As
filed with the Securities and Exchange Commission on December 31, 2024
Registration
No. 333-283927
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
PRE-EFFECTIVE
AMENDMENT NO. 1
TO
FORM
S-1
REGISTRATION
STATEMENT
UNDER
THE
SECURITIES ACT OF 1933
ORAGENICS,
INC.
(Exact
name of registrant as specified in its charter)
| Florida |
|
59-3410522 |
(State
or other jurisdiction of
incorporation
or organization) |
|
(I.R.S.
Employer
Identification
No.) |
1990
Main Street, Suite 750
Sarasota,
Florida 34236
(813)
286-7900
(Address,
including zip code, and telephone number, including area code, of principal executive offices)
Janet
Huffman, Chief Financial Officer
Oragenics,
Inc.
1990
Main Street, Suite 750
Sarasota,
Florida 34236
(813)
286-7900
(Name,
address, including zip code, and telephone number, including area code, of agent for service)
With
copies to:
Mark
A. Catchur, Esq.
Julio
C. Esquivel, Esq.
Shumaker,
Loop & Kendrick, LLP
101
East Kennedy Boulevard
Suite
2800
Tampa,
Florida 33602
Telephone:
(813) 229-7600
Facsimile:
(813) 229-1660 |
|
Ralph
V. DeMartino, Esq.
Marc
Rivera, Esq.
ArentFox
Schiff LLP
1717
K Street NW
Washington,
DC 20006
Telephone:
(202) 350-3643
Facsimile:
(202) 857-6395 |
Approximate
date of commencement of proposed sale to the public: From time to time after this registration statement becomes effective.
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the
Securities Act of 1933, other than securities offered only in connection with dividend or interest reinvestment plans, check the following
box. ☒
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the
following box and list the Securities Act registration statement number of the earlier effective registration statement for the same
offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting
company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,”
“smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large
accelerated filer |
☐ |
Accelerated
filer |
☐ |
| |
|
|
|
| Non-accelerated
filer |
☒ |
Smaller
reporting company |
☒ |
| |
|
|
|
| |
|
Emerging
growth company |
☐ |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act. ☐
The
Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the
Registrant shall file a further amendment which specifically states that the Registration Statement shall thereafter become effective
in accordance with Section 8(A) of the Securities Act of 1933, or until this registration statement shall become effective on such date
as the Commission, acting pursuant to section 8(a), may determine.
The
information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement
filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and is not soliciting
an offer to buy these securities in any state where the offer or sale is not permitted.
| PRELIMINARY
PROSPECTUS |
|
SUBJECT
TO COMPLETION |
|
DATED,
DECEMBER 31, 2024 |
Up
to 15,151,515 Units, consisting of One Share of Common Stock
or
One Pre-Funded Warrant and
One
Common Warrant to Purchase One Share of Common
Stock
Up
to 15,151,515 Shares of Common Stock underlying such Common Warrants
Up
to 15,151,515 Shares of Common Stock underlying such Pre-Funded Warrants
Placement
Agent Warrants to Purchase an Aggregate of up to 757,576 Shares of Common Stock
Up
to 757,576 Shares of Common Stock Underlying the Placement Agent Warrants

This
is a reasonable best efforts public offering of up to 15,151,515 units consisting of one share of common stock, par value $0.001 per
share (“Common Stock”) (or one Pre-Funded Warrant to purchase one share of our Common Stock in lieu thereof), and one common
warrant to purchase one share of our Common Stock (the “Common Warrants” and together with the shares of Common Stock, the
“Units”) at an assumed public offering price of $0.33 per Unit, the last reported sale price of our common stock as reported
on NYSE American, or the “NYSE American,” on December 13, 2024. The actual public offering price per Unit will be determined
between us and the Placement Agent at the time of pricing and may be at a discount to this assumed offering price. Therefore, the assumed
public offering price used throughout this prospectus may not be indicative of the final offering price.
The
Units have no stand-alone rights and will not be certificated or issued as stand-alone securities. Each Common Warrant offered hereby
is exercisable upon issuance at an initial exercise price of $0.33 (assuming an offering price of $0.33 per Unit) per share of common
stock, and will expire five (5) years from the date of issuance.
We
are also offering to each purchaser of Units whose purchase of shares of our Common Stock in this offering would otherwise result in
the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of
the holder, 9.99%) of our outstanding shares of Common Stock immediately following consummation of this offering, the opportunity to
purchase, if the purchaser so chooses, Units consisting of one Pre-Funded Warrant to purchase one share of Common Stock (“Pre-Funded
Warrants”), and one Common Warrant. Each Pre-Funded Warrant will be exercisable immediately upon issuance, will expire when exercised
in full, and will be exercisable for one share of our Common Stock. The purchase price of each Unit including a Pre-Funded Warrant will
equal the price per Unit of the Units including one share of Common Stock being sold to the public in this offering, minus $0.001, and
the exercise price of each Pre-Funded Warrant will be $0.001 per share. For each Unit including a Pre-Funded Warrant that we sell, the
number of Units including one share of our Common Stock that we are offering will be decreased on a one-for-one basis.
The
Common Stock and Prefunded Warrants can each be purchased in this offering only with the accompanying Common Warrants that are part of
a Unit, but the components of the Units will be immediately separable and will be issued separately in this offering. See “Description
of Securities” in this prospectus for more information.
This
offering also relates to the shares of Common Stock issuable upon exercise of the Common Warrants (the “Common Warrant Shares”),
the shares of Common Stock issuable upon exercise of the Pre-Funded Warrants (the “Pre-Funded Warrant Shares”), and the shares
of Common Stock issuable upon exercise of Placement Agent Warrants (defined below).
There
is no established public trading market for the Units, the Common Warrants or the Pre-Funded Warrants, and we do not expect a
market to develop. We do not intend to apply for listing of the Units, the Common Warrants or the Pre-Funded Warrants on any securities
exchange or other nationally recognized trading system. Without an active trading market, the liquidity of the Units, the Common Warrants
and the Pre-Funded Warrants will be limited.
Our
Common Stock is listed on NYSE American under the symbol “OGEN.” On December 13, 2024, the closing sale price of our common
stock was $0.33 per share.
Investing
in our securities involves a high degree of risk. You should review carefully the disclosures described under the heading “Risk
Factors” beginning on page 14 of this prospectus and in documents that are incorporated by reference into this prospectus
for a discussion of the risks and uncertainties that should be considered in connection with an investment in our securities.
We
have engaged Dawson James Securities, Inc. to act as our placement agent (the “Placement Agent”) in connection with the securities
offered by this prospectus. The Placement Agent has agreed to use its reasonable best efforts to arrange for the sale of the securities
offered by this prospectus. The Placement Agent is not purchasing or selling any of the securities we are offering, and the Placement
Agent is not required to arrange the purchase or sale of any specific number or dollar amount of securities. We have agreed to pay to
the Placement Agent the Placement Agent fees set forth in the table below, which assumes that we sell all of the securities offered by
this prospectus. There is no arrangement for funds to be received in escrow, trust or similar arrangement. There is no minimum number
of shares of securities or minimum aggregate amount of proceeds that is a condition for this offering to close. We may sell fewer than
all of the securities offered hereby, which may significantly reduce the amount of proceeds received by us, and investors in this offering
will not receive a refund if we do not sell all of the securities offered hereby. Because there is no escrow account and no minimum number
of securities or amount of proceeds, investors could be in a position where they have invested in us, but we have not raised sufficient
proceeds in this offering to adequately fund the intended uses of the proceeds as described in this prospectus. We will bear all costs
associated with the offering. See “Plan of Distribution” on page 29 of this prospectus for more information regarding
these arrangements. This offering will terminate no later than January 31, 2025, unless we decide to terminate the offering (which we
may do at any time in our discretion) prior to that date.
We
have agreed to pay the Placement Agent a fee based on the aggregate proceeds raised in this offering as set forth in the table below:
| | |
Per Unit | | |
Total (3) | |
| Public offering price (1) | |
$ | 0.33 | | |
$ | 5,000,000 | |
| Placement Agent fees (2) | |
$ | .0231 | | |
$ | 350,000 | |
| Proceeds, before expenses, to us (4) | |
$ | 0.3069 | | |
$ | 4,650,000 | |
| (1) |
Assumes
the sale of Units including one share of Common Stock. The public offering price of Units
including one Pre-Funded Warrant will be $0.339.
|
| (2) |
We
will pay the Placement Agent a cash fee equal to seven percent (7%) of the aggregate gross proceeds raised in this offering. In addition,
we have agreed to reimburse the Placement Agent for certain offering-related expenses and have agreed to issue to the Placement Agent
warrants to purchase up to five percent 5% of the shares of our Common Stock (including the shares of Common Stock underlying any
Pre-Funded Warrants) sold in the offering with an exercise price equal to 125% of the per share offering price (the “Placement
Agent Warrants”). We refer you to “Plan of Distribution” beginning on page 29 for additional information
regarding compensation to be received by the Placement Agent. |
| |
|
| (3) |
Assuming
the maximum offering amount is sold in this offering. |
| |
|
| (4) |
Because
there is no minimum number of securities or amount of proceeds required as a condition to closing in this offering, the actual public
offering amount, placement agent fees, and proceeds to us, if any, are not presently determinable and may be substantially less than
the total maximum offering amounts set forth above. We estimate the total expenses of this offering payable by us, excluding the
placement agent fee, will be approximately $350,000. |
The
delivery of the shares of Common Stock, Pre-Funded Warrants and Common Warrants to purchasers is expected to be made no later than January
__, 2025.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed
upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
Dawson
James Securities, Inc.
The
date of this prospectus is , 2024.
TABLE
OF CONTENTS
ABOUT
THIS PROSPECTUS
We
incorporate by reference important information into this prospectus. You may obtain the information incorporated by reference without
charge by following the instructions under “Where You Can Find More Information.” You should carefully read this prospectus
as well as additional information described under “Incorporation of Certain Information by Reference,” before deciding to
invest in our securities.
You
should rely only on the information contained in this prospectus. We have not, and the Placement Agent has not, authorized anyone to
provide any information or to make any representations other than those contained in this prospectus or in any free writing prospectuses
prepared by or on behalf of us or to which we have referred you, and we take no responsibility for any other information others may give
you. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give
you.
This
prospectus is an offer to sell only the securities offered hereby, and only under circumstances and in jurisdictions where it is lawful
to do so. We are not making an offer to sell these securities in any jurisdiction where an offer or sale is not permitted. The information
contained in this prospectus or in any applicable free writing prospectus is current only as of its date, regardless of its time of delivery
or any sale of our securities. Our business, financial condition, results of operations and prospects may have changed since that date.
The
information incorporated by reference or provided in this prospectus contains statistical data and estimates, including those relating
to market size and competitive position of the markets in which we participate, that we obtained from our own internal estimates and
research, as well as from industry and general publications and research, surveys and studies conducted by third parties. Industry publications,
studies and surveys generally state that they have been obtained from sources believed to be reliable. While we believe our internal
company research is reliable and the definitions of our market and industry are appropriate, neither this research nor these definitions
have been verified by any independent source.
For
investors outside the United States: We have not, and the Placement Agent has not, done anything that would permit this offering or possession
or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons
outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating
to, the offering of the securities and the distribution of this prospectus outside the United States.
This
prospectus and the information incorporated by reference into this prospectus contain references to our trademarks and to trademarks
belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus and the information incorporated
by reference into this prospectus, including logos, artwork, and other visual displays, may appear without the ® or TM symbols, but
such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights
or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies’
trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other company.
The
representations, warranties and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated
by reference herein were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating
risk among the parties to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such
representations, warranties or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and
covenants should not be relied on as accurately representing the current state of our affairs.
As
used in this prospectus, unless the context indicates or otherwise requires, “the Company,” “our Company,” “we,”
“us,” and “our” refer to Oragenics, Inc., a Florida corporation, and its consolidated subsidiaries.
CAUTIONARY
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus and the documents incorporated by reference herein contain forward-looking statements. These are based on our management’s
current beliefs, expectations and assumptions about future events, conditions and results and on information currently available to us.
Discussions containing these forward-looking statements may be found, among other places, in the sections entitled “Business,”
“Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations”
contained in the documents incorporated by reference herein.
Any
statements in this prospectus, or incorporated herein, about our expectations, beliefs, plans, objectives, assumptions or future events
or performance are not historical facts and are forward-looking statements. Within the meaning of Section 27A of the Securities Act and
Section 21E of the Exchange Act, these forward-looking statements include statements regarding:
| |
● |
Our
need to raise additional capital to continue to implement our business strategy; |
| |
|
|
| |
● |
Our
financial capacity and performance, including our ability to obtain funding, non-dilutive or otherwise, necessary to do the research,
development, manufacture, and commercialization of any one or all of our product candidates; |
| |
|
|
| |
● |
Our
ability to maintain our listing on the NYSE American and the trading market of our common stock; |
| |
|
|
| |
● |
The
timing, progress and results of clinical trials of our product candidates; |
| |
|
|
| |
● |
Uncertainties
regarding submission, approval and scope of filings for regulatory approval of our product candidates and our ability to obtain and
maintain regulatory approvals for our product candidates for any indication; |
| |
|
|
| |
● |
Uncertainties
regarding the potential benefits, activity, effectiveness and safety of our product candidates including as to administration, distribution
and storage; |
| |
|
|
| |
● |
Uncertainties
regarding the size of the patient populations, market acceptance and opportunity for and clinical utility of our product candidates,
if approved for commercial use; |
| |
|
|
| |
● |
Our
manufacturing capabilities and strategy, including the scalability and commercial viability of our manufacturing methods and processes,
and those of our contractual partners; |
| |
|
|
| |
● |
Our
ability to successfully commercialize our product candidates; |
| |
|
|
| |
● |
The
potential benefits of, and our ability to maintain, our relationships and collaborations with the NIAID, the NIH, the NRC and other
potential collaboration or strategic relationships; |
| |
|
|
| |
● |
Uncertainties
regarding our expenses, ongoing losses, future revenue, capital requirements; |
| |
|
|
| |
● |
Our
ability to identify, recruit and retain key personnel and consultants; |
| |
|
|
| |
● |
Our
ability to obtain, retain, protect, and enforce our intellectual property position for our product candidates, and the scope of such
protection; |
| |
|
|
| |
● |
Our
ability to advance the development of our new and existing product candidate under the timelines and in accord with the milestones
projected; |
| |
|
|
| |
● |
Our
need to comply with extensive and costly regulation by worldwide health authorities, who must approve our product candidates prior
to substantial research and development and could restrict or delay the future commercialization of certain of our product candidates; |
| |
● |
Our
ability to successfully complete pre-clinical and clinical development of, and obtain regulatory approval of our product candidates
and commercialize any approved products on our expected timeframes or at all; |
| |
|
|
| |
● |
The
safety, efficacy, and benefits of our product candidates; |
| |
|
|
| |
● |
The
effects of government regulation and regulatory developments, and our ability and the ability of the third parties with whom we engage
to comply with applicable regulatory requirements; |
| |
|
|
| |
● |
The
capacities and performance of our suppliers and manufacturers and other third parties over whom we have limited control; and |
| |
|
|
| |
● |
Our
competitive position and the development of and projections relating to our competitors or our industry. |
In
some cases, you can identify forward-looking statements by the words “may,” “might,” “can,” “will,”
“to be,” “could,” “would,” “should,” “expect,” “intend,” “plan,”
“objective,” “anticipate,” “believe,” “estimate,” “predict,” “project,”
“potential,” “likely,” “continue” and “ongoing,” or the negative of these terms, or other
comparable terminology intended to identify statements about the future, although not all forward-looking statements contain these words.
These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity,
performance or achievements to be materially different from the information expressed or implied by these forward-looking statements.
You
should refer to the “Risk Factors” section contained in this prospectus and any related free writing prospectus, and under
similar headings in the other documents that are incorporated by reference into this prospectus, for a discussion of important factors
that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. Given these
risks, uncertainties and other factors, many of which are beyond our control, we cannot assure you that the forward-looking statements
in this prospectus will prove to be accurate, and you should not place undue reliance on these forward-looking statements. Furthermore,
if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in
these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that
we will achieve our objectives and plans in any specified time frame, or at all.
Except
as required by law, we assume no obligation to update these forward-looking statements publicly, or to revise any forward-looking statements
to reflect events or developments occurring after the date of this prospectus, even if new information becomes available in the future.
PROSPECTUS
SUMMARY
This
summary highlights certain information appearing elsewhere in this prospectus. Because it is only a summary, it does not contain all
of the information that you should consider before investing in our common stock and it is qualified in its entirety by, and should be
read in conjunction with, the more detailed information appearing elsewhere in this prospectus. Before you decide to invest in our common
stock, you should read the entire prospectus carefully, including “Risk Factors” beginning on page 14 and the financial statements
and related notes incorporated by reference to this prospectus.
Our
Company
We
are a development-stage company dedicated to the research and development of nasal delivery pharmaceutical medications in neurology.
Our lead product, the ONP-002 drug, is a fully synthetic, non-naturally occurring neurosteroid, is lipophilic, and it has been shown
in animal models that it can cross the blood-brain barrier rapidly to reduce swelling, oxidative stress and inflammation while restoring
proper blood flow through gene amplification.
