0001980845false00-000000000019808452024-09-262024-09-260001980845engn:WarrantsMember2024-09-262024-09-260001980845us-gaap:CommonStockMember2024-09-262024-09-26
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): September 26, 2024 |
enGene Holdings Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
British Columbia |
001-41854 |
Not applicable |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
4868 Rue Levy, Suite 220 |
|
Saint-Laurent, Quebec, Canada |
|
H4R 2P1 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: 514 332-4888 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Shares |
|
ENGN |
|
The Nasdaq Stock Market LLC |
Warrants, each exercisable for one Common Share, at an exercise price of $11.50 per Share |
|
ENGNW |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Item 7.01 Regulation FD Disclosure.
On September 26, 2024, enGene Holdings Inc. (the “Company”) issued a press release announcing preliminary data from the ongoing pivotal cohort of the LEGEND study of the Company’s non-viral lead investigational product detalimogene voraplasmid, (also known as detalimogene, and previously EG-70) in patients with high-risk, Bacillus Calmette-Guérin (“BCG”)-unresponsive, non-muscle invasive bladder cancer (“NMIBC”) with carcinoma in situ (“Cis”), a copy of which is furnished herewith as Exhibit 99.1 to this Current Report on Form 8-K. In addition, on September 26, 2024, the Company announced that it intends to host a conference call to discuss the preliminary data. A form of the slide presentation to be used during that conference call is being furnished as Exhibit 99.2 to this Current Report on Form 8-K.
The information in this Item 7.01, including Exhibit 99.1 and Exhibit 99.2, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing.
Item 8.01 Other Events.
The LEGEND study is a multi-cohort study to establish efficacy of detalimogene in high-risk BCG-unresponsive, NMIBC patients with Cis, as well as those with papillary-only NMIBC. The ongoing pivotal cohort is an approximately 100 patient, open-label study evaluating detalimogene in BCG-unresponsive NMIBC patients with Cis. Patients receive 800µg/mL of detalimogene intravesically at weeks one, two, five, and six during a 12-week cycle, for up to four cycles, with assessments at three, six, nine, and 12 months. The study’s primary efficacy endpoint is the percentage of patients with a complete response at 12 months, based on cystoscopy, urine cytology and biopsy.
On September 26, 2024, the Company announced preliminary data from the ongoing pivotal cohort of the LEGEND study. As of the September 13, 2024 data cutoff date (the “data cutoff date”), 21 patients in the pivotal cohort had been assessed for response at three months, including 17 patients that had also been assessed for response at six months. Of the 21 patients, 15 were male and six were female, with a median age of 74 years (range 59-92). The patients had a median of 11 prior doses of BCG treatment (range 8-33) and their tumor stages were T1 plus Cis (three patients, 14.3%), Ta plus Cis (three patients, 14.3%) and Cis only (15 patients, 71.4%).
As of the data cutoff date, 71% of patients (15 of 21) achieved a complete response (“CR”) at any time, 67% of patients (14 of 21) achieved a CR at three months and 47% of patients (8 of 17) achieved a CR at six months. In the 21 patients, the complete response rate at six months was estimated using a Kaplan-Meier analysis to be 51%.
Detalimogene was generally well-tolerated by patients with no discontinuations due to treatment-related adverse events (“TRAEs”). Of the 42 patients in the safety analysis, which included all patients dosed in the LEGEND Phase 2 cohorts as of the data cutoff date, 20 patients (47.6%) experienced any grade TRAE, 17 patients (40.5%) experienced a Grade 1 TRAE, nine patients experienced a Grade 2 TRAE and two patients experienced a Grade 3 TRAE (peripheral edema and urosepsis). There were no Grade 4 or Grade 5 TRAEs reported. The most common TRAEs (≥10%) were dysuria (eight Grade 1; one Grade 2), bladder spasm (four Grade 1; four Grade 2), pollakiuria (five Grade 1; no Grade 2) and fatigue (three Grade 1; two Grade 2).
Item 9.01 Financial Statements and Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
ENGENE HOLDINGS INC. |
|
|
|
|
Date: |
September 26, 2024 |
By: |
/s/ Ronald H. W. Cooper |
|
|
Name: Title: |
Ronald H. W. Cooper
Chief Executive Officer |
Exhibit 99.1

Detalimogene Demonstrates 71% Complete Response Rate at Any Time in Preliminary Analysis of LEGEND Pivotal Cohort
Data from pivotal cohort demonstrate compelling clinical activity, consistent with Phase 1 results
Favorable tolerability profile with no drug-related discontinuations
Detalimogene’s profile to date supports its potential as a foundational therapy for NMIBC
enGene to host a conference call to discuss preliminary data today at 8:00 a.m. ET
BOSTON and MONTREAL, September 26, 2024 – enGene Holdings Inc. (Nasdaq: ENGN or “enGene” or the “Company”), is a clinical-stage genetic medicines company whose non-viral lead investigational product detalimogene voraplasmid (also known as detalimogene, and previously EG-70) is in an ongoing pivotal study in patients with high-risk, Bacillus Calmette-Guérin (BCG)-unresponsive, non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (Cis). Today, the Company announced preliminary data from 21 patients assessed at three months, including 17 patients who were also assessed at six months, in the ongoing pivotal cohort of the LEGEND study. The Complete Response (CR) rate at any time was 71%, the CR rate at three months was 67% and the CR rate at six months was 47%. Detalimogene was generally well-tolerated by patients and no patients discontinued due to treatment-related adverse events.
