false 0001693011 0001693011 2025-01-10 2025-01-10
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 10, 2025
INOZYME PHARMA, INC.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
| Delaware |
|
001-39397 |
|
38-4024528 |
(State or Other Jurisdiction
of Incorporation) |
|
(Commission File Number) |
|
(IRS Employer
Identification No.) |
|
|
|
|
|
| 321 Summer Street Suite 400 |
|
|
|
|
| Boston, Massachusetts |
|
|
|
02210 |
| (Address of Principal Executive Offices) |
|
|
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: (857) 330-4340
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange
on which registered |
| Common stock, par value $0.0001 per share |
|
INZY |
|
Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02 Results of Operations and Financial Condition.
Inozyme Pharma, Inc. (the “Company”) expects to report cash, cash equivalents, and short-term investments of approximately $113.1 million as of December 31, 2024.
The financial statements for the Company for the year ended December 31, 2024 are not yet available. The estimated cash, cash equivalents, and short-term investments amount as of December 31, 2024 is preliminary and unaudited, represents management’s estimate as of the date of this report, is subject to completion of the Company’s financial closing procedures for the year ended December 31, 2024, and does not present all necessary information for a complete understanding of the Company’s financial condition as of December 31, 2024, or the Company’s results of operations for the year ended December 31, 2024. The actual financial results may differ materially from the preliminary estimated financial information.
The information in this Item 2.02 shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended (the “Securities Act”), or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Item 7.01 Regulation FD Disclosure.
On January 10, 2025, the Company issued a press release announcing positive interim data for INZ-701 in infants and young children with ENPP1 Deficiency and key program updates. A copy of the press release is attached as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference. On January 10, 2025, the Company also posted a presentation related to interim clinical and safety data of INZ-701 treatment in infants and young children with ENPP1 Deficiency and key program updates under “Events and Presentations” on the “Investors” section of the Company’s website (www.inozyme.com). The information contained in, or that can be accessed through, the Company’s website is not a part of this filing. A copy of the presentation is attached as Exhibit 99.2 to this Current Report on Form 8-K and is incorporated herein by reference.
The information in this Item 7.01, including Exhibits 99.1 and 99.2, shall not be deemed “filed” for purposes of Section 18 of the Exchange Act or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Item 8.01 Other Events.
On January 10, 2025, the Company announced positive interim data from its ENERGY 1 trial and Expanded Access Program (“EAP”) evaluating INZ-701 in infants and young children with ENPP1 Deficiency, completion of enrollment in the Company’s ENERGY 3 pivotal trial in pediatric patients with ENPP1 Deficiency and regulatory guidance for the Company’s planned ASPIRE pivotal trial in children with ABCC6 Deficiency.
ENERGY 1 Trial and Expanded Access Program
Interim data from the ENERGY 1 trial (three infants) and the EAP (two infants and one child - 2.5 years old) evaluated patients with generalized arterial calcification of infancy (“GACI”), a severe manifestation of ENPP1 Deficiency. Patients were treated with INZ-701 for periods of three weeks to 22 months. Key results include:
| |
• |
|
Improved Survival: 80% of infants treated with INZ-701 survived beyond their first year, compared to a historical survival rate of approximately 50%. |
| |
• |
|
Reduction in Arterial Calcifications: Substantial reductions or stabilization of arterial calcifications were observed in all surviving patients, including complete resolution in some instances. There was no evidence of progression of arterial calcification in any patient. |
| |
• |
|
Improved Heart Function: Stabilization or improvement in left ventricular ejection fraction was noted in all surviving patients. |
| |
• |
|
Reduced Risk of Rickets: No radiographic evidence of rickets was observed in patients evaluated beyond one year of age and at-risk of rickets development (n=3), supported by stabilization or increases in serum phosphate levels. |
| |
• |
|
Favorable Safety Profile: INZ-701 was well-tolerated, with no serious treatment-related adverse events in infants and young children. Observed treatment-related events were limited to mild injection site reactions. Across studies to-date, low, often transient, anti-drug antibody (“ADA”) levels were noted in some children and adults, with no impact on pharmacokinetics (“PK”) or pharmacodynamics (“PD”). In the ENERGY 1 trial and EAP, higher ADA levels in some infants significantly |
| |
affected PK and PD. In infants with high ADA levels, data collected pre- and post-dosing demonstrated substantial transient increases in PPi and drug exposure following INZ-701 administration, consistent with the clinical effects observed. ADAs were not associated with adverse events in any patient. |
Enrollment Complete in ENERGY 3 Pivotal Trial
The Company today announced completion of enrollment in its ENERGY 3 pivotal trial of INZ-701 in patients with ENPP1 Deficiency aged >1 to <13 years. Based on recommendations from the U.S. Food and Drug Administration (“FDA”), the primary endpoint of plasma PPi should be supported by consistent trends in appropriate clinical endpoints, such as radiographic global impression of change (“RGI-C”), a measure for progression or improvement of rickets. As per agreement with the European Medicines Agency (“EMA”), plasma PPi and RGI-C are co-primary endpoints, with a relaxed p-value of <0.2 for RGI-C.
With 25 patients enrolled, the trial’s 2:1 randomized design provides >90% power to detect meaningful differences in RGI-C between treatment and control groups. Strong patient interest and scheduled screenings may result in the enrollment of additional participants in January 2025. The Company anticipates completing the one-year dosing period for all patients by January 2026, with topline data expected in early 2026.
Regulatory Progress for Planned ASPIRE Pivotal Trial in Children with ABCC6 Deficiency
The Company is advancing the development of INZ-701 in ABCC6 Deficiency. In April 2024, the Company reported topline data from an open-label, dose-escalation study in adults, along with findings from a natural history study documenting the significant disease burden in patients with the early-onset form of the disease, known as GACI Type 2 (GACI-2). The adult study demonstrated positive improvements in vascular and retinal pathology after 48 weeks of treatment with INZ-701, as well as normalization of PPi levels at the highest dose tested, supporting further development in additional age groups. The natural history study revealed a high disease burden characterized by childhood strokes, arteriopathy, cardiovascular complications, and early mortality. Further research has identified a substantial pediatric population with ABCC6 Deficiency, underscoring the significant unmet medical need in this group.
The natural history study data, supplemented by literature reports, has informed the design of the Company’s planned randomized, controlled ASPIRE trial of INZ-701 in children with ABCC6 Deficiency. The proposed primary endpoint, comprising major adverse clinical events over a two-year treatment period, has been reviewed and received preliminary support from U.S. and EU regulators. The trial is expected to enroll approximately 70 patients from infancy up to <18 years old with biallelic or monoallelic ABCC6 Deficiency. The Company is currently refining the trial design to harmonize feedback from the FDA and EMA.
The Company plans to continue regulatory engagement over the coming months to finalize the trial protocol. Pending ongoing regulatory review and the availability of financial resources, the Company aims to initiate the ASPIRE trial in early 2026.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits:
Cautionary Note Regarding Forward-Looking Statements
Statements in this Current Report on Form 8-K about future expectations, plans, and prospects, as well as any other statements regarding matters that are not historical facts, may constitute “forward-looking statements” within the meaning of The Private Securities Litigation Reform Act of 1995. These statements include, but are not limited to, statements relating to the initiation, enrollment, timing, and design of the Company’s planned clinical trials, enrollment and availability of data from clinical trials, the potential benefits of INZ-701 and the Company’s regulatory strategy. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Any forward-looking statements are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in, or implied by, such forward-looking statements. These risks and uncertainties include, but are not limited to, risks associated with the Company’s ability
to conduct its ongoing clinical trials of INZ-701 for ENPP1 Deficiency, ABCC6 Deficiency and calciphylaxis; enroll patients in ongoing and planned trials; obtain and maintain necessary approvals from the FDA and other regulatory authorities; continue to advance its product candidates in preclinical studies and clinical trials; replicate in later clinical trials positive results found in preclinical studies and early-stage clinical trials of its product candidates; advance the development of its product candidates under the timelines it anticipates in planned and future clinical trials; obtain, maintain, and protect intellectual property rights related to its product candidates; manage expenses; comply with the covenants under its outstanding loan agreement; and raise the substantial additional capital needed to achieve its business objectives. For a discussion of other risks and uncertainties, and other important factors, any of which could cause the Company’s actual results to differ from those contained in the forward-looking statements, see the “Risk Factors” section in the Company’s most recent Annual Report on Form 10-K and Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission, as well as discussions of potential risks, uncertainties, and other important factors, in the Company’s most recent filings with the Securities and Exchange Commission. In addition, the forward-looking statements included in this Current Report on Form 8-K represent the Company’s views as of the date hereof and should not be relied upon as representing the Company’s views as of any date subsequent to the date hereof. The Company anticipates that subsequent events and developments will cause the Company’s views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, the Company specifically disclaims any obligation to do so.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
| Date: January 10, 2025 |
|
|
|
By: |
|
/s/ Douglas A. Treco |
|
|
|
|
|
|
Name: Douglas A. Treco
Title: Chief Executive Officer |
Exhibit 99.1
Inozyme Pharma Announces Positive Interim Data for INZ-701 in Infants and Young Children with ENPP1
Deficiency and Key Program Updates
- Positive interim results in infants and young children with ENPP1 Deficiency
showed improvements from baseline in multiple measures of disease, including survival, heart function, and stabilization or reduction in ectopic calcification and hypophosphatemia, with no radiographic evidence of rickets –
- Enrollment complete in ENERGY 3 pivotal trial in pediatric patients with ENPP1 Deficiency; on track to complete dosing in
January 2026, with topline data to follow in early 2026 –
- Regulatory guidance from FDA and EMA supports planned
ASPIRE pivotal trial focused on addressing severe complications of ABCC6 Deficiency in children –
BOSTON, Jan. 10, 2025 – Inozyme Pharma, Inc.
(Nasdaq: INZY) (“the Company” or “Inozyme”), a clinical-stage biopharmaceutical company developing innovative therapeutics for rare diseases that affect bone health and blood vessel function, today announced positive interim data
from its ENERGY 1 trial and Expanded Access Program (EAP) evaluating INZ-701 in infants and young children with ENPP1 Deficiency, completion of enrollment in the ENERGY 3 pivotal trial in pediatric patients
with ENPP1 Deficiency and regulatory guidance for the ASPIRE pivotal trial in children with ABCC6 Deficiency.
“We believe these highly encouraging
outcomes in infants and young children, combined with previously reported data from adult studies, provide strong support for the potential impact of INZ-701 on rickets, a key clinical endpoint in the ongoing
pivotal ENERGY 3 trial, and underscore its potential to address the significant needs of pediatric patients,” said Douglas A. Treco, Ph.D., CEO and Chairman of Inozyme Pharma.
Matt Winton, Ph.D., Senior Vice President and COO of Inozyme Pharma added, “Our team and global collaborators worked tirelessly to identify and diagnose
these rare patients and initiate treatment as quickly as possible. Tragically, in some cases, we have been unable to begin treatment before the infant passed. This only deepens our commitment to the patient community and strengthens our resolve to
address unmet needs across all populations as we advance INZ-701.”
Positive Interim Data from the ENERGY
1 trial and Expanded Access Program
Interim data from the ENERGY 1 trial (three infants) and the EAP (two infants and one child -2.5 years old) evaluated patients with generalized arterial calcification of infancy (GACI), a severe manifestation of ENPP1 Deficiency. Patients were treated with INZ-701
for periods of three weeks to 22 months. The data presentation can be accessed here on Inozyme’s Investor Relations site. Key results include:
| |
• |
|
Improved Survival: 80% of infants treated with INZ-701
survived beyond their first year, compared to a historical survival rate of approximately 50%. |
| |
• |
|
Reduction in Arterial Calcifications: Substantial reductions or stabilization of arterial
calcifications were observed in all surviving patients, including complete resolution in some instances. There was no evidence of progression of arterial calcification in any patient. |
| |
• |
|
Improved Heart Function: Stabilization or improvement in left ventricular ejection fraction (LVEF)
was noted in all surviving patients. |
| |
• |
|
Reduced Risk of Rickets: No radiographic evidence of rickets was observed in patients evaluated
beyond one year of age and at-risk of rickets development (n=3), supported by stabilization or increases in serum phosphate levels. |
| |
• |
|
Favorable Safety Profile: INZ-701 was well-tolerated, with no
serious treatment-related adverse events in infants and young children. Observed treatment-related events were limited to mild injection site reactions. Across studies to-date low, often transient, anti-drug
antibody (ADA) levels were noted in some children and adults, with no impact on pharmacokinetics (PK) or pharmacodynamics (PD). In the ENERGY 1 trial and EAP, higher ADA levels in some infants significantly affected PK and PD. In infants with high
ADA levels, data collected pre- and post-dosing demonstrated substantial transient increases in PPi and drug exposure following INZ-701 administration, consistent with
the clinical effects observed. ADAs were not associated with adverse events in any patient. |
Enrollment Complete in ENERGY 3 Pivotal
Trial
The Company today announced completion of enrollment in its ENERGY 3 pivotal trial of INZ-701 in
patients with ENPP1 Deficiency aged >1 to <13 years. Based on recommendations from the U.S. Food and Drug Administration (FDA), the primary endpoint of plasma PPi should be supported by consistent trends in appropriate clinical endpoints, such
as radiographic global impression of change (RGI-C), a measure for progression or improvement of rickets. As per agreement with the European Medicines Agency (EMA), plasma PPi and RGI-C are co-primary endpoints, with a relaxed p-value of <0.2 for RGI-C.
