Xilio to Host Virtual Investor Conference Call and Webcast on Monday, November 6, 2023 to Review Progress Across Pipeline, Including Phase 1/2 Clinical Data for XTX202, a Tumor-Activated, Engineered, Beta-Gamma IL-2, Presented at the SITC Annual Meeting
31 Octubre 2023 - 7:15AM

Xilio Therapeutics, Inc. (Nasdaq: XLO), a clinical-stage
biotechnology company discovering and developing tumor-activated
immuno-oncology therapies for people living with cancer, today
announced that it will host an investor conference call and webcast
on November 6, 2023 at 8:00 a.m. ET to review progress across its
pipeline of tumor-activated molecules, including Phase 1/2 clinical
data for XTX202, an investigational tumor-activated, engineered,
beta-gamma IL-2, which will be presented at the Society for
Immunotherapy of Cancer (SITC) 38th Annual Meeting, taking place in
San Diego, California from November 1-5, 2023.
The call will feature members of Xilio’s
management team and Howard Kaufman, M.D., FACS, a clinical
investigator on Xilio’s Phase 1/2 clinical trial for XTX202 and a
leading authority of local tumor immunotherapy and oncolytic
viruses.
Conference Call and Webcast
Information
The webcast may be accessed by clicking here.
The webcast of the conference call will also be available under
“Events and Presentations” in the Investors & Media section of
the Xilio Therapeutics website at https://ir.xiliotx.com/. The
archived webcast will be available on the Xilio Therapeutics
website approximately two hours after the conference call and will
be available for 30 days following the call.
About Xilio Therapeutics
Xilio Therapeutics is a clinical-stage
biotechnology company discovering and developing tumor-activated
immuno-oncology (I-O) therapies with the goal of significantly
improving outcomes for people living with cancer without the
systemic side effects of current I-O treatments. The company is
using its proprietary geographically precise solutions (GPS)
platform to build a pipeline of novel, tumor-activated molecules,
including antibodies, cytokines and other biologics, which are
designed to optimize their therapeutic index and localize
anti-tumor activity within the tumor microenvironment. Xilio is
currently advancing multiple programs for tumor-activated I-O
treatments in clinical development, as well as programs in
preclinical development. Learn more by visiting www.xiliotx.com and
follow us on LinkedIn (Xilio Therapeutics, Inc.).
Cautionary Note Regarding
Forward-Looking Statements
This press release contains forward-looking
statements within the meaning of the Private Securities Litigation
Reform Act of 1995, as amended, including, without limitation,
statements regarding plans to present progress across Xilio’s
pipeline of tumor-activated molecules, including Phase 1/2 clinical
data for XTX202; the potential benefits of any of Xilio’s current
or future product candidates in treating patients; and Xilio’s
strategy, goals and anticipated financial performance, milestones,
business plans and focus. The words “aim,” “may,” “will,” “could,”
“would,” “should,” “expect,” “plan,” “anticipate,” “intend,”
“believe,” “estimate,” “predict,” “project,” “potential,”
“continue,” “seek,” “target” and similar expressions are intended
to identify forward-looking statements, although not all
forward-looking statements contain these identifying words. Any
forward-looking statements in this press release are based on
management’s current expectations and beliefs and are subject to a
number of important risks, uncertainties and other factors that may
cause actual events or results to differ materially from those
expressed or implied by any forward-looking statements contained in
this press release, including, without limitation, risks and
uncertainties related to ongoing and planned research and
development activities, including initiating, conducting or
completing preclinical studies and clinical trials and the timing
and results of such preclinical studies or clinical trials; the
delay of any current or planned preclinical studies or clinical
trials or the development of Xilio’s current or future product
candidates; Xilio’s ability to obtain and maintain sufficient
preclinical and clinical supply of current or future product
candidates; Xilio’s advancement of multiple early-stage programs;
interim or preliminary preclinical or clinical data or results,
which may not be predictive of future preclinical or clinical data
or results; Xilio’s ability to successfully demonstrate the safety
and efficacy of its product candidates and gain approval of its
product candidates on a timely basis, if at all; results from
preclinical studies or clinical trials for Xilio’s product
candidates, which may not support further development of such
product candidates; actions of regulatory agencies, which may
affect the initiation, timing and progress of current or future
clinical trials; Xilio’s ability to obtain, maintain and enforce
patent and other intellectual property protection for current or
future product candidates; Xilio’s ability to obtain and maintain
sufficient cash resources to fund its operations beyond the end of
the second quarter of 2024; the impact of international trade
policies on Xilio’s business, including U.S. and China trade
policies; and Xilio’s ability to maintain its clinical trial
collaboration with Roche to develop XTX101 in combination with
atezolizumab. These and other risks and uncertainties are described
in greater detail in the sections entitled “Risk Factor Summary”
and “Risk Factors” in Xilio’s filings with the U.S. Securities and
Exchange Commission (SEC), including Xilio’s most recent Quarterly
Report on Form 10-Q and any other filings that Xilio has made
or may make with the SEC in the future. Any forward-looking
statements contained in this press release represent Xilio’s views
only as of the date hereof and should not be relied upon as
representing its views as of any subsequent date. Except as
required by law, Xilio explicitly disclaims any obligation to
update any forward-looking statements.
This press release contains hyperlinks to
information that is not deemed to be incorporated by reference in
this press release.
For Investor and Media Inquiries:
Julissa VianaVice President, Head of Investor Relations and
Corporate Communicationsinvestors@xiliotx.com
Melissa ForstArgot PartnersXilio@argotpartners.com
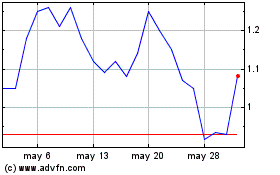
Xilio Therapeutics (NASDAQ:XLO)
Gráfica de Acción Histórica
De Nov 2024 a Dic 2024
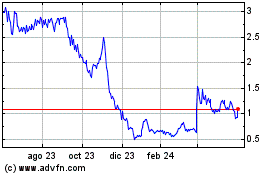
Xilio Therapeutics (NASDAQ:XLO)
Gráfica de Acción Histórica
De Dic 2023 a Dic 2024