“We are pleased with this preliminary dataset, which clearly demonstrates that detalimogene is highly active and generally well tolerated. The data are consistent with what was observed in Phase 1 and give us increased confidence in the emerging profile,” said Raj Pruthi, M.D., Chief Medical Officer of enGene. “We are also planning protocol refinements in LEGEND, which we believe will provide patients with additional clinical benefit.”
“The promising initial efficacy and safety data observed in LEGEND’s pivotal cohort, combined with detalimogene’s ease of handling, administration, minimal storage requirements, and lack of post-procedural patient restrictions underscore its potential to become a broadly utilized treatment option for NMIBC patients, especially in the community practice setting,” remarked Suzanne Merrill, M.D., a urologist specializing in bladder cancer with the United Urology Group in Colorado.
Bladder cancer is a top 10 cancer by incidence in the US with high annual treatment cost. NMIBC represents more than 75% of bladder cancer diagnosis and over 70% of urologists practice in the community setting where the majority of these patients are treated.
“Detalimogene was designed to be the most practical therapy for urologists to manage NMIBC,” said Ron Cooper, Chief Executive Officer of enGene. “The early results from our pivotal LEGEND study clearly demonstrate that detalimogene has the potential to offer a highly differentiated profile by striking an optimal balance between efficacy, tolerability, and ease of use.”
Safety Information
The overall tolerability profile associated with detalimogene was favorable, and there have been no drug-related discontinuations in the study. Of the 42 patients assessed for safety, inclusive of all Phase 2 cohorts, 20 patients (48%) experienced at least one treatment-related adverse event (TRAE), which were mainly Grade 1/2 in severity, except for two Grade 3 TRAEs (peripheral edema and urosepsis). The most common TRAEs (≥10%) were dysuria, bladder spasm, pollakiuria and fatigue. There were no Grade 4 or Grade 5 TRAEs reported.
About LEGEND
The LEGEND study is a multi-cohort study to establish efficacy of detalimogene in high-risk NMIBC patients with Cis, as well as those with papillary-only NMIBC. The pivotal cohort is an approximately 100 patient, open-label study evaluating detalimogene in BCG-unresponsive NMIBC patients with Cis. Patients receive 800µg/mL of detalimogene intravesically at weeks one, two, five, and six during a 12-week cycle, for up to four cycles, with assessments at three, six, nine, and 12 months. The study’s primary efficacy endpoint is the percentage of patients with a complete response at 12 months, based on cystoscopy, urine cytology and biopsy.
In addition to LEGEND’s pivotal cohort, the Company plans to initiate enrollment of three additional cohorts in the LEGEND study: (i) high-risk BCG-exposed patients with Cis and BCG-naïve patients with Cis in cohorts 2a and 2b, respectively, and (ii) high-risk BCG-unresponsive NMIBC patients with papillary-only disease in cohort 3. Enrollment for cohorts 2a, 2b, and 3 is expected to begin in the fourth quarter of 2024. The Company remains on track to file a Biologics License Application (BLA) for detalimogene in its pivotal cohort in mid-2026.
Investor Conference Call
enGene will host a conference call and live webcast at 8:00 a.m. ET today, September 26, 2024. Individuals interested in listening to the conference call may do so by using the webcast link available at the “Investors" section of the Company's website at www.engene.com/investors. Following the live webcast, an archived version of the call will also be available on the website.
About enGene
enGene is a clinical-stage biotechnology company mainstreaming genetic medicines through the delivery of therapeutics to mucosal tissues and other organs, with the goal of creating new ways to address diseases with high clinical needs. enGene’s lead program is detalimogene voraplasmid, (also known as detalimogene, and previously EG-70) for patients with Non-Muscle Invasive Bladder Cancer (NMIBC) – a disease with a high clinical burden. Detalimogene is being evaluated in the ongoing multi-cohort LEGEND Phase 2 study, which includes a pivotal cohort studying detalimogene in Bacillus Calmette Guérin (BCG)-unresponsive patients with carcinoma in situ (Cis). Detalimogene was developed using enGene’s proprietary Dually Derivatized Oligochitosan (DDX) platform, which enables penetration of mucosal tissues and delivery of a wide range of sizes and types of cargo, including DNA and various forms of RNA. For more information, visit enGene.com.