With 25 patients enrolled, the trial’s 2:1 randomized design provides >90% power to detect meaningful differences in
RGI-C between treatment and control groups. Strong patient interest and scheduled screenings may result in the enrollment of additional participants in January 2025. Inozyme anticipates completing the one-year dosing period for all patients by January 2026, with topline data expected in early 2026.
Regulatory Progress for ASPIRE Pivotal Trial in Children with ABCC6 Deficiency: Preliminary Support from
U.S. and EU Regulators
Inozyme is advancing the development of INZ-701 in ABCC6 Deficiency. In April 2024, the
Company reported topline data from an open-label, dose-escalation study in adults, along with findings from a natural history study documenting the significant disease burden in patients with the early-onset form of the disease, known as GACI
Type 2 (GACI-2). The adult study demonstrated positive improvements in vascular and retinal pathology after 48 weeks of treatment with INZ-701, as well as normalization
of PPi levels at the highest dose tested, supporting further development in additional age groups. The natural history study revealed a high disease burden characterized by childhood strokes, arteriopathy, cardiovascular complications, and early
mortality. Further research has identified a substantial pediatric population with ABCC6 Deficiency, underscoring the significant unmet medical need in this group.
The natural history study data, supplemented by literature reports, has informed the design of the Company’s planned randomized, controlled ASPIRE trial
of INZ-701 in children with ABCC6 Deficiency. The proposed primary endpoint, comprising major adverse clinical events over a two-year treatment period, has been reviewed
and received preliminary support from U.S. and EU regulators. The trial is expected to enroll approximately 70 patients from infancy up to <18 years old with biallelic or monoallelic ABCC6 Deficiency. Inozyme is currently refining the study
design to harmonize feedback from the FDA and EMA.
The Company plans to continue regulatory engagement over the coming months to finalize the trial
protocol. Pending ongoing regulatory review and the availability of financial resources, Inozyme aims to initiate the ASPIRE trial in early 2026.
About ENPP1 Deficiency
ENPP1 Deficiency is a serious and
progressive rare disease that affects blood vessels, soft tissues, and bones. Individuals who present in utero or in infancy are typically diagnosed with generalized arterial calcification of infancy (GACI Type 1), with about 50% of these infants
not surviving beyond six months. Children with this condition typically develop rickets, specifically autosomal-recessive hypophosphatemic rickets type 2 (ARHR2), while adolescents and adults may develop osteomalacia, or softened bones. ARHR2 and
osteomalacia cause pain and difficulty with movement. Additionally, patients may experience hearing loss, calcification in arteries and joints, and heart problems.Biallelic ENPP1 Deficiency affects approximately 1 in 64,000 pregnancies worldwide.
Initially, it was believed to only impact individuals with two copies of the mutated gene. However, many individuals with just one copy of the mutated gene (monoallelic ENPP1 Deficiency) also exhibit severe symptoms. This suggests that the worldwide
prevalence of ENPP1 Deficiency may be much higher than current estimates, which are based solely on biallelic cases. Currently, there are no approved therapies for ENPP1 Deficiency.
About ABCC6 Deficiency
ABCC6 Deficiency is a progressive and debilitating rare disease that affects blood vessels and soft tissues. Infants with ABCC6 Deficiency are diagnosed with
generalized arterial calcification of infancy (GACI Type 2), which is similar to GACI Type 1, the infant form of ENPP1 Deficiency. Pediatric patients who survive beyond the first year of life may develop neurological disease, including strokes, and
cardiovascular diseases due to ongoing vascular calcification and stenosis. In older individuals, ABCC6 Deficiency manifests as pseudoxanthoma elasticum (PXE), characterized by abnormal mineralization in blood vessels and soft tissues, affecting the
skin, visual function, and vascular system. Biallelic ABCC6 Deficiency is estimated to affect 1 in 25,000 to 1 in 50,000 individuals worldwide. Initially, it was believed to only impact individuals with two copies of the mutated gene. However, many
people with just one copy of the mutated gene (monoallelic ABCC6 Deficiency) also exhibit severe symptoms. This suggests that the worldwide prevalence of ABCC6 Deficiency may be much higher than current estimates, which are based solely on biallelic
cases. Currently, there are no approved therapies for ABCC6 Deficiency.
About Inozyme Pharma
Inozyme Pharma is a pioneering clinical-stage biopharmaceutical company dedicated to developing innovative therapeutics for rare diseases that affect bone
health and blood vessel function. We are experts in the PPi-Adenosine Pathway, where the ENPP1 enzyme generates inorganic pyrophosphate (PPi), which regulates mineralization, and adenosine, which controls intimal proliferation (the overgrowth of
smooth muscle cells inside blood vessels). Disruptions in this pathway impact the levels of these molecules, leading to severe musculoskeletal, cardiovascular, and neurological conditions, including ENPP1 Deficiency, ABCC6 Deficiency, calciphylaxis,
and ossification of the posterior longitudinal ligament (OPLL).
Our lead candidate, INZ-701, is an ENPP1 Fc
fusion protein enzyme replacement therapy (ERT) designed to increase PPi and adenosine, enabling the potential treatment of multiple diseases caused by deficiencies in these molecules. It is currently in clinical development for the treatment of
ENPP1 Deficiency, ABCC6 Deficiency, and calciphylaxis. By targeting the PPi-Adenosine Pathway, INZ-701 aims to correct pathological mineralization and intimal proliferation, addressing the significant
morbidity and mortality in these devastating diseases.
For more information, please visit https://www.inozyme.com/ or follow Inozyme
on LinkedIn, X, and Facebook.
Cautionary Note Regarding Forward-Looking Statements
Statements in this press release about future expectations, plans, and prospects, as well as any other statements regarding matters that are not historical
facts, may constitute “forward-looking statements” within the meaning of The Private Securities Litigation Reform Act of 1995. These statements include, but are not limited to, statements relating to the initiation, timing, and design of
our planned clinical trials, enrollment and availability of data from clinical trials, the potential benefits of INZ-701 and our regulatory strategy. The
words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,”
“potential,” “predict,” “project,” “should,” “target,” “will,” “would,” and similar expressions are intended to identify forward-looking statements, although not all
forward-looking statements contain these identifying words. Any forward-looking statements are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to
differ materially and adversely from those set forth in, or implied by, such forward-looking statements. These risks and uncertainties include, but are not limited to, risks associated with the Company’s ability to conduct its ongoing clinical
trials of INZ-701 for ENPP1 Deficiency, ABCC6 Deficiency, and calciphylaxis; enroll patients in ongoing and planned trials; obtain and maintain necessary approvals from the FDA and other regulatory
authorities; continue to advance its product candidates in preclinical studies and clinical trials; replicate in later clinical trials positive results found in preclinical studies and early-stage clinical trials of its product candidates; advance
the development of its product candidates under the timelines it anticipates in planned and future clinical trials; obtain, maintain, and protect intellectual property rights related to its product candidates; manage expenses; comply with covenants
under its outstanding loan agreement; and raise the substantial additional capital needed to achieve its business objectives. For a discussion of other risks and uncertainties, and other important factors, any of which could cause the Company’s
actual results to differ from those contained in the forward-looking statements, see the “Risk Factors” section in the Company’s most recent Annual Report on Form 10-K filed with the Securities
and Exchange Commission, as well as discussions of potential risks, uncertainties, and other important factors, in the Company’s most recent filings with the Securities and Exchange Commission. In addition, the forward-looking statements
included in this press release represent the Company’s views as of the date hereof and should not be relied upon as representing the Company’s views as of any date subsequent to the date hereof. The Company anticipates that subsequent
events and developments will cause the Company’s views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, the Company specifically disclaims any obligation to do so.
