Filed
Pursuant to Rule 424(b)4
Registration
333-259833
PROSPECTUS
Warrants
to Purchase 959,005 Shares of Common Stock
Nutriband
Inc.
Nasdaq
trading symbol for the common stock: NTRB
This
prospectus covers the issuance by Nutriband Inc. (the “Company”) of 959,005 shares of common stock, par value $0.001 per
share (“common stock”)’ upon the exercise of warrants (the “Warrants”), issued in a public offering on
October 1, 2021 of 1,232,000 units (each a “Unit”) of common stock and warrants that were offered in the IPO on The Nasdaq
Capital Market, each Unit consisting of one share of common stock and one warrant (each a “Warrant”) at a price of $5.36
per Unit. The underwriters received 184,800 warrants. Each Warrant is immediately exercisable, entitles the holder to purchase one share
of common stock at an exercise price of $6.43 and expires five (5) years from the date of issuance. As of July 25, 2024, 457,795 Warrants
issued in the IPO have been exercised, with net proceeds to the Company of $2,942,970, and 959,005 Warrants are unexercised and outstanding.
A
prospectus supplement may also add, update, or change information included in this prospectus. You should rely only on the information
contained in this prospectus, any applicable prospectus supplement or any related free writing prospectus. See “Where You Can
Find More Information.”
Our common stock and Warrants are traded on the
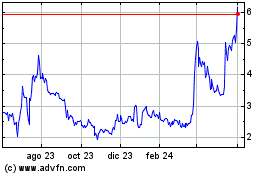
NASDAQ Capital Market under the symbols “NTRB” and “NTRBW,” respectively. On July 25, 2024, the last reported
sale price of our common stock was $8.47 per share, and the last reported sale price for the Warrants was $2.02 per Warrant.
Investing
in our common stock involves risks. See “Risk Factors” beginning on page 4.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined
if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The
date of this prospectus is July 26, 2024.
TABLE OF CONTENTS
You should rely only on the information contained
in this prospectus and in any free writing prospectus prepared by or on behalf of us and delivered or made available to you. Neither
we nor the underwriters have authorized anyone to provide you with additional or different information. We are offering to sell, and
seeking offers to buy, shares of our common stock only in jurisdictions where offers and sales are permitted. The information contained
in this prospectus or a free writing prospectus is accurate only as of its date, regardless of its time of delivery or of any sale of
shares of our common stock. Our business, financial condition, operating results, and prospects may have changed since that date, and
neither the delivery of this prospectus nor any sale made in connection with this prospectus shall, under any circumstances, create any
implication that there has been no change in our affairs since the date of this prospectus or that the information contained by reference
to this prospectus is correct as of any time after its date.
References to “we,” “us,”
“our” and words of like import refer to us and our subsidiaries, including 4P Therapeutics LLC following our acquisition
of 4P Therapeutics on August 1, 2018, and certain assets of Pocono Coated Products, LLC, on August 31, 2020, unless the context indicates
otherwise. References to 4P Therapeutics or Pocono Coated Products, LLC refer to the business and operations of 4P Therapeutics or Pocono
Coated Products, LLC, as the case may be, prior to acquisition by us unless the context indicates otherwise.
Industry and Market Data
The market data and certain other statistical
information used throughout this prospectus are based on independent industry publications, government publications and other published
independent sources. Some data is also based on our good faith estimates. The industry in which we operate is subject to a high degree
of uncertainty and risk due to a variety of factors, including those described in the section entitled “Risk Factors.” These
and other factors could cause results to differ materially from those expressed in these publications.
EXPLANATORY NOTE
This Post-Effective Registration Statement on
Form S-1 is being filed by Nutriband Inc. (the “Company”) with the U.S. Securities and Exchange Commission (the “SEC”)
in connection with the Company’s 959,005 unexercised outstanding Warrants to purchase common stock issued in the October 1, 2021
public offering of the Company’s common stock. The Warrants are exercisable for cash at any time after their original issuance
and at any time up to October 1, 2026, five years after the date of their original issuance. At any time a registration statement registering
the issuance of the shares of common stock underlying the Warrants under the Securities Act of 1933, as amended (the “Securities
Act”) is effective and available for the issuance of such shares, or an exemption from registration under the Securities Act is
available for the issuance of such shares, the Warrants may be exercised by payment of the exercise price in full in immediately available
funds for the number of shares of common stock purchased upon such exercise. If a registration statement registering the issuance of
the shares of common stock underlying the Warrants under the Securities Act is not effective or available and an exemption from registration
under the Securities Act is not available for the issuance of such shares, the Warrant holder may, in its sole discretion, elect to exercise
the Warrant through a cashless exercise, in which case the holder would receive upon such exercise the net number of shares of common
stock determined according to the formula set forth in the Warrant. No fractional shares of common stock will be issued in connection
with the exercise of a Warrant. In lieu of fractional shares, the Company will pay the holder an amount in cash equal to the fractional
amount multiplied by the exercise price.
PROSPECTUS SUMMARY
This summary highlights information contained
elsewhere in this prospectus. This summary does not contain all the information you should consider before investing in the securities.
However, you should read the entire prospectus carefully, including the “Risk Factors,” “Management’s Discussion
and Analysis of Financial Condition and Results of Operations,” and our financial statements, including the notes thereto, appearing
elsewhere in this prospectus.
This summary highlights information contained
elsewhere in this prospectus This summary does not contain all the information you should consider before investing in the securities.
However, you should read the entire prospectus carefully, including the “Risk Factors,” “Management’s Discussion
and Analysis of Financial Condition and Results of Operations,” and our financial statements, including the notes thereto, appearing
elsewhere in this report.
Overview
Nutriband Inc. (the “Company”, “Nutriband”,
“we” or “us”), was incorporated in Nevada in January 2016. Our primary business is the development of a portfolio
of transdermal pharmaceutical products. Our development pipeline consists of transdermal products that are based on our proprietary AVERSA™
abuse deterrent transdermal technology that we believe can be incorporated into existing transdermal patches that contain drugs
that are susceptible to abuse and misuse such as opioid and stimulant drugs.
The Company’s revenues are based on providing
services through our subsidiaries Pocono Pharmaceuticals operating as Active Intelligence and 4P Therapeutics. Pocono Pharmaceuticals
provides contract manufacturing services for health, wellness and over-the-counter pharmaceutical customers and 4P Therapeutics performs
contract research and development related services for pharmaceutical and medical devices customers. We manage and evaluate our operations,
and report our financial results, through these two separate subsidiaries.
Our principal offices are located in Orlando,
Florida, and our subsidiary, Pocono Pharmaceuticals, has a manufacturing facility in Cherryville, North Carolina. We primarily operate
and derive most of our revenues in the United States.
Selected Risks Associated with our Business
and Operations
Our business is subject to significant risks,
which are disclosed in more detail under “Risk Factors,” which begins on page 4, as a result of which an investment
in our common stock is highly speculative and could result in the loss of your entire investment. Significant risks include, but are
not limited to, the following:
| |
● |
The
FDA regulatory process may take longer and be more expensive than we anticipate without any assurance that we will obtain FDA approval. |
| |
|
|
| |
● |
If
we are not able to obtain FDA approval for our lead product, we may not have the resources to develop any other product, and we may
not be able to continue in business. |
| |
|
|
| |
● |
We
may not be able to launch any products for which we receive FDA marketing approval. |
| |
● |
We
may not be able to establish a distribution network for the marketing and sale of any products for which we receive FDA approval. |
| |
● |
We
may not be able to establish manufacturing facilities in compliance with FDA good manufacturing practices or to enter into manufacturing
agreements for the manufacture of our products in an FDA approved manufacturing facility. |
| |
|
|
| |
● |
It
will be necessary to us to enter into a joint venture or other strategic relationship in order to develop, perform clinical testing
for, manufacture or market any of our proposed products. We may not be able to enter into such a relationship, and any relationship
may not be successful, and the other party may have business interests and priorities that are different from ours. |
| |
|
|
| |
● |
We
may not be able to protect our rights in our intellectual property, and we may be subject to intellectual property litigation which
would be expensive and disruptive of our operations even if we eventually prevail on the merits. |
| |
|
|
| |
● |
Unanticipated
side effects or other adverse events resulting from the use of our product could require a recall of our products and, even if no
recall is required, our reputation could be impaired by side effects. |
| |
|
|
| |
● |
We
may fail to comply with all applicable laws and regulations relating to our product. We may have to change or adapt our operations
in the event of changes in national, regional and local government regulations, taxation, controls and political and economic developments
that affect our products and the market for our products; |
| |
|
|
| |
● |
We
may be unable to accurately estimate anticipated expenses, capital requirements and needs for additional financing; |
| |
|
|
| |
● |
The
terms of our recent financing, including the antidilution provisions of the warrants, may impair our ability to raise funds for our
operations during the term of the warrants. |
Our Organization
We are a Nevada corporation, incorporated on
January 4, 2016. In January 2016, we acquired Nutriband Ltd, an Irish company which was formed by Gareth Sheridan, our chief executive
officer, in 2012 to enter the health and wellness market by marketing transdermal patches. Our corporate headquarters are located at
121 S. Orange Ave. Suite 1500, Orlando, Florida 32765, telephone (407) 377-6695. Our website is www.nutriband.com. Information
contained on or available through our website or any other website does not constitute a portion of this prospectus.
Recent Financings
On October 5, 2021, the Company consummated a
public offering (the “IPO”) of units (the “Units”), of common stock and warrants that were offered in the IPO
on The Nasdaq Capital Market, which included 1,231,200 (each a “Unit”), each Unit consisting of one share of common stock
and one warrant (each a “Warrant”) at a price of $5.36 per Unit. Each Warrant is immediately exercisable, entitles the holder
to purchase one share of common stock at an exercise price of $6.43 and expires five (5) years from the date of issuance. The underwriters’
over-allotment option was exercised for 184,800 warrants to purchase shares of common stock bringing the total net proceeds to the Company
from the IPO to $5,836,230. The shares of common stock and Warrants were separately transferable immediately upon issuance. As of April
30, 2024, 457,795 warrants issued in the IPO have been exercised, with net proceeds to the Company of $ 2,942,970, and 959,005 warrants
are unexercised and outstanding.
Recent Development
On April 19, 2024, the Company completed an $8,400,000
equity financing with European investors (the “Offering”) of 2,100,000 units (“Units”), at a price of $4.00 per
Unit, each Unit consisting of one share of common stock (“Shares”) and a Warrant to purchase two Shares of common stock.
The Warrants have an initial exercise price of $6.43, are exercisable by payment of the exercise price in cash only and expire April
19, 2029, five years from the date of issuance. The Offering was made solely to investors resident outside the United States and was
not registered under the Securities Act of 1933, as amended (the “Securities Act”), or the securities laws of any jurisdiction,
including any jurisdiction outside the United States, but was made privately by the Company pursuant to the exemptions from registration
provided in the SEC’s Regulation S and other exemptions under the Securities Act.
SELECTED CONSOLIDATED FINANCIAL
DATA
The following information as of January 31, 2024
and 2023, and for years then ended, has been derived from our audited consolidated financial statements which appear elsewhere in this
prospectus. The following information as of and for the three months ended April 30, 2024 and 2023, has been derived from our unaudited
consolidated financial statements which appear elsewhere in this prospectus.
Statement of Operations Information:
| | |
Three
Months Ended
April 30, | | |
Year
Ended
January 31, | |
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| | |
(Unaudited) | | |
| | |
| |
| Revenue | |
$ | 408,532 | | |
| 476,932 | | |
$ | 2,085,314 | | |
$ | 2,079,609 | |
| Cost of revenue | |
| 243,746 | | |
| 254,648 | | |
| 1,223,209 | | |
| 1,329,200 | |
| Selling, general and administrative expenses | |
| 1,079,728 | | |
| 839,732 | | |
| 3,773,606 | | |
| 3,916,041 | |
| Net (loss) | |
| (1,898,077 | ) | |
| (1,015,229 | ) | |
| (5,485,314 | ) | |
| (4,483,474 | ) |
| Net (loss) per share of common stock (basic and diluted) | |
$ | (0.21 | ) | |
| (0.13 | ) | |
| (0.69 | ) | |
| (0.53 | ) |
| Weighted average shares of common stock outstanding (basic
and diluted) | |
| 9,159,869 | | |
| 7,833,150 | | |
| 7,954,105 | | |
| 8,459,547 | |
Balance Sheet Information:
| | |
April 30, | | |
January 31, | |
| | |
2024 | | |
2024 | | |
2023 | |
| | |
(Unaudited) | | |
| | |
| |
| Current assets | |
$ | 8,729,940 | | |
$ | 1,021,863 | | |
$ | 2,693,745 | |
| Working capital surplus (deficiency) | |
| 7,313,082 | | |
| 22,770 | | |
| 1,945,132 | |
| Accumulated deficit | |
| (29,878,096 | ) | |
| (27,980,019 | ) | |
| (22,494,705 | ) |
| Stockholders’ equity | |
| 13,363,113 | | |
| 6,438,235 | | |
| 8.572,990 | |
RISK FACTORS
An investment in our common stock involves
a high degree of risk. You should carefully consider the risks described below together with all of the other information included in
this prospectus before making an investment decision with regard to our securities. The statements contained in this prospectus include
forward-looking statements that are subject to risks and uncertainties that could cause actual results to differ materially from those
set forth in or implied by forward-looking statements. The risks set forth below are not the only risks facing us. Additional risks and
uncertainties may exist that could also adversely affect our business, prospects or operations. If any of the following risks actually
occurs, our business, financial condition or results of operations could be harmed. In that case, the trading price of our common stock
could decline, and you may lose all or a significant part of your investment.
Risks Concerning our Business
Because we are an early-stage company with
minimal revenue and a history of losses and we expect to continue to incur substantial losses for the foreseeable future, we cannot assure
you that we can or will be able to operate profitably.
We are subject to the risks common to start-up,
pre-revenue enterprises, including, among other factors, undercapitalization, cash shortages, limitations with respect to personnel,
financial and other resources and lack of revenues. Drug development companies typically incur substantial losses during the product
development and FDA testing phase of the business and do not generate revenues until after the drug has received FDA approval, which
cannot be assured, and until the company has started to sell the product. We can give no assurance that we can or will ever be successful
in achieving profitability and the likelihood of our success must be considered in light of our early stage of operations. We cannot
assure you that we will be able to operate profitably or generate positive cash flow. If we cannot achieve profitability, we may be forced
to cease operations and you may suffer a total loss of your investment.
Because
we do not have a product we can market in the United States, we cannot predict when or whether
we will operate profitably.
Our
lead product, which is our abuse deterrent fentanyl transdermal system, is currently in development
and is not yet approved by the FDA in the United States or by any other regulatory agency
in any other country. Because of the numerous risks and uncertainties associated with product
development, we cannot assure you that we will be able to develop and market any products
or achieve or attain profitability. If we are able to obtain financing for our operations,
we expect that we will incur substantial expenses as we continue with our product development
programs and clinical trials. Further, if we are required by applicable regulatory authorities,
including the FDA as well as the comparable regulatory agencies in other countries in which
we may seek to market product, to perform studies in addition to those we currently anticipate,
our expenses will increase beyond expectations and the timing of any potential product approval
may be delayed. As a result, we expect to continue to incur substantial losses and negative
cash flow for the foreseeable future.
A number of factors, including, but not limited to the following,
may affect our ability to develop our business and operate profitably:
| |
● |
our
ability to obtain necessary funding to develop our proposed products; |
| |
● |
the
success of clinical trials for our products; |
| |
● |
our
ability to obtain FDA approval for us to market any proposed product in our pipeline in the United States; |
| |
● |
any
delays in regulatory review and approval of product in development; |
| |
● |
if
we obtain FDA approval to market our product, our ability to establish manufacturing and distribution operations or entering into
manufacturing and distribution agreements with qualified third parties; |
| |
● |
market
acceptance of our products; |
| |
● |
our
ability to establish an effective sales and marketing infrastructure; |
| |
● |
our
ability to protect our intellectual property; |
| |
● |
competition
from existing products or new products that may emerge; |
| |
● |
the
ability to commercialize our products; |
| |
● |
potential
product liability claims and adverse events; |
| |
● |
our
ability to adequately support future growth; and |
| |
● |
our
ability to attract and retain key personnel to manage our business effectively. |
Our failure to develop our abuse deterrent
fentanyl transdermal system will impair our ability to continue in business.
Our lead product is our abuse deterrent fentanyl
transdermal system, and we are devoting our resources primarily to developing this product to enable us to obtain FDA approval and to
market the product. If we are not able to obtain necessary financing to develop, obtain FDA marketing approval and market this product
successfully, we may not have the resources to develop additional products, and we may not be able to continue in business.
Before we can market in the United States
any product which is classified by the FDA as a drug, we must obtain FDA marketing approval.
Our proposed transdermal products are drug-device
combinations that are considered by the FDA to be drugs, which require approval by the FDA. In order to obtain FDA approval, it is necessary
to conduct a series of preclinical and clinical tests to confirm that the product is safe and effective. Even though the medication that
is being delivered through our transdermal patch may have already received FDA approval, because we are changing the dosage form or route
of administration, we will need to complete, to the FDA’s satisfaction, all of the studies required to demonstrate safety and efficacy.
At any point, the FDA could ask us to perform additional tests or to refine and redo a test that we had previously completed. The process
of obtaining FDA approval could take many years, with no assurance that the FDA will approve the product. The FDA also will need to approve
the manufacturing process and the manufacturing facility.
We may need to rely on a contract research organization to conduct
our preclinical and clinical trials.
Although we believe
that we, through 4P Therapeutics, have the capabilities to conduct certain preclinical studies and early-stage clinical studies in house,
we may need to rely on third party contract research organizations to conduct our pivotal preclinical and clinical trials. Our failure
or the failure of the contract research organization to conduct the trials in compliance with FDA regulations could possibly derail our
obtaining FDA approval and could require us to redo any preclinical or clinical trials which we or the contract research organization
administered.
We may encounter delays in completing clinical
trials, which would increase our costs and delay market entry.
We may experience delays in completing the clinical
trials necessary for FDA approval. These delays may result from a number of factors which could prevent us from starting the trial on
time or completing the study in a timely manner, which may include factors out of our control. Since we may need to rely on third parties
for supplying us with the drug and transdermal patches used in the trials, there may be various reasons for us to experience a delay
in obtaining the clinical materials required to start each clinical trial, which may include factors out of our control. Clinical trials
can be delayed or terminated for a number of reasons, including delay or failure to:
| |
● |
obtain
necessary financing; |
| |
● |
obtain
regulatory approval to commence a trial; |
| |
● |
reach
agreement on acceptable terms with prospective contract research organizations, investigators and clinical trial sites, the terms
of which may be subject to extensive negotiation and vary significantly among different research organizations and trial sites; |
| |
● |
obtain
institutional review board approval at each site; |
| |
● |
enlist
suitable patients to participate in a trial; |
| |
● |
have
patients complete a trial or return for post-treatment follow-up; |
| |
● |
ensure
clinical sites observe trial protocol or continue to participate in a trial; |
| |
● |
address
any patient safety concerns that arise during the course of a trial; |
| |
● |
address
any conflicts with new or existing laws or regulations; |
| |
● |
add
a sufficient number of clinical trial sites; or |
| |
● |
manufacture
sufficient quantities of the product candidate for use in clinical trials. |
Patient enrollment is also a significant factor
in the timely completion of clinical trials and is affected by many factors, including the size and nature of the patient population,
the proximity of patients to clinical sites, the eligibility criteria for the trial, the design of the clinical trial, competing clinical
trials and clinicians’ and patients’ perceptions as to the potential advantages of the drug being studied in relation to
available alternatives, including any new drugs or treatments that may be approved for the indications we are investigating.
We may also encounter delays if a clinical trial
is suspended or terminated by us, by the independent review boards of the institutions in which such trials are being conducted, by the
trial’s data safety monitoring board, or by the FDA. Such authorities may suspend or terminate one or more of our clinical trials
due to a number of factors, including our failure to conduct the clinical trial in accordance with relevant regulatory requirements or
clinical protocols, inspection of the clinical trial operations or trial site by the FDA resulting in the imposition of a clinical hold,
unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from using a drug, changes in governmental regulations
or administrative actions or lack of adequate funding to continue the clinical trial.
If we experience delays in carrying out or completing
clinical trials for any product candidates, the commercial prospects of our product candidates may be harmed, and our ability to generate
revenues from any of these product candidates will be delayed. In addition, any delays in completing our clinical trials will increase
our costs, slow down the product development and approval process and jeopardize our ability to commence product sales and generate revenues.
Any of these occurrences may significantly harm our business and financial condition. In addition, many of the factors that cause, or
lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of
our product candidates.
Our ability to finance our operations and
generate revenues depends on the clinical and commercial success of our abuse deterrent fentanyl transdermal system and our other related
product candidates and failure to achieve such success will negatively impact our business.
Our prospects, including our ability to finance
our operations and generate revenues, depend on the successful development, regulatory approval and commercialization of our abuse deterrent
fentanyl transdermal system, which itself requires substantial financing, as well as our other product candidates. The clinical and commercial
success of our product candidates depends on a number of factors, many of which are beyond our control, including:
| |
● |
the
FDA’s acceptance of our parameters for regulatory approval relating to our product candidates, including our proposed indications,
primary endpoint assessments, primary endpoint measurements and regulatory pathways; |
| |
● |
the
FDA’s acceptance of the number, design, size, conduct and implementation of our clinical trials, our trial protocols and the
interpretation of data from preclinical studies or clinical trials; |
| |
● |
the
FDA’s acceptance of the sufficiency of the data we collect from our preclinical studies and pivotal clinical trials to support
the submission of a New Drug Application, known as an NDA, without requiring additional preclinical or clinical trials; |
| |
● |
the
FDA’s acceptance of our abuse deterrent labelling relating to our products, including our abuse deterrent fentanyl transdermal
system; |
| |
● |
when
we submit our NDA upon completion of our clinical trials, the FDA’s willingness to schedule an advisory committee meeting,
if applicable, in a timely manner to evaluate and decide on the approval of our NDA; |
| |
● |
the
recommendation of the FDA’s advisory committee, if applicable, to approve our application without limiting the approved labelling,
specifications, distribution, or use of the products, or imposing other restrictions; |
| |
● |
our
ability to satisfy any issued raised by the FDA in response to our test data; |
| |
● |
the
FDA’s satisfaction with the safety and efficacy of our product candidates; |
| |
● |
the
prevalence and severity of adverse events associated with our product candidates; |
| |
● |
the
timely and satisfactory performance by third party contractors of their obligations in relation to our clinical trials; |
| |
● |
if
we receive FDA approval, our success in educating physicians and patients about the benefits, administration and use our product
candidates; |
| |
● |
our
ability to raise additional capital on acceptable terms in order to achieve conduct the necessary clinical trials; |
| |
● |
the
availability, perceived advantages and relative cost of alternative and competing treatments; |
| |
● |
the
effectiveness of our marketing, sales and distribution strategy and operations; |
| |
● |
our
ability to develop, validate and maintain a commercially viable manufacturing process that is compliant with current good manufacturing
practices; |
| |
● |
our
ability to obtain, protect and enforce our intellectual property rights; |
| |
● |
our
ability to bring an action timely for patent infringement arising out of the filing of ANDAs by generic companies seeking approval
to market generic versions of our products, if applicable, before the expiry of our patents; and |
| |
● |
our
ability to avoid third party claims of patent infringement or intellectual property violations. |
If we fail to achieve these objectives or to
overcome the challenges presented above, many of which are beyond our control, in a timely manner, we could experience significant delays
or an inability to successfully commercialize our product candidates. Accordingly, even if we obtain FDA approval to market our products,
we may not be able to generate sufficient revenues through the sale of our products to enable us to continue our business.
Since we do not have commercial manufacturing
capability, if we are unable to establish manufacturing facilities, we may have to enter into a manufacturing agreement with a manufacturer
that has been approved by the FDA.
Any commercial manufacturer of our products and
the manufacturing facilities where we make our commercial products will be subject to FDA inspection. Part of the process of seeking
FDA approval to market our products is the FDA’s approval of the manufacturing process and facility. Although we may establish
our own manufacturing facilities, the establishment of a manufacturing facility is very costly, and, unless we obtain funding for that
purpose, it would be necessary for us to engage a contract manufacturer who has experience is manufacturing FDA-approved transdermal
products. By relying on a contract manufacturer, we will be dependent upon the manufacturer, whose interests may be different from ours.
Any contract manufacturer will be responsible for product quality and for meeting regulatory requirements. If the manufacturer does not
meet our quality standards and delivers products that do not meet our specifications, we may both incur liability for breach of our warranty
to our customer, as well as liability for any adverse events, including death, that may result from the use, abuse or accidental misuse
of the product. Regardless of whether we are able to make a claim against the contract manufacturer, our reputation may be harmed and
we may lose business as a result. Further, the contract manufacturer may have other customers and may allocate its resources based on
the contract manufacturer’s interest rather than our interest. Furthermore, we may not be able to assure ourselves that we will
get favorable pricing.
If we or any third-party
manufacturer fails to comply with FDA current good manufacturing practices, we may not be able to sell our products until and unless
the manufacturer becomes compliant.
All FDA approved drugs, including our proposed
transdermal products, must be manufactured in accordance with good manufacturing practices. All manufacturing facilities are inspected
by the FDA as a matter of routine inspection or for a specific cause. If a manufacturer fails to comply with all applicable regulations,
the FDA can prohibit us from distributing products manufactured in those facilities, whether they are a contract manufacturer or own
facility. Failure to be in compliance with good manufacturing practices could result in the FDA closing the facilities or limiting our
use of the facilities.
If the FDA implements Risk Evaluation and
Mitigation Strategies policies for any of our proposed products, we will need to comply with such policies before we can obtain FDA approval
or the product.
The Food and Drug Administration Amendments Act
of 2007 gave FDA the authority to require a Risk Evaluation and Mitigation Strategy (REMS) from manufacturers to ensure that the benefits
of a drug or biological product outweigh its risks. If one of our proposed product candidates does receive regulatory approval, the approval
may be limited to specific conditions and dosages or the indications for use may otherwise be limited, which could restrict the commercial
value of the product. The FDA may require a REMS, which can include a medication guide, patient package insert, a communication plan,
elements to assure safe use and implementation system, and include a timetable for assessment of the REMS. Further, the FDA may require
that certain contraindications, warnings or precautions be included in the product labeling and may require testing and surveillance
programs to monitor the safety of approved products that have been commercialized. In addition, the FDA may require post-approval testing
which involves clinical trials designed to further assess a drug product’s safety and effectiveness after the NDA.
Depending on the extent of the REMS requirements,
any U.S. launch may be delayed, the costs to commercialize may increase substantially and the potential commercial market could be restricted.
Furthermore, risks that are not adequately addressed through the proposed REMS program may also prevent or delay its approval for commercialization.
Our products will continue to be subject to FDA review after
FDA approval is given.
Discovery of previously unknown problems with
our products or unanticipated problems with the manufacturing processes and facilities, even after FDA and other regulatory approvals
of the product for commercial sale, may result in the imposition of significant restrictions, including withdrawal of the product from
the market.
The FDA and other regulatory agencies continue
to review products even after the products receive agency approval. If and when the FDA approves one of our products, its manufacture
and marketing will be subject to ongoing regulation, which could include compliance with current good manufacturing practices, adverse
event reporting requirements and general prohibitions against promoting products for unapproved or “off-label” uses. We are
also subject to inspection and market surveillance by the FDA for compliance with these and other requirements. Any enforcement action
resulting from the failure, even by inadvertence, to comply with these requirements could affect the manufacture and marketing of our
products. In addition, the FDA or other regulatory agencies could withdraw a previously approved product from the market upon receipt
of newly discovered information. The FDA or another regulatory agency could also require us to conduct additional, and potentially expensive,
studies in areas outside our approved indicated uses.
We must continually monitor the safety
of our products once approved and marketed for potential adverse events which could jeopardize our ability to continue marketing the
products.
As with all medical products, the use of our
products could sometimes produce undesirable side effects or adverse reactions or events (referred to cumulatively as adverse events).
For the most part, we expect these adverse events to be known and occur at some predicted frequency based on our experience in the clinical
development program. When adverse events are reported to us, we are required to investigate each event and the circumstances surrounding
it to determine whether it was caused by our product and whether a previously unrecognized safety issue exists. We will also be required
to periodically report summaries of these events to the applicable regulatory authorities. If the adverse effects are significant, we
may be required to recall our product. We cannot assure you that our transdermal products will not cause skin irritation or other adverse
events. Our ability to market our products may be impaired by unanticipated adverse events and any recall of our product. Because we
are an early-stage company, our reputation, and our ability to market products, could be affected more severely than a major pharmaceutical
company.
In addition, the use of our products could be
associated with serious and unexpected adverse events, or with less serious reactions at a greater than expected frequency. Such issues
may arise when our products are used in critically ill or otherwise compromised patient populations. When unexpected events are reported
to us, we are required to make a thorough investigation to determine causality and the implications for product safety. These events
must also be specifically reported to the applicable regulatory authorities. If our evaluation concludes, or regulatory authorities perceive,
that there is an unreasonable risk associated with the product, we would be obligated to withdraw the impacted lot(s) of that product
or recall the product and discontinue marketing until all problems are satisfactorily resolved. Furthermore, an unexpected adverse event
of a new product could be recognized only after extensive use of the product, which could expose us to product liability risks, enforcement
action by regulatory authorities and damage to our reputation and public image.
A serious adverse finding concerning the risk
of any of our products by any regulatory authority could adversely affect our reputation, business and financial results.
If we obtain FDA approval to market our
products, we expect to spend considerable time and money complying with federal and state laws and regulations governing their sale,
and, if we are unable to fully comply with such laws and regulations, we could face substantial penalties.
Health care providers, physicians and others
will play a primary role in the recommendation and prescription of our proposed products. Further, if we use third-party sales and marketing
providers, they may expose us to broadly applicable fraud and abuse and other health care laws and regulations that may constrain the
business or financial arrangements and relationships through which we market, sell and distribute our products. Applicable federal and
state health care laws and regulations are expected to include, but not be limited to, the following:
| ● | The
federal anti-kickback statute is a criminal statute that makes it a felony for individuals
or entities knowingly and willfully to offer or pay, or to solicit or receive, direct or
indirect remuneration, in order to induce the purchase, order, lease, or recommending of
items or services, or the referral of patients for services, that are reimbursed under a
federal health care program, including Medicare and Medicaid; |
| ● | The
federal False Claims Act imposes liability on any person who knowingly submits, or causes
another person or entity to submit, a false claim for payment of government funds. Penalties
include three times the government’s damages plus civil penalties of $5,500 to $11,000
per false claim. In addition, the False Claims Act permits a person with knowledge of fraud,
referred to as a qui tam plaintiff, to file a lawsuit on behalf of the government against
the person or business that committed the fraud, and, if the action is successful, the qui
tam plaintiff is rewarded with a percentage of the recovery; |
| ● | Health
Insurance Portability and Accountability Act, known as HIPAA, imposes obligations, including
mandatory contractual terms, with respect to safeguarding the privacy, security and transmission
of individually identifiable health information; |
| ● | The
Social Security Act contains numerous provisions allowing the imposition of a civil money
penalty, a monetary assessment, exclusion from the Medicare and Medicaid programs, or some
combination of these penalties; and |
| ● | Many
states have analogous state laws and regulations, such as state anti-kickback and false claims
laws. In some cases, these state laws impose more strict requirements than the federal laws.
Some state laws also require pharmaceutical companies to comply with certain price reporting
and other compliance requirements. |
Our failure to comply with any of these federal
and state health care laws and regulations, or health care laws in foreign jurisdictions, could have a material adverse effect on our
business, financial condition, result of operations and cash flows.
Before we can market our products outside
of the United States, we will need to obtain regulatory approval in each country in which we propose to sell our products.
In order to market and sell our products in jurisdictions
other than the United States, we must obtain separate marketing approvals and comply with numerous and varying regulatory requirements.
The regulatory approval process outside the United States generally includes all of the risks associated with obtaining FDA and can involve
additional testing.
In addition, in many countries worldwide, it
is required that the product be approved for reimbursement before the product can be approved for sale in that country. We may not obtain
approvals from regulatory authorities outside the United States on a timely basis, if at all. Even if we were to receive approval in
the United States, approval by the FDA for marketing in the United States does not ensure approval by regulatory authorities in other
countries. Similarly, approval by one regulatory authority outside the United States would not ensure approval by regulatory authorities
in other countries. We may not be able to file for marketing approvals and may not receive necessary approvals to commercialize our products
in any market. If we are unable to obtain approval of our product candidates by regulatory authorities in foreign jurisdictions, the
commercial prospects of those product candidates may be significantly diminished and our business prospects could be impaired.
Outside the United States, particularly in member
states of the European Union, the pricing of prescription drugs is subject to governmental control. In these countries, pricing negotiations
or the successful completion of health technology assessment procedures with governmental authorities can take considerable time after
receipt of marketing approval for a product. In addition, there can be considerable pressure by governments and other stakeholders on
prices and reimbursement levels, including as part of cost containment measures. Certain countries allow companies to fix their own prices
for medicines but monitor the pricing.
In addition to regulations in the United States,
if we market outside of the United States, we will be subject to a variety of regulations governing, among other things, clinical trials
and any commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, we must obtain the requisite
approvals from regulatory authorities in foreign countries prior to the commencement of clinical trials or marketing of the product in
those countries.
If we do not have sufficient product liability
insurance, we may be subject to claims that are in excess of our net worth.
Before we market any pharmaceutical product,
we will need to purchase significant product liability insurance. However, in the event of major claims from the use of our products,
it is possible that our product liability insurance will not be sufficient to cover claims against us. We cannot assure you that we will
not face liability arising out of the use of our products which is significantly in excess of the limits of our product liability insurance.
In such event, if we do not have the funds or access to the funds necessary to satisfy such liability, we may be unable to continue in
business.
Because some of the patches we are developing,
such as our abuse deterrent fentanyl patch, have potential severe side effects, we may face liability in the event patients suffer serious,
possibly life-threatening, side effects from our products.
Fentanyl patches have known side effects and
may cause serious or life-threatening breathing problems due to opioid-induced respiratory depression. In addition, taking certain medications
with fentanyl may increase the risk of serious or life-threatening breathing problems, sedation or coma. Because of the seriousness of
the side effects, fentanyl patches should only be used in accordance labelling approved by the FDA or by the applicable regulatory authorities
outside of the United States. Fentanyl patches are only indicated for the treatment of people who are tolerant to opioid medications
because they have taken this type of medication for at least one week and should not be used to treat mild or moderate pain, short-term
pain, pain after an operation or medical or dental procedure, or pain that can be controlled by medication that is taken on an as-needed
basis. Although we will include all warnings on the packaging that are required by the FDA or foreign regulatory authorities, claims
may be made against us in the event that death or serious side effects result from the use of our abuse deterrent fentanyl transdermal
system, even if prescribed for a patient for whom fentanyl patches should not be prescribed. We cannot assure you that we will not face
significant liability as a result of such side effects and we may not have sufficient product liability insurance to cover any damages
that may be assessed against us.
We may decide not to continue developing
or commercializing any products at any time during development or after approval, which would reduce or eliminate our potential return
on investment for those product candidates.
We may decide to discontinue the development
of our abuse deterrent fentanyl transdermal system or any other product in our pipeline or not to continue to commercialize any potential
product for a variety of reasons, such as the appearance of new technologies that make our product less commercially viable, an increase
in competition, changes in or failure to comply with applicable regulatory requirements, changes in the regulatory or public policy environment,
the discovery of unforeseen side effects during clinical development or after the approved product has been marketed or the occurrence
of adverse events at a rate or severity level that is greater than experienced in prior clinical trials. If we discontinue a program
in which we have invested significant resources, we will not receive any return on our investment.
If any of our potential products are approved
for marketing but fail to achieve the broad degree of physician or market acceptance necessary for commercial success, our operating
results and financial condition will be adversely affected.
If any of the products in our pipeline receives
FDA approval thereby allowing us to market the product in the United States, it will be necessary for us to generate acceptance of our
product for the indications covered by the FDA approval. In order to generate acceptance in the marketplace, we will need to demonstrate
to physicians, patients and payors that our product provides a distinct advantage or better outcome at a price that reflects the value
of our product as compared with existing products. We will need to develop and implement a marketing program directed at both physicians
and the general public. Since we do not presently have the resources necessary to develop or implement an in-house marketing program
and we may not have the funds to do so if and when we obtain FDA approval to market our product, we will need to establish a distribution
network though license and distribution agreements with third parties who have the capability to market our product to physicians, and
we will be dependent upon the ability of these third parties to market our products effectively. We cannot assure you that we will be
able to negotiate license and distribution agreements with terms that are acceptable to us. Since we do not have an established track
record and our product pipeline is relatively small, we may be at a disadvantage in negotiating the terms of license and distribution
agreements. Further, we may have little control over the development and implementation of our licensee’s marketing program, and
our licensees may have interests that are inconsistent with ours with respect to the allocation of resources and implementation of the
marketing program. We cannot assure you that a marketing program for any of our products can or will be implemented effectively or that
we will be successful in developing physician and emergency service acceptance of our products.
The drug delivery industry is subject to
rapid technological change and, our failure to keep up with technological developments may impair our ability to market our products.
Our products use technology which we developed
for the transdermal delivery of drugs. The field of drug delivery is subject to rapid technological changes. Our future success will
depend upon our ability to keep abreast of the latest developments in the industry and to keep pace with advances in technology and changing
customer requirements. If we cannot keep pace with such changes and advances, our proposed products could be rendered obsolete, which
would result in our having to cease its operations.
If we obtain FDA approval, we will face
significant competition from better known and better capitalized companies.
If we obtain FDA approval for any of our products,
we expect to face significant competition from existing companies, which are better known and already have developed relationships with
physicians within the healthcare system. Any product we may develop will compete with existing medications performing the same medicinal
functions, which may include transdermal patches. We cannot assure you that we will be able to compete successfully. In addition, even
if we are able to commercialize our product candidates, we may not be able to price them competitively with current standard of care
products or their price may drop considerably due to factors outside our control. If this happens or the price of materials and manufacture
increases dramatically, our ability to continue to operate our business would be materially harmed and we may be unable to commercialize
any products successfully. In addition, other pharmaceutical companies may be engaged in developing, patenting, manufacturing and marketing
products that compete with those that we are developing. These potential competitors may include large and experienced companies that
enjoy significant competitive advantages over us, such as greater financial, research and development, manufacturing, personnel and marketing
resources, greater brand recognition and more experience and expertise in obtaining marketing approvals from the FDA and foreign regulatory
authorities.
Healthcare reforms by governmental authorities,
court decisions affecting health care policies and related reductions in pharmaceutical pricing, reimbursement and coverage by third-party
payors may adversely affect our business.
We expect the healthcare industry to face increased
limitations on reimbursement, rebates and other payments as a result of healthcare reform, which could adversely affect third-party coverage
of our proposed products and how much or under what circumstances healthcare providers will prescribe or administer our products, if
approved.
In both the U.S. and other countries, sales of
our products, if approved for marketing, will depend in part upon the availability of reimbursement from third-party payors, which include
governmental authorities, managed care organizations and other private health insurers. Third-party payors are increasingly challenging
the price and examining the cost effectiveness of medical products and services.
Increasing expenditures for healthcare have been
the subject of considerable public attention in the United States. Both private and government entities are seeking ways to reduce or
contain healthcare costs. Numerous proposals that would effect changes in the United States healthcare system have been introduced or
proposed in Congress and in some state legislatures, including reducing reimbursement for prescription products and reducing the levels
at which consumers and healthcare providers are reimbursed for purchases of pharmaceutical products.