Forward-Looking Statements
Some of the statements contained in this press release may constitute forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, and “forward-looking information” within the meaning of Canadian securities laws (collectively, “forward-looking statements”). enGene’s forward-looking statements include, but are not limited to, statements regarding enGene’s expectations, hopes, beliefs, intentions, goals, strategies, forecasts and projections. The words “anticipate”, “appear”, “approximate”, “believe”, “continue”, “could”, “estimate”, “expect”, “foresee”, “intend”, “may”, “might”, “plan”, “possible”, “potential”, “predict”, “project”, “seek”, “should”, “would”, and similar expressions may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking. Forward-looking statements may include, for example, statements about: our beliefs as to the potential benefits of detalimogene, our plans to amend the LEGEND protocol, our plans regarding expansion and modification of the LEGEND study for potential additional bladder cancer indications for detalimogene, our expectations regarding timing of the enrollment of patients in the additional cohorts of the LEGEND study, and our expectations regarding regulatory submissions.
Many factors, risks, uncertainties and assumptions could cause the Company’s actual results, performance or achievements to differ materially from those expressed or implied by the forward-looking statements, including, without limitation, that preliminary clinical data may not accurately reflect the complete results of a particular study and remain subject to audit and verification, and final data may differ materially from preliminary data; the Company’s ability to recruit and retain qualified scientific and management personnel; establish clinical trial sites and enroll patients in its clinical trials; execute on the Company’s clinical development plans and ability to secure regulatory approval on anticipated timelines; and other risks and uncertainties detailed in filings with Canadian securities regulators on SEDAR+ and with the U.S. Securities and Exchange Commission (“SEC”) on EDGAR, including those described in the “Risk Factors” section of the Company’s Annual Report on Form 10-K for the fiscal year ended October 31, 2023 and most recent Quarterly Report on Form 10-Q for the fiscal quarter ended July 31, 2024 (copies of which may be obtained at www.sedarplus.ca or www.sec.gov).