Contacts
Investors:
Inozyme Pharma
Stefan Riley, Senior Director of IR and Corporate
Communications
(617) 461-2442
stefan.riley@inozyme.com
Media:
Biongage Communications
Todd Cooper
(617) 840-1637
todd@biongage.com

Exhibit 99.2 Interim clinical and safety data of INZ-701 treatment in
infants and young children with ENPP1 Deficiency and key program updates January 2025 Ella Living with ENPP1 Deficiency

Legal disclaimer This presentation and any statements made orally during
this presentation contain estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to
give undue weight to such data and estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and
risk. Neither Inozyme Pharma, Inc. nor its affiliates, advisors or representatives make any representations as to the accuracy or completeness of that data or undertakes to update such data after the date of this presentation. Forward-Looking
Statement Disclaimer Statements in this presentation about future expectations, plans, and prospects, as well as any other statements regarding matters that are not historical facts, may constitute forward-looking statements that involve substantial
risks and uncertainties. These statements include, but are not limited to, statements relating to the initiation, timing, and design of our planned clinical trials, the potential benefits of INZ-701 and our regulatory strategy. The words
“anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “potential,”
“predict,” “project,” “should,” “target,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking
statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results
or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements. For a discussion of risks and uncertainties, and other important factors, any of which could cause our actual results to
differ from those contained in the forward-looking statements, see the “Risk Factors” section in our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission, as well as discussions of potential risks,
uncertainties and other important factors, in the Company’s most recent filings with the Securities and Exchange Commission. In addition, the forward-looking statements included in this presentation represent our views as of the date of this
presentation. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements in the future, we specifically disclaim any obligation to do so. These
forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this presentation. 2
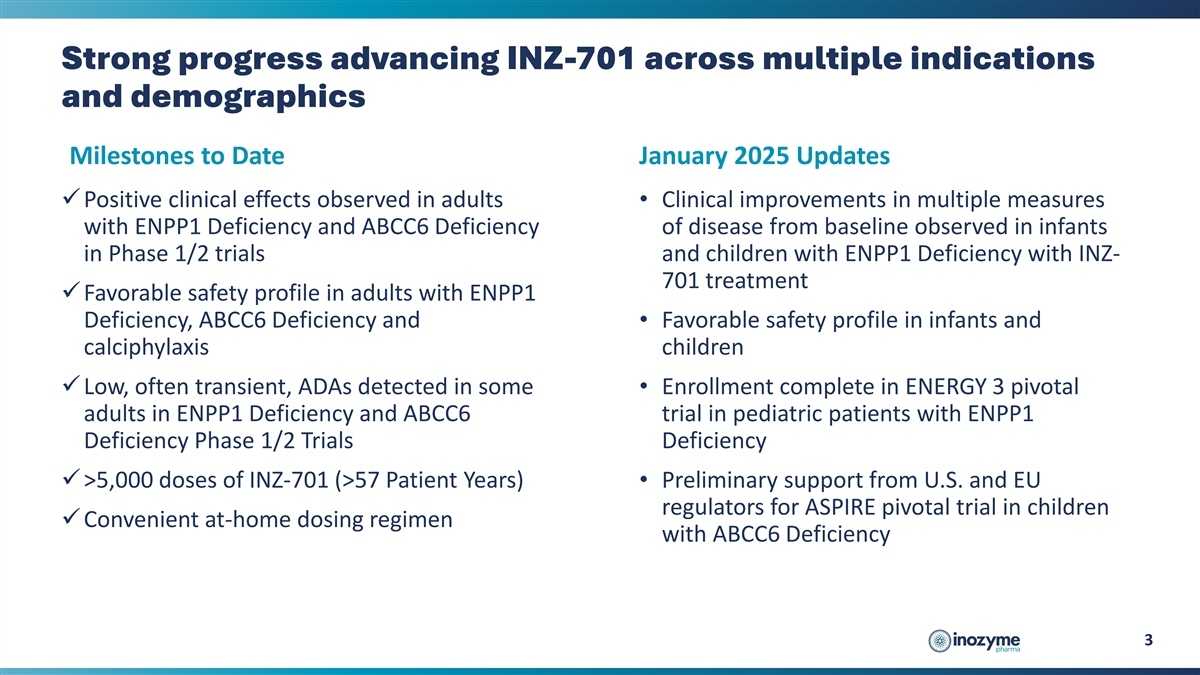
Strong progress advancing INZ-701 across multiple indications and
demographics Milestones to Date January 2025 Updates üPositive clinical effects observed in adults • Clinical improvements in multiple measures with ENPP1 Deficiency and ABCC6 Deficiency of disease from baseline observed in infants in
Phase 1/2 trials and children with ENPP1 Deficiency with INZ- 701 treatment üFavorable safety profile in adults with ENPP1 Deficiency, ABCC6 Deficiency and • Favorable safety profile in infants and calciphylaxis children üLow, often
transient, ADAs detected in some • Enrollment complete in ENERGY 3 pivotal adults in ENPP1 Deficiency and ABCC6 trial in pediatric patients with ENPP1 Deficiency Phase 1/2 Trials Deficiency ü >5,000 doses of INZ-701 (>57 Patient