On
December 28, 2023, we successfully consummated our previously announced Asset Purchase Agreement with Odyssey Health, Inc. (“Odyssey”),
pursuant to which we purchased all of Odyssey’s assets related to the segment of Odyssey’s business focused on developing
medical products that treat brain related illnesses and diseases (the “Neurology Assets”). The Neurology Assets include drug
candidates for treating mild traumatic brain injury (mTBI), also known as concussion, and for treating Niemann Pick Disease Type C (NPC),
as well as Odyssey’s novel proprietary nasal formulation and its novel breath-powered intranasal delivery device.
As
a result of the acquisition of the Neurology Assets, we expect that, in the near- and mid-terms, we will focus our resources and efforts
on the continued development of the Neurology Assets and primarily ONP-002, which, as discussed further below, has successfully completed
Phase 1 clinical trials. The acquisition is expected to build on our expertise in intranasal platforms and expand our portfolio into
more areas of unmet medical needs. Nasal delivery offers many advantages over standard systemic delivery systems, such as its non-invasive
character, a fast onset of action and in many cases reduced side effects due to a more targeted delivery.
Accordingly,
given our limited resources, we anticipate, for the time being, placing the development of our nasal COVID-19 product candidate and our
lantibiotics program on hold.
In
conjunction with the Neurology Asset acquisition, we paid Odyssey a total of $1,000,000 in cash, $500,000 of which was paid in October,
2023 and $500,000 of which was paid on December 11, 2023. In addition, at the closing, we issued Odyssey 8,000,000 shares of our newly
created Series F Non-Voting Convertible Preferred Stock, which are convertible into our common stock on a one-to-one basis (subject to
certain adjustments). Odyssey converted 511,308 of those shares into our common stock on December 28, 2023. Pursuant to the Certificate
of Designation creating the Series F Preferred Stock, the remainder of the shares are not convertible until the occurrence of all of
the following: (i) Oragenics’ shall have applied for and been approved for initial listing on the NYSE American or another national
securities exchange or shall have been delisted from the NYSE American, which Oragenics’ does not anticipate undertaking until
it meets the NYSE American’s initial listing standards, and (ii) if required by the rules of the NYSE American, Oragenics’
shareholders shall have approved any change of control that could be deemed to occur upon the conversion of the Series F Preferred Stock
into common stock, based on the fact and circumstances existing at such time.
About
Mild Traumatic Brain Injury (mTBI)
Concussions
are a serious unmet medical need that affects millions worldwide. Repetitive concussions are thought to increase the risk of developing
Chronic Traumatic Encephalopathy (“CTE”) and other neuropsychiatric disorders. It is estimated that 5 million concussions
occur in the U.S. annually and that as many as 50% go unreported. The worldwide incidence of concussion is estimated at 69 million. The
global market for concussion treatment was valued at $6.9 billion in 2020 and is forecast to reach $8.9 billion by 2027, according to
Grandview Research. Common settings for concussion include contact sports, military training and operations, motor vehicle accidents,
children at play and elderly falls.
Our
ONP-002 Neurology Asset for Brain Related Illness and Injury
Our
lead product and focus is on the development and commercialization of ONP-002 for the treatment of mild traumatic brain injury (“mTBI”
or “concussion”). ONP-002 to date has been shown to be stable up to 104 degrees for 18-months. The ONP-002 drug candidate
is used in conjunction with Oragenic’s novel breath-powered intranasal device. In use, breath power drives the ONP-002 drug candidate
from the novel intranasal device through the nasal passage and directly into the brain for mTBI or concussion treatment. The novel intranasal
device is lightweight and uniquely designed for easy and simple use in the field.
We
believe the proprietary nasal formulation and intranasal administration allows for rapid and direct accessibility to the brain. The novel
intranasal device is breath propelled and is designed to drive and concentrate the ONP-002 drug into the brain, which then easily crosses
the blood brain barrier. In operation, when patients blow into the intranasal device, the soft palate closes in the back of the nasopharynx.
This mechanism prevents the flow of the ONP-002 drug into the lungs or esophagus, minimizes systemic ONP-002 drug exposure and side effects,
and concentrates the ONP-002 drug flow into the brain. In other words, this mechanism traps the ONP-002 drug in the nasal cavity allowing
for more abundant and faster drug availability in the traumatized brain.
Expected
ONP-002 Product Development Timeline:
| Pre-clinical
Animal Studies |
|
Phase
1 |
|
Phase
2a |
|
Phase
2b |
|
Phase
3 |
| Complete |
|
Complete |
|
Estimated
Q1 2025 start |
|
Estimated
Q3 2025 start |
|
Estimated
Q4 2026 start |
This
product development plan is an estimate and is subject to change based on funding, technical risks and regulatory approvals.
Validation
and Stability of ONP-002
A
Certificate of Analysis (“COA”) was issued by the manufacturer of the drug, indicating that testing methods were standard
and include appearance, identification by 1H NMR, identification by Mass Spectroscopy (MS), optical purity by HPLC, residual solvent
analysis, elemental impurities, percent water, and residue on ignition. The manufacturer has shown both the specifications and the results,
indicating that the material supplied passes all criteria. The ONP-002 drug is supplied in essentially pure form. As such, no excipients
are believed to be present. Stability studies were performed by storing the ONP-002 drug samples under carefully controlled conditions
with respect to temperature and humidity. The stability testing protocol included storage at about 25 °C± 2 °C at about
60% relative humidity ± 5% relative humidity for about 24 months and at about 40 °C± 2 °C at about 75% relative
humidity ± 5% for about 18 months. The ONP-002 drug samples were pulled at essentially the scheduled time and analyzed for appearance,
purity, assay, optical purity, and water content. No changes in ONP-002 were observed.
Intellectual
Property
Domestic
and foreign patents applications on the ONP-002 compound have been filed and to date, several have been issued. Domestic and foreign
patent applications have also been filed on the novel breath-powered intranasal delivery device as follows:
| |
● |
New
chemical entity IP filings – USPTO pending, granted in Europe and Canada |
| |
○ |
C-20
steroid compounds, compositions and uses thereof to treat traumatic brain injury (TBI), including concussion. |
| |
○ |
The
invention relates to ONP-002 drug compound, compositions and methods of use thereof to treat, minimize and/or prevent traumatic brain
injury (TBI), including severe TBI, moderate TBI, and mild TBI, including concussions. |
| |
|
|
| |
○ |
Patent
expiration with max patent term extension – 9/17/2040 |
| |
|
|
| |
○ |
Patent
expiration with no patent term extension – 9/17/2035 |
| |
● |
Method
of intranasal delivery and device components – Domestic and Foreign patent applications pending |
| |
|
|
| |
● |
Breath-powered
intranasal device and use thereof – Domestic and Foreign patent applications pending |
ONP-002
Pre-Clinical Trials
The
ONP-002 drug has completed toxicology studies in rats and dogs. Those studies show that the ONP-002 drug has a large safety margin of
its predicted efficacious dose. In preclinical animal studies, the ONP-002 drug demonstrated rapid and broad biodistribution throughout
the brain while simultaneously reducing swelling, inflammation, and oxidative stress, along with an excellent safety profile.
Results
from the preclinical studies suggest that the ONP-002 drug has an equivalent, and potentially superior, neuroprotective effect compared
to related neurosteroids. The animals treated with the ONP-002 drug post-concussion showed positive behavioral outcomes using various
testing platforms including improved memory and sensory-motor performance, and reduced depression/anxiety like behavior.
ONP-002
Drug Induction of PXR
The
induction of the human CYP450 enzymes, CYP2B6, and CYP3A4 by ONP-002, as measured by mRNA expression, was tested in human hepatocytes
from 3 donors at 3 concentrations: about 1 μM, about 10 μM and about 100 μM. Results reflected that the ONP-002 drug through
the known PXR-mechanism produced a modest induction of CYP3A4, up to about 17% of the positive control, and a greater induction of CYP2B6,
of up to about 59% of the positive control, both at a concentration of about 100 μM. Past data reflected that the ONP-001 drug candidate
(ent-Progesterone) and Progesterone induce the PXR receptor. Receptor binding studies have been performed showing neither the
ONP-001 drug candidate or the ONP-002 drug activate the classical Progesterone Receptor.
ONP-002
Drug Animal Studies
All
surgical animals (male Sprague-Dawley rats approx. 250 grams) were anesthetized with an initial isoflurane induction for about 4 min-the
minimum time necessary to sedate the animal. The scalp was shaved and cleaned with isopropanol and betadine. During the stereotaxic surgery,
anesthesia was maintained with isoflurane. A medial incision was made, and the scalp was pulled back over the medial frontal cortex.
An approximate 6-mm diameter craniotomy was performed exposing the brain tissue. An electrically controlled injury device using a 5 mm
metal impactor was positioned over the exposed brain. An impact speed of about 1.6 m/s at about a 90-degree angle from vertical was used
to produce an open head injury at a depth of 1mm to create a milder TBI. All treatments were given intranasal (IN) as a liquid solution
with a micro atomizer. Vehicle for all administrations was about 22.5% Hydroxy-Propyl-β-cyclodextrin (HPβCD).
Molecular
Studies - Brain tissue was taken from the penumbral region of injury.
Cerebral
Edema
In
Figure 2, we show that the ONP-002 drug reduces swelling in rats compared to vehicle-treated at 24-hrs after brain injury by measure
of brain water content through speed-vacuum dehydration and tissue weight comparisons. The ONP-002 drug-treated (about 4mg/kg) and vehicle-treated
were compared to sham which was set at zero. Local edema can occur after mTBI. Severe cerebral edema is associated with poor outcomes
including increased mortality after mTBI with Second Impact Syndrome (2). *Denotes significance at p<0.05, n=6

Inflammation
mTBI
causes vascular and neuronal stress. Microglia and reactive astrocytes infiltrate the areas of injury and release inflammatory mediators,
like TNF-alpha. We show that the ONP-002 drug (about 4mg/kg) reduces TNF-alpha-mediated neuroinflammation in brain tissue of rats compared
to vehicle at approximately 24-hrs after mTBI (ELISA).
Pharmacokinetics
and Safety of IN ONP-002 Drug in Dog
This
pivotal GLP 14-day study used repeat dosing of the ONP-002 drug, 3X a day, approximately about 4 hours apart, for approximately 14 consecutive
days at concentrations of about 0, 3, 10 or 23 mg/mL at a volume of about 1 mL/nostril to beagle dogs (both nostrils had drug administered).
The intranasal treatment was given as a liquid solution using a micro atomizer using about 22.5% HPβCD as the vehicle. Intranasal
ONP-002 drug dosing revealed that the ONP-002 drug was well tolerated up to the highest dose of about 23 mg/ml or about 46mg in total
per dosing. Clinical observations were limited to increased salivation in dogs which occurred in a dose-dependent manner. There were
no effects on body weight, food consumption, ophthalmic parameters, clinical chemistry, haematology, or organ weights at any of the doses
tested. Microscopic analysis revealed purulent exudates in the nasal turbinate and evidence of inflammatory infiltrates and fibrin deposition
in the lungs. All of these events were classified as mild, reversed during the recovery period, and did not appear to show any dose dependency.
Similar findings were evident in vehicle control treated dogs indicating the findings were vehicle related. The highest dose of about
23 mg/ml was thus determined to be the NOAEL which is equivalent to a ONP-002 dose of about 1.5mg/kg and about 2.3mg/kg in male and female
dogs, respectively. Testing shows the dose-dependent increase in plasma exposure of the ONP-002 drug in male and female dogs following
IN administration. Plasma exposure levels were similar in males and females and there did not appear to be any evidence of drug accumulation
following multiple doses.
Cardiopulmonary
Safety Pharmacology
The
effect of the ONP-002 drug on the human ether-a-go-go related gene (hERG) tail currents was assessed in a non-Good Laboratory Practice
(GLP) study using manual whole-cell patch clamp. The ONP-002 drug tested at a single concentration of about 10 μM inhibited hERG tail
currents by about 42.6% (n=3). In order to achieve a safety factor of about 30-fold between in vitro hERG IC50 and free plasma levels
of the ONP-002 drug in clinical studies, the Cmax (maximum concentration) should not exceed a free drug concentration of about 0.33 μM
(about 99 ng/ml). The ONP-002 drug is about 97.2% human plasma protein bound and is estimated to reach a plasma Cmax of about 12.5 nM,
the highest dose of about 0.533 mg/kg to be administered in the planned first in human (FIH) study, which provides a safety factor of
about 800-fold. A GLP study has been conducted at Charles River, Inc. and will be incorporated into the IND submission.
ONP-002
Drug Clinical Trials
The
ONP-002 drug has completed a Phase 1 clinical trial in healthy human subjects showing it is safe and well tolerated.
Safety
studies have established a dosing regimen of 2X/day for fourteen days. The Phase 1 clinical trial was performed in Melbourne, Australia
with a Contract Research Organization (CRO), Avance Clinical Pty Ltd and Nucleus Network Pty Ltd. The country of Australia provides a
currency exchange advantage and a tax rebate at the end of our fiscal year from the Australian government on all Research and Development
performed in Australia.
The
Phase 1 study was double-blinded, randomized and placebo controlled (3:1, drug:placebo). Phase 1 used a Single Ascending/Multiple Ascending
(SAD/MAD) drug administration design. The SAD component was a 1X treatment (low, medium, or high dose) and the MAD component was a 1X/day
treatment for five consecutive days (low and medium dose). Blood and urine samples were collected at multiple time points for safety
pharmacokinetics. Standard safety monitoring was provided for each body system.
Forty
human subjects (31 males, 9 females) were successfully enrolled in Phase 1. The Safety Review Board, made up of medical doctors, has
reviewed the trial data and has determined the drug is safe and well tolerated at all dosing levels.
We
anticipate preparing for Phase 2 clinical trials to further evaluate the ONP-002 drug’s safety and efficacy. Based on the Phase
1 data, we plan to apply for an Investigational New Drug application with the FDA and conduct a Phase 2 trial in the United States.
We
anticipate a Phase 2 clinical trial will be performed administering the ONP-002 drug intranasally in concussed patients 2x a day for
up to fourteen days. The Phase 2a feasibility study is expected to be performed in Australia with a target initiation date in the third
quarter of 2024 to be followed closely by a Phase 2b proof of concept study in the US.
We
have entered an agreement with one of the leading Contract Research Organization (CRO) in Australia, to conduct a Phase 2 clinical trial
in Australia. This trial aims to evaluate the ONP-002 drug for TBI. This Australian CRO, renowned for its clinical trial management capabilities
and quality of service in Australia, New Zealand, and North America, brings over two decades of expertise in navigating the Therapeutic
Goods Administration, Food and Drug Administration, and European Medicines Agency regulatory landscapes. Other key third party well-respected
vendors also have been engaged to advance and monitor our progress.
On
July 10, 2024, we announced that we had developed a new proprietary formulation for the novel ONP-002 neurosteroid. We believe the nasal
cavity provides access for our novel neurosteroid formulation to enter the brain in minutes. Given the difficulty of getting neurosteroids
into solution, unique formulations must be developed to achieve therapeutic levels. We believe that our recent work has increased the
final dose levels significantly while also providing for improved intranasal drug delivery and adhesion and, thus, longer absorption
times. We further believe we have successfully completed an improved proprietary formulation of the ONP-002 drug that should significantly
increase the bioavailability of the intranasal drug formulation. The enhanced drug percentages in this novel proprietary formulation
have been developed as part of our platform for acute-field delivery of the drug. Our newly developed proprietary intranasal drug formulation
is intended to reduce the duration of initial concussion symptoms and prevent long-lasting symptoms that can be debilitating after a
concussion.
On
August 8, 2024, we announced our candidate for treating concussion successfully completed a study that indicates the ONP-002 drug does
not cause cardiotoxicity. Prior to conducting a clinical trial, the U.S. Food and Drug Administration (FDA) requires pharmaceuticals
to be tested on cardiac receptors to ensure that they do not show any causes of electrical malformations. Further, on August 30, 2024,
we announced we successfully completed a study that indicates the ONP-002 drug does not cause DNA damage and genotoxicity in an animal
model. Prior to conducting a clinical trial, the U.S. Food and Drug Administration (FDA) requires that pharmaceuticals be tested on cells
and animals to ensure they do not cause damage affecting cell division.
Our
Medical Advisors
Dr.
James “Jim” Kelly, Neurologist, serves as our Chief Medical Officer, and oversees our upcoming Phase 2 clinical trial for
treating concussion. In the recent past, Dr. Kelly served as the Executive Director of the Marcus Institute for Brain Health (MIBH) and
Professor of Neurology at the University of Colorado Anschutz Medical Campus in Aurora, Colorado. The MIBH specialized treatment program
is funded by the Marcus Foundation to care for US military veterans with persistent symptoms of TBI. Dr. Kelly was also National Director
of the Avalon Action Alliance TBI Programs for which the MIBH serves as the clinical coordinating center. Prior to these recent positions,
Dr. Kelly was the Director of the National Intrepid Center of Excellence (NICoE) at Walter Reed National Military Medical Center in Bethesda,
MD. As its founding Director, he led the creation of an innovative interdisciplinary team of healthcare professionals who blended high-tech
diagnosis and treatment with complementary and alternative medical interventions in a holistic, integrative approach to the care of US
military personnel with the complex combination of TBI and psychological conditions, such as post-traumatic stress, depression, and anxiety.