You should not place undue reliance on any forward-looking statements, which speak only as of the date on which they are made. enGene anticipates that subsequent events and developments will cause enGene’s assessments to change. While enGene may elect to update these forward-looking statements at some point in the future, enGene specifically disclaims any obligation to do so, unless required by applicable law. Nothing in this press release should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved.
Contact:
For media contact: media@engene.com
For investor contact: investors@engene.com

Non-Viral Genetic Medicine Preliminary Data from LEGEND Pivotal Cohort September 26, 2024 Exhibit 99.2

Cautionary Statement Regarding Forward-Looking Statements This Presentation contains certain forward-looking statements within the meaning of the federal securities laws and "forward-looking information" within the meaning of Canadian securities laws (collectively, "forward-looking statements"). Forward-looking statements may be identified by the use of the words such as “plan”, “forecast”, “intend”, “development”, “expect”, “anticipate”, “become”, “believe”, “continue”, “could”, “estimate”, “expect”, “intends”, “may”, “might”, “plan”, “possible”, “project”, “should”, “would”, “strategy”, “future”, “potential”, “opportunity”, “target”, “term”, “will”, “would”, “will be” or similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These forward-looking statements include, but are not limited to, statements regarding the potential benefits of detalimogene, the anticipated market acceptance of detalimogene, plans for research and development, estimates and forecasts of financial and performance metrics, projections of market opportunity and market share, expectations and timing related to regulatory submissions and commercial product launches and the prospects for regulatory approval of detalimogene. These forward-looking statements are based on various estimates and assumptions, whether or not identified in this presentation, and on the current expectations of the management of enGene Holdings Inc. ("enGene"), are not predictions of annual performance, and are subject to risks and uncertainties. These forward-looking statements are subject to a number