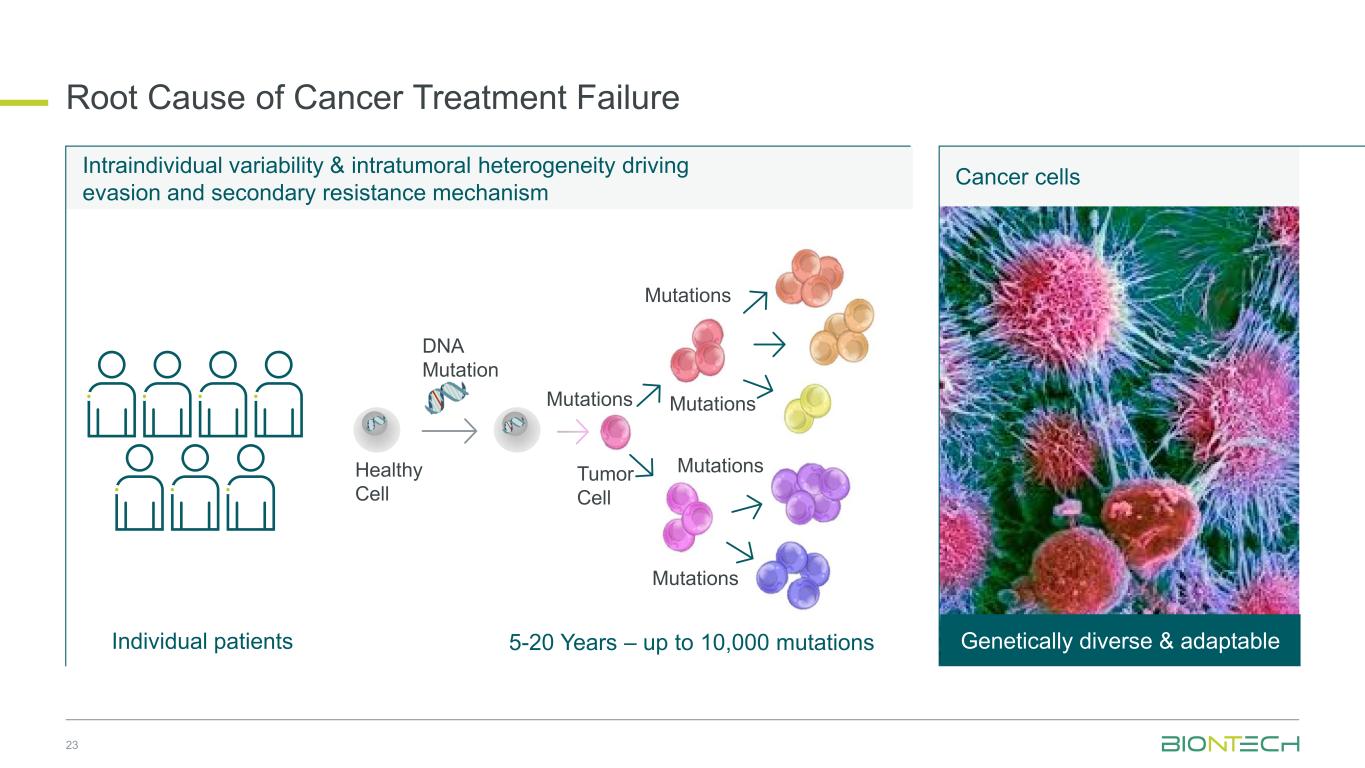
Intraindividual variability & intratumoral heterogeneity driving evasion and secondary resistance mechanism Root Cause of Cancer Treatment Failure Cancer cells Genetically diverse & adaptable5-20 Years – up to 10,000 mutations Mutations DNA Mutation Healthy Cell Mutations Mutations Mutations Mutations Individual patients 23 Tumor Cell

Our Oncology Approach 24 Strategy Portfolio strategy covering compound classes with synergistic mechanism of actions • Immunomodulators • Targeted therapies • Personalized mRNA vaccines Programs across a wide range of solid tumors and stages of treatment Programs with first-in-class and / or best-in-class potential Unique therapeutic combinations Goals Address the continuum of cancer treatment Bring novel therapies to cancer patients and establish new treatment paradigms Open up novel options to combine platforms and therapies