Years) • Preliminary support from U.S. and EU regulators for ASPIRE pivotal trial in children üConvenient at-home dosing regimen with ABCC6 Deficiency 3
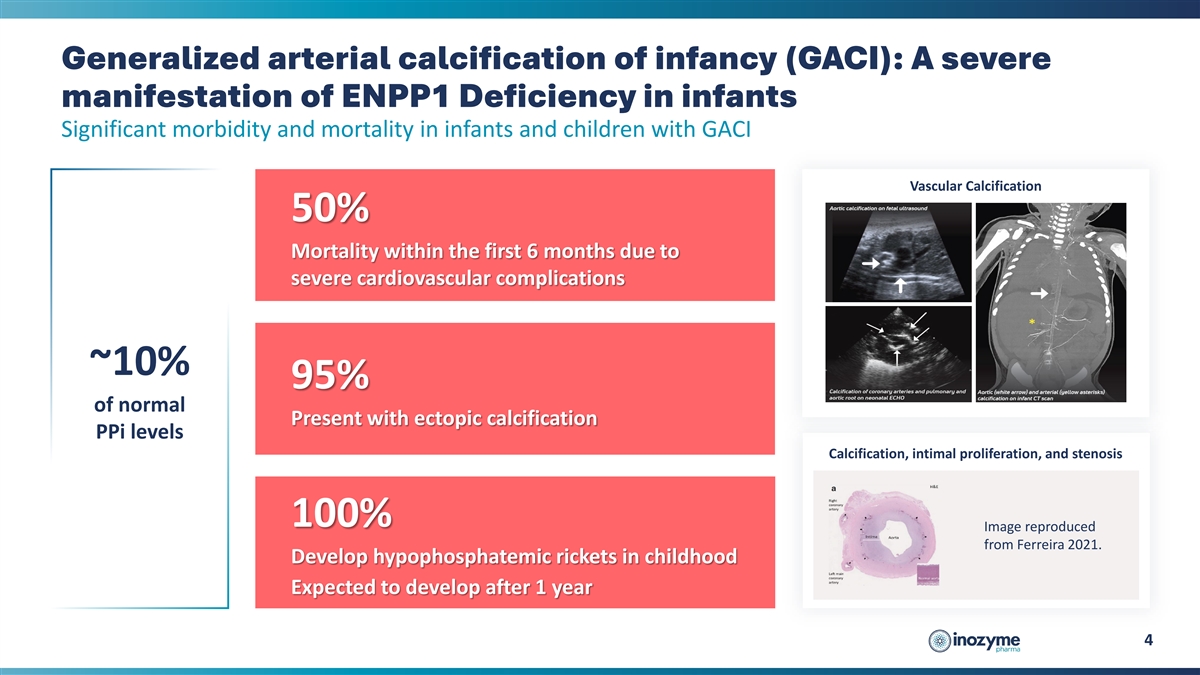
Generalized arterial calcification of infancy (GACI): A severe
manifestation of ENPP1 Deficiency in infants Significant morbidity and mortality in infants and children with GACI Vascular Calcification 50% Mortality within the first 6 months due to severe cardiovascular complications ~10% 95% of normal Present
with ectopic calcification PPi levels Calcification, intimal proliferation, and stenosis 100% Image reproduced from Ferreira 2021. Develop hypophosphatemic rickets in childhood Expected to develop after 1 year 4
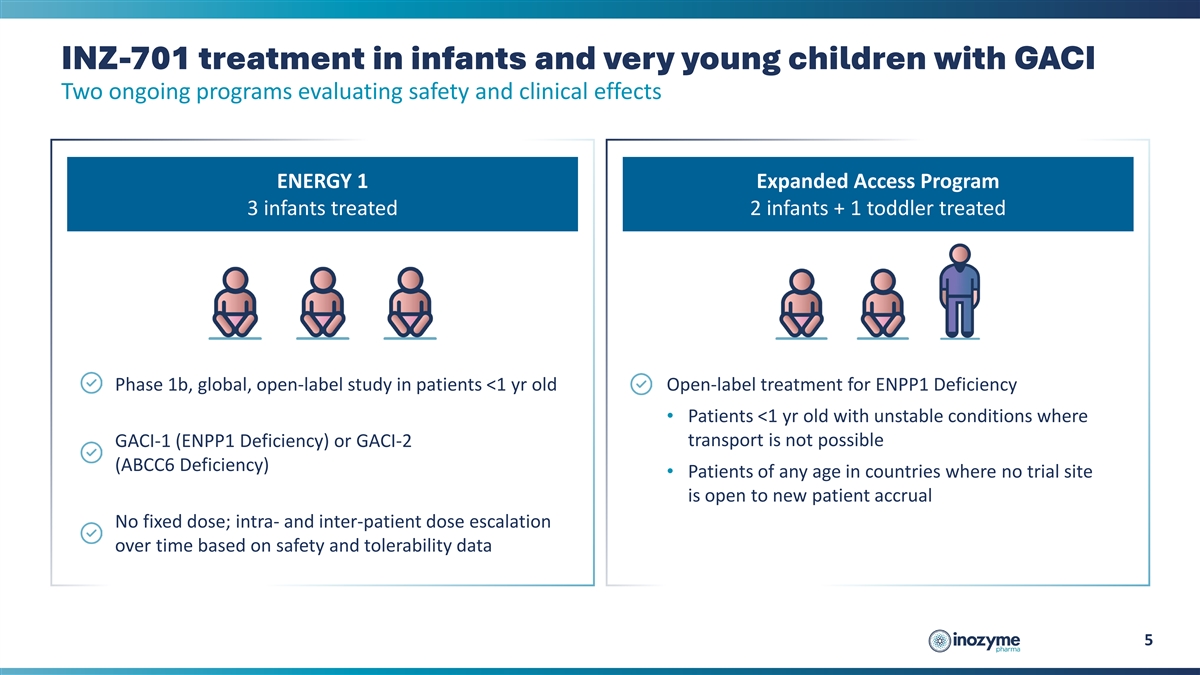
INZ-701 treatment in infants and very young children with GACI Two
ongoing programs evaluating safety and clinical effects ENERGY 1 Expanded Access Program 3 infants treated 2 infants + 1 toddler treated Phase 1b, global, open-label study in patients <1 yr old Open-label treatment for ENPP1 Deficiency •
Patients <1 yr old with unstable conditions where transport is not possible GACI-1 (ENPP1 Deficiency) or GACI-2 (ABCC6 Deficiency) • Patients of any age in countries where no trial site is open to new patient accrual No fixed dose; intra-
and inter-patient dose escalation over time based on safety and tolerability data 5
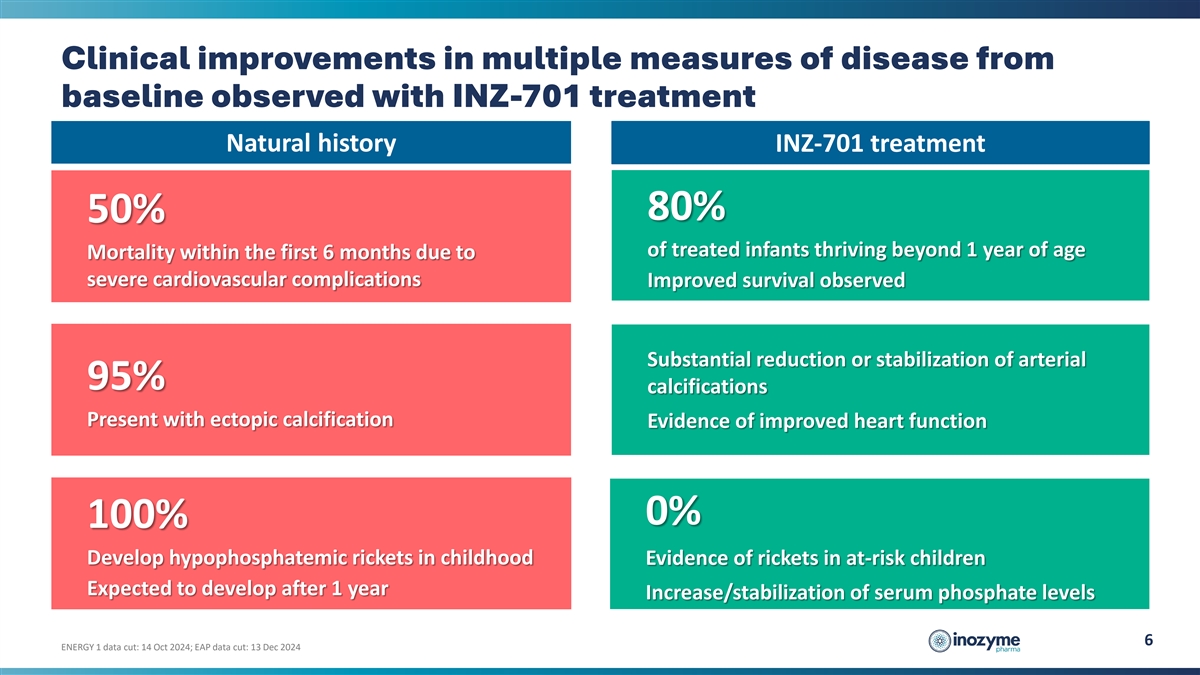
Clinical improvements in multiple measures of disease from baseline
observed with INZ-701 treatment Natural history INZ-701 treatment 80% 50% of treated infants thriving beyond 1 year of age Mortality within the first 6 months due to severe cardiovascular complications Improved survival observed Substantial
reduction or stabilization of arterial 95% calcifications Present with ectopic calcification Evidence of improved heart function 0% 100% Develop hypophosphatemic rickets in childhood Evidence of rickets in at-risk children Expected to develop after
1 year Increase/stabilization of serum phosphate levels 6 ENERGY 1 data cut: 14 Oct 2024; EAP data cut: 13 Dec 2024
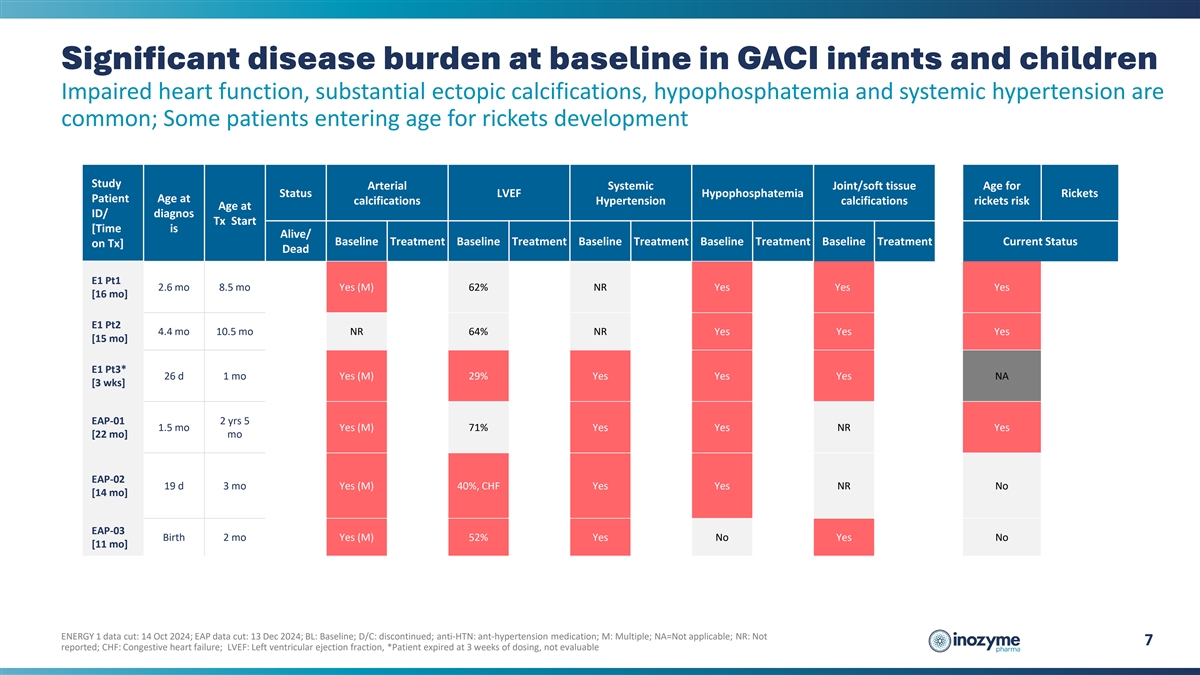
Significant disease burden at baseline in GACI infants and children
Impaired heart function, substantial ectopic calcifications, hypophosphatemia and systemic hypertension are common; Some patients entering age for rickets development Study Arterial Systemic Joint/soft tissue Age for Status LVEF Hypophosphatemia
Rickets Patient Age at calcifications Hypertension calcifications rickets risk Age at ID/ diagnos Tx Start [Time is Alive/ Baseline Treatment Baseline Treatment Baseline Treatment Baseline Treatment Baseline Treatment Current Status on Tx] Dead E1
Pt1 2.6 mo 8.5 mo Yes (M) 62% NR Yes Yes Yes [16 mo] E1 Pt2 4.4 mo 10.5 mo NR 64% NR Yes Yes Yes [15 mo] E1 Pt3* 26 d 1 mo Yes (M) 29% Yes Yes Yes NA [3 wks] EAP-01 2 yrs 5 1.5 mo Yes (M) 71% Yes Yes NR Yes [22 mo] mo EAP-02 19 d 3 mo Yes (M) 40%,
CHF Yes Yes NR No [14 mo] EAP-03 Birth 2 mo Yes (M) 52% Yes No Yes No [11 mo] ENERGY 1 data cut: 14 Oct 2024; EAP data cut: 13 Dec 2024; BL: Baseline; D/C: discontinued; anti-HTN: ant-hypertension medication; M: Multiple; NA=Not applicable; NR: Not
7 reported; CHF: Congestive heart failure; LVEF: Left ventricular ejection fraction, *Patient expired at 3 weeks of dosing, not evaluable