of risks and uncertainties, including but not limited to, those described in the “Risk Factors” sections of enGene’s Annual Report on Form 10-K for the fiscal year ended October 31, 2023, Quarterly Reports on Form 10-Q for the fiscal quarters ended January 31, 2024, April 30, 2024 and July 31, 2024, each of which has been filed with the Securities and Exchange Commission (“SEC”) and Canadian Securities Regulators (copies of which may be obtained at www.sedarplus.ca or www.sec.gov). You should carefully consider the risks and uncertainties described in the “Risk Factors” section of such Annual Report and Quarterly Reports, as well as other documents if and when filed by enGene from time to time with the SEC and Canadian securities regulators. If any of these risks materialize or our assumptions prove incorrect, actual events and results could differ materially from those contained in the forward-looking statements. There may be additional risks that enGene presently knows or that enGene currently believes are immaterial that could also cause actual events and results to differ. In addition, forward-looking statements reflect enGene’s expectations, plans, or forecasts of future events and views as of the date of this presentation. enGene anticipates that subsequent events and developments will cause enGene’s assessments to change. While enGene may elect to update these forward-looking statements at some point in the future, enGene specifically disclaim any obligation to do so, unless required by applicable law. These forward-looking statements should not be relied upon as representing enGene’s assessments as of any date subsequent to the date of this presentation. Accordingly, undue reliance should not be placed upon the forward-looking statements contained herein Intellectual Property This Presentation contains trademarks, service marks, trade names, copyrights, and products of enGene and other companies, which are the property of their respective owners. The use or display of third parties’ trademarks, service marks, trade names, copyrights, or products in this Presentation is not intended to, and does not, imply a relationship with enGene, or an endorsement of or sponsorship by enGene. Solely for convenience, the trademarks, service marks, and trade names referred to in this Presentation may appear without the ®, TM or