In this role, Dr. Kelly was frequently called upon by leaders of the Military Health System at the Pentagon, the US Congress, the Department
of Veterans Affairs, and numerous military facilities in the continental US and abroad. Dr. Kelly has interacted with the FDA and clinical
trials for brain injury throughout his esteemed career. He is a strong advocate for treatments in the acute phase of brain injury and
understands the value of protecting the brain early on from inflammation, swelling and oxidative stress to gain better clinical outcomes.
Dr.
William “Frank” Peacock serves as our Chief Clinical Officer, and will conduct our anticipated Phase 2 clinical trial for
treating concussion in emergency departments. Dr. Peacock is currently the Vice Chair for Emergency Medicine Research at Baylor College
of Medicine and a past Professor at the Cleveland Clinic Lerner College of Medicine. He is also the Principal Investigator of a trial
for a company developing blood biomarkers for the identification of concussion in the emergency department, which is analyzing acute
blood markers that are elevated after concussion to not only ensure concussion is identified but also as a predictor of potential severity
and longer-term complications. Dr. Peacock is a world-renowned speaker and researcher. He has been instrumental in the approval and use
of high sensitivity blood troponins for acute coronary syndrome failure in emergency settings, which can be seen in the JAMA Cardiology
publication, Efficacy of High-Sensitivity Troponin T in Identifying Very-Low-Risk Patients with Possible Acute Coronary Syndrome,
and he is the editor of the first book of “Biomarkers of Traumatic Brain Injury”.
Our
SARS-CoV-2 Vaccine Product Candidate – NT-CoV2-1
Prior
to the purchase of the Neurology Assets, starting in May 2020 with the acquisition of one hundred percent (100%) of the total issued
and outstanding common stock of Noachis Terra, Inc. (“Noachis Terra”) and through December 31, 2023, we were focused on the
development and commercialization of a vaccine produce candidate to provide long-lasting immunity from SARS-CoV-2, which causes COVID-19.
During that time, we conducted testing in animal models, including SARS-CoV-2 challenge studies in hamsters, using specific formulations
for intramuscular administration and intranasal administration, both based on the NIAID pre-fusion stabilized spike protein antigens.
In
June of 2021, we initiated an immunogenicity study in mice and on August 30, 2021, we announced the successful completion of the mouse
studies that supported further development using either intramuscular or intranasal routes of administration. In September of 2021, we
initiated a hamster challenge to assess inhibition of viral replication using adjuvants specific for intramuscular and intranasal administration.
In December of 2021, we announced that both formulations generated robust immune responses and reduced the SARS-CoV-2 viral loads to
undetectable levels in the nasal passages and lungs five days following a viral challenge. On June 14, 2022, we announced that the results
of these studies were published in Nature Scientific Reports.
In
March of 2022, following a positive assessment of a rabbit-based pilot study, we initiated a Good Laboratory Practice toxicology study
to evaluate the safety profile and immunogenicity of NT-CoV2-1 in rabbits. This preclinical study was designed to provide data required
to advance our intranasal vaccine candidate into human clinical studies.
Following
the successful results of the animal studies previously referenced and a Type B Pre-IND Meeting with the FDA we determined to focus our
development efforts and financial resources on the intranasal delivery vaccine produce candidate, NT-CoV2-1. As part of this intranasal
development focus, during 2023 we entered into strategic license agreements and announced an award of a grant from CQDM.
However,
due to lack of financial resources our research and development activities for our NT-CoV2-1 vaccine product were suspended as of December
31, 2023, and are not currently active. We will continue to evaluate opportunities and funding resources for our SARS-CoV-2 and NT-CoV2-1
candidate products in the future of which there can be no assurances. These opportunities and funding resources could include, without
limitation, sublicensing agreements, joint ventures or partnerships, sales or licensing of technology, government grants and public or
private financings, through the sale of debt or equity securities or by securing a line of credit or other loan. There can be no assurances
that we will be able to secure any such opportunity or funding.
In
March of 2022, following a positive assessment of a rabbit-based pilot study, we initiated a Good Laboratory Practice toxicology study
to evaluate the safety profile and immunogenicity of NT-CoV2-1 in rabbits. This preclinical study was designed to provide data required
to advance our intranasal vaccine candidate into human clinical studies.
Following
the successful results of the animal studies previously referenced and a Type B Pre-IND Meeting with the FDA we determined to focus our
development efforts and financial resources on the intranasal delivery vaccine produce candidate, NT-CoV2-1. As part of this intranasal
development focus, during 2023 we entered into strategic license agreements and announced an award of a grant from CQDM.
However,
due to lack of financial resources our research and development activities for our NT-CoV2-1 vaccine product were suspended as of December
31, 2023, and are not currently active. We will continue to evaluate opportunities and funding resources for our SARS-CoV-2 and NT-CoV2-1
candidate products in the future of which there can be no assurances. These opportunities and funding resources could include, without
limitation, sublicensing agreements, joint ventures or partnerships, sales or licensing of technology, government grants and public or
private financings, through the sale of debt or equity securities or by securing a line of credit or other loan. There can be no assurances
that we will be able to secure any such opportunity or funding.
Our
Lantibiotic Product Candidate
Members
of our scientific team discovered that a certain bacterial strain of Streptococcus mutans, produces Mutacin 1140 (MU1140), a molecule
belonging to the novel class of antibiotics known as lantibiotics. Lantibiotics, such as MU1140, are highly modified peptide antibiotics
made by a small group of Gram-positive bacterial species. Over 60 lantibiotics have been discovered, to date. We believe lantibiotics
are generally recognized by the scientific community to be potent antibiotic agents. In nonclinical testing, MU1140 has shown activity
against all Gram-positive bacteria against which it has been tested, including those responsible for a number of healthcare associated
infections, or HAIs. A high percentage of hospital-acquired infections are caused by highly antibiotic-resistant bacteria such as methicillin-resistant
Staphylococcus aureus (MRSA) or multidrug-resistant Gram-negative bacteria. We believe the need for novel antibiotics is increasing because
of the growing resistance of target pathogens to existing FDA approved antibiotics on the market.
While
lantibiotics are promising, in 2023 we concluded we needed to make several changes to reduce the cash used in operations. In September
of 2023, we terminated our lease for the building where some of the research and development activities for the lantibiotic program were
undertaken. The closing of the laboratory was part of the continued focus on preserving cash resources while seeking additional funding
through various mechanisms. As of December 31, 2023, research and development activities related to the lantibiotic program are inactive.
We will evaluate opportunities for the lantibiotic program; however, moving forward our focus is to strengthen our focus and expertise
on developing our intranasal drug delivery platform and drug candidates that treat brain related illnesses and diseases.
Our
Business Development Strategy
Success
in the biopharmaceutical and product development industry relies on the continuous development of novel product candidates. Most product
candidates do not make it past the clinical development stage, which forces companies to look externally for innovation. Accordingly,
we expect, from time to time, to seek strategic opportunities through various forms of business development, which can include strategic
alliances, licensing deals, joint ventures, collaborations, equity or debt-based investments, dispositions, mergers, and acquisitions.
We view these business development activities as a necessary component of our strategies, and we seek to enhance shareholder value by
evaluating business development opportunities both within and complementary to our current business, as well as opportunities that may
be new and separate from the development of our existing product candidates.
As
discuss elsewhere, our current focus is on advancing our ONP-002 product candidate to treat concussion. Work on our other project candidates
currently is not active. As part of the focus on ONP-002, and to conserve resources, we have made several changes to reduce cash used
in operations until additional capital can be obtained. As previously announced, we exercised our option under our lease with Hawley-Wiggins,
LLC (the “Landlord”), for the building located in Progress Park and known as 13700 Progress Boulevard, Alachua, Florida 32615
(the “Lease”) to terminate the Lease by paying nine (9) months of advance rent, plus prorated rent for the month of September,
2023, plus applicable sales tax. In addition to the termination of the Lease, the Company eliminated two staff positions and Dr. Martin
Handfield transitioned from an employee of the Company to a consultant. Dr. Handfield continues to be available to provide support services
on an hourly basis through a consulting agreement. Dr. Handfield’s employment agreement was terminated in accordance with its terms.
The Alachua lease contained the laboratory where some of the research and development for the lantibiotic program was undertaken.
Corporate
and Other Information
We
were incorporated in November 1996 and commenced operations in 1999. We consummated our initial public offering in June 2003. Our executive
office is located at, 1990 Main Street, Suite 750, Sarasota, Florida 34236. Our telephone number is (813) 286-7900 and our website is
http://www.oragenics.com. We make available free of charge on our website our annual report on Form 10-K, quarterly reports on Form 10-Q,
current reports on Form 8-K and amendments to those reports as soon as reasonably practicable after we electronically file or furnish
such materials to the Securities and Exchange Commission (the “SEC”). The reports are also available at www.sec.gov.
Implications
of Being a Smaller Reporting Company
We
are a “smaller reporting company” as defined in Rule 10(f)(1) of Regulation S-K. We will remain a smaller reporting company
until the last day of the fiscal year in which (1) the market value of our shares of Common Stock held by non-affiliates exceeds $250
million or (2) our annual revenues exceeded $100 million during such completed fiscal year and the market value of our shares of Common
Stock held by non-affiliates exceeds $700 million, each as determined on an annual basis. A smaller reporting company may take advantage
of relief from some of the reporting requirements and other burdens that are otherwise applicable generally to public companies. These
provisions include:
| |
● |
being
permitted to provide only two years of audited financial statements, in addition to any required unaudited interim financial statements,
with correspondingly reduced “Management’s Discussion and Analysis of Financial Condition and Results of Operations”
disclosure; |
| |
|
|
| |
● |
not
being required to comply with the auditor attestation requirements in the assessment of our internal control over financial reporting;
and |
| |
|
|
| |
● |
reduced
disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements. |
SUMMARY
OF RISK FACTORS
Our
business is subject to a number of risks of which you should be aware of before making an investment decision. These risks are discussed
more fully in the “Risk Factors” section of this prospectus immediately following this prospectus summary. Some of
these risks include the following:
| ● |
We
have incurred significant losses since our inception, have limited financial resources, do not generate any revenues and will need
to raise additional capital in the future. |
| |
|
| ● |
We
may not be able to secure additional funding. |
| |
|
| ● |
Our
auditor has expressed substantial doubt about our ability to continue as a going concern. |
| |
|
| ● |
We
may not be able to satisfy the continued listing standards of the NYSE American and may be delisted from the NYSE American. |
| |
|
| ● |
We
have limited neurology-specific research, development, manufacturing, testing, regulatory, commercialization, sales, distribution,
and marketing experience, and we may need to invest significant financial and management resources to establish these capabilities. |
| |
|
| ● |
None
of our product candidates have been approved for sale and if we are unable to successfully develop our product candidates, we may
not be able to continue as a going concern. |
| |
|
| ● |
Our
product candidates, if approved, will face significant competition; many of our competitors have significantly greater resources
and experience. |
| |
|
| ● |
Our
ONP-002 concussion candidate may face competition from biosimilars approved through an abbreviated regulatory pathway. |
| |
|
| ● |
The
market opportunities for our neurology product candidates may be smaller than we believe them to be and we cannot assure you that
the market and consumers will accept our products or product candidates. |
| |
|
| ● |
If
our manufacturers and suppliers fail to meet our requirements and the requirements of regulatory authorities, our research and development
may be materially adversely affected. |
| |
|
| ● |
We
rely on the significant experience and specialized expertise of our senior management and scientific team and the loss of any of
our key personnel or our inability to successfully hire their successors could harm our business. |
| |
|
| ● |
If
any of our product candidates are shown to be ineffective or harmful in humans, we will be unable to generate revenues from these
product candidates. |
| |
|
| ● |
We
might not be successful at acquiring, investing in or integrating businesses, entering into joint ventures or divesting businesses. |
| |
|
| ● |
Our
concussion and neurology related research and development efforts are to a large extent dependent upon our intellectual property
and biologicals materials licenses. |
| |
|
| ● |
We
may not be able to protect our intellectual property and if we are unable to protect our trademarks or other intellectual property
from infringement, our business prospects may be harmed. |
| |
|
| ● |
We
may be subject to claims challenging the inventorship of our patents and other intellectual property rights. |
| |
|
| ● |
If
we are sued for infringing intellectual property rights of third parties, it will be costly and time-consuming and an unfavorable
outcome in that litigation could have a material adverse effect on our business. |
| |
|
| ● |
Our
success will depend on our ability to partner or sub-license our product candidates. |
| ● |
Security
breaches and other disruptions to our information technology systems or those of the vendors on whom we rely on could compromise
our information and expose us to liability, reputational damage, or other costs. |
| |
|
| ● |
Our
product candidates are subject to substantial government regulation and will be subject to ongoing and continued regulatory review
and we may also be subject to healthcare laws, regulation and enforcement. |
| |
|
| ● |
We
may be unable to obtain regulatory approval for our product candidates under applicable regulatory requirements. |
| |
|
| ● |
Delays
or difficulties in the enrollment of patients in clinical trials may result in additional costs and delays. |
| |
|
| ● |
Our
product candidates may cause serious or undesirable side effects. |
| |
|
| ● |
Our
employees, independent contractors, principal investigators, consultants, vendors and CROs may engage in misconduct or other improper
activities, including noncompliance with regulatory standards and requirements. |
| |
|
| ● |
Even
if our current product candidates or any future product candidates obtain regulatory approval, they may fail to achieve the broad
degree of health care payers, physician and patient adoption and use necessary for commercial success. |
| |
|
| ● |
The
issuance of additional equity securities by us in the future will result in dilution and the conversion of our outstanding preferred
stock will result in significant dilution. |
| |
|
| ● |
Our
Series B preferred stock, if not converted into common stock, has a distribution and liquidation preference senior to our common
stock in liquidation which could negatively affect the value of our common stock and impair our ability to raise additional capital. |
| |
|
| ● |
Certain
provisions of our articles of incorporation, bylaws, executive employment agreements and stock option plan may prevent a change of
control of our company that a shareholder may consider favorable. |
| |
|
| ● |
The
price and volume of our common stock has been volatile and fluctuates substantially. |
| |
|
| ● |
The
requirements of being a public company may strain our resources, divert management’s attention and affect our ability to attract
and retain qualified members for our Board of Directors. |
| |
|
| ● |
If
we fail to maintain an effective system of internal controls, we may not be able to accurately report our financial results or prevent
fraud. |
THE
OFFERING
| Issuer: |
|
Oragenics,
Inc. |
| |
|
|
| Units
Offered: |
|
We
are offering 15,151,515 Units. Each Unit consists of one share of Common Stock (or one Pre-Funded
Warrant to purchase one share of our Common Stock in lieu thereof) and a Common Warrant to
purchase one share of our Common Stock (together with the shares of Common Stock underlying
such warrants). The Units have no stand-alone rights and will not be certificated or issued
as stand-alone securities.
The
Common Stock and Prefunded Warrants can each be purchased in this offering only with the accompanying Common Warrants that are part
of a Unit, but the components of the Units will be immediately separable and will be issued separately in this offering. See “Description
of Securities” in this prospectus for more information. |
| |
|
|
| Pre-Funded
Warrants: |
|
We
are also offering to each purchaser of Units whose purchase of shares of our Common Stock
in this offering would otherwise result in the purchaser, together with its affiliates and
certain related parties, beneficially owning more than 4.99% (or, at the election of the
holder, 9.99%) of our outstanding shares of Common Stock immediately following consummation
of this offering, the opportunity to purchase, if the purchaser so chooses, Units consisting
of one Pre-Funded Warrant to purchase one share of Common Stock (“Pre-Funded Warrants”),
and one Common Warrant. Each Pre-Funded Warrant will be exercisable immediately upon issuance,
will expire when exercised in full, and will be exercisable for one share of our Common Stock.
For each Unit including a Pre-Funded Warrant that we sell, the number of Units including
one share of our Common Stock that we are offering will be decreased on a one-for-one basis.