Cost reduction initiatives and changes in coverage
implemented through legislation or regulation could decrease utilization of and reimbursement for any approved products, which in turn
would affect the price we can receive for those products. Any reduction in reimbursement that results from federal legislation or regulation
may also result in a similar reduction in payments from private payors, since private payors often follow Medicare coverage policy and
payment limitations in setting their own reimbursement rates.
Significant developments that may adversely affect
pricing in the United States include the enactment of federal healthcare reform laws and regulations, including the Affordable Care Act,
or ACA, which is popularly known as Obamacare, and the Medicare Prescription Drug Improvement and Modernization Act of 2003. A
recent district court decision which struck down Obamacare, if upheld, could have a material adverse effect upon reimbursement and payment
for products such as our proposed products. Changes to the healthcare system enacted as part of any healthcare reform in the United States,
as well as the increased purchasing power of entities that negotiate on behalf of Medicare, Medicaid, and private sector beneficiaries,
may result in increased pricing pressure by influencing, for instance, the reimbursement policies of third-party payors. Regulatory changes
which have the effect of decreasing the use of opioids has resulted in a decrease in the size of the market for opioid products, including
fentanyl, could impact the market for our abuse deterrent fentanyl transdermal system or any other opioid-based transdermal product we
may develop.
It is difficult and costly to protect our
proprietary rights, and we may not be able to ensure their protection.
Our commercial success will depend in part on
obtaining and maintaining patent protection and trade secret protection for our technology which is incorporated in our products as well
as successfully defending these patents against third-party challenges, should any be brought. 4P Therapeutics originally filed an international
patent application under the Patent Cooperation Treaty for worldwide prosecution of the abuse deterrent transdermal technology intellectual
property used in our lead product, the abuse deterrent fentanyl transdermal system.
The AVERSA abuse deterrent
technology utilized in our AVERSA product pipeline is covered by an international intellectual property portfolio with patents issued
in 45 countries including the United States, Europe, Japan, Korea, Russia, Mexico, Canada and Australia. Patent prosecution is still
pending in China and Hong Kong. These patents provide patent coverage to 2035. We continue to build on our proprietary positions in the
United States and internationally for our product candidates AVERSA Fentanyl, AVERSA buprenorphine and AVERSA methylphenidate as well
as other products and technology that we may have in development. Our policy is to pursue, maintain and defend patent rights developed
internally or acquired externally and to protect the technology, inventions and improvements that are commercially important to the development
of our business. We cannot be sure that patents will be granted with respect to any of our pending patent applications or with respect
to any patent applications filed by us in the future, nor can we be sure that any of our existing patents or any patents granted to us
in the future will be commercially useful in protecting our technology. We also rely on trade secrets to protect our commercial products
and product candidates. Our commercial success also depends in part on our non-infringement of the patents or proprietary rights of third
parties.
Our ability to stop third parties from making,
using, selling, offering to sell or importing products utilizing our proprietary or patented technology is dependent upon the extent
to which we have rights under valid and enforceable patents or trade secrets that cover these activities. The patent positions of pharmaceutical
and biopharmaceutical companies can be highly uncertain and involve complex legal and factual questions for which important legal principles
remain unresolved. No consistent policy regarding the breadth of claims allowed in biopharmaceutical patents has emerged to date in the
United States. The biopharmaceutical patent situation outside the United States varies from country to country and is even more uncertain.
Changes in either the patent laws or in interpretations of patent laws in the United States and other countries may diminish the value
of our intellectual property. Accordingly, we cannot predict the breadth of claims that may be allowed or enforced in any patents we
may be granted. Further, if any patents are granted and are subsequently deemed invalid and unenforceable, it could impact our ability
to license our technology and, as noted previously, fend off competitive challenges. Patent litigation is very expensive and we may not
have sufficient funds to defend our proprietary technology from infringement, either as a plaintiff in an action seeking to stop infringers
from using our technology, or as a defendant in an action against us alleging infringement by us.
The degree of future protection for our proprietary
rights is uncertain because legal means afford only limited protection and may not adequately protect our rights or permit us to gain
or keep our competitive advantage. For example:
| ● | others
may be able to make compositions or formulations that are similar to our product s but that
are not covered by the claims of our patents; |
| ● | other
persons may have filed patents covering inventions, technology or processes that we use,
with the result that we may infringe upon the prior patents; |
| ● | others
may independently develop similar or alternative technologies or duplicate any of our technologies; |
| ● | our
pending patent applications may not result in the grant of patents; |
| ● | any
patents which may be issued may not provide us with any competitive advantages, or may be
held invalid or unenforceable as a result of legal challenges by third parties; |
| ● | our
inability to fund any litigation to defend our proprietary rights, either in defense of an
action against us or a plaintiff to seek to prevent infringement. |
| ● | our
failure to develop additional proprietary technologies that are patentable. |
If we seek to expand our business through
acquisition, we may not be successful in identifying acquisition targets or integrating their businesses with our existing business.
We have expanded our
business by acquisition, and we may make acquisitions in the future. In 2017, we issued 1,458,333 shares of common stock, valued at $2,500,000,
in connection with our proposed acquisition of Advanced Health Brands, Inc., but the stock of Advanced Health Brands was never transferred
to us and the value of the intellectual property we were to have acquired did not have the value we anticipated, with the result that
we incurred a $2,500,000 impairment loss in the year ended January 31, 2018. In September 2018, we entered into an agreement to acquire
Carmel Biosciences Inc., and in November 2018, we terminated the agreement. We previously entered into another acquisition agreement
which was rescinded shortly after the agreement was executed. We cannot assure you that any acquisition we complete will be successful
or that any acquisition agreement we may enter into will result in an acquisition. An acquisition can be unsuccessful for a number of
reasons, including the following:
| ● | We
may incur significant expenses and devote significant management time to the acquisition
and we may be unable to consummate the acquisition on acceptable terms. |
| ● | The
integration of any acquisition with our existing business may be difficult and, if we are
not able to integrate the business successfully, we may not only be unable to operate the
business profitably, but management may be unable to devote the necessary time to the development
of our existing business; |
| ● | The
key employees who operated the acquired business successfully prior to the acquisition may
not be happy working for us and may resign, thus leaving the business without the necessary
continuity of management. |
| ● | Even
if the business is successful, our senior executive officers may need to devote significant
time to the acquired business, which may distract them from their other management activities. |
| ● | If
the business does not operate as we expect, we may incur an impairment charge based on the
value of the assets acquired. |
| ● | The
products or proposed products of the acquired company may have regulatory problems with the
FDA or any other regulatory agency, including the need for additional and unanticipated testing
or the need for a recall or a change in labeling. |
| ● | We
may have difficulty maintaining the necessary quality control over the acquired business
and its products and services. |
| ● | To
the extent that an acquired company operates at a loss prior to our acquisition, we may not
be able to develop profitable operations following the acquisition. |
| ● | The
acquired company may have liabilities or obligations which were not disclosed to us, or the
acquired assets, including any intellectual property, may not have the value we anticipated. |
| ● | The
assets, including intellectual property, of the acquired company may not have the value that
we anticipated. |
| ● | We
may require significant capital both to acquire and to operate the business, and the capital
requirements of the business may be greater than we anticipated. Our failure to obtain funds
on reasonable terms may impair the value of the acquisition. |
| ● | The
acquired company may not operate at the revenue level or with the gross margin shown in the
financial statements or projections. |
| ● | Patents
may not be granted for patent applications which the acquired company filed or patents may
be successfully challenged. |
| ● | There
may be conflicts in management styles that prevent us from integrating the acquired company
with us. |
| ● | The
business of the acquired company may have problems of which management was unaware and which
do not become evident until after the acquisition and we may require significant funding
to remedy the problem. |
| ● | The
indemnification obligations of the seller under the purchase agreement, if any, may be inadequate
to compensate us for any loss, damage or expense which we may sustain, including undisclosed
claims or liabilities. |
| ● | To
the extent that the acquired company is dependent upon its management to maintain relationships
with existing customers, we may have difficulty in retaining the business of these customers
if there is a change in management. |
| ● | Government
agencies may seek damages after we make the acquisition for conduct which occurred prior
to the acquisition and we may not have adequate recourse against the seller. |
If any of the foregoing or any other events which
we do not contemplate happen, we may incur significant expenses, which we may not be able to cover, and the development of our business
can be impaired. We cannot assure you that any acquisition we will make will be successful.
We are dependent on third party distributors
for the international marketing of our consumer products and complying with applicable laws.
We do not currently sell or market our consumer
transdermal products domestically, or for our international sales, directly to international consumers, and we rely on distributors to
sell and market these products. We cannot market our consumer transdermal patch products in the United States without first obtaining
FDA approval. We do not plan to seek FDA approval or market these products in the United States at this time. We plan to sell our transdermal
consumer products to distributors in those countries in which the products can be sold in compliance with all applicable regulations
without our spending significant monies for preclinical and clinical studies to obtain regulatory approval.
We are dependent upon our chief executive
officer, our president and our chief operating officer.
We are dependent upon
Gareth Sheridan, our chief executive officer, Serguei Melnik, our president and Dr. Alan Smith, our chief operating officer who is president
of 4P Therapeutics. Although Mr. Sheridan and Mr. Melnik have employment agreements with us, the employment agreements do not guarantee
that the officer will continue with us. We do not have an employment agreement with Dr. Smith. The loss of Mr. Sheridan, Mr. Melnik or
Dr. Smith would materially impair our ability to conduct our business.
If we are unable to attract, train and
retain technical and financial personnel, our business may be materially and adversely affected.
Our future success depends, to a significant
extent, on our ability to attract, train and retain key management, technical, regulatory and financial personnel. Recruiting and retaining
capable personnel with experience in pharmaceutical product development is vital to our success. There is substantial competition for
qualified personnel, and competition is likely to increase. We cannot assure you we will be able to attract or retain the personnel we
require. Our financial condition is likely to impair our ability to attract qualified candidates. If we are unable to attract and retain
qualified employees, our business may be materially and adversely affected.
Risks Concerning our Securities
Our lack of internal controls over financial
reporting may affect the market for and price of our common stock.
Pursuant to Section 404 of the Sarbanes-Oxley
Act, we are required to file a report by our management on our internal control over financial reporting. Our disclosure controls and
our internal controls over financial reporting are not effective. We do not have the financial resources or personnel to develop or implement
systems that would provide us with the necessary information on a timely basis so as to be able to implement financial controls The absence
of internal controls over financial reporting may inhibit investors from purchasing our stock and may make it more difficult for us to
raise capital or borrow money. Implementing any appropriate changes to our internal controls may require specific compliance training
of our directors and employees, entail substantial costs in order to modify our existing accounting systems, take a significant period
of time to complete and divert management’s attention from other business concerns. These changes may not, however, be effective
in developing or maintaining internal control.
The market price for our common stock may
be volatile and your investment in our common stock could suffer a decline in value.
The trading volume in our stock is low, which
may result in volatility in our stock price. As a result, any reported prices may not reflect the price at which you would be able to
sell shares of common stock if you want to sell any shares you own or buy if you wish to buy shares. Further, stocks with a low trading
volume may be more subject to manipulation than a stock that has a significant public float and is actively traded. The price of our
stock may fluctuate significantly in response to a number of factors, many of which are beyond our control. These factors include, but
are not limited to, the following, in addition to the risks described above and general market and economic conditions:
| ● | the
market’s perception as to our ability to generate positive cash flow or earnings; |
| ● | changes
in our or any securities analysts’ estimate of our financial performance; |
| ● | the
perception of our ability to raise the necessary financing to complete the product development
activities including preclinical and clinical testing required for FDA approval and our ability
to generate revenue and cash flow from our products; |
| ● | the
anticipated or actual results of our operations; |
| ● | changes
in market valuations of other companies in our industry; |
| ● | litigation
or changes in regulations and insurance company reimbursement policies affecting prescription
drugs; |
| ● | concern
that our internal controls are ineffective; |
| ● | any
discrepancy between anticipated or projected results and actual results of our operations; |
| ● | actions
by third parties to either sell or purchase stock in quantities which would have a significant
effect on our stock price; and |
| ● | other
factors not within our control. |
Raising funds by issuing equity or convertible
debt securities could dilute the net tangible book value of the common stock and impose restrictions on our working capital.
We anticipate that we
will require funds for our business. If we were to raise capital by issuing equity securities, either alone or in connection with a non-equity
financing, the net tangible book value of the then outstanding common stock could decline. If the additional equity securities were issued
at a per share price less than the market price, which is customary in the private placement of equity securities, the holders of the
outstanding shares would suffer dilution, which could be significant. Further, if we are able to raise funds from the sale of debt securities,
the lenders may impose restrictions on our operations and may impair our working capital as we service any such debt obligations.
Stockholders may experience significant
dilution as a result of future equity offerings and other issuances of our common stock or other securities.
We will need to raise substantial funds in order
to develop our products. In order to raise additional capital, we may in the future offer additional shares of our common stock or other
securities convertible into or exchangeable for our common stock at prices that may not which is less than the market price and which
may be based on a discount from market at the time of issuance. Stockholders will incur dilution upon exercise of any outstanding stock
options, warrants or upon the issuance of shares of common stock under our present and future stock incentive programs. In addition,
the sale of shares and any future sales of a substantial number of shares of our common stock in the public market, or the perception
that such sales may occur, could adversely affect the price of our common stock. We cannot predict the effect, if any, that market sales
of those shares of common stock or the availability of those shares of common stock for sale will have on the market price of our common
stock.
Our failure to
meet the continued listing requirements of Nasdaq could result in a de-listing of our common stock.
If we fail to satisfy
the continued listing requirements of Nasdaq, such as the corporate governance requirements or the minimum closing bid price requirement,
Nasdaq may take steps to de-list our securities. Such a de-listing would likely have a negative effect on the price of our common stock
and would impair your ability to sell or purchase our Common Stock when you wish to do so. In the event of a de-listing, we would take
actions to restore our compliance with Nasdaq’s listing requirements, but we can provide no assurance that any such action taken
by us would allow our common stock to become listed again, stabilize the market price or improve the liquidity of our common stock, prevent
our common stock from dropping below the Nasdaq minimum bid price requirement or prevent future non-compliance with Nasdaq’s listing
requirements.
We and our senior executive officers settled
an SEC investigation, which may affect the market for and the market price of our common stock and our ability to list on a stock exchange.
Following an investigation into the accuracy
of statements in our Form 10 registration statement filed June 2, 2016, as amended, and our Form 10-K annual report filed May 8, 2017
that did not accurately reflect the FDA’s jurisdiction over our consumer products and did not disclose that we could not legally
market these products in the United States, a Wells notice which we, our chief executive officer and our chief financial officer received
on August 10, 2017 and a Wells submission which we and the officers submitted in response to the Wells notice, the SEC, on December 26,
2018, announced that it has accepted our settlement offer and instituted settled an administrative cease-and-desist proceeding against
us and our chief executive officer and chief financial officer. The SEC’s administrative order, dated December 26, 2018, finds
that we and the officers consented – without admitting or denying any findings by the SEC — to cease-and-desist orders against
them for violations by us of Sections 12(g) and 13(a) of the Securities Exchange Act of 1934 and Rules 12b-20 and 13a-1 thereunder, which
require issuers to file accurate registration statements and annual reports with the Commission; violations by the officers for causing
our violations of the above issuer reporting provisions; and violations by the officers of Rule 13a-14 of the Exchange Act, which requires
each principal executive and principal financial officer of issuers to attest that annual reports filed with the SEC do not contain any
untrue statements of material fact. In addition to consenting to the cease-and-desist orders, the officers have each agreed to pay a
$25,000 civil penalty to resolve the investigation. The administrative order does not impose a civil penalty or any other monetary relief
against us. The settlement may affect the market for and the market price of our common stock.
The market price for our common stock may
be volatile and your investment in our common stock could suffer a decline in value.
The trading volume in our stock is low, which
may result in volatility in our stock price. As a result, any reported prices may not reflect the price at which you would be able to
sell shares of common stock if you want to sell any shares you own or buy if you wish to buy shares. Further, stocks with a low trading
volume may be more subject to manipulation than a stock that has a significant public float and is actively traded. The price of our
stock may fluctuate significantly in response to a number of factors, many of which are beyond our control. These factors include, but
are not limited to, the following, in addition to the risks described above and general market and economic conditions:
| ● | concern
about the effects of our settlement with the SEC; |
| ● | the
market’s reaction to our financial condition and its perception of our ability to raise
necessary funding or enter into a joint venture, as well as its perception of the possible
terms of any financing or joint venture; |
| ● | the
market’s perception as to our ability to generate positive cash flow or earnings; |
| ● | changes
in our or any securities analysts’ estimate of our financial performance; |
| ● | the
perception of our ability to raise the necessary financing to complete the product development
activities including preclinical and clinical testing required for FDA approval and our ability
to generate revenue and cash flow from our products; |
| ● | the
anticipated or actual results of our operations; |
| ● | changes
in market valuations of other companies in our industry; |
| ● | litigation
or changes in regulations and insurance company reimbursement policies affecting prescription
drugs; |
| ● | concern
that our internal controls are ineffective; |
| ● | any
discrepancy between anticipated or projected results and actual results of our operations; |
| ● | actions
by third parties to either sell or purchase stock in quantities which would have a significant
effect on our stock price; and |
| ● | other
factors not within our control. |
Because of our executive officers’
stock ownership and stock ownership of certain other stockholders that have invested in the company, these stockholders have the power
to elect all directors and to approve any action requiring stockholder approval.
Our
officers and directors as a group beneficially own approximately 46% of our common stock
as of July 18, 2024. As a result, they have the effective power using their contacts with
a limited number of other shareholders to elect all of our directors and to approve any action
requiring stockholder approval.
Raising funds
by issuing equity or convertible debt securities could dilute the net tangible book value of the common stock and impose restrictions
on our working capital.
We anticipate that we
will require funds for our business. If we were to raise capital by issuing equity securities, either alone or in connection with a non-equity
financing, the net tangible book value of the then outstanding common stock could decline. If the additional equity securities were issued
at a per share price less than the market price, which is customary in the private placement of equity securities, the holders of the
outstanding shares would suffer dilution, which could be significant. Further, if we are able to raise funds from the sale of debt securities,
the lenders may impose restrictions on our operations and may impair our working capital as we service any such debt obligations.
We may issue preferred
stock whose terms could adversely affect the voting power or value of our common stock.
Our articles of incorporation
authorize us to issue, without the approval of our stockholders, one or more classes or series of preferred stock having such designations,
preferences, limitations and relative rights, including preferences over our common stock respecting dividends and distributions, as
our board of directors may determine. The terms of one or more classes or series of preferred stock could adversely impact the voting
power or value of our common stock. For example, we might grant holders of preferred stock the right to elect a number of our directors
in all events or on the happening of specified events or the right to veto specified transactions. Similarly, the repurchase or redemption
rights or liquidation preferences we might assign to holders of preferred stock could affect the residual value of the common stock.
We do not intend
to pay any cash dividends in the foreseeable future.
We have not paid any
cash dividends on our common stock and do not intend to pay cash dividends on our common stock in the foreseeable future.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains “forward-looking
statements,” within the meaning of the Private Securities Litigation Reform Act of 1995, all of which are subject to risks and
uncertainties. Forward-looking statements can be identified by the use of words such as “expects,” “plans,” “will,”
“forecasts,” “projects,” “intends,” “estimates,” and other words of similar meaning.
One can identify them by the fact that they do not relate strictly to historical or current facts. These statements are likely to address
our growth strategy, financial results and product and development programs. One must carefully consider any such statement and should
understand that many factors could cause actual results to differ from our forward-looking statements. These factors may include inaccurate
assumptions and a broad variety of other risks and uncertainties, including some that are known and some that are not. No forward-looking
statement can be guaranteed and actual future results may vary materially.
These risks and uncertainties, many of which
are beyond our control, include, and are not limited to:
| |
● |
Our
ability to raise the necessary financing for the development of our business and the terms of any financing which we are able to
raise; |
| |
● |
Our
ability to receive FDA marketing approval for any products we may develop; |
| |
● |
Our
ability to obtain and enforce any United States and foreign patent we may seek; |
| |
● |
Our
ability to design and execute clinical trials to the satisfaction of regulatory authorities; |
| |
● |
Our
ability to engage, if and when necessary, an independent preclinical or clinical testing organization to design and implement our
trials; |
| |
● |
Our
ability to launch any products for which we receive FDA marketing approval; |
| |
● |
Our
ability to generate sufficient revenue from our contract services to cover our operating expenses; |
| |
● |
Our
ability to establish a distribution network for the marketing and sale of any products for which we receive FDA approval; |
| |
● |
Our
ability to establish manufacturing facilities in compliance with FDA good manufacturing practices or to enter into manufacturing
agreements for the manufacture of our products in an FDA approved manufacturing facility; |
| |
● |
Our
ability to enter into joint venture or other strategic relationship with respect to any of our proposed products; |
| |
● |
The
ability of the other party to any joint venture or strategic relationship to implement successfully any plans for the development,
clinical testing, manufacturing and marketing of the products subject to the joint venture or strategic relationship; |
| |
● |
Our
ability to evaluate potential acquisitions, and the consequences of our failure to accurately evaluate the acquisitions; |
| |
● |
Our
ability to integrate any business we acquire with our business; |
| |
● |
Changes
in national, regional and local government regulations, taxation, controls and political and economic developments that the market
for our products; |
| |
● |
Our
ability to develop and market products with the most current technology; |
| |
● |
Our
ability to obtain and maintain any permits or licenses necessary for our business; |
| |
● |
Our
ability to identify, hire and retain qualified executive, administrative, regulatory, research and development, and other personnel; |
| |
● |
Our
ability to negotiate licenses on favorable terms with companies that have experience in marketing products such as ours; |
| |
● |
The
costs associated with defending and resolving pending and potential legal claims, even if such claims are without merit; |
| |
● |
The
effects of the SEC settlement; |
| |
● |
The
effects of competition on our and our licensee’s ability to price, market and sell our product; |
| |
● |
Our
ability to achieve favorable pricing for our products with third party reimbursement parties with respect to our products; |
| |
● |
Our
ability to accurately estimate anticipated expenses, capital requirements and needs for additional financing; |
| |
● |
Our
ability to accurately estimate the timing, cost or other aspects of the commercialization of our product candidates; |
| |
● |
Actions
by third parties to either sell or purchase our common stock in quantities that would have a significant effect on our stock price; |
| |
● |
Risks
generally associated with pre-revenue development stage companies in the pharmaceutical industry; |
| |
● |
Current
and future economic and political conditions; |
| |
● |
The
impact of changes in accounting rules on our financial statements; |
| |
● |
Other
assumptions described in this prospectus; and |
| |
● |
Other
matters that are not within our control. |
Information regarding market and industry statistics
contained in this prospectus is included based on information available to us that we believe is accurate. It is generally based on industry
and other publications that are not produced for purposes of securities offerings or economic analysis. We have not reviewed or included
data from all sources. Forecasts and other forward-looking information obtained from these sources are subject to the same qualifications
and the additional uncertainties accompanying any estimates of future market size, revenue and market acceptance of products and services.
We do not assume any obligation to update any forward-looking statement. As a result, you should not place undue reliance on these forward-looking
statements.
The forward-looking statements in this prospectus
speak only as of the date of this prospectus and you should not to place undue reliance on any forward-looking statements. Forward-looking
statements are subject to certain events, risks, and uncertainties that may be outside of our control. When considering forward-looking
statements, you should carefully review the risks, uncertainties and other cautionary statements in this prospectus as they identify
certain important factors that could cause actual results to differ materially from those expressed in or implied by the forward-looking
statements. These factors include, among others, the risks described under in this prospectus, including those described under “Business,”
“Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations”
as well as in other reports and documents we file with the SEC. We undertake no obligation to revise or publicly release the results
of any revision to these forward-looking statements, except as required by law. Given these risks and uncertainties, you are cautioned
not to place undue reliance on such forward-looking statements.
USE OF PROCEEDS
Any proceeds that we receive upon exercise of
Warrants will be used for working capital. There is no assurance that the holders of our Warrants will elect to exercise any or all of
such Warrants.
CAPITALIZATION
The following table sets forth our capitalization
as of April 30, 2024.
| | |
April
30,
2024 | |
| Common stock | |
$ | 10,960 | |
| Additional paid-in capital | |
| 43,263,194 | |
| Accumulated other comprehensive loss | |
| (304 | ) |
| Treasury Stock | |
| (32,641 | ) |
| Accumulated deficit | |
| (29,878,096 | ) |
| Stockholders’ equity | |
| 13,363,113 | |
You should read the foregoing table in connection
with “Prospectus Summary — Selected Consolidated Financial Data,” “Management’s Discussion and Analysis
of Financial Condition and Results of Operations,” and our consolidated financial statements and related notes.
Common Stock and Warrants Listing and Trading
Since our initial public offering on October
1, 2021, our common stock has traded on The NASDAQ Capital Market under the symbol “NTRB”, and our Warrants are traded on
that exchange under the symbol “NTRBW”.
As of July 25 2024, we had approximately 110
holders of record of our common stock.
The transfer agent for the common stock is Equiniti
Trust Company, LLC, 6201 15th Ave, Brooklyn, NY, telephone (800) 937-5449.
MANAGEMENT’S DISCUSSION
AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of financial
condition and results of operations should be read in conjunction with our consolidated financial statements and related notes included
elsewhere in this report. This discussion contains forward-looking statements that involve risks, uncertainties and assumptions. See
“Note Regarding Forward-Looking Statements.” Our actual results could differ materially from those anticipated in the forward-looking
statements as a result of certain factors discussed in “Risk Factors” and elsewhere in this prospectus.
Overview
AVERSA™ Abuse Deterrent Transdermal Products
Our primary business is the development of a
portfolio of transdermal pharmaceutical products. Our lead product under development is AVERSA Fentanyl, our abuse deterrent fentanyl
transdermal system which will require approval from the Food and Drug Administration (“FDA”) and substantial capital for
research and development. AVERSA Fentanyl has the potential to provide clinicians and patients with an extended-release transdermal fentanyl
product for use in managing chronic pain requiring around the clock opioid therapy combined with properties designed to deter the abuse
and misuse of fentanyl patches. In addition, we believe that our abuse deterrent technology can be broadly applied to various other transdermal
products and our strategy is to follow the development of our abuse deterrent fentanyl transdermal system with the development of abuse
deterrent transdermal products for pharmaceuticals that have a risk of abuse, misuse or accidental exposure.
On September 19, 2023, the United States Patent
and Trademark Office (USPTO) granted US Patent No. 11,759,431 for Nutriband’s proprietary AVERSA abuse deterrent technology utilizing
taste aversion to address the primary routes of abuse of opioid based transdermal patches. The issuance of this patent, entitled, “Abuse
and Misuse Deterrent Transdermal Systems,” further expands Nutriband’s intellectual property protection in the United States
for its portfolio of AVERSA abuse deterrent transdermal products.
Transdermal Pharmaceutical Products
Through October 31, 2018, our business was the
development of a line of consumer and health products that are delivered through a transdermal or topical patch. Following our acquisition
of 4P Therapeutics on August 1, 2018, our focus expanded to include prescription pharmaceuticals, and we are seeking to develop and seek
FDA approval on a number of transdermal pharmaceutical products under development by 4P Therapeutics.
Most of our planned consumer products require
FDA approval for sale in the United States, and we have not sought to obtain, and we do not plan to seek to obtain, FDA approval to market
these products in the United States at this time. Following our acquisition of selected assets from Pocono Coated Products, LLC (“Pocono”),
we are primarily focused on providing contract manufacturing services and consulting services to third party brands with no intention
at this time to launch our own consumer products.
4P Therapeutics has not generated any revenue
from any of its products under development. Rather, prior to our acquisition, 4P Therapeutics generated revenue to provide cash for its
operations through contract research and development and related services for a small number of clients in the life sciences field on
an as-needed basis. We are, for the near term, continuing this activity, although we do not anticipate that it will generate significant
revenues and, since our acquisition, it has generated minor gross margins. We have no long-term contractual obligations, and either party
can terminate at any time.
With the change in our focus, our capital requirements
increased substantially. The process of developing pharmaceutical products and submitting them for FDA approval is both time consuming
and expensive, with no assurance of obtaining approval from the FDA to market our product in the United States. We will require approximately
$13 million for research and development of our abuse deterrent fentanyl transdermal system, including clinical manufacturing and clinical
trials that need to be completed in order to obtain FDA approval. However, the total cost could be substantially in excess of that amount.
On August 31, 2020, the Company entered into
a Purchase Agreement (“Agreement”), with Pocono Coated Products (“PCP”), pursuant to which PCP agreed to sell
the Company all of the assets associated with its Transdermal, Topical, Cosmetic and Nutraceutical business (the “Assets”).
PCP was the manufacturer of our transdermal consumer products, and we bought that business from them. The purchase price for the Assets
was (i) $6,000,000 paid in shares of the Company’s common stock at a value of the average price of the previous 90 days at the
date of Closing (the “Shares”); (ii) a promissory note of the Company in the principal amount of $1,500,000, which is due
upon the earlier of (a) twelve (12) months from issuance, or (b) immediately following a capital raise of no less than $4,000,000 and/or
a public offering of no less than $4,000,000. The note was repaid in full in October 2021. Subsequent to the repayment of the note, the
Shares were released from escrow.
On November 1, 2021, The Board of Directors adopted
the 2021 Employee Stock Option Plan (the “Plan”). The Company has reserved 408,333 shares to issue and sell upon the exercise
of stock options issued under the Plan. On November 3, 2021, the Company filed a Registration Statement on Form S-8, to register under
the Securities Act of 1933, as amended, the 408,333 shares of common stock reserved for issuance under the Plan, and on October 12, 2022,
a Post-Effective Amendment to the Form S-8 was filed with the SEC.
Forward Split of
our Common Stock.
On July 26, 2022, our
Board of Directors approved the amendment to our Articles of Incorporation to effect a 7 for 6 forward stock split (the “Stock
Split”) of our outstanding common stock. We filed the amendment set forth in a Certificate of Change with the Secretary of State
of Nevada on August 4, 2022. The 7:6 forward split was effective for trading purposes on the Nasdaq Capital Market on August 12, 2022.
Each shareholder of record as of the August 15, 2022 record date received one (1) additional share of common stock for each six (6) shares
held as of the record date. No fractional shares of common stock were issued in connection with the Stock Split. Instead, all shares
were rounded up to the next whole share. In connection with the Stock Split, which did not require shareholder approval under the Nevada
corporation law, the number of authorized shares of common stock of the Company was increased in the same ratio as the shares of outstanding
common stock were increased in the Stock Split, from 250,000,000 authorized shares to 291,666,666 authorized shares.
On December 15, 2023, the Company filed the Proxy
Statement with the SEC for its Annual Meeting of Stockholders, held January 21, 2024, in Orlando, Florida. This Proxy Statement is available
on our website at HTTPS://Nutriband.com/proxy.
On March 20, 2024, our Board of Directors adopted
an amendment to the Company’s 2021 Employees Stock Option Plan (the “Plan”) increasing the number of shares of
common stock subject to the plan (as of March 20, 2024 875,000 shares) to 1,400,00 shares (the “Amendment”). The plan adopted
by the Board on November 1, 2021, provided for an initial 350,000 shares to issue and sell upon the exercise of stock options issued
under the Plan. The Plan provides for an automatic annual increase to be added on February 1 of each year equal to the lesser of
(i) 250,000 shares of Common Equity or (ii) five percent (5%) of the total shares of Common Stock outstanding on such
date (including for this purpose any shares of Common Stock issuable upon conversion of any outstanding capital equity of the Company)
or (iii) such lesser number as determined by the Board. We will submit the Amendment to the Plan to our stockholders for adoption
and approval at the 2025 Annual Meeting. If the Amendment is not approved by stockholders within one year of adoption by the increase
in shares subject to the Plan will be void, together with any options issued following March 20, 2024 in the period pending approval
of the Plan by our stockholders.
On April 19, 2024, the Company completed an $8,400,000
equity financing with European investors (the “Offering”) of 2,100,000 units (“Units”), at a price of $4.00 per
Unit, each Unit consisting of one share of common stock (“Shares”) and a Warrant to purchase two Shares of common stock,
the Warrants having an initial exercise price of $6.43, are exercisable by payment of the exercise price in cash only and expire April
19, 2029, five years from the date of issuance (“Warrants”). The Offering was made solely to investors resident outside the
United States and was not registered under the Securities Act of 1933, as amended (the “Securities Act”), or the securities
laws of any jurisdiction, including any jurisdiction outside the United States, but was made privately by the Company pursuant to the
exemptions from registration provided in the SEC’s Regulation S and other exemptions under the Securities Act.
Results of Operations
Years Ended January 31, 2024 and 2023
For the year ended January 31, 2024, we generated
revenue of $2,085,314 and our costs of revenue were $1,223,209. For the year ended January 31, 2023, we generated revenue of $2,079,609
and our costs of revenue were $1,329,200. Our revenue for the year ended January 31, 2024, included sales of $1,920,280 from contract
manufacturing services performed in our Pocono Pharmaceuticals (Active Intelligence) segment and $165,034 from contract research and
development services from our 4P Therapeutics segment. The revenue from the Transdermal Patches segment remained relatively constant
from the prior year. An increase in demand is expected in the subsequent year. Our cost of revenue for our contract research and
development services represents our labor cost plus a modest amount of material costs which we passed on to the client. Our cost of sales
during the year for our contract services in comparison to the prior year as our main contract has been completed and the balance of
the contract is being recognized with limited additional costs.
For the year ended January 31, 2024, our selling,
general and administrative expenses were $3,773,606, primarily legal, accounting, administrative salaries non-cash compensation from
the issuance of warrants and employee stock options, compared to $3,916,041 for the year ended January 31, 2023. The decrease from 2023
is primarily due to a decrease in salaries and wages to executives of the Company.
During the years ended January 31, 2024 and 2023,
the Company recorded an impairment expense of $-0- and $327,326, respectively, due to a write down of Goodwill in connection with its
Pocono acquisition. The write down of goodwill for the year ended January 31, 2023, was attributable primarily to the effects of the
pandemic. As of January 31, 2024, the valuation of the reporting unit exceeds the carrying amount of goodwill using the value in use
or the going concern premise.
During the year ended January 31, 2024, the Company
incurred research and development expenses for its Aversa Fentanyl product of $1,960,425, primarily due to labor and material costs incurred
at our contract manufacturer, Kindeva Drug Delivery, as compared to $982,227 for the year ended January 31, 2023.
During the year ended January 31, 2024, the Company
incurred a loss on extinguishment of debt of $554,423, consisting primarily of the loss on the conversion of $2,000,000 of credit line
note into 1,026,750 shares of the Company’s common stock. There was no gain or loss on extinguishment of debt during the year ended
January 31, 2023.
We incurred interest expense of $75,815 for the
year ended January 31, 2024, as compared to $6,289 for the year ended January 31, 2023. The increase is primarily due to interest on
the Company’s related party credit line note.
As a result of the foregoing, we sustained a
net loss of $5,485,314, or $(0.69) per share (basic and diluted) for the year ended January 31, 2024, compared with a loss of $4,483,474,
or $(0.53) per share (basic and diluted) for the year ended January 31, 2023.
Liquidity and Capital
Resources January 31, 2024
As of January 31, 2024,
we had $492,942 in cash and cash equivalents and working capital of $22,770, as compared with cash and cash equivalents of $1,985,440
and working capital of $1,945,132 as of January 31, 2023. During the year ended January 31, 2024, the Company on March 19, 2023, entered
a three-year Credit Line Note facility for $2 million, to fund its research and development of its Aversa Fentayl product and an amendment
thereto on July 13, 2023, increasing the amount under the credit line to $5 million. During 2024, the Company drew down a total of $2,000,000
under the credit line. In December 2023, the $2,000,000 was converted into shares of the Company’s common stock. On April 19, 2024,
the Company completed an $8,400,000 equity financing with European investors (the “Offering”) of 2,100,000 units (“Units”),
at a price of $4.00 per Unit, each Unit consisting of one share of common stock (“Shares”) and a Warrant to purchase two
Shares of common stock.
For the year ended January
31, 2024, we used cash of $3,527,509 in our operations. The principal adjustments to our net loss of $5,485,314 were depreciation and
amortization of $287,722, net loss on extinguishment of debt of $554,423 and stock-based compensation of $742,696.
For the year ended January
31, 2024, we used cash in investing activities of $51,761 primarily for the purchase of equipment.
For the year ended January
31, 2024, we provided cash in financing activities of $2,086,772, primarily from the proceeds of $2,000,000 from the proceeds of $2,000,000
from its line of credit and $106,528 from a factoring arrangement, offset from the payment on notes of $19,756. For the year ended January
31, 2023, we had cash flows of $160,074 from financing activities, primarily of $296,875 from the exercise of warrants, offset by a payment
on notes and the repurchase of treasury stock.
Off Balance Sheet Arrangements
We have no off-balance sheet
arrangements that have or are reasonably likely to have a current or future material effect on our financial condition, changes in financial
condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources.
Three Months Ended April 30, 2024 and 2023
For the three months
ended April 30, 2024, we generated revenue of $408,532 and our costs of revenue were $243,746. For the three months ended April 30, 2023,
we generated revenue of $478,942 and our costs of revenue were $254,648. Our revenue for April 30, 2024, was derived from sales from
contract manufacturing services performed in our Pocono Pharmaceuticals (Active Intelligence) segment $-0- from contract research and
development services from our 4P Therapeutics segment. The revenue from the Transdermal Patches segment remained relatively constant
from the prior year. An increase in demand is expected in the balance of the current year. The Company’s contract with Sorrento
Therapeutics was completed and 4P Therapeutics devoted most of its time to the development of its Aversa product, our cost of revenue
for our contract research and development services represents our labor cost plus a modest amount of material costs which we passed on
to the client.
For the three months
ended April 30, 2024, our selling, general and administrative expenses were $1,078,728 primarily legal, accounting and administrative
salaries and non-cash compensation from the issuance of employee stock options compared to $422,955 for the three months ended April
30, 2024.The increase from 2023 is primarily attributable to increases in non-cash equity-based expenses.
During the three months
ended April 30, 2024, the Company incurred research and development expenses of its Aversa Fentanyl product of $974,535, primarily of
salaries and increases in development costs from Kindeva as compared to $400,430 for the three months ended April 30, 2023.
We incurred interest
expense of $8,618 for the three months ended April 30, 2024, as compared to $3,166 for the three months ended April 30, 2023. The increase
is primarily due to interest in the Company’s related party loans.
As a result of the foregoing,
we sustained a net loss of $1,898,077 or $(0.21) per share (basic and diluted) for the three months ended April 30, 2024, compared with
a loss of $1,015,235, or $(0.13) per share (basic and diluted) for the three months ended April 30, 2023.
Liquidity and Capital
Resources April 30, 2024
As of April 30, 2024,
we had $8,347,740 in cash and cash equivalents and working capital of $7,313,072, as compared with cash and cash equivalents of $492,942
and working capital of $22,870 as of January 31, 2024.
For the three months
ended April 30, 2024, we used cash of $833,926 in our operations. The principal adjustments to our net loss of $1,898,077 were depreciation
and amortization of $69,101, and the issuance of employee stock options for services in the amount of $422,955.
For the three months
ended April 30, 2024, we used cash in investing activities of $6,195 primarily for the purchase of equipment.
For the three months
ended April 30, 2024, we provided cash in financing activities of $8,694,919. During the three months ended April 30, 2024, the Company
entered into an equity financing agreement with European investors and received proceeds of $8,400,00, of which $7.12 million is from
related parties, to fund its research and development of its Aversa Fentanyl product. The Company also received proceeds of $300,000
from its Credit Line Promissory Note.
Off Balance Sheet
Arrangements
We have no off-balance
sheet arrangements that have or are reasonably likely to have a current or future material effect on our financial condition, changes
in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources.