SM symbols, but such references are not intended to indicate, in any way, that enGene will not assert, to the fullest extent permitted under applicable law, their rights or the right of the applicable licensor in such trademarks, service marks and trade names. Industry and Market Data This Presentation relies on and refers to certain information and statistics based on estimates by enGene’s management and/or obtained from third party sources which enGene believes to be reliable. enGene has not independently verified the accuracy or completeness of any such third party information, which involves elements of subjective judgment and analysis that may or may not prove to be accurate. None enGene, or its affiliates or any third parties that provide information to enGene or its affiliates, such as market research firms, guarantees the accuracy, completeness, timeliness, or availability of any information. None enGene, or its affiliates, or any third parties that provide information to enGene, and its affiliates, such as market research firms, is responsible for any errors or omissions (negligent or otherwise), regardless of the cause, or the results obtained from the use of such content. enGene may have supplemented such information where necessary, taking into account publicly available information about other industry participants and enGene management’s best view as to information that is not publicly available. Neither enGene nor its affiliates give any express or implied warranties with respect to the information included herein, including, but not limited to, any warranties regarding its accuracy or of merchantability or fitness for a particular purpose or use, and they expressly disclaim any responsibility or liability for direct, indirect, incidental, exemplary, compensatory, punitive, special, or consequential damages, costs, expenses, legal fees, or losses (including lost income or profits and opportunity costs) in connection with the use of the information herein. Lead Program (detalimogene voraplasmid) The lead program described herein is an investigational drug therapy, which has not been approved for marketing by the U.S. Food and Drug Administration or any other regulatory agency and that has not been subject to testing designed to demonstrate that the therapy is effective in humans or to provide a basis to predict