Towards a Potentially Curative Approach to Cancer: Differentiated Combinations of Multiplatform Assets Space for curative approaches Immunomodulators Novel checkpoint inhibitors, cytokines, immune agonists mRNA vaccines Targeted therapy ADCs, CAR-T, TCR-T, small molecules SynergySynergy Synergy 25 CAR = chimeric antigen receptor; ADC = antibody-drug conjugate; IO = immuno-oncology; TCR-T = T-cell receptor engineered T cell. Immunomodulators • Our modality agnostic armamentarium aims to focus on the most relevant and crucial IO pathways • Targeting different but complementary players in the complex cancer immunity cycle may promote a thorough and durable anti-tumoral effect mRNA cancer vaccines • Could eliminate polyclonal residual disease with individualized vaccines for potential long-term impact • Polyspecific activity by targeting multiple antigens at once Targeted therapy • Potent and precise therapies could rapidly reduce tumor burden • Efficacy across the entire disease continuum including late lines

Towards a Potentially Curative Approach to Cancer: Differentiated Combinations of Multiplatform Assets Space for curative approaches Immunomodulators Novel checkpoint inhibitors, cytokines, immune agonists mRNA vaccines Targeted therapy ADCs, CAR-T, TCR-T, small molecules SynergySynergy Synergy 26 CAR = chimeric antigen receptor; ADC = antibody-drug conjugate; IO = immuno-oncology; TCR-T = T-cell receptor engineered T cell. Immunomodulators • Our modality agnostic armamentarium aims to focus on the most relevant and crucial IO pathways • Targeting different but complementary players in the complex cancer immunity cycle may promote a thorough and durable anti-tumoral effect mRNA cancer vaccines • Could eliminate polyclonal residual disease with individualized vaccines for potential long-term impact • Polyspecific activity by targeting multiple antigens at once Targeted therapy • Potent and precise therapies could rapidly reduce tumor burden • Efficacy across the entire disease continuum including late lines

27 1. Partnered with Genmab; 2. Partnered with OncoC4; 3. Partnered with Biotheus. CTLA4 = Cytotoxic T-Lymphocyte-Associated Protein 4; CD27, CD40, 4-1BB = members of the tumor necrosis factor receptor superfamily; PDL-1 =Programmed cell death ligand 1; HER2 = human epidermal growth factor receptor 2; ADCC = Antibody dependent cell-mediated cytotoxicity; ADCP = Antibody dependent cellular phagocytosis; PROC = platinum-resistant ovarian cancer; NSCLC = non-small cell lung cancer; EC = endometrial cancer APC = antigen presenting cells; VEGF = vascular endothelial growth factor; TME = tumor microenvironment; CTx = chemotherapy; IND = investigational new drug application; FIH = first in human. Therapeutic IO Candidates with Novel Mode of Action Across Multiple Solid Tumors BNT313/ GEN10531 Clinical status • Ph1/2 in multiple solid tumors BNT316/ ONC-3922 (gotistobart) Clinical status • Ph1/2 in multiple solid tumors • Ph2 in PROC • Ph3 in 2L+ mNSCLC . BNT311/ GEN10461 (acasunlimab) Clinical status • Ph1/2 in multiple solid tumors • Ph2 in mNSCLC • Ph2 in 2L mEC BNT312/ GEN10421 Clinical status • Ph1/2 trials in multiple solid tumors BNT314/ GEN10591 Clinical status • IND approved • FIH planned BNT327/ PM80023 Clinical status • Several Ph2/3 in patients in China ongoing • Investigational New Drug Application accepted for further studies in the U.S. Anti-CD27Anti-CTLA4 Anti-4-1BBAnti-PD-L1 Anti-4-1BBAnti CD40 Anti-4-1BBAnti-EpCAM Anti-VEGF Anti-PD-L1 VHH Inert Fc Optimized Fc BNT315/ GEN10551 Clinical status • IND approved • FIH planned Anti-OX40 Multiple trial starts and data readouts planned in 2024 Inert Fc Inert Fc Inert FcInert Fc Inert Fc