This
offering also relates to the shares of Common Stock issuable upon exercise of the Common Warrants (the “Common Warrant Shares”),
the shares of Common Stock issuable upon exercise of the Pre-Funded Warrants (the “Pre-Funded Warrant Shares”), and the
shares of Common Stock issuable upon exercise of Placement Agent Warrants (defined below). |
| |
|
|
| Description
of warrants: |
|
The
Common Warrants will be exercisable beginning on the closing date and expire on the fifth anniversary of the closing date and have
an initial exercise price per share equal to $0.33 per share, subject to appropriate adjustment in the event of recapitalization
events, stock dividends, stock splits, stock combinations, reclassifications, reorganizations or similar events affecting our Common
Stock. |
| |
|
|
| Common
stock outstanding prior to this offering: |
|
12,570,100
shares of Common Stock, as of December 13, 2024 |
| |
|
|
| Common
Stock to be outstanding after this offering: |
|
27,386,723
shares assuming we sell only Units and no Pre-Funded Warrants and assuming no exercise of the Common Warrants being offered in this
offering. To the extent Pre-Funded Warrants are sold, it will reduce the number of shares of Common Stock that we are offering on
a one-for-one basis. |
| Offering
Price: |
|
The
assumed offering price is $0.33 per Unit including one share of Common Stock, the last reported sale price of our Common Stock as
reported on NYSE American on December 13, 2024. The purchase price of each Unit including a Pre-Funded Warrant will equal the price
per Unit of the Units including one share of Common Stock being sold to the public in this offering, minus $0.001, and the exercise
price of each Pre-Funded Warrant will be $0.001 per share. |
| |
|
|
| Use
of Proceeds: |
|
We
estimate that we will receive net proceeds of approximately $4.3 million from our sale of Common Stock in this offering. We intend
to use the net proceeds from this offering, along with our existing cash and cash equivalents, to fund our ongoing ONP-2 concussion
clinical trials, along with other related research and development activities, as well as for working capital and other general corporate
purposes. See “Use of Proceeds” in this prospectus for a more complete description of the intended use of proceeds
from this offering. |
| |
|
|
| Trading
market and symbol: |
|
Our
Common Stock is listed on NYSE American, or the “NYSE American,” under the symbol “OGEN.” |
| |
|
|
| Risk
Factors: |
|
Investing
in our Common Stock involves a high degree of risk. See “Risk Factors” beginning on page 14 and the other information
in this prospectus for a discussion of the factors you should consider carefully before you decide to invest in our common |
| |
|
|
| Lock-Up: |
|
Our
directors and officers have agreed, subject to certain exceptions, not to offer, pledge, sell, contract to sell, grant, lend, or
otherwise transfer or dispose of, directly or indirectly, or enter into any swap or other arrangement that transfers to another,
in whole or in part, any of the economic consequences of ownership of any shares of our capital stock or any securities convertible
into or exercisable or exchangeable for shares of our Common Stock, for a period of three (3) months from the date of this Offering. |
The
number of shares of our Common Stock to be outstanding after this offering is based on shares of our Common Stock outstanding as of December
13, 2024, and excludes the following:
●
917,353 shares of our Common Stock issuable upon the exercise of outstanding options under our equity incentive plans at a weighted
average exercise price of $4.97 per share;
●
736,574 shares of Common Stock reserved for issuance under outstanding warrants with a weighted average exercise price of $20.57 per
share;
●
2,004,164 additional shares of Common Stock reserved for future issuance under our 2021 equity incentive plan;
●
1,139,705 shares of common stock reserved for issuance under outstanding pre-funded warrants as of December 13, 2024 with a weighted
average exercise price of $0.001 per share; and
●
approximately 7,488,692 shares of Common Stock reserved for issuance under conversion of 7,488,692 outstanding shares of Series F Non-Voting,
Convertible Preferred Stock.
Unless
we indicate otherwise or unless the context otherwise requires, all information in this prospectus assumes the following:
| |
● |
no
exercise of outstanding options or warrants; |
| |
● |
assumes
no exercise of the Pre-Funded Warrants issued in this offering; and |
| |
● |
no
exercise of the Placement Agent’s warrants to be issued upon consummation of this offering. |
RISK
FACTORS
An
investment in our securities involves a high degree of risk. Before deciding whether to purchase our securities, including the shares
of Common Stock offered by this prospectus, you should carefully consider the risks and uncertainties described under “Risk Factors”
in our Annual Report on Form 10-K for the fiscal year ended December 31, 2023, any subsequent Quarterly Report on Form 10-Q and our other
filings with the SEC, all of which are incorporated by reference herein. If any of these risks actually occur, our business, financial
condition and results of operations could be materially and adversely affected and we may not be able to achieve our goals, the value
of our securities could decline and you could lose some or all of your investment. Additional risks not presently known to us or that
we currently believe are immaterial may also significantly impair our business operations. If any of these risks occur, our business,
results of operations or financial condition and prospects could be harmed. In that event, the market price of our Common Stock and the
value of the warrants could decline, and you could lose all or part of your investment.
Risks
Relating to this Offering
The
market price of our Common Stock has been, and may continue to be volatile and fluctuate significantly, which could result in substantial
losses for investors.
The
trading price for our Common Stock has been, and we expect it to continue to be, volatile. The price at which our Common Stock trades
depends upon a number of factors, including our historical and anticipated operating results, our financial situation, announcements
by us or our competitors, our ability or inability to raise the additional capital we may need and the terms on which we raise it, and
general market and economic conditions. Some of these factors are beyond our control. Broad market fluctuations may lower the market
price of our Common Stock and affect the volume of trading in our stock, regardless of our financial condition, results of operations,
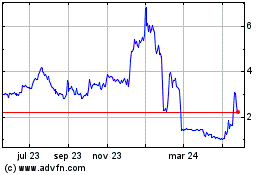
business or prospects. The closing price of our Common Stock as reported on the NYSE American had a high price of $9.00 and a low price
of $2.62 in the 52-week period ended December 31, 2023 and a high price of $6.84 and a low price of $0.27 from January 1, 2024 through
December 13, 2024. Among the factors that may cause the market price of our Common Stock to fluctuate are the risks described in this
“Risk Factors” section and other factors, including:
| |
● |
results
of preclinical and clinical studies of our product candidates or those of our competitors; |
| |
|
|
| |
● |
regulatory
or legal developments in the U.S. and other countries, especially changes in laws and regulations applicable to our product candidates; |
| |
|
|
| |
● |
actions
taken by regulatory agencies with respect to our product candidates, clinical studies, manufacturing process or sales and marketing
terms; |
| |
|
|
| |
● |
introductions
and announcements of new products by us or our competitors, and the timing of these introductions or announcements; |
| |
|
|
| |
● |
announcements
by us or our competitors of significant acquisitions or other strategic transactions or capital commitments; |
| |
|
|
| |
● |
fluctuations
in our quarterly operating results or the operating results of our competitors; |
| |
|
|
| |
● |
variance
in our financial performance from the expectations of investors; |
| |
|
|
| |
● |
changes
in the estimation of the future size and growth rate of our markets; |
| |
|
|
| |
● |
changes
in accounting principles or changes in interpretations of existing principles, which could affect our financial results; |
| |
|
|
| |
● |
failure
of our products to achieve or maintain market acceptance or commercial success; |
| |
● |
conditions
and trends in the markets we serve; |
| |
|
|
| |
● |
changes
in general economic, industry and market conditions; |
| |
|
|
| |
● |
changes
in legislation or regulatory policies, practices or actions; |
| |
|
|
| |
● |
the
commencement or outcome of litigation involving our company, our general industry or both; |
| |
|
|
| |
● |
recruitment
or departure of key personnel; |
| |
|
|
| |
● |
changes
in our capital structure, such as future issuances of securities, redemption or conversion of preferred stock or the incurrence of
additional debt; |
| |
|
|
| |
● |
actual
or expected sales of our Common Stock by our stockholders; |
| |
|
|
| |
● |
acquisitions
and financings; and |
| |
|
|
| |
● |
the
trading volume of our Common Stock. |
In
addition, the stock markets, in general, NYSE American and the market for biotech companies in particular, may experience a loss of investor
confidence. Such loss of investor confidence may result in extreme price and volume fluctuations in our Common Stock that are unrelated
or disproportionate to the operating performance of our business, financial condition or results of operations. These broad market and
industry factors may materially harm the market price of our Common Stock and expose us to securities class action litigation. Such litigation,
even if unsuccessful, could be costly to defend and divert management’s attention and resources, which could further materially
harm our financial condition and results of operations.
This
offering may cause the trading price of our Common Stock to decrease.
The
shares of Common Stock we propose to issue and ultimately will issue if this offering is completed may result in an immediate decrease
in the market price of our Common Stock. This decrease may continue after the completion of this offering.
We
cannot assure you that we will continue to be listed on the NYSE American.
Our
Common Stock commenced trading on the NYSE American (formerly the NYSE MKT) on April 10, 2013, and we are subject to certain NYSE American
continued listing requirements and standards. On April 18, 2024, we received notification (the “Notice”) from the NYSE American
that we were no longer in compliance with NYSE American’s continued listing standards. Specifically, the letter stated that the
Company was not in compliance with the continued listing standards set forth in Sections 1003(a)(ii) and 1003(a)(iii) of the NYSE American
Company Guide (the “Company Guide”). Section 1003(a)(ii) requires a listed company to have stockholders’ equity of
$4 million or more if the listed company has reported losses from continuing operations and/or net losses in three of its four most recent
fiscal years. Section 1003(a)(iii) requires a listed company to have stockholders’ equity of $6 million or more if the listed company
has reported losses from continuing operations and/or net losses in our five most recent fiscal years. We reported shareholders’
equity of $3.2 million as of December 31, 2023, and losses from continuing operations and/or net losses in its five most recent fiscal
years ended December 31, 2023. On May 17, 2024, we submitted a plan of compliance (the “Plan”) to the NYSE American. On June
18, 2024, the NYSE American accepted our Plan; the Company will be able to continue its listing during the Plan period and will be subject
to continued periodic review by the NYSE American staff. If we are not in compliance with the continued listing standards by October
18, 2025 or if the Company does not make progress consistent with the Plan during the Plan period, the Company will be subject to delisting
procedures as set forth in the NYSE American Company Guide. The Company is committed to undertaking a transaction or transactions in
the future to achieve compliance with the NYSE American’s requirements. However, there can be no assurance that the Company will
be able to achieve compliance with the NYSE American’s continued listing standards within the required timeframe. If the Common
Stock ultimately were to be delisted for any reason, it could negatively impact the Company by (i) reducing the liquidity and market
price of the Company’s Common Stock; (ii) reducing the number of investors willing to hold or acquire the Common Stock, which could
negatively impact the Company’s ability to raise equity financing; (iii) limiting the Company’s ability to use a registration
statement to offer and sell freely tradable securities, thereby preventing the Company from accessing the public capital markets; and
(iv) impairing the Company’s ability to provide equity incentives to its employees.
This
is a reasonable best efforts offering, no minimum amount of securities is required to be sold, and we may not raise the amount of capital
we believe is required for our business plans, including our near-term business plans.
The
Placement Agent has agreed to use its reasonable best efforts to solicit offers to purchase the securities in this offering. The Placement
Agent has no obligation to buy any of the securities from us or to arrange for the purchase or sale of any specific number or dollar
amount of the securities. There is no required minimum number of securities that must be sold as a condition to completion of this offering.
Because there is no minimum offering amount required as a condition to the closing of this offering, the actual offering amount, Placement
Agent fees and proceeds to us are not presently determinable and may be substantially less than the maximum amounts set forth above.
We may sell fewer than all of the securities offered hereby, which may significantly reduce the amount of proceeds received by us, and
investors in this offering will not receive a refund in the event that we do not sell an amount of securities sufficient to support our
continued operations, including our near-term continued operations. Thus, we may not raise the amount of capital we believe is required
for our operations in the short-term and may need to raise additional funds, which may not be available or available on terms acceptable
to us.
You
will experience immediate and substantial dilution as a result of this offering and may experience additional dilution in the future.
You
will incur immediate and substantial dilution as a result of this offering. After giving effect to the sale by us of shares offered in
this offering at the public offering price of $0.33 per share, and after deducting the placement agent fees and estimated offering expenses
payable by us, investors in this offering can expect an immediate dilution of approximately $0.06 per share (without assigning any value
to the Warrants). The exercise of outstanding stock options and warrants and the conversion of our outstanding preferred stock may result
in further dilution of your investment and, with regard to our Series F Convertible Preferred Stock, will result in a material further
dilution of your investment. See “Dilution” below for a more detailed discussion of the dilution you will incur if you purchase
our securities in the offering.
Future
sales of our Common Stock in the public market could cause our stock price to fall.
Sales
of a substantial number of shares of our Common Stock, or the perception by the market that those sales could occur, could cause the
market price of our Common Stock to decline or could make it more difficult for us to raise funds through the sale of equity in the future.
Future issuances of Common Stock could further depress the market for our Common Stock. We expect to continue to incur drug development
and selling, general and administrative costs, and to satisfy our funding requirements, we will need to sell additional equity securities,
which may include sales of significant amounts of Common Stock to strategic investors, and which Common Stock may be subject to registration
rights and warrants with anti-dilutive protective provisions. The sale or the proposed sale of substantial amounts of our Common Stock
or other equity securities in the public markets or in private transactions may adversely affect the market price of our Common Stock
and our stock price may decline substantially. Our stockholders may experience substantial dilution and a reduction in the price that
they are able to obtain upon sale of their shares. Also, new equity securities issued may have greater rights, preferences or privileges
than our existing Common Stock. In addition, we have a significant number of shares of restricted stock, stock options and warrants outstanding.
To the extent that outstanding stock options or warrants have been or may be exercised or other shares issued, investors purchasing our
Common Stock in this offering may experience further dilution.
If
we make one or more significant acquisitions in which the consideration includes stock or other securities, our stockholders’ holdings
may be significantly diluted. In addition, stockholders’ holdings may also be diluted if we enter into arrangements with third
parties permitting us to issue shares of Common Stock in lieu of certain cash payments upon the achievement of milestones.
The
issuance of shares of our Common Stock under our 2021 Equity Incentive Plan is covered by Form S-8 registration statements we filed with
the Securities and Exchange Commission, or SEC, and upon exercise of the options, such shares may be resold into the market. We have
also issued shares of Common Stock and warrants in connection with previous private placements. Such shares are available for resale
as well as certain of the shares of Common Stock issuable upon exercise of the warrants. We have also issued shares of our Common Stock
in the private placement and financing transaction, which are deemed to be “restricted securities,” as that term is defined
in Rule 144 promulgated under the Securities Act of 1933, as amended, or Securities Act, and such shares may be resold pursuant to the
provisions of Rule 144. The resale of shares acquired from us in private transactions could cause our stock price to decline significantly.
In addition, the conversion of outstanding shares preferred stock into Common Stock and the subsequent sale of shares of Common Stock
could also cause our stock price to decline significantly.
In
addition, from time to time, certain of our shareholders may be eligible to sell all or some of their restricted shares of Common Stock
by means of ordinary brokerage transactions in the open market pursuant to Rule 144, subject to certain limitations. In general, pursuant
to Rule 144, after satisfying a six-month holding period: (i) affiliated shareholders, or shareholders whose shares are aggregated, may,
under certain circumstances, sell within any three-month period a number of securities which does not exceed the greater of 1% of the
then-outstanding shares of Common Stock or the average weekly trading volume of the class during the four calendar weeks prior to such
sale and (ii) non-affiliated shareholders may sell without such limitations, in each case provided we are current in our public reporting
obligations. Rule 144 also permits the sale of securities by non-affiliates that have satisfied a one-year holding period without any
limitation or restriction.
We
are unable to estimate the number of shares that may be sold because this will depend on the market price for our Common Stock, the personal
or business circumstances of sellers and other factors.
You
may experience future dilution as a result of future equity offerings.
To
raise additional capital, we may in the future offer additional shares of our Common Stock or other securities convertible into or exchangeable
for our Common Stock at prices that may not be the same as the prices per share in this offering. We may sell shares or other securities
in any other offering at a price per share that is less than the prices per share paid by investors in this offering, and investors purchasing
shares of our Common Stock or other securities in the future could have rights superior to existing stockholders. The price per share
at which we sell additional shares of our Common Stock, or securities convertible or exchangeable into Common Stock, in future transactions
may be higher or lower than the prices per share paid by investors in this offering.
The
issuance of additional equity securities by us in the future would result in dilution to our existing common shareholders.
Our
Board of Directors has authority, without action or vote of our shareholders, to issue all or a part of our authorized but unissued shares,
except where shareholder approval is required by law or the rules of any exchange on which our shares are listed. Any issuance of additional
equity securities by us in the future could result in dilution to our existing common shareholders. Such issuances could be made at a
price that reflects a discount or a premium to the then-current trading price of our Common Stock. In addition, our business strategy
may include expansion through internal growth by acquiring complementary businesses, acquiring or licensing additional products or brands,
or establishing strategic relationships with targeted customers and suppliers. In order to do so, or to finance the cost of our other
activities, we may issue additional equity securities that could result in further dilution to our existing common shareholders. These
issuances would dilute the percentage ownership interest of our existing common shareholders, which would have the effect of reducing
their influence on matters on which our shareholders vote and might dilute the book value of our Common Stock. For example, our outstanding
shares of Common Stock at December 31, 2023 were 3,080,693 and, due to additional Common Stock issuances related to capital raises
and the conversion of Series A and Series B preferred stock, at December 13, 2024 our outstanding shares of Common Stock were
12,570,100. Furthermore, if holders of our outstanding preferred stock convert their preferred shares into Common Stock, an additional
7,488,692 shares of Common Stock could be issued resulting in dilution to our existing common shareholders.
Our
management team may invest or spend the proceeds of this offering in ways with which you may not agree or in ways which may not yield
a significant return.
Our
management will have broad discretion over the use of proceeds from this offering. We intend to use the net proceeds from this offering
to fund a portion of our ONP-002 research and clinical trials, and for working capital and general corporate purposes. Our management
will have considerable discretion in the application of the net proceeds, and you will not have the opportunity, as part of your investment
decision, to assess whether the proceeds are being used appropriately. The net proceeds may be used for corporate purposes that do not
increase our operating results or enhance the value of our Common Stock.