in advance whether an adequate level of efficacy in humans will be demonstrated in further testing. Although deemed sufficient to permit further testing, the limited, early Phase 1 testing to date is not a sufficient basis on which to predict efficacy or safety and no representation is made as to detalimogene’s efficacy or safety. Although the FDA has indicated that the Phase 2 portion of the current LEGEND study may potentially support BLA approval, that outcome will depend entirely on the results of Phase 2 clinical testing, which are not expected to be available until 2026. Disclaimers
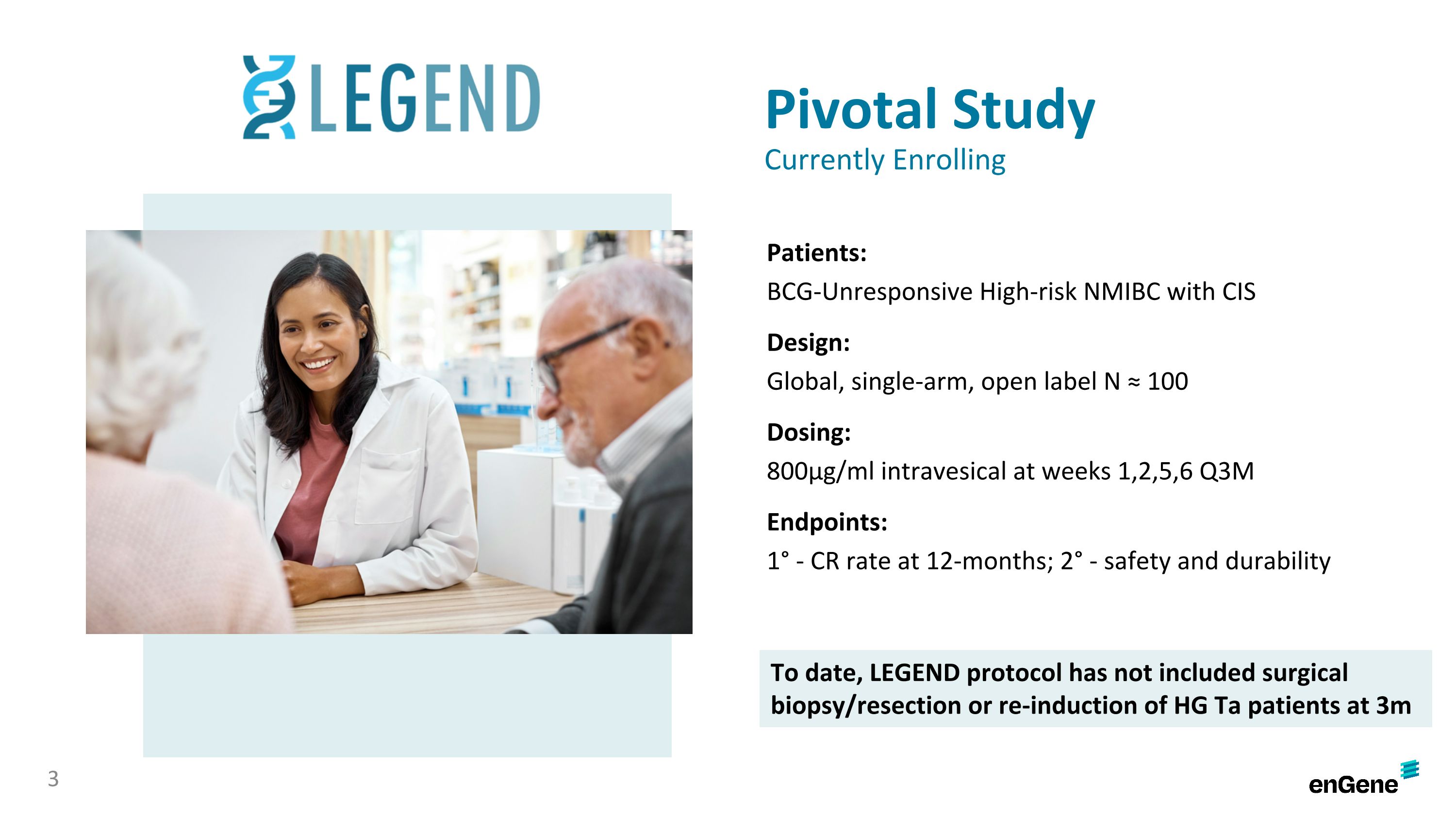
Pivotal Study Currently Enrolling Patients: BCG-Unresponsive High-risk NMIBC with CIS Design: Global, single-arm, open label N ≈ 100 Dosing: 800μg/ml intravesical at weeks 1,2,5,6 Q3M Endpoints: 1° - CR rate at 12-months; 2° - safety and durability 3 To date, LEGEND protocol has not included surgical biopsy/resection or re-induction of HG Ta patients at 3m
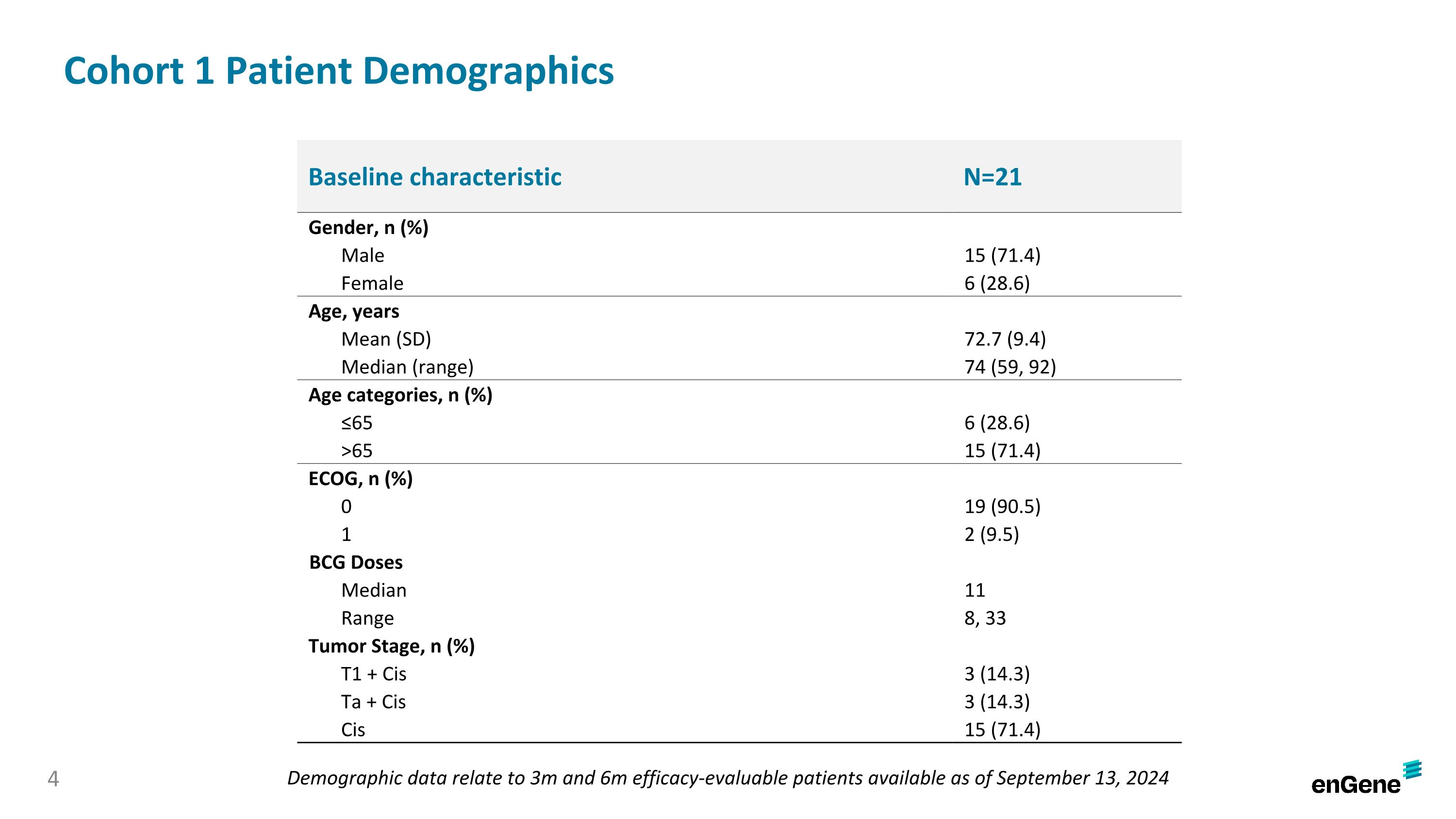
Cohort 1 Patient Demographics Baseline characteristic N=21 Gender, n (%) Male 15 (71.4) Female 6 (28.6) Age, years Mean (SD) 72.7 (9.4) Median (range) 74 (59, 92) Age categories, n (%) ≤65 6 (28.6) >65 15 (71.4) ECOG, n (%) 0 19 (90.5) 1 2 (9.5) BCG Doses Median 11 Range 8, 33 Tumor Stage, n (%) T1 + Cis 3 (14.3) Ta + Cis 3 (14.3) Cis 15 (71.4) Demographic data relate to 3m and 6m efficacy-evaluable patients available as of September 13, 2024 4
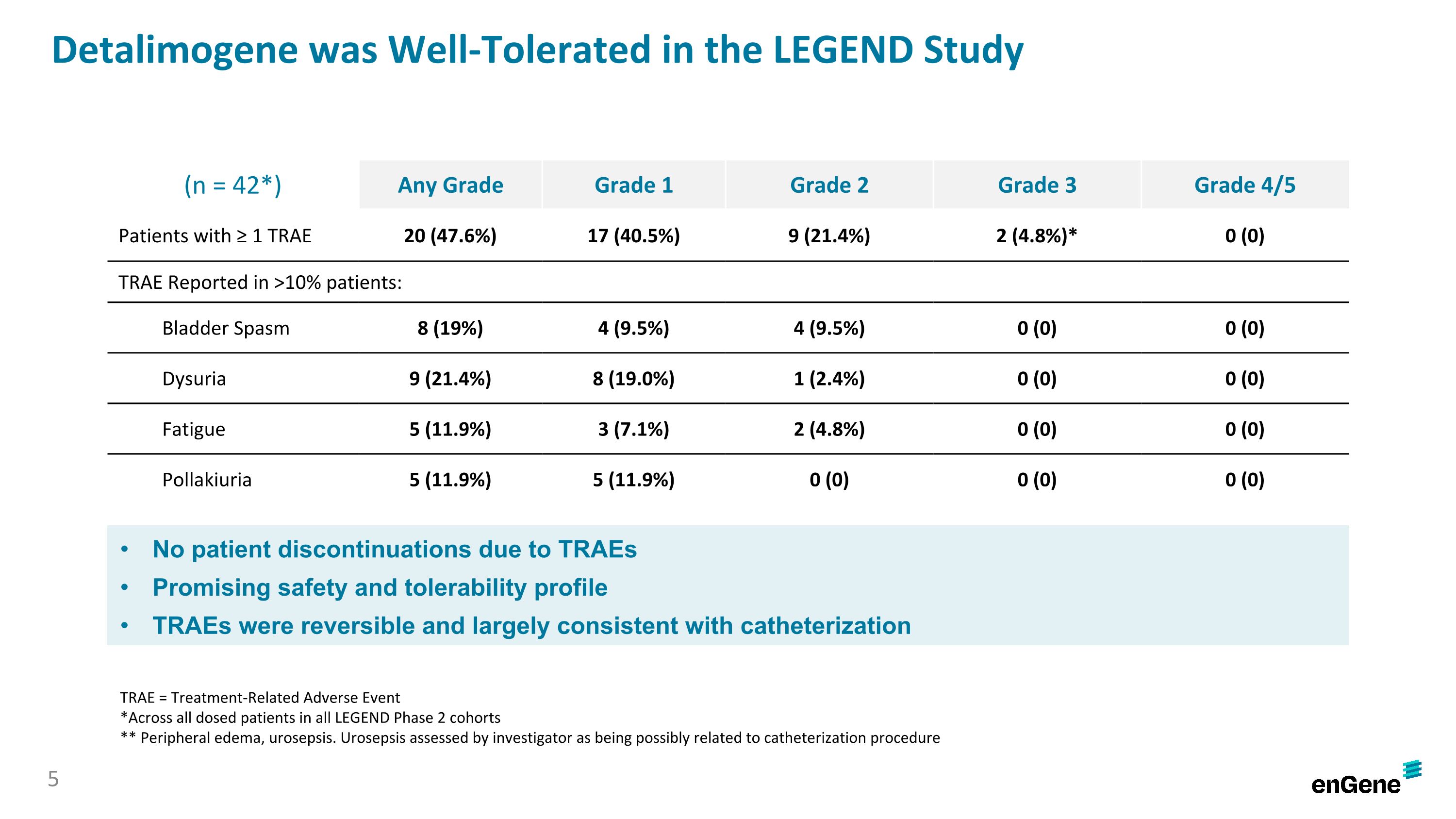
Detalimogene was Well-Tolerated in the LEGEND Study (n = 42*) Any Grade Grade 1 Grade 2 Grade 3 Grade 4/5 Patients with ≥ 1 TRAE 20 (47.6%) 17 (40.5%) 9 (21.4%) 2 (4.8%)* 0 (0) TRAE Reported in >10% patients: Bladder Spasm 8 (19%) 4 (9.5%) 4 (9.5%) 0 (0) 0 (0) Dysuria 9 (21.4%) 8 (19.0%) 1 (2.4%) 0 (0) 0 (0) Fatigue 5 (11.9%) 3 (7.1%) 2 (4.8%) 0 (0) 0 (0) Pollakiuria 5 (11.9%) 5 (11.9%) 0 (0) 0 (0) 0 (0) No patient discontinuations due to TRAEs Promising safety and tolerability profile TRAEs were reversible and largely consistent with catheterization TRAE = Treatment-Related Adverse Event *Across all dosed patients in all LEGEND Phase 2 cohorts ** Peripheral edema, urosepsis. Urosepsis assessed by investigator as being possibly related to catheterization procedure
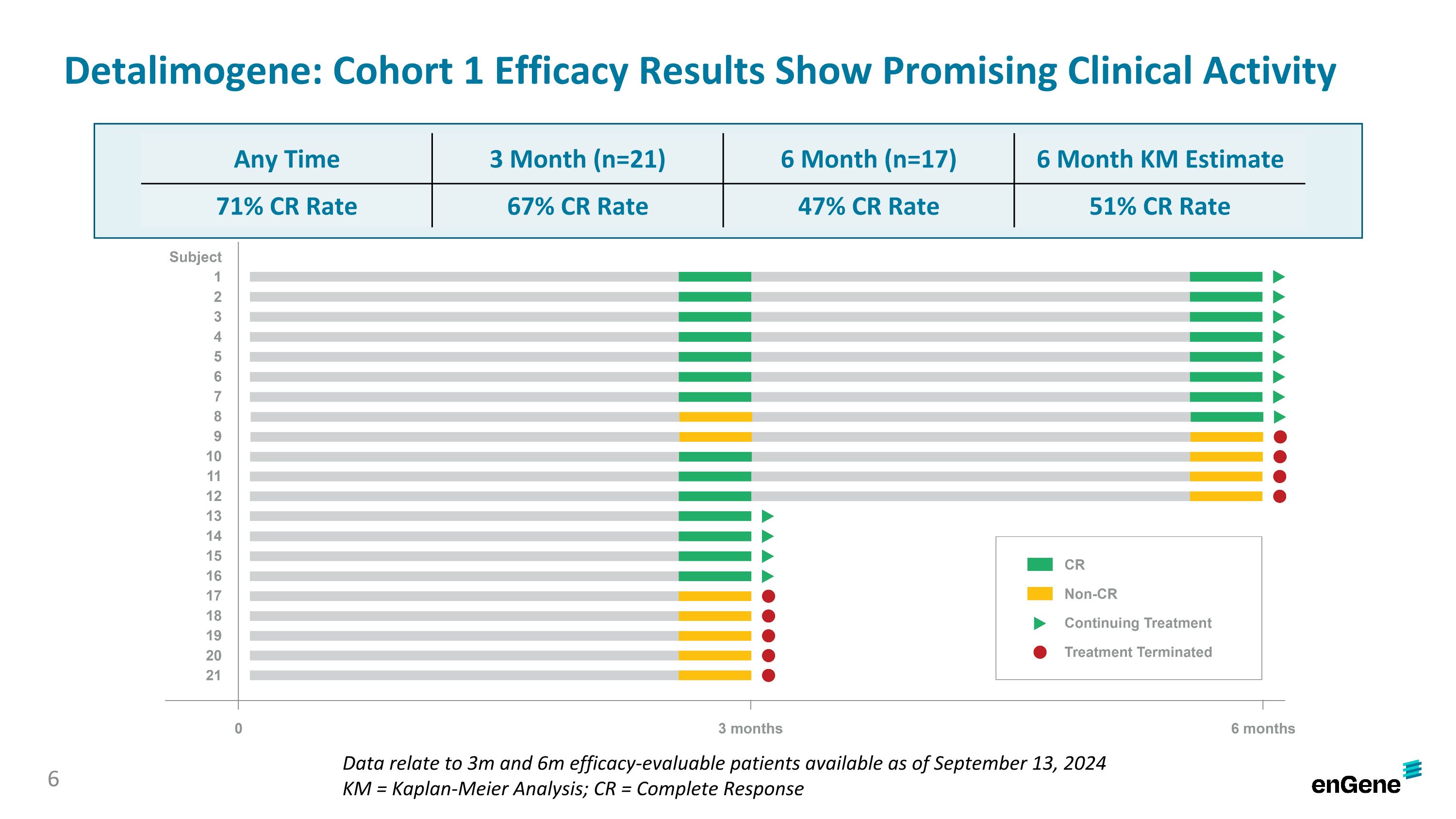