BNT327/PM80021 Combined with Nab-Paclitaxel: Antitumor Activity as First Line Therapy in Patients with TNBC 28 1. Partnered with Biotheus; TNBC = triple negative breast cancer; ORR = objective response rate; DCR = disease control rate, DoR = duration of response; PFS = progression free survival; PD = progressive disease; SD = stable disease; PR = partial response; CR = complete response -100 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 20 0 -20 -40 -60 -80 Phase 2 (NCT05879068): clinical activity of BNT327/PM8002 in combination with nab-paclitaxel Jiong Wu et al. Presented at SABCS 2023. Poster#PS08-06 PR SD PD CR Non-CR/Non-PD On Treatment Study Months C ha ng e fr om B as el in e (% ) C ha ng e fr om B as el in e (% ) -100 60 40 0 -20 -40 -80 20 -60 PR SD PD CR Anti-tumor activity observed in patients with locally advanced or metastatic triple-negative breast cancer (n=42) Manageable adverse eventsORR: 78.6 DCR: 95.2%

Towards a Potentially Curative Approach to Cancer: Differentiated Combinations of Multiplatform Assets Space for curative approaches Immunomodulators Novel checkpoint inhibitors, cytokines, immune agonists mRNA vaccines Targeted therapy ADCs, CAR-T, TCR-T, small molecules SynergySynergy Synergy 29 CAR = chimeric antigen receptor; ADC = antibody-drug conjugate; IO = immuno-oncology; TCR-T = T-cell receptor engineered T cell. Immunomodulators • Our modality agnostic armamentarium aims to focus on the most relevant and crucial IO pathways • Targeting different but complementary players in the complex cancer immunity cycle may promote a thorough and durable anti-tumoral effect mRNA cancer vaccines • Could eliminate polyclonal residual disease with individualized vaccines for potential long-term impact • Polyspecific activity by targeting multiple antigens at once Targeted therapy • Potent and precise therapies could rapidly reduce tumor burden • Efficacy across the entire disease continuum including late lines

30 ADCs: The Innovation Cycle is Just Beginning Differentiated ADC linker technology • Stability improving safety profile • Higher efficacy Novel mechanisms of actions • Tumor specific activation • Improved and novel payloads Novel targets and novel epitopes • Targeting broader spectrum of tumors • Higher specificity BioNTech plans to develop ADCs against novel targets Linker • Conjugates the payload to the antibody Antibody • Binds to a specific antigen on the surface of cancer cellsPayload • Highly potent cytotoxic compounds BioNTech is driving the development of next-generation ADCs ADC = antibody-drug conjugate. Our deep understanding of ADC targets and immunology distinctively positions us to consolidate and maximize the substantial therapeutic window offered by the next-gen ADC technology

Targeting TROP2, cleavable linker and topoisomerase I inhibitor DAR: 4 Targeting B7H3, cleavable linker and topoisomerase I inhibitor DAR: 6 Targeting HER3, cleavable linker allows for intracellular and extracellular release of topoisomerase I inhibitor DAR: 8 Targeting HER2, cleavable linker and topoisomerase I inhibitor DAR: 8 Clinical Stage ADC Portfolio 1. Partnered with DualityBio; 2. Partnered with MediLink; The completion of the agreement is subject to customary closing conditions, including clearance under the Hart-Scott-Rodino ("HSR") Antitrust Improvements Act. ADC = antibody-drug conjugates; DAR = drug-to-antibody ratio; HR = hormone receptor; HER2/3 = human epidermal growth factor receptor 2/3; TROP2 = trophoblast cell-surface antigen 2; mBC = metastatic breast cancer BNT324/ DB-13111 BNT323/ DB-13031 BNT325/ DB-13051 BNT326/ YL2022 Clinical status • Ph3 in HR+ HER2-low mBC • Ph1/2 in multiple solid tumors Clinical status • Ph1/2 in multiple solid tumors Clinical status • Ph1/2 in multiple solid tumors Clinical status • Ph1 in multiple solid tumors HER2 B7H3 TROP2 HER3 31 Additional trials are planned to start in 2024 and beyond