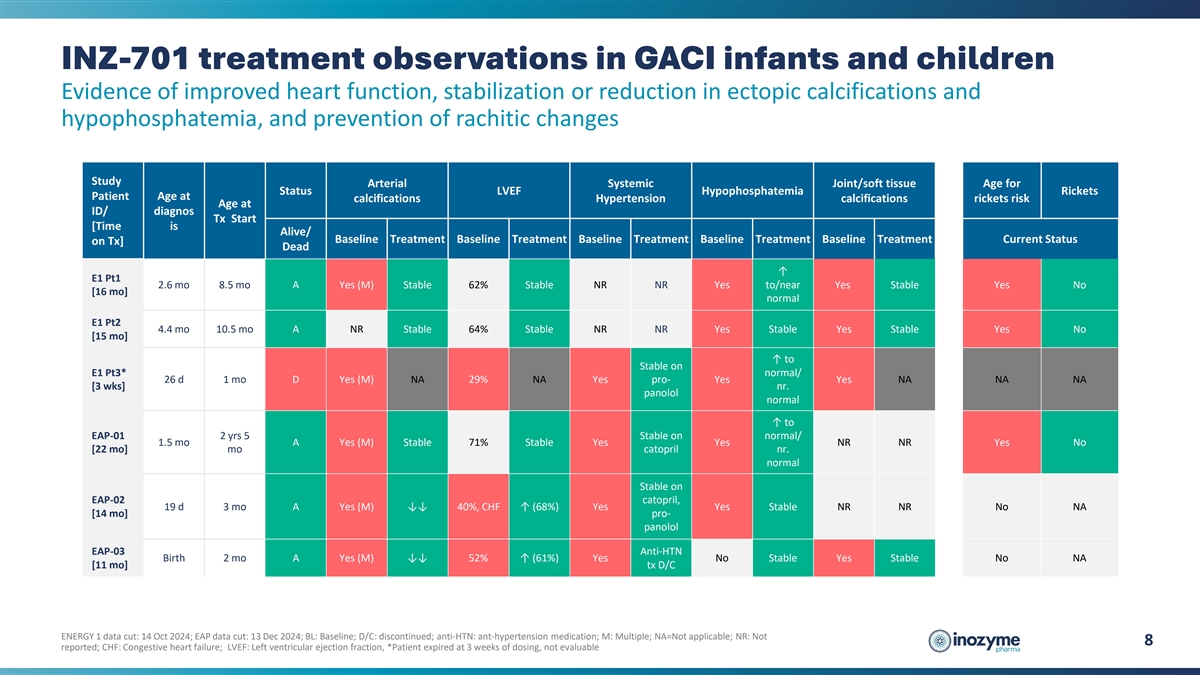
INZ-701 treatment observations in GACI infants and children Evidence of
improved heart function, stabilization or reduction in ectopic calcifications and hypophosphatemia, and prevention of rachitic changes Study Arterial Systemic Joint/soft tissue Age for Status LVEF Hypophosphatemia Rickets Patient Age at
calcifications Hypertension calcifications rickets risk Age at ID/ diagnos Tx Start [Time is Alive/ Baseline Treatment Baseline Treatment Baseline Treatment Baseline Treatment Baseline Treatment Current Status on Tx] Dead ↑ E1 Pt1 2.6 mo 8.5
mo A Yes (M) Stable 62% Stable NR NR Yes to/near Yes Stable Yes No [16 mo] normal E1 Pt2 4.4 mo 10.5 mo A NR Stable 64% Stable NR NR Yes Stable Yes Stable Yes No [15 mo] ↑ to Stable on E1 Pt3* normal/ 26 d 1 mo D Yes (M) NA 29% NA Yes pro- Yes
Yes NA NA NA [3 wks] nr. panolol normal ↑ to EAP-01 2 yrs 5 Stable on normal/ 1.5 mo A Yes (M) Stable 71% Stable Yes Yes NR NR Yes No [22 mo] mo catopril nr. normal Stable on EAP-02 catopril, 19 d 3 mo A Yes (M) ↓↓ 40%, CHF ↑
(68%) Yes Yes Stable NR NR No NA [14 mo] pro- panolol EAP-03 Anti-HTN Birth 2 mo A Yes (M) ↓↓ 52% ↑ (61%) Yes No Stable Yes Stable No NA [11 mo] tx D/C ENERGY 1 data cut: 14 Oct 2024; EAP data cut: 13 Dec 2024; BL: Baseline; D/C:
discontinued; anti-HTN: ant-hypertension medication; M: Multiple; NA=Not applicable; NR: Not 8 reported; CHF: Congestive heart failure; LVEF: Left ventricular ejection fraction, *Patient expired at 3 weeks of dosing, not evaluable
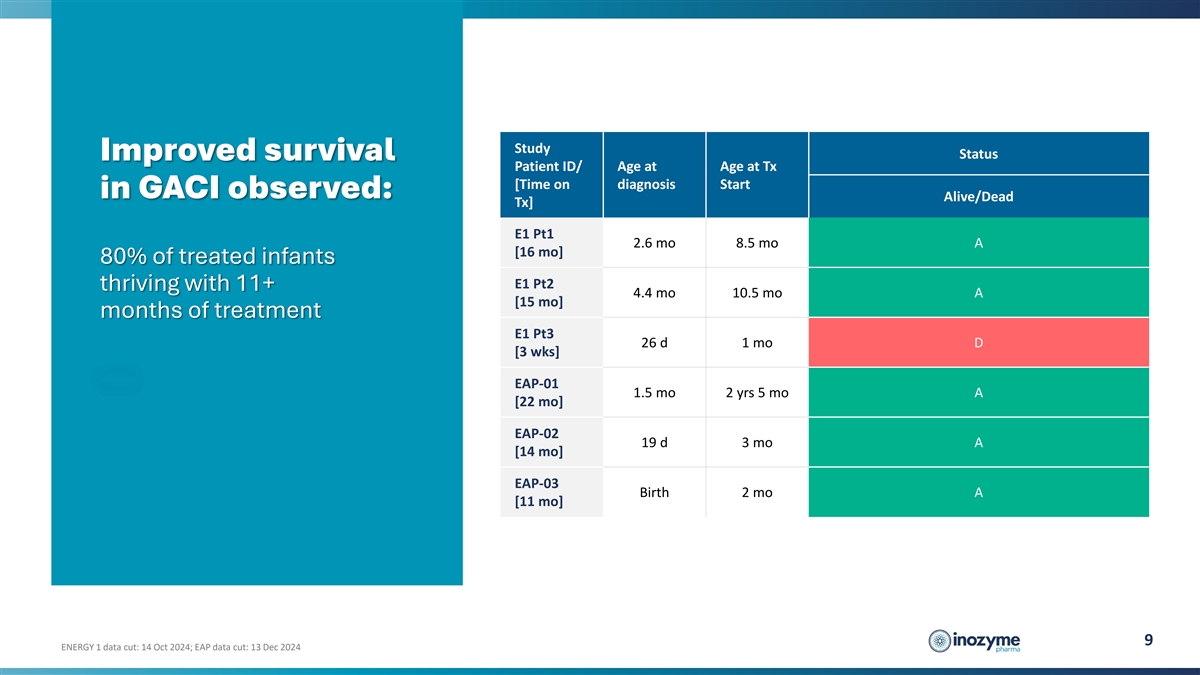
Study Status Improved survival Patient ID/ Age at Age at Tx [Time on
diagnosis Start in GACI observed: Alive/Dead Tx] E1 Pt1 2.6 mo 8.5 mo A [16 mo] 80% of treated infants E1 Pt2 thriving with 11+ 4.4 mo 10.5 mo A [15 mo] months of treatment E1 Pt3 26 d 1 mo D [3 wks] EAP-01 1.5 mo 2 yrs 5 mo A [22 mo] EAP-02 19 d 3
mo A [14 mo] EAP-03 Birth 2 mo A [11 mo] 9 ENERGY 1 data cut: 14 Oct 2024; EAP data cut: 13 Dec 2024
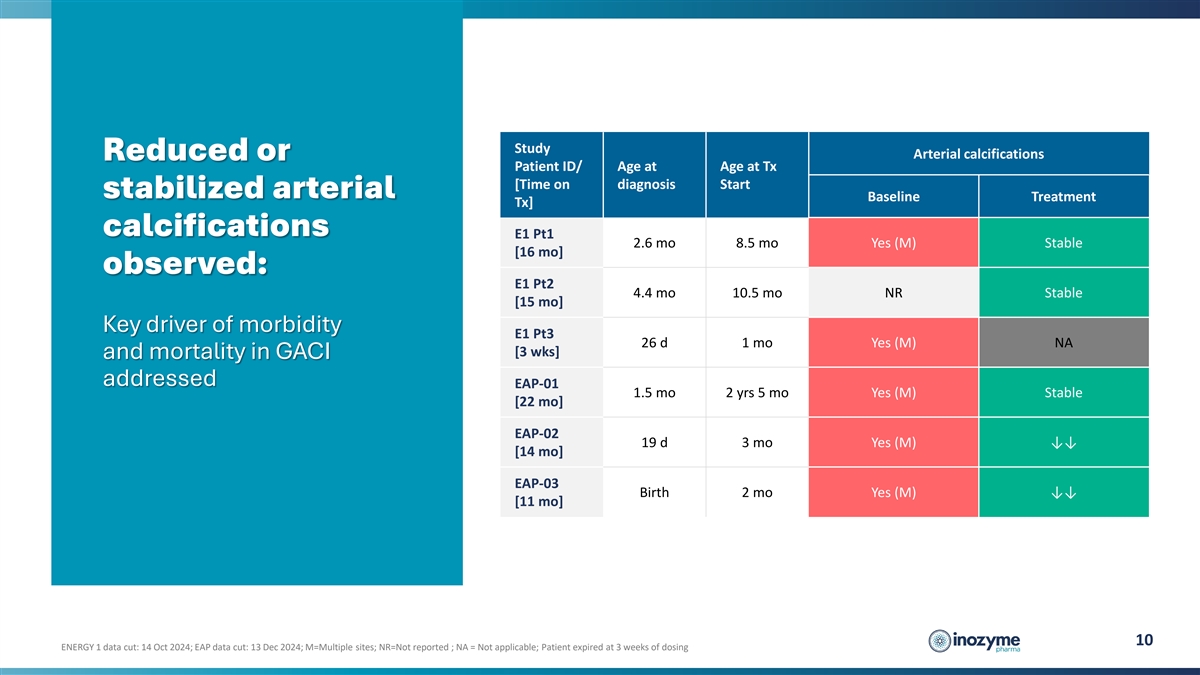
Study Arterial calcifications Reduced or Patient ID/ Age at Age at Tx
[Time on diagnosis Start stabilized arterial Baseline Treatment Tx] calcifications E1 Pt1 2.6 mo 8.5 mo Yes (M) Stable [16 mo] observed: E1 Pt2 4.4 mo 10.5 mo NR Stable [15 mo] Key driver of morbidity E1 Pt3 26 d 1 mo Yes (M) NA [3 wks] and
mortality in GACI addressed EAP-01 1.5 mo 2 yrs 5 mo Yes (M) Stable [22 mo] EAP-02 19 d 3 mo Yes (M) ↓↓ [14 mo] EAP-03 Birth 2 mo Yes (M) ↓↓ [11 mo] 10 ENERGY 1 data cut: 14 Oct 2024; EAP data cut: 13 Dec 2024; M=Multiple
sites; NR=Not reported ; NA = Not applicable; Patient expired at 3 weeks of dosing
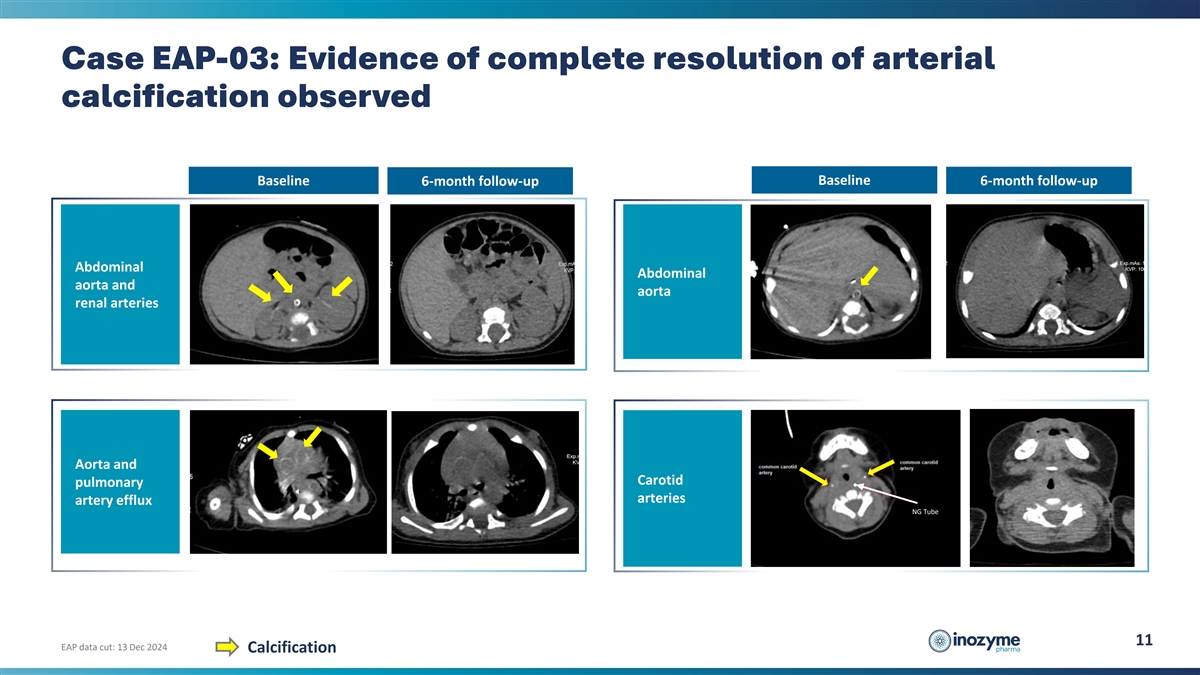
Case EAP-03: Evidence of complete resolution of arterial calcification
observed Baseline Baseline 6-month follow-up 6-month follow-up Abdominal Abdominal aorta and aorta renal arteries Aorta and Carotid pulmonary arteries artery efflux NG Tube 11 EAP data cut: 13 Dec 2024 Calcification
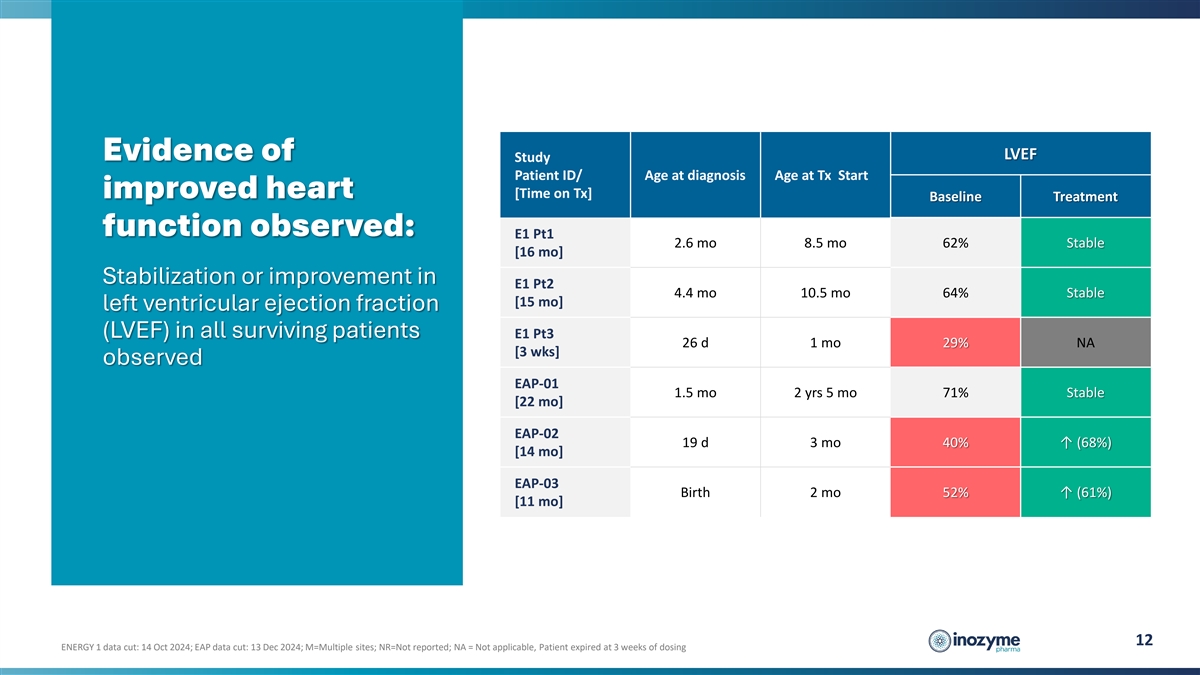
LVEF Evidence of Study Patient ID/ Age at diagnosis Age at Tx Start
improved heart [Time on Tx] Baseline Treatment function observed: E1 Pt1 2.6 mo 8.5 mo 62% Stable [16 mo] Stabilization or improvement in E1 Pt2 4.4 mo 10.5 mo 64% Stable [15 mo] left ventricular ejection fraction (LVEF) in all surviving patients E1