Critical Accounting Policies
Going Concern Assessment
Management assesses liquidity
and going concern uncertainty in the Company’s condensed financial statements to determine whether there is sufficient cash on
hand and working capital, including available borrowings on loans, to operate for a period of at least one year from the date the consolidated
financial statements are issued or available to be issued, which is referred to as the “look-forward period”, as defined
in GAAP. As part of this assessment, based on conditions that are known and reasonably knowable to management, management will consider
various scenarios, forecasts, projections, estimates and will make certain key assumptions, including timing and nature of projected
cash expenditures or programs, its ability to delay or curtail expenditures or programs and its ability to raise additional capital,
if necessary, among other factors. Based on this assessment, as necessary or applicable, management makes certain assumptions around
implementing curtailments or delays in the nature and timing of programs and expenditures to the extent it deems probable those implementations
can be achieved and management has the proper authority to execute them within the look-forward period.
As of April 30, 2024,
the Company had cash and cash equivalents of $8,347,740 and working capital of $7,313,082. For the three months ended April 30, 2024,
the Company incurred a net loss from operations of $1,898,077 and used cash flow from operations of $833,926. The Company has generated
operating losses since its inception and has relied on sales of securities and issuance of third-party and related-party debt to support
cash flow from operations. The Company has used these proceeds from the sales of securities and issuance of third-party and related party
debt to fund operations and will continue to use the funds as needed. In March 2023, the Company entered into a three-year $2,000,000
Credit Line Note facility with a related party, amended on July 13, 2023, to $5,000,000, which will permit the Company to draw down on
the credit line to fund the Company’s research and development of its Aversa product. On April 19, 2024, the Company received proceeds
of $8,400,000 from equity financing with European investors, of which $7.12 million is from related parties.
Management has prepared
estimates of operations for the next twelve months and believes that sufficient funds will be generated from operations to fund its operations
for one year from the date of the filing of these condensed consolidated financial statements, which indicates improved operations and
the Company’s ability to continue operations as a going concern.
Management believes the
substantial doubt about the ability of the Company to continue as a going concern is alleviated by the above assessment.
Principles of Consolidation
The consolidated financial
statements of the Company include the Company and its wholly owned subsidiaries. All material intercompany balances and transactions
have been eliminated. The operations of 4P Therapeutics are included in the Company’s financial statements from the date of acquisition
of August 1, 2018, and the operations of Pocono and Active Intelligence are included in the Company’s financial statements from
the date of acquisition of September 1, 2020 under Pocono Pharmaceuticals Inc. The wholly owned subsidiaries are as follows:
Nutriband
Ltd.
4P
Therapeutics LLC
Pocono
Pharmaceuticals Inc.
Use of Estimates
The preparation of the
consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires
the Company to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses, and related
disclosure of contingent assets and liabilities. On an ongoing basis, the Company evaluates its estimates including, but not limited
to, those related to such items as income tax exposures, accruals, depreciable/useful lives, allowance for doubtful accounts and valuation
allowances. The Company bases its estimates on historical experience and on other various assumptions that are believed to be reasonable
under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities
that are not readily apparent from other sources. Actual results could differ from those estimates.
Revenue Recognition
In May 2014, the FASB
issued ASU No. 2014-09, “Revenue from Contracts with Customers (Topic 606) (“ASU 2014-09”), which amends the accounting
standards for revenue recognition. ASU 2014-09 is based on principles that govern the recognition of revenue at an amount an entity expects
to be entitled when products are transferred to a customer. The Company recognizes revenue based on the five criteria for revenue recognition
established under Topic 606: 1) identify the contract, 2) identify separate performance obligations, 3) determine the transaction price,
4) allocate the transaction price among the performance obligations, and 5) recognize revenue as the performance obligations are satisfied.
Revenue
Types
The following is a description
of the Company’s revenue types, which include professional services and sale of goods:
| |
● |
Contract
development and manufacturing services for consumer health transdermal, topical and tape products with revenues listed under sale
of goods. |
| |
● |
Product
revenues derived from the sale of the Company’s consumer transdermal, topical and tape products with sales listed under sale
of goods. |
| |
● |
Contract
research and development services for pharmaceutical and medical devices for life sciences customers with revenues listed under services. |
Contracts with Customers
A contract
with a customer exists when (i) we enter into an enforceable contract with a customer that defines each party’s rights regarding
the goods or services to be transferred and identifies the payment terms related to these goods or services, (ii) the contract has commercial
substance and, (iii) we determine that collection of substantially all consideration for services that are transferred is probable based
on the customer’s intent and ability to pay the promised consideration.
Contract Liabilities
Deferred
revenue is a liability related to a revenue producing activity for which revenue has not been recognized. The Company records deferred
revenue when it receives consideration from a contract before achieving certain criteria that must be met for revenue to be recognized
in conformity with GAAP.
Performance Obligations
A performance
obligation is a promise in a contract to transfer a distinct good or service to the customer and is the unit of account in the new revenue
standard. The contract transaction price is allocated to each distinct performance obligation and recognized as revenue when, or as,
the performance obligation is satisfied. For the Company’s different revenue service types, the performance obligation is satisfied
at different times. The Company’s performance obligations include providing products and professional services in the area of research.
The Company recognizes product revenue performance obligations in most cases when the product has shipped to the customer. When we perform
professional service work, we recognize revenue when we have the right to invoice the customer for the work completed, which typically
occurs over time on a monthly basis for the work performed during that month.
All revenue recognized
in the income statement is considered to be revenue from contracts with customers.
Cash and cash equivalents.
Cash and cash equivalents
include cash on hand, cash on deposit in money market accounts. The Company considers short-term highly liquid investments with an original
maturity date of three months or less that are not part of an investment pool to be cash equivalents. As of April 30, 2024, the Company
had $7,879,000 that exceeded federally insured limits.
Accounts receivable
Trade accounts receivables
are recorded at the net invoice value and are not interest bearing. The Company maintains allowances for doubtful accounts for estimated
losses from the inability of its customers to make required payments. The Company determines its allowances by both specific identification
of customer accounts where appropriate and the application of historical loss to non-applicable accounts. For the three months ended
April 30, 2024, and 2023, the Company recorded bad debt expenses of $1,200 and $-0-, respectively, for doubtful accounts related to accounts
receivable. During the year ended January 31, 2024, the Company entered into an accounts receivable sale agreement for one of its subsidiaries.
The Company received $106,528 in funds against an account receivable that is currently a claim in bankruptcy. The net accounts receivable
remain on the books of the Company and a corresponding amount has been included as a secured borrowing liability under Notes payable.
As of April 30, 2024, the receivable has been reserved in full. If the bankruptcy claim is not paid in full by the debtor, Company is
obligated to pay any difference to the factor. The loan bears interest at 10%. The Company adopted ASU 2016-13 during 2013, and implemented
the guidance on expected credit losses.
Inventories
Inventories are valued
at the lower of cost and reasonable value determined using the first-in, first-out (FIFO) method. Net realized value is the estimated
selling price in the ordinary course of business, less applicable variable selling expenses. The cost of finished goods and work in process
is comprised of material costs, direct labor costs and other direct costs and related production overheads (based on normal operating
capacity). As of April 30, 2024, total inventory was $168,505, consisting of work-in-process of $27,447, finished goods of $26,751 and
raw materials of $114,307. As of January 31, 2024, total inventory was $168,605, consisting of work-in-process of $7,466, finished goods
of $8,707 and raw materials of $134,691.
Property, Plant
and Equipment
Property and equipment
represent an important component of the Company’s assets. The Company depreciates its plant and equipment on a straight-line basis
over the estimated useful life of the assets. Property, plant and equipment is stated at historical cost. Expenditures for minor repairs,
maintenance and replacement parts which do not increase the useful lives of the assets are charged to expense as incurred. All major
additions and improvements are capitalized. Depreciation is computed using the straight-line method. The lives over which the fixed assets
are depreciated range from 3 to 20 years as follows:
| Lab
Equipment |
|
5-10
years |
| Furniture
and fixtures |
|
3
years |
| Machinery
and equipment |
|
10-20
years |
Intangible Assets
Intangible assets include
trademarks, intellectual property and customer base acquired through business combinations. The Company accounts for Other Intangible
Assets under the guidance of ASC 350, “Intangibles-Goodwill and Other.” The Company capitalizes certain costs related to
patent technology. A substantial component of the purchase price related to the Company’s acquisitions have also been assigned
to intellectual property and other intangibles. Under the guidance, other intangible assets with definite lives are amortized over their
estimated useful lives. Intangible assets with indefinite lives are tested annually for impairment. Trademarks, intellectual property
and customer base are being amortized over their estimated useful lives of ten years.
Goodwill
Goodwill represents the
difference between the total purchase price and the fair value of assets (tangible and intangible) and liabilities at the date of acquisition.
Goodwill is reviewed for impairment annually on January 31, and more frequently as circumstances warrant, and written down only in the
period in which the recorded value of such assets exceeds their fair value. The Company does not amortize goodwill in accordance with
ASC 350. In connection with the Company’s acquisition of 4P Therapeutics LLC in 2018, the Company recorded Goodwill of $1,719,235.
On August 31, 2020, in connection with the Company’s acquisition of Pocono Coated Products LLC and Active Intelligence LLC, the
Company recorded Goodwill of $5,810,640. During the years ended January 31, 2024, and 2023, the Company recorded an impairment charge
of $-0- and $327,326, respectively, reducing the Active Intelligence LLC Goodwill to $3,302,478. As of April 30, 2024, and January 31,
2024, Goodwill amounted to $5,021,713 and $5,021,713, respectively.
Long-lived Assets
Management reviews long-lived
assets for potential impairment whenever significant events or changes in circumstances indicate that the carrying amount of an asset
may not be recoverable. An impairment exists when the carrying amount of the long-lived asset is not recoverable and exceeds its fair
value. The carrying amount of a long-lived asset is not recoverable if it exceeds the sum of the estimated undiscounted cash flows expected
to result from the use and eventual disposition of the asset. If an impairment exists, the resulting write-down would be the difference
between the fair market value of the long-lived asset and the related book value.
Earnings per Share
Basic earnings per share
of common stock is computed by dividing net earnings by the weighted average number of shares of common stock outstanding during the
period. Diluted earnings per share is computed by dividing net earnings by the weighted average number of shares of common stock
and potential shares of common stock outstanding during the period. Potential shares of common stock consist of shares issuable
upon the exercise of outstanding options and common stock purchase warrants. As of April 30, 2024, and 2023, there were 6,747,873 and
1,783,373 common stock equivalents outstanding, that were not included in the calculation of dilutive earnings per share as their effect
would be anti-dilutive.
Stock-Based Compensation
ASC 718, “Compensation
- Stock Compensation,” prescribes accounting and reporting standards for all share-based payment transactions in which employee
services, and, since February 1, 2019, non-employees, are acquired. Transactions include incurring liabilities, or issuing or offering
to issue shares, options and other equity instruments such as employee stock ownership plans and stock appreciation rights. Share-based
payments to employees, including grants of employee stock options, are recognized as compensation expense in the financial statements
based on their fair values. That expense is recognized over the period during which an employee is required to provide services in exchange
for the award, known as the requisite service period (usually the vesting period). As of February 1, 2019, pursuant to ASC 2018-07, ASC
718 was applied to stock-based compensation for both employees and non-employees.
Business
Combinations
The
Company recognizes the assets acquired, the liabilities assumed, and any non-controlling interest in the acquired entity at the acquisition
date, measured at their fair values as of that date, with limited exceptions specified in the accounting literature. In accordance with
this guidance, acquisition-related costs, including restructuring costs, must be recognized separately from the acquisition and will
generally be expensed as incurred. That replaces the cost-allocation process detailed in previous accounting literature, which required
the cost of an acquisition to be allocated to the individual assets acquired and liabilities assumed based on their estimated fair value.
Leases
In
February 2016, the FASB issued ASU 2016-02, “Leases” (Topic 842), to provide a new comprehensive model for lease accounting
under this guidance, lessees and lessors should apply a “right-of-use” model in accounting for all leases (including subleases)
and eliminate the concept of operating leases and off-balance-sheet leases. Recognition, measurement and presentation of expenses will
depend on classification as a finance or operating lease. Similar modifications have been made to lessor accounting in-line with revenue
recognition guidance.
The
Company applies the guidance for right-of-use accounting for all leases and records the operating lease liabilities on its balance sheet.
The Company completed the necessary changes to its accounting policies, processes, disclosure and internal control over financial reporting.
Research
and Development Expenses
Research
and development costs are expensed as incurred.
Income
Taxes
Taxes
are calculated in accordance with taxation principles currently effective in the United States and Ireland.
The
Company accounts for income taxes under the asset and liability method, which requires the recognition of deferred tax assets and liabilities
for the expected future tax consequences of events that have been included in the financial statements. Under this method, deferred tax
assets and liabilities are determined based on the differences between the financial statements and tax basis of assets and liabilities
using enacted tax rates in effect for the year in which the differences are expected to reverse. The effect of a change in tax rates
on deferred tax assets and liabilities is recognized in income in the period that includes the enactment date.
The Company records net deferred
tax assets to the extent they believe these assets will more-likely-than-not be realized. In making such determination, the Company considers
all available positive and negative evidence, including future reversals of existing taxable temporary differences, projected future
taxable income, tax planning strategies and recent financial operations. In the event the Company was to determine that it would be able
to realize its deferred income tax assets in the future in excess of its net recorded amount, the Company would make an adjustment to
the valuation allowance which would reduce the provision for income taxes.
Business
Nutriband Inc. (the “Company”, “Nutriband”,
“we” or “us”), was incorporated in Nevada in January 2016. Our primary business is the development of a portfolio
of transdermal pharmaceutical products. Our development pipeline consists of transdermal products that are based on our proprietary AVERSA™
abuse deterrent transdermal technology that we believe can be incorporated into existing transdermal patches that contain drugs
that are susceptible to abuse and misuse such as opioid and stimulant drugs.
The Company’s revenues are based on providing
services through our subsidiaries Pocono Pharmaceuticals operating as Active Intelligence and 4P Therapeutics. Pocono Pharmaceuticals
provides contract manufacturing services for health, wellness and over-the-counter pharmaceutical customers and 4P Therapeutics performs
contract research and development related services for pharmaceutical and medical devices customers. We manage and evaluate our operations,
and report our financial results, through these two separate subsidiaries.
Our principal offices are located in Orlando,
Florida, and our subsidiary, Pocono Pharmaceuticals, has a manufacturing facility in Cherryville, North Carolina. We primarily operate
and derive most of our revenues in the United States.
Recent Development
On April 19, 2024, the Company completed an $8,400,000
equity financing with European investors (the “Offering”) of 2,100,000 units (“Units”), at a price of $4.00 per
Unit, each Unit consisting of one share of common stock (“Shares”) and a Warrant to purchase two Shares of common stock,
the Warrants having an initial exercise price of $6.43, are exercisable by payment of the exercise price in cash only and expire April
19, 2029, five years from the date of issuance (“Warrants”). The Offering was made solely to investors resident outside the
United States and was not registered under the Securities Act, or the securities laws of any jurisdiction, including any jurisdiction
outside the United States, but was made privately by the Company pursuant to the exemptions from registration provided in the SEC’s
Regulation S and other exemptions under the Securities Act.
Our Business
AVERSA Abuse Deterrent Transdermal Products
Our lead product under development is AVERSA
Fentanyl, an abuse deterrent fentanyl transdermal system that combines an approved generic fentanyl patch with our AVERSA abuse deterrent
transdermal technology to reduce the abuse and misuse of fentanyl patches. We believe that our AVERSA technology can be broadly applied
to various transdermal products, and our plan is to follow the development of AVERSA Fentanyl with the development of additional abuse
deterrent transdermal products for pharmaceuticals that have a risk or history of abuse, misuse or accidental exposure. Specifically,
we have expanded our development pipeline to include AVERSA Buprenorphine and AVERSA Methylphenidate. In addition, we are developing
a portfolio of transdermal pharmaceutical products to deliver already approved drugs or biologics that are typically delivered by injection
but with the potential to improve compliance and therapeutic outcomes through transdermal delivery.
In January 2024, we signed a commercial development
and clinical supply agreement with Kindeva Drug Delivery, formerly 3M Drug Delivery (“Kindeva”), for the development of AVERSA
Fentanyl using Kindeva’s FDA-approved fentanyl patch. This agreement replaced the previous feasibility agreement between the two
companies which was focused on establishing the feasibility of incorporating our AVERSA abuse deterrent transdermal technology into Kindeva’s
commercial transdermal manufacturing process. The commercial development and clinical supply agreement is focused on developing the commercial
manufacturing process for AVERSA Fentanyl.
The product development program for AVERSA Fentanyl
includes performing preclinical and clinical studies to demonstrate the abuse deterrent properties of the product. The development program
is based on the fact that the fentanyl transdermal system is already approved and the only change to the approved product will be to
incorporate the AVERSA technology into the patch design with no change being made to the fentanyl drug matrix or its demonstrated safety,
patch performance or drug release characteristics. Preclinical studies to be performed consist of laboratory-based in vitro manipulation
and chemical extraction studies per FDA guidance. Clinical evaluation consists of a Phase 1 human abuse potential study to demonstrate
the abuse potential of the product per FDA guidance. The regulatory path to FDA approval is planned to be a 505(b)(2) NDA submission
to access the safety and efficacy information on file for the Duragesic® fentanyl transdermal system as the reference-listed
drug and to be able to obtain approval for abuse deterrent claims as a branded pharmaceutical product.
The product development program for the additional
AVERSA pipeline products, AVERSA Buprenorphine and AVERSA Methylphenidate, are similar to that of AVERSA Fentanyl, assuming that the
AVERSA technology is incorporated into an already approved transdermal patch.
Acquisition of 4P Therapeutics
Pursuant to an acquisition agreement dated April
5, 2018 between us and 4P Therapeutics, on August 1, 2018, we acquired all of the equity interest in 4P Therapeutics from Steven Damon,
the owner of 4P Therapeutics. The purchase price of $2,250,000, consisting of 62,500 shares of common stock, valued at $1,850,000, and
cash of $400,000, and are to pay Mr. Damon a 6% royalty on any revenue we receive or derive from our utilization or sale of the abuse
deterrent intellectual property that we acquired as a part of the assets 4P Therapeutics, including partner license milestones and development
payments. The royalty is payable pursuant to the acquisition agreement and continues as long as we generate revenue from our utilization
or sale of the abuse deterrent intellectual property we acquired as part of the acquisition of 4P Therapeutics. The 62,500 shares were
issued to Mr. Damon (41,750 shares pre-split) and Dr. Alan Smith (20,750 shares pre-split). In connection with the acquisition, Mr. Damon
retained any cash and accounts receivable and assumed any liabilities other than those relating to the ongoing business. Pursuant to
the acquisition agreement, we appointed Mr. Damon to our board of directors in April 2018, when we signed the acquisition agreement.
Mr. Damon resigned as a director in January 2022.
As a result of the acquisition, the focus of
our business changed from the development and marketing outside of the U.S. of consumer transdermal products to the development of 4P
Therapeutics’ portfolio of pharmaceutical transdermal products. Our lead product under development is AVERSA® Fentanyl
(abuse deterrent fentanyl transdermal system) which we plan to develop to deter the abuse and accidental misuse of fentanyl transdermal
patches. Fentanyl is a potent synthetic opioid that is marketed as a transdermal patch for chronic pain management. There are currently
several generic fentanyl patches on the market but none of them have abuse deterrent properties. We believe that AVERSA Fentanyl, once
approved by the US FDA will significantly deter the abuse and accidental misuse of fentanyl transdermal patches.
With the acquisition of 4P Therapeutics, we acquired
a research pipeline of other transdermal products, including novel transdermal products that involve delivery of peptides and proteins
through the skin. These drugs are off patent but are currently only available as injections, and we are evaluating the possibility of
developing a transdermal delivery system for these drugs as an alternative to injection but with improved compliance and safety. In addition,
we may develop certain generic passive transdermal products where we think we can make an improvement to existing patches and where we
believe we can take significant market share with good profit margins. The prioritization of our portfolio product candidates will be
reviewed on an ongoing basis and will take into account technical progress, market potential and R&D funding available. We cannot
assure you that we will be able to develop and obtain FDA approval for any of these potential products or that we can be successful in
marketing any such products. The FDA approval process can take many years to complete successfully, and we will require substantial funding
for each product that goes through the process. We cannot assure you that we will obtain FDA marketing approval for any of our products.
In addition to performing research and development
for its own products, 4P Therapeutics performs contract research and development services for a small number of clients in the life sciences
field to help support its ongoing operations. The work includes conducting early-stage drug and device clinical and preclinical studies
and providing clinical-regulatory and formulation/analytical consulting services. Neither we nor current clients have any long-term commitments,
and either party can terminate at any time. We do not expect to generate significant revenues from these services.
Acquisition of Pocono Coated Products
On August 25, 2020, the Company formed Pocono
Pharmaceuticals Inc.(“Pocono”), a wholly owned subsidiary of the Company. Effective August 31, 2020, the Company entered
into a Purchase Agreement (“Agreement”) with Pocono Coated Products (“PCP”), a manufacturer of topical and transdermal
products, pursuant to which PCP agreed to sell the Company certain of the assets and liabilities associated with its Transdermal, Topical,
Cosmetic and Nutraceutical business (the “Business”), including all related equipment, intellectual property and trade secrets,
cash balances, receivables, bank accounts and inventory. The net assets were contributed to Pocono. Included in the transaction, the
Company acquired 100% of the membership interests of Active Intelligence LLC (“Active Intelligence”). The purchase price
for the assets of the Business is (i) $6,000,000 paid in 608,519 shares of the Company’s common stock, based on the average price
for the Company’s common stock for the previous 90 days as of the date of Closing; (ii) a promissory note of the Company in the
principal amount of $1,500,000, which has been paid in full as of October 1, 2021.
Our Organization
We are a Nevada corporation, incorporated on
January 4, 2016. In January 2016, we acquired Nutriband Ltd, an Irish company which was formed by Gareth Sheridan, our chief executive
officer, in 2012, to enter the health and wellness market by marketing transdermal patches. Our corporate headquarters are located at
121 S. Orange Ave. Suite 1500, Orlando, Florida 32801, telephone (407) 377-6695. Our website is www.nutriband.com. Information
contained on or available through our website or any other website does not constitute a portion of this annual report.
Pharmaceutical Products in Development
We have a pipeline of transdermal pharmaceutical
products that are primarily in the early stages of development. Our current focus is on developing our AVERSA abuse deterrent transdermal
patch products. Our lead product is AVERSA Fentanyl for which we have a commercial development agreement with Kindeva Drug Delivery,
a contract development and manufacturing organization. We plan to follow on from this with the development of additional products utilizing
the AVERSA abuse deterrent transdermal technology, namely, AVERSA Buprenorphine and AVERSA Methylphenidate.
Transdermal patches containing opioid and stimulant
drugs are designed to provide an alternative route of administration for treatment of conditions such as chronic pain, opioid use disorder
or attention deficit/hyperactivity disorder. Although transdermal versions offer improved pharmacokinetic delivery as well as patient
convenience with wear times of up to 7 days, they contain an increased drug payload which can often be a target for recreational drug
abusers or subject to accidental pediatric exposure, particularly with infants and toddlers. Abuse of opioids in general, and in particular
fentanyl abuse and overdose, continues to be an epidemic which can lead to the abuse of prescription transdermal fentanyl and other opioid
containing transdermal products.
AVERSA Fentanyl is an abuse deterrent fentanyl
patch for the treatment of chronic pain. As the United States faces an epidemic of opioid abuse, fentanyl transdermal patches have become
an attractive target for recreational drug abusers due to the high potency of fentanyl, the high drug content contained in patches designed
for delivery over three days, and its ease of abuse by the oral route. We are looking to utilize our proprietary approach to incorporate
aversive agents into the transdermal patch to deter the abuse of fentanyl patches by the oral, buccal and inhaled routes, which represent
as much as 70% of all transdermal fentanyl abuse. The technology is based on the incorporation of taste and sensory aversive agents into
the patch that are intended to make abuse a very unpleasant experience thereby deterring the recreational abuse of fentanyl patches.
These aversive agents have high potency, established safety, and the potential to prevent accidental misuse by children and pets. The
aversive agents are coated onto the backing of the transdermal patch in a controlled release formulation that provides immediate and
sustained release of aversive agents. This provides several advantages including having a physical separation of the aversive agents
from the drug matrix, availability of aversive agents even after the patch is used and making it difficult to separate the aversive agents
from the drug by extraction. The aversive agents are not contained in the drug matrix and are not delivered to the skin during patch
wear. In addition to the fentanyl patch, this technology has broad applicability to any patch where deterring abuse as well as accidental
misuse by children and pets are valuable attributes.
According to the FDA1, accidental
exposure to medication is a leading cause of poisoning in children. Young children, in particular, have died or become seriously ill
after being exposed to a skin patch containing fentanyl, a powerful opioid pain reliever. Children can overdose on new and used fentanyl
patches by putting them in their mouth or sticking the patches on their skin. This can cause death by slowing the child’s breathing
and decreasing the levels of oxygen in their blood.
We believe that our abuse deterrent technology
can be broadly applied to various transdermal products and our strategy is to follow the development of our AVERSA Fentanyl with the
development of additional products for pharmaceuticals that have a risk or history of abuse, misuse or accidental exposure. For example,
we believe that our technology can be utilized in other transdermal products to deter the abuse of other drugs such as buprenorphine,
an opioid, and methylphenidate, a central nervous system stimulant. Buprenorphine is an opioid used to treat opioid addiction, acute
pain and chronic pain. It can be used under the tongue, by injection, as a skin patch, or as an implant. For opioid addiction, it is
typically only started when withdrawal symptoms have begun and for the first two days of treatment under direct observation of a health
care provider. For longer term treatment of addiction, a combination formulation of buprenorphine/naloxone is recommended to prevent
misuse by injection. Methylphenidate, sold under various trade names, such as Ritalin in oral form, and in transdermal patch form known
as Daytrana, is a central nervous system stimulant that is used in the treatment of attention deficit hyperactivity disorder and narcolepsy.
We plan to develop transdermal delivery systems for buprenorphine and methylphenidate after we make significant progress on our abuse
deterrent fentanyl transdermal system.
Our research pipeline consists primarily of drug
compounds which have been previously approved by the FDA and are now off-patent. In some cases, we are developing a non-injectable version
of the drug utilizing our transdermal technology which represents a new route of administration. We believe that transdermal delivery
has the potential to improve compliance, which can lead to improved therapeutic outcomes associated with these treatments. In most cases,
we plan to utilize the 505(b) (2) NDA regulatory pathway provided by the FDA which allows us to reference the safety information
on file at FDA for the approved drug or to reference the published literature instead of having to generate new safety information that
would typically be required for new chemical entities. However, we cannot assure you that the FDA will concur with our approach or that
we will be able to receive FDA approval to market any of products that we develop.
| 1 |
https://www.fda.gov/consumers/consumer-updates/accidental-exposures-fentanyl-patches-continue-be-deadly-children |
In addition, we may seek to develop certain generic
transdermal products where we think we can efficiently make an improvement to existing patches and potentially take significant market
share with good profit margins.
The prioritization of our portfolio of product
candidates will be reviewed on an ongoing basis and will take into account technical progress, market potential, available funding and
commercial interest. Our ability to take any meaningful steps to the development of any of these products is determined by our ability
to provide sufficient funding for such activities.
Pharmaceutical Manufacturing and Supply
Manufacturing of our pharmaceutical transdermal
products in development will be performed in compliance with FDA current Good Manufacturing Practices (cGMP) and all applicable local
regulations by contract manufacturers. All manufacturing processes and facilities will be subject to review by the FDA during development,
prior to approval and during subsequent routine FDA inspections. We plan to continue to rely on contract manufacturers and, potentially,
collaboration partners to manufacture commercial quantities of our products, if and when approved for marketing by the FDA.
Government Regulation and Regulatory Path
United States
The pharmaceutical business is subject to extensive
government regulation. In the United States, we must comply with the rules and regulations of the FDA. In other countries, we must comply
with the laws and regulations of each country to legally market and sell our products. Obtaining FDA approval does not mean that the
product will be approved in other countries. Each country may require that additional clinical and nonclinical studies be conducted prior
to approval.
The process required by the FDA to receive approval
prior to marketing and distributing a drug in the United States generally involves a preclinical phase followed by three phases of clinical
trials. The definition of drug is broadly defined and includes the pharmaceutical products we have in development. Even though the drug
used in each of our proposed products is currently approved by the FDA in other dosage forms, we will still need to conduct a development
program that will include preclinical and clinical trials before we receive FDA marketing approval. The FDA also has a number of abbreviated
approval pathways which, if we are eligible, could shorten the time for approval. For example, the regulatory path for the AVERSA products
in development is intended to follow a 505(b)(2) NDA regulatory pathway which may reduce the amount of clinical work that needs to be
performed to a single trial to evaluate the abuse potential of the product as the safety and efficacy of the drug has already been established.
However, we cannot be certain that we will be able to use any abbreviated approval pathway, in which event we will need to comply with
the full regulatory pathway as described below.
In general, the full NDA product development
program required for new drugs for FDA approval consists of the following phases of development listed below. The full NDA pathway is
not expected to be required for products incorporating AVERSA technology into an already approved transdermal patch.
| ● | Preclinical
phase. Before a drug company can test an experimental treatment in humans, it must prove
the drug is safe and effective in animals. Scientists run tests in various animals before
presenting the data to the FDA as an investigational new drug application. For already approved
drugs, an animal study may not be required prior to testing in humans. In most cases, the
company must file an Investigational New Drug (IND) submission to get clearance to test the
product in humans. |
| ● | Phase
one clinical trial. In the first round of clinical trials, the drug company attempts
to establish the drug’s safety in humans. Drug researchers administer the treatment
to healthy individuals — instead of patients suffering from the disease or condition
the drug is intended to treat — and gradually increase the dose to see if the drug
is toxic at higher levels or if any possible side effects occur. These drug trials are usually
small, containing about 20 to 80 participants, according to the FDA. For drug delivery products
incorporating already approved drugs, Phase 1 studies involve measuring blood levels of the
drug to understand the pharmacokinetics for a new route of administration. |
| ● | Phase
two clinical trial. In the second round of clinical trials, researchers give the treatment
to patients who have the disease to assess the drug’s efficacy. The trial is randomized,
meaning half of the study participants receive the drug and half receive a placebo. These
trials usually contain hundreds of participants, according to the FDA. There is about a 30
percent chance of a drug moving on to a phase three clinical trial, according to data from
the biotech trade organization BIO. For already approved drugs, as is the case with drug
delivery products, a Phase 2 trial may not be necessary as the therapeutic drug doses and
blood concentrations are already known. However, a Phase 2 may be conducted to inform the
design of the Phase 3 clinical trial in regards to the safety and efficacy of the product
when used by patients. |
| ● | Phase
three clinical trial. In the third phase of clinical trials, researchers work with the
FDA to design a larger trial to test the drug’s ideal dosage, patient population and
other factors that could decide whether the drug is approved, according to the report. These
trials usually contain a few hundred to thousands of participants. In the case of drug delivery
products that utilize an approved drug, Phase 3 trials will typically include a comparison
to the already approved reference product. For example, a transdermal patch may be compared
to an injection. |
| ● | New
drug application (NDA). Once a drug company collects and analyzes all data from the clinical
trials, it submits a new drug application to the FDA. The application includes trial data,
preclinical information and details on the drug’s manufacturing process. If the FDA
accepts the application for review, the agency typically has ten months, or six months if
the drug has priority review status, to make a decision whether to approve the drug or not.
The FDA can hold an advisory committee meeting where independent experts assess the data
and recommend whether to approve the drug. From there, the FDA will either approve the drug
or give the applicant a complete response letter, which explains why the drug did not get
approved and what steps the applicant must take before resubmitting the application for approval. |
Before approving an NDA, the FDA may inspect
the facilities where the product is being manufactured or facilities that are significantly involved in the product development and distribution
process and will not approve the product unless they determine that compliance with current good manufacturing practices is satisfactory.
The FDA may deny approval of an NDA if applicable statutory or regulatory criteria are not satisfied, or may require additional testing
or information, which can delay the approval process. In pursuing FDA approval there may be various delays and it is possible that approval
may never be granted. In addition, new government requirements may be established that could delay or prevent regulatory approval of
our product candidates under development.
If a product is approved, the FDA may impose
limitations on the indications for use for which the product may be marketed, may require that warning statements be included in the
product labeling, may require that additional studies or trials be conducted following approval as a condition of the approval, may impose
restrictions and conditions on product distribution, prescribing or dispensing in the form of a risk management plan, or impose other
limitations.
Once a product receives FDA approval, marketing
the product for other indicated uses or making certain manufacturing or other changes related to the product will require FDA review
and approval of a supplemental NDA or a new NDA, which may require additional clinical safety and efficacy data and may require additional
review fees. In addition, further post-marketing testing and surveillance to monitor the safety or efficacy of a product may be required.
Also, product approvals may be withdrawn if compliance with regulatory standards is not maintained or if safety or manufacturing problems
occur following initial marketing.
With respect to the labeling for our abuse deterrent
transdermal fentanyl system or any other opioid transdermal patch we develop, it is likely that the FDA will require us to disclose the
risks of improper use or abuse using language required by the FDA upon approval.
FDA Approval Pathways
The FDA has several pathways that can be followed
to obtain FDA approval.
| ● | A
stand-alone NDA is an application submitted under Section 505(b)(1) of the Food, Drug and
Cosmetic Act (“FD&C Act”) and approved under Section 505(c) of the FD&C
Act that contains full reports of investigations of safety and effectiveness that were conducted
by or for the applicant or for which the applicant has a right of reference or use. This
is typically the pathway used for new chemical entities. |
| ● | A
505(b)(2) application is a limited NDA submitted under Section 505(b)(1) and approved under
Section 505(c) of the FD&C Act that contains full reports of investigations of safety
and effectiveness, where at least some of the information required for approval comes from
studies not conducted by or for the applicant and for which the applicant has not obtained
a right of reference or use. This is the pathway typically taken for off-patent drugs that
are being development into alternate dosage forms or routes of administration. |
| ● | An
ANDA is an application for a duplicate of a previously approved drug product that was submitted
and approved under Section 505(j) of the FD&C Act. An ANDA relies on the FDA’s
finding that the previously approved drug product is safe and effective. An ANDA generally
must contain information to show that the proposed generic product (1) is the same as the
drug with respect to the active ingredients, conditions of use, route of administration,
dosage form, strength and labeling (with certain permissible differences) and (2) is bioequivalent
to the referenced drug. An ANDA may not be submitted if studies are necessary to establish
the safety and effectiveness of the proposed product. This is the pathway taken for generic
drugs. |
Nutriband plans to utilize the 505(b)(2) New
Drug Application (NDA) regulatory pathway which limits the development required for products that contain drugs that have already been
approved, and allows applicants to reference data already on file at the FDA. As a result, the NDA application will be primarily based
on a single Phase 1 human abuse potential clinical study with no Phase 2 or 3 clinical trials needed. A clinical abuse potential study
is typically performed in recreational drug abusers and is designed to demonstrate that the abuse-deterrent product is less preferable
to recreational drug abusers than conventional fentanyl patches which contain no abuse-deterrent technology.
Following a successful Phase 1 clinical abuse
potential study, Nutriband intends to file a 505(b)(2) NDA to the FDA for marketing approval of AVERSA™ Fentanyl, which has the
potential to be the first and only abuse deterrent patch approved anywhere in the world. The AVERSA™ Fentanyl NDA has the potential
to receive an expedited review by FDA as has been granted for certain abuse-deterrent oral opioid products, which shortens the regulatory
review period to six months from the conventional 10-month FDA review cycle for NDAs.
Combined, the clinical development and regulatory
path for AVERSA Fentanyl is substantially limited compared to conventional pharmaceutical product development, requiring only a single
clinical trial and, following a limited NDA pathway, undergoing an expedited review by the FDA.
We cannot assure you that we will be able to
take advantage of any of the available abbreviated approval pathways for any of our proposed products.
Post-approval requirements
Any drug products for which we receive FDA approval
will be subject to continuing regulation by the FDA. Certain requirements include, among other things, record-keeping requirements, reporting
of adverse events with the product, providing the FDA with updated safety and efficacy information on an annual basis or more frequently
for specific events, product sampling and distribution requirements, complying with certain electronic records and signature requirements
and complying with FDA promotion and advertising requirements. These promotion and advertising requirements include, among others, standards
for direct-to-consumer advertising, prohibitions against promoting drugs for uses or patient populations that are not described in the
drug’s approved labeling, known as “off-label use,” and other promotional activities, such as those considered to be
false or misleading. Failure to comply with FDA regulations can have negative consequences, including the immediate discontinuation of
noncomplying materials, adverse publicity, enforcement letters from the FDA, mandated corrective advertising or communications with doctors,
and civil or criminal penalties. Such enforcement may also lead to scrutiny and enforcement by other government and regulatory bodies.
Although physicians may prescribe legally available
drugs for off-label uses, manufacturers may not encourage, market or promote such off-label uses. As a result, “off-label promotion”
has formed the basis for litigation under the Federal False Claims Act, violations of which are subject to significant civil fines and
penalties. In addition, manufacturers of prescription products are required to disclose annually to the Center for Medicaid and Medicare
any payments made to physicians and teaching hospitals in the U.S. under the federal Physician Payment Sunshine Act. Reportable payments
may be direct or indirect, in cash or kind, for any reason, and are required to be disclosed even if the payments are not related to
the approved product. Failure to fully disclose or not in time reporting could lead to penalties up to $1.15 million per year.
The manufacturing of any of our products will
be required to comply with the FDA’s current Good Manufacturing Practices (cGMP) regulations. These regulations require, among
other things, quality control and quality assurance, as well as the corresponding maintenance of comprehensive records and documentation.
Drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are also required to register with
the FDA their establishments and list any products they make and to comply with related requirements in certain states. These entities
are further subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with current good manufacturing
practices and other laws. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality
control to maintain cGMP compliance.
Discovery of problems with a product after approval
may result in serious and extensive restrictions on a product, manufacturer or holder of an approved NDA, as well as lead to potential
market disruptions. These restrictions may include recalls, suspension of a product until the FDA is assured that quality standards can
be met, and continuing oversight of manufacturing by the FDA under a “consent decree,” which frequently includes the imposition
of costs and continuing inspections over a period of many years, as well as possible withdrawal of the product from the market. In addition,
changes to the manufacturing process generally require prior FDA approval before being implemented. Other types of changes to the approved
product, such as adding new indications and additional labeling claims, are also subject to further FDA review and approval.
The FDA also may require post-marketing testing,
or Phase IV testing, as well as risk minimization action plans and surveillance to monitor the effects of an approved product or place
conditions on an approval that could otherwise restrict the distribution or use of our products.
Other Government Regulations
We may be subject to government regulations that
are applicable to businesses generally, including those relating to workers’ health and safety, environmental and waste disposal,
wage and hour and labor practices, including sexual harassment laws and regulations, and anti-discrimination laws and regulations.
In addition, we must comply with the laws and
regulations governing the research and manufacture of products containing controlled substances such as fentanyl and other opioids. We
or our contract manufacturer must be licensed by the Drug Enforcement Agency (DEA) and the state(s) in which we conduct research and
development activities.
Europe and Other Countries
If we market our products in any countries other
than the United States, we would be subject to the laws of those countries. To obtain market access for our products in other countries
we must comply with numerous and varying regulatory requirements of such countries regarding the demonstration of safety and efficacy
for authorization and governing, among other things, clinical trials and commercial sales, pricing and distribution of our products.