ADC Portfolio Constructed with Thoughtful Considerations 1. RNAseq data from AACR Project GENIE; 2. Partnered with DualityBio. *The completion of the agreement with MediLink is subject to customary closing conditions, including clearance under the Hart-Scott-Rodino Antitrust Improvements Act. ADC = antibody-drug conjugate; IO = immuno-oncology; MoA = mode of action; HR = hormone receptor; HER = human epidermal growth factor receptor; TROP2 = trophoblast cell-surface antigen; UC = uretherial cancer; EC = endometrial cancer Target Program Stage Indications Partner Ph1/2 Ph3 HER2 BNT323/DB-1303 HR+ HER2-low mBC DualityBio Solid tumors with HER2 expression TROP2 BNT325/DB-1305 Solid tumors DualityBio B7H3 BNT324/DB-1311 Solid tumors DualityBio HER3 BNT326/YL202 Solid tumors MediLink* Target NSCLC SCLC HER2+ BC HR+ BC TNBC CRC Gastric Ovarian PDAC HNSCC Prostate HER2 TROP2 B7-H3 HER3 High Medium / / Low Very low / No-expression • ADC combinations that are based on non- overlapping tumor antigens and different payload MoAs • ADC + IO to advance towards (neo)adjuvant and frontline settings • Four clinical stage ADCs with broad, yet minimal overlapping, indication opportunities • Innovative trial design to open leapfrog path • Fast-follower potential in large indications • BNT323/DB-13032 in multiple pivotal studies Expression level by indication1 Unique indication selection strategy Wider therapeutic window may enable novel combinations in earlier lines 32 Advanced asset on path to registration
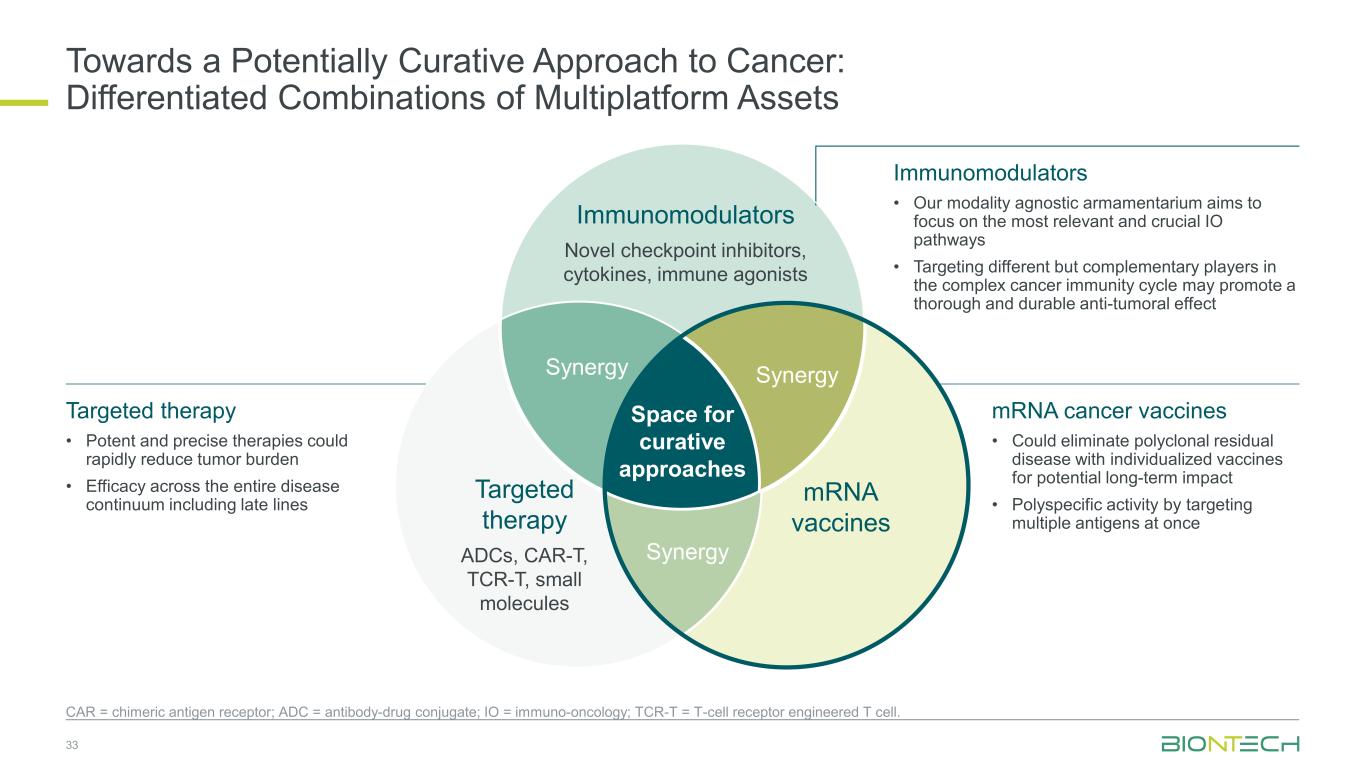
Towards a Potentially Curative Approach to Cancer: Differentiated Combinations of Multiplatform Assets Space for curative approaches Immunomodulators Novel checkpoint inhibitors, cytokines, immune agonists mRNA vaccines Targeted therapy ADCs, CAR-T, TCR-T, small molecules SynergySynergy Synergy 33 CAR = chimeric antigen receptor; ADC = antibody-drug conjugate; IO = immuno-oncology; TCR-T = T-cell receptor engineered T cell. Immunomodulators • Our modality agnostic armamentarium aims to focus on the most relevant and crucial IO pathways • Targeting different but complementary players in the complex cancer immunity cycle may promote a thorough and durable anti-tumoral effect mRNA cancer vaccines • Could eliminate polyclonal residual disease with individualized vaccines for potential long-term impact • Polyspecific activity by targeting multiple antigens at once Targeted therapy • Potent and precise therapies could rapidly reduce tumor burden • Efficacy across the entire disease continuum including late lines

