Futura Medical Says FDA Agrees to Design of Study on MED3000 Erectile Dysfunction Drug
19 Julio 2021 - 1:44AM
Noticias Dow Jones
By Matteo Castia
Futura Medical PLC said Monday that the Food and Drug
Administration has agreed to the design of the non-clinical human
factor study on the group's MED3000 treatment for erectile
dysfunction.
The U.K. pharmaceutical developer, which focuses on sexual
health and pain relief, said the study will test the ability of
subjects to self-diagnose their erectile dysfunction, correctly
select the product based on label information and test their
ability to correctly use the product without supervision of a
doctor.
The company said it aims to launch the product on the market
during 2022 and that manufacturing scale-up and capacity to meet
projected demand is progressing well.
Write to Matteo Castia at matteo.castia@dowjones.com
(END) Dow Jones Newswires
July 19, 2021 02:43 ET (06:43 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
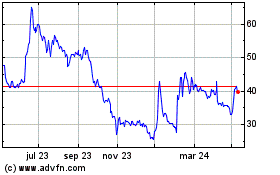
Futura Medical (LSE:FUM)
Gráfica de Acción Histórica
De Abr 2024 a May 2024
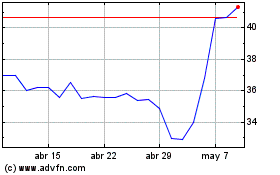
Futura Medical (LSE:FUM)
Gráfica de Acción Histórica
De May 2023 a May 2024