The
precise amount and timing of the application of these proceeds will depend upon a number of factors, such as the timing and progress
of our research and development efforts, our funding requirements and the availability and costs of other funds. As of the date of this
prospectus, we cannot specify with certainty all of the particular uses for the net proceeds to us from this offering. Depending on the
outcome of our efforts and other unforeseen events, our plans and priorities may change and we may apply the net proceeds of this offering
in different manners than we currently anticipated.
The
failure by our management to apply these funds effectively could harm our business, financial condition and results of operations. Pending
their use, we may invest the net proceeds from this offering in short-term, interest-bearing instruments. These investments may not yield
a favorable return to our stockholders.
We
do not intend to pay cash dividends.
We
have not declared or paid any cash dividends on our Common Stock, and we do not anticipate declaring or paying cash dividends for the
foreseeable future. Any future determination as to the payment of cash dividends on our Common Stock will be at our Board of Directors’
discretion and will depend on our financial condition, operating results, capital requirements and other factors that our Board of Directors
considers to be relevant.
There
is no public market for the Pre-Funded Warrants being offered by us in this offering.
There
is no established public trading market for the Pre-Funded Warrants, and we do not expect a market to develop. In addition, we do not
intend to apply to list the Pre-Funded Warrants on any national securities exchange or other nationally recognized trading system. Without
an active market, the liquidity of the Pre-Funded Warrants will be limited.
The
Common Warrants and Pre-Funded Warrants are speculative in nature.
The
Common Warrants and Pre-Funded Warrants offered hereby do not confer any rights of share of Common Stock ownership on their holders,
such as voting rights or the right to receive dividends, but rather merely represent the right to acquire shares of Common Stock at a
fixed price. Holders of the warrants and Pre-Funded Warrants may acquire the shares of Common Stock issuable upon exercise of such warrants.
Following this offering, the market value of the warrants and Pre-Funded Warrants is uncertain and there can be no assurance that the
market value of the warrants and Pre-Funded Warrants will equal or exceed its public offering price.
Certain
provisions in our existing warrants could discourage an acquisition of us by a third party.
Certain
provisions of our existing warrants could make it more difficult or expensive for a third party to acquire us. Our warrants prohibit
us from engaging in certain transactions constituting “fundamental transactions” unless, among other things, the surviving
entity assumes our obligations under the warrants and Pre-Funded Warrants. Further, the warrants provide that, in the event of certain
transactions constituting “fundamental transactions,” with some exception, holders of such warrants will have the right,
at their option, to require us to repurchase such warrants at a price described in such warrants. These and other provisions of the warrants
offered by this prospectus could prevent or deter a third party from acquiring us even where the acquisition could be beneficial to you.
USE
OF PROCEEDS
We
estimate that the net proceeds from the offering will be approximately $4.3 million. “Net proceeds” are what we expect to
receive after deducting the placement agent fees and estimated offering expenses payable by us.
We
intend to use the net proceeds from this offering, along with our existing cash and cash equivalents, to fund our ongoing ONP-2 concussion
clinical trials, along with other related research and development activities, as well as for working capital and other general corporate
purposes.
The
net proceeds from this offering, together with our cash, will not be sufficient for us to fund our ONP-002 product candidate through
regulatory approval, and we will need to raise additional capital to complete the development and commercialization of our ONP-002 product
candidate. We may satisfy our future cash needs through the sale of equity securities, debt financings, working capital lines of credit,
corporate collaborations or license agreements, grant funding, interest income earned on invested cash balances or a combination of one
or more of these sources, but there can be no assurances that such future sources will be available to us. This expected use of the net
proceeds from this offering represents our intentions based upon our current plans and business conditions. As of the date of this prospectus,
we cannot predict with certainty all of the particular uses for the net proceeds to be received upon the closing of this offering or
the amounts that we will actually spend. The amounts and timing of our actual expenditures and the extent of clinical development may
vary significantly depending on numerous factors, including the progress of our development efforts, the clinical trials we may commence
in the future, as well as any collaborations that we may enter with third parties for our product candidates and any unforeseen cash
needs. As a result, our management will have significant discretion in the use of any net proceeds and Investors will be relying on the
judgment of our management regarding the application of the proceeds. See, “Risk Factors.”
DIVIDEND
POLICY
We
have never paid cash dividends on our Common Stock. Moreover, we do not anticipate paying periodic cash dividends on our Common Stock
for the foreseeable future. We intend to use all available cash and liquid assets in the operation and growth of our business. Any future
determination about the payment of dividends will be made at the discretion of our board of directors and will depend upon our earnings,
if any, capital requirements, operating and financial conditions and on such other factors as our board of directors deems relevant.
CAPITALIZATION
The
following table sets forth our cash and capitalization as of September 30, 2024:
| |
● |
on
an actual basis; |
| |
|
|
| |
● |
on
a pro forma as adjusted basis to give effect to the sale of 15,151,515 shares of Common Stock in this offering at an assumed offering
price of $0.33 per share, and after deducting the estimated placement agent fees and estimated offering expenses payable by us, and
assuming no sale of Pre-Funded Warrants and no exercise of warrants. |
The
information below is illustrative only, and our capitalization following the completion of this offering will be adjusted based on the
actual offering price and other terms of this offering determined at pricing. You should read the information in this table together
with our financial statements and accompanying notes incorporated by reference to this prospectus and the “Description
of Capital Stock” section of this prospectus.
| | |
September 30,
2024 | | |
Pro Forma as
adjusted | |
| | |
(Unaudited) | | |
| |
| Assets | |
| | | |
| | |
| Current assets: | |
| | | |
| | |
| Cash and cash equivalents | |
$ | 3,144,049 | | |
$ | 7,444,049 | |
| Prepaid expenses and other current assets | |
| 969,294 | | |
| 969,294 | |
| Total current assets | |
| 4,113,343 | | |
| 8,413,343 | |
| Prepaid research and development expense | |
| 1,090,750 | | |
| 1,090,750 | |
| Total assets | |
$ | 5,204,093 | | |
| 9,504,093 | |
| Liabilities and Shareholders’ Equity | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | |
| Accounts payable and accrued expenses | |
$ | 1,607,114 | | |
| 1,607,11 4 | |
| Short-term notes payable | |
| 519,392 | | |
| 519,392 | |
| Total liabilities | |
| 2,126,506 | | |
| 2,126,506 | |
| | |
| | | |
| | |
| Shareholders’ equity: | |
| | | |
| | |
| Preferred stock, no par value; 50,000,000 shares authorized; 5,417,000 Series A shares, 4,050,000 Series B shares, -0- Series C shares, 7,488,692 Series F shares outstanding at September 30, 2024 | |
| 1,592,723 | | |
| — | |
| Common stock, $0.001 par value; 350,000,000 shares authorized and 9,387,547 actual shares issued and outstanding at September 30, 2024 | |
| 9,388 | | |
| 26,132 | |
| Additional paid-in capital | |
| 214,912,523 | | |
| 220,877,502 | |
| Accumulated Deficit | |
| (213,437,047 | ) | |
| (213,437,047 | ) |
| Total shareholders’ equity | |
| 3,077,587 | | |
| 7,377,587 | |
| Total liabilities and shareholders’ equity | |
$ | 5,204,093 | | |
| 9,504,093 | |
The
number of shares of our Common Stock to be outstanding after this offering is based on shares of our Common Stock outstanding as of December
13, 2024, and excludes the following:
●
917,353 shares of our Common Stock issuable upon the exercise of outstanding options under our equity incentive plans at a weighted
average exercise price of $4.97 per share;
●
736,574 shares of Common Stock reserved for issuance under outstanding warrants with a weighted average exercise price of $20.57 per
share;
●
2,004,164 additional shares of Common Stock reserved for future issuance under our 2021 equity incentive plan;
●
1,139,705 shares of common stock reserved for issuance under outstanding pre-funded warrants as of December 13, 2024 with a weighted
average exercise price of $0.001 per share; and
●
approximately 7,488,692 shares of Common Stock reserved for issuance under conversion of 7,488,692 outstanding shares of Series F Non-Voting,
Convertible Preferred Stock.
Unless
we indicate otherwise or unless the context otherwise requires, all information in this prospectus assumes the following:
| |
● |
no
exercise of outstanding options or warrants; |
| |
|
|
| |
● |
assumes
no exercise of the Pre-Funded Warrants issued in this offering; and |
| |
|
|
| |
● |
no
exercise of the Placement Agent’s warrants to be issued upon consummation of this offering. |
DILUTION
If
you purchase Common Stock in this offering, your interest will be diluted immediately to the extent of the difference between the assumed
offering price of $0.33 per share and the net tangible book value per share of our Common Stock immediately upon the consummation of
this offering.
Our
net tangible book value as of September 30, 2024 was approximately $3.1 million, or approximately $0.24 per share, based upon 12,570,100
shares of Common Stock outstanding as of December 13, 2024. Net tangible book value per share of our Common Stock represents our total
tangible assets (total assets less intangible assets) less total liabilities divided by the number of shares of Common Stock outstanding
as of that date. Dilution in net tangible book value per share represents the difference between the amount per share paid by purchasers
of shares of Common Stock in this offering and the net tangible book value per share of Common Stock immediately after this offering.
After
giving effect to the sale by us of shares of our Common Stock in this offering at the assumed offering price of $0.33 per share and after
deducting the placement agent fees and estimated offering expenses payable by us, our as adjusted, pro forma net tangible book value
would be approximately $7.4 million, or approximately $.0.27 per share of Common Stock, as of September 30, 2024. This represents an
immediate increase in our adjusted, pro forma net tangible book value of approximately $0.02 per share to existing stockholders and
an immediate dilution of approximately $0.06 per share to new investors. The following table illustrates this calculation on a per share
basis:
| Offering price per share | |
$ | 0.33 | |
| Historical net tangible book value per share as of September 30, 2024 | |
$ | 0.24 | |
| As adjusted, pro forma net tangible book value per share after giving effect to this offering | |
$ | 0.27 | |
| Increase in adjusted, pro forma net tangible book value per share attributable to new investors | |
$ | 0.02 | |
| As adjusted, pro forma dilution per share to investors in this offering | |
$ | 0.06 | |
The
number of shares of our Common Stock to be outstanding after this offering is based on shares of our Common Stock outstanding as of December
13, 2024, and excludes the following:
●
917,353 shares of our Common Stock issuable upon the exercise of outstanding options under our equity incentive plans at a weighted
average exercise price of $4.97 per share;
●
736,574 shares of Common Stock reserved for issuance under outstanding warrants with a weighted average exercise price of $20.57 per
share;
●
2,004,164 additional shares of Common Stock reserved for future issuance under our 2021 equity incentive plan;
●
1,139,705 shares of common stock reserved for issuance under outstanding pre-funded warrants as of December 13, 2024 with a weighted
average exercise price of $0.001 per share; and
●
approximately 7,488,692 shares of Common Stock reserved for issuance under conversion of 7,488,692 outstanding shares of Series F Non-Voting,
Convertible Preferred Stock.
Importantly,
the conversion of our outstanding preferred stock, and in particular our Series F Convertible Preferred Stock, will result in a material
further dilution of your investment. After giving effect to the sale of our Common Stock in this offering at the assumed offering price
of $0.33 per share and after deducting the placement agent fees and estimated offering expenses per share, and assuming the conversion
of all 7,488,692 outstanding shares of Series F Convertible Preferred Stock, which shares are entitled to convert into shares of our
Common Stock on a one-to-one basis, subject to certain restrictions and adjustments, our as adjusted, pro forma net tangible book value
per share of Common Stock would be approximately $0.21, as of September 30, 2024. This represents an immediate dilution of approximately
$0.12 per share to new investors. See “Risk Factors”.
In
addition, we may choose to raise additional capital due to market conditions or strategic considerations, even if we believe we have
sufficient funds for our current or future operating plans. To the extent that additional capital is raised through the sale of equity
or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
DESCRIPTION
OF CAPITAL STOCK
The
following descriptions are summaries of the material terms that are included in our amended and restated articles of incorporation (as
amended) and our bylaws (as amended) as well as the specific agreements such descriptions relate to. This summary is qualified in its
entirety by the specific terms and provisions contained in our restated articles of incorporation, bylaws and the specific agreements
described herein, copies of which we have filed as exhibits to the registration statement of which this prospectus is a part, and by
the provisions of applicable law.
Overview
Authorized
Capital Stock
Our
authorized capital stock consists of 350,000,000 shares of Common Stock, par value $0.001, and 50,000,000 shares of preferred stock,
without par value.
Common
Stock
Voting
Holders
of our Common Stock are entitled to one vote for each share on all matters submitted to a stockholder vote. Holders of our Common Stock
do not have cumulative voting rights. Therefore, holders of a majority of the shares of our Common Stock voting for the election of directors
collectively hold the voting power to elect all of our directors. Holders of our Common Stock representing one third of the voting power
of our capital stock issued, outstanding and entitled to vote, represented in person or by proxy, are necessary to constitute a quorum
at any meeting of stockholders.
Dividends
Subject
to preferences that may be applicable to any outstanding preferred stock, the holders of our Common Stock are entitled to receive ratably
all dividends, if any, as may be declared from time to time by our Board of Directors out of the funds legally available.
Rights
upon Liquidation
Upon
our liquidation, dissolution or winding-up, after payment in full of our liabilities and the amounts required to be paid to holders of
any outstanding shares of preferred stock, if any, all holders of our Common Stock, along with the holders of our Series A Convertible
Preferred Stock and Series B Convertible Preferred Stock on an “as if” converted basis, will be entitled to receive a pro
rata distribution of all of our assets and funds legally available for distribution.
Redemption
and Pre-Emptive Rights
No
shares of our Common Stock are subject to redemption or have preemptive rights to purchase additional shares of our Common Stock or any
of our other securities.
Fully
Paid and Nonassessable
All
of our outstanding shares of Common Stock are, and the shares of Common Stock to be issued in this offering will be, fully paid and nonassessable.
Listing
of Common Stock
Our
Common Stock is currently listed on the NYSE American under the trading symbol “OGEN”.
Preferred
Stock
Our
Board of Directors has the authority, without action by our shareholders, to designate and issue up to 50,000,000 shares of preferred
stock in one or more series or classes and to designate the rights, preferences and privileges of each series or class, which may be
greater than the rights of our Common Stock. These rights, preferences and privileges could include dividend rights, conversion rights,
voting rights, redemption rights, liquidation preferences, the number of shares constituting any class or series and the designation
of the class or series. Terms selected by our Board of Directors in the future could decrease the amount of earnings and assets available
for distribution to holders of shares of Common Stock or adversely affect the rights and powers, including voting rights, of the holders
of shares of Common Stock without any further vote or action by the stockholders. As a result, the rights of holders of our Common Stock
will be subject to, and may be adversely affected by, the rights of the holders of the Series F Convertible Preferred Stock or any other
preferred stock that may be issued by us in the future, which could have the effect of decreasing the market price of our Common Stock.
The Company’s previously issued shares of Series A, B, C, D and E Preferred Stock have all either been cancelled or converted and
are no longer outstanding.
Series
F Convertible Preferred Stock
On
December 28, 2023, we issued 8,000,000 shares of convertible preferred stock, designated as the Series F Convertible Preferred Stock
(“Series F Preferred Stock”) pursuant to the certificate of designation and rights filed by the Company with the Secretary
of State of the State of Florida (“Series F Certificate of Designation”), as partial consideration for the purchase of certain
assets of Odyssey Health, Inc. On December 28, 2023 and pursuant to the Series F Certificate of Designation, 511,308 shares of Series
F Preferred were converted to Common Stock and, as a result of such conversion, 7,488,692 shares of Series F Convertible Preferred Stock
remain outstanding.
The
following description is a summary of the material provisions of the Series F Convertible Preferred Stock.
Liquidation
Preference
The
Series F Preferred Stock is economically equivalent to the Company’s Common Stock. Upon liquidation, it is at parity with the Common
Stock and junior to any class or series of capital stock of the Corporation created specifically ranking by its terms senior to the Series
F Preferred Stock.
Dividends
No
dividends shall be paid on shares of the Series F Preferred Stock.
Voting
The
Series F Preferred Stock has no voting rights, except as required by applicable law and except for limited protective voting rights specifically
set forth in Certificate of Designation.
Conversion
The
Series F Preferred Stock is convertible commencing with the date of its issuance into Common Stock on a 1 for 1 basis (subject to customary
adjustments). However, pursuant to the Series F Certificate of Designation, the holder of the Series F Preferred Stock cannot convert
shares of Series F Preferred Stock into more than 19.9% of the Company’s Common Stock outstanding as of October 4, 2023 until (i)
the Company shall have applied for and been approved for initial listing on the NYSE American or another national securities exchange
or shall have been delisted from the NYSE American, and (ii) if required by the rules of the NYSE American, the Company’s shareholders
shall have approved any change of control that could be deemed to occur upon the conversion of the Series F Preferred Stock into Common
Stock, based on the facts and circumstances existing at such time.