mRNA Cancer Vaccines May Become the Next Tangible Transformation in Oncology 34 Individual patient samples (blood and tissue) AI-driven neoantigen prediction On-demand tailored RNA manufacturing Individualized immuno- therapy Mapping of mutations Fixed combination of shared tumor antigens** Multi-antigen approach tailored to each indication Neo- antigens Individualized therapy Multiple shared antigens Off-the-shelf therapy Cancer vaccine platforms iNeST1,* FixVac individualized Neoantigen-Specific immunoTherapy Fixed Antigen Vaccine ANTIGEN 1 ANTIGEN 2 ANTIGEN 3 ANTIGEN 4 1. iNeST is being developed in collaboration with Genentech, a member of the Roche Group. *autogene cevumeran/BNT122; ** Amount of tumor antigens varies across programs. AI = artificial intelligence.

Personalized mRNA Cancer Vaccines: Key Takeaways 35 Aim to bring personalized cancer vaccines into the adjuvant setting in multiple indications including tumors with low mutational burden and cold tumor types Low tumor mass, with residual cancer cells Tumor resistance mechanisms not fully established Healthier immune system allows for functional T-cell responses High unmet need, not addressed by approved immunotherapies Demonstrated ability to generate durable de novo neoantigen specific polyepitope T-cell responses in multiple cold tumor types Colorectal Cancer 20-35% relapse rate within 4 years after adjuvant therapy • 5-year survival rates of locoregional disease are ~70% • ctDNA is a potential marker for minimal residual disease and is under evaluation to identify patients at high risk of disease recurrence1-3 Randomized Phase 2 trial in adjuvant setting initiated and recruiting Adjuvant Setting Low Mutational Burden 1. Kotani et al. Nat Med. 2023, 2. Vesterman Henriksen et al. Clin Cancer Res.2022; 3. Chidharla et al. Int J Mol Sci. 2023; 4. Oettle, H. et al. JAMA 2013; 5. Neoptolemos, J. P. et al. NEJM 2004. CPI = checkpoint inhibitor. Pancreatic Ductal Adenocarcinoma 69−75% relapse rate within 5 years after adjuvant therapy • Expected to become the 2nd leading cause of cancer-related death in the US by 2030 • 5-year survival rates after resection alone are ~10%4,5 • CPI resistant due to low mutation burden and consecutively few mutation-derived neoantigens Phase 1 trial completed & randomized Phase 2 trial in adjuvant setting recruiting

Contribute to a Potentially Curative Approach to Cancer: Differentiated Combinations of Multiplatform Assets 36 CAR = chimeric antigen receptor; ADC = antibody-drug conjugate; IO = immuno-oncology; TCR-T = T-cell receptor engineered T cell. Space for curative approaches Immunomodulators Novel checkpoint inhibitors, cytokines, immune agonists mRNA vaccines Targeted therapy ADCs, CAR-T, TCR-T, small molecules SynergySynergy Synergy Immunomodulators • Our modality agnostic armamentarium aims to focus on the most relevant and crucial IO pathways • Targeting different but complementary players in the complex cancer immunity cycle may promote a thorough and durable anti-tumoral effect mRNA cancer vaccines • Could eliminate polyclonal residual disease with individualized vaccines for potential long-term impact • Polyspecific activity by targeting multiple antigens at once Targeted therapy • Potent and precise therapies could rapidly reduce tumor burden • Efficacy across the entire disease continuum including late lines