The European medicines regulatory system is based
on a network of around 50 regulatory authorities from the 31 countries in the European Economic Area, the European Commission and
the European Medicines Agency. All medicines must be authorized before they can be placed on the market in the European Union. The European
system offers different routes for authorization. A centralized procedure allows the marketing of a medicine on the basis of a single
European Union assessment and marketing authorization which is valid throughout the European Union. However, a majority of medicines
authorized in the European Union do not fall within the scope of the centralized procedure, and we do not know whether our proposed products
will fall within the centralized authorization. We also do not know how the withdrawal of Great Britain from the European Union will
affect the procedure for approval of medicines in the United Kingdom. If we are not able to use the centralized procedure, we would need
to use one of the following procedures. One method is the decentralized procedure where we would apply for simultaneous authorization
in more than one European Union member. The second method is the mutual-recognition procedure where we would have a medicine authorized
in one European Union country apply for authorization to be recognized in other European Union countries. In either case, we would be
required to complete clinical trials to demonstrate the safety and efficacy of the medicine and show that the medicine is manufactured
in accordance with good manufacturing practices based upon European Union standards.
In countries other than the United States and
the European Union, we would be required to comply with the applicable laws of those countries, which may require us to perform additional
clinical testing.
Failure to obtain regulatory approval in any
country would prevent our product candidates from being marketed in those countries. In order to market and sell our products in jurisdictions
other than the United States and the European Union, we must obtain separate marketing approvals and comply with numerous and varying
regulatory requirements. The regulatory approval process outside the United States and the European Union generally includes all of the
risks associated with obtaining FDA and European Union approval but can involve additional testing.
In addition, in many countries worldwide, it
is required that the product be approved for reimbursement before the product can be approved for sale in that country. We may not obtain
approvals from regulatory authorities outside the United States on a timely basis, if at all. Even if we were to receive approval in
the United States or the European Union, approval by the FDA or the European Medicines Agency does not ensure approval by regulatory
authorities in other countries or jurisdictions. Similarly, approval by one regulatory authority outside the United States would not
ensure approval by regulatory authorities in other countries or jurisdictions. We may not be able to file for marketing approvals and
may not receive necessary approvals to commercialize our products in any market. If we are unable to obtain approval of our product candidates
by regulatory authorities in other foreign jurisdictions, the commercial prospects of those product candidates may be significantly diminished
and our business prospects could decline.
Outside the United States, particularly in member
states of the European Union, the pricing of prescription drugs is subject to governmental control. In these countries, pricing negotiations
or the successful completion of health technology assessment procedures with governmental authorities can take considerable time after
receipt of marketing approval for a product. In addition, there can be considerable pressure by governments and other stakeholders on
prices and reimbursement levels, including as part of cost containment measures.
In addition to regulations in the United States,
if we market outside of the United States, we will be subject to a variety of regulations governing, among other things, clinical trials
and any commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, we must obtain the requisite
approvals from regulatory authorities in foreign countries prior to the commencement of clinical trials or marketing of the product in
those countries.
Intellectual Property
The AVERSA abuse deterrent technology utilized
in our AVERSA product pipeline is covered by an international intellectual property portfolio with patents issued in 45 countries including
the United States, Europe, Japan, Korea, Russia, Mexico, Canada, and Australia and pending in China and Hong Kong. These patents provide
patent coverage to 2035. We continue to build on our proprietary positions in the United States and internationally for our product candidates
AVERSA Fentanyl, AVERSA Buprenorphine and AVERSA Methylphenidate as well as other products and technology that we may have in development.
Our policy is to pursue, maintain and defend patent rights developed internally or acquired externally and to protect the technology,
inventions and improvements that are commercially important to the development of our business. We cannot be sure that patents will be
granted with respect to any of our pending patent applications or with respect to any patent applications filed by us in the future,
nor can we be sure that any of our existing patents or any patents granted to us in the future will be commercially useful in protecting
our technology. We also may rely on trade secrets to protect our commercial products and product candidates. Our commercial success also
depends in part on our non-infringement of the patents or proprietary rights of third parties.
On September 19, 2023, the United States Patent
and Trademark Office (USPTO) granted US Patent No. 11,759,431 for Nutriband’s proprietary AVERSA abuse deterrent technology utilizing
taste aversion to address the primary routes of abuse of opioid based transdermal patches. The issuance of this patent, entitled, “Abuse
and Misuse Deterrent Transdermal Systems,” further expands Nutriband’s intellectual property protection in the United States
for its portfolio of AVERSA abuse deterrent transdermal products.
Further, we plan to seek trademark protection
in the United States and internationally where available and when appropriate. We have registered the name Nutriband in the United States.
We have filed an intent to use Trademark application for AVERSA. The USPTO will require us to show the mark used in commerce prior to
fully registering the trademark.
Publications
On March 8, 2024, Nutriband presented data on
the incidence of transdermal patch abuse and accidental pediatric exposure as a scientific poster at the 2024 American Academy of Pain
Medicine (AAPM) Annual Meeting2. The American Academy of Pain Medicine (AAPM) is dedicated to advancing multidisciplinary
pain care, education, advocacy, and research.
The company engaged Rocky Mountain Poison &
Drug Safety (RMPDS), a division of Denver Health and Hospital Authority, Denver, Colorado, to determine the incidence of abuse and accidental
pediatric exposure of transdermal patches containing drugs of abuse (fentanyl, buprenorphine, and methylphenidate) in the United States
based on poison center data for the surveillance period 2018-2022. RMPDS utilized the Researched Abuse, Diversion and Addiction-Related
Surveillance (RADARS®) System, a surveillance system that collects real-world safety and effectiveness data about prescription drugs
(https://www.radars.org/).
| 2 |
Olsen,
H, Mogusu, E, Black, JC, Sumbundu, K, Dart, RC. Poison center exposure calls involving fentanyl, buprenorphine, and methylphenidate
transdermal patches in the United States. Poster presented at the 40th Annual Meeting of the American Academy of Pain Medicine; 2024
Mar 7-10; Scottsdale, Arizona. https://www.radars.org/system/publications/39.%20Olsen.pdf |
The data indicate that transdermal patch abuse
and accidental pediatric exposures to patches continues to be a serious problem resulting in major medical outcomes and death, suggesting
an unmet need for safer abuse-deterrent versions of transdermal patches containing drugs with a risk of abuse, misuse or accidental exposure.
Key findings from the study showed that major medical outcome or death resulted from a notable proportion of fentanyl and buprenorphine
patch intentional and accidental pediatric exposures with two deaths reported due to abuse of fentanyl transdermal patches. The oral
route accounted for the majority of fentanyl patch abuse with 62.5% of all intentional abuse/misuse event reports for fentanyl patches
(85.3% of non-dermal routes of abuse). Furthermore, there was a notable proportion of accidental pediatric exposures to transdermal formulations
that resulted in major medical outcomes (fentanyl patches: 10.1%, buprenorphine patches: 16.7%). Abuse and overdose are a real problem
with transdermal fentanyl as well as other transdermal opioid and stimulant products. In addition, there continues to be an alarming
amount of accidental pediatric exposures, resulting in major negative health outcomes. We believe our AVERSA abuse-deterrent technology
will have a substantial impact on both of these unfortunate and preventable situations and will help reduce the risk of harm from opioid
and stimulant patches by providing taste aversion agents in every patch.
Market Assessment
The company engaged leading healthcare consulting
company Health Advances to assess the market opportunity and commercial strategy for AVERSA Fentanyl and AVERSA Buprenorphine.
AVERSA Fentanyl is the lead AVERSA product under
development and has the potential to be the world’s first fentanyl transdermal systems with abuse deterrent properties. Once approved
by the United States FDA, Aversa Fentanyl will be priced competitively with the non-abuse deterrent patch and has the potential to reach
peak annual US sales of $80-200 million according to the assessment performed by Health Advances in January 2022. This assessment did
not include the impact of the revised CDC Opioid Prescribing Guidelines which were published in November 2022 that encouraged prescribers
to implement comprehensive and holistic pain management including responsible opioid use particularly for patients with moderate to severe
chronic pain. Health Advances was able to confirm the significant unmet patient need for AVERSA Fentanyl based on rigorous primary and
secondary market research accompanied with deep experience in the abuse deterrence pain space. Nutriband is also considering developing
the product for strategic international markets as protected by its global abuse deterrent patent portfolio.
AVERSA Buprenorphine is the second AVERSA product
under development and has the potential to be the world’s first buprenorphine transdermal systems with abuse deterrent properties.
Once approved by the United States FDA, Aversa Buprenorphine will be priced competitively with non-abuse deterrent options and has the
potential to reach peak annual US sales of $70-130 million according to the assessment performed by Health Advances in October 2023.
Health Advances was able to confirm the significant unmet patient need for Aversa™ Buprenorphine based on rigorous primary and
secondary market research accompanied with deep experience in the abuse deterrence pain space. Nutriband is also considering developing
the product for strategic international markets as protected by its global abuse deterrent patent portfolio.
Competition
The pharmaceutical industry is highly competitive
and subject to rapid change as new products are developed and marketed. Potential competitors include large pharmaceutical and biotechnology
companies, specialty pharmaceutical and generic drug companies, and medical technology companies. We believe the key competitive factors
that will affect the development and commercial success of our products are product performance including safety and efficacy, level
of patient compliance, healthcare professional acceptance, and the extent of insurance reimbursement of our products.
As our development pipeline includes products
that contain opioids (AVERSA Fentanyl and AVERSA Buprenorphine), we continually monitor the market for opioid products, particularly
in the United States. Pharmaceutical companies engaged in the distribution and sale of opioids, in particular for the treatment of chronic
pain, are promoting responsible opioid use. In 2022, the CDC revised its clinical practice guideline for prescribing opioids to ease
the restrictions on prescribers and encourage responsible opioid use particularly for patients with moderate to severe pain. Our abuse
deterrent opioid products potentially offer a unique proposition to meet the unmet needs of patients by deterring the abuse and misuse
of opioids while making opioids accessible to those patients who need them. If approved, our AVERSA pipeline products will compete with
the currently marketed products that do not contain abuse deterrent features as well as other products that may employ different abuse
deterrent technology. We may also have to compete with products that do not contain opioids or other drugs that are susceptible to abuse.
We are not aware of any abuse deterrent transdermal products that are in development or being marketed at this time. If we obtain regulatory
approval to market our products, we cannot assure you that we will be successful in the marketplace.
Properties
We do not own any real property. We lease under
a one year lease an office for $ 2,500 per month at 121 South Orange Street, Orlando, Florida. With the office lease, we have access
to boardrooms, kitchen facilities and administrative support services. We lease manufacturing space in Cherryville, North Carolina, for
$3,000 per month under a three-year lease entered into on February 1, 2022, with a renewal option.
Legal Proceedings
The Company is currently a defendant in a lawsuit
initiated by Joseph Gunnar, LLC (“Gunnar”) and Lucosky Brookman LLP (“LB”) in the Supreme Court of the State
of New York, New York County, under Index No.654633/2023. The lawsuit alleges multiple allegations such as breach of contract, fraudulent
activities, and tortious interference and seeks damages following the Company’s termination of an engagement letter for assistance
with a public stock offering. Gunnar is seeking over $500,000 in damages plus punitive damages, while LB is demanding reimbursement of
legal fees.
In response, the Company denies all allegations,
alleging that the engagement letter was unenforceable, and its termination was legally justified. The Company has also initiated counterclaims
against Joseph Gunnar & Co., accusing them of intentional interference and breach of fiduciary duty, and is seeking $1,000,000 for
each claim along with a declaratory judgment affirming the legality and justification of the termination. The plaintiffs have denied
these counterclaims.
Currently, there are no pending hearings or motions
as both parties are engaged in discovery and are attempting to resolve the matter amicably.
MANAGEMENT
Set forth below are the name, age, position of
and biographical information about each nominee, all of whom are currently directors and compromise our entire Board as of the record
date.
| Name |
|
Age |
|
Position |
| Gareth
Sheridan |
|
34 |
|
Chief
Executive Officer and Director |
| Serguei
Melnik |
|
51 |
|
Chairman
of the Board, President and Secretary |
| Sergei Glinka |
|
58 |
|
Director |
| Mark
Hamilton(1)(3) |
|
37 |
|
Director |
| Radu
Bujoreanu(1)(2)(3) |
|
54 |
|
Director |
| Stefani
Mancas(2)(3) |
|
47 |
|
Director |
| Irina
Gram(2)(1) |
|
36 |
|
Director |
| Gerald
Goodman |
|
76 |
|
Chief
Financial Officer |
| Alan
Smith, Ph.D. |
|
57 |
|
Chief
operating officer and president of 4P Therapeutics |
| Jeff
Patrick, Pharm.D. |
|
55 |
|
Chief
scientific officer |
| (1) |
Member
of the Audit Committee. |
| (2) |
Member
of the Compensation Committee. |
| (3) |
Member
of the Nominating and Corporate Governance Committee. |
Gareth Sheridan, our founder, has been chief
executive officer and a director since our organization in 2016. In 2012, Mr. Sheridan founded Nutriband Ltd., an Irish company which
we acquired in 2016. Mr. Sheridan was named Ireland’s ‘Young Entrepreneur of the Year’ in 2014 in the National Bank
of Ireland Startup Awards for establishing Nutriband Ltd. Mr. Sheridan has further business awards from S. Dublin’s Best Young
Entrepreneur and Nutriband Ltd as S. Dublin’s Best Startup Company. Mr. Sheridan has also worked as a Business Mentor with 100
Minds, a social enterprise founded in 2013, that brings together some of Ireland’s top college students and connects them with
one cause to achieve large charitable goals in a short space of time. Mr. Sheridan is also a past Nissan Generation Next Ambassador,
receiving the acknowledgement in 2015 by Nissan Ireland as one of Ireland’s future generational leaders. In 2019 Mr. Sheridan served
on the Board of the St. James Hospital foundation, the charitable foundation for Ireland’s largest public hospital. Mr. Sheridan
received a B.Sc. in Business and Management from Dublin Institute of Technology in 2012 where he concentrated on international economics,
venture creation and entrepreneurship.
Serguei Melnik, who was elected by the Board
as President on October 8, 2021, serves as a member of the board of directors and is a co-founder of Nutriband Inc. Mr. Melnik
has previously served as our chief financial officer and a director since January 2016. Mr. Melnik has been involved in general
business consulting for companies in the U.S. financial markets and setting up a legal and financial framework for operations of
foreign companies in the U.S. Mr. Melnik advised UNR Holdings, Inc. with regard to the initiation of the trading of its stock
in the over-the-counter markets in the U.S. and has provided general advice with respect to the U.S. financial markets for
companies located in the U.S. and abroad. From February 2003 to May 2005, he was the Chief Operations Officer and a Board
member of Asconi Corporation, Winter Park, Florida, with regard to restructuring the company and listing it on the American Stock Exchange.
Mr. Melnik from June 1995 to December 1996 was a lawyer in the Department of Foreign Affairs, JSC Bank “Inteprinzbanca,”,
Chisinau, Moldova, and prior thereto practiced law in Moldova in various positions. Mr. Melnik is fluent in Russian, Romanian, English
and Spanish.
Sergei Glinka, an investor
in our April 17, 2024 private offshore financing, joined our Board of Directors on May 15, 2024. Mr. Glinka has been the Commercial Manager
of TG Biochemicals Limited, Cyprus. since 2019. He has been a shareholder and member of the Board of GST Investments OÜ, Estonia
since 2019. From 2000 to 2019, Mr. Glinka was a shareholder and member of the Board of Transgroup Invest AS, Commencing in 1973 Mr. Glinka
attended secondary school in Moldova, graduating in 1981, and graduated from the Tallinn Merchant Marine School, Estonia, in 1986.
Mark Hamilton, an independent director since
July 2018, is an experienced director level professional who joined global consulting firm, Korn Ferry in 2020 as a Managing Consultant.
Prior to moving into organizational consulting, Mark qualified as a Chartered Accountant in global advisory firm, BDO, where he spent
12 years advising some of Ireland’s most successful businesses. His work originated in corporate finance/corporate recovery
and more recently, he spent 5 years leading BDO’s client management and sales function, as Head of Business Development.
Mr. Hamilton is a Member of the Association
of Chartered Accountants (ACA), since 2012. Mr. Hamilton’s accounting/consulting background and experience in corporate finance,
restructuring, sales and talent assists us in his role as an independent Board member and Committee Chair. Mr. Hamilton has a very
strong presence in the business community across jurisdictions, along with an accomplished track record in project management and business
development. Educated at Terenure College, Mark went on to study a B.Sc. degree in Business & Management at Dublin Institute
of Technology and subsequently received First Class Honours in his postgraduate degree, for which he specialized in Accountancy
in 2009. In addition to his ACA qualification, Mark has also recently completed a diploma in Corporate Governance and is now a member
of the Corporate Governance Institute which will assist him in his role as Independent Director, alongside his recent approval by the
Central Bank of Ireland to act as an Independent Director to regulated entities.
Radu Bujoreanu has been a director since June
2019. Mr Bujoreanu is a real estate agent and investor since 2019 and currently he is with Samson Properties LLC. Mr. Bujoreanu
has been the owner and executive director of Consular Assistance, Inc., which provided assistance in obtaining visas, travel documents,
other national and foreign documents and related services since December 2002 to December 2020. From 2003 to 2005 he served as an independent
director and member of the Board of Directors of Asconi Corporation. From August 1999 to August 2002 Mr. Bujoreanu worked as a consular
officer at the Embassy of the Republic of Moldova to the United States. Before that from May 1994 to August 1999 he was Chief of Bilateral
Treaties section in the International Law and Treaties Department of the Ministry of Foreign Affairs of the Republic of Moldova.
Mr. Bujoreanu received his bachelor degree in international public law from the University of Moldova.
Dr. Stefani Mancas graduated Summa cum Laude
from the Military Navy College in Constanta, Romania. After attending the faculty of Cybernetics from the Academy of Economic Studies
in Bucharest, Stefani transferred to University of Central Florida, and graduated with a dual B.Sc. in Mathematics/ Aerospace Engineering,
a Master’s Degree in Applied Mathematics, and a Ph.D. in Mathematical Sciences from the Department of Mathematics. The Ph.D. dissertation
topic was “Dissipative solitons in the cubic-quintic complex Ginzburg-Landau equation: Bifurcations and Spatiotemporal Structure”,
for which Stefani received the UCF Outstanding Dissertation Award.
Currently, Stefani is a tenured full Professor,
and a researcher in the Department of Mathematics at Embry-Riddle Aeronautical University in Daytona Beach, Florida. Stefani’s
research areas deal with finding analytical solutions to nonlinear dissipative equations that can be reduced through Darboux transformations
to Riccati or Abel equations. The main focus is on Schrödinger equation, for which Stefani is using methods based on factorization,
and variational formulation together with ansatz reduction with global minimizers of objective functions, applied to supersymmetric quantum
mechanics. Another important area of interest is the theory of elliptic functions with applications to nonlinear optics, soliton theory,
general relativity, as well as optimization of the blockchain, and quantum cryptography.
Irina
Gram was elected as a director of the Company at the January 21, 2022 stockholders meeting.
Irina is a Senior Financial Analyst at Thales IFEC, Melbourne, Florida. There she is responsible
for financial planning, analysis and risk and opportunities reviews of multiple development
and customer programs. From 2016 to 2017, she was a Project Engineering Coordinator at Thales
IFEC, where she executed budgeting and forecasting activities with specialized focus on SFRD
spending, interfaced with engineering team to monitor and report the performance of the financial
impact of projects. From 2013 to 2016, she held various project management, accounting and
reporting positions with Siemens Building Technology, Inc., Winter Park, Florida. She received
a Bachelor’s Degree in Finance from the University of Central Florida, Orlando, Florida,
where she graduated in May 2015, with honors, and received a Masters Degree in business
administration from the University of Central Florida, Orlando, Florida, in May 2019.
Gerald
Goodman has been our chief accounting officer since July 31, 2018 and was elected our
Chief Financial Officer on November 12, 2020. Mr. Goodman is a certified public
accountant and, since 2014, has practiced with his own firm, Gerald Goodman CPA P.C. From
January 1, 2010 until December 31, 2014, Mr. Goodman practiced with Madsen &
Associates, CPA’s Inc., Murray, Utah, and was a non-equity partner and managed the
firm’s SEC practice. Mr. Goodman is a director of Lifestyle Medical Network, Inc.,
which provides management services to healthcare providers. From 1971 to 2010, Mr. Goodman
was a partner in the accounting firm of Wiener, Goodman & Company P.C. Mr. Goodman
is a 1970 graduate of Pennsylvania State University where he received a B.S. Degree
in Accounting.
Alan Smith, Ph.D., serves as Chief Operating
Officer of Nutriband and President of 4P Therapeutics, a wholly owned subsidiary of Nutriband. He joined the Company after Nutriband
acquired 4P Therapeutics in 2018. Dr. Smith co-founded 4P Therapeutics in 2011 to develop drug-device and biologic-device combination
products to meet the needs of patients, physicians, and payers, and was Vice President, Clinical, Regulatory, Quality and Operations
at the time of the acquisition. Dr. Smith is co-inventor of the Company’s Aversa™ abuse deterrent transdermal system technology.
Dr. Smith has over 20 years of experience in the research and development of drug and biologic delivery systems, diagnostics and medical
devices for treatment and management of chronic pain, diabetes, and cardiovascular disease. Previously, he was with Altea Therapeutics,
a venture capital funded company focused on novel transdermal drug and biologic delivery, most recently serving as Vice President, Product
Development and Head of Clinical R&D, Regulatory Affairs, and Project Management. Prior to joining Altea Therapeutics, he led the
development of transdermal glucose monitoring systems at SpectRx, Inc., a publicly traded noninvasive diagnostics company. Dr. Smith
received Ph.D. and M.S. degrees in Biomedical Engineering from Rutgers University and the University of Medicine and Dentistry of New
Jersey. He currently serves on the Editorial Advisory Board of Expert Opinion on Drug Delivery.
Jeff Patrick Pharm.D. currently serves as Director
of Drug Development Institute at the Ohio State University Comprehensive Cancer Center. Dr. Patrick most recently serving as Chief Scientific
Officer for New Haven Pharmaceuticals. Prior roles included global vice president of professional affairs at Mallinckrodt Pharmaceuticals,
Inc.; and roles with ascending responsibilities at Dyax, Myogen/Gilead, Actelion and Sanofi-Synthelabo, Inc. Dr. Patrick is a residency-trained
clinical pharmacist with approximately 20 years of pharmaceutical industry experience. He brings expertise in executive leadership, scientific
and medical strategy, drug development and commercialization to the company. Prior to pursuing a career in research and development,
Patrick was an ambulatory care clinical pharmacist at the University of Tennessee Medical Center and a clinical assistant professor of
pharmacy at the University of Tennessee College of Pharmacy, where he earned his doctorate in pharmacy. He also completed the Wharton
School of Business Pharmaceutical Executive Program. Dr. Patrick works for us on a part-time basis.
CORPORATE GOVERNANCE AND THE BOARD OF DIRECTORS
Board Leadership Structure and Risk Oversight
Gareth Sheridan serves as Chief Executive Officer
and Serguei Melnik is serving as our Chairman and President. Our Chairman leads the Board of Directors in its discussions and has such
other duties as are prescribed by the Board. As Chief Executive Officer, Mr. Sheridan is responsible for implementing the Company’s
strategic and operating objectives and day-to-day decision-making related to such implementation.
The Board of Directors currently has three standing
committees (audit, compensation, and nominating and corporate governance) that are chaired and composed entirely of directors who are
independent under Nasdaq and SEC rules. Given the role and scope of authority of these committees, and that a majority of the members
of the Board are independent, the Board of Directors believes that its leadership structure is appropriate. We select directors as members
of these committees with the expectation that they will be free of relationships that might interfere with the exercise of independent
judgement.
Our Board of Directors is our Company’s
ultimate decision-making body, except with respect to those matters reserved to the stockholders. Our Board of Directors selects our
senior management team, which is charged with the conduct of our business. Our Board of Directors also acts as an advisor and counselor
to senior management and oversees its performance.
Board Composition
Our business and affairs are managed under the
direction of our Board of Directors. The number of directors is determined by our board of directors, subject to the terms of our certificate
of incorporation and bylaws. Our board of directors currently consists of six members, four of which are independent directors.
Meetings
Our Board of Directors held two meetings and
acted by written consent eight times during fiscal 2024.
Committees of the Board of Directors
The board of directors has created three committees — the
audit committee, the compensation committee and the nominating and corporate governance committee. Each of the committees has a charter
which meets the Nasdaq Stock Market requirements and is composed of three independent directors.
Audit Committee
The audit committee is comprised of Mr. Hamilton,
as chairman, Mr. Bujoreanu and Irina Gram. We believe that Mark Hamilton qualifies as an “audit committee financial expert”
under the rules of the Nasdaq Stock Market. The audit committee oversees, reviews, acts on and reports on various auditing and accounting
matters to the board, including: the selection of our independent accountants, the scope of our annual audits, fees to be paid to the
independent accountants, the performance of our independent accountants and our accounting practices, all as set forth in our audit committee
charter. The Audit Committee met three times in fiscal 2024.
Compensation Committee
The compensation committee is comprised of Irina
Gram, Chairperson, Mr. Bujoreanu and Dr. Mancas. The compensation committee oversees the compensation of our chief executive
officer and our other executive officers and reviews our overall compensation policies for employees generally as set forth in the audit
committee charter. If so authorized by the board, the compensation committee may also serve as the granting and administrative committee
under any option or other equity-based compensation plans which we may adopt. The compensation committee will not delegate its authority
to fix compensation; however, as to officers who report to the chief executive officer, the compensation committee will consult with
the chief executive officer, who may make recommendations to the compensation committee. Any recommendations by the chief executive officer
are accompanied by an analysis of the basis for the recommendations. The committee will also discuss with the chief executive officer
and other responsible officers the compensation policies for employees who are not officers. The compensation committee has the responsibilities
and authority relating to the retention, compensation, oversight and funding of compensation consultants, legal counsel and other compensation
advisers. The compensation committee members will consider the independence of such advisors before selecting or receiving advice from
such advisors. The compensation committee met three times in fiscal 2024.
Nominating and Corporate Governance Committee
The nominating and corporate governance committee,
which is comprised of Dr. Mancas, Mark Hamilton and Mr. Bujoreanu, will identify, evaluate and recommend qualified nominees
to serve on our board; develop and oversee our internal corporate governance processes, and maintain a management succession plan. The
nominating and corporate governance committee met two times in fiscal 2024.
Risk Management
The Board has an active role, as a whole and
also at the committee level, in overseeing the management of our risks. The Compensation Committee of our Board is responsible for overseeing
the management of risks relating to our executive compensation plans and arrangements. The Audit Committee of our Board oversees management
of financial risks, under its charter it is to meet periodically and at least four times per year with management to review and assess
the Company’s major financial risk exposures and the manner in which such risks are being monitored and controlled. The Nominating
and Corporate Governance Committee of our Board is responsible for the management of risks associated with the independence of the Board
members and potential conflicts of interest. While each committee is responsible for evaluating certain risks and overseeing the management
of such risks, the entire Board of Directors is informed about such risks.
Independent Directors
Five of our directors, Sergei Glinka, Mark Hamilton,
Radu Bujoreanu, Stefani Mancas and Irina Gram are independent directors based on the NASDAQ definition of independent director.
Family Relationships
There are no family relationships among our directors
and executive officers.
Compensation Committee Interlocks and Insider Participation
None of our executive officers serve on the board
of directors or compensation committee of a company that has an executive officer who serves on our Board or compensation committee.
No member of our Board is an executive officer of a company in which one of our executive officers serves as a member of the board of
directors or compensation committee of that company.
Conflicts of Interest
Certain conflicts of interest exist and may continue
to exist between the Company and its officers and directors due to the fact that each has other business interests to which they devote
their primary attention. Each officer and director may continue to do so notwithstanding the fact that management time should be devoted
to the business of the Company.
Certain conflicts of interest may exist between
the Company and its management, and conflicts may develop in the future. The Company has not established policies or procedures for the
resolution of current or potential conflicts of interest between the Company, its officers and directors or affiliated entities. There
can be no assurance that management will resolve all conflicts of interest in favor of the Company, and conflicts of interest may arise
that can be resolved only through the exercise by management their best judgment as may be consistent with their fiduciary duties. Management
will try to resolve conflicts to the best advantage of all concerned.
Compliance with Section 16(a) of
the Securities Exchange Act of 1934
Section 16(a) of the Exchange Act
requires our officers and directors, and persons who beneficially own more than ten percent of our Common Stock, to file reports of ownership
and changes of ownership of such securities with the SEC. Mr. Goodman, Dr. Smith, Dr. Patrick, Mr. Bujoreanu,
and Ms. Gram have not yet filed their Form 3 reports.
Gareth Sheridan and Serguei Melnik have not filed
Form 4’s reporting receipt of compensation in fiscal years 2024 and 2025; With the exception of Gerald Goodman, who has filed Form
5’s to catch up on Form 4’s due over the past three fiscal years. Mr. Goodman is late with respect to Form 4’s required
to be filed for stock option compensation issuances for fiscal 2024 and 2025. No other officer or director has filed any ownership reports.
EXECUTIVE
COMPENSATION
The
table below shows the compensation for services in all capacities we paid during the years
ended January 31, 2024 and 2023 to the individuals serving as our principal executive
officers during the last completed fiscal year and our other two most highly paid executive
officers at the end of the last completed fiscal year (whom we refer to collectively as our
“named executive officers”);
| Name and Principal Position | |
Year | | |
Salary
$ | | |
Bonus
Awards
$ | | |
Stock
Awards
$ | | |
Option/
Awards(1)
$ | | |
Incentive
Plan
Compensation
$ | | |
Nonqualified
Deferred
Earnings
$ | | |
All Other
Compensation
$ | | |
Total
$ | |
| Gareth Sheridan, | |
2024 | | |
| 150,000 | | |
| | | |
| | | |
| 82,110 | | |
| | | |
| | | |
| 25,000 | | |
| 257,110 | |
| CEO(1) | |
2023 | | |
| 200,000 | | |
| | | |
| 38,000 | | |
| 140,672 | | |
| | | |
| | | |
| | | |
| 378,672 | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Serguei Melnik | |
2024 | | |
| 150,000 | | |
| | | |
| | | |
| 82,110 | | |
| | | |
| | | |
| 25,000 | | |
| 257,110 | |
| President | |
2023 | | |
| 200,000 | | |
| | | |
| | | |
| 146,672 | | |
| | | |
| | | |
| | | |
| 346,672 | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Alan Smith | |
2024 | | |
| 154,000 | | |
| | | |
| | | |
| 42,720 | | |
| | | |
| | | |
| 5,000 | | |
| 201,720 | |
| Chief Operating Officer | |
2023 | | |
| 179,000 | | |
| | | |
| | | |
| 57,490 | | |
| | | |
| | | |
| | | |
| 236,490 | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Gerald Goodman | |
2024 | | |
| 110,000 | | |
| | | |
| | | |
| 52,866 | | |
| | | |
| | | |
| 30,000 | | |
| 192,866 | |
| Chief Financial Officer | |
2023 | | |
| 160,000 | | |
| — | | |
| | | |
| 114,976 | | |
| — | | |
| | | |
| — | | |
| 274,976 | |
| (1) |
During
the year ended January 31, 2023, we issued to Gareth Sheridan, our CEO, 11,667 shares of common stock valued at $38,000, representing
compensation for the year ended January 31, 2023. |
| | |
Directors
Compensation | |
| | |
Fees Earned
or Paid in
Cash | | |
Stock
Awards | | |
Option
Awards | | |
Non-Equity
Incentive
Plan
Compensation’ | | |
Change in
Pension
Value and
NonQualified Deferred
Compensation
Earnings | | |
All
Other Compensation | | |
Total | |
| Name | |
($) | | |
($) | | |
($) | | |
($) | | |
($) | | |
($) | | |
($) | |
| (a) | |
(b) | | |
(c) | | |
(d) | | |
(e) | | |
(f) | | |
(g) | | |
(h) | |
| Mark Hamilton | |
$ | 5,000 | | |
$ | - | | |
$ | 10,146 | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | 15,146 | |
| Radu Bujorneau | |
$ | 5,000 | | |
$ | - | | |
$ | 11,214 | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | 16,214 | |
| Stefani Mancas | |
$ | 5,000 | | |
$ | - | | |
$ | 10,146 | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | 15,146 | |
| Irina Gram | |
$ | 5,000 | | |
$ | - | | |
$ | 10,146 | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | 15,146 | |
Employment Agreements with Company Officers
The Company entered into a three-year employment
agreement with Gareth Sheridan, our CEO, and Serguei Melnik, our President, effective February 1, 2022. The agreement also provides that
the executives will continue as a director. The agreement provides for an initial term, commencing on the effective date of the agreement
and ending on January 31, 2025, and continuing on a year-to-year basis thereafter unless terminated by either party on not less than
30 days’ notice given prior to the expiration of the initial term or any one-year extension. For their services to the Company
during the term of the agreement, Mr. Sheridan and Mr. Melnik will receive an annual salary of $250,000 per annum, commencing on the
effective date of the agreement. Mr. Sheridan and Mr. Melnik will also receive a performance Pbonus of 3.5% of net income before income
taxes. As of July 31, 2022, the Company and Mr. Sheridan and Mr. Melnik mutually agreed to reduce their annual salary to $150,000.
The Company entered into a three-year employment
agreement with Gerald Goodman, our CFO, effective February 1, 2022. The agreement provides for an initial term, commencing on the effective
date of the agreement and ending on January 31, 2025, and continuing on a year-to-year basis thereafter unless terminated by either party
on not less than 30 days’ notice given prior to the expiration of the initial term or any one-year extension. For his services
to the Company during the term of the agreement, Mr. Goodman will receive an annual salary of $210,000 per annum, commencing on the effective
date of the agreement. As of July 31, 2022, the Company and Mr. Goodman mutually agreed to reduce his annual salary to $110,000.
The Employment Agreements
provide for incentive payments as established by the Board of Directors, and the Employment Agreements with Mr. Sheridan and Mr. Melnik
provide for a performance bonus as follows:
| Net Operating Profit Before Income Taxes | |
Performance
Bonus | |
| On the First $10 Million | |
| 3.5 | % |
| On the Next $40 Million | |
| 3.5 | % |
| On the Next $50 Million | |
| 3.5 | % |
| On all Amounts Over $100 Million | |
| 3.5 | % |
Each of the Employment
Agreements contains similar provisions for discharge for “cause”, including breach of the Employment Agreement or specified
detrimental conduct by the employee, in which cases accrued compensation would payable as provided in the Employment Agreements. The
Agreements also provide for termination by the executives for “good reason”, comprising events such as breach of the Agreement
by the Company, assignment of duties inconsistent with the Executive’s position, , or in the event of a change in control of the
Company. In the event of a termination by the Company without cause, or by the executive for “good reason”, the Company is
required to pay to the Executive in a lump sum in cash within 30 days after the date of termination the aggregate of the following amounts:
| |
A. |
the
sum of (1) the executive’s annual minimum salary through the date of termination to the extent not theretofore paid, (2) any
annual incentive payment earned by the executive for a prior period to the extent not theretofore paid and not theretofore deferred,
(3) any annual performance bonus payment earned by the executive for a prior period to the extent not theretofore paid and not theretofore
deferred,(4) any accrued and unused vacation pay and (5) any business expenses incurred by the executive that are unreimbursed
as of the date of termination; |
| |
B. |
The
product of (1) the performance bonus payment and (2) a fraction, the numerator of which is the number of days that have elapsed in
the fiscal year of the Company in which the date of termination occurs as of the date of termination, and the denominator of which
is 365; |
| |
C. |
the
amount equal to the sum of (1) three (3) times the executive’s annual minimum salary; (2) one (1) times the performance
bonus payment and (3) one (1) times the incentive payment; |
| |
D. |
In
the event executive is not fully vested in any retirement benefits with the Company from pension, profit sharing or any other qualified
or non-qualified retirement plan, the difference between the amounts executive would have been paid if he or she had been vested
on the date his/her employment was terminated and the amounts paid or owed to the executive pursuant to such retirement plans; |
| |
E. |
The
product of (1) the incentive payment and (2) a fraction, the numerator of which is the number of days that have elapsed in the fiscal
year of the Company in which the date of termination occurs as of the date of termination, and the denominator of which is 365; and |
| |
F. |
If
applicable, the present value of the amount equal to the sum of five (5) years’ Performance Bonus pay with such amount being
calculated based on the Performance Bonus paid to the Employee the year prior to Termination. |
In addition, all stock options and warrants outstanding
as of the date of termination and held by the executive shall vest in full and become immediately exercisable for the remainder of their
full term; all restricted stock shall no longer be restricted to the extent permitted by law, and the Company will use its best efforts,
at its sole cost to register such restricted stock as expeditiously as possible.
Gross-up Reimbursement on Excise Taxes Paid
by Employee on Certain Payments received from Company
The Employment Agreements
of Mr. Sheridan and Mr. Melnik provide that, to the extent any payment under the Employment Agreement to the executive is subject to
the excise tax imposed by section 4999 of the Internal Revenue Code, the executive is entitled to a gross-up payment from the Company
to reimburse the executive for additional federal, state and local taxes imposed on executive by reason of the excise tax and the Company’s
payment of the initial taxes on such amount. The Company is also required to bear the costs and expenses of any proceeding with any taxing
authority in connection with the imposition of any such excise tax.
Employment Agreement with Alan Smith
The Company entered into a three-year employment
agreement with Alan Smith, our Chief Operating Officer, effective October 1, 2021, for an initial term of three years through September
30, 2024. For his services to the Company during the term of the agreement, Mr. Smith receives a fixed base salary of $204,000 per year,
payable no less frequently than monthly. This base salary is reviewed not later than the end of each calendar year that Mr. Smith is
employed by the Company. As of July 31, 2022, the Company and Mr. Smith mutually agreed to reduce his annual salary to $154,000.
Pension Benefits
We currently have no plans that provide for payments
or other benefits at, following, or in connection with retirement of our officers.
OUTSTANDING EQUITY AWARDS
AT FISCAL YEAR-END
| | |
Option
Awards | | |
| |
Stock
Awards | |
| | |
Number of
Shares of
Common Stock
Underlying
Unexercised
Options
Exercisable | | |
Number of
Securities
Underlying
Unexercised
Options
Unexercisable | | |
Equity
Incentive Plan
Awards:
Number of
Securities
Underlying
Unexercised
Unearned Options | | |
Options Exercise
Price | | |
Options
Expiration
Date | |
Number of
Shares or
Units of
Stock
that
Have
Not
Vested | | |
Market
Value of
Shares or
Units of Stock
That
Have
Not
Vested | | |
Equity
Incentive Plan
Awards:
Number of
Unearned
Shares, Units
or Other Rights That
Have
Not Vested | | |
Incentive
Plan
Awards:
Market
or
Payout
Value of
Unearned
Shares, Units or
Other
Rights That | |
| Name | |
(#) | | |
(#) | | |
(#) | | |
($) | | |
($) | |
(#) | | |
($) | | |
(#) | | |
($) | |
| (a) | |
(b) | | |
(c) | | |
(d) | | |
(e) | | |
(f) | |
(g) | | |
(h) | | |
(i) | | |
(j) | |
| Gareth Sheridan, CEO | |
| 23,333 | | |
| - | | |
| - | | |
$ | 4.58 | | |
January
21, 2025 | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
| 29,167 | | |
| - | | |
| - | | |
$ | 4.50 | | |
August 2, 2025 | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
| 25,000 | | |
| - | | |
| - | | |
$ | 4.12 | | |
December 8, 2025 | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
| 70,000 | | |
| - | | |
| - | | |
$ | 2.12 | | |
October 27, 2026 | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| |
| | | |
| | | |
| | | |
| | |
| Serguei Melnik, President | |
| 23,333 | | |
| - | | |
| - | | |
$ | 4.58 | | |
January 21, 2025 | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
| 29,167 | | |
| - | | |
| - | | |
$ | 4.50 | | |
August 2, 2025 | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
| 25,000 | | |
| - | | |
| - | | |
$ | 4.12 | | |
December 8, 2025 | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
| 70,000 | | |
| - | | |
| - | | |
$ | 2.12 | | |
October 27, 2026 | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| |
| | | |
| | | |
| | | |
| | |
| Alan Smith, COO | |
| 11,667 | | |
| - | | |
| - | | |
$ | 4.16 | | |
January 21, 2025 | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
| 11,667 | | |
| - | | |
| - | | |
$ | 4.09 | | |
August 2, 2025 | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
| 10,000 | | |
| - | | |
| - | | |
$ | 3.75 | | |
December 8, 2025 | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
| 40,000 | | |
| - | | |
| - | | |
$ | 1.93 | | |
October 27, 2026 | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| |
| | | |
| | | |
| | | |
| | |
| Gerald Goodman, CFO | |
| 11,667 | | |
| - | | |
| - | | |
$ | 4.16 | | |
January 21, 2025 | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
| 23,333 | | |
| - | | |
| - | | |
$ | 4.09 | | |
August 2, 2025 | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
| 20,000 | | |
| - | | |
| - | | |
$ | 3.75 | | |
December 8, 2025 | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
| 49,500 | | |
| - | | |
| - | | |
$ | 1.93 | | |
October 27, 2026 | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
| 87,500 | (1) | |
| - | | |
| - | | |
$ | 1.93 | | |
October 27, 2026 | |
| - | | |
| - | | |
| - | | |
| - | |
| (1) |
This
option held by Mr. Goodman is in the form of a common stock purchase warrant. |
Bonuses
Any bonuses granted
in the future will relate to meeting certain performance criteria that are directly related to areas within the named executive’s
responsibilities with the Company. As we continue to grow, more defined bonus programs may be established to attract and retain our employees
at all levels.