Pt3 26 d 1 mo 29% NA [3 wks] observed EAP-01 1.5 mo 2 yrs 5 mo 71% Stable [22 mo] EAP-02 19 d 3 mo 40% ↑ (68%) [14 mo] EAP-03 Birth 2 mo 52% ↑ (61%) [11 mo] 12 ENERGY 1 data cut: 14 Oct 2024; EAP data cut: 13 Dec 2024; M=Multiple sites;
NR=Not reported; NA = Not applicable, Patient expired at 3 weeks of dosing
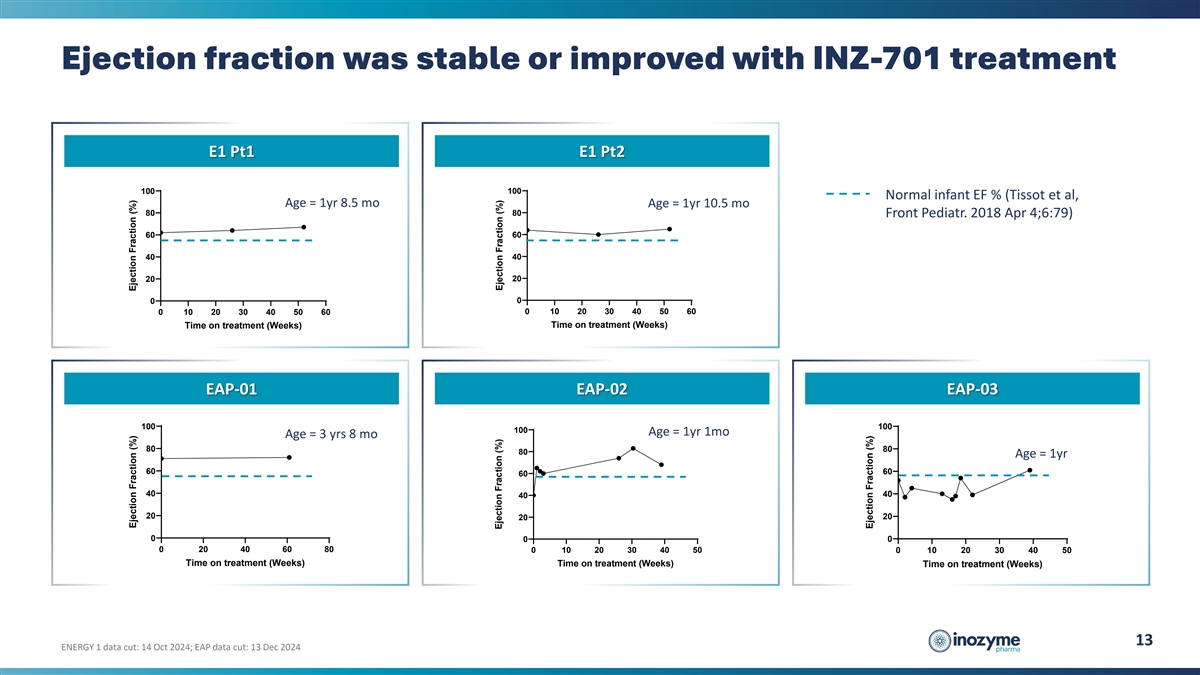
Ejection fraction was stable or improved with INZ-701 treatment E1 Pt1
E1 Pt2 100 100 Normal infant EF % (Tissot et al, Age = 1yr 8.5 mo Age = 1yr 10.5 mo 80 80 Front Pediatr. 2018 Apr 4;6:79) 60 60 40 40 20 20 0 0 0 10 20 30 40 50 60 0 10 20 30 40 50 60 Time on treatment (Weeks) Time on treatment (Weeks) EAP-01 EAP-02
EAP-03 100 100 100 Age = 1yr 1mo Age = 3 yrs 8 mo 80 80 80 Age = 1yr 60 60 60 40 40 40 20 20 20 0 0 0 0 20 40 60 80 0 10 20 30 40 50 0 10 20 30 40 50 Time on treatment (Weeks) Time on treatment (Weeks) Time on treatment (Weeks) 13 ENERGY 1 data cut:
14 Oct 2024; EAP data cut: 13 Dec 2024 Ejection Fraction (%) Ejection Fraction (%) Ejection Fraction (%) Ejection Fraction (%) Ejection Fraction (%)
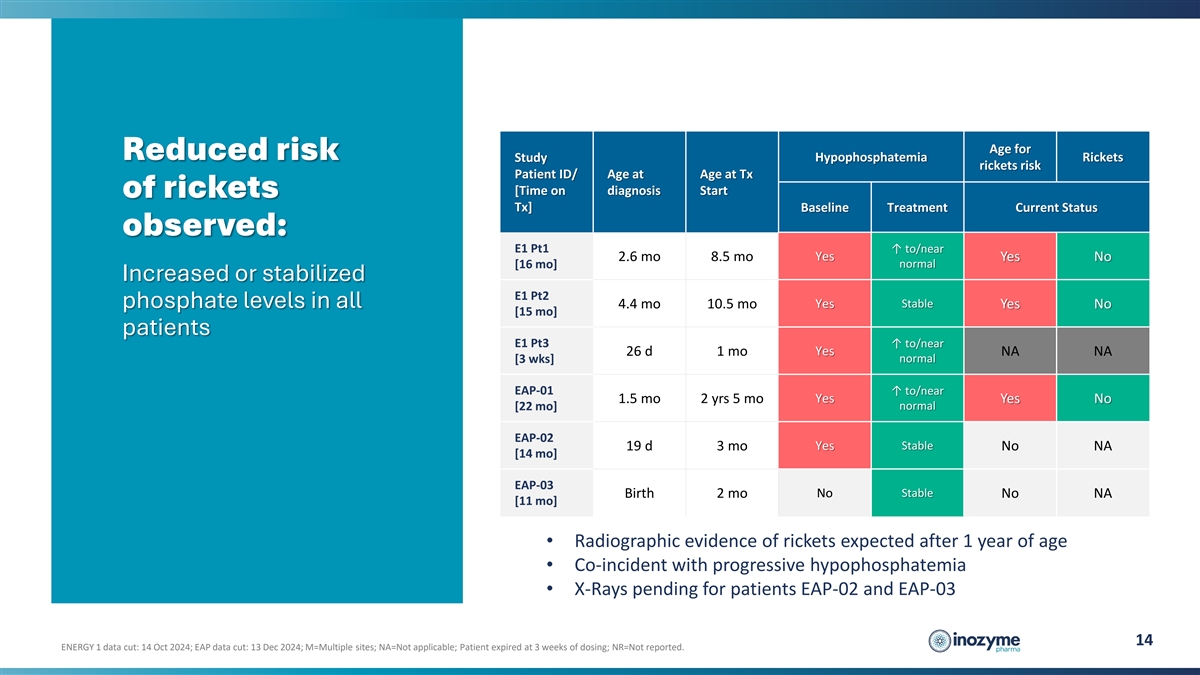
Age for Reduced risk Hypophosphatemia Rickets Study rickets risk
Patient ID/ Age at Age at Tx [Time on diagnosis Start of rickets Tx] Baseline Treatment Current Status observed: E1 Pt1 ↑ to/near 2.6 mo 8.5 mo Yes Yes No normal [16 mo] Increased or stabilized E1 Pt2 Stable phosphate levels in all 4.4 mo 10.5
mo Yes Yes No [15 mo] patients E1 Pt3 ↑ to/near Yes 26 d 1 mo NA NA normal [3 wks] EAP-01 ↑ to/near Yes 1.5 mo 2 yrs 5 mo Yes No [22 mo] normal EAP-02 Yes Stable 19 d 3 mo No NA [14 mo] EAP-03 Birth 2 mo No Stable No NA [11 mo] •
Radiographic evidence of rickets expected after 1 year of age • Co-incident with progressive hypophosphatemia • X-Rays pending for patients EAP-02 and EAP-03 14 ENERGY 1 data cut: 14 Oct 2024; EAP data cut: 13 Dec 2024; M=Multiple sites;
NA=Not applicable; Patient expired at 3 weeks of dosing; NR=Not reported.
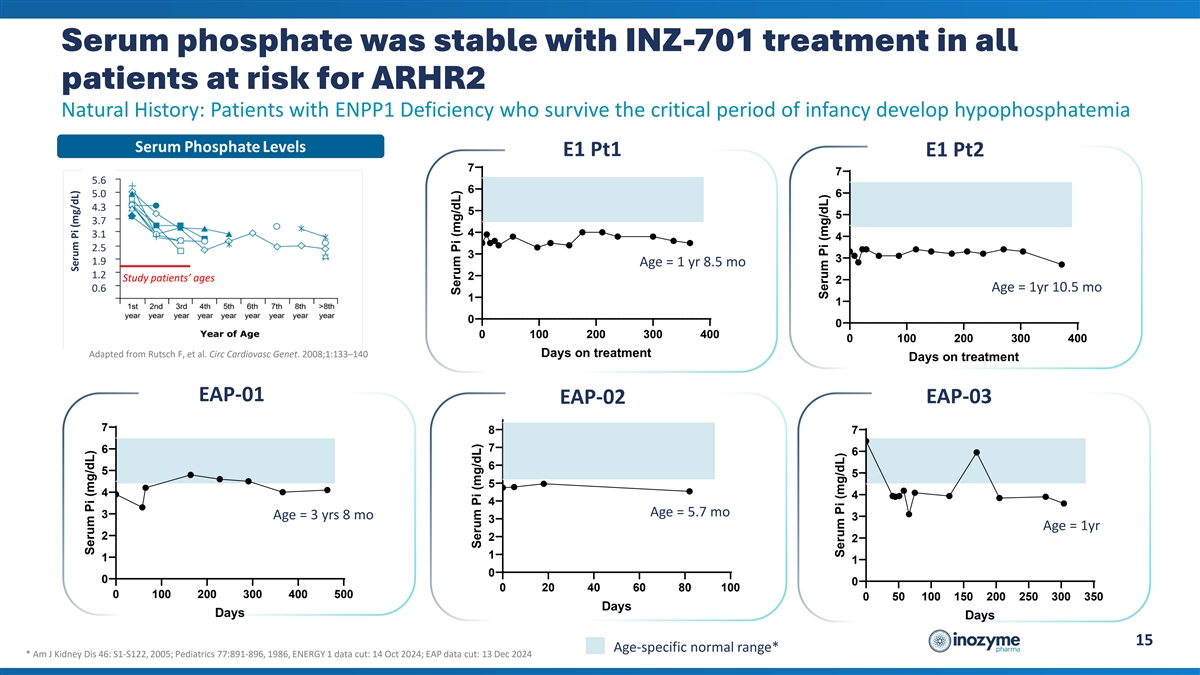
Serum phosphate was stable with INZ-701 treatment in all patients at
risk for ARHR2 Natural History: Patients with ENPP1 Deficiency who survive the critical period of infancy develop hypophosphatemia E1 Pt1 E1 Pt2 7 7 5.6 6 5.0 6 4.3 5 5 3.7 4 3.1 4 2.5 3 3 1.9 Age = 1 yr 8.5 mo 1.2 2 Study patients’ ages 2 Age
= 1yr 10.5 mo 0.6 1 1 0 0 0 100 200 300 400 0 100 200 300 400 Adapted from Rutsch F, et al. Circ Cardiovasc Genet. 2008;1:133–140 Days on treatment Days on treatment EAP-01 EAP-02 EAP-03 7 8 7 7 6 6 6 5 5 5 4 4 4 Age = 5.7 mo 3 Age = 3 yrs 8
mo 3 3 Age = 1yr 2 2 2 1 1 1 0 0 0 0 20 40 60 80 100 0 100 200 300 400 500 0 50 100 150 200 250 300 350 Days Days Days 15 Age-specific normal range* * Am J Kidney Dis 46: S1-S122, 2005; Pediatrics 77:891-896, 1986, ENERGY 1 data cut: 14 Oct 2024;
EAP data cut: 13 Dec 2024 Serum Pi (mg/dL) Serum Pi (mg/dL) Serum Pi (mg/dL) Serum Pi (mg/dL) Serum Pi (mg/dL) Serum Pi (mg/dL)
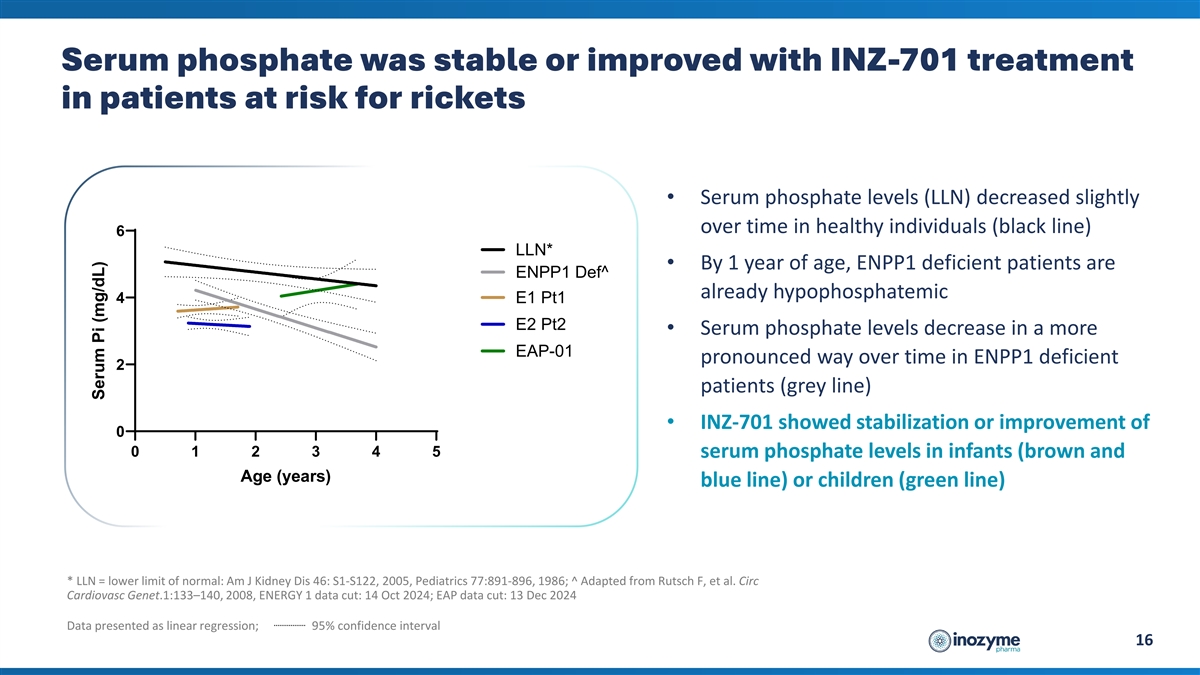
Serum phosphate was stable or improved with INZ-701 treatment in
patients at risk for rickets • Serum phosphate levels (LLN) decreased slightly over time in healthy individuals (black line) 6 LLN* • By 1 year of age, ENPP1 deficient patients are ENPP1 Def^ already hypophosphatemic 4 E1 Pt1 E2 Pt2