Our Pipeline Holds Potential for Synergistic Drug Combinations 1. Reinhard, K. et al. Science. 2020. CAR = chimeric antigen receptor; IO = immuno-oncology; ADC = antibody-drug conjugates; MoA = mechanism of action. 37 CAR Cancer vaccine+ IO Cancer vaccine+ IO ADC+ Cancer vaccineADC + IO IO+ ADCs deliver cytotoxic drugs directly to cancer cells while immunomodulators activate the immune system to recognize and destroy cancer cells Potentially converging checkpoint inhibition and improved immune cell trafficking and ADC penetration ADCs could quickly debulk tumors while cancer vaccines potentially boost the immune system to eradicate multi-conal micrometastases; hence potentially lifting the long-term survival curve Immunomodulators could activate the immune system supporting vaccine-induced tumor-specific T cell responses Complementary and/or potentially synergistic MoA of immunomodulators could enhance T cell priming and sustain activation mRNA cancer vaccine mediates in vivo stimulation and controlled expansion of CAR-T cells, induces a memory T cell phenotype and increases target sensitivity1
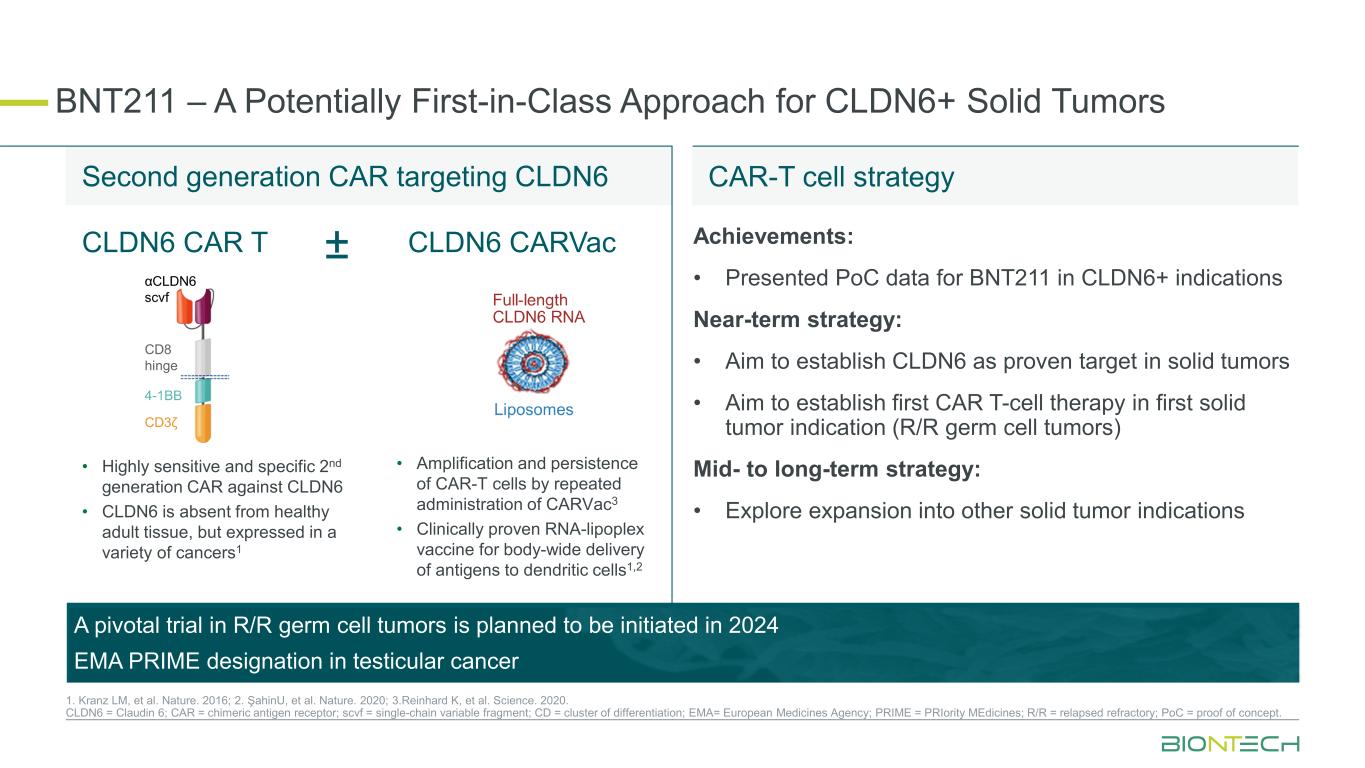
BNT211 – A Potentially First-in-Class Approach for CLDN6+ Solid Tumors 1. Kranz LM, et al. Nature. 2016; 2. ŞahinU, et al. Nature. 2020; 3.Reinhard K, et al. Science. 2020. CLDN6 = Claudin 6; CAR = chimeric antigen receptor; scvf = single-chain variable fragment; CD = cluster of differentiation; EMA= European Medicines Agency; PRIME = PRIority MEdicines; R/R = relapsed refractory; PoC = proof of concept. CLDN6 CAR T ± • Highly sensitive and specific 2nd generation CAR against CLDN6 • CLDN6 is absent from healthy adult tissue, but expressed in a variety of cancers1 • Amplification and persistence of CAR-T cells by repeated administration of CARVac3 • Clinically proven RNA-lipoplex vaccine for body-wide delivery of antigens to dendritic cells1,2 CLDN6 CARVac Second generation CAR targeting CLDN6XXXX CAR-T cell strategy Achievements: • Presented PoC data for BNT211 in CLDN6+ indications Near-term strategy: • Aim to establish CLDN6 as proven target in solid tumors • Aim to establish first CAR T-cell therapy in first solid tumor indication (R/R germ cell tumors) Mid- to long-term strategy: • Explore expansion into other solid tumor indications A pivotal trial in R/R germ cell tumors is planned to be initiated in 2024 EMA PRIME designation in testicular cancer