Preemptive
Rights
No
holders of Series F Preferred Stock will, as holders of Series F Preferred Stock, have any preemptive rights to purchase or subscribe
for our Common Stock or any of our other securities.
Redemption
The
Series F Preferred Stock is not redeemable by the Company.
Trading
Market
There
is no established trading market for any of the Series F Preferred Stock, and the Company does not expect a market to develop. The Company
does not intend to apply for a listing for any of the Series F Preferred Stock on any securities exchange or other nationally recognized
trading system.
The
following descriptions are summaries of the material terms that are included in our amended and restated articles of incorporation (as
amended) and our bylaws (as amended) as well as the specific agreements such descriptions relate to. This summary is qualified in its
entirety by the specific terms and provisions contained in our restated articles of incorporation, bylaws and the specific agreements
described herein, copies of which we have filed as exhibits to our Form 10-K.
Series
A, B, C, D and E Preferred Stock
The
Company’s previously had issued shares of Series A, B, C, D and E Preferred Stock. All of the shares of the Series A and Series
B Preferred Stock were converted into Common Stock. All of the shares of Series C Non-Voting, Non-Convertible Preferred Stock were redeemed
by the Company in accordance with their terms and no shares of Series C Non-Voting, Non-Convertible Preferred Stock remain outstanding.
All of the shares of Series D Preferred Stock-Converted to Common Stock were converted to Common Stock and as such, the Company no longer
has any Series D Preferred Stock outstanding. Pursuant to the terms of the Series E Certificate of Designation, upon effectiveness of
an amendment to the Amended and Restated Articles of Incorporation of the Company to effect an increase in the shares of Common Stock
the Company was authorized to issue from 4,166,666 shares of Common Stock to 350,000,000 shares of Common Stock (the “Amendment”),
each share of Series E Preferred Stock would be automatically transferred to the Company and cancelled for no consideration with no action
on behalf of the holders of Series E Preferred Stock. The Company’s shareholders approved the Amendment on December 14, 2023, and
accordingly, all of the shares of Series E Preferred Stock resumed the status of authorized but unissued preferred stock and are no longer
designated as Series E Preferred Stock.
Certain
Anti-Takeover Provisions
Florida
Law
We
are not subject to the statutory anti-takeover provisions under Florida law because in our articles of incorporation we have specifically
elected to opt out of both the “control-share acquisitions” (F.S. 607.0902) and the “affiliated transactions”
(F.S. 607.0901) statutes. Since these anti-takeover statutes do not apply to a corporation that has specifically elected to opt out of
such provisions, we would not be able to invoke the protection of such statutes in the event of a hostile takeover attempt.
Articles
of Incorporation and Bylaw Provisions
Our
articles of incorporation and bylaws contain provisions that could have an anti-takeover effect. These provisions include
| |
● |
authorization
of the issuance of “blank check” preferred stock that could be issued by our Board of Directors without shareholder approval
and that may be substantially dilutive or contain preferences or rights objectionable to an acquiror; |
| |
|
|
| |
● |
the
ability of the Board of Directors to amend the bylaws without shareholder approval; |
| |
|
|
| |
● |
vacancies
on our board may only be filled by the remaining Directors and not our shareholders; and |
| |
|
|
| |
● |
requirements
that only our Board, our President or holders of more than 10% of our shares can call a special meeting of shareholders. |
These
provisions in our articles of incorporation and bylaws could delay or discourage transactions involving an actual or potential change
in control of us, including transactions in which shareholders might otherwise receive a premium for their shares over their current
prices. Such provisions could also limit the ability of shareholders to approve transactions that shareholders may deem to be in their
best interests and could adversely affect the price of our Common Stock.
Transfer
Agent and Registrar
The
transfer agent and registrar of our Common Stock is Continental Stock Transfer & Trust Company, 1 State Street 30th Floor, New York,
New York 10004, telephone: (212) 509-4000.
DESCRIPTION
OF SECURITIES WE ARE OFFERING
Common
Stock
We
are offering of up to 15,151,515 units consisting of one share of common stock, par value $0.001 per share (“Common Stock”)
(or one Pre-Funded Warrant to purchase one share of our Common Stock in lieu thereof), and one warrant to purchase one share of our Common
Stock (the “Common Warrants” and together with the shares of Common Stock, the “Units”). The Units have no stand-alone
rights and will not be certificated or issued as stand-alone securities. Each Common Warrant offered hereby is exercisable upon issuance
at an initial exercise price of $0.33 (assuming an offering price of $0.33 per Unit) per share of common stock, and will expire five
(5) years from the date of issuance.
We
are also offering to each purchaser of Units whose purchase of shares of our Common Stock in this offering would otherwise result in
the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of
the holder, 9.99%) of our outstanding shares of Common Stock immediately following consummation of this offering, the opportunity to
purchase, if the purchaser so chooses, Units consisting of one Pre-Funded Warrant to purchase one share of Common Stock (“Pre-Funded
Warrants”), and one Common Warrant. Each Pre-Funded Warrant will be exercisable immediately upon issuance, will expire when exercised
in full, and will be exercisable for one share of our Common Stock.
The
Common Stock and Prefunded Warrants can each be purchased in this offering only with the accompanying Common Warrants that are part of
a Unit, but the components of the Units will be immediately separable and will be issued separately in this offering.
This
offering also relates to the shares of Common Stock issuable upon exercise of the Common Warrants (the “Common Warrant Shares”),
the shares of Common Stock issuable upon exercise of the Pre-Funded Warrants (the “Pre-Funded Warrant Shares”), and the shares
of Common Stock issuable upon exercise of Placement Agent Warrants (defined below).
We
are authorized to issue 350,000,000 shares of our Common Stock at $0.001 par value per share and 50,000,000 shares of our preferred stock,
with no par value.
Common
Stock
The
material terms and provisions of our Common Stock are described under the caption “Description of Capital Stock” in this
prospectus and are incorporated herein by reference.
Common
Warrants
The
following summary of certain terms and provisions of the Common Warrants that are being offered hereby is not complete and is subject
to, and qualified in its entirety by, the provisions of the Common Warrant, the form of which will be filed as an exhibit to the registration
statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the form of
Common Warrant for a complete description of the terms and conditions of the Common Warrants.
Duration
and Exercise Price
Each
Common Warrant is exercisable upon issuance at an initial exercise price of $0.33 per share (assuming an offering price of $0.33 per
Unit), and will expire five (5) years from the date of issuance. The exercise price and number of shares of Common Stock issuable upon
exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting
our Common Stock and the exercise price. The Common Warrants will be issued separately from the Common Stock and may be transferred separately
immediately thereafter.
Exercisability
The
Common Warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise
notice accompanied by payment in full for the number of shares of our Common Stock purchased upon such exercise (except in the case of
a cashless exercise as discussed below). Generally, a holder (together with its affiliates) may not exercise any portion of such holder’s
Common Warrants to the extent that the holder would own more than 4.99% of the outstanding Common Stock (or at the election of a holder
prior to the date of issuance, 9.99%) immediately after exercise, except that upon at least 61 days’ prior notice from the holder
to us, the holder may increase the amount of ownership of outstanding stock after exercising the holder’s warrants up to 9.99%
of the number of shares of our Common Stock outstanding immediately after giving effect to the exercise, as such percentage ownership
is determined in accordance with the terms of the Common Warrants.
Cashless
Exercise
If,
at the time a holder exercises its Common Warrants, a registration statement registering the issuance of the shares of Common Stock underlying
the Common Warrants under the Securities Act is not then effective or available for the issuance of such shares, then in lieu of making
the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may
elect instead to receive upon such exercise (either in whole or in part) the net number of shares of Common Stock determined according
to a formula set forth in the Common Warrant.
Fundamental
Transactions
In
the event of a fundamental transaction, as described in the Common Warrant and generally including any reorganization, recapitalization
or reclassification of our Common Stock, the sale, transfer or other disposition of all or substantially all of our properties or assets,
our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding Common Stock, or any person
or group becoming the beneficial owner of 50% of the voting power represented by our outstanding Common Stock, the holders of the Common
Warrants will be entitled to receive upon exercise of the Common Warrants the kind and amount of securities, cash or other property that
the holders would have received had they exercised the Common Warrants immediately prior to such fundamental transaction. In addition,
in certain circumstances, upon a fundamental transaction, the holder of a Common Warrant will have the right to require us to repurchase
its Common Warrants at the Black-Scholes value; provided, however, that, if the fundamental transaction is not within our control, including
not approved by our Board, then the holder will only be entitled to receive the same type or form of consideration (and in the same proportion),
at the Black-Scholes value of the unexercised portion of the Common Warrant that is being offered and paid to the holders of our Common
Stock in connection with the fundamental transaction.
Transferability
Subject
to applicable laws, a Common Warrant may be transferred at the option of the holder upon surrender of the Common Warrant to us together
with the appropriate instruments of transfer.
Fractional
Shares
No
fractional shares of Common Stock will be issued upon the exercise of the Common Warrants. Rather, the number of shares of Common Stock
to be issued will, at our election, either be rounded up to the next whole share or we will pay a cash adjustment in respect of such
final fraction in an amount equal to such fraction multiplied by the exercise price.
Trading
Market
There
is no established trading market for the Common Warrants, and we do not expect an active trading market to develop. We do not intend
to apply to list the Common Warrants on any securities exchange or other trading market. Without a trading market, the liquidity of the
Common Warrants will be extremely limited.
Right
as a Stockholder
Except
as otherwise provided in the Common Warrants or by virtue of such holder’s ownership of our shares of Common Stock, the holder
of a Common Warrant does not have the rights or privileges of a holder of our Common Stock, including any voting rights, until the holder
exercises the Common Warrant.
Waivers
and Amendments
The
Common Warrants may be modified or amended, or the provisions thereof waived with the written consent of the Company and the respective
holder.
Governing
Law.
The
Common Warrants are governed by New York law.
Pre-Funded
Warrants
The
following summary of certain terms and provisions of Pre-Funded Warrants that are being offered hereby is not complete and is subject
to, and qualified in its entirety by, the provisions of the Pre-Funded Warrant, the form of which will be filed as an exhibit to a Current
Report on Form 8-K that we will file with the SEC and that will be incorporated by reference into the registration statement of which
this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the form of Pre-Funded Warrant
for a complete description of the terms and conditions of the Pre-Funded Warrants.
Duration
and Exercise Price
Each
Pre-Funded Warrant offered hereby will have an initial exercise price of $0.001 per share. The Pre-Funded Warrants will be immediately
exercisable and may be exercised at any time until the Pre-Funded Warrants are exercised in full. The exercise price and number of shares
of Common Stock issuable upon exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations
or similar events affecting our Common Stock and the exercise price.
Exercisability
The
Pre-Funded Warrants will be exercisable, at the option of each holder, in whole or in part, by delivering a duly executed exercise notice
accompanied by payment in full for the number of purchased upon such exercise (except in the case of a cashless exercise as discussed
below). A holder (together with its affiliates) may not exercise any portion of the Pre-Funded Warrant to the extent that the holder
would own more than 4.99% (or, at the election of a purchaser, 9.99%) of the outstanding Common Stock immediately after exercise, except
that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of ownership of outstanding
stock after exercising the holder’s Pre-Funded Warrants up to 9.99% of the number of shares of Common Stock outstanding immediately
after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Pre-Funded Warrants.
No fractional shares of Common Stock will be issued in connection with the exercise of a Pre-Funded Warrant. In lieu of fractional shares,
we will pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price.
Cashless
Exercise
In
lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price,
the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of Common Stock determined
according to a formula set forth in the Pre-Funded Warrants.
Fundamental
Transaction
In
the event of a fundamental transaction, as described in the Pre-Funded Warrants and generally including any reorganization, recapitalization
or reclassification of our Common Stock, the sale, transfer or other disposition of all or substantially all of our properties or assets,
our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding voting securities, the holders
of the Pre-Funded Warrants will be entitled to receive upon exercise of the Pre-Funded Warrants the kind and amount of securities, cash
or other property that the holders would have received had they exercised the Pre-Funded Warrants immediately prior to such fundamental
transaction, other than one in which a successor entity that is a publicly traded corporation (whose stock is quoted or listed for trading
on a national securities exchange, including, but not limited to, the New York Stock Exchange, the NYSE American, the Nasdaq Global Select
Market, the Nasdaq Global Market or the Nasdaq Capital Market) assumes the Common Warrant such that the warrant shall be exercisable
for the publicly traded Common Stock of such successor entity.
Transferability
Subject
to applicable laws, a Pre-Funded Warrant may be transferred at the option of the holder upon surrender of the Pre-Funded Warrant to us
together with the appropriate instruments of transfer.
Exchange
Listing
We
do not intend to list the Pre-Funded Warrants on any securities exchange or nationally recognized trading system.
Rights
as a Stockholder
Except
as otherwise provided in the Pre-Funded Warrants or by virtue of such holder’s ownership of, the holders of the Pre-Funded Warrants
do not have the rights or privileges of holders of our Common Stock, including any voting rights, until they exercise their Pre-Funded
Warrants.
Governing
Law.
The
Common Warrants are governed by New York law.
Listing
of Common Stock
Our
Common Stock is currently listed on the NYSE American under the trading symbol “OGEN”.
Transfer
Agent and Registrar
The
transfer agent and registrar of our Common Stock is Continental Stock Transfer & Trust Company, 1 State Street 30th Floor, New York,
New York 10004, telephone: (212) 509-4000.
PLAN
OF DISTRIBUTION
We
engaged Dawson James Securities, Inc. (“Dawson James” or the “Placement Agent”) to act as our exclusive placement
agent to solicit offers to purchase the securities offered by this prospectus on a reasonable best efforts basis. Dawson James is not
purchasing or selling any securities, nor are they required to arrange for the purchase and sale of any specific number or dollar amount
of securities, other than to use their “reasonable best efforts” to arrange for the sale of the securities by us. Therefore,
we may not sell the entire amount of securities being offered. There is no minimum amount of proceeds that is a condition to closing
of this offering. The Placement Agent does not guarantee that it will be able to raise new capital in this offering. The terms of this
offering were subject to market conditions and negotiations between us and prospective investors in consultation with the Placement Agent.
The Placement Agent will have no authority to bind us. This offering will terminate no later than January 31, 2025, unless
we decide to terminate the offering (which we may do at any time in our discretion) prior to that date. We will have one closing for
all the securities purchased in this offering. The combined public offering price per share (or Pre-Funded Warrant) will be fixed for
the duration of this offering. Dawson James may engage one or more sub-placement agents or selected dealers to assist with the offering.
We
will enter into a securities purchase agreement directly with the institutional investors, at the investor’s option, who purchase
our securities in this offering. Investors who do not enter into a securities purchase agreement shall rely solely on this prospectus
in connection with the purchase of our securities in this offering.
We
will deliver the securities being issued to the investor upon receipt of such investor’s funds for the purchase of the securities
offered pursuant to this prospectus. We expect to deliver the securities being offered pursuant to this prospectus on or about [__________],
2025.
We
have agreed to indemnify the Placement Agent against specified liabilities, including liabilities under the Securities Act, and to contribute
to payments the Placement Agent may be required to make in respect thereof.
Placement
Agent Fees and Expenses
The
following table shows the per share and per Pre-Funded Warrant and total placement agent fees we will pay in connection with the sale
of the securities in this offering.
| | |
Per Unit | | |
Per Pre-Funded
Warrant | | |
Total | |
| Public offering price | |
$ | 0.33 | | |
$ | | | |
$ | 5,000,000 | |
| Placement Agent fees (1) | |
$ | .0231 | | |
$ | | | |
$ | 350,000 | |
| Proceeds to us, before expenses(2) | |
$ | 0.3069 | | |
$ | | | |
$ | 465,000 | |
1.
We have agreed to pay to the placement agent a cash fee equal to 7% of the aggregate gross proceeds raised in this offering subject to
a partial adjustment in the event certain investors participate. Because there is no minimum offering amount required as a condition
to closing in this offering, the actual aggregate cash placement fee, if any, is not presently determinable and may be substantially
less than the maximum amount set forth above.
2.
We have also agreed to reimburse the Placement Agent at closing for legal and other expenses incurred by the placement agent in connection
with this offering in an amount up to $125,000; provided that if the that if the Offering does not result in the Company receiving at
least $3 million in gross proceeds, the cap on such expenses shall be $75,000.
We
estimate that the total expenses of the offering, including registration, filing and listing fees, printing fees and legal and accounting
expenses, but excluding placement agent fees, will be approximately $350,000, all of which are payable by us. This figure includes the
Placement Agent’s accountable expenses, including, but not limited to, legal fees for Placement Agent’s legal counsel, that
we have agreed to pay at the closing of the offering up to an aggregate expense reimbursement of $125,000, assuming we receive at least
$3 million of gross proceeds in the offering. If we receive less than $3 million in gross proceeds in the offering, the cap on
expense reimbursements will be $75,000.
Placement
Agent Warrants
In
addition, we have agreed to issue to the Placement Agent or its designees warrants to purchase up to 757,576shares of Common Stock (which
represents 5.0% of the aggregate number of shares of shares of common stock issued in this offering) with an exercise price of $0.45
per share (representing 125% of the public offering price per share) and exercisable for five years from the date of the commencement
of sales in this offering.