Other Director Compensation
There are no agreements
or arrangements by which any directors or nominees are to receive compensation or other payments from third parties in return for serving
on the Board of Directors.
PRINCIPAL STOCKHOLDERS
The
following table provides information concerning the beneficial ownership of the Company’s
common Stock by each director, certain executive officers, by all directors and officers
of the Company as a group as of July 25, 2024. In addition, the table provides information
concerning the current beneficial owners, if any, known to the Company to hold more than
five percent (5%) of the outstanding common stock of the Company.
The amounts and percentage of stock beneficially
owned are reported based on regulations of the SEC governing the determination of beneficial ownership of securities. Under the rules
of the SEC, a person is deemed to be a “beneficial owner” of a security if that person has or shares “voting power,”
which includes the power to dispose of or to direct the disposition of such security. A person is also deemed to be a beneficial owner
of any securities of which that person has a right to acquire beneficial ownership within 60 days after July 25,d 2024. Under these
rules, more than one person may be deemed a beneficial owner of the same securities and a person may be deemed a beneficial owner of
securities in which he has no economic interest. The percentage of common stock beneficially owned is based on 11,106,185 shares of common
stock outstanding as of July 25, 2024.
Name
and Address(1) of Beneficial Owner
(Management and Directors) |
|
Shares
of
Common
Stock
Owned
Directly |
|
|
Shares
of
Derivative
Securities
Owned
Beneficially |
|
|
Total
Beneficial
Ownership
Including
Option
Grants |
|
|
Percentage of
Issued and
Outstanding
Common
Stock |
|
| Gareth
Sheridan |
|
|
1,761,667 |
|
|
|
245,000 |
|
|
|
2,006,667 |
|
|
|
17.68 |
% |
| Serguei
Melnik(2) |
|
|
820,418 |
|
|
|
245,000 |
|
|
|
1,065,418 |
|
|
|
9.39 |
% |
| Stefani
Mancas |
|
|
14,125 |
|
|
|
25,583 |
|
|
|
39,708 |
|
|
|
* |
|
| Mark
Hamilton |
|
|
17,208 |
|
|
|
28,500 |
|
|
|
45,708 |
|
|
|
* |
|
| Radu
Bujoreanu |
|
|
15,750 |
|
|
|
29,333 |
|
|
|
45,083 |
|
|
|
* |
|
| Irina
Gram |
|
|
1,167 |
|
|
|
18,000 |
|
|
|
19,167 |
|
|
|
* |
|
| Dr. Jeff
Patrick |
|
|
36,612 |
|
|
|
160,000 |
|
|
|
220,000, |
|
|
|
2.27 |
% |
| Alan
Smith |
|
|
48,893 |
|
|
|
143,334 |
|
|
|
185,242 |
|
|
|
1.71 |
% |
| Gerald
Goodman(3) |
|
|
86,335 |
|
|
|
179,500 |
|
|
|
265,835 |
|
|
|
2.36 |
% |
| Sergei
Glinka(5) |
|
|
825,000 |
|
|
|
1,650,000 |
|
|
|
2,475,000 |
|
|
|
19.40 |
% |
| All
officers and directors as a group (10 individuals) |
|
|
3,627,175 |
|
|
|
2,784,250 |
|
|
|
6,4
11,425 |
|
|
|
46.16 |
% |
| Other
Beneficial Owners |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Vitalie
Botgros(4) |
|
|
2,370,774 |
|
|
|
1,808,288 |
|
|
|
4,179,002 |
|
|
|
32.36 |
% |
| * | Less
than One (1%) Percent. |
| (1) |
The
address for each director and officer, unless indicated otherwise, is c/o Nutriband, Inc., 121 South Orange Ave., Suite 1500, Orlando,
FL 32801. |
| (2) |
Includes
29,167 shares owned by Mr. Melnik’s wife, as to which Mr. Melnik disclaims beneficial ownership, and 58,334 shares
held under the UGMA for the benefit of his minor children. |
| (3) |
Gerald
Goodman holds 86,335 shares directly and has been granted three-year options under the Company’s 2021 Employee Stock Option
Plan to purchase an aggregate of 179,500 shares of common stock at exercise prices ranging from $1.93 per share to $4.16 per share. |
| (4) |
Mr. Botgros, to the knowledge of the Company, is
the ultimate beneficial owner of (1) 1,323,177 shares of common stock held by TII Jet Services Ltd. (“Jet Services”), and
(2) 1,047,597 shares of common stock held by Nociata Holdings. Jet Services holds warrants to purchase 547,095 shares of common stock
(of which warrants 134,635 are the publicly-traded Company warrants (NTRB).
Jet Services is 100% owned by Nociata Holding
Limited, a Cyprus company (“Nociata”). Nociata is 100% owned by Mr. Botgros and holds owns 100% of the equity in Jet
Services, so that Mr. Botros is the ultimate beneficial owner of all securities held by Jet Services and Nociata received 525,000
of its shares held, and 1,050,000 warrants from its investment in the Company’s private offshore financing closed April 19,
2024. Nociata holds 211,133 of the Nutriband publicly-traded warrants.
Mr. Botgros’ address is c/o Nociata
Holding Limited, 1Apriliou, 47 Demetriou Bldg., 2,1st Floor, Flat/Office 12, 3117 Limassol, Cyprus. |
| (5) |
Mr.
Glinka purchased 825,000 shares of common stock and 1,650,000 warrants in Nutriband’s equity financing that was completed April
19, 2024. Mr. Glinka’s address is 13 Morfu Str., Matina Court FL 402, 3012 Limassol, Cyprus. Mr. Glinka’s son, Daniil
Glinka, purchased 125,000 shares of common stock at the offering price of $4.00 per share with warrants to purchase 250,000 shares
of common stock in the Company’s April 19, 2024 private offshore financing. |
To our knowledge, all beneficial owners named
in this table have sole voting and investment power with respect to all shares shown as beneficially owned by them.
CERTAIN
RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
On February 1, 2023,
the Board of Directors ratified and authorized the issuance of Option Award Agreements with respect option grants approved February 1,
2023, by the Compensation Committee, to officers and directors as set forth in the table below.
| Name | |
No. of
Shares | | |
| | |
|
| Jeff Patrick, Chief Scientific Officer | |
| 30,000 | | |
$ | 3.98 | | |
Services Rendered in fiscal
2024 |
On September 18, 2023,
the Board of Directors ratified and authorized the issuance of Option Award Agreements with respect option grants approved September
18, 2023, by the Compensation Committee, to officers and directors as set forth in the table below.
| Name | |
No. of
Shares | | |
| | |
|
| Jeff Patrick, Chief Scientific Officer | |
| 20,000 | | |
$ | 2.65 | | |
Services Rendered in fiscal
2024 |
On October 19, 2023,
the Board of Directors ratified and authorized the issuance of Option Award Agreements with respect option grants approved October 19,
2023, by the Compensation Committee modified a common stock purchase warrant issued to Gerald Goodman, as set forth in the table below.
| Name | |
No. of
Shares | | |
No. of
Warrants |
| Gareth Sheridan, CEO | |
| 70,000 | | |
| - | | |
$ | 2.12 | | |
Services Rendered in fiscal
2024 |
| Serguei Melnik, Chairman & President | |
| 70,000 | | |
| - | | |
$ | 2.12 | | |
Services Rendered in fiscal 2024 |
| Gerald Goodman, CFO | |
| 49,500 | | |
| - | | |
$ | 1.93 | | |
Services Rendered in fiscal 2024 |
| Alan Smith, Chief Operating Officer | |
| 40,000 | | |
| - | | |
$ | 1.93 | | |
Services Rendered in fiscal 2024 |
| Jeff Patrick, Chief Scientific Officer | |
| 40,000 | | |
| - | | |
$ | 1.93 | | |
Services Rendered in fiscal 2024 |
| Gerald Goodman, CFO | |
| - | | |
| 87,500 | | |
$ | 1.93 | | |
Services Rendered in fiscal 2024 |
On March 20, 2024, our Board of Directors authorized
the issuance of stock option award agreements to the following options to Board members and officers, and a consultant.
| Date |
|
|
Title
and Amount (1) |
|
Purchaser |
|
Principal
Underwriter |
|
Total
Offering Price/
Underwriting
Discounts |
| March
20, 2024 |
|
|
Option
to purchase 97,500 shares of common stock. |
|
Gareth
Sheridan, Chief Executive Officer |
|
NA |
|
$2.61
per share/NA |
| March
20, 2024 |
|
|
Option
to purchase 97,500 shares of common stock. |
|
Serguei
Melnik, President |
|
NA |
|
$2.61
per share/NA |
| March
20, 2024 |
|
|
Option
to purchase 75,000 shares of common stock. |
|
Gerald
Goodman, Chief Financial Officer |
|
NA |
|
$2.37
per share/NA |
| March
20, 2024 |
|
|
Option
to purchase 70,000 shares of common stock. |
|
Alan
Smith, Chief Operating Officer |
|
NA |
|
$2.37
per share/NA |
| March
20, 2024 |
|
|
Option
to purchase 25,000 shares of common stock. |
|
Jeff
Patrick, Chief Scientific Officer |
|
NA |
|
$2.37
per share/NA |
| March
20, 2024 |
|
|
Option
to purchase 2,500 shares of common stock. |
|
Dianna
Mather |
|
NA |
|
$2.37
per share/NA |
| March
20, 2024 |
|
|
Option
to purchase 12,500 |
|
Olet
Burea, consultant |
|
NA |
|
$2.37
per share/NA |
On April 19, 2024, the Company completed an $8,400,000
equity financing with European investors which included related parties. The related parties invested a total of $7,120,000 and received
1,780,000 shares of common stock and warrants to purchase 3,560,000 shares of common stock @ $6.43 per share. One related party, a director
of the Company, invested $4.5 million which included $500,000 from his son and $700,000 from an entity he controls. The other related
party invested $2.62 million from entities controlled by the investor.
The
Company agreed on May 14, 2024, to convert $300,000, which as of that date of all the outstanding
principal on the Credit line Promissory Note of the Company held by TII Jet Services LDA,
a related party, (the “Holder”). The conversion was made pursuant to the terms
of a Conversion Agreement dated May 14, 2024, which provided that the conversion of $300,000
of principal and $4,922 of accrued interest would be made at a price of $4.00 per share,
resulting in the Company issuing on May 14, 2024, a total of 76,230 shares of common stock
at the conversion price of $4.00 per share. On May 22 Company agreed on May 14, 2024, to
convert $300,000, which as of that date of all the outstanding principal on the Credit line
Promissory Note of the Company held by TII Jet Services LDA, a related party, (the “Holder”).
The conversion was made pursuant to the terms of a Conversion Agreement dated May 14, 2024,
which provided that the conversion of $300, 2024, the Conversion Agreement was amended allowing
the lender to purchase an additional 152,460 shares of common stock at a rate of two warrants
per converted share, with an exercise price of $6.43 per share and a term of five years from
the conversion date (May 14, 2024).
Independent Directors
Five of our directors, Sergei Glinka, Mark Hamilton,
Radu Bujoreanu, Stefani Mancas and Irina Gram are independent directors based on the NASDAQ definition of independent director.
DESCRIPTION OF SECURITIES
Our authorized capital stock consists of 10,000,000
shares of preferred stock, par value $0.001 per share, none of which have been issued, and 291,666,666 shares of common stock, par value
$0.001 per share, of which 11,106.185 shares are issued and outstanding. Holders of our common stock are entitled to equal voting rights,
consisting of one vote per share on all matters submitted to a stockholder vote. Holders of common stock do not have cumulative voting
rights. Therefore, holders of a majority of the shares of common stock can elect all of our directors. The presence, in person or by
proxy duly authorized, of the holders of a majority of the outstanding shares of stock entitled to vote are necessary to constitute a
quorum at any meeting of our stockholders. A vote by the holders of a majority of our outstanding shares is required to effectuate certain
fundamental corporate changes such as liquidation, merger or an amendment to our articles of incorporation. In the event of liquidation,
dissolution or winding up of our company, either voluntarily or involuntarily, each outstanding share of the common stock is entitled
to share equally in our assets.
Holders of our common stock have no pre-emptive
rights, no conversion rights and there are no redemption provisions applicable to our common stock. They are entitled to receive dividends
when and as declared by our board of directors, out of funds legally available therefore. We have not paid cash dividends in the past
and do not expect to pay any within the foreseeable future.
The board of directors has broad powers to create
one or more series of preferred stock and to designate the voting powers, designations, preferences, limitations, restrictions and relative
rights of each series.
Warrants
Warrants Issued in the Public Offering
The following summary of certain terms and provisions
of the warrants (“Warrants”) included in the Units offered sold in our October 1, 2021 public offering (“IPO”)
is not complete and is subject to, and qualified in its entirety by, the provisions of the warrant agent agreement between us and Equinity
Trust Company, LLC, as warrant agent. As of June 28, 2024, 457,795 Warrants issued in the IPO have been exercised, with net proceeds
to the Company of $2,942,970, and 959,005 Warrants are unexercised and outstanding.
Exercisability. The Warrants are
exercisable at any time after their original issuance and at any time up to the date that is five years after their original issuance.
The Warrants are exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice and,
at any time a registration statement registering the issuance of the shares of common stock underlying the Warrants under the Securities
Act is effective and available for the issuance of such shares, or an exemption from registration under the Securities Act is available
for the issuance of such shares, by payment in full in immediately available funds for the number of shares of common stock purchased
upon such exercise. If a registration statement registering the issuance of the shares of common stock underlying the Warrants under
the Securities Act is not effective or available and an exemption from registration under the Securities Act is not available for the
issuance of such shares, the holder may, in its sole discretion, elect to exercise the Warrant through a cashless exercise, in which
case the holder would receive upon such exercise the net number of shares of common stock determined according to the formula set forth
in the Warrant. No fractional shares of common stock will be issued in connection with the exercise of a Warrant. In lieu of fractional
shares, we will pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price.
Exercise Limitation. A holder does not
have the right to exercise any portion of the warrant if the holder (together with its affiliates) would beneficially own in excess of
4.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership
is determined in accordance with the terms of the Warrants. However, any holder may increase or decrease such percentage to any other
percentage not in excess of 9.99%, provided that any increase in such percentage shall not be effective until 61 days following notice
from the holder to us.
Exercise Price. The exercise price
per whole share of common stock purchasable upon exercise of the Warrants is $6.43 per share, which is 120% of public offering price
of the common stock adjusted for the August 12, 2022 forward stock split. The exercise price is subject to appropriate adjustment in
the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting
our common stock and also upon any distributions of assets, including cash, stock or other property to our stockholders.
Transferability. Subject to applicable
laws, the Warrants may be offered for sale, sold, transferred or assigned without our consent.
Exchange Listing. We were approved for
listing of our common stock and the Warrants on The NASDAQ Capital Market under the symbols “NTRB,” and “NTRBW,”
respectively.
Warrant Agent. The transfer agent for
the common stock and warrant agent for the warrants is Equinity Trust Company, LLC (“Equiniti”), 6201 15th Ave,
Brooklyn, NY 11219, telephone (800) 937-5449.
The Warrants have been issued in registered form
under a warrant agent agreement between AST, as warrant agent, and us. The Warrants were initially be represented only by one or more
global warrants deposited with the warrant agent, as custodian on behalf of The Depository Trust Company (DTC) and registered in the
name of Cede & Co., a nominee of DTC, or as otherwise directed by DTC.
Fundamental Transactions. In the
event of a fundamental transaction, as described in the Warrants and generally including any reorganization, recapitalization or reclassification
of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation
or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming
the beneficial owner of 50% of the voting power represented by our outstanding common stock, the holders of the warrants will be entitled
to receive upon exercise of the warrants the kind and amount of securities, cash or other property that the holders would have received
had they exercised the warrants immediately prior to such fundamental transaction.
Rights as a Stockholder. Except as
otherwise provided in the Warrants or by virtue of such holder’s ownership of shares of our common stock, the holder of a Warrant
does not have the rights or privileges of a holder of our common stock, including any voting rights, until the holder exercises the Warrant.
Governing Law. The Warrants and the warrant
agent agreement are governed by Delaware law.
Representative’s Warrants
As additional compensation to the underwriters
in the public offering, upon consummation of our 2021 public offering, we issued to the designees of the underwriters warrants to purchase
an aggregate of 123,200 shares of our common stock (the “Underwriters’ Warrants”). The Underwriters’ Warrants
are exercisable, in whole or in part, and will expire on the fifth anniversary of the effective date of that registration statement.
Warrants Issued in Recent Private Offshore Financing
On April 19, 2024, the
Company completed an $8,400,000 equity financing with European investors (the “Offering”) of 2,100,000 units (“Units”),
at a price of $4.00 per Unit, each Unit consisting of one share of common stock (“Shares”) and a warrant to purchase two
shares of common stock (the “warrants”). The warrants are exercisable by payment of the exercise price in cash only and expire
April 19, 2029, five years from the date of issuance. In this Offering, 4,200,000 warrants were issued to the investors, which have an
exercise price of $6.43, The Offering was made solely to investors resident outside the United States and was not registered under the
Securities Act, or the securities laws of any jurisdiction, including any jurisdiction outside the United States, but was made privately
by the Company pursuant to the exemptions from registration provided in the SEC’s Regulation S and other exemptions under the Securities
Act.
Nevada Law Provisions Relating to Certain
Transactions
Sections 78.378 through 78.3793 of the Nevada
Revised Statutes contains voting limitations on certain acquisitions of control shares. Sections 78.411 through 78.444 contain restrictions
of combinations with interested stockholders. The Nevada law defines an interested stockholder as a beneficial owner (directly or indirectly)
of 10% or more of the voting power of the outstanding shares of the corporation. In addition, combinations with an interested stockholder
remain prohibited for three years after the person became an interested stockholder unless (i) the transaction is approved by the board
of directors or the holders of a majority of the outstanding shares not beneficially owned by the interested party, or (ii) the interested
stockholder satisfies certain fair value requirements.
Limitation on liability of officers and directors
Nevada law provides that subject to certain very
limited statutory exceptions, a director or officer is not individually liable to the corporation or its stockholders or creditors for
any damages as a result of any act or failure to act in his or her capacity as a director or officer, unless it is proven that the act
or failure to act constituted a breach of his or her fiduciary duties as a director or officer and such breach of those duties involved
intentional misconduct, fraud or a knowing violation of law. The statutory standard of liability established by NRS Section 78.138 controls
even if there is a provision in the corporation’s articles of incorporation unless a provision in the corporation’s articles
of incorporation provides for greater individual liability.
Indemnification
Nevada law permits broad provisions for indemnification
of officers and directors.
Our bylaws provide that each person who was or
is made a party or is threatened to be made a party to or is involved (including, without limitation, as a witness) in any threatened,
pending, or completed action, suit or proceeding, whether formal or informal, civil, criminal, administrative or investigative (hereinafter
a “proceeding”), by reason of the fact that he or she is or was a director of or who is or was serving at our request as
a director, officer, employee or agent of this or another corporation or of a partnership, joint venture, trust, other enterprise, or
employee benefit plan (a “covered person”), whether the basis of such proceeding is alleged action in an official capacity
as a covered person shall be indemnified and held harmless by us to the fullest extent permitted by applicable law, as then in effect,
against all expense, liability and loss (including attorneys’ fees, costs, judgments, fines, ERISA excise taxes or penalties and
amounts to be paid in settlement) reasonably incurred or suffered by such person in connection therewith, and such indemnification shall
continue as to a person who ceased to be a covered person and shall inure to the benefit of his or her heirs, executors and administrators.
However, no indemnification shall be provided
hereunder to any covered person to the extent that such indemnification would be prohibited by Nevada state law or other applicable law
as then in effect, nor, with respect to proceedings seeking to enforce rights to indemnification, shall we indemnify any covered person
seeking indemnification in connection with a proceeding (or part thereof) initiated by such person except where such proceeding (or part
thereof) was authorized by our board of directors, nor shall we indemnify any covered person who shall be adjudged in any action, suit
or proceeding for which indemnification is sought, to be liable for any negligence or intentional misconduct in the performance of a
duty.
SEC Policy on Indemnification for Securities
Act liabilities
Insofar as indemnification for liabilities arising
under the Securities Act of 1933 may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions,
we have been informed that in the opinion of the Securities and Exchange Commission, such indemnification is against public policy as
expressed in the Securities Act and is therefore unenforceable.
Transfer Agent and Warrant Agent
The transfer agent for the common stock and warrant
agent for the warrants is Equiniti Trust Company, LLC, 6201 15th Ave, Brooklyn, NY 11219, telephone (800) 937-5449.
SHARES ELIGIBLE FOR FUTURE
SALE
Sale of Restricted Securities
We have 11,106,185 shares of common stock outstanding.
Of these shares, approximately 4,250,000 shares are freely tradable, except that any shares purchased by our affiliates or by investors
in the Company in a private offering may generally only be sold in compliance with Rule 144, which is described below. Of the remaining
outstanding shares, the shares beneficially owned by our officers and directors and by recent investors in the Company will be deemed
“restricted securities” under the Securities Act.
Rule 144
Any shares of our common stock held by an “affiliate”
of ours may not be resold publicly except in compliance with the registration requirements of the Securities Act or under an exemption
under Rule 144 or otherwise. Rule 144 permits our common stock that has been acquired by a person who is an affiliate of ours, or has
been an affiliate of ours within the three months of the date of sale, to be sold into the market in an amount that does not exceed,
during any three-month period, the greater of:
| |
● |
1%
of the total number of shares of our common stock outstanding; or |
| |
● |
the
average weekly reported trading volume of our common stock for the four calendar weeks prior to the sale. |
Such sales are also subject to specific manner
of sale provisions, a six-month holding period requirement, notice requirements and the availability of current public information about
us.
We estimate that approximately 4,700,000 shares
of our common stock are eligible for sale under Rule 144.
Rule 144 also provides that a person who is not
deemed to have been an affiliate of ours at any time during the three months preceding a sale, and who has for at least six months beneficially
owned shares of our common stock that are restricted securities, will be entitled to freely sell such shares of our common stock subject
only to the availability of current public information regarding us. A person who is not deemed to have been an affiliate of ours at
any time during the three months preceding a sale, and who has beneficially owned for at least one year shares of our common stock that
are restricted securities, will be entitled to freely sell such shares of our common stock under Rule 144 without regard to the current
public information requirements of Rule 144.
EXPERTS
Our financial statements included in this prospectus
as of January 31, 2024 and 2023, have been included in reliance on the reports of Sadler, Gibb & Associates, LLC, an independent
registered public accounting firm, given on the authority of such firm as experts in accounting and
auditing.
WHERE
YOU CAN FIND MORE INFORMATION
The
Securities and Exchange Commission maintains an Internet site which contains reports, proxy and information statements, and other information
regarding registrants that file electronically with the Commission at the address: sec.gov.
NUTRIBAND
INC.
January
31, 2024
Index
to Consolidated Financial Statements
REPORT OF INDEPENDENT
REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of Nutriband Inc.:
Opinion on the Financial Statements
We have audited the accompanying consolidated
balance sheets of Nutriband Inc. (“the Company”) as of January 31, 2024 and 2023, the related consolidated statements of
operations, stockholders’ equity, and cash flows for each of the years in the two-year period ended January 31, 2024 and the related
notes (collectively referred to as the “financial statements”). In our opinion, the financial statements referred to above
present fairly, in all material respects, the financial position of the Company as of January 31, 2024 and 2023, and the results of its
operations and its cash flows for each of the years in the two-year period ended January 31, 2024, in conformity with accounting principles
generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility
of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our
audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”)
and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable
rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the
standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial
statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged
to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding
of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s
internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to
assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that
respond to those risks. Such procedures included examining on a test basis, evidence regarding the amounts and disclosures in the financial
statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well
as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below
are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to
the audit committee and that: (1) related to accounts or disclosures that are material to the financial statements and (2) involved our
especially challenging, subjective, or complex judgements. The communication of critical audit matters does not alter in any way our
opinion on the financial statements, taken as a whole, and we are not, by communicating the critical matter below, providing separate
opinions on the critical audit matter or on the accounts or disclosures to which they relate.
Long-Lived Asset Impairment Assessment
Critical Audit Matter Description
As described in note 2 to the consolidated
financial statements, the Company performs impairment testing for its long-lived assets when events or changes in circumstances indicate
that its carrying amount may not be recoverable and exceeds its fair value. Due to challenging industry and economic conditions, the
Company tested its long-lived assets during the year ended January 31, 2024. The Company’s evaluation of the recoverability of
these long-lived asset groups involved comparing the undiscounted future cash flows expected to be generated by these long-lived asset
groups to their respective carrying amounts. The Company’s recoverability analysis requires management to make significant estimates
and assumptions related to cash flows over the remaining useful life of these long-lived asset groups.
We identified the evaluation of the
recoverability analysis for the long-lived assets in the 4P Therapeutics asset group as a critical audit matter because of the significant
estimates and assumptions management used in the related cash flow analysis. Performing audit procedures to evaluate the reasonableness
of these estimates and assumptions required a high degree of auditor judgment and an increased extent of effort.
How the Critical Audit Matter was
Addressed in the Audit
Our audit procedures related to the
following:
| ● | Testing
management’s process for developing the undiscounted cash flow model. |
| ● | Evaluating
the appropriateness of the undiscounted cash flow models used by management. |
| ● | Testing
the completeness and accuracy of underlying data used in the undiscounted cash flow model. |
| ● | Evaluating
the significant assumptions used by management, including assumptions related to current
and planned costs, future revenues, gross margin and other operating expenses to discern
whether they are reasonable considering (i) the current and past performance of the entity;
(ii) the consistency with external market and industry data; and (iii) whether these assumptions
were consistent with evidence obtained in other areas of the audit. |
| ● | Professionals
with specialized skill and knowledge were utilized by the Firm to assist in the evaluation
of the undiscounted cash flow model and underlying assumptions. |
/s/ Sadler, Gibb & Associates, LLC
We have served as the Company’s auditor since 2016.
Draper, UT
April 30, 2024
NUTRIBAND
INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED BALANCE
SHEETS
|
| | |
January
31, | |
| | |
2024 | | |
2023 | |
| ASSETS | |
| | |
| |
| CURRENT ASSETS: | |
| | | |
| | |
| Cash and cash equivalents | |
$ | 492,942 | | |
$ | 1,985,440 | |
| Accounts receivable | |
| 148,649 | | |
| 113,045 | |
| Inventory | |
| 168,605 | | |
| 229,335 | |
| Prepaid expenses | |
| 211,667 | | |
| 365,925 | |
| Total Current
Assets | |
| 1,021,863 | | |
| 2,693,745 | |
| | |
| | | |
| | |
| PROPERTY & EQUIPMENT-net | |
| 774,924 | | |
| 897,735 | |
| | |
| | | |
| | |
| OTHER ASSETS: | |
| | | |
| | |
| Goodwill | |
| 5,021,713 | | |
| 5,021,713 | |
| Operating lease right of use asset | |
| 31,374 | | |
| 62,754 | |
| Intangible assets-net | |
| 667,280 | | |
| 780,430 | |
| | |
| | | |
| | |
| TOTAL ASSETS | |
$ | 7,517,154 | | |
$ | 9,456,377 | |
| | |
| | | |
| | |
| LIABILITIES AND STOCKHOLDERS’
EQUITY | |
| | | |
| | |
| | |
| | | |
| | |
| CURRENT LIABILITIES: | |
| | | |
| | |
| Accounts payable and accrued expenses | |
$ | 680,132 | | |
$ | 534,679 | |
| Deferred revenue | |
| 157,502 | | |
| 162,903 | |
| Operating lease liability-current portion | |
| 34,276 | | |
| 31,291 | |
| Notes payable-current
portion | |
| 127,183 | | |
| 19,740 | |
| Total Current
Liabilities | |
| 999,093 | | |
| 748,613 | |
| | |
| | | |
| | |
| LONG-TERM LIABILITIES: | |
| | | |
| | |
| Note payable-net of current portion | |
| 79,826 | | |
| 100,497 | |
| Note payable-related party | |
| - | | |
| - | |
| Operating lease
liability-net of current portion | |
| - | | |
| 34,277 | |
| Total Liabilities | |
| 1,078,919 | | |
| 883,387 | |
| | |
| | | |
| | |
| Commitments and Contingencies | |
| - | | |
| - | |
| | |
| | | |
| | |
| STOCKHOLDERS’ EQUITY: | |
| | | |
| | |
| Preferred stock, $.001 par value,
10,000,000 shares authorized, -0- outstanding | |
| - | | |
| - | |
| Common stock, $.001 par value,
291,666,666 shares authorized; 8,869,870 and 7,843,150 shares issued at January 31,2024 and 2023, respectively, 8,859,870 and 7,833,150
shares outstanding as of January 31, 2024 and 2023, respectively | |
| 8,860 | | |
| 7,833 | |
| Additional paid-in-capital | |
| 34,442,339 | | |
| 31,092,807 | |
| Accumulated other comprehensive loss | |
| (304 | ) | |
| (304 | ) |
| Treasury stock, 10,000 and 10,000 shares
at cost, respectively | |
| (32,641 | ) | |
| (32,641 | ) |
| Accumulated
deficit | |
| (27,980,019 | ) | |
| (22,494,705 | ) |
| Total Stockholders’
Equity | |
| 6,438,235 | | |
| 8,572,990 | |
| | |
| | | |
| | |
| TOTAL LIABILITIES
AND STOCKHOLDERS’ EQUITY | |
$ | 7,517,154 | | |
$ | 9,456,377 | |
See notes to consolidated financial statements
NUTRIBAND
INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS
OF OPERATIONS
| | |
For the
Years Ended
January 31, | |
| | |
2024 | | |
2023 | |
| | |
| | |
| |
| Revenue | |
$ | 2,085,314 | | |
$ | 2,079,609 | |
| | |
| | | |
| | |
| Costs and expenses: | |
| | | |
| | |
| Cost of revenues | |
| 1,223,209 | | |
| 1,329,200 | |
| Research and development | |
| 1,960,425 | | |
| 982,227 | |
| Goodwill impairment | |
| - | | |
| 327,326 | |
| Selling, general
and administrative | |
| 3,773,606 | | |
| 3,916,041 | |
| Total Costs and
Expenses | |
| 6,957,240 | | |
| 6,554,794 | |
| | |
| | | |
| | |
| Loss from operations | |
| (4,871,926 | ) | |
| (4,475,185 | ) |
| | |
| | | |
| | |
| Other income (expense): | |
| | | |
| | |
| Interest income | |
| 16,850 | | |
| - | |
| Loss on extinguishment of debt | |
| (554,423 | ) | |
| - | |
| Interest expense | |
| (75,815 | ) | |
| (8,289 | ) |
| Total other income
(expense) | |
| (613,388 | ) | |
| (8,289 | ) |
| | |
| | | |
| | |
| Loss before provision for income taxes | |
| (5,485,314 | ) | |
| (4,483,474 | ) |
| | |
| | | |
| | |
| Provision for income taxes | |
| - | | |
| - | |
| | |
| | | |
| | |
| Net loss | |
$ | (5,485,314 | ) | |
$ | (4,483,474 | ) |
| | |
| | | |
| | |
| Net loss per share of common stock-basic
and diluted | |
$ | (0.69 | ) | |
$ | (0.53 | ) |
| |
| | | |
| | |
| Weighted average shares of common stock
outstanding - basic and diluted | |
| 7,954,105 | | |
| 8,459,547 | |
See notes to consolidated financial statements
NUTRIBAND
INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS
OF STOCKHOLDERS’ EQUITY
| | |
| | |
| | |
| | |
| | |
Accumulated | | |
| | |
| |
| | |
| | |
Common
Stock | | |
Additional | | |
Other | | |
| | |
| |
| | |
| | |
Number of | | |
| | |
Paid In | | |
Comprehensive | | |
Accumulated | | |
Treasury | |
| Year
Ended January 31, 2024 | |
Total | | |
shares | | |
Amount | | |
Capital | | |
Income(Loss) | | |
Deficit | | |
Stock | |
| Balance, February
1, 2023 | |
$ | 8,572,990 | | |
| 7,833,150 | | |
$ | 7,833 | | |
$ | 31,092,807 | | |
$ | (304 | ) | |
$ | (22,494,705 | ) | |
$ | (32,641 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Warrants issued
for services | |
| 242,840 | | |
| | | |
| | | |
| 242,840 | | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Options issued
for services | |
| 499,856 | | |
| - | | |
| - | | |
| 499,856 | | |
| - | | |
| - | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Issuance of
common stock for note payable and interest | |
| 2,607,863 | | |
| 1,026,720 | | |
| 1,027 | | |
| 2,606,836 | | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net loss for
the year ended January 31, 2024 | |
| (5,485,314 | ) | |
| - | | |
| - | | |
| - | | |
| - | | |
| (5,485,314 | ) | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance,
January 31, 2024 | |
$ | 6,438,235 | | |
| 8,859,870 | | |
$ | 8,860 | | |
$ | 34,442,339 | | |
$ | (304 | ) | |
$ | (27,980,019 | ) | |
$ | (32,641 | ) |
| | |
| | |
| | |
| | |
| | |
Accumulated | | |
| | |
| |
| | |
| | |
Common
Stock | | |
Additional | | |
Other | | |
| | |
| |
| | |
| | |
Number of | | |
| | |
Paid In | | |
Comprehensive | | |
Accumulated | | |
Treasury | |
| Year
Ended January 31, 2023 | |
Total | | |
shares | | |
Amount | | |
Capital | | |
Income(Loss) | | |
Deficit | | |
Stock | |
| Balance, February
1, 2022 | |
$ | 11,859,285 | | |
| 9,154,846 | | |
$ | 9,155 | | |
$ | 29,966,132 | | |
$ | (304 | ) | |
$ | (18,011,231 | ) | |
$ | (104,467 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Exercise of warrants | |
| 296,875 | | |
| 55,417 | | |
| 56 | | |
| 296,819 | | |
| - | | |
| - | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Common stock
returned in settlement | |
| - | | |
| (1,400,000 | ) | |
| (1,400 | ) | |
| 1,400 | | |
| - | | |
| - | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Treasury stock
issued for services | |
| 113,155 | | |
| 33,471 | | |
| 32 | | |
| 3,746 | | |
| - | | |
| - | | |
| 109,377 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Treasury stock
and warrants issued for termination agreement | |
| 174,025 | | |
| 25,000 | | |
| 25 | | |
| 92,545 | | |
| | | |
| | | |
| 81,455 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Treasury stock
repurchased | |
| (119,006 | ) | |
| (35,584 | ) | |
| (35 | ) | |
| 35 | | |
| - | | |
| - | | |
| (119,006 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Options issued
for services | |
| 732,130 | | |
| | | |
| | | |
| 732,130 | | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net
loss for the year ended January 31, 2023 | |
| (4,483,474 | ) | |
| - | | |
| - | | |
| - | | |
| - | | |
| (4,483,474 | ) | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance,
January 31, 2023 | |
$ | 8,572,990 | | |
| 7,833,150 | | |
$ | 7,833 | | |
$ | 31,092,807 | | |
$ | (304 | ) | |
$ | (22,494,705 | ) | |
$ | (32,641 | ) |
See notes to consolidated financial statements
NUTRIBAND
INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS
OF CASH FLOWS
| | |
For the
Years Ended
January 31, | |
| | |
2024 | | |
2023 | |
| Cash flows from operating activities: | |
| | |
| |
| Net loss | |
$ | (5,485,314 | ) | |
$ | (4,483,474 | ) |
| Adjustments to reconcile net loss to net cash used in operating
activities: | |
| | | |
| | |
| Depreciation and amortization | |
| 287,722 | | |
| 330,143 | |
| Operating lease expense | |
| 31,380 | | |
| 38,813 | |
| Loss on extinguishment of debt | |
| 554,423 | | |
| - | |
| Reserve for doubtful accounts | |
| 118,365 | | |
| - | |
| Treasury stock issued for services | |
| - | | |
| 113,155 | |
| Treasury stock and warrants issued for
termination agreement | |
| - | | |
| 174,025 | |
| Goodwill impairment | |
| - | | |
| 327,326 | |
| Stock-based compensation-warrants | |
| 242,840 | | |
| - | |
| Stock-based compensation-options | |
| 499,856 | | |
| 732,130 | |
| Changes in operating assets and liabilities: | |
| | | |
| | |
| Accounts receivable | |
| (153,969 | ) | |
| (41,665 | ) |
| Prepaid expenses | |
| 154,258 | | |
| 4,547 | |
| Inventories | |
| 60,730 | | |
| (97,687 | ) |
| Deferred revenue | |
| (5,401 | ) | |
| 56,636 | |
| Operating lease liability | |
| (31,292 | ) | |
| (36,287 | ) |
| Accounts payable
and accrued expenses | |
| 198,893 | | |
| (104,860 | ) |
| Net Cash Used
In Operating Activities | |
| (3,527,509 | ) | |
| (2,987,198 | ) |
| | |
| | | |
| | |
| Cash flows from investing activities: | |
| | | |
| | |
| Purchase of equipment | |
| (51,761 | ) | |
| (79,304 | ) |
| Net Cash Used
in Investing Activities | |
| (51,761 | ) | |
| (79,304 | ) |
| | |
| | | |
| | |
| Cash flows from financing activities: | |
| | | |
| | |
| Proceeds from note payable-related
party | |
| 2,000,000 | | |
| - | |
| Proceeds from secured borrowing liability | |
| 106,528 | | |
| - | |
| Proceeds from exercise of warrants | |
| - | | |
| 296,875 | |
| Payment on note payable | |
| (19,756 | ) | |
| (17,795 | ) |
| Purchase of
treasury stock | |
| - | | |
| (119,006 | ) |
| Net Cash Provided
by Financing Activities | |
| 2,086,772 | | |
| 160,074 | |
| | |
| | | |
| | |
| Net change in cash | |
| (1,492,498 | ) | |
| (2,906,428 | ) |
| Cash and cash equivalents - Beginning
of period | |
| 1,985,440 | | |
| 4,891,868 | |
| Cash and cash equivalents - End of period | |
$ | 492,942 | | |
$ | 1,985,440 | |
| | |
| | | |
| | |
| Supplementary information: | |
| | | |
| | |
| | |
| | | |
| | |
| Cash paid for: | |
| | | |
| | |
| Interest | |
$ | 7,352 | | |
$ | 4,266 | |
| Income taxes | |
$ | - | | |
$ | - | |
| | |
| | | |
| | |
| Supplemental disclosure of non-cash investing and financing
activities: | |
| | | |
| | |
| Adoption of ASC
842 Operating lease asset and liability | |
$ | - | | |
$ | 94,134 | |
| Promissory note
on equipment purchase | |
$ | - | | |
$ | 22,794 | |
| Common stock returned
in settlement | |
$ | - | | |
$ | 1,400 | |
| Issuance of common
stock for extinguishment of debt | |
$ | 2,607,863 | | |
$ | - | |
See notes to consolidated financial statements
NUTRIBAND
INC. AND SUBSIDIARIES
Notes to Consolidated Financial
Statements
as of and for the Years
Ended January 31, 2024 and 2023
| 1. | ORGANIZATION
AND DESCRIPTION OF BUSINESS |
Organization
Nutriband
Inc. (the “Company”) is a Nevada corporation, incorporated on January 4, 2016. In January 2016, the Company acquired Nutriband
Ltd, an Irish company which was formed by the Company’s chief executive officer in 2012 to enter the health and wellness market
by marketing transdermal patches. References to the Company relate to the Company and its subsidiaries unless the context indicates otherwise.