Detalimogene: Cohort 1 Efficacy Results Show Promising Clinical Activity Data relate to 3m and 6m efficacy-evaluable patients available as of September 13, 2024 KM = Kaplan-Meier Analysis; CR = Complete Response Any Time 3 Month (n=21) 6 Month (n=17) 6 Month KM Estimate 71% CR Rate 67% CR Rate 47% CR Rate 51% CR Rate 6
Detalimogene: Designed To Be the First-Choice Therapy 71% CR rate at any time highlights promising activity Promising safety profile observed; no disconnections due to tolerability Convenient product attributes support community use Evolving care paradigm Global expansion expected to further enrollment (US, Canada, EU, Asia Pacific) + + + 7
v3.24.3
Document And Entity Information
|
Sep. 26, 2024 |
| Document Information [Line Items] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Sep. 26, 2024
|
| Entity Registrant Name |
enGene Holdings Inc.
|
| Entity Central Index Key |
0001980845
|
| Entity Emerging Growth Company |
true
|
| Entity File Number |
001-41854
|
| Entity Incorporation, State or Country Code |
A1
|
| Entity Tax Identification Number |
00-0000000
|
| Entity Address, Address Line One |
4868 Rue Levy, Suite 220
|
| Entity Address, City or Town |
Saint-Laurent
|
| Entity Address, State or Province |
QC
|
| Entity Address, Country |
CA
|
| Entity Address, Postal Zip Code |
H4R 2P1
|
| City Area Code |
514
|
| Local Phone Number |
332-4888
|
| Entity Information, Former Legal or Registered Name |
Not Applicable
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity Ex Transition Period |
true
|
| Common Stock [Member] |
|
| Document Information [Line Items] |
|
| Title of 12(b) Security |
Common Shares
|
| Trading Symbol |
ENGN
|
| Security Exchange Name |
NASDAQ
|
| Warrants [Member] |
|
| Document Information [Line Items] |
|
| Title of 12(b) Security |
Warrants, each exercisable for one Common Share, at an exercise price of $11.50 per Share
|
| Trading Symbol |
ENGNW
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionISO 3166-1 alpha-2 country code.
| Name: |
dei_EntityAddressCountry |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:countryCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=engn_WarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
enGene (NASDAQ:ENGNW)
Gráfica de Acción Histórica
De Oct 2024 a Nov 2024

enGene (NASDAQ:ENGNW)
Gráfica de Acción Histórica
De Nov 2023 a Nov 2024