• Serum phosphate levels decrease in a more EAP-01 pronounced way over time in ENPP1 deficient 2 patients (grey line) • INZ-701 showed stabilization or improvement of 0 0 1 2 3 4 5 serum phosphate levels in infants (brown and Age (years)
blue line) or children (green line) * LLN = lower limit of normal: Am J Kidney Dis 46: S1-S122, 2005, Pediatrics 77:891-896, 1986; ^ Adapted from Rutsch F, et al. Circ Cardiovasc Genet.1:133–140, 2008, ENERGY 1 data cut: 14 Oct 2024; EAP data
cut: 13 Dec 2024 Data presented as linear regression; 95% confidence interval 16 Serum Pi (mg/dL)
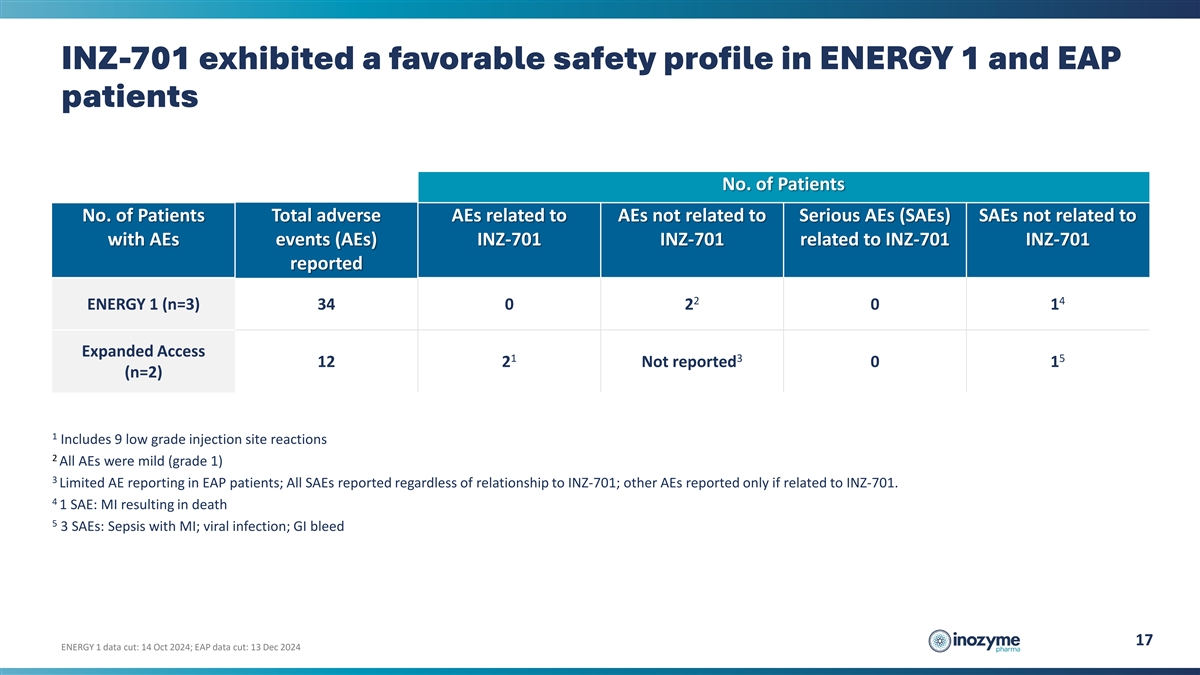
INZ-701 exhibited a favorable safety profile in ENERGY 1 and EAP
patients No. of Patients No. of Patients Total adverse AEs related to AEs not related to Serious AEs (SAEs) SAEs not related to with AEs events (AEs) INZ-701 INZ-701 related to INZ-701 INZ-701 reported 2 4 ENERGY 1 (n=3) 34 0 2 0 1 Expanded Access 1
3 5 12 2 Not reported 0 1 (n=2) 1 Includes 9 low grade injection site reactions 2 All AEs were mild (grade 1) 3 Limited AE reporting in EAP patients; All SAEs reported regardless of relationship to INZ-701; other AEs reported only if related to
INZ-701. 4 1 SAE: MI resulting in death 5 3 SAEs: Sepsis with MI; viral infection; GI bleed 17 ENERGY 1 data cut: 14 Oct 2024; EAP data cut: 13 Dec 2024
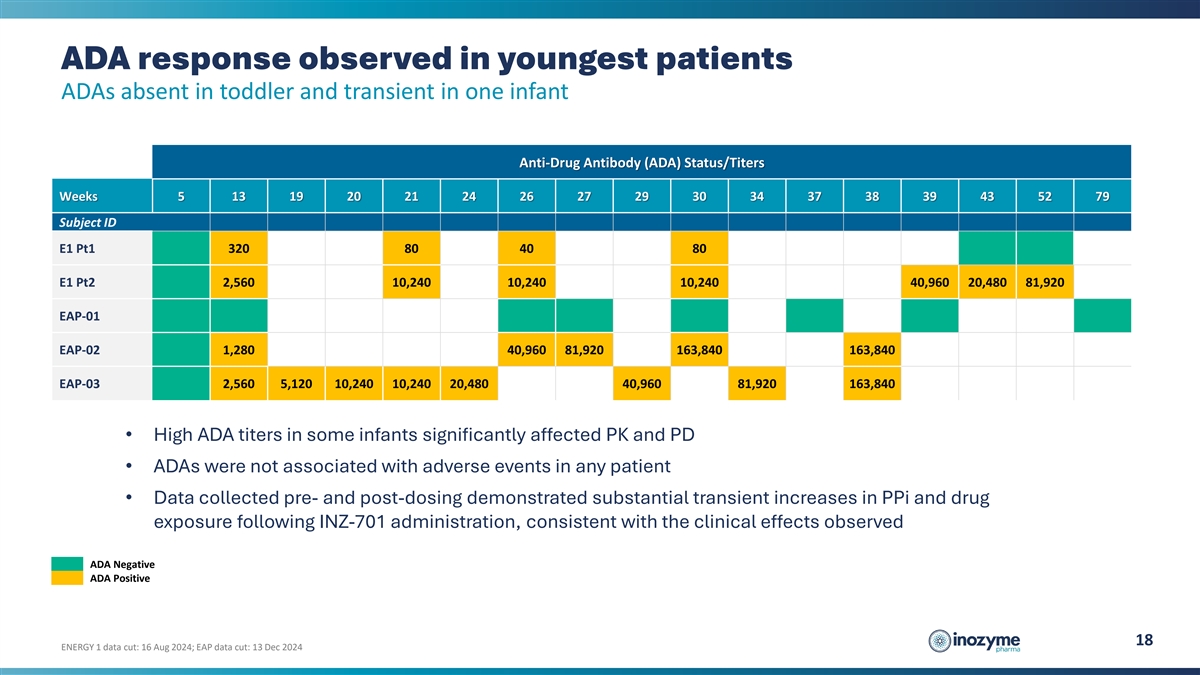
ADA response observed in youngest patients ADAs absent in toddler and
transient in one infant Anti-Drug Antibody (ADA) Status/Titers Weeks 5 13 19 20 21 24 26 27 29 30 34 37 38 39 43 52 79 Subject ID E1 Pt1 320 80 40 80 E1 Pt2 2,560 10,240 10,240 10,240 40,960 20,480 81,920 EAP-01 EAP-02 1,280 40,960 81,920 163,840
163,840 EAP-03 2,560 5,120 10,240 10,240 20,480 40,960 81,920 163,840 • High ADA titers in some infants significantly affected PK and PD • ADAs were not associated with adverse events in any patient • Data collected pre- and
post-dosing demonstrated substantial transient increases in PPi and drug exposure following INZ-701 administration, consistent with the clinical effects observed ADA Negative ADA Positive 18 ENERGY 1 data cut: 16 Aug 2024; EAP data cut: 13 Dec
2024
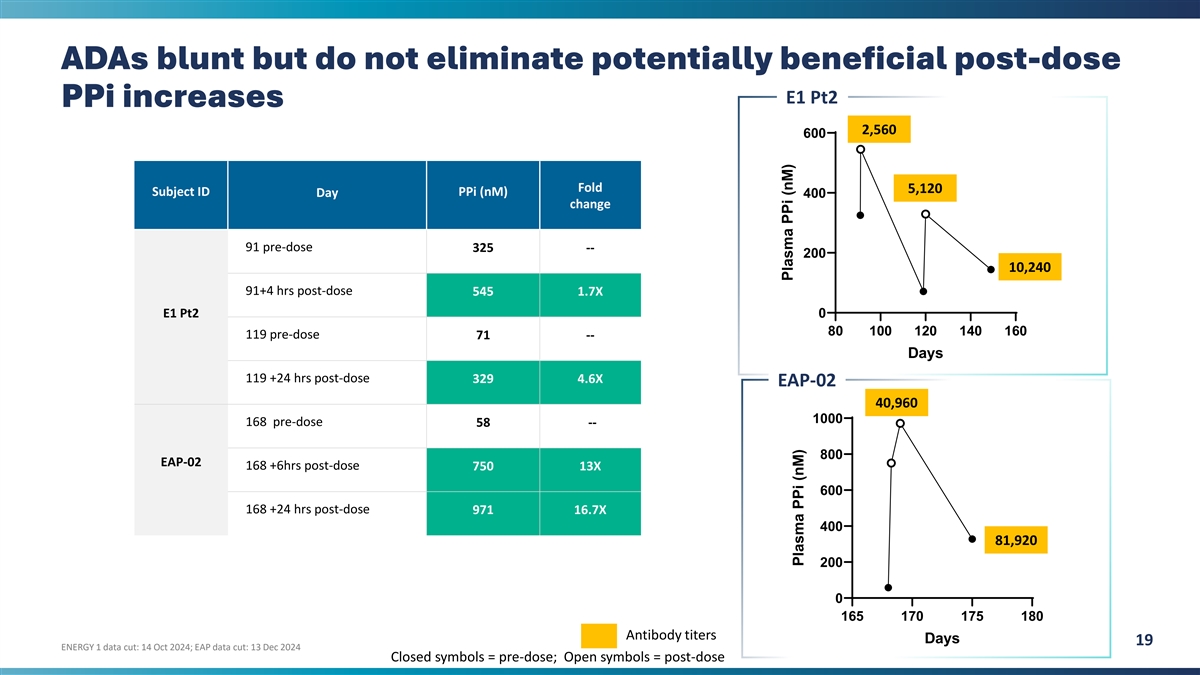
ADAs blunt but do not eliminate potentially beneficial post-dose E1 Pt2
PPi increases 2,560 600 Fold 5,120 Subject ID PPi (nM) Day 400 change 91 pre-dose 325 -- 200 10,240 91+4 hrs post-dose 545 1.7X E1 Pt2 0 80 100 120 140 160 119 pre-dose 71 -- Days 119 +24 hrs post-dose 329 4.6X EAP-02 40,960 1000 168 pre-dose 58 ---
800 EAP-02 168 +6hrs post-dose 750 13X 600 168 +24 hrs post-dose 971 16.7X 400 81,920 200 0 165 170 175 180 Antibody titers Days 19 ENERGY 1 data cut: 14 Oct 2024; EAP data cut: 13 Dec 2024 Closed symbols = pre-dose; Open symbols = post-dose Plasma
PPi (nM) Plasma PPi (nM)
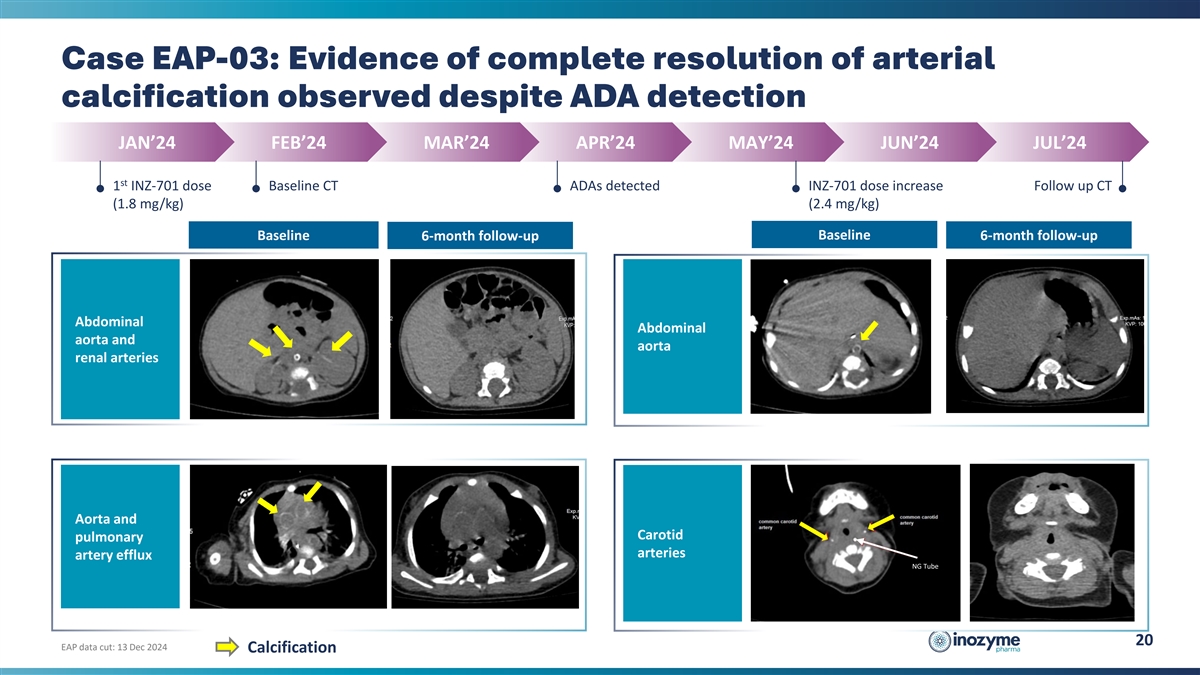
Case EAP-03: Evidence of complete resolution of arterial calcification
observed despite ADA detection JAN’24 FEB’24 MAR’24 APR’24 MAY’24 JUN’24 JUL’24 st 1 INZ-701 dose Baseline CT ADAs detected INZ-701 dose increase Follow up CT (1.8 mg/kg) (2.4 mg/kg) Baseline Baseline