On August
1, 2018, the Company acquired 4P Therapeutics LLC (“4P Therapeutics”) for $2,250,000, consisting of 250,000 shares of common
stock, valued at $1,850,000, and $400,000, and a royalty of 6% on all revenue generated by the Company from the abuse deterrent intellectual
property that had been developed by 4P Therapeutics payable to the former owner of 4P Therapeutics. The former owner of 4P Therapeutics
has been a director of the Company since April 2018, when the Company entered into an agreement to acquire 4P Therapeutics. The former
owner resigned as a director in January 2022.
4P Therapeutics
is engaged in the development of a series of transdermal pharmaceutical products, that are in the preclinical stage of development. Prior
to the acquisition of 4P Therapeutics, the Company’s business was the development and marketing of a range of transdermal consumer
patches. Most of these products are considered drugs in the United States and cannot be marketed in the United States without approval
by the Food and Drug Administration (the “FDA”). The Company entered a feasibility agreement as an initial step to seek FDA
approval of its consumer transdermal products and its consumer products which are not being marketed in the United States.
With the acquisition
of 4P Therapeutics, 4P Therapeutics’ drug development business became the Company’s principal business. The Company’s
approach is to use generic drugs that are off patent and incorporate them into the Company’s transdermal drug delivery system.
Although these medications have received FDA approval in oral or injectable form, the Company needs to conduct a transdermal product
development program which will include the preclinical and clinical trials that are necessary to receive FDA approval before we can market
any of our pharmaceutical products.
On August
25, 2020, the Company formed Pocono Pharmaceuticals Inc. (“Pocono Pharmaceuticals”), a wholly owned subsidiary of the Company.
On August 31, 2020, the Company acquired certain assets and liabilities associated with the Transdermal, Topical, Cosmetic, and Nutraceutical
business of Pocono Coated Products LLC (“PCP”). The net assets were contributed to Pocono Pharmaceuticals. Included in the
transaction, Pocono Pharmaceuticals also acquired 100% of the membership interests of Active Intelligence LLC (“Active Intelligence”).
Pocono Pharmaceuticals
is a contract development and manufacturing organization with unique process capabilities and experience focused on coated product manufacturing.
Pocono helps their customers with product design and development along with manufacturing to bring new products to market with minimal
capital investment. Pocono Pharmaceutical’s competitive edge is a low-cost manufacturing base: a result of its unique processes
and state-of-the-art material technology. Active Intelligence manufactures activated kinesiology tape for transdermal or topical use.
| 2. | SUMMARY
OF SIGNIFICANT ACCOUNTING POLICIES |
Forward
Stock Split
On July 26,
2022, our Board of Directors approved the amendment to our Articles of Incorporation to effect a 7- for- 6 forward stock split (the “Stock
Split”) of our outstanding common stock. The Company filed the amendment set forth in a Certificate of Change with the Secretary
of State of Nevada on August 4, 2022. The 7:6 forward stock split was effective for trading purposes on the Nasdaq Capital Market on
August 12, 2022. Each shareholder of record as of the August 15, 2022 record date received one (1) additional share for each six (6)
shares held as of the record date. No fractional shares of common stock were issued in connection with the Stock Split. Instead, all
shares were rounded up to the next whole share. In connection with the Stock Split, which did not require shareholder approval under
the Nevada corporation law, the number of shares of common stock of the Company was increased in the same ratio as the shares of outstanding
common stock were increased in the Stock Split, from 250,000,000 authorized shares to 291,666,666 authorized shares.
All share
and per share information in these financial statements retroactively reflect the forward stock split.
Going
Concern Assessment
Management
assesses liquidity and going concern uncertainty in the Company’s condensed financial statements to determine whether there is
sufficient cash on hand and working capital, including available borrowings on loans, to operate for a period of at least one year from
the date the consolidated financial statements are issued or available to be issued, which is referred to as the “look-forward
period”, as defined in GAAP. As part of this assessment, based on conditions that are known and reasonably knowable to management,
management will consider various scenarios, forecasts, projections, estimates and will make certain key assumptions, including timing
and nature of projected cash expenditures or programs, its ability to delay or curtail expenditures or programs and its ability to raise
additional capital, if necessary, among other factors. Based on this assessment, as necessary or applicable, management makes certain
assumptions around implementing curtailments or delays in the nature and timing of programs and expenditures to the extent it deems probable
those implementations can be achieved and management has the proper authority to execute them within the look-forward period.
As of January
31, 2024, the Company had cash and cash equivalents of $492,942 and working capital of $22,770. For the year ended January 31, 2024,
the Company incurred a loss from operations of $4,871,926 and used cash flow from operations of $3,527,509. The Company has generated
operating losses since its inception and has relied on sales of securities and issuance of third-party and related-party debt to support
cash flow from operations. In October 2021, the Company consummated a public offering and received net proceeds of $5,836,230. The Company
has also received to date $3,239,845 in proceeds from the exercise of warrants. The Company has used these proceeds to fund operations
and will continue to use the funds as needed. In March 2023, the Company entered into a three-year $2,000,000 Credit Line Note facility
with a related party, amended on July 13, 2023, to $5,000,000, which will permit the Company to draw down on the credit line to fund
the Company’s research and development of its Aversa product. The Company was advanced $2,000,000, all of which was settled by
the issuance of common stock during the year ended January 31, 2024. The $2,000,000 debt and accrued interest was converted into 1,026,720
shares of the Company’s common stock. On April 19, 2024, the Company received proceeds of $8,400,000 from a private placement of
its common stock.
Management
has prepared estimates of operations for the next twelve months and believes that sufficient funds will be generated from operations
to fund its operations for one year from the date of the filing of these condensed consolidated financial statements, which indicates
improved operations and the Company’s ability to continue operations as a going concern.
Management
believes the substantial doubt about the ability of the Company to continue as a going concern is alleviated by the above assessment.
Principles
of Consolidation
The consolidated
financial statements of the Company include the Company and its wholly owned subsidiaries. All material intercompany balances and transactions
have been eliminated. The operations of 4P Therapeutics are included in the Company’s financial statements from the date of acquisition
of August 1, 2018, and the operations of Pocono and Active Intelligence are included in the Company’s financial statements from
the date of acquisition of September 1, 2020 under Pocono Pharmaceuticals Inc. The wholly owned subsidiaries are as follows:
Nutriband
Ltd.
4P Therapeutics
LLC
Pocono Pharmaceuticals
Inc.
Use
of Estimates
The preparation
of the consolidated financial statements in conformity with accounting principles generally accepted in the United States of America
requires the Company to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses,
and related disclosure of contingent assets and liabilities. On an ongoing basis, the Company evaluates its estimates including, but
not limited to, those related to such items as income tax exposures, accruals, depreciable/useful lives, allowance for doubtful accounts
and valuation allowances. The Company bases its estimates on historical experience and on other various assumptions that are believed
to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets
and liabilities that are not readily apparent from other sources. Actual results could differ from those estimates.
Revenue
Recognition
In May 2014,
the FASB issued ASU No. 2014-09, “Revenue from Contracts with Customers (Topic 606) (“ASU 2014-09”), which amends the
accounting standards for revenue recognition. ASU 2014-09 is based on principles that govern the recognition of revenue at an amount
an entity expects to be entitled when products are transferred to a customer. The Company recognizes revenue based on the five criteria
for revenue recognition established under Topic 606: 1) identify the contract, 2) identify separate performance obligations, 3) determine
the transaction price, 4) allocate the transaction price among the performance obligations, and 5) recognize revenue as the performance
obligations are satisfied.
Revenue
Types
The
following is a description of the Company’s revenue types, which include professional services and sale of goods:
| ● | Contract
development and manufacturing services for consumer health transdermal, topical and tape
products with revenues listed under sale of goods |
| ● | Product
revenues derived from the sale of the Company’s consumer transdermal, topical and tape
products with sales listed under sale of goods |
| ● | Contract
research and development services for pharmaceuticals and medical devices for life sciences
customers with revenues listed under services |
Contracts with Customers
A contract with a customer exists when
(i) we enter into an enforceable contract with a customer that defines each party’s rights regarding the goods or services to be
transferred and identifies the payment terms related to these goods or services, (ii) the contract has commercial substance and, (iii)
we determine that collection of substantially all consideration for services that are transferred is probable based on the customer’s
intent and ability to pay the promised consideration.
Contract Liabilities
Deferred revenue is a liability related
to a revenue producing activity for which revenue has not been recognized. The Company records deferred revenue when it receives consideration
from a contract before achieving certain criteria that must be met for revenue to be recognized in conformity with GAAP.
Performance Obligations
A performance obligation is a promise
in a contract to transfer a distinct good or service to the customer and is the unit of account in the new revenue standard. The contract
transaction price is allocated to each distinct performance obligation and recognized as revenue when, or as, the performance obligation
is satisfied. For the Company’s different revenue service types, the performance obligation is satisfied at different times. The
Company’s performance obligations include providing products and professional services in the area of research. The Company recognizes
product revenue performance obligations in most cases when the product has shipped to the customer. When we perform professional service
work, we recognize revenue when we have the right to invoice the customer for the work completed, which typically occurs over time on
a monthly basis for the work performed during that month.
All revenue
recognized in the income statement is considered to be revenue from contracts with customers.
Disaggregation of Revenues
The Company
disaggregates its revenue from contracts with customers by type and by geographical location. See the tables:
| | |
Years Ended | |
| | |
January
31, | |
| | |
2024 | | |
2023 | |
| Revenue by type | |
| | |
| |
| Sale of goods | |
$ | 1,920,280 | | |
$ | 1,785,507 | |
| Services | |
| 165,034 | | |
| 294,102 | |
| Total | |
$ | 2,085,314 | | |
$ | 2,079,609 | |
| | |
Years Ended | |
| | |
January
31, | |
| | |
2024 | | |
2023 | |
| Revenue by geographic location: | |
| | |
| |
| United States | |
$ | 2,085,314 | | |
$ | 2,079,609 | |
| Foreign | |
| - | | |
| - | |
| | |
$ | 2,085,314 | | |
$ | 2,079,609 | |
Cash and cash equivalents.
Cash and cash equivalents include cash
on hand, cash on deposit in money market accounts. The Company considers short-term highly liquid investments with an original maturity
date of three months or less that are not part of an investment pool to be cash equivalents. As of January 31, 2024, the Company has
no balances that exceed federally insured limits.
Accounts
receivable
Trade accounts
receivables are recorded at the net invoice value and are not interest bearing. The Company maintains allowances for doubtful accounts
for estimated losses from the inability of its customers to make the required payments. The Company determines its allowances by both
the specific identification of customer accounts where appropriate and the application of historical loss to non-applicable accounts.
For the years ended January 31, 2024, and 2023, the Company recorded bad debt expenses of $118,364 and $-0-, respectively, for doubtful
accounts related to accounts receivable. During the year ended January 31, 2024, the Company entered into an accounts receivable sale
agreement for one of its subsidiaries. The Company received $106,528 in funds against an account receivable that is currently a claim
in bankruptcy. The net accounts receivable remain on the books of the Company and a corresponding amount has been included as a secured
borrowing liability under Notes payable. As of January 31, 2024, the receivable has been reserved in full. If the bankruptcy claim is
not paid in full by the debtor, Company is obligated to pay any difference to the factor. The loan bears interest at 10%. The Company
adopted ASU 2016-13 during 2023 and implemented the guidance on expected credit losses.
Inventories
Inventories
are valued at the lower of cost and reasonable value determined using the first-in, first-out (FIFO) method. Net realized value is the
estimated selling price in the ordinary course of business, less applicable variable selling expenses. The cost of finished goods and
work in process is comprised of material costs, direct labor costs and other direct costs and related production overheads (based on
normal operating capacity). As of January 31, 2024, total inventory was $168,605, consisting of work-in-process of $7,466, finished goods
of $8,707 and raw materials of $134,691. As of January 31, 2023, total inventory was $229,335, consisting of work-in-process of $11,021
and raw materials of $218,334.
Property,
Plant and Equipment
Property and
equipment represent an important component of the Company’s assets. The Company depreciates its plant and equipment on a straight-line
basis over the estimated useful life of the assets. Property, plant and equipment is stated at historical cost. Expenditures for minor
repairs, maintenance and replacement parts which do not increase the useful lives of the assets are charged to expense as incurred. All
major additions and improvements are capitalized. Depreciation is computed using the straight-line method. The lives over which the fixed
assets are depreciated range from 3 to 20 years as follows:
| Lab Equipment | |
| 5-10
years | |
| Furniture and fixtures | |
| 3
years | |
| Machinery and equipment | |
| 10-20
years | |
Intangible
Assets
Intangible
assets include trademarks, intellectual property and customer base acquired through business combinations. The Company accounts for Other
Intangible Assets under the guidance of ASC 350, “Intangibles-Goodwill and Other.” The Company capitalizes certain costs
related to patent technology. A substantial component of the purchase price related to the Company’s acquisitions have also been
assigned to intellectual property and other intangibles. Under the guidance, other intangible assets with definite lives are amortized
over their estimated useful lives. Intangible assets with indefinite lives are tested annually for impairment. Trademarks, intellectual
property and customer base are being amortized over their estimated useful lives of ten years.
Goodwill
Goodwill represents
the difference between the total purchase price and the fair value of assets (tangible and intangible) and liabilities at the date of
acquisition. Goodwill is reviewed for impairment annually on January 31, and more frequently as circumstances warrant, and written down
only in the period in which the recorded value of such assets exceeds their fair value. The Company does not amortize goodwill in accordance
with ASC 350. In connection with the Company’s acquisition of 4P Therapeutics LLC in 2018, the Company recorded Goodwill of $1,719,235.
On August 31, 2020, in connection with the Company’s acquisition of Pocono Coated Products LLC and Active Intelligence LLC, the
Company recorded Goodwill of $5,810,640. During the years ended January 31, 2024, and 2023, the Company recorded an impairment charge
of $-0- and $327,326, respectively, reducing the Active Intelligence LLC Goodwill to $3,302,478. As of January 31, 2024, and 2023, Goodwill
amounted to $5,021,713 and $5,021,713, respectively.
Long-lived
Assets
Management
reviews long-lived assets for potential impairment whenever significant events or changes in circumstances indicate that the carrying
amount of an asset may not be recoverable. An impairment exists when the carrying amount of the long-lived asset is not recoverable and
exceeds its fair value. The carrying amount of a long-lived asset is not recoverable if it exceeds the sum of the estimated undiscounted
cash flows expected to result from the use and eventual disposition of the asset. If an impairment exists, the resulting write-down would
be the difference between the fair market value of the long-lived asset and the related book value.
Earnings
per Share
Basic earnings
per share of common stock is computed by dividing net earnings by the weighted average number of shares of common stock outstanding during
the period. Diluted earnings per share is computed by dividing net earnings by the weighted average number of shares of common
stock and potential shares of common stock outstanding during the period. Potential shares of common stock consist of shares issuable
upon the exercise of outstanding options and common stock purchase warrants. As of January 31, 2024, and 2023, there were 2,157,873 and
1,778,006 common stock equivalents outstanding, that were not included in the calculation of dilutive earnings per share as their effect
would be anti-dilutive.
Stock-Based
Compensation
ASC 718, “Compensation
- Stock Compensation,” prescribes accounting and reporting standards for all share-based payment transactions in which employee
services, and, since February 1, 2019, non-employees, are acquired. Transactions include incurring liabilities, or issuing or offering
to issue shares, options and other equity instruments such as employee stock ownership plans and stock appreciation rights. Share-based
payments to employees, including grants of employee stock options, are recognized as compensation expense in the financial statements
based on their fair values. That expense is recognized over the period during which an employee is required to provide services in exchange
for the award, known as the requisite service period (usually the vesting period). As of February 1, 2019, pursuant to ASC 2018-07, ASC
718 was applied to stock-based compensation for both employees and non-employees.
Business
Combinations
The Company
recognizes the assets acquired, the liabilities assumed, and any non-controlling interest in the acquired entity at the acquisition date,
measured at their fair values as of that date, with limited exceptions specified in the accounting literature. In accordance with this
guidance, acquisition-related costs, including restructuring costs, must be recognized separately from the acquisition and will generally
be expensed as incurred. That replaces the cost-allocation process detailed in previous accounting literature, which required the cost
of an acquisition to be allocated to the individual assets acquired and liabilities assumed based on their estimated fair value.
Leases
In February
2016, the FASB issued ASU 2016-02, “Leases” (Topic 842), to provide a new comprehensive model for lease accounting under
this guidance, lessees and lessors should apply a “right-of-use” model in accounting for all leases (including subleases)
and eliminate the concept of operating leases and off-balance-sheet leases. Recognition, measurement and presentation of expenses will
depend on classification as a finance or operating lease. Similar modifications have been made to lessor accounting in-line with revenue
recognition guidance.
The Company
applies guidance for right-of-use accounting for all leases and records the operating lease liabilities on its balance sheet. The Company
completed the necessary changes to its accounting policies, processes, disclosure and internal control over financial reporting.
Research
and Development Expenses
Research and
development costs are expensed as incurred.
Income
Taxes
Taxes are
calculated in accordance with taxation principles currently effective in the United States and Ireland.
The Company
accounts for income taxes under the asset and liability method, which requires the recognition of deferred tax assets and liabilities
for the expected future tax consequences of events that have been included in the financial statements. Under this method,
deferred tax assets and liabilities are determined based on the differences between the financial statements and tax basis of assets
and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. The effect
of a change in tax rates on deferred tax assets and liabilities is recognized in income in the period that includes the enactment date.
The Company
records net deferred tax assets to the extent they believe these assets will more-likely-than-not be realized. In making such
determination, the Company considers all available positive and negative evidence, including future reversals of existing taxable temporary
differences, projected future taxable income, tax planning strategies and recent financial operations. In the event the Company
was to determine that it would be able to realize its deferred income tax assets in the future in excess of its net recorded amount,
the Company would make an adjustment to the valuation allowance which would reduce the provision for income taxes.
Fair
Value Measurements
FASB ASC
820, “Fair Value Measurements and Disclosure” (“ASC 820”), defines fair value as the exchange price that would
be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or
liability in an orderly transaction between participants on the measurement date. ASC 820 also establishes a fair value hierarchy which
requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC
820 describes three levels of inputs that may be used to measure fair value.
The Company
utilizes the accounting guidance for fair value measurements and disclosures for all financial assets and liabilities and nonfinancial
assets and liabilities that are recognized or disclosed at fair value in the consolidated financial statements on a recurring basis during
the reporting period. The fair value is an exit price, representing the price that would be received to sell an asset or paid to transfer
a liability in an orderly transaction between market participants based upon the best use of the asset or liability at the measurement
date. The Company utilizes market data or assumptions that market participants would use in pricing the asset or liability. ASC 820 establishes
a three-tier value hierarchy, which prioritizes the inputs used in measuring fair value. These tiers are defined as follows:
| |
Level
1 |
-Observable
inputs such as quoted market prices in active markets. |
| |
|
|
| |
Level
2 |
-Inputs
other than quoted prices in active markets that are either directly or indirectly observable. |
| |
|
|
| |
Level
3 |
-Unobservable
inputs about which little or no market data exists, therefore requiring an entity to develop its own assumptions. |
The carrying
value of the Company’s financial instruments, including accounts receivable, prepaid expenses, accounts payable and accrued expenses,
and deferred revenue approximate their fair value due to the short maturities of these financial instruments.
Recent
Accounting Standards
In June
2016, the FASB issued ASU 2016-13, Financial Instruments-Credit Losses (Topic 326), The ASU introduces a new credit loss methodology.
Current Expected Credit Loss (“CECL”), which requires earlier recognition of credit losses, which also provides additional
transparency about credit risk. Since its original issuance in 2016, the FASB has issued several updates to the original ASU. The Company
adopted ASU 2016-13 during the year ended January 31, 2024. The adoption of ASU 2016-13 did not have a material impact on the Company’s
balance sheet or statement of operations.
The Company
has reviewed all other FASB-issued ASU accounting pronouncements and interpretations thereof that have effective dates during the period
reported and in future periods. The Company has carefully considered the new pronouncements that alter previous GAAP and does not believe
that any new or modified principles will have a material impact on the Company’s reported financial position or operations in the
near term. The applicability of any standard is subject to the formal review of the Company’s financial management and certain
standards are under consideration.
| | |
January
31, | |
| | |
2024 | | |
2023 | |
| Lab equipment | |
$ | 144,585 | | |
$ | 144,585 | |
| Machinery and equipment | |
| 1,292,389 | | |
| 1,240,628 | |
| Furniture and fixtures | |
| 19,643 | | |
| 19,643 | |
| | |
| 1,456,617 | | |
| 1,404,856 | |
| Less: Accumulated depreciation | |
| (681,693 | ) | |
| (507,121 | ) |
| Net Property and Equipment | |
$ | 774,924 | | |
$ | 897,735 | |
Depreciation expenses amounted to $174,572
and $183,660 for the years ended January 31, 2024, and 2023, respectively. During the years ended January 31, 2024, and 2023, depreciation
expenses of $131,360 and $139,689, respectively, have been allocated to cost of goods sold.
The Company adopted the provisions
of ASC 740, “Income Taxes, (“ASC 740”). As a result of the implementation of ASC 740, the Company recognized no adjustment
in the net liability for unrecognized income tax benefits. The Company believes there are no potential uncertain tax positions, and all
tax returns are correct as filed. Should the Company recognize a liability for uncertain tax positions, the Company will separately recognize
the liability for uncertain tax positions on its balance sheet. Included in any liability or uncertain tax positions, the Company will
also set up a liability for interest and penalties. The Company’s policy is to recognize interest and penalties related to uncertain
tax positions as a component of the current
provision for income taxes.
There is no U.S. tax provision due
to losses from U.S. operations for the years ended January 31, 2024 and 2023. Deferred income taxes are provided for the temporary differences
between the financial reporting and tax basis of the Company’s assets and liabilities. The principal item giving rise to deferred
taxes is the net operating loss carryforward in the U.S. Valuation allowances are established when necessary to reduce deferred tax assets
to the amount expected to be realized. The Company has set up a valuation allowance for losses for certain carryforwards that it believes
may not be realized.
The provision for income taxes consists of the following:
| | |
| Years
Ended
January 31, | |
| | |
| 2024 | | |
| 2023 | |
| Current | |
| | | |
| | |
| Federal | |
$ | - | | |
$ | - | |
| Foreign | |
| - | | |
| - | |
| | |
| | | |
| | |
| Deferred | |
| | | |
| | |
| Federal | |
| - | | |
| - | |
| Foreign | |
| - | | |
| - | |
A reconciliation of taxes on income computed at the federal
statutory rate to amounts provided is as follows:
| | |
Years
Ended
January 31, | |
| | |
2024 | | |
2023 | |
| Book Income (loss from operations) | |
$ | (1,151,916 | ) | |
$ | (941,530 | ) |
| Common stock issued for services | |
| 155,966 | | |
| 168,768 | |
| Impairment expense | |
| - | | |
| 68,738 | |
| Unused operating losses | |
| 995,950 | | |
| 704,024 | |
| Income tax expense | |
$ | - | | |
$ | - | |
As of January 31, 2024, the Company
recorded a deferred tax asset associated with a net operating loss (“NOL”) carryforward of approximately $15,800,000 that
was fully offset by a valuation allowance due to the determination that it was more likely than not that the Company would be unable
to utilize those benefits in the foreseeable future. The Company’s NOL expires in 2041. The tax effect of the valuation allowance
increased by approximately $1,151,916 during the year ended January 31, 2024. On December 22, 2017, the Tax Cuts and Jobs Act (the “Tax
Act”) significantly revised U.S. corporate income tax law by, among other things, reducing the corporate rate from 34% to 21%.
Because the Company recognizes a valuation allowance for the entire balance, there is no net impact on the Company’s balance sheet
or results of operations.
The types of temporary differences
between tax basis of assets and liabilities and their financial reporting amounts that give rise to the deferred tax liability and deferred
tax asset and their approximate tax effects are as follows:
| | |
January
31, | |
| | |
2024 | | |
2023 | |
| Net operating loss carryforward (expire through
2040) | |
$ | (3,312,698 | ) | |
$ | (2,316,748 | ) |
| Stock issued for services | |
$ | (1,455,848 | ) | |
| (1,299,882 | ) |
| Intangible impairment expense | |
$ | (1,051,714 | ) | |
| (1,051,714 | ) |
| Valuation allowance | |
$ | 5,820,260 | | |
| 4,668,344 | |
| Net deferred taxes | |
$ | - | | |
$ | - | |
Notes Payable
Active Intelligence,
entered into an agreement with the Carolina Small Business Development Fund for a line of credit of $160,000 due October 16, 2028, with
interest of 5% per year. The amount assumed was $139,184. The loan requires monthly payments of principal and interest of $1,697. During
the year ended January 31, 2024, the Company made $15,378 of principal payments. As of January 31, 2024, the amount due was $85,249,
of which $16,129 is current. As of January 31, 2023, the amount due was $100,627.
On April 3,
2022, the Company entered into a retail installment agreement for the purchase of an automobile. The contract price was $32,274, of which
$22,795 was financed. The agreement is for five years bearing interest at 2.95% per annum with payments of $410 per month. The loan is
secured by automobile. As of January 31, 2024, the amount due was $15,232 of which $4,456 is current. As of January 31, 2023, the amount
due was $19,610.
Note payable-related
party.
On July 17,
2023, the Company entered an amended Credit Line Note agreement, for an increased $5,000,000 credit line facility Note, with TII Jet
Services LDA, a shareholder of the Company (replacing the $2,000,000 facility with the same lender that the Company entered on March
17, 2023). Outstanding advances under the Note bears interest at 7% per annum. The promissory note is due and payable in full on March
19, 2026. Interest is payable annually on December 31 of each year during the term of the note. During the year ended January 31, 2024,
the Company received $2,000,000 on the Note. In December 2023, the Company converted the balance of the credit facility of $2,000,000
and $53,476 of accrued interest into 1,026,520 shares of common stock. The fair value of the common stock was $2,554,423 resulting in
a $554,423 loss on extinguishment. As of January 31, 2024, the balance due was $-0-. The Company recorded interest expense of $60,453
for the year ended January 31, 2024.
Secured
borrowing liability.
The Company
entered into an accounts receivable sale agreement for one of its subsidiaries in connection with a bankruptcy claim. The Company received
$106,528 and recorded the transaction as a secured loan payable against the account receivable. The sale of the account receivable balance
was to an outside third party, whereby if the bankruptcy court does not pay the balance in full, the Company will owe back the unpaid
portion. The loan is classified as a current liability as the Company expects the bankruptcy will be resolved in the next twelve months.
The loan bears interest at 10%. For the year ended January 31, 2024, the Company recorded interest expense of $5,470.
Interest expenses
for the year ended January 31, 2024, and 2023, were $75,815 and $6,289, respectively.
As of January 31, 2024, and 2023,
intangible assets consisted of intellectual property and trademarks, customer base, and license agreement, net of amortization, as follows:
| | |
January
31, | |
| | |
2024 | | |
2023 | |
| Customer base | |
$ | 314,100 | | |
$ | 314,100 | |
| Intellectual property and trademarks | |
| 817,400 | | |
| 817,400 | |
| | |
| | | |
| | |
| Total | |
| 1,131,500 | | |
| 1,131,500 | |
| | |
| | | |
| | |
| Less: Accumulated amortization | |
| (464,220 | ) | |
| (351,070 | ) |
| | |
| | | |
| | |
| Net Intangible Assets | |
$ | 667,280 | | |
$ | 780,430 | |
In February
2021, the Company acquired an IP license from Rambam Med-Tech Ltd. for $50,000. The value of the intangible assets, consisting of intellectual
property, license agreement and customer base has been recorded at their fair value by the Company and are being amortized over a period
of three to ten years. The Company terminated the license agreement in October 2022. The Company issued 25,000 shares of its common stock
from its treasury shares held by the Company and warrants to purchase 25,000 shares at an exercise price of $7.50 per share as part of
the termination agreement. The Company recorded a termination expense of $174,025 during the year ended January 31, 2023. Which is included
in selling, general and administrative expenses. The Company expensed the balance of the agreement of $33,334 during the year ended January
31, 2023, which is included in selling, general and administrative expenses. Amortization expenses for the years ended January 31, 2024,
and 2023 amounted to $113,150 and $146,483, respectively.
| Year Ended January 31, | |
| |
| 2025 | |
$ | 113,109 | |
| 2026 | |
| 113,109 | |
| 2027 | |
| 113,109 | |
| 2028 | |
| 113,109 | |
| 2029 | |
| 113,109 | |
| 2030 and thereafter | |
| 101,735 | |
| | |
$ | 667,280 | |
| 7. | RELATED
PARTY TRANSACTIONS |
Activity during the year ended January 31, 2024
| a) | On
February 1, 2023, options to purchase 30,000 shares of the Company’s common stock were
issued to an executive of the Company at a price of $3.975 per share. The options vest immediately
and expire in three years. The fair value of the options issued for services amounted to
$75,030 and was expensed during the year ended January 31, 2024. |
| b) | In
September and October 2023, options to purchase 374,500 shares of common stock to executives
and directors of the Company at a price of $1.93, $2.12 and $2.65 per share. The options
vest immediately and expire in three years. The fair value of the options issued amounted
to $424,826 and was expensed during the year ended January 31, 2024. |
| | | |
| c) | On
October 26, 2023, warrants to purchase 87,500 shares of the Company’s common stock
were issued to the Chief Financial Officer at a price of $1.93 per share. The warrant expires
in three years. The fair value of the warrants issued amounted to $93,450 and was expensed
during the year ended January 31, 2024. |
| | | |
| d) | On
July 17, 2023, the Company entered an amended Credit Line Note facility with TII Jet Services
LDA, a shareholder of the Company, for a credit facility of $5 million (replacing the $2,000,000
facility with the same lender that the Company entered on March 17, 2023). See Note 5 for
further information. TII Jet Services LDA is owned 100% by a shareholder of the Company.
During the year ended January 31, 2024, the Company received $2,000,000 from the credit facility.
In December 2023, TII Jet Services LDA converted the balance of the credit facility of $2,000,000
and $53,436 of accrued interest into 1,026,520 shares of the Company’s common stock. |
Activity during
the year ended January 31, 2023
| a) | In
May 2022, the Company issued stock awards to the Company’s CEO and the independent
members of the Board of Directors. The CEO received 11,667 shares and the four directors
received 1,167 shares each. The Company recorded a compensation expense of $53,200 in connection
with the issuance of the shares. |
| | | |
| b) | On
August 2, 2022, 137,084 options to purchase shares of the Company’s common stock were
issued to executives of the Company at prices of $4.09 and $4.50 per share. The options vest
immediately and expire in three years. The fair value of the options issued for services
amounted to $399,075 and was expensed during the year ended January 31, 2023. |
| | | |
| c) | On
September 30, 2022, 35,000 options to purchase shares of the Company’s common stock
were issued to the independent directors of the Company at a price of $3.59 per share. The
options vest immediately and expire in five years. The fair value of the options issued for
services amounted to $85,995 and was expensed during the year ended January 31, 2023 |
| | | |
| d) | On
December 7, 2022, options to purchase 107,500 shares of the Company’s common stock
were issued to executives of the Company at prices of $3.53 and $3.88 per share. The options
vest immediately and expire in three years. The fair value of the options issued amounted
to $245,170 and was expensed during the year ended January 31, 2023. |
Preferred Stock
On January 15, 2016, the board of directors
of the Company approved a certificate of amendment to the articles of incorporation and changed the authorized capital stock of the Company
to include and authorize 10,000,000 shares of Preferred Stock, par value $0.001 per share.
On May 24, 2019, the board of directors
created a series of preferred stock consisting of 2,500,000 shares designated as the Series A Convertible Preferred Stock (“Series
A Preferred Stock”). On June 20, 2019, the Series A preferred Stock was terminated, and the 2,500,000 shares were restored to the
status of authorized but unissued shares of Preferred Stock, without designation as to series, until such stock is once more designated
as part of a particular series by the board of directors.
Common Stock
On June 25, 2019, the Company effected
a one-for-four reverse stock split, pursuant to which each outstanding share of common stock was changed into 0.25 shares of common stock,
and the Company decreased its authorized common stock in the same ratio from 100,000,000 to 25,000,000 shares.
On January 27, 2020, the Company amended
its Articles of Incorporation to increase its authorized common shares from 25,000,000 authorized shares to 250,000,000 authorized shares.
On July 26, 2022, the Board of Directors
of the Company approved a 7-for-6 forward stock split, effective for trading purposes as of August 12, 2022, pursuant to which each shareholder
as of the August 15, 2022 record date received one (1) additional share for each six (6) shares held as of the record date. Pursuant
to the operation of the amendment providing for the forward stock split filed with the Secretary of State of Nevada on August 4, 2022,
the authorized common stock of the Company was increased from 250,000,000 shares to 291,666,666 shares in connection with the forward
split.
Activity during
the Year Ended January 31, 2024
| (a) | As
of January 31, 2024, the Company holds 10,000 of its shares comprising $32,641 of treasury
stock. There was no activity during the year ended January 31, 2024. |
| | | |
| (b) | In
December 2024, TII Jet Services LDA converted $2,000,000 of its outstanding credit facility
and $53,436 of accrued interest into 1,026,720 shares of the Company’s common stock.
The fair value of the common stock at the date of issuance was $2,554,423, resulting in a
$554,423 loss on extinguishment. |
Activity during the Year Ended January
31, 2023
| (a) | In
March and May 2022, the Company purchased 35,584 shares of its common stock for $119,006
and recorded the purchase as Treasury Stock. In May and December 2022, the Company issued
33,397 shares of stock awards to management, directors and employees from the treasury shares
and recorded compensation expense of $113,155 In December 2022, the Company issued 25,000
shares from the treasury shares to non-employees in connection of the termination of the
Rambam license agreement. As of January 31, 2023, the Company held 10,000 of its shares comprising
$32,641 of treasury stock. |
| | | |
| (b) | On
July 29, 2022, the Company received proceeds of $296,875 from the exercise of warrants and
issued 55,417 shares of common stock. |
| | | |
| (c) | In
July 2022, the Company cancelled 1,400,000 shares received in connection with the settlement
of a lawsuit. See Note 11 for further information. |
Warrants
The following table summarizes the
changes in warrants outstanding and the related price of the shares of the Company’s common stock issued to non-employees of the
Company during the year ended January 31, 2024. On March 7, 2023, the Company issued 30,000 warrants to purchase the Company’s
common shares to Barandnic Holdings Ltd. for services provided. The warrants are exercisable at a price of $4.00 per share and expire
five years from the date of issuance. On October 27, 2023, the Company issued 145,833 warrants to purchase the Company’s common
shares to management (87,500 warrants were issued to the Chief Financial Officer) and non-employees of the Company. The warrants are
exercisable at a price of $1.93 per share and expire in three years from the date of issuance. These warrants replace previously issued
warrants that have now been cancelled. The Company used the Black-Scholes valuation model to record the fair value. The valuation model
used a dividend rate of 0%; expected term of 1.5 years; volatility rates of 152.10-174.45%; and a risk-free rate of 4.31%-4.84%. Non-cash
compensation for the year ended January 31, 2024, amounted to $242,840.
| | |
| | |
Exercise | | |
Remaining | | |
Intrinsic | |
| | |
Shares | | |
Price | | |
Life | | |
Value | |
| Outstanding, January 31, 2022 | |
| 1,435,622 | | |
$ | 6.91 | | |
| 3.93
years | | |
$ | - | |
| | |
| | | |
| | | |
| | | |
| | |
| Granted | |
| 25,000 | | |
| 7.50 | | |
| 5.00
years | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | |
| Expired/Cancelled | |
| (97,534 | ) | |
| 5.36 | | |
| - | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | |
| Exercised | |
| (55,417 | ) | |
| 5.36 | | |
| - | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | |
| Outstanding, January 31, 2023 | |
| 1,307,671 | | |
| 6.43 | | |
| 3.34
years | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | |
| Granted | |
| 175,833 | | |
| 2.28 | | |
| 2.97
years | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | |
| Expired/Cancelled | |
| (200,466 | ) | |
| 6.33 | | |
| - | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | |
| Exercised | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | |
| Outstanding - January 31, 2024 | |
| 1,283,038 | | |
$ | 5.88 | | |
| 2.97
years | | |
$ | 99,166 | |
| | |
| | | |
| | | |
| | | |
| | |
| Exercisable - January 31, 2024 | |
| 1,283,038 | | |
$ | 5.88 | | |
| 2.97
years | | |
$ | 99,166 | |
The following
table summarizes additional information relating to the warrants outstanding as of January 31, 2024:
Range of
Exercise
Prices | | |
Number
Outstanding | | |
Weighted
Average
Remaining
Contractual
Life(Years) | | |
Weighted
Average
Exercise Price for
Shares
Outstanding | | |
Number
Exercisable | | |
Weighted Average
Exercise Price for
Shares
Exercisable | | |
Intrinsic
Value | |
| $ | 4.00 | | |
| 30,000 | | |
| 4.10 | | |
$ | 4.00 | | |
| 30,000 | | |
$ | 4.00 | | |
$ | - | |
| $ | 6.43 | | |
| 1,082,205 | | |
| 2.68 | | |
$ | 6.43 | | |
| 1,082,205 | | |
$ | 6.43 | | |
$ | - | |
| $ | 1.93 | | |
| 145,833 | | |
| 2.74 | | |
$ | 1.93 | | |
| 145,833 | | |
$ | 1.93 | | |
$ | 99,166 | |
| $ | 7.50 | | |
| 25,000 | | |
| 3.77 | | |
$ | 7.50 | | |
| 25,000 | | |
$ | 7.50 | | |
$ | - | |
Options
The following table summarizes the
changes in options outstanding and the related price of the shares of the Company’s common stock issued to employees of the Company.
See Note 7 for the issuance of related party options.
On November 1, 2021, the Board of Directors
adopted the 2021 Employee Stock Option Plan (the “Plan”). The Company has reserved 408,333 shares for issuance and sale upon
the exercise of stock options. In accordance with the Plan, on February 1, 2022, the Company reserved an additional 233,333 shares and
on February 1, 2023, the Company reserved an additional 233,333 shares. The options vest immediately and expire in three years. Under
the Plan, options may be granted which are intended to qualify as Incentive Stock Options (“ISO’s”) under Section 422
of the Internal Revenue Code of 1986 (the “Code”) or which are not (“non-ISO’s”) intended to qualify as
Incentive Stock Options thereunder. The Plan also provides for restricted stock awards representing shares of common stock that are issued
subject to such restrictions on transfer and other incidents of ownership and such forfeiture conditions as the Board of Directors, or
the committee administering the Plan composed of directors who qualify as “independent” under Nasdaq rules, may determine.