The
Placement Agent warrants will be exercisable upon issuance, will have a cashless exercise provision and will terminate on the fifth anniversary
of the commencement date of sales in this offering. The Placement Agent warrants are not exercisable or convertible for more than five
years from the commencement date of sales in this offering. The Placement Agent warrants also provide for customary anti-dilution provisions,
demand and “piggyback” registration rights with respect to the registration of the shares of common stock underlying the
warrants for a period not to exceed five years from the commencement of sales in the offering. We have registered the Placement Agent
warrants and the shares underlying the Placement Agent warrants in this offering.
The
Placement Agent warrants and the underlying shares will be deemed to be compensation by FINRA, and therefore will be subject to FINRA
Rule 5110(e)(1). In accordance with FINRA Rule 5110(e)(1), neither the Placement Agent warrants nor any of our shares of common stock
issued upon exercise of the Placement Agent warrants may be sold, transferred, assigned, pledged or hypothecated, or be the subject of
any hedging, short sale, derivative, put or call transaction that would result in the effective economic disposition of such securities
by any person, for a period of 180 days immediately following the commencement date of sales in this offering, subject to certain exceptions.
The Placement Agent warrants to be received by the Placement Agent and related persons in connection with this offering: (i) fully comply
with lock-up restrictions pursuant to FINRA Rule 5110(e)(1); and (ii) fully comply with transfer restrictions pursuant to FINRA Rule
5110(e)(2).
Tail
In
the event the closing of this offering results in the Company receiving at least $3 million of gross proceeds, we have also agreed to
pay the Placement Agent a tail fee in an amount equal to 7% of the aggregate gross proceeds raised in any public or private offering
or other financing consummated by the Company in the three month period following the expiration or termination of our engagement of
the Placement Agent to the extent that such financing or capital is provided to the Company by investors introduced to this offering
by the Placement Agent.
Other
Relationships
The
Placement Agent and certain of its affiliates are full service financial institutions engaged in various activities, which may include
securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment,
hedging, financing and brokerage activities. The Placement Agent and certain of its affiliates have, from time to time, performed, and
may in the future perform, various commercial and investment banking and financial advisory services for us and our affiliates, for which
they received or will receive customary fees and expenses.
In
the ordinary course of their various business activities, the Placement Agent and certain of its affiliates may make or hold a broad
array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including
bank loans) for their own account and for the accounts of their customers, and such investment and securities activities may involve
securities and/or instruments issued by us and our affiliates. If the Placement Agent or its affiliates have a lending relationship with
us, they routinely hedge their credit exposure to us consistent with their customary risk management policies. The Placement Agent and
its affiliates may hedge such exposure by entering into transactions that consist of either the purchase of credit default swaps or the
creation of short positions in our securities or the securities of our affiliates, including potentially the Common Stock offered hereby.
Any such short positions could adversely affect future trading prices of the Common Stock offered hereby. The Placement Agent and certain
of its affiliates may also communicate independent investment recommendations, market color or trading ideas and/or publish or express
independent research views in respect of such securities or instruments and may at any time hold, or recommend to clients that they acquire,
long and/or short positions in such securities and instruments.
Determination
of Offering Price
The
public offering price per share and the public offering price per Pre-Funded Warrant we are offering and the exercise prices and other
terms of the warrants were negotiated between us and the investors, in consultation with the Placement Agent based on the trading of
our Common Stock prior to this offering, among other things. Other factors considered in determining the public offering prices of the
securities we are offering and the exercise prices and other terms of the Pre-Funded Warrants include the history and prospects of our
company, the stage of development of our business, our business plans for the future and the extent to which they have been implemented,
an assessment of our management, general conditions of the securities markets at the time of the offering and such other factors as were
deemed relevant.
Lock-Up
Agreements
Each
of our officers and directors and five percent (5%) shareholders have agreed to be subject to a lock-up period of 90 days following the
date of this prospectus. This means that, during the applicable lock-up period, they may not offer for sale, contract to sell, or sell
any shares of our Common Stock or any securities convertible into, or exercisable or exchangeable for, shares of our Common Stock subject
to certain customary exceptions. In addition, provided that the Company receives at least $3 million in gross proceeds in this offering,
we have agreed to not issue any shares of Common Stock or securities exercisable or convertible into shares of Common Stock or file any
registration statement with the Commission relating to the offering of any of our securities, subject to certain exceptions, for a period
of 90 days following the closing date of this offering; provided that following the closing date of this offering we will be permitted
to enter into an agreement in connection with an “at the market” offering under Rule 415(a)(4) under the Securities Act and
make sales thereunder.
Indemnification
We
have agreed to indemnify the placement agent against certain liabilities, including certain liabilities arising under the Securities
Act, or to contribute to payments that the placement agent may be required to make for these liabilities.
Regulation
M
The
Placement Agent may be deemed to be an underwriter within the meaning of Section 2(a)(11) of the Securities Act and any fees received
by it and any profit realized on the sale of the securities by it while acting as principal might be deemed to be underwriting discounts
or commissions under the Securities Act. The placement agent will be required to comply with the requirements of the Securities Act and
the Exchange Act of 1934, as amended (the “Exchange Act”), including, without limitation, Rule 10b-5 and Regulation M under
the Exchange Act. These rules and regulations may limit the timing of purchases and sales of our securities by the placement agent. Under
these rules and regulations, the placement agent may not (i) engage in any stabilization activity in connection with our securities;
and (ii) bid for or purchase any of our securities or attempt to induce any person to purchase any of our securities, other than as permitted
under the Exchange Act, until they have completed their participation in the distribution.
Electronic
Distribution
A
prospectus in electronic format may be made available on a website maintained by the placement agent and the placement agent may distribute
prospectuses electronically. Other than the prospectus in electronic format, the information on these websites is not part of this prospectus
or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or the placement agent
and should not be relied upon by investors.
The
NYSE American Listing
Our
Common Stock is currently listed on the NYSE American under the symbol “OGEN.” On December 13, 2024, the reported closing
price per share of our Common Stock was $0.33. The final public offering price will be determined between us, the placement agent and
the investors in the offering, and may be at a discount to the current market price of our Common Stock. Therefore, the assumed public
offering price used throughout this prospectus may not be indicative of the final public offering price. There is no established public
trading market for the Pre-Funded Warrants, and we do not expect such markets to develop. In addition, we do not intend to apply for
a listing of the Pre-Funded Warrants on any national securities exchange or other nationally recognized trading system.
Transfer
Agent and Registrar
The
transfer agent and registrar of our Common Stock is Continental Stock Transfer & Trust Company, 1 State Street 30th Floor, New York,
New York 10004, telephone: (212) 509-4000.
Offer
and Sale Restrictions Outside the United States
Other
than in the United States, no action has been taken by us or the Placement Agent that would permit a public offering of the securities
offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may
not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection
with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will
result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes
are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus.
This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in
any jurisdiction in which such an offer or a solicitation is unlawful.
EXPERTS
The
consolidated financial statements for the year ended December 31, 2023, appearing in the Company’s Annual Report on Form 10-K for
the year ended December 31, 2023, filed with the SEC on March 29, 2024, have been audited by Cherry Bekaert LLP, independent registered
public accounting firm, as set forth in their report, which report includes an explanatory paragraph about the existence of substantial
doubt concerning the Company’s ability to continue as a going concern, and have been incorporated herein by reference in reliance
upon such report given on the authority of such firm as experts in accounting and auditing, in giving said reports.
The
consolidated financial statements for the year ended December 31, 2022, appearing in the Company’s Annual Report on Form 10-K for
the year ended December 31, 2023, filed with the SEC on March 29, 2024, have been audited by CBIZ CPAs P.C., formerly known as Mayer
Hoffman McCann P.C., independent registered public accounting firm, as set forth in their report, which report includes an explanatory
paragraph about the existence of substantial doubt concerning the Company’s ability to continue as a going concern, and have been
incorporated herein by reference in reliance upon such report given on the authority of such firm as experts in accounting and auditing,
in giving said reports.
LEGAL
MATTERS
The
validity of the issuance of the securities offered hereby has been passed upon for us by Shumaker, Loop & Kendrick, LLP, Tampa, Florida.
ArentFox Schiff LLP, Washington, D.C., has acted as counsel for the Placement Agent.
INFORMATION
INCORPORATED BY REFERENCE
In
this document, we “incorporate by reference” certain information we file with the SEC, which means that we can disclose important
information to you by referring to that information. The information incorporated by reference is considered to be a part of this prospectus.
Any statement contained in a document incorporated by reference herein shall be deemed to be modified or superseded for all purposes
to the extent that a statement contained in this prospectus or in any other subsequently filed document that is also incorporated or
deemed to be incorporated by reference, modifies or supersedes such statement. Any statement so modified or superseded shall not be deemed,
except as so modified or superseded, to constitute a part of this prospectus. We incorporate by reference the documents listed below
(other than, in each case, documents or information deemed to be furnished and not filed in accordance with SEC rules):
| ●
|
Our
Annual Report on Form 10-K for the year ended December 31, 2023, filed with the SEC on March 29, 2024; |
| |
|
| ●
|
Our
Quarterly Reports on Form 10-Q for the quarter ended March 31, 2024, filed with the SEC on May 15, 2024, for the quarter ended June 30, 2024, filed with the SEC on August 9, 2024, for the quarter ended September 30, 2024 filed with the SEC on November 13, 2024;
|
| |
|
| ●
|
Our
Definitive Proxy Statement on Schedule 14A, filed with the SEC on October 31, 2024; |
| |
|
| ●
|
Our
Current Reports on Form 8-K filed January 2, 2024, January 16, 2024, January 23, 2024, February 5, 2024, February 7, 2024, February 12, 2024, February 28, 2024, February 28, 2024, March 1, 2024, March 18, 2024, April 16, 2024, April 22, 2024, May 7, 2024, May 16, 2024, May 17, 2024, May 22, 2024, May 23, 2024, June 20, 2024; June 26, 2024, July 10, 2024, July 22, 2024, August 8, 2024, August 12, 2024, August 14, 2024, August 16, 2024, August 21, 2024, September 5, 2024, September 20, 2024, October 9, 2024, October 15, 2024, October 16, 2024, October 18, 2024, November 29, 2024, December 16, 2024. |
| |
|
| ●
|
The
description of our Common Stock set forth in Exhibit 4.2 of our Current Report on Form 8-K filed March 1, 2024. |
We
also incorporate by reference into this prospectus and the accompanying prospectus all documents (other than current reports furnished
under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on such form that are related to such items) that are filed by us with the
SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, after the
date of this prospectus until we sell all of the securities covered by this prospectus and the accompanying prospectus or the sale of
securities by us pursuant to this prospectus and the accompanying prospectus is terminated.
A
statement contained in a document incorporated by reference into this prospectus shall be deemed to be modified or superseded for purposes
of this prospectus to the extent that a statement contained in this prospectus or in any other subsequently filed document which is also
incorporated by reference in this prospectus modifies or replaces such statement. Any statements so modified or superseded shall not
be deemed, except as so modified or superseded, to constitute a part of this prospectus.
We
hereby undertake to provide without charge to each person, including any beneficial owner, to whom a copy of this prospectus is delivered,
upon written or oral request of any such person, a copy of any and all of the information that has been or may be incorporated by reference
in this prospectus, including any exhibits that are specifically incorporated by reference in such documents. Requests for such copies
should be directed as follows: Oragenics, Inc., 1990 Main St Suite 750 Sarasota, Florida 34236, Attention: Investor Relations, Phone:
(813) 276-7900.
This
prospectus is part of a registration statement we filed with the SEC. That registration statement and the exhibits filed along with the
registration statement contain more information about us and the shares in this offering. Because information about documents referred
to in this prospectus is not always complete, you should read the full documents which are filed as exhibits to the registration statement.
You may read and copy the full registration statement and its exhibits at the SEC’s website.
WHERE
YOU CAN FIND MORE INFORMATION
We
are a public company and file annual, quarterly and current reports, proxy statements and other information with the Securities and Exchange
Commission (“SEC”). You can request copies of these documents by writing to the SEC and paying a fee for the copying cost.
Our SEC filings are also available to the public at the SEC’s web site at http://www.sec.gov.
In
addition, we maintain a web site that contains information regarding our company, including copies of reports, proxy statements and other
information we file with the SEC. The address of our web site is www.oragenics.com. Except for the documents specifically incorporated
by reference into this prospectus, information contained on our website or that can be accessed through our website does not constitute
a part of this prospectus. We have included our website address only as an inactive textual reference and do not intend it to be an active
link to our website.
Up
to 15,151,515 Units, consisting of One Share of Common Stock and One Warrant to Purchase One Share of Common Stock
Up
to 15,151,515 Shares of Common Stock underlying such Warrants
Pre-Funded
Warrants to Purchase up to 15,151,515 Shares of Common Stock
Up
to 15,151,515 Shares of Common Stock underlying such Pre-Funded Warrants
Up
to 757,576 Shares of Common Stock Underlying the Placement Agent Warrants

PRELIMINARY
PROSPECTUS
Dawson James Securities, Inc.
,
2024
PART
II
INFORMATION
NOT REQUIRED IN PROSPECTUS
Item
13. Other Expenses of Issuance and Distribution.
The
following table sets forth an estimate of the fees and expenses payable by the registrant in connection with the issuance and distribution
of the securities being registered. All amounts are estimated except the SEC registration filing fee. All of the expenses below will
be paid by us.
| SEC registration fee | |
$ | 765.50 | |
| FINRA filing fee | |
$ | 1,250.00 | |
| Accounting fees and expenses | |
$ | | |
| Legal fees and expenses | |
$ | | |
| Transfer agent and registrar fees | |
$ | * | |
| Printing and engraving expenses | |
$ | * | |
| Miscellaneous | |
$ | * | |
| Total | |
$ | | |
| * |
These
fees cannot be estimated at this time as they are calculated based on the securities offered and the number of issuances. An estimate
of the aggregate expenses in connection with the sale and distribution of the securities being offered will be included in the applicable
prospectus. |
Item
14. Indemnification of Directors and Officers.
Under
our Bylaws, each of our directors has the right to be indemnified by us to the maximum extent permitted by law against (i) reasonable
expenses incurred in connection with any threatened, pending or completed civil, criminal, administrative, investigative or arbitrative
action, suit or proceeding seeking to hold the director liable by reason of his or her actions in such capacity and (ii) reasonable payments
made by the director in satisfaction of any judgment, money decree, fine, penalty or settlement for which he or she became liable in
such action, suit or proceeding. This right to indemnification includes the right to the advancement of reasonable expenses by us, to
the maximum extent permitted by law. Under our Bylaws, each of our officers who are not directors is entitled to the same indemnification
rights, including the right to the advancement of reasonable expenses, which are provided to our directors.
Pursuant
to the Florida Business Corporation Act, a Florida corporation has the power to indemnify its directors and officers provided that they
act in good faith and reasonably believe that their conduct was lawful and in the corporate interest (or not opposed thereto), as set
forth in the Business Corporation Act. Under the Business Corporation Act, unless limited by its articles of incorporation, a corporation
must indemnify a director or officer who is wholly successful, on the merits or otherwise, in the defense of any proceeding to which
he or she was a party because he or she is or was a director or officer, against reasonable expenses incurred by the director or officer
in connection with the proceeding. Our Articles of Incorporation do not contain any such limitations. The Business Corporation Act permits
a corporation to pay for or reimburse reasonable expenses in advance of final disposition of an action, suit or proceeding only upon
(i) the director’s certification that he or she acted in good faith and in the corporate interest (or not opposed thereto), (ii)
the director furnishing a written undertaking to repay the advance if it is ultimately determined that he or she did not meet this standard
of conduct, and (iii) a determination is made that the facts then known to those making the determination would not preclude indemnification
under the Business Corporation Act.
Under
our Articles of Incorporation, no director will be liable to us or our shareholders for monetary damages for breach of his or her fiduciary
duty as a director, to the maximum extent permitted by law.
The
Florida Business Corporation Act also empowers a corporation to provide insurance for directors and officers against liability arising
out of their positions, even though the insurance coverage may be broader than the corporation’s power to indemnify. We maintain
directors’ and officers’ liability insurance for the benefit of our directors and officers.
In
our employment agreements with Michael Redmond, our President, and Janet Huffman, our Chief Financial Officer, we agreed to indemnify
them for all claims arising out of performance of their duties, other than those arising out of their breach of the agreement or their
gross negligence or willful misconduct.
At
present, there is no pending litigation or proceeding involving any of the registrant’s directors or executive officers as to which
indemnification is being sought nor is the registrant aware of any threatened litigation that may result in claims for indemnification
by any executive officer or director.
The
Placement Agency agreement, if any, entered into with respect to an offering of securities registered hereunder will provide for indemnification
by any Placement Agents of any offering, our directors and officers who sign the registration statement and our controlling persons for
some liabilities, including liabilities arising under the Securities Act.