6-month follow-up 6-month follow-up Abdominal Abdominal aorta and aorta renal arteries Aorta and Carotid pulmonary arteries artery efflux NG Tube 20 EAP data cut: 13 Dec 2024 Calcification
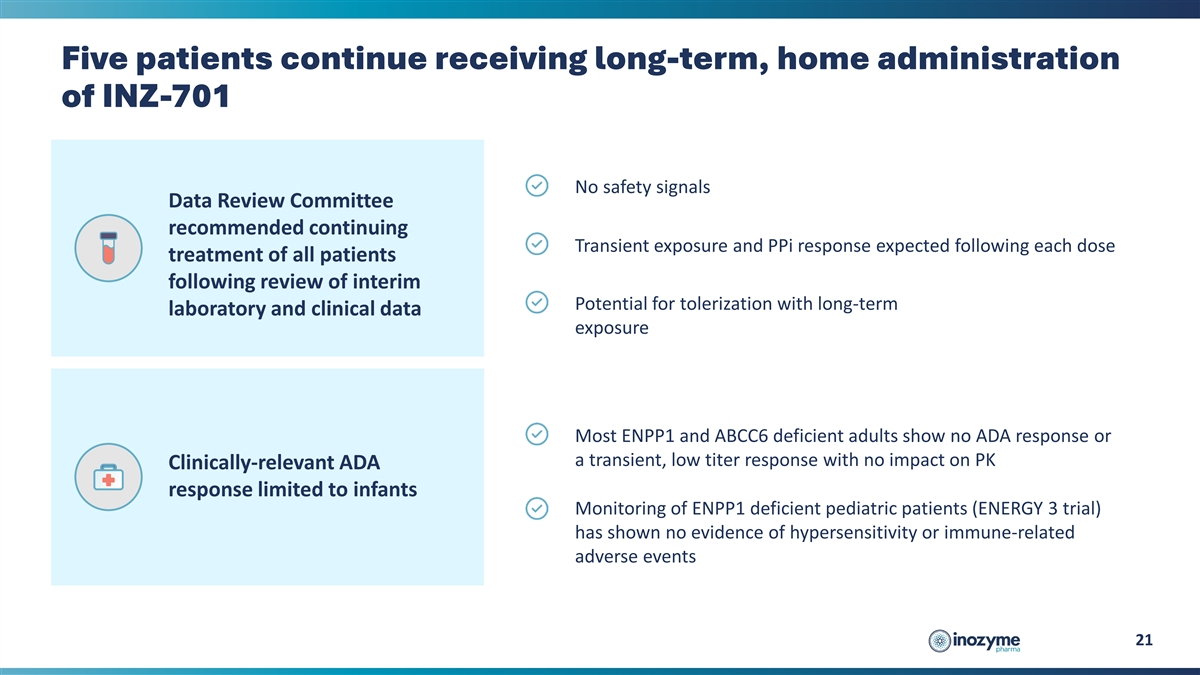
Five patients continue receiving long-term, home administration of
INZ-701 No safety signals Data Review Committee recommended continuing Transient exposure and PPi response expected following each dose treatment of all patients following review of interim Potential for tolerization with long-term laboratory and
clinical data exposure Most ENPP1 and ABCC6 deficient adults show no ADA response or a transient, low titer response with no impact on PK Clinically-relevant ADA response limited to infants Monitoring of ENPP1 deficient pediatric patients (ENERGY 3
trial) has shown no evidence of hypersensitivity or immune-related adverse events 21
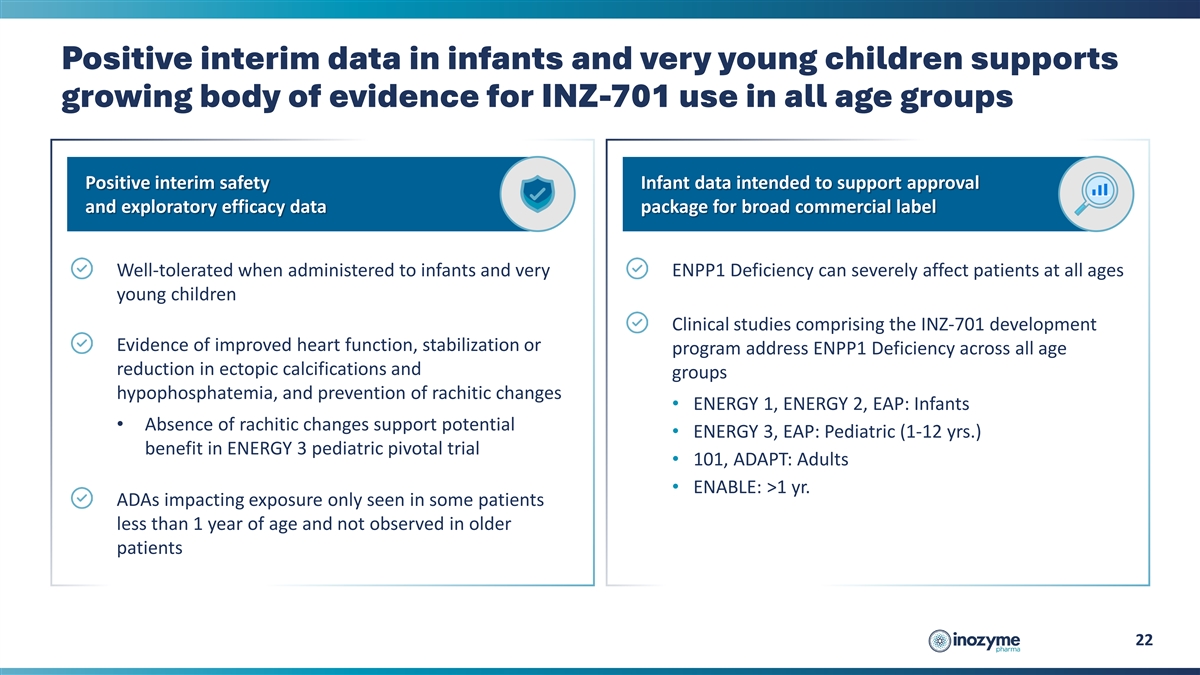
Positive interim data in infants and very young children supports
growing body of evidence for INZ-701 use in all age groups Positive interim safety Infant data intended to support approval and exploratory efficacy data package for broad commercial label Well-tolerated when administered to infants and very ENPP1
Deficiency can severely affect patients at all ages young children Clinical studies comprising the INZ-701 development Evidence of improved heart function, stabilization or program address ENPP1 Deficiency across all age reduction in ectopic
calcifications and groups hypophosphatemia, and prevention of rachitic changes • ENERGY 1, ENERGY 2, EAP: Infants • Absence of rachitic changes support potential • ENERGY 3, EAP: Pediatric (1-12 yrs.) benefit in ENERGY 3 pediatric
pivotal trial • 101, ADAPT: Adults • ENABLE: >1 yr. ADAs impacting exposure only seen in some patients less than 1 year of age and not observed in older patients 22

ASPIRE: Planned Pivotal Study in Pediatric Patients with ABCC6
Deficiency
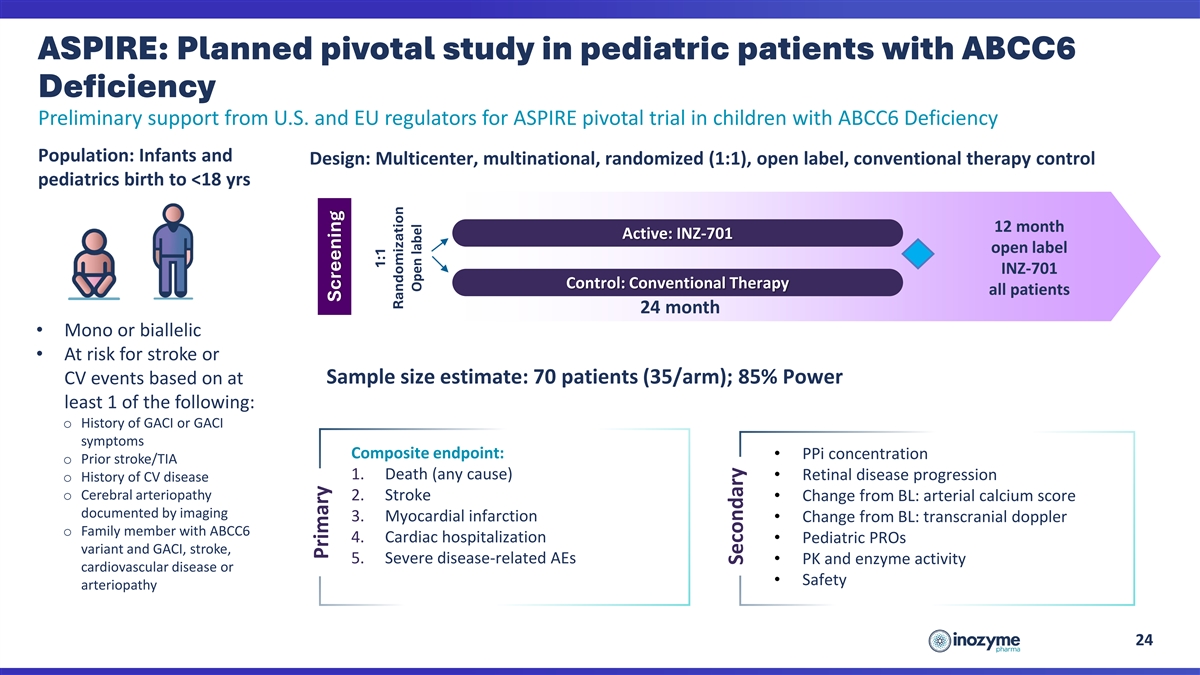
ASPIRE: Planned pivotal study in pediatric patients with ABCC6
Deficiency Preliminary support from U.S. and EU regulators for ASPIRE pivotal trial in children with ABCC6 Deficiency Population: Infants and Design: Multicenter, multinational, randomized (1:1), open label, conventional therapy control pediatrics
birth to <18 yrs 12 month Active: INZ-701 open label INZ-701 Control: Conventional Therapy all patients 24 month • Mono or biallelic • At risk for stroke or Sample size estimate: 70 patients (35/arm); 85% Power CV events based on at
least 1 of the following: o History of GACI or GACI symptoms Composite endpoint: • PPi concentration o Prior stroke/TIA 1. Death (any cause) • Retinal disease progression o History of CV disease o Cerebral arteriopathy 2. Stroke •
Change from BL: arterial calcium score documented by imaging 3. Myocardial infarction • Change from BL: transcranial doppler o Family member with ABCC6 4. Cardiac hospitalization • Pediatric PROs variant and GACI, stroke, 5. Severe
disease-related AEs • PK and enzyme activity cardiovascular disease or • Safety arteriopathy 24 Primary Screening 1:1 Randomization Open label Secondary

Thank you
v3.24.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Inozyme Pharma (NASDAQ:INZY)
Gráfica de Acción Histórica
De Dic 2024 a Ene 2025

Inozyme Pharma (NASDAQ:INZY)
Gráfica de Acción Histórica
De Ene 2024 a Ene 2025