On November 3, 2021, the Company filed a Registration Statement on Form S-8, to register under the Securities Act of 1933, as amended
the 408,333 shares of common stock reserved for issuance under the Plan. As of January 31, 2024, 166 shares remain available and issuance
under the Plan.
During the year ended January 31, 2024,
404,500 options to purchase shares of the Company’s common stock were issued to executive officers and employees at prices of $1.93-$3.975
per share. The options vest immediately and expire three years from the date of issuance. The fair value of the options issued for services
amounted to $499,856 and was recorded during the year ended January 31, 2024. The Company used the Black-Scholes valuation model to record
the fair value. The valuation model used a dividend rate of 0%; expected term of 1.5 years; volatility rates of 121.52-143.54%; and a
risk-free rate of 3.00-4.5%.
During the year ended January 31, 2023,
279,584 options to purchase shares of the Company’s common stock were issued to executive officers and directors of the Company
at prices of $3.59 to $4.50 per share. The options vest immediately and expire three years from the date of issuance. The fair value
of the options issued for services amounted to $732,130 and was recorded during the year ended January 31, 2023. The Company used the
Black-Scholes valuation model to record the fair value. The valuation model used a dividend rate of 0%; expected term of 1.5 years; volatility
rate of 152.10-174.45%; and a risk-free rate of 3%.
The following table summarizes additional
information relating to the options outstanding as of January 31, 2024.
| | |
Shares | | |
Exercise
Price | | |
Remaining
Life | | |
Intrinsic
Value | |
| Outstanding, January 31, 2022 | |
| 190,751 | | |
$ | 4.26 | | |
| 2.97
years | | |
| | |
| | |
| | | |
| | | |
| | | |
| | |
| Granted | |
| 279,584 | | |
| 3.93 | | |
| 3.00
years | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | |
| Expired/Cancelled | |
| - | | |
| - | | |
| - | | |
| | |
| | |
| | | |
| | | |
| | | |
| | |
| Exercised | |
| - | | |
| - | | |
| - | | |
| | |
| | |
| | | |
| | | |
| | | |
| | |
| Outstanding, January 31, 2023 | |
| 470,335 | | |
| 4.13 | | |
| 2.53
years | | |
| | |
| | |
| | | |
| | | |
| | | |
| | |
| Granted | |
| 404,500 | | |
| 2.18 | | |
| 2.68
years | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | |
| Expired/Cancelled | |
| - | | |
| - | | |
| - | | |
| | |
| | |
| | | |
| | | |
| | | |
| | |
| Exercised | |
| - | | |
| - | | |
| - | | |
| | |
| | |
| | | |
| | | |
| | | |
| | |
| Outstanding- January 31, 2024 | |
| 874,835 | | |
$ | 3.23 | | |
| 2.31
years | | |
$ | 214,460 | |
| | |
| | | |
| | | |
| | | |
| | |
| Exercisable - January 31, 2024 | |
| 874,835 | | |
$ | 3.23 | | |
| 2.31
years | | |
$ | 214,460 | |
The following table summarizes additional
information relating to the options outstanding as of January 31, 2024:
| Prices | | |
Outstanding | | |
Life(Years) | | |
Shares
Outstanding | | |
Exercisable | | |
Shares
Exercisable | | |
Value | |
| | | |
| | |
| | |
| | |
| | |
| | |
| |
| $ | 1.93 | | |
| 214,500 | | |
| 2.74 | | |
$ | 1.93 | | |
| 214,500 | | |
$ | 1.93 | | |
$ | 145,860 | |
| $ | 2.12 | | |
| 140,000 | | |
| 2.74 | | |
$ | 2.12 | | |
| 140,000 | | |
$ | 2.12 | | |
$ | 68,600 | |
| $ | 2.65 | | |
| 20,000 | | |
| 2.63 | | |
$ | 2.65 | | |
| 20,000 | | |
$ | 2.65 | | |
$ | - | |
| $ | 3.59 | | |
| 35,000 | | |
| 3.67 | | |
$ | 3.59 | | |
| 35,000 | | |
$ | 3.59 | | |
$ | - | |
| $ | 3.75 | | |
| 57,500 | | |
| 1.85 | | |
$ | 3.75 | | |
| 57,500 | | |
$ | 3.75 | | |
$ | - | |
| $ | 3.98 | | |
| 30,000 | | |
| 2.01 | | |
$ | 3.98 | | |
| 30,000 | | |
$ | 3.98 | | |
$ | - | |
| $ | 4.09 | | |
| 78,750 | | |
| 1.50 | | |
$ | 4.09 | | |
| 78,750 | | |
$ | 4.09 | | |
$ | - | |
| $ | 4.12 | | |
| 50,000 | | |
| 1.85 | | |
$ | 4.12 | | |
| 50,000 | | |
$ | 4.12 | | |
$ | - | |
| $ | 4.16 | | |
| 144,083 | | |
| 0.97 | | |
$ | 4.16 | | |
| 144,083 | | |
$ | 4.16 | | |
$ | - | |
| $ | 4.50 | | |
| 58,334 | | |
| 1.50 | | |
$ | 4.50 | | |
| 58,334 | | |
$ | 4.50 | | |
$ | - | |
| $ | 4.58 | | |
| 46,668 | | |
| 0.97 | | |
$ | 4.58 | | |
| 46,668 | | |
$ | 4.58 | | |
$ | - | |
| | | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| | | | |
| 874,835 | | |
| 2.06 | | |
$ | 3.23 | | |
| 874,835 | | |
$ | 3.23 | | |
$ | 214,460 | |
We organize and manage our business
by the following two segments which meet the definition of reportable segments under ASC280-10, Segment Reporting: Sales of Goods and
Services. These segments are based on the type of products or services provided and are the same as our business units. Separate financial
information is available and regularly reviewed by our chief officer decision maker, in making resource allocation decisions for our
segments. Our chief officer decision maker evaluates segment performance to the GAAP measure of gross profit.
| | |
Years
Ended January 31, | |
| | |
2024 | | |
2023 | |
| Net sales | |
| | |
| |
| Pocono Pharmaceuticals | |
$ | 1,920,280 | | |
$ | 1,785,597 | |
| 4P Therapeutics | |
| 165,034 | | |
| 294,102 | |
| | |
| 2,085,314 | | |
| 2,079,699 | |
| Gross profit | |
| | | |
| | |
| Pocono Pharmaceuticals | |
| 744,391 | | |
| 726,702 | |
| 4P Therapeutics | |
| 117,714 | | |
| 23,702 | |
| | |
| 862,105 | | |
| 750,404 | |
| Operating expenses | |
| | | |
| | |
| Selling, general and administrative-Pocono
Pharmaceuticals | |
| 606,275 | | |
| 577,930 | |
| Selling, general and administrative-4P
Therapeutics | |
| 236,953 | | |
| 103,181 | |
| Selling, general and administrative-Corporate | |
| 2,930,378 | | |
| 3,234,930 | |
| Research and development-4P Therapeutics | |
| 1,960,425 | | |
| 982,227 | |
| Goodwill impairment-Pocono
Pharmacueticals | |
| - | | |
| 327,326 | |
| | |
| 5,734,031 | | |
| 5,225,594 | |
| Depreciation and Amortization | |
| | | |
| | |
| Pocono Pharmaceuticals | |
$ | 222,159 | | |
$ | 264,156 | |
| Corporate | |
| 13,986 | | |
| - | |
| 4P Therapeutics | |
| 51,577 | | |
| 65,987 | |
| | |
$ | 287,722 | | |
$ | 330,143 | |
The following table presents information
about net sales and property and equipment, net of accumulated depreciation, in the United States and elsewhere.
| | |
Years Ended
January 31, | |
| | |
2024 | | |
2023 | |
| Net sales | |
| | |
| |
| United States | |
$ | 2,085,314 | | |
$ | 2,079,699 | |
| Outside the
United States | |
| - | | |
| - | |
| | |
$ | 2,085,314 | | |
$ | 2,079,699 | |
| | |
| January
31, | | |
| January
31, | |
| | |
| 2024 | | |
| 2023 | |
| Property and equipment, net of accumulated depreciation | |
| | | |
| | |
| United States | |
$ | 774,924 | | |
$ | 897,735 | |
| Outside the
United States | |
| - | | |
| - | |
| | |
$ | 774,924 | | |
$ | 897,735 | |
| Assets | |
| | | |
| | |
| Corporate | |
$ | 344,192 | | |
$ | 1,745,731 | |
| Pocono Pharmaceuticals | |
| 5,079,293 | | |
| 5,400,814 | |
| 4P Therapeutics | |
| 2,093,369 | | |
| 2,309,832 | |
| | |
$ | 7,516,854 | | |
$ | 9,456,377 | |
| 11. | COMMITMENTS
AND CONTIGENCIES |
Employment
Agreements
The Company entered into three-year
employment agreements with Gareth Sheridan, our CEO, and Serguei Melnik, our President, effective February 1, 2022. The agreement also
provides that the executives will continue as directors and officers of the Company for the respective terms thereof. The agreement provides
for an initial term, commencing on the effective date of the agreement and ending on January 31, 2025, and continuing on a year-to-year
basis thereafter unless terminated by either party on not less than 30 days’ notice given prior to the expiration of the initial
term or any one-year extension. For their services to the Company during the term of the agreement, Mr. Sheridan and Mr. Melnik will
receive an annual salary of $250,000 per annum, commencing on the effective date of the agreement. Mr. Sheridan and Mr. Melnik will also
receive a performance bonus of 3.5% of net income before income taxes. As of July 31, 2022, the Company and Mr. Sheridan and Mr. Melnik
mutually agreed to reduce their annual salary to $150,000.
The Company entered into a three-year
employment agreement with Gerald Goodman, our CFO, effective February 1, 2022. The agreement provides for an initial term, commencing
on the effective date of the agreement and ending on January 31, 2025, and continuing on a year-to-year basis thereafter unless terminated
by either party on not less than 30 days’ notice given prior to the expiration of the initial term or any one-year extension. For
his services to the Company during the term of the agreement, Mr. Goodman will receive an annual salary of $210,000 per annum, commencing
on the effective date of the agreement. As of July 31, 2022, the Company and Mr. Goodman mutually agreed to reduce his annual salary
to $110,000.
Kindeva Drug Delivery
Agreement
On January 4, 2022, the Company signed
a feasibility agreement with Kindeva Drug Delivery, L.P. (“Kindeva”) to develop Nutriband’s lead product, AVERSA Fentanyl,
based on its proprietary AVERSA abuse deterrent transdermal technology and Kindeva’s FDA-approved transdermal fentanyl patch (fentanyl
transdermal system). The feasibility agreement provides for adapting Kindeva’s commercial transdermal manufacturing process to
incorporate AVERSA technology in the fentanyl transdermal system.
The agreement will remain in force
until the earlier of: (1) the completion of the work and deliverables under the Workplan; or (2) two (2) years after the Effective Date,
after which time the agreement will expire. The feasibility Workplan was completed in February 2024.
The estimated cost to complete the
feasibility Workplan was approximately $2.5 million. Nutriband made an advance deposit of $250,000 in January 2022, to be applied against
the final invoices. As of January 31, 2024, Nutriband has incurred expenses of $2,369,508 and the net deposit of $138,250 after application
to final invoices is included in prepaid expenses.
In January 2024, Nutriband signed a
commercial development and clinical supply agreement with Kindeva Drug Delivery for development of AVERSA Fentanyl using Kindeva’s
FDA-approved fentanyl patch. Kindeva will perform commercial manufacturing process development and clinical supplies manufacturing for
the human abuse potential clinical study required by the FDA in support of a New Drug Application. The agreement replaces the previous
feasibility agreement between the two companies which was focused on adapting Kindeva’s commercial transdermal manufacturing process
to incorporate AVERSA abuse deterrent transdermal technology. The estimated cost to complete the commercial process development and clinical
supplies manufacturing is approximately $8.1 million and the expected timing of FDA submission is twelve to eighteen months.
Lease Agreement
On February 1, 2022, Pocono Pharmaceuticals
entered into a lease agreement with Geometric Group, LLC for 12,000 square feet of warehouse space currently occupied by Active Intelligence.
The monthly rental is $3,000 and the lease expires on January 31, 2025. The lease can be extended for an additional three years at the
same monthly rental. The Company recorded a Right of Use asset in the amount of $94,134 in connection with the valuation.
MDM Worldwide Agreement
In September 2022, the Company entered
into a public relations agreement with MDM Worldwide. In connection with the agreement, the Company agreed to issue 20,000 options to
MDM Worldwide. In October 2023, the contract was mutually terminated, and no options were issued. For the year ended January 31, 2024,
the Company paid MDM Worldwide $190,000.
Money Channel Agreement
On March 13, 2023, the Company entered
into a media advertising agreement with Money Channel Inc. The Company will pay a monthly fee and after ninety days can cancel the agreement.
The Company, after 90 days, will also issue options to purchase 50,000 shares of common stock to Money Channel Inc. at an exercise price
of $4.00 per share. In June 2023, the parties agreed to terminate the agreement by mutual consent. No options were issued. For the year
ended January 31, 2024, the Company paid the Money Channel $100,000.
Sorrento Therapeutics, Inc.
Agreement
4P Therapeutics had unpaid account
receivables related to a contract clinical research services agreement in place with Sorrento Therapeutics. On February 13, 2023, Sorrento
declared Chapter 11 bankruptcy. On July 25, 2023, 4P Therapeutics assigned its claim under the bankruptcy proceedings from Sorrento Therapeutics
Inc. and received proceeds of $106,528. The amount due under the claim was $118,675 and 4P Therapeutics recorded a reserve for bad debts
of $118,675 during the year ended January 31, 2024. Under the agreement with the buyer of the claim, 4P Therapeutics will make proportional
restitution and/or repayment of the purchase amount to the extent the claim is disallowed, reduced or not paid at the same time or distribution
rate as other general unsecured claims against the Debtor are paid. The Company has recorded the amount of the proceeds as a secured
loan payable to the factor as of January 31, 2024.
Upstream Termination
On May 24, 2023, the Company sent
notice of the termination of the Securities Facility Services Agreement, dated January 3, 2023, by and between MERJ DEP Ltd. And the
Company (“Agreement”), which provided for the dual listing of the Company’s common stock on the MERJ Upstream exchange
(“Upstream”), which is operated as a fully registered and licensed integrated securities exchange, clearing system and depository
for digital and non-digital securities under the Seychelles security laws. The termination was effective May 31, 2023.
Legal Proceedings
The Company is currently a defendant
in a lawsuit initiated by Joseph Gunnar, LLC (“Gunnar”) and Lucosky Brookman LLP (“LB”) in the Supreme Court
of the State of New York, New York County, under Index No.654633/2023. The lawsuit alleges multiple allegations such as breach of contract,
fraudulent activities, and tortious interference and seeks damages following the Company’s termination of an engagement letter
for assistance with a public stock offering. Gunnar is seeking over $500,000 in damages plus punitive damages, while LB is demanding
reimbursement of legal fees.
In response, the Company denies all
allegations, alleging that the engagement letter was unenforceable, and its termination was legally justified. The Company has also initiated
counterclaims against Joseph Gunnar & Co., accusing them of intentional interference and breach of fiduciary duty, and is seeking
$1,000,000 for each claim along with a declaratory judgment affirming the legality and justification of the termination. The plaintiffs
have denied these counterclaims.
Currently, there are no pending hearings
or motions as both parties are engaged in discovery and are attempting to resolve the matter amicably.
| (a) | In
February and April 2024, the Company received proceeds of $300,000 from its Credit Line Facility. |
| | | |
| (b) | On
March 20, 2024, 390,000 options to purchase shares of the Company’s common stock were
issued to executive officers and employees at prices of $2.37-$2.61 per share. The options
vest immediately and expire three years from the date of issuance. The fair value of the
options issued amounted to $450,000. |
| | | |
| (c) | On
March 20, 2024, our Board of Directors adopted an amendment to the Company’s Employee
Stock Option Plan (the “Plan”) increasing the number of shares of common stock
subject to the Plan (as of March 20, 2024, 875,000 shares) to 1,400,000 shares (the “Amendment”).
The Company will submit the Amendment to the Plan to our stockholders for adoption and approval
at the 2025 Annual Meeting. If the Amendment is not approved by stockholders within one year
of adoption, the increase in shares subject to the Plan will be void, together with any options
issued following March 20,2024, in the period pending approval of the Plan by our stockholders. |
| | | |
| (d) | On
April 19, 2024, the Company completed an $8,400,000 equity financing with European investors
(the “Offering”) of 2,100,000 units (“Units”), at a price of $4.00
per Unit, each Unit consisting of one share of common stock (“Shares”) and a
Warrant to purchase two Shares of common stock, the Warrants having an initial exercise price
of $6.43, are exercisable by payment of the exercise price in cash only and expire April
19,2029, five years from the date of issuance (“Warrants”). The Offering was
made solely to investors residing outside the United States and was not registered under
the Security Act of 1933, as amended, (the “Security Act”), or the securities
law of any jurisdiction, including outside the United States, but was made privately by the
Company pursuant to the exemptions from registration provided in the SEC’s Regulation
S and other exemptions under the Securities Act. |
Unaudited Financial Statements for the Three
and Six Months Ended April 30, 2024
NUTRIBAND INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED BALANCE SHEETS
| | |
April 30, | | |
January 31, | |
| | |
2024 | | |
2024 | |
| | |
(Unaudited) | | |
| |
| ASSETS | |
| | |
| |
| | |
| | |
| |
| CURRENT ASSETS: | |
| | |
| |
| Cash
and cash equivalents | |
$ | 8,347,740 | | |
$ | 492,942 | |
| Accounts receivable | |
| 85,144 | | |
| 148,649 | |
| Inventory | |
| 168,505 | | |
| 168,605 | |
| Prepaid
expenses | |
| 128,551 | | |
| 211,667 | |
| Total
Current Assets | |
| 8,729,940 | | |
| 1,021,863 | |
| | |
| | | |
| | |
| PROPERTY
& EQUIPMENT-net | |
| 740,305 | | |
| 774,924 | |
| | |
| | | |
| | |
| OTHER ASSETS: | |
| | | |
| | |
| Goodwill | |
| 5,021,713 | | |
| 5,021,713 | |
| Operating lease
right of use asset | |
| 23,529 | | |
| 31,374 | |
| Intangible
assets-net | |
| 638,993 | | |
| 667,280 | |
| | |
| | | |
| | |
| TOTAL
ASSETS | |
$ | 15,154,480 | | |
$ | 7,517,154 | |
| | |
| | | |
| | |
| LIABILITIES
AND STOCKHOLDERS’ EQUITY | |
| | | |
| | |
| | |
| | | |
| | |
| CURRENT LIABILITIES: | |
| | | |
| | |
| Accounts payable
and accrued expenses | |
$ | 994,106 | | |
$ | 680,132 | |
| Deferred revenue | |
| 269,345 | | |
| 157,502 | |
| Operating lease
liability-current portion | |
| 25,988 | | |
| 34,276 | |
| Notes
payable-current portion | |
| 127,419 | | |
| 127,183 | |
| Total
Current Liabilities | |
| 1,416,858 | | |
| 999,093 | |
| | |
| | | |
| | |
| LONG-TERM LIABILITIES: | |
| | | |
| | |
| Note payable-net
of current portion | |
| 74,509 | | |
| 79,826 | |
| Note
payable-related party | |
| 300,000 | | |
| - | |
| Total
Liabilities | |
| 1,791,367 | | |
| 1,078,919 | |
| | |
| | | |
| | |
| Commitments
and Contingencies | |
| - | | |
| - | |
| | |
| | | |
| | |
| STOCKHOLDERS’
EQUITY: | |
| | | |
| | |
| Preferred stock, $.001 par value,
10,000,000 shares authorized, -0- outstanding | |
| - | | |
| - | |
| Common stock, $.001 par value,
291,666,666 shares authorized; 10,969,870 and 8,869,870 shares issued at April 30, 2024 and January 31,2024, respectively, 10,959,870
and 8,859,870 shares outstanding as of April 30, 2024 and January 31, 2024, respectively | |
| 10,960 | | |
| 8,860 | |
| Additional paid-in-capital | |
| 43,263,194 | | |
| 34,442,339 | |
| Accumulated other
comprehensive loss | |
| (304 | ) | |
| (304 | ) |
| Treasury stock,
10,000 and 10,000 shares at cost, respectively | |
| (32,641 | ) | |
| (32,641 | ) |
| Accumulated
deficit | |
| (29,878,096 | ) | |
| (27,980,019 | ) |
| Total
Stockholders’ Equity | |
| 13,363,113 | | |
| 6,438,235 | |
| | |
| | | |
| | |
| TOTAL
LIABILITIES AND STOCKHOLDERS’ EQUITY | |
$ | 15,154,480 | | |
$ | 7,517,154 | |
See notes to unaudited consolidated financial
statements
NUTRIBAND INC. AND SUBSIDIARIES
UNAUDITED CONSOLIDATED STATEMENTS OF OPERATIONS
| | |
For the Three Months Ended | |
| | |
April 30, | |
| | |
2024 | | |
2023 | |
| | |
| | |
| |
| Revenue | |
$ | 408,532 | | |
$ | 476,932 | |
| | |
| | | |
| | |
| Costs and expenses: | |
| | | |
| | |
| Cost of revenues | |
| 243,746 | | |
| 254,648 | |
| Research and development | |
| 974,535 | | |
| 400,430 | |
| Selling, general
and administrative | |
| 1,079,728 | | |
| 839,732 | |
| Total Costs and
Expenses | |
| 2,298,009 | | |
| 1,494,810 | |
| | |
| | | |
| | |
| Loss from operations | |
| (1,889,477 | ) | |
| (1,017,878 | ) |
| | |
| | | |
| | |
| Other income (expense): | |
| | | |
| | |
| Interest income | |
| 18 | | |
| 5,815 | |
| Interest expense | |
| (8,618 | ) | |
| (3,166 | ) |
| Total other income
(expense) | |
| (8,600 | ) | |
| 2,649 | |
| | |
| | | |
| | |
| Loss before provision for income taxes | |
| (1,898,077 | ) | |
| (1,015,229 | ) |
| | |
| | | |
| | |
| Provision for income taxes | |
| - | | |
| - | |
| | |
| | | |
| | |
| Net loss | |
$ | (1,898,077 | ) | |
$ | (1,015,229 | ) |
| | |
| | | |
| | |
| Net loss per share of common stock-basic
and diluted | |
$ | (0.21 | ) | |
$ | (0.13 | ) |
| | |
| | | |
| | |
| Weighted average shares of common stock outstanding | |
| | | |
| | |
| - basic and diluted | |
| 9,159,869 | | |
| 7,833,150 | |
See notes to unaudited consolidated financial
statements
NUTRIBAND INC. AND SUBSIDIARIES
UNAUDITED CONSOLIDATED STATEMENTS OF STOCKHOLDERS’
EQUITY
Three Months Ended April 30, 2024
| | |
| | |
| | |
| | |
| | |
Accumulated | | |
| | |
| |
| | |
| | |
Common Stock | | |
Additional | | |
Other | | |
| | |
| |
| | |
| | |
Number of | | |
| | |
Paid In | | |
Comprehensive | | |
Accumulated | | |
Treasury | |
| | |
Total | | |
shares | | |
Amount | | |
Capital | | |
Income(Loss) | | |
Deficit | | |
Stock | |
| Balance, February
1, 2024 | |
$ | 6,438,235 | | |
| 8,859,870 | | |
$ | 8,860 | | |
$ | 34,442,339 | | |
$ | (304 | ) | |
$ | (27,980,019 | ) | |
$ | (32,641 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Options issued
for services | |
| 422,955 | | |
| - | | |
| - | | |
| 422,955 | | |
| - | | |
| - | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Proceeds from
sale of common stock and warrants | |
| 8,400,000 | | |
| 2,100,000 | | |
| 2,100 | | |
| 8,397,900 | | |
| - | | |
| - | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net loss
for the three months ended April 30, 2024 | |
| (1,898,077 | ) | |
| - | | |
| - | | |
| - | | |
| - | | |
| (1,898,077 | ) | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance,
April 30, 2024 | |
$ | 13,363,113 | | |
| 10,959,870 | | |
$ | 10,960 | | |
$ | 43,263,194 | | |
$ | (304 | ) | |
$ | (29,878,096 | ) | |
$ | (32,641 | ) |
Three Months Ended April 30, 2023
| | |
| | |
| | |
| | |
| | |
Accumulated | | |
| | |
| |
| | |
| | |
Common Stock | | |
Additional | | |
Other | | |
| | |
| |
| | |
| | |
Number of | | |
| | |
Paid In | | |
Comprehensive | | |
Accumulated | | |
Treasury | |
| | |
Total | | |
shares | | |
Amount | | |
Capital | | |
Income(Loss) | | |
Deficit | | |
Stock | |
| Balance, February
1, 2023 | |
$ | 8,572,990 | | |
| 7,833,150 | | |
$ | 7,833 | | |
$ | 31,092,807 | | |
$ | (304 | ) | |
$ | (22,494,705 | ) | |
$ | (32,641 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Warrants issued
for services | |
| 87,090 | | |
| - | | |
| - | | |
| 87,090 | | |
| - | | |
| - | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Options issued
for services | |
| 75,030 | | |
| - | | |
| - | | |
| 75,030 | | |
| - | | |
| - | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net loss
for the three months ended April 31, 2023 | |
| (1,015,229 | ) | |
| - | | |
| - | | |
| - | | |
| - | | |
| (1,015,229 | ) | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance,
April 30, 2023 | |
$ | 7,719,881 | | |
| 7,833,150 | | |
$ | 7,833 | | |
$ | 31,254,927 | | |
$ | (304 | ) | |
$ | (23,509,934 | ) | |
$ | (32,641 | ) |
See notes to unaudited consolidated financial
statements
NUTRIBAND INC. AND SUBSIDIARIES
UNAUDITED CONSOLIDATED STATEMENTS OF CASH FLOWS
| | |
For the Three Months Ended | |
| | |
April 30, | |
| | |
2024 | | |
2023 | |
| Cash flows from operating activities: | |
| | |
| |
| Net loss | |
$ | (1,898,077 | ) | |
$ | (1,015,229 | ) |
| Adjustments to reconcile net loss to net cash used in operating
activities: | |
| | | |
| | |
| Depreciation and amortization | |
| 69,101 | | |
| 75,201 | |
| Operating lease expense | |
| 7,845 | | |
| 7,845 | |
| Stock-based compensation-warrants | |
| - | | |
| 87,090 | |
| Stock-based compensation-options | |
| 422,955 | | |
| 75,030 | |
| Changes in operating assets and liabilities: | |
| | | |
| | |
| Accounts receivable | |
| 63,505 | | |
| (51,596 | ) |
| Prepaid expenses | |
| 84,631 | | |
| (3,354 | ) |
| Inventories | |
| (1,415 | ) | |
| 47,838 | |
| Deferred revenue | |
| 111,843 | | |
| 25,794 | |
| Operating lease liability | |
| (8,288 | ) | |
| (7,557 | ) |
| Accounts payable
and accrued expenses | |
| 313,974 | | |
| 9,074 | |
| Net Cash Used
In Operating Activities | |
| (833,926 | ) | |
| (749,864 | ) |
| | |
| | | |
| | |
| Cash flows from investing activities: | |
| | | |
| | |
| Purchase of equipment | |
| (6,195 | ) | |
| (2,624 | ) |
| Net Cash Used
in Investing Activities | |
| (6,195 | ) | |
| (2,624 | ) |
| | |
| | | |
| | |
| Cash flows from financing activities: | |
| | | |
| | |
| Proceeds from note payable-related
party | |
| 300,000 | | |
| 50,000 | |
| Proceeds from sale of common stock
and warrants | |
| 8,400,000 | | |
| - | |
| Payment on note
payable | |
| (5,081 | ) | |
| (4,877 | ) |
| Net Cash Provided
by Financing Activities | |
| 8,694,919 | | |
| 45,123 | |
| | |
| | | |
| | |
| Net change in cash | |
| 7,854,798 | | |
| (707,365 | ) |
| | |
| | | |
| | |
| Cash and cash equivalents - Beginning
of period | |
| 492,942 | | |
| 1,985,440 | |
| | |
| | | |
| | |
| Cash and cash equivalents - End of period | |
$ | 8,347,740 | | |
$ | 1,278,075 | |
| | |
| | | |
| | |
| Supplementary information: | |
| | | |
| | |
| | |
| | | |
| | |
| Cash paid for: | |
| | | |
| | |
| Interest | |
$ | 611 | | |
$ | 1,725 | |
| | |
| | | |
| | |
| Income taxes | |
$ | - | | |
$ | - | |
See notes to unaudited consolidated financial
statements
NUTRIBAND INC. AND SUBSIDIARIES
Notes to Unaudited Consolidated Financial Statements
as of and for the Three Months Ended April 30,
2024 and 2023
| 1. | ORGANIZATION
AND DESCRIPTION OF BUSINESS |
Organization
Nutriband Inc. (the “Company”)
is a Nevada corporation, incorporated on January 4, 2016. In January 2016, the Company acquired Nutriband Ltd, an Irish company which
was formed by the Company’s chief executive officer in 2012 to enter the health and wellness market by marketing transdermal patches.
References to the Company relate to the Company and its subsidiaries unless the context indicates otherwise.
On August 1, 2018, the Company acquired
4P Therapeutics LLC (“4P Therapeutics”) for $2,250,000, consisting of 250,000 shares of common stock, valued at $1,850,000,
and $400,000, and a royalty of 6% on all revenue generated by the Company from the abuse deterrent intellectual property that had been
developed by 4P Therapeutics payable to the former owner of 4P Therapeutics. The former owner of 4P Therapeutics has been a director
of the Company since April 2018, when the Company entered into an agreement to acquire 4P Therapeutics. The former owner resigned as
a director in January 2022.
4P Therapeutics is engaged in the
development of a series of transdermal pharmaceutical products, that are in the preclinical stage of development. Prior to the acquisition
of 4P Therapeutics, the Company’s business was the development and marketing of a range of transdermal consumer patches. Most of
these products are considered drugs in the United States and cannot be marketed in the United States without approval by the Food and
Drug Administration (the “FDA”). The Company entered a feasibility agreement as an initial step to seek FDA approval of its
consumer transdermal products and its consumer products which are not being marketed in the United States.
With the acquisition of 4P Therapeutics,
4P Therapeutics’ drug development business became the Company’s principal business. The Company’s approach is to use
generic drugs that are off patent and incorporate them into the Company’s transdermal drug delivery system. Although these medications
have received FDA approval in oral or injectable form, the Company needs to conduct a transdermal product development program which will
include the preclinical and clinical trials that are necessary to receive FDA approval before we can market any of our pharmaceutical
products.
On August 25, 2020, the Company formed
Pocono Pharmaceuticals Inc. (“Pocono Pharmaceuticals”), a wholly owned subsidiary of the Company. On August 31, 2020, the
Company acquired certain assets and liabilities associated with the Transdermal, Topical, Cosmetic, and Nutraceutical business of Pocono
Coated Products LLC (“PCP”). The net assets were contributed to Pocono Pharmaceuticals. Included in the transaction, Pocono
Pharmaceuticals also acquired 100% of the membership interests of Active Intelligence LLC (“Active Intelligence”).
Pocono Pharmaceuticals is a coated
products manufacturing entity organized to take advantage of its unique process capabilities and experience. Pocono helps their customers
with product design and development along with manufacturing to bring new products to market with minimal capital investment. Pocono
Pharmaceutical’s competitive edge is a low-cost manufacturing base: a result of its unique processes and state-of-the-art material
technology. Active Intelligence manufactures activated kinesiology tape. The tape has transdermal and topical properties. This tape is
used the same as traditional kinesiology tape.
| 2. | SUMMARY
OF SIGNIFICANT ACCOUNTING POLICIES |
Unaudited Financial Statements
The consolidated balance sheet as
of April 30, 2024, and the consolidated statements of operations, stockholders’ equity, and cash flows for the periods presented
have been prepared by the Company and are unaudited. In the opinion of management, all adjustments (consisting solely of normal recurring
adjustments) necessary to prepare fairly the financial position, results of operations, changes in stockholders’ equity and cash
flows for all periods presented have been made. The results for the three months ended April 30, 2024, are not necessarily indicative
of the results to be expected for the full year. The consolidated financial statements should be read in conjunction with the consolidated
financial statements and footnotes thereto included in Nutriband’s Annual Report on Form 10-K for the year ended January 31, 2024.
Certain information and footnote disclosures
required under generally accepted accounting principles in the United States of America (U.S. GAAP”) have been condensed or omitted
from these consolidated financial statements pursuant to the rules and regulations, including interim reporting requirements of the U.S.
Securities and Exchange Commission (“SEC”). The preparation of consolidated financial statements in accordance with U.S.
GAAP requires management to make estimates and assumptions that affect the reported amounts and the disclosures of contingent amounts
in our consolidated financial statements and accompanying footnotes. Actual results could differ from estimates.
The Company’s significant accounting
policies in Note 2 in the Company’s Annual Report on Form 10-K for the year ended January 31, 2024. There were no significant changes
to these accounting policies during the three months ended April 30, 2024.
Forward Stock Split
On July 26, 2022, our Board of Directors
approved the amendment to our Articles of Incorporation to effect a 7- for- 6 forward stock split (the “Stock Split”) of
our outstanding common stock. The Company filed the amendment set forth in a Certificate of Change with the Secretary of State of Nevada
on August 4, 2022. The 7:6 forward stock split was effective for trading purposes on the Nasdaq Capital Market on August 12, 2022. Each
shareholder of record as of the August 15, 2022 record date received one (1) additional share for each six (6) shares held as of the
record date. No fractional shares of common stock were issued in connection with the Stock Split. Instead, all shares were rounded up
to the next whole share. In connection with the Stock Split, which did not require shareholder approval under the Nevada corporation
law, the number of shares of common stock of the Company was increased in the same ratio as the shares of outstanding common stock were
increased in the Stock Split, from 250,000,000 authorized shares to 291,666,666 authorized shares.
All share and per share information
in these financial statements retroactively reflect the forward stock split.
Going Concern Assessment
Management assesses liquidity and
going concern uncertainty in the Company’s condensed financial statements to determine whether there is sufficient cash on hand
and working capital, including available borrowings on loans, to operate for a period of at least one year from the date the consolidated
financial statements are issued or available to be issued, which is referred to as the “look-forward period”, as defined
in GAAP. As part of this assessment, based on conditions that are known and reasonably knowable to management, management will consider
various scenarios, forecasts, projections, estimates and will make certain key assumptions, including timing and nature of projected
cash expenditures or programs, its ability to delay or curtail expenditures or programs and its ability to raise additional capital,
if necessary, among other factors. Based on this assessment, as necessary or applicable, management makes certain assumptions around
implementing curtailments or delays in the nature and timing of programs and expenditures to the extent it deems probable those implementations
can be achieved and management has the proper authority to execute them within the look-forward period.
As of April 30, 2024, the Company
had cash and cash equivalents of $8,347,740 and working capital of $7,313,082. For the three months ended April 30, 2024, the Company
incurred a net loss from operations of $1,898,077 and used cash flow from operations of $833,926. The Company has generated operating
losses since its inception and has relied on sales of securities and issuance of third-party and related-party debt to support cash flow
from operations. The Company has used these proceeds from the sales of securities and issuance of third-party and related party debt
to fund operations and will continue to use the funds as needed. In March 2023, the Company entered into a three-year $2,000,000 Credit
Line Note facility with a related party, amended on July 13, 2023, to $5,000,000, which will permit the Company to draw down on the credit
line to fund the Company’s research and development of its Aversa product. On April 19, 2024, the Company received proceeds of
$8,400,000 from equity financing with European investors, of which $7.12 million is from related parties.
Management has prepared estimates
of operations for the next twelve months and believes that sufficient funds will be generated from operations to fund its operations
for one year from the date of the filing of these condensed consolidated financial statements, which indicates improved operations and
the Company’s ability to continue operations as a going concern.
Management believes the substantial
doubt about the ability of the Company to continue as a going concern is alleviated by the above assessment.
Principles of Consolidation
The consolidated financial statements
of the Company include the Company and its wholly owned subsidiaries. All material intercompany balances and transactions have been eliminated.
The operations of 4P Therapeutics are included in the Company’s financial statements from the date of acquisition of August 1,
2018, and the operations of Pocono and Active Intelligence are included in the Company’s financial statements from the date of
acquisition of September 1, 2020 under Pocono Pharmaceuticals Inc. The wholly owned subsidiaries are as follows:
Nutriband
Ltd.
4P Therapeutics
LLC
Pocono
Pharmaceuticals Inc.
Use of Estimates
The preparation of the consolidated
financial statements in conformity with accounting principles generally accepted in the United States of America requires the Company
to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure
of contingent assets and liabilities. On an ongoing basis, the Company evaluates its estimates including, but not limited to, those related
to such items as income tax exposures, accruals, depreciable/useful lives, allowance for doubtful accounts and valuation allowances.
The Company bases its estimates on historical experience and on other various assumptions that are believed to be reasonable under the
circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not
readily apparent from other sources. Actual results could differ from those estimates.
Revenue Recognition
In May 2014, the FASB issued ASU No.
2014-09, “Revenue from Contracts with Customers (Topic 606) (“ASU 2014-09”), which amends the accounting standards
for revenue recognition. ASU 2014-09 is based on principles that govern the recognition of revenue at an amount an entity expects to
be entitled when products are transferred to a customer. The Company recognizes revenue based on the five criteria for revenue recognition
established under Topic 606: 1) identify the contract, 2) identify separate performance obligations, 3) determine the transaction price,
4) allocate the transaction price among the performance obligations, and 5) recognize revenue as the performance obligations are satisfied.
Revenue
Types
The following is a description of
the Company’s revenue types, which include professional services and sale of goods:
| ● | Contract
development and manufacturing services for consumer health transdermal, topical and tape
products with revenues listed under sale of goods. |
| ● | Product
revenues derived from the sale of the Company’s consumer transdermal, topical and tape
products with sales listed under sale of goods. |
| ● | Contract
research and development services for pharmaceutical and medical devices for life sciences
customers with revenues listed under services. |
Contracts
with Customers
A contract with a customer exists
when (i) we enter into an enforceable contract with a customer that defines each party’s rights regarding the goods or services
to be transferred and identifies the payment terms related to these goods or services, (ii) the contract has commercial substance and,
(iii) we determine that collection of substantially all consideration for services that are transferred is probable based on the customer’s
intent and ability to pay the promised consideration.
Contract Liabilities
Deferred revenue is a liability related
to a revenue producing activity for which revenue has not been recognized. The Company records deferred revenue when it receives consideration
from a contract before achieving certain criteria that must be met for revenue to be recognized in conformity with GAAP.
Performance
Obligations
A performance obligation is a promise
in a contract to transfer a distinct good or service to the customer and is the unit of account in the new revenue standard. The contract
transaction price is allocated to each distinct performance obligation and recognized as revenue when, or as, the performance obligation
is satisfied. For the Company’s different revenue service types, the performance obligation is satisfied at different times. The
Company’s performance obligations include providing products and professional services in the area of research. The Company recognizes
product revenue performance obligations in most cases when the product has shipped to the customer. When we perform professional service
work, we recognize revenue when we have the right to invoice the customer for the work completed, which typically occurs over time on
a monthly basis for the work performed during that month.
All revenue recognized in the income
statement is considered to be revenue from contracts with customers.