BNT211-01: Antitumoral Activity at Dose Level 2 39 Data cut-off: 10 Sep 2023. Waterfall plot showing best percent change from baseline in sum of target lesion diameters and spider plot showing percent change in target sum from baseline over time for patients treated with CLDN6 CAR-T ± CLDN6 CARVac at DL2 (N = 22). * Patient had non-measurable disease per RECIST 1.1 and BOR was assessed by tumor marker response. ** Patient achieved complete response after surgical removal of tumors. Response data was pending for 5 patients at the data cut-off. Dotted lines show standard response evaluation criteria used to determine objective tumor response for target lesions per RECIST 1.1 (CR = –100%, PR = 30 to –100%, SD = –30 to 20%, and PD = 20% or higher). Graphs contains additional data entered manually into the database following the data cut-off date that was not available in formal outputs. BOR = best overall response; CR = complete response; DCR = disease control rate; DL = dose level; EOC = epithelial ovarian cancer; GCT = germ cell tumor; PD = progressive disease; ORR = objective response rate; PR = partial response; SD = stable disease. CLDN6 CAR-T <DL2 DL2 >DL2 Total Safety evaluable patients, n 10 27 7 44 Efficacy evaluable patients, n 9 22 7 38 Patients with PR/CR, n 1 13 3 17 Patients with SD, n 1 8 2 11 Patients with PD, n 7 1 2 10 ORR, % 11.1 59.1 42.9 44.7 DCR, % 22.2 95.5 71.4 73.7 Days post-infusionGCT EOC Others C ha ng e in ta rg et s um [% ] C ha ng e in ta rg et s um [% ] 60 30 0 -30 -60 -90 * ** 60 40 0 -20 -60 -100 20 -40 -80 0 50 100 150 200 Best response and change in target sum (DL2 only± CLDN6 CARVac ) Phase 1/2 FIH study (NCT04503278): Efficacy at all dose levels Haanen J. et al. Presented at ESMO 2023. Abstract #LBA35.