Item
15. Recent Sales of Unregistered Securities.
In
connection with a public offering the Company conducted in February 2024, the Company issued to ThinkEquity LLC, as representative of
the underwriters in such offering, and certain of its designees, a warrant, exercisable one hundred eighty (180) days after February
27, 2024 and expiring on February 27, 2029, to purchase up to 5% of the Shares sold in the Offering, equating to 70,000 Shares
of Common Stock at an exercise price of $1.875 per share. The Representative’s Warrants were not
registered under the Securities Act and were instead offered pursuant to the exemption provided in Section 4(a)(2) under the Securities
Act and Rule 506(b) promulgated thereunder.
On
December 23, 2023, in connection with the closing of the purchase of the Neurology Assets from Odyssey Health, Inc., and in partial consideration
for such assets, the Company issued 8 million shares of convertible Series F Preferred Stock to Odyssey. The
Series F Preferred Stock was not registered under the Securities Act and was instead offered pursuant to the exemption provided in Section
4(a)(2) under the Securities Act and Rule 506(b) promulgated thereunder.
On
August 4, 2023, the Company entered into a Securities Purchase Agreement (the “Purchase Agreement”) with two healthcare-focused
investors, pursuant to which the Company issued in a private placement an aggregate of (i) 404,728 shares of the Company’s Common
Stock and (ii) 404,728 shares of Series E Mirroring Preferred Stock (the “Series E Preferred Stock”). The
Common Stock and Series E Preferred Stock were not registered under the Securities Act and were instead offered pursuant to the exemption
provided in Section 4(a)(2) under the Securities Act and Rule 506(b) promulgated thereunder.
Item
16. Exhibits and Financial Statement Schedules.
| 10.10 |
|
Form of Employee Stock Option Agreement. + |
|
10-K |
|
001-32188 |
|
10.26 |
|
3/26/13 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.11 |
|
Form of Consultant Stock Option Agreement. + |
|
10-K |
|
001-32188 |
|
10.27 |
|
3/26/13 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.12 |
|
Form of Notice of Grant of Stock Options and Stock Option Award Agreement (Employee). + |
|
8-K |
|
001-32188 |
|
10.1 |
|
3/18/15 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.13 |
|
Form of Notice of Grant of Stock Options and Stock Option Award Agreement (Directors). + |
|
10-K |
|
001-32188 |
|
10.23 |
|
3/4/20 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.14 |
|
Form of Director Restricted Stock Award Agreement. + |
|
8-K |
|
001-32188 |
|
10.3 |
|
3/18/15 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.15 |
|
Executive Employment Agreement between the Company and Martin Handfield dated May 11, 2010. + |
|
10-Q |
|
001-32188 |
|
10.16 |
|
11/14/11 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.16 |
|
Executive Employment Agreement between the Company and Janet Huffman dated effective March 8, 2023. + |
|
8-K |
|
001-32188 |
|
10.1 |
|
3/8/23 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.17 |
|
Executive Employment Agreement for Mr. Redmond dated December 28, 2023 |
|
8-K |
|
001-32188 |
|
10.1 |
|
12/29/23 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.18 |
|
2021 Equity Incentive Plan+ |
|
8-K |
|
001-32188 |
|
10.1 |
|
2/28/22 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.19 |
|
First Amendment to 2021 Equity Incentive Plan |
|
8-K |
|
001-32188 |
|
4.2 |
|
12/15/23 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.20 |
|
Second Amendment to 2021 Equity Incentive Plan |
|
8-K |
|
001-32188 |
|
4.3 |
|
12/13/24 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.21 |
|
Form Stock Option Award Agreement (Directors)+ |
|
8-K |
|
001-32188 |
|
10.2 |
|
2/28/22 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.22 |
|
Form Stock Option Award Agreement (Employees)+ |
|
8-K |
|
001-32188 |
|
10.3 |
|
2/28/22 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.23 |
|
Form Stock Option Award Agreement (Consultants)+ |
|
8-K |
|
001-32188 |
|
10.4 |
|
2/28/22 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.24 |
|
Placement Agency Agreement dated June 25, 2024 between Oragenics, Inc. and Dawson James Securities, Inc. |
|
8-K |
|
001-32188 |
|
1.1 |
|
6/26/24 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.25 |
|
At-The-Market Issuance Sales Agreement between the Company and Ascendiant Capital Markets, LLC dated August 8, 2024 |
|
10-Q |
|
001-32188 |
|
10.1 |
|
8/9/24 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 21.1 |
|
Subsidiaries of Registrant |
|
10-K |
|
001-3288 |
|
21.1 |
|
3/24/22 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 23.1 |
|
Consent of Cherry Bekaert LLP, an Independent Public Accounting Firm |
|
|
|
|
|
|
|
|
|
X |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 23.2 |
|
Consent of CBIZ CPAs P.C., an independent public accounting firm. |
|
|
|
|
|
|
|
|
|
X |
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 24.1 |
|
Powers of Attorney (included on signature page). *** |
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 107 |
|
Filing Fee Table *** |
|
|
|
|
|
|
|
|
|
|
| |
* |
Confidential
treatment has been granted as to certain portions of this exhibit pursuant to Rule 406 of the Securities Act of 1933, as amended,
or Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
|
| |
|
|
| |
+ |
Executive
management contract or compensatory plan or arrangement. |
| |
|
|
| |
** |
Furnished
herewith and not deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the
“Exchange Act”), and shall not be deemed to be incorporated by reference into any filing under the Securities Act of
1933, as amended, or the Exchange Act (whether made before or after the date of the Form 10-Q), irrespective of any general incorporation
language contained in such filing. |
| |
|
|
| |
*** |
Previously filed. |
Item
17. Undertakings.
(1)
The undersigned registrant hereby undertakes:
a.
To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
i.
To include any prospectus required by Section 10(a)(3) of the Securities Act;
ii.
To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective
amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration
statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities
offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range
may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and
price represent no more than 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration
Fee” table in the effective registration statement; and
iii.
To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or
any material change to such information in the registration statement;
provided,
however, that paragraphs (1)(i), (1)(ii) and (1)(iii) above do not apply if the information required to be included in a post-effective
amendment by those paragraphs is contained in reports filed with or furnished to the Securities and Exchange Commission by the registrant
pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration
statement.
b.
That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be
a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed
to be the initial bona fide offering thereof.
c.
To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the
termination of the offering.
d.
That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant to Rule
424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other
than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the
date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus
that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration
statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such
first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration
statement or made in any such document immediately prior to such date of first use.
e.
That, for the purpose of determining liability of the registrant under the Securities Act to any purchaser in the initial distribution
of the securities, the undersigned registrant hereby undertakes that in a primary offering of securities of the undersigned registrant
pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities
are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to
the purchaser and will be considered to offer or sell such securities to such purchaser:
i.
Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule
424 (§ 230.424 of this chapter);
ii.
Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by
the undersigned registrant;
iii.
The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant
or its securities provided by or on behalf of the undersigned registrant; and
iv.
Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(2)
The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act, each filing of
the registrant’s annual report pursuant to Section 13(a) or Section 15(d) of the Exchange Act (and, where applicable, each filing
of an employee benefit plan’s annual report pursuant to Section 15(d) of the Exchange Act) that is incorporated by reference in
the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering
of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3)
The undersigned registrant hereby undertakes that:
a.
For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part
of this registration statement in reliance on Rule 430A and contained in a form of prospectus filed by the undersigned registrant pursuant
to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time
it was declared effective; and
b.
For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus
shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at
that time shall be deemed to be the initial bona fide offering thereof. (4) Insofar as indemnification for liabilities arising
under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing
provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy
as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities
(other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant
in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection
with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling
precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as
expressed in the Securities Act and will be governed by the final adjudication of such issue.
SIGNATURES
Pursuant
to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this registration statement to be signed
on its behalf by the undersigned, thereunto duly authorized in the City of Sarasota, State of Florida on December 31, 2024.
| |
ORAGENICS,
INC. |
| |
|
|
| |
By: |
/s/
Janet Huffman |
| |
|
Janet
Huffman |
| |
|
President
(Interim Principal Executive Officer) and Chief Financial Officer Interim (Principal Financial Officer) |
Pursuant
to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed below by the following persons
in the capacities indicated and on December 31, 2024.
| Signature |
|
Title |
| /s/
Janet Huffman |
|
President
(Interim Principal Executive Officer) and Chief Financial Officer Interim (Principal Financial Officer) |
| Janet
Huffman |
|
|
| |
|
|
| * |
|
|
| Charles
L. Pope |
|
Executive
Chairman and Director |
| |
|
|
| * |
|
|
| Robert
C. Koski |
|
Director |
| |
|
|
| * |
|
|
| Frederick
W. Telling |
|
Director |
| |
|
|
| * |
|
|
| Alan
W. Dunton |
|
Director |
| |
|
|
| * |
|
|
| John
Gandolfo |
|
Director |
| |
|
|
| * |
|
|
| Bruce
Cassidy |
|
Director |
| *By: |
/s/ Janet Huffman |
|
| |
Janet Huffman |
|
| |
Attorney-in-fact |
|
Exhibit
5.1
 |
|
Bank
of America Plaza 813.229.7600
101
East Kennedy Boulevard 813.229.1660 fax
Suite
2800
Tampa,
Florida 33602
_____________________________________________
www.shumaker.com |
December
31, 2024
Oragenics,
Inc.
1990
Main Street, Suite 750
Sarasota,
FL 34236
Re:
Oragenics, Inc.
Ladies
and Gentlemen:
We
are acting as counsel to Oragenics, Inc., a Florida corporation (the “Company”), in connection with the preparation
and filing of the in connection with the preparation and filing with the U.S. Securities and Exchange Commission (the “Commission”),
pursuant to the Securities Act of 1933, as amended (the “Securities Act”), of the Registration Statement on Form S-1 of the
Company (including all exhibits thereto, the “Registration Statement”), including a related prospectus filed with the Registration
Statement (the “Prospectus”) relating to the proposed public offering (the “Offering”) of (i) up to 15,151,515
units consisting of one share (the “Common Shares”) of the Company’s Common Stock, $0.001 par value per share (“Common
Stock”) (or one Pre-Funded Warrant to purchase one share of our Common Stock in lieu thereof) and one warrant (the “ Common
Warrant”) to purchase one share of Common stock (“collectively, the “Units”); (ii) Pre-Funded Warrants to purchase
up to 15,151,515 shares of Common Stock (the “Pre-Funded Warrants”); (iii) up to 15,151,515 shares of Common Stock issuable
from time to time upon exercise of the Common Warrants (the “Common Warrant Shares”); and (iv) ) up to 15,151,515 shares
of Common Stock issuable from time to time upon exercise of the Pre-Funded Warrants (the Pre-Funded Warrant Shares” and collectively
with the Common Warrant Shares, the “Warrant Shares” and collectively with the Units and Pre-Funded Warrants the “Securities”).
The Securities are to be sold to the several purchasers pursuant to securities purchase agreements (each, a “Securities Purchase
Agreement” and collectively, the “Securities Purchase Agreements”) among the Company and the purchasers’
signatory thereto (collectively, the “Purchasers”).
As
such counsel and for purposes of our opinions set forth herein, we have examined and relied upon originals or copies, certified or otherwise
identified to our satisfaction, of such documents, resolutions, certificates and other instruments of the Company and corporate records
furnished to us by the Company, and have reviewed certificates of public officials, statutes, records and such other instruments and
documents as we have deemed necessary or appropriate as a basis for the opinion set forth below, including without limitation (A) the
Registration Statement, Prospectus and the respective exhibits thereto, (B) the form of Securities Purchase Agreement and the Pre-funded
Warrants (collectively, the “Transaction Documents”); (C) the articles of incorporation, as amended and bylaws, as
amended of the Company; and (D) such agreements, instruments, resolutions of the board of directors of the Company and committees thereof
and other corporate records, and such other documents as we have deemed necessary or appropriate for the purpose of issuing this opinion
letter, and we have obtained from officers and other representatives and agents of the Company and from public officials, and have relied
(with your permission) upon, such certificates, representations and assurances, and public filings, as we have deemed necessary or appropriate.
In our examination, we have assumed: (i) the authenticity of original documents and the genuineness of all signatures; (ii) the conformity
to the originals of all documents submitted to us as copies; (iii) the truth, accuracy and completeness of the information, representations
and warranties contained in the instruments, documents, certificates and records we have reviewed; (iv) that the Warrants have been duly
authorized; and (v) the legal capacity for all purposes relevant hereto of all natural persons and, with respect to all parties to agreements
or instruments relevant hereto other than the Company, that such parties had the requisite power and authority (corporate or otherwise)
to execute, deliver and perform such agreements or instruments, that such agreements or instruments have been duly authorized by all
requisite action (corporate or otherwise), executed and delivered by such parties and that such agreements or instruments are the valid,
binding and enforceable obligations of such parties. As to any facts material to the opinions expressed herein that were not independently
established or verified, we have relied upon oral or written statements and representations of officers and other representatives of
the Company.
Based
upon and subject to the foregoing, we are of the opinion that:
| |
1. |
The
Common Shares have been duly authorized by the Company and if, when and to the extent any Common Shares are issued and sold in accordance
with all applicable terms and conditions set forth in, and in the manner contemplated by, the relevant Transaction Documents, and
as described in the Registration Statement and Prospectus (including payment in full of all consideration required for such Common
Shares), such Common Shares will be validly issued, fully paid and nonassessable; and |
| |
|
|
| |
2. |
The
Common Warrants have been duly authorized by the Company and if, when and to the extent any Common Warrants are issued and sold in
accordance with all applicable terms and conditions set forth in, and in the manner contemplated by, the relevant Transaction Documents
(including due and proper exercise of the relevant Common Warrant(s) in accordance with such Common Warrant(s) and payment in full
of all consideration required thereunder for such Common Warrant), and as described in the Registration Statement and Prospectus,
such Common Warrants will be validly issued, fully paid and nonassessable. |
| |
|
|
| |
3. |
The
Pre-funded Warrants have been duly authorized by the Company and if, when and to the extent any Pre-funded Warrants are issued and
sold in accordance with all applicable terms and conditions set forth in, and in the manner contemplated by, the relevant Transaction
Documents (including due and proper exercise of the relevant Pre-funded Warrant(s) in accordance with such Pre-funded Warrant(s)
and payment in full of all consideration required thereunder for such Pre-funded Warrant), and as described in the Registration Statement
and Prospectus, such Pre-funded Warrants will be validly issued, fully paid and nonassessable. |
We
are admitted to practice in the State of Florida. This opinion letter is limited to the laws of the State of Florida, as such laws presently
exist and to the facts as they presently exist. We express no opinion as to the effect of the law of any other jurisdiction. We
assume no obligation to revise or supplement this opinion letter should the laws of such jurisdictions be changed after the date hereof
by legislative action, judicial decision or otherwise. Without limiting the generality of the foregoing, we express no opinion with respect
to (i) state securities or “Blue Sky” laws or (ii) state or federal antifraud laws.
We
hereby consent to the inclusion of this opinion as Exhibit 5.1 to the Registration Statement in accordance with the requirements of Item 601(b)(5) of
Regulation S-K promulgated under the Securities Act and to the references to our firm therein and in the Prospectus under the caption
“Legal Matters.” In giving this consent, we do not thereby admit that we are an “expert” within the meaning of
the Securities Act of 1933, as amended.
| |
Very
truly yours, |
| |
|
| |
/s/
Shumaker, Loop & Kendrick, LLP |
| |
|
| |
SHUMAKER,
LOOP & KENDRICK, LLP |
Exhibit
23.1
Consent
of Independent Registered Public Accounting Firm
We
hereby consent to the incorporation by reference in this Registration Statements on Form S-1 of Oragenics, Inc. (the “Company”)
of the report dated March 28, 2024 relating to the consolidated financial statements of the Company and its subsidiaries as of and for
the year ended December 31, 2023 (which report includes an explanatory paragraph relating to a going concern uncertainty) appearing in
the Company’s Annual Report on Form 10-K for the year ended December 31, 2023, as filed with the Securities and Exchange Commission.
We also consent to the reference to us under the heading “Experts” in such Registration Statement.
| /s/
Cherry Bekaert, LLP. |
|
| |
|
| Tampa,
Florida |
|
| |
|
| December
31, 2024 |
|
Exhibit
23.2
CONSENT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We
consent to the incorporation by reference in this Pre-Effective Amendment No. 1 to the Registration Statement on Form S-1 and
related prospectus of our report dated April 17, 2023, with respect to the consolidated financial statements of Oragenics, Inc. (the
“Company”) as of December 31, 2022 and for the year then ended (which report includes an explanatory paragraph regarding
the existence of substantial doubt about the Company’s ability to continue as a going concern), included in the Annual Report on
Form 10-K for the year ended December 31, 2023, and to the reference to us under the heading “Experts” in the prospectus
which is part of this Registration Statement.
/s/
CBIZ CPAs P.C. 1
San
Diego, California
December
31, 2024
1 In
certain jurisdictions, CBIZ CPAs P.C. operates under its previous name, Mayer Hoffman McCann P.C.
Oragenics (AMEX:OGEN)
Gráfica de Acción Histórica
De Dic 2024 a Ene 2025
Oragenics (AMEX:OGEN)
Gráfica de Acción Histórica
De Ene 2024 a Ene 2025