Disaggregation
of Revenues
The Company disaggregates its revenue
from contracts with customers by type and by geographical location. See the tables:
| | |
Three Months Ended | |
| | |
April 30, | |
| | |
2024 | | |
2023 | |
| Revenue by type | |
| | |
| |
| Sale of goods | |
$ | 408,532 | | |
$ | 401,057 | |
| Services | |
| - | | |
| 75,875 | |
| Total | |
$ | 408,532 | | |
$ | 476,932 | |
| | |
Three Months Ended | |
| | |
April 30, | |
| | |
2024 | | |
2023 | |
| Revenue by geographic location: | |
| | | |
| | |
| United States | |
$ | 408,532 | | |
$ | 476,932 | |
| Foreign | |
| - | | |
| - | |
| | |
$ | 408,532 | | |
$ | 476,932 | |
Cash and cash equivalents.
Cash and cash equivalents include
cash on hand, cash on deposit in money market accounts. The Company considers short-term highly liquid investments with an original maturity
date of three months or less that are not part of an investment pool to be cash equivalents. As of April 30, 2024, the Company had $7,879,000
that exceeded federally insured limits.
Accounts receivable
Trade accounts receivables are recorded
at the net invoice value and are not interest bearing. The Company maintains allowances for doubtful accounts for estimated losses from
the inability of its customers to make the required payments. The Company determines its allowances by both the specific identification
of customer accounts where appropriate and the application of historical loss to non-applicable accounts. For the three months ended
April 30, 2024, and 2023, the Company recorded bad debt expenses of $1,200 and $-0-, respectively, for doubtful accounts related to accounts
receivable. During the year ended January 31, 2024, the Company entered into an accounts receivable sale agreement for one of its subsidiaries.
The Company received $106,528 in funds against an account receivable that is currently a claim in bankruptcy. The net accounts receivable
remain on the books of the Company and a corresponding amount has been included as a secured borrowing liability under Notes payable.
As of April 30, 2024, the receivable has been reserved in full. If the bankruptcy claim is not paid in full by the debtor, Company is
obligated to pay any difference to the factor. The loan bears interest at 10%. The Company adopted ASU 2016-13 during 2013 and implemented
the guidance on expected credit losses.
Inventories
Inventories are valued at the lower
of cost and reasonable value determined using the first-in, first-out (FIFO) method. Net realized value is the estimated selling price
in the ordinary course of business, less applicable variable selling expenses. The cost of finished goods and work in process is comprised
of material costs, direct labor costs and other direct costs and related production overheads (based on normal operating capacity). As
of April 30, 2024, total inventory was $168,505, consisting of work-in-process of $27,447, finished goods of $26,751 and raw materials
of $114,307. As of January 31, 2024, total inventory was $168,605, consisting of work-in-process of $7,466, finished goods of $8,707
and raw materials of $134,691.
Property, Plant and Equipment
Property and equipment represent an
important component of the Company’s assets. The Company depreciates its plant and equipment on a straight-line basis over the
estimated useful life of the assets. Property, plant and equipment is stated at historical cost. Expenditures for minor repairs, maintenance
and replacement parts which do not increase the useful lives of the assets are charged to expense as incurred. All major additions and
improvements are capitalized. Depreciation is computed using the straight-line method. The lives over which the fixed assets are depreciated
range from 3 to 20 years as follows:
| Lab Equipment | |
5-10 years |
| Furniture and fixtures | |
3 years |
| Machinery and equipment | |
10-20 years |
Intangible Assets
Intangible assets include trademarks,
intellectual property and customer base acquired through business combinations. The Company accounts for Other Intangible Assets under
the guidance of ASC 350, “Intangibles-Goodwill and Other.” The Company capitalizes certain costs related to patent technology.
A substantial component of the purchase price related to the Company’s acquisitions have also been assigned to intellectual property
and other intangibles. Under the guidance, other intangible assets with definite lives are amortized over their estimated useful lives.
Intangible assets with indefinite lives are tested annually for impairment. Trademarks, intellectual property and customer base are being
amortized over their estimated useful lives of ten years.
Goodwill
Goodwill represents the difference
between the total purchase price and the fair value of assets (tangible and intangible) and liabilities at the date of acquisition. Goodwill
is reviewed for impairment annually on January 31, and more frequently as circumstances warrant, and written down only in the period
in which the recorded value of such assets exceeds their fair value. The Company does not amortize goodwill in accordance with ASC 350.
In connection with the Company’s acquisition of 4P Therapeutics LLC in 2018, the Company recorded Goodwill of $1,719,235. On August
31, 2020, in connection with the Company’s acquisition of Pocono Coated Products LLC and Active Intelligence LLC, the Company recorded
Goodwill of $5,810,640. During the years ended January 31, 2024, and 2023, the Company recorded an impairment charge of $-0- and $327,326,
respectively, reducing the Active Intelligence LLC Goodwill to $3,302,478. As of April 30, 2024, and January 31, 2024, Goodwill amounted
to $5,021,713 and $5,021,713, respectively.
Long-lived Assets
Management reviews long-lived assets
for potential impairment whenever significant events or changes in circumstances indicate that the carrying amount of an asset may not
be recoverable. An impairment exists when the carrying amount of the long-lived asset is not recoverable and exceeds its fair value.
The carrying amount of a long-lived asset is not recoverable if it exceeds the sum of the estimated undiscounted cash flows expected
to result from the use and eventual disposition of the asset. If an impairment exists, the resulting write-down would be the difference
between the fair market value of the long-lived asset and the related book value.
Earnings per Share
Basic earnings per share of common
stock is computed by dividing net earnings by the weighted average number of shares of common stock outstanding during the period. Diluted
earnings per share is computed by dividing net earnings by the weighted average number of shares of common stock and potential shares
of common stock outstanding during the period. Potential shares of common stock consist of shares issuable upon the exercise of
outstanding options and common stock purchase warrants. As of April 30, 2024, and 2023, there were 6,747,873 and 1,783,373 common stock
equivalents outstanding, that were not included in the calculation of dilutive earnings per share as their effect would be anti-dilutive.
Stock-Based Compensation
ASC 718, “Compensation - Stock
Compensation,” prescribes accounting and reporting standards for all share-based payment transactions in which employee services,
and, since February 1, 2019, non-employees, are acquired. Transactions include incurring liabilities, or issuing or offering to issue
shares, options and other equity instruments such as employee stock ownership plans and stock appreciation rights. Share-based payments
to employees, including grants of employee stock options, are recognized as compensation expense in the financial statements based on
their fair values. That expense is recognized over the period during which an employee is required to provide services in exchange for
the award, known as the requisite service period (usually the vesting period). As of February 1, 2019, pursuant to ASC 2018-07, ASC 718
was applied to stock-based compensation for both employees and non-employees.
Business Combinations
The Company recognizes the assets
acquired, the liabilities assumed, and any non-controlling interest in the acquired entity at the acquisition date, measured at their
fair values as of that date, with limited exceptions specified in the accounting literature. In accordance with this guidance, acquisition-related
costs, including restructuring costs, must be recognized separately from the acquisition and will generally be expensed as incurred.
That replaces the cost-allocation process detailed in previous accounting literature, which required the cost of an acquisition to be
allocated to the individual assets acquired and liabilities assumed based on their estimated fair value.
Leases
In February
2016, the FASB issued ASU 2016-02, “Leases” (Topic 842), to provide a new comprehensive model for lease accounting under
this guidance, lessees and lessors should apply a “right-of-use” model in accounting for all leases (including subleases)
and eliminate the concept of operating leases and off-balance-sheet leases. Recognition, measurement and presentation of expenses will
depend on classification as a finance or operating lease. Similar modifications have been made to lessor accounting in-line with revenue
recognition guidance.
The Company
applies the guidance for right-of-use accounting for all leases and records the operating lease liabilities on its balance sheet. The
Company completed the necessary changes to its accounting policies, processes, disclosure and internal control over financial reporting.
Research and Development Expenses
Research and development costs are
expensed as incurred.
Income Taxes
Taxes are calculated in accordance
with taxation principles currently effective in the United States and Ireland.
The Company accounts for income taxes
under the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax
consequences of events that have been included in the financial statements. Under this method, deferred tax assets and liabilities
are determined based on the differences between the financial statements and tax basis of assets and liabilities using enacted tax rates
in effect for the year in which the differences are expected to reverse. The effect of a change in tax rates on deferred tax assets and
liabilities is recognized in income in the period that includes the enactment date.
The Company records net deferred tax
assets to the extent they believe these assets will more likely than not be realized. In making such determination, the Company
considers all available positive and negative evidence, including future reversals of existing taxable temporary differences, projected
future taxable income, tax planning strategies and recent financial operations. In the event the Company was to determine
that it would be able to realize its deferred income tax assets in the future in excess of its net recorded amount, the Company would
make an adjustment to the valuation allowance which would reduce the provision for income taxes.
Fair Value Measurements
FASB ASC 820, “Fair Value Measurements
and Disclosure” (“ASC 820”), defines fair value as the exchange price that would be received for an asset or paid to
transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction
between participants on the measurement date. ASC 820 also establishes a fair value hierarchy which requires an entity to maximize the
use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 820 describes three levels of inputs
that may be used to measure fair value.
The Company utilizes the accounting
guidance for fair value measurements and disclosures for all financial assets and liabilities and nonfinancial assets and liabilities
that are recognized or disclosed at fair value in the consolidated financial statements on a recurring basis during the reporting period.
The fair value is an exit price, representing the price that would be received to sell an asset or paid to transfer a liability in an
orderly transaction between market participants based upon the best use of the asset or liability at the measurement date. The Company
utilizes market data or assumptions that market participants would use in pricing the asset or liability. ASC 820 establishes a three-tier
value hierarchy, which prioritizes the inputs used in measuring fair value. These tiers are defined as follows:
| |
Level
1 |
- |
Observable
inputs such as quoted market prices in active markets. |
| |
|
|
|
| |
Level
2 |
- |
Inputs
other than quoted prices in active markets that are either directly or indirectly observable. |
| |
|
|
|
| |
Level
3 |
- |
Unobservable
inputs about which little or no market data exists, therefore requiring an entity to develop its own assumptions. |
The carrying value of the Company’s
financial instruments, including accounts receivable, prepaid expenses, accounts payable and accrued expenses, and deferred revenue approximate
their fair value due to the short maturities of these financial instruments.
Recent Accounting Standards
In June 2016, the FASB issued ASU
2016-13, Financial Instruments-Credit Losses (Topic 326), The ASU introduces a new credit loss methodology. Current Expected Credit Loss
(“CECL”), which requires earlier recognition of credit losses, which also provides additional transparency about credit risk.
Since its original issuance in 2016, the FASB has issued several updates to the original ASU. The Company adopted ASU 2016-13 during
the year ended January 31, 2024. The adoption of ASU 2016-13 did not have a material impact on the Company’s balance sheet or statement
of operations.
The Company has reviewed all other
FASB-issued ASU accounting pronouncements and interpretations thereof that have effective dates during the period reported and in future
periods. The Company has carefully considered the new pronouncements that alter previous GAAP and does not believe that any new or modified
principles will have a material impact on the Company’s reported financial position or operations in the near term. The applicability
of any standard is subject to the formal review of the Company’s financial management and certain standards are under consideration.
| | |
April 30, | | |
January 31, | |
| | |
2024 | | |
2024 | |
| Lab equipment | |
$ | 144,585 | | |
$ | 144,585 | |
| Machinery and equipment | |
| 1,298,584 | | |
| 1,292,389 | |
| Furniture and fixtures | |
| 19,643 | | |
| 19,643 | |
| | |
| 1,462,812 | | |
| 1,456,617 | |
| Less: Accumulated depreciation | |
| (722,507 | ) | |
| (681,693 | ) |
| Net Property and Equipment | |
$ | 740,305 | | |
$ | 774,924 | |
Depreciation expenses amounted to
$40,814 and $46,914 for the three months ended April 30, 2024, and 2023, respectively. During the three months ended April 30, 2024,
and 2023, depreciation expenses of $30,242 and $36,179, respectively, have been allocated to cost of goods sold.
Notes Payable
Active Intelligence, entered into
an agreement with the Carolina Small Business Development Fund for a line of credit of $160,000 due October 16, 2028, with interest of
5% per year. The amount assumed was $139,184. The loan requires monthly payments of principal and interest of $1,697. During the three
months ended April 30, 2024, the Company made $3,959 of principal payments. As of April 30, 2024, the amount due was $81,290, of which
$16,331 is current. As of January 31, 2024, the amount due was $85,249.
On April 3, 2022, the Company entered
into a retail installment agreement for the purchase of an automobile. The contract price was $32,274, of which $22,795 was financed.
The agreement is for five years bearing interest at 2.95% per annum with payments of $410 per month. The loan is secured by automobile.
As of April 30, 2024, the amount due was $14,860 of which $4,560 is current. As of January 31, 2024, the amount due was $15,232.
Note payable-related party.
On July 17, 2023, the Company entered
an amended Credit Line Note agreement, for an increased $5,000,000 credit line facility t the Company entered on March 17, 2023). Outstanding
advances under the Note bears interest at 7% per annum. The promissory note is due and payable in full on March 19, 2026. Interest is
payable annually on December 31 of each year during the term of the note. During the year ended January 31, 2024, the Company received
$2,000,000 on the Note. In December 2023, the Company converted the balance of the credit facility of $2,000,000 and $53,476 of accrued
interest into 1,026,520 shares of common stock. The fair value of the common stock was $2,554,423 resulting in a $554,423 loss on extinguishment.
The Company received advances of $300,000 during the three months ended April 30, 2024. As of April 30, 2024, the balance due was $300,000.
The Company recorded interest expense of $4,163 for the three months ended April 30, 2024.
Secured borrowing liability.
The Company entered into an accounts
receivable sale agreement for one of its subsidiaries in connection with a bankruptcy claim. The Company received $106,528 and recorded
the transaction as a secured loan payable against the account receivable. The sale of the account receivable balance was to an outside
third party, whereby if the bankruptcy court does not pay the balance in full, the Company will owe back the unpaid portion. The loan
is classified as a current liability as the Company expects the bankruptcy will be resolved in the next twelve months. The loan bears
interest at 10%. For the three months ended April 30, 2024, the Company recorded interest expense of $2,578.
Interest expenses for the three months
ended April 30, 2024, and 2023, were $8,618 and $3,166, respectively.
As of April 30, 2024, and January
31, 2024, intangible assets consisted of intellectual property and trademarks, customer base, and license agreement, net of amortization,
as follows:
| | |
April 30, | | |
January 31, | |
| | |
2024 | | |
2024 | |
| Customer base | |
$ | 314,100 | | |
$ | 314,100 | |
| Intellectual property and trademarks | |
| 817,400 | | |
| 817,400 | |
| | |
| | | |
| | |
| Total | |
| 1,131,500 | | |
| 1,131,500 | |
| | |
| | | |
| | |
| Less: Accumulated amortization | |
| (492,507 | ) | |
| (464,220 | ) |
| | |
| | | |
| | |
| Net Intangible Assets | |
$ | 638,993 | | |
$ | 667,280 | |
Amortization expenses for the three
months ended April 30, 2024, and 2023 amounted to $28,287 and $28,287, respectively.
| Year Ended January 31, | |
| |
| 2025 | |
$ | 84,863 | |
| 2026 | |
| 113,148 | |
| 2027 | |
| 113,148 | |
| 2028 | |
| 113,148 | |
| 2029 | |
| 94,226 | |
| 2030 and thereafter | |
| 120,460 | |
| | |
$ | 638,993 | |
| 6. | RELATED
PARTY TRANSACTIONS |
Activity during the year ended April
30, 2024
| a) | In
March 2024, options to purchase 390,000 shares of common stock to executives and employees
of the Company at a price of $2.37 and $2.61 per share. The options vest immediately and
expire in three years. The fair value of the options issued amounted to $422,955 and was
expensed during the three months ended April 30, 2024. |
| b) | On
April 19, 2024, the Company completed an $8,400,000 equity financing with European investors
which included related parties. The related parties invested a total of $7,120,000 and received
1,780,000 shares of common stock and warrants to purchase 3,560,000 shares of common stock
@ $6.43 per share. One related party, a director of the Company, invested $4.5 million which
included $500,000 from his son and $700,000 from an entity he controls. The other related
party invested $2.62 million from entities controlled by the investor. |
| | | |
| c) | During
the three months ended April 30, 2024, the Company received $300,000 from the credit line
facility with TII Jet Services LDA. See Note 4 for further information. |
Activity during
the three months ended April 30, 2023
| a) | On
February 1, 2023, options to purchase 30,000 shares of the Company’s common stock were
issued to an executive of the Company at a price of $3.975 per share. The options vest immediately
and expire in three years. The fair value of the options issued for services amounted to
$75,030 and was expensed during the three months ended April 30, 2023. |
Preferred Stock
On January 15, 2016, the board of
directors of the Company approved a certificate of amendment to the articles of incorporation and changed the authorized capital stock
of the Company to include and authorize 10,000,000 shares of Preferred Stock, par value $0.001 per share.
On May 24, 2019, the board of directors
created a series of preferred stock consisting of 2,500,000 shares designated as the Series A Convertible Preferred Stock (“Series
A Preferred Stock”). On June 20, 2019, the Series A preferred Stock was terminated, and the 2,500,000 shares were restored to the
status of authorized but unissued shares of Preferred Stock, without designation as to series, until such stock is once more designated
as part of a particular series by the board of directors.
Common Stock
On June 25, 2019, the Company effected
a one-for-four reverse stock split, pursuant to which each outstanding share of common stock was changed into 0.25 shares of common stock,
and the Company decreased its authorized common stock in the same ratio from 100,000,000 to 25,000,000 shares.
On January 27, 2020, the Company amended
its Articles of Incorporation to increase its authorized common shares from 25,000,000 authorized shares to 250,000,000 authorized shares.
On July 26, 2022, the Board of Directors
of the Company approved a 7-for-6 forward stock split, effective for trading purposes as of August 12, 2022, pursuant to which each shareholder
as of the August 15, 2022 record date received one (1) additional share for each six (6) shares held as of the record date. Pursuant
to the operation of the amendment providing for the forward stock split filed with the Secretary of State of Nevada on August 4, 2022,
the authorized common stock of the Company was increased from 250,000,000 shares to 291,666,666 shares in connection with the forward
split.
Activity during
the Three Months Ended April 30, 2024
| (a) | As
of April 30, 2024, the Company holds 10,000 of its shares comprising $32,641 of treasury
stock. There was no activity during the three months ended April 30, 2024. |
| | | |
| (b) | On
April 19, 2024, the Company completed an $8,400,000 equity financing with European investors,
of which $7.12 million is from related parties, (the “Offering”) of 2,100,000
units (“Units”), at a price of $4.00 per Unit, consisting of one share of common
stock (“Shares”) and a Warrant to purchase two Shares of common stock, the Warrant
having an exercise price of $6.43, are exercisable by payment of the exercise price in cash
only and expire April 19, 2029, five years from the date of issuance (“Warrants”).
The offering was made solely to investors residing outside the United States and was not
registered under the Security Act of 1933, as amended, (the “Security Act”),
or the security law of any jurisdiction, including outside the United States, but was made
privately by the Company pursuant to the exemptions from registration provided in the SEC’s
Regulation S and other exemptions under the Securities Act. See Note 6 for further information. |
Activity during the Three Months
Ended January 31, 2023
| (a) | As
of April 30, 2023, the Company held 10,000 of its shares comprising $32,641 of treasury stock.
There was no activity during the three months ended April 30, 2023. |
Warrants
The following table summarizes the
changes in warrants outstanding and the related price of the shares of the Company’s common stock issued to non-employees of the
Company during the year ended January 31, 2024. On March 7, 2023, the Company issued 30,000 warrants to purchase the Company’s
common shares to Barandnic Holdings Ltd. for services provided. The warrants are exercisable at a price of $4.00 per share and expire
five years from the date of issuance. On October 27, 2023, the Company issued 145,833 warrants to purchase the Company’s common
shares to management (87,500 warrants were issued to the Chief Financial Officer) and non-employees of the Company. The warrants are
exercisable at a price of $1.93 per share and expire in three years from the date of issuance. These warrants replace previously issued
warrants that have now been cancelled. The Company used the Black-Scholes valuation model to record the fair value. The valuation model
used a dividend rate of 0%; expected term of 1.5 years; volatility rates of 152.10-174.45%; and a risk-free rate of 4.31%-4.84%. Non-cash
compensation for the year ended January 31, 2024, amounted to $242,840.
On April 19, 2024, in connection with
a private placement of the Company’s common stock, the Company issued 4,200,000 warrants. The warrants are exercisable at a price
of $6.43 per share and expire five years from the date of issuance.
| | |
Shares | | |
Exercise
Price | | |
Remaining
Life | |
Intrinsic
Value | |
| Outstanding, January 31, 2023 | |
| 1,307,671 | | |
$ | 6.43 | | |
3.34 years | |
$ | - | |
| | |
| | | |
| | | |
| |
| | |
| Granted | |
| 175,833 | | |
| 2.28 | | |
2.97 years | |
| - | |
| | |
| | | |
| | | |
| |
| | |
| Expired/Cancelled | |
| (200,466 | ) | |
| 6.33 | | |
- | |
| - | |
| | |
| | | |
| | | |
| |
| | |
| Exercised | |
| - | | |
| - | | |
- | |
| - | |
| | |
| | | |
| | | |
| |
| | |
| Outstanding, January 31, 2024 | |
| 1,283,038 | | |
| 5.88 | | |
2.97 years | |
| - | |
| | |
| | | |
| | | |
| |
| | |
| Granted | |
| 4,200,000 | | |
| 6.43 | | |
5.00 years | |
| - | |
| | |
| | | |
| | | |
| |
| | |
| Expired/Cancelled | |
| - | | |
| - | | |
- | |
| - | |
| | |
| | | |
| | | |
| |
| | |
| Exercised | |
| - | | |
| - | | |
- | |
| - | |
| | |
| | | |
| | | |
| |
| | |
| Outstanding- April 30, 2024 | |
| 5,483,038 | | |
$ | 6.30 | | |
4.25 years | |
$ | 233,125 | |
| | |
| | | |
| | | |
| |
| | |
| Exercisable - April 30, 2024 | |
| 5,483,038 | | |
$ | 6.30 | | |
4.25 years | |
$ | 233,125 | |
The following
table summarizes additional information relating to the warrants outstanding as of April 30, 2024:
Range of Exercise
Prices | | |
Number
Outstanding | | |
Weighted
Average
Remaining
Contractual
Life(Years) | | |
Weighted
Average
Exercise Price
for Shares
Outstanding | | |
Number
Exercisable | | |
Weighted
Average
Exercise Price
for Shares
Exercisable | | |
Intrinsic
Value | |
| | | |
| | |
| | |
| | |
| | |
| | |
| |
| $ | 4.00 | | |
| 30,000 | | |
| 4.10 | | |
$ | 4.00 | | |
| 30,000 | | |
$ | 4.00 | | |
$ | - | |
| $ | 6.43 | | |
| 5,282,205 | | |
| 2.68 | | |
$ | 6.43 | | |
| 5,282,205 | | |
$ | 6.43 | | |
$ | - | |
| $ | 1.93 | | |
| 145,833 | | |
| 2.74 | | |
$ | 1.93 | | |
| 145,833 | | |
$ | 1.93 | | |
$ | 233,125 | |
| $ | 7.50 | | |
| 25,000 | | |
| 3.77 | | |
$ | 7.50 | | |
| 25,000 | | |
$ | 7.50 | | |
$ | - | |
| | | | |
| 5,483,038 | | |
| | | |
| | | |
| 5,483,038 | | |
| | | |
$ | 233,125 | |
Options
The following table summarizes the
changes in options outstanding and the related price of the shares of the Company’s common stock issued to employees of the Company.
See Note 7 for the issuance of related party options.
On November 1, 2021, the Board of
Directors adopted the 2021 Employee Stock Option Plan (the “Plan”). The Company has reserved 408,333 shares for issuance
and sale upon the exercise of stock options. In accordance with the Plan, on February 1, 2022, the Company reserved an additional 233,333
shares and on February 1, 2023, the Company reserved an additional 233,333 shares. The options vest immediately and expire in three years.
Under the Plan, options may be granted which are intended to qualify as Incentive Stock Options (“ISO’s”) under Section
422 of the Internal Revenue Code of 1986 (the “Code”) or which are not (“non-ISO’s”) intended to qualify
as Incentive Stock Options thereunder. The Plan also provides for restricted stock awards representing shares of common stock that are
issued subject to such restrictions on transfer and other incidents of ownership and such forfeiture conditions as the Board of Directors,
or the committee administering the Plan composed of directors who qualify as “independent” under Nasdaq rules, may determine.
On November 3, 2021, the Company filed a Registration Statement on Form S-8, to register under the Securities Act of 1933, as amended
the 408,333 shares of common stock reserved for issuance under the Plan.
On March 20, 2024, our Board of Directors
adopted an amendment to the Company’s Employee Stock Option Plan (the “Plan”) increasing the number of shares of common
stock subject to the Plan (as of March 20, 2024, 875,000 shares) to 1,400,000 shares (the “Amendment”). The Company will
submit the Amendment to the Plan to our stockholders for adoption and approval at the 2025 Annual Meeting. If the Amendment is not approved
by stockholders within one year of adoption, the increase in shares subject to the Plan will be void, together with any options issued
following March 20, 2024, in the period pending approval of the Plan by our stockholders. As of April 30, 2024, 135,165 shares remain
available for issuance of options under the Plan.
During the three months ended April
30, 2024, 390,000 options to purchase shares of the Company’s common stock were issued to executive officers and employees at prices
of $2.37- $2.61 per share. The options vest immediately and expire three years from the date of issuance. The fair value of the options
issued for services amounted to $422,955 and was recorded during the three months ended April 30, 2024. The Company used the Black-Scholes
valuation model to record the fair value. The valuation model used a dividend rate of 0%; expected term of 1.5 years; volatility rate
of 97.83%; and a risk-free rate of 4.87%.
During the year ended January 31,
2024, 404,500 options to purchase shares of the Company’s common stock were issued to executive officers and employees at prices
of $1.93-$3.975 per share. The options vest immediately and expire three years from the date of issuance. The fair value of the options
issued for services amounted to $499,856 and was recorded during the year ended January 31, 2024. The Company used the Black-Scholes
valuation model to record the fair value. The valuation model used a dividend rate of 0%; expected term of 1.5 years; volatility rates
of 121.52-143.54%; and a risk-free rate of 3.00-4.5%.
| | |
Shares | | |
Exercise
Price | | |
Remaining
Life | |
Intrinsic
Value | |
| Outstanding, January 31, 2023 | |
| 470,335 | | |
$ | 4.13 | | |
2.53 years | |
| | |
| | |
| | | |
| | | |
| |
| | |
| Granted | |
| 404,500 | | |
| 2.18 | | |
2.68 years | |
| - | |
| | |
| | | |
| | | |
| |
| | |
| Expired/Cancelled | |
| - | | |
| - | | |
- | |
| | |
| | |
| | | |
| | | |
| |
| | |
| Exercised | |
| - | | |
| - | | |
- | |
| | |
| | |
| | | |
| | | |
| |
| | |
| Outstanding, January 31, 2024 | |
| 874,835 | | |
| 3.23 | | |
2.31 years | |
| | |
| | |
| | | |
| | | |
| |
| | |
| Granted | |
| 390,000 | | |
| 2.49 | | |
2.80 years | |
$ | 378,300 | |
| | |
| | | |
| | | |
| |
| | |
| Expired/Cancelled | |
| - | | |
| - | | |
- | |
| | |
| | |
| | | |
| | | |
| |
| | |
| Exercised | |
| - | | |
| - | | |
- | |
| | |
| | |
| | | |
| | | |
| |
| | |
| Outstanding- April 30, 2024 | |
| 1,264,835 | | |
$ | 2.63 | | |
2.31 years | |
$ | 910,285 | |
| | |
| | | |
| | | |
| |
| | |
| Exercisable - April 30, 2024 | |
| 1,264,835 | | |
$ | 2.63 | | |
2.31 years | |
$ | 910,285 | |
The following table summarizes additional
information relating to the options outstanding as of April 30, 2024:
Range of Exercise
Prices | | |
Number
Outstanding | | |
Weighted
Average
Life(Years) | | |
Weighted
Average
Exercise Price
for Shares
Outstanding | | |
Number
Exercisable | | |
Weighted
Average
Exercise Price
for Shares
Exercisable | | |
Intrinsic
Value | |
| | | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| $ | 1.93 | | |
| 214,500 | | |
| 2.74 | | |
$ | 1.93 | | |
| 214,500 | | |
$ | 1.93 | | |
$ | 328,185 | |
| $ | 2.12 | | |
| 140,000 | | |
| 2.74 | | |
$ | 2.12 | | |
| 140,000 | | |
$ | 2.12 | | |
$ | 187,600 | |
| $ | 2.37 | | |
| 195,000 | | |
| 2.37 | | |
$ | 2.37 | | |
| 195,000 | | |
$ | 2.37 | | |
$ | 212,550 | |
| $ | 2.61 | | |
| 195,000 | | |
| 2.61 | | |
$ | 2.61 | | |
| 195,000 | | |
$ | 2.61 | | |
$ | 165,750 | |
| $ | 2.65 | | |
| 20,000 | | |
| 2.63 | | |
$ | 2.65 | | |
| 20,000 | | |
$ | 2.65 | | |
$ | 16,200 | |
| $ | 3.59 | | |
| 35,000 | | |
| 3.67 | | |
$ | 3.59 | | |
| 35,000 | | |
$ | 3.59 | | |
$ | - | |
| $ | 3.75 | | |
| 57,500 | | |
| 1.85 | | |
$ | 3.75 | | |
| 57,500 | | |
$ | 3.75 | | |
$ | - | |
| $ | 3.98 | | |
| 30,000 | | |
| 2.01 | | |
$ | 3.98 | | |
| 30,000 | | |
$ | 3.98 | | |
$ | - | |
| $ | 4.09 | | |
| 78,750 | | |
| 1.50 | | |
$ | 4.09 | | |
| 78,750 | | |
$ | 4.09 | | |
$ | - | |
| $ | 4.12 | | |
| 50,000 | | |
| 1.85 | | |
$ | 4.12 | | |
| 50,000 | | |
$ | 4.12 | | |
$ | - | |
| $ | 4.16 | | |
| 144,083 | | |
| 0.97 | | |
$ | 4.16 | | |
| 144,083 | | |
$ | 4.16 | | |
$ | - | |
| $ | 4.50 | | |
| 58,334 | | |
| 1.50 | | |
$ | 4.50 | | |
| 58,334 | | |
$ | 4.50 | | |
$ | - | |
| $ | 4.58 | | |
| 46,668 | | |
| 0.97 | | |
$ | 4.58 | | |
| 46,668 | | |
$ | 4.58 | | |
$ | - | |
We organize and manage our business
by the following two segments which meet the definition of reportable segments under ASC280-10, Segment Reporting: Sales of Goods and
Services. These segments are based on the customer type of products or services provided and are the same as our business units. Separate
financial information is available and regularly reviewed by our chief officer decision maker, who is our chief executive officer, in
making resource allocation decisions for our segments. Our chief officer decision maker evaluates segment performance to the GAAP measure
of gross profit.
| | |
Three Months
Ended
April 30, | |
| | |
2024 | | |
2023 | |
| Net sales | |
| | |
| |
| Pocono Pharmaceuticals | |
$ | 408,532 | | |
$ | 401,057 | |
| 4P Therapeutics | |
| - | | |
| 75,875 | |
| | |
| 408,532 | | |
| 476,932 | |
| Gross profit | |
| | | |
| | |
| Pocono Pharmaceuticals | |
| 164,786 | | |
| 169,308 | |
| 4P Therapeutics | |
| - | | |
| 52,976 | |
| | |
| 164,786 | | |
| 222,284 | |
| Operating expenses | |
| | | |
| | |
| Selling, general and administrative-Pocono
Pharmaceuticals | |
| 154,394 | | |
| 136,863 | |
| Selling, general and administrative-4P
Therapeutics | |
| 24,354 | | |
| 16,921 | |
| Selling, general and administrative-Corporate | |
| 900,980 | | |
| 685,948 | |
| Research and
development-4P Therapeutics | |
| 974,535 | | |
| 400,430 | |
| | |
| 2,054,263 | | |
| 1,240,162 | |
| Depreciation and Amortization | |
| | | |
| | |
| Pocono Pharmaceuticals | |
$ | 56,823 | | |
$ | 55,208 | |
| Corporate | |
| 3,011 | | |
| 3,497 | |
| 4P Therapeutics | |
| 9,267 | | |
| 16,496 | |
| | |
$ | 69,101 | | |
$ | 75,201 | |
The following table presents information
about net sales and property and equipment, net of accumulated depreciation, in the United States and elsewhere.
| | |
Three Months
Ended
April 30, | |
| | |
2024 | | |
2023 | |
| Net sales | |
| | |
| |
| United States | |
$ | 408,532 | | |
$ | 401,057 | |
| Outside the
United States | |
| - | | |
| 75,875 | |
| | |
$ | 408,532 | | |
$ | 476,932 | |
| | |
April 30, | | |
January 31, | |
| | |
2024 | | |
2024 | |
| Property and equipment, net of accumulated depreciation | |
| | |
| |
| United States | |
$ | 740,305 | | |
$ | 774,924 | |
| Outside the
United States | |
| - | | |
| - | |
| | |
$ | 740,305 | | |
$ | 774,924 | |
| Assets | |
| | | |
| | |
| Corporate | |
$ | 8,210,779 | | |
$ | 344,192 | |
| Pocono Pharmaceuticals | |
| 5,044,569 | | |
| 5,079,293 | |
| 4P Therapeutics | |
| 1,899,132 | | |
| 2,093,369 | |
| | |
$ | 15,154,480 | | |
$ | 7,516,854 | |
| 10. | COMMITMENTS
AND CONTIGENCIES |
Employment
Agreements
The Company entered into three-year
employment agreements with Gareth Sheridan, our CEO, and Serguei Melnik, our President, effective February 1, 2022. The agreement also
provides that the executives will continue as directors and officers of the Company for the respective terms thereof. The agreement provides
for an initial term, commencing on the effective date of the agreement and ending on January 31, 2025, and continuing on a year-to-year
basis thereafter unless terminated by either party on not less than 30 days’ notice given prior to the expiration of the initial
term or any one-year extension. For their services to the Company during the term of the agreement, Mr. Sheridan and Mr. Melnik will
receive an annual salary of $250,000 per annum, commencing on the effective date of the agreement. Mr. Sheridan and Mr. Melnik will also
receive a performance bonus of 3.5% of net income before income taxes. As of July 31, 2022, the Company and Mr. Sheridan and Mr. Melnik
mutually agreed to reduce their annual salary to $150,000.
The Company entered into a three-year
employment agreement with Gerald Goodman, our CFO, effective February 1, 2022. The agreement provides for an initial term, commencing
on the effective date of the agreement and ending on January 31, 2025, and continuing on a year-to-year basis thereafter unless terminated
by either party on not less than 30 days’ notice given prior to the expiration of the initial term or any one-year extension. For
his services to the Company during the term of the agreement, Mr. Goodman will receive an annual salary of $210,000 per annum, commencing
on the effective date of the agreement. As of July 31, 2022, the Company and Mr. Goodman mutually agreed to reduce his annual salary
to $110,000.
Kindeva Drug
Delivery Agreement
On January 4, 2022, the Company signed
a feasibility agreement with Kindeva Drug Delivery, L.P. (“Kindeva”) to develop Nutriband’s lead product, AVERSA Fentanyl,
based on its proprietary AVERSA abuse deterrent transdermal technology and Kindeva’s FDA-approved transdermal fentanyl patch (fentanyl
transdermal system). The feasibility agreement provides for adapting Kindeva’s commercial transdermal manufacturing process to
incorporate AVERSA technology in the fentanyl transdermal system.
The agreement will remain in force
until the earlier of: (1) the completion of the work and deliverables under the Workplan; or (2) two (2) years after the Effective Date,
after which time the agreement will expire. The feasibility Workplan was completed in February 2024.
The estimated cost to complete the
feasibility Workplan was approximately $2.5 million. Nutriband made an advance deposit of $250,000 in January 2022, to be applied against
the final invoices. As of April 30, 2024, Nutriband has incurred expenses of $2,950,998 and the deposit of $250,000 has been applied
to the final invoices.
On January 15, 2024, the Nutriband
signed a commercial development and clinical supply agreement for their lead product with Kindeva. Kindeva will perform commercial manufacturing
process development and manufacturing clinical supplies for the human abuse liability clinical study required by the FDA in support of
a New Drug Application. The new agreement replaces the previous feasibility agreement between the two companies which was focused on
adapting Kindeva’s commercial transdermal manufacturing process to incorporate AVERSA abuse deterrent transdermal technology. The
estimated cost to complete is approximately $8.1 million and the expected timing of FDA submission is twelve to eighteen months.
Lease Agreement
On February 1, 2022, Pocono Pharmaceuticals
entered into a lease agreement with Geometric Group, LLC for 12,000 square feet of warehouse space currently occupied by Active Intelligence.
The monthly rental is $3,000 and the lease expires on January 31, 2025. The lease can be extended for an additional three years at the
same monthly rental. The Company recorded a Right of Use asset in the amount of $94,134 in connection with the valuation.
Sorrento Therapeutics, Inc.
Agreement
On July 25, 2023, 4P Therapeutics
assigned its claim under the bankruptcy proceedings from Sorrento Therapeutics Inc. and received proceeds of $106,528. The amount due
under the claim was $118,675 and 4P Therapeutics recorded a reserve for bad debts of $118,675 during the year ended January 31, 2024.
Under the agreement with the buyer of the claim, 4P Therapeutics will make proportional restitution and/or repayment of the purchase
amount to the extent the claim is disallowed, reduced or not paid at the same time or distribution rate as other general unsecured claims
against the Debtor are paid. The Company has recorded the amount of the proceeds as a secured loan payable to the factor as of April
30, 2024.
Legal Proceedings
The Company is currently a defendant
in a lawsuit initiated by Joseph Gunnar, LLC (“Gunnar”) and Lucosky Brookman LLP (“LB”) in the Supreme Court
of the State of New York, New York County, under Index No.654633/2023. The lawsuit alleges multiple allegations such as breach of contract,
fraudulent activities, and tortious interference and seeks damages following the Company’s termination of an engagement letter
for assistance with a public stock offering. Gunnar is seeking over $500,000 in damages plus punitive damages, while LB is demanding
reimbursement of legal fees.
In response, the Company denies all
allegations, alleging that the engagement letter was unenforceable, and its termination was legally justified. The Company has also initiated
counterclaims against Joseph Gunnar & Co., accusing them of intentional interference and breach of fiduciary duty, and is seeking
$1,000,000 for each claim along with a declaratory judgment affirming the legality and justification of the termination. The plaintiffs
have denied these counterclaims.
Currently, there are no pending hearings
or motions as both parties are engaged in discovery and are attempting to resolve the matter amicably.
| (a) | The
Company agreed on May 14, 2024, to convert $300,000, which as of that date of all the outstanding
principal on the Credit line Promissory Note of the Company held by TII Jet Services LDA,
a related party, (the “Holder”). The conversion was made pursuant to the terms
of a Conversion Agreement dated May 14, 2024, which provided that the conversion of $300,000
of principal and $4,922 of accrued interest would be made at a price of $4.00 per share,
resulting in the Company issuing on May 14, 2024, a total of 76,230 shares of common stock
at the conversion price of $4.00 per share. On May 22, 2024, the Conversion Agreement was
amended allowing the lender to purchase an additional 152,460 shares of common stock at a
rate of two warrants per converted share, with an exercise price of $6.43 per share and a
term of five years from the conversion date (May 14, 2024). |
Nutriband (NASDAQ:NTRB)
Gráfica de Acción Histórica
De Nov 2024 a Dic 2024
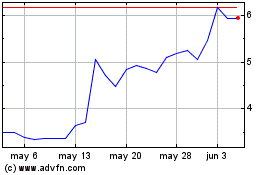
Nutriband (NASDAQ:NTRB)
Gráfica de Acción Histórica
De Dic 2023 a Dic 2024