BNT211-01: CARVac Improves CAR-T Persistence at Dose Level 2 Data cut-off: 1 Sep 2023. BioNTech data on file derived from peripheral blood applying semi-quantitative PCR directed against CAR transgene. Displayed as copies of transgene per µg of DNA input of isolated PBMC. Pending data up to day 50: 2 patients each in monotherapy and combination cohort. Pending data up to day 90: 3 patients for monotherapy, and 4 patients for combination cohort. CAR = chimeric antigen receptor; CARVac = CAR T-cell amplifying RNA vaccine; DL = dose level; EOC = epithelial ovarian cancer; GCT = germ cell tumor; LLOQ = lower limit of quantification; PBMC = peripheral blood mononuclear cells. Phase 1/2 FIH study (NCT04503278): Pharmacokinetic data Haanen J. et al. Presented at ESMO 2023. Abstract #LBA35. 1 x 108 (DL2) CAR – T only 1 x 108 (DL2) CAR – T + CARVac C LD N 6 C AR – T C on c. (c op ie s/ µg ) Time (Day)GCT EOC Others 106 105 104 103 102 101 100 0 25 50 75 100 0 25 50 75 100 LLOQLLOQ 40

Our Achievements in 2023 Pave Way for the Next Stage of Growth in Oncology 41 NSCLC = non-small cell lung cancer; HR = hormone receptor; HER = human epidermal growth receptor; BC = breast cancer; CRC = colorectal cancer; PDAC = pancreatic ductal adenocarcinoma. Prioritizing lead late-stage programs to accelerate path-to-market Plan to build fully integrated global oncology organization by the end of 2025 to discover, develop, and commercialize a multi-product portfolio Accessed and continue to access external innovation to accelerate pipeline maturation in a capital-efficient manner 10+ potentially registrational trials running for at least 6 programs, plan to start combination trials 2023 2024 2025 Ongoing mid- & late-stage trials in multiple indications, including NSCLC, HR+ HER2-low BC, CRC, PDAC

Advancing Our Vision: A Once in a Generation Opportunity to Transform Medicine . 1. Partnered with Pfizer. YE = Year end; IND = Investigational new drug. Infectious diseases Globally marketed COVID-19 vaccine franchise1 Maintain and deepen COVID-19 vaccine leadership Launch multiple oncology products from 2026 onwards Mid-term goals 2025-2029Driving transformation today Long-term vision 2030 Launch next-generation and combination COVID-19 vaccines Approved products across oncology and infectious disease portfolio Cardiovascular diseases Neurodegenerative diseases Autoimmune diseases Oncology Initiating additional potentially registrational trials We aim to be a multi-product global biotechnology leader, working to address the world’s most pressing health challenges with pioneering, disruptive technologies delivered at scale 42 9 Phase 2 trials 2 Phase 3 trials 7 programs in 9 clinical trials 20 programs in 30 clinical trials Innovation engine producing multiple INDs per year Potential new disease areas

Thank you

Appendix

Advancing our Pipeline: Select Data Milestones in 2024 45 1. Partnered with Genmab; 2. Partnered with OncoC4; 3. Partnered with DualityBio; 4. Partnered with Biotheus; 5. Partnered with Pfizer. NSCLC = non-small cell lung cancer, R/R = relapsed/refractors. Program Indication Targeted Milestone Oncology BNT311/GEN1046 (acasunlimab)1 R/R met. NSCLC, +/- pembrolizumab Phase 2 data BNT312/GEN10421 Multiple solid tumors Ph1/2 expansion cohort data BNT316/ONC-392 (gotistobart)2 Multiple solid tumors Ph1/2 expansion cohort data BNT323/DB-13033 Multiple solid tumors Ph1/2 expansion cohort data BNT325/DB-13053 Multiple solid tumors Ph1/2 data BNT327/PM80024 Multiple solid tumors Phase 2 data Infectious disease BNT162b25 COVID-19, Omicron XBB.1.5 monovalent vaccine Phase 2/3 data BNT1675 Shingles Phase 1 trial update
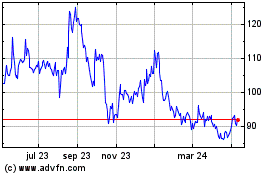
BioNTech (NASDAQ:BNTX)
Gráfica de Acción Histórica
De Abr 2024 a May 2024
BioNTech (NASDAQ:BNTX)
Gráfica de Acción Histórica
De May 2023 a May 2024