Dermata Therapeutics to Present at the Life Sciences Investor Forum on Thursday, November 14, 2024
11 Noviembre 2024 - 7:35AM

Dermata Therapeutics, Inc. (Nasdaq: DRMA; DRMAW) (“Dermata” or the
“Company”), a late-stage biotechnology company focused on the
treatment of medical and aesthetics skin diseases and conditions,
today announced that Gerry Proehl, Chairman, President, and
Chief Executive Officer, will present live at the Life Sciences
Investor Forum hosted by VirtualInvestorConferences.com, on
Thursday, November 14, 2024 at 12:00 PM ET.
Date: November 14, 2024 Time: 12:00pm – 12:30pm ET Link:
https://bit.ly/3NuUDa4Available for 1x1 meetings: November 15 and
18, 2024
This will be a live, interactive online event where investors
are invited to ask the Company questions in real-time. If attendees
are not able to join the event live on the day of the conference,
an archived webcast will also be made available after the
event.
It is recommended that online investors pre-register and run the
online system check to expedite participation and receive event
updates.
Learn more about the event at
www.virtualinvestorconferences.com.
Recent Dermata Highlights
- Outstanding efficacy, safety and
tolerability results in a Phase 2b clinical trial in moderate to
severe acne
- Approaching completion of
enrollment in its DMT310 Phase 3 Spongilla Treatment of Acne
Research (STAR-1) clinical trial with topline results expected in
Q1 2025
- Large, addressable market with
approximately 30 million patients in the US alone seeking
treatments for acne
- Continues discussions with
potential botulinum toxin partners to further develop DMT410 for
topical delivery of botulinum toxin
- Raised $7.8M in gross proceeds from
financings completed during 2024
About Dermata Therapeutics
Dermata Therapeutics, Inc. is a late-stage biotechnology company
focusing on the treatment of medical and aesthetic skin diseases
and conditions. The Company's lead product candidate, DMT310, is
the Company’s first product candidate being developed from its
Spongilla technology platform and is currently being evaluated in a
Phase 3 program. DMT310 is a once-weekly topical product candidate
derived from a naturally sourced freshwater sponge with multiple
unique mechanisms of action. DMT310 has been studied for the
treatment of acne, rosacea, and psoriasis. The Company's second
product candidate, DMT410, uses its Spongilla technology as a new
method for topical intradermal delivery of botulinum toxin for the
treatment of hyperhidrosis and multiple aesthetic skin conditions.
Dermata is headquartered in San Diego, California. For more
information, please visit http://www.dermatarx.com/.
About Virtual Investor Conferences®
Virtual Investor Conferences (“VIC”) is the leading proprietary
investor conference series that provides an interactive forum for
publicly traded companies to seamlessly present directly to
investors.
Providing a real-time investor engagement solution, VIC is
specifically designed to offer companies more efficient investor
access. Replicating the components of an on-site investor
conference, VIC offers companies enhanced capabilities to connect
with investors, schedule targeted one-on-one meetings and enhance
their presentations with dynamic video content. Accelerating the
next level of investor engagement, Virtual Investor Conferences
delivers leading investor communications to a global network of
retail and institutional investors.
Forward-Looking Statements
Statements in this press release that are not strictly
historical in nature are forward-looking statements. These
statements are based on the Company's current beliefs and
expectations and new risks may emerge from time to time.
Forward-looking statements are subject to known and unknown risks,
uncertainties, assumptions, and other factors including, but are
not limited to, statements related to: expectations with regard to
the timing of meetings and/or responses from submissions with
regulatory bodies; expectations with regard to the timing of
submission of an NDA; the uncertainties inherent in clinical trials
including enrolling an adequate number of patients on time or be
completed on schedule, if at all; timing and ability to generate
clinical data; expectations with regard to any potential
partnership opportunities for any of the Company's product
candidates; the Company's expectations with regard to current cash
and cash equivalents and the amount of time it will fund
operations; the success, cost, and timing of its product candidates
DMT310 and DMT410 development activities and ongoing and planned
clinical trials; and whether the results of any ongoing or planned
clinical trials of DMT310 or DMT410 will lead to future product
development. These statements are only predictions based on current
information and expectations and involve a number of risks and
uncertainties. Actual events or results may differ materially from
those projected in any of such statements due to various factors,
including the risks and uncertainties inherent in drug development,
approval, and commercialization, and the fact that past results of
clinical trials may not be indicative of future trial results. For
a discussion of these and other factors, please refer to Dermata's
filings with the Securities and Exchange Commission. You are
cautioned not to place undue reliance on these forward-looking
statements, which speak only as of the date hereof. This caution is
made under the safe harbor provisions of the Private Securities
Litigation Reform Act of 1995. All forward-looking statements are
qualified in their entirety by this cautionary statement and
Dermata undertakes no obligation to revise or update this press
release to reflect events or circumstances after the date hereof,
except as required by law.
CONTACTS:
Clay ChaseInvestor Relations619-917-6771cc@sdthc.com
Virtual Investor Conferences John M. ViglottiSVP Corporate
Services, Investor AccessOTC Markets Group (212)
220-2221johnv@otcmarkets.com
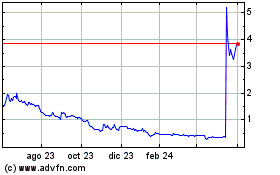
Dermata Therapeutics (NASDAQ:DRMA)
Gráfica de Acción Histórica
De Nov 2024 a Dic 2024
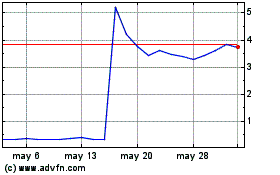
Dermata Therapeutics (NASDAQ:DRMA)
Gráfica de Acción Histórica
De Dic 2023 a Dic 2024