Bausch Health Gets FDA Approval for Acne Treatment Cabtreo
20 Octubre 2023 - 5:05PM
Noticias Dow Jones
By Ben Glickman
Bausch Health Cos. has received approval from the Food and Drug
Administration for Cabtreo, its topical gel for acne.
The Laval, Quebec-based pharmaceutical company said the
treatment, which combines clindamycin phosphate, adapalene and
benzoyl peroxide, is expected to become available in the first
quarter of 2024.
The approved new drug application allows for the use of Cabtreo
in treating acne vulgaris in patients 12 years and older.
Write to Ben Glickman at ben.glickman@wsj.com
(END) Dow Jones Newswires
October 20, 2023 17:50 ET (21:50 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
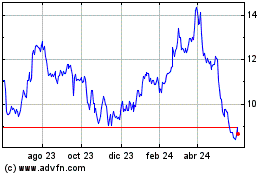
Bausch Health Companies (TSX:BHC)
Gráfica de Acción Histórica
De Abr 2024 a May 2024
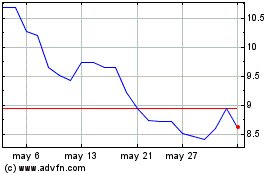
Bausch Health Companies (TSX:BHC)
Gráfica de Acción Histórica
De May 2023